Abstract
Current treatment for schizophrenia-spectrum disorders focusses primarily on psychotropic medication to treat symptoms, though their efficacy varies between patients and psychotropic medication is often accompanied by severe side effects. Nutritional interventions to prevent and treat mental illness have received considerable attention over recent years. However, evidence for nutritional interventions in schizophrenia-spectrum disorders remains limited in quantity and quality. Pathways currently in focus include: i) nutritional deficits and impairments in glucose metabolism, ii) inflammation and immune dysregulation (also known from the mild encephalitis hypothesis), and iii) altered gut microbiota. All of which appear to be interconnected. Key limiting factors for advancing research in this field are research challenges associated with assessing and interpreting inflammatory profiles, microbiota and subjective nutritional assessments, which is further complicated by illness characteristics. This review describes the state of evidence for key hypotheses, including underlying mechanisms, implicated in schizophrenia-spectrum disorders, the challenges in nutritional psychiatry research and the current state of nutrition interventions in mental healthcare.
Keywords: Nutritional psychiatry, Diet, Mental health, Gut-brain axis, Research challenges, Psychosis
1. Introduction
Schizophrenia-spectrum disorders are of unknown aetiology and current treatments can often be suboptimal. Treatment is based on antipsychotic medication which target positive symptoms of psychosis, however negative symptoms, and cognitive and functional impairment, fail to adequately improve. In addition, antipsychotic medications have significant side effects (Patel et al., 2014). Novel strategies to prevent and treat these disorders are of significant interest.
Nutritional interventions in psychiatry have received considerable attention over recent years including both nutrient supplementation and specific dietary strategies to prevent and treat mental illness (Firth et al., 2019a, 2019b). The vast majority has focussed on high-prevalence mental illness (depression and anxiety), however attention is turning to disorders such as schizophrenia and related psychoses. Varying hypotheses have been presented, some of which may be interrelated, however require further research for understanding. These include the microbiota-gut-brain axis (MGBA), immune dysregulation and dietary inflammation potential, nutritional deficits and impairments in glucose metabolism. It is notable that furthering the understanding in this field has been limited by a range of challenges in completing, interpreting and demonstrating clinical applicability of research studies in nutritional psychiatry.
Despite the evidence for effects on symptomatology and cognition being in its infancy, nutrition interventions are now accepted as a core strategy in mental healthcare to combat the physical health inequalities and life-expectancy gap in people with psychotic-spectrum disorders (World Health Organisation (WHO), 2018). Physical health has long been neglected in people with schizophrenia-spectrum leading to significantly higher rates of non-communicable diseases such as obesity, diabetes and cardiovascular disease, fuelling a significant reduction in life-expectancy (Firth et al., 2019c). This has resulted from a complex interplay of medication side-effects, symptoms, cognitive impairments, unhealthy lifestyle, lack of physical health support and diagnostic overshadowing (Firth et al., 2019c). The recent movement to provide adequate physical health support to people with schizophrenia-spectrum disorders has clearly defined a role for nutrition interventions in mental health services (World Health Organisation (WHO), 2018). This presents an ideal scenario to test the effects of nutrition interventions as adjunctive treatments for positive and negative symptoms, and cognitive impairments in people with schizophrenia-spectrum disorders. This review describes key hypothesised underlying cellular and molecular mechanisms implicated in schizophrenia-spectrum disorders, the challenges in nutritional psychiatry research and the current state of nutrition interventions in mental healthcare.
2. Pathways
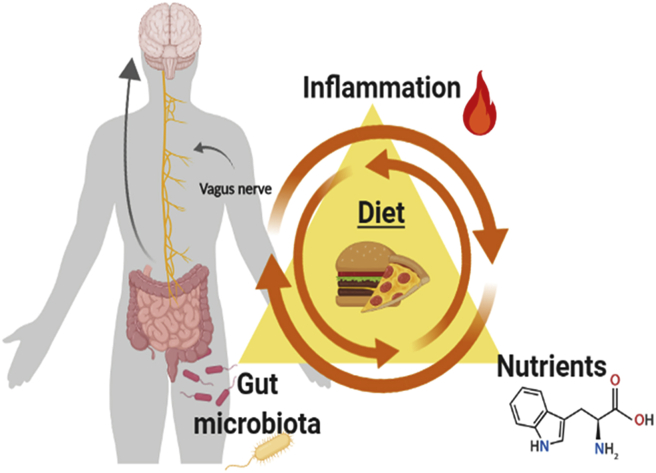
There are several potential pathways how diet might be associated with psychosis: 1st nutrients, 2nd inflammation and 3rd gut microbiota (see Fig. 1). Certain serum nutrients are lower in in people with schizophrenia-spectrum disorders, possibly due to poor dietary quality or issues with absorption. Inflammation is associated with schizophrenia-spectrum disorders and the dietary intake of this population appears to have high potential to increase inflammation. The MGBA is a bi-directional pathway system connecting the intestinal flora and central nervous system (Sharon et al., 2016). People with schizophrenia have been found to have disturbances of the MGBA. Several interactions between the gut and the brain have been observed, including vagal nerve activation, immune system modification, the synthesis of neurotransmitters and alterations of neurotransmitter pathways (Wang et al., 2019; Kaelberer et al., 2018).
Fig. 1.
Triangle of Nutrients, Inflammation and gut-microbiota in schizophrenia-spectrum disorders (This figure was created with biorender.com).
2.1. Nutrients in psychosis-spectrum disorders
Nutrient deficiencies, as a result of insufficient dietary intake or inadequate absorption, are a risk factor for mental disorders and may play a role in developing a psychotic illness and enduring schizophrenia (Sarris et al., 2015). Numerous meta-analyses have explored whether there are serum vitamin and mineral level deficits in people with schizophrenia compared to people without mental illness (Wang et al., 2016; Cao et al., 2016a, 2016b; Valipour et al., 2014; Firth et al., 2018a).
Folate (B9), B6 and B12 are vitamins important for DNA synthesis and cell division as well as RNA transcription and subsequent protein synthesis. These B vitamins are thought to be implicated in the development of schizophrenia, primarily through the altered one-carbon metabolism cycle hypothesis (Kale et al., 2010). This pathway depends on these three vitamins to create precursors to DNA. Altered one-carbon metabolism is primarily due to altered levels of these nutrients and results in increased levels of homocysteine altering DNA methylation. Hyperhomocysteinemia leads to vasculopathies such as disruption of the blood brain barrier, neurodegeneration and schizophrenia (Kalani et al., 2014).
A 2016 meta-analysis of case-control studies and a cross-sectional study found that decreased serum folate was associated with schizophrenia (26 studies, WMD = −1.57, 95% CI -2.11 to −1.02, p < 0.001) (Wang et al., 2016). This was confirmed in a second meta-analysis of case-control studies limited to the English language which found significantly lower levels of serum folate in people with schizophrenia (n = 1463) compared to nonpsychiatric controls (n = 1276), (20 studies, SMD = −0.57, 95% CI -0.76 to −0.37, p < 0.001) (Cao et al., 2016a). Further, an 2018 umbrella review of meta-analyses of associations between non-genetic risk factors and schizophrenia spectrum disorders found robust evidence for low serum folate level as a risk factor for schizophrenia (Belbasis et al., 2018). A meta-analysis of case-control studies and Mendelian randomisation analysis of serum B6 levels in people with schizophrenia compared to nonpsychiatric controls found B6 levels were significantly lower in people with schizophrenia (n = 840) compared to controls (n = 1285), (SMD = 0.48, 95% CI -0.57 to −0.39), however the Mendelian randomisation analysis was unable to find a causal relationship between B6 and schizophrenia (Tomioka et al., 2018). The evidence for serum B12 deficits in people with schizophrenia is less clear. A 2016 meta-analysis of case-control and cohort studies found no statistically significant difference in serum levels between people with schizophrenia (n = 1092) and nonpsychiatric controls (n = 1021), (13 studies, SMD = 0.09, 95% CI -0.03 to 0.22, p = 0.067) (Cao et al., 2016b). In fact, the mean serum level of B12 in people with schizophrenia was numerically higher than in the nonpsychiatric controls. This suggests that while vitamin B12 plays a key role in brain development and function, the evidence with which vitamin B12 deficiencies are implicated in the development of schizophrenia is limited.
The same umbrella review which found robust evidence for low folate as a risk factor for schizophrenia, also found robust evidence for low vitamin D status as a risk factor for schizophrenia (Belbasis et al., 2018).
