Abstract
Children with ASD are more likely to experience gastrointestinal (GI) symptoms than typically-developed children. Numerous studies have reported immune abnormalities and inflammatory profiles in the majority of individuals with ASD. Immune dysfunction is often hypothesized as a driving factor in many GI diseases and it has been suggested that it is more apparent in children with ASD that exhibit GI symptoms. In this study we sought to characterize peripheral T cell subsets in children with and without GI symptoms, compared to healthy typically-developing children. Peripheral blood mononuclear cells were isolated from participants, who were categorized into three groups: children with ASD who experience GI symptoms (n = 14), children with ASD who do not experience GI symptoms (n = 10) and typically-developing children who do not experience GI symptoms (n = 15). In order to be included in the GI group, GI symptoms such as diarrhea, constipation, and/or pain while defecating, had to be present in the child regularly for the past 6 months; likewise, in order to be placed in the no GI groups, bowel movements could not include the above symptoms present throughout development. Cells were assessed for surface markers and intracellular cytokines to identify T cell populations. Children with ASD and GI symptoms displayed elevated TH17 populations (0.757% ± 0.313% compared to 0.297% ± 0.197), while children with ASD who did not experience GI symptoms showed increased frequency of TH2 populations (2.02% ± 1.08% compared to 1.01% ± 0.58%). Both ASD groups showed evidence of reduced gut homing regulatory T cell populations compared to typically developing children (ASDGI:1.93% ± 0.75% and ASDNoGI:1.85% ± 0.89 compared to 2.93% ± 1.16%). Children with ASD may have deficits in immune regulation that lead to differential inflammatory T cell subsets that could be linked to associated co-morbidities.
Keywords: ASD, Autism, Cytokines, Gastrointestinal, Immune, Mucosal immunity, Regulatory T-cells, TH17, TH2
Highlights
-
•
Children with ASD and co-morbid GI symptoms displayed elevated TH17 populations.
-
•
Children with ASD without GI symptoms displayed elevated TH13 populations.
-
•
Both ASD groups had decreased frequencies of gut homing regulatory T cells.
1. Introduction
Neurodevelopmental disorders, such as autism spectrum disorders (ASD) are often considered disorders of the brain, particularly since they are defined by behavioral traits including repetitive and stereotyped behaviors and by impairments in communication and social interactions (Association, 2013). However, many individuals with ASD also suffer from one or more medical comorbidities including epilepsy, sleep disorders, asthma and gastrointestinal (GI) dysfunction (Levisohn, 2007; Bauman, 2010; Kohane et al., 2012; Maenner et al., 2012; Chen et al., 2013; Zerbo et al., 2015b; Mannion and Leader, 2016). Reports of GI related symptoms in autism have occurred since the disorder was first described in 1943 (Kanner, 1943) and studies since have continued to report symptoms of constipation, diarrhea, vomiting, abdominal pain, gas and bloating associated with ASD. The full extent of GI symptoms among individuals with ASD has been debated, with differences in study designs, report biases, or population studies vs. clinic based studies, all leading to a wide range (20–90%) in the reported prevalence rates (Horvath and Perman, 2002; Molloy and Manning-Courtney, 2003; Niehus and Lord, 2006; Valicenti-McDermott et al., 2006; Xue et al., 2008; Buie et al., 2010; Coury et al., 2012). One large cohort study including close to 1000 participants reported children with ASD are 6–8 times more likely to suffer from GI symptoms compared to age-matched typically-developing children; in addition, GI symptoms were associated with poorer behavioral assessment scores (Chaidez et al., 2014).
Immune dysfunction has been reported in approximately 60% of children with ASD (Careaga et al., 2017). Although immune abnormalities in ASD have been reported since the late 1970’s (Stubbs and Crawford, 1977) a consensus for differences in cellular activation has not yet been reached (Rose and Ashwood, 2014). In mainly mixed cultures of peripheral blood mononuclear cells (PBMC), upon stimulation some studies have observed elevated T-helper (TH)1 cytokines (Singh, 1996; Croonenberghs et al., 2002a; Ashwood et al., 2011b; Ricci et al., 2013; Careaga et al., 2017) while others found increased TH2 cell associated cytokines (Molloy et al., 2006; Ashwood et al., 2011c; Goines et al., 2011b; Careaga et al., 2017; Krakowiak et al., 2017); in addition to elevated innate cytokines (Jyonouchi et al. 2001, 2005, 2014; Croonenberghs et al., 2002a; Enstrom et al., 2010; Ashwood et al., 2011b; Malik et al., 2011; Careaga et al., 2017). A number of studies have found altered frequencies or altered activation status of specific immune cells or cell subsets (Gupta et al., 1998; Sweeten et al., 2003; Enstrom et al. 2009b, 2010; Mostafa et al., 2010; Ashwood et al., 2011a; Lopez-Cacho et al., 2016; Ahmad et al., 2017), elevated chemokines concentrations (Ashwood et al., 2011d; Manzardo et al., 2012; Al-Ayadhi and Mostafa, 2013; Zerbo et al., 2014), decreased soluble TNF receptors (Ashwood, 2018), altered immunoglobulin titers (Croonenberghs et al., 2002b; Heuer et al., 2008; Enstrom et al., 2009a) and the presence of autoantibodies to brain or CNS proteins (Singh et al. 1993, 1997; Singer et al., 2006; Cabanlit et al., 2007; Braunschweig et al., 2008; Wills et al., 2009; Goines et al., 2011a; Frye et al., 2016). A recent study seeking to better characterize immune endophenotypes in ASD identified a subgroup of individuals within the ASD population who exhibited elevated production of inflammatory cytokines from stimulated immune cells. Using cluster analyses, those with enhanced immune activation following immune challenge displayed more severe core ASD features and impaired behaviors (Careaga et al., 2017). This was true for both children with ASD who displayed increased TH1 cytokine profiles and those with increased TH2 cytokine profiles.
We hypothesize that immune activation involving several arms of the immune system is observed in ASD, and that the unifying denominator among these studies is in fact a decrease in immune regulation and inability to control immune responses. Corroborating this hypothesis, decreased levels of the regulatory cytokines transforming growth factor (TGF)β1 (Okada et al., 2007; Ashwood et al., 2008; Abdallah et al., 2013b), interleukin (IL)-10 (Jyonouchi et al. 2005, 2012, 2014; Abdallah et al., 2012) and IL-35 (Rose and Ashwood, 2019) have been reported from blood components or in stimulated immune cell cultures from individuals with ASD. In addition, decreased frequency of regulatory T cells (Tregs) have also been found in individuals with ASD (Ashwood et al., 2004; Mostafa et al., 2010; Ahmad et al., 2017). On a background of decreased regulation, the varying pro-inflammatory immune abnormalities reported in ASD may reflect the heterogeneity of ASD, genetic background or environmental exposures, and illustrate the need to find common subgroups within ASD, that may help define more targeted treatments to benefit more individuals across the spectrum (Critchfield et al., 2011; Coury et al., 2012; Ousley and Cermak, 2014).
We previously reported altered immune responses in children with ASD who experience GI symptoms. Peripheral blood mononuclear cells from children with ASD and GI symptoms produced increased mucosa-related cytokines but decreased TGFβ after stimulation in vitro (Rose et al., 2018), suggesting a net imbalance away from a regulated response. In the current study we seek to expand upon these findings and further characterize the cellular mediators that may be driving these differential profiles. We utilized flow cytometry to identify and characterize T cells in children with ASD who experience GI symptoms, children with ASD who do not experience GI symptoms and in typically-developing children who do not experience GI symptoms. T cells were identified and grouped based on their expression of CD3, CD4, CD8, and CD25 and further characterized by intracellular expression of interferon gamma (IFNγ), IL-10, IL-13, IL-17. Furthermore, we also evaluated populations of T cells expressing the gut-homing integrin α4β7.
