Abstract
Varying degrees of liver injuries have been reported in patients infected with the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). In general, oxidative stress is actively involved in initiation and progression of liver damage. The liver metabolizes various compounds that produce free radicals. Maintaining the oxidative/antioxidative balance is important in coronavirus disease 2019 (COVID-19) patients. Antioxidant vitamins, essential trace elements and food compounds, such as polyphenols, appear to be promising agents, with effects in oxidative burst. Deficiency of these nutrients suppresses immune function and increases susceptibility to COVID-19. Daily micronutrient intake is necessary to support anti-inflammatory and antioxidative effects but for immune function may be higher than current recommended dietary intake. Antioxidant supplements (β-carotene, vitamin A, vitamin C, vitamin E, and selenium) could have a potential role in patients with liver damage. Available evidence suggests that supplementing the diet with a combination of micronutrients may help to optimize immune function and reduce the risk of infection. Clinical trials based on the associations of diet and SARS-CoV-2 infection are lacking. Unfortunately, it is not possible to definitively determine the dose, route of administration and best timing to intervene with antioxidants in COVID-19 patients because clinical trials are still ongoing. Until then, hopefully, this review will enable clinicians to understand the impact of micronutrient dietary intake and liver status assessment in COVID-19 patients.
Keywords: SARS-CoV-2, COVID-19, Liver injury, Antioxidants, Nutrients, Treatment
Core Tip: In this review, we highlight the importance of an optimal micronutrient intake and status to boost the immune system, providing special emphasis on liver injury during the coronavirus disease 2019 (commonly known as COVID-19) crisis and focusing on the most relevant nutrients that reduce oxidative stress.
INTRODUCTION
Since its first appearance in December 2019 in Wuhan, China, coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), has spread swiftly around the world. The angiotensin converting enzyme 2 (ACE2) receptor has been identified as the target vehicle for the virus entrance into the host cell[1]. After binding of SARS-CoV-2, the bioavailability of ACE2 is reduced and angiotensin 2 becomes extremely available to promote oxidative stress cascades and inflammatory responses, which in turn contribute to the pathological host responses and the severity of disease[2,3]. The multiple organ involvement is mainly attributed to the wide distribution of ACE2 receptors[4]. Incidence of liver injury in SARS-CoV-2–infected patients is about 36% (range: 21%–52%)[1].
Any liver damage occurring during the COVID-19 course or treatment, in patients with or without previous liver diseases, could be considered as COVID-19-associated liver injury[4]. The presence of ACE2 receptors in hepatic cholangiocytes has pointed out the possibility of direct infection and active SARS-CoV-2 replication in the liver[5]. Many studies have also shown that COVID-19-associated liver injuries can be induced and/or exacerbated by the inflammatory response, endothelial changes, hypoxia and coagulopathy characteristic of severe disease course[4]. Accordingly, higher rates of liver dysfunction have been detected in critically ill COVID-19 patients[6]. In addition, compatibility between histological alternations and impaired blood flow has been found through the post-mortem analysis of COVID-19 patients’ liver samples. Marked derangement of intrahepatic blood vessels, including portal vein fibrosis and wall inflammation, herniated portal vein with activated Kupffer cells containing large necrotic debris, and vascular thrombosis accompanied with high D-dimer (≥ 500 ng/dL) and high platelet count, have been observed[7].
In addition to impaired blood flow, more than a 50% of post-mortem liver samples of COVID-19 patients have shown the presence of large, small or mixed fatty droplets in hepatic parenchyma, a condition known as hepatic steatosis[7]. More recently, the hepatic steatosis, which is commonly associated with comorbidities such as metabolic syndrome, diabetes, obesity and hypertension, has also been recognized as an independent risk factor for severe disease in patients with COVID-19, significantly contributing to greater incidence of critical illness and lethality[8].
POSSIBLE MECHANISMS OF LIVER INJURY IN COVID-19
The main biochemical findings in COVID-19-affected liver are elevated transaminase aspartate aminotransferase (commonly referred to as AST) and alanine transaminase (ALT), which are above referent range in 20.0%-22.5% and 14.6%-20.1% COVID-19 cases, respectively. The increase of AST and ALT is followed by slightly increased total bilirubin in 35% of cases, as well as elevated alkaline phosphatase and gamma-glutamyl transferase in 6.1% and 21.1% of COVID-19 patients, respectively[4]. A meta-analysis of 25 relevant studies showed that COVID-19 survivors accounted for a lower percent of patients and lower increase of these parameters, compared with non-survivors. Moreover, cured patients had lower levels of total bilirubin and lactate dehydrogenase, higher level of albumin and lower proportion of markedly decreased albumin than patients who succumbed to the disease[9].
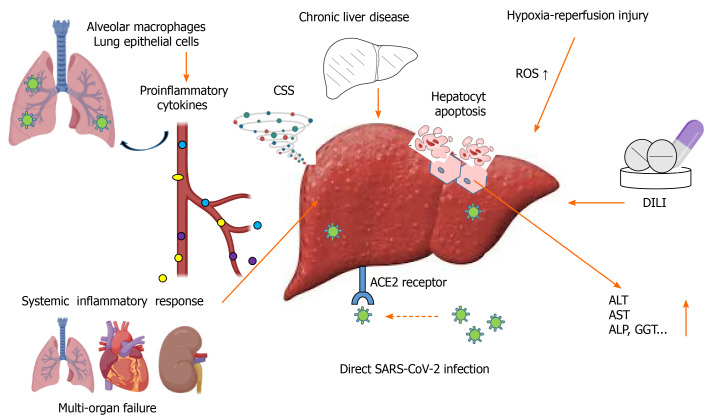
Until now, proposed mechanisms of COVID-19-associated liver injuries are: (1) Systemic inflammatory response; (2) Hypoxia–reperfusion injury; (3) Drug-induced liver injury; (4) Direct viral infection; and (5) Coexisting chronic liver disease [non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease (commonly referred to as ALD), hepatitis B virus and/or hepatitis C virus infection, hepatocellular carcinoma, etc.] (Figure 1).
Figure 1.
Pathophysiological mechanisms of coronavirus disease 2019-associated liver injuries. Created with BioRender.com. ACE2: Angiotensin converting enzyme 2; ALP: Alkaline phosphatase; ALT: Alanine transaminase; AST: Aspartate aminotransferase; CSS: Cytokine storm syndrome; DILI: Drug-induced liver injury; GGT: Gamma-glutamyl transferase; ROS: Reactive oxygen species.
Systemic inflammatory response is an extensive body inflammatory activity. It begins when SARS-CoV-2 lung infection activates alveolar macrophages and lung epithelial cells to promote massive release of proinflammatory cytokines, particularly interleukin (IL)-6, tumor necrosis factor (TNF), and IL-1β[10]. Acting locally, these cytokines increase permeability of the lung endothelium and support further dissemination of the pathogen, but after reaching the circulation they spread through the body and induce excessive immune response, with deleterious impact on many organs (Figure 1). Proinflammatory cytokines further hyperactivate monocytes, macrophages and T lymphocytes, generating a self-producing loop which results in cytokines overwhelming the system [i.e. cytokine storm syndrome (also known as CSS)]. Thus, the cytokine storm can result in multiorgan damage, including to the liver[2].
In addition to CSS, hypoxia, as a consequence of pulmonary failure, is one of the most important factors causing secondary liver injury in COVID-19 patients[11]. Low or no circulation, reduced oxygen supply and lipid accumulation in hepatocytes during shock, and the hypoxic state lead to marked increases in reactive oxygen species (ROS) and their peroxidation products[12]. These hypoxia-induced products are directly involved in hepatic cell damage and death (Figure 1); while, on the other hand, they can act as second messengers to activate redox-sensitive transcription factors. They exaggerate the release of multiple proinflammatory factors and cause hepatocyte apoptosis, followed by further infiltration of inflammatory cells into hepatic tissue and consequent liver damage/failure[13]. However, the main part of liver injury caused by disturbed blood flow occurs during reperfusion, rather than at the time of the hypoxic state[14].
During the course of the COVID-19 epidemic, infected patients have been treated with different antipyretics (e.g., paracetamol, ibuprofen), analgetics (e.g., caffeine), anti-inflammatory agents (e.g., aminosalicylates, corticosteroids), antirheumatics (e.g., chloroquine, hydroxychloroquine) and antiviral agents (e.g., baricitinib, abidol, lopinavir, ritonavir, remdesivir), and many of these drugs are hepatotoxic[15]. However, despite reports on more frequent liver injuries in critically ill COVID-19 patients[11,16,17], there is no evidence on liver dysfunction induced exclusively by drugs in severe SARS-CoV-2 infection.