Vitamin D has a major role in calcium and phosphate homeostasis and metabolism and thus, various mechanisms of action are conceivable. Vitamin D is an essential neurosteroid which modulates neurotransmitters and neurotrophic factors, hence influencing brain plasticity (Garcion et al., 2002). Epidemiological data demonstrates that the risk of developing schizophrenia is higher in winter or spring season of birth, living in high latitude versus low latitude, early life urban residency and migrant status, which is in line with the risks for vitamin D deficiency (Eyles et al., 2018; Saha et al., 2006; Vassos et al., 2012). Vitamin D deficiency, which is highly prevalent in schizophrenia, is associated with a range of relevant outcomes, most importantly, neurochemical and behavioural alterations (Ryan et al., 2015). The suggested biological mechanism by which vitamin D deficiency is involved in the development of schizophrenia focusses on the expression of vitamin D receptors in relevant areas of the brain including the dopaminergic system (Ryan et al., 2015; Chiang et al., 2016). A 2014 systematic review and meta-analysis of studies assessing serum vitamin D in people with schizophrenia had three key findings (Valipour et al., 2014). First, mean serum vitamin D levels were significantly lower in people with schizophrenia compared to non-psychiatric controls (13 studies, mean difference = −5.91 ng/mL, 95% CI -10.68 to −1.14). Second, the overall prevalence of vitamin D deficiency in people with schizophrenia was 65.3% (95% CI 46.4% to 84.2%). Third, vitamin D deficient people were 2.16 times (95% CI 1.32 to 3.56) more likely to have schizophrenia compared to people with sufficient vitamin D (Valipour et al., 2014). The research has been furthered with evidence of neonatal vitamin D deficiency being associated with the development of schizophrenia later in life. A recent study found that people with neonatal vitamin D deficiency (<20.4 nmol/L) had a 44% increased risk of developing schizophrenia later in life compared to the reference quintile (40.1–53.5 nmol/L) (Eyles et al., 2018). There is also evidence to suggest that deficits exist in serum antioxidant levels (including vitamins C and E) (Flatow et al., 2013).
To explore whether nutrient deficits are present early in the course of illness, a meta-analysis of 28 studies examined blood levels of 6 vitamins and 10 minerals in 1221 individuals with first-episode psychosis and 1391 nonpsychiatric controls was conducted (Firth et al., 2018a). This meta-analysis found lower levels of folate (6 studies, g = 0.624, 95% CI 0.072 to 1.176, p = 0.027) and vitamin D (7 studies, g = 1.055, 95% CI 0.119 to 1.99, p = 0.027), preliminary evidence for vitamin C (2 studies, g = 2.207, 95% CI 0.71 to 3.71, p = 0.004) and no evidence for the other vitamins and minerals subjected to meta-analysis (vitamin A, B12, E, zinc, magnesium, sodium, potassium, calcium, copper, chromium, iron, manganese and selenium), although many had a small number of studies (Firth et al., 2018a). In addition, exploration of correlates found significant inverse relationships for both folate and vitamin D and symptomatology in people with first-episode psychosis (Firth et al., 2018a).
A current research gap is that it is unclear whether nutrient deficits are related to poor dietary intake (e. g. due to unfavourable food choices) or poor absorption possibly linked to the gut microbiome. A 2019 systematic review and meta-analysis of studies assessing dietary intake in people with severe mental illness found that people with schizophrenia and related psychoses have more excessive and less healthy dietary intakes compared to people without a mental illness (Teasdale et al., 2019a). The diets of people with schizophrenia were characterised by large intakes of highly processed food, non-nutritive foods, and low intakes of core foods such as fruits and vegetables, which suggests that low micronutrient intake is conceivable, however, this review found mixed results relating to micronutrient intake (Teasdale et al., 2019a). The reasons behind the excessive and unhealthy dietary intake appear to be multifactorial and can include increased appetite secondary to psychotropic medication, particularly antipsychotics such as olanzapine and clozapine, disinhibition for food restraint secondary to cognitive impairments, and financial and motivation constraints leading to a reliance of convenience foods (Teasdale et al., 2017a).
Aside from nutrient deficits, hypotheses exist for: i) disturbances to glucose metabolism and mitochondrial dysfunction, and ii) gluten intolerance in the pathophysiology of schizophrenia. Energy metabolism dysfunction and oxidative stress commonly coincide with the development of schizophrenia, suggesting it is directly implicated in the pathophysiology (Martins-de-Souza et al., 2011). Glucose is the main energy source for the brain and is used for the development of dendritic spines and neurotransmitter processes enabling normal synaptic communication, however in schizophrenia, glucose and energy metabolism are impaired and alter synaptic communication (Pillinger et al., 2017). It has also been hypothesised that mitochondrial dysfunction leads to oxidative stress which may trigger immune-inflammatory responses, degenerative neurodegenerative processes and subsequently schizophrenia (Rajasekaran et al., 2015). Human studies targeting multiple domains (including biochemical, genetic, gene expression and imaging) have found changes to energy metabolism within the brain, activity of the electron transport chain and expression of genes which involve mitochondrial function.
It has been proposed that following a ketogenic diet (low in carbohydrate, moderate in protein and high in fat) can restore normal synaptic communication and improve psychiatric and neurocognitive outcomes in people with schizophrenia. The ketogenic diet forces the brain to utilise an alternative energy substrate, ketone bodies, and is thought to normalise energy metabolism within the brain. The ketogenic diet may present a novel therapeutic strategy in schizophrenia, however the current evidence base is limited to animal models, case reports and a small, uncontrolled study of 10 people with schizophrenia (Bostock et al., 2017; Sarnyai et al., 2019). Research is now turning to larger intervention studies, with the first RCT to explore the effects of following a ketogenic diet on symptomatology and functioning in people with schizophrenia currently being conducted (Ruusunen, 2019).
The gluten intolerance hypothesis is based on mechanism linking gluten intake, immune reactions and inflammation, and the development of schizophrenia. In this model it is proposed that within the subgroup of people with schizophrenia and related psychoses with inflammation, consumption of gluten drives an immune response and is implicated in the pathophysiology of the illness. Elevated Anti-Gliadin antibodies (AGA) are more frequent in people with schizophrenia than people without, and AGA has been positively associated with peripheral inflammatory markers (Kelly et al., 2018) in people with schizophrenia. Case reports in human subjects with elevated AGA have suggested improvements in symptomatology following a gluten-free diet (Tomaka et al., 2017), however similar to the ketogenic diet, there is a paucity of RCTs and therefore a lack of evidence for cause and effect.
2.2. Immune modulation in schizophrenia-spectrum disorders
Recent literature has highlighted an important link between inflammation and the pathogenesis of schizophrenia-spectrum disorders. People with psychosis show alterations in inflammatory parameters including total white blood cell count (Jackson and Miller, 2019) and subsequent immune dysregulation (Jackson and Miller, 2019; Brown and Derkits, 2009; Wang and Miller, 2017). Further, inflammatory alterations in the cerebrospinal fluid such as a higher serum albumin ratio, and elevated interleukin levels have been described in this population (Orlovska-Waast et al., 2019). Positive and negative symptoms of schizophrenia occur in the context of inflammatory disorders, infections and autoimmune diseases (Chiveri et al., 2003; Hiroshi et al., 2003; Mercadante et al., 2000) and infections have been found to be a risk factor for schizophrenia in a large 30-year epidemiological register study in Denmark (Nielsen et al., 2013). Infections in the early stages of pregnancy also seem to be of importance. Epidemiological research has shown prenatal microbial infections increase the likelihood of schizophrenia and other psychosis spectrum disorders by the factor of 10 to 20 (Babulas et al., 2006). These findings match with the Mild Encephalitis hypothesis saying that mild neuroinflammation may represent the core of the pathology in a subgroup of patients with psychosis (Bechter, 2013). Further, some antipsychotics, such as risperidone, possess immunomodulatory properties (Mantere et al., 2019), and anti-inflammatory treatments seem to be beneficial in individuals with psychotic disorders (Akhondzadeh et al., 2007).
However, it remains unclear where this inflammation comes from. Immune dysregulation is partly attributed to psychological stressors, which is considered a common risk factor for many psychiatric illnesses (Fagundes et al., 2013). Stress activates the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis (HPA axis). For example, an altered cortisol immune function and HPA axis impairment are described in patients with schizophrenia undergoing stress paradigms (Glassman et al., 2018). This important interaction of stress and the immune system is described within the vulnerability-stress-inflammation model. During stress, individuals with psychosis express inflammation-promoting risk genes (Uher and Zwicker, 2017). A range of stressors could, therefore, inflict typical alterations of dopaminergic, serotonergic, noradrenergic and glutamatergic neurotransmission and cause concomitant neuroinflammation including microglial activation (Müller, 2018; Hafizi et al., 2018).
