Abstract
Fatigue is a persistent and debilitating symptom following radiation therapy for prostate cancer. However, it is not well-understood how radiation targeted to a small region of the body can lead to broad changes in behavior. In this study, we used targeted pelvic irradiation of healthy male mice to test whether inflammatory signaling mediates changes in voluntary physical activity levels. First, we tested the relationship between radiation dose, blood cell counts, and fatigue-like behavior measured as voluntary wheel-running activity. Next, we used oral minocycline treatments to reduce inflammation and found that minocycline reduces, but does not eliminate, the fatigue-like behavioral changes induced by radiation. We also used a strain of mice lacking the MyD88 adaptor protein and found that these mice also showed less fatigue-like behavior than the wild-type controls. Finally, using serum and brain tissue samples, we determined changes in inflammatory signaling induced by irradiation in wild-type, minocycline treated, and MyD88 knockout mice. We found that irradiation increased serum levels of IL-6, a change that was partially reversed in mice treated with minocycline or lacking MyD88. Overall, our results suggest that inflammation plays a causal role in radiation-induced fatigue and that IL-6 may be an important mediator.
Keywords: Inflammation, Cytokines, Fatigue, Cancer-related fatigue, Radiotherapy, Voluntary wheel-running activity, Minocycline, myd88
Abbreviations: CRF, cancer-related fatigue; CFS, chronic fatigue syndrome; VWRA, voluntary wheel running activity; RBC, red blood cell; WBC, white blood cell; MCV, mean corpuscular volume; MyD88, myeloid differentiation primary response 88 protein; TLR, toll-like receptor; CCL, chemokine (CC) ligand; CD30 L, CD30 ligand; CXCL, chemokine (CXC) ligand; FGF, fibroblast growth factor; Fas-L, Fas Ligand; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; LIF, leukemia inhibitory factor; M-CSF, macrophage colony-stimulating factor; PDGF-bb, platelet-derived growth factor subunit B; RANTES, regulated on activation normal T cell expressed and secreted; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor
Highlights
-
•
Pelvic irradiation leads to fatigue-like behavior that is dose-dependent.
-
•
Radiation increases serum levels of many cytokines, including IL-6.
-
•
Minocycline treatment can reduce radiation-induced fatigue and serum IL-6.
-
•
MyD88 gene deletion can reduce radiation-induced fatigue and serum IL-6.
1. Introduction
Fatigue is a highly distressing and prevalent symptom of cancer and cancer treatment that has a high cost to society and can significantly worsen quality of life (Hofman et al., 2007). Fatigue is commonly reported by patients with prostate cancer during and after radiotherapy, affecting decision-making and daily physical activity (Langston et al., 2013). It can be associated with cognitive performance deficits, despite both the disease and the treatment being localized outside the central nervous system (Feng et al., 2019). The mechanism by which the localized tumors or treatments affect fatigue behavior is not well-understood, although it may include combinations of anemia, inflammation, HPA dysregulation, or bioenergetic impairments (Feng et al., 2020; Grossberg et al., 2020; Bower, 2014; Hsiao et al., 2016). A better understanding of the mechanisms of cancer-related fatigue (CRF) might identify avenues for the development of therapies to combat this distressing symptom.
Inflammation has been proposed as an important mechanism for CRF, which is supported by multiple lines of evidence describing an associations among fatigue, inflammation, and cytokine levels (Bower, 2014; Hsiao et al., 2016; O'Higgins et al., 2018). Fatigue symptoms are also commonly observed in other diseases that are marked by inflammation including rheumatoid arthritis (Louati and Berenbaum, 2015) and lupus (Bakshi et al., 2018). Additionally, there is evidence of elevated levels of proinflammatory cytokines in chronic fatigue syndrome (Montoya et al., 2017; Yang et al., 2019). Fatigue can be part of the symptom cluster known as sickness behavior, which encompasses a wide range of symptoms and behaviors that occur during infection and may be a behavioral response to large elevations in brain cytokine levels (Dantzer, 2009). A strong inflammatory response may be present in CRF, as cancer can elicit a persistent inflammatory response and produce cytokines in the tumor microenvironment (Dranoff, 2004; Todoric et al., 2016; Diakos et al., 2014), and cancer treatments like radiotherapy can induce inflammation (Schaue et al., 2015). Together, these lines of evidence point to a potential role for inflammatory processes in CRF.
In this study, we used a previously established mouse model of CRF induced by targeted peripheral radiation therapy (Wolff et al., 2017). We targeted irradiation to the pelvic region of male mice to model radiotherapy for prostate cancer and induce a fatigue-like behavior measured as a decline in voluntary wheel-running activity (VWRA). The radiation of tissues causes breaks in DNA, resulting in cell death and inflammation predominantly in the irradiated region of the pelvis (Schaue et al., 2015). Our main hypothesis is that the peripheral damage triggers expression of circulating inflammatory signals that mediate the decrease in voluntary activity, or in other words, that inflammation is causing fatigue. Our approach was to first test the dose-dependence of irradiation on fatigue-like behavior, and then to test whether interfering with inflammation would affect both circulating cytokine levels and fatigue-like behavior.
We used two different methods for reducing inflammatory response. First, we added oral minocycline into the drinking water of wild-type mice. Minocycline is a semi-synthetic tetracycline antibiotic with microbicidal effects on both gram-negative and gram-positive bacteria (Garrido-Mesa et al., 2013). Significantly, minocycline also displays multiple non-antibiotic properties, including various anti-inflammatory properties. For instance, minocycline has been found to reduce LPS-induced cytokine release in human monocytes (Pang et al., 2012) and inhibit the activation of microglia in the brain (Moller et al., 2016). Second, as an additional method of reducing inflammation, we used a genetic knockout mouse model and compared the effect of irradiation on mice lacking the gene for MyD88 (Hou et al., 2008) to the effect on wild-type controls. MyD88 is an adaptor protein in toll-like receptor (TLR) and interleukin-1 receptor (IL1-R) signaling and is important in both innate and adaptive immune responses (Deguine and Barton, 2014).
The most important result of our study was that minocycline treatment or MyD88 gene deletion reversed part of the radiation-induced decline in VWRA. We also evaluated changes in the levels of cytokines in serum and brain tissue samples, and most notably found that post-irradiation serum levels of IL-6 were increased in irradiated animals, reduced in minocycline-treated mice, and in one measure were reduced in the MyD88 knockout mice as well. Together, these results suggest that irradiation-induced inflammation plays a causal role in the development of fatigue-like behavior.
