Abstract
Background
There are limited data from clinical trials describing preoperative MRI features and performance in the evaluation of mammographically detected ductal carcinoma in situ (DCIS).
Purpose
To report qualitative MRI features of DCIS, MRI performance in the identification of additional disease, and associations of imaging features with pathologic, genomic, and surgical outcomes from the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN) E4112 trial.
Materials and Methods
Secondary analyses of a multicenter prospective clinical trial from the ECOG-ACRIN Cancer Research Group included women with DCIS diagnosed with conventional imaging techniques (mammography and US), confirmed via core-needle biopsy (CNB), and enrolled between March 2015 and April 2016 who were candidates for wide local excision (WLE) based on conventional imaging and clinical examination results. DCIS MRI features and pathologic features from CNB and excision were recorded. Each woman without invasive upgrade of the index DCIS at WLE received a 12-gene DCIS score. MRI performance metrics were calculated. Associations of imaging features with invasive upgrade, dichotomized DCIS score (<39 vs ≥39), and single WLE success were estimated in uni- and multivariable analyses.
Results
Among 339 women (median age, 60 years; interquartile range, 51–66 years), most DCIS cases showed nonmass enhancement (NME) (195 of 339 [58%]) on MRI scans with larger median size than on mammograms (19 mm vs 12 mm; P < .001). Positive predictive value of MRI-prompted CNBs was 32% (21 of 66) (95% CI: 22, 44), yielding an additional cancer detection rate of 6.2% (21 of 339) (95% CI: 4.1, 9.3). MRI false-positive rate was 14.2% (45 of 318) (95% CI: 10.7, 18.4). No imaging features were associated with invasive upgrade or DCIS score (P = .05 to P = .95). Smaller size and focal NME distribution at MRI were linked to single WLE success (P < .001).
Conclusion
Preoperative MRI depicted ductal carcinoma in situ (DCIS) diagnosed with conventional imaging most commonly as nonmass enhancement, with larger median span than mammography, and additional cancer detection rate of 6.2%. MRI features of this subset of DCIS did not enable prediction of pathologic or genomic outcomes.
Clinical trial registration no. NCT02352883
© RSNA, 2021
Online supplemental material is available for this article.
See also the editorial by Kuhl in this issue.
An earlier incorrect version of this article appeared online. This article was corrected on August 4, 2021.
Summary
Breast MRI depicts ductal carcinoma in situ diagnosed with conventional imaging (mammography with or without US), with a larger median span than mammography, has an additional cancer detection rate of 6.2%, and could assist with wide local excision.
Key Results
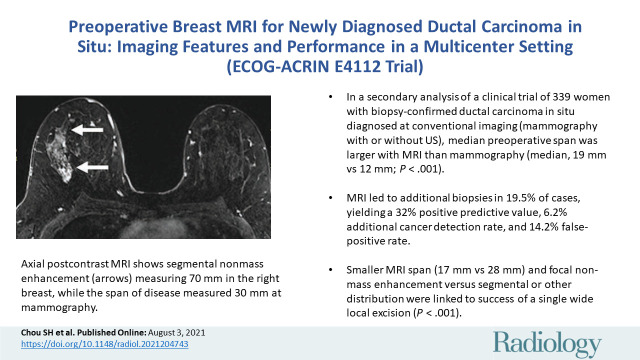
■ In a secondary analysis of a clinical trial of 339 women with biopsy-confirmed ductal carcinoma in situ diagnosed at conventional imaging (mammography with or without US), median preoperative span was larger with MRI than with mammography (median, 19 mm vs 12 mm; P < .001).
■ MRI led to additional biopsies in 19.5% of cases, yielding a 32% positive predictive value, 6.2% additional cancer detection rate, and 14.2% false-positive rate.
■ Smaller MRI span (17 mm vs 28 mm) and focal nonmass enhancement (vs segmental or other distribution) were linked to success of a single wide local excision (P < .001).
Introduction
Ductal carcinoma in situ (DCIS) is a stage 0 noninvasive breast cancer that accounts for 20%–25% of new breast cancer diagnoses in the United States (1). Although women with DCIS have an overall 20-year breast cancer–specific mortality of 3.3% with standard treatment, their risk of breast cancer death remains 1.8 times higher than that of the general U.S. population (2). Because of ambiguity in optimal treatment, DCIS is often treated more aggressively than its true biology warrants (3–5).
Prior research has established that contrast-enhanced MRI has the highest sensitivity in the detection of DCIS (6,7) and the greatest accuracy for depiction of its extent (8–10). There are conflicting data on whether this translates to improved surgical outcomes, such as lower re-excision rates (11–15). Published studies have reported variable associations of MRI features with DCIS features, such as nuclear grade and comedonecrosis (6,8,16–18). However, most of these MRI studies had retrospective or single-center designs, which are subject to selection bias. These MRI studies also often had younger study samples with larger mammographic disease extent than did the non-MRI studies (19,20).
In response to the need to optimize DCIS treatment and to clarify how MRI affects surgeries, the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network (ECOG-ACRIN) Cancer Research Group conducted the E4112 phase II single-arm multicenter trial (21). All enrolled women were candidates for wide local excision (WLE) based on conventional imaging (mammography with or without US) results prior to MRI. Primary results indicated that overall mastectomy conversion was 19.2% after MRI (MRI findings accounted for 38.5% conversions), with a WLE success rate of 96.1%. The study protocol included prospective collection of MRI interpretations and pathologic assessments, allowing for unbiased secondary analyses of imaging features. In this article, we report prespecified secondary objectives to assess the relationship between baseline clinical covariates, qualitative MRI features, and DCIS score and to assess the diagnostic accuracy of MRI. We also report post hoc analyses aimed at assessment of the diagnostic performance of MRI-prompted core needle biopsy (CNB) and the relationship between baseline clinical covariates and MRI features with an upgrade to invasive cancer and single WLE success.
Materials and Methods
Exact Sciences (successor of Genomic Health) supported the Oncotype DX DCIS Score Test for study participants. The authors had full control of the data and information submitted for publication. No authors were employees of or consultants to Exact Sciences.
Study Participants
A detailed account of the multicenter trial from the ECOG-ACRIN Cancer Research Group (ClinicalTrials.gov identifier, NCT02352883) has been published previously (21). Briefly, the trial enrolled 368 women with newly diagnosed pure DCIS from 75 institutions between March 2015 and April 2016 (institutional review board approval was obtained at each site). Written informed consent was obtained from all women, and the trial was compliant with the Health Insurance and Portability and Accountability Act. Exclusion criteria included ineligibility, participant withdrawal, MRI intolerance, MRI completed more than 30 days after registration, no documented surgical plan, and no documented surgery. Those who met all eligibility criteria underwent preoperative MRI and had known final surgery. Each site reported the demographic, mammographic, MRI, and pathologic features of the index DCIS. Here we report methods used for the secondary objectives of the trial protocol. The participants in our study overlapped those in a previously published study in JAMA Oncology in 2019 (21). The prior study reported on the primary outcomes and measures of the E4112 trial, which were the mastectomy conversion rate after MRI and the reasons for those conversions among 339 women with a DCIS diagnosis. The current study reports the secondary and post hoc analyses of the E4112 trial, including MRI features of DCIS, MRI performance in the identification of additional disease, and associations of MRI features with meaningful outcome.