Dietary induced inflammation has recently come into focus in schizophrenia research. In this context, poor dietary quality could, at least in part, pave the way for immune dysregulation and mental disorders. There have been several studies revealing a modest effect of dietary composition on some inflammatory markers in general population. For example, the Nurses’ Health Study of 732 women found that a healthier dietary pattern (higher intakes of fruit, vegetables, legumes, fish, poultry and wholegrains) was inversely associated with inflammatory markers C-reactive protein (CRP) and E-selectin, and a Western dietary pattern (higher intakes of red and processed meats, sweets, desserts, French fries and refined grains) was positively associated with CRP, E-selectin, sICAM-1 and sVCAM-1 (Lopez-Garcia et al., 2004). Further, studies have found that a Western dietary pattern correlates significantly with inflammatory markers such as CRP and interleukin (IL)-6 and could potentially lead to a disruption of the epithelial barrier of the gut layer, again leading to inflammation by forming a vicious cycle (Kelly et al., 2015; Noble et al., 2017). Meta-analyses of intervention trials have demonstrated causal evidence for improving dietary intake, whether it be by following a healthy dietary pattern, such as the Mediterranean diet, or modifying macronutrient composition on reducing inflammation, improving endothelial function and improving metabolic components (Schwingshackl and Hoffmann, 2014a, 2014b; Kastorini et al., 2011).
The effect of dietary components on inflammatory markers led to the development of the Dietary Inflammatory Index (DII), which calculates a score based on inflammatory potential of 45 different food parameters (Hébert et al., 2019). The DII has been validated against a number of inflammatory markers including CRP, IL-1β, IL-4, IL-6, IL- 10 and TNF-α (Hébert et al., 2019).
Studies of dietary inflammation potential in schizophrenia-spectrum disorders are limited. Possibly the first study to do this was a population-based study of the UK Biobank data (Firth et al., 2018b). This study demonstrated that people with schizophrenia showed least beneficial dietary patterns corresponding to a western-style diet rich in sugars and saturated fats (even after correction for co-factors such as age, gender, education, BMI, social deprivation and ethnicity) compared to nonpsychiatric controls, major depressive disorder and bipolar affective disorder. Further, calculation of the DII demonstrated significantly higher inflammatory potential in people with schizophrenia compared to nonpsychiatric controls (B = 0.220, SE = 0.084, p = 0.009) (Firth et al., 2018b). Therefore, further research is warranted for the hypothesis that unhealthy dietary behaviour of people experiencing psychosis leads to inflammation and subsequently, positive and negative symptoms or cognitive impairments in people with schizophrenia.
2.3. Gut microbiota in schizophrenia-spectrum disorders
The gut microbiome is the collective genome of microbes, archaea, fungi and viruses residing in the gastrointestinal tract. The number of bacteria in the human gut is approximately 3.8 × 1013 meaning that the body consists of slightly more bacterial cells than human cells (Sender et al., 2016). People living with schizophrenia and first-episode psychosis show alterations in gut microbial composition when compared to nonpsychiatric controls (Xu et al., 2019; Yuan et al., 2019; Schwarz et al., 2018). However, these differences are not consistent as studies are finding alterations of bacterial counts on different levels of phylogeny (Shen et al., 2018a; Zheng et al., 2019). A recent study which included 63 patients with schizophrenia and 69 nonpsychiatric controls, found that both medicated and unmedicated people with schizophrenia showed a decreased alpha-diversity and an altered microbial composition in comparison to healthy controls (Zheng et al., 2019). Further, some bacterial taxa (for example Veillonellaceae) were negatively correlated with the severity of schizophrenia. In first-episode psychosis, a Finnish research group compared the gut microbiota of 28 patients with 16 matched controls. The Lactobacillus group bacteria were elevated in the first-episode psychosis group, which correlated with symptom severity (Schwarz et al., 2018). A recent study from China of 41 people with first-episode psychosis and 41 healthy controls found that drug naive people with first-episode psychosis exhibit lower numbers of faecal Bifidobacterium spp., Escherichia coli, Lactobacillus spp. compared with healthy controls (HC) (all p’s < 0.001) (Yuan et al., 2018). A further study from China analysed faecal microbiota along with magnetic resonance spectroscopy in people with a high risk for psychosis. In this study, the phylogenetic orders Clostridiales, Lactobacillales and Bacteroidales and genera Lactobacillus and Prevotella were increased in the ultra-high risk group compared to both the high risk group and nonpsychiatric controls (He et al., 2018).
The important role of the MGBA, linking the gut microbiota and the central nervous system, is underlined by mechanistic studies showing that schizophrenia-like behaviour could be transferred to pathogen-free mice by faecal microbiota transplantation (FMT) originating from people with schizophrenia (Zhu et al., 2019). The FMT resulted in a range of behavioural abnormalities such as psychomotor hyperactivity, impaired learning and memory in the animals along with alterations of the tryptophan-kynurenine pathway and increased levels of extracellular dopamine in both the prefrontal cortex and the hippocampus (Zhu et al., 2019).
The underlying pathways rely on the gut microbiotas’ important part in the synthesis of short chain fatty acids, the regulation of neurotransmission and immune function. The intestines are covered by a mucus layer and gastrointestinal mucin is secreted by gut mucosal epithelial cells depending on the colonization with gut microbes. The gut microbiota constantly produce short chain fatty acids (SCFAs) such as butyrate and propionate, which serve to strengthen the gut barrier (Shen et al., 2018b). SCFAs modulate a range of immune and epigenetic pathways (such as IL-6 and TNF-alpha release from macrophages) (Kim et al., 2014). Some of these aforementioned pathways are also dysregulated in people with schizophrenia (Fan et al., 2007; Miller et al., 2011). Bacteria, which produce high levels of butyrate, improve the function of the intestinal barrier and improve the blood brain barrier as well (Braniste et al., 2014; Chen et al., 2017). Blood brain barrier dysfunction is thought to be involved in the pathogenesis of schizophrenia (Yuan et al., 2019). Due to dysfunctions of the blood brain barrier, microglia are activated and the microglia hypothesis of schizophrenia states that the disease is caused by activated microglia (Monji et al., 2009, 2013). Activated microglia produce inflammatory cytokines and reactive oxygen species, leading to neuroinflammation and altered brain plasticity due to a failure in synaptic pruning (Marques et al., 2019). Gut microbiota further produce and consume key central neurotransmitters including dopamine, noradrenaline, serotonin and gamma-aminobutyric acid (GABA) which influence not only the enteric nervous system but also the gut-brain axis (Strandwitz, 2018).
Although microbiome research is nascent, gut microbiota appear to harbour potential as a modifiable risk factor in schizophrenia-spectrum disorders (Dinan et al., 2014). As an example it has been shown, that unfavourable dietary changes which affect the intestinal bacterial communities lead to a thinning of the mucus layer, and a decline of SCFAs, and subsequent impairment of the intestinal barrier (Singh et al., 2018). Further, dietary intake impacts on neurotransmitters such as dopamine (Briguglio et al., 2018). This leads to the hypothesis that the dietary behaviour of people experiencing psychosis alters gut microbiota composition and thus, has an influence on the course of the disease. The authors are unaware of human studies that explored dietary intake, microbiome and related effects on psychopathology and neurocognition in people experiencing psychosis, but this research gap is likely to be a priority in the coming years.
3. Research challenges in the field
Each pathway discussed above is complex and, at present, much of the research is limited to animal models. The field has developed rapidly over recent years however remains in its infancy relative to other fields of research. A complicating and limiting factor to the progress of research in this field is that the proposed pathways are intertwined. This section reviews research challenges reported by others and discusses potential strategies to assist in progressing this field of research.
