Abstract
Steroid has recently been reported as a treatment for new coronavirus disease (COVID-19). The incidence of oropharyngeal candidiasis due to the inhaled steroid ciclesonide is lower than that due to other inhaled steroids. We report the first case of oral candidiasis with COVID-19 pneumonia using ciclesonide. A 75-year-old man was hospitalized for COVID-19 pneumonia. After admission, an oral combination of lopinavir/ritonavir was administered, and ciclesonide was inhaled for 7 days. On the 14th day of hospitalization, white plaque was found in his oral mucosa. Candida albicans was identified by oral bacterial tests, and amphotericin B was initiated. On the 35th hospital day, negative result for C. albicans was confirmed. Intraoral monitoring and intervention by dental care workers are considered important for the prevention of infectious complications induced by corticosteroids.
Keywords: COVID-19, SARS-CoV-2, ciclesonide, oral candidiasis, Candida albicans
Introduction
Immune dysregulation caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been suggested as a causative pathway for oral manifestations in coronavirus disease (COVID-19).1 Multidrug therapy and invasive treatment may cause a decrease in immune function and worsen oral conditions, especially oropharyngeal candidiasis (OPC).2 The inhaled steroid ciclesonide has recently been reported as a treatment for new COVID-19.3 The incidence of OPC due to ciclesonide is lower than that due to other inhaled steroids;4 however, there are no reports of OPC in patients with COVID-19 pneumonia using ciclesonide. We report a case of oral candidiasis caused by ciclesonide with COVID-19 pneumonia, according to CAse REport (CARE) guidelines,4 and conduct a literature review.
Case report
The patient was a 75-year-old man patient with a medical history of atrial fibrillation taking warfarin. Fever and impaired consciousness were noted in mid-March 2020, and the patient was transferred to our hospital as an emergency. COVID-19 pneumonia was suspected due to a history of close contact with spouse who had COVID-19, increased fever and respiratory rate, decreased permeability of the right whole lung field on chest X-ray, and accumulation of pleural effusion. He was urgently admitted via the emergency department. A positive polymerase chain reaction test result for COVID-19 was confirmed, and a definitive diagnosis of COVID-19 pneumonia was made. After admission, an oral combination of lopinavir/ritonavir (800 mg/200 mg/ day, twice daily) was administered, and ciclesonide (800 µg/day, twice daily) was inhaled for 7 days. On the 7th day of hospitalization, aspiration pneumonia was observed. On the 14th day of hospitalization, white plaque began to appear in the oral cavity. At the first visit to our department on the 14th day, white plaque was found on both buccal mucosa and the back of the tongue, but there was no oral pain or dysgeusia. Candida albicans was identified by oral bacterial tests (oral swab culture), and a diagnosis of oral candidiasis was made. No fungi such as C. albicans were identified in blood cultures and sputum cultures. An intraoral examination revealed multiple pseudomembranous structures with white plaque on both buccal mucosa and tongue (Figure 1). The white plaque was easily wiped with gauze and exhibited an erythematous mucosa, suggesting oral candidiasis. Oral administration of syrup containing amphotericin B (400 mg /day) was initiated 4 times per day, and negative result for C. albicans was confirmed on the 35th hospital day. The mucosal surface of the oral cavity was also normalized (Figure 2).
Figure 1.
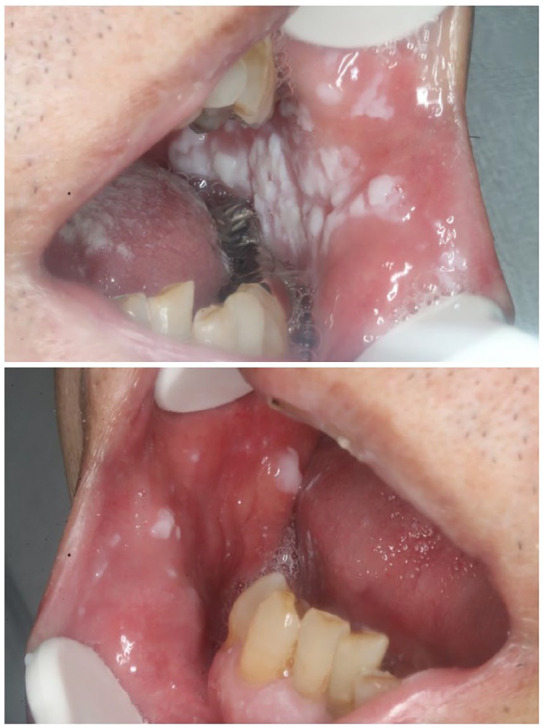
Oral candidiasis in a patient with COVID-19 14 days after admission. White plaque on the tongue and buccal mucosa is visible.
