Abstract
Background
The function of high‐density lipoprotein can change from protective to proatherosclerotic under inflammatory conditions. Herein, we studied whether inflammation could modify the relationship between high‐density lipoprotein level and risk of adverse outcomes in patients with chronic kidney disease .
Methods and Results
In total, 1864 patients from the prospective KNOW‐CKD (Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease) were enrolled. The main predictor was high‐density lipoprotein cholesterol (HDL‐C) level. Presence of inflammation was defined by hs‐CRP (high‐sensitivity C‐reactive protein) level of ≥1.0 mg/L. The primary outcome was extended major adverse cardiovascular events. During 9231.2 person‐years of follow‐up, overall incidence of the primary outcome was 15.8 per 1000 person‐years. In multivariable Cox analysis after adjusting for confounders, HDL‐C level was not associated with the primary outcome. There was a significant interaction between the inflammatory status and HDL‐C for risk of extended major adverse cardiovascular events (P=0.003). In patients without inflammation, the hazard ratios (HRs) (95% CIs) for HDL‐C levels <40, 50 to 59, and ≥60 mg/dL were 1.10 (0.50–1.82), 0.95 (0.50–1.82), and 0.42 (0.19–0.95), respectively, compared with HDL‐C of 40 to 49 mg/dL. However, the significant association for HDL‐C ≥60 mg/dL was not seen after Bonferroni correction. In patients with inflammation, we observed a trend toward increased risk of extended major adverse cardiovascular events in higher HDL‐C groups (HRs [95% CIs], 0.73 [0.37–1.43], 1.24 [0.59–2.61], and 1.56 [0.71–3.45], respectively), but without statistical significance.
Conclusions
The association between HDL‐C level and adverse cardiovascular outcomes showed reverse trends based on inflammation status in Korean patients with chronic kidney disease.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01630486.
Keywords: chronic kidney disease, high‐density lipoprotein cholesterol, high‐sensitivity C‐reactive protein, inflammation, major adverse cardiovascular events
Subject Categories: Cardiovascular Disease, Lipids and Cholesterol, Nephrology and Kidney
Nonstandard Abbreviations and Acronyms
- eMACE
extended major adverse cardiovascular event
- MACE
major adverse cardiovascular event
Clinical Perspective
What Is New?
In this Korean cohort study including 1864 patients with chronic kidney disease, there was a significant interaction between the inflammatory status and high‐density lipoprotein cholesterol for risk of cardiovascular outcomes.
In patients without inflammation, a higher high‐density lipoprotein cholesterol level was nonlinearly associated with a lower risk of cardiovascular outcomes, although this association was not significant after statistical adjustment with Bonferroni correction.
In contrast, the opposite graded association was observed in patients with inflammation.
What Are the Clinical Implications?
In patients with chronic kidney disease, clinical implications of high‐density lipoprotein cholesterol level can be differently interpreted on the basis of the inflammatory status.
Cardiovascular disease (CVD) is the leading cause of mortality worldwide. Besides the traditional risk factors, such as hypertension, diabetes mellitus, and hypercholesterolemia, chronic kidney disease (CKD) is considered as a nontraditional risk factor for CVD. The risk of adverse atherosclerotic vascular events increases, even in patients with mildly decreased kidney function.1 Notably, other nontraditional risk factors, such as inflammation, oxidative stress, and CKD‐related bone and mineral disorders, are highly prevalent among patients with CKD. These factors can collectively lead to remarkably high mortality rates.
Among the traditional risk factors, dyslipidemia, which is characterized by the elevated levels of triglycerides and low‐density lipoprotein (LDL) cholesterol and decreased levels of high‐density lipoprotein cholesterol (HDL‐C), is commonly encountered in patients with CKD. The role of LDL as a major culprit in the development of atherosclerosis has been well established. The lowering of LDL cholesterol levels reduces the occurrence of major adverse cardiovascular events (MACEs). The beneficial effects of LDL cholesterol lowering therapy have been shown in nondialysis patients with CKD.2 In contrast, high‐density lipoprotein (HDL), known as the “good cholesterol,” provides antiatherogenic effects mostly attributed to its potent anti‐inflammatory, antioxidative, and antithrombotic properties. It also plays a role in reverse cholesterol transport,3 through which surplus cholesterol is removed from lipid‐laden macrophages and peripheral tissues, thereby providing atheroprotection. An inverse linear relationship has been established between HDL‐C level and CVD risk.4
In contrast to the traditional concept of HDL‐C, recent Mendelian studies showed no causality between HDL‐C and CVD.5, 6 In addition, several randomized controlled studies failed to demonstrate the beneficial effects of HDL‐C on the prevention of CVD.7, 8 Moreover, there has been evidence suggesting contrasting effects of HDL in certain conditions, such as diabetes mellitus and/or CKD, wherein HDL can induce endothelial dysfunction and arterial hypertension through the generation of systemic oxidative stress rather than conferring vascular protection.9 In addition, there is growing evidence that the composition of HDL, rather than the circulating HDL‐C level, determines its functional properties.10 In contrast to the conventional notion about the inverse linear relationship between the HDL‐C levels and adverse outcomes, a U‐shaped association of HDL‐C levels with mortality has been reported in a large population‐based study.11 Therefore, the discrepancy in the clinical implications of HDL under various conditions should be clarified.
It is well established that inflammation greatly contributes to the pathogenesis of atherosclerosis. Low‐grade inflammation is a key feature of CKD, and persistent inflammation can accelerate the decline in kidney function and worsen vascular disease in patients with CKD.12 The functional properties of HDL can be influenced by inflammation,10 and its antioxidative and antithrombotic actions become less effective during kidney failure.13, 14 Therefore, we hypothesized that inflammation can alter the conventional relationship between the HDL‐C level and CVD risk in patients with CKD. In this study, we examined the clinical implications of HDL‐C levels for predicting MACEs in the presence and absence of inflammation among Korean patients with CKD.
METHODS
Because of ethical issues and data protection regulations, data that support the findings of the present study cannot be made publicly available.
