Abstract
Melissa officinalis L. and Origanum majorana L., within Lamiaceae family, and Calendula officinalis L. and Achillea millefolium L., within the Asteraceae, have been considered a good source of bioactive ingredients with health benefits. In this study, the supercritical fluid extraction (SFE) using pure CO2, and the ultrasound assisted extraction (UAE) were proposed as green techniques to obtain plant-based extracts with potential antioxidant and anti-inflammatory activities. Higher values of total phenolic content and antioxidant activity were achieved in UAE ethanol:water (50:50, v/v) extracts. Meanwhile, UAE pure ethanol extracts showed greater anti-inflammatory activity. RP-HPLC-PAD-ESI-QTOF-MS/MS analysis showed a vast number of phenolic compounds in the extracts, including unreported ones. O. majorana ethanol:water extract presented the highest content of phenolics and antioxidant activity; among its composition, both rosmarinic acid and luteolin glucoside derivatives were abundant. The pure ethanol extract of A. millefolium resulted in an important content of caffeoylquinic acid derivatives, luteolin-7-O-glucoside and flavonoid aglycones, which could be related to the remarkable inhibition of TNF-α, IL-1β and IL-6 cytokines. Besides, borneol and camphor, found in the volatile fraction of A. millefolium, could contributed to this latter activity. Thus, this study points out that O. majorana and A. millefolium are considered a promising source of bioactive ingredients with potential use in health promotion.
Keywords: Achillea millefolium, Origanum majorana, anti-inflammatory activity, antioxidant activity, sustainable extraction, phenolic composition
1. Introduction
In the last decades, an increasing number of studies have focused on finding new sources of food ingredients for applications in pharmaceuticals, cosmetics, the food industry and, of course, nutrition [1]. Several compounds, such as phenolic compounds, terpenes, carotenoids, saponins and peptides are considered to possess interesting biological activities [2]. The first step in the development of food ingredients involves finding out the most suitable plant matrix to draw those bioactivities. In this regard, the Lamiaceae and Asteraceae families represent a great source of valued plant species. These species are often used as flavoring ingredients in many culinary preparations, but are also considered as medicinal herbs, where their beneficial effects have been mainly related to phenolic compounds [3,4].
Phenolic compounds, which are the secondary metabolites of plants, have been extensively tested as antibacterial, antitumor, antioxidant and anti-inflammatory agents [5]. Because of their structural characteristics, phenolic compounds are considered as natural antioxidant and anti-inflammatory chemicals which are able to avoid cellular damage related to oxidative stress. Moreover, phenolic compounds are considered to be capable of reducing the occurrence of many chronic pathologies and diseases, such as cardiovascular and neurodegenerative disease, and cancer [6].
Melissa officinalis L., commonly known as lemon balm, is a species of Lamiaceae typically used for the prevention and treatment of nervous disturbances and gastrointestinal disorders. Different extracts of M. officinalis have exhibited antioxidant, antimicrobial and anti-inflammatory activities [7,8]. In particular, hydroxycinnamic acids, such as rosmarinic acid and luteolin glycosylated derivatives, were found as the main phenolic constituents [9]. Origanum majorana L., also known as marjoram, is another species of Lamiaceae that has been appreciated because of its therapeutic properties against gastrointestinal, respiratory and neurological disorders. Extracts from marjoram, including its essential oil, have been reported to possess antimicrobial, antioxidant, anti-proliferative and anti-inflammatory activities [4,6]. Caffeic acid derivatives and flavonoids, along with ursolic acid, carvacrol and terpineol, have been associated with these biological activities [1,6].
The Asteraceae species Achillea millefolium L., usually known as yarrow, is consumed worldwide for the treatment of gastrointestinal disorders and hepatobiliary complaints, as well as for wound/ulcer healing and skin inflammation [10]. Essential oil from A. millefolium has been associated with antimicrobial and anti-inflammatory activities [3,11]. Moreover, alcoholic and water extracts have shown antioxidant and antitumor properties [12,13]. Caffeic acid derivatives, mainly caffeoylquinic acids and flavones, have been reported as parts of the yarrow’s composition [10]. Calendula officinalis L., (marigold) has been frequently used for healing skin diseases, wounds and duodenal ulcers [14,15]. Extracts of this Asteraceae species has been reported for their anti-inflammatory, antibacterial, antioxidant and antitumor activity [16,17]. Quercetin derivatives, mainly their glycosylated and methylated forms, have been described within the phenolic composition of marigold [18].
Nowadays, according to the sustainable development goals launched by the United Nations, the use of sustainable extraction techniques to obtain plant-based bioactive ingredients is essential [19]. For that purpose, extraction techniques run by the Green Chemistry principles should be used. Supercritical Fluid Extraction (SFE) has been widely used for the extraction of hydrophobic compounds in a more efficient and green environment [1]. SFE primarily uses CO2 as carrier, as well as a green and GRAS (Generally Recognized as Safe) solvent, which allows the recovery of high-quality extracts. Besides, SFE represents a minimizing solvent-consuming process due to CO2 recirculation. Ultrasound Assisted Extraction (UAE) applies ultrasonic vibrations generated at high frequencies to enhance the extraction of plant bioactives. Compared to conventional techniques, UAE has been considered as more efficient due to its solvent-reduction consumption and shorter-extraction times [20]. Therefore, minor solvent and energy waste are achieved. Methanol and/or its water mixtures have also been frequently used. Nevertheless, this toxic solvent should be replaced with other GRAS solvents, such as ethanol, to obtain high-quality food ingredients.
Therefore, this study focused on the use of advanced and sustainable extraction methodologies (SFE-CO2 and UAE using ethanol and water) to optimize bioactive secondary metabolite extraction from Melissa officinalis L. (MEL), Origanum majorana L. (MAJ), Achillea millefolium L. (MIL) and Calendula officinalis L. (CAL). Moreover, a screening of the antioxidant and anti-inflammatory activities, along with a comprehensive HPLC-PAD-ESI-QTOF-MS/MS and GC-MS analysis of the extracts, was carried out to correlate the composition and bioactivities.
2. Materials and Methods
2.1. Reagents
HPLC grade acetonitrile was obtained from Macron Fine Chemicals (Madrid, Spain) and formic acid (99%) from Acros Organics (Madrid, Spain). Pure ethanol (99.5%) was purchased from Panreac (Barcelona, Spain). 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH), 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), fluorescein, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), phorbol 12-myristate 13-acetate (PMA), and (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox, 97%) were purchased from Sigma-Aldrich (Madrid, Spain). Phenolic acids authentic standards (HPLC purity ≥ 95%) were purchased from Sigma-Aldrich, Phytolab (Madrid, Spain), Cymit Química SL (Madrid, Spain) and Extrasynthese S.A. (Genay, France) as detailed in Supplementary Material (Table S1).
2.2. Plant Material and Extraction
Origanum majorana L. (leaves), Melissa officinalis L. (leaves), Achillea millefolium L. (inflorescences and leaves), and Calendula officinalis L. (flowers) were purchased as dry raw material from a local herbal company (Murciana Herboristería, Murcia, Spain). The samples were ground at 9000 rpm for 60 s to diminish the particle size (Grindomix GM200, Retsch GmbH, Haan, Germany), preserved under vacuum and stored at room temperature until their use.
2.2.1. Ultrasound Assisted Extraction (UAE)
UAE plant extracts were obtained using a Branson 250 digital device (Branson Ultrasonics, Danbury, CT, USA) with an electric power of 200 W and frequency of 60 Hz. A corresponding solvent volume of ethanol or ethanol:water (50:50, v/v) was added to 35 g of ground plant material in a ratio of 1:10 (plant/solvent, w/v). The mixture was submitted to ultrasounds for 30 min and 70% amplitude using a probe of ½′ diameter. Next, samples were filtered, and ethanol was removed under vacuum at 30 °C (IKA RV 10, Madrid, Spain). Samples were freeze-dried when required. Dried extracts were maintained at −20 °C protected from light until use. Extractions were carried out in triplicate.
2.2.2. Supercritical Fluid Extraction (SFE)
SFE assays were carried out in a pilot-plant supercritical fluid extractor (Model SF2000, Thar Technology, Pittsburgh, PA, USA) equipped with a 2 L cylinder extraction cell. This cell was fully filled with plant material, i.e., 713 g for M. officinalis L., 550 g for O. majorana L., 500 g for C. officinalis L. and 383 g for A. millefolium L. To obtain the SFE extracts, a CO2 flow was established at 70 g/min at 140 bar and 40 °C. After the extraction process (180 min), a precipitated oleoresin-type extract was recovered from the extraction vessel with ethanol. Then, ethanol was removed under vacuum (30 °C) to obtain a final solid residue, which was kept at −20 °C until use. Extractions were conducted in triplicate.
2.3. Total Phenolic Content and Antioxidant Activity
The total phenolic content (TPC) was determined by the Folin-Ciocalteu colorimetric method, as described by Singleton et al. [21]. A calibration curve of gallic acid was used, and results are expressed as mg of gallic acid equivalents (GAE) per gram of extract.
To determine the antioxidant activity of the extracts, two methodologies were used. The ABTS•+ radical scavenging capacity was performed at four different concentrations of each extract, following the procedure described by Re et al. [22]. Results are expressed in mmol of Trolox equivalents/g of extract. The Oxygen Radical Absorbance Capacity (ORAC) assay was carried out according to Huang y col. [23]. The reaction occurred in a 96-well black round-bottom plate containing 150 µL of fluorescein stock solution (8 × 10−8, PBS 0.075 M), 25 µL of plant extract, PBS (blank samples) or Trolox solution (reference standard), and 25 µL of AAPH radical fresh solution (165.94 mM). Excitation and emission wavelength were set at 485 nm and 520 nm, respectively (Infinite M200, Tecan, Madrid, Spain), and the fluorescence intensity was recorded every 1 min at 37 °C until the value was <5% of the initial reading. Results are expressed as mmol of Trolox/g of extract. Analyses were done in triplicate.
2.4. Cell Culture and Anti-Inflammatory Activity
Human THP-1 monocytes (ATCC, Manassas, VA, USA) were maintained and cultured in supplemented RPMI 1640 media (Gibco, Paisley, UK) until confluence to perform the cytotoxicity and anti-inflammatory assays as described by Villalva et al. [24]. For differentiation of THP-1 monocytes to macrophages, cells were seeded in 24-well plates (5 × 105 cells/mL) with 100 ng/mL of PMA for 48 h. The cytotoxicity of the extracts was tested (10, 20 and 50 using µg/mL) on differentiated macrophages using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium Bromide (MTT). For anti-inflammatory assays, macrophages were incubated with or in absence of 0.05 µg/mL of bacterial lipopolysaccharide (LPS from E. coli O55:B5, Sigma-Aldrich, Madrid, Spain) in the presence of a non-cytotoxic extract concentration for 24 h. The secretion of the pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, was measured in the cell’s supernatants using ELISA assay kits (BD Biosciences, Aalst, Belgium), according to the manufacturer’s instructions. Results were expressed as mean of three determinations ± standard deviation.