Abundant expression of ACE2 receptors makes the liver susceptible to direct infection by SARS-CoV-2[1,11]. Up-regulated expression of ACE2 receptors in hepatocytes of COVID-19 patients that suffer from severe hypoxia has also been found. Yet, there is no evidence of viral replication inside hepatocytes. On the other side, the liver harbors the body’s largest macrophage pool, indicating that the liver damage may be caused by lymphocytes recognizing infected hepatocytes and consequently inducing a cell death process[1].
OXIDATIVE STRESS, LIVER DISEASES AND COVID-19
Oxidative stress plays an important role in liver diseases. Although both ROS and reactive nitrogen species (RNS) are involved in normal physiological processes, excessive amounts of either can destroy the major cellular components, proteins, lipids, and DNA. Chronic liver diseases are one of the biggest risk factors for liver complications in COVID-19, and commonly these diseases are marked by increased oxidative stress[18]. In chronic liver diseases, function in all types of cells is altered including hepatocyte, stellate, endothelial and Kupffer cells and their impaired gene expression led to increased liver damage[19]. Oxidative stress and mitochondrial dysfunction have a key role in the development of NAFLD. Mitochondrial dysfunction implies not only the formation of ROS and RNS, glutathione (GSH) depletion, and protein oxidation but also inhibition of the β-oxidation of lipids, increased steatosis, and lipid accumulation in hepatocytes[18]. ROS are generated in a lipid-rich environment, leading to lipid peroxidation (LPO) and further production and release of very reactive products of LPO. These LPO products impair the respiratory chain in hepatocytes, leading to impaired oxidative phosphorylation, reduced ATP synthesis, and further increase ROS production[20]. Moreover, ROS, along with products of LPO, induce activation of several cytokines, including TNF-α, IL-6 and IL-8, as well as cell death and fibrosis[21]. Furthermore, decreased activity of antioxidative enzymes, particularly superoxide dismutase and catalase, and lower level of coenzyme Q10 had been noticed in patients with liver diseases[22].
Most mechanisms of tissue damage due to SARS-CoV-2 infection are directly associated with oxidative stress[23]. Nevertheless, according to our knowledge, there are no studies about the parameters of oxidative stress in patients with SARS-CoV-2 infection. However, Polonikov et al[24] recently showed that people who had lower GSH levels before infection developed a more severe form of the disease than those with optimal GSH levels and suggested the crucial role of GSH in determining individual responsiveness to SARS-CoV-2 infection and disease pathogenesis.
In addition to producing O2 and H2O2, the cell infected by SARS-CoV-2 also produces IL-18 and IL-1β[2]. Besides, T lymphocytes and natural killer (NK) cells produce TNF-α, interferon (IFN)-γ, and IL-1β and stimulate the production of other proinflammatory cytokines, including IL-6 which contributes to the cytokine storm[25]. Additionally, IL-6 acts on liver cells, which in turn increase the synthesis of fibrinogen, plasminogen activator inhibitor-1, and C-reactive protein (CRP). The levels of these molecules rise consistently in COVID-19 patients[26]. Since liver diseases are associated with disturbed redox balance and inflammation, SARS-CoV-2 infection can lead to serious manifestations and lethality in COVID-19 patients.
Considering that the more recent studies recognize oxidative stress as a key player in COVID-19 severity, the free radical scavenging by specific natural and/or synthetic antioxidants would be beneficial in preventing the progression of COVID-19[23]. Among others, some natural plant compounds, such as polyphenols, are known to have anti-inflammatory, antioxidative and antiapoptotic properties[27,28] that make them potentially beneficial in the effort to improve therapies for the most vulnerable SARS-CoV-2-infected patients.
ANTIOXIDANT NUTRIENTS, DIET AND COVID-19
Vitamins and minerals are actively involved in neutralizing the harmful effects of oxidative species that induce liver damages. Their deficiency suppresses immune function and increases to the risk of infection. Patients with liver steatosis have increased LPO markers and proinflammatory cytokines as well as reduced total plasma antioxidant capacity (TAC)[29]. Literature data presented that vitamins (A, C, D, E, B6, B12, and folate), omega-3 fatty acids and minerals such as zinc, selenium, iron, magnesium and copper have important and common roles in maintaining the immune system[30]. Inadequate dietary intake and/ or status of these micronutrients contribute to a decrease in resistance to infections, and consequently increased disease burden[31]. Zhang and Liu[32] pointed out that in the assessment of micronutrients besides vitamins A, D, B, C and omega-3 fatty acids in COVID-19 patients, trace elements (selenium, zinc, and iron) should also be considered. Anti-inflammatory dietary index and dietary TAC are emphasized as a concept of the association between nutrients, dietary sources, eating habits, inflammation, and oxidative stress[33,34]. Omega-3 fatty acids, vitamin A and vitamin C, as well as polyphenols (widely present in plant-based foods) are known nutritional ingredients with anti-inflammatory and antioxidant properties[35]. There is evidence that supplementation with higher daily doses of nutrients (vitamins D, C, E, zinc and omega-3 fatty acids) than recommended might have a beneficial effect in patients with COVID-19, potentially reducing the viral load and the duration of hospitalization[31,36-38]. Oxidative stress contributes to the onset and progression of liver damage through related pathological mechanism[35]. According to a recent study by de Oliveira et al[39] NAFLD patients with lower hepatic injury have higher dietary TAC. It suggests a that increased consumption of foods with naturally high TAC leads to reduced production of LPO[39].
Vitamin A
Liver injury is associated with impaired vitamin A homeostasis. Vitamin A plays a role in regulating the production of IL-2 (promoting T cell growth) and proinflammatory TNF-α, which activates the microbial action of macrophages[31]. Also, vitamin A is involved in the phagocytic and oxidative explosive activities of macrophages activated during inflammation[40]. Vitamin A and some other retinoids show important immunomodulatory properties, by increasing the efficiency of actions of type 1 IFNs (e.g., IFN-I), which are important antiviral cytokines[41]. Thus, retinoids promoted increase antiviral responses mediated by IFN-I. There is evidence that activation of retinoid signaling can strongly inhibit coronaviruses[42]. Thus, retinoids could possibly suppress viral replication in COVID-19 patients and may interfere with the cellular uptake of SARS-CoV-2 by inhibiting ACE2[43].
Dietary plant sources of vitamin A are carotenoids, such as β-carotene, while animal sources are rich in retinyl esters. Major food sources of vitamin A are presented in Table 1. β-carotene is known as a scavenger of ROS[44]. It also enhances T lymphocyte response, NK cell activity, and IL-2 production[45]. Liver represents the largest pool of retinyl esters in hepatic stellate cells. In hepatitis C-infected patients, vitamin A deficiency is associated with a lack of response to IFN as antiviral therapy[46]. Further, NAFLD is associated with declining circulating and hepatic retinol levels[30], and patients with NAFLD have higher risk for SARS-CoV-2 infections[47]. Adequate daily intake (about 650 μg/d for women and 750 μg/d for men) and liver storage below 80% in a healthy person are required to keep retinol levels in plasma around 2 µmol/L[45]. Data from 10 western European countries, demonstrated that low levels of vitamin A are associated with the incidence and mortality of COVID-19[41]. Vitamin A deficiency can occur during SARS-CoV-2 infection due to effects on the lung and liver body stores caused by inflammation process[48], suggesting importance of supplementation during this disease[49].
Table 1.