Study Design and Participants
The KNOW‐CKD (Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease) is a nationwide multicenter prospective study aimed at investigating renal and cardiovascular outcomes in Korean patients with predialysis CKD, stages 1 through 5. The detailed design, methods, study rationale, participant enrollment, and protocol summary have been described previously (NCT01630486 at http://www.clinicaltrials.gov).15 In total, 2238 adults, aged 20 to 75 years, were enrolled from 9 tertiary‐care hospitals between 2011 and 2016. Among the participants, 58 patients who were lost to follow‐up after baseline visit were excluded. In addition, 316 patients with missing data on demographics, including body mass index, socioeconomic status based on income, and educational background; behavioral patterns, including history of smoking; laboratory test results, including fasting blood glucose, serum albumin, phosphate, and HDL‐C levels; and baseline renal functions, including glomerular filtration rate and urine protein/creatinine ratio, were excluded. Finally, 1864 participants were included in the analysis (Figure S1). Detailed description on data collection is in Data S1. We used the creatinine method, which requires calibration traceable to isotope dilution mass spectrometry, and calculated the estimated glomerular filtration rate using the CKD Epidemiologic Collaboration equation.16 Urine protein/creatinine ratio was calculated as the urine protein concentration divided by the urine creatinine concentration (g/g).
This study was conducted in accordance with the principles of Declaration of Helsinki, and the study protocol was approved by the institutional review board at each participating clinical center, as follows: Yonsei University Severance Hospital (4‐2011‐0163), Seoul National University Hospital (1104‐089‐359), Seoul National University Bundang Hospital (B‐1106/129‐008), Kangbuk Samsung Medical Center (2011‐01‐076), Seoul St. Mary's Hospital (KC11OIMI0441), Gil Hospital (GIRBA2553), Eulji General Hospital (201105‐01), Chonnam National University Hospital (CNUH‐2011‐092), and Pusan Paik Hospital (11‐091) in 2011. All participants provided the written informed consent.
Exposure and Outcome Ascertainment
The exposures of interest were serum HDL‐C level and presence of inflammation, which was defined as hs‐CRP (high‐sensitivity C‐reactive protein) level ≥1.0 mg/L, following the recommendations proposed by the Centers for Disease Control and Prevention and the American Heart Association.17 Considering a possible nonlinear relationship with the outcomes, the serum HDL‐C levels were categorized into the following 4 groups with 10‐mg/dL increments: <40, 40 to 49 (reference), 50 to 59, and ≥60 mg/dL. The primary outcome was extended MACEs (eMACEs), including fatal and nonfatal cardiovascular events, such as myocardial infarction, unstable angina, coronary intervention/surgery, hospitalization for heart failure, symptomatic arrhythmia, and/or cardiac death. Secondary end points included separate outcomes of nonfatal MACEs and all‐cause mortality. Participants were followed up until March 31, 2019, and were censored at the date of the last visit or the occurrence of events, as applicable.
Statistical Analysis
Data from descriptive analyses are presented as mean±SD, median (interquartile range), or proportion, as appropriate. Data were compared using the Student t test, analysis of variance, Kruskal‐Wallis test, and χ2 test. Cox proportional‐hazards model was used to evaluate the associations between serum HDL‐C category and subsequent cardiovascular outcomes based on the presence of inflammation. This association was assessed with HDL‐C being treated as a continuous variable per SD increase. On the basis of the confounding factors, 3 models were constructed for each analysis. Factors that were included in a stepwise manner for adjustment included the following: factors known to affect the risk of CVD, factors that significantly differed among 4 HDL‐C groups, and factors shown to have significant association with the primary outcome (ie, P<0.10) in the unadjusted model. Model 1 was adjusted for sociodemographic factors and anthropometric data, including age, sex, smoking status, socioeconomic status, educational level, body mass index, systolic blood pressure, presence of coronary artery disease, and diabetes mellitus. Model 2 was adjusted for the laboratory parameters, including estimated glomerular filtration rate, urine protein/creatinine ratio, levels of fasting blood glucose, LDL cholesterol, triglyceride, serum albumin, and hs‐CRP, in addition to variables adjusted in model 1. Model 3 was further adjusted for medication use, including renin‐angiotensin system blockers, diuretics, and lipid‐lowering drugs (statins). We further performed multiple comparisons among HDL categories after statistical adjustment using Bonferroni method. For sensitivity analyses to assess robustness of our findings, study participants were stratified into quartiles according to the serum levels of HDL‐C. In addition, another different cutoff value for the status of inflammation was examined using the median value of hs‐CRP. Furthermore, we constructed the time‐varying model with lipid‐lowering drugs treated as time‐varying covariate. For secondary analyses for nonfatal MACEs, we used a cause‐specific hazard function for competing risk model. In this analysis, noncardiac death that occurred before nonfatal MACE was treated as a competing risk and was censored. Kaplan‐Meier curve analysis for the cumulative incidence of primary outcome was used to derive the incidence rates, and differences among groups were compared by log‐rank test. We explored the continuous and nonlinear relationship between serum HDL‐C level and study outcomes using fully adjusted restricted cubic spline model with 3 knots placed at the 25th, 50th, and 75th percentiles. To examine the effect modification of the relationship between HDL‐C level and inflammation, we performed subgroup analyses stratified by age (<60 versus ≥60 years), sex, body mass index (<25 versus ≥25 kg/m2), medical history of diabetes mellitus (presence versus absence), systolic blood pressure (<140 versus ≥140 mm Hg), serum albumin (<4.0 versus ≥4.0 g/dL), baseline estimated glomerular filtration rate (<50 versus ≥50 mL/min per 1.73 m2), and urine protein/creatinine ratio (<1.0 versus ≥1.0 g/g). All exposure‐event associations are expressed as hazard ratios (HRs) and 95% CIs. The differences with P<0.05 were considered statistically significant. All statistical analyses were performed using Stata, version 14.2 (StataCorp, College Station, TX), and R, version 3.4.3 (www.r‐project.org; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline Characteristics of the Participants
The baseline characteristics of the participants, stratified by the HDL‐C categories, are presented in Table 1 and Table S1. The mean HDL‐C level was 33.6±4.6 mg/dL. The histogram and kernel density plot, showing the distribution of HDL‐C, are presented in Figure S2. Most of the patients with higher HDL‐C levels were women, young, and nonsmokers, had lower body mass index, and had fewer comorbid conditions, such as hypertension, diabetes mellitus, coronary artery disease, and cerebrovascular disease. These patients had lower hs‐CRP and fasting blood glucose levels, more preserved kidney function, and decreased urinary protein excretion. A significant inverse relationship between the HDL‐C and hs‐CRP levels (γ=−0.234; P<0.001) was observed (Figure S3).
Table 1.