2. Materials and methods
2.1. Ethics
This study was approved by the National Heart Lung and Blood Institute (NHLBI) Animal Care and Use Committee (protocol H-0288) of the National Institutes of Health (NIH), Bethesda, Maryland, USA. All aspects of animal care in this study were compliant with The Guide for the Care and Use of Laboratory Animals (The Guide for the Care an, 2011).
2.2. Animals
A total of 154 mice were used in this study, and all were approximately 7-week-old male mice bred on a C57BL6 background. Ninety-six wild-type C57BL6 mice were acquired from Charles River Laboratories (Wilmington, MA, USA), and an additional 28 MyD88 knockout mice and 28 wild-type controls were purchased from Jackson Labs (Bar Harbor, ME, USA). Animals were individually housed on a 12:12 h light-dark cycle at roughly 22.2 °C and 50% humidity, and all mouse handling and experimental procedures were conducted during the light cycle. Mice had ad libitum access to food and water throughout the study.
2.3. Experimental timeline
Study 1: Fatigue and radiation dose. First, 40 mice were housed in running wheel cages for one week of baseline recording (days −7 through −1). Next, mice received 8 Gy radiation (n = 12), 4 Gy radiation (n = 12), or sham (n = 16) treatments for three consecutive days (days 0–2). The day after finishing irradiation (day 3), all animals had three more days in running wheel cages (days 3–5). Animals were euthanized in the morning of day 6; blood was taken for CBC analysis, and serum and brain tissues were isolated for multiplex or ELISA analysis.
Study 2: Minocycline. First, 56 mice were housed in running wheel cages for one week of baseline recording (days −14 through −8). Wheel running continued for one subsequent week (days −7 through −1), during which 28 mice received minocycline and 28 did not. Next, mice received 8 Gy radiation or sham treatments for three consecutive days (days 0–2). The day after finishing irradiation (day 3), all animals had three more days in running wheel cages (days 3–5). A Y-maze test for spontaneous alternation was administered on 28 mice on day 5. Animals were euthanized in the morning of day 6, and serum and brain tissues were isolated for multiplex or ELISA analysis.
Study 3: MyD88 knockout mice. First, the 28 MyD88 knockout mice and 28 wild-type controls were housed in running wheel cages for one week of baseline recording (days −7 through −1). A Y-maze test for spontaneous alternation was administered on 14 on day −4. Next, mice received 8 Gy radiation or sham treatments for three consecutive days (days 0–2). The day after finishing irradiation (day 3), all animals had three more days in running wheel cages (days 3–5). Another Y-maze test was administered on 40 mice between 13:00 and 17:00 on day 5. Animals were euthanized in the morning of day 6, and serum and brain tissues were isolated for multiplex or ELISA analysis.
2.4. Voluntary wheel running activity (VWRA)
Running wheels (Lafayette Neuroscience, Indiana, USA) recorded wheel rotations in 1-min intervals, and VWRA was quantified as the number of minutes during which the wheel rotated, as previous studies have shown that time is a more consistent measure than distance or speed (Wolff et al., 2018). We excluded data from wheels that did not accurately count revolutions, which was verified by manually rotating the wheel and verifying that it matched the recorded counts.
2.5. Irradiation
This method is described in detail in a previous publication (Wolff et al., 2017). In summary, mice were divided into irradiated or sham groups in a way that distributed bodyweight evenly across groups but did not take any other factors into account. Each day between 9:00 and 13:00, mice were anesthetized with an intraperitoneal injection of a mixture of 100 mg/kg ketamine (MWI Animal Health, Boise, ID, USA) and 10 mg/kg xylazine (Akorn Animal Health, Lake Forest, IL, USA). Mice were then placed inside a custom-built lead shielding device within a GammaCell 40 Exactor irradiator (Best Theratronics, Ottowa, Ontario, Canada). The lead shielding targeted a 4 or 8 Gy radiation dose at approximately 1 Gy per minute to a region around the pelvis. Mice in the sham groups underwent the same procedure as those in the irradiated groups, except that they were left inside of lead shielding but outside of the irradiator. After irradiation, mice recovered on a heated pad placed underneath their individual home cages.
2.6. Minocycline treatment
Minocycline-treated mice (n = 28) received 1 mg/ml minocycline HCl diluted into their drinking water. Assuming 2 ml of water consumption per day, this would result in a final dose ~100 mg/kg per day. Once started, minocycline treatment continued throughout the duration of the study. Control mice (n = 28) received normal drinking water.
2.7. Blood and tissue samples
Tissues were collected between 9:00 and 11:00 on day 6 from all mice. Mice were anesthetized with an intraperitoneal injection of a mixture of 100 mg/kg ketamine and 10 mg/kg xylazine, and they were then exsanguinated via blood collection from the inferior vena cava. For complete blood count (CBC), blood was collected into Sarstedt EDTA microtubes and tested on an Advia 120 analyzer (Siemens Healthineers, Forchheim, Bavaria, Germany). Serum samples were collected by centrifuging whole blood in Sarsedt Z-Gel microtubes at 17,000×g for 15 min.
Whole brains were extracted and flash frozen in liquid nitrogen immediately after exsanguination and decapitation. Brains were homogenized using a bead-mill homogenizer (Thermo Fischer Scientific, Waltheim, Massachusetts, USA) in ice-cold RIPA buffer (#89901, Thermo Fischer) with freshly added protease inhibitor cocktail (Millipore Sigma, Burlington, Massachusetts, USA) and centrifuged at 14,000×g for 15 min at 4 °C. Protein concentrations of lysate supernatants were determined using a Pierce BCA kit (#23227, Thermo Fischer Scientific).
2.8. Multiplex and ELISA
Serum and brain tissue samples were run on multiplex arrays for a range of targets using one of two arrays: Bio-Plex Pro Mouse Cytokine 23-Plex Immunoassay (Bio-Rad, Hercules, California, USA), which tested 23 targets in duplicate, or Quantibody® Mouse Inflammation Array 1 Kit (RayBiotech, Peachtree Corners, GA, USA), which tested 40 targets in quadruplicate. The lists of target molecules for the two arrays substantially overlap but are not identical, and all targets are displayed in the figures and supplementary material. Validation of multiplex experiments were performed using ELISA with most samples run in duplicate except where otherwise indicated. Mouse Quantikine ELISA kits (R&D Systems, Minneapolis, Minnesota, USA) were used to measure eotaxin (MME00), IL-5 (M5000), and IL-6 (M6000B) following the manufacturer's instructions.
