Abstract
Background
Blood-brain barrier (BBB) pathology may be associated with mental disorders. The aim of this systematic review and meta-analysis is to identify, evaluate and summarize available evidence on whether potential biomarkers of BBB pathology are altered in patients with schizophrenia spectrum disorders, major depression and bipolar disorder compared to healthy controls.
Methods
The primary outcome is blood S100B, while secondary outcomes include biomarkers in blood and/or cerebrospinal fluid, i.e. albumin ratio, fibrinogen, immunoglobulin G, glial fibrillary acidic protein, amyloid beta (Aβ), matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases, endothelial glycocalyx constituents, and cell adhesion molecules (CAMs). A systematic search in PubMed, Embase and PsycINFO resulted in 131 eligible studies, of which 93 were included in the meta-analysis. Meta- and subgroup analyses were undertaken using random-effects modelling. The protocol was a priori registered on PROSPERO (CRD42020152721).
Results
S100B was increased in schizophrenia spectrum disorders (24 studies; 1107 patients; standardized mean difference (SMD) = 0.82; 95% confidence interval (CI) = 0.51 to 1.13; I2 = 90%), major depression (13 studies; 584 patients; SMD = 0.57; 95% CI = 0.31 to 0.83; I2 = 73%) and bipolar disorder (4 studies; 142 patients; SMD = 0.55; 95% CI = 0.16 to 0.94; I2 = 48%). Similarly, numerous secondary outcomes, including albumin ratio, fibrinogen, Aβ, MMPs and CAMs, were altered. Results of the included studies varied considerably, and important confounders were often not accounted for.
Conclusions
The findings implicate occurrence of BBB pathology in patients with schizophrenia spectrum disorders, major depression and bipolar disorder compared to healthy controls. However, definite conclusions cannot be drawn, mainly because the investigated biomarkers are indirect measures of BBB pathology.
Keywords: Blood-brain barrier, Schizophrenia, Major depression, Bipolar disorder, S100B, Albumin ratio
Highlights
-
•
The blood-brain barrier (BBB) can be studied indirectly through markers in blood and CSF.
-
•
Markers of BBB pathology were altered in schizophrenia, depression and bipolar disorder.
-
•
The findings implicate occurrence of BBB pathology in patients compared to controls.
-
•
BBB pathology is expected to contribute to the pathogenesis of severe mental disorders.
1. Introduction
Severe mental disorders, i.e. schizophrenia spectrum disorders, major depression and bipolar disorder, account for a significant proportion of the global burden of disease (Murray et al., 2012) and, to varying degrees, share characteristic traits and specific genetic risk factors (Martin et al., 2018). Although traditionally considered to be disorders purely of the brain, abnormalities outside the central nervous system (CNS) are commonly seen (Benros et al., 2012; Correll et al., 2017; Krogh et al., 2014). In line with this, a growing body of research suggests that the blood-brain barrier (BBB), which separates the CNS from peripheral tissues, may be involved in the pathogenesis of severe mental disorders (Pollak et al., 2018). However, while it is well established that BBB pathology, which is defined as changes in the structural integrity and function of the BBB, is present in several neurological disorders (Larochelle et al., 2011; Marchi et al., 2012; Sweeney et al., 2018; Yang and Rosenberg, 2011), the evidence for BBB pathology in mental disorders has not yet been systematically reviewed and quantified.
Through complex structural and functional mechanisms, the BBB plays an important role in brain protection and homeostasis (Abbott et al., 2010). Consequences of BBB pathology comprise brain entry of toxins and pathogens that can injure neuronal tissue directly or indirectly, e.g. by causing oedema, which can induce hypoperfusion and hypoxia, or by activating microglia and astrocytes, which can lead to reactive gliosis and neuroinflammation (Zlokovic, 2011). Our primary outcome, S100B, is a calcium-binding protein abundant in glial cells of the CNS, predominantly in astrocytes. Under normal conditions, levels of S100B are low in blood and high in brain tissue (Fig. 1). During BBB disruption, S100B is released into blood in significant amounts and has thus been suggested to be a good biomarker of BBB disruption as well as brain injury in general (Marchi et al., 2003). Our secondary outcomes include compounds in blood and/or cerebrospinal fluid (CSF) that might otherwise be implicated in BBB pathology (Pollak et al., 2018; Sweeney et al., 2018), i.e. albumin ratio, albumin, fibrinogen, immunoglobulin G (IgG), glial fibrillary acidic protein (GFAP), amyloid beta (Aβ), matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), endothelial glycocalyx constituents, and cell adhesion molecules (CAMs) (Fig. 1).
Fig. 1.
Overview of the blood-brain barrier and the investigated biomarkers.
Figure information: The BBB separates brain tissue from blood and is composed of brain microvascular endothelial cells, pericytes and astrocyte endfeet. Tight junctional complexes restrict paracellular diffusion between the brain microvascular endothelial cells, while the luminal and abluminal surfaces are coated by the glycoprotein-rich glycocalyx layer and the basement membrane, respectively (Abbott et al., 2010; Kutuzov et al., 2018). In a similar, but not identical way, the BCSFB separates CSF from blood and is formed by the epithelial cells of the choroid plexus. Associations between study outcomes and BBB pathology are explained in the figure. Abbreviations: BBB = blood-brain barrier; BCSFB = blood-cerebrospinal fluid barrier; CSF = cerebrospinal fluid; GFAP = glial fibrillary acidic protein; ICAM1 = intercellular adhesion molecule 1; IgG = immunoglobulin G; MMPs = matrix metalloproteinases; TIMPs = tissue inhibitor of metalloproteinases; VCAM1 = vascular cell adhesion molecule 1.
In this comprehensive systematic review and meta-analysis, we aim to identify, evaluate and summarize available evidence on whether biomarkers of BBB pathology are altered in patients with schizophrenia spectrum disorders, major depression and bipolar disorder compared to healthy controls.
2. Methods
Our review follows Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and the study protocol was a priori registered on PROSPERO (CRD42020152721).
