Abstract
Repetitive transcranial magnetic stimulation (rTMS) is a safe and well-tolerated intervention for major depressive disorder (MDD). Over 150 randomized controlled trials (RCTs) have been carried out, and its efficacy has been confirmed in dozens of meta-analyses. Real world data has also confirmed the effectiveness of rTMS for MDD in clinical practice, with the most recent literature indicating response rates of 40–50% and remission rates of 25–30%. In this review, we first offer an historical perspective, followed by a review of basic principles, such as putative mechanisms, procedures and protocols, stimulation targets, efficacy and durability of response, side effects, and the placebo controversy. In the second part of this review, we first discuss solutions to increase accessibility to rTMS, such as modifications to treatment equipment, protocols and setting. We continue with possible means to further increase effectiveness, such as treatment personalization and extension. We conclude by addressing the scheduling issue, with accelerated rTMS (arTMS) as a possible solution.
Keywords: major depression, TMS
Background
Major depressive disorder (MDD) is a major public health problem, now ranked as the leading cause of disability worldwide. According to Friedrich and colleagues, well over 300 million people suffer from this condition at any time.1 Disability rates are also high, with data from 2010 estimating that MDD represents 2.5% of global disability-adjusted life years (DALYs) worldwide.2 First-line approaches to MDD include pharmacotherapy and psychotherapy. Although effective, these approaches still leave a significant proportion of patients with incomplete remission, that often leads to treatment-resistant depression (TRD). TRD is classically defined as the lack of response to two separate antidepressant trials3 and is associated with significant morbidity and a high suicide risk.4 A number of alternative treatments have therefore been developed to target TRD.
Historically, only electroconvulsive therapy (ECT) was available for patients with TRD. ECT was the first brain stimulation therapy developed and has been in continuous use for over 80 years. Because of the need for general anesthesia, cognitive side-effects, and the negative perception around the treatment generated in the media, this method has suffered from low patient acceptability.5 Other methods have therefore been explored to harness the power of electrotherapy, while minimizing side-effects and treatment complexity. Repetitive transcranial magnetic stimulation (rTMS) has been at the forefront of such approaches.
rTMS is a tool used to therapeutically deliver electromagnetic pulses to specific brain regions, inducing an electrical current in the neural tissue. This has been shown to induce long-lasting after-effects capitalizing on the brain’s capacity for neuroplasticity. Unlike ECT, rTMS does not induce a therapeutic seizure, and therefore does not require anesthesia. It is safe, well-tolerated, and does not cause adverse cognitive side effects.3,6–9 rTMS has been mainly studied for MDD, but new indications have appeared in the last few years, such as for obsessive-compulsive disorder (OCD) and nicotine use disorder. rTMS has also shown preliminary evidence for post-traumatic stress disorder (PTSD) and schizophrenia. rTMS is also used for disorders outside the field of psychiatry, with definite efficacy for motor stroke, and preliminary evidence for Parkinson’s disease, fibromyalgia, post-stroke aphasia, multiple sclerosis, and Alzheimer’s disease.6
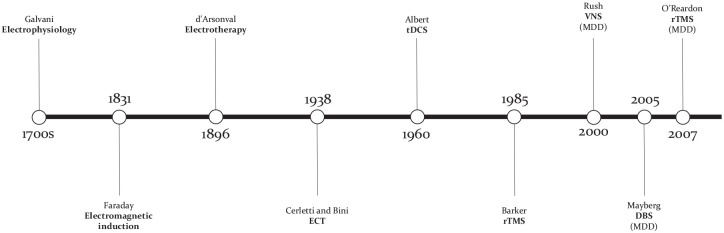
Historical perspective
Luigi Galvani (1737–1798) laid the foundations that created the field of electrophysiology and Michael Faraday (1791–1867) first demonstrated the principle of electromagnetic induction by showing that an electrical current in a coil could induce a magnetic field and vice versa (Figure 1). Later, French physician and physicist Jacques-Arsène d’Arsonval (1851–1940) pioneered the therapeutic use of high frequency current applied to the body, effectively creating the field of electrotherapy. In the 1930s, the field of psychiatry was revolutionized by Ugo Cerletti (1877–1963) and Lucio Bini (1908–1964). Following the work of Meduna with convulsive therapy, the two Italian physicians perfected what they then called ‘electroshock treatment’, now known as ECT.10 The success of ECT stimulated research in the therapeutic use of electricity to treat mental illness in order to find alternatives with less side effects. An early form of transcranial direct current stimulation (tDCS) was pioneered in the 1960s by Albert, but later abandoned given the success of psychiatric drugs, only to reexperience a resurgence as a treatment for MDD in the late 1990s.11 The 1980s saw the development of several brain stimulation technologies, such as deep brain stimulation (DBS) for Parkinson’s disease and vagus nerve stimulation (VNS) for epilepsy, which would both later be studied as treatments for MDD (unlike DBS, VNS eventually received FDA approval in 2005 for patients with chronic or recurrent MDD that have not experienced an adequate response to four or more antidepressant trials).12,13 In the early 1980s, Merton and Morton developed transcranial electrical stimulation (tES), which was associated with high pain levels. In response to this, the English engineer Anthony Barker developed transcranial magnetic stimulation (TMS) in 1985. Initially, TMS was only able to deliver single pulses and was used as a neurodiagnostic tool. Eventually, TMS machines were optimized to deliver pulses repetitively, and rTMS was born. Researchers then became interested in this technology and studied its effect on different cortical regions in the late 1980s and early 1990s.
Figure 1.
Important milestones in the development of electric therapies. Abbreviations: DBS, deep brain stimulation; ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; VNS, vagus nerve stimulation.
The discovery of rTMS use for MDD followed the observation of transient mood elevations following motor cortex stimulation in healthy controls.14 Affective reactions were also observed in studies of hemispheric language dominance, especially following left prefrontal cortex (dominant) stimulation.15 At the same time, converging evidence suggested that left dorsolateral prefrontal cortex (DLPFC) dysfunction was relevant in the pathophysiology of MDD16 which ultimately led to the study of left DLPFC rTMS in MDD.17,18
Through time, accumulating evidence for the efficacy of rTMS in MDD led to US Food and Drug Administration (FDA) approval in 2008, specifically for the treatment of MDD that did not respond to one medication trial. More specifically, this approval followed a 2007 industry sponsored RCT (N = 301), the largest at the time.19 Those findings were subsequently confirmed in a large (N = 190) National Institute of Mental Health (NIMH)-funded RCT published in 2010.20 Since then, rTMS has gained acceptance as a safe, well-tolerated and effective treatment for MDD. The latest 2016 Canadian Network for Mood and Anxiety Treatment (CANMAT) guidelines recommends rTMS as a first line treatment for MDD patients who have failed at least 1 antidepressant, with level 1 evidence for safety, tolerability and acute efficacy.3
Review of basic principles
Putative mechanisms
rTMS is delivered through the use of a powerful and focused electromagnetic coil (1.0-2.5 Tesla). Through Faraday’s law of induction, the magnetic field generated from the coil induces an electrical field in a conducting substance. Since the cortex is a conducting substance, the magnetic field generated from the coil induces a regional electrical current in the underlying neural tissue that also induces changes in regions functionally connected to the locally stimulated region. When applied repetitively, it has been shown that rTMS can modulate brain activity by invoking lasting changes.21 Experimental evidences from preclinical studies indicate that rTMS produces complex neurobiochemical effects causing changes in different systems, including neurotransmitters release, gene expressions, neuroendocrine systems changes and glutamate AMPA receptor/NMDA receptor expression (calcium ion dynamics). These effects may have neuroprotective actions by reducing oxidative stress and activating neurotrophic factors.22 Altogether, these could modify the electrophysiological properties of neurons, leading to long-lasting synaptic plasticity-related through long-term potentiation (LTP) and depression (LTD) phenomena.