2.9. Pathology
Thirty-two animals were designated for pathology study before beginning the experiment, all of which were to undergo 8 Gy irradiation. Immediately after euthanasia, mouse tissues were fixed with 10% buffered formalin and embedded in paraffin. Sternums were fixed and a decalcification procedure was applied before paraffin embedding. Paraffin sections were cut and stained with routine Haemotoxylin and Eosin (H&E). All slides were digital scanned with NDP Nanozoomer (Hamamatsu, Shizuoka, Japan) for histology evaluations. Pathology findings were scored as 0 = normal, 1 = mild damage, 2 = moderate damage, 3 = severe damage.
2.10. Data analysis
The nominal level of statistical significance for all tests was set at 0.05. All p-values are from two-tailed tests unless otherwise specified.
Analysis of variance (ANOVA) and mixed models were conducted in R version 3.6.1. Levene's test was used to check for homogeneity of variance (“leveneTest” in car_3.0–3), and q-q plots were used to assess normality of residuals. Linear mixed models (“lmer” in lme4_1.1–21) were used to test repeated-measures data (VWRA or body weight) for main effects of treatment group and time, and all mixed models used a random intercept for each animal.
Analysis of covariance, univariate (ANCOVA) or multivariate (MANCOVA), was used to assess the significance of effects in the CBC data, and models were fit using SAS v 9.4, TS1M6 (SAS Institute, Cary NC, USA). See the supplemental materials for details.
All other statistical analyses were conducted in python version 3.7.1. All t-tests used Welch's correction for unequal variance. The statistical significance of differences in multiplex data were calculated using pairwise t-tests, and a Holm-Sidak correction was used for multiple comparisons correction. More details are available in the supplemental materials.
3. Results
3.1. Fatigue and radiation dose
As described in previous papers (JOVE), male mice received three consecutive days of irradiation targeted to the pelvic region (Wolff et al., 2017). To focus on the behaviors most like fatigue symptoms in humans, we are mainly displaying VWRA as a fraction of baseline activity, rather than as an absolute number. This metric more accurately depicts the variability that is attributable to the irradiation procedure itself, which is our outcome of interest, and it reduces the variability attributable to different baseline behavioral tendencies, which are not relevant to this study.
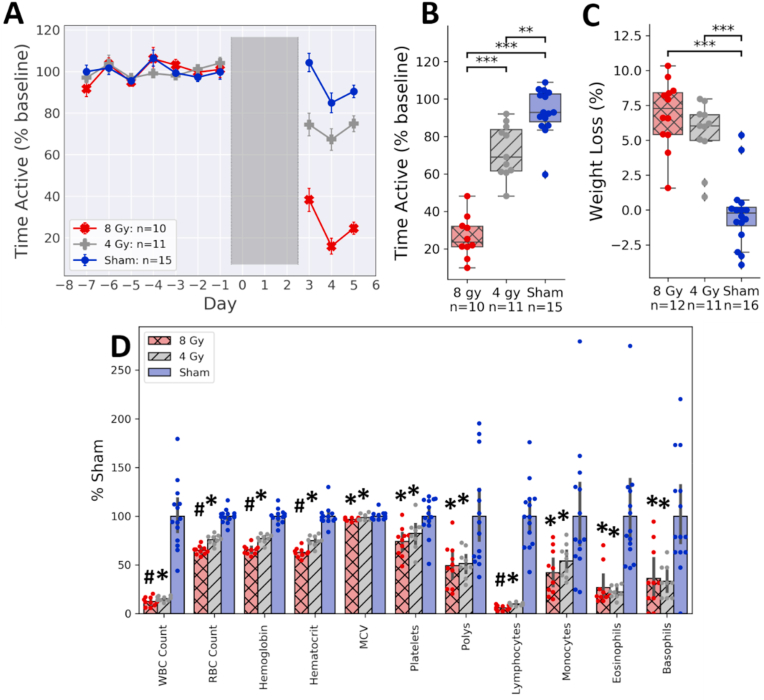
We found that both 4 and 8 Gy doses of radiation induced fatigue-like behavior measured as a decline in VWRA (Fig. 1A). A linear mixed model with a random intercept from each animal showed a statistically significant effect of irradiation (p = 10−4), with significant differences between the 4 and 8 Gy groups (p < 10−8) and between the sham and 4 Gy groups (p = 10−3). The dose-dependent effect did not appear to be linear; the mean effect of 8 Gy irradiation on VWRA was more than twice that of 4 Gy. Body weight was also measured on days 0 and 2, and there was a significant effect of irradiation on bodyweight (Fig. 1C, F1,37 = 56.25, p = 10−8). Pairwise comparisons did not show a statistically significant difference between bodyweight changes in the 4 Gy and 8 Gy dose groups (d = 0.59, t22 = 1.41, p = 0.17).
Fig. 1.
Dose-dependent effects of pelvic irradiation on VWRA. (A) Changes in VWRA over the 15-day study, with total time active on the running wheels expressed as a percentage of the 7-day baseline average. Irradiation took place over three days (0–2), during which animals did not have access to the running wheels. (B) Change in VWRA averaged across the entire post-irradiation period (days 3–5). (C) Bodyweight decreased from day 0 to day 2. (D) CBC results normalized to the sham mean. ∗∗p < 0.005, ∗∗∗p < 0.0005. ∗Significantly different from the sham group (p < 0.05). #Significantly different from both the 4 Gy and sham groups (p < 0.05).
We performed a complete blood count (CBC) on the mice in these experiments. Compared to the sham group, both the 4 Gy and 8 Gy doses of radiation had a statistically significant effect on all CBC measurements (Fig. 1D). Comparing the 4 Gy to the 8 Gy dose groups, we found a statistically significant effect of irradiation on red blood cells (RBC), hemoglobin, hematocrit, and lymphocytes; however, we did not find a significant effect on mean corpuscular volume (MCV), total white blood cells (WBC), platelets, neutrophils, monocytes, basophils, or eosinophils. These findings suggest that the decreases in VWRA beyond those seen in the 4 Gy group are more likely related to a decrease in erythropoietic markers (e.g., RBC, hemoglobin) or lymphocytes, and less likely to be a result of a decrease in the other cell types measured. Means and standard deviations for raw counts are displayed in Supplementary Table S1 and the statistical analysis in Table S2.
