Abstract
The gut–liver axis plays an important role in the pathogenesis of various liver diseases. Probiotics are living bacteria that may be used to correct disorders of this axis. Notable progress has been made in the study of probiotic drugs for the treatment of various liver diseases in the last decade. It has been proven that probiotics are useful for hepatic encephalopathy, but their effects on other symptoms and syndromes of cirrhosis are poorly studied. Their effectiveness in the treatment of metabolic associated fatty liver disease has been shown both in experimental models and in clinical trials, but their effect on the prognosis of this disease has not been described. The beneficial effects of probiotics in alcoholic liver disease have been shown in many experimental studies, but there are very few clinical trials to support these findings. The effects of probiotics on the course of other liver diseases are either poorly studied (such as primary sclerosing cholangitis, chronic hepatitis B and C, and autoimmune hepatitis) or not studied at all (such as primary biliary cholangitis, hepatitis A and E, Wilson's disease, hemochromatosis, storage diseases, and vascular liver diseases). Thus, despite the progress in the study of probiotics in hepatology over the past decade, there are many unexplored and unclear questions surrounding this topic.
Keywords: Gut-liver axis, Pathogenesis, Gut dysbiosis, Gut microbiota, Gut microbiome, Liver disease, Probiotics, Hepatic encephalopathy, Cirrhosis, Metabolic associated fatty liver disease, Alcoholic liver disease, Primary sclerosing cholangitis
Core Tip: Probiotics are useful for hepatic encephalopathy, but their effects on other symptoms and syndromes of cirrhosis are poorly studied. Their effectiveness in the treatment of metaboliс associated fatty liver disease has been shown both in experimental models and in clinical trials, but their effect on the prognosis of this disease has not been described. The beneficial effects of probiotics in alcoholic liver disease have been shown in many experimental studies, but there are very few clinical trials to support these findings. The effects of probiotics on the course of other liver diseases are either poorly studied or not studied at all.
INTRODUCTION
It has been 10 years since the World Journal of Gastroenterology published an article titled “Probiotics in Hepatology”[1]. The following decade was marked by tremendous progress in the study of the gut-liver axis[2,3]. It was shown that the gut microbiota plays an important role in the development of various liver diseases. Probiotics are drugs that target it[4]. The aim of this review is to describe the current data on the use of probiotics for the treatment of liver diseases.
SCIENTIFIC BASIS FOR THE USE OF PROBIOTICS IN LIVER DISEASES
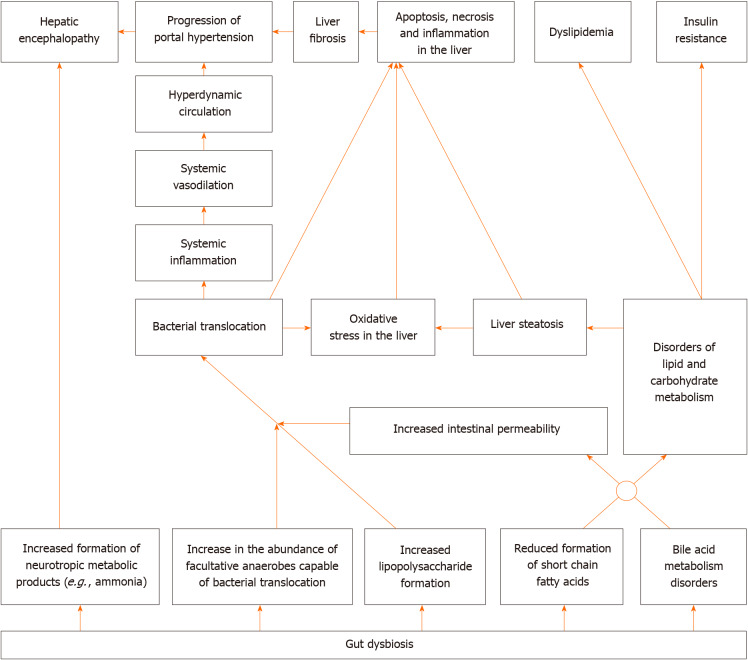
Gut dysbiosis[5-7], small intestinal bacterial overgrowth[8,9] and an increase in the permeability of the intestinal wall[10] leads to bacterial translocation in cirrhosis[11,12]. The latter leads to systemic and liver inflammatory reaction, as well as hemodynamic changes[13], and contributes to the development of complications of cirrhosis, such as ascites, esophageal varices, and hepatorenal syndrome[2,11,12]. In addition, the gut microbiota produces a variety of neuroactive products of protein metabolism, which are normally removed by the liver and abundantly enter the bloodstream, leading to the development of hepatic encephalopathy, in cirrhosis[14].
The gut microbiota plays an important role in the regulation of metabolism in our body. It modifies bile acids (deconjugation, conversion of primary into secondary), which through their receptors [farnesoid X receptor (FXR) and Takeda G-protein receptor 5], have a variety of effects on the metabolism[15,16]. In addition, the gut microbiota forms short-chain fatty acids (SCFA), which through their receptors, also have a complex effect on metabolism and maintain intestinal barrier integrity[17]. Gut dysbiosis leads to disorders of these regulatory functions, which can result in metabolic changes.
Alterations in gut microbiota and increased intestinal permeability were also described in alcoholic liver disease[18,19], metabolic associated fatty liver disease (MAFLD)[20], primary sclerosing cholangitis[21,22], and autoimmune hepatitis[23]. Gut dysbiosis was also reported in primary biliary cholangitis[24], Wilson’s disease[25], hepatitis B[26] and hepatitis C[27] recently.
At the same time, probiotics have shown their ability to correct gut dysbiosis[28], increase production of SCFA[29], and reduce the increased permeability of the intestinal barrier[30]. All this constitutes the scientific basis for their use in the treatment of liver diseases.
A simplified diagram of the gut-liver axis is shown in Figure 1.
Figure 1.
Simplified diagram of the gut-liver axis.
PROBIOTICS FOR CIRRHOSIS
According to the latest meta-analysis of randomized controlled trials (RCT), the use of probiotics is effective in the treatment of minimal hepatic encephalopathy and prevents the development of overt hepatic encephalopathy. Probiotics are as effective in treating minimal hepatic encephalopathy as rifaximin, lactulose, and L-orinithin-L-aspartate. There was no effect of probiotics on mortality. The addition of lactulose to probiotics did not significantly affect the effectiveness of the treatment. Probiotics lower blood ammonium levels more than lactulose. The addition of lactulose to probiotics paradoxically increases blood ammonium levels. The use of probiotics was not accompanied by the development of significant side effects[31]. Other recent meta-analyses have reached similar conclusions[32,33].