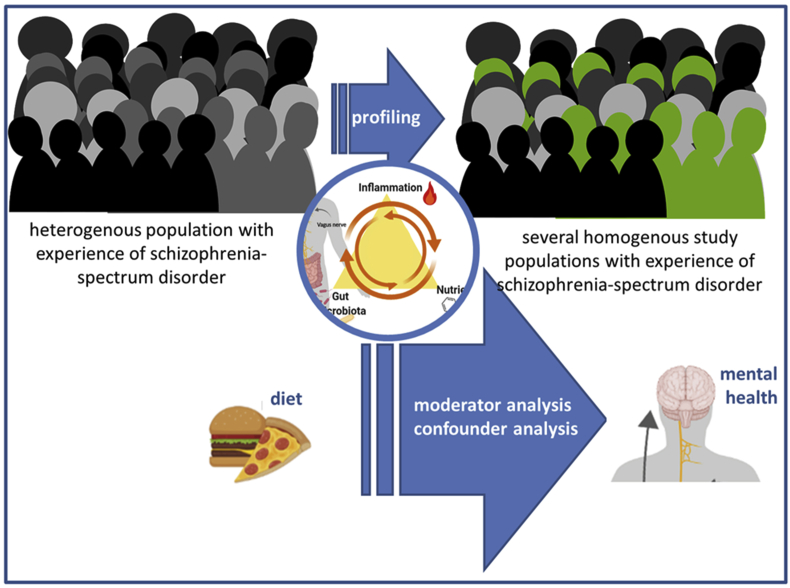
It is expected that contradictory or ambiguous results in research on schizophrenia may arise, at least in part, due to the heterogeneous study populations (Barron et al., 2017). As for the majority of chronic diseases, the aetiology of schizophrenia is based on interplay of complex genetic and environmental risk factors (Radhakrishnan et al., 2017; Joseph et al., 2017; Arroll et al., 2014). Fundamental differences in pathogenesis lead to different symptoms, and thus it is a spectrum disorder, rather than a unitary disorder, characterised by heterogeneous illness presentation, course, and outcomes (Joseph et al., 2017; Khandaker and Dantzer, 2016). There are efforts to distinguish psychosis subgroups based on symptomatology (Kay and Opler, 1987; Strik et al., 2010) and on underlying pathology and endophenotypes (Barron et al., 2017; Garcia-Rizo et al., 2012). A complicating factor is that phenotypes of schizophrenia-spectrum disorders are also mutable over time (Wigand et al., 2018) and depend on treatment status (Radhakrishnan et al., 2017). Also, the Mild Encephalitis hypothesis applies only to a subgroup of patients with psychosis and neuroinflammation and even only in certain stages of the disease (Bechter, 2013). As a consequence, treatment effects should be investigated in specific homogenous populations that are well-characterised, for example, by immune phenotypes (Kirkpatrick and Miller, 2013; Khandaker et al., 2015).
The complexity of the disorder is only one factor impeding research on diet and schizophrenia-spectrum disorders, the complexities extend through each of pathways that have been described: nutrients and metabolism, inflammation and gut-microbiota.
First, there is no consensus on which markers best reflect inflammation in human trials (Arroll et al., 2014; Calder et al., 2009, 2013). There are almost one thousand candidate biomarkers for inflammation (Minihane et al., 2015). In addition, uncertainty exists regarding the place of measurement (peripheral vs. central markers) (Radhakrishnan et al., 2017; Kirkpatrick and Miller, 2013), and the measurements for concentration change of inflammatory molecules in response to challenges (Minihane et al., 2015). As a consequence, differences in study methodology exist and comparisons that can be made between studies are limited. Moreover, challenges of assessing mild inflammatory conditions (Bechter, 2013; Minihane et al., 2015), as is the case with Mild Encephalitis, complicate research in this field and delivers the basis for ongoing discussion in psychoneuroimmunology (Pollak et al., 2020).
Second, there is wide variation in inflammatory profiles with several modifying factors that affect the concentration of an inflammatory marker at a given time. These include study factors, such as fasting conditions, time of blood draw and chronobiology (Severance et al., 2017) and individual factors, such as genetics, age, body fat, diet and physical activity (Calder et al., 2013). Importantly, clinical status of people with psychosis (i.e. acutely unwell versus clinically stable) and treatment with psychotropic medications alter inflammatory markers (Kirkpatrick and Miller, 2013). Thus, study factors should be standardised for clinical trials and individual factors should be taken into account through analysis of confounder variables.
There is even more uncertainty about gut-microbiota. Gut-microbiota research is a rapidly evolving field however there is still a large knowledge gap with regard to microbial composition and functional capacity in a ‘healthy’ population (Eisenstein, 2020). The gut-microbiota is extremely dynamic (Rea et al., 2020), and microbial composition is individualised with microbiome function varying considerably between people (Dash et al., 2015) and influenced by factors including genetics, age, lifestyle factors and medical conditions (Rea et al., 2020). Advancements in research methodology for gut-microbiota have evolved in line with the exponential growth of research in the field in the last two decades (Dash et al., 2015). Innovation in ‘omics’ technologies provide enormous opportunities for the analysis of biological samples. Though, this rapid evolvement for research methodology has driven the problem of comparability of research data. Multidimensional data were generated using these new technologies and analysis tools were developed to analyse these complex data (Rea et al., 2020). However, the interpretation of these ‘big data’ remains challenging (Minihane et al., 2015).
A substantial knowledge gap exists for the nutrient pathway in schizophrenia. Early detection of nutritional deficits and clinical deficiencies in the blood may be impeded due to the body’s ability to regulate nutrient homeostasis. Furthermore, only one fourth of dietary trials in people with SMI have conducted any objective dietary assessment (Teasdale et al., 2017b). It has been demonstrated that people with severe mental illness have a more excessive, and lower quality, dietary intake compared to people without mental illness, consuming a diet high ultra-processed food and low in core foods (Teasdale et al., 2019a). However, the validity of these available data can be scrutinised, as so far no nutritional assessment tool or method has undergone validity testing in people with severe mental illness (Teasdale et al., 2017c). Further, the Digit.Diet study (Mueller-Stierlin, 2016), has shown the difficulties in feasibility and acceptability of varying dietary assessment methods in people with severe mental illness (currently unpublished). One hypothesised reason for this is cognitive impairment, a persistent feature of schizophrenia-spectrum disorders (Gold and Harvey, 1993; O’Carroll, 2000; Green et al., 2000), which impedes memory required for retrospective assessment and complicates future planning, required for prospective dietary records (written or photographic).
Dietary interventions remain underexplored in the context of psychiatric disorders, especially schizophrenia and related psychoses. As there are plenty of confounding factors, randomized controlled trials are worthwhile. When RCTs are not feasible, sufficiently sized cohort studies could be conducted with confounder analysis for dietary and psychiatric data alongside biomarkers of inflammation and gut microbiota (Firth et al., 2018b). In addition, dietary intake should be considered in nutrient supplementation studies, due to its strong potential for confounding.
To attain homogenous study populations, profiling of study participants is recommended. However, this can complicate the recruitment of study participants and lead to the necessity for multi-site trials. Furthermore, research should aim to identify population subgroups that may particularly benefit from dietary interventions (Minihane et al., 2015). Since, for example, it has been shown that benefits of gluten-free diet are limited to a subpopulation of people with schizophrenia who demonstrate sensitivity to gluten (Joseph et al., 2017; Levinta et al., 2018; Kalaydjian et al., 2006). Therefore, it is discussed to individualise treatment based on diagnosed nutritional deficiencies and physiological abnormalities (Joseph et al., 2017; Arroll et al., 2014), on the unique microbiome signature (Rea et al., 2020), or on inflammatory markers (Minihane et al., 2015).
A number of methodological issues have been raised that need to be addressed in future studies. These have been brought together in a multi-component approach, which could guide further research on the role of diet in schizophrenia-spectrum disorders (see Fig. 2).
Fig. 2.
A multi-component approach for investigating the role of diet in schizophrenia-spectrum disorders (This figure has partly been created with biorender.com).
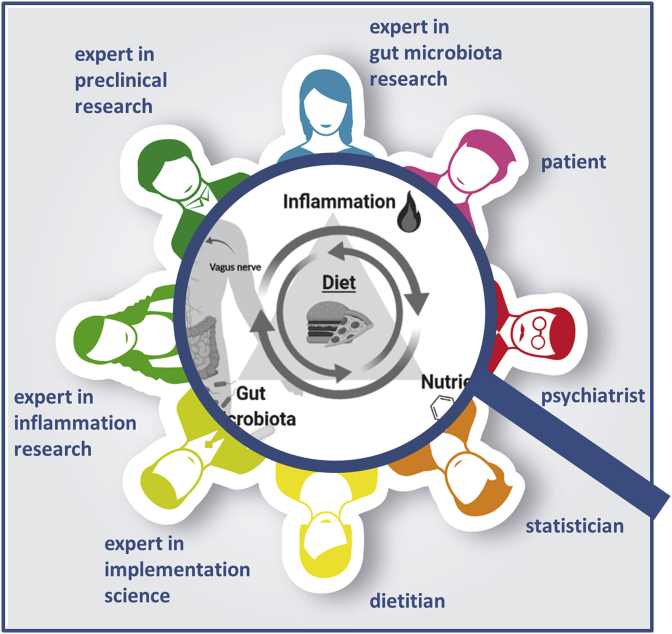
Research would benefit from studies adopting this approach where experts from different fields collaborate to disentangle the complexity of this research subject. This kind of research is only possible by multi-professional consortia (see Fig. 3), including:
-
•
People experiencing schizophrenia-spectrum disorders are the focus of the research team and future research should include co-design with people who have had lived experience of a psychotic illness.
-
•
Mental health professionals, especially psychiatrists, are fundamental in this team for diagnostic reasons but also their responsibility for drug treatment and in this way, they can assist in managing one of the most important confounding factors.