2.5. RP-HPLC-PAD-ESI-QTOF-MS/MS Analysis
Mass spectrometric detection was performed using an HPLC system (1100 series, Agilent Technologies, Santa Clara, CA, USA) connected to an ultra-high-resolution QTOF instrument (MAXIS II, Bruker, Bremen, Germany). Electrospray ionization source was used in the negative mode for all the analyses, and the parameters were adjusted as follows: capillary voltage 3400 V, end plate offset 500 V, in-source Collision Induced Dissociation energy (isCID) 30 eV for MS/MS spectra. Nitrogen was used as the nebulizer gas (pressure of 4 Bar) and drying gas (heated to 250 °C, flow 8 L/min). For accurate high-resolution, mass spectrometry (HRMS) internal calibration was performed after each chromatographic run using a mixture of phosphazenes. The accurate obtained masses were processed using the elemental composition calculator incorporated in the data analysis software (Bruker, Bremen, Germany). Prior to mass detection, dry extracts were dissolved at 2.5 mg/mL in ethanol:water (60:40, v/v) and filtered using a 0.45 μm polyvinylidene fluoride (PVDF) filter. Separation was achieved in a reversed-phase ACE Excell 3 Super C18 column (150 mm × 4.6 mm, 3 μm, ACT, Aberdeen, Scotland) protected by a pre-column (ACE 3 C18-AR, 10 mm × 3 mm), as described by Villalva et al. [24], with slight modifications. Briefly, the chromatographic separation was performed using solvent A (water with 0.1% formic acid, v/v) and solvent B (pure acetonitrile), following the next gradient elution: 0–1 min, 5% B; 6 min, 15% B; 21 min, 25% B; 26 min, 35% B; 36–41 min, 50% B; 43–48 min, 100% B; 50 min, 5% B. Identification of phenolic compounds was performed by contrasting the accurate mass and MS/MS fragmentation pattern with the literature, and by comparison of its retention time and UV-Vis max absorption pattern with the available analytical standards. Phenolic compounds quantification was carried out in a HPLC-PAD system (1260 Infinity series, Agilent Technologies, Santa Clara, CA, USA) using a five-level calibration curve of authentic phenolics standards. Those compounds for which standards were not available were quantified with the calibration curve of that compound with the greater structural affinity, e.g., apigenin-7-O-glucoside was used for apigenin glycosylated derivative, and chlorogenic acid was used for caffeoylquinic acid derivative. In addition, ethyl gallate was used as internal standard. Analyses were carried out by triplicate, and the results are expressed as mg phenolic compound/g of extract.
2.6. GC-MS Identification
The volatile fraction of the extracts was determined by GC-MS in an Agilent Technologies 7890A system (Agilent Technologies, Santa Clara, CA, USA) coupled to a 5975C triple-axis mass spectrometer detector. The column used was a HP-5MS (5% Phenyl methyl siloxane, Agilent 19091S-433) and the chromatographic conditions for separation were followed as described by García-Risco et al. [25]. The components were identified based on their relative retention time and mass spectrum compared to the library data of the GC-MS system (NIST MS 2.0). Analyses were carried out in triplicate.
2.7. Statistical Analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Fisher’s least significance differences (LSD) test to discriminate among means (p < 0.05). To determine the correlation between the different experimental data, a Pearson test was carried out (p < 0.05). Statgraphics Centurion XVI (Statpoint Technologies Inc., Warrenton, VA, USA) software was used for this purpose.
3. Results
3.1. Evaluation of TPC and Antioxidant Activity of the Plant Extracts
Two different green extraction methodologies involving SFE and UAE were used in this study to obtain the bioactive compounds from the Lamiaceae and Asteraceae species, following the extraction conditions previously studied by our research group [25]. As shown in Table 1, the extraction yield was influenced by the solvent polarity regardless of the plant used. Overall, the SFE extracts demonstrated a lower mass recovery in comparison with the UAE. Moreover, within the UAE extracts, those obtained with pure ethanol achieved significantly inferior yield values than the extracts obtained with 50% ethanol.
Table 1.
Extraction yield, total phenolic content (TPC) and antioxidant activity of the plant extracts, obtained by supercritical fluids extraction (SFE) and ultrasound assisted extraction (UAE) techniques.
| MEL | MAJ | CAL | MIL | ||
|---|---|---|---|---|---|
| Yield 1 | SFE | 0.6 ± 0.1 C d | 1.5 ± 0.2 C b | 2.8 ± 0.2 C a | 0.8 ± 0.1 C c |
| UAE-100 | 4.2 ± 0.2 B c | 5.9 ± 0.4 B a | 6.7 ± 0.7 B a | 5.1 ± 0.1 B b | |
| UAE-50 | 22.7 ± 0.6 A b | 15.6 ± 0.1 A c | 27.9 ± 0.6 A a | 14.8 ± 0.1 A d | |
| TPC 2 | SFE | 16 ± 1 C b | 56 ± 1 C a | 13 ± 1 C c | 14 ± 2 B bc |
| UAE-100 | 70 ± 1 B c | 148 ± 3 B a | 36 ± 1 B d | 111 ± 2 A b | |
| UAE-50 | 112 ± 2 A b | 247 ± 5 A a | 82 ± 2 A d | 106 ± 3 A c | |
| TEAC 3 | SFE | 0.03 ± 0.01 Cc | 0.64 ± 0.02 C a | 0.05 ± 0.02 B c | 0.08 ± 0.01 C b |
| UAE-100 | 0.24 ± 0.01 B c | 0.71 ± 0.01 B a | 0.06 ± 0.01 B d | 0.29 ± 0.03 B b | |
| UAE-50 | 0.71 ± 0.02 A b | 1.46 ± 0.03 A a | 0.33 ± 0.02 A d | 0.52 ± 0.04 A c | |
| ORAC 4 | SFE | 0.76 ± 0.01 C b | 1.59 ± 0.04 C a | 0.38 ± 0.02 C c | 0.73 ± 0.02 C b |
| UAE-100 | 0.94 ± 0.03 B c | 2.57 ± 0.14 B a | 0.48 ± 0.01 B d | 1.86 ± 0.11 B b | |
| UAE-50 | 2.71 ± 0.12 A b | 5.17 ± 0.09 A a | 1.32 ± 0.10 A d | 2.16 ± 0.02 A c |
1 Yield expressed in percentage (%). 2 TPC as mg GAE/g extract. 3 TEAC (Trolox Equivalent Antioxidant Capacity) as mmol Trolox/g extract. 4 ORAC (Oxygen Radical Absorbance Capacity) as mmol Trolox/g extract. A–D Different superscript letters denote significant differences within a column (p < 0.05). a–c Different subscript letters denote significant differences within a line (p < 0.05). MEL, Melissa officinalis. MAJ, Origanum majorana. MIL, Achillea millefolium. CAL, Calendula officinalis.
Two complementary assays, ABTS radical scavenging and ORAC, were used to evaluate the antioxidant activity of the SFE and UAE extracts (Table 1). Overall, the solvent polarity seemed to exert a clear influence on the antioxidant activity despite the plant matrix. Thus, an increment in the solvent polarity allowed us to obtain extracts with higher antioxidant activity. In this context, the greatest TEAC and ORAC values were achieved using 50% ethanol (UAE-50), followed by 100% ethanol (UAE-100) extracts. In contrast, SFE extracts, carried out with pure CO2, presented a limited antioxidant activity. Moreover, both the UAE-50 extracts from Lamiaceae species, MAJ and MEL, presented a higher antioxidant activity than those from the Asteraceae species, highlighting O. majorana as the most effective and C. officinalis as the least effective.
A similar behavior was found regarding the TPC, as can be observed in Table 1. For all the plants studied, the extracts obtained by UAE presented higher TPC values than those obtained by SFE. Moreover, the UAE-50 extracts exhibited a significantly higher recovery of phenolic compounds (mg GAE/g extract) compared to 100% ethanol, as reported by Rodríguez-Pérez et al. [20] regarding the UAE plant extracts. Therefore, we confirmed the close correlation between the total content of TPC and the antioxidant activity (TEAC or ORAC value) (Supplementary Material, Figure S1). Moreover, despite of the extraction technique and the solvent used, the greatest TPC value corresponded to MAJ, while the lowest was found in CAL.
Overall, within the studied extracts, the Lamiacea species exhibited a greater performance regarding the TPC and antioxidant activity properties. The radical scavenging capacities of extracts from O. majorana and M. officinalis have already been related to their high phenolic content [9,26].
3.2. Anti-Inflammatory Activity of the Plant Extracts
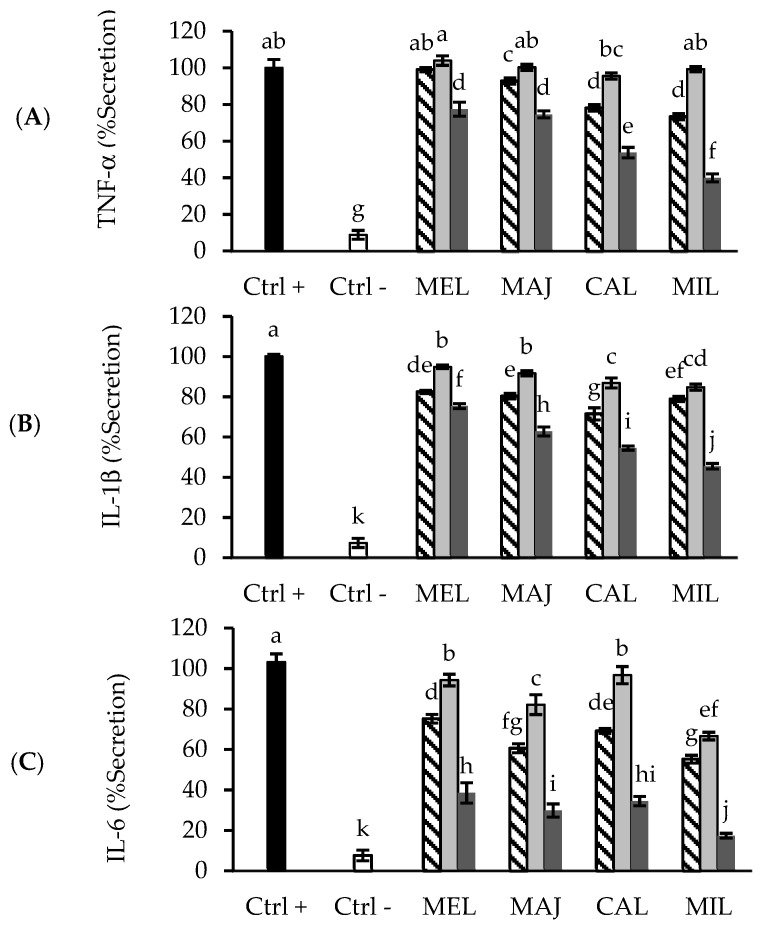
To evaluate the anti-inflammatory activity of the Lamiaceae and Asteraceae species, LPS-activated THP-1 macrophages were exposed to 10 μg/mL of SFE and UAE extracts, which represented a non-cytotoxic concentration (data not shown). A significant release of the studied proinflammatory cytokines was observed in the stimulated THP-1 macrophages (positive control) in contrast to the non-stimulated cells (negative control) (Figure 1).