2.1. Inclusion and exclusion criteria
We included studies fulfilling the following criteria: 1) Case-control study. 2) Investigation of markers of BBB pathology, as defined in section 2.2. 3) Inclusion of patients diagnosed with schizophrenia spectrum disorders, major depression or bipolar disorder versus healthy controls. Patients must have been diagnosed according to Diagnostic and Statistical Manual of Mental Disorders (DSM) (American Psychiatric Association, 2013), International Classification of Disease (ICD) (World Health Organization, 1992) or similar classifications that might have been used before implementation of DSM and ICD. 4) In case of a mixed patient population (e.g. inclusion of both patients with schizophrenia spectrum disorders and bipolar disorder), we only included the study if results for patient groups were available separately. 5) Published in a peer-reviewed journal. Studies written in other languages than English were excluded. No restrictions were imposed on publication period, age, gender or ethnicity of study participants.
2.2. Outcomes
2.2.1. Primary outcome
-
1.
S100b in blood
2.2.2. Secondary outcomes
-
1.
Markers exclusively in blood: GFAP.
-
2.
Markers exclusively in CSF: Albumin, fibrinogen, plasminogen, Aβ and IgG.
-
3.
Markers in both blood and CSF: Albumin ratio, MMPs, TIMPs, CAMs (i.e. soluble intercellular adhesion molecule 1 (sICAM1), soluble vascular cell adhesion molecule 1 (sVCAM1) and sE-, sL- and sP-selectin) and endothelial glycocalyx constituents (i.e. syndecan 1, heparan sulfate, hyaluronan and chondroitin sulfate).
2.3. Search strategy
References were identified through searches of PubMed, Embase and PsycINFO for articles published from inception to September 27, 2019, by the use of text words and, when appropriate, MeSH terms or similar, as described in detail in the search protocol (Table S1). Furthermore, reference lists of relevant reviews were carefully searched for additional studies. One investigator (JF) examined titles and abstracts and subsequently reviewed full-text articles for eligibility. If there were any doubts about a particular study, it was included for further assessment by a senior investigator (JK). Any disagreements were solved by discussion between investigators.
2.4. Data extraction and bias assessment
Two authors (JF and RM) independently extracted data using a pre-piloted form. In case of missing or unclear data, authors were contacted by email. If no response was received, a follow-up email was sent. Similarly, two authors (JF and RM) independently assessed the risk of bias in each study using a modified version of the Newcastle-Ottawa Scale (NOS) for case-control studies (Table S5).
2.5. Statistical analysis
Meta-analyses were conducted using RevMan (v5.0) and Comprehensive Meta-Analysis (v2) and performed separately for patients with schizophrenia spectrum disorders, bipolar disorder and major depression. Since we expected differences in techniques, e.g. assays, we estimated differences between patients and controls using the random-effects approach and reported the results as standardized mean difference (SMD). The SMD is the mean difference between cases and controls divided by the pooled standard deviation (SD). The result is a unit free effect size and, by convention, SMDs of 0.2, 0.5 and 0.8 are considered small, medium and large effect sizes, respectively. The degree of heterogeneity was quantified using the I2-statistic, which can be interpreted as the percentage of variation observed between the studies attributable to between study differences rather than stochastic variation. If SDs were not reported, we calculated SDs based on median and range as described by Hozo et al. (2005).
For the primary outcome, S100B, trial sequential analysis was applied to calculate the diversity-adjusted required information size and trial sequential monitoring boundaries for benefit and futility (Wetterslev et al., 2009). This approach allows to differentiate significant results into ‘spuriously significant’ (type I error) caused by sparse data or repetitive testing and ‘truly significant’ results, as well as neutral results into ‘spuriously insignificant’ (type II error) caused by lack of power and ‘truly neutral’ results. Based on 1545 healthy controls from 41 studies, the mean S100B was estimated to 104.4 (SD = 283) based on a random-effects analysis. A priori, we set the least significant change to 0.2 SD, which in this dataset corresponded to a difference of S100B of 57 ng/L, the two-sided alpha-values was set to 5%, and beta to 10%. Variance and heterogeneity correction were derived from each meta-analysis.
Furthermore, to determine whether heterogeneity of effect estimates was explained by population characteristics, we conducted meta-regression analyses of the following factors, when reported in ≥3 studies: 1) BMI (mean), 2) illness duration (mean), 3) age (mean), 4) psychotropic medications (% treated), 5) gender (% of males), and 6) cigarette smoking (% of smokers) (Table S6). In addition, we conducted subgroup analyses based on whether S100B was measured in serum or plasma. P-values of 0.05, or below, were considered significant for the primary outcome. Due to multiplicity, for the secondary outcomes and the meta-regression and subgroup analyses, p-values between 0.05 and 0.01 were considered ‘potentially significant’, while p-values below 0.01 were considered significant.
We also conducted stratified analyses and meta-regression in an attempt to determine whether particular clinical or study-design characteristic s influence the relationship bet ween tight glycemic control and patient outcomes.