-
•
Dietitians or clinical nutritionists performing dietary assessment and are responsible for designing and delivering dietary interventions
-
•
Experts in implementation science are very important when it comes to the successful implementation of rather complex dietary interventions.
-
•
Experts in the field of gut microbiota and inflammation are needed for the extensive profiling of study participants and for moderator analysis.
-
•
Experts in applied fundamental research and preclinical research should be incorporated in order to make optimal use of the knowledge gained from preclinical studies.
-
•
Experts with excellent skills in statistical analysis are needed to interpret generated data. E.g. pathway-based analysis could be used for hypothesis-driven investigation of the statistical interaction between diet and mental health via nutrients, inflammation or microbiota.
Fig. 3.
Multi-professional research consortia investigating the role of diet in schizophrenia-spectrum disorders (This figure has partly been created with biorender.com).
In short, strong interprofessional networks are needed to deliver high-quality nutritional psychiatry research to realise the clinical potential of this rapidly expanding field. To date, consolidation of results from different studies is impeded by the different research methodologies that have been used. In order to gain maximum knowledge from current research activities, it is recommended that extensive discussion between international experts occurs in a structure format, e. g. Delphi approach. The result of the discussion should then be presented in a guideline, as has already been done for omega-3 supplementation (Guu et al., 2019).
4. Nutritional interventions
4.1. Role of diet in mental health care
Current treatment for psychotic disorders focusses primarily on antipsychotic medication with or without other psychotropic medication. Psychotropic medications, which are often an essential component of treatment, have limited efficacy in some patients and are often accompanied by severe side effects which can compromise treatment compliance (Arroll et al., 2014). Further, antipsychotic medication primarily targets the reduction of positive symptoms, and does little for other domains such as negative symptoms and cognitive impairments. The transition to a multidisciplinary approach providing psychosocial interventions allows a more holistic model of care. Given a healthy diet can reduce inflammation and promote gut microbiota composition, thought to be beneficial for health, dietary interventions represent an attractive therapeutic possibility for people with psychosis, with minimal risk of adverse effects (Joseph et al., 2017; Rea et al., 2020; Dinan and Cryan, 2020).
Despite the call for “nutritional medicine as mainstream in psychiatry” (Sarris et al., 2015), currently, nutritional interventions are rarely considered for improving mental health outcomes in routine care. One of the main reasons for this is the lack of evidence of its effectiveness. In addition to weight-loss, there is strong evidence that a healthy dietary pattern can reduce inflammation and improve endothelial function (Schwingshackl and Hoffmann, 2014a), however there is paucity of evidence demonstrating the effect of dietary interventions on microbiome, inflammatory makers and symptomatology in people with schizophrenia-spectrum disorders. A 2017 systematic review and meta-analysis of nutrition intervention RCTs for people with severe mental illness found that inflammatory markers and mental health outcomes were infrequently assessed, and no conclusions could be made regarding the effects of nutrition interventions on either of these domains, and no intervention included microbiome as an outcome (Teasdale et al., 2017b).
Regarding nutrient supplementation, a recent meta-review of 33 meta-analyses of nutrient supplementation in mental disorders came to the following conclusions regarding schizophrenia-spectrum disorders (Firth et al., 2019b): First, there was no evidence for nutrient supplementation as a standalone treatment for any mental disorder, and second, although nutrient supplementation was found to be safe and there was no indication of adverse effects or contraindications with psychotropic medications, there was limited evidence of effectiveness as adjunctive treatment in people with schizophrenia-spectrum disorders (Firth et al., 2019b). The most promising evidence was for methylfolate, the most bioactive form of folate, in reducing negative symptoms and N-acetylcysteine (NAC), a nutraceutical form of the amino acid cysteine, for reducing total symptom scores, which appeared to be driven by reductions in negative symptoms (Firth et al., 2019b).
Folate may be best administered in combination with other B-vitamins (e.g. B12 and B6) which may be effective through the one-carbon metabolism cycle. A meta-analysis which included seven RCTs using B-vitamins, found that B-vitamins were most effective in combination for schizophrenia symptomatology, and that B-vitamin effectiveness was associated with shorter illness duration (Firth et al., 2017a). The theory that the combination of B-vitamins may work through the one-carbon metabolism cycle is supported by an RCT in people with schizophrenia and hyperhomocysteinemia, which found reductions in homocysteine levels and psychiatric symptomatology (Levine et al., 2006). Further an RCT of vitamins B12, B6 and folate in people with first-episode psychosis reduced homocysteine levels but did not improve psychopathology or neurocognition, however significant differences were noted for secondary outcomes - attention and vigilance (Allott et al., 2019). In current clinical practice guidelines, folate and B12 levels are recommended to be assessed in first-episode psychosis to rule out deficiencies (Early Psychosis Guidelines Writing Group and EPPIC National Support Program, 2016). Further research may describe a role for assessing homocysteine levels and more specific nutrient supplementation guidelines in clinical practice.
4.2. Role of diet in physical health care for psychosis
Despite an absence of trials assessing dietary interventions on psychopathology and neurocognition, dietary interventions are becoming routine care in people with schizophrenia-spectrum disorder given the 15-year mortality gap, predominantly driven by cardiometabolic complications (Lawrence et al., 2013). People experiencing severe mental illness have higher rates of abdominal obesity (OR 4.43), hypertriglyceridemia (OR 2.73), metabolic syndrome (OR 2.35), low HDL (OR 2.35), diabetes (OR 1.99) and hypertension (1.36) when compared to people in the general population (Vancampfort et al., 2015). The 2019 Lancet Psychiatry Commission: A blueprint for protecting physical health in people with mental illness highlighted dietary interventions, in combination with physical activity, smoking cessation, best-practice psychotropic prescribing and the use of metformin in prevention and treatment as key strategies to reduce these health inequalities and mortality gap, and that these components should be provided right from the start of treatment and recovery (Firth et al., 2019c).
An example of program meeting the recommendations of the Lancet Commission is the ‘Keeping the Body in Mind’ program, which is a multidisciplinary program composed of specialist clinicians, embedded within the mental health service, delivery lifestyle intervention (nutrition and physical activity interventions) from the early stages of treatment of a psychotic illness (Curtis et al., 2016). The dietitian uses an individualised approach in providing education, behaviour change processes, motivation and goal setting, combined with practical life-skills building around shopping, meal planning, cooking and food budgeting (Teasdale et al., 2016). The ‘Keeping the Body in Mind’ program has demonstrated that protection of physical health in people with schizophrenia-spectrum disorders is possible (Curtis et al., 2016, 2018; Teasdale et al., 2019b). Moreover, the integration of nutrition interventions into routine mental healthcare for physical health reasons provides the opportune platform to test the effects of different strategies on symptomatology, cognition and quality of life, and may assist in growing the evidence base more efficiently.
An important consideration as the evidence base grows is that nutrition interventions in real world settings are rarely delivered as the sole intervention, they are frequently combined with other lifestyle components, most notably physical activity. The evidence base for physical activity is robust for improving physical health components (Firth et al., 2015), and the evidence for improving mental health outcomes in schizophrenia-spectrum disorders has grown significantly over the last decade (Firth et al., 2015, 2017b). If efficacy of nutritional strategies for mental health outcomes in schizophrenia-spectrum disorders is demonstrated, real world interventions should look to deliver both nutrition and physical activity interventions to people with schizophrenia-spectrum disorders for both physical and mental health as a combination rather than in independent silos.
5. Conclusion
In conclusion, there is a strong rationale for the critical role of diet in psychosis for protecting physical health. However, the complexity of the topic appears to be delaying the evidence base on therapeutic benefits for symptomatology and cognition. A multi-component approach by interdisciplinary research consortia using profiling and extensive moderator and confounder analysis for dietary intervention studies on mental health outcomes is recommended. Ideally, independent studies targeting specific nutritional psychiatry questions with adequate funding will increase in the coming decades. However, given dietitians and clinical nutritionists are becoming more frequent in mental health services to target physical health, it may be beneficial to make greater use of these available services by designing nutritional psychiatry studies around their dietary intervention programs. In either case research methods of different studies should be well aligned. It is anticipated that ongoing and greater collaborations in nutritional psychiatry research will lead to consensus on research methods including intervention protocols and outcome measurements, such as the measurement of diet, inflammation and gut microbiota.
Authors’ contributions
S.M., S.T. and A.S.M-S. provided the conception of the article during an onsite meeting, drafted simultaneously different parts of the article, revised it critically for important intellectual content, and gave final approval of the version to be submitted.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgements
None.
Contributor Information
Scott Teasdale, Email: scott.teasdale@unswalumni.com.
Sabrina Mörkl, Email: sabrina.moerkl@medunigraz.at.