Several RCTs that studied the effect of probiotics on other indicators in cirrhosis been published.
The use of probiotics (Clostridium butyricum combined with Bifidobacterium infantis) in minimal hepatic encephalopathy led to a decrease in the abundance of harmful Enterococcus and Enterobacteriaceae in the gut microbiome. The blood levels of markers of bacterial translocation [lipopolysaccharide (LPS)], intestinal permeability (D-lactate) and damage to the intestinal epithelium (diamine oxidase) also decreased in these patients[34]. The use of probiotic beverage Yakult 400 also led to a decrease in the abundance of Enterobacteriaceae in the gut microbiome[35]. In another RCT, administration of Lactobacillus GG for 8 wk led to an increase in the proportion of beneficial bacteria (Lachnospiracea and Clostridia XIV) and a decrease in the proportion of harmful ones (Enterobacteriaceae). Moreover, this was accompanied by a decrease in endotoxemia and systemic inflammation[36].
Administration of a probiotic for cirrhosis leads to an improvement in cognitive functions and an increase in gait speed, but does not significantly affect the risk of falling and the hand grip muscular strength[37].
A recent meta-analysis showed that administration of probiotics for cirrhosis does not significantly affect C-reactive protein (CRP) and interleukin (IL)-6 Levels, but leads to a decrease in tumor necrosis factor alpha (TNF-α) level[38].
Probiotic Lactobacillus casei (L. casei) Shirota application for 6 mo did not have a significant effect on neutrophil function, the blood level of LPS and most cytokines, frequency of bacterial DNA detection in blood, intestinal permeability (but it was baseline normal), quality of life, indicators of the complete blood count, or liver and kidney function in non-severe cirrhosis (Child-Pugh scores < 11)[39].
The use of a multi-strain probiotic containing several species of Bifidobacterium and Lactobacillus for non-severe cirrhosis (Child-Pugh scores < 12) showed similar results[40]. However, the intake of this probiotic led to an increase in the abundance of Faecalibacterium prausnitzii, Syntrophococcus sucromutans, Bacteroidetes vulgatus, Prevotella, and Alistipes shahii in the fecal microbiome. At the same time, the abundance of Bifidobacterium bifidum, Lactobacillus acidophilus (L. acidophilus), and L. casei remained unchanged[41].
One of the most studied probiotics for cirrhosis is VSL#3, a mixture containing eight bacterial strains. Its use for 6 mo led to a decrease in the Child-Pugh and MELD scale values, the blood level of IL-1b and IL-6, TNF-α, aldosterone, renin, brain natriuretic peptide, ammonia, and indole, as well as the risk of hospitalization, but did not significantly affect mortality[42]. Its use for 2 mo in patients with large esophageal varices without a history of bleeding improves their response to propanolol[43]. Administration of this probiotic for 28 d did not lead to any significant change in the blood content of the plasminogen activator inhibitor and vascular endothelial growth factor, but led to an increase in the blood levels of large endothelin and nitric oxide and a decrease in the blood levels of thromboxane B2[44]. In addition, the use of this probiotic for 6 wk led to a decrease in the hepatic venous pressure gradient, cardiac output, and heart rate and an increase in systemic vascular resistance and sodium levels in the blood, but did not significantly affect the mean pulmonary artery pressure[45]. However, the last two studies were not controlled. VSL#3 also prevents the development of endothelial dysfunction in experimental models of cirrhosis[46].
Probiotics reduce the risk of development of re-bleeding from esophageal varices after endoscopic treatment in cirrhosis according to a retrospective study. Moreover, the larger the dose of the probiotic, the more pronounced the effect[47].
The probiotic tolerance was excellent and there were no significant side effects in any of the cited studies. However, cases of the development of spontaneous bacterial periotinitis[48] and fatal endocarditis[49] caused by probiotic strains, which was consumed by a patient with cirrhosis for a long time, are described.
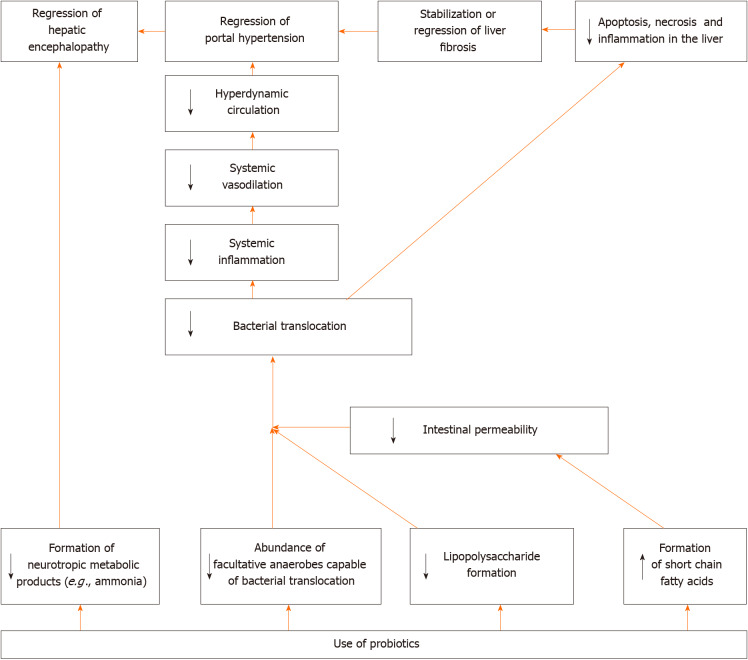
Summarizing these data, we can deduce the aforementioned facts. The effectiveness of probiotics in the treatment of minimal hepatic encephalopathy and in the prevention of development of overt hepatic encephalopathy has been confirmed by a series of meta-analyses and is beyond doubt. In addition, most studies have shown an improvement in the profile of the gut microbiota after following administration. At the same time, the influence of probiotics on other characteristics of patients with cirrhosis (intestinal permeability, bacterial translocation, systemic inflammation and others) differs from study to study. Perhaps this is due to the fact that different probiotic strains were used, which had different effects on these indicators. It would be helpful to conduct studies that directly compare probiotics that have shown and not shown an effect on these biomarkers.
The suggested mechanism of action of probiotics in cirrhosis is shown in Figure 2.