Ongoing clinical trials of antioxidant supplementation in coronavirus disease 2019 patients
|
Antioxidants and nutrients
|
Plant and/or animal sources
|
Potential target pathways
|
Recommended dietary intake; Effective dose for respiratory infection)
|
Registered clinical trials of COVID-19, rial number
|
Intervention dose
|
Primary outcome
|
| Vitamin A | Carrots (raw); Cantaloupe; Mango; Salmon; Eggs | Role in immune competence; Immunomodulatory action | 900/700 μg M/F | IRCT20200319046819N1[49] | 25000 IU vitamin A and 600000 IU vitamin D per day, 300 IU vitamin E twice a week, 2000 mg vitamin C 4 times in a day, amp vitamin B; Soluvit in a day, for 1 wk | Weight, height, BMI; Severity of pulmonary involvement (CT scan); Respiratory support (invasive or non-invasive); WBC, CRP, IL6, TNF-α, IFN-γ, ESR; Body temperature; Organ involvement; Hospital duration; Mortality |
| Vitamin D | Portabella mushrooms; Dairy products; Eggs; Fish | Maintenance of cell physical barrier integrity; Increased antimicrobial protein production; Anti-inflammatory state; ACE2 and other members of the RAS expression | 5- 15 μg; 20-50 μg[60] | NCT04334005[61] | 25000 IU vitamin D | Mortality, all-cause mortality within 14 d |
| NCT04344041[62] | 400000 IU/or 50000 IU | Serum 25(OH)D3 changes during treatment | ||||
| NCT04636086[63] | 50000 IU vitamin D ; 25000 IU vitamin D ; Days 1-4, 8,15, 22, 29 and 36 | Vitamin D serum concentration, clinical improvement, biological markers | ||||
| NCT04621058[64] | D deficiency (< 30 ng/mL) 0.532 mg vitamin D (< 40 ng/mL): 0.266 mg vitamin D | Mortality reduction, lent of hospital reduction | ||||
| NCT04483635[65] | 100000 IU vitamin D tab. baseline + 10000 IU/wk; 16 wk | Change in incidence laboratory-confirmed COVID-19 infection | ||||
| NCT04386850[66] | 25 µg 25(OH)D3; 2 mo | Hospitalization, disease duration, death, mortality, ventilation | ||||
| Vitamin C | Oranges; Lemon; Green and red peppers; Tomato; Broccoli; Brussel sprouts; Cabbage; Cauliflower; Spinach; Sweet potato; Winter squash | Antioxidant capacity; Immunomodulatory effects on interferon production; Cytokine production down-regulation | 40-80 mg; 6–8 g[77] | NCT04323514[74] | 10 g vitamin C infusion | Hospital mortality |
| NCT04530539[75] | 1000 mg vitamin C | Severity of symptoms | ||||
| NCT04682574[76] | 30 g/d 2 d with standard treatment | Partial pressure of oxygen in arterial blood to fraction of inspired oxygen | ||||
| Vitamin E | Sunflower seeds; Nuts; Almonds; Blueberries; Kivi; Broccoli | Antioxidant action; Immunomodulatory effects | 7–10 mg; 30–200 mg[82] | |||
| Zinc | Pumpkin and squash seeds nuts; Soybean; Beef; Mollusks; Lamb | Reduces ROS in viral infections; Replication inhibition; Immunomodulatory effects; Antibody production; NK cell activity; Cytokine production by mononuclear cells; Chemotaxis response reduction; Neutrophil respiratory burst reduction | 8–14 mg; 30–50 mg[43] | NCT04335084[90] | Zinc, vitamin D, vitamin C 12 wk and hydroxychloroquine for 1 d | Prevention of COVID-19 symptoms recorded in a daily diary |
| Selenium | Sunflower seeds; Coconut meat; Mollusks; Salmon; Turkey; Ham | Antioxidant balanceROS balance in inflammatory processes | 60–70 mg | |||
| Copper | Cashew nuts; Tofu; Mushrooms; Beef; Oyster; Cereals; Roots, tubers: Sweet potato; Quinoa | Role in immunity antimicrobial action due to copper toxicity; Enhance macrophage activity in lung infection | 900 μg | |||
| Curcumin | Rhizome of turmeric (Curcuma longa) | Antioxidant capacity; Virus-ACE2 interaction reduction; ACE2 level increase; Antiviral activities SARS-CoV-2 protease, spike glycoprotein-RBD and PD-ACE2 binding | IRCT20200611047735N1[114] | 160 mg curcumin; 2 wk | Immune responses (IFN-γ, IL-17, IL-4 and TGF-β) | |
| IRCT20121216011763N46[115] | 1000 mg curcumin + 10 mg piperine twice/day, 2 wk | Clinical symptoms, duration, severity, inflammatory mediators | ||||
| Silymarin | Milk thistle (Silybum marianum) | Antioxidant capacity; Anti-inflammatory; p38 MAPK pathway antiviral | NCT04394208[116] | 420 mg/d; 3 divided doses | Clinical outcome duration of mechanical ventilation hospitalization |
ACE2: Angiotensin converting enzyme 2; COVID-19: Coronavirus disease 2019; CRP: C-reactive protein; CT: Computed tomography; ESR: Erythrocyte sedimentation rate; IFN-γ: Interferon-gamma; IL: Interleukin; NK: Natural killer; PD: Protease domain; RAS: Renin-angiotensin system; RBD: Receptor binding domain; ROS: Reactive oxygen species; SARS-CoV-2: Severe acute respiratory syndrome-coronavirus-2; tab.: Tablet; TGF-β: Transforming growth factor-beta; TNF-α: Tumor necrosis factor-alpha; WBC: White blood cell.
Vitamin D
Vitamin D is involved in several functions in the body, including increasing the oxidative burst potential of macrophages, and biosynthesis of superoxide dismutase. Furthermore, it suppresses the expression of proinflammatory cytokines, while promotes the expression of anti-inflammatory cytokines. In patients with chronic liver diseases, vitamin D deficiency is often reported[50], due to impaired synthesis of vitamin D–binding protein and hydroxylation of vitamin D. In spite of available in vitro and in vivo evidence, the therapeutic effect of vitamin D in liver disease still remain controversial[51]. Further studies will be required to clarify whether vitamin D have beneficial effects on liver diseases.
Vitamin D is actively involved in antioxidant, immunomodulatory, and antiviral responses. It induces the expression of enzymes involved in the antioxidant defense system. Also, it increases the levels of GSH, and suppresses the expression of NADPH oxidase. This way, vitamin D affects the reduction of oxidative stress and cellular oxidation[2]. Since deficiency of vitamin D is a global health challenge, the need for vitamin D supplementation has been a notable topic of research and discussion during the COVID-19 pandemic. Further, vitamin D deficiency elevates the vulnerability to acute viral respiratory infections[52]. Thus, supplementation with vitamin D enhances the innate immune responses to influenza and viral hepatitis C[53,54]. Vitamin D receptor is also expressed in pulmonary tissue.
The immunomodulatory effect of this vitamin are well established, and the supplements seems to be useful in the prevention or treatment of COVID-19. Vitamin D supplementation stimulates the binding of angiotensin-II to the ACE2 receptor, thereby diminishing the number of SARS-CoV-2 particles that can bind to ACE2 receptors and enter the cells[55,56]. Two meta-analysis reported that COVID-19 patients with a low blood vitamin D levels have worse prognoses[57] and that the risk of SARS-CoV-2 infection was significantly increased in patients with low levels of vitamin D[58]. CRP, as marker of inflammation and the cytokine storm, was highly expressed in COVID-19 patients with severe symptoms and correlate with vitamin deficiency[59]. Therefore, vitamin D could act as an adjuvant therapeutic for the treatment of this novel virus[60]. Ongoing clinical trials of vitamin D supplementation[61-66] in patients with COVID-19 are presented in Table 1.
Vitamin C
Vitamin C has a key antioxidant role, maintains cellular redox balance and protects against free radicals (ROS and RNS) during oxidative fight[67]. Also, vitamin C regenerates oxidized vitamin E, GSH and carotenoids, modifies pro-inflammatory cytokine production, and lower blood histamine. The meta-analysis reported that administration of high doses of vitamin C was associated with a reduction in-hospital mortality among patients with severe sepsis[68]. The hepatoprotective property of water soluble vitamin C is attributed to its antioxidant action, Animal models have lent strong evidence that vitamin C supplementation significantly decreases hepatic markers of oxidative stress, hepatocellular ballooning, and inflammation[69]. The prooxidant potential of vitamin C is enhanced with the presence of iron and copper catalysts in vitro. Through the Fenton reaction, the antiviral activity of vitamin C increases, resulting in the formation of hydroxyl radicals from hydrogen peroxide. High intake of vitamin C may be an effective choice in the early treatment of COVID-19[70,71]. Several clinical studies evaluating vitamin C infusion in patients with COVID-19 are in progress (Table 1)[72-76]. It is indisputable that consumption of citrus fruits and vegetables that contain vitamin C is important in supporting the immune system during the COVID-19 pandemic[77].