Standard rTMS protocols can be divided in two broad categories, with high-frequency (HF) stimulation having mainly excitatory effects (usually delivered at 10 or 20 Hz) and low-frequency (LF) stimulation having inhibitory effects (usually delivered at 1 Hz). Newer theta burst stimulation (TBS) protocols have also been introduced, based on the brain endogenous theta rhythm occurring in the hippocampus.23 In TBS protocols, TMS pulses are delivered in triplets that occur 5 times per second. Huang and colleagues were the first to study the effects of TBS on the human motor cortex, and TBS has been studied as a therapeutic stimulation protocol more recently.24 The two main TBS protocols are intermittent TBS (iTBS) and continuous TBS (cTBS), with iTBS demonstrated to be mostly excitatory and cTBS inhibitory. TBS protocols have been suggested to produce more powerful, consistent and longer-lasting effects on cortical physiology than regular rTMS protocols, while requiring a shorter application period.24 Recent research highlighted the variability in response to theta burst protocols with some individuals exhibiting no response, expected responses (ie, facilitation after iTBS and inhibition after cTBS) or paradoxical responses. Indeed, a group recently published a study where healthy subjects received various doses of TBS protocols.25 In this study, the only TBS protocols which caused statistically significant changes in cortical excitability were 1200 pulses of iTBS, which paradoxically had an inhibitory effect, and 3600 pulses of cTBS, which paradoxically had an excitatory effect. These surprising results highlight the need to better understand and predict individual responses to TBS protocols to try and optimize clinical response to therapeutic protocols.
Procedures and protocols
rTMS is usually delivered by operators (either technicians or nurses) under physician supervision. For MDD, standard protocols involve daily sessions on weekdays. A conventional course of rTMS is usually anywhere between 20 and 30 daily sessions, delivered over 4 to 6 weeks.3 Conventional figure-8 coils that can stimulate neural tissue down to 1.1 cm beneath the cortical surface are generally used. Deep TMS using newer helmet-shaped “H-coils” have been developed that can reach deeper structures up to 2.8 cm below the cortical surface.26 Treatment intensity is determined using the resting motor threshold (rMT), which is the minimum intensity necessary to induce a muscle contraction in the contralateral hand 50% of the time. A stimulation intensity of 120% of the rMT is most commonly used for standard rTMS, since higher stimulation intensity has been linked with stronger widespread cortical activation,27 and because the prefrontal cortex lies deeper in the cranial vault than the motor cortex.28,29 For TBS, the appropriate stimulation intensity still remains an active area of research. Indeed, Chung and colleagues found an inverse U-shape relationship between intensity and iTBS plastic effects, where iTBS stimulation at 75% of the rMT yielded the largest neurophysiological changes compared to 50% and 100%.30 Subsequently, Alkhasli and colleagues showed differential effects of iTBS delivered at 90% vs 120%, with the former increasing fronto-striatal activity and the latter having no such effect.31
The first approved FDA protocol involved 10 Hz stimulation (3000 pulses, 37.5 min duration) at an intensity of 120% rMT to the left DLPFC.19,20 Deep TMS was subsequently approved in 2013 using an 18 Hz protocol (1980 pulses, ~20 min duration).32 More recently, a large study compared the initial FDA-approved rTMS protocol in the U.S. head-to-head with a new 3 min iTBS protocol (600 pulses)33 using the same stimulation intensity of 120% rMT. This iTBS protocol was shown to be non-inferior to the 10 Hz protocol, allowing it to also receive FDA approval.
Stimulation targets
The conventional stimulation target for rTMS in MDD is the left DLPFC. That target was initially chosen based on studies showing hypometabolism in this area in patients suffering from MDD.17,34 The most recent 2016 CANMAT guidelines recommend either left-DLPFC (L-DLPFC) HF rTMS or right-DLPFC (R-DLPFC) LF rTMS as first line protocols. Switching between these protocols in non-responders, bilateral rTMS (L-DLPFC HF and R-DLPFC LF) and theta-burst (TBS) protocols are considered second line (of note, little actual evidence supports the recommendation of switching between HF and LF protocols). Finally, HF rTMS bilaterally to the dorsomedial prefrontal cortex (DMPFC) is considered third line. New targets such as the lateral orbitofrontal cortex (of) are also being investigated.
In order to reliably stimulate these different structures, different targeting methods have been developed. Scalp-based methods are widely used, simple to apply and cost-effective.35 Neuronavigation using anatomical MRI has also been a popular method, although more complex and expensive.36 More recently, Fox and colleagues introduced a neuronavigation method using functional MRI (fMRI), where the region of the DLPFC most anticorrelated to the subgenual anterior cingulate cortex (sgACC) is stimulated.37
Efficacy, response prediction, durability of response and maintenance
Over 360 studies have reported on the efficacy of rTMS for MDD, including over 150 RCTs and 45 meta-analyses. Most studies enrolled patients who had 2 antidepressant trials for their current MDD episodes. So far, HF and LF protocols have shown similar efficacy, but they have yet to be compared in a large scale RCT with adequate blinding. RCTs of HF rTMS have reported response rates varying between 40–50% and remission rates of 25–30%, with a weighted mean difference of 2.31 and an effect size of 0.33. A well-design recent systematic review with network meta-analysis of 81 studies (N = 4233) suggested superior efficacy of bilateral rTMS (OR = 3.96; 95% CI = 2.37–6.60) over HF (OR = 3.07; 95% CI = 2.24–4.21) and LF (OR = 2.37; 95% CI = 1.52–3.68) protocols.38
No clear consensus currently exists regarding response prediction. Suggested positive predictors include less severe and shorter duration depressive episodes, recurrent depression (vs chronic depression), previous response to rTMS and concurrent antidepressant use during treatment.39 Negative predictors include higher baseline symptom severity, refractoriness level and chronicity, benzodiazepine use, shorter rTMS courses and psychotic depression. Biological markers of response are currently being explored,40 but reliable and scalable biomarkers still remain elusive.