Figure 1.
Levels of (A) TNF-α, (B) IL-1β and (C) IL-6 secreted by THP-1 macrophages, activated with LPS, in presence of 10 μg/mL of SFE (stripped bars), UAE-50 (light grey bars) and UAE-100 (dark grey bars) plant extracts. Ctrl+, positive control (cells stimulated with LPS without extract). Ctrl −, negative control (cells non-stimulated). MEL, Melissa officinalis L., MAJ, Origanum majorana L., CAL, Calendula officinalis L, MIL, Achillea millefolium L. Each bar is the mean of three determinations ± SD. a–k Different letters indicate statistical differences among LSD procedure (p < 0.05).
The addition of plant extracts from both techniques, SFE or UAE, resulted in dissimilar results, regarding the release inhibition of the three cytokines studied. Thus, the most relevant inhibition of TNF-α was observed in presence of the UAE-100 extracts, for all plant species (Figure 1A). Moreover, the highest inhibition was exhibited by MIL UAE-100 followed by CAL UAE-100, demonstrating a reduction approx. 60% and 50%, respectively, compared with the positive control. However, none of the UAE-50 extracts inhibited this cytokine secretion. Concerning SFE extracts, only CAL and MIL presented a secretion reduction of approx. 30%.
The obtained results for IL-1β release in presence of plant extracts (Figure 1B) were similar to those obtained for TNF-α, since UAE-100 extracts were the most active. In particular, MIL UAE-100 produced an important decrease in the IL-1β secretion (approx. 55%) compared with the positive control. Finally, UAE-100 extracts also exhibited an important inhibition of IL-6 (Figure 1C), especially MIL UAE-100 demonstrated approx. an 80% inhibition. In addition, for this cytokine, all SFE extracts presented a moderate inhibition. Overall, these results bring to light the higher anti-inflammatory potential of the studied Asteraceae species compared to the Lamiaceae ones, mainly UAE-100 extracts.
According to the above results, a trend for proinflammatory cytokines inhibition could be delineated, i.e., UAE-100 > SFE > UAE 50 extracts. Thus, contrary to the antioxidant activity, the anti-inflammatory activity of these extracts was not completely related to their TPC. Therefore, the observed anti-inflammatory properties of the extracts could be linked to the presence of specific phenolic compounds [14]. However, the contributions of other types of compounds cannot be discarded, since the anti-inflammatory effect of terpenes, presented in the essential oil of these plants, has been described [4,11].
3.3. RP-HPLC-PAD-ESI-QTOF-MS/MS Identification and RP-HPLC-PAD Quantification of Selected UAE Extracts
As mentioned previously, a close relationship between TPC and antioxidant activity has been established. Nevertheless, to better understand which specific compounds are behind this activity, a comprehensive analysis of such extracts with a remarkable activity, i.e., UAE-50 extracts, was carried out. Although several studies regarding the phenolic composition of the four studied plants have already published, in this study, 146 compounds were tentatively identified, of which 26 compounds have not been previously described for these plant matrices. Figure S2 shows the base peak chromatogram of the A. millefolium UAE-50 extract, where each peak is numbered according to its elution order. Furthermore, Table 2 shows the overall results of the retention time (Rt), theoretical mass (m/z), accurate mass (m/z) and MS/MS fragmentation patterns of all identified compounds, i.e., organic acids, phenolic acids (hydroxybenzoic and hydroxycinnamic acids and their derivatives), flavonoids (flavones, flavonols, flavanones and their derivatives), other polar compounds and saponins.
Table 2.
Compounds identified by HPLC-PAD-ESI-QTOF-MS/MS (negative ionization mode) in the selected UAE 50% ethanol extracts from the Lamiaceae and Asteraceae genera.
| Peak No. | Rt (min) | Compound | Theoretical Mass (m/z) | Accurate Mass (m/z) | MS/MS Product Ions (m/z) | Extract |
|---|---|---|---|---|---|---|
| Organic acids | ||||||
| 1 | 2.5 | Gluconic acid | 195.0510 | 195.0511 | 129 (100) | M, O, C, A |
| 2 | 3.0 | Quinic acid | 191.0561 | 191.0564 | 127 (100), 111 (30) | M, O, C, A |
| 3 | 3.3 | Tartaric acid | 149.0092 | 149.0093 | 72 (100) | M, O |
| 4 | 3.5 | Malic acid | 133.0142 | 133.0144 | 115 (100) | M, O, C, A |
| 5 | 3.7 | Citric acid | 191.0197 | 191.0192 | 111 (100), 87 (40) | O, C, A |
| 6 | 4.3 | Isocitric acid | 191.0197 | 191.0192 | 111 (100), 87 (40) | O |
| 7 | 5.1 | Succinic acid | 117.0930 | 117.0950 | 73 (100) | M |
| Hydroxybenzoic acids and derivatives | ||||||
| 9 | 5.3 | Gallic acid * | 169.0142 | 169.0144 | 125 (100) | M, O |
| 10 | 5.4 | Protocatechuic acid pentoside | 285.0616 | 285.0622 | 153 (100), 109 (80) | C |
| 11 | 5.5 | 3,4-dihydroxyphenillactic acid—hexoside | 359.0984 | 359.0987 | 197 (100), 179 (60) | M, O |
| 13 | 5.7 | Dihydroxybenzoic acid hexoside | 315.0722 | 315.0721 | 153 (98), 109 (80) | M, O, A |
| 15 | 6.1 | 3,4-dihydroxyphenil-lactic acid | 197.0455 | 197.0456 | 179 (80), 135 (100) | M, O |
| 17 | 6.4 | Hydroxybenzoic acid hexoside | 299.0772 | 299.0774 | 137 (100) | M, O, C |
| 20 | 8.2 | Protocatechuic acid * | 153.0193 | 153.0197 | 109 (100) | M, O, A |
| 49 | 19.4 | Syringic acid derivative | 313.0565 | 313.0572 | 197 (100), 121 (25) | O, C |
| Hydroxyccinamic acids and derivatives | ||||||
| 12 | 5.6 | Vainillic acid hexoside | 329.0772 | 329.0767 | 167(100), 152 (20) | O |
| 14 | 6.0 | Hydroxyferulic acid hexoside isomer I | 371.0620 | 371.0627 | 209 (100), 191 (55) | C |
| 16 | 6.2 | Hydroxyferulic acid hexoside isomer II | 371.0620 | 371.0627 | 209 (100), 191 (40) | C |
| 18 | 6.8 | Neochlorogenic acid * | 353.0878 | 353.0877 | 191 (100), 179 (76), 135 (40) | O, C, A |
| 19 | 7.1 | Hydroxyferulic acid hexoside isomer III | 371.0620 | 371.0627 | 209 (100), 191 (25) | C |
| 21 | 8.5 | Caftaric acid isomer | 311.0409 | 311.0410 | 179 (14), 149 (100) | A |
| 22 | 8.6 | Caffeic acid dihexoside | 503.1406 | 503.1410 | 179 (14), 149 (100) | M |
| 23 | 8.8 | Caftaric acid * | 311.0409 | 311.0410 | 179 (14), 149 (100) | M, A |
| 25 | 9.5 | Caffeoylquinic acid isomer I | 353.0878 | 353.0877 | 191 (100), 179 (20) | A |
| 26 | 9.6 | Caffeic acid hexoside I | 341.0878 | 341.0882 | 179 (100) 135 (60) | M, C |
| 27 | 9.8 | Coumaric acid hexoside | 325.0929 | 325.0934 | 163 (100), 119 (20) | M, O |
| 28 | 10.2 | Chlorogenic acid * | 353.0878 | 353.0877 | 191 (100), 161 (10) | C, A |
| 29 | 10.3 | Cryptochlorogenic acid * | 353.0878 | 353.0877 | 191 (100), 161 (11) | O, C, A |
| 30 | 10.8 | Caffeic acid hexuronide | 355.0671 | 355.0678 | 191 (100) | C |
| 32 | 13.9 | Caffeic acid hexoside II | 341.0878 | 341.0882 | 179 (100), 135 (65) | M, O |
| 33 | 14.1 | Coumaric acid pentoside | 295.0459 | 295.0464 | 163 (80), 119 (63) | M |
| 34 | 14.3 | Coumaroylquinic acid | 337.0929 | 337.0936 | 191 (100) | C |
| 35 | 14.5 | Caffeoylshikimic acid | 335.0772 | 335.0780 | 179 (100), 135 (77) | C |
| 36 | 14.8 | Caffeoylquinic acid isomer II | 353.0878 | 353.0879 | 191 (100), 161 (10) | C, A |
| 37 | 15.2 | Feruloyl tartaric acid | 325.0565 | 325.0571 | 193, 161, 134 | M |
| 39 | 15.8 | Caffeic acid * | 179.0350 | 179.0353 | 135 (100) | M, O, C, A |
| 46 | 18.8 | Yunaneic acid E isomer I | 571.1093 | 571.1099 | 329 (31), 197 (100), 179 (24), 135 (39) | M |
| 48 | 19.2 | Yunaneic acid E isomer II | 571.1093 | 571.1099 | 329 (31), 197 (100), 179 (24), 135 (39) | M |
| 50 | 19.7 | Feruloylquinic acid | 367.1035 | 367.1042 | 191 (100), 173 (40) | C |