Vitamin E
Vitamin E is a well-known fat-soluble vitamin, with a high antioxidant potential, which combats free radicals by a chain-breaking effect and protects cells from oxidative damage. Also, vitamin E enhances production of IL-2 and reduces production of prostaglandin E. Thus, vitamin E indirectly saves T cell function[32]. In addition, blood level of vitamin E inversely correlates with the number of products of oxidative stress, which is in line with the degree of liver injury. The potential mechanisms of action of vitamin E against COVID-19 involve the antioxidant properties of vitamin E derivatives that increase the integrity of the cell membrane and improve the adaptive response of the immune system to viral infections of the respiratory tract[78]. Natural isomers of vitamin E, α and γ tocopherols also have ROS scavenging potential. Taking vitamin E with vitamin C or alone, facilitates the removal of free radicals formed in the liver tissue[50]. The results of a meta-analysis[79] revealed that vitamin E supplementation significantly reduced AST, ALT, steatosis, inflammation, and hepatocellular ballooning. Vitamin E is also shown to be effective for normalizing ALT levels in patients with chronic liver injury in doses of 600 mg, while 800 IU is recommended for nondiabetic patients with nonalcoholic steatohepatitis (commonly known as NASH)[80,81]. As reviewed by Sahin et al[43], 200 IU vitamin E has no effect on lower respiratory infection but treatment with 800 IJ increases resistance to respiratory infections. Vitamins E and C in combination have been suggested as a useful antioxidant therapy in treating critically ill COVID-19 patients with gastric cardia complications. Major food sources of vitamin E and recommended dietary intake[82] are presented in Table 1.
Vitamins B6 and B12
Vitamin B6 is essential cofactor for metabolism of amino acids, which are structural components of cytokines. It has been shown that higher levels of the active form of vitamin B6, pyridoxal 5'-phosphate (PLP) in plasma with parallel reduction in liver PLP is linked to the lower inflammation rate[32]. Adequate vitamin B6 (and B12) supplementation in COVID-19 patients has been included in current protocols for the treatment[83], in order to regulate the altered homocysteine metabolism caused by the corona virus. Vitamin B6 administration at higher doses can decrease levels of inflammatory markers such as the TNF-α and IL-6, as well as D-dimer values, preventing coagulopathy in COVID-19 patients[84]. Methylcobalamin supplements may improve clinical deterioration during SARS-CoV-2 infection and decrease COVID-19-related symptoms and organ damage[85]. Combination of vitamin B12 (500 μg), vitamin D (1000 IU) and magnesium supplements was also shown to reduce COVID-19 severity symptoms and reduce the need for oxygen and intensive care support[86].
Zinc
Zinc enhances the function of antioxidant proteins and it is well known on its antioxidant activity, including protection against ROS and RNS[43]. It also has a strong anti-inflammatory action, reduces proliferation of proinflammatory Th17 and Th9 cells, and thus decreases release of proinflammatory cytokines such as TNF-α, IL-2 and IL-6. Zinc is potentially considered a major mineral during SARS-CoV-2 infection due to its dual immunomodulatory and antiviral effects. Indirect data suggest that zinc may decrease the activity of ACE2[43]. In NAFLD patients, with comorbidities such as old age, overweight, immune disturbance, diabetes and atherosclerosis, zinc deficiency and low body status may increase the risk of severe COVID-19 illness[34]. However, in the latest meta-analyses of 12 randomized control studies, the decrease of IL-6, increase of IL-2 and no ameliorative effects on TNF-α level due to zinc supplementation has been found[87].
Recent studies have shown that zinc supplementation is able to decrease SARS-CoV-2 infections, due to inhibition of viral uncoating, binding, and replication. It is reported by Finzi et al[88] that the clinical respiratory symptoms were improved in COVID-19 patients supplemented with high dose of zinc citrate, zinc gluconate or zinc acetate. Roy et al[89] suggested the combined use of curcumin and zinc treatment. The authors speculated that zinc in synergy with curcumin might promote the synthesis of ionophore complexes, leading to the synchronized and improved antiviral activity. Curcumas exert an antiviral effect by inhibiting entry of the virus into the cell, while zinc inhibits the RNA polymerase. Major food sources of zinc, recommended dietary intake[43], and ongoing study that examines the effect of zinc supplementation in preventing COVID-19 symptoms[90] are presented in Table 1.
Selenium
As a part of selenoproteins, selenium has essential role in regulation of redox reactions, particularly in suppression of ROS produced during oxidative stress. Deficiency of selenium in the diet, concomitant with elevated oxidative stress in the host tissue, may lead to alteration of viral genome. The activity of selenoproteins in antioxidant defense, affects function of host leukocytes and NK cells[43], while selenium deficiency alters immune response by reducing proliferation of T cells, the lymphocyte-mediated toxicity, and NK cell activity[91]. Besides being pointed out as a promising treatment for COVID-19[92], crucial role of selenium in the SARS-CoV-2 emergence and spread has been suggested. Low selenium status in the host affects the viral genome[93], leading to the virus becoming more virulent[92]. Dietary supplementation of selenium can stimulate the activity of cytotoxic effector cells in COVID-19[94].
The meta-analysis of antioxidants effects in COVID-19 patients revealed decreased mortality and the requirement of mechanical ventilation, but no shorter time in intensive care unit (ICU) due to antioxidant therapies (predominantly based in supplementation of selenium)[95,96]. However, in meta-analysis performed by Allingstrup and Afshari[97], no clear results supporting beneficial effect of selenium supplementation in critically ill patients, including less days on a ventilation and shorter ICU and hospital stay, was shown. The elevated ratio of thromboxane A2 to prostacyclin I2 followed by vasoconstriction and blood coagulation, has been found in animal models deficient in selenium[93]. The same mechanism possible explains the venous thromboembolism in COVID-19 patients with selenium deficiency.
Iron
Among many other physiological roles, iron is known to regulate production and action of cytokines. It is also involved in the generation of ROS in neutrophils, which presents pathogen-killing activity during oxidative burst[31]. In hepatitis C, enhanced hepcidin production favors viral persistence, while the inflammation during SARS-CoV-2 infection might be associated with promotion of hepcidin production. Elevated hepcidin reduces iron uptake in the gut, thus diminishing the effect of iron supplementation. Even more, the sequestration of iron in macrophages, hepatocytes and enterocytes, can be reduced by hepcidin[98]. When SARS-CoV-2 colonizes these cells, the accumulated iron is used for viral replication. Evidence in the context of SARS-CoV, based on the previous studies, indicates that iron is important for viral replication, which is why iron is a promising adjuvant therapeutic agent in treating the SARS-CoV-2 infection[99].
Copper
Being a part of copper/zinc-superoxide dismutase, an enzyme essential for anti-ROS defense, copper accumulates at sites of inflammation and acts as a free-radical scavenger[100]. Due to its activity in maintaining intracellular antioxidant balance, an important role for copper in the inflammatory response has been suggested. In general, it is important for IL-2 production and response, as well as for antibody production. Between 80% and 95% of copper is transported by ceruloplasmin, the levels of which are increased in response to inflammation and infection. Changes in copper homeostasis are a crucial component of respiratory burst. Copper has distinctive antimicrobial properties and helps to destroy a broad range of microorganisms[101]. It competes with zinc for the same absorption site, and the copper deficiency is likely to occur when zinc orally supplemented at high doses. Copper deficiency has been identified in liver disease, in NAFLD associated with insulin resistance, steatosis, and an accelerated progression of NASH[102,103]. Copper has proven to be capable of inactivating different types of viruses, such as influenza A/WSN/33 and recently SARS-CoV-2[104]. SARS-CoV-2 is very sensitive to the copper surface[105], thus copper may be proposed for supplementation during COVID-19 treatment[106].
Magnesium
Magnesium is involved in several functions in the body, including antioxidant defense. It assists in DNA protection against oxidative damage and diminishes the production of superoxide anion radical[31]. It is hypothesized that a low magnesium status may encourage transition from mild to critical clinical manifestations of the SARS-CoV-2 infection[107]. Magnesium also maintains proper lung function and reduces the risk of airway hyper-reactivity. It has been noticed that magnesium deficiency is related to reduced activity of immune cells and increased levels of IL-6, which is recognized as a key player in the COVID-19 associated cytokine storm[108]. According to a recent study by Tan et al[86] , the concomitant oral supplementation of vitamin D, magnesium, and vitamin B12 decreases clinical deterioration in patients with COVID-19. It should be noted that magnesium has a relationship to vitamin D physiology. This suggests that magnesium could contribute to the beneficial effects of vitamin D on COVID-19 outcomes.
Polyphenols
Polyphenols are a large class of bioactive compounds from plants, which exert protective effects against oxidative stress, ultraviolet radiations, and different pathogens. Although there are more than 8000 polyphenolic compounds, all of them contain one or more phenolic rings with hydroxyl groups. The most common polyphenols are flavonoids (including, flavonols, isoflavones, flavones, flavanones, flavan-3-ols and anthocyanins), while nonflavonoids are phenolic acids (gallic, ellagic, chlorogenic or hydoxycinnamic), stilbenes (resveratrol), and lignans[109]. The antiviral activities of some polyphenolic compounds are well documented in the literature. Among the targeted viruses are hepatitis B and C viruses, herpes simplex virus-1, influenza A virus, and Epstein-Barr virus[110]. However, data on their effects on coronaviruses are still sparse.