The durability of response varies greatly among studies, with relapse rates initially as high as 80% at 6–12 months.41 With more modern protocols, a 2014 multisite naturalistic observational study over a 1-year follow-up period concluded that rTMS demonstrated a statistically and clinically meaningful durability of effect.42 In that study (N = 120), 62.5% of patients continued to meet response criteria after the acute course over the follow-up period, and 36.2% received retreatment (average of 16.2 ± 21.1 additional treatment days over the follow-up period). Finally, a recent systematic review and meta-analysis of 18 studies, found that 66.5% of patients still maintained response at 3 months, which decreased to 52.9% at 6 months and 46.3% at 12 months.43 Longer durability of response was associated with female sex and maintenance treatment.
No consensus currently exists on whether maintenance is needed to prevent relapse and what the optimal maintenance regimen is. Initially, a common approach was to taper down to 3 sessions a week, then 2 sessions per week, then 1 session per week, and finally 1 session every 2 weeks.44 Several case series and open-label studies did suggest increased durability of response with a maintenance course following an acute course.45 Only one RCT (N = 49) has compared 1 session per month to observation only and did not find any statistically significant difference in relapse rates.46 Others have favored “clustered maintenance,” with 5–10 sessions delivered over a 3–5 days period every month.35 Such a study in N = 281 participants found a decreased relapse rate for maintenance rTMS (24.2%) or combination (15.9%) over antidepressant treatment alone (44.4%).47 A recent review suggested a conservative maintenance course over 3 months, with 3 sessions per week for the first 2 weeks following the acute course, followed by 2 sessions per week for another 2 weeks, and 1 session per week for 8 weeks.48 Overall, maintenance rTMS has not been well-studied, and much work remains to be done to clarify both the need for it and the optimal schedule.
Side effects
The most recent and up to date safety expert guidelines have reiterated the high safety and tolerability profile of rTMS.7 The most common side effect of rTMS is scalp discomfort or pain during treatment (~40%), followed by headaches after treatment (20–30%) and fatigue (15–20%).3 rTMS has also been associated, albeit rarely, with more severe adverse events. Seizure risk is currently estimated to be “very low.” A large polling study led in 174 rTMS clinics around the world reported 24 seizures in 300,000 rTMS sessions (standardized risk of 7/100,000 sessions).49 Of those 24 seizures, 19 happened in patients with pre-existing risk factors (medication, neurological condition, epilepsy). Seizures have been reported with TMS (single-pulse and paired-pulse) and rTMS. HF rTMS may be more likely to cause seizures than other protocols, but seizures occurrence is too rare to allow for valid statistical comparison. Finally, it is possible that many reported seizures actually were nonepileptic seizures or syncopal events, which can be difficult distinguish from true seizures.49 Regarding hearing risk, rTMS can be associated with high decibel levels at higher stimulation intensities. Even though risk of inducing auditory problems is very low, guidelines recommend that all patients and operators wear hearing protection during treatment.49 Any type of ferromagnetic implants near or in the cranium have also been considered to be a contraindication for rTMS, and it is important for patients to be assessed with an MRI safety questionnaire before proceeding with treatment. Finally, there is no evidence of negative cognitive effects of rTMS, with some evidence of potential pro-cognitive effects.50
Placebo effect
Even to this day, some groups still debate the true efficacy of rTMS.51,52 Non-specific effects such as behavioral activation are unavoidable in rTMS, given the fact that patients need to travel daily to a treatment center for several weeks. Patients also interact daily with rTMS operators, which can provide some amount psychological support. The initial 2007 industry-sponsored RCT that led to the FDA-approval in the United States was criticized since it was unable to show significant statistical differences on their primary outcome, with the positive results reported being from secondary analyses.19 Two other studies also failed to show superiority over sham, one from 2007,46 and a more recent one from 2018.53 Finally, a recent systematic review and meta-analysis estimated the rTMS placebo response to be large (g = 0.8, 95% CI = 0.65–0.95, p < 0.01).54
Even though these observations can be concerning, it is important to contextualize these findings, since large placebo responses are common in antidepressant and psychotherapy trials.54 It is also important to note that a lot of meta-analytic results about rTMS are being driven by many older trials which used older, less effective protocols and inferior sham techniques. Newer sham rTMS techniques include specially designed coils that contain both active and sham capacity, scalp electrodes to reproduce somatic sensations, and EMLA cream.55,56 Overall, the most recent meta-analysis (81 RCTs, 4233 patients) unequivocally shows superiority of rTMS over sham (HF rTMS: OR 3.07; 95% CI = 2.24–4.21, iTBS OR 2.54; 95% CI = 1.07–6.05).38 Also, the largest and most recent international guidelines for rTMS give a level A rating of “definite efficacy” to both HF and iTBS in MDD.6
Other depressive disorders
Finally, it is worth mentioning other depressive disorders for which rTMS has been studied. Regarding bipolar depression, a recent meta-analysis identified eight14 sham-controlled RCTs (N = 257) and reported a small but significant improvement in depression scores (standardized mean difference = 0.302, p < 0.05), higher remission rates (risk difference = 0.104 ± 0.044, p < 0.05, NNT = 10) and a trend for greater response (risk difference = 0.074 ± 0.039, p = 0.06).57 Of note, a recent sham-controlled iTBS RCT for bipolar depression was negative.58 This study can be criticized for its small sample size (N = 37), recruitment issues and low total number of sessions.27 Participants were also not asked to discontinue anticonvulsants prior to treatment, which have been shown to alter cortical excitability in preclinical and clinical studies.59–62 The results of this trial also question the prevailing notion that HF rTMS and TBS protocols can be used interchangeably and also highlight the potential confounding effects of anticonvulsants on the intended therapeutic outcomes when using rTMS.
Little data is currently available regarding MDD with comorbid anxiety (also known as anxious depression or MDD with anxious distress). A recent secondary analysis from the THREE-D study assessed comorbid anxiety symptoms using the anxiety/somatization items from the 17-item Hamilton Depression Rating Scale (HRSD-17).63 Interestingly, they did find a statistically significant reduction in anxiety symptoms, irrespective of the stimulation protocol. The authors concluded that rTMS might have a clinically meaningful effect on anxiety symptoms in MDD, but that further research is required. Finally, we are not aware of any specific RCT for persistent depressive disorder (dysthymia) and atypical depression.