3.2. Fatigue and inflammation
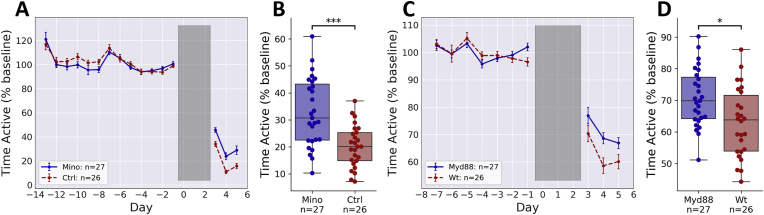
To test whether inflammation was mediating the changes in fatigue-like behavior, we diluted minocycline into the drinking water of half of the animals, with the control group receiving normal drinking water. A small increase in VWRA for the minocycline-treated group relative to the control group was observed. To test for significance, we used a linear mixed model of VWRA over the full seven days prior to irradiation. We did not observe a statistically significant effect of minocycline treatment (p = 0.75) nor a significant interaction between treatment and time (p = 0.15).
After irradiation, there was a clear separation between minocycline-treated and control mice (Fig. 2A). A linear mixed model showed significant effects of treatment (p = 10−3) and time (p = 10−15). In the control group with normal drinking water, the post-irradiation median VWRA dropped to about 20% of the baseline average (Fig. 2A), similar to the previously observed value (Fig. 1A). The minocycline-treated group showed higher post-irradiation VWRA, with a median of about 30% of the baseline average, which is a statistically significant difference from the control group (Fig. 2B, d = 1.06, t52 = 4.58, p < 10−4).
Fig. 2.
Effect of inflammatory modulation on post-irradiation VWRA. All mice underwent 8 Gy irradiation daily on days 0–2. (A) Changes in VWRA over the course of the study. Minocycline was administered into the drinking water of mice in the “Mino” group starting on day −7. (B) Change in VWRA averaged across the post-irradiation period (days 3–5). (C) Changes in VWRA over the course of the study for MyD88 knockout mice (“Myd88”) and wild-type controls (“Wt”). (D) Change in VWRA averaged across the post-irradiation period (days 3–5). ∗p < 0.05, ∗∗∗p < 0.0005.
Minocycline has many effects besides those on inflammation, most notably its antibiotic properties, which could affect the gut microbiome or confer resistance to infection. The latter could be particularly important in irradiated mice with reduced WBC counts. To reduce inflammation more directly, we next tested mice with a deletion of the MyD88 gene, which encodes an adaptor protein involved in the immune response to bacterial infection. In contrast to minocycline-treated mice, these mice should be more susceptible to infection than wild-type controls (Takeuchi et al., 2000; Serbina et al., 2003). The experiment design was the same as the previous experiment, except here half of the mice lacked the gene for MyD88 and the other half were wild-type controls. Our results with MyD88 knockout mice were strikingly similar to those from the minocycline-treated mice; there was a small, but statistically significant increase in post-irradiation VWRA in the MyD88 deficient mice relative to the wild-type controls (Fig. 2C and D). A linear mixed model showed a significant effect of genotype on VWRA after irradiation (p < 0.05) but not before (p = 0.86).
Comparing the time active in the minocycline-treated and Myd88 knockout mice (y-axes for Fig. 2B and D) showed a marked and surprising difference in the effects of irradiation on the wild-type control groups. This difference is most likely due to substrain differences: the MyD88 knockout mice and their controls were bred by Jackson Labs on a C57BL/6J (B6J) background, while all other mice in the study were C57BL/6NCrl (B6NCrl) mice bred by Charles River Labs. Directly comparing the untreated, wild-type groups from both substrains shows a very large and statistically significant difference in susceptibility to irradiation-induced fatigue (Supplementary Fig. S3B, d = 1.83, t51 = 17.40, p = 10−20). The effect of substrain was much larger than the effect of the minocycline or MyD88 deletion, and slightly larger than the difference between 4 Gy and 8 Gy doses shown in Fig. 1B. There was also an effect of substrain on weight change during irradiation, with weight loss being statistically significant in the B6NCrl mice (d = 1.14, t53 = 4.97, p = 10−5) but not in the B6J mice (d = 0.44, t53 = 1.63, p = 0.11).
When modeling fatigue-like behavior, it is important that the induced changes in locomotor activity are not due to overt tissue damage. To assess this, we did pathology studies in portions of the minocycline-treated animals, the MyD88 knockout animals, and their respective controls (Supplementary Fig S2, Table S3). While a previous study found no overt tissue damage two weeks after completing three days of 8 Gy irradiation (Renner et al., 2016), we observed that irradiated animals showed signs of lost hematopoietic cells in bone marrow. This observation is consistent with our CBC results, which showed fewer RBCs in irradiated mice. We also saw centered nuclei in muscle cells and very mild signs of damage in the lungs, but we did not see any consistent signs of damage to the heart, liver, kidney, skin, spleen, or gastrointestinal tract. Nonetheless, it is still possible that undetectable levels of damage influenced behavior. No differences in irradiation-induced pathology findings were observed when comparing minocycline-treated or MyD88 knockout mice to their control groups.