Polyphenols could exert their anti-SARS-CoV-2 potential in several ways. The first one is inhibition of virus entry into the host cells. Since the spike (S) protein is the sole protein of coronaviruses responsible for its fusion with host cells, it is a meaningful target for antiviral therapies. Several polyphenols have demonstrated the interference with the S protein in vitro. In particular, luteolin and quercetin prevented SARS-CoV-2 infection of Vero E6 cells[111], while emodin interfered with the interaction between S protein and its receptor ACE2 in the same cells[112]. Hesperidin has shown a similar effect on the S protein-ACE2 interaction as emodin, and curcumin and several polyphenols from Citrus species also possess a high affinity to S protein[110].
The fusion of S protein and ACE2 could also be prevented by targeting ACE2. Some polyphenols, such as curcumin and resveratrol, down-regulate expression and function of ACE2, thereby affecting severity of COVID-19. Further, Jena et al[113] recently published a computational study that showed flavonoids such as catechin and curcumin could bind the ACE2, impeding its interaction with the virus, but these implications have not been confirmed in vivo so far. Ongoing studies that examine the effect of curcumin and silymarin supplementation on clinical outcomes in COVID-19 patients[114-116] are presented in Table 1.
Considering that ACE2 is abundantly expressed in the liver, this organ represents a potential target for direct infection and cytotoxicity caused by an active viral replication in hepatic cells[117]. Accordingly, persons with chronic liver disease can have serious consequences on health after SARS-CoV-2 infection, and polyphenols dietary intake could diminish these complications.
Aloe vera is one of the nutraceuticals with the strongest antiviral potential, with quercetin and kaempferol as the most active components against viruses. They both effectively reduce the viral replication of the influenza virus in vitro, and also reduce oxidative damage in the lungs and liver of infected mice[118]. Therefore, quercetin and kaempferol are good candidates for preclinical and clinical studies on the liver protection in SARS-CoV-2 infection.
Green tea and coffee are commonly consumed beverages worldwide and important sources of polyphenols. The polyphenols from green tea: Gallocatechin-3-gallate, epigallocatechin gallate, and epicatechingallate are recognized as potential weapon to battle SARS-CoV-2[119]. Moreover, both coffee and green tea have been shown to lower prevalence of metabolic syndrome, type 2 diabetes, and NAFLD[120].
Another mechanism for the beneficial effects of polyphenols in COVID-19 is the suppression of inflammatory response of the host. The above-mentioned cytokine storm, that has been responsible for lethal outcome in many patients worldwide, may be reduced by several polyphenols, including curcumin, resveratrol, emodin, epigallocatechin, gallate, naringenin, apigenin, and kaempferol[121]. All these polyphenols have shown potential to reduce level of proinflammatory cytokines in vitro and in vivo; although, their beneficial effects against SARS-CoV-2 need to be confirmed in clinical studies.
The severe inflammatory response could also lead to immune-mediated damage of the liver[118]. In line with this, polyphenol mediates the body’s fight against the cytokine storm, which would protect the liver as well. Nevertheless, clinical studies on the hepatoprotective potential of different polyphenols in patients with COVID-19 are urgently needed to confirm this hypothesis.
Omega-3 fatty acids
Fatty acids are important players in hepatic lipid metabolism. Omega-3 fatty acids promote fat oxidation and decrease endogenous lipid synthesis by altering gene expression[122]. Patients with liver ALD have low levels of omega-3 fatty acids[123]. Despite genetic and anthropometric factors that could influence their benefits, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) possess a strong anti-inflammatory potential. Thus, a dietary intake of 250 mg/d is recommended for maintaining optimal health[36]. A study by Zheng et al[124] showed a statistically significant association between NAFLD and the severity of COVID-19 in obese patients and those with metabolic syndrome and fatty liver, while a study by Mahamid et al[47] showed that NAFLD is a significant risk factor for severe COVID-19 independently of both sex or metabolic syndrome. Also, NAFLD patients, in particular those with associated obesity, commonly have an impaired omega-3 fatty acid level and an increased omega-6/omega-3 ratio that can be ameliorated by a nutritional intervention[125,126].
The European Society for Parenteral and Enteral Nutrition has recently declared that omega-3 fatty acids can increase oxygenation in COVID-19 patients[127,128]. Bistrian[129] proposed parenteral supplementation with high doses of EPA and DHA (4–6 g/d), for treatment of patients with severe COVID-19, to inhibit cytokine storm and reduce the inflammation. The possible benefits and risk of omega-3 fatty acid intervention for patients with COVID-19 have been reviewed[130]. The authors concluded that although EPA and DHA may positively affect resolution of inflammation and recovery in patients with COVID-19, excess of EPA and/or DHA can induce LPO of cell membranes, resulting in cell damage due to elevated oxidative stress. This could be prevented by a concomitant supplementation with antioxidants, such as vitamin C and E[30,36]. However, based on the clinical trials that have been completed to date, it is still difficult to determine the optimal dose, route and timing of omega-3 fatty acids administration in COVID-19 patients. Until randomized clinical test data are validated, omega-3 fatty acid supplementation, especially at high doses, must be performed carefully in patients with COVID-19.
Dietary patterns
The COVID-19 pandemic provides nutrition researchers with new opportunities to keep the public, especially those in high-risk groups, constantly informed about the potential benefits of good nutrition and healthy eating habits for prevention and therapy in COVID-19[40,131]. The most vulnerable are the elderly, due to a declined gastrointestinal uptake of micronutrients and macronutrients[132]. Many studies have shown association between a high intake of plant foods, fruit and vegetables and low levels of inflammatory parameters.
There is strong evidence of a favorable impact of the Mediterranean diet on IL-6 and CRP values[133]. Traditionally, the Mediterranean diet is characterized by low intake of simple carbohydrates, high consumption of olive oil, fiber, fresh fruit and vegetables, legumes, whole grains, olives, nuts and fish, moderate consumption of dairy products and poultry, low intake of red meat, many condiments and spices, and regular but moderate wine intake. Oleic acid consumption, which is abundant in olive oil, is also a feature. These components directly affect the synthesis of various antioxidant enzymes and may reduce liver tissue damage generated by oxidative stress[134].
Numerous studies have also shown the anti-inflammatory effect of the dietary approach stop hypertension (referred to as DASH) diet[135]. The ability of nutrients rich in antioxidants (bayberry juice, chocolate, onion, lettuce, and tomato products) to increase TAC in plasma and thereby reduce NAFLD through modifications of oxidative stress is well documented[34]. However, proper nutrition and nutrition support can help battle against COVID-19 by improving immune responses and aiding inflammatory processes[40,136].
CONCLUSION
Foods rich in vitamins, minerals, polyphenols and other bioactive compounds may decrease inflammatory pathway activity and prevent liver damage in COVID-19 patients. An important additional aspect is their ability to control oxidative stress. Antioxidants supplementation during the COVID-19 pandemic crisis should respect nutritional status and serum levels of relevant nutrients in patients. It is essential to recognize nutritional deficiencies in COVID-19 patients, and to intervene to improve their status. Constant public informing about balanced diet and healthy eating habits is important in the prevention of SARS-CoV-2 infection.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
Manuscript source: Invited manuscript
Peer-review started: January 29, 2021
First decision: March 29, 2021
Article in press: August 17, 2021
Specialty type: Nutrition and dietetics
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sakina NL S-Editor: Liu M L-Editor: A P-Editor: Li JH
Contributor Information
Danijela Ristic-Medic, Group for Nutritional Biochemistry and Dietology, Centre of Research Excellence in Nutrition and Metabolism, National Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia. dristicmedic@gmail.com.
Snjezana Petrovic, Group for Nutritional Biochemistry and Dietology, Centre of Research Excellence in Nutrition and Metabolism, National Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia.
Aleksandra Arsic, Group for Nutritional Biochemistry and Dietology, Centre of Research Excellence in Nutrition and Metabolism, National Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia.
Vesna Vucic, Group for Nutritional Biochemistry and Dietology, Centre of Research Excellence in Nutrition and Metabolism, National Institute for Medical Research, University of Belgrade, Belgrade 11129, Serbia.