Obstacles to the current care delivery model and effectiveness issues
Background
Currently, delivery of rTMS is mostly occurs in specialized care centers or clinics, which precludes its widespread use in the community. The reasons for this are manifold. First, current stimulation protocols require costly and complex rTMS devices; acquisition and operation costs are thus high. With each session costing anywhere between USD$200-300 in private clinics, a full course of rTMS may cost as much as USD$5000-USD$10,000 in some jurisdictions. Stimulators able to deliver the new iTBS protocols start at around USD$50,000 and can go up to USD$200,000. Furthermore, the cooled coils needed for those kinds of intensive protocols cost around USD$10,000. Even with the use of briefer TBS protocols, it is estimated that the average cost per remission is USD$3695.64 Efforts are therefore underway to find novel ways of decreasing rTMS costs and will be discussed in section 4.2. Second, effectiveness remains to be optimized. Indeed, even though rTMS consistently achieves remission rates of 25–30%, it is still below what is reported for ECT (~50%).65,66 Solutions are discussed in sections 4.3 and 4.4. Moreover, the current once-daily paradigm necessitates that patients travel every weekday to a treatment center over 6 weeks in order to receive a full 30 sessions course, which can be discouraging for some. Shortened “accelerated” rTMS courses are discussed in section 4.5.
Treatment equipment, protocols, and setting
Current conventional rTMS procedures are complex, which stems from several aspects. First, figure-8 coils do not allow direct visualization of the underlying scalp landmarks. A lot of experience and training is thus required for correct coil positioning, which is still not guaranteed. Parabolic coils could be a solution to this and have just started to be studied using safe and effective 1 Hz protocols.67,68 Their central opening allows for direct visualization of scalp landmarks and therefore easier and more reliable positioning. Their shape also fits the natural curvature of the head and their large stimulation area could decrease the need for precise positioning. 1 Hz stimulation also has the advantage to only require basic stimulators that do not require cooling, further decreasing costs. Still, figure-8 and parabolic coils alike have limited stimulation depth. Other large coils, such as the H-coils used for Deep TMS, have a special design where electric coil elements are dispersed over the head rather than densely organized and reduce the electrostatic charge on the brain surface, allowing electric field to reach deeper brain regions.69,70 Deep TMS has been well studied for the treatment of MDD and could be more effective than figure-8 coils, even though the cost is still high.71
In addition, LF 1 Hz protocols can simplify rTMS delivery and help decrease costs.72 So far, 1 Hz rTMS has been shown superior to sham and the bulk of the literature suggest similar efficacy to HF protocols,72,73 even though a large scale multicentric sham-controlled trial still remains to be performed. R-DLPFC 1 Hz rTMS has received level I evidence from the most recent 2016 CANMAT, which ranks it as a first-line protocol. Some have suggested that the 1 Hz rTMS may be better tolerated than HF protocols, while also having a better safety profile.74–76 Low frequency, 1 Hz rTMS can also be delivered on more affordable equipment than HF protocols, which could decrease costs and therefore potentially increase accessibility to rTMS.73
Furthermore, rTMS has classically been delivered by an operator attending to a single patient in a closed-room concept. Patients are treated sequentially, one after the other, and the rTMS operators are thus not continually active. Other areas of medicine have addressed this productivity issue and have optimized their workflow to increase accessibility. One example is the area of oncology, where patients are treated in an open area setting, with nurses caring for multiple patients are the same time. This could potentially increase the rTMS treatment center efficacy and accessibility while decreasing costs.
Effectiveness optimization through treatment personalization
The DLPFC is classically localized using the international standardized 10-20 EEG system. The Beam F3 system is another calculation system taking into account individual variations of head circumferences for more precision in localizing the F3 position.35 Such scalp-based methods of navigation are the most common, but several clinics use anatomical MRI guidance to increase precision, as some studies have demonstrated that the DLPFC has a high level of inter-individual variability.77 Some have suggested the superiority of neuronavigated rTMS.36 Conversely, a recent RCT compared neuronavigated to non-neuronavigated rTMS and did not find any benefits from neuronavigation, concluding that any potential benefit from this approach is too small to justify the extra costs, time and efforts.78 Even more novel approaches using functional connectivity-based imaging are currently being studied.79,80
Moreover, rTMS has been shown to modulate the autonomic nervous system by decreasing blood pressure and heart rate in animal models, theorized to be mediated through trans-synaptic modulation of the subgenual anterior cingulate cortex (sgACC). Several studies in human subjects have also reported that DLPFC stimulation reliably modulates HR compared to other neighboring cortical areas, such as the motor cortex, that had no such effect on cardiac activity.75,81,82 As such, neuro-cardiac-guided TMS (NCG-TMS) could show promise to improve target selection and optimize treatment response.
Furthermore, the question of whether symptom provocation during treatment can increase effectiveness still remains unanswered. The fact that motor cortex stimulation shows phase variance (different effects whether stimulation is delivered at rest vs during muscle contraction) suggests that a similar phenomenon could take place in other cortical regions. Deep TMS for OCD has been specifically approved using symptom provocation,83 and an rTMS study for MDD where some stimulation sessions were delivered concurrently with psychotherapy reported response and remission rates of 66% and 56%, respectively.84 This suggests that cognitive state during rTMS should be controlled, and also could increase effectiveness.
Finally, some studies have shown better outcomes the closer the stimulation frequency is to endogenous alpha rhythm, bringing up the question of whether rTMS frequency should be personalized based on individual EEG signatures.85,86
In summary, several aspects of rTMS delivery could be optimized to decrease costs and therefore potentially increase accessibility, such as parabolic coils, LF 1 Hz stimulation and open-room settings. Deep TMS and neuro-navigation also hold the promise of increased efficacy, although at a higher cost. NCG-TMS could be a novel approach for better functional targeting. The effects of symptom provocation during stimulation and frequency personalization based on individual EEG activity remain to be studied more extensively.
Effectiveness optimization through treatment extension
Some studies have suggested that one way to increase rTMS efficacy would be to increase the number of overall treatment sessions beyond what conventional protocols usually deliver. In a first report,87 Avery and colleagues studied the effects of an open-label treatment extension of the original 2007 industry sponsored trial.19 In that study, a 3-week treatment extension allowed an additional 26.0% and 11.0% response and remission rates in patients who had not responded to the initial 3 weeks of treatment. In another report,88 McDonald and colleagues made a similar follow-up study of the landmark 2010 NIMH-funded rTMS trial cohort.20 Patient who had failed to meet minimal response criteria were invited to extend treatment for an additional 3–6 weeks. Of those, 30.5% eventually meet criteria for remission. These studies are part of the reason why modern rTMS protocols now offer a minimum of 6 weeks of treatment.
Data from the landmark Deep TMS trial also supports this observation.32 In that trial, 212 MDD patients received an initial 4-week phase of active or sham Deep TMS (20 sessions), followed by a continuation phase of two sessions per week for an additional 12 weeks (up to 16 weeks / 44 sessions total). 38.4% of patients responded to the initial 4 weeks phase. In a subsequent report, a post hoc analysis was carried to characterize the response outcomes among acute-phase non-responders who had received the active treatment.89 61% of these individuals ended up responding to the post-acute continuation phase.
Subsequently, another group went further than anyone and treated patients (N = 58) up to 21 weeks in an open-label bilateral neuro-navigated 20 Hz iTBS study and reported response and remission rates of 83% and 72%, respectively.90 Average time to remission was 7.3 treatment weeks (SD = 4.5, 0.6–21.2), which is beyond the 6 weeks usually offered. A major limitation of that study was the lack of control group, preventing us from excluding that many patients who ended up responding did so simply because of the natural evolution of their condition.
