Abstract
We have analyzed the in vivo importance of different regions of Rap1p, a yeast transcriptional regulator and telomere binding protein. A yeast strain (SCR101) containing a regulatable RAP1 gene was used to test functional complementation by a range of Rap1p derivatives. These experiments demonstrated that the C terminus of the protein, containing the putative transcriptional activation domain and the regions involved in silencing and telomere function, is not absolutely essential for cell growth, a result confirmed by sporulation of a diploid strain containing a C terminal deletion derivative of RAP1. Northern analysis with cells that expressed Rap1p lacking the transcriptional activation domain revealed that this region is important for the expression of only a subset of Rap1p-activated genes. The one essential region within Rap1p is the DNA binding domain. We have investigated the possibility that this region has additional functions. It contains two Myb-like subdomains separated by a linker region. Individual point mutations in the linker region had no effect on Rap1p function, although deletion of the region abolished cell growth. The second Myb-like subdomain contains a large unstructured loop of unknown function. Domain swap experiments with combinations of elements from DNA binding domains of Rap1p homologues from different yeasts revealed that major changes can be made to the amino acid composition of this region without affecting Rap1p function.
Rap1p is a yeast multifunctional protein involved in transcriptional activation, transcriptional silencing, and telomere function (13, 29, 30, 34, 35, 46, 47). It has a complicated organization with several apparently independent functional domains. The 827-amino-acid primary sequence can be subdivided conveniently into three regions, a central DNA binding domain plus N-terminal and C-terminal domains of approximately equal size (46). The DNA binding domain is located between amino acids 361 and 596 and consists of two Myb-like subdomains, each based on a helix-turn-helix motif (24, 27). The first subdomain (domain 1) contains three alpha helices, the second and third of which comprise the helix-turn-helix motif (H1B and H1C). The second subdomain (domain 2) contains four alpha helices; again, the second and third helices form a helix-turn-helix motif (H2B and H2C). In each subdomain, the third helix is the DNA recognition helix and the other helices are important in maintaining the overall architecture of the structure (27). The two subdomains are connected by a linker of approximately 30 amino acids that may be important in determining their relative positions. This linker contains two turns, each composed of six amino acids. An additional feature of interest in the second subdomain is a relatively large region of more than 50 amino acids that has been described as a partially unstructured loop. This is located between helices 2A and 2B, and its function, if any, is not apparent (27). The C terminus of Rap1p contains regions implicated in transcriptional activation (positions 630 to 695), as well as mating type and telomeric silencing and telomeric length control (positions 665 to 827) (20, 30, 32, 49). It is also important for the mechanism that results in transcriptional repression of ribosomal protein genes when there is a defect in the protein secretion pathway (37). The C terminus is the target for all of the protein-protein interactions involving Rap1p characterized to date, including interactions with the Sir proteins Sir3p and Sir4p and competing interactions with the Rif proteins Rif1p and Rif2p (21, 23, 38, 53). The N terminus of Rap1p is a large region that is not essential for cell viability, although it may be involved in regulating the activity of Rap1p through a putative BRCT domain (6, 38). It has also been shown to potentiate DNA bending by Rap1p in vitro (39).
Rap1p has been implicated in transcriptional activation of many genes, including the mating-type genes MATα1 and MATα2, ribosomal protein genes, and glycolytic genes (3–5, 10, 16, 40, 43, 44, 54). At the MATα locus, the MATα1 and MATα2 genes are activated by a bidirectional upstream activation sequence (UAS) consisting of a single Rap1p site (16). UAS found upstream of some of the ribosomal protein genes are more complex, containing one or more Rap1p binding sites and a T-rich DNA element approximately 25 bp in length (17, 18, 43, 54). A third class of Rap1p UAS are found upstream of many glycolytic genes. These contain binding sites for several other transcription factors, including Gcr1p, a glycolytic gene-specific factor (1, 12, 26). It is possible that Rap1p performs a single function at these diverse UAS or that it works in different ways in the different situations. The current view in the literature is that Rap1p contains a single transcriptional activation domain analogous to conventional transcription factors (20, 38, 46). At the simple MATα UAS, Rap1p may make direct contact with the general transcriptional machinery via this activation domain. However, at the more complex UAS, it may not work in this way. At the UAS of the HIS4 gene, a Rap1p binding site is required for both basal transcription and stimulated transcription in response to amino acid starvation (14). It must be present for the formation of micrococcal nuclease-sensitive regions corresponding to binding sites for the transcriptional activators Gcn4p, Bas1p, and Bas2p (14). The role of Rap1p in this situation may be to modify the chromatin structure around its binding site to allow other transcription factors access to the DNA. The idea that Rap1p is an accessory factor that facilitates the roles of other activators has been supported by studies on the promoters of ribosomal protein genes, although these do not provide evidence that chromatin effects are important. At these promoters, although a single Rap1p site on its own can activate transcription, significantly greater effects are seen when it is combined with a T-rich element, which on its own is also a poor activator (17). At the UAS of the glycolytic genes ENO1, TPI, and PYK1, Rap1p functions to promote the binding of Gcr1p, a protein that interacts with DNA only very weakly on its own (2, 15, 52). This could be achieved via a direct protein-protein interaction or by some effect of Rap1p on the surrounding DNA (44, 51). There may also be some redundancy of function within the N- and C-terminal domains of Rap1p because it can promote DNA binding by Gcr1p in vitro as long as the DNA binding domain plus either the N terminus or the C terminus is present (33).
To gain more insights into the activation role of Rap1p, we have undertaken a detailed functional analysis of the protein in vivo. Our aim was to define the regions of Rap1p that are required for cell survival, based on the premise that the transcriptional activation function is likely to be essential. In the second part of the analysis, we used Rap1p homologues from other yeast species. Studies of homologues of important proteins from related organisms often reveal key information about their functional organization. The only homologue of Rap1p characterized to date was identified in Kluyveromyces lactis (31). This protein is smaller than the budding yeast protein, largely because the N-terminal domain is reduced in size. The DNA binding domain is relatively highly conserved (69% identity), as are other regions within the C-terminal domain. The K. lactis protein does not provide the essential function or functions of budding-yeast Rap1p in complementation experiments (31). Homologues of Rap1p appear to be present in other budding yeasts (42), and we have recently used degenerate PCR to clone DNA fragments encoding the DNA binding domains of two such homologues (see Results). There is also a partial sequence encoding a putative Rap1p homologue in the Candida albicans genome database (Stanford University). We have used these sequences in combination with the crystal structure of the Saccharomyces cerevisiae DNA binding domain to investigate further the functions of the DNA binding domain of Rap1p.
MATERIALS AND METHODS
Strains and media.
Plasmid manipulations were carried out with Escherichia coli MC1061 [F− araD139 Δ(ara-leu)7696 Δ(lac)174 galU galK hsdR strA (Strr)]. Rap1p derivatives were analyzed in the conditional rap1 strain SCR101 (18) grown in synthetic complete (SC) medium (22) containing either 2% (wt/vol) galactose or 2% (wt/vol) glucose, supplemented with 0.2% (wt/vol) adenine, tryptophan, and histidine. The rap1ΔC strains were constructed with the diploid strain 842 a/α ade2-1/ade2-1 trp1-1/trp1-1 leu2/leu2 his3-11/his3-11 ura3/ura3 can1/CAN1. Zygosaccharomyces rouxii NCYC 564 and Saccharomyces unisporus NCYC 971 were obtained from the National Collection of Yeast Cultures, Norwich, United Kingdom.
Plasmid construction.