Baseline Characteristics of Participants Based on HDL‐C Categories
| Characteristics | HDL‐C Categories, mg/dL | Total (N=1864) | P Value | |||
|---|---|---|---|---|---|---|
|
<40 (N=514) |
40–49 (N=537) |
50–59 (N=407) |
≥60 (N=406) |
|||
| Demographic data | ||||||
| Age, y | 56.2±11.3 | 53.6±12.1 | 52.6±12.7 | 51.2±12.7 | 53.6±12.3 | <0.001 |
| Men, n (%) | 405 (78.8) | 354 (65.9) | 222 (54.5) | 152 (37.4) | 1133 (60.8) | <0.001 |
| BMI, kg/m2 | 25.29±3.12 | 25.02±3.24 | 24.38±3.51 | 23.16±3.34 | 24.55±3.39 | <0.001 |
| SBP, mm Hg | 128.5±16.9 | 127.9±14.7 | 127.1±16.7 | 126.5±15.3 | 127.58±15.9 | 0.22 |
| DBP, mm Hg | 76.2±11.2 | 76.8±10.4 | 77.7±11.7 | 77.1±11.0 | 76.9±11. | 0.25 |
| Economic status, n (%) | 0.02 | |||||
| ≥$4905/mo | 95 (18.5) | 135 (25.1) | 94 (23.1) | 111 (27.3) | 435 (23.3) | |
| $1635–$4905/mo | 282 (54.9) | 277 (51.6) | 218 (53.6) | 218 (53.7) | 995 (53.4) | |
| <$1635/mo | 137 (26.7) | 125 (23.3) | 95 (23.3) | 77 (19.0) | 434 (23.3) | |
| Education, n (%) | 0.39 | |||||
| <9 y | 69 (13.4) | 64 (11.9) | 56 (13.8) | 40 (9.9) | 229 (12.3) | |
| 9–12 y | 69 (13.4) | 66 (12.3) | 44 (10.8) | 44 (10.8) | 223 (12.0) | |
| ≥12 y | 376 (73.2) | 407 (75.8) | 307 (75.4) | 322 (79.3) | 1412 (75.8) | |
| Smoking status, n (%) | <0.001 | |||||
| Never | 192 (37.4) | 282 (52.5) | 236 (58.0) | 290 (71.4) | 1000 (53.6) | |
| Current | 113 (22.0) | 93 (17.3) | 60 (14.7) | 34 (8.4) | 300 (16.1) | |
| Former | 209 (40.7) | 162 (30.2) | 111 (27.3) | 82 (20.2) | 564 (30.3) | |
| Comorbidities | ||||||
| Hypertension, n (%) | 509 (99.0) | 525 (97.8) | 389 (95.6) | 369 (90.9) | 1792 (96.1) | <0.001 |
| Diabetes mellitus, n (%) | 231 (44.9) | 200 (37.2) | 97 (23.8) | 94 (23.2) | 622 (33.4) | <0.001 |
| MI, n (%) | 20 (3.9) | 8 (1.5) | 1 (0.2) | 1 (0.2) | 30 (1.6) | <0.001 |
| CVD, n (%) | 40 (7.8) | 31 (5.8) | 29 (7.1) | 11 (2.7) | 111 (6.0) | 0.008 |
| Charlson comorbidity index | 4.1±2.1 | 3.5±2.1 | 3.0±2.3 | 2.7±2.1 | 3.4±2.2 | <0.001 |
| Medication, n (%) | 226 (36.8) | 173 (27.3) | 73 (15.9) | 87 (16.4) | 559 (25.0) | |
| ACE inhibitor | 52 (10.1) | 69 (12.8) | 39 (9.6) | 43 (10.6) | 203 (10.9) | 0.36 |
| ARB | 431 (83.9) | 434 (80.8) | 323 (79.4) | 303 (74.6) | 1491 (80.0) | 0.006 |
| Diuretics | 210 (40.9) | 189 (35.2) | 104 (25.6) | 79 (19.5) | 582 (31.2) | <0.001 |
| Statin | 0.04 | |||||
| No statin | 229 (44.6) | 257 (47.9) | 199 (48.9) | 209 (51.5) | 894 (48.0) | |
| Low intensity | 39 (7.6) | 58 (10.8) | 21 (5.2) | 26 (6.4) | 144 (7.7) | |
| Moderate intensity | 235 (45.7) | 208 (38.7) | 177 (43.8) | 160 (39.4) | 780 (41.8) | |
| High intensity | 11 (2.1) | 14 (2.6) | 10 (2.5) | 11 (2.7) | 46 (2.5) | |
| Niacin | 0 (0.0) | 3 (0.6) | 0 (0.0) | 0 (0.0) | 3 (0.2) | 0.06 |
| Laboratory parameters | ||||||
| eGFR, mL/min per 1.73 m2 | 41.4±24.9 | 52.6±30.0 | 57.9±31.1 | 64.2±33.3 | 53.3±30.9 | <0.001 |
| BUN, mg/dL | 32.5±17.0 | 28.1±15.1 | 26.4±15.2 | 24.6±14.4 | 28.2±15.8 | <0.001 |
| Albumin, g/dL | 4.1±0.4 | 4.2±0.4 | 4.2±0.4 | 4.2±0.4 | 4.2±0.4 | 0.07 |
| Fasting glucose, mg/dL | 114.3±42.5 | 112.8±40.8 | 108.2±37.2 | 103.5±30.4 | 110.2±38.7 | <0.001 |
| hs‐CRP, mg/L | 0.9 (0.4–2.3) | 0.7 (0.3–1.7) | 0.5 (0.2–1.5) | 0.3 (0.1–1.0) | 0.6 (0.2–1.64) | <0.001 |
| Tchol, mg/dL | 158.3±37.0 | 173.1±37.8 | 177.9±36.0 | 191.0±37.2 | 173.9±38.7 | <0.001 |
| HDL‐C, mg/dL | 33.6±4.6 | 44.3±2.8 | 54.2±3.0 | 72.1±13.0 | 49.6±15.5 | <0.001 |
| Triglyceride, mg/dL | 176.0 (130.0–244.0) | 141.5 (101.0–197.0) | 112.0 (85.0–161.0) | 95.0 (73.0–135.5) | 132.0 (92.0–192.0) | <0.001 |
| LDL‐C, mg/dL | 88.6±29.7 | 100.3±32.2 | 100.2±30.6 | 100.0±31.7 | 96.9±31.5 | <0.001 |
| uPCR, g/g | 0.6 (0.2–1.8) | 0.5 (0.2–1.5) | 0.4 (0.1–1.3) | 0.4 (0.1–1.4) | 0.5 (0.1–1.5) | 0.002 |
Data are presented as mean±SD, number (percentage), or median (interquartile range). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; BUN, blood urea nitrogen; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; SBP, systolic blood pressure; Tchol, total cholesterol; and uPCR, urine protein/creatinine ratio.