3.3. Fatigue and cytokines
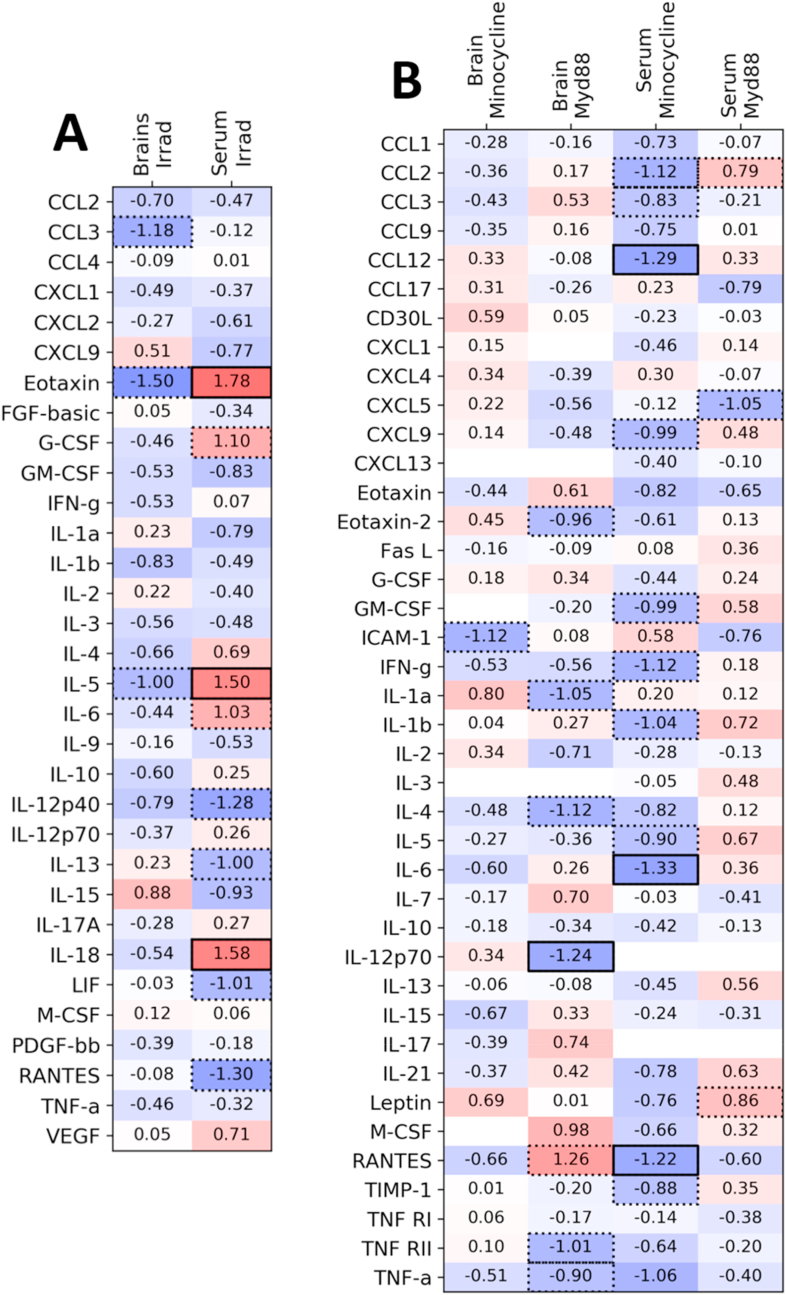
Since we found that the fatigue-like behavior was partially reversed by the anti-inflammatory effects of minocycline treatment or MyD88 gene deletion, we anticipated seeing that the changes in behavior were accompanied by changes in levels of circulating cytokines. We used multiplex kits to test both serum samples and whole-brain homogenates taken while the animals were still showing fatigue-like behavior. The resulting effect sizes and significance calculations are displayed in Fig. 3, with mean concentrations and other statistics for each cytokine listed in Supplementary Table S4–6.
Fig. 3.
Serum and brain multiplex. Heatmap of results from multiplex kits on serum samples and whole-brain homogenates. Different multiplex kits were used for comparing (A) irradiated to sham animals and for comparing (B) minocycline-treated or myd88 knockout mice to their controls. All target molecules are listed as rows, and the treatment and sample are listed as columns. Effect size (Cohen's d) is written in the center of each cell, which compares the treatment listed on the left with its control (irradiation treatment relative to sham, minocycline relative to plain water, and MyD88 knockout mice compared to wild-type). If a cell has no number, then measured concentrations were outside of the range of the standards. Cells are shaded in darker blue when the effect size is more negative (i.e., when the values for the treatment group are lower than the corresponding control group), and darker red when the effect size is more positive (i.e., the treatment group shows higher levels). Cells are outlined with a dotted line when the difference in group means is significant (Welch's t-test, p < 0.05), and outlined with a solid line when the difference in means is significant after a Holm-Sidak correction for multiple comparisons. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
First, we compared cytokine levels in irradiated (8 Gy) or sham-irradiated mice. In the serum samples, 10 of 32 cytokines showed significant differences: Eotaxin, G-CSF, IL-5, IL-6, and IL-18 were significantly higher in the irradiated mice, while IL-12(p40), IL-13, LIF, and RANTES were significantly lower.
Next, we compared cytokine levels between experimental and control groups for both the minocycline and MyD88 experiments. In the serum samples, there were significantly lower concentrations of a number of serum cytokines in minocycline-treated mice relative to control mice, including CCL2, CCL3, CCL12, CXCL9, GM-CSF, IFN-γ, IL-1b, IL-5, and IL-6. MyD88 deletion resulted in fewer serum differences (only CXCL5 was significantly lower than in controls, with leptin and CCL2 significantly higher) and remarkably little overlap with the difference seen in minocycline-treated mice. In brain samples, we saw only a statistically significant difference in ICAM-1 when comparing the minocycline-treated group to its control group. MyD88 deletion resulted in broader post-irradiation differences in whole brain samples, with statistically significant decreases in Eotaxin-2, IL-4, IL-12(p70), RANTES, TNF RII, and TNF-α.