A fragment of the RAP1 gene (positions −436 to +1809) was amplified by PCR (primers U/S1 and U/S2 [Table 1]) to introduce novel BglII sites at positions −429 and +1799 and a SmaI site at position +1793. This PCR product was cut with BglII and cloned into the BamHI site of pRS415 (48) to generate pAJ826. A SphI-SalI fragment of pPE711 (10) was inserted into pAJ826 to regenerate the entire 3′ end of the RAP1 gene (pRAP1). Plasmid pRAPΔC was constructed by the insertion of a blunt-ended BglII-EcoRI fragment containing the PGK terminator fragment (36) into the SmaI site of pAJ826. pRAPΔNΔC and pRAPΔN were made by deleting the region between the two NsiI sites in the RAP1 gene (positions +52 to +1024) from pRAPΔC and pRAP1, respectively. A fragment of the RAP1 gene from positions +1033 to +1902 was amplified by PCR (primers 1797+ and 2666−) to introduce a novel HindIII site at position +1891. This PCR product was cleaved with HindIII and used to replace the HindIII fragment of pRAP1 (positions +1080 to +2074), deleting positions +1891 to +2074 (pRAPΔAct). To construct pRAPΔSil, a BclI fragment of pRAP1 (positions +1203 to +2395) was replaced with a BclI-BglII fragment (positions +1203 to +2101) from pPE711. pRAPΔTox was made by PCR amplification of a region of the RAP1 gene from positions +1876 to +2497 (primers 2640+ and 3262−), introducing a novel BamHI site at position +1886. This PCR product was cloned into pGEM T (Promega) and reisolated as a BamHI-SalI fragment. This was blunt ended and subcloned into the SmaI site of pAJ826, thus deleting positions +1793 to +1885 from the RAP1 sequence. A 902-bp HindIII fragment of this construct was used to replace the 994-bp HindIII fragment from the wild-type RAP1 gene in pRAP1, giving pRAPΔTox. Plasmid pAJ90, used to generate the rap1ΔC strain, was constructed as follows. A URA3 selectable marker was isolated as a HindIII-SmaI fragment from plasmid pYEUra3 (Clontech), and the HindIII end was made blunt. The resulting fragment was cloned into an end-filled XbaI site (422 bp downstream of the stop codon of RAP1) in the downstream region of the RAP1 gene. PCR was then used to amplify the region between the stop codon of RAP1 and position +1001, containing the URA3 gene, incorporating SmaI sites at both ends of the fragment (primers RDSF and RDSR2). The PCR product was cut with SmaI and ligated into SmaI-digested pAJ826. This introduced a DNA sequence encoding 4 amino acids (TRDE) and a stop codon immediately downstream of the region encoding the DNA binding domain of Rap1p followed by the RAP1 downstream region containing the selectable marker.
TABLE 1.
Synthetic oligonucleotides used in this study
| Name | Sequence a | Position (RAP1)b | Site(s)c |
|---|---|---|---|
| U/S1 | AGTACGAGATCTAGAAGGGGCAATATG | BglII | |
| U/S2 | ACTATAAGATCTTGCCCGGGTGGCGGCAGA | 1780–1809 (rev) | BglII, SmaI |
| 2666− | TGGTATAGCGAAGCTTATGGAAGCAGC | 1876–1902 (rev) | HindIII |
| 2640+ | GCTGCTTCCGGATCCTACGCTATACC | 1876–1901 | BamHI |
| 3262− | CTTAATTCAATCGATCATAACAGG | 1274–2497 (rev) | ClaI |
| pRH1 | CGTTTATGAGGTTAACAAGTTTGG | 1251–1274 | |
| pRH2 | CCAAACTTGTTAACCTCATAAACG | 1251–1274 (rev) | |
| pRH3 | GAGGTTGACGCGTTTGGTAAATTG | 1258–1281 | |
| pRH4 | CAATTTACCAAACGCGTCAACCTC | 1258–1281 (rev) | |
| 1797+ | GGGCAAAGGTCCATGATTTCGAGG | 1033–1058 | |
| pRH5 | CCAAACTTGTCGACCTCATAAACG | 1251–1274 (rev) | SalI |
| pRH6 | GACGATGGAGTCGACATAAAGACT | 1291–1314 | SalI |
| AD695− | TCTGTTGTTGAGATCTATGGTGGAAA | 2077–2102 (rev) | BglII |
| pRHKL1 | CATTCTACCGAAATCGATAAAGAG | ClaI | |
| pRHKL2 | GAATGGGTTGCATGCTCAAGTGCG | (rev) | SphI |
| pRHSUZR | CTACCACCATCGATAAA | ClaI | |
| pRHSU2 | GTGTGTTGCATGCATCTCTGC | (rev) | SphI |
| pRHZR2 | GTGGGTTGCATGCGATTCACC | (rev) | SphI |
| pRHKL4 | GGTAGATCTCTCCCGGGTTTTTAGGG | (rev) | SmaI |
| pRH7 | GTTTGCCACCATCGATTAAAAGG | 1318–1341 | ClaI |
| pRH8 | CCTTTAATCGATGGTGGCAAAAC | 1318–1341 (rev) | ClaI |
| 2500− | TTGGTGAGGTTCTTCATC | 1719–1736 (rev) | |
| 2544+ | TCTGCCGCCACCCGGGCAAGAAAT | 1779–1802 | SmaI |
| 628− | GTATAGCGTAAGCCCGGGAAGCAGC | 1875–1899 (rev) | SmaI |
| 662− | GTGGATACTCCCGGGGCAAAC | 1978–1998 (rev) | SmaI |
| RDSF | GAAGGACCCGGGATGAGTAATTG | 2469–2491 | |
| RDSR2 | CTTGCCCGGGTCGTAGGAATTCTAGTAG | (rev) |
The DNA sequences are shown in the 5′ to 3′ direction.
The position indicated corresponds to the position of the sequence in the RAP1 coding region, relative to the ATG. (rev), reverse PCR primer.
Where a restriction enzyme site has been introduced by use of the primer, the site is indicated.
Plasmids pAJ96 and pAJ97 were used to produce strains expressing Rap1p truncated at amino acids 628 and 662, respectively. Plasmid pAJ90 was digested with EcoRI, and the largest fragment was isolated and religated. This produced a derivative containing a single EcoRI site (pAJ94). pAJ94 was cut with SmaI, and PCR fragments encoding the required C terminal portions of the RAP1 gene were inserted. The fragments were generated with primers 2544+ and either 628− or 662−. These primers introduced SmaI sites at both ends of the PCR product. Each product was cut with SmaI before being ligated into pAJ94. The resulting plasmids encoded Rap1p in which amino acid 597 had been changed to a threonine residue; this was followed by the required amino acids from Rap1p plus the amino acids RDE and a stop codon.
Generation of the RAP1/rap1ΔC strains.
A HindIII fragment of pAJ90 encoding the DNA binding domain of Rap1p and also containing the RAP1 downstream region plus the URA3 selectable marker was transformed into the diploid strain 842 by the one-step method (11). Plasmids pAJ96 and pAJ97 were digested with BamHI and EcoRI to produce similar fragments for transformation. Transformants were selected on SC medium lacking uracil. Colonies that grew were screened for the required gene replacement by using gel retardation assays and Southern blotting.
Generation of specific mutations in the DNA binding domain of Rap1p.
Site-specific mutations were introduced into the RAP1 gene by using the QuikChange site-specific mutagenesis kit as specified by the manufacturer (Stratagene). Primers pRH1 and pRH2 were used to create the D422N mutation, and primers pRH3 and pRH4 were used to create the K423A mutation. To delete the linker region from the DNA binding domain, two fragments of the RAP1 gene (positions +1033 to +1274 and positions +1291 to +2102) were amplified by PCR (with primers 1797+, pRH5, pRH6, and AD695−), such that a novel SalI site was introduced into each fragment (positions +1263 and +1301). Both fragments were digested with SalI and ligated to create a larger RAP1 fragment. This fragment was digested with HindIII and used to replace the corresponding HindIII fragment of pRAP1. The resulting plasmid encoded Rap1p lacking the two short turns within the DNA binding domain.
