Abstract
Autoimmune hemolytic anemia (AIHA) is a very rare presentation of COVID-19, and AIHA due to COVID-19 alone (i.e., in the absence of an associated underlying disorder) is extremely rare. Warm agglutinin disease accounts for the majority of AIHA in general. Here, we report a case of a 23-year-old male with bronchial asthma who was referred to our hospital with SARS-COV-2 infection and severe anemia presenting as acute immune-mediated hemolytic crisis due to warm autoimmune hemolytic anemia (AIHA). Extensive laboratory testing was performed, including polyspecific direct antiglobulin test, complete autoimmune workup and common infections leading to AIHA were ruled out by serology and molecular methods. The patient required multiple blood transfusions and other therapeutic interventions before clinical stabilization. Treatment of new-onset AIHA is always challenging in the presence of an active viral replication; combining immunosuppression with active COVID-19 infection creates extremely difficult diagnostic and management settings, as this case illustrates.
Keywords: Autoimmune hemolytic anemia, COVID-19, hemolysis, SARS-COV-2, warm antibody
INTRODUCTION
The spectrum of disease due to SARS-COV-2 infection includes acute infection, post-acute hyper inflammatory illness, late inflammatory and virological sequelae.[1,2] These three overlapping stages define the temporal course of SARS-CoV-2 infection and capture the distinct phases of host–viral interaction. Although COVID-19 has a wide spectrum of manifestations,[3] the pathophysiology of severe COVID-19 remains poorly understood. The mechanism mainly implicated in the pathogenesis of multiorgan dysfunction is hyperinflammatory syndrome. Inflammatory stimulus causes fulminant and fatal cytokine release, which is associated with disease severity and poor outcome.[4] The spectrum of complications also includes various autoimmune disorders such as autoimmune thrombocytopenia, Guillain–Barré syndrome and antiphospholipid antibody syndrome.[5,6,7]
Autoimmune hemolytic anemia (AIHA) is a rare autoimmune disorder characterized by autoantibodies against red blood cells (RBCs), and it can be triggered by various viral infections. In COVID-19, both cold agglutinin disease (CAD) and warm AIHA have been reported.[8,9] There is growing evidence that COVID-19 patients with severe diseases may have a higher risk of hematologic anomalies such as coagulopathies. Here, we present the case of a young male to highlight that COVID-19 can present as hemolytic anemia. Through the clinical experience of this case, we propose the management techniques in such cases while acknowledging the current gaps in understanding.
CASE REPORT
A 23-year-old male with a history of bronchial asthma on metered dose inhaler for the past 5 years was referred to our hospital as a case of SARS-COV-2 infection with severe anemia (hemoglobin [Hb] level of 3.6 gm/dl). He presented with fever, cough, malaise and myalgia of 5-day duration and high-colored urine of 1-day duration. The patient was admitted to our critical care unit for detailed evaluation. On examination, he was pale, icteric and febrile and with the following vital signs: temperature, 38.7°C; blood pressure, 121/65 mm Hg; heart rate, 88 beats/minute; respiratory rate, 18/min; and oxygen saturation, 99% at room air. He had no clubbing, cyanosis, lymphadenopathy, edema, arthritis or rash. Cutaneous stigmata of chronic liver disease were not present. Respiratory, cardiovascular, gastrointestinal and central nervous system were normal on examination. SARS-COV-2 infection was confirmed by rtPCR of nasopharyngeal swab. Other laboratory values at the time of admission are given in Table 1.
Table 1.