Figure 2.
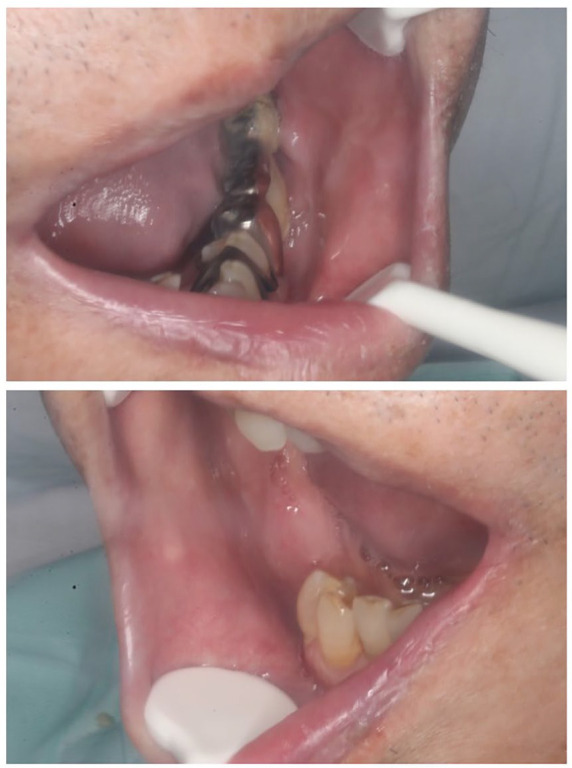
Oral candidiasis in a patient with COVID-19 (35 days after admission) after oral treatment. The white plaque on the oral mucosa is no longer visible.
Discussion
A literature review of oral candidiasis in COVID-19 patients was carried out from inception to August 2021. Inclusion criteria comprised cases diagnosed as oral candidiasis and COVID-19 patients, affecting the population aged more than 20 years. Two criteria were used: (1) clinical manifestations and associated systemic findings, and (2) delivered treatment in oral candidiasis. The following databases were assessed: PubMed (National Library of Medicine) and Web of Science (Thomson Reuters). The search was performed in August 2021 using the following keywords: [(COVID-19) AND (oral) AND (candidiasis)]. The electronic searches yielded 32 articles (PubMed = 20 and Web of Science = 12). Following the removal of 12 duplicates, inclusion and exclusion criteria were applied on 20 articles. In this mini-review, six articles were selected. And we also searched for related articles in the reference lists of these articles. Finally, we selected nine articles involved with 91 cases (Table 1).1,5–12 The included studies originated in seven countries: Iran (53 cases), Italy (28 cases), Czech Republic (4 cases), Greece (2 cases), Brazil (2 cases), Colombia (1 case), and Spain (1 case). There was no article that originated in Asia. Most cases had white membranous patches on the tongue dorsum as oral clinical manifestations. Most patients had burning sensation and/or pain in oral area. As the associated systemic findings, some of the patients were complicated by candidiasis in the esophagus and vagina. Topical antifungal agents were prescribed for oral candidiasis in COVID-19 patients. Nystatin, fluconazole, and miconazole were often used. This case had a history of atrial fibrillation and so it was unable to use azole antifungal drugs because he was taking anticoagulants (warfarin). Therefore, we chose amhotensin B as the antifungal agent.
Table 1.