A recent secondary analysis from the THREE-D trial33 has shown different patients’ response trajectories to rTMS.85 Using a group-based trajectory modeling statistical approach, the authors identified a “nonresponse” group (11%) with minimal improvement by the end of treatment, a “rapid response” group (19%) with almost complete improvement by mid-treatment followed by a plateau, and 2 additional groups (70%) showing steady and linear improvement all throughout treatment and no apparent plateau by the end. This important majority of patients may have required more than 30 sessions to achieve maximum benefits. In a follow-up study, the same group applied their trajectory analysis to two recent RCTs91,92 that compared arTMS to conventional once-daily rTMS.93 They made similar observations regarding the existence of 4 distinct response trajectories, while failing to find an association between protocol and trajectory.
Overall, it appears that treatment extension could be beneficial for a substantial number of patients, and potentially help increase the response and remission rates.
Treatment acceleration
A conventional 30 session rTMS course is usually delivered over 6 weeks. Patients have to come every weekday to the treatment center, which can be a problem for working individuals, patients who have families, and people who live far away from the treatment center.94 Recently, several studies have explored “accelerated” rTMS (arTMS), in which multiple sessions are given daily in order to shorten the treatment course. Some evidence suggests that this approach allows comparable effectiveness to standard once-daily rTMS, while shortening treatment length.91 Neurophysiological experiments have also suggested that multiple daily rTMS sessions may have greater effect on cortical excitability compared to single daily sessions,95 although this is contested.92
arTMS has been the subject of several studies,56,79,91,95–100 RCTs.90,101–107 A recent arTMS meta-analysis93,104 identified 11 trials (6 RCTs and 5 open-label) and retained 3 of these RCTs102,106 in their main analysis. Cumulative main effect size was 0.39 (95% CI 0.005–0.779). Average decrease on the HRSD-17 was 6.28 (± 0.78 SE) for the active group and 3.63 (90% CI ± 0.74 SE) for the sham group, which reached significance (p = 0.041). Overall, the number of available studies were small and heterogeneous regarding the protocols used, which makes interpretation difficult. Still, the authors reported that the data provided preliminary support for arTMS efficacy for MDD.
More recently, high-dosage highly-accelerated and personalized intermittent theta-burst (iTBS) arTMS feasibility studies have reported remission rates of up to ~90%, while delivering treatment over only 5 days.78,79 These unprecedented results garnered international attention, and the group recently reported having completed a sham-controlled RCT.108 Active treatment was significantly superior to the sham group, with 78.6% remission rate in the active group (N = 14) and 13.3% remission rate in the sham group (N = 15).
In summary, arTMS may lead to shorter treatment duration while maintaining or improving overall effectiveness, which could make it easier for patients to attend a full course of treatment. Preliminary data regarding novel high-dose intensive courses also hold the promise of significantly increasing effectiveness.
Conclusion
In conclusion, rTMS is one of the latest developments in the long history of electrotherapy. rTMS is believed to induce long-lasting neuroplastic changes which could be responsible for its therapeutic effect, even though many unknowns remain, especially around the proper stimulation intensity in TBS protocols. We covered the usual treatment protocols and discussed the data supporting the efficacy, effectiveness and durability of effects of rTMS for MDD. The evidence for the effectiveness of rTMS for MDD is clear, as supported in dozens of well powered RCTs and meta-analyses. rTMS is also safe and generally well tolerated, with few side effects. It is clear that rTMS carries a considerable embedded placebo effect that needs to be harnessed in clinical practice but managed carefully in future clinical trials. In the second part of this review, we discussed the limitations to the current care delivery model of rTMS for MDD as well as the effectiveness that remains to be optimized. To increase accessibility, potential solutions include easy-to-use parabolic coils, 1 Hz protocols and open-room settings. In terms of effectiveness optimization, treatment personalization and extension could both help increase response and remission rates, arTMS protocols could help shorten courses and therefore simplify logistics for patients, and high-dosage highly-accelerated personalized courses could dramatically increase effectiveness. Finally, to support those recommendations, the development of large databases appear to be increasingly required. Such databases are easy to implement and could help better clarify the short and long term outcomes of rTMS for MDD.
Acknowledgments
JPM would like to thank the Brain & Behavior Research Foundation and the Branch Out Neurological Foundation for their financial support.
Footnotes
Author contributions: JPM wrote the manuscript and VDJ, PL and DMB reviewed the manuscript.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare no financial interests relative to this work. JPM reports research grants from the Brain & Behavior Research Foundation NARSAD Young Investigator Award, the Réseau Québécois sur le Suicide, les troubles de l’Humeur et les troubles Associés (RQSHA), the Canadian Research Initiative in Substance Misuse (CRISM), and salary support for his graduate studies from the Branch Out Neurological Foundation (BONF). VDJ and PL do not report any conflict of interest. DMB receives research support from CIHR, NIH, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Family Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. He is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He also receives in-kind equipment support from MagVenture for investigator-initiated research. He received medication supplies for an investigator-initiated trial from Indivior.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jean-Philippe Miron  https://orcid.org/0000-0002-5277-541X
https://orcid.org/0000-0002-5277-541X
Contributor Information
Jean-Philippe Miron, Centre de Recherche du CHUM (CRCHUM), Centre Hospitalier de l’Université de Montréal (CHUM) and Département de Psychiatrie, Faculté de Médecine, Université́ de Montréal, Montréal, QC, Canada Institute of Medical Science and Department of Psychiatry, Faculty of Medicine, University of Toronto, Toronto, ON, Canada CHUM, 1051 Sanguinet, Montréal, QC, H2X 3E4, Canada.
Véronique Desbeaumes Jodoin, CRCHUM, CHUM and Département de Psychiatrie, Faculté de Médecine, Université́ de Montréal, Montréal, QC, Canada.
Paul Lespérance, CRCHUM, CHUM and Département de Psychiatrie, Faculté de Médecine, Université́ de Montréal, Montréal, QC, Canada.
Daniel M. Blumberger, Institute of Medical Science and Department of Psychiatry, Faculty of Medicine, University of Toronto, Toronto, ON, Canada Temerty Centre for Therapeutic Brain Intervention, Centre for Addiction and Mental Health, Toronto, ON, Canada.