Breast MRI Technique and Reporting
All participating sites performed bilateral breast MRI (1.5 T or 3.0 T) using dedicated breast coils in accordance with the American College of Radiology Breast MRI Accreditation Program, which mandates in-plane pixel size of 1 mm or smaller for the dynamic series and a fluid-sensitive series (22). Interpreting radiologists were instructed to report the following American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) (23) descriptors of the index DCIS: morphology (mass, nonmass enhancement [NME], or focus), internal enhancement, mass shape and margin, NME distribution, signal on fluid-sensitive series, initial and delayed-phase kinetics, and maximum size. Radiologists also recorded background parenchymal enhancement (BPE) level (none or minimal, mild, moderate, or marked) and final BI-RADS assessments (23). The protocol provided radiologists with guidance to recommend biopsy for additional suspicious MRI findings located more than 2 cm from the index DCIS or in the contralateral breast or if additional MRI extent would convert the participant to mastectomy.
Pathologic Analysis Features and DCIS Score
The status of hormone receptor, nuclear grade, and central necrosis at baseline CNB pathologic analysis was reported in the trial. The data collection also included obtaining pathologic results from additional MRI-prompted CNBs. Women with pure DCIS and successful WLE (margins ≥2 mm) as the final surgery received a 12-gene DCIS score evaluation (Oncotype DX Breast DCIS Score assay; Exact Sciences) of their index tumor surgical specimens, assessed on a continuous scale (score of 0–100) and dichotomized as low (<39) or intermediate or high (≥39) (24).
Reference Standard and Definitions
The MRI scan was considered test positive for additional disease beyond the known DCIS when there was an examination-level BI-RADS category 4 or 5 designation; all other BI-RADS assessments were considered test negative. The reference standard for clinically relevant disease beyond the known DCIS was based on CNB and surgical specimen pathologic analysis after MRI combined with 1-year clinical or imaging follow-up. The absence of a new cancer diagnosis within 1 year after the final surgery represented a negative reference standard result. For the reference standard, any CNB yielding DCIS or invasive cancer was considered a true-positive outcome, and no CNB performed or no malignancy seen at CNB pathologic analysis (including high-risk disease) was considered a true-negative outcome. Positive predictive value (PPV) was defined as the proportion of participants with a positive reference standard result on any additional CNB samples among the cohort of examinations or interventions in question. PPV2 was defined as the proportion of participants with a positive reference standard result for any additional CNB samples among all examinations with a positive final BI-RADS assessment. PPV3 was defined as the proportion of participants with a positive reference standard result for any additional CNB samples among all participants who underwent additional CNB. Cancer detection rate (CDR) was calculated as the proportion of women who had malignancy at CNB among all participants. False-positive rate was defined as the proportion of women that underwent additional CNB and yielded negative results among all participants without any additional disease, as defined by the negative reference standard.
Statistical Analysis
Statistical analyses were conducted by the statisticians of the ECOG-ACRIN Cancer Research Group (J.R., C.G., and B.S.S., with 5, 40, and 20 years of experience, respectively). This article reports results from the analysis of two prespecified secondary objectives of the trial, namely assessment of (a) the relationship between baseline clinical covariates, qualitative MRI features, and DCIS score and (b) the diagnostic accuracy of MRI in determination of DCIS extent. Results from several post hoc analyses also are reported. All analyses were based on complete data, except as indicated. Descriptive statistics of MRI features for the index DCIS were summarized by frequencies and percentages for categorical data and by median and interquartile range (IQR) for continuous data. Comparison of index DCIS size on mammograms and MRI scans was performed by using the Wilcoxon signed-rank test.
To address the first of the two secondary objectives, the dichotomized DCIS score results were compared between baseline DCIS characteristics by using the Wald test after fitting a simple logistic regression model for continuous variables and the Fisher exact test for categorical variables. The following baseline characteristics were analyzed: nuclear grade, central necrosis, BPE, MRI size, difference between MRI size and mammography size, morphology, mass shape and margins, NME distribution, and internal enhancement. As an elaboration of this analysis, multiple logistic regression modeling was used to assess the relationship between the dichotomized DCIS score results and prespecified clinical covariates and MRI features, including baseline nuclear grade, central necrosis, MRI size, BPE, and morphology. A similar approach was used in a post hoc analysis to assess the relationship between clinical covariates and MRI features, including NME distribution, with a successful single WLE.
To address the second prespecified secondary objective, we derived estimates of MRI sensitivity, specificity, and PPV2 at the participant level with Wilson 95% CIs.
In the post hoc analyses, we derived estimates of PPV3 at the participant and breast levels and CDR and false-positive rate at the participant level with Wilson 95% CIs. Participants with missing additional biopsy data were considered to have not had an additional biopsy; participants without additional biopsies were defined as having negative results with the reference standard.
All reported P values are two sided and are reported without adjustment for multiplicity. P < .05 was considered to indicate a significant difference. Analyses were performed with SAS/STAT, version 9.4 (SAS Institute).