2. Methods
2.1. Subjects
Children with ASD and GI symptoms of irregular bowel movements (ASDGI) (n = 14), or children with ASD and no GI symptoms (ASDNoGI) (n = 10), and typically developing children without GI symptoms (TD) (n = 15) were recruited into this study. Participants had previously been enrolled in the Childhood Autism Risk from Genetics and Environment (CHARGE) study (Hertz-Picciotto et al., 2006). A diagnosis of autism spectrum disorder was confirmed at the UC Davis MIND Institute by trained staff using the Autism Diagnostic Interview-Revised (ADI-R), and the Autism Diagnostic Observation Schedule (ADOS). The Social Communication Questionnaire (SCQ) was used to screen for behavioral and developmental characteristics of ASD in the typically-developing group. Participants in the TD groups had to score within the typical range, i.e. below 15, on the SCQ and above 70 on the Mullen Scales of Early Learning (MSEL) and Vineland Adaptive Behavior Score (VABS). All participants were assessed using the Aberrant Behavior Checklist (ABC) to assess impairments within the domains of irritability, lethargy, social withdrawal, stereotypic behavior, hyperactivity and inappropriate speech. Participants were randomly recruited from the CHARGE database based on inclusion/exclusion criteria. A telephone interview, along with GI and health questionnaires were used to assess participant eligibility into the study. Medications and/or behavioral therapies used at the time of enrollment or within the previous year were collected and recorded. Participants were excluded if they had a known diagnosis of other GI pathology (e.g. celiac disease or Inflammatory Bowel Disease), use of antibiotics or antifungal medications within the prior month, medications affecting GI transit (stool softeners), and/or recent evidence of a GI infection based on stool laboratory tests. In addition, participants were excluded if there was evidence of a seizure disorder, genetic disorders (i.e. Fragile X syndrome, Tuberous Sclerosis Complex), liver or pancreatic disease, cystic fibrosis, or chronic infection. Children receiving clinically monitored and prescribed dietary interventions under the guidance of trained nutritionists/clinicians, medications, or complementary alternative treatments such as supplements other than a standard daily multivitamin/mineral tablet were also excluded. However, for children who were receiving nutritional modifications that were not overseen by trained nutritionists/clinicians, the dietary changes were documented but the participants not excluded from the study. Parental reports of suspected food sensitivities/intolerances that had not been diagnosed through clinical assessment were also not grounds for exclusion but were documented.
2.2. GI symptom evaluation
CHARGE GI history (GIH) survey and GI symptom survey (GISS), based upon Rome III Diagnostic Questionnaire for the Pediatric Functional GI Disorders (Walker et al., 2006) were obtained from parent/legal guardians. The GIH (Chaidez et al., 2014) and GISS (Rose et al., 2018) assessments have been reported previously. The GIH scores the frequency; abdominal pain, blood in stool/vomit, constipation, diarrhea, difficulty swallowing, gaseousness/bloating, pain on stooling, sensitivity to foods, vomiting, on a Likert scale [(0) = never, (1) = rarely, (2) = sometimes, (3) = frequently and (4) = always)]. The GIH assessed any allergies to foods, if any foods caused or worsened symptoms, reported dietary restrictions, by whom (child, parent or doctor) and for what reason; food aversions and what they were, and; if a clinical GI diagnosis had ever been given. The assessment was for both current (within the past three months) and previous experiences. The GISS consisted of 7 sections, each section had 1 to 6 questions to determine if the participant met the criteria for constipation, diarrhea or irritable bowel syndrome (IBS) (see (Chaidez et al., 2014) and (Rose et al., 2018) for more information about the GIH and GISS).
Participants who met the criteria for constipation, diarrhea or IBS on the GI history and symptom surveys were placed in their corresponding GI group), those that did not meet criteria for irregular bowel movements, GI symptoms and had consistent stooling patterns for the past 6 months were placed in the corresponding no GI group. Participants who did not meet the criteria for constipation, diarrhea or IBS, but had inconsistent stooling patterns during the last 6 months were excluded from this study.
This study was approved by institutional review boards for the State of California and the University of California, Davis. Both written and informed consent was obtained from a legal guardian for all study participants prior to data collection in accordance with the UC Davis IRB protocol.
2.3. Blood collection and cellular assays
Peripheral blood was collected from each participant in acid-citrate dextrose Vacutainers (BD Biosciences; San Jose, Ca). Each tube was centrifuged at 2100 rpm for 10 min, plasma was removed and the remaining blood components were layered onto lymphocyte separation medium (Corning; Manassas, VA), and centrifuged at 1700 rpm for 30 min. PBMC from the buffy layer were collected and washed with Hanks balanced salt solution (Corning; Manassas, VA). After isolation, PBMC were allowed to rest overnight in complete media (RPMI 1640 (Invitrogen; Carlsbad, CA) with 10% Fetal Bovine Serum (FBS) (Corning; Manassas, VA), 100 IU/ml penicillin (Invitrogen; Carlsbad, CA) and 100 IU/ml streptomycin (Invitrogen; Carlsbad, CA)). PMBC were then divided and plated in complete media containing 10.6 μM Brefeldin A and 2 μM Monesin (protein transport inhibitor cocktail (ebioscience; San Diego,CA)) or in complete media containing 10.6 μM Brefeldin A, 2 μM Monesin, 81 nM Phorbol 12-Myristate 13-Acetate (PMA) and 1.34 μM Ionomycin (Cell Stimulation Cocktail (plus protein transport inhibitors) (ebioscience; San Diego, CA)) for 4 h. Following stimulation, PBMC were washed with PBS (Corning; Manassas, VA), 1 × 106 cells per well were plated for each condition and stained with 100 μL of Live/dead amine dye (LIVE/DEAD Fixable Aqua Dead Cell Strain Kit, for 405 nm excitation (Invitrogen; Carlsbad, CA) for 20 min in the dark. PBMC were washed with PBS, then reconstituted in 100 μL of PBMC wash (PBS (Corning), FBS (Corning), sodium azide (Sigma-Aldrich; St. Louis, MO)) containing 10% FcR block (Miltenyi Biotec; San Diego, CA). Cells were incubated in the dark for 10 min at 4 °C, antibody cocktails (see below) were added and cells were incubated in the dark for another 30 min at 4 °C. Following staining, cells were washed 3 times with PBMC wash and resuspended in 100 μL of 1x Fix/Perm (BD Cytofix/Cytoperm solution; BD Bioscience, San Jose, CA)) and incubated in the dark for 20 min at 4 °C. Cells were washed twice with 1x perm wash buffer (BD Perm/Wash buffer (BD bioscience)) and resuspended in 50 μL of perm wash buffer and intracellular antibody cocktail, followed by a 30 min incubation in the dark at room temperature. Cells were subsequently washed twice with 1x Perm wash buffer then resuspended in 100 μL 1% paraformaldehyde (Sigma-Aldrich). Cells were stored in the dark at 4 °C until analysis. Flow cytometric acquisition was performed on a LSR II flow cytometer (BD Biosciences) using FACSDiva software (BD Biosciences) and 100,000 acquired events were captured for each staining tube. Flow cytometry data was analyzed with Flowjo software (Tree Star, Inc; Ashland, OR). Lymphocytes were gated using forward scatter and side scatter parameters. Dead cells were excluded and live cells were gated for presence of CD3 (T cells).
2.3.1. Antibodies
Antibodies were purchased from BioLegend: anti-human CD3 (clone UCHT1)-brilliant Violet 421; anti-human integrin β7 (clone FIB27); anti-human FoxP3 (clone 206D)-PE. The following antibodies were purchased from eBioscience: anti-human CD8α (clone OKT8)-Alexa Fluor 700; anti-human IL-17A (clone eBio64DEC17)-FITC; anti-human IL-13 (clone 85BRD)-FITC; anti-human CD25 (clone BC96)-Alexa Fluor 488; anti-human IL-10 (clone JES3-9D7)-PE. Anti-human IFNγ (clone B27)-PE was purchased from BD Bioscience.
2.4. Statistical analysis
One-way ANOVA were used to determine statistical significance of cell population frequencies. Multiple comparisons were corrected for using Tukey’s multiple comparison test; the adjusted probability (p)-values were reported, except where noted, p-values < 0.05 were considered statistically significant. Outliers were removed in Prism using ROUT with the standard of Q = 1%. Behavioral data (ABC subscales) correlations to cell frequencies were analyzed utilizing using Spearman’s correlation, p-values < 0.05 were considered statistically significant.