Association of HDL‐C Level With the Risk of eMACEs
During 9231.2 person‐years of follow‐up (median, 5.1 years), the primary outcome of eMACEs was observed in 140 (7.5%) patients with an incidence rate of 15.8 per 1000 person‐years. There were 44 (8.6%), 44 (8.2%), 31 (7.6%), and 21 (5.2%) primary outcome events in patients with HDL‐C levels of <40, 40 to 49, 50 to 59, and ≥60 mg/dL, respectively (Table 2). In multivariable Cox model after sequential adjustments, no association of HDL‐C level with the risk of eMACEs was observed (Table 3 and Figure 1). Compared with the HDL‐C category of 40 to 49 mg/dL, the HDL‐C categories of <40, 50 to 59, and ≥60 mg/dL showed adjusted HRs (95% CIs) of 0.93 (0.59–1.44), 1.05 (0.65–1.68), and 0.72 (0.41–1.24), respectively. In the additional analysis with HDL‐C as a continuous variable, 1‐SD increase in HDL‐C level was not associated with the primary outcome.
Table 2.
Rates of Adverse Outcome Events Based on the HDL‐C Categories Overall and in the Absence and Presence of Inflammation
| Variable | Total | HDL‐C Categories | |||
|---|---|---|---|---|---|
| <40 mg/dL | 40–49 mg/dL | 50–59 mg/dL | ≥60 mg/dL | ||
| All | |||||
| No. of participants | 1864 | 514 | 537 | 407 | 406 |
| Person‐years | 8888.3 | 2323.6 | 2549.8 | 1922.2 | 2092.7 |
| eMACE* | |||||
| Events, n (%) | 140 (7.5) | 44 (8.6) | 44 (8.2) | 31 (7.6) | 21 (5.2) |
| Incidence rate per 1000 person‐years | 15.8 | 18.9 | 17.3 | 16.1 | 10.0 |
| Non‐fatal MACE | |||||
| Events, n (%) | 122 (6.5) | 39 (7.6) | 37 (6.9) | 28 (6.9) | 18 (4.4) |
| Incidence rate per 1000 person‐years | 13.7 | 16.8 | 14.5 | 14.6 | 8.6 |
| All‐cause mortality | |||||
| Events, n (%) | 96 (5.2) | 37 (7.2) | 25 (4.7) | 19 (4.7) | 15 (3.7) |
| Incidence rate per 1000 person‐years | 10.4 | 15.1 | 9.5 | 9.5 | 7.0 |
| In the absence of inflammation | |||||
| No. of participants | 1142 | 260 | 314 | 267 | 301 |
| Person‐years | 5495.5 | 1141.9 | 1473.1 | 1291.2 | 1589.4 |
| eMACE | |||||
| Events, n (%) | 76 (6.7) | 24 (9.2) | 25 (8.0) | 18 (6.7) | 9 (3.0) |
| Incidence rate per 1000 person‐years | 13.8 | 21.0 | 17.0 | 13.9 | 5.7 |
| Non‐fatal MACE | |||||
| Events, n (%) | 67 (5.9) | 21 (8.1) | 22 (7.0) | 16 (6.0) | 8 (2.7) |
| Incidence rate per 1000 person‐years | 12.2 | 18.4 | 14.9 | 12.4 | 5.0 |
| All‐cause mortality | |||||
| Events, n (%) | 45 (3.9) | 18 (6.9) | 11 (3.5) | 9 (3.4) | 7 (2.3) |
| Incidence rate per 1000 person‐years | 7.9 | 14.9 | 7.3 | 6.7 | 4.3 |
| In the presence of inflammation | |||||
| No. of participants | 722 | 254 | 223 | 140 | 105 |
| Person‐years | 3392.8 | 1181.7 | 1076.7 | 631.0 | 503.3 |
| eMACE | |||||
| Events, n (%) | 64 (8.9) | 20 (7.9) | 19 (8.5) | 13 (9.3) | 12 (11.4) |
| Incidence rate per 1000 person‐years | 18.9 | 16.9 | 17.6 | 20.6 | 23.8 |
| Non‐fatal MACE | |||||
| Events, n (%) | 55 (7.6) | 18 (7.1) | 15 (6.7) | 12 (8.6) | 10 (9.5) |
| Incidence rate per 1000 person‐years | 16.2 | 15.2 | 13.9 | 19.0 | 19.9 |
| All‐cause mortality | |||||
| Events, n (%) | 51 (7.1) | 19 (7.5) | 14 (6.3) | 10 (7.1) | 8 (7.6) |
| Incidence rate per 1000 person‐years | 14.3 | 15.2 | 12.6 | 15.1 | 14.8 |
eMACE indicates extended major adverse cardiovascular events; and HDL‐C, high‐density lipoprotein cholesterol.
eMACE included both fatal and non‐fatal major cardiovascular events, such as myocardial infarction, unstable angina, coronary intervention/surgery, hospitalization for heart failure, symptomatic arrhythmia, and/or cardiac death.
Table 3.