Results
Participant and Index DCIS Characteristics
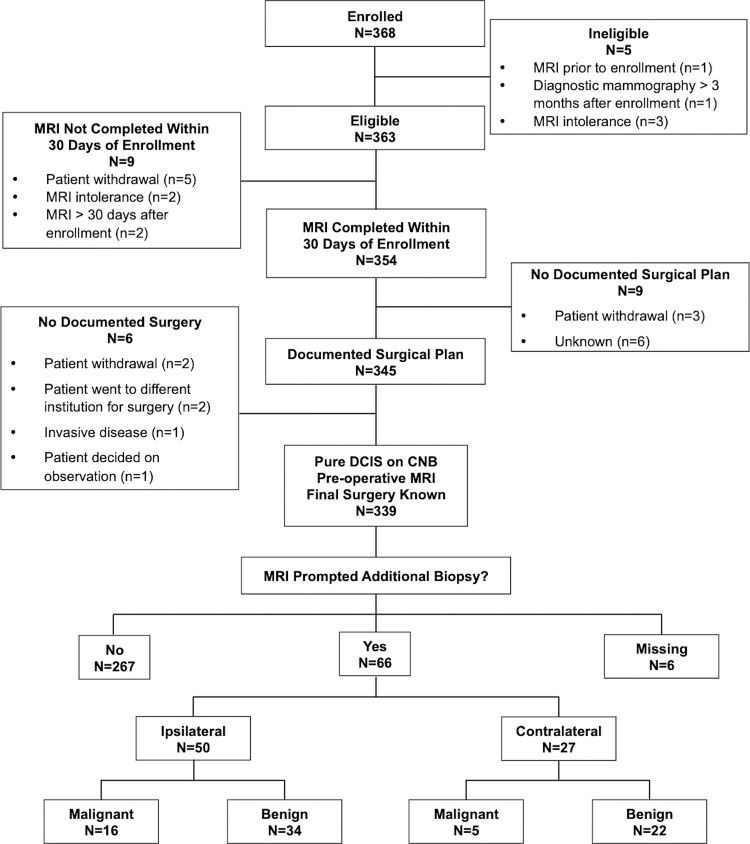
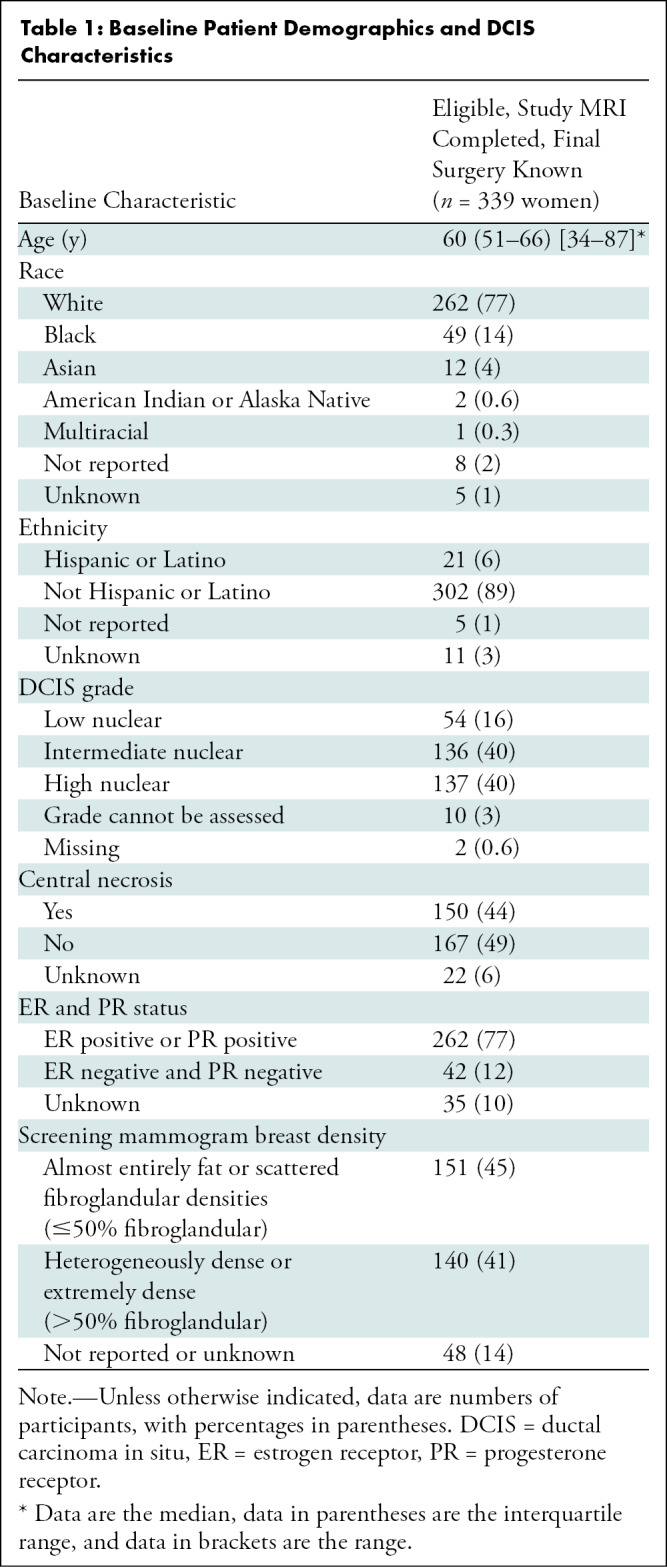
Among the 368 enrolled women, 29 were excluded due to ineligibility (n = 5), participant withdrawal (n = 8), MRI intolerance (n = 2), MRI completed more than 30 days after registration (n = 2), no documented surgical plan (n = 6), or no documented surgery (n = 6) (21). After exclusions, 339 women (median age, 60 years; IQR, 51–66 years) with pure DCIS at CNB were included. They underwent preoperative breast MRI and had known final surgical outcomes, with MRI-prompted additional biopsy data also summarized in the Standards for Reporting of Diagnostic Accuracy diagram (Fig 1). Demographics and index DCIS characteristics were previously reported in the primary article and are summarized in Table 1 (21). Notably, less than half of participants (41%, 140 of 339) had heterogeneously or extremely dense breasts on mammograms, while the majority had DCIS that was either intermediate (136 of 339, 40%) or high (137 of 339, 40%) nuclear grade and was hormone receptor positive (262 of 339, 77%).
Figure 1:
Standards for Reporting of Diagnostic Accuracy flow diagram. CNB = core needle biopsy, DCIS = ductal carcinoma in situ.