Laboratory values at the time of admission
| Parameter | Results |
|---|---|
| Hemoglobin | 3.6 g/dl |
| Hematocrit | 19% |
| Total leukocyte count | 10,930/mm3 |
| Differential count | Neutrophil 8000/mm3 |
| Lymphocyte 1200/mm3 | |
| Platelet count | 237,000/mm3 |
| Serum creatinine | 1.1 mg/dl |
| Lactate dehydrogenase | 878 U/L (135-248) |
| C-reactive protein | 1.7 mg/L (0-5) |
| Serum ferritin | 1428 ug/L (64-434) |
| Total bilirubin | 6.95 mg/dl (0-1.2) |
| Conjugated bilirubin | 1.27 mg/dl (0-1.2) |
| Serum haptoglobin | 15 mg/dl (40-200) |
| International normalized ratio | 1.08 (0.9-1.2) |
| Creatine phosphokinase | 50 U/L (26-308) |
| Serum iron | 223 mcg/dl (37-181) |
| Total iron-binding capacity | 261 mcg/dl (250-450) |
| Transferrin saturation | 85.4% (20-45) |
| D-dimer | 3.9 mcg/ml (<0.5) |
The work up of anemia revealed reticulocytosis of 13.2% (0.60%–1.83%) and elevated lactate dehydrogenase (878 U/L). Peripheral blood smear showed numerous polychromatic cells with many spherocytes and nucleated red cells suggestive of hemolytic anemia [Figure 1]. Polyspecific direct antiglobulin test was 4+ for IgG, suggesting autoimmune etiology. Indirect antiglobulin test was not performed. Autoimmune disorders leading to AIHA such as systemic lupus erythematosus, rheumatoid arthritis, antiphospholipid (APLA) syndrome and connective tissue disorders were ruled out by an antinuclear antibody and APLA profile. Rheumatoid factor was normal. The patient was not on any medication that could lead to drug-induced AIHA.
Figure 1.
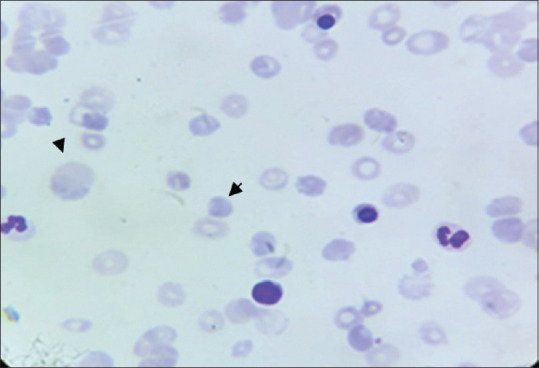
Peripheral blood smear showing polychromatic cells and spherocytes
Common infections leading to AIHA were ruled out by serology and molecular methods. Multiplex PCRs of sputum for adenovirus, Chlamydia pneumonia, Mycoplasma pneumonia, Legionella pneumophila and influenza were negative. Serology testing for HIV, HCV, and HBV was negative. IgM and IgG antibodies against M. pneumonia, Epstein–Barr virus (EBV) and cytomegalovirus were also negative. The osmotic fragility test was negative. Glucose-6-phosphate dehydrogenase level was normal. Ultrasound abdomen showed moderate splenomegaly. Antimyeloperoxidase and antiproteinase-3 antibodies were negative. Kappa and lambda immunofixation electrophoresis for monoclonal proteins and immunofixation electrophoresis for cryoglobulins were negative.
The temporal association of COVID-19 infection with hemolysis in this patient suggests that SARS-CoV-2 could have been the trigger for hemolysis leading to secondary AIHA. Treatment was started with oral azithromycin (500 mg/day) for 5 days, intravenous methylprednisolone at 1 g/day for 3 days followed by oral prednisolone 1 mg/kg/day. Two units of packed RBC transfusion was given on Days 4 and 6. Following steroid administration, the patient's hemoglobin levels progressively increased, and he became transfusion independent.
Steroid pulse therapy was well tolerated by the patient. He tested negative for SARS-CoV-2 on rtPCR on Day 14 of admission, and thus was discharged. One week later, his hemoglobin had risen to 11.3 g/dl and steroids were tapered off over the next 3 months. At the time of reporting this case, the patient had no evidence of hemolysis 3 months after discontinuation of steroids, confirming the diagnosis of secondary AIHA due to COVID-19 [Figures 2 and 3].