HRs for the eMACE Outcomes Based on the HDL‐C Categories Overall and in the Absence and Presence of Inflammation
| Variable | HDL‐C per SD | HDL‐C Category, mg/dL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <40 | 40–49 | 50–59 | ≥60 | |||||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| All | ||||||||||
| Model 1* | 0.90 (0.74–1.10) | 0.31 | 0.90 (0.59–1.37) | 0.63 | Reference | 1.05 (0.66–1.67) | 0.83 | 0.75 (0.44–1.29) | 0.30 | |
| Model 2† | 0.85 (0.68–1.06) | 0.15 | 0.94 (0.60–1.46) | 0.77 | Reference | 1.03 (0.64–1.65) | 0.91 | 0.71 (0.41–1.23) | 0.23 | |
| Model 3‡ | 0.86 (0.70–1.07) | 0.18 | 0.93 (0.59–1.44) | 0.74 | Reference | 1.05 (0.65–1.68) | 0.85 | 0.72 (0.41–1.24) | 0.23 | |
| In the absence of inflammation | ||||||||||
| Model 1 | 0.73 (0.56–0.97) | 0.03 | 0.96 (0.54–1.69) | 0.88 | Reference | 0.95 (0.51–1.76) | 0.86 | 0.43 (0.20–0.95) | 0.04 | |
| Model 2 | 0.67 (0.49–0.92) | 0.01 | 1.07 (0.59–1.95) | 0.82 | Reference | 0.92 (0.48–1.75) | 0.79 | 0.43 (0.19–0.96) | 0.04 | |
| Model 3 | 0.67 (0.48–0.92) | 0.01 | 1.10 (0.60–2.00) | 0.76 | Reference | 0.95 (0.50–1.82) | 0.88 | 0.42 (0.19–0.95) | 0.04 | |
| In the presence of inflammation | ||||||||||
| Model 1 | 1.25 (0.96–1.63) | 0.10 | 0.81 (0.43–1.55) | 0.53 | Reference | 1.25 (0.61–2.54) | 0.55 | 1.71 (0.80–3.64) | 0.16 | |
| Model 2 | 1.19 (0.90–1.58) | 0.22 | 0.69 (0.35–1.36) | 0.29 | Reference | 1.16 (0.55–2.44) | 0.70 | 1.55 (0.70–3.43) | 0.28 | |
| Model 3 | 1.19 (0.90–1.57) | 0.22 | 0.73 (0.37–1.43) | 0.35 | Reference | 1.24 (0.59–2.61) | 0.58 | 1.56 (0.71–3.45) | 0.27 | |
eMACE indicates extended major adverse cardiovascular event; HDL‐C, high‐density lipoprotein cholesterol; and HR, hazard ratio.
Model 1: adjusted for age, sex, body mass index, smoking status, socioeconomic status, educational status, systolic blood pressure, presence of coronary artery disease, and diabetes mellitus.
Model 2: model 1+laboratory parameters, including fasting blood glucose, low‐density lipoprotein cholesterol, triglyceride, serum albumin, hs‐CRP (high‐sensitivity C‐reactive protein), estimated glomerular filtration rate, and urine protein/creatinine ratio.
Model 3: model 2+medications' use, including renin‐angiotensin system blockers, diuretics, and statins.
Figure 1. Association of high‐density lipoprotein cholesterol (HDL‐C) level with the primary outcome.
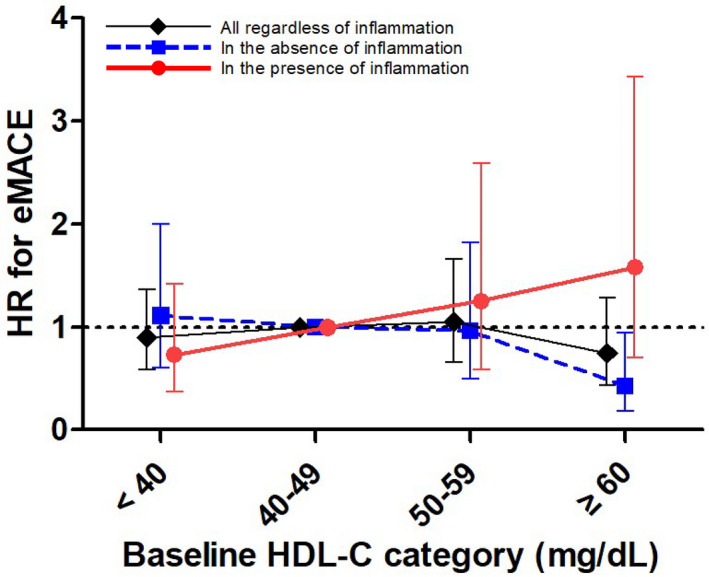
In multivariable Cox model after sequential adjustments, the relationship of HDL‐C level with cardiovascular diseases differed depending on the inflammatory status in patients with chronic kidney disease. In the absence of inflammation, HDL‐C level ≥60 mg/dL was associated with a lower risk of extended major adverse cardiovascular events (eMACEs), whereas this relationship was reversed in the presence of inflammation. The model is adjusted for age, sex, body mass index, smoking status, socioeconomic status, educational status, systolic blood pressure, presence of coronary artery disease, diabetes mellitus, laboratory parameters, including fasting blood glucose, low‐density lipoprotein cholesterol, triglycerides, serum albumin, hs‐CRP (high‐sensitivity C‐reactive protein), estimated glomerular filtration rate, and urine protein/creatinine ratio, and medications' use, including renin‐angiotensin system blockers, diuretics, and statins. Bars represent 95% CIs. HR indicates hazard ratio.
Risk‐Modifying Effect of Inflammation on the Relationship Between HDL‐C Level and Cardiovascular Outcome
We examined whether the association between the HDL‐C level and risk of eMACEs differed according to the inflammatory status. There was a significant interaction between the inflammatory status and HDL‐C for risk of eMACEs (P=0.003). Among the patients without inflammation (hs‐CRP <1.0 mg/L), the incidence rate of eMACEs decreased gradually in patients with increasing HDL‐C concentrations. A nonlinear inverse relationship was observed between the HDL‐C level and risk of eMACEs in multivariable Cox analysis. Adjusted HRs (95% CIs) for the HDL‐C categories of <40, 50 to 59, and ≥60 mg/dL were 1.10 (0.60–2.00), 0.95 (0.50–1.82), and 0.42 (0.19–0.95), respectively, compared with the HDL‐C category of 40 to 49 mg/dL (Table 3 and Figure 1). However, this association was not significant after statistical adjustment with Bonferroni correction (Table S2). Additional analysis with HDL‐C as a continuous variable showed that 1‐SD increase in the HDL‐C level was significantly associated with a 33% lower risk of eMACEs in patients without inflammation (Table 3). In contrast, this relationship disappeared among patients with inflammation (hs‐CRP ≥1.0 mg/L). Multivariable Cox models revealed a graded increase in the HRs for the risk of eMACEs, but these did not reach statistical significance. Adjusted HRs (95% CIs) for the HDL‐C categories of <40, 50 to 59, and ≥60 mg/dL were 0.73 (0.37–1.43), 1.24 (0.59–2.61), and 1.56 (0.71–3.45), respectively, compared with the HDL‐C category of 40 to 49 mg/dL (Table 3 and Figure 1). The opposite direction of the association between HDL‐C and eMACEs in patients with inflammation was also observed when HDL‐C was treated as a continuous variable (Table 3). As shown in Figure 2, the unadjusted cumulative incidence of the primary outcome was notably lower in patients without inflammation and with HDL‐C levels of ≥60 mg/dL, whereas this pattern disappeared in those with inflammation. Restricted cubic spline curves for the adjusted HRs for eMACEs also corroborated these findings (Figure S4). Additional sensitivity analyses with HDL‐C quartiles (Table S3) and with the different cutoff of hs‐CRP (Table S4) all showed that the different association of HDL‐C with eMACEs by the inflammatory status remained consistent. Furthermore, in a time‐varying model to account for time‐dependent effects of statins on outcomes, such a bidirectional association persisted (Table S5).