3. Results
PBMC were cultured with either a stimulation cocktail or a control cocktail for 4 h prior to staining. Cell surface markers and intracellular cytokines or transcription markers were assessed to identify and characterize T cell populations. We did not find any significant differences between live/dead cell populations among groups, nor did we observe any differences among the frequencies of total T cell (CD3+) population, CD4 T cell population, or CD8 T cell sub-population. The frequencies of α4β7 (as assessed by β7+) T cell also did not differ between groups.
3.1. Intracellular cytokine profiles
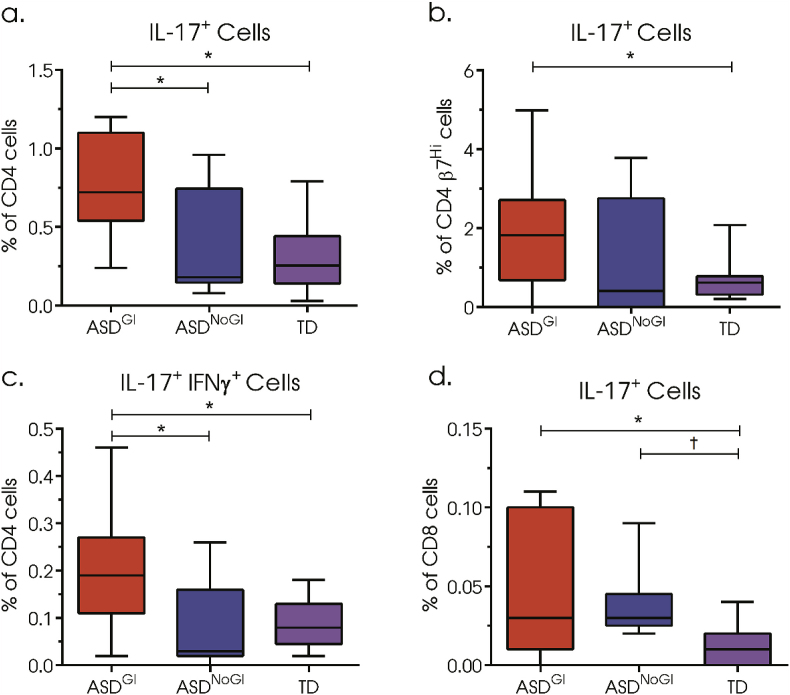
In children with ASD who experience GI symptoms (ASDGI), there was over a 2-fold increased frequency of IL-17+ CD4 T cells (mean: 0.757%, Standard Deviation: (0.313%)) (Fig. 1a) compared to both children with ASDNoGI (0.39% (0.34%); adjusted p-value = 0.0134) or to TD children (0.297% (0.197%): p = 0.0004). In cells that were β7Hi, elevated frequencies of IL-17+ CD4 T cells were also observed (Fig. 1b) in ASDGI (1.85% (1.42%)) compared to TD (0.70% (0.52%) p = 0.0384). In ASDGI there were also elevated populations of IL-17.1 CD4+ T cells that were double positive for both IL-17 and IFNγ (0.20% (0.12%)) (Fig. 1c) compared to both ASDNoGI (0.09% (0.09%); p = 0.0162) and TD (0.09% (0.05%); p = 0.0062). In addition to elevated IL-17+CD4+ populations, we observed increased IL-17+ CD8 T cell populations in ASDGI (0.05% (0.04%)) compared to TD (0.01% (0.01%); p = 0.0040). ASDNoGI (0.04% (0.02%)) CD8+ IL-17+ cells were similarly elevated compared to TD, however, significance was lost after correcting for multiple comparisons (adjusted p-value = 0.0632, uncorrected p-value = 0.0251) (Fig. 1d).
Fig. 1.
Frequency of cytokine positive CD4 T cells after 4h stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin and the protein transport inhibitor, brefeldin A. (a) Frequency of IL-17 positive CD4 T cells, (b) frequency of IL-17 positive β7Hi CD4 T cells, (c) frequency of double-positive IL-17 and IFNγ CD4 T cells. (d) frequency of IL-17 positive CD8 T cells. Data depicted as box and whisker graphs. *denotes adjusted p-value < 0.05, † denotes uncorrected p-value < 0.05.
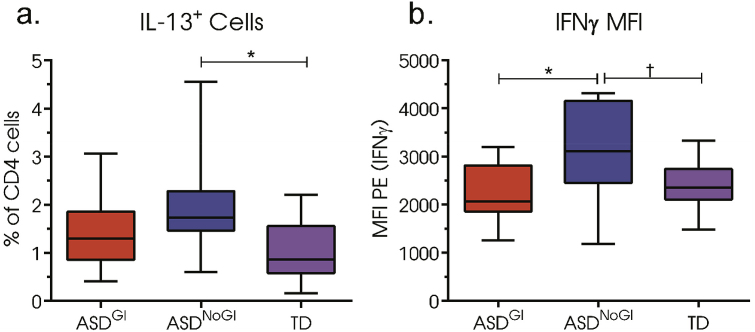
We observed a 2-fold increased frequency of IL-13+ CD4 T in children with ASDNoGI (2.02% (1.08%)) compared to TD (1.01% (0.58%); p = 0.0147) (Fig. 2a). The differences in frequency of IL-13+ CD4 T cells were not observed for ASDGI compared to TD (p = ns). Interestingly, no differences in the frequency of canonically-defined TH1 (CD4+IFNγ+) were identified between groups; however, we did observe an increase in median fluorescent intensity (MFI) for IFNγ for ASDNoGI (3112 (1017)) (Fig. 2b) compared to ASDGI (2241 (637); p = 0.0266) and there was a trend in TD that did not reach significance after correction (2398 (518); adjusted p = 0.0580; uncorrected p-value = 0.0229).
Fig. 2.
T cell frequencies and median fluorescent intensities (MFI) of CD4 T cells after 4 h stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin and the protein transport inhibitor, brefeldin A. (a) IL-13 positive CD4 T cells (b) MFI of phycoerythrin (PE) conjugated to IFNγ. Data depicted as box and whisker graphs. *denotes p-value < 0.05, † denotes uncorrected p-value < 0.05.
3.2. Regulatory T cells
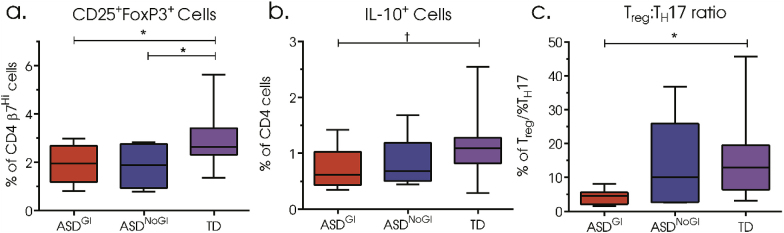
To determine the frequency of regulatory T cells we used CD25 (IL-2 receptor) and Forkhead P3 (FoxP3) transcription factor. These are thought to define one of the major subsets of T cells with regulatory function in humans; other cells with regulatory function do exist but were not assessed here. We found significantly decreased frequencies of β7hiCD25+FoxP3+ CD4 T cells in both ASDGI (1.93% (0.75%); p = 0.0473) and ASDNoGI (1.85% (0.89%); p = 0.0448) compared to TD (2.93% (1.16%)) (Fig. 3a). In addition, there were trends for decreased frequencies of IL-10+ CD4 T cells in children with ASDGI compared to TD, however, significance was lost after correcting for multiple comparisons (adjusted p-value = 0.0946; uncorrected p-value = 0.0386) (Fig. 3b). Considering the increased frequency of TH17 cells we identify in ASDGI we examined the ratio of Treg/TH17 (CD4+CD25+FoxP3+)/(CD4+IL-17+) populations among our groups. ASDGI had the lowest ratio of Treg to TH17 cells (4.28, 1.96) compared to TD (15.89, 12.63; p = 0.0230) (Fig. 3c).
Fig. 3.
Frequency of regulatory CD4 T cell populations after 4 h stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin and the protein transport inhibitor, brefeldin A. (a) Frequency of double-positive, CD25 and FoxP3, β7Hi expressing CD4 T cells, (b) frequency of IL-10 positive CD4 T cells, (c) ratio of CD25+Foxp3+ cells to CD4+IL-17+ cells. Data depicted as box and whisker graphs. *denotes p-value < 0.05, † denotes uncorrected p-value < 0.05.