Figure 2.
Suggested mechanism of action of probiotics in cirrhosis.
PROBIOTICS FOR ALCOHOLIC LIVER DISEASE
The use of probiotics led to a decrease in the level of steatosis, inflammation, oxidative stress, and cell death in the liver, a decrease in the level of biomarkers of systemic inflammation, bacterial translocation, gut dysbiosis, dyslipidemia, damage to the intestinal epithelium, and intestinal permeability in experimental alcoholic liver disease (Table 1)[50-54]. Probiotics restore the alcohol-damaged epithelial barrier in the intestines by epidermal growth factor receptor activation[55]. Functioning of this receptor is also required for the protective effect of probiotics in alcoholic liver disease[55]. Probiotics suppress alcohol-induced apoptosis of hepatocytes[56].
Table 1.
Effects of probiotics on different disorders in experimental alcoholic liver disease
|
Disorder
|
Biomarker changes
|
Ref.
|
| Liver steatosis | ↓ Liver mass, ↓ content of triglycerides, free fatty acids, and cholesterol in the liver tissues | [50-52,54] |
| Liver inflammation | ↓ Myeloperoxidase activity, expression of tumor necrosis factor alpha gene and neutrophil infiltration in the liver | [54] |
| Oxidative stress in liver | ↓ Level of nitric oxide and malondialdehyde and ↑ level of glutathione and catalase in the liver tissue | [50,51,54] |
| Death of hepatocytes | ↓ Serum aminotransferases | [50-54] |
| Systemic inflammation | ↓ Serum IL-6 and tumor necrosis factor alpha | [51-53] |
| Bacterial translocation | ↓ Serum lipopolysaccharide | [51-54] |
| Gut dysbiosis | ↑ Firmicutes, Clostridiales and Lactobacillales; ↓ Proteobacteria and Campylobacterales | [51,53] |
| Damage to the intestinal epithelium | ↓ Serum diamine oxidase | [53] |
| Increased intestinal permeability | ↓ Serum D-lactate, ↑ the amount of occludin and other protein of tight junction in the gut epithelium, ↓ intestinal permeability for dyes | [50,52-54] |
| Dyslipidemia | ↓ Serum cholesterol and triglycerides | [50,52-54] |
These effects are not just due to the living bacteria themselves, which are part of the probiotics, but also the supernatant of their culture[57].
However, unlike many published experimental results, there are very few clinical trials on the effectiveness of probiotics in alcoholic liver disease. There was no effect of the probiotics (Lactobacillus subtilis and Streptococcus faecium) on total protein, cholesterol, or IL-1b levels in the blood according to RCT. The probiotics blocked the growth of blood LPS level in alcoholic hepatitis, but only in the cirrhosis subgroup. The number of Escherichia coli decreased in the feces in the probiotics groups. Changes in the levels of other biomarkers were not compared between the probiotic and placebo groups in this RCT[58].
It was shown that probiotics led to a more pronounced decrease in the activity of transaminases in the blood than standard therapy while significantly having no effects on the level of total bilirubin and GGT in alcoholic steatohepatitis in an earlier RCT[59].
Thus, the encouraging results of the use of probiotics in the treatment of alcoholic liver disease, obtained in experimental models, need to be confirmed by a large number of clinical trials.
PROBIOTICS FOR METABOLIC ASSOCIATED FATTY LIVER DISEASE
The use of probiotics led to a decrease in the level of steatosis, lipogenesis, oxidative stress, and inflammation in the liver, a decrease in the level of biomarkers of insulin resistance, bacterial translocation, gut permeability, and systemic inflammation and a decrease in blood level of lipids and glucose and in expression of the inflammation activator receptor genes (toll-like receptors 4 and 9, and NLRP3) in the liver in experimental MAFLD (Table 2)[60-66]. It also leads to a decrease in the LPS content and an increase in the bile acid content in feces[62,67], increases the content of cholesterol 7α-hydroxylase, which converts cholesterol to bile acids, and transporters of bile acids into bile in the liver[62], enhances the transfer of Nrf2 (transcription factor of antioxidant defense genes) to the nucleus[66], transfers metabolism from carbohydrate utilization to fat utilization[63], increases the acetate and butyrate level in feces[68], improves gut microbiome structure by increasing the abundance of gram-positive bacteria such as Firmicutes and decreasing gram-negative bacteria such as Bacteroidetes, Proteobacteria, and Fusobacteria[69], but does not affect the degree of cholesterol reabsorption[63].
Table 2.
Effects of probiotics on different disorders in experimental metabolic associated fatty liver disease
|
Disorder
|
Biomarker changes
|
Ref.
|
| Liver steatosis | ↓ Liver mass, the size and number of lipid droplets, the content of triglycerides, free fatty acids, and cholesterol in the liver tissues | [60-66] |
| Obesity | ↓ Body mass, subcutaneous fat | [61-63,65,66] |
| Intensified lipogenesis | ↓ Expression of the genes of sterol regulatory element-binding protein 1c (SREBP-1c), 3-hydroxy-3-methylglutaryl-CoA reductase, acetyl-CoA carboxylase 1, acetyl-CoA acetyltransferase 2, and fatty acid synthase, ↑ activated 5' adenosine monophosphate-activated protein kinase (SREBP-1c inhibitor) | [60,62,65,66] |
| Reduced lipolysis | ↑ Expression of the gene of peroxisome proliferator-activated receptor alpha (fatty acid catabolism enhancer) and acyl-CoA oxidase | [60,62] |
| Bile acid metabolism disorders | ↑ Expression of the gene of bile salt export pump, farnesoid X recetor, cholesterol 7a-hydroxylase, sodium taurocholate cotransporting polypeptide, ↓ the content of bile acids in the liver tissues | [62] |
| Oxidative stress | ↓ Total reactive oxygen species, lipid peroxidates, and malondialdehyde and ↑ glutathione, superoxide dismutase, and catalase in the liver tissue | [60,61,65,66] |
| Liver inflammation | ↓ Expression of the genes of tumor necrosis factor alpha, interleukin 1-beta and 6 and the content of NF-κB in the liver | [60] |
| Death of hepatocytes | ↓ Serum aminotransferases | [60,61,65] |
| Insulin resistance | ↓ HOMA-IR, insulin, resistin | [63,64] |
| Systemic inflammation | ↓ Serum tumor necrosis factor alpha, interleukin 1-beta and 6 | [60,64] |
| Bacterial translocation | ↓ Serum lipopolysaccharide | [64] |
| Increased gut permeability | ↑ Amount of proteins of tight junction in the gut | [64] |
| Disorders of the metabolism of carbohydrates and lipids | ↓ Serum total cholesterol, low density lipoprotein cholesterol, glucose, triglycerides, and free fatty acids, ↑ expression of the gene of low-density lipoprotein receptor | [60-62,65,66] |
Some of these effects can be achieved using the supernatants of the cultures of live probiotics[70].