3. Results
3.1. Search results and study characteristics
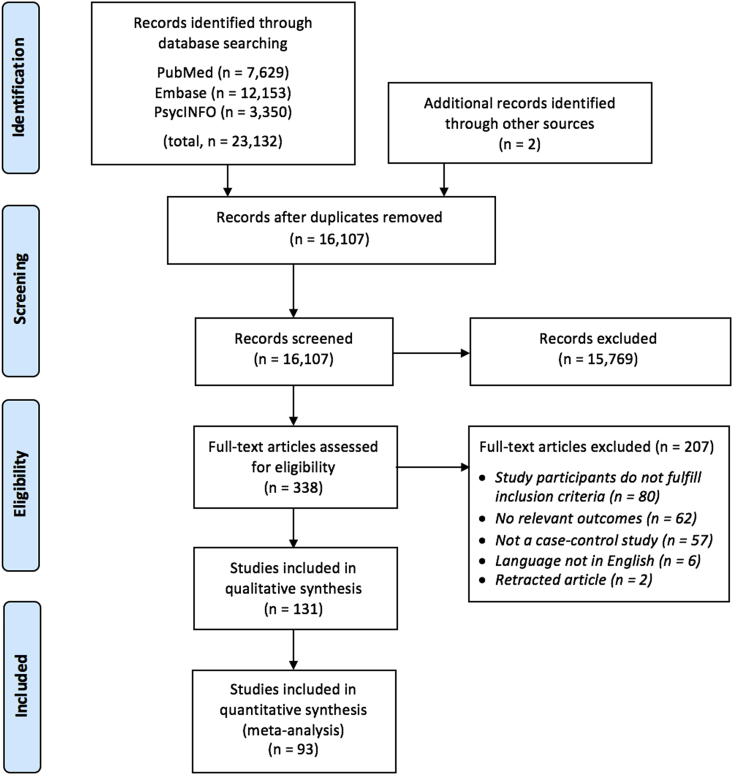
As outlined in the PRISMA flow diagram (Fig. 2), we identified 23,132 citations through the database search and two studies through other sources. A total of 131 studies were eligible for inclusion, and 93 of these reported data necessary for conducting the meta-analysis, while 38 studies were only included for qualitative synthesis either due to missing data (Table S3) or due to study participants overlapping with studies already included in the meta-analysis (Table S4). Of the 93 studies included in the meta-analysis (Table S2), 45 included patients with schizophrenia spectrum disorders, 36 included patients with major depression and 15 included patients with bipolar disorder. The median study sample size was 74 (range = 20–728), mean age ranged from 15 to 76 years, mean illness duration ranged from less than one to 35 years, and use of psychotropic medications varied regarding status, type and duration. Furthermore, studies varied regarding gender distribution.
Fig. 2.
PRISMA flow diagram of literature search and study selection.
3.2. Bias assessment
All studies were biased regarding at least one of the three assessed domains (i.e. selection, comparability and outcome) (Table S5). 15/93 (16%) studies stated that patients were consecutively enrolled, 88/93 (95%) studies included both males and females, 46/93 (49%) studies matched cases and controls on (or adjusted the results for) at least two relevant factors, 19/93 (20%) studies assessed outcomes blindly and 17/93 (18%) studies had more than 5% missing data (e.g. due to patient drop-out or assay issues).
3.3. Primary outcome
S100B was increased in patients with schizophrenia (24 studies; 1107 patients; SMD = 0.82; 95% CI = 0.51 to 1.13; I2 = 90%), major depression (13 studies; 584 patients; SMD = 0.57; 95% CI = 0.31 to 0.83; I2 = 73%) and bipolar disorder (4 studies; 142 patients; SMD = 0.55; 95% CI = 0.16 to 0.94; I2 = 48%) compared to controls (Fig. 3). Due to missing data, six S100B studies could be included in the qualitative synthesis only. Five of these reported increased levels of S100B in patients compared to controls (Arostegui et al., 2016; Deng et al., 2017; Haenisch et al., 2014; Schroeter et al., 2002; Xiong et al., 2014), while one reported no difference between groups (Haenisch et al., 2015).
Fig. 3.
Forest plots on the primary outcome, S100B.
3.3.1. Trial sequential analysis
The required information size was reached for S100B in schizophrenia spectrum disorders, major depression and bipolar disorder (Figure S1), suggesting that the observed results are not due to random error.
3.3.2. Meta-regression and subgroup analyses
Increased duration of illness potentially predicted increased SMDs of S100B levels in patients with schizophrenia spectrum disorders (17 studies; patients; SMD = 0.05, 95% CI 0.00 to 0.10, p = 0.03) and major depression (3 studies; 106 patients; SMD = 0.21, 95% CI 0.00 to 0.43, p = 0.05), while illness duration was only reported in two studies including patients with bipolar disorder (Table S6). Differences in mean age, mean BMI, percentages of males, percentages of smokers and percentages of patients receiving psychotropic medications did not explain heterogeneity of effect estimates. Furthermore, subgroup analyses revealed that the effect estimates did not depend on whether S100B was measured in plasma or serum. In a post-hoc analysis, we pooled results from the three patient groups (i.e. schizophrenia, major depression and bipolar disorder) to investigate if heterogeneity was explained by disease category. The result (p = 0.45) suggests that levels of S100B were independent of disease category.
3.4. Secondary outcomes
3.4.1. Schizophrenia spectrum disorders
The albumin ratio (2 studies; 69 patients; SMD = 0.71; 95% CI = 0.37 to 1.05; I2 = 0%) and blood levels of MMP9 (7 studies; 533 patients; SMD = 0.71; 95% CI = 0.34 to 1.08, I2 = 85%) and sP-selectin (3 studies; 98 patients; SMD = 4.45; 95% CI = 1.17 to 7.73, I2 = 98%) were increased in patients compared to controls. Levels of the following Aβ isoforms: Aβ1-17, −18, −19, −33, −34, −35, −36, −37, −38, −39, −40, −42 and Aβ11-40 were decreased, while blood levels of MMP2 (2 studies; 300 patients, SMD = −0.67; 95% CI = −0.49 to −0.09; I2 = 31%) were potentially decreased in patients compared to controls. Levels of Aβ10-40, Aβ11-42, albumin, fibrinogen, IgG, MMP3, TIMP1, sICAM1, sVCAM1, sE-, and sL-selectin did not differ between patients and controls (Table 1 and Figure S2). For the secondary outcomes, findings of studies included for the qualitative synthesis are presented in Table S3 exclusively.
Table 1.