Annabel Sandra Müller-Stierlin, Email: annabel.mueller-stierlin@uni-ulm.de.
References
- Akhondzadeh S., Tabatabaee M., Amini H., Abhari S.A.A., Abbasi S.H., Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr. Res. 2007;90(1–3):179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Allott K., McGorry P.D., Yuen H.P., Firth J., Proffitt T.-M., Berger G. The Vitamins in Psychosis study: a randomized, double-blind, placebo-controlled trial of the effects of vitamins B12, B6, and folic acid on symptoms and neurocognition in first-episode psychosis. Biol. Psychiatr. 2019;86(1):35–44. doi: 10.1016/j.biopsych.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Arroll M.A., Wilder L., Neil J. Nutritional interventions for the adjunctive treatment of schizophrenia: a brief review. Nutr. J. 2014;13(1):91. doi: 10.1186/1475-2891-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babulas V., Factor-Litvak P., Goetz R., Schaefer C.A., Brown A.S. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am. J. Psychiatr. 2006;163(5):927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- Barron H., Hafizi S., Andreazza A.C., Mizrahi R. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int. J. Mol. Sci. 2017;18(3):651. doi: 10.3390/ijms18030651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechter K. Updating the mild encephalitis hypothesis of schizophrenia. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2013;42:71–91. doi: 10.1016/j.pnpbp.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Belbasis L., Köhler C.A., Stefanis N., Stubbs B., van Os J., Vieta E. Risk factors and peripheral biomarkers for schizophrenia spectrum disorders: an umbrella review of meta-analyses. Acta Psychiatr. Scand. 2018;137(2):88–97. doi: 10.1111/acps.12847. [DOI] [PubMed] [Google Scholar]
- Bostock E., Kirkby K.C., Taylor B.V.M. The current status of the ketogenic diet in psychiatry. Front. Psychiatr. 2017;8:43. doi: 10.3389/fpsyt.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6(263):158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briguglio M., Dell’Osso B., Panzica G., Malgaroli A., Banfi G., Zanaboni Dina C. Dietary neurotransmitters: a narrative review on current knowledge. Nutrients. 2018;10(5):591. doi: 10.3390/nu10050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S., Derkits E.J. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatr. 2009;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C., Albers R., Antoine J.M., Blum S., Bourdet-Sicard R., Ferns G.A., Rabet L., Serafini M., Van Eden W., Van Loo J., Vas Dias W., Vidry S., Winklhofer-Roob B.M., Zhao J. Special issue: inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009;101:S1–S45. doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
- Calder P.C., Ahluwalia N., Albers R., Bosco N., Bourdet-Sicard R., Haller D. A consideration of biomarkers to be used for evaluation of inflammation in human nutritional studies. Br. J. Nutr. 2013;109(S1):S1–S34. doi: 10.1017/S0007114512005119. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang D.-F., Xu M.-Y., Liu Y.-Q., Yan L.-L., Wang J.-Y. Lower folate levels in schizophrenia: a meta-analysis. Psychiatr. Res. 2016;245:1–7. doi: 10.1016/j.psychres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang D.-F., Xu M.-Y., Liu Y.-Q., Yan L.-L., Wang J.-Y. Vitamin B12 and the risk of schizophrenia: a meta-analysis. 2016;172(1–3):216–217. doi: 10.1016/j.schres.2016.01.050. [DOI] [PubMed] [Google Scholar]
- Chen T., Kim C.Y., Kaur A., Lamothe L., Shaikh M., Keshavarzian A. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017;8(3):1166–1173. doi: 10.1039/c6fo01532h. [DOI] [PubMed] [Google Scholar]
- Chiang M., Natarajan R., Fan X. Vitamin D in schizophrenia: a clinical review. Evid. Base Ment. Health. 2016;19(1):6–9. doi: 10.1136/eb-2015-102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiveri L., Sciacco M., Prelle A. Schizophreniform disorder with cerebrospinal fluid PCR positivity for herpes simplex virus type 1. Eur. Neurol. 2003;50(3):182. doi: 10.1159/000073062. [DOI] [PubMed] [Google Scholar]
- Curtis J., Watkins A., Rosenbaum S., Teasdale S.B., Kalucy M., Samaras K. Evaluating an individualized lifestyle and life skills intervention to prevent antipsychotic-induced weight gain in first-episode psychosis. Early Interven. Psychiatr. 2016;10(3):267–276. doi: 10.1111/eip.12230. [DOI] [PubMed] [Google Scholar]
- Curtis J., Watkins A., Teasdale S.B., Lederman O., Kalucy M., Lappin J. 2 year follow-up: still keeping the body in Mind. Aust. N. Z. J. Psychiatr. 2018;52:602–603. doi: 10.1177/0004867417753553. [DOI] [PubMed] [Google Scholar]
- Dash S., Clarke G., Berk M., Jacka F.N. The gut microbiome and diet in psychiatry: focus on depression. Curr. Opin. Psychiatr. 2015;28(1):1–6. doi: 10.1097/YCO.0000000000000117. [DOI] [PubMed] [Google Scholar]
- Dinan T.G., Cryan J.F. Gut microbiota: a missing link in psychiatry. World Psychiatr. 2020;19(1):111–112. doi: 10.1002/wps.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T., Borre Y., Cryan J. Genomics of schizophrenia: time to consider the gut microbiome? Mol. Psychiatr. 2014;19(12):1252. doi: 10.1038/mp.2014.93. [DOI] [PubMed] [Google Scholar]
- Early Psychosis Guidelines Writing Group and EPPIC National Support Program . second ed. Orygen, the National Centre for Excellence in Youth Mental Health; Melbourne: 2016. Australia Clinical Guidelines for Early Psychosis. update. [Google Scholar]
- Eisenstein M. The hunt for a healthy microbiome. Nature. 2020;577:S6–S8. doi: 10.1038/d41586-020-00193-3. [DOI] [PubMed] [Google Scholar]
- Eyles D.W., Trzaskowski M., Vinkhuyzen A.A.E., Mattheisen M., Meier S., Gooch H. The association between neonatal vitamin D status and risk of schizophrenia. Sci. Rep. 2018;8(1):1–8. doi: 10.1038/s41598-018-35418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes C.P., Glaser R., Kiecolt-Glaser J.K. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Goff D.C., Henderson D.C. Inflammation and schizophrenia. Expert Rev. Neurother. 2007;7(7):789–796. doi: 10.1586/14737175.7.7.789. [DOI] [PubMed] [Google Scholar]
- Firth J., Cotter J., Elliott R., French P., Yung A.R. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol. Med. 2015;45(7):1343–1361. doi: 10.1017/S0033291714003110. [DOI] [PubMed] [Google Scholar]
- Firth J., Stubbs B., Sarris J., Rosenbaum S., Teasdale S., Berk M. The effects of vitamin and mineral supplementation on symptoms of schizophrenia: a systematic review and meta-analysis. Psychol. Med. 2017;47(9):1515–1527. doi: 10.1017/S0033291717000022. [DOI] [PubMed] [Google Scholar]
- Firth J., Stubbs B., Rosenbaum S., Vancampfort D., Malchow B., Schuch F. Aerobic exercise improves cognitive functioning in people with schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 2017;43(3):546–556. doi: 10.1093/schbul/sbw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J., Carney R., Stubbs B., Teasdale S., Vancampfort D., Ward P. Nutritional deficiencies and clinical correlates in first-episode psychosis: a systematic review and meta-analysis. Schizophr. Bull. 2018;44(6):1275–1292. doi: 10.1093/schbul/sbx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J., Stubbs B., Teasdale S.B., Ward P.B., Veronese N., Shivappa N. Diet as a hot topic in psychiatry: a population-scale study of nutritional intake and inflammatory potential in severe mental illness. World Psychiatr. 2018;17(3):365–367. doi: 10.1002/wps.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J., Marx W., Dash S., Carney R., Teasdale S.B., Solmi M. The effects of dietary improvement on symptoms of depression and anxiety: a meta-analysis of randomized controlled trials. Psychosom. Med. 2019;81(3):265. doi: 10.1097/PSY.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J., Teasdale S.B., Allott K., Siskind D., Marx W., Cotter J. The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatr. 2019;18(3):308–324. doi: 10.1002/wps.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J., Siddiqi N., Koyanagi A., Siskind D., Rosenbaum S., Galletly C. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatr. 2019;6(8):675–712. doi: 10.1016/S2215-0366(19)30132-4. [DOI] [PubMed] [Google Scholar]
- Flatow J., Buckley P., Miller B.J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatr. 2013;74(6):400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rizo C., Fernandez-Egea E., Oliveira C., Justicia A., Bernardo M., Kirkpatrick B. Inflammatory markers in antipsychotic-naïve patients with nonaffective psychosis and deficit vs. nondeficit features. Psychiatr. Res. 2012;198(2):212–215. doi: 10.1016/j.psychres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcion E., Wion-Barbot N., Montero-Menei C.N., Berger F., Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metabol. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- Glassman M., Wehring H.J., Pocivavsek A., Sullivan K.M., Rowland L.M., McMahon R.P. Peripheral cortisol and inflammatory response to a psychosocial stressor in people with schizophrenia. J. Neuropsychiatr. 2018;2(2):4. doi: 10.21767/2471-8548.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.M., Harvey P.D. Cognitive deficits in schizophrenia. Psychiatr. Clin. 1993;16(2):295–312. [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr. Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Guu T.-W., Mischoulon D., Sarris J., Hibbeln J., McNamara R.K., Hamazaki K. International society for nutritional psychiatry research practice guidelines for omega-3 fatty acids in the treatment of major depressive disorder. Psychother. Psychosom. 2019;88(5):263–273. doi: 10.1159/000502652. [DOI] [PubMed] [Google Scholar]