Butarate, formed by probiotic strains, enhances the formation of tight junction proteins, as well as activates 5' adenosine monophosphate-activated protein kinase (inhibits lipogenesis) and increases the lifetime of Nrf2 in cell culture[71].
Consuming yogurt four times or more per week reduces the risk of developing MAFLD[72].
A number of systematic reviews with meta-analyses describing the effect of probiotics on the course of MAFLD were published recently. The meta-analysis, including 105 studies of patients with MAFLD and/or its underlying disorders (obesity and/or diabetes), showed that administration of probiotics leads to a decrease in body weight, body mass index, waist circumference, body fat mass, visceral and subcutaneous adipose tissue mass, fasting glucose, glycated hemoglobin, insulin, HOMA-IR, ALT, AST, triglycerides and CRP[73]. A meta-analysis that included 22 studies of patients with MAFLD showed that probiotics lower weight, body mass index, ALT, AST, GGT, ALP, total cholesterol, LDL-C, triglycerides, glucose, insulin, TNF-α, leptin, and liver steatosis and do not significantly affect waist circumference, waist-to-hip ratio, fat mass, serum albumin, HDL-C, HOMA-IR, CRP, or IL-6[74]. Other meta-analysis came to broadly similar conclusions[75]. The fourth meta-analysis showed that administration of probiotics for MAFLD resulted in a decrease in liver fibroscan stiffness[76].
Several new RCTs have been published following these meta-analyses.
The use of a multi-strain probiotic for 1 year in patients with metabolic associated steatohepatitis (MASH) resulted in a decrease in the severity of ballooning necrosis and fibrosis, without significantly affecting steatosis and inflammatory infiltration of liver compared to the placebo. Moreover, the level of bilirubin, ALT, ALP, leptin, TNF-α, IL-1b, IL-6, and LPS decreased in the blood, without significant difference in HOMA-IR and body weight[77].
The use of another multi-strain probiotic for 12 wk led to, among other things, a decrease in liver fat content according to MRI data and an increase in the Bacteroidetes/Firmicutes ratio[78].
A combined probiotic (Bifidobacterium, Lactobacillus and Enterococcus, 1 g two times per day, 3 mo) in histologically verified MAFLD lowered the serum levels of ALT, AST, GGT, total cholesterol, triglycerides, and HOMA-IR and the value of the histological scale of steatohepatitis activity NAS, and proportion of patients with dysbiosis, but did not significantly affect the serum levels of total bilirubin and high density lipoprotein cholesterol[79].
The use of a probiotic (L. casei, L. rhamnosus, L. acidophilus, Bifidobacterium longum, and B. breve) for MASH led to a decrease in the serum levels of triglycerides, ALT, AST, GGT, and ALP, but did not significantly affect fasting blood sugar, the serum levels of cholesterol and its fractions, CRP, weight, body mass index, percent body fat, waist circumference, and waist-to-hip ratio[80].
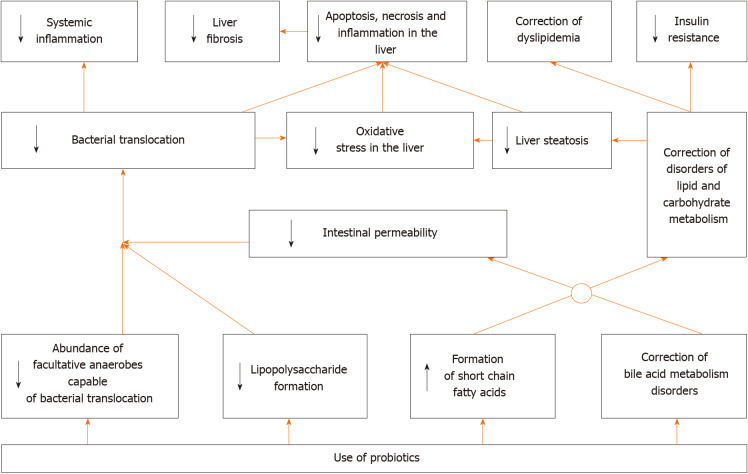
In general, it can be argued that RCTs and their meta-analyses have confirmed most of the results obtained in experimental models of MAFLD. However, we did not find any studies that described the effect of probiotics on prognosis in this disease, which is a challenge for future researchers. Based on the data obtained, the following mechanism of the development of positive effect of probiotics in MAFLD can be assumed (Figure 3).
Figure 3.
Suggested mechanism of action of probiotics in metabolic associated fatty liver disease.
PROBIOTICS FOR VIRAL HEPATITIS
Unlike for cirrhosis, alcoholic and MAFLD, probiotics have hardly been researched as drugs for viral hepatitis. Perhaps this is due to the fact that, unlike these diseases, effective therapy for viral hepatitis already exists.
Long-term use of a probiotic Enterococcus fecalis strain FK-23 in chronic viral hepatitis C led to a decrease in ALT and AST levels, with no significant effect on viral load, blood total protein, urea and hemoglobin levels, and platelet count in an uncontrolled clinical study[81].
Bifidobacterium adolescentis SPM0212 showed antiviral effects against hepatitis B virus in cell culture[82].
PROBIOTICS FOR CHOLESTATIC DISEASES
The use of L. rhamnosus GG reduced the biochemical and histological signs of hepatitis, cholestasis, and fibrosis after ligation of the common biliary duct in mice. Perhaps the reason for this is that probiotics increases the activity of FXR in the intestine. This receptor enhances the formation of fibroblast growth factor 15 (FGF15) in response to stimulation with bile acids. FGF15 reduces the production of bile acids in the liver due to negative feedback. With cholestasis, few bile acids enter the intestine, this receptor is not activated enough, the FGF15 content in the blood decreases, and the formation of bile acids in the liver increases. The latter, with cholestasis, have a toxic effect on the liver, which is manifested by its inflammation and fibrosis. The intake of this probiotic led to an increase in the activity of FXR in the intestine and FGF15 in the blood, which close this feedback, protecting the liver from autointoxication with bile acids. This hypothesis is supported by the fact that the use of powerful antagonists of FXR blocks the positive effect of the probiotic in this case and the culture supernatant of this probiotic increases the activity of this receptor in tissue cultures[83].