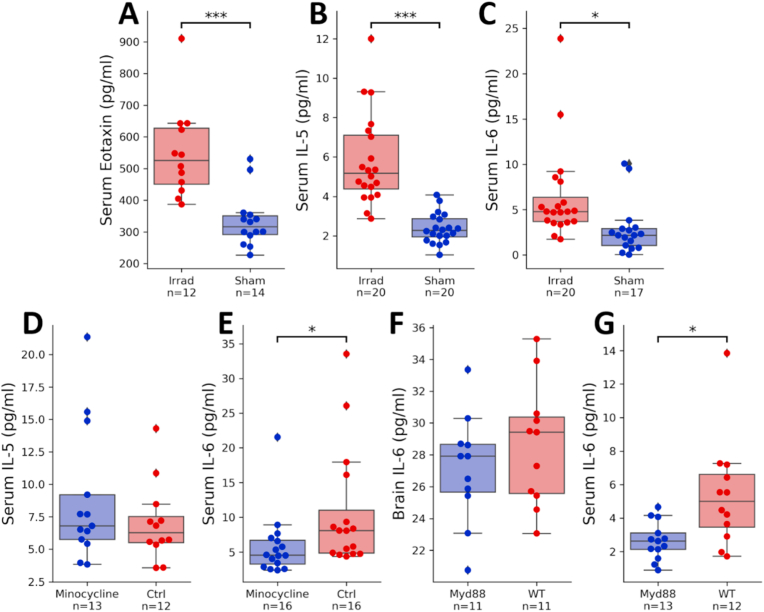
Although multiple comparisons corrections are not appropriate for data that we would expect to be highly correlated due to mutual feedback and common regulatory mechanisms, it is nonetheless very likely that many comparisons would generate false positives. To validate results, we did separate ELISAs on several targets. Because we were only interested in group differences that matched what was observed in the multiplex, analysis of these results used one-tailed p values. We first tested three targets comparing irradiation vs. sham (Eotaxin, IL-5, and IL-6). All three reproduced the results from the multiplex, showing a significant elevation in the irradiated group (Fig. 4A, IL-6: d = 0.80, t36 = 2.67, p = 0.0060; Fig. 4B, Eotaxin: d = 1.39, t25 = 4.51, p = 10−4; Fig. 4C, IL-5: d = 1.43, t39 = 6.28, p = 10−6). Next, we conducted follow-up ELISA experiments to confirm findings from minocycline treatment and MyD88 deletion. The decrease in IL-6 in minocycline-treated mice was again significant (Fig. 4D, d = 0.68, t31 = 1.97, p = 0.031), though the decrease in serum IL-5 was not (Fig. 4E, d = 0.43, t24 = 1.08, p = 0.29). In MyD88 knockout mice, we did not see a replication of the multiplex results in whole-brain IL-6 (Fig. 4F, d = 0.42, t21 = 0.97, p = 0.17).
Fig. 4.
ELISA follow-up to multiplex. Target molecules selected based on significance in the multiplex, with an addition of serum IL-6 in myd88 knockout mice vs controls. Concentrations of (A) eotaxin, (B) IL-5, and (C) IL-6 in serum samples taken from 8 Gy irradiated mice and sham-irradiated mice. Concentrations of (D) IL-5 and (E) IL-6 in serum samples taken from minocycline-treated mice compared to control mice with normal drinking water. Concentration of IL-6 in (F) whole-brain homogenates and (G) serum samples taken from MyD88 knockouts and wild-type mice. ∗p < 0.05, ∗∗∗p < 0.0005.
Multiplex data may also produce false negatives. Due to the robustness of the changes in serum IL-6 seen in the irradiated mice and reversed in minocycline-treated mice, we conducted an additional follow-up ELISA experiment to look for differences in serum IL-6 levels between MyD88 knockout mice and wild-type mice, even though the decrease shown by the multiplex experiment was not significant. In this follow-up measurement, the decrease in serum IL-6 was large and significant (Fig. 4H, d = 1.03, t24 = 2.77, two-tailed p = 0.015).
4. Discussion
In previous studies (Wolff et al., 2018), we used a mouse model of radiation therapy for treatment of prostate cancer to show that pelvic irradiation alone is sufficient to induce a profound fatigue-like behavior that can be measured as a decline in VWRA or home cage locomotor activity. This fatigue-like behavior is different from radiation sickness, which is induced by total-body irradiation and can consequently trigger local inflammatory responses across the entire organism. In this study, we used minocycline treatments and MyD88 knockout mice to show that inflammation is a cause of the fatigue-like behavior induced by targeted peripheral irradiation. The two inflammatory modulations each reversed only a small portion of the total VWRA decline from the irradiation procedure, but it is important to note that we do not know how much of the total inflammation is reversed by these modulations. Because of that, it is not possible to quantitatively evaluate the relationship between inflammation and fatigue; we can only say that the former is to some degree causing the latter.
Whole-body irradiation is often used in research to eliminate WBCs from mice, and in this study, our CBC results show very few WBCs remain even when using an irradiation procedure targeted to a pelvic region. Only lymphocytes showed a significant difference between the 4 Gy and 8 Gy doses, which might suggest a connection between the loss of lymphocytes and the decline in VWRA after irradiation, though any specific mechanism would be unclear. The decline may more likely be a consequence of inflammatory cytokine signaling, as we found that many cytokine plasma concentrations are substantially altered by the irradiation procedure.
As wheel running is a voluntary behavior, the choice of whether to use the running wheel may be influenced by circulating cytokines signaling to the brain. Since we did not find evidence of substantial changes in cytokine concentrations inside the brain itself, the cytokines may not signal directly inside the brain, but instead signal from the periphery. The evidence presented here has important limitations, however. First, the accuracy of the measurements could be compromised by blood contamination in the brain samples, which were taken from animals that were not perfused. Second, cytokine signaling may be localized to specific brain regions and therefore undetectable in our samples of the entire brain. We used whole-brain homogenates here as a first step towards understanding changes in the brain, but future studies would benefit from a look at more specific regions. For example, there is evidence that peripheral inflammatory cytokines including IL-6 can induce sickness behaviors through vagal circuits (Schweighofer et al., 2016) and particularly through activation of neuronal populations within the paraventricular nucleus of the hypothalamus (Belevych et al., 2010). These could be potential mediators of fatigue-like declines in locomotor activity and would be good targets for future study.
Our results point to circulating IL-6 as a likely candidate for mediating irradiation-induced fatigue, as it was the only cytokine in our serum samples that, like fatigue, was increased by irradiation and decreased by minocycline treatment. This suggests that the peripheral irradiation may induce both inflammatory signaling and fatigue-like behavior that is closely related to sickness. LPS is a strong inducer of IL-6 (Bluthé et al., 2000) and can also induce sickness behavior, including weight loss and decreased VWRA (Harden et al., 2006, 2011). However, studies that have used multiplex arrays to measure cytokines in the serum of LPS-injected mice also consistently find elevated IL-1b (Banks et al., 2015; Bobrowski et al., 2005; Erickson and Banks, 2011; Fourrier et al., 2017; Yang et al., 2018), which we did not see in our experiments. This suggests that there may also be important differences between the inflammatory signals induced by LPS and those induced by the irradiation procedure in our mouse model. However, it is important to note that our cytokine data are all correlational, so future experiments should specifically test whether IL-6 or other cytokines may be causally related to the fatigue behavior.