Figure 2.
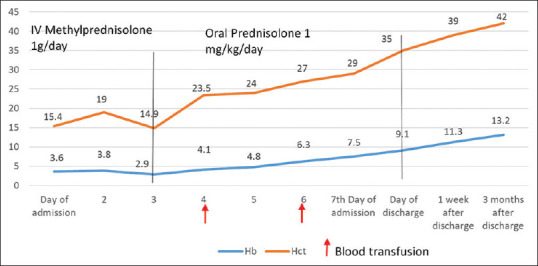
Hemoglobin and hematocrit values from day of admission to 3 months after discharge (red arrows indicate days on which blood transfusion was administered; black line indicates duration of administration of steroids)
Figure 3.
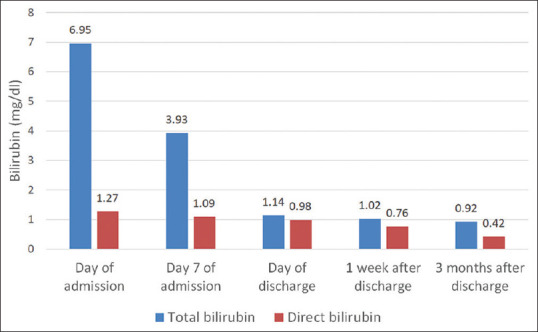
Total bilirubin and direct bilirubin of patient from day of admission to 3 months after discharge
DISCUSSION
AIHA is a rare disease that affects 1 to 3/100,000 persons per year and can affect a wide age range. It is an acquired, clinically heterogeneous disorder characterized by the presence of autoantibodies that bind with self-antigens expressed on the RBC membrane, leading to accelerated hemolysis with or without complement activation.[10] The most common form of AIHA is caused by warm autoantibodies (w-AIHA) that react with self-antigens on RBC membrane at 37°C. w-AIHA accounts for 70% to 80% of all AIHA adult cases and 50% of pediatric cases.[10]
w-AIHA is classified as primary or secondary, depending on the etiology.[11] Secondary AIHA can be due to lymphoproliferative syndromes; chronic lymphoblastic leukemia; non-Hodgkin's lymphoma; autoimmune diseases such as systemic lupus erythematosus; APLA; infections such as cytomegalovirus, HIV, hepatitis C or EBV infection; mycoplasma infection or due to drugs such as cephalosporins. Although rare, SARS-CoV-2 infection has now been established as a secondary cause of AIHA based on cases reported in the literature.[8,9]
Pallor, weakness, jaundice, dark urine and splenomegaly can occur following acute AIHA. Anemia, reticulocytosis, elevated unconjugated bilirubin and lactate dehydrogenase, serum aspartate aminotransferase comparatively higher than serum alanine aminotransferase, and low haptoglobin are all common laboratory findings observed in AIHA.[10] Our patient also presented with these features, which was suggestive of hemolytic anemia, but presence of IgG >4 by direct antiglobulin test confirmed the diagnosis of AIHA.
Extrapulmonary complications of COVID-19 are increasingly being reported, but secondary AIHA is very rare. The exact immunopathogenic mechanism leading to hemolysis in SARS-CoV-2 infection has not yet been clearly understood. Seven cases of warm and cold AIHA associated with COVID-19 were reported by Lazarian et al.,[8] in which the median time between AIHA onset and the first COVID-19 symptoms was 9 days (range: 4–13 days) and all patients had presented with marked signs of hemolysis. In our case, AIHA developed 5 days after the onset of symptoms of COVID-19. Lopez et al.[9] reported direct Coombs test positivity for IgG and C3 in a COVID-19 patient who had a history of congenital thrombocytopenia. Warm antibodies were detected in four of the seven cases reported by Lazarian et al.[8] Similarly, our case had a direct antiglobulin test positivity for IgG. In the majority of SARS-CoV-2-associated AIHA cases, inflammatory markers such as C-reactive protein and D-dimer levels were elevated, suggesting the cardinal role of hyperinflammatory state in immune hemolysis.[11] In our case as well, inflammatory markers were elevated, further indicating the role of the hyperinflammatory state.