3.3. Behavior correlations
Regulatory T cell populations were negatively correlated with the aberrant behavior checklist (ABC) subscale stereotypy (Spearman r = −0.336, p = 0.0072) suggesting the fewer the numbers of Tregs the worse the behavior. CD8+IL17+ cell populations positively correlated with increased stereotypy (0.245, p = 0.0454) (Table 1).
Table 1.
Aberrant behavior checklist (ABC) and cell population frequencies correlations. Spearman’s rho (r) value and p-values are listed for ABC subscale hyperactivity and stereotypy; p-values <0.05 are in bold.
| Aberrant behavior Checklist Correlations | ||||
|---|---|---|---|---|
| Cell Population | Hyperactivity |
Stereotypy |
||
| Spearman r | p value | Spearman r | p value | |
| CD4 T cell: β7HiCD25+ | −0.177 | 0.1528 | −0.336 | 0.0072 |
| CD4 T cell β7HiCD25+Foxp3+ | −0.036 | 0.7657 | −0.114 | 0.3549 |
| CD4 IL10+ | −0.149 | 0.1841 | −0.095 | 0.4037 |
| CD4 IL-13+ | 0.075 | 0.5112 | −0.090 | 0.4320 |
| CD8 IL17+ | 0.187 | 0.1214 | 0.245 | 0.0454 |
4. Discussion
In this study we provide evidence of altered immune populations in children with ASD who experience GI symptoms and in children with ASD without GI symptoms compared to typically-developing children. In the ASDGI group we found elevated frequencies of TH17 and TH17.1 populations, and coupled to this were decreased populations of IL-10 producing CD4 T cells, reductions in regulatory T cells, and decreased ratios of regulatory T cells compared to inflammatory TH17 subsets. Considering our previous findings of increased mucosa-related cytokines and decreased regulatory cytokines, mainly active TGFβ1, in children with ASD who experience GI symptoms (Rose et al., 2018), these data are suggestive of impairments in immune regulation. Alterations in the ASDNoGI group included elevated populations of IL-13+ CD4 T cells that could represent a shift to a TH2 response. However, an increase in median fluorescent intensity for PE-IFNγ in ASDNoGI children may suggest activation could be different within this group compared to children with ASDGI, or that it is more heterogeneous.
The main aim of this study was to identify differences in inflammatory and regulatory T cell populations in ASD with and without GI symptoms compared to typically-developing children. Children with ASD and GI symptoms displayed the largest number of differences in cell frequencies and our major finding of increased populations of IL-17+ T cells. TH17 cells play a central role in combating extracellular pathogens, particularly in mucosal tissues (Kleinewietfeld and Hafler, 2013) where they orchestrate immunity to extracellular pathogens by producing cytokines that help to recruit, support, and promote neutrophils, monocytes and other lymphocytes (Kolls and Khader, 2010; Onishi and Gaffen, 2010; Peck and Mellins, 2010). The major cytokine mediators produced by TH17 cells include its eponymic cytokine, IL-17A, as well as IL-17F, IL-21 and IL-22 (Kolls and Khader, 2010; Onishi and Gaffen, 2010; Peck and Mellins, 2010). These cytokines, IL-17A in particular, act on epithelial, mesenchymal and immune cells to produce an array of inflammatory mediators including neutrophil chemoattractants (CXCL1 CXCL2, CXCL5, CXCL8), monocyte chemoattractants and growth factors (GM-CSF, CCL1, CCL2, CCL20), antimicrobial peptides, and acute phase proteins, among others (Kolls and Khader, 2010; Onishi and Gaffen, 2010; Peck and Mellins, 2010). While these inflammatory mediators help to maintain an effective defense against pathogens, disruption in regulation can lead to excessive inflammation and the TH17 pathway has been implicated in many autoimmune disorders including those that affect the GI such as Crohn’s disease and ulcerative colitis (UC) (Onishi and Gaffen, 2010; Galvez, 2014); as such, balance between TH17 and regulatory T cell (Treg) responses are particularly crucial for maintaining equilibrium between protection against pathogens and tolerating commensal microbes (Omenetti and Pizarro, 2015).
Studies regarding IL-17 producing cells and IL-17 related cytokines in ASD have reported mixed findings. While some studies report elevated levels of IL-17 in serum/plasma (Suzuki et al., 2011; Al-Ayadhi and Mostafa, 2012), others have found no differences in circulating IL-17 or by production of stimulated PBMC (Enstrom et al., 2008; Onore et al., 2009; Jyonouchi et al., 2012). A handful of studies have begun to address frequencies of IL-17 producing cells, particularly CD4+ helper T cells. Ahmad et al. reported elevated CD4+RORγT+, CD4+ GATA-3+, and CD4+T-bet+ cells while simultaneously finding decreased CD4+FoxP3+ populations (Ahmad et al., 2017). A more recent study also reported elevated TH17 cells along with increased production of IL-17 but decreased Treg populations and related cytokine production of IL-10 and TGF-β (Moaaz et al., 2019). Another study examined frequencies of TH1, TH2, and TH17 populations and while they did not find differences in total TH17 frequencies they did report elevated frequencies of activated TH17 cells, defined as CD3+CD4+CXCR3−CCR6+HLA-DR+CD38+ cells in ASD compared to controls (Basheer et al., 2018). One explanation for the discrepancies concerning IL-17 in ASD may involve the heterogeneity of ASD and the co-morbidities that may accompany individuals with ASD. A 2017 study examining a non-overlapping cohort of the CHARGE study reported elevated production of IL-17 by phytohemagglutinin-stimulated PBMC in ASD compared to TD. Furthermore, when ASD and TD groups where further stratified by a diagnosis of asthma, ASD with asthma produced more IL-17 than any other group, including the TD plus asthma group (Akintunde et al., 2015). This suggest that some groups may find elevated TH17 cells and IL-17 when the study population is knowingly or unknowingly enriched with co-morbidities that involve TH17 pathways, such as asthma, autoimmunity, or GI related symptoms, such as we report in the current study. Along these same lines we previously reported finding no differences in TH17 populations between ASD and TD in an earlier cohort of the CHARGE study (Onore et al., 2009), which was not enriched for any particular co-morbidities but contained more children with autism that had an early onset of symptoms vs. those that undergo regression of autism.
Given the importance of balance between TH17 and Treg populations, it is noteworthy to mention that in addition to increased TH17 populations found in children with ASDGI, we also found evidence of decreased Treg populations. Specifically, we found decreased frequencies of IL-10+ CD4 T cells and β7+CD25HiFoxP3+ CD4 T cells, suggesting that both peripheral and gut related Treg populations may be reduced in this group. Our previous study also found lower production of active TGFβ1 from PBMC from ASDGI which supports the idea that immune regulation is impaired in ASDGI (Rose et al., 2018). The stability of Treg is complex and likely varies based on a number of factors including type of regulatory T cell. There are 2 main subsets of Treg: natural Treg (nTreg) derived from the thymus and induced Treg (iTreg) that are induced in the periphery (Lin et al., 2013). While nTreg appear to be very stable, iTreg may be more plastic and may possess the ability to become inflammatory under certain conditions (Kleinewietfeld and Hafler, 2013). Stability of Foxp3 is in part managed by epigenetic regulation and is essential for Treg stability; in particular, demethylation of the Treg-specific demethylated region (TSDR) at FOXP3 is required for differentiation and results in FOXP3 expression and the suppressive function of Treg (Fontenot et al., 2003; Floess et al., 2007; Toker et al., 2013; Feng et al., 2014). The lack of appropriate demethylation or the gain of methylation at the FOXP3 TSDR triggers Treg instability, allowing for loss of Treg suppressive functions and gain of effector T cell functions, such as IL-17 production (Hoffmann et al., 2009; Wang et al., 2013). Future studies are needed to further explore the plasticity and stability of Treg in ASD, and how the microbiota and other environmental influences contribute.