Markers of BBB pathology in patients compared to healthy controls.
| Schizophrenia spectrum disorders vs. healthy controls | |||||||
|---|---|---|---|---|---|---|---|
| Marker | Studies | Cases | Controls | SMD | 95% CI | p-value | I2 |
| Serum/plasma S100B | 24 | 1107 | 873 | 0.82 | 0.51 to 1.13 | <0.001 | 90% |
| Albumin ratio | 2 | 69 | 72 | 0.71 | 0.37 to 1.05 | <0.001 | 0% |
| CSF albumin | 3 | 101 | 103 | 0.30 | −0.10 to 0.70 | 0.14 | 44% |
| CSF fibrinogen | 1 | 46 | 35 | −0.15 | −0.59 to 0.29 | 0.51 | NA |
| CSF Aβ1-38 | 1 | 11 | 20 | −1.22 | −2.03 to −0.42 | 0.003 | NA |
| CSF Aβ1-40 | 1 | 11 | 20 | −1.47 | −2.31 to −0.64 | <0.001 | NA |
| CSF Aβ1-42 | 1 | 11 | 20 | −1.57 | −2.42 to −0.73 | <0.001 | NA |
| CSF IgG | 3 | 101 | 103 | 0.14 | −0.56 to 0.84 | 0.70 | 81% |
| CSF sICAM1 | 2 | 56 | 47 | −0.19 | −0.81 to 0.44 | 0.56 | 48% |
| CSF sVCAM1 | 2 | 61 | 50 | −0.17 | −1.13 to 0.79 | 0.73 | 80% |
| CSF TIMP1 | 1 | 46 | 35 | 0.36 | −0.09 to 0.80 | 0.12 | NA |
| CSF MMP3 | 1 | 46 | 35 | 0.16 | −0.28 to 0.60 | 0.47 | NA |
| Serum/plasma sICAM1 | 12 | 833 | 941 | 0.20 | −0.07 to 0.47 | 0.15 | 83% |
| Serum/plasma sVCAM1 | 5 | 529 | 542 | −0.44 | −1.15 to 0.27 | 0.23 | 97% |
| Serum/plasma TIMP1 | 2 | 300 | 313 | 0.54 | −0.25 to 1.33 | 0.18 | 94% |
| Serum/plasma MMP2 | 2 | 300 | 313 | −0.67 | −0.49 to −0.09 | 0.02 | 31% |
| Serum/plasma MMP3 | 2 | 300 | 313 | −0.01 | −0.17 to 0.15 | 0.91 | 0% |
| Serum/plasma MMP9 | 7 | 533 | 509 | 0.71 | 0.34 to 1.08 | <0.001 | 85% |
| Serum/plasma sP-selectin | 3 | 98 | 92 | 4.45 | 1.17 to 7.73 | 0.008 | 98% |
| Serum/plasma sE-selectin | 3 | 112 | 105 | 0.79 | −0.32 to 1.90 | 0.16 | 93% |
| Serum/plasma sL-selectin | 2 | 62 | 55 | −134.33 | −401.9 to 133.3 | 0.33 | 99% |
|
| |||||||
| Major depression vs. healthy controls | |||||||
|
Marker |
Studies |
Cases |
Controls |
SMD |
95% CI |
p-value |
I2 |
| Serum/plasma S100B | 13 | 584 | 571 | 0.57 | 0.31 to 0.83 | <0.001 | 73% |
| Albumin ratio | 4 | 82 | 124 | 0.13 | −0.29 to 0.55 | 0.55 | 43% |
| CSF albumin | 2 | 36 | 42 | 0.02 | −0.51 to 0.55 | 0.94 | 0% |
| CSF fibrinogen | 2 | 66 | 60 | 0.56 | 0.20 to 0.91 | 0.002 | 0% |
| CSF Aβ1-38 | 1 | 28 | 38 | −0.86 | −1.37 to −0.35 | 0.001 | NA |
| CSF Aβ1-40 | 3 | 71 | 81 | −0.80 | −1.14 to −0.46 | <0.001 | 0% |
| CSF Aβ1-42 | 9 | 205 | 297 | 0.13 | −0.32 to 0.59 | 0.57 | 81% |
| CSF IgG | 2 | 36 | 42 | −0.22 | −0.75 to 0.31 | 0.41 | 0% |
| CSF MMP9 | 1 | 22 | 13 | −0.20 | −0.89 to 0.49 | 0.57 | NA |
| Serum/plasma sICAM1 | 5 | 338 | 345 | 0.73 | 0.14 to 1.31 | 0.014 | 86.1% |
| Serum/plasma sVCAM1 | 5 | 336 | 340 | 0.28 | −0.03 to 0.59 | 0.08 | 52% |
| Plasma TIMP1 | 1 | 245 | 254 | 0.08 | −0.09 to 0.26 | 0.36 | NA |
| Serum/plasma MMP2 | 2 | 261 | 294 | −0.56 | −1.35 to 0.23 | 0.16 | 84% |
| Plasma MMP3 | 1 | 245 | 254 | −0.01 | −0.18 to 0.17 | 0.95 | NA |
| Serum/plasma MMP9 | 3 | 330 | 372 | 0.19 | −0.12 to 0.50 | 0.24 | 64% |
| Serum sP-selectin | 2 | 166 | 166 | 0.20 | −0.22 to 0.62 | 0.36 | 52% |
| Serum sE-selectin | 2 | 32 | 51 | 0.54 | 0.08 to 1.00 | 0.02 | 0% |
| Serum sL-selectin | 1 | 17 | 36 | −0.01 | −0.59 to 0.56 | 0.97 | NA |
|
| |||||||
| Bipolar disorder vs. healthy controls | |||||||
|
Marker |
Studies |
Cases |
Controls |
SMD |
95% CI |
p-value |
I2 |
| Serum/plasma S100B | 4 | 142 | 101 | 0.55 | 0.16 to 0.94 | 0.006 | 48% |
| Albumin ratio | 1 | 134 | 85 | 0.42 | 0.15 to 0.70 | 0.003 | NA |
| CSF Aβ1-38 | 1 | 139 | 71 | −0.11 | −0.39 to 0.18 | 0.47 | NA |
| CSF Aβ1-40 | 1 | 139 | 71 | −0.18 | −0.46 to 0.11 | 0.23 | NA |
| CSF Aβ1-42 | 2 | 155 | 96 | −0.17 | −0.70 to 0.37 | 0.54 | 60% |
| CSF TIMP1 | 1 | 125 | 87 | 0.18 | −0.10 to 0.45 | 0.21 | NA |
| CSF TIMP2 | 1 | 125 | 87 | 0.23 | −0.04 to 0.51 | 0.10 | NA |
| Serum/plasma sICAM1 | 3 | 157 | 144 | 0.41 | 0.18 to 0.64 | <0.001 | 0% |
| Serum/plasma sVCAM1 | 3 | 157 | 144 | 0.37 | −2.08 to 2.82 | 0.77 | 99% |
| Serum MMP1 | 1 | 24 | 21 | 0.39 | −0.20 to 0.98 | 0.20 | NA |
| Serum MMP2 | 1 | 12 | 40 | −0.82 | −1.49 to −0.16 | 0.02 | NA |
| Serum MMP3 | 1 | 24 | 21 | −0.16 | −0.74 to 0.43 | 0.60 | NA |
| Serum MMP7 | 2 | 119 | 162 | 0.80 | 0.55 to 1.05 | <0.001 | 0% |
| Serum MMP9 | 3 | 328 | 224 | 0.11 | −0.06 to 0.29 | 0.20 | 0% |
| Serum MMP10 | 1 | 24 | 21 | 0.16 | −0.42 to 0.75 | 0.59 | NA |
| Serum TIMP1 | 2 | 245 | 133 | 0.16 | −0.09 to 0.42 | 0.21 | 12% |
| Serum TIMP2 | 1 | 221 | 112 | 0.05 | −0.18 to 0.27 | 0.70 | NA |