Figure 2. Kaplan‐Meier curves for the cumulative incidence of the primary outcome based on the high‐density lipoprotein cholesterol (HDL‐C) categories.
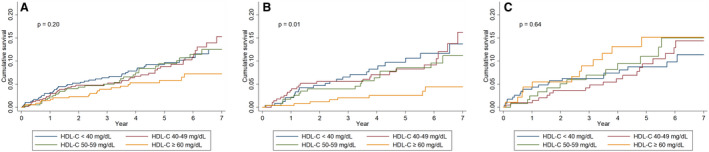
A, Regardless of inflammation. B, In the absence of inflammation. C, In the presence of inflammation. The cumulative incidence of extended major adverse cardiovascular events was notably lower in patients without inflammation and with HDL‐C level ≥60 mg/dL, whereas this pattern disappeared in those with inflammation. Statistical analysis was performed using the log‐rank test.
Secondary Outcome Analysis
Nonfatal MACEs occurred in 122 (6.5%) patients; the incidence rate was lower in patients with higher HDL‐C levels (Table 2). In line with the results of primary outcome analysis, the relationship between the HDL‐C level and nonfatal MACEs varied significantly, depending on the inflammatory status. Overall, the HDL‐C level was not associated with the risk of nonfatal MACEs. However, an inverse association was observed for this outcome among patients without inflammation; in particular, the HDL‐C level of ≥60 mg/dL was associated with a 59% (95% CI, 0.17–0.97) lower risk of nonfatal MACEs than the HDL‐C level of 40 to 49 mg/dL. Notably, this association was reversed in patients with inflammation, but the difference was not statistically significant (Table S6 and Figure S5).
No significant difference was observed in the risk of all‐cause death among the patients stratified by HDL‐C categories (Table S7 and Figure S6). In a separate analysis based on the inflammatory status, the adjusted HRs were lower in patients without inflammation and with higher HDL‐C level compared with the reference group of HDL‐C 40 to 49 mg/dL; however, the results were not statistically significant. The cumulative incidence curves for nonfatal MACEs (Figure S7) and all‐cause death (Figure S8) yielded similar findings.
Subgroup Analysis
We tested the effect modification on the relationship between HDL‐C level and inflammation for eMACEs in the prespecified subgroups (Figure S9). Overall, the risk‐modifying effect of inflammation on the relationship between HDL‐C level and the risk of eMACEs existed among most subgroups. However, significant interaction was observed between serum albumin and HDL‐C levels for the primary outcome based on the inflammatory status. In patients with serum albumin level <4.0 g/dL, the adjusted HR per SD increase in HDL‐C was 0.32 (95% CI, 0.10–1.00) in the absence of inflammation, whereas it was 2.38 (95% CI, 1.19–4.77) in the presence of inflammation.
DISCUSSION
In the present study, we showed that the relationship of HDL‐C level with CVD differed depending on the inflammatory status in patients with CKD. There was a significant interaction between inflammation and HDL‐C level for the primary outcome. In the absence of inflammation, HDL‐C level ≥60 mg/dL was associated with a lower risk of eMACEs, although statistical significance was lost after Bonferroni correction. In contrast, the opposite trend was observed for the association between HDL‐C and adverse CVD in the presence of inflammation. We showed a similar relationship using various analytical models. The difference in the nature of HDL‐C based on inflammation was particularly evident for nonfatal cardiovascular events and more pronounced in patients with lower albumin levels. Our findings suggest that inflammation modifies the relationship between HDL‐C level and the risk of eMACEs, and the beneficial association of high HDL‐C levels is lost under the influence of inflammation in patients with CKD.
The notion of HDL‐C being a “good cholesterol” was questioned in recent studies that reported nonlinear associations between the HDL‐C concentrations and mortality.18, 19 A recent cohort study reported a U‐shaped relationship between the HDL‐C level and mortality.11 In addition to Mendelian randomization studies that failed to find the causal genetic association of HDL‐C with CVD,5, 6 some genetic variants associated with higher HDL‐C concentrations have been reported to have paradoxically increased the CVD risk.20, 21 Herein, we sought to examine if such discrepancy in the clinical implications of HDL‐C might be attributed to inflammation. Our findings suggest that inflammation had a risk‐modifying effect on the relationship between the HDL‐C level and adverse outcomes in patients with CKD.
We particularly focused on inflammation because inflammatory responses affect metabolism and composition of HDL,22 thereby suggesting a possible link between altered HDL function and inflammation under noxious medical conditions. This was suggested in previous studies, which indicated the loss of beneficial effects of HDL‐C in patients with diabetes mellitus and coronary artery disease, who generally present with increased inflammation.23, 24 CKD is also a condition wherein HDL‐mediated vasoprotective effects are significantly impaired.9 As patients with CKD are burdened with uremia, inflammation, and oxidative stress,25, 26 HDL may impart the pro‐oxidant and proinflammatory effects in the setting of renal failure. Consistent with previous investigations that analyzed the inflammation‐related association between elevated HDL‐C and the cardiovascular risk in nondiabetic postinfarction patients and a male population‐based cohort,27, 28 we showed that higher HDL concentrations were associated with a lower risk for eMACEs and nonfatal MACEs in the absence of inflammation, whereas opposite association was observed in the presence of inflammation. These findings were supported by subgroup analysis. Notably, the risk‐modifying effect of inflammation was observed in patients with lower serum albumin level. Albumin is a negative acute‐phase reactant; its concentration represents the nutritional status and is inversely correlated with inflammation. In the present study, a beneficial association of higher HDL‐C level was observed with eMACEs among patients with albumin level <4.0 g/dL in the absence of inflammation, whereas the association was reversed in its presence. Malnutrition interacts with inflammation, which can modify the relationship between cholesterol and CVD.29 These findings can explain the difference in relationship between HDL‐C level and adverse outcomes, depending on the inflammatory status.