| 54 | 20.5 | Salvianolic acid I | 537.1038 | 537.1043 | 493 (25), 339(100), 295(22), 197(60) | M, O |
| 64 | 21.9 | Yunnaneic acid D isomer | 539.1195 | 539.1198 | 197 (80), 135 (60) | M |
| 76 | 24.9 | Rosmarinic acid hexoside | 521.1301 | 521.1305 | 359 (21), 197 (100), 179 (12), 161 (29) | M, O |
| 81 | 26.5 | Chicoric acid | 473.0725 | 473.0730 | 311 (50), 179 (80), 149 (100), 135 (10) | M |
| 82 | 26.9 | 3,4-dicaffeoylquinic acid * | 515.1195 | 515.1189 | 353 (100), 335 (30), 179 (69), 173 (80) | A |
| 86 | 28.0 | 1,5-dicaffeoylquinic acid * | 515.1195 | 515.1190 | 353 (100), 191 (40) | C, A |
| 88 | 28.1 | Sagerinic acid | 719.1618 | 719.1619 | 539 (20), 521 (7), 359 (100), 197 (20) | M |
| 89 | 28.3 | 3,5-dicaffeoylquinic acid * | 515.1195 | 515.1190 | 353 (100), 191 (55), 179 (35), 135 (21) | C, A |
| 90 | 28.4 | Salvianolic acid E isomer | 717.1456 | 717.1463 | 537 (28), 519 (100), 339 (88), 295 (22) | M, O |
| 95 | 28.9 | Isorosmarinic acid | 359.0772 | 359.0771 | 197 (35), 179 (30), 161 (100), 135 (20) | M |
| 98 | 29.4 | 4,5-dicaffeoylquinic acid * | 515.1195 | 515.1190 | 353 (100), 191 (10), 179 (30), 173 (40) | C, A |
| 101 | 30.1 | Rosmarinic acid * | 359.0772 | 359.0771 | 197 (80), 179 (50), 161 (100), 135 (30) | M, O |
| 104 | 30.5 | Lithospermic acid * | 537.1038 | 537.1041 | 493 (44), 359 (43), 295 (93), 161 (100) | O |
| Hydroxyccinamic acids and derivatives (continued) | ||||||
| 105 | 30.7 | Lithospermic acid isomer I | 537.1038 | 537.1041 | 493 (24), 295 (80), 197 (18), 161 (90) | M, O |
| 106 | 30.8 | Salvianolic acid B * | 717.1461 | 717.1445 | 519 (30), 359 (100), 295 (10), 179 (10) | M, O |
| 109 | 31.5 | Dicaffeoylquinic acid isomer | 515.1195 | 515.1191 | 353 (100), 191 (15),179 (35), 173 (30) | C, A |
| 110 | 31.7 | Lithospermic acid isomer II | 537.1038 | 537.1040 | 493 (32), 359 (30), 295 (100) | M, O |
| 111 | 31.9 | Feruloyl-O-caffeoylquinic acid | 529.1351 | 529.1350 | 367 (100), 193 (55), 191 (22) | A |
| 112 | 32.2 | Salvianolic acid L isomer I | 717.1461 | 717.1464 | 519 (100), 359 (30), 339 (15), 149 (18) | M, O |
| 113 | 32.4 | Sagecoumarin caftaride | 829.1258 | 829.1260 | 667 (88), 535 (80), 311 (50), 135 (47) | M |
| 114 | 32.5 | Salvianolic acid L hydroxycaffeide | 895.1730 | 895.1728 | 519 (77), 369 (73), 161 (100) | M |
| 115 | 32.6 | Salvianolic acid A isomer | 493.1140 | 493.1145 | 359 (100), 295 (10), 197 (20), 161 (39) | M, O |
| 116 | 32.9 | Methylrosmarinic acid | 373.0929 | 373.0932 | 179 (100), 161 (20), 135 (82) | M |
| 118 | 33.4 | Sagecoumarin isomer | 535.0882 | 535.0886 | 359 (8), 197 (10), 177 (100), 161 (19) | M, O |
| 119 | 33.7 | Tricaffeoylquinic acid | 677.1512 | 677.1499 | 515 (100), 353 (68) | C, A |
| 120 | 33.7 | Salvianolic acid C derivative | 715.1305 | 715.1304 | 535 (100), 491 (11), 311 (9), 135 (7) | M |
| 123 | 34.4 | Salvianolic acid L isomer II | 717.1461 | 717.1462 | 519 (100), 339 (13) | M, O |
| 128 | 36.3 | Rosmarinic acid derivative I | 565.1351 | 565.1353 | 359 (60), 197 (28), 179 (20), 161 (100) | M, O |
| 130 | 37.9 | Rosmarinic acid derivative II | 565.1351 | 565.1353 | 359 (100), 197 (30), 179 (13), 135 (36) | M |
| 136 | 39.7 | Salvianolic acid C isomer | 491.0984 | 491.0986 | 267 (16), 179 (100), 161 (21) | M, O |
| 139 | 40.0 | Salvianolic acid F isomer | 313.0718 | 313.0720 | 161 (100) | M |
| 140 | 40.2 | Rosmarinic acid derivative III | 565.1351 | 565.1353 | 359 (94), 197 (24), 179 (20), 161 (100) | M, O |
| 143 | 42.7 | Salvianolic acid C caffeoylhydroxycaffeide | 849.1672 | 849.1674 | 359 (100), 179 (6), 161 (20), 135 (40) | M |
| Flavone derivatives | ||||||
| 24 | 9.3 | Luteolin 6,8-C-dihexoside | 609.1461 | 609.1453 | 489 (100), 325 (40) | O, A |
| 31 | 13.7 | Vicenin 2 * | 593.1512 | 593.1507 | 473 (100) | O, A |
| 38 | 15.5 | Apigenin-hexoside-pentoside I | 563.1406 | 563.1401 | 473 (10), 443 (20) | A |
| 40 | 17.9 | Schaftoside isomer | 563.1406 | 563.1401 | 473 (10), 443 (20) | A |
| 41 | 18.1 | Schaftoside * | 563.1406 | 563.1401 | 473 (10), 443 (20) | O, A |
| 42 | 18.2 | Luteolin-C-hexoside | 447.0933 | 447.0925 | 357 (38), 327 (100) | O |
| 44 | 18.4 | Luteolin diglucuronide | 637.1046 | 637.1042 | 285(100) | O, A |
| 45 | 18.8 | Homoorientin * | 447.0933 | 447.0930 | 429 (30), 357 (100), 327 (80) | A |
| 47 | 19.2 | Luteolin-hexoside-hexuronide | 623.1254 | 623.1246 | 447 (90), 285 (100), 112 (60) | A |
| 51 | 19.7 | Apigenin-hexoside-pentoside II | 563.1406 | 563.1401 | 473 (10), 443 (20) | A |
| 52 | 19.8 | Luteolin dihexoside I | 609.1461 | 609.1451 | 447 (100), 357 (26), 327 (70), 285 (10) | A |
| 53 | 20.2 | 6-Hydroxyluteolin-7-O-glucoside | 463.0882 | 463.0880 | 301 (100) | O, A |
| 56 | 20.7 | Luteolin hexoside | 447.0871 | 447.0873 | 285 (100) | O |
| 55 | 20.8 | Apigenin dihexoside | 593.1512 | 593.1511 | 269 (100) | A |
| 62 | 21.8 | Luteolin dihexoside II | 609.1745 | 609.1750 | 447 (20), 285(10) | O, A |
| 63 | 21.9 | Luteolin rutinoside | 593.1432 | 593.1430 | 285 (100) | O |
| 70 | 24.0 | Apigenin deoxylhexoside | 577.1563 | 577.1557 | 269 (100) | A |
| 72 | 24.4 | Luteolin-O-glucoside | 447.0931 | 447.0932 | 285 (100), 151 (20) | O |
| 73 | 24.5 | Apigenin glycosylated derivative | 445.1140 | 445.1136 | 269 (100) | A |
| 74 | 24.8 | Luteolin-7-O-β-glucoside * | 447.0933 | 447.0928 | 285 (100) | M, O, A |
| 77 | 25.2 | Luteolin-7-O-glucuronide * | 461.0725 | 461.0722 | 285 (100) | O, A |
| 78 | 25.4 | Apigenin hexoside | 431.0928 | 431.0928 | 269 (100) | O |
| 91 | 28.6 | Diosmin * | 607.1663 | 607.1668 | 607 (10), 299 (100), 284 (10) | O |
| 93 | 28.7 | Luteolin acetylglucoside | 489.0970 | 489.0973 | 447 (30), 285 (100) | O |
| 94 | 28.8 | Apigenin-7-O-glucoside * | 431.0984 | 431.0980 | 269 (100) | O, A |
| 96 | 29.0 | Luteolin-O-malonylglucoside | 533.0937 | 533.0931 | 489 (100), 285 (15) | A |
| 97 | 29.4 | Apigenin-7-O-glucuronide * | 445.0776 | 445.0768 | 269 (100) | O |
| 100 | 29.8 | Apigenin-O-hexuronide | 445.0776 | 445.0775 | 269 (100) | A |
| 102 | 30.3 | Luteolin-O-hexuronide | 461.0725 | 461.0727 | 285 (100) | M |
| 121 | 34.1 | Luteolin dimer | 569.0725 | 569.0718 | 285 (100), 112 (80) | A |
| 122 | 34.8 | Luteolin * | 285.0405 | 285.0400 | 175 (80), 151 (100), 107 (51) | M, O, A |
| 132 | 38.1 | Apigenin * | 269.0455 | 269.0454 | 112 (100) | O, A |
| 137 | 39.7 | Diosmetin * | 299.0561 | 299.0554 | 112 (100) | A |
| 138 | 39.8 | Trihydroxy dimethoxyflavone I | 329.0667 | 329.0665 | 314 (20), 299 (100) | O |
| 141 | 40.6 | Trihydroxy dimethoxyflavone II | 329.0667 | 329.0665 | 299 (100) | A |
| 144 | 43.1 | Methoxyacacetin | 313.0718 | 313.0716 | 283 (100), 112 (60) | O, A |
| 145 | 43.4 | Dihydroxy trimethoxyflavone | 343.0823 | 343.0820 | 328 (100), 313 (20) | O, A |
| Flavonol derivatives | ||||||
| 43 | 18.2 | Quercetin-3-O-rhmanosylrutinoside | 755.2040 | 755.2052 | 301 (100), 271 (23) | C |
| 57 | 20.8 | Quercetin-3-neohesperidoside | 609.1461 | 609.1468 | 301 (100) | C |
| 58 | 21.0 | Isorhamnetin-3-O-rhamnosylrutinoside* | 769.2197 | 769.224 | 315 (100), 300 (20) | C |
| 60 | 21.4 | Quercetin hexoside I | 463.0882 | 463.0880 | 301 (100) | A |
| 61 | 21.7 | Quercetin-O-pentosylhexoside | 595.1305 | 595.1310 | 301 (100) | C |
| 65 | 22.9 | Rutin * | 609.1097 | 609.1093 | 301 (100) | C, A |
| 66 | 23.1 | Isovitexin | 431.0984 | 431.0981 | 311 (100) | A |
| 67 | 23.2 | Vitexin * | 431.0984 | 431.0981 | 311 (100) | A |
| 68 | 23.4 | Quercetin-malonylhexosyl-rhamnoside | 695.1465 | 695.1472 | 651 (100), 301 (23) | C |