Characteristics of COVID-19 patients with oral candidiasis in adult.
| Author(s) | Country | No. of patients | Age (years) | Sex | Oral clinical manifestations | Location on oral mucosa | Oral symptoms | Associated systemic findings | Delivered treatment |
|---|---|---|---|---|---|---|---|---|---|
| Baraboutis et al.5 | Greece | 2 | NA | F | NA | NA | NA | Esophageal candidiasis (one of the two) | NA |
| Corchuelo and Ulloa6 | Colombia | 1 | 40 | F | Whitish spots | Tongue dorsum | Dry mouth | NA | Topical nystatin Oral hygiene control Mouth rinses (chlorhexidine gluconate 0.12%) |
| Díaz Rodríguez et al.7 | Spain | 1 | 78 | F | 1. Pseudomembranous candidiasis 2. Angular cheilitis |
Tongue Palate |
Dry mouth | NA | 1. Topical nystatin 2. Ointment (neomycin, nystatin, and triamcinolone acetonide) |
| Riad et al.8 | Czech Republic | 1 | 47 | F | Pseudomembranous structures with white and painful plaques | Tongue dorsum Palate |
Burning sensation Pain |
NA | NA |
| Salehi et al.9 | Iran | 53 | 63.1 ± 16.4 (27–90) |
30 F 23 M |
Pseudomembranous structures White plaques |
NA | NA | NA | Did not receive any treatment (1 patient) Fluconazole (21 patients) Nystatin (13 patients) Caspofungin (1 patient) |
| Amorim Dos Santos et al.10 | Brazil | 1 | 67 | M | White plaque | Tongue dorsum | NA | NA | Intravenous fluconazole Topical nystatin Chlorhexidine digluconate (0.12%) alcohol-free mouth rinses Hydrogen peroxide Oral health care |
| Favia et al.11 | Italy | 28 | NA | NA | 1. Red forms (median rhomboid glossitis-like
appearance) 2. White forms |
1. Tongue 2. Palate |
Burning sensation Pain |
NA | Topical miconazole |
| Riad et al.1 | Czech Republic | 3 | 70 | F | White membranous patches | Tongue dorsum Mouth floor Soft plate Oropharynx region Buccal mucosa |
Burning sensation Pain |
Dysphagia Vaginal Candida infection |
Topical nystatin Mouthwash (chlorhexidine 0.2%) |
| 25 | F | Erythematous candidiasis | Tongue dorsum | Burning sensation | NA | Topical miconazole | |||
| 56 | F | White membranous patches | Labial mucosa Soft palate Tongue dorsum |
NA | Dysphagia | Systemic fluconazole Topical miconazole |
|||
| Teixeira et al.12 | Brazil | 1 | 70 | F | NA | Oral mucosa | Pain | Bacterial infection Fungal infections in the skin |
Topical nystatin |
COVID-19: coronavirus disease; F: female; NA: not available; M: male.
Ciclesonide is an inhaled corticosteroid that is converted in the lungs to its active metabolite, desisobutyryl-ciclesonide, which produces a strong antiviral effect at the alveoli. It has been reported that the deposition and activation of ciclesonide in the oropharynx are low, and the incidence of local side effects is low.13–15 The incidence of oral candidiasis is considered to be the lowest among inhaled steroids.14–16 As in this case, elderly patients with COVID-19 pneumonia with underlying diseases may develop fungal infection due to the compromised status. According to a recent report,17 the risk factor of COVID-19 patients with fungal co-infection, for example, oral candidiasis, was the prolonged use of antibiotics. Therefore, appropriate antimicrobial stewardship interventions specific for the COVID-19 pandemic are urgently required.18
The most common fungal infection of the oral cavity is candidiasis. Other fungal infections include aspergillosis, cryptococcosis, histoplasmosis, and others.19 Oral fungal infections are rare, but they are associated with discomfort, including dysgeusia, pain, and redness. Once a fungal infection is confirmed, it is easy to treat it accordingly. Intraoral monitoring and intervention by dental care workers are considered important for the prevention of infectious complications induced by corticosteroids.