The paradoxical association of HDL‐C in patients with inflammation can be further explained by the modified function of HDL in the CKD setting. Reactive oxygen species, uremia, and systemic inflammation impair the cholesterol uptake capability of HDL.30, 31 Its capacity of unloading cholesterol is decreased with the disturbance of scavenger receptor‐B1 by the accumulated oxidation products in renal disease.30, 32 Elevated HDL‐C level owing to the accumulation of malfunctioning leftovers does not necessarily indicate improved HDL function; rather, it is suggestive of defective reverse cholesterol transport. Impaired HDL function has been demonstrated ex vivo in patients undergoing hemodialysis.33
Our findings may aid in interpreting the Janus‐faced role of HDL‐C. The clinicians performing risk assessment with HDL‐C must identify the deleterious factors, such as CKD and inflammation, in which the beneficial relationship of HDL‐C is uncertain. Several studies have reported failure in reducing cardiovascular events and/or unexpectedly increasing mortality in subjects with intentionally elevated HDL‐C levels.34 The participants in these studies had previously experienced CVD; therefore, they were presumed to have inflammation, and patients with reduced kidney function were also included. In this regard, a customized approach is needed for incorporating HDL‐C as a factor in cardiovascular risk stratification in the context of CKD and inflammation.
Our study has several limitations. First, because this was an observational study, the potential confounding factors might not have been thoroughly controlled. To minimize the bias, we constructed Cox models after rigorous adjustment of several variables and used numerous analytical methods. In addition, our cohort purely included patients with CKD, with ≥65% of patients in CKD stage 3 and above, which enabled us to examine the relationship between the HDL‐C level and risk of MACEs in this unique population. Nevertheless, it cannot be overlooked that the interpretation of the data is limited because of the relatively small size of the cohort, consisting of only Korean population with a limited number of events. Second, the significant association of HDL‐C ≥60 mg/dL with a lower risk of eMACEs disappeared after Bonferroni correction, and there were wide CIs for the HRs, suggesting this relationship was not strong. This finding implies that besides inflammation, other factors can affect the relationship between HDL‐C and CVD in patients with CKD. Third, serum HDL‐C levels were not measured at the central laboratory. However, all participating centers used identical direct enzymatic assays for estimating the HDL‐C concentration, and all measurements were performed within 24 hours of sampling. Finally, the qualitative assessment of the composition and function of HDL‐C was not feasible in this study. As mentioned above, dysfunctional HDL may be more important than HDL‐C concentration itself. However, although impaired HDL function has been proved in patients undergoing dialysis, Bauer et al reported that HDL‐C efflux capacity cannot predict cardiovascular events in patients with CKD.35 Future studies should address this issue on the altered composition and function of HDL‐C using a standardized assay, and be replicated in other ethnic populations.
In conclusion, the findings of the present study showed that the conventional beneficial association of HDL‐C was preserved in patients with CKD without inflammation, whereas the relationship of HDL‐C differed in its presence. Our hypothesis generated in this study should be confirmed in larger sample cohorts and requires future research investigating the underlying pathophysiologic mechanisms.
Sources of Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (grant numbers 2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, 2019E320101, and 2019E320102). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
None.
Supporting information
Appendix S1
Data S1
Tables S1–S7
Figures S1–S9
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.021731
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1.Go AS,Chertow GM,Fan D,McCulloch CE,Hsu C‐Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. DOI: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C,Landray MJ,Reith C,Emberson J,Wheeler DC,Tomson C,Wanner C,Krane V,Cass A,Craig J, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011;377:2181–2192. DOI: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X,Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Curr Opin Cardiol. 2007;22:368–372. DOI: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors Collaboration ,Di Angelantonio E,Sarwar N,Perry P,Kaptoge S,Ray KK,Thompson A,Wood AM,Lewington S,Sattar N,Packard CJ, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. DOI: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes MV,Asselbergs FW,Palmer TM,Drenos F,Lanktree MB,Nelson CP,Dale CE,Padmanabhan S,Finan C,Swerdlow DI, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–550. DOI: 10.1093/eurheartj/eht571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voight BF,Peloso GM,Orho‐Melander M,Frikke‐Schmidt R,Barbalic M,Jensen MK,Hindy G,Hólm H,Ding EL,Johnson T, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet. 2012;380:572–580. DOI: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowman L,Hopewell JC,Chen F,Wallendszus K,Stevens W,Collins R,Wiviott SD,Cannon CP,Braunwald E,Sammons E, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–1227. DOI: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 8.Lincoff AM,Nicholls SJ,Riesmeyer JS,Barter PJ,Brewer HB,Fox KAA,Gibson CM,Granger C,Menon V,Montalescot G, et al. Evacetrapib and cardiovascular outcomes in high‐risk vascular disease. N Engl J Med. 2017;376:1933–1942. DOI: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 9.Moradi H,Pahl MV,Elahimehr R,Vaziri ND. Impaired antioxidant activity of high‐density lipoprotein in chronic kidney disease. Transl Res. 2009;153:77–85. DOI: 10.1016/j.trsl.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Marsche G,Saemann MD,Heinemann A,Holzer M. Inflammation alters HDL composition and function: implications for HDL‐raising therapies. Pharmacol Ther. 2013;137:341–351. DOI: 10.1016/j.pharmthera.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Bowe B,Xie Y,Xian H,Balasubramanian S,Zayed MA,Al‐Aly Z. High density lipoprotein cholesterol and the risk of all‐cause mortality among U.S. veterans. Clin J Am Soc Nephrol. 2016;11:1784–1793. DOI: 10.2215/CJN.00730116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz MI,Carrero JJ,Axelsson J,Lindholm B,Stenvinkel P. Low‐grade inflammation in chronic kidney disease patients before the start of renal replacement therapy: sources and consequences. Clin Nephrol. 2007;68:1–9. DOI: 10.5414/CNP68001. [DOI] [PubMed] [Google Scholar]