| 71 | 24.1 | Isorhamnetin-3-O-neohesperoside | 623.1618 | 623.1621 | 315 (100), 300 (10) | C |
| 75 | 24.9 | Quercetin hexoside II | 463.0882 | 463.0889 | 301 (100) | C |
| 79 | 25.5 | Quercetin hexuronide | 477.0675 | 477.0671 | 301 (100) | A |
| 80 | 25.9 | Kaempferol-3-O-rutinoside * | 593.1512 | 593.1521 | 285 (100) | C |
| 83 | 27.4 | Ishoramnetin-3-O-rutinoside * | 623.1618 | 623.1627 | 315 (100) | C |
| 84 | 27.7 | Quercetin-3-O-acetyl-glucoside | 505.0988 | 505.0996 | 463 (30), 301 (100) | C |
| 85 | 27.8 | Isorhamnetin hexoside I | 477.1038 | 477.1035 | 315 (100) | O, A |
| 87 | 28.0 | Quercetin pentoside | 433.072 | 433.0718 | 301 (100) | O |
| 92 | 28.7 | Isorhamnetin-3-O-glucoside * | 477.1038 | 477.1045 | 315 (100) | C |
| 99 | 29.8 | Kaempferide glucuronide | 475.0819 | 475.0821 | 299.0522 (100) | O |
| 103 | 30.3 | Ishoramnetin-3-O-acetylglucoside | 519.1144 | 519.1150 | 315 (100), 300(15) | C |
| 107 | 30.9 | Isorhamnetin hexoside II | 477.1038 | 477.1035 | 315 (100) | A |
| 125 | 35.0 | Quercetin * | 301.0354 | 301.0352 | 151 (60) | O, A |
| 126 | 36.1 | Methoxyquercetin isomer | 315.0510 | 315.0508 | 301 (100) | O, A |
| 127 | 36.2 | Quercetin dimethyl ether | 329.0624 | 329.0625 | 314 (100), 299 (70) | O |
| 129 | 37.5 | Jaceidin isomer | 359.0767 | 359.0727 | 344 (57), 329 (100) | O |
| 133 | 38.3 | Dihydroxyquercetin dimethyl ether | 331.0823 | 331.0817 | 299 (100) | O |
| 134 | 38.3 | Isorhamnetin * | 315.0510 | 315.0511 | 301 (100), 209 (15) | C |
| 142 | 41.0 | Centaureidin | 359.0772 | 359.0770 | 344 (59), 229 (100) | A |
| 146 | 45.8 | Casticin * | 373.0929 | 373.0923 | 358 (43), 343 (90) | A |
| Flavanone derivatives | ||||||
| 59 | 21.2 | Eriocitrin | 595.1585 | 595.1591 | 287(100) | O |
| 69 | 23.5 | Eriodyctiol hexoside | 449.1029 | 449.1028 | 287.0524 (100) | O |
| 117 | 33.3 | Eriodictyol | 287.0561 | 287.0555 | 151 (100), 135 (85) | O |
| 131 | 37.9 | Naringenin * | 271.0612 | 271.0607 | 151 (100) | O |
| Other compounds | ||||||
| 8 | 5.2 | Arbutin * | 271.0823 | 271.0819 | 108 (100) | O |
| 108 | 31.3 | Calendasaponin B | 971.4857 | 971.4855 | 971 (100), 809 (40) | C |
| 122 | 34.2 | Calendasaponin A | 1117.5436 | 1117.5439 | 1117 (100), 955 (10) | C |
| 135 | 39.5 | Calenduloside G | 793.4373 | 793.4376 | 631 (100), 613 (30) | C |
* Comparison with standard. Rt, retention time. M, Melissa officinalis; O, Origanum majorana; C, Calendula officinalis; A, Achillea millefolium.
Although some similarities were found among all UAE-50 extracts, such as the presence of organic acids and caffeic acid, we observed many differences. Within the Lamiaceae species, 49 compounds were identified in M. officinalis, mostly rosmarinic acid (RA) and its derivatives, either dimers, trimers or tetramers. In total, 70 compounds were identified in O. majorana showing a multivariate composition of caffeic acid derivatives and flavonoids, mainly glycosylated flavones. Besides, flavanones were only detected in this latter plant. With respect to the Asteraceae species, 41 compounds were identified in C. officinalis. Glycosylated and methylated derivatives of flavonols, along with caffeic acid derivatives, were particularly exhibited in this extract. Finally, within the 59 compounds identified in A. millefolium, the presence of several mono- and di- caffeoylquinic acid (CQA) derivatives represented a special characteristic in this plant, along with a great variety of methoxylated and glycosylated flavones.
Different studies have already published the compositions, mainly the phenolic composition, of M. officinalis [7,8,9] and O. majorana [5,26,27]. However, an extended characterization of the ethanol:water extracts, including the unreported compounds, was undertaken the present article for these matrices (Table 2). Regarding the phenolic acid constituents, some yunnaneic acid isomers have been described for M. officinalis [7,8]. Nevertheless, to the best of our knowledge, yunnaneic acid D (peak 64) is still unreported. Similarly, sagecoumarins have been identified in Lamiaceae species, mainly in S. officinalis and M. officinalis [8,28], but not in O. majorana. Thus, peak 118 was tentatively identified as a sagecoumarin isomer in MAJ-50 according to its fragmentation pattern [8].
This study also contributes to the understanding of an extended flavonoid composition of O. majorana. To the best of our knowledge, 11 compounds were not yet referenced in marjoram, despite the fact that they were found in other Lamiaceae spp. For instance, the presence of luteolin diglucuronide (peak 44), eriodictyol hexoside (peak 69) and isorhamnetin hexoside (peak 85) were described in T. vulgaris [29]; eriocitrin (peak 59) and kaempferide glucururonide (peak 99) were reported in Origanum spp. [30,31]; and 6-hydroxyluteolin-7-O-glucoside (peak 53) and luteolin acetylglucoside (peak 93) were detected in S. officinalis [28,32]. Furthermore, in the MAJ-50 extract, a group of methoxylated flavonoids was detected and tentatively identified as dihydroxyquercetin dimethyl ether (peak 133), trihydroxy dimethoxyflavone (peak 138), methoxyacacetin (peak 144) and dihydroxy trimethoxyflavone (peak 145). This type of methoxylated compounds has been also characterized in thyme using LC–MS/MS techniques [33].
Regarding the Asteraceae species, the composition of C. officinalis agrees with the published literature [15,17,18]. However, no previous records were found for kaempferol-3-O-rutinoside (peak 80), which was also identified by the comparison with its authentic standard. Moreover, according to our results, a considerable number of phenolic compounds remained unreported in C. officinalis (peaks 10, 14, 16, 19, 34, 35, 49, 50 and 68). Nevertheless, it is worth mentioning that these phenolic compounds have recently been recognized in other Calendula spp and Asteracea spp. [13,16,34]. In CAL-50, the presence of saponins derivatives is a noteworthy characteristic, since calendasaponins B, A and G have recently been reported in other Calendula spp. [16], where peaks 108, 122 and 135 were tentatively identified, respectively. The composition of A. millefolium, has been extensively addressed due to a wide range of bioactivities found in this plant. Thus, most of the compounds derived from our study were in accordance with those found previously [10,12,13]. In addition, new CQA derivatives were defined in the A. millefolium ethanol:water extract, namely feruloyl-O-caffeoylquinic acid (peak 111) and tricaffeoylquinic acid (peak 119). In this sample, the presence of a new flavone derivate was also observed, corresponding with 6-hydroxyluteolin-7-O-glucoside (peak 53). This derivate has not been reported specifically in A. millefolium, but it was characterized within the Asteraceae genera [32].
From all tentatively identified compounds, 107 phenolic compounds were quantified in the ethanol:water extracts, as can be observed in Table 3. Within the Lamiaceae species, rosmarinic acid (RA) was particularly abundant in MEL-50, while for MAJ-50 extract, RA and 6-hydroxyluteolin-7-O-glucoside were found as the main phenolics. With respect to Asteraceae, isorhamnetin-3-O-rhamnosylrutinoside was the most representative constituent for CAL-50, whereas 3,5-dicaffeoylquinic acid (3,5-DCQA) was the most representative for MIL-50.
Table 3.
Phenolic composition of the selected UAE extracts. MEL, Melissa officinalis L., MAJ, Origanum majorana L., CAL, Calendula officinalis L., MIL, Achillea millefolium L. Results are expressed in mean ± S.D. (mg/g extract).