Table 1:
Baseline Patient Demographics and DCIS Characteristics
MRI Features
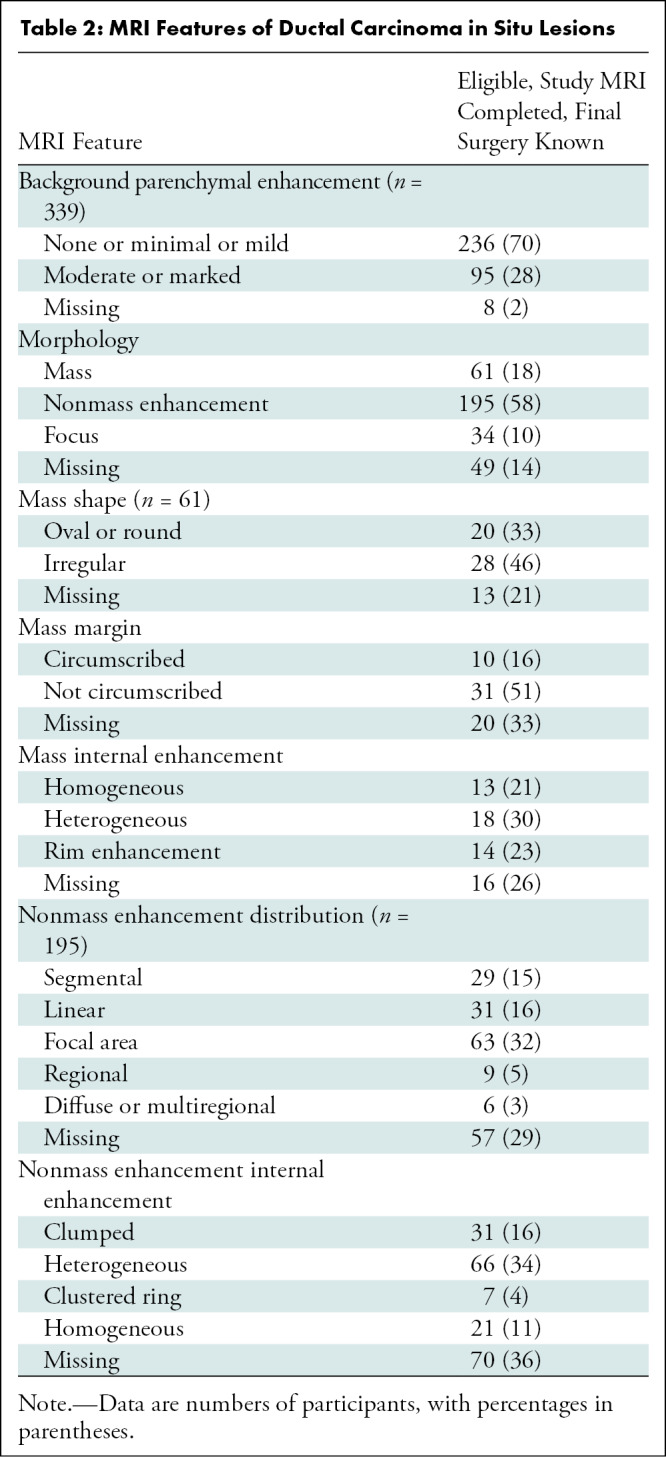
The median size on MRI scans for all index DCIS lesions was 19 mm (IQR, 12–32 mm; 102 missing). Of the 155 index DCIS lesions for which both mammographic and MRI sizes were available, the median MRI size was larger (19 mm; IQR, 11–30 mm) than the mammographic size (12 mm; IQR, 8–22 mm; P < .001). MRI characteristics of DCIS lesions are summarized in Table 2, with most DCIS lesions appearing as NME (58%, 195 of 339), 18% appearing as masses (61 of 339), 10% appearing as foci (34 of 339), and 14% (49 of 339) not recorded or missing. Of the 195 DCIS lesions appearing as NME, focal (n = 63, 32%), linear (n = 31, 16%), and segmental (n = 29, 15%) distributions were most frequently described, and heterogeneous (n = 66, 34%) or clumped (n = 31, 16%) internal enhancement patterns were more common than homogeneous (n = 21, 11%) or clustered ring (n = 7, 4%) patterns.
Table 2:
MRI Features of Ductal Carcinoma in Situ Lesions
MRI Performance in Diagnosis of Additional Malignancies
Nearly 16% (53 of 339) of cases were missing data documenting additional MRI findings or additional malignancy at subsequent biopsy or surgery (Table E1 [online]). Among the 286 women without these missing data, 43 (15%) had a positive final BI-RADS assessment. Among these 43 women, 11 were confirmed to have a positive reference standard results, yielding a PPV2 of 26% (95% CI: [15, 40]). The sensitivity of MRI in the detection of additional disease was 79% (11 of 14; 95% CI: 52, 92) and the specificity was 88.2% (240 of 272; 95% CI: 83.9, 91.5). Three MRI examinations were deemed false negative for additional malignancy based on their BI-RADS 6 assessments and subsequent pathologic analysis: one BI-RADS 6 examination had an additional incidental cancer diagnosed in a quadrant different from the index DCIS at mastectomy, enabling confirmation of the false-negative designation, while the other two BI-RADS 6 examinations yielded a diagnosis of contralateral malignancy via CNB after MRI, which suggests BI-RADS assessment errors.
At the participant level, 66 women (19%) underwent CNB after MRI in either breast, which yielded 21 additional malignancies (six invasive cancers, 15 DCIS lesions) in 21 women for a PPV3 of 32% (95% CI: 22, 44), a CDR of 6.2% (21 of 339; 95% CI: 4.1, 9.3), and a false-positive rate of 14.2% (45 of 318; 95% CI: 10.7, 18.4). At the breast level, 50 women underwent CNB of the breast ipsilateral to the known DCIS, which yielded 16 malignancies for a PPV3 of 32% (95% CI: 21, 46). Twenty-seven women underwent CNB of the contralateral breast, which yielded five contralateral malignancies for a CDR of 1.5% (five of 339; 95% CI: 0.6, 3.4) and a PPV3 of 18.5% (95% CI: 8.2, 36.7).
DCIS Score and Final Pathologic Analysis
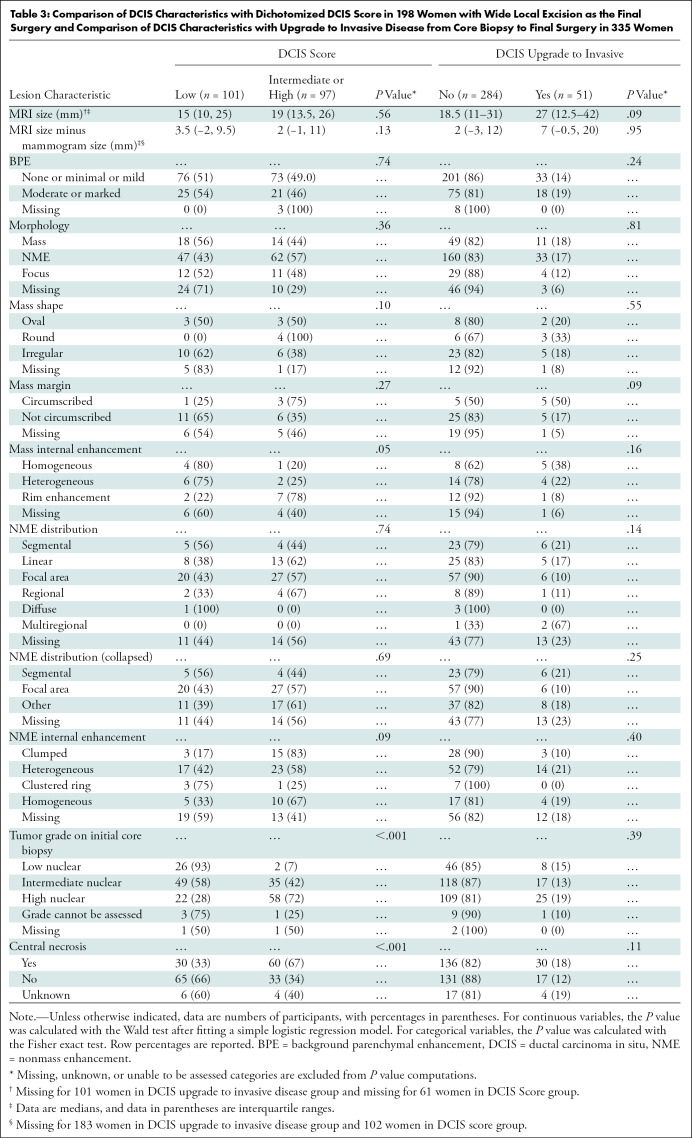
DCIS score results were available in 198 of the 274 women in whom successful WLE was the final surgery. Dichotomized DCIS score result was low (<39) in 101 (51%) women and intermediate or high in 97 (49%) women. We used prespecified univariable analysis and did not find evidence of any association between imaging features and dichotomized DCIS score (P = .05 to P = .74) (Table 3). Baseline DCIS characteristics of higher nuclear grade and the presence of central necrosis were associated with higher DCIS score (P < .001). These results held true in multivariable analysis after adjusting for MRI size, BPE, and morphology (Table E2 [online]).