Construction of hybrid DNA binding domain expression plasmids.
The RAP1 promoter and coding region (positions −429 to +2709) was isolated as a NotI-SalI fragment from pRAP1 and cloned into the corresponding sites of plasmid pRS413 (48) to create pAJ928. A silent point mutation (A to G) was introduced at position +1332 in the RAP1 gene by using the QuikChange kit (primers pRH7 and pRH8). This mutation created a ClaI site (position +1331) upstream of helix H2A of the DNA binding domain. This plasmid, pAJ930, also contained novel SphI and SmaI sites at positions +1601 and +1793, respectively, within the RAP1 gene. Plasmid pAJ930 was used to create the hybrid DNA binding domain expression plasmids. Fragments of the RAP1 genes encoding the three different unstructured loop regions flanked by helices H2A and H2B were generated by PCR. The K. lactis sequence was isolated with primers pRHKL1 and pRHKL2, the S. unisporus sequence was isolated with primers pRHSUZR and pRHSU2, and the Z. rouxii sequence was isolated with primers pRHSUZR and pRHZR2. The RAP1 fragments were digested with ClaI and SphI and cloned into the corresponding sites in pAJ930. The region of the K. lactis RAP1 gene encoding subdomain 2 was generated by PCR with primers pRHKL1 and pRHKL4, digested with ClaI and SmaI, and cloned into the corresponding sites of pAJ930.
Isolation of RAP1 homologues by degenerate PCR.
Yeast genomic DNA prepared by the “ten minute” method (25) was subjected to PCR with degenerate primers homologous to conserved regions within the DNA binding domain of S. cerevisiae and K. lactis Rap1p. Primer 1 corresponds to the amino acid sequence EEDEFILD (amino acids 366 to 373 in S. cerevisiae Rap1p) and contained the degenerate DNA sequence 5′GARGARGAYGARTTYATHYTNGA 3′ (N = A, C, G, or T; K = G or T; R = A or G; Y = C or T; H = A, C, or T). Primer 2 was the reverse complement of the amino acid sequence ENAWRDRF (amino acids 538 to 545 in S. cerevisiae Rap1p) and contained the degenerate DNA sequence 5′ AANCKRTCCKCCANGCRTTYTC 3′. PCR with successively lower annealing temperatures in the initial cycles (touchdown PCR) was used for 35 cycles of 94°C for 1 min, annealing at 70°C initially and then a decrease of 2°C every two cycles to a final temperature of 56°C, and extension at 72°C for 2 min 30 s.
Complementation of conditional rap1 strains.
SCR101 cells were transformed with each of the RAP1-containing plasmids by the one-step method (11). Transformants were selected and restreaked on SC agar containing galactose. For colony dilution assays, transformed cells were suspended in 0.5 ml of 25 mM sodium phosphate buffer (pH 7), and serial dilutions containing 106, 105, 104, and 103 cells/ml were made. Aliquots of each dilution (10 μl) were spotted onto SC plates containing glucose or galactose. Growth was carried out for 4 days at 30°C. Growth curves were generated by inoculating transformants at a cell density of 104 per ml into SC medium containing glucose and taking optical density readings at 600 nm.
Isolation of probes used in Northern analysis.
PCR was performed on total yeast genomic DNA isolated from S. cerevisiae LL20 (NCYC 1445), using primers specific for the following loci: PYK1 (positions +292 to +771), MATα1 (+91 to +490), MATα2 (+34 to +600), RPS10 (+491 to +950), RPL19 (+425 to +919), and RPL45 (+30 to +314). The PGK mRNA and 18S rRNA probes have been described previously (8).
Northern analysis.
Transformed SCR101 cells were grown for approximately six generations (to a cell density of 107 cells per ml) in SC medium containing glucose. Total cellular RNA was extracted from 20 ml of each culture by using the PureScript system (Gentra Systems, Inc.). A 10-μg portion of each RNA sample was electrophoresed through a 1.5% agarose gel containing 20 mM morpholinepropanesulfonic acid (MOPS), 5 mM sodium acetate, 0.1 mM EDTA, and 0.66 M formaldehyde. The separated RNA samples were then transferred to Zeta Probe GT nylon membrane (Bio-Rad Laboratories Ltd.) and probed with radioactively labelled fragments of the relevant genes. Probes were removed from the membrane by soaking in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.5% sodium dodecyl sulfate at 95°C prior to subsequent probings. The signals from each probe were quantified with a Molecular Dynamics PhosphorImager and compensated for background. After adjusting the figures for differences in loading, the RNA level in the RAP1+ strain was designated 100% and the RNA levels in the other strains were calculated relative to this percentage.
Production of Rap1p derivatives by in vitro transcription and translation.
All Rap1p mutants were prepared by in vitro transcription-translation with the TNT reagents as specified by the manufacturer (Promega). Control templates were used to produce either the isolated DNA binding domain of Rap1p or the full-length protein. Reaction mixtures containing no added DNA template were used in parallel.
Gel retardation assays.
SCR101 transformants were grown to mid-log phase in SC medium containing glucose, and total protein extracts were made as described previously (19). Gel retardation assays were performed with 2 μg of total protein extract and probes specific for Rap1p and for Abf1p, as described previously (9). Sites 1 and 2 in Fig. 6 were two weak Rap1p binding sites isolated previously (19). Site 1 contained the sequence 5′CGTACACCCACCAGAT3′, and site 2 contained the sequence 5′GAGCCTAACACCC3′.
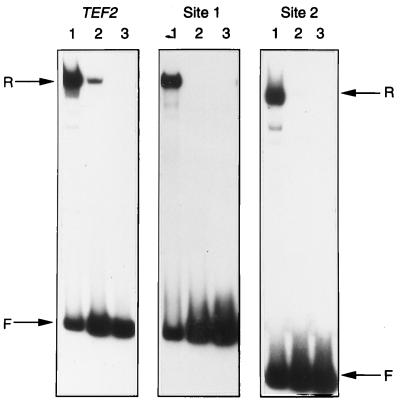
FIG. 6.
DNA binding by Rap1pΔ423–435 (delDT). Rap1p and Rap1pΔ423–435 were synthesized in vitro and tested in gel retardation assays with three DNA probe fragments. These were the strong binding site from the TEF2 gene promoter (TEF2) and two weak binding sites (site 1 and site 2 [see Materials and Methods]). In each case, lane 1 contains the wild-type Rap1p, lane 2 contains the deleted version, and lane 3 contains a control in vitro transcription-translation lysate not primed with DNA.
Western blotting.
Aliquots (10 μg) of each total protein extract were electrophoresed in a 10% polyacrylamide gel containing sodium dodecyl sulfate, then transferred to 0.2-μm-pore-size nitrocellulose. The filter was blocked in 2.5% (wt/vol) dried milk powder–0.05% (vol/vol) lauryl dimethylamine oxide (LDAO) in Tris-buffered saline (buffer I). Rabbit anti-Rap1p antibody (generously provided by Judith Berman) was used at a 1:5,000 dilution in buffer I, and the filter was washed for three periods of 30 min in buffer I. The secondary antibody used was horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G at a dilution of 1:2000 in buffer I. The filter was washed three times in Tris-buffered saline, prior to detection using the enhanced chemiluminescence system (Amersham), according to the manufacturer’s instructions.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the cloned S. unisporus and Z. rouxii sequences are AF043217 and AF043218, respectively.