| Compound | MEL-50 | MAJ-50 | CAL-50 | MIL-50 | MIL-100 |
|---|---|---|---|---|---|
| Hydroxybenzoic acids and derivatives | |||||
| Protocatechuic acid pentoside | nd | nd | 0.14 ± 0.03 | nd | nd |
| 3,4-dihydroxyphenil lactic acid | 0.61 ± 0.04 | 1.29 ± 0.06 | nd | nd | nd |
| Protocatechuic acid * | 0.19 ± 0.07 | 0.22 ± 0.10 | nd | 0.35 ± 0.09 1 | 0.11 ± 0.03 |
| Syringic acid derivative | nd | <LOQ | 0.38 ± 0.04 | nd | nd |
| Hydroxyccinamic acids and derivatives | |||||
| Hydroxyferulic acid hexoside isomer I | nd | nd | 0.16 ± 0.05 | nd | nd |
| Hydroxyferulic acid hexoside isomer II | nd | nd | 0.20 ± 0.08 | nd | nd |
| Neochlorogenic acid * | nd | 0.27 ± 0.05 | 0.60 ± 0.10 | 0.43 ± 0.10 1 | 0.26 ± 0.05 |
| Hydroxyferulic acid hexoside isomer III | nd | nd | 0.24 ± 0.01 | nd | nd |
| Caftaric acid isomer | nd | nd | nd | 0.08 ± 0.03 | 0.06 ± 0.03 |
| Caffeic acid dihexoside | 0.32 ± 0.05 | nd | nd | nd | nd |
| Caftaric acid * | 0.38 ± 0.08 | nd | nd | 0.16 ± 0.03 1 | 0.08 ± 0.03 |
| Caffeoylquinic acid isomer I | nd | nd | nd | 0.22 ± 0.04 | 0.19 ± 0.04 |
| Caffeic acid hexoside I | <LOQ | nd | 0.20 ± 0.01 | nd | nd |
| Chlorogenic acid * | nd | nd | 7.92 ± 0.39 | 7.84 ± 0.57 1 | 6.41 ± 0.33 |
| Cryptochlorogenic acid * | nd | 0.75 ± 0.11 | 0.16 ± 0.04 | 0.47 ± 0.091 | 0.13 ± 0.06 |
| Caffeic acid hexoside II | 0.21 ± 0.08 | 0.29 ± 0.07 | nd | nd | nd |
| Hydroxyccinamic acids and derivatives (continued) | |||||
| Coumaroylquinic acid | nd | nd | 0.09 ± 0.03 | nd | nd |
| Caffeoylshikimic acid | nd | nd | 0.20 ± 0.05 | nd | nd |
| Caffeoylquinic acid isomer II | nd | nd | <LOQ | 0.64 ± 0.10 1 | 0.10 ± 0.06 |
| Caffeic acid * | 0.57 ± 0.09 | 0.78 ± 0.10 | 0.19 ± 0.04 | 0.40 ± 0.07 | 0.33 ± 0.04 |
| Yunnaneic acid E isomer I | 1.20 ± 0.15 | nd | nd | nd | nd |
| Yunnaneic acid E isomer II | 1.94 ± 0.14 | nd | nd | nd | nd |
| Salvianolic acid I | 0.77 ± 0.13 | <LOQ | nd | nd | nd |
| Yunnaneic acid D isomer | 0.37 ± 0.05 | nd | nd | nd | nd |
| Rosmarinic acid hexoside | 7.19 ± 0.84 | <LOQ | nd | nd | nd |
| Chicoric acid | 0.75 ± 0.06 | nd | nd | nd | nd |
| 3,4-dicaffeoylquinic acid * | nd | nd | nd | 1.42 ± 0.09 | 1.43 ± 0.07 |
| 1,5-dicaffeoylquinic acid * | nd | nd | 0.31 ± 0.09 | 2.57 ± 0.10 1 | 1.73 ± 0.09 |
| Sagerinic acid | 2.22 ± 0.18 | nd | nd | nd | nd |
| 3,5-dicaffeoylquinic acid * | nd | nd | 5.37 ± 0.96 | 15.30 ± 1.02 | 21.93 ± 1.21 1 |
| Salvianolic acid E | 0.43 ± 0.05 | <LOQ | nd | nd | nd |
| Isorosmarinic acid | 0.46 ± 0.05 | nd | nd | nd | nd |
| 4,5-dicaffeoylquinic acid * | nd | nd | 2.61 ± 0.15 | 5.70 ± 0.20 1 | 4.41 ± 0.13 |
| Rosmarinic acid * | 19.21 ± 1.02 | 37.61 ± 1.90 | nd | nd | nd |
| Lithospermic acid * | nd | 10.52 ± 0.71 | nd | nd | nd |
| Lithospermic acid isomer I | 1.84 ± 0.09 | 21.30 ± 1.02 | nd | nd | nd |
| Salvianolic acid B * | 0.49 ± 0.02 | 2.06 ± 0.08 | nd | nd | nd |
| Dicaffeoylquinic acid isomer | nd | nd | 0.08 ± 0.03 | 0.18 ± 0.06 1 | 0.08 ± 0.02 |
| Lithospermic acid isomer II | 8.65 ± 0.52 | 3.56 ± 0.23 | nd | nd | nd |
| Feruloyl-O-caffeoylquinic acid | nd | nd | nd | 0.13 ± 0.03 | 0.12 ± 0.02 |
| Salvianolic acid L isomer I | 1.75 ± 0.08 | 4.32 ± 0.15 | nd | nd | nd |
| Sagecoumarin caftaride | 0.64 ± 0.06 | nd | nd | nd | nd |
| Sagecoumarin isomer | 0.40 ± 0.05 | 1.33 ± 0.09 | nd | nd | nd |
| Tricaffeoylquinic acid | nd | nd | 0.13 ± 0.02 | 0.30 ± 0.06 | 0.39 ± 0.07 |
| Salvianolic acid C derivative | 2.19 ± 0.09 | nd | nd | nd | nd |
| Salvianolic acid L isomer II | 0.95 ± 0.06 | 0.98 ± 0.07 | nd | nd | nd |
| Rosmarinic acid derivative I | <LOQ | 0.27 ± 0.07 | nd | nd | nd |
| Rosmarinic acid derivative II | 0.30 ± 0.03 | nd | nd | nd | nd |
| Salvianolic acid F isomer | 1.16 ± 0.09 | nd | nd | nd | nd |
| Rosmarinic acid derivative III | 0.46 ± 0.03 | 0.35 ± 0.04 | nd | nd | nd |
| Salvianolic acid C caffeoylhydroxycaffeide | 3.32 ± 0.10 | nd | nd | nd | nd |
| Flavone derivatives | |||||
| Vicenin 2 * | nd | 2.56 ± 0.17 | 0.11 ± 0.06 | 4.00 ± 0.33 1 | 2.35 ± 0.13 |
| Apigenin-hexoside-pentoside I | nd | nd | nd | 0.55 ± 0.21 1 | 0.44 ± 0.12 |
| Schaftoside isomer | nd | nd | nd | 3.20 ± 0.14 1 | 1.38 ± 0.10 |
| Schaftoside * | nd | <LOQ | nd | 2.73 ± 0.12 1 | 2.04 ± 0.12 |
| Luteolin-C-hexoside | nd | 2.19 ± 0.15 | nd | nd | nd |
| Homoorientin * | nd | nd | nd | 1.00 ± 0.17 | 2.26 ± 0.22 1 |
| Apigenin-hexoside-pentoside II | nd | nd | nd | 2.02 ± 0.18 1 | 1.22 ± 0.12 |
| Luteolin dihexoside I | nd | nd | nd | 1.82 ± 0.10 | 3.01 ± 0.12 1 |
| 6-hydroxyluteolin-7-O-glucoside | nd | 35.80 ± 2.11 | nd | 1.99 ± 0.09 | 2.48 ± 0.10 |
| Apigenin dihexoside | nd | nd | nd | 0.31 ± 0.07 | 0.23 ± 0.06 |
| Luteolin dihexoside II | nd | <LOQ | nd | 0.27 ± 0.04 | 0.26 ± 0.06 |
| Apigenin deoxylhexoside | nd | nd | nd | 0.26 ± 0.09 | 0.36 ± 0.04 |
| Luteolin-O-glucoside | nd | 17.52 ± 1.10 | nd | nd | nd |
| Apigenin glycosylated derivative | nd | nd | nd | 3.21 ± 0.13 1 | 2.42 ± 0.09 |
| Luteolin-7-O-β-glucoside * | 0.21 ± 0.03 | 14.61 ± 1.01 | nd | 4.96 ± 0.35 | 8.23 ± 0.72 1 |
| Flavone derivatives (continued) | |||||
| Luteolin-7-O-glucuronide * | nd | 4.09 ± 0.11 | nd | 0.69 ± 0.07 | 0.82 ± 0.07 |
| Diosmin * | nd | 3.77 ± 0.19 | nd | nd | nd |
| Apigenin-7-O-glucoside * | nd | 2.17 ± 0.09 | nd | 1.05 ± 0.21 | 2.28 ± 0.32 1 |
| Luteolin-O-malonylglucoside | nd | nd | nd | 0.26 ± 0.07 | 0.55 ± 0.09 1 |
| Apigenin-7-O-glucuronide * | nd | 1.70 ± 0.09 | nd | nd | nd |
| Luteolin-O-hexuronide | 1.64 ± 0.11 | nd | nd | nd | nd |
| Luteolin * | <LOQ | 1.10 ± 0.08 | nd | 1.70 ± 0.07 | 1.89 ± 0.09 1 |
| Apigenin * | nd | 0.09 ± 0.02 | nd | 0.42 ± 0.04 | 0.59 ± 0.05 1 |
| Diosmetin * | nd | nd | nd | 0.34 ± 0.03 | 0.45 ± 0.05 1 |
| Trihydroxy dimethoxyflavone I | nd | 0.69 ± 0.07 | nd | nd | nd |
| Trihydroxy dimethoxyflavone II | nd | nd | nd | <LOQ | 0.24 ± 0.06 |
| Methoxyacacetin | nd | 0.04 ± 0.02 | nd | <LOQ | 0.22 ± 0.05 |
| Dihydroxy trimethoxyflavone | nd | nd | nd | 0.13 ± 0.03 | 0.31 ± 0.04 1 |
| Flavonol derivatives | |||||
| Quercetin-3-O-rhmanosylrutinoside | nd | nd | 1.17 ± 0.73 | nd | nd |
| Quercetin 3-neohesperidoside | nd | nd | 0.18 ± 0.06 | nd | nd |
| Isorhamnetin-3-O-rhamnosylrutinoside * | nd | nd | 14.22 ± 1.30 | nd | nd |
| Quercetin hexoside I | nd | nd | nd | 0.72 ± 0.09 | 1.34 ± 0.90 1 |
| Quercetin-O-pentosylhexoside | nd | nd | 0.36 ± 0.07 | nd | nd |
| Rutin * | nd | nd | 0.57 ± 0.06 | 0.99 ± 0.09 | 1.12 ± 0.11 |
| Isovitexin | nd | nd | nd | 0.50 ± 0.09 | 0.46 ± 0.07 |
| Vitexin * | nd | nd | nd | 0.46 ± 0.09 | 0.66 ± 0.11 |
| Quercetin-malonylhexosyl-rhamnoside | nd | nd | 0.65 ± 0.12 | nd | nd |
| Isorhamnetin-3-O-neohesperidoside | nd | nd | 1.89 ± 0.14 | nd | nd |
| Quercetin hexoside II | nd | nd | 0.19 ± 0.06 | nd | nd |
| Quercetin hexuronide | nd | nd | nd | 0.45 ± 0.04 1 | 0.21 ± 0.03 |
| Kaempferol-3-O-rutinoside* | nd | nd | 0.19 ± 0.07 | nd | nd |
| Ishoramnetin-3-O-rutinoside * | nd | nd | 7.23 ± 0.62 | nd | nd |
| Quercetin-3-O-acetyl-glucoside | nd | nd | 0.64 ± 0.04 | nd | nd |
| Isorhamnetin hexoside I | nd | <LOQ | nd | 0.48 ± 0.07 | 1.18 ± 0.10 1 |
| Isorhamnetin-3-O-glucoside* | nd | nd | 0.67 ± 0.09 | nd | nd |
| Ishoramnetin-3-O-acetylglucoside | nd | nd | 1.12 ± 0.09 | nd | nd |
| Isorhamnetin hexoside II | nd | nd | nd | 0.17 ± 0.04 | 0.53 ± 0.07 1 |
| Quercetin * | nd | 0.36 ± 0.03 | nd | 0.33 ± 0.04 | 0.64 ± 0.05 1 |
| Methoxyquercetin isomer | nd | <LOQ | nd | 0.58 ± 0.08 | 0.82 ± 0.11 1 |
| Quercetin dimethyl ether | nd | 0.81 ± 0.10 | nd | nd | nd |
| Jaceidin isomer | nd | 0.62 ± 0.08 | nd | nd | nd |
| Dihydroxyquercetin dimethyl ether | nd | 0.61 ± 0.07 | nd | nd | nd |
| Centaureidin | nd | nd | nd | 0.27 ± 0.06 | 1.90 ± 0.10 1 |
| Casticin * | nd | nd | nd | 0.35 ± 0.03 | 2.53 ± 0.11 1 |
| Flavanone derivatives | |||||
| Eriodictyol | nd | 0.67 ± 0.05 | nd | nd | nd |
| Naringenin * | nd | 0.94 ± 0.10 | nd | nd | nd |
| Σ Phenolic compounds | 60.8 ± 1.30 | 176.2 ± 2.5 | 48.2 ± 0.9 | 72.4 ± 1.0 | 83.2 ± 1.0 1 |
* Identified and quantified via comparison with authentic standards. nd, not detected. < LOQ, below limit of quantification. 1 Indicates statistical differences between MIL-50 and MIL-100 extracts (p < 0.05).