Table 3:
Comparison of DCIS Characteristics with Dichotomized DCIS Score in 198 Women with Wide Local Excision as the Final Surgery and Comparison of DCIS Characteristics with Upgrade to Invasive Disease from Core Biopsy to Final Surgery in 335 Women
Final surgery of the ipsilateral breast enabled confirmation of pure DCIS in 284 (84%) of 339 women and invasive upgrade in 51 (15%) women; in four women, final pathology findings were unavailable (Table 3). We did not find evidence that median DCIS size was larger at MRI than at mammography in women whose status was upgraded to invasive disease versus those whose status was not upgraded (27 mm vs 18.5 mm, P = .09). No DCIS clinical covariates or imaging features were associated with invasive upgrade (P = .09 to P = .95).
Single WLE Success
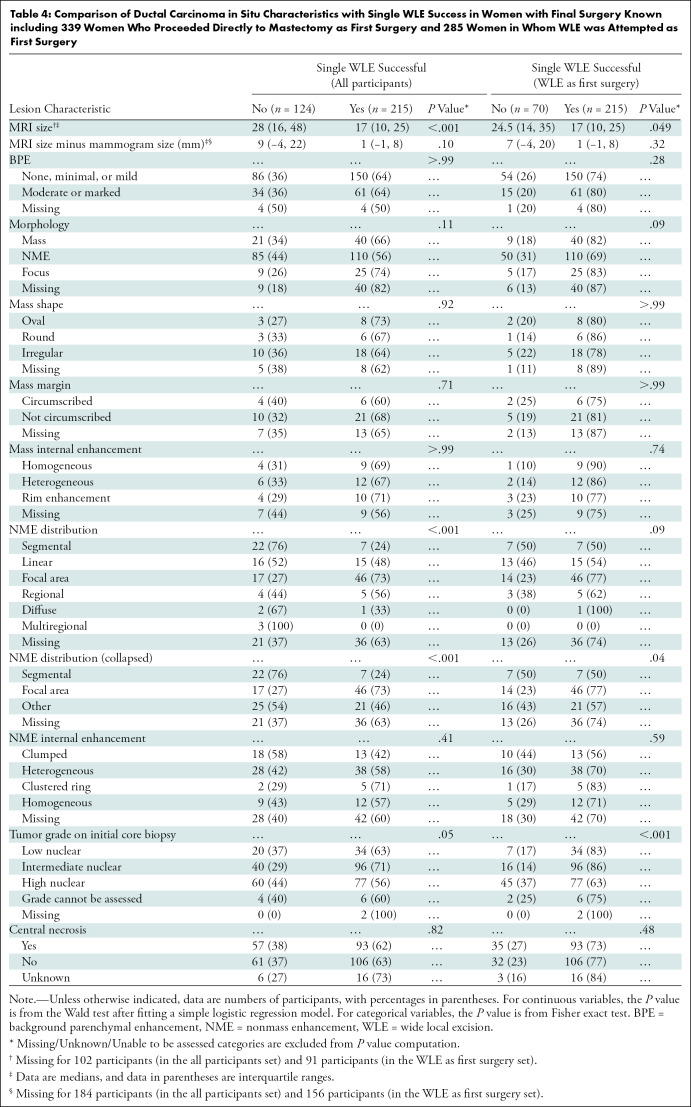
Detailed surgical outcomes of the E4112 trial were previously reported (21). WLE was the first surgery performed in 285 of 339 (84%) women, and the rate of single successful WLE (no subsequent re-excision or mastectomy) was 63% when considering the entire sample (215 of 339) and 75% when considering only those women in whom WLE was first attempted (215 of 285). When considering all 339 participants, women who underwent one successful WLE surgery had smaller DCIS lesions on MRI scans (median, 17 mm; IQR, 10–25 mm) than those who underwent re-excision or mastectomy (median, 28 mm; IQR, 16–48 mm; P < .001) (Table 4). This association remained significant when we excluded the 54 women who underwent mastectomy as their first surgery (P = .049).
Table 4:
Comparison of Ductal Carcinoma in Situ Characteristics with Single WLE Success in Women with Final Surgery Known including 339 Women Who Proceeded Directly to Mastectomy as First Surgery and 285 Women in Whom WLE was Attempted as First Surgery
Among patients with DCIS manifesting as NME, focal distribution was associated with greater single WLE success compared with segmental or other distributions (P < .001 for all participants, P = .04 for WLE as first surgery). This association was not significant in multivariable analysis adjusting for MRI size, BPE, nuclear grade, and central necrosis at CNB among women with WLE as their first surgery (P = .07 to P = .84) (Table E3 [online]). Similarly, low and intermediate nuclear grades were associated with greater single WLE success compared with high nuclear grade (P = .048 for all participants, P < .001 for WLE as first surgery). Moderate or marked BPE versus none or minimal BPE or mild BPE (odds ratio, 3.7; 95% CI: 1.2, 11.3; P = .02) and intermediate versus high nuclear grade DCIS (odds ratio, 2.5; 95% CI: 1.1, 6.0; P = .03) were significantly associated with greater odds of single WLE success among women with WLE as their first surgery. Examples of MRI findings and corresponding surgical outcomes are provided in Figures 2–4. When WLE was first attempted, a higher proportion (43%, 26 of 60) of women who underwent two WLE surgeries required more than one wire or seed for needle localization compared with participants who underwent successful single WLE (23%, 51 of 222) (Table E4 [online]).
Figure 2:
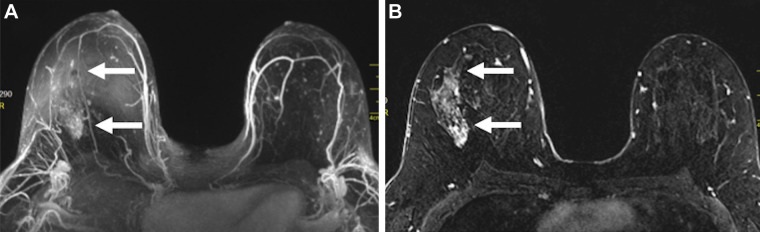
Images in a 46-year-old woman with right breast ductal carcinoma in situ who underwent mastectomy after a greater extent of disease was seen on MRI scans. (A) Maximum intensity projection and (B) first postcontrast axial T1-weighted subtraction images show segmental nonmass enhancement (arrows) measuring 70 mm in the upper outer quadrant of the right breast. Span of disease measured 30 mm at mammography.