Plasticity of TH17 is not only limited to TH17/Treg relationships, reports of TH17 cells gaining a TH1-like phenotype have also been described (Harrington et al., 2006). While some TH17 cells that gain TH1-like features and secrete IFNγ retain expression of IL-17, generally they lose the ability to produce IL-17 and irreversibly transdifferentiate into a TH1-like cell (Lee et al., 2009; Omenetti and Pizarro, 2015). TH17 cells that transdifferentiate have been termed ex-TH17 cells (Hirota et al., 2011). Stability of TH17 cells is dependent on environmental signals, importantly, TGFβ is required; in the absence of TGFβ and with exposure to IL-12, TH17 cells can also express IFNγ (Kleinewietfeld and Hafler, 2013). Like Treg, TH17 stability is also linked to its epigenetic status, which is controlled by both histone and DNA methylation regulation. The TH1 transcription factor gene promoter, tbx21, has a bivalent status in TH17 cells characterized by dual-positive status of both H3K4me3 and H3K27me3 leading to TH17 instability and the inclination of TH17 cells to obtain a TH1-like phenotype after exposure to IL-12 (Wei et al., 2009). Functionally, TH1-like, ex-TH17 cells have been characterized as highly pathogenic, produce more inflammatory cytokines, have a higher proliferative capacity than TH17 or TH1 cells, (Basdeo et al., 2017; Gartlan et al., 2017) and have been reported to be elevated in rodent models of autoimmune disorders. In experimental autoimmune encephalomyelitis (EAE), fate-mapping experiments showed that the majority of myelin-specific CD4 T cells in the spinal cord were ex-TH17 cells (Hirota et al., 2011). Our finding of elevated IL-17+IFNγ+ CD4 T cell in children with ASD who experience GI symptoms, lends further evidence to the disruption of Treg/TH17 balance in ASDGI and is a commonality shared with patients with IBD and other autoimmune disorders in which increased populations of IL-17+IFNγ+ CD4 T cells have also been identified (Annunziato et al., 2007; Galvez, 2014; Ueno et al., 2018), and in addition to being described as more inflammatory, they may also be more resistant to suppression by Tregs, at least in Crohn’s disease (Annunziato et al., 2007). While we know these cells play a role in disease pathogenesis, it is currently unknown if these cells have a role in heathy individuals. One explanation for the flexibility for TH17 cells to easily transdifferentiate into a TH1-like phenotype is to enable a quick shift from combating extracellular pathogens to intracellular pathogens (Zhou et al., 2009).
As well as a change in the balance of immune activation in ASDGI we also noted decreased regulation in ASDNoGI. In this context, IL-13+ CD4 T cells but not IL-17 cells were increased in ASDNoGI. These data may suggest that both ASDGI and ASDNoGI have decreased regulation and increased inflammatory responses but that inflammatory signals may be different and lead to different co-morbidities. IL-4, IL-5 and IL-13 are the classical cytokines associated with TH2 responses (Nakayama et al., 2017) and are the major cytokines involved with mediating type 2 responses. TH2 cells are important for humoral responses, protection against extracellular parasites and are drivers of inflammation in asthma and atopy (Paul and Zhu, 2010; Nakayama et al., 2017). ASD are often accompanied with co-morbidities, including an increased proclivity towards allergies and asthma (Mostafa et al., 2008; Sacco et al., 2012; Chen et al., 2013; Kotey et al., 2014; Zerbo et al., 2015a). Epidemiology studies have found associations between maternal asthma and increased risk for developing a neurodevelopmental disorder (Croen et al., 2005; Leonard et al., 2006; May-Benson et al., 2009; Langridge et al., 2013; Lyall et al., 2014; Instanes et al., 2015; Gidaya et al., 2016; Theoharides et al., 2016), furthermore, findings of elevated cytokines associated with a TH2 response have also been reported from mid-gestational maternal sera, amniotic fluid, and in neonatal blood spots from children who later developed ASD (Goines et al., 2011b; Abdallah et al., 2013a; Krakowiak et al., 2015). Interestingly, studies in mice lacking T cells were shown to have impairments in cognitive function (Kipnis et al., 2004; Brynskikh et al., 2008), and in particular, IL-4 producing T cells in the meninges were shown to be important in learning and memory (Derecki et al., 2010). Mice who had undergone Morris water training had an increase in IL-4 producing meningeal T cells, moreover, mice who were deficient in IL-4 (IL-4−/− mice) displayed learning defects that could be restored upon adoptive transfer of T cells from wildtype mice (Derecki et al., 2010). The impact of IL-4 on neurodevelopment is likely complex, dependent on concentration, stage of development and region.
4.1. Study limitations
Our study does have several limitations, mostly revolving around small sample sizes which restricted how we could stratify our study population. In the future we would like to further stratify the GI populations based on specific symptoms (constipation vs. diarrhea vs. IBS). A fourth group of typically-developing children with GI symptoms of irregular bowel movements (TDGI) were also recruited but due to a statistically lower median of age for the group TDGI as well as low recruitment numbers (n = 5) we were not able to include this group in statistical analysis. Furthermore, our population age range was wide and included children 3–12 years of age, larger sample sizes would have made it possible to compare data across age. Lastly, the smaller population size made it difficult to study gender differences as our study is primarily composed of males, which is consistent with the gender ratio of ASD diagnosis, however, resulted in too few females to perform statistical analysis. In order to analyze cytokine profiles of T cells, the cells first required stimulation, for this we choose to use a standard stimulant and timeframe to stimulate with (PMA/Ionomycin for 4 h), however, it is possible that other routes of activation may reveal variation in results or that a longer stimulation may show more dramatic results. IL-10 and IL-17, for example, take longer to reach peak cytokine production. Despite these limitations we feel that this study provides valuable insights on immune regulation in children with ASD and GI symptoms.
5. Conclusions
Considering the heterogeneity of ASD and the varying types of immune dysfunction reported in ASD, we sought to investigate differences in T cell profiles in children with ASD who experience GI symptoms compared to ASD without GI symptoms. We were able to find differences in dominant T cell lineages based on whether or not GI symptoms were present. One commonality among our ASD groups compared to the TD group was evidence of decreased immune regulation; both ASDGI and ASDNoGI displayed lower frequencies of B7+CD25+FoxP3+ CD4 T cells. Moreover, this decrease in regulation was accompanied by an increase in differential inflammatory T cell lineages; for ASDGI, this was elevated TH17 populations and for ASDNoGI, increased TH2 populations. Elevated TH17 populations have frequently been identified in autoimmune and GI disorders and may provide a target for future treatments that may help alleviate GI inflammation. The role TH2 cells play in ASD is currently unknown and should be investigated further.
Funding
This study was supported by the Autism Speaks Foundation [Grant #7567], The National Institutes of Health [grant #R21HD086669, R01MH118209, R01HD090214, R01ES015359, U54HD079125]; NARSAD Foundation, Michael and Barbara Bass Foundation, the Jane Botsford Johnson Foundation, Jonty Foundation and the Peter Emch Foundation.
Declaration of competing interest
There are no conflicts of interest for any of the authors.
Acknowledgements
We would like to thank the participants and their families for their participation in the study and the staff of both the University of California Davis M.I.N.D. Institute and the CHARGE project for their technical support.