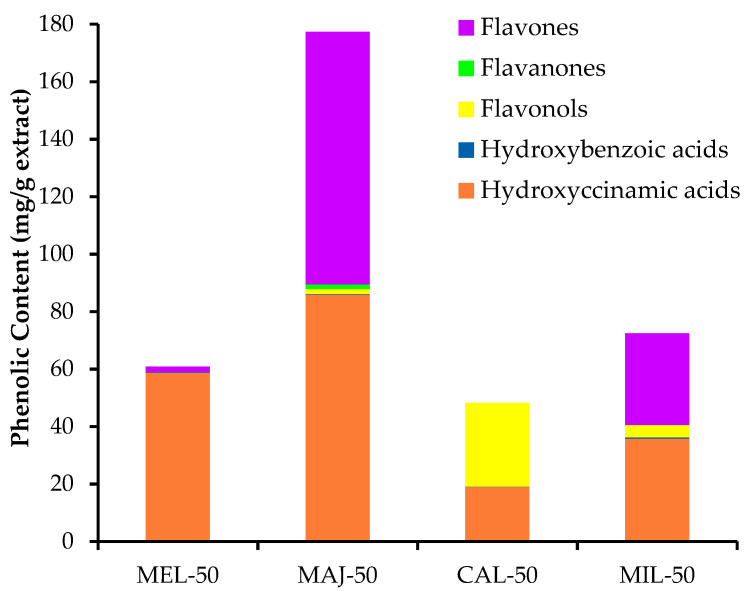
Nevertheless, in an attempt to better understanding the quantitative differences within the studied samples, the cumulative concentration of phenolic compounds in the four extracts was plot, as shown in Figure 2. Considering these results, the antioxidant activity of the ethanol:water extracts could be attributable to the sum of the phenolic compounds (mg/g extract), but also to the presence of specific phenolic compounds. Thus, the greatest antioxidant activity of MAJ-50 was clearly associated to its maximum content of phenolic compounds (Figure 2), mainly hydroxycinnamic acids and flavones, compared to the other extracts, but probably influenced by those most representative compounds in this sample, such as RA and its dimers, i.e., lithosphermic acid isomer, and luteolin glucosylated derivatives, i.e. 6-hydroxyluteolin-7-O-glucoside, luteolin-O-glucoside and luteolin-7-O-β-glucoside, which also have been recognized for its antioxidant activity [9,32].
Figure 2.
Concentration by type of phenolic compounds in the studied UAE-50 extracts, expressed as mg/g extract. MEL, Melissa officinalis L., MAJ, Origanum majorana L., CAL, Calendula officinalis L., MIL, Achillea millefolium L.
Although MEL-50 showed a smaller total accumulation of phenolics than MIL-50 (Figure 2), M. officinalis showed better results than A. millefolium in terms of antioxidant activity (Table 1). In this regard, the main difference was the greater concentration of hydroxycinnamic acids in MEL-50. Therefore, its better antioxidant effect could be related. Thus, the presence of a high content of RA and its derivatives in MEL-50 could be considered the principal factor contributing to its antioxidant activity.
Regarding MIL-50 and CAL-50, although both plants belong to the same family, their individual phenolic composition showed quite different results (Figure 2). MIL-50 was distinguished by the presence of a similar content of both hydroxycinnamic acids and flavones, whereas in CAL-50, the abundance of flavonols was relevant. A greatest content of 3,5-DCQA, along with chlorogenic acid, was found in A. millefolium ethanol:water extract. This may be primarily attributed to its antioxidant activity [35]. Although this type of antioxidant component, i.e., CGA and DCQAs, was also found in CAL-50, its lack of antioxidant activity could be attributed to its low content of phenolic compounds overall.
In addition, the total phenolic composition of the MIL-100 extract (Table 3), which exhibited the highest anti-inflammatory activity, displayed a quite superior content compared to the A. millefolium ethanol:water extract. In this context, significant increases were found in certain compounds, such as 3,5-DCQA, luteolin-7-O-glucoside, casticin and centaureidin. In agreement with our results, Ali et al. [10] reported the anti-inflammatory activity of an enriched fraction in DCQAs and flavonoids from A. millefolium. At the same time, centaureidin and casticin have been associated with anti-inflammatory activity [36,37]. Furthermore, other aglycones that were enriched in the MIL-100 extract, such as apigenin, quercetin, luteolin and diosmetin, seemed to reduce the IL-6 and TNF-α secretion in the LPS-stimulated RAW macrophages [38]. Thus, the cytokines release inhibition shown by the MIL-100 extract could be attributed, at least in part, to the abovementioned phenolic compounds. On the other hand, since pure ethanol was used as the solvent extract, volatile compounds typically found in A. millefolium essential oil with powerful anti-inflammatory properties may have also been extracted [3,11]. Therefore, a GC-MS analysis of the MIL-100 extract was also conducted.
3.4. GC-MS Composition of Ethanolic Extracts
The composition of the volatile fraction of MIL UAE-100 extract, shown in Table 4, demonstrated the presence of a wide range of monoterpenes and sesquiterpenes [3], found in a greater or lesser extent. As can be observed, borneol (23.8%) was the most abundant compound so far, although camphor (10.4%), and β-eudesmol (8.6%) were also representative in the extract. As mentioned by other authors, borneol and camphor, some of the mainly constituents in several Asteracea spp. essential oil, significantly inhibited nitric oxide production, CCL2 (Chemokine (C-C motif) ligand 2) release and cytokines (TNF-α, IL-1β and IL-6) secretion in macrophages [4,11].
Table 4.
Chemical composition of the peak area contribution of Achillea millefolium L. ethanolic extract (UAE-100 extract) of the volatile fraction identified by GC-MS.
| Rt (min) | RI | Compound | % Area |
|---|---|---|---|
| 4.6 | 997 | Yomogi alcohol | 6.1 |
| 5.1 | 1028 | Eucalyptol | 5.0 |
| 5.5 | 1058 | Artemisia ketone | 4.3 |
| 5.8 | 1079 | Artemisia alcohol | 5.6 |
| 6.2 | 1101 | Thujone | 2.9 |
| 6.9 | 1138 | Camphor | 10.4 |
| 7.2 | 1160 | Borneol | 23.8 |
| 7.6 | 1174 | Terpinene-4-ol | 1.9 |
| 8.7 | 1261 | (5E)-5,9-Dimethyl-5,8-decadien-2-one | 2.9 |
| 9.3 | 1299 | Carvacrol | 2.9 |
| 12.1 | 1478 | α-curcumene | 0.9 |
| 13.5 | 1569 | Spathulenol | 2.7 |
| 13.6 | 1578 | Caryophillene oxide | 2.6 |
| 13.8 | 1589 | Viridiflorol | 5.1 |
| 14.3 | 1630 | δ-Cadinol | 4.6 |
| 14.5 | 1640 | β-Eudesmol | 8.6 |
| 15.6 | 1718 | Chamazulene | 5.9 |
| 16.8 | 1890 | Corymbolone | 3.8 |
| ∑ AUC | 4.10 × 106 |
Rt: retention time. RI: retention index. AUC: area under curve.
With respect to the the anti-inflammatory properties of A. millefolium ethanol extract, an exclusive influence of one group of compounds cannot be completely established. However, some accumulative factors, such as (i) a greater abundance of some specific phenolics, (ii) the presence of terpenoid compounds in the volatile fraction and (iii) the accumulative, or synergistic, effect of minor compounds, could contribute to this activity.
4. Conclusions
This study highlights that the application of innovative and sustainable techniques for obtaining a specific group of bioactive compounds, together with the use of advance chemical analysis of complex plant extracts, makes it possible to obtain new healthy ingredients. Thereby, UAE with ethanol:water or pure ethanol proved to be a better sustainable methodology than SFE with pure CO2 for obtaining compounds with antioxidant and/or anti-inflammatory activities from the four studied plants. Moreover, Origanum majorana (Lamiaceae) was displayed as the best source of antioxidant compounds, whereas Achillea millefolium (Asteraceae) showed a remarkable anti-inflammatory activity on LPS-stimulated macrophages. A deep high-resolution MS/MS analysis of the plant extracts constituents, particularly phenolic compounds, allowed the tentative identification of the compounds responsible of these bioactivities. Thus, the antioxidant activity of O. majorana extracts may be manly linked to its high content of phenolic compounds, especially rosmarinic acid and luteolin glucoside derivates. On the other hand, the anti-inflammatory activity of A. millefolium could, in part, be explained by its richness of specific phenolics, i.e., 3,5-DCQA, luteolin-7-O-glucoside, casticin and centaureidin, but also to the presence of volatile compounds, such as borneol and camphor. Thus, both, O. majorana and A. millefolium should be considered as good sources of potent bioactive ingredients with promising applications in multiple industry, especially the food and pharmaceutical industries.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/foods10092067/s1, Table S1: Authentic commercial standards (HPLC purity ≥ 95%), Figure S1: Antioxidant activity of Asteraceae and Lamiaceae plant extracts as a function of TPC (mg GAE/g). (A) TEAC value (mmol trolox/g); (B) ORAC (mmol trolox/g). Figure S2: Base-peak chromatogram of Achillea millefolium obtained by UAE (ethanol-water, 50:50, v/v) analysed by RP-HPLC-ESI-QTOF-MS in negative ionization mode. * IS, internal standard (ethyl gallate).
Author Contributions
Conceptualization, M.V. and L.J.; methodology, L.J. and S.S.; formal analysis, M.V., L.S.-P. and M.d.l.N.S.-S.; investigation, M.V.; data curation, M.V. and L.J.; writing—original draft preparation, M.V. and L.J.; writing—review and editing, L.J. and S.S.; supervision, T.F., M.R.G.-R. and G.R.; funding acquisition, G.R., S.S. and L.J. All authors have read and agreed to the published version of the manuscript.