Figure 4:
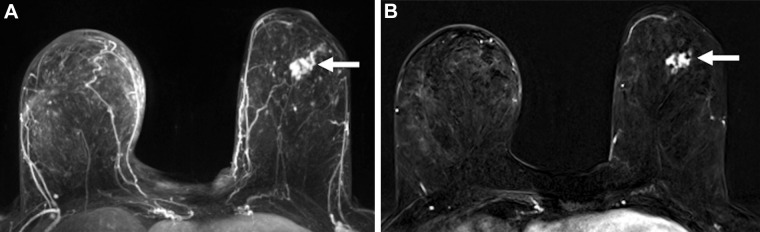
Images in a 60-year-old woman with left breast ductal carcinoma in situ who underwent successful wide local excision after similar extent of disease was seen on MRI scans. (A) Maximum intensity projection and (B) first postcontrast axial T1-weighted subtraction images show an enhancing mass (arrow) measuring 27 mm in the lower outer quadrant. Span of disease measured 27 mm at mammography.
Figure 3:
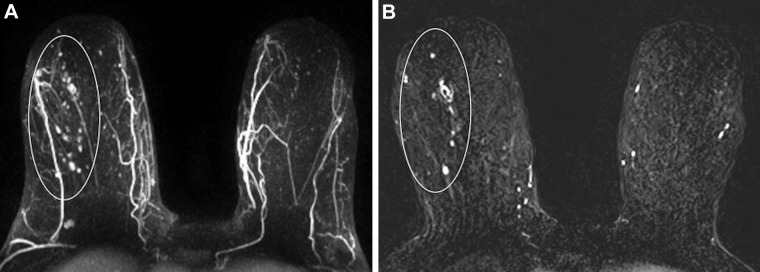
Images in a 72-year-old woman with right breast ductal carcinoma in situ who underwent successful wide local excision after greater extent of disease was seen on MRI scans. (A) Maximum intensity projection and (B) first postcontrast axial T1-weighted subtraction images show segmental nonmass enhancement (oval outline) measuring 100 mm in the upper outer quadrant. Span of disease measured 48 mm at mammography.
Discussion
In this secondary analysis of the multicenter Eastern Cooperative Oncology Group–American College of Radiology Imaging Network E4112 trial, we confirmed that preoperative MRI performed to evaluate the extent of ductal carcinoma in situ (DCIS) diagnosed with conventional imaging most commonly depicts DCIS as nonmass enhancement (NME) with a larger median size. We also found that 6.2% of preoperative MRI-prompted additional biopsies revealed additional disease, with a positive predictive value (PPV) among all participants who underwent additional core-needle biopsy (CNB) of 32% (ipsilateral, 32%; contralateral, 19%) and a false-positive rate of 14.2%. Smaller MRI size (17 mm vs 28 mm) and focal NME distribution were associated with greater single wide local excision success (both P < .001). While basic DCIS features of nuclear grade and central necrosis were highly associated with DCIS score (both P < .001), we found no evidence of qualitative imaging features that enabled prediction of DCIS score or upgrade to invasive disease at surgery (P = .05 to P = .95).
Identification of MRI features associated with meaningful pathologic, genomic, and surgical outcomes is of paramount importance. Multiple ongoing trials are investigating the safety of de-escalating DCIS treatment, including the option of active surveillance in patients at low risk (3–5). However, as many as 25% of women with pure DCIS at CNB are reclassified as having invasive cancer at surgical excision (25). Accurate preoperative prediction of DCIS biology and extent would assist with individualized risk assessment and treatment optimization. A particular strength of our study is the clearly defined entry criteria without specific restrictions based on demographics, mammographic density, DCIS characteristics, or a combination thereof, which mitigate against potential selection biases in prior investigations (8–10,14,16–19). Nevertheless, our specific entry criteria resulted in selection of only a subset (mammography detected) of the spectrum of DCIS.
In support of prior research (6,8,9), we confirmed prospective MRI visualization of most (85.5%) pure DCIS across all nuclear grades after CNB sampling. We corroborated previous findings that pure DCIS commonly manifests as NME on MRI scans and that MRI often depicts larger disease extent than mammography (10,16,17,26). When we assessed the performance measures of preoperative MRI in the detection of additional findings outside the index DCIS in our study, we estimated a specificity of 88.2% and a PPV2 of 25.6%, which are in the expected ranges for benchmarks established for screening MRI (23). The sensitivity of 78.6% is slightly below the breast MRI screening benchmark of more than 80%. However, as mentioned in the Results, two of the examinations with false-negative findings may have been incorrectly designated as false-negative MRI scans due to suspected BI-RADS coding error. If these were deemed true-positive results, then MRI sensitivity would improve to 93% (13 of 14). Also, we demonstrated a favorable PPV3 of 32%, also concordant with benchmarks (range, 20%–50% PPV3) (23). In the absence of established benchmarks for diagnostic MRI, we also found our performance results comparable to reported measures for diagnostic MRI from a single institution, where they showed both PPV2 and PPV3 of 32% and proposed diagnostic MRI benchmarks of 20.5%–37.5% for PPV2 and 24.5%–44.3% for PPV3 (27). Our additional CDR of 6.2% probably impacted surgical planning, as these cancers likely were located at least 2 cm from the index DCIS or in the contralateral breast based on study protocol guidance. Notably, our CDR (1.5%, 95% CI: 0.6, 3.4) and PPV3 (18.5%, 95% CI: 8.2, 36.7) for detection of contralateral disease with MRI after negative conventional imaging and clinical breast examinations were slightly lower than in a prior landmark study by Lehman et al (28) that included both invasive cancers and DCIS as index malignancy (CDR, 3.1%; 95% CI: 2.0, 4.2; PPV3, 21%; 95% CI: 14, 27), although the differences are unlikely to be clinically meaningful.
The upgrade rate of DCIS to invasive cancer at surgical excision in our study was 15%. This is lower than a previously reported estimate of 25.9% from a meta-analysis (25). Our lower upgrade rate may be due to the fact that lesions must be depicted mammographically and must be eligible for WLE before MRI; thus, they may be smaller than those in other studies included in the meta-analysis (25). Among pure DCIS lesions tested in our study, approximately half had a low DCIS score result, which is consistent with previously reported results (29). Qualitative MRI and clinical features did not yield significant associations with DCIS score results and invasive upgrade rates. Qualitative features were limited to the BI-RADS lexicon, and there were substantial missing data, limiting power to identify associations. Furthermore, our study did not assess quantitative or radiomics features to predict these features, which have shown promise in small DCIS cohorts in the prediction of outcomes and pathologic features (30–32) and in larger invasive breast cancer cohorts in the prediction of genomic signatures (33–35).
We report two findings that, to our knowledge, have not been previously described. Both focal NME distribution and smaller DCIS size on MRI scans were associated with single successful WLE. This supports a potential role of preoperative MRI in the prediction of DCIS at risk for requiring re-excisions and suggests bracketed localization approaches may be warranted in such lesions. Our re-excision rate of 25% among participants in whom WLE was first attempted underscores the importance of translating knowledge of disease extent to the surgical suite via preoperative multiple or bracketed wire or seed needle localization to achieve successful WLE.