References
- Abdallah M.W., Larsen N., Grove J., Norgaard-Pedersen B., Thorsen P., Mortensen E.L., Hougaard D.M. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. World J. Biol. Psychiatr. 2013;14(7):528–538. doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- Abdallah M.W., Larsen N., Mortensen E.L., Atladottir H.O., Norgaard-Pedersen B., Bonefeld-Jorgensen E.C., Grove J., Hougaard D.M. Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J. Neuroimmunol. 2012;252(1–2):75–82. doi: 10.1016/j.jneuroim.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Abdallah M.W., Mortensen E.L., Greaves-Lord K., Larsen N., Bonefeld-Jorgensen E.C., Norgaard-Pedersen B., Hougaard D.M., Grove J. Neonatal levels of neurotrophic factors and risk of autism spectrum disorders. Acta Psychiatr. Scand. 2013;128(1):61–69. doi: 10.1111/acps.12020. [DOI] [PubMed] [Google Scholar]
- Ahmad S.F., Zoheir K.M.A., Ansari M.A., Nadeem A., Bakheet S.A., Al-Ayadhi L.Y., Alzahrani M.Z., Al-Shabanah O.A., Al-Harbi M.M., Attia S.M. Dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in children with autism. Mol. Neurobiol. 2017;54(6):4390–4400. doi: 10.1007/s12035-016-9977-0. [DOI] [PubMed] [Google Scholar]
- Akintunde M.E., Rose M., Krakowiak P., Heuer L., Ashwood P., Hansen R., Hertz-Picciotto I., Van de Water J. Increased production of IL-17 in children with autism spectrum disorders and co-morbid asthma. J. Neuroimmunol. 2015;286:33–41. doi: 10.1016/j.jneuroim.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ayadhi L.Y., Mostafa G.A. Elevated serum levels of interleukin-17A in children with autism. J. Neuroinflammation. 2012;9:158. doi: 10.1186/1742-2094-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ayadhi L.Y., Mostafa G.A. Elevated serum levels of macrophage-derived chemokine and thymus and activation-regulated chemokine in autistic children. J. Neuroinflammation. 2013;10:72. doi: 10.1186/1742-2094-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F., Giudici F., Romagnani P., Parronchi P., Tonelli F., Maggi E., Romagnani S. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P. Differential T cell levels of tumor necrosis factor receptor-II in children with autism. Front. Psychiatr. 2018;9(543) doi: 10.3389/fpsyt.2018.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P., Anthony A., Torrente F., Wakefield A.J. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J. Clin. Immunol. 2004;24(6):664–673. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- Ashwood P., Corbett B.A., Kantor A., Schulman H., Van de Water J., Amaral D.G. In search of cellular immunophenotypes in the blood of children with autism. PloS One. 2011;6(5) doi: 10.1371/journal.pone.0019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P., Enstrom A., Krakowiak P., Hertz-Picciotto I., Hansen R.L., Croen L.A., Ozonoff S., Pessah I.N., Van de Water J. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J. Neuroimmunol. 2008;204(1–2):149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I.N., Van de Water J. Altered T cell responses in children with autism. Brain Behav. Immun. 2011;25(5):840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I.N., Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2011;232(1–2):196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A.P. American Psychiatric Publishing; Washington, D.C.: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- Basdeo S.A., Cluxton D., Sulaimani J., Moran B., Canavan M., Orr C., Veale D.J., Fearon U., Fletcher J.M. Ex-Th17 (nonclassical Th1) cells are functionally distinct from classical Th1 and Th17 cells and are not constrained by regulatory T cells. J. Immunol. 2017;198(6):2249–2259. doi: 10.4049/jimmunol.1600737. [DOI] [PubMed] [Google Scholar]
- Basheer S., Venkataswamy M.M., Christopher R., Van Amelsvoort T., Srinath S., Girimaji S.C., Ravi V. Immune aberrations in children with Autism Spectrum Disorder: a case-control study from a tertiary care neuropsychiatric hospital in India. Psychoneuroendocrinology. 2018;94:162–167. doi: 10.1016/j.psyneuen.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Bauman M.L. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. 2010;7(3):320–327. doi: 10.1016/j.nurt.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D., Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Croen L.A., Pessah I.N., Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29(2):226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynskikh A., Warren T., Zhu J., Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav. Immun. 2008;22(6):861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Buie T., Campbell D.B., Fuchs G.J., 3rd, Furuta G.T., Levy J., Vandewater J., Whitaker A.H., Atkins D., Bauman M.L., Beaudet A.L., Carr E.G., Gershon M.D., Hyman S.L., Jirapinyo P., Jyonouchi H., Kooros K., Kushak R., Levitt P., Levy S.E., Lewis J.D., Murray K.F., Natowicz M.R., Sabra A., Wershil B.K., Weston S.C., Zeltzer L., Winter H. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl. 1):S1–S18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Cabanlit M., Wills S., Goines P., Ashwood P., Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann. N. Y. Acad. Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Careaga M., Rogers S., Hansen R.L., Amaral D.G., Van de Water J., Ashwood P. Immune endophenotypes in children with autism spectrum disorder. Biol. Psychiatr. 2017 doi: 10.1016/j.biopsych.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaidez V., Hansen R.L., Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 2014;44(5):1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.-H., Su T.-P., Chen Y.-S., Hsu J.-W., Huang K.-L., Chang W.-H., Chen T.-J., Bai Y.-M. Comorbidity of allergic and autoimmune diseases in patients with autism spectrum disorder: a nationwide population-based study. Res. Autism Spectr. Disord. 2013;7(2):205–212. doi: 10.1016/j.rasd.2012.08.008. [DOI] [Google Scholar]
- Coury D.L., Ashwood P., Fasano A., Fuchs G., Geraghty M., Kaul A., Mawe G., Patterson P., Jones N.E. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics. 2012;130(Suppl. 2):S160–S168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- Critchfield J.W., van Hemert S., Ash M., Mulder L., Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol. Res. Pract. 2011. 2011:161358. doi: 10.1155/2011/161358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen L.A., Grether J.K., Yoshida C.K., Odouli R., Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch. Pediatr. Adolesc. Med. 2005;159(2):151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J., Bosmans E., Deboutte D., Kenis G., Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45(1):1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J., Wauters A., Devreese K., Verkerk R., Scharpe S., Bosmans E., Egyed B., Deboutte D., Maes M. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol. Med. 2002;32(8):1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- Derecki N.C., Cardani A.N., Yang C.H., Quinnies K.M., Crihfield A., Lynch K.R., Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010;207(5):1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom A., Krakowiak P., Onore C., Pessah I.N., Hertz-Picciotto I., Hansen R.L., Van de Water J.A., Ashwood P. Increased IgG4 levels in children with autism disorder. Brain Behav. Immun. 2009;23(3):389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom A., Onore C., Hertz-Picciotto I., Hansen R., Croen L., Van De Water J., Ashwood P. Detection of IL-17 and IL-23 in plasma samples of children with autism. Am. J. Biochem. Biotechnol. 2008;2(2):7. doi: 10.3844/ajbbsp.2008.114.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom A.M., Lit L., Onore C.E., Gregg J.P., Hansen R.L., Pessah I.N., Hertz-Picciotto I., Van de Water J.A., Sharp F.R., Ashwood P. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav. Immun. 2009;23(1):124–133. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom A.M., Onore C.E., Van de Water J.A., Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav. Immun. 2010;24(1):64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Arvey A., Chinen T., van der Veeken J., Gasteiger G., Rudensky A.Y. Control of the inheritance of regulatory T cell identity by a cis element in the Foxp3 locus. Cell. 2014;158(4):749–763. doi: 10.1016/j.cell.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floess S., Freyer J., Siewert C., Baron U., Olek S., Polansky J., Schlawe K., Chang H.D., Bopp T., Schmitt E., Klein-Hessling S., Serfling E., Hamann A., Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5(2):e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Frye R.E., Delhey L., Slattery J., Tippett M., Wynne R., Rose S., Kahler S.G., Bennuri S.C., Melnyk S., Sequeira J.M., Quadros E. Blocking and binding folate receptor alpha autoantibodies identify novel autism spectrum disorder subgroups. Front. Neurosci. 2016;10:80. doi: 10.3389/fnins.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm. 2014;2014:928461. doi: 10.1155/2014/928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartlan K.H., Varelias A., Koyama M., Robb R.J., Markey K.A., Chang K., Wilkinson A.N., Smith D., Ullah M.A., Kuns R.D., Raffelt N.C., Olver S.D., Lineburg K.E., Teal B.E., Cheong M., Teng M.W.L., Smyth M.J., Tey S.K., MacDonald K.P.A., Hill G.R. Th17 plasticity and transition toward a pathogenic cytokine signature are regulated by cyclosporine after allogeneic SCT. Blood Adv. 2017;1(6):341–351. doi: 10.1182/bloodadvances.2016002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidaya N.B., Lee B.K., Burstyn I., Michael Y., Newschaffer C.J., Mortensen E.L. In utero exposure to β-2-adrenergic receptor agonist drugs and risk for autism spectrum disorders. Pediatrics: peds. 2016;137(2) doi: 10.1542/peds.2015-1316. e20151316. [DOI] [PubMed] [Google Scholar]