References
- 1.Friedrich MJ.Depression is the leading cause of disability around the world. JAMA 2017; 317: 1517. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 2013; 10: e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milev RV, Giacobbe P, Kennedy SH, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder. Can J Psychiatry 2016; 61: 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olin B, Jayewardene AK, Bunker M, et al. Mortality and suicide risk in treatment-resistant depression: an observational study of the long-term impact of intervention. PLoS One 2012; 7: e48002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maughan D, Molodynski A.An international perspective on the acceptability and sustainability of electroconvulsive therapy. BJPsych Int 2016; 13: 10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefaucheur J-P, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol 2020; 131: 474–528. [DOI] [PubMed] [Google Scholar]
- 7.Rossi S, Antal A, Bestmann S, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert Guidelines. Clin Neurophysiol 2020; 132: 269–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perera T, George MS, Grammer G, et al. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul 2016; 99: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wassermann EM.Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 1998; 108: 1–16. [DOI] [PubMed] [Google Scholar]
- 10.Endler NS.The origins of electroconvulsive therapy (ECT). Convulsive Ther 1988; 4: 5–23. [PubMed] [Google Scholar]
- 11.Sarmiento CI, San-Juan D, Prasath VBS. Letter to the Editor: brief history of transcranial direct current stimulation (tDCS): from electric fishes to microcontrollers. Psychol Med 2016; 46: 3259–3261. [DOI] [PubMed] [Google Scholar]
- 12.Troster AI.Neuropsychology of deep brain stimulation in neurology and psychiatry. Front Biosci 2009; 14: 1857. [DOI] [PubMed] [Google Scholar]
- 13.George MS, Rush AJ, Sackeim HA, et al. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol 2003; 6: 73–83. [DOI] [PubMed] [Google Scholar]
- 14.Bickford RG, Guidi M, Fortesque P, et al. Magnetic stimulation of human peripheral nerve and brain: response enhancement by combined magnetoelectrical technique. Neurosurgery 1987; 20: 110–116. [DOI] [PubMed] [Google Scholar]
- 15.Pascual-Leone A, Catala MD, Pascual-Leone Pascual A.Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology 1996; 46: 499–502. [DOI] [PubMed] [Google Scholar]
- 16.George MS, Ketter TA, Post RM.Prefrontal cortex dysfunction in clinical depression. Depression 1994; 2: 59–72. [Google Scholar]
- 17.George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995; 6: 1853–1856. [DOI] [PubMed] [Google Scholar]
- 18.Kolbinger HM, Höflich G, Hufnagel A, et al. Transcranial magnetic stimulation (TMS) in the treatment of major depression—a pilot study. Hum Psychopharmacol Clin Exp 1995; 10: 305–310. [Google Scholar]
- 19.O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007; 62: 1208–1216. [DOI] [PubMed] [Google Scholar]
- 20.George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 2010; 67: 507–516. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald PB, Fountain S, Daskalakis ZJ.A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 2006; 117: 2584–2596. [DOI] [PubMed] [Google Scholar]
- 22.Soundara Rajan T, Ghilardi MFM, Wang HY, et al. Mechanism of action for rTMS: a working hypothesis based on animal studies. Front Physiol 2017; 8: 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond D, Dunwiddie T, Rose G.Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J Neurosci 1988; 8: 4079–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y-Z, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron 2005; 45: 201–206. [DOI] [PubMed] [Google Scholar]
- 25.McCalley DM, Lench DH, Doolittle JD, et al. Determining the optimal pulse number for theta burst induced change in cortical excitability. Sci Rep 2021; 11: 8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filipčić I, Filipčić IŠ, Milovac Sučić ŽS, et al. Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-Coil in treatment of major depressive disorder; A randomized clinical trial. J Psychiatr Res 2019; 114: 113–119. [DOI] [PubMed] [Google Scholar]
- 27.Nahas Z, Lomarev M, Roberts DR, et al. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry 2001; 50: 712–720. [DOI] [PubMed] [Google Scholar]
- 28.Stokes MG, Chambers CD, Gould IC, et al. Distance-adjusted motor threshold for transcranial magnetic stimulation. Clin Neurophysiol 2007; 118: 1617–1625. [DOI] [PubMed] [Google Scholar]
- 29.Stokes MG, Chambers CD, Gould IC, et al. Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. J Neurophysiol 2005; 94: 4520–4527. [DOI] [PubMed] [Google Scholar]
- 30.Chung SW, Rogasch NC, Hoy KE, et al. The effect of single and repeated prefrontal intermittent theta burst stimulation on cortical reactivity and working memory. Brain Stimul 2018; 11: 566–574. [DOI] [PubMed] [Google Scholar]
- 31.Alkhasli I, Sakreida K, Mottaghy FM, et al. Modulation of fronto-striatal functional connectivity using transcranial magnetic stimulation. Front Hum Neurosci 2019; 13: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levkovitz Y, Isserles M, Padberg F, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 2015; 14: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 2018; 391: 1683–1692. [DOI] [PubMed] [Google Scholar]
- 34.Pascual-Leone A, Rubio B, Pallardó F, et al. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996; 348: 233–237. [DOI] [PubMed] [Google Scholar]
- 35.Beam W, Borckardt JJ, Reeves ST, et al. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul 2009; 2: 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modak A, Fitzgerald PB.Personalising transcranial magnetic stimulation for depression using neuroimaging: a systematic review. World J Biological Psychiatry. Epub ahead of print 21April2021. DOI: 10.1080/15622975.2021.1907710. [DOI] [PubMed] [Google Scholar]
- 37.Weigand A, Horn A, Caballero R, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry 2018; 84: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiat 2016; 74: 143. [DOI] [PubMed] [Google Scholar]
- 39.Kar SK.Predictors of response to repetitive transcranial magnetic stimulation in depression: a review of recent updates. Clin Psychopharm Neu 2019; 17: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 2017; 23: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen RB, Boggio PS, Fregni F.Risk factors for relapse after remission with repetitive transcranial magnetic stimulation for the treatment of depression. Depress Anxiety 2009; 26: 682–688. [DOI] [PubMed] [Google Scholar]
- 42.Dunner DL, Aaronson ST, Sackeim HA, et al. A Multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry 2014; 75: 1394–1401. [DOI] [PubMed] [Google Scholar]
- 43.Senova S, Cotovio G, Pascual-Leone A, et al. Durability of antidepressant response to repetitive transcranial magnetic stimulation: systematic review and meta-analysis. Brain Stimul 2019; 12: 119–128. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald PB.Is Maintenance repetitive transcranial magnetic stimulation for patients with depression a valid therapeutic strategy. Clin Pharmacol Ther 2019; 106: 723–725. [DOI] [PubMed] [Google Scholar]
