Abstract
Background
Although methamphetamine abuse is associated with the development of heart failure (HF), nationwide data on methamphetamine‐associated HF (MethHF) hospitalizations are limited. This study evaluates nationwide HF hospitalizations associated with substance abuse to better understand MethHF prevalence trends and the clinical characteristics of those patients.
Methods and Results
This cross‐sectional period‐prevalence study used hospital discharge data from the National Inpatient Sample to identify adult primary HF hospitalizations with a secondary diagnosis of abuse of methamphetamines, cocaine, or alcohol in the United States from 2002 to 2014. All 2014 MethHF admissions were separated by regional census division to evaluate geographical distribution. Demographics, payer information, and clinical characteristics of MethHF hospitalizations were compared with all other HF hospitalizations. Total nationwide MethHF hospitalizations increased from 547 in 2002 to 6625 in 2014 with a predominance on the West Coast. Methamphetamine abuse was slightly more common among primary HF hospitalizations compared with all‐cause hospitalizations (7.4 versus 6.4 per 1000; Cohen h=0.012; P<0.001). Among HF hospitalizations, patients with MethHF were younger (mean age, 48.9 versus 72.4 years; Cohen d=1.93; P<0.001), more likely to be on Medicaid (59.4% versus 8.8%; Cohen h=1.16; P<0.001) or uninsured (12.0% versus 2.6%; Cohen h=0.36; P<0.001), and more likely to present to urban hospitals (43.8% versus 28.3%; Cohen h=0.32; P<0.001) than patients with non‐methamphetamine associated HF. Patients with MethHF had higher rates of psychiatric comorbidities and were more likely to leave the hospital against medical advice.
Conclusions
MethHF hospitalizations have significantly increased in the United States, particularly on the West Coast. Coordinated public health policies and systems of care are needed to address this rising epidemic.
Keywords: alcohol, cardiac hospitalization, cardiotoxicity, cocaine, heart failure
Subject Categories: Heart Failure, Risk Factors
Nonstandard Abbreviations and Acronyms
- MethHF
methamphetamine‐associated heart failure
- NIS
National Inpatient Sample
- non‐Meth HF
heart failure not associated with methamphetamine
Clinical Perspective
What Is New?
This cross‐sectional study demonstrates an increase in methamphetamine‐associated heart failure (HF) hospitalizations from 2002 to 2014 in the United States, predominantly on the West Coast.
Compared with other inpatients with HF, inpatients with methamphetamine‐associated HF were more likely to be younger, male, present to urban hospitals, on Medicaid or uninsured, and leave the hospital prematurely.
What Are the Clinical Implications?
Methamphetamine‐associated HF prevalence trends are unlikely to decline in the near future and greater efforts should be made by providers to diagnose and document methamphetamine abuse in patients with HF, especially since methamphetamine abuse is a potentially reversible cause of HF.
While cocaine and alcohol have long been associated with non‐ischemic cardiomyopathy and heart failure (HF), methamphetamine has garnered less attention despite its known cardiotoxicity and rising rates of abuse.1, 2, 3, 4, 5, 6, 7 Also known as “meth” or “crystal,” methamphetamine is an illicit addictive stimulant within the amphetamine class that can be orally ingested, snorted, smoked, vaporized, or injected intravenously to achieve a feeling of euphoria. By stimulating the release of endogenous catecholamines (dopamine and norepinephrine), methamphetamine has both α‐ and β‐ adrenergic agonist effects that modulate heart rate, heart contractility, and vasoconstriction, resulting in adverse cardiovascular consequences that include tachycardia, hypertension, pulmonary arterial hypertension, and dilated cardiomyopathy.8, 9, 10, 11, 12 Clinically, individuals who abuse methamphetamine may present with methamphetamine‐associated heart failure (MethHF). Previous studies have discussed the association between amphetamine abuse and HF while others have demonstrated the rising number of amphetamine‐related hospitalizations and costs.7, 8, 9, 10, 11, 12, 13 The Drug Enforcement Agency recognizes the growing methamphetamine threat in the United States, noting annual increases in criminal methamphetamine seizures, lethal overdoses, and positive urine chemistry tests over the past decade.14, 15, 16, 17 Despite such widespread prevalence and known cardiovascular harm, the epidemiology and clinical characteristics of methamphetamine abusers admitted for decompensated HF in the United States remains undefined. Such investigation is particularly relevant as hospitalizations for decompensated HF are associated with higher rates of subsequent readmission or mortality,18, 19 and cardiovascular mortality related to HF has seen a recent increase that is more pronounced in younger individuals.20
Methods
Detailed results of the analyses reported herein are available from the corresponding author upon reasonable request.
Data Sources
Data were obtained from the National Inpatient Sample (NIS), which is part of the Healthcare Cost and Utilization Project and sponsored by the Agency