| Serum E-selectin | 2 | 74 | 71 | −0.18 | −0.51 to 0.16 | 0.31 | 4% |
| Serum P-selectin | 1 | 130 | 130 | 0.33 | 0.09 to 0.58 | 0.008 | NA |
Table information:For space-saving purposes, results on CSF Aβ isoforms other than Aβ1-38, -40 and -42 are illustrated inFigure S2exclusively. Abbreviations: Aβ = amyloid beta; CI = confidence interval; CSF = cerebrospinal fluid; IgG = immunoglobulin G; NA = not available; MMP = matrix metalloproteinase; sICAM1 = soluble intercellular adhesion molecule 1; SMD = standardized mean difference; sVCAM1 = soluble vascular cell adhesion molecule 1; TIMP = tissue inhibitor of metalloproteinases.
3.4.2. Major depression
Levels of fibrinogen (1 study; 66 patients; SMD = 0.56; 95% CI = 0.20 to 0.91) were increased, while blood levels of sICAM1 (5 studies; 338 patients; SMD = 0.73; 95% CI = 0.14 to 1.31; I2 = 86%) and sE-selectin (2 studies; 32 patients; SMD = 0.54; 95% CI = 0.08 to 1.00; I2 = 0%) were potentially increased in patients compared to controls. In contrast, levels of Aβ1-38 (1 study; 28 patients; SMD = −0.86; 95% CI = −1.37 to −0.35) and Aβ1-40 (3 studies; 71 patients; SMD = −0.8; 95% CI = −1.14 to −0.46; I2 = 0%) were decreased in patients compared to controls. The albumin ratio, Aβ1-42, albumin, IgG, MMP2, -3, -9, TIMP1, sVCAM1, sP- and sL-selectin did not differ between groups (Table 1 and Figure S2).
3.4.3. Bipolar disorder
The albumin ratio (1 study; 134 patients; SMD = 0.42; 95% CI = 0.15 to 0.70) and blood levels of sICAM1 (3 studies; 157 patients; SMD = 0.41; 95% CI = 0.18 to 0.64; I2 = 0%), sP-selectin (1 study; 130 patients; SMD = 0.33; 95% CI = 0.09 to 0.58) and MMP7 (2 studies; 119 patients; SMD = 0.80; 95% CI = 0.55 to 1.05; I2 = 0%) were increased in patients compared to controls. Meanwhile, serum levels of MMP2 (1 study; 12 patients; SMD = −0.82; 95% CI = −1.49 to −0.16) were potentially decreased in patients compared to controls, and levels of Aβ1-38, −40, −42, MMP1, -3, -9, -10, TIMP1, -2, sVCAM1 and sE-selectin did not differ between groups (Table 1 and Figure S2).
4. Discussion
This is the first systematic review and meta-analysis gathering current evidence on whether biomarkers of BBB pathology are altered in patients with severe mental disorders, i.e. schizophrenia spectrum disorders, major depression and bipolar disorder, compared to healthy controls. A subset of the included data is previously unpublished and was kindly provided to us by study authors (Bruno et al., 2017; Cai et al., 2018; Coughlin et al., 2013; Graham et al., 2008; Hayes et al., 2014; Pomara et al., 2014; Yamamori et al., 2013). The primary outcome, S100B, was increased in all patient groups. Similarly, numerous secondary outcomes, including the albumin ratio, fibrinogen, Aβ, MMPs and CAMs, were altered. Meta-regression and subgroup analyses revealed that heterogeneity of effect estimates was not explained by population characteristics. Together, the findings of the present study implicate occurrence of BBB pathology in patients with severe mental disorders compared to healthy controls.
The uncertainty regarding the extent to which the investigated biomarkers are associated with BBB pathology denotes the first and foremost limitation of the present study. Nonetheless, the investigated biomarkers all provide insights into the BBB, and more superior blood and CSF biomarkers have not been discovered yet. Secondly, important information (e.g. regarding use of psychotropic medications, illness duration, recent head trauma, BMI and smoking status) was not consistently reported in the included studies (Table S2 and -S3). Thirdly, 47/93 (51%) studies neither matched cases and controls, nor adjusted the results, for relevant confounding factors. Fourthly, publication bias, either due to non-publication of studies with non-significant results, or to inadequate reporting within the included studies, may pose a threat to the validity of the current review. To minimize publication bias, we systematically contacted study authors to request unreported results. Fifthly, the techniques used to analyse biomarkers varied across studies (Table S2 and -S3). Sixthly, considerable between-study heterogeneity of effect estimates was evident (Table 1). Finally, the investigated biomarkers were analysed separately, and uncertainty exists regarding the extent to which the biomarkers are interdependent.