- Hafizi S., Guma E., Koppel A., Da Silva T., Kiang M., Houle S. S169. Microglial activation and morphological brain alterations in psychosis and psychosis risk. Schizophr. Bull. 2018;44(Suppl. 1):S390. [Google Scholar]
- He Y., Kosciolek T., Tang J., Zhou Y., Li Z., Ma X. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. Eur. Psychiatr. 2018;53:37–45. doi: 10.1016/j.eurpsy.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Hébert J.R., Shivappa N., Wirth M.D., Hussey J.R., Hurley T.G. Perspective: the dietary inflammatory Index (DII)—lessons learned, improvements made, and future directions. Adv. Nutr. 2019;10(2):185–195. doi: 10.1093/advances/nmy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroshi H., Seiji K., Toshihiro K., Nobuo K. An adult case suspected of recurrent measles encephalitis with psychiatric symptoms. Seishin Shinkeigaku Zasshi. 2003;105(10):1239–1246. [PubMed] [Google Scholar]
- Jackson A.J., Miller B.J. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr. Scand. 2019 doi: 10.1111/acps.13140. Epub ahead of print 18 Dec. [DOI] [PubMed] [Google Scholar]
- Joseph J., Depp C., Shih P-aB., Cadenhead K.S., Schmid-Schönbein G. Modified mediterranean diet for enrichment of short chain fatty acids: potential adjunctive therapeutic to target immune and metabolic dysfunction in schizophrenia? Front. Neurosci. 2017;11:155. doi: 10.3389/fnins.2017.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408):eaat5326. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani A., Kamat P.K., Givvimani S., Brown K., Metreveli N., Tyagi S.C. Nutri-epigenetics ameliorates blood–brain barrier damage and neurodegeneration in hyperhomocysteinemia: role of folic acid. J. Mol. Neurosci. 2014;52(2):202–215. doi: 10.1007/s12031-013-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaydjian A.E., Eaton W., Cascella N., Fasano A. The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatr. Scand. 2006;113(2):82–90. doi: 10.1111/j.1600-0447.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- Kale A., Naphade N., Sapkale S., Kamaraju M., Pillai A., Joshi S. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatr. Res. 2010;175(1–2):47–53. doi: 10.1016/j.psychres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Kastorini C.-M., Milionis H.J., Esposito K., Giugliano D., Goudevenos J.A., Panagiotakos D.B. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011;57(11):1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Opler L.A. The positive-negative dimension in schizophrenia: its validity and significance. Psychiatr. Dev. 1987;5(2):79–103. [PubMed] [Google Scholar]
- Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D.L., Demyanovich H.K., Eaton W.W., Cascella N., Jackson J., Fasano A. Anti gliadin antibodies (AGA IgG) related to peripheral inflammation in schizophrenia. Brain Behav. Immun. 2018;69:57–59. doi: 10.1016/j.bbi.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Dantzer R. Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacology. 2016;233(9):1559–1573. doi: 10.1007/s00213-015-3975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Cousins L., Deakin J., Lennox B.R., Yolken R., Jones P.B. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatr. 2015;2(3):258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Park J., Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Network. 2014;14(6):277–288. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B., Miller B.J. Inflammation and schizophrenia. Schizophr. Bull. 2013;39(6):1174–1179. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D., Hancock K.J., Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ. 2013;346 doi: 10.1136/bmj.f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Stahl Z., Sela B.-A., Ruderman V., Shumaico O., Babushkin I. Homocysteine-reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemia. Biol. Psychiatr. 2006;60(3):265–269. doi: 10.1016/j.biopsych.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Levinta A., Mukovozov I., Tsoutsoulas C. Use of a gluten-free diet in schizophrenia: a systematic review. Adv. Nutr. 2018;9(6):824–832. doi: 10.1093/advances/nmy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia E., Schulze M.B., Fung T.T., Meigs J.B., Rifai N., Manson J.E. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2004;80(4):1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- Mantere O., Trontti K., Garcia-Gonzalez J., Balcells I., Saarnio S., Mäntylä T. Immunomodulatory effects of antipsychotic treatment on gene expression in first-episode psychosis. J. Psychiatr. Res. 2019;109:18–26. doi: 10.1016/j.jpsychires.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Marques T.R., Ashok A.H., Pillinger T., Veronese M., Turkheimer F.E., Dazzan P. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol. Med. 2019;49(13):2186–2196. doi: 10.1017/S0033291718003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-de-Souza D., Harris L.W., Guest P.C., Bahn S. The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxidants Redox Signal. 2011;15(7):2067–2079. doi: 10.1089/ars.2010.3459. [DOI] [PubMed] [Google Scholar]
- Mercadante M.T., Busatto G.F., Lombroso P.J., Prado L., Rosário-Campos M.C., do Valle R. The psychiatric symptoms of rheumatic fever. Am. J. Psychiatr. 2000;157(12):2036–2038. doi: 10.1176/appi.ajp.157.12.2036. [DOI] [PubMed] [Google Scholar]
- Miller B.J., Buckley P., Seabolt W., Mellor A., Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol. Psychiatr. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minihane A.M., Vinoy S., Russell W.R., Baka A., Roche H.M., Tuohy K.M. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br. J. Nutr. 2015;114(7):999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A., Kato T., Kanba S. Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatr. Clin. Neurosci. 2009;63(3):257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Monji A., Kato T.A., Mizoguchi Y., Horikawa H., Seki Y., Kasai M. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2013;42:115–121. doi: 10.1016/j.pnpbp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Mueller-Stierlin Annabel Sandra. 2016. Evaluation of the digital method to assess the nutrient intake of patients with mental disorders with regard to the method’s practicability.https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00010104 German Clinical Trials Register, 2016 Mar 24 [cited 2020 Feb 20], Identifier: DRKS00010104. [6 pages]. Available from: [Google Scholar]
- Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 2018;44(5):973–982. doi: 10.1093/schbul/sby024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P.R., Benros M.E., Mortensen P.B. Hospital contacts with infection and risk of schizophrenia: a population-based cohort study with linkage of Danish national registers. Schizophr. Bull. 2013;40(6):1526–1532. doi: 10.1093/schbul/sbt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble E.E., Hsu T.M., Kanoski S.E. Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome, and cognitive impairment. Front. Behav. Neurosci. 2017;11:9. doi: 10.3389/fnbeh.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll R. Cognitive impairment in schizophrenia. Adv. Psychiatr. Treat. 2000;6(3):161–168. [Google Scholar]
- Orlovska-Waast S., Köhler-Forsberg O., Brix S.W., Nordentoft M., Kondziella D., Krogh J. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol. Psychiatr. 2019;24(6):869–887. doi: 10.1038/s41380-018-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K.R., Cherian J., Gohil K., Atkinson D. Schizophrenia: overview and treatment options. Pharm. Therap. 2014;39(9):638. [PMC free article] [PubMed] [Google Scholar]
- Pillinger T., Beck K., Gobjila C., Donocik J.G., Jauhar S., Howes O.D. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74(3):261–269. doi: 10.1001/jamapsychiatry.2016.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak T.A., Prüss H., van Elst L.T., Vincent A., Najjar S., Bechter K. Autoimmune psychosis–Authors’ reply. Lancet Psychiatr. 2020;7(2):123–125. doi: 10.1016/S2215-0366(19)30527-9. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R., Kaser M., Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr. Bull. 2017;43(4):693–697. doi: 10.1093/schbul/sbx057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran A., Venkatasubramanian G., Berk M., Debnath M. Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci. Biobehav. Rev. 2015;48:10–21. doi: 10.1016/j.neubiorev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Rea K., Dinan T.G., Cryan J.F. Gut microbiota: a perspective for psychiatrists. Neuropsychobiology. 2020;79(1–2):50–62. doi: 10.1159/000504495. [DOI] [PubMed] [Google Scholar]