RESULTS
Analysis of N-terminal and C-terminal deletions.
To investigate the in vivo importance of different regions of Rap1p, we used yeast strain SCR101 (18). In this strain the chromosomal copy of the RAP1 gene is under the control of the GAL UAS, allowing Rap1p expression to be turned on and off by growing yeast cells on minimal medium containing either galactose (gal medium) or glucose (glu medium). The ability of a series of Rap1p derivatives to allow the growth of SCR101 in the absence of endogenous Rap1p has been determined. Each derivative was expressed from the authentic RAP1 promoter on a single-copy plasmid. Transformants were selected on gal medium (chromosomal RAP1 gene on) and then plated at a series of dilutions on glu medium (Fig. 1A). Transformants containing either the positive or negative control plasmid (pRS415 and pRAP1) behaved as expected. The other plasmids expressed Rap1p derivatives lacking defined regions of the N and/or C terminus. To confirm that the approach generated results consistent with previous work from other laboratories, these included regions thought to be nonessential for Rap1p function. For example, pRAPΔN expressed Rap1p lacking most of the N terminus (amino acids 19 to 340), a region thought not to be required for normal growth (38). Our results confirmed this observation. The C terminus of Rap1p contains regions of the protein implicated in transcriptional activation, silencing, and telomere length regulation and is the target for interactions with Rif1p, Rif2p, Sir3p, and Sir4p (20, 21, 32, 38, 49, 53). We found that deletion of either the transcriptional activation domain (pRAPΔAct, deletion of amino acids 629 to 690) or the C-terminal silencing domain (pRAPΔSil, deletion of amino acids 700 to 798) resulted in a slow-growth phenotype but was not lethal. The slow-growth phenotype of cells expressing these deletion derivatives is consistent with the behavior of yeast strains containing the rap1t alleles, which truncate the protein within the C terminus (30). When the entire C terminus of the protein was absent (pRAPΔC), either alone or in combination with a deletion within the N terminus (pRAPΔNΔC), growth was compromised even further and only microcolonies were produced. A deletion that removed most of the N terminus of the protein and approximately half of the DNA binding domain (pRAPΔDBD; deletion of amino acids 20 to 497) completely abolished the ability of the cells to grow.
FIG. 1.
Effect on cell growth of deletions within the RAP1 gene. (A) Plate assays showing the growth of yeast strains expressing deletion derivatives of Rap1p. In each case, transformed colonies selected on gal plates were diluted and single spots containing 104, 103, 102, and 10 cells were plated onto gal and glu plates. The plates were incubated for 4 days at 30°C and photographed. The diagrams on the right show the Rap1p derivatives expressed in each strain. The numbers indicate which amino acids from the Rap1p primary sequence were present. The large shaded area in the middle of each sequence indicates the DNA binding domain, and the smaller shaded area indicates the C-terminal activation domain. (B) Growth curves of Rap1p deletion strains in glu medium. Each strain was inoculated at a cell density of 104 cells/ml. The cultures were incubated with shaking at 30°C, and aliquots taken at intervals for optical density at 600 nm (OD600) readings. (C) Western blot showing the expression of Rap1p derivatives in the transformed yeast strains. Cultures were grown to mid-log phase in glu medium and used to prepare protein extracts. Approximately equal amounts of each extract were loaded per lane. Rap1p derivatives were detected with an anti-Rap1p antibody. The identities of the derivatives are indicated above the lanes. The positions of molecular mass markers and their sizes in kilodaltons are shown on the left of the figure.
To quantify the key observations from the plate assays, we performed growth rate experiments with cells inoculated into glu medium (Fig. 1B). The results confirmed that cells expressing full-length Rap1p, Rap1pΔN, or Rap1pΔTox (deletion of amino acids 597 to 629) exhibited very similar growth characteristics whereas cells expressing Rap1pΔAct or Rap1pΔSil showed slower growth during the exponential phase. The remaining Rap1p derivatives resulted in even slower growth which was difficult to quantify because prolonged growth in liquid culture selected cells in which recombination between the chromosomal and plasmid-borne RAP1 genes had occurred (data not shown).
To establish that the Rap1p derivatives were produced correctly and were the only source of Rap1p in cells growing in glucose medium, protein extracts were prepared from mid-log-phase cultures and tested in Western blots with an anti-Rap1p antibody (Fig. 1C). The transformants expressing full-length Rap1p, Rap1pΔTox, Rap1pΔC, Rap1pΔSil, and Rap1pΔAct each contained a Rap1p derivative of the predicted size. Rap1pΔNΔC was not detected by the assay, probably because this derivative lacked the epitopes recognized by the antibody. The presence of this protein was confirmed by a gel retardation assay with the strong Rap1p binding site from the TEF2 promoter as a probe (data not shown).
Generation of a rap1ΔC haploid strain.
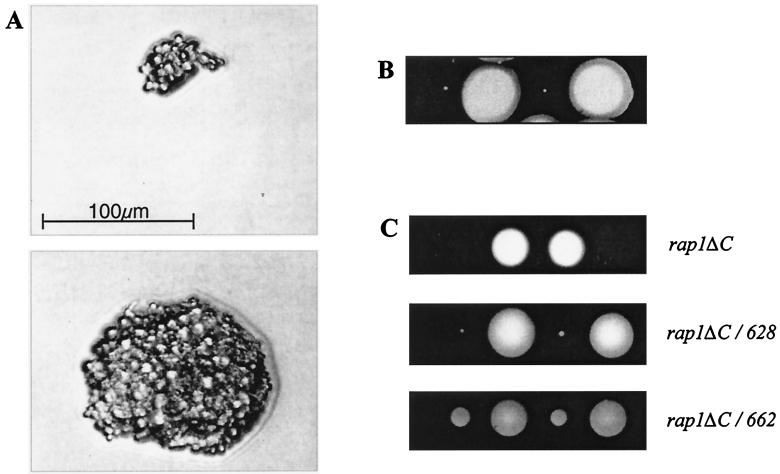
To confirm the observation that the C terminus of Rap1p is not essential for cell growth, we constructed a rap1ΔC haploid strain by sporulation of a heterozygous diploid. One copy of the RAP1 gene in the diploid strain was replaced with a deleted version that lacks the region encoding the entire C terminus of Rap1p. This diploid strain produced the full-length and deleted versions of Rap1p in approximately equal amounts as judged by gel retardation assays (data not shown). The strain was then sporulated, and tetrads were dissected onto yeast extract-peptone-dextrose (YPD) medium. This resulted in a 2:2 segregation of normally growing and very-slow-growing colonies. The very-slow-growing colonies became visible to the naked eye only after about 10 days of incubation at 30°C. However, the development of individual colonies could be observed microscopically, and it was clear that the cells were surviving and dividing at a very low but constant rate (Fig. 2). This confirmed the observation from the SCR101 experiments that the C terminus of Rap1p is important for normal growth but is not absolutely essential.
FIG. 2.
Growth of rap1ΔC, rap1ΔC/628, and rap1ΔC/662 haploid strains. Tetrads from the RAP1/ rap1 diploid strains were dissected onto YPD medium. Typically, two of the four spores developed into colonies at a normal rate and the other two grew more slowly. (A) Microscopic views of a slow-growing rap1ΔC colony after 2 days (top) and 9 days (bottom). Such colonies became visible to the naked eye only after about 10 days of growth. (B) The 2:2 segregation between normal and slow-growing rap1ΔC colonies after 14 days of growth. (C) Comparison between rap1ΔC, rap1ΔC/628, and rap1ΔC/662 colonies after 5 days of growth.
Where is the key region for cell growth in the C terminus of Rap1p?