In addition, L. rhamnosus GG increases the content of Firmicutes and Actinobacteria in the gut microbiota, which convert primary bile acids into secondary ones, which are poorly absorbed, and therefore, removed with feces. There is a significant increase in the content of bile acids due to secondary ones, with an absolute and relative decrease in the content of primary bile acids in the feces of such animals. That is, administration of this probiotic for cholestasis leads not only to a decrease in the formation of new bile acids, but also to an increase in the removal of already formed ones with the feces[83].
L. rhamnosus GG has a similar protective effect in another model of cholestatic liver damage, in which the excretion of bile acids is blocked due to the knockout of the gene of their transporter multidrug resistance protein 2[83]. The use of L. casei rhamnosus was as effective as neomycin in preventing cholangitis in patients with biliary atresia who underwent Kasai operation[84].
The use of probiotics for primary sclerosing cholangitis combined with inflammatory bowel diseases did not have a significant effect on pruritus, fatigue, serum level of bilirubin, ALP, GGT, AST, ALT, prothrombin, albumin, and bile salts in a very small RCT that included 14 patients[85].
We did not find any other clinical trial of probiotics in chronic cholestatic diseases. Given the very encouraging results of the experimentary study, a large RCT on this topic seems to be very interesting.
PROBIOTICS FOR AUTOIMMUNE HEPATITIS
We found only one study describing the effect of probiotics on liver condition in experimental autoimmune hepatitis. It was shown that they lead to a decrease in the formation of TNF-α, IL-6, and IL-1b in the liver, as well as in the proportion of Th-17 cells among CD4+ lymphocytes in the liver and spleen[86]. Further experimental studies and clinical trials are needed to clarify the usefulness of probiotics in the treatment of this disease.
CONCLUSION
In conclusion, the study of probiotics in hepatology is uneven. It has been proven that they are useful in hepatic encephalopathy, but their effect on other symptoms and syndromes of cirrhosis is poorly studied. Their effectiveness in the treatment of MAFLD has been proven both in experimental models and in clinical trials, but their effect on the prognosis of this disease has not been described. The beneficial effects of probiotics in alcoholic liver disease have been well shown in many experimental studies, but there are very few clinical trials to support them. The effect of probiotics on the course of other liver diseases is either poorly studied (primary sclerosing cholangitis, chronic hepatitis B and C, autoimmune hepatitis), or not studied at all (primary biliary cholangitis, hepatitis A and E, Wilson's disease, hemochromatosis, storage diseases, vascular liver diseases, etc.).
Footnotes
Conflict-of-interest statement: Dr. Maslennikov reports a grant from by Biocodex Microbiota Foundation (National Research Grant Russia 2019) outside the submitted work.
Manuscript source: Invited manuscript
Peer-review started: May 19, 2021
First decision: June 4, 2021
Article in press: August 16, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen F S-Editor: Yan JP L-Editor: A P-Editor: Guo X
Contributor Information
Roman Maslennikov, Department of Internal Medicine, Gastroenterology and Hepatology, Sechenov University, Moscow 119435, Russia; Scientific Community for Human Microbiome Research, Moscow 119435, Russia; Department of Internal Medicine, Consultative and Diagnostic Center of the Moscow City Health Department, Moscow 107564, Russia. mmmm00@yandex.ru.
Vladimir Ivashkin, Department of Internal Medicine, Gastroenterology and Hepatology, Sechenov University, Moscow 119435, Russia; Scientific Community for Human Microbiome Research, Moscow 119435, Russia.
Irina Efremova, Department of Internal Medicine, Gastroenterology and Hepatology, Sechenov University, Moscow 119435, Russia.
Elena Poluektova, Department of Internal Medicine, Gastroenterology and Hepatology, Sechenov University, Moscow 119435, Russia; Scientific Community for Human Microbiome Research, Moscow 119435, Russia.
Elena Shirokova, Department of Internal Medicine, Gastroenterology and Hepatology, Sechenov University, Moscow 119435, Russia.
References
- 1.Lata J, Jurankova J, Kopacova M, Vitek P. Probiotics in hepatology. World J Gastroenterol. 2011;17:2890–2896. doi: 10.3748/wjg.v17.i24.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Simbrunner B, Mandorfer M, Trauner M, Reiberger T. Gut-liver axis signaling in portal hypertension. World J Gastroenterol. 2019;25:5897–5917. doi: 10.3748/wjg.v25.i39.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trush EA, Poluektova EA, Beniashvilli AG, Shifrin OS, Poluektov YM, Ivashkin VT. The Evolution of Human Probiotics: Challenges and Prospects. Probiotics Antimicrob Proteins. 2020;12:1291–1299. doi: 10.1007/s12602-019-09628-4. [DOI] [PubMed] [Google Scholar]
- 5.Maslennikov R, Ivashkin V, Efremova I, Alieva A, Kashuh E, Tsvetaeva E, Poluektova E, Shirokova E, Ivashkin K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J Hepatol 2021; 13: 557-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949–955. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int. 2018;12:567–576. doi: 10.1007/s12072-018-9898-2. [DOI] [PubMed] [Google Scholar]