- 45.Rachid F.Maintenance repetitive transcranial magnetic stimulation (rTMS) for relapse prevention in with depression: a review. Psychiatry Res 2018; 262: 363–372. [DOI] [PubMed] [Google Scholar]
- 46.Philip NS, Dunner DL, Dowd SM, et al. Can medication free, treatment-resistant, depressed patients who initially respond to tms be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimul 2016; 9: 251–257. [DOI] [PubMed] [Google Scholar]
- 47.Wang H-N, Wang X-X, Zhang R-G, et al. Clustered repetitive transcranial magnetic stimulation for the prevention of depressive relapse/recurrence: a randomized controlled trial. Transl Psychiatry 2017; 7: 1292, http://www.nature.com/articles/s41398-017-0001-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang J, Chu Y, Ren Y, et al. Maintenance treatment of transcranial magnetic stimulation (TMS) for treatment-resistant depression patients responding to acute TMS treatment. Int J Physiol Pathophysiol Pharmacol 2020; 12: 128–133. [PMC free article] [PubMed] [Google Scholar]
- 49.Lerner AJ, Wassermann EM, Tamir DI.Seizures from transcranial magnetic stimulation 2012–2016: results of a survey of active laboratories and clinics. Clin Neurophysiol 2019; 130: 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin DM, McClintock SM, Forster JJ, et al. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress Anxiety 2017; 34: 1029–1039. [DOI] [PubMed] [Google Scholar]
- 51.Amad A, Jardri R, Rousseau C, et al. Excess significance bias in repetitive transcranial magnetic stimulation literature for neuropsychiatric disorders. Psychother Psychosom 2019; 88: 363–370. [DOI] [PubMed] [Google Scholar]
- 52.Amad A, Naudet F, Fovet T.Repetitive transcranial magnetic stimulation for depression: the non-inferiority extrapolation. J Affect Disorders 2019; 254: 124–126. [DOI] [PubMed] [Google Scholar]
- 53.Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans. JAMA Psychiatry 2018; 75: 884–893, http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/jamapsychiatry.2018.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Razza LB, Moffa AH, Moreno ML, et al. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog Neuropsychopharmacol Biol Psychiatry 2018; 81: 105–113. [DOI] [PubMed] [Google Scholar]
- 55.Mennemeier MS, Triggs WJ, Chelette KC, et al. Sham transcranial magnetic stimulation using electrical stimulation of the scalp. Brain Stimul 2009; 2: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGirr A, Eynde FV, den Tovar-Perdomo S, et al. Effectiveness and acceptability of accelerated repetitive transcranial magnetic stimulation (rTMS) for treatment-resistant major depressive disorder:aAn open label trial. J Affect Disord 2015; 173: 216–220. [DOI] [PubMed] [Google Scholar]
- 57.Tee MMK, Au CH. A systematic review and meta-analysis of randomized sham-controlled trials of repetitive transcranial magnetic stimulation for bipolar disorder. Psychiatr Q 2020; 91: 1225–1247. [DOI] [PubMed] [Google Scholar]
- 58.McGirr A, Vila-Rodriguez F, Cole J, et al. Efficacy of active vs sham intermittent theta burst transcranial magnetic stimulation for patients with bipolar depression. JAMA Netw Open 2021; 4: e210963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HW, Seo HJ, Cohen LG, et al. Cortical excitability during prolonged antiepileptic drug treatment and drug withdrawal. Clin Neurophysiol 2005; 116: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 60.Darmani G, Bergmann TO, Zipser C, et al. Effects of antiepileptic drugs on cortical excitability in humans: a TMS-EMG and TMS-EEG study. Hum Brain Mapp 2019; 40: 1276–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunter AM, Minzenberg MJ, Cook IA, et al. Concomitant medication use and clinical outcome of repetitive Transcranial Magnetic Stimulation (rTMS) treatment of major depressive disorder. Brain Behav 2019; 9: e01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ziemann U, Lönnecker S, Steinhoff BJ, et al. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res 1996; 109: 127–135. [DOI] [PubMed] [Google Scholar]
- 63.Trevizol AP, Downar J, Vila-Rodriguez F, et al. Effect of repetitive transcranial magnetic stimulation on anxiety symptoms in patients with major depression: an analysis from the THREE-D trial. Depress Anxiety 2021; 38: 262–271. [DOI] [PubMed] [Google Scholar]
- 64.Mendlowitz AB, Shanbour A, Downar J, et al. Implementation of intermittent theta burst stimulation compared to conventional repetitive transcranial magnetic stimulation in patients with treatment resistant depression: a cost analysis. PLoS One 2019; 14: e0222546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dierckx B, Heijnen WT, van den Broek WW, et al. Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 2012; 14: 146–150. [DOI] [PubMed] [Google Scholar]
- 66.Eranti S, Mogg A, Pluck G, et al. A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. Am J Psychiatry 2007; 164: 73–81. [DOI] [PubMed] [Google Scholar]
- 67.Miron J-P, Voetterl H, Mansouri F, et al. A case series of a novel 1 Hz right-sided dorsolateral prefrontal cortex rTMS protocol in major depression. Brain Stimul 2019; 13: 372–374. [DOI] [PubMed] [Google Scholar]
- 68.Miron J-P, Voetterl H, Fox L, et al. Optimized repetitive transcranial magnetic stimulation techniques for the treatment of major depression: a proof of concept study. Psychiatry Res 2021; 298: 113790. [DOI] [PubMed] [Google Scholar]
- 69.Roth Y, Zangen A, Hallett M.A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol 2002; 19: 361–370. [DOI] [PubMed] [Google Scholar]
- 70.Zibman S, Pell GS, Barnea-Ygael N, et al. Application of transcranial magnetic stimulation for major depression: coil design and neuroanatomical variability considerations. Eur Neuropsychopharmacol 2021; 45: 73–88. [DOI] [PubMed] [Google Scholar]
- 71.Gellersen HM, Kedzior KK.Antidepressant outcomes of high-frequency repetitive transcranial magnetic stimulation (rTMS) with F8-coil and deep transcranial magnetic stimulation (DTMS) with H1-coil in major depression: a systematic review and meta-analysis. Bmc Psychiatry 2019; 19: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miron J-P, Sheen J, Mansouri F, et al. The role of low-frequency repetitive transcranial magnetic stimulation in major depression: a call to increase the evidence base. Brain Stimul 2020; 13: 1296–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berlow YA, Zandvakili A, Philip NS.Low frequency right-sided and high frequency left-sided repetitive transcranial magnetic stimulation for depression: the evidence of equivalence. Brain Stimul 2020; 13: 1793–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loo CK, McFarquhar TF, Mitchell PB.A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. Int J Neuropsychopharmacol 2008; 11: 131–147. [DOI] [PubMed] [Google Scholar]
- 75.Kaur M, Michael JA, Hoy KE, et al. Investigating high- and low-frequency neuro-cardiac-guided TMS for probing the frontal vagal pathway. Brain Stimul 2020; 13: 931–938. [DOI] [PubMed] [Google Scholar]