Conclusion
Oral candidiasis associated with COVID-19 patients is not common but may occur due to immune dysregulation or prolonged use of antibiotics. Intervention by dental care workers from the early stage of onset may be effective in preventing complications. It is important to promote medical and dental cooperation to provide safe and secure medical care.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for English language editing.
Footnotes
Author contributions: N.O., Y.I, A.T., T.I, M.K., and K.M. were responsible for the treatment and care of the patient. N.O., Y.I, A.T., T.I, M.K., and K.M. drafted the original manuscript. N.O. was involved in the conception of this report. All authors critically reviewed this report. A.M. contributed to the final drafting of the manuscript. All authors have read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
ORCID iD: Nobuhide Ohashi  https://orcid.org/0000-0002-2345-4350
https://orcid.org/0000-0002-2345-4350
References
- 1.Riad A, Gomaa E, Hockova B, et al. Oral candidiasis of COVID-19 patients: case report and review of evidence. J Cosmet Dermatol 2021; 20(6): 1580–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dziedzic A, Wojtyczka R.The impact of coronavirus infectious disease 19 (COVID-19) on oral health. Oral Dis 2021; 27(Suppl. 3): 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwabuchi K, Yoshie K, Kurakami Y, et al. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J Infect Chemother 2020; 26(6): 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013; 2(5): 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baraboutis IG, Gargalianos P, Aggelonidou E, et al. Initial real-life experience from a designated COVID-19 centre in Athens, Greece: a proposed therapeutic algorithm. SN Compr Clin Med 2020; 2: 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corchuelo J, Ulloa FC.Oral manifestations in a patient with a history of asymptomatic COVID-19: case report. Int J Infect Dis 2020; 100: 154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Díaz Rodríguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID-19. Oral Dis. Epub ahead of print 22 July 2020. DOI: 10.1111/odi.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riad A, Gad A, Hockova B, et al. Oral candidiasis in non-severe COVID-19 patients: call for antibiotic stewardship. Oral Surg. Epub ahead of print 21 September 2020. DOI: 10.1111/ors.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses 2020; 63(8): 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amorim Dos Santos J, Normando AGC, Carvalho Da, Silva RL, et al. Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations? Int J Infect Dis 2020; 97: 326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favia G, Tempesta A, Barile G, et al. Covid-19 Symptomatic patients with oral lesions: clinical and histopathological study on 123 cases of the University Hospital Policlinic of Bari with a purpose of a new classification. J Clin Med 2021; 10(4): 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teixeira IS, Leal FS, Tateno RY, et al. Photobiomodulation therapy and antimicrobial photodynamic therapy for orofacial lesions in patients with COVID-19: a case series. Photodiagnosis Photodyn Ther 2021; 34: 102281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeks ED, Perry CM.Ciclesonide: a review of its use in the management of asthma. Drugs 2008; 68(12): 1741–1770. [DOI] [PubMed] [Google Scholar]
- 14.Newman S, Salmon A, Nave R, et al. High lung deposition of 99mTc-labeled ciclesonide administered via HFA-MDI to patients with asthma. Respir Med 2006; 100(3): 375–384. [DOI] [PubMed] [Google Scholar]
- 15.Richter K, Kanniess F, Biberger C, et al. Comparison of the oropharyngeal deposition of inhaled ciclesonide and fluticasone propionate in patients with asthma. J Clin Pharmacol 2005; 45(2): 146–152. [DOI] [PubMed] [Google Scholar]
- 16.Nave R, Zech K, Bethke TD.Lower oropharyngeal deposition of inhaled ciclesonide via hydrofluoroalkane metered-dose inhaler compared with budesonide via chlorofluorocarbon metered-dose inhaler in healthy subjects. Eur J Clin Pharmacol 2005; 61(3): 203–208. [DOI] [PubMed] [Google Scholar]
- 17.Hocková B, Riad A, Valky J, et al. Oral complications of ICU patients with COVID-19: case-series and review of two hundred ten cases. J Clin Med 2021; 10: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; 71(9): 2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santosh ABR, Muddana K, Bakki SR.Fungal infections of oral cavity: diagnosis, management, and association with COVID-19. SN Compr Clin Med 2021; 3(6): 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]