- 9.Maslennikov R, Pavlov C, Ivashkin V. Is small intestinal bacterial overgrowth a cause of hyperdynamic circulation in cirrhosis? Turk J Gastroenterol. 2019;30:964–975. doi: 10.5152/tjg.2019.18551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, Gasbarrini A, Miele L, Grieco A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol. 2019;25:4814–4834. doi: 10.3748/wjg.v25.i33.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsiaoussis GI, Assimakopoulos SF, Tsamandas AC, Triantos CK, Thomopoulos KC. Intestinal barrier dysfunction in cirrhosis: Current concepts in pathophysiology and clinical implications. World J Hepatol. 2015;7:2058–2068. doi: 10.4254/wjh.v7.i17.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 13.Maslennikov R, Driga A, Ivashkin K, Ivashkin V. NT-proBNP as a biomarker for hyperdynamic circulation in decompensated cirrhosis. Gastroenterol Hepatol Bed Bench. 2018;11:325–332. [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe A, Lim JK, Jakab SS. Pathophysiology of Hepatic Encephalopathy. Clin Liver Dis. 2020;24:175–188. doi: 10.1016/j.cld.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–171. doi: 10.1080/19490976.2019.1674124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174–1182. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao S, Jiang S, Qian D, Duan J. Modulation of microbially derived short-chain fatty acids on intestinal homeostasis, metabolism, and neuropsychiatric disorder. Appl Microbiol Biotechnol. 2020;104:589–601. doi: 10.1007/s00253-019-10312-4. [DOI] [PubMed] [Google Scholar]
- 18.Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:235–246. doi: 10.1038/s41575-018-0099-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Z, Zhong W. Targeting the gut barrier for the treatment of alcoholic liver disease. Liver Res. 2017;1:197–207. doi: 10.1016/j.livres.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD) Cell Mol Life Sci. 2019;76:1541–1558. doi: 10.1007/s00018-019-03011-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little R, Wine E, Kamath BM, Griffiths AM, Ricciuto A. Gut microbiome in primary sclerosing cholangitis: A review. World J Gastroenterol. 2020;26:2768–2780. doi: 10.3748/wjg.v26.i21.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhillon AK, Kummen M, Trøseid M, Åkra S, Liaskou E, Moum B, Vesterhus M, Karlsen TH, Seljeflot I, Hov JR. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 2019;39:371–381. doi: 10.1111/liv.13979. [DOI] [PubMed] [Google Scholar]
- 23.Lin R, Zhou L, Zhang J, Wang B. Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int J Clin Exp Pathol. 2015;8:5153–5160. [PMC free article] [PubMed] [Google Scholar]
- 24.Tang R, Wei Y, Li Y, Chen W, Chen H, Wang Q, Yang F, Miao Q, Xiao X, Zhang H, Lian M, Jiang X, Zhang J, Cao Q, Fan Z, Wu M, Qiu D, Fang JY, Ansari A, Gershwin ME, Ma X. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67:534–541. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
- 25.Cai X, Deng L, Ma X, Guo Y, Feng Z, Liu M, Guan Y, Huang Y, Deng J, Li H, Sang H, Liu F, Yang X. Altered diversity and composition of gut microbiota in Wilson's disease. Sci Rep. 2020;10:21825. doi: 10.1038/s41598-020-78988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun Y, Chang Y, Kim HN, Ryu S, Kwon MJ, Cho YK, Kim HL, Cheong HS, Joo EJ. Alterations of the Gut Microbiome in Chronic Hepatitis B Virus Infection Associated with Alanine Aminotransferase Level. J Clin Med. 2019;8 doi: 10.3390/jcm8020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M, Yoshiji H, Tanaka Y. Gut Dysbiosis Associated With Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:869–877. doi: 10.1093/cid/ciy205. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Niu Z, Zou M, Liu S, Wang M, Gu X, Lu H, Tian H, Jha R. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J Dairy Sci. 2020;103:5816–5829. doi: 10.3168/jds.2019-18003. [DOI] [PubMed] [Google Scholar]
- 29.Markowiak-Kopeć P, Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12 doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Yu Z, Tian F, Zhao J, Zhang H, Zhai Q, Chen W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb Cell Fact. 2020;19:23. doi: 10.1186/s12934-020-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhiman RK, Thumburu KK, Verma N, Chopra M, Rathi S, Dutta U, Singal AK, Taneja S, Duseja A, Singh M. Comparative Efficacy of Treatment Options for Minimal Hepatic Encephalopathy: A Systematic Review and Network Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:800–812.e25. doi: 10.1016/j.cgh.2019.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Cao Q, Yu CB, Yang SG, Cao HC, Chen P, Deng M, Li LJ. Effect of probiotic treatment on cirrhotic patients with minimal hepatic encephalopathy: A meta-analysis. Hepatobiliary Pancreat Dis Int. 2018;17:9–16. doi: 10.1016/j.hbpd.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev 2017; 2: CD008716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, Wang Y, Ling Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596–3604. doi: 10.1177/0300060518776064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga H, Tamiya Y, Mitsuyama K, Ishibashi M, Matsumoto S, Imaoka A, Hara T, Nakano M, Ooeda K, Umezaki Y, Sata M. Probiotics promote rapid-turnover protein production by restoring gut flora in patients with alcoholic liver cirrhosis. Hepatol Int. 2013;7:767–774. doi: 10.1007/s12072-012-9408-x. [DOI] [PubMed] [Google Scholar]
- 36.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs M, Thacker LR, Wade JB, Daita K, Sistrun S, White MB, Noble NA, Thorpe C, Kakiyama G, Pandak WM, Sikaroodi M, Gillevet PM. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39:1113–1125. doi: 10.1111/apt.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Román E, Nieto JC, Gely C, Vidal S, Pozuelo M, Poca M, Juárez C, Guarner C, Manichanh C, Soriano G. Effect of a Multistrain Probiotic on Cognitive Function and Risk of Falls in Patients With Cirrhosis: A Randomized Trial. Hepatol Commun. 2019;3:632–645. doi: 10.1002/hep4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazemi A, Soltani S, Ghorabi S, Keshtkar A, Daneshzad E, Nasri F, Mazloomi SM. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: A systematic review and meta-analysis of clinical trials. Clin Nutr. 2020;39:789–819. doi: 10.1016/j.clnu.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Macnaughtan J, Figorilli F, García-López E, Lu H, Jones H, Sawhney R, Suzuki K, Fairclough S, Marsden J, Moratella A, Cox IJ, Thomas L, Davies N, Williams R, Mookerjee R, Wright G, Jalan R. A Double-Blind, Randomized Placebo-Controlled Trial of Probiotic Lactobacillus casei Shirota in Stable Cirrhotic Patients. Nutrients. 2020;12 doi: 10.3390/nu12061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horvath A, Durdevic M, Leber B, di Vora K, Rainer F, Krones E, Douschan P, Spindelboeck W, Durchschein F, Zollner G, Stauber RE, Fickert P, Stiegler P, Stadlbauer V. Changes in the Intestinal Microbiome during a Multispecies Probiotic Intervention in Compensated Cirrhosis. Nutrients. 