The absence of some commonly seen signs of sickness behavior like elevated IL-1b could also be related to the time after irradiation, as here we have only a single “snapshot” of serum concentrations taken four days after completing the irradiation procedure. We chose this day as it is shortly after the peak of fatigue-like behavior that occurs on day 4 or 5 but well before the behavior typically normalizes around day 9 (Wolff et al., 2017, 2018). It is likely that cytokine levels all have their own temporal expression patterns across many days after irradiation, and the relative levels of each cytokine could vary substantially depending on the timing of sample collection.
The CBC results suggest that in addition to inflammation, another factor contributing to the decline in VWRA could be the loss of erythropoietic markers (RBCs and hemoglobin). This finding is consistent with results from fatigue-like behavior induced by chemotherapeutic drugs that also reduce RBC counts (Mahoney et al., 2013; Zombeck et al., 2013). It is also consistent with clinical studies, where radiation treatments for prostate cancer are associated with anemia that correlates with subjective levels of fatigue (Feng et al., 2020). There is also evidence that anemia may be a prognostic indicator of cancer treatment outcomes (Harrison et al., 2002) and that anemia may exacerbate cancer symptoms in patients concurrently treated with therapies that can inhibit hematopoiesis such as anti-androgens (Feng et al., 2020). Our experiments suggest that in our model, the loss of oxygen transport by RBCs may be of particular importance at higher doses of radiation, as increasing the dose from 4 Gy to 8 Gy significantly affected both RBC counts and VWRA, but did not significantly affect WBC counts nor bodyweight. The fatigue-like behavior still present in minocycline-treated and MyD88 knockout mice after irradiation is likely due at least in part to RBC loss, though there may be other mechanisms at play as well, including changes in muscle metabolism and mitochondrial function seen in recent studies of radiation-induced fatigue (Feng et al., 2020; Kim et al., 2019).
The results of this study suggest that some CRF symptoms in patients that receive radiation therapy may be caused by inflammation, although what we can conclude from this study is limited beyond merely the use of mice as a model organism. Most notably, our study shows the effects only of radiation, as unlike clinical populations, the mice in our study did not have tumors. There is evidence in humans and in mice that tumors can cause elevated inflammatory signaling that may contribute to fatigue and similar symptoms or behaviors (Dranoff, 2004). In cancer patients, radiation also may be accompanied by other treatments like chemotherapy or hormone therapy, and additional treatments may interact in ways that we cannot predict from studying the effects of radiation in isolation, as we did in this study. Additionally, in mice as in humans, it can be difficult to attribute behavioral changes to any single causes. Fatigue is commonly associated with other symptoms, for example pain, and the reduced wheel running in mice we saw here may similarly have other causes, such as pain. Nonetheless, to fully understand the effects of cancer treatments on behavior, it will be important to have mechanistic study of cancer and its treatments both in isolation and combined, and we believe this study is an important early step.
Funding
This study was supported by the Divisions of Intramural Research of the National Institute of Nursing Research and the National Institute of Mental Health of the NIH, Bethesda, Maryland, USA.
Author contributions
BSW: conceptualization, formal analysis, software, supervision, writing – original draft. SAA: investigation, methodology, writing – original draft. LRF: conceptualization, methodology, writing – reviewing and editing. PLJ: formal analysis, writing – original draft. LNS: resources, conceptualization, methodology, project administration, writing – reviewing and editing.
Declaration of competing of interest
There are no conflicts of interest to report.
Acknowledgments
We would like to thank the NHLBI Murine Phenotyping Core for their invaluable help with experiments, including Dr. Danielle Springer, Michele Allen, and Heather Potts. We would also like to thank the NHLBI Animal Surgery and Resources Core for help with taking blood and tissue samples. Also, Alicia A. Livinski, NIH Library for manuscript editing and formatting assistance. Finally, we would like to thank Dr. Zu-Xi Yu and the NHLBI Pathology Core for their pathology work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100264. Data are publically available at https://doi.org/10.17605/OSF.IO/BJZ57.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bakshi J., Segura B.T., Wincup C., Rahman A. Unmet needs in the pathogenesis and treatment of systemic lupus erythematosus. Clin. Rev. Allergy Immunol. 2018;55(3):352–367. doi: 10.1007/s12016-017-8640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks W.A., Gray A.M., Erickson M.A., Salameh T.S., Damodarasamy M., Sheibani N. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation. 2015;12:223. doi: 10.1186/s12974-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belevych N., Buchanan K., Chen Q., Bailey M., Quan N. Location-specific activation of the paraventricular nucleus of the hypothalamus by localized inflammation. Brain Behav. Immun. 2010;24(7):1137–1147. doi: 10.1016/j.bbi.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé R.M., Michaud B., Poli V., Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol. Behav. 2000;70(3–4):367–373. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Bobrowski W.F., McDuffie J.E., Sobocinski G., Chupka J., Olle E., Bowman A. Comparative methods for multiplex analysis of cytokine protein expression in plasma of lipopolysaccharide-treated mice. Cytokine. 2005;32(5):194–198. doi: 10.1016/j.cyto.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bower J.E. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguine J., Barton G.M. MyD88: a central player in innate immune signaling. F1000prime reports. 2014;6:97. doi: 10.12703/P6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakos C.I., Charles K.A., McMillan D.C., Clarke S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Canc. 2004;4(1):11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- Erickson M.A., Banks W.A. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav. Immun. 2011;25(8):1637–1648. doi: 10.1016/j.bbi.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.R., Regan J., Shrader J.A., Liwang J., Ross A., Kumar S. Cognitive and motor aspects of cancer-related fatigue. Cancer Med. 2019;8(13):5840–5849. doi: 10.1002/cam4.