- 76.Sun W, Mao W, Meng X, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study. Epilepsia 2012; 53: 1782–1789. [DOI] [PubMed] [Google Scholar]
- 77.Fox MD, Buckner RL, White MP, et al. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 2012; 72: 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hebel T, Göllnitz A, Schoisswohl S, et al. A direct comparison of neuronavigated and non-neuronavigated intermittent theta burst stimulation in the treatment of depression. Brain Stimul 2021; 14: 335–343. [DOI] [PubMed] [Google Scholar]
- 79.Cole EJ, Stimpson KH, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry 2020; 177: 716–726. [DOI] [PubMed] [Google Scholar]
- 80.Williams NR, Sudheimer KD, Bentzley BS, et al. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain 2018; 141: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iseger TA, Padberg F, Kenemans JL, et al. Neuro-Cardiac-Guided TMS (NCG-TMS): probing DLPFC—sgACC—vagus nerve connectivity using heart rate—first results. Brain Stimul 2017; 10: 1006–1008. [DOI] [PubMed] [Google Scholar]
- 82.Iseger TA, Arns M, Downar J, et al. Cardiovascular differences between sham and active iTBS related to treatment response in MDD. Brain Stimul 2020; 13: 167–174. [DOI] [PubMed] [Google Scholar]
- 83.Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry 2019; 176: 931–938. [DOI] [PubMed] [Google Scholar]
- 84.Donse L, Padberg F, Sack AT, et al. Simultaneous rTMS and psychotherapy in major depressive disorder: clinical outcomes and predictors from a large naturalistic study. Brain Stimul 2018; 11: 337–345. [DOI] [PubMed] [Google Scholar]
- 85.Roelofs CL, Krepel N, Corlier J, et al. Individual alpha frequency proximity associated with repetitive transcranial magnetic stimulation outcome: an independent replication study from the ICON-DB consortium. Clin Neurophysiol 2021; 132: 643–649. [DOI] [PubMed] [Google Scholar]
- 86.Corlier J, Carpenter LL, Wilson AC, et al. The relationship between individual alpha peak frequency and clinical outcome with repetitive Transcranial Magnetic Stimulation (rTMS) treatment of Major Depressive Disorder (MDD). Brain Stimul 2019; 12: 1572–1578. [DOI] [PubMed] [Google Scholar]
- 87.Avery DH, Isenberg KE, Sampson SM, et al. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry 2008; 69: 441–451. [DOI] [PubMed] [Google Scholar]
- 88.McDonald WM, Durkalski V, Ball ER, et al. Improving the antidepressant efficacy of transcranial magnetic stimulation: maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress Anxiety 2011; 28: 973–980, https://onlinelibrary.wiley.com/doi/full/10.1002/da.20885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yip AG, George MS, Tendler A, et al. 61% of unmedicated treatment resistant depression patients who did not respond to acute TMS treatment responded after four weeks of twice weekly deep TMS in the Brainsway pivotal trial. Brain Stimul 2017; 10: 847–849. [DOI] [PubMed] [Google Scholar]
- 90.Stubbeman WF, Zarrabi B, Bastea S, et al. Bilateral neuronavigated 20Hz theta burst TMS for treatment refractory depression: an open label study. Brain Stimul 2018; 11: 953–955. [DOI] [PubMed] [Google Scholar]
- 91.Fitzgerald PB, Hoy KE, Elliot D, et al. Accelerated repetitive transcranial magnetic stimulation in the treatment of depression. Neuropsychopharmacology 2018; 43: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fitzgerald PB, Chen L, Richardson K, et al. A pilot investigation of an intensive theta burst stimulation protocol for patients with treatment resistant depression. Brain Stimul 2020; 13: 137–144. [DOI] [PubMed] [Google Scholar]
- 93.Kaster TS, Chen L, Daskalakis ZJ, et al. Depressive symptom trajectories associated with standard and accelerated rTMS. Brain Stimul 2020; 13: 850–857. [DOI] [PubMed] [Google Scholar]
- 94.Sonmez AI, Camsari DD, Nandakumar AL, et al. Accelerated TMS for depression: a systematic review and meta-analysis. Psychiat Res 2018; 273: 770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maeda F, Keenan JP, Tormos JM, et al. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol 2000; 111: 800–805. [DOI] [PubMed] [Google Scholar]
- 96.Dardenne A, Baeken C, Crunelle CL, et al. Accelerated HF-rTMS in the elderly depressed: a feasibility study. Brain Stimul 2017; 11: 247–248. [DOI] [PubMed] [Google Scholar]
- 97.Holtzheimer PE, 3rd, McDonald WM, Mufti M, et al. Accelerated repetitive transcranial magnetic stimulation for treatment-resistant depression. Depress Anxiety 2010; 27: 960–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Desbeaumes Jodoin V, Miron JP, Lespérance P. Safety and efficacy of accelerated repetitive transcranial magnetic stimulation protocol in elderly depressed unipolar and bipolar patients. Am J Geriatr Psychiatry 2019; 27: 548–558. [DOI] [PubMed] [Google Scholar]
- 99.Modirrousta M, Meek B, Wikstrom SL.Efficacy of twice-daily vs once-daily sessions of repetitive transcranial magnetic stimulation in the treatment of major depressive disorder: a retrospective study. Neuropsychiatr Dis Treat 2018; 14: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schulze L, Feffer K, Lozano C, et al. Number of pulses or number of sessions? An open-label study of trajectories of improvement for once-vs. twice-daily dorsomedial prefrontal rTMS in major depression. Brain Stimul 2017; 11: 327–336, http://linkinghub.elsevier.com/retrieve/pii/S1935861X17309580 [DOI] [PubMed] [Google Scholar]
- 101.Tor P-C, Gálvez V, Goldstein J, et al. Pilot study of accelerated low-frequency right-sided transcranial magnetic stimulation for treatment-resistant depression. J ECT 2016; 32: 180–182, http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00124509-201609000-00007 [DOI] [PubMed] [Google Scholar]
- 102.Baeken C.Accelerated rTMS: a potential treatment to alleviate refractory depression. Front Psychol 2018; 9: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baeken C, Vanderhasselt M-A, Remue J, et al. Intensive HF-rTMS treatment in refractory medication-resistant unipolar depressed patients. J Affect Disord 2013; 151: 625–631. [DOI] [PubMed] [Google Scholar]
- 104.Desmyter S, Duprat R, Baeken C, et al. Accelerated intermittent theta burst stimulation for suicide risk in therapy-resistant depressed patients: a randomized, sham-controlled trial. Front Hum Neurosci 2016; 10: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duprat R, Desmyter S, Rudi de R, et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: a fast road to remission. J Affect Disord 2016; 200: 6–14. [DOI] [PubMed] [Google Scholar]
- 106.George MS, Raman R, Benedek DM, et al. A two-site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatients. Brain Stimul 2014; 7: 421–431. [DOI] [PubMed] [Google Scholar]
- 107.Loo CK, Mitchell PB, McFarquhar TF, et al. A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychol Med 2007; 37: 341–349. [DOI] [PubMed] [Google Scholar]
- 108.Phillips AL, Cole EJ, Bentzley BS, et al. Stanford accelerated intelligent neuromodulation therapy (SAINT-TRD) induces rapid remission from treatment-resistant depression in a double-blinded, randomized, and controlled trial. Brain Stimul 2020; 13: 1859–1860. [Google Scholar]