2020;12 doi: 10.3390/nu12061874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horvath A, Leber B, Schmerboeck B, Tawdrous M, Zettel G, Hartl A, Madl T, Stryeck S, Fuchs D, Lemesch S, Douschan P, Krones E, Spindelboeck W, Durchschein F, Rainer F, Zollner G, Stauber RE, Fickert P, Stiegler P, Stadlbauer V. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment Pharmacol Ther. 2016;44:926–935. doi: 10.1111/apt.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, Khattri A, Malhotra S, Duseja A, Chawla YK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–37.e3. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Gupta N, Kumar A, Sharma P, Garg V, Sharma BC, Sarin SK. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int. 2013;33:1148–1157. doi: 10.1111/liv.12172. [DOI] [PubMed] [Google Scholar]
- 44.Marlicz W, Wunsch E, Mydlowska M, Milkiewicz M, Serwin K, Mularczyk M, Milkiewicz P, Raszeja-Wyszomirska J. The effect of short term treatment with probiotic VSL#3 on various clinical and biochemical parameters in patients with liver cirrhosis. J Physiol Pharmacol. 2016;67:867–877. [PubMed] [Google Scholar]
- 45.Rincón D, Vaquero J, Hernando A, Galindo E, Ripoll C, Puerto M, Salcedo M, Francés R, Matilla A, Catalina MV, Clemente G, Such J, Bañares R. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 2014;34:1504–1512. doi: 10.1111/liv.12539. [DOI] [PubMed] [Google Scholar]
- 46.Rashid SK, Idris-Khodja N, Auger C, Alhosin M, Boehm N, Oswald-Mammosser M, Schini-Kerth VB. Probiotics (VSL#3) prevent endothelial dysfunction in rats with portal hypertension: role of the angiotensin system. PLoS One. 2014;9:e97458. doi: 10.1371/journal.pone.0097458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Q, Gao F, Yang X, Hu Y, Liu Y, Hou Y, Li Y, Zhu B, Niu S, Huang Y, Wang X. Protective Effect of Probiotics against Esophagogastric Variceal Rebleeding in Patients with Liver Cirrhosis after Endoscopic Therapy. Med Sci Monit. 2020;26:e924040. doi: 10.12659/MSM.924040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran L, Dontaraju VS, Patel K. Lactobacillus-Associated Spontaneous Bacterial Peritonitis in a Liver Cirrhosis Patient on Probiotics. Cureus. 2020;12:e11896. doi: 10.7759/cureus.11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naqvi SSB, Nagendra V, Hofmeyr A. Probiotic related Lactobacillus rhamnosus endocarditis in a patient with liver cirrhosis. IDCases. 2018;13:e00439. doi: 10.1016/j.idcr.2018.e00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai YS, Lin SW, Chen YL, Chen CC. Effect of probiotics Lactobacillus paracasei GKS6, L. plantarum GKM3, and L. rhamnosus GKLC1 on alleviating alcohol-induced alcoholic liver disease in a mouse model. Nutr Res Pract. 2020;14:299–308. doi: 10.4162/nrp.2020.14.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Z, Wu Y, Wang Y, Sun H, You Y, Piao C, Liu J. Lactobacillus rhamnosus Granules Dose-Dependently Balance Intestinal Microbiome Disorders and Ameliorate Chronic Alcohol-Induced Liver Injury. J Med Food. 2020;23:114–124. doi: 10.1089/jmf.2018.4357. [DOI] [PubMed] [Google Scholar]
- 52.Zheng TX, Pu SL, Tan P, Du YC, Qian BL, Chen H, Fu WG, Huang MZ. Liver Metabolomics Reveals the Effect of Lactobacillus reuteri on Alcoholic Liver Disease. Front Physiol. 2020;11:595382. doi: 10.3389/fphys.2020.595382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang H, Lin Z, Zeng Y, Lin X, Zhang Y. Probiotic and glutamine treatments attenuate alcoholic liver disease in a rat model. Exp Ther Med. 2019;18:4733–4739. doi: 10.3892/etm.2019.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim BK, Lee IO, Tan PL, Eor JY, Hwang JK, Kim SH. Protective Effect of Lactobacillus fermentum LA12 in an Alcohol-Induced Rat Model of Alcoholic Steatohepatitis. Korean J Food Sci Anim Resour. 2017;37:931–939. doi: 10.5851/kosfa.2017.37.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shukla PK, Meena AS, Manda B, Gomes-Solecki M, Dietrich P, Dragatsis I, Rao R. Lactobacillus plantarum prevents and mitigates alcohol-induced disruption of colonic epithelial tight junctions, endotoxemia, and liver damage by an EGF receptor-dependent mechanism. FASEB J. 2018:fj201800351R. doi: 10.1096/fj.201800351R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu J, Ren T, Zhou M, Cheng M. The combination of blueberry juice and probiotics reduces apoptosis of alcoholic fatty liver of mice by affecting SIRT1 pathway. Drug Des Devel Ther. 2016;10:1649–1661. doi: 10.2147/DDDT.S102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G32–G41. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD, Cheon GJ, Choi DH, Ham YL, Shin DH, Kim EJ. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27:1300–1306. doi: 10.1097/MEG.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 59.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan Y, Liu C, Zhao S, Wang X, Wang J, Zhang H, Wang Y, Zhao G. Probiotic Bifidobacterium lactis V9 attenuates hepatic steatosis and inflammation in rats with non-alcoholic fatty liver disease. AMB Express. 2020;10:101. doi: 10.1186/s13568-020-01038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Azarang A, Farshad O, Ommati MM, Jamshidzadeh A, Heydari R, Abootalebi SN, Gholami A. Protective Role of Probiotic Supplements in Hepatic Steatosis: A Rat Model Study. Biomed Res Int. 2020;2020:5487659. doi: 10.1155/2020/5487659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M, Guo WL, Li QY, Xu JX, Cao YJ, Liu B, Yu XD, Rao PF, Ni L, Lv XC. The protective mechanism of Lactobacillus plantarum FZU3013 against non-alcoholic fatty liver associated with hyperlipidemia in mice fed a high-fat diet. Food Funct. 2020;11:3316–3331. doi: 10.1039/c9fo03003d. [DOI] [PubMed] [Google Scholar]
- 63.Naudin CR, Maner-Smith K, Owens JA, Wynn GM, Robinson BS, Matthews JD, Reedy AR, Luo L, Wolfarth AA, Darby TM, Ortlund EA, Jones RM. Lactococcus lactis Subspecies cremoris Elicits Protection Against Metabolic Changes Induced by a Western-Style Diet. Gastroenterology. 2020;159:639–651.e5. doi: 10.1053/j.gastro.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Z, Chen L, Zhao Y, Wang C, Duan C, Yang G, Niu C, Li S. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl Microbiol Biotechnol. 2020;104:5273–5282. doi: 10.1007/s00253-020-10633-9. [DOI] [PubMed] [Google Scholar]