References
- 1.Lizardo-Thiebaud MJ, Cervantes-Alvarez E, Limon-de la Rosa N, Tejeda-Dominguez F, Palacios-Jimenez M, Méndez-Guerrero O, Delaye-Martinez M, Rodriguez-Alvarez F, Romero-Morales B, Liu WH, Huang CA, Kershenobich D, Navarro-Alvarez N. Direct or Collateral Liver Damage in SARS-CoV-2-Infected Patients. Semin Liver Dis. 2020;40:321–330. doi: 10.1055/s-0040-1715108. [DOI] [PubMed] [Google Scholar]
- 2.Beltrán-García J, Osca-Verdegal R, Pallardó FV, Ferreres J, Rodríguez M, Mulet S, Sanchis-Gomar F, Carbonell N, García-Giménez JL. Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression. Antioxidants (Basel) 2020;9:936. doi: 10.3390/antiox9100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laforge M, Elbim C, Frère C, Hémadi M, Massaad C, Nuss P, Benoliel JJ, Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20:515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soldo J, Heni M, Königsrainer A, Häring HU, Birkenfeld AL, Peter A. Increased Hepatic ACE2 Expression in NAFL and Diabetes-A Risk for COVID-19 Patients? Diabetes Care. 2020;43:e134–e136. doi: 10.2337/dc20-1458. [DOI] [PubMed] [Google Scholar]
- 5.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan K, Samuel K, Vandeputte M, Hayes PC, Plevris JN. SARS-CoV-2 Infection and the Liver. Pathogens. 2020;9:430. doi: 10.3390/pathogens9060430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palomar-Lever A, Barraza G, Galicia-Alba J, Echeverri-Bolaños M, Escarria-Panesso R, Padua-Barrios J, Halabe-Cherem J, Hernandez-Molina G, Chargoy-Loustaunau TN, Kimura-Hayama E. Hepatic steatosis as an independent risk factor for severe disease in patients with COVID-19: A computed tomography study. JGH Open. 2020;4:1102–1107. doi: 10.1002/jgh3.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye L, Chen B, Wang Y, Yang Y, Zeng J, Deng G, Deng Y, Zeng F. Prognostic value of liver biochemical parameters for COVID-19 mortality. Ann Hepatol. 2021;21:100279. doi: 10.1016/j.aohep.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. doi: 10.1038/s41392-020-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Li Q, Xu W, Chen L. Molecular and cellular mechanisms of liver dysfunction in COVID-19. Discov Med. 2020;30:107–112. [PubMed] [Google Scholar]
- 14.Henrion J. Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol Belg. 2000;63:336–347. [PubMed] [Google Scholar]
- 15.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43:615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grattagliano I, de Bari O, Bernardo TC, Oliveira PJ, Wang DQ, Portincasa P. Role of mitochondria in nonalcoholic fatty liver disease--from origin to propagation. Clin Biochem. 2012;45:610–618. doi: 10.1016/j.clinbiochem.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Zhu R, Wang Y, Zhang L, Guo Q. Oxidative stress and liver disease. Hepatol Res. 2012;42:741–749. doi: 10.1111/j.1872-034X.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 20.Ucar F, Sezer S, Erdogan S, Akyol S, Armutcu F, Akyol O. The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013;18:127–133. doi: 10.1179/1351000213Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M, Musabak U, Erbil MK, Karaeren N, Dagalp K. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2005;100:850–855. doi: 10.1111/j.1572-0241.2005.41500.x. [DOI] [PubMed] [Google Scholar]
- 23.Schönrich G, Raftery MJ, Samstag Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv Biol Regul. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polonikov A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect Dis. 2020;6:1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 25.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC Jr. Fibrinolysis Shutdown Correlation with Thromboembolic Events in Severe COVID-19 Infection. J Am Coll Surg. 2020;231:193–203.el. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vučić V, Grabež M, Trchounian A, Arsić A. Composition and Potential Health Benefits of Pomegranate: A Review. Curr Pharm Des. 2019;25:1817–1827. doi: 10.2174/1381612825666190708183941. [DOI] [PubMed] [Google Scholar]
- 28.Petrovic S, Arsic A, Ristic-Medic D, Cvetkovic Z, Vucic V. Lipid Peroxidation and Antioxidant Supplementation in Neurodegenerative Diseases: A Review of Human Studies. Antioxidants (Basel) 2020;9:1128. doi: 10.3390/antiox9111128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coelho JM, Cansanção K, Perez RM, Leite NC, Padilha P, Ramalho A, Peres W. Association between serum and dietary antioxidant micronutrients and advanced liver fibrosis in non-alcoholic fatty liver disease: an observational study. PeerJ. 2020;8:e9838. doi: 10.7717/peerj.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12:1181. [Google Scholar]
- 31.Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohouli MH, Fatahi S, Sayyari A, Olang B, Shidfar F. Associations between dietary total antioxidant capacity and odds of non-alcoholic fatty liver disease (NAFLD) in adults: a case-control study. J Nutr Sci. 2020;9:e48. doi: 10.1017/jns.2020.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, Bohn T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients. 2020;12:1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020;3:74–92. doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemilä H, Chalker E. Vitamin C Can Shorten the Length of Stay in the ICU: A Meta-Analysis. Nutrients. 2019;11:708. doi: 10.3390/nu11040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Oliveira DG, de Faria Ghetti F, Moreira APB, Hermsdorff HHM, de Oliveira JM, de Castro Ferreira LEVV. Association between dietary total antioxidant capacity and hepatocellular ballooning in nonalcoholic steatohepatitis: a cross-sectional study. Eur J Nutr. 2019;58:2263–2270. doi: 10.1007/s00394-018-1776-0. [DOI] [PubMed] [Google Scholar]
- 40.Budhwar S, Sethi K, Chakraborty M. A Rapid Advice Guideline for the Prevention of Novel Coronavirus Through Nutritional Intervention. Curr Nutr Rep. 2020;9:119–128. doi: 10.1007/s13668-020-00325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galmés S, Serra F, Palou A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients. 2020;12:2738. doi: 10.3390/nu12092738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trasino SE. A role for retinoids in the treatment of COVID-19? Clin Exp Pharmacol Physiol. 2020;47:1765–1767. doi: 10.1111/1440-1681.13354. [DOI] [PubMed] [Google Scholar]
- 43.Sahin E, Orhan C, Uckun FM, Sahin K. Clinical Impact Potential of Supplemental Nutrients as Adjuncts of Therapy in High-Risk COVID-19 for Obese Patients. Front Nutr. 2020;7:580504. doi: 10.3389/fnut.2020.580504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stipp M. SARS-CoV-2: Micronutrient Optimization in Supporting Host Immunocompetence. Int J Clin Case Rep Rev IJCCR. 2020;2 [Google Scholar]
- 45.Saeed A, Dull aart RPF, Schreuder TCMA, Blokzijl H, Faber KN. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD) Nutrients. 2017;10 doi: 10.3390/nu10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bitetto D, Bortolotti N, Falleti E, Vescovo S, Fabris C, Fattovich G, Cussigh A, Cmet S, Fornasiere E, Ceriani E, Pirisi M, Toniutto P. Vitamin A deficiency is associated with hepatitis C virus chronic infection and with unresponsiveness to interferon-based antiviral therapy. Hepatology. 2013;57:925–933. doi: 10.1002/hep.26186. [DOI] [PubMed] [Google Scholar]
- 47.Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2020:epub ahead of print. doi: 10.1097/MEG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stephensen CB, Lietz G. Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2. Br J Nutr. 2021:1–10. doi: 10.1017/S0007114521000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bitarafan S. Impact of vitamin B, A, D, E, C supplementation on improvement and mortality rate in patients with COVID-19 admitted in intensive care unit. [accessed 2020 Dec 15]. In: IRCT.ir/trial/ [Internet]. Teheran: Iranian Registry of Clinical Trails. Available from: https://www.irct.ir/trial/46838. IRCT registration number: IRCT20200319046819N1.
- 50.Kozeniecki M, Ludke R, Kerner J, Patterson B. Micronutrients in Liver Disease: Roles, Risk Factors for Deficiency, and Recommendations for Supplementation. Nutr Clin Pract. 2020;35:50–62. doi: 10.1002/ncp.10451. [DOI] [PubMed] [Google Scholar]
- 51.Elangovan H, Chahal S, Gunton JE. Vitamin D in liver disease: Current evidence and potential directions. Biochim Biophys Acta Mol Basis Dis. 2017;1863:907–916. doi: 10.1016/j.bbadis.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A, Aaseth J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients. 2020;12:2358. doi: 10.3390/nu12082358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27 doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 54.Abu-Mouch S, Fireman Z, Jarchovsky J, Zeina AR, Assy N. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol. 2011;17:5184–5190. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rafiullah M. Can a Combination of AT1R Antagonist and Vitamin D Treat the Lung Complication of COVID-19? Am J Med Sci. 2020;360:338–341. doi: 10.1016/j.amjms.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanham-New SA, Webb AR, Cashman KD, Buttriss JL, Fallowfield JL, Masud T, Hewison M, Mathers JC, Kiely M, Welch AA, Ward KA, Magee P, Darling AL, Hill TR, Greig C, Smith CP, Murphy R, Leyland S, Bouillon R, Ray S, Kohlmeier M. Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutr Prev Health. 2020;3:106–110. doi: 10.1136/bmjnph-2020-000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghasemian R, Shamshiria A, Heydari , K Malekan M; Alizadeh-Navaei R; Ebrahimzadeh MA; Jafarpour H; Shahmirzadi AR; Khodabandeh M; Seyfari B; Motamedzadeh A, Dadgostar E, Aalinezhad M, Sedaghat M, Behnamfar N, Asadi A, Zarandi B, Razzaghi N, Vahid Yaghoubi Naei V, Hessami A, Azizi A, Mohseni R, Skamsirian D. The Role of Vitamin D in The Age of COVID-19: A Systematic Review and Meta-Analysis Along with an Ecological Approach. 2020 Preprint. Available from: medRxiv: 20123554.