The rap1ΔC strain grew very slowly, with dissected spores giving visible colonies only after 10 days of incubation at 30°C (see above). This is significantly poorer growth than that observed previously for a strain containing the rap1-17 allele, one of the rap1t alleles that encodes a derivative of Rap1p truncated at amino acid 662, approximately halfway through the activation domain (30). To test which region in the C terminus of Rap1p is responsible for this difference in growth, we constructed two new haploid strains. The first of these expressed a Rap1p derivative truncated at amino acid 662 (rap1ΔC/662) and is similar to the rap1-17 strain. The second expressed a Rap1p derivative truncated at amino acid 628 (rap1ΔC/628). This strain lacked the entire activation domain but contained the region between amino acids 598 and 628. Spores containing each of these rap1 alleles were dissected onto YPD medium, and their growth was compared with that of isogenic wild-type spores and of spores containing Rap1p lacking the entire C terminus (Fig. 2C). The rap1ΔC/662 spores produced colonies that grew more slowly than the wild type but significantly faster than the rap1ΔC spores, which, after only 5 days of growth, have not given rise to visible colonies. This result is consistent with previously published data (30). The rap1ΔC/628 spores gave rise to colonies that were clearly visible after 5 days but were much smaller than the rap1ΔC/662 colonies. Each of the truncated proteins was present at approximately the same level in transformed cells (data not shown). These results suggest that the main reason for the growth rate difference between rap1-17 and rap1ΔC strains is the presence or absence of the region between amino acids 629 and 662. They also suggest that in the absence of the rest of the C terminus, the region between amino acids 598 and 628 may play a functional role.
Differential effects of activation domain and silencing-domain deletions on transcription of diverse genes.
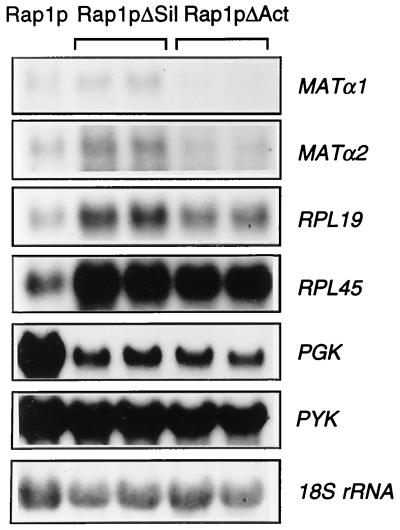
Because SCR101 transformants expressing either Rap1pΔSil or Rap1pΔAct grew at reasonable rates in liquid culture, it was possible to investigate the effects of these deletions on the expression of Rap1p-activated genes (Fig. 3 and Table 2). At the MATα locus, the results were not identical for MATα1 and MATα2, although they are activated by a single bidirectional UAS (16). In the Rap1pΔAct strain, expression of MATα1 was reduced by more than 50% whereas expression of MATα2 showed little change. Expression of both MATα genes was increased in the Rap1pΔSil strain (see Discussion). RPL19A and RPL19B are two ribosomal protein genes located on chromosome II that encode identical proteins. Each gene has a promoter containing a Rap1p binding site and a T-stretch. We compared the expression of these genes with the expression of RPL45, a ribosomal protein gene activated by Abf1p (18). RPL19 and RPL45 mRNA levels both increased in the Rap1p deletion strains, suggesting that these ribosomal protein genes can respond to secondary consequences of the Rap1p deletions. Although this complicates the interpretation of the data, the results show clearly that the activation domain of Rap1p is not absolutely required for expression of RPL19. Similar experiments with a second pair of ribosomal protein genes with the Rap1p binding site plus T-stretch promoter organization, RPS10-1 and RPS10-2 (43), gave similar results (data not shown), suggesting that the results for RPL19 are typical of this class of genes. Finally, we determined the effects of the RAP1 deletions on expression of two glycolytic genes, PGK and PYK1. These genes have UAS that are complex and contain binding sites for several different transcription factors, including Rap1p (7–10, 15, 40). In both Rap1pΔSil and Rap1pΔAct strains, PGK expression was reduced by over 80%. PYK1 expression showed little change in the Rap1pΔSil strain and a small decrease in the Rap1pΔAct strain.
FIG. 3.
Expression of Rap1p-activated genes in Rap1p deletion strains. The strains were inoculated into glucose liquid medium and grown to mid-log phase. Cells were harvested and used to prepare RNA for Northern blots. Specific mRNAs were detected in multiple probings of the same blot. In each case, the Rap1p derivative present is shown at the top of the lanes and the mRNA detected in each panel is shown on the right. For the deletion strains, the two lanes represent mRNA isolated from different independent transformants.
TABLE 2.
Quantification of relative mRNA levels of Rap1p-activated genes in strains expressing deletion derivatives of Rap1p
| Strain | Relative mRNA level ofa
|
|||||
|---|---|---|---|---|---|---|
| MAT α1 | MAT α2 | RPL19 | RPL45 | PGK | PYK | |
| RAPI+ | 100 | 100 | 100 | 100 | 100 | 100 |
| Rap1ΔSil-1 | 140 | 191 | 329 | 367 | 16 | 101 |
| Rap1ΔSil-2 | 115 | 170 | 312 | 319 | 15 | 103 |
| Rap1ΔAct-1 | 37 | 95 | 204 | 284 | 12 | 70 |
| Rap1ΔAct-2 | 44 | 115 | 251 | 366 | 12 | 73 |
Relative to the level in the RAP1+ strain.
Analysis of conserved regions within the DNA binding domain.
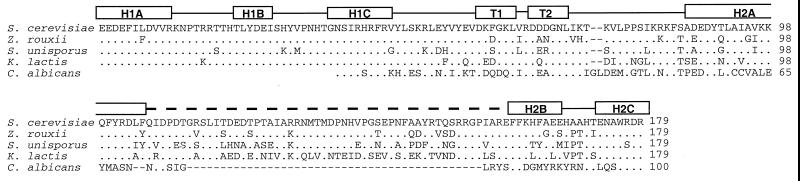
Because the DNA binding domain is the only essential region within Rap1p, we considered the possibility that it has extra functions in addition to DNA recognition. To investigate this, we isolated sequences encoding DNA binding domains of RAP1 homologues from other yeasts and used these in a functional analysis. Degenerate PCR was used to clone genomic DNA fragments from the yeasts Z. rouxii and S. unisporus that encode DNA binding domains of putative Rap1p homologues. We compared the amino acid sequences encoded by these fragments with the DNA binding domains of S. cerevisiae and K. lactis Rap1p and with the same region from a putative C. albicans Rap1p homologue described in the C. albicans genome database (Fig. 4). The Rap1p DNA binding domain is well conserved between the homologues with the highest degree of conservation in domain 1. The double-turn region separating the two subdomains is also generally well conserved, although in the C. albicans protein the first turn differs to some extent. The unstructured loop shows less conservation in sequence but is conserved in length, except for in the putative C. albicans Rap1p, which has 44 amino acids fewer than the other proteins in this region.
FIG. 4.
Rap1p homologues in other yeasts. The figure shows an alignment of part of the DNA binding domain of Rap1p with the known homologue from K. lactis and putative homologues from Z. rouxii, S. unisporus, and C. albicans. The alignment begins at a position corresponding to amino acid 366 in S. cerevisiae Rap1p and was produced with Clustal W (50). The diagram above the sequences indicates the positions of structural features in the S. cerevisiae protein. The thick dotted line indicates the position of the large loop between H2A and H2B in subdomain 2. Dots represent amino acids that are identical to those found in S. cerevisiae Rap1p. The C. albicans sequence represents the hypothetical translation product of a 421-bp DNA sequence (384031G07) in the Candida genome database.