- 65.Park EJ, Lee YS, Kim SM, Park GS, Lee YH, Jeong DY, Kang J, Lee HJ. Beneficial Effects of Lactobacillus plantarum Strains on Non-Alcoholic Fatty Liver Disease in High Fat/High Fructose Diet-Fed Rats. Nutrients. 2020;12 doi: 10.3390/nu12020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Z, Wang C, Zhang L, Zhao Y, Duan C, Zhang X, Gao L, Li S. Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl Microbiol Biotechnol. 2019;103:5843–5850. doi: 10.1007/s00253-019-09703-4. [DOI] [PubMed] [Google Scholar]
- 67.Lee NY, Joung HC, Kim BK, Kim BY, Park TS, Suk KT. Lactobacillus lactis CKDB001 ameliorate progression of nonalcoholic fatty liver disease through of gut microbiome: addendum. Gut Microbes. 2020;12:1829449. doi: 10.1080/19490976.2020.1829449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li H, Liu F, Lu J, Shi J, Guan J, Yan F, Li B, Huo G. Probiotic Mixture of Lactobacillus plantarum Strains Improves Lipid Metabolism and Gut Microbiota Structure in High Fat Diet-Fed Mice. Front Microbiol. 2020;11:512. doi: 10.3389/fmicb.2020.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang W, Li Q, Chai W, Sun C, Zhang T, Zhao C, Yuan Y, Wang X, Liu H, Ye H. Lactobacillus paracasei Jlus66 extenuate oxidative stress and inflammation via regulation of intestinal flora in rats with non alcoholic fatty liver disease. Food Sci Nutr. 2019;7:2636–2646. doi: 10.1002/fsn3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Q, Liu Y, Li F, Gu Z, Liu M, Shao T, Zhang L, Zhou G, Pan C, He L, Cai J, Zhang X, Barve S, McClain CJ, Chen Y, Feng W. Probiotic culture supernatant improves metabolic function through FGF21-adiponectin pathway in mice. J Nutr Biochem. 2020;75:108256. doi: 10.1016/j.jnutbio.2019.108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One. 2013;8:e63388. doi: 10.1371/journal.pone.0063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang S, Fu J, Zhang Q, Liu L, Lu M, Meng G, Yao Z, Wu H, Xia Y, Bao X, Gu Y, Sun S, Wang X, Zhou M, Jia Q, Song K, Wu Y, Xiang H, Niu K. Association between habitual yogurt consumption and newly diagnosed non-alcoholic fatty liver disease. Eur J Clin Nutr. 2020;74:491–499. doi: 10.1038/s41430-019-0497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koutnikova H, Genser B, Monteiro-Sepulveda M, Faurie JM, Rizkalla S, Schrezenmeir J, Clément K. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019;9:e017995. doi: 10.1136/bmjopen-2017-017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang Y, Huang J, Zhang WY, Qin S, Yang YX, Ren H, Yang QB, Hu H. Effects of probiotics on nonalcoholic fatty liver disease: a systematic review and meta-analysis. Therap Adv Gastroenterol. 2019;12:1756284819878046. doi: 10.1177/1756284819878046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. 2019;110:139–149. doi: 10.1093/ajcn/nqz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan MY, Mihali AB, Rawala MS, Aslam A, Siddiqui WJ. The promising role of probiotic and synbiotic therapy in aminotransferase levels and inflammatory markers in patients with nonalcoholic fatty liver disease - a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31:703–715. doi: 10.1097/MEG.0000000000001371. [DOI] [PubMed] [Google Scholar]
- 77.Duseja A, Acharya SK, Mehta M, Chhabra S Shalimar, Rana S, Das A, Dattagupta S, Dhiman RK, Chawla YK. High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol. 2019;6:e000315. doi: 10.1136/bmjgast-2019-000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahn SB, Jun DW, Kang BK, Lim JH, Lim S, Chung MJ. Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Sci Rep. 2019;9:5688. doi: 10.1038/s41598-019-42059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cai GS, Su H, Zhang J. Protective effect of probiotics in patients with non-alcoholic fatty liver disease. Medicine (Baltimore) 2020;99:e21464. doi: 10.1097/MD.0000000000021464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Behrouz V, Aryaeian N, Zahedi MJ, Jazayeri S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: A randomized clinical trial. J Food Sci. 2020;85:3611–3617. doi: 10.1111/1750-3841.15367. [DOI] [PubMed] [Google Scholar]
- 81.Oo KM, Lwin AA, Kyaw YY, Tun WM, Fukada K, Goshima A, Shimada T, Okada S. Safety and long-term effect of the probiotic FK-23 in patients with hepatitis C virus infection. Biosci Microbiota Food Health. 2016;35:123–128. doi: 10.12938/bmfh.2015-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee DK, Kang JY, Shin HS, Park IH, Ha NJ. Antiviral activity of Bifidobacterium adolescentis SPM0212 against Hepatitis B virus. Arch Pharm Res. 2013;36:1525–1532. doi: 10.1007/s12272-013-0141-3. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y, Chen K, Li F, Gu Z, Liu Q, He L, Shao T, Song Q, Zhu F, Zhang L, Jiang M, Zhou Y, Barve S, Zhang X, McClain CJ, Feng W. Probiotic Lactobacillus rhamnosus GG Prevents Liver Fibrosis Through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology. 2020;71:2050–2066. doi: 10.1002/hep.30975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lien TH, Bu LN, Wu JF, Chen HL, Chen AC, Lai MW, Shih HH, Lee IH, Hsu HY, Ni YH, Chang MH. Use of Lactobacillus casei rhamnosus to Prevent Cholangitis in Biliary Atresia After Kasai Operation. J Pediatr Gastroenterol Nutr. 2015;60:654–658. doi: 10.1097/MPG.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 85.Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: a randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol. 2008;20:688–692. doi: 10.1097/MEG.0b013e3282f5197e. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H, Liu M, Liu X, Zhong W, Li Y, Ran Y, Guo L, Chen X, Zhao J, Wang B, Zhou L. Bifidobacterium animalis ssp. Lactis 420 Mitigates Autoimmune Hepatitis Through Regulating Intestinal Barrier and Liver Immune Cells. Front Immunol. 2020;11:569104. doi: 10.3389/fimmu.2020.569104. [DOI] [PMC free article] [PubMed] [Google Scholar]