- Goines P., Haapanen L., Boyce R., Duncanson P., Braunschweig D., Delwiche L., Hansen R., Hertz-Picciotto I., Ashwood P., Van de Water J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav. Immun. 2011;25(3):514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P.E., Croen L.A., Braunschweig D., Yoshida C.K., Grether J., Hansen R., Kharrazi M., Ashwood P., Van de Water J. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol. Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Aggarwal S., Rashanravan B., Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J. Neuroimmunol. 1998;85(1):106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- Harrington L.E., Mangan P.R., Weaver C.T. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 2006;18(3):349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I., Croen L.A., Hansen R., Jones C.R., van de Water J., Pessah I.N. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ. Health Perspect. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer L., Ashwood P., Schauer J., Goines P., Krakowiak P., Hertz-Picciotto I., Hansen R., Croen L.A., Pessah I.N., Van de Water J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1(5):275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K., Duarte J.H., Veldhoen M., Hornsby E., Li Y., Cua D.J., Ahlfors H., Wilhelm C., Tolaini M., Menzel U., Garefalaki A., Potocnik A.J., Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011;12(3):255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P., Boeld T.J., Eder R., Huehn J., Floess S., Wieczorek G., Olek S., Dietmaier W., Andreesen R., Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 2009;39(4):1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- Horvath K., Perman J.A. Autism and gastrointestinal symptoms. Curr. Gastroenterol. Rep. 2002;4(3):251–258. doi: 10.1007/s11894-002-0071-6. [DOI] [PubMed] [Google Scholar]
- Instanes J.T., Halmøy A., Engeland A., Haavik J., Furu K., Klungsøyr K. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol. Psychiatr. 2015;81(5):452–459. doi: 10.1016/j.biopsych.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H., Geng L., Davidow A.L. Cytokine profiles by peripheral blood monocytes are associated with changes in behavioral symptoms following immune insults in a subset of ASD subjects: an inflammatory subtype? J. Neuroinflammation. 2014;11:187. doi: 10.1186/s12974-014-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H., Geng L., Ruby A., Zimmerman-Bier B. Dysregulated innate immune responses in young children with autism spectrum disorders: their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology. 2005;51(2):77–85. doi: 10.1159/000084164. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H., Geng L., Streck D.L., Toruner G.A. Immunological characterization and transcription profiling of peripheral blood (PB) monocytes in children with autism spectrum disorders (ASD) and specific polysaccharide antibody deficiency (SPAD): case study. J. Neuroinflammation. 2012;9:4. doi: 10.1186/1742-2094-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H., Sun S., Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J. Neuroimmunol. 2001;120(1–2):170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nerv. Child. 1943;2:33. [PubMed] [Google Scholar]
- Kipnis J., Cohen H., Cardon M., Ziv Y., Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl. Acad. Sci. U. S. A. 2004;101(21):8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M., Hafler D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013;25(4):305–312. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane I.S., McMurry A., Weber G., MacFadden D., Rappaport L., Kunkel L., Bickel J., Wattanasin N., Spence S., Murphy S., Churchill S. The Co-morbidity burden of children and young adults with autism spectrum disorders. PloS One. 2012;7(4) doi: 10.1371/journal.pone.0033224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls J.K., Khader S.A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21(6):443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotey S., Ertel K., Whitcomb B. Co-occurrence of autism and asthma in a nationally-representative sample of children in the United States. J. Autism Dev. Disord. 2014;44(12):3083–3088. doi: 10.1007/s10803-014-2174-y. [DOI] [PubMed] [Google Scholar]
- Krakowiak P., Goines P.E., Tancredi D.J., Ashwood P., Hansen R.L., Hertz-Picciotto I., Van de Water J. Neonatal cytokine profiles associated with autism spectrum disorder. Biol. Psychiatr. 2015 doi: 10.1016/j.biopsych.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P., Goines P.E., Tancredi D.J., Ashwood P., Hansen R.L., Hertz-Picciotto I., Van de Water J. Neonatal cytokine profiles associated with autism spectrum disorder. Biol. Psychiatr. 2017;81(5):442–451. doi: 10.1016/j.biopsych.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge A.T., Glasson E.J., Nassar N., Jacoby P., Pennell C., Hagan R., Bourke J., Leonard H., Stanley F.J. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PloS One. 2013;8(1) doi: 10.1371/journal.pone.0050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard H., de Klerk N., Bourke J., Bower C. Maternal health in pregnancy and intellectual disability in the offspring: a population-based study. Ann. Epidemiol. 2006;16(6):448–454. doi: 10.1016/j.annepidem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Levisohn P.M. The autism-epilepsy connection. Epilepsia. 2007;48(Suppl. 9):33–35. doi: 10.1111/j.1528-1167.2007.01399.x. [DOI] [PubMed] [Google Scholar]
- Lin X., Chen M., Liu Y., Guo Z., He X., Brand D., Zheng S.G. Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int. J. Clin. Exp. Pathol. 2013;6(2):116–123. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Cacho J.M., Gallardo S., Posada M., Aguerri M., Calzada D., Mayayo T., Lahoz C., Cardaba B. Characterization of immune cell phenotypes in adults with autism spectrum disorders. J. Invest. Med. 2016;64(7):1179–1185. doi: 10.1136/jim-2016-000070. [DOI] [PubMed] [Google Scholar]
- Lyall K., Ashwood P., Van de Water J., Hertz-Picciotto I. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J. Autism Dev. Disord. 2014;44(7):1546–1555. doi: 10.1007/s10803-013-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner M.J., Arneson C.L., Levy S.E., Kirby R.S., Nicholas J.S., Durkin M.S. Brief report: association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. J. Autism Dev. Disord. 2012;42(7):1520–1525. doi: 10.1007/s10803-011-1379-6. [DOI] [PubMed] [Google Scholar]
- Malik M., Sheikh A.M., Wen G., Spivack W., Brown W.T., Li X. Expression of inflammatory cytokines, Bcl2 and cathepsin D are altered in lymphoblasts of autistic subjects. Immunobiology. 2011;216(1–2):80–85. doi: 10.1016/j.imbio.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Mannion A., Leader G. An investigation of comorbid psychological disorders, sleep problems, gastrointestinal symptoms and epilepsy in children and adolescents with autism spectrum disorder: a two year follow-up. Res. Autism Spectr. Disord. 2016;22:20–33. doi: 10.1016/j.rasd.2015.11.002. [DOI] [Google Scholar]
- Manzardo A.M., Henkhaus R., Dhillon S., Butler M.G. Plasma cytokine levels in children with autistic disorder and unrelated siblings. Int. J. Dev. Neurosci. 2012;30(2):121–127. doi: 10.1016/j.ijdevneu.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Benson T.A., Koomar J., Teasdale A. Incidence of pre-, peri-, and post-natal birth and developmental problems of children with sensory processing disorder and children with autism spectrum disorder. Front. Integr. Neurosci. 2009;3:31. doi: 10.3389/neuro.07.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaaz M., Youssry S., Elfatatry A., El Rahman M.A. Th17/Treg cells imbalance and their related cytokines (IL-17, IL-10 and TGF-beta) in children with autism spectrum disorder. J. Neuroimmunol. 2019;337:577071. doi: 10.1016/j.jneuroim.2019.577071. [DOI] [PubMed] [Google Scholar]