Our meta-analysis includes ten studies on S100B (Arora et al., 2019; Ayyildiz et al., 2018; Chen et al., 2017; Falcone et al., 2010; Goff et al., 2018; Hendouei et al., 2016; Hong et al., 2016; Milleit et al., 2016; Morera-Fumero et al., 2017; Tao et al., 2019) that were neither included in previous meta-analyses on S100B in schizophrenia (Aleksovska et al., 2014; Schroeter et al., 2009; Schumberg et al., 2016) or affective disorders (da Rosa et al., 2016; Kroksmark and Vinberg, 2018; Schroeter et al., 2011). Nonetheless, our findings of increased blood levels of S100B are congruous with the previous meta-analyses. Since S100B is also secreted by adipose tissue, one may hypothesize that increased levels of S100B can be explained by the increased prevalence of obesity in severe mental disorders (Steiner et al., 2010). However, our meta regression analysis revealed that BMI did not predict the between-study heterogeneity in S100B effects estimates (Table S6). Yet, only 13 of the included studies reported BMI, and the lack of clarity on this matter gives evidence of the importance of considering confounding factors when investigating S100B in future studies. Similar to S100B, GFAP is almost exclusively expressed by astrocytes, and BBB disruption is a prerequisite for entry of GFAP into blood (Marchi et al., 2003). However, evidence to support the validity of GFAP as a biomarker of BBB disruption is limited compared to S100B. Only one study investigating GFAP was eligible for inclusion, but found decreased serum GFAP and increased serum S100B in patients with schizophrenia compared to controls (Xiong et al., 2014). These contrasting results as well as the limited amount of available evidence confirms that uncertainty remains associated with GFAP regarding its potential as a biomarker of BBB disruption.
A few more eligible studies have investigated the albumin ratio, which is widely considered to be a valid measure of BBB disruption. The albumin ratio was increased in patients with schizophrenia spectrum disorders and bipolar disorder, while it did not differ significantly between patients with major depression and controls. The latter finding may be due to fewer eligible cases with major depression. A previous meta-analysis, which gathered affective disorders into one group, found an increased albumin ratio in patients with affective disorders compared to healthy controls (Orlovska-Waast et al., 2019). In addition, the above mentioned previous meta-analysis investigated CSF albumin and IgG and made the same findings as the current study (Orlovska-Waast et al., 2019).
Our meta-analysis revealed that multiple CSF Aβ isoforms were decreased in patients with schizophrenia spectrum disorders and major depression. In Alzheimer’s disease and other conditions, brain amyloid deposition, which is inversely related to CSF Aβ, has been identified as a potential contributor to BBB pathology (Biron et al., 2011; Gosselet et al., 2013; Hartz et al., 2012). Specifically, Aβ may trigger cerebral angiogenesis and affect the expression of tight junctions and MMPs, thereby causing BBB disruption (Biron et al., 2011; Gosselet et al., 2013; Hartz et al., 2012). No previous meta-analyses have examined MMPs and TIMPs in severe mental disorders, and the finding of increased levels of certain MMPs in patients with schizophrenia spectrum disorders and bipolar disorder may prove important, since MMPs contribute to BBB pathology and neuroinflammation (Rempe et al., 2016). As part of a complex system of regulation, TIMPs inhibit MMP activity and prevent excessive tissue degradation. The current study revealed that levels of TIMPs did not differ between patients and controls. The fact that the increases in levels of certain MMPs are not accompanied by increases in TIMPs suggests that MMP activity, and not just levels of MMPs, are increased. Similarly, this is the first meta-analysis examining CAMs in severe mental disorders. We found increased blood levels of sP-selectin in patients with schizophrenia spectrum disorders, increased blood levels of sICAM1 and sP-selectin in patients with bipolar disorder and potentially increased blood levels of sICAM1 and sE-selectin in patients with major depression. Although CAMs are not specific for the endothelial cells of the BBB, they reflect migration of immune cells across the BBB and serve as markers of both peripheral and central inflammation (Muller, 2019; Pollak et al., 2018).
Interestingly, the endothelial glycocalyx layer, which mainly consists of glycoproteins and proteoglycans, exerts an important role in maintaining BBB integrity (Ando et al., 2018; Kutuzov et al., 2018). However, we did not find any eligible studies investigating biomarkers of degradation of the endothelial glycocalyx layer. Considering the potential role of inflammation in the pathogenesis of severe mental disorders, it is notable that inflammatory conditions have been associated with degradation of the endothelial glycocalyx layer (Kolarova et al., 2014).
4.1. Conclusion and perspectives
The current systematic review and meta-analysis implicates occurrence of BBB pathology in patients with severe mental disorders compared to healthy controls. Dysfunctional brain function and structure have previously been identified as substrate for severe mental disorders (Drevets et al., 2008). Accordingly, considering the functions of the BBB in maintaining CNS homeostasis and protecting the brain from toxins and pathogens, BBB pathology is expected to contribute to the pathogenesis of severe mental disorders. However, definite conclusions cannot be drawn since findings of the included studies varied considerably and since important confounders were often not accounted for. Furthermore, the investigated biomarkers are indirect measures of BBB pathology and therefore have a degree of uncertainty. This uncertainty may be reduced in future studies thanks to technological progress in neuroimaging techniques (Veksler et al., 2014), which can now be used in combination with blood and CSF biomarkers to improve the understanding of the role of the BBB in severe mental disorders.
Funding
Michael E. Benros’ work was funded by the Independent Research Fund Denmark (grant number 7025-00078B) and by an unrestricted grant from The Lundbeck Foundation (grant number R268-2016-3925).