Funding
Authors thanks to the Spanish Government (Project: PID2019-110183RB-C22/AEI/10.13039/501100011033) and Comunidad Autónoma de Madrid (ALIBIRD2020-CM (S2018/BAA-4343)) for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chiriac E.R., Chiţescu C.L., Geană E.-I., Gird C.E., Socoteanu R.P., Boscencu R. Advanced Analytical Approaches for the Analysis of Polyphenols in Plants Matrices—A Review. Separations. 2021;8:65. doi: 10.3390/separations8050065. [DOI] [Google Scholar]
- 2.Poletto P., Alvarez-Rivera G., Torres T.M., Mendiola J.A., Ibañez E., Cifuentes A. Compressed fluids and phytochemical profiling tools to obtain and characterize antiviral and anti-inflammatory compounds from natural sources. Trends Analyt. Chem. 2020;129:115942. doi: 10.1016/j.trac.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farhadi N., Babaei K., Farsaraei S., Moghaddam M., Pirbalouti A.G. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crop. Prod. 2020;152:112570. doi: 10.1016/j.indcrop.2020.112570. [DOI] [Google Scholar]
- 4.Miguel M.G., da Silva C.I., Farah L., Castro Braga F., Figueiredo A.C. Effect of Essential Oils on the Release of TNF-α and CCL2 by LPS-Stimulated THP-1 Cells. Plants. 2021;10:50. doi: 10.3390/plants10010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallverdú-Queralt A., Regueiro J., Alvarenga J.F.R., Martinez-Huelamo M., Leal L.N., Lamuela-Raventos R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: Caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015;35:189–195. doi: 10.1590/1678-457X.6580. [DOI] [Google Scholar]
- 6.Sharifi-Rad M., Berkay Yılmaz Y., Antika G., Salehi B., Tumer T.B., Kulandaisamy Venil C., Gitishree D., Kumar Patra J., Kazahan N., Akram M., et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phytother. Res. 2021;35:95–121. doi: 10.1002/ptr.6785. [DOI] [PubMed] [Google Scholar]
- 7.Carocho M., Barros L., Calhelha R.C., Ćirić A., Soković M., Santos-Buelga C., Morales P., Ferreira I.C. Melissa officinalis L. decoctions as functional beverages: A bioactive approach and chemical characterization. Food Funct. 2015;6:2240–2248. doi: 10.1039/C5FO00309A. [DOI] [PubMed] [Google Scholar]
- 8.Ozarowski M., Mikolajczak P.L., Piasecka A., Kachlicki P., Kujawski R., Bogacz A., Bartkowiak-Wieczorek J., Szulc M., Kaminska E., Kujawska M., et al. Influence of the Melissa officinalis leaf extract on long-term memory in scopolamine animal model with assessment of mechanism of action. Evid. Based. Complement. Alternat. 2016;2016:9729818. doi: 10.1155/2016/9729818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miron T.L., Herrero M., Ibáñez E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chromatogr. A. 2013;1288:1–9. doi: 10.1016/j.chroma.2013.02.075. [DOI] [PubMed] [Google Scholar]
- 10.Ali S.I., Gopalakrishnan B., Venkatesalu V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A review. Phytother. Res. 2017;31:1140–1161. doi: 10.1002/ptr.5840. [DOI] [PubMed] [Google Scholar]
- 11.Abdossi V., Kazemi M. Bioactivities of Achillea millefolium essential oil and its main terpenes from Iran. Int. J. Food Prop. 2016;19:1798–1808. doi: 10.1080/10942912.2015.1086787. [DOI] [Google Scholar]
- 12.Dias M.I., Barros L., Dueñas M., Pereira E., Carvalho A.M., Alves R.C., Oliveira M.B., Santos-Buelga C., Ferreira I.C. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013;141:4152–4160. doi: 10.1016/j.foodchem.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Pereira J.M., Peixoto V., Teixeira A., Sousa D., Barros L., Ferreira I.C.F.R., Vasconcelos M.H. Achillea millefolium L. hydroethanolic extract inhibits growth of human tumor cells lines by interfering with cell cycle and inducing apoptosis. Food Chem. Toxicol. 2018;118:635–644. doi: 10.1016/j.fct.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Dinda M., Mazumdar S., Das S., Ganguly D., Dasgupta U.B., Dutta A., Jana K., Karmakar P. The water fraction of Calendula officinalis hydroethanol extract stimulates in vitro and in vivo proliferation of dermal fibroblasts in wound healing. Phytother. Res. 2016;30:1696–1707. doi: 10.1002/ptr.5678. [DOI] [PubMed] [Google Scholar]
- 15.Miguel M., Barros L., Pereira C., Calhelha R.C., Garcia P.A., Castro M.Á., Santos-Buelga C., Ferreira I.C. Chemical characterization and bioactive properties of two aromatic plants: Calendula officinalis L. (flowers) and Mentha cervina L.(leaves) Food Funct. 2016;7:2223–2232. doi: 10.1039/C6FO00398B. [DOI] [PubMed] [Google Scholar]
- 16.Faustino M.V., Pinto D.C., Gonçalves M.J., Salgueiro L., Silveira P., Silva A.M. Calendula L. species polyphenolic profile and in vitro antifungal activity. J. Funct. Foods. 2018;45:254–267. doi: 10.1016/j.jff.2018.04.013. [DOI] [Google Scholar]
- 17.Pires T.C., Dias M.I., Barros L., Calhelha R.C., Alves M.J., Oliveira M.B.P., Santos-Buelga C., Ferreira I.C. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018;105:580–588. doi: 10.1016/j.foodres.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Hernández-Saavedra D., Pérez-Ramírez I.F., Ramos-Gómez M., Mendoza-Díaz S., Loarca-Pina G., Reynoso-Camacho R. Phytochemical characterization and effect of Calendula officinalis, Hypericum perforatum, and Salvia officinalis infusions on obesity-associated cardiovascular risk. Med. Chem. Res. 2016;25:163–172. doi: 10.1007/s00044-015-1454-1. [DOI] [Google Scholar]
- 19.United Nations: Sustainable Development Goals. [(accessed on 2 March 2021)]. Available online: https://sustainabledevelopment.un.org/sdgs.
- 20.Rodríguez-Pérez C., Quirantes-Piné R., Fernández-Gutiérrez A., Segura-Carretero A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crop. Prod. 2015;66:246–254. doi: 10.1016/j.indcrop.2015.01.002. [DOI] [Google Scholar]
- 21.Singleton V.L., Orthofer R., Lamuela-Reventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 22.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 23.Huang D., Ou B., Hampsch-Woodill M., Flanagan J.A., Prior R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 24.Villalva M., Jaime L., Aguado E., Nieto J.A., Reglero G., Santoyo S. Anti-inflammatory and antioxidant activities from the basolateral fraction of Caco-2 cells exposed to a rosmarinic acid enriched extract. J. Agric. Food Chem. 2018;66:1167–1174. doi: 10.1021/acs.jafc.7b06008. [DOI] [PubMed] [Google Scholar]
- 25.García-Risco M.R., Mouhid L., Salas-Pérez L., López-Padilla A., Santoyo S., Jaime L., Ramírez de Molina A., Reglero G., Fornari T. Biological activities of Asteraceae (Achillea millefolium and Calendula officinalis) and Lamiaceae (Melissa officinalis and Origanum majorana) plant extracts. Plant Foods Hum. Nutr. 2017;72:96–102. doi: 10.1007/s11130-016-0596-8. [DOI] [PubMed] [Google Scholar]
- 26.Hossain M.B., Camphuis G., Aguiló-Aguayo I., Gangopadhyay N., Rai D.K. Antioxidant activity guided separation of major polyphenols of marjoram (Origanum majorana L.) using flash chromatography and their identification by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2014;37:3205–3213. doi: 10.1002/jssc.201400597. [DOI] [PubMed] [Google Scholar]
- 27.Taamalli A., Arráez-Román D., Abaza L., Iswaldi I., Fernández-Gutiérrez A., Zarrouk M., Segura-Carretero A. LC-MS-based metabolite profiling of methanolic extracts from the medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015;26:320–330. doi: 10.1002/pca.2566. [DOI] [PubMed] [Google Scholar]
- 28.Martins N., Barros L., Santos-Buelga C., Henriques M., Silva S., Ferreira I.C. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015;170:378–385. doi: 10.1016/j.foodchem.2014.08.096. [DOI] [PubMed] [Google Scholar]
- 29.Martins N., Barros L., Santos-Buelga C., Silva S., Henriques M., Ferreira I.C. Decoction, infusion and hydroalcoholic extract of cultivated thyme: Antioxidant and antibacterial activities, and phenolic characterization. Food Chem. 2015;167:131–137. doi: 10.1016/j.foodchem.2014.06.094. [DOI] [PubMed] [Google Scholar]
- 30.Martins N., Barros L., Santos-Buelga C., Henriques M., Silva S., Ferreira I.C. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014;158:73–80. doi: 10.1016/j.foodchem.2014.02.099. [DOI] [PubMed] [Google Scholar]
- 31.Tuttolomondo T., La Bella S., Licata M., Virga G., Leto C., Saija A., Trombetta D., Tomaino A., Speciale A., Napoli E.M., et al. Biomolecular characterization of wild sicilian oregano: Phytochemical screening of essential oils and extracts, and evaluation of their antioxidant activities. Chem. Biodivers. 2013;10:411–433. doi: 10.1002/cbdv.201200219. [DOI] [PubMed] [Google Scholar]
- 32.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 33.Pacifico S., Piccolella S., Papale F., Nocera P., Lettieri A., Catauro M. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods. 2016;20:253–266. doi: 10.1016/j.jff.2015.11.008. [DOI] [Google Scholar]
- 34.Olennikov D.N., Chirikova N.K., Kashchenko N.I., Nikolaev V.M., Kim S.W., Vennos C. Bioactive Phenolics of the Genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS Profile of the Siberian Species and Their Inhibitory Potential Against α-Amylase and α-Glucosidase. Front. Pharmacol. 2018;9:756. doi: 10.3389/fphar.2018.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang N., Kitts D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2016;8:16. doi: 10.3390/nu8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan E.W.C., Wong S.K., Chan H.T. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018;16:147–152. doi: 10.1016/j.joim.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Jachak S.M., Gautam R., Selvam C., Madhan H., Srivastava A., Khan T. Anti-inflammatory, cyclooxygenase inhibitory and antioxidant activities of standardized extracts of Tridax procumbens L. Fitoterapia. 2011;82:173–177. doi: 10.1016/j.fitote.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Mueller M., Hobiger S., Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122:987–996. doi: 10.1016/j.foodchem.2010.03.041. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.