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.R., Wolff B.S., Liwang J., Regan J.M., Alshawi S., Raheem S. Cancerrelated fatigue during combined treatment of androgen deprivation therapy and radiotherapy is associated with mitochondrial dysfunction. Int. J. Mol. Med. 2020;45(2):485–496. doi: 10.3892/ijmm.2019.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourrier C., Remus-Borel J., Greenhalgh A.D., Guichardant M., Bernoud-Hubac N., Lagarde M. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflammation. 2017;14(1):170. doi: 10.1186/s12974-017-0939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N., Zarzuelo A., Galvez J. Minocycline: far beyond an antibiotic. Br. J. Pharmacol. 2013;169(2):337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg A.J., Vichaya E.G., Gross P.S., Ford B.G., Scott K.A., Estrada D. Interleukin 6-independent metabolic reprogramming as a driver of cancer-related fatigue. Brain Behav. Immun. 2020;88:230–241. doi: 10.1016/j.bbi.2020.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden L.M., du Plessis I., Poole S., Laburn H.P. Interleukin-6 and leptin mediate lipopolysaccharide-induced fever and sickness behavior. Physiol. Behav. 2006;89(2):146–155. doi: 10.1016/j.physbeh.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Harden L.M., du Plessis I., Roth J., Loram L.C., Poole S., Laburn H.P. Differences in the relative involvement of peripherally released interleukin (IL)-6, brain IL-1beta and prostanoids in mediating lipopolysaccharide-induced fever and sickness behavior. Psychoneuroendocrinology. 2011;36(5):608–622. doi: 10.1016/j.psyneuen.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Harrison L.B., Shasha D., Homel P. Prevalence of anemia in cancer patients undergoing radiotherapy: prognostic significance and treatment. Oncology. 2002;63(Suppl. 2):11–18. doi: 10.1159/000067147. [DOI] [PubMed] [Google Scholar]
- Hofman M., Ryan J.L., Figueroa-Moseley C.D., Jean-Pierre P., Morrow G.R. Cancer-related fatigue: the scale of the problem. Oncol. 2007;12(Suppl. 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- Hou B., Reizis B., DeFranco A.L. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29(2):272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C.P., Daly B., Saligan L.N. The Etiology and management of radiotherapy-induced fatigue. Expert Rev Qual Life Cancer Care. 2016;1(4):323–328. doi: 10.1080/23809000.2016.1191948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Lee M., Kim D.Y., Kim K.I., Yi J.Y. Mechanisms of energy metabolism in skeletal muscle mitochondria following radiation exposure. Cells. 2019;8(9) doi: 10.3390/cells8090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston B., Armes J., Levy A., Tidey E., Ream E. The prevalence and severity of fatigue in men with prostate cancer: a systematic review of the literature. Support. Care Canc. 2013;21(6):1761–1771. doi: 10.1007/s00520-013-1751-5. [DOI] [PubMed] [Google Scholar]
- Louati K., Berenbaum F. Fatigue in chronic inflammation - a link to pain pathways. Arthritis Res. Ther. 2015;17:254. doi: 10.1186/s13075-015-0784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney S.E., Davis J.M., Murphy E.A., McClellan J.L., Gordon B., Pena M.M. Effects of 5-fluorouracil chemotherapy on fatigue: role of MCP-1. Brain Behav. Immun. 2013;27(1):155–161. doi: 10.1016/j.bbi.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller T., Bard F., Bhattacharya A., Biber K., Campbell B., Dale E. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia. 2016;64(10):1788–1794. doi: 10.1002/glia.23007. [DOI] [PubMed] [Google Scholar]
- Montoya J.G., Holmes T.H., Anderson J.N., Maecker H.T., Rosenberg-Hasson Y., Valencia I.J. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc. Natl. Acad. Sci. U. S. A. 2017;114(34) doi: 10.1073/pnas.1710519114. E7150-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Higgins C.M., Brady B., O'Connor B., Walsh D., Reilly R.B. The pathophysiology of cancer-related fatigue: current controversies. Support. Care Canc. 2018;26(10):3353–3364. doi: 10.1007/s00520-018-4318-7. [DOI] [PubMed] [Google Scholar]
- Pang T., Wang J., Benicky J., Saavedra J.M. Minocycline ameliorates LPS-induced inflammation in human monocytes by novel mechanisms including LOX-1, Nur77 and LITAF inhibition. Biochim. Biophys. Acta. 2012;1820(4):503–510. doi: 10.1016/j.bbagen.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner M., Feng R., Springer D., Chen M.K., Ntamack A., Espina A. A murine model of peripheral irradiation-induced fatigue. Behav. Brain Res. 2016;307:218–226. doi: 10.1016/j.bbr.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaue D., Micewicz E.D., Ratikan J.A., Xie M.W., Cheng G., McBride W.H. Radiation and inflammation. Semin. Radiat. Oncol. 2015;25(1):4–10. doi: 10.1016/j.semradonc.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer H., Rummel C., Roth J., Rosengarten B. Modulatory effects of vagal stimulation on neurophysiological parameters and the cellular immune response in the rat brain during systemic inflammation. Intensive Care Med Exp. 2016;4(1):19. doi: 10.1186/s40635-016-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina N.V., Kuziel W., Flavell R., Akira S., Rollins B., Pamer E.G. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19(6):891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Hoshino K., Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 2000;165(10):5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- The Guide for the Care and Use of Laboratory Animals. eighth ed. National Research Council of the National Academies; Washington, D.C.: 2011. [Google Scholar]
- Todoric J., Antonucci L., Karin M. Targeting inflammation in cancer prevention and therapy. Canc. Prev. Res. 2016;9(12):895–905. doi: 10.1158/1940-6207.CAPR-16-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff B.S., Renner M.A., Springer D.A., Saligan L.N. A mouse model of fatigue induced by peripheral irradiation. JoVE. 2017;121 doi: 10.3791/55145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff B.S., Raheem S.A., Saligan L.N. Comparing passive measures of fatigue-like behavior in mice. Sci. Rep. 2018;8(1):14238. doi: 10.1038/s41598-018-32654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Boza-Serrano A., Dunning C.J.R., Clausen B.H., Lambertsen K.L., Deierborg T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J. Neuroinflammation. 2018;15(1):168. doi: 10.1186/s12974-018-1204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Yang Y., Wang D., Li C., Qu Y., Guo J. The clinical value of cytokines in chronic fatigue syndrome. J. Transl. Med. 2019;17(1):213. doi: 10.1186/s12967-019-1948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck J.A., Fey E.G., Lyng G.D., Sonis S.T. A clinically translatable mouse model for chemotherapy-related fatigue. Comp. Med. 2013;63(6):491–497. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.