- 13.Holzer M,Birner‐Gruenberger R,Stojakovic T,El‐Gamal D,Binder V,Wadsack C,Heinemann A,Marsche G. Uremia alters HDL composition and function. J Am Soc Nephrol. 2011;22:1631–1641. DOI: 10.1681/ASN.2010111144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weichhart T,Kopecky C,Kubicek M,Haidinger M,Döller D,Katholnig K,Suarna C,Eller P,Tölle M,Gerner C, et al. Serum amyloid A in uremic HDL promotes inflammation. J Am Soc Nephrol. 2012;23:934–947. DOI: 10.1681/ASN.2011070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh K‐H,Park SK,Park HC,Chin HJ,Chae DW,Choi KH,Han SH,Yoo TH,Lee K,Kim Y‐S, et al. KNOW‐CKD (KoreaN cohort study for Outcome in patients With Chronic Kidney Disease): design and methods. BMC Nephrol. 2014;15:80. DOI: 10.1186/1471-2369-15-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS,Stevens LA,Schmid CH,Zhang Y,Castro AF,Feldman HI,Kusek JW,Eggers P,Van Lente F,Greene T, et al; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson TA,Mensah GA,Alexander RW,Anderson JL,Cannon RO,Criqui M,Fadl YY,Fortmann SP,Hong Y,Myers GL, et al. Markers of inflammation and cardiovascular disease. Circulation. 2003;107:499–511. DOI: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 18.Madsen CM,Varbo A,Nordestgaard BG. Extreme high high‐density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38:2478–2486. DOI: 10.1093/eurheartj/ehx163. [DOI] [PubMed] [Google Scholar]
- 19.Ko DT,Alter DA,Guo H,Koh M,Lau G,Austin PC,Booth GL,Hogg W,Jackevicius CA,Lee DS, et al. High‐density lipoprotein cholesterol and cause‐specific mortality in individuals without previous cardiovascular conditions: the CANHEART Study. J Am Coll Cardiol. 2016;68:2073–2083. DOI: 10.1016/j.jacc.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 20.Agerholm‐Larsen B,Nordestgaard BG,Steffensen R,Jensen G,Tybjaerg‐Hansen A. Elevated HDL cholesterol is a risk factor for ischemic heart disease in white women when caused by a common mutation in the cholesteryl ester transfer protein gene. Circulation. 2000;101:1907–1912. DOI: 10.1161/01.CIR.101.16.1907. [DOI] [PubMed] [Google Scholar]
- 21.Andersen RV,Wittrup HH,Tybjaerg‐Hansen A,Steffensen R,Schnohr P,Nordestgaard BG. Hepatic lipase mutations, elevated high‐density lipoprotein cholesterol, and increased risk of ischemic heart disease: the Copenhagen City Heart Study. J Am Coll Cardiol. 2003;41:1972–1982. DOI: 10.1016/S0735-1097(03)00407-8. [DOI] [PubMed] [Google Scholar]
- 22.de la Llera Moya M,McGillicuddy FC,Hinkle CC,Byrne M,Joshi MR,Nguyen V,Tabita‐Martinez J,Wolfe ML,Badellino K,Pruscino L, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222:390–394. DOI: 10.1016/j.atherosclerosis.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorrentino SA,Besler C,Rohrer L,Meyer M,Heinrich K,Bahlmann FH,Mueller M,Horváth T,Doerries C,Heinemann M, et al. Endothelial‐vasoprotective effects of high‐density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended‐release niacin therapy. Circulation. 2010;121:110–122. DOI: 10.1161/CIRCULATIONAHA.108.836346. [DOI] [PubMed] [Google Scholar]
- 24.Riwanto M,Rohrer L,Roschitzki B,Besler C,Mocharla P,Mueller M,Perisa D,Heinrich K,Altwegg L,von Eckardstein A, et al. Altered activation of endothelial anti‐ and proapoptotic pathways by high‐density lipoprotein from patients with coronary artery disease: role of high‐density lipoprotein‐proteome remodeling. Circulation. 2013;127:891–904. DOI: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 25.Himmelfarb J,Stenvinkel P,Ikizler TA,Hakim RM. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. DOI: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta J,Mitra N,Kanetsky PA,Devaney J,Wing MR,Reilly M,Shah VO,Balakrishnan VS,Guzman NJ,Girndt M, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. DOI: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corsetti JP,Gansevoort RT,Sparks CE,Dullaart RP. Inflammation reduces HDL protection against primary cardiac risk. Eur J Clin Invest. 2010;40:483–489. DOI: 10.1111/j.1365-2362.2010.02287.x. [DOI] [PubMed] [Google Scholar]
- 28.Corsetti JP,Zareba W,Moss AJ,Rainwater DL,Sparks CE. Elevated HDL is a risk factor for recurrent coronary events in a subgroup of non‐diabetic postinfarction patients with hypercholesterolemia and inflammation. Atherosclerosis. 2006;187:191–197. DOI: 10.1016/j.atherosclerosis.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Contreras G,Hu BO,Astor BC,Greene T,Erlinger T,Kusek JW,Lipkowitz M,Lewis JA,Randall OS,Hebert L, et al. Malnutrition‐inflammation modifies the relationship of cholesterol with cardiovascular disease. J Am Soc Nephrol. 2010;21:2131–2142. DOI: 10.1681/ASN.2009121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiss AB,Voloshyna I,De Leon J,Miyawaki N,Mattana J. Cholesterol metabolism in CKD. Am J Kidney Dis. 2015;66:1071–1082. DOI: 10.1053/j.ajkd.2015.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanktree MB,Thériault S,Walsh M,Paré G. HDL cholesterol, LDL cholesterol, and triglycerides as risk factors for CKD: a Mendelian randomization study. Am J Kidney Dis. 2018;71:166–172. DOI: 10.1053/j.ajkd.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol. 2016;12:37–47. DOI: 10.1038/nrneph.2015.180. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto S,Yancey PG,Ikizler TA,Jerome WG,Kaseda R,Cox B,Bian A,Shintani A,Fogo AB,Linton MF, et al. Dysfunctional high‐density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60:2372–2379. DOI: 10.1016/j.jacc.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz GG,Olsson AG,Abt M,Ballantyne CM,Barter PJ,Brumm J,Chaitman BR,Holme IM,Kallend D,Leiter LA, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. DOI: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 35.Bauer L,Kern S,Rogacev KS,Emrich IE,Zawada A,Fliser D,Heinemann A,Heine GH,Marsche G. HDL cholesterol efflux capacity and cardiovascular events in patients with chronic kidney disease. J Am Coll Cardiol. 2017;69:246–247. DOI: 10.1016/j.jacc.2016.10.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data S1
Tables S1–S7
Figures S1–S9