- Molloy C.A., Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003;7(2):165–171. doi: 10.1177/1362361303007002004. [DOI] [PubMed] [Google Scholar]
- Molloy C.A., Morrow A.L., Meinzen-Derr J., Dawson G., Bernier R., Dunn M., Hyman S.L., McMahon W.M., Goudie-Nice J., Hepburn S., Minshew N., Rogers S., Sigman M., Spence M.A., Tager-Flusberg H., Volkmar F.R., Lord C. Familial autoimmune thyroid disease as a risk factor for regression in children with Autism Spectrum Disorder: a CPEA Study. J. Autism Dev. Disord. 2006;36(3):317–324. doi: 10.1007/s10803-005-0071-0. [DOI] [PubMed] [Google Scholar]
- Mostafa G.A., Al Shehab A., Fouad N.R. Frequency of CD4+CD25high regulatory T cells in the peripheral blood of Egyptian children with autism. J. Child Neurol. 2010;25(3):328–335. doi: 10.1177/0883073809339393. [DOI] [PubMed] [Google Scholar]
- Mostafa G.A., Hamza R.T., El-Shahawi H.H. Allergic manifestations in autistic children: relation to disease severity. J. Pediatr. Neurol. 2008;6(2):115–123. [Google Scholar]
- Nakayama T., Hirahara K., Onodera A., Endo Y., Hosokawa H., Shinoda K., Tumes D.J., Okamoto Y. Th2 cells in health and disease. Annu. Rev. Immunol. 2017;35(1):53–84. doi: 10.1146/annurev-immunol-051116-052350. [DOI] [PubMed] [Google Scholar]
- Niehus R., Lord C. Early medical history of children with autism spectrum disorders. J. Dev. Behav. Pediatr. 2006;27(2 Suppl. l):S120–S127. doi: 10.1097/00004703-200604002-00010. [DOI] [PubMed] [Google Scholar]
- Okada K., Hashimoto K., Iwata Y., Nakamura K., Tsujii M., Tsuchiya K.J., Sekine Y., Suda S., Suzuki K., Sugihara G., Matsuzaki H., Sugiyama T., Kawai M., Minabe Y., Takei N., Mori N. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2007;31(1):187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Omenetti S., Pizarro T.T. The Treg/Th17 Axis: a dynamic balance regulated by the gut microbiome. Front. Immunol. 2015;6(639) doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi R.M., Gaffen S.L. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129(3):311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C., Enstrom A., Krakowiak P., Hertz-Picciotto I., Hansen R., Van de Water J., Ashwood P. Decreased cellular IL-23 but not IL-17 production in children with autism spectrum disorders. J. Neuroimmunol. 2009;216(1–2):126–129. doi: 10.1016/j.jneuroim.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousley O., Cermak T. Autism spectrum disorder: defining dimensions and subgroups. Curr. Dev. Disord. Rep. 2014;1(1):20–28. doi: 10.1007/s40474-013-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W.E., Zhu J. How are TH2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010;10:225. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck A., Mellins E.D. Precarious balance: Th17 cells in host defense. Infect. Immun. 2010;78(1):32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci S., Businaro R., Ippoliti F., Lo Vasco V.R., Massoni F., Onofri E., Troili G.M., Pontecorvi V., Morelli M., Rapp Ricciardi M., Archer T. Altered cytokine and BDNF levels in autism spectrum disorder. Neurotox. Res. 2013;24(4):491–501. doi: 10.1007/s12640-013-9393-4. [DOI] [PubMed] [Google Scholar]
- Rose D., Ashwood P. Potential cytokine biomarkers in autism spectrum disorders. Biomarkers Med. 2014;8(9):1171–1181. doi: 10.2217/bmm.14.39. [DOI] [PubMed] [Google Scholar]
- Rose D., Ashwood P. Rapid communication: plasma interleukin-35 in children with autism. Brain Sci. 2019;9(7):152. doi: 10.3390/brainsci9070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D.R., Yang H., Serena G., Sturgeon C., Ma B., Careaga M., Hughes H.K., Angkustsiri K., Rose M., Hertz-Picciotto I., Van de Water J., Hansen R.L., Ravel J., Fasano A., Ashwood P. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav. Immun. 2018;70:354–368. doi: 10.1016/j.bbi.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco R., Lenti C., Saccani M., Curatolo P., Manzi B., Bravaccio C., Persico A.M. Cluster Analysis of autistic patients based on principal pathogenetic components. Autism Res. 2012;5(2):137–147. doi: 10.1002/aur.1226. [DOI] [PubMed] [Google Scholar]
- Singer H.S., Morris C.M., Williams P.N., Yoon D.Y., Hong J.J., Zimmerman A.W. Antibrain antibodies in children with autism and their unaffected siblings. J. Neuroimmunol. 2006;178(1–2):149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Singh V.K. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J. Neuroimmunol. 1996;66(1–2):143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Singh V.K., Singh E.A., Warren R.P. Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biol. Psychiatr. 1997;41(6):753–755. doi: 10.1016/S0006-3223(96)00522-7. [DOI] [PubMed] [Google Scholar]
- Singh V.K., Warren R.P., Odell J.D., Warren W.L., Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav. Immun. 1993;7(1):97–103. doi: 10.1006/brbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- Stubbs E.G., Crawford M.L. Depressed lymphocyte responsiveness in autistic children. J. Autism Child. Schizophr. 1977;7(1):49–55. doi: 10.1007/BF01531114. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Matsuzaki H., Iwata K., Kameno Y., Shimmura C., Kawai S., Yoshihara Y., Wakuda T., Takebayashi K., Takagai S., Matsumoto K., Tsuchiya K.J., Iwata Y., Nakamura K., Tsujii M., Sugiyama T., Mori N. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PloS One. 2011;6(5) doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeten T.L., Posey D.J., McDougle C.J. High blood monocyte counts and neopterin levels in children with autistic disorder. Am. J. Psychiatr. 2003;160(9):1691–1693. doi: 10.1176/appi.ajp.160.9.1691. [DOI] [PubMed] [Google Scholar]
- Theoharides T., Tsilioni I., Patel A., Doyle R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry. 2016;6(6):e844. doi: 10.1038/tp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A., Engelbert D., Garg G., Polansky J.K., Floess S., Miyao T., Baron U., Duber S., Geffers R., Giehr P., Schallenberg S., Kretschmer K., Olek S., Walter J., Weiss S., Hori S., Hamann A., Huehn J. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J. Immunol. 2013;190(7):3180–3188. doi: 10.4049/jimmunol.1203473. [DOI] [PubMed] [Google Scholar]
- Ueno A., Jeffery L., Kobayashi T., Hibi T., Ghosh S., Jijon H. Th17 plasticity and its relevance to inflammatory bowel disease. J. Autoimmun. 2018;87:38–49. doi: 10.1016/j.jaut.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Valicenti-McDermott M., McVicar K., Rapin I., Wershil B.K., Cohen H., Shinnar S. Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. J. Dev. Behav. Pediatr. 2006;27(2 Suppl. l):S128–S136. doi: 10.1097/00004703-200604002-00011. [DOI] [PubMed] [Google Scholar]
- Walker L.S., C-D A., Rasquin-Weber A. Rome III: the Functional Gastrointestinal Disorders. Degnon Associates, Inc; McLean, Virginia: 2006. Questionnaire on pediatric gastrointestinal symptoms, Rome III version (QPGS-RIII) [Google Scholar]
- Wang L., Liu Y., Han R., Beier U.H., Thomas R.M., Wells A.D., Hancock W.W. Mbd2 promotes foxp3 demethylation and T-regulatory-cell function. Mol. Cell Biol. 2013;33(20):4106–4115. doi: 10.1128/mcb.00144-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Wei L., Zhu J., Zang C., Hu-Li J., Yao Z., Cui K., Kanno Y., Roh T.-Y., Watford W.T., Schones D.E., Peng W., Sun H.-w., Paul W.E., O’Shea J.J., Zhao K. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30(1):155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S., Cabanlit M., Bennett J., Ashwood P., Amaral D.G., Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav. Immun. 2009;23(1):64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Brimacombe M., Chaaban J., Zimmerman-Bier B., Wagner G.C. Autism spectrum disorders: concurrent clinical disorders. J. Child Neurol. 2008;23(1):6–13. doi: 10.1177/0883073807307102. [DOI] [PubMed] [Google Scholar]
- Zerbo O., Leong A., Barcellos L., Bernal P., Fireman B., Croen L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav. Immun. 2015;46:232–236. doi: 10.1016/j.bbi.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O., Leong A., Barcellos L., Bernal P., Fireman B., Croen L.A. Immune mediated conditions in autism spectrum disorders. Brain Behav. Immun. 2015;46:232–236. doi: 10.1016/j.bbi.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O., Yoshida C., Grether J.K., Van de Water J., Ashwood P., Delorenze G.N., Hansen R.L., Kharrazi M., Croen L.A. Neonatal cytokines and chemokines and risk of autism spectrum disorder: the early markers for autism (EMA) study: a case-control study. J. Neuroinflammation. 2014;11:113. doi: 10.1186/1742-2094-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Chong M.M.W., Littman D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]