Declaration of competing interests
The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100102.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Aleksovska K., Leoncini E., Bonassi S., Cesario A., Boccia S., Frustaci A. Systematic review and meta-analysis of circulating S100B blood levels in schizophrenia. PloS One. 2014;9 doi: 10.1371/journal.pone.0106342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Ando Y., Okada H., Takemura G., Suzuki K., Takada C., Tomita H. Brain-Specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain Barrier. Sci. Rep. 2018;8:17523. doi: 10.1038/s41598-018-35976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P., Sagar R., Mehta M., Pallavi P., Sharma S., Mukhopadhyay A.K. Serum S100B levels in patients with depression. Indian J. Psychiatr. 2019;61:70–76. doi: 10.4103/psychiatry.IndianJPsychiatry_391_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arostegui S., Horrillo I., Sanz-Arzuaga C., Intxauspe L., Del Rio D., Ballesteros J. Biochemical parameters associated with neuroinflammation in depressed patients: relationship with clinical intensity [abstract]. In: 29th ECNP; Austria. Eur. Neuropsychopharmacol. 2016;26:S208. [Google Scholar]
- Ayyildiz H., Eren N., Aslan B., Turgay F., Cigerli S., Karamustafalioglu O. Serum S100B levels in patients with major depression and panic disorder. Nobel Medicus. 2018;14:39–44. [Google Scholar]
- Benros M.E., Mortensen P.B., Eaton W.W. Autoimmune diseases and infections as risk factors for schizophrenia. Ann. N. Y. Acad. Sci. 2012;1262:56–66. doi: 10.1111/j.1749-6632.2012.06638.x. [DOI] [PubMed] [Google Scholar]
- Biron K.E., Dickstein D.L., Gopaul R., Jefferies W.A. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PloS One. 2011;6 doi: 10.1371/journal.pone.0023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno D., Nierenberg J., Grothe M.J., Pratico D., Pillai A., Csernansky J. A neurobiological model of memory impairment in late-life major depressive disorder [abstract] AAIC UK Alzheimer Dement. 2017;13:1332–1333. [Google Scholar]
- Cai H.Q., Catts V.S., Webster M.J., Galletly C., Liu D., O’Donnell M. Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol. Psychiatr. 2018 doi: 10.1038/s41380-018-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Tian L., Chen N., Xiu M., Wang Z., Yang G. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatr. Res. 2017;247:6–11. doi: 10.1016/j.psychres.2016.09.029. [DOI] [PubMed] [Google Scholar]
- Correll C.U., Solmi M., Veronese N., Bortolato B., Rosson S., Santonastaso P. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatr. 2017;16:163–180. doi: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin J.M., Ishizuka K., Kano S.I., Edwards J.A., Seifuddin F.T., Shimano M.A. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol. Psychiatr. 2013;18:10–11. doi: 10.1038/mp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rosa M.I., Simon C., Grande A.J., Barichello T., Oses J.P., Quevedo J. Serum S100B in manic bipolar disorder patients: systematic review and meta-analysis. J. Affect. Disord. 2016;206:210–215. doi: 10.1016/j.jad.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Deng H., Kahlon R.S., Mohite S., Amin P.A., Zunta-Soares G., Colpo G.D. Elevated plasma s100b, psychotic symptoms, and cognition in schizophrenia. Psychiatr. Q. 2017;89:53–60. doi: 10.1007/s11126-017-9514-y. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone T., Fazio V., Lee C., Simon B., Franco K., Marchi N. Serum S100B: a potential biomarker for suicidality in adolescents? PloS One. 2010;5 doi: 10.1371/journal.pone.0011089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff D.C., Zeng B., Ardekani B.A., Diminich E.D., Tang Y., Fan X. Association of hippocampal atrophy with duration of untreated psychosis and molecular biomarkers during initial antipsychotic treatment of first-episode psychosis. JAMA Psychiatry. 2018;75:370–378. doi: 10.1001/jamapsychiatry.2017.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselet F., Saint-Pol J., Candela P., Fenart L. Amyloid-beta peptides, Alzheimer’s disease and the blood-brain barrier. Curr. Alzheimer Res. 2013;10:1015–1033. doi: 10.2174/15672050113106660174. [DOI] [PubMed] [Google Scholar]
- Graham K.A., Cho H., Brownley K.A., Harp J.B. Early treatment-related changes in diabetes and cardiovascular disease risk markers in first episode psychosis subjects. Schizophr. Res. 2008;101:287–294. doi: 10.1016/j.schres.2007.12.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch F., Alsaif M., Guest P.C., Rahmoune H., Dickerson F., Yolken R. Multiplex immunoassay analysis of plasma shows prominent upregulation of growth factor activity pathways linked to GSK3beta signaling in bipolar patients. J. Affect. Disord. 2014;156:139–143. doi: 10.1016/j.jad.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Haenisch F., Alsaif M., Guest P.C., Rahmoune H., Yolken R.H., Dickerson F. Multiplex immunoassay analysis of plasma shows differences in biomarkers related to manic or mixed mood states in bipolar disorder patients. J. Affect. Disord. 2015;185:12–16. doi: 10.1016/j.jad.2015.05.065. [DOI] [PubMed] [Google Scholar]
- Hartz A.M., Bauer B., Soldner E.L., Wolf A., Boy S., Backhaus R. Amyloid-beta contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43:514–523. doi: 10.1161/strokeaha.111.627562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes L.N., Severance E.G., Leek J.T., Gressitt K.L., Rohleder C., Coughlin J.M. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr. Bull. 2014;40:963–972. doi: 10.1093/schbul/sbu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendouei N., Hosseini S.H., Panahi A., Khazaeipour Z., Barari F., Sahebnasagh A. Negative correlation between serum S100B and leptin levels in schizophrenic patients during treatment with clozapine and risperidone: preliminary evidence. Iran. J. Pharm. Res. (IJPR) 2016;15:323–330. [PMC free article] [PubMed] [Google Scholar]