Our study had limitations. First, this trial was designed to determine preoperative MRI performance in routine practice. Therefore, we could not assess the benefit of newer approaches, such as ultrafast technique, or evaluate MRI performance and correlation with mammography-occult DCIS, which constitutes approximately 50% of all high-grade DCIS lesions (6). Additionally, 80.5% of DCIS lesions in our study were of intermediate or high nuclear grade; therefore, our results might not be generalizable to low-grade DCIS lesions. Second, we could not assess associations of clinical covariates with qualitative mammographic features, which have demonstrated value in predicting DCIS score in one single-site study (36). Third, due to missing pathologic data, we were unable to determine MRI accuracy to depict final pathologic span. Fourth, our analyses were limited by substantial missing data for MRI features, which may have led to type II errors of false rejection of associations of MRI features with pathologic, DCIS score result, and surgical outcomes.
In conclusion, our study confirms that ductal carcinoma in situ (DCIS) diagnosed with conventional imaging is typically depicted as nonmass enhancement (NME) on MRI scans and yields benchmark performance measures in the DCIS preoperative setting. We also report that focal distribution of NME may portend greater single wide local excision success, which has implications for optimal surgical management and warrants further study. Although qualitative MRI features failed to enable prediction of invasive upgrade and DCIS score result of DCIS diagnosed with conventional imaging, quantitative MRI radiomic analyses not limited by the Breast Imaging Reporting and Data System lexicon should be pursued.
Acknowledgments
Acknowledgment
The authors acknowledge the contributions of the late Dr Lawrence J. Solin, whose work inspired this study. He received international recognition for his research on the management of DCIS, including integration of biomarkers and advanced imaging to better individualize therapy.
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health (UG1CA189828, UG1CA233180, UG1CA189822, UG1CA189829, UG1CA189854, UG1CA189859, UG1CA233160, UG1CA233196, UG1CA233290, UG1CA233320, UG1CA233328, and R01CA203883).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government.
Disclosures of Conflicts of Interest: S.H.S.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from GE Healthcare. Other relationships: disclosed no relevant relationships. J.R. disclosed no relevant relationships. C.D.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant to GE Healthcare and Clairity; institution received grants from Hologic and GE Healthcare. Other relationships: disclosed no relevant relationships. S.A.K. disclosed no relevant relationships. R.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: was reimbursed for travel related to role as board of selection committee chair. Other relationships: disclosed no relevant relationships. S.S.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Dako Agilent for Ki67 analysis; gave lectures for Dako (part of Agilent Technologies), Ventana (part of Roche Diagnostics) Targos, and Bristol Myers Squibb; institution holds a patent for three-gene signature for progression of ductal carcinoma in situ. Other relationships: disclosed no relevant relationships. J.X. disclosed no relevant relationships. R.L.C. disclosed no relevant relationships. S.H.J. disclosed no relevant relationships. D.W.S. disclosed no relevant relationships. L.K.H. disclosed no relevant relationships. J.L.S. disclosed no relevant relationships. J.R.B. disclosed no relevant relationships. I.F.G. disclosed no relevant relationships. B.S.S. disclosed no relevant relationships. C.G. disclosed no relevant relationships. L.I.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant to Celgene and Athenex. Other relationships: disclosed no relevant relationships. A.C.W. disclosed no relevant relationships. K.D.M. disclosed no relevant relationships. J.A.S. disclosed no relevant relationships. C.E.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: gave lectures for Bracco Diagnostics. Other relationships: disclosed no relevant relationships. H.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from GE Healthcare. Other relationships: disclosed no relevant relationships.
Abbreviations:
- BI-RADS
- Breast Imaging Reporting and Data System
- BPE
- background parenchymal enhancement
- CDR
- cancer detection rate
- CNB
- core-needle biopsy
- DCIS
- ductal carcinoma in situ
- ECOG-ACRIN
- Eastern Cooperative Oncology Group–American College of Radiology Imaging Network
- IQR
- interquartile range
- NME
- nonmass enhancement
- PPV
- positive predictive value
- WLE
- wide local excision
References
- 1. DeSantis CE , Ma J , Gaudet MM , et al . Breast cancer statistics, 2019 . CA Cancer J Clin 2019. ; 69 ( 6 ): 438 – 451 . [DOI] [PubMed] [Google Scholar]
- 2. Narod SA , Iqbal J , Giannakeas V , Sopik V , Sun P . Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ . JAMA Oncol 2015. ; 1 ( 7 ): 888 – 896 . [DOI] [PubMed] [Google Scholar]
- 3. Elshof LE , Tryfonidis K , Slaets L , et al . Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study . Eur J Cancer 2015. ; 51 ( 12 ): 1497 – 1510 . [DOI] [PubMed] [Google Scholar]
- 4. Hwang ES , Hyslop T , Lynch T , et al . The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS) . BMJ Open 2019. ; 9 ( 3 ): e026797 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Francis A , Thomas J , Fallowfield L , et al . Addressing overtreatment of screen detected DCIS; the LORIS trial . Eur J Cancer 2015. ; 51 ( 16 ): 2296 – 2303 . [DOI] [PubMed] [Google Scholar]
- 6. Kuhl CK , Schrading S , Bieling HB , et al . MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study . Lancet 2007. ; 370 ( 9586 ): 485 – 492 . [DOI] [PubMed] [Google Scholar]
- 7. Kuhl CK , Strobel K , Bieling H , et al . Impact of Preoperative Breast MR Imaging and MR-guided Surgery on Diagnosis and Surgical Outcome of Women with Invasive Breast Cancer with and without DCIS Component . Radiology 2017. ; 284 ( 3 ): 645 – 655 . [DOI] [PubMed] [Google Scholar]
- 8. Baur A , Bahrs SD , Speck S , et al . Breast MRI of pure ductal carcinoma in situ: sensitivity of diagnosis and influence of lesion characteristics . Eur J Radiol 2013. ; 82 ( 10 ): 1731 – 1737 . [DOI] [PubMed] [Google Scholar]
- 9. Hwang ES , Kinkel K , Esserman LJ , Lu Y , Weidner N , Hylton NM . Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity . Ann Surg Oncol 2003. ; 10 ( 4 ): 381 – 388 . [DOI] [PubMed] [Google Scholar]
- 10. Menell JH , Morris EA , Dershaw DD , Abramson AF , Brogi E , Liberman L . Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging . Breast J 2005. ; 11 ( 6 ): 382 – 390 . [DOI] [PubMed] [Google Scholar]