In vitro and in vivo effects of mutagenesis of the double-turn region.
In our functional analysis of the DNA binding domain, we focused on the regions that are not involved directly in binding to DNA. Specific conserved amino acids in the double-turn motif within the linker region of the S. cerevisiae Rap1p were subjected to site-directed mutagenesis. The first turn in all the Rap1p homologues starts with an aspartic acid residue (D422). This was changed to an asparagine. The crystal structure of the Rap1p DNA binding domain suggested that the second amino acid in the first turn (lysine, K423) could be at a key position if the double-turn region is involved in protein-protein interactions (27). To investigate this possibility, the lysine was mutated to an alanine. To test the effects of these mutations on the function of Rap1p in vivo, each mutation was introduced into the full-length RAP1 gene. The ability of each mutant protein to provide the essential function(s) of Rap1p was determined by using complementation in yeast strain SCR101 (Fig. 5A). Transformants containing either mutant protein grew normally on glu medium, suggesting that neither mutation had affected any essential function mediated through the DNA binding domain. Gel retardation assays with each of the mutant DNA binding domains produced in vitro demonstrated that neither mutation had affected the ability of Rap1p to bind to DNA (data not shown).
FIG. 5.
Growth of yeast strains expressing DNA binding domain mutants of Rap1p. The results of plate assays indicating the results of colony dilution experiments are shown. In each case, transformed colonies selected on gal plates were diluted and single spots containing 104, 103, 102, and 10 cells were plated onto gal and glu plates. The plates were incubated for 4 days at 30°C and photographed. (A) Mutations within the double-turn region. Rap1p D422N and Rap1p K423A are the two point mutations. Rap1p delDT is the deletion derivative lacking amino acids 423 to 435. (B) Hybrid DNA binding domains. Rap1p/Kl-UL, Rap1p/Zr-UL, and Rap1p/Su-UL contain the unstructured loop region from K. lactis, Z. rouxii, and S. unisporus, respectively, replacing the same region in S. cerevisiae Rap1p. Rap1p/KlD2 contains sub-domain 2 from K. lactis Rap1p in place of the same subdomain of the S. cerevisiae protein.
To investigate further the role of the double-turn region, similar experiments were performed with a RAP1 gene in which almost the entire region had been deleted (Δ423–435). This was predicted to result in the two halves of the DNA binding domain being brought closer together and to abolish any protein-protein interactions involving the double-turn region. The in vivo function of Rap1pΔ423–435 was tested by using strain SCR101. Transformants expressing this mutant version of Rap1p were unable to grow on glu medium (Fig. 5A). To investigate why this derivative was nonfunctional, we tested the effect of the deletion on DNA binding activity in vitro (Fig. 6). Equal amounts of Rap1p containing either the mutant or wild-type DNA binding domain were tested for binding to a strong Rap1p binding site (TEF2) and two weaker binding sites isolated previously when Rap1p was used to select binding sites from a random pool of oligonucleotides (19). Rap1pΔ423–435 bound less strongly to the TEF2 binding site than did the wild-type Rap1p. Binding of the mutant protein to either of the two weaker binding sites was not detected. We concluded that the double-turn deletion mutation abolished Rap1p function in vivo because the affinity of Rap1p for its binding site was reduced.
Domain swap experiments.
Another region that could be involved in additional functions is the large unstructured loop within the second Myb-like subdomain. This was found to be relatively poorly conserved between the Rap1p homologues, allowing us to test a range of mutations by swapping domains between the different homologues and the S. cerevisiae protein. Previous workers have shown that the whole K. lactis RAP1 gene cannot complement a defect in the S. cerevisiae RAP1 gene (31), a result confirmed in our test system (data not shown). The region encoding the unstructured loop plus helices 2A and 2B from each of the three budding-yeast RAP1 genes was used to replace precisely the corresponding region of the S. cerevisiae gene. The hybrid genes were then tested for their ability to complement a RAP1 deficiency in SCR101 (Fig. 5B). All three hybrid genes allowed good growth of the strain on glucose medium. When each of the hybrid proteins was synthesized in vitro, each was able to bind strongly and specifically to the TEF2 Rap1p binding site in gel retardation assays (data not shown). These experiments confirmed that even big changes within the unstructured loop region of Rap1p are tolerated without compromising the function of the protein. Since differences in the unstructured loop region could not account for the failure of K. lactis Rap1p to function in S. cerevisiae, we tested the effect of replacing the whole of subdomain 2 of the S. cerevisiae protein with the corresponding subdomain from the K. lactis protein (Fig. 5B). The hybrid protein complemented the RAP1 deficiency in SCR101, although the cells grew significantly more slowly than when wild-type Rap1p was present. We also tested the ability of the complete DNA binding domain of the K. lactis protein to replace the function of the same domain of the S. cerevisiae protein. Complementation experiments demonstrated that this hybrid protein was functional, although, again, growth was compromised significantly (data not shown).
DISCUSSION
Functional analysis in vivo has demonstrated that the C terminus of Rap1p is not essential for vegetative growth, despite being the target for all of the protein-protein interactions involving Rap1p characterized to date (21, 38, 53). This observation was confirmed in two test systems, one involving functional complementation of a galactose-repressible RAP1 gene and the other involving sporulation and growth of a rap1ΔC strain. The C terminus contains the putative transcriptional activation domain (20), deletion of which also resulted in slow growth. It was known previously that neither the silencing nor the telomere function of Rap1p is essential for cell growth, and so it was assumed that the transcriptional activation function of Rap1p is essential (30, 49). Our data suggest either that this is not the case or that the previously mapped activation domain is not necessary for transcriptional activation in all contexts. We investigated this by measuring the mRNA levels of a sample of genes activated by Rap1p in strains containing Rap1p lacking either the activation or the silencing domain. Interpretation of these experiments is complicated by the fact that the strains grew more slowly than the wild type and that expression of the genes that we tested can be influenced by the growth rate (10, 28). Nevertheless, the results demonstrated clearly that the activation domain of Rap1p is more important in some contexts than in others. The Matα UAS is a bidirectional UAS consisting of a single Rap1p binding site, located in the region between the divergently transcribed MATα1 and MATα2 genes (16). We found that the activation domain of Rap1p is important at this UAS but that its deletion affected MATα1 more severely than it affected MATα2. This may be because there is a directionality to the way that Rap1p functions in this context. Rap1p sites in UAS are usually orientated with the AC-rich strand running in the 5′ to 3′ direction towards the transcription start site. The site in the MATα intergenic region is orientated in this way with respect to the MATα2 gene, and expression of this gene is higher than that of MATα1. Perhaps deletion of the activation domain had more effect on MATα1 because Rap1p was in a suboptimal position with respect to the basal promoter. Deletion of the silencing domain resulted in increased levels of mRNA from these genes, probably as a result of partial derepression of the silenced copies at the HML locus. Expression of the RPL19 ribosomal protein genes increased in both deletion strains. This was probably a secondary consequence of the Rap1p deletions, because it was also observed for RPL45, a gene that is activated by Abf1p, not Rap1p (18). Despite this complication, the results show clearly that the activation domain of Rap1p is not essential for high-level gene expression in the context of ribosomal protein gene promoters. The two glycolytic genes tested, PGK and PYK1, were chosen because the involvement of Rap1p at their UAS has been well characterized (10, 15, 40). PGK expression was reduced in both deletion strains, perhaps as a secondary consequence of growth rate changes. However, there was little difference between the activation domain and silencing-domain deletion strains, suggesting that either both or neither of these domains is important in transcriptional activation of PGK. PYK1 expression was only mildly affected in both deletion strains, perhaps because expression of this gene is less susceptible to growth rate effects. The small decrease in expression in the activation domain deletion strain indicates that the activation domain could play some role in transcriptional activation at this UAS, but again it is not essential for high-level gene expression.