- Hong W., Zhao M., Li H., Peng F., Wang F., Li N. Higher plasma S100B concentrations in schizophrenia patients, and dependently associated with inflammatory markers. Sci. Rep. 2016;6:27584. doi: 10.1038/srep27584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarova H., Ambruzova B., Svihalkova Sindlerova L., Klinke A., Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediat. Inflamm. 2014 doi: 10.1155/2014/694312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh J., Benros M.E., Jorgensen M.B., Vesterager L., Elfving B., Nordentoft M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav. Immun. 2014;35:70–76. doi: 10.1016/j.bbi.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Kroksmark H., Vinberg M. Does s100b have a potential role in affective disorders? A literature review. Nord. J. Psychiatr. 2018;72:462–470. doi: 10.1080/08039488.2018.1472295. [DOI] [PubMed] [Google Scholar]
- Kutuzov N., Flyvbjerg H., Lauritzen M. Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc. Natl. Acad. Sci. U.S.A. 2018;115:e9429–9438. doi: 10.1073/pnas.1802155115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle C., Alvarez J.I., Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585:3770–3780. doi: 10.1016/j.febslet.2011.04.066. [DOI] [PubMed] [Google Scholar]
- Marchi N., Granata T., Ghosh C., Janigro D. Blood-brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia. 2012;53:1877–1886. doi: 10.1111/j.1528-1167.2012.03637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N., Rasmussen P., Kapural M., Fazio V., Kight K., Mayberg M.R. Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor. Neurol. Neurosci. 2003;21:109–121. [PMC free article] [PubMed] [Google Scholar]
- Martin J., Taylor M.J., Lichtenstein P. Assessing the evidence for shared genetic risks across psychiatric disorders and traits. Psychol. Med. 2018;48:1759–1774. doi: 10.1017/s0033291717003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milleit B., Smesny S., Rothermundt M., Preul C., Schroeter M.L., von Eiff C. Serum S100B protein is specifically related to white matter changes in schizophrenia. Front. Cell. Neurosci. 2016;10:33. doi: 10.3389/fncel.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morera-Fumero A.L., Díaz-Mesa E., Abreu-Gonzalez P., Fernandez-Lopez L., Cejas-Mendez M.d.R. Day/night changes in serum S100B protein concentrations in acute paranoid schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;75:207–212. doi: 10.1016/j.pnpbp.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Muller N. The role of intercellular adhesion molecule-1 in the pathogenesis of psychiatric disorders. Front. Pharmacol. 2019;10:1251. doi: 10.3389/fphar.2019.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/s0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Orlovska-Waast S., Kohler-Forsberg O., Brix S.W., Nordentoft M., Kondziella D., Krogh J. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol. Psychiatr. 2019;24:869–887. doi: 10.1038/s41380-018-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak T.A., Drndarski S., Stone J.M., David A.S., McGuire P., Abbott N.J. The blood-brain barrier in psychosis. Lancet Psychiatr. 2018;5:79–92. doi: 10.1016/s2215-0366(17)30293-6. [DOI] [PubMed] [Google Scholar]
- Pomara N., Reichert C., Han Lee S., Nierenberg J., Halliday M.R., Sagare A.P. vol. 39. 2014. Increased CSF matrix metalloproteinase-9 (MMP-9) and reduced white matter integrity with increasing age in late-life major depression [abstract] pp. S324–S325. (ACNP 53rd Annual Meeting; United States. Neuropsychopharmacology). [Google Scholar]
- Rempe R.G., Hartz A.M.S., Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J. Cerebr. Blood Flow Metabol. 2016;36:1481–1507. doi: 10.1177/0271678x16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Abdul-Khaliq H., Diefenbacher A., Blasig I.E. S100B is increased in mood disorders and may be reduced by antidepressive treatment. Neuroreport. 2002;13:1675–1678. doi: 10.1097/00001756-200209160-00021. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Abdul-Khaliq H., Krebs M., Diefenbacher A., Blasig I.E. Neuron-specific enolase is unaltered whereas S100B is elevated in serum of patients with schizophrenia - original research and meta-analysis. Psychiatr. Res. 2009;167:66–72. doi: 10.1016/j.psychres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Steiner J., Mueller K. Glial pathology is modified by age in mood disorders - a systematic meta-analysis of serum S100B in vivo studies. J. Affect. Disord. 2011;134:32–38. doi: 10.1016/j.jad.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Schumberg K., Polyakova M., Steiner J., Schroeter M.L. Serum S100B is related to illness duration and clinical symptoms in schizophrenia-A Meta-regression analysis. Front. Cell. Neurosci. 2016;10:46. doi: 10.3389/fncel.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J., Myint A.M., Schiltz K., Westphal S., Bernstein H.G., Walter M. S100B serum levels in schizophrenia are presumably related to visceral obesity and insulin resistance. Cardiovasc. Psychiatry Neurol. 2010 doi: 10.1155/2010/480707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R., Fu Z., Xiao L. Chronic food antigen-specific IgG-mediated hypersensitivity reaction as a risk factor for adolescent depressive disorder. Dev. Reprod. Biol. 2019;17:183–189. doi: 10.1016/j.gpb.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veksler R., Shelef I., Friedman A. Blood-brain barrier imaging in human neuropathologies. Arch. Med. Res. 2014;45:646–652. doi: 10.1016/j.arcmed.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterslev J., Thorlund K., Brok J., Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med. Res. Methodol. 2009;9:86. doi: 10.1186/1471-2288-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Clinical descriptions and diagnostic guidelines; Geneva: 1992. The ICD-10 Classification of Mental and Behavioural Disorders. [Google Scholar]
- Xiong P., Zeng Y., Wu Q., Huang D.X.H., Zainal H., Xu X. Combining serum protein concentrations to diagnose schizophrenia: a preliminary exploration. J. Clin. Psychiatr. 2014;75:e794–801. doi: 10.4088/JCP.13m08772. [DOI] [PubMed] [Google Scholar]
- Yamamori H., Hashimoto R., Ishima T., Kishi F., Yasuda Y., Ohi K. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci. Lett. 2013;556:37–41. doi: 10.1016/j.neulet.2013.09.059. [DOI] [PubMed] [Google Scholar]
- Yang Y., Rosenberg G.A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/strokeaha.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.