- 11. Balleyguier C , Dunant A , Ceugnart L , et al . Preoperative Breast Magnetic Resonance Imaging in Women With Local Ductal Carcinoma in Situ to Optimize Surgical Outcomes: Results From the Randomized Phase III Trial IRCIS . J Clin Oncol 2019. ; 37 ( 11 ): 885 – 892 . [DOI] [PubMed] [Google Scholar]
- 12. Fancellu A , Turner RM , Dixon JM , Pinna A , Cottu P , Houssami N . Meta-analysis of the effect of preoperative breast MRI on the surgical management of ductal carcinoma in situ . Br J Surg 2015. ; 102 ( 8 ): 883 – 893 . [DOI] [PubMed] [Google Scholar]
- 13. Lam DL , Smith J , Partridge SC , et al . The Impact of Preoperative Breast MRI on Surgical Management of Women with Newly Diagnosed Ductal Carcinoma In Situ . Acad Radiol 2020. ; 27 ( 4 ): 478 – 486 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pilewskie M , Olcese C , Eaton A , et al . Perioperative breast MRI is not associated with lower locoregional recurrence rates in DCIS patients treated with or without radiation . Ann Surg Oncol 2014. ; 21 ( 5 ): 1552 – 1560 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoon GY , Choi WJ , Kim HH , Cha JH , Shin HJ , Chae EY . Surgical Outcomes for Ductal Carcinoma in Situ: Impact of Preoperative MRI . Radiology 2020. ; 295 ( 2 ): 296 – 303 . [DOI] [PubMed] [Google Scholar]
- 16. Esserman LJ , Kumar AS , Herrera AF , et al . Magnetic resonance imaging captures the biology of ductal carcinoma in situ . J Clin Oncol 2006. ; 24 ( 28 ): 4603 – 4610 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jansen SA , Newstead GM , Abe H , Shimauchi A , Schmidt RA , Karczmar GS . Pure ductal carcinoma in situ: kinetic and morphologic MR characteristics compared with mammographic appearance and nuclear grade . Radiology 2007. ; 245 ( 3 ): 684 – 691 . [DOI] [PubMed] [Google Scholar]
- 18. Rahbar H , Partridge SC , Demartini WB , et al . In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters . Radiology 2012. ; 263 ( 2 ): 374 – 382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Itakura K , Lessing J , Sakata T , et al . The impact of preoperative magnetic resonance imaging on surgical treatment and outcomes for ductal carcinoma in situ . Clin Breast Cancer 2011. ; 11 ( 1 ): 33 – 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pilewskie M , Kennedy C , Shappell C , et al . Effect of MRI on the management of ductal carcinoma in situ of the breast . Ann Surg Oncol 2013. ; 20 ( 5 ): 1522 – 1529 . [DOI] [PubMed] [Google Scholar]
- 21. Lehman CD , Gatsonis C , Romanoff J , et al . Association of Magnetic Resonance Imaging and a 12-Gene Expression Assay With Breast Ductal Carcinoma In Situ Treatment . JAMA Oncol 2019. ; 5 ( 7 ): 1036 – 1042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeMartini WB , Rahbar H . Breast magnetic resonance imaging technique at 1.5 T and 3 T: requirements for quality imaging and American College of Radiology accreditation . Magn Reson Imaging Clin N Am 2013. ; 21 ( 3 ): 475 – 482 . [DOI] [PubMed] [Google Scholar]
- 23. Morris EA , Comstock CE , Lee CH , et al . ACR BI-RADS Magnetic Resonance Imaging . In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System . Reston, Va: : American College of Radiology, 2013. . [Google Scholar]
- 24. Solin LJ , Gray R , Baehner FL , et al . A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast . J Natl Cancer Inst 2013. ; 105 ( 10 ): 701 – 710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brennan ME , Turner RM , Ciatto S , et al . Ductal carcinoma in situ at core-needle biopsy: meta-analysis of underestimation and predictors of invasive breast cancer . Radiology 2011. ; 260 ( 1 ): 119 – 128. 10.1148/radiol.11102368 . [DOI] [PubMed] [Google Scholar]
- 26. Nori J , Meattini I , Giannotti E , et al . Role of preoperative breast MRI in ductal carcinoma in situ for prediction of the presence and assessment of the extent of occult invasive component . Breast J 2014. ; 20 ( 3 ): 243 – 248 . [DOI] [PubMed] [Google Scholar]
- 27. Sedora Román NI , Mehta TS , Sharpe RE , et al . Proposed biopsy performance benchmarks for MRI based on an audit of a large academic center . Breast J 2018. ; 24 ( 3 ): 319 – 324 . [DOI] [PubMed] [Google Scholar]
- 28. Lehman CD , Gatsonis C , Kuhl CK , et al . MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer . N Engl J Med 2007. ; 356 ( 13 ): 1295 – 1303 . [DOI] [PubMed] [Google Scholar]
- 29.Rakovitch EK, Koch A, Grimard L, et al. Refined local recurrence risk estimates based on a multigene expression assay combined with clinicopathological features significantly impacts radiotherapy recommendation in patients with low/intermediate risk DCIS treated with breast-conserving surgery [abstract]. In: Proceedings of the 2019 San Antonio Breast Cancer Symposium, San Antonio, Tex, December 10–14, 2019. Philadelphia, Pa: American Association for Cancer Research, 2019;P6-16-01. [Google Scholar]
- 30. Chou SS , Gombos EC , Chikarmane SA , Giess CS , Jayender J . Computer-aided heterogeneity analysis in breast MR imaging assessment of ductal carcinoma in situ: Correlating histologic grade and receptor status . J Magn Reson Imaging 2017. ; 46 ( 6 ): 1748 – 1759 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim SA , Cho N , Ryu EB , et al . Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery . Radiology 2014. ; 270 ( 3 ): 699 – 707 . [DOI] [PubMed] [Google Scholar]
- 32. Luo J , Johnston BS , Kitsch AE , et al . Ductal Carcinoma in Situ: Quantitative Preoperative Breast MR Imaging Features Associated with Recurrence after Treatment . Radiology 2017. ; 285 ( 3 ): 788 – 797 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ha R , Chang P , Mutasa S , et al . Convolutional Neural Network Using a Breast MRI Tumor Dataset Can Predict Oncotype Dx Recurrence Score . J Magn Reson Imaging 2019. ; 49 ( 2 ): 518 – 524 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li H , Zhu Y , Burnside ES , et al . MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays . Radiology 2016. ; 281 ( 2 ): 382 – 391 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nam KJ , Park H , Ko ES , Lim Y , Cho HH , Lee JE . Radiomics signature on 3T dynamic contrast-enhanced magnetic resonance imaging for estrogen receptor-positive invasive breast cancers: Preliminary results for correlation with Oncotype DX recurrence scores . Medicine (Baltimore) 2019. ; 98 ( 23 ): e15871 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woodard GA , Price ER . Qualitative Radiogenomics: Association Between BI-RADS Calcification Descriptors and Recurrence Risk as Assessed by the Oncotype DX Ductal Carcinoma In Situ Score . AJR Am J Roentgenol 2019. ; 212 ( 4 ): 919 – 924 . [DOI] [PubMed] [Google Scholar]