- Ruusunen Anu. ClinicalTrials.gov. National Library of Medicine (US); Bethesda (MD): 2019. Ketogenic Diet for Psychotic Disorders (PsyDiet)https://clinicaltrials.gov/ct2/show/NCT03873922 Identifier - NCT03873922; registered: 2019 Mar 14. [Google Scholar]
- Ryan J.W., Anderson P.H., Morris H.A. Pleiotropic activities of vitamin D receptors–adequate activation for multiple health outcomes. Clin. Biochem. Rev. 2015;36(2):53. [PMC free article] [PubMed] [Google Scholar]
- Saha S., Chant D.C., Welham J.L., McGrath J.J. The incidence and prevalence of schizophrenia varies with latitude. Acta Psychiatr. Scand. 2006;114(1):36–39. doi: 10.1111/j.1600-0447.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z., Kraeuter A.-K., Palmer C.M. Ketogenic diet for schizophrenia: clinical implication. Curr. Opin. Psychiatr. 2019;32(5):394–401. doi: 10.1097/YCO.0000000000000535. [DOI] [PubMed] [Google Scholar]
- Sarris J., Logan A.C., Akbaraly T.N., Amminger G.P., Balanzá-Martínez V., Freeman M.P. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatr. 2015;2(3):271–274. doi: 10.1016/S2215-0366(14)00051-0. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Maukonen J., Hyytiainen T., Kieseppa T., Oresic M., Sabunciyan S. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr. Res. 2018;192:398–403. doi: 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Schwingshackl L., Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr. Metabol. Cardiovasc. Dis. 2014;24(9):929–939. doi: 10.1016/j.numecd.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Schwingshackl L., Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr. Metabol. Cardiovasc. Dis. 2014;24(9):929–939. doi: 10.1016/j.numecd.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8) doi: 10.1371/journal.pbio.1002533. e1002533-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance E.G., Gressitt K.L., Stallings C.R., Katsafanas E., Schweinfurth L.A., Savage C.L.G. Probiotic normalization of Candida albicans in schizophrenia: a randomized, placebo-controlled, longitudinal pilot study. Brain Behav. Immun. 2017;62:41–45. doi: 10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The central nervous system and the gut microbiome. Cell. 2016;167(4):915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Xu J., Li Z., Huang Y., Yuan Y., Wang J. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: a cross-sectional study. Schizophr. Res. 2018;197:470–477. doi: 10.1016/j.schres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Shen Z.-H., Zhu C.-X., Quan Y.-S., Yang Z.-Y., Wu S., Luo W.-W. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018;24(1):5. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D.P., Singh S., Bijalwan V., Kumar V., Khare P., Baboota R.K. Co-supplementation of isomalto-oligosaccharides potentiates metabolic health benefits of polyphenol-rich cranberry extract in high fat diet-fed mice via enhanced gut butyrate production. Eur. J. Nutr. 2018;57(8):2897–2911. doi: 10.1007/s00394-017-1561-5. [DOI] [PubMed] [Google Scholar]
- Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strik W., Wopfner A., Horn H., Koschorke P., Razavi N., Walther S. The Bern psychopathology scale for the assessment of system-specific psychotic symptoms. Neuropsychobiology. 2010;61(4):197–209. doi: 10.1159/000297737. [DOI] [PubMed] [Google Scholar]
- Teasdale S.B., Ward P.B., Rosenbaum S., Watkins A., Curtis J., Kalucy M. A nutrition intervention is effective in improving dietary components linked to cardiometabolic risk in youth with first-episode psychosis. Br. J. Nutr. 2016;115(11):1987–1993. doi: 10.1017/S0007114516001033. [DOI] [PubMed] [Google Scholar]
- Teasdale S.B., Samaras K., Wade T., Jarman R., Ward P.B. A review of the nutritional challenges experienced by people living with severe mental illness: a role for dietitians in addressing physical health gaps. J. Hum. Nutr. Diet. 2017;30(5):545–553. doi: 10.1111/jhn.12473. [DOI] [PubMed] [Google Scholar]
- Teasdale S.B., Ward P.B., Rosenbaum S., Samaras K., Stubbs B. Solving a weighty problem: systematic review and meta-analysis of nutrition interventions in severe mental illness. Br. J. Psychiatr. 2017;210:110–118. doi: 10.1192/bjp.bp.115.177139. [DOI] [PubMed] [Google Scholar]
- Teasdale S.B., Ward P.B., Samaras K. Dietary intervention in the dystopian world of severe mental illness: measure for measure, then manage. Acta Psychiatr. Scand. 2017;135(2):180. doi: 10.1111/acps.12670. [DOI] [PubMed] [Google Scholar]
- Teasdale S.B., Ward P.B., Samaras K., Firth J., Stubbs B., Tripodi E. Dietary intake of people with severe mental illness: systematic review and meta-analysis. Br. J. Psychiatr. 2019;214(5):251–259. doi: 10.1192/bjp.2019.20. [DOI] [PubMed] [Google Scholar]
- Teasdale S.B., Curtis J., Ward P.B., Watkins A., Lederman O., Rosenbaum S. The effectiveness of the Keeping the Body in Mind Xtend pilot lifestyle program on dietary intake in first-episode psychosis: two-year outcomes. Obes. Res. Clin. Pract. 2019;13(2):214–216. doi: 10.1016/j.orcp.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Tomaka J., Karakuła-Juchnowicz H., Morylowska-Topolska J., Dzikowski M., Juchnowicz D., Flis M. Gluten-related disorders and schizophrenia-potential linking mechanisms, diagnostic and therapeutic challenge. Curr. Prob. Psychiatr. 2017;18(1):9–24. [Google Scholar]
- Tomioka Y., Numata S., Kinoshita M., Umehara H., Watanabe S-y, Nakataki M. Decreased serum pyridoxal levels in schizophrenia: meta-analysis and Mendelian randomization analysis. J. Psychiatry Neurosci. 2018;43(3):194. doi: 10.1503/jpn.170053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R., Zwicker A. Etiology in psychiatry: embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatr. 2017;16(2):121–129. doi: 10.1002/wps.20436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valipour G., Saneei P., Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. J. Clin. Endocrinol. Metab. 2014;99(10):3863–3872. doi: 10.1210/jc.2014-1887. [DOI] [PubMed] [Google Scholar]
- Vancampfort D., Stubbs B., Mitchell A., De Hert M., Wampers M., Ward P.B. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatr. 2015;14(3):339–347. doi: 10.1002/wps.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassos E., Pedersen C.B., Murray R.M., Collier D.A., Lewis C.M. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr. Bull. 2012;38(6):1118–1123. doi: 10.1093/schbul/sbs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.K., Miller B.J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 2017;44(1):75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhai J.-X., Liu D.-W. Serum folate levels in schizophrenia: a meta-analysis. Psychiatr. Res. 2016;235:83–89. doi: 10.1016/j.psychres.2015.11.045. [DOI] [PubMed] [Google Scholar]
- Wang Y., Yuan X., Kang Y., Song X. Tryptophan-kynurenine pathway as a novel link between gut microbiota and schizophrenia: a review. Trop. J. Pharmaceut. Res. 2019;18(4):897–905. [Google Scholar]
- Wigand M.E., Lang F.U., Müller-Stierlin A.S., Reichhardt L., Trif S., Schulze T.G. Psychosis is mutable over time: a longitudinal psychopathology study. Psychopathology. 2018;51:186–191. doi: 10.1159/000486897. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) WHO; Geneva: 2018. Management of Physical Health Conditions in Adults with Severe Mental Disorders: WHO Guidelines. [PubMed] [Google Scholar]
- Xu R., Wu B., Liang J., He F., Gu W., Li K. 27 Jun 2019. Altered Gut Microbiota and Mucosal Immunity in Patients with Schizophrenia. Brain, Behavior, and Immunity. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Yuan X., Zhang P., Wang Y., Liu Y., Li X., Kumar B.U. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naive, normal weight patients with first episode schizophrenia. Schizophr. Res. 2018;201:299–306. doi: 10.1016/j.schres.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Yuan X., Kang Y., Zhuo C., Huang X.-F., Song X. The gut microbiota promotes the pathogenesis of schizophrenia via multiple pathways. Biochem. Biophys. Res. Commun. 2019;512(2):373–380. doi: 10.1016/j.bbrc.2019.02.152. [DOI] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Liu M., Chen J., Pan J., Han Y. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019;5(2) doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Guo R., Wang W., Ju Y., Wang Q., Ma Q. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatr. 07 Aug 2019 doi: 10.1038/s41380-019-0475-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]