w-AIHA can be fatal due to acute presentation or treatment refractoriness leading to acute relapses that require multiple treatment modalities. It is associated with a mortality rate of 11% in adults and 4% in children.[12] The various treatment modalities used for w-AIHA includes steroids, intravenous immunoglobulin and rituximab.[12] About 80% of the patients with w-AIHA clinically respond to steroids within 1 to 3 weeks, with sustained value of hemoglobin >10g/dl. The level of hemoglobin at presentation is predictive of relapse risk: the lower the hemoglobin at presentation, the more the risk of relapse.[11] Severe cases have more than threefold increased risk of relapse compared to mild cases (Hb >10 g/dl).[11]
To prevent relapse, steroids should be slowly tapered over a 6-month period after complete or partial remission. In the long-term follow up (>2 years), relapses are observed in around 50–80% of the patients. They require prolonged use of low dose of steroids (≤15 mg/day prednisone) to maintain Hb >10g/dl. In our case, despite severe hemolysis, steroids were tapered off over a shorter period (i.e., in 3 months), as the response to steroids were satisfactory.
When response to steroids is not satisfactory, high-dose intravenous immunoglobulin or plasmapheresis should be considered. Patients with AIHA refractory to steroids require diagnostic re-evaluation to rule out a secondary cause such as an underlying undiagnosed malignancy or rheumatologic disease.[12] Some cases of steroid refractory AIHA may require treatment with rituximab (anti-CD 20 monoclonal antibody targeting B-lymphocyte) or splenectomy.[12] In some patients with brisk hemolysis and severe anemia, RBC transfusion can be lifesaving, which was also the case for our patient. The presence of active SARS-CoV-2 infection makes treatment of AIHA challenging, as steroids are the mainstay of treatment.[4] Careful risk–benefit assessment is required before administering steroids during the viral replication phase, but they can be safely administered when the patient progresses to the hyperinflammation stage. Risk of relapse with COVID-19 associated secondary w-AIHA has not yet been studied.
CONCLUSION
Secondary AIHA triggered by COVID-19 is extremely rare, and only few COVID-19-related w-AIHA cases have been reported in the literature. This case highlights the importance of considering COVID-19 as a differential diagnosis for secondary AIHA. Clinical heterogeneity is the hallmark of w-AIHA, and thus therapeutic decisions need to be individualized, taking into consideration the high chance of relapse and steroid refractoriness.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his clinical information to be reported in the journal. The patient understands that his name and initials will not be published, and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Peer review
This article was peer-reviewed by three independent and anonymous reviewers.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–32. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 4.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–6. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zulfiqar AA, Lorenzo-Villalba N, Hassler P, Andrès E. Immune Thrombocytopenic purpura in a patient with Covid-19. N Engl J Med. 2020;382:e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190:29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez C, Kim J, Pandey A, Huang T, DeLoughery TG. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190:31–2. doi: 10.1111/bjh.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gehrs BC, Friedberg RC. Autoimmune hemolyticanemia. Am J Hematol. 2002;69:258–71. doi: 10.1002/ajh.10062. [DOI] [PubMed] [Google Scholar]
- 11.Kalfa TA. Warm antibody autoimmune hemolyticanemia. Hematology Am Soc HematolEduc Program. 2016;2016:690–7. doi: 10.1182/asheducation-2016.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodsky RA. Warm autoimmune hemolytic anemia. N Engl J Med. 2019;381:647–54. doi: 10.1056/NEJMcp1900554. [DOI] [PubMed] [Google Scholar]