The data on gene expression and functional complementation by deletion derivatives of Rap1p are consistent with the idea that there is some redundancy of function between the C terminus of Rap1p and other domains of the protein (33). This may include redundancy in both the interaction with Gcr1p and other possible roles in transcriptional activation. In the light of these observations, we focused on the possibility of additional functions for the central DNA binding domain. This is a large region of the protein that could be involved in protein-protein interactions that have not yet been characterized (24, 27). The double-turn motif between the two subdomains appeared to be a good candidate for involvement in such an interaction. Site-directed mutagenesis of two conserved amino acid residues failed to affect the function of Rap1p in vivo, suggesting that this motif is unlikely to be a target for specific protein-protein interactions. Deletion of the double-turn region did affect Rap1p function in vivo, probably as a consequence of an effect on the DNA binding properties of Rap1p. Interestingly, even this big perturbation of the domain did not completely abolish the ability of Rap1p to interact with a strongly recognized target site. In addition to the double-turn region, a second potential target for protein-protein interactions is the large region in the second subdomain described as the unstructured loop (27). The domain swap experiments with different combinations of subelements from the DNA binding domains of Rap1p homologues demonstrated that even big changes within the unstructured loop of the S. cerevisiae protein do not prevent Rap1p function in vivo. Of the 54 amino acids in this region, 30 are different between the S. cerevisiae and K. lactis proteins, but the hybrid protein was still functional. This suggests that the unstructured loop is unlikely to play a key role in any additional functions of the DNA binding domain. Taken together, these analyses suggest that if the DNA binding domain does have another function, this function is more likely to be a consequence of the DNA binding domain interacting with DNA, perhaps involving DNA bending or untwisting, than of a protein-protein interaction.
The data presented has demonstrated that the activation domain of Rap1p, identified by hybrid protein experiments (20), can be important in the context of the whole protein at authentic UAS. However, Rap1p is not a simple transcription factor, because this activation domain appears to be important at only some of the promoters where Rap1p functions. Our data supports the idea that there is functional redundancy between the C terminus and other regions of Rap1p, including the DNA binding domain. It may be this redundancy that has thwarted previous attempts to understand the transcriptional activation function of this protein.
ACKNOWLEDGMENTS
This work was supported by a Project Grant from the BBSRC (U.K.) and by the University of Nottingham Research Opportunities Fund. K.A.H. is a M.Phil. student funded by the European Union Social Fund.
We thank Paula Gonçalves, Willem Mager, and Rudi Planta (Amsterdam) for generously providing yeast strain SCR101; Judith Berman (Minnesota) for the anti-Rap1p antibody; Sue Miles for technical assistance; Stuart Ingleston for help with figures; and Paul Sharp for advice on protein alignments.
REFERENCES
- 1.Baker H V. Glycolytic gene expression in Saccharomyces cerevisiae: nucleotide sequence of GCR1, null mutants, and evidence for expression. Mol Cell Biol. 1986;6:3774–3784. doi: 10.1128/mcb.6.11.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker H V. GCR1 of Saccharomyces cerevisiae encodes a DNA binding protein whose binding is abolished by mutations in the CTTCC sequence motif. Proc Natl Acad Sci USA. 1991;88:9443–9447. doi: 10.1073/pnas.88.21.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitter G A, Chang K K H, Egan K M. A multi-component upstream activation sequence of the Saccharomyces cerevisiae glyceraldehyde-3-phosphate dehydrogenase gene promoter. Mol Gen Genet. 1991;231:22–32. doi: 10.1007/BF00293817. [DOI] [PubMed] [Google Scholar]
- 4.Brindle P K, Holland J P, Willett C E, Innis M A, Holland M J. Multiple factors bind the upstream activation sites of the yeast enolase genes ENO1 and ENO2: ABF1 protein, like repressor activator protein RAP1, binds cis-acting sequences which modulate repression or activation of transcription. Mol Cell Biol. 1990;10:4872–4885. doi: 10.1128/mcb.10.9.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler G, Dawes I W, McConnell D J. TUF factor binds to the upstream region of the pyruvate decarboxylase structural gene (PDC1) of Saccharomyces cerevisiae. Mol Gen Genet. 1990;223:449–456. doi: 10.1007/BF00264453. [DOI] [PubMed] [Google Scholar]
- 6.Callebaut I, Mornon J P. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. doi: 10.1016/s0014-5793(96)01312-9. [DOI] [PubMed] [Google Scholar]
- 7.Chambers A, Packham E A, Graham I R. Control of glycolytic gene expression in the budding yeast (Saccharomyces cerevisiae) Curr Genet. 1995;29:1–9. doi: 10.1007/BF00313187. [DOI] [PubMed] [Google Scholar]
- 8.Chambers A, Stanway C, Kingsman A J, Kingsman S M. The UAS of the yeast PGK gene is composed of multiple functional elements. Nucleic Acids Res. 1988;16:8245–8260. doi: 10.1093/nar/16.17.8245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers A, Stanway C, Tsang J S H, Henry Y, Kingsman A J, Kingsman S M. ARS binding factor 1 binds adjacent to RAP1 at the UASs of the yeast glycolytic genes PGK and PYK1. Nucleic Acids Res. 1990;18:5393–5399. doi: 10.1093/nar/18.18.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers A, Tsang J H, Stanway C, Kingsman A J, Kingsman S M. Transcriptional control of the Saccharomyces cerevisiae PGK gene by RAP1. Mol Cell Biol. 1989;9:5516–5524. doi: 10.1128/mcb.9.12.5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D-Z, Yang B C, Kuo T T. One-step transformation of yeast in stationary phase. Curr Genet. 1992;21:83–84. doi: 10.1007/BF00318659. [DOI] [PubMed] [Google Scholar]
- 12.Clifton D, Fraenkel D G. The gcr (glycolysis regulation) mutation of Saccharomyces cerevisiae. J Biol Chem. 1981;256:13074–13078. [PubMed] [Google Scholar]
- 13.Conrad M N, Wright J H, Wolf A J, Zakian V A. RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell. 1990;63:739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- 14.Devlin C, Tice-Baldwin K, Shore D, Arndt K T. RAP1 is required for BAS1/BAS2- and GCN4-dependent transcription of the yeast HIS4 gene. Mol Cell Biol. 1991;7:3642–3651. doi: 10.1128/mcb.11.7.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drazinic C M, Smerage J B, Lopez M C, Baker H V. Activation mechanism of the multifunctional transcription factor repressor-activator protein 1 (Rap1) Mol Cell Biol. 1996;16:3187–3196. doi: 10.1128/mcb.16.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giesman D, Best L, Tatchell K. The role of RAP1 in the regulation of the Matα locus. Mol Cell Biol. 1991;11:1069–1079. doi: 10.1128/mcb.11.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonçalves P M, Griffioen G, Minnee R, Bosma M, Kraakman L S, Mager W H, Planta R J. Transcriptional activation of yeast ribosomal protein genes requires additional elements apart from binding sites for Abf1p or Rap1p. Nucleic Acids Res. 1995;23:1475–1480. doi: 10.1093/nar/23.9.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves P M, Maurer K, Amerongen G V N, Bergkamp-Steffens K, Mager W H, Planta R J. C-terminal domains of general regulatory factors Abf1p and Rap1p in Saccharomyces cerevisiae display functional similarity. Mol Microbiol. 1996;19:535–543. doi: 10.1046/j.1365-2958.1996.404939.x. [DOI] [PubMed] [Google Scholar]