- 58.Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, Youssef MR, Omar M, Attia AS, Fawzy MS, Killackey M, Kandil E, Duchesne J. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2021;93:733–740. doi: 10.1002/jmv.26360. [DOI] [PubMed] [Google Scholar]
- 59.Daneshkhah A, Eshein A, Subramanian H, Roy HK, Backman V. The role of vitamin d in suppressing cytokine storm in COVID-19 patients and associated mortality. 2020 Preprint. Available from: medRxiv: 20058578.
- 60.McCartney DM, Byrne DG. Optimisation of Vitamin D Status for Enhanced Immuno-protection Against Covid-19. Ir Med J. 2020;113:58. [PubMed] [Google Scholar]
- 61.Garzón MC. Vitamin D on Prevention and Treatment of COVID-19 (COVITD-19). [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04334005. ClinicalTrials.gov Identifier: NCT04334005.
- 62.Annweiler C. COVID-19 and Vitamin D Supplementation: a Multicenter Randomized Controlled Trial of High Dose Versus Standard Dose Vitamin D3 in High-risk COVID-19 Patients (CoVitTrial). [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD) U.S.National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04344041. ClinicalTrials.gov Identifier: NCT04344041.
- 63.Rousseau AF. Effect of Vitamin D on Hospitalized Adults With COVID-19 Infection. [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04636086. ClinicalTrials.gov Identifier: NCT04636086.
- 64.Duran-Cantolla C. Efficacy of Vitamin D Treatment in Mortality Reduction Due to COVID-19. [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04621058. ClinicalTrials.gov Identifier: NCT04621058.
- 65.Ducharme FM. PRevention of COVID-19 With Oral Vitamin D Supplemental Therapy in Essential health Care Teams (PROTECT). [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04483635. ClinicalTrials.gov Identifier: NCT04483635.
- 66.Sahraian M. Oral 25-hydroxyvitamin D3 and COVID-19. [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04386850. ClinicalTrials.gov Identifier: NCT04386850.
- 67.Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, Apostolopoulos V, Stojanovska L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. 2021;143:1–9. doi: 10.1016/j.maturitas.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carr AC. Vitamin C administration in the critically ill: a summary of recent meta-analyses. Crit Care. 2019;23:265. doi: 10.1186/s13054-019-2538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rezazadeh A, Yazdanparast R, Molaei M. Amelioration of diet-induced nonalcoholic steatohepatitis in rats by Mn-salen complexes via reduction of oxidative stress. J Biomed Sci. 2012;19:26. doi: 10.1186/1423-0127-19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Erol A. High-dose intravenous vitamin C treatment for COVID-19. 2020 Preprint. Available from: OSF Preprints.
- 71.Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020;12:3760. doi: 10.3390/nu12123760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu F, Zhu Y, Zhang J, Li Y, Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10:e039519. doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waqas Khan HM, Parikh N, Megala SM, Predeteanu GS. Unusual Early Recovery of a Critical COVID-19 Patient After Administration of Intravenous Vitamin C. Am J Case Rep. 2020;21:e925521. doi: 10.12659/AJCR.925521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corrao S. Use of Ascorbic Acid in Patients with COVID 19. [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04323514. ClinicalTrials.gov Identifier: NCT04323514.
- 75.Fogleman C. The Effect of Melatonin and Vitamin C on COVID-19. [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04530539. ClinicalTrials.gov Identifier: NCT04530539.
- 76.Hafeez MM. Role of Mega Dose of Vitamin C in Critical COVID-19 Patients. [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04682574. ClinicalTrials.gov Identifier: NCT04682574.
- 77.Hemilä H. Vitamin C and Infections. Nutrients. 2017;9:339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could Vitamins Help in the Fight Against COVID-19? Nutrients. 2020;12:2550. doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, Nakade Y, Ito K, Fukuzawa Y, Yoneda M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31:923–930. doi: 10.1016/j.nut.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 80.Ji HF, Sun Y, Shen L. Effect of vitamin E supplementation on aminotransferase levels in patients with NAFLD, NASH, and CHC: results from a meta-analysis. Nutrition. 2014;30:986–991. doi: 10.1016/j.nut.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 81.Kawanaka M, Mahmood S, Niiyama G, Izumi A, Kamei A, Ikeda H, Suehiro M, Togawa K, Sasagawa T, Okita M, Nakamura H, Yodoi J, Yamada G. Control of oxidative stress and reduction in biochemical markers by Vitamin E treatment in patients with nonalcoholic steatohepatitis: a pilot study. Hepatol Res. 2004;29:39–41. doi: 10.1016/j.hepres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Hemilä H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging. 2016;11:1379–1385. doi: 10.2147/CIA.S114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shakoor H, Feehan J, Mikkelsen K, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, Stojanovska L, Apostolopoulos V. Be well: A potential role for vitamin B in COVID-19. Maturitas. 2021;144:108–111. doi: 10.1016/j.maturitas.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desbarats J. Pyridoxal 5'-phosphate to mitigate immune dysregulation and coagulopathy in COVID-19. 2020 Preprint. Available from: Preprints: 202005.0144.
- 85.dos Santos LMJ. Can vitamin B12 be an adjuvant to COVID-19 treatment? GSC Bio Pharm Sci. 2020;11:01–05. [Google Scholar]
- 86.Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, Wong HM, Tern PJW, Chandran M, Chay JWM, Nagarajan C, Sultana R, Low JGH, Ng HJ. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;79-80:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faghfouri AH, Baradaran B, Khabbazi A, Khaje Bishak Y, Zarezadeh M, Tavakoli-Rouzbehani OM, Faghfuri E, Payahoo L, Alipour M, Alipour B. Invited Letter to Editor in response to Profiling Inflammatory Cytokines Following Zinc Supplementation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Br J Nutr. 2021:1–5. doi: 10.1017/S0007114521001070. [DOI] [PubMed] [Google Scholar]
- 88.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int J Infect Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roy A, Sarkar B, Celik C, Ghosh A, Basu U, Jana M, Jana A, Gencay A, Can Sezgin G, Ildiz N, Dam P, Mandal AK, Ocsoy I. Can concomitant use of zinc and curcumin with other immunity-boosting nutraceuticals be the arsenal against COVID-19? Phytother Res. 2020;34:2425–2428. doi: 10.1002/ptr.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hazan S. A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection (HELPCOVID-19). [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04335084. ClinicalTrials.gov Identifier: NCT04335084.
- 91.Ivory K, Prieto E, Spinks C, Armah CN, Goldson AJ, Dainty JR, Nicoletti C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin Nutr. 2017;36:407–415. doi: 10.1016/j.clnu.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bae M, Kim H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules. 2020;25:5346. doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kieliszek M, Lipinski B. Selenium supplementation in the prevention of coronavirus infections (COVID-19) Med Hypotheses. 2020;143:109878. doi: 10.1016/j.mehy.2020.109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park SJ, Yim GW, Paik H, Lee N, Lee S, Lee M, Kim HS. Efficacy and safety of intravenous administration of high-dose selenium for preventing chemotherapy-induced peripheral neuropathy in platinum-sensitive recurrent ovarian, fallopian or primary peritoneal cancer: study protocol for a phase III, double-blind, randomized study. J Gynecol Oncol. 2021:epub ahead of print. doi: 10.3802/jgo.2021.32.e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Y, Yang M, Mao Z, Yuan R, Wang L, Hu X, Zhou F, Kang H. The clinical outcomes of selenium supplementation on critically ill patients: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019;98:e15473. doi: 10.1097/MD.0000000000015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allingstrup M, Afshari A. Selenium supplementation for critically ill adults. Cochrane Database Syst Rev. 2015:CD003703. doi: 10.1002/14651858.CD003703.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260–272. doi: 10.3324/haematol.2019.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu W, Zhang S, Nekhai S, Liu S. Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic Against Viral Survival. Curr Clin Microbiol Rep. 2020:1–7. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fedele D, De Francesco A, Riso S, Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: An overview. Nutrition. 2021;81:111016. doi: 10.1016/j.nut.2020.111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hutasoit N, Kennedy B, Hamilton S, Luttick A, Rahman Rashid RA, Palanisamy S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf Lett. 2020;25:93–97. doi: 10.1016/j.mfglet.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chakravarthy MV, Waddell T, Banerjee R, Guess N. Nutrition and Nonalcoholic Fatty Liver Disease: Current Perspectives. Gastroenterol Clin North Am. 2020;49:63–94. doi: 10.1016/j.gtc.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 103.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu L, Liou IW, Biggins SW, Yeh M, Jalikis F, Chan LN, Burkhead J. Copper Deficiency in Liver Diseases: A Case Series and Pathophysiological Considerations. Hepatol Commun. 2019;3:1159–1165. doi: 10.1002/hep4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Altarelli M, Ben-Hamouda N, Schneider A, Berger MM. Copper Deficiency: Causes, Manifestations, and Treatment. Nutr Clin Pract. 2019;34:504–513. doi: 10.1002/ncp.10328. [DOI] [PubMed] [Google Scholar]
- 106.Raha S, Mallick R, Basak S, Duttaroy AK. Is copper beneficial for COVID-19 patients? Med Hypotheses. 2020;142:109814. doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wallace TC. Combating COVID-19 and Building Immune Resilience: A Potential Role for Magnesium Nutrition? J Am Coll Nutr. 2020;39:685–693. doi: 10.1080/07315724.2020.1785971. [DOI] [PubMed] [Google Scholar]
- 108.Iotti S, Wolf F, Mazur A, Maier JA. The COVID-19 pandemic: is there a role for magnesium? Magnes Res. 2020;33:21–27. doi: 10.1684/mrh.2020.0465. [DOI] [PubMed] [Google Scholar]
- 109.Annunziata G, Jiménez-García M, Capó X, Moranta D, Arnone A, Tenore GC, Sureda A, Tejada S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem Toxicol. 2020;139:111248. doi: 10.1016/j.fct.2020.111248. [DOI] [PubMed] [Google Scholar]
- 110.Utomo RY, Ikawati M, Meiyanto E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. 2020 Preprint. Available from: Preprints: 2020030214.
- 111.Yi L, Li Z, Yuan K, Qu X, Chen J, Wang G, Zhang H, Luo H, Zhu L, Jiang P, Chen L, Shen Y, Luo M, Zuo G, Hu J, Duan D, Nie Y, Shi X, Wang W, Han Y, Li T, Liu Y, Ding M, Deng H, Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jena AB, Kanungo N, Nayak V, Chainy GBN, Dandapat J. Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational studies. Sci Rep. 2021;11:2043. doi: 10.1038/s41598-021-81462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nikpoor AR. Evaluation of the effect of nano micelles containing curcumin (Sina Ccurcumin) as a therapeutic supplement in patients with COVID-19 and investigating of immune responses balance changes following treatment: A randomized double blind clinical trial. [accessed 2020 Dec 15] In: IRCT.ir/trial/ [Internet]. Teheran: Iranian Registry of Clinical Trails. Available from: https://www.irct.ir/trial/48843. IRCT registration number: IRCT20200611047735N1.
- 115.Askari G. Effect of curcumin-piperine supplementation on disease duration, severity and clinical signs, and inflammatory factors in patients with coronavirus (COVID-19): A randomized, double-blind, placebo-controlled clinical trial study. [accessed 2020 Dec 15] In: IRCT.ir/trial/ [Internet]. Teheran: Iranian Registry of Clinical Trails. Available from: https://www.irct.ir/trial/47529. IRCT registration number: IRCT20121216011763N46.
- 116.Salem K. Silymarin in COVID-19 Pneumonia (SCOPE). [accessed 2020 Dec 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04394208. ClinicalTrials.gov Identifier: NCT04394208.
- 117.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278–1281. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 118.Limanaqi F, Busceti CL, Biagioni F, Lazzeri G, Forte M, Schiavon S, Sciarretta S, Frati G, Fornai F. Cell Clearing Systems as Targets of Polyphenols in Viral Infections: Potential Implications for COVID-19 Pathogenesis. Antioxidants (Basel) 2020;9:1105. doi: 10.3390/antiox9111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Semiz S, Serdarevic F. Prevention and Management of Type 2 Diabetes and Metabolic Syndrome in the Time of COVID-19: Should We Add a Cup of Coffee? Front Nutr. 2020;7:581680. doi: 10.3389/fnut.2020.581680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paraiso IL, Revel JS, Stevens JF. Potential use of polyphenols in the battle against COVID-19. Curr Opin Food Sci. 2020;32:149–155. doi: 10.1016/j.cofs.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, Banach M, Sahebkar A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;34:2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abenavoli L, Greco M, Milic N, Accattato F, Foti D, Gulletta E, Luzza F. Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study. Nutrients. 2017;9:870. doi: 10.3390/nu9080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ristić-Medić D, Takić M, Vučić V, Kandić D, Kostić N, Glibetić M. Abnormalities in the serum phospholipids fatty acid profile in patients with alcoholic liver cirrhosis - a pilot study. J Clin Biochem Nutr. 2013;53:49–54. doi: 10.3164/jcbn.12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.López-Bautista F, Barbero-Becerra VJ, Ríos MY, Ramírez-Cisneros MÁ, Sánchez-Pérez CA, Ramos-Ostos MH, Uribe M, Chávez-Tapia NC, Juárez-Hernández E. Dietary consumption and serum pattern of bioactive fatty acids in NAFLD patients. Ann Hepatol. 2020;19:482–488. doi: 10.1016/j.aohep.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 126.Ristic-Medic D, Kovacic M, Takic M, Arsic A, Petrovic S, Paunovic M, Jovicic M, Vucic V. Calorie-Restricted Mediterranean and Low-Fat Diets Affect Fatty Acid Status in Individuals with Nonalcoholic Fatty Liver Disease. Nutrients. 2020;13:15. doi: 10.3390/nu13010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Darwesh AM, Bassiouni W, Sosnowski DK, Seubert JM. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol Ther. 2021;219:107703. doi: 10.1016/j.pharmthera.2020.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M, Singer P endorsed by the ESPEN Council. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bistrian BR. Parenteral Fish-Oil Emulsions in Critically Ill COVID-19 Emulsions. JPEN J Parenter Enteral Nutr. 2020;44:1168. doi: 10.1002/jpen.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rogero MM, Leão MC, Santana TM, Pimentel MVMB, Carlini GCG, da Silveira TFF, Gonçalves RC, Castro IA. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic Biol Med. 2020;156:190–199. doi: 10.1016/j.freeradbiomed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fernández-Quintela A, Milton-Laskibar I, Trepiana J, Gómez-Zorita S, Kajarabille N, Léniz A, González M, Portillo MP. Key Aspects in Nutritional Management of COVID-19 Patients. J Clin Med. 2020;9:2589. doi: 10.3390/jcm9082589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jaggers GK, Watkins BA, Rodriguez RL. COVID-19: repositioning nutrition research for the next pandemic. Nutr Res. 2020;81:1–6. doi: 10.1016/j.nutres.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Billingsley HE, Carbone S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: an in-depth review of the PREDIMED. Nutr Diabetes. 2018;8:13. doi: 10.1038/s41387-018-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bhattacharjee B, Pal PK, Chattopadhyay A, Bandyopadhyay D. Oleic acid protects against cadmium induced cardiac and hepatic tissue injury in male Wistar rats: A mechanistic study. Life Sci. 2020;244:117324. doi: 10.1016/j.lfs.2020.117324. [DOI] [PubMed] [Google Scholar]
- 135.Ardestani ME, Onvani S, Esmailzadeh A, Feizi A, Azadbakht L. Adherence to Dietary Approaches to Stop Hypertension (DASH) Dietary Pattern in Relation to Chronic Obstructive Pulmonary Disease (COPD): A Case-Control Study. J Am Coll Nutr. 2017;36:549–555. doi: 10.1080/07315724.2017.1326858. [DOI] [PubMed] [Google Scholar]
- 136.Morais AHA, Aquino JS, da Silva-Maia JK, Vale SHL, Maciel BLL, Passos TS. Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Br J Nutr. 2021;125:851–862. doi: 10.1017/S0007114520003311. [DOI] [PMC free article] [PubMed] [Google Scholar]