- 19.Graham I R, Chambers A. Use of a selection technique to identify the diversity of binding sites for the yeast RAP1 transcription factor. Nucleic Acids Res. 1994;22:124–130. doi: 10.1093/nar/22.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy C F J, Balderes D, Shore D. Dissection of a carboxy-terminal region of the yeast regulatory protein RAP1 with effects on both transcriptional activation and silencing. Mol Cell Biol. 1992;12:1209–1217. doi: 10.1128/mcb.12.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy C F J, Sussel L, Shore D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 22.Hawthorne D C, Mortimer R K. Chromosome mapping in Saccharomyces cerevisiae: centromere-linked genes. Genetics. 1960;45:1085–1110. doi: 10.1093/genetics/45.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hecht A, Strahl-Bolsinger S, Grunstein M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature. 1996;383:92–95. doi: 10.1038/383092a0. [DOI] [PubMed] [Google Scholar]
- 24.Henry Y A L, Chambers A, Tsang J S H, Kingsman A J, Kingsman S M. Characterisation of the DNA binding domain of the yeast RAP1 protein. Nucleic Acids Res. 1990;18:2617–2623. doi: 10.1093/nar/18.9.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 26.Holland M J, Yokoi T, Holland J P, Myambo K, Innis M A. The GCR1 gene encodes a positive transcriptional regulator of the enolase and glyceraldehyde-3-phosphate dehydrogenase gene families in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:813–820. doi: 10.1128/mcb.7.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konig P, Giraldo R, Chapman L, Rhodes D. The crystal structure of the DNA-binding domain of yeast RAP1 in complex with telomeric DNA. Cell. 1996;85:125–136. doi: 10.1016/s0092-8674(00)81088-0. [DOI] [PubMed] [Google Scholar]
- 28.Kraakman L S, Griffioen G, Zerp S, Groeneveld P, Thevelein J M, Mager W M, Planta R J. Growth-related expression of ribosomal protein genes in Saccharomyces cerevisiae. Mol Gen Genet. 1993;238:196–204. doi: 10.1007/BF00281618. [DOI] [PubMed] [Google Scholar]
- 29.Kurtz S, Shore D. RAP1 protein activates and silences transcription of mating-type genes in yeast. Genes Dev. 1991;5:616–628. doi: 10.1101/gad.5.4.616. [DOI] [PubMed] [Google Scholar]
- 30.Kyrion G, Boakye K A, Lustig A J. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:5159–5173. doi: 10.1128/mcb.12.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson G P, Castanotto D, Rossi J J, Malafa M P. Isolation and functional analysis of a Kluyveromyces lactis RAP1 homologue. Gene. 1994;150:35–41. doi: 10.1016/0378-1119(94)90854-0. [DOI] [PubMed] [Google Scholar]
- 32.Liu C, Mao X, Lustig A J. Mutational analysis defines a C-terminal tail domain of RAP1 essential for telomeric silencing in Saccharomyces cerevisiae. Genetics. 1994;138:1025–1040. doi: 10.1093/genetics/138.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez M C, Smerage J B, Baker H V. Multiple domains of repressor activator protein 1 contribute to facilitated binding of glycolysis regulatory protein 1. Proc Natl Acad Sci USA. 1998;95:14112–14117. doi: 10.1073/pnas.95.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lustig A J, Kurtz S, Shore D. Involvement of the silencer and UAS binding protein RAP1 in regulation of telomere length. Science. 1990;250:549–553. doi: 10.1126/science.2237406. [DOI] [PubMed] [Google Scholar]
- 35.Marcand S, Gilson E, Shore D. A protein-counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 36.Mellor J, Dobson M J, Roberts N A, Tuite M F, Emtage J S, White S, Lowe P A, Patel T, Kingsman A J, Kingsman S M. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene. 1983;24:1–14. doi: 10.1016/0378-1119(83)90126-9. [DOI] [PubMed] [Google Scholar]
- 37.Mizuta K, Tsujii R, Warner J R, Nishiyama M. The C-terminal silencing domain of Rap1p is essential for the repression of ribosomal protein genes in response to a defect in the secretory pathway. Nucleic Acids Res. 1998;26:1063–1069. doi: 10.1093/nar/26.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moretti P, Freeman K, Coodly L, Shore D. Evidence that a complex of SIR proteins interacts with the silencer and telomere binding protein RAP1. Genes Dev. 1994;8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- 39.Muller T, Gilson E, Schmidt R, Giraldo R, Sogo J, Gross H, Gasser S M. Imaging the asymmetrical DNA bend induced by repressor activator protein 1 with scanning tunneling microscopy. J Struct Biol. 1994;113:1–12. doi: 10.1006/jsbi.1994.1027. [DOI] [PubMed] [Google Scholar]
- 40.Nishizawa M, Araki R, Teranishi Y. Identification of an upstream activating sequence and an upstream repressible sequence of the pyruvate kinase gene of the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:442–451. doi: 10.1128/mcb.9.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogden J E, Stanway C, Kim S, Mellor J, Kingsman A J, Kingsman S M. Efficient expression of the Saccharomyces cerevisiae PGK gene depends on an upstream activating sequence, but does not require TATA sequences. Mol Cell Biol. 1986;6:4335–4343. doi: 10.1128/mcb.6.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pretorius G H J, Muller H E. Conservation of binding site specificity of three yeast DNA binding proteins. FEBS Lett. 1992;298:203–205. doi: 10.1016/0014-5793(92)80057-n. [DOI] [PubMed] [Google Scholar]
- 43.Rotenberg M O, Woolford J R. Tripartite upstream promoter element essential for expression of Saccharomyces cerevisiae ribosomal protein genes. Mol Cell Biol. 1986;6:674–687. doi: 10.1128/mcb.6.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santangelo G M, Tornow J. Efficient transcription of the glycolytic gene ADH1 and three translational component genes requires the GCR1 product, which can act through TUF/GRF/RAP binding sites. Mol Cell Biol. 1990;10:859–862. doi: 10.1128/mcb.10.2.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 46.Shore D. RAP1: a protean regulator in yeast. Trends Genet. 1994;10:408–412. doi: 10.1016/0168-9525(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 47.Shore D, Nasmyth K. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell. 1987;51:721–732. doi: 10.1016/0092-8674(87)90095-x. [DOI] [PubMed] [Google Scholar]
- 48.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sussel L, Shore D. Separation of transcriptional activation and silencing functions of the RAP1-encoded repressor/activator protein 1: isolation of viable mutants affecting both silencing and telomere length. Proc Natl Acad Sci USA. 1991;88:7749–7753. doi: 10.1073/pnas.88.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson J D, Higgins J D, Gibson T J. Clustal-W-improving the sensitivity of progressive multiple sequence alignment through sequence weighting position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tornow J, Zeng X, Gao W, Santangelo G M. GCR1, a transcriptional activator in Saccharomyces cerevisiae, complexes with RAP1 and can function without its DNA binding domain. EMBO J. 1993;12:2431–2437. doi: 10.1002/j.1460-2075.1993.tb05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uemura H, Koshio M, Inoue Y, Cecilia Lopez M, Baker H V. The role of Gcr1p in the transcriptional activation of glycolytic genes in yeast Saccharomyces cerevisiae. Genetics. 1997;147:521–532. doi: 10.1093/genetics/147.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 1997;11:748–760. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- 54.Woudt L P, Mager W H, Nieuwint R T M, Wassenaar G M, van der Kuyl A C, Murre J J, Hoekman M F M, Brockhoff P G M, Planta R J. Analysis of upstream activation sites of yeast ribosomal protein genes. Nucleic Acids Res. 1987;15:6037–6048. doi: 10.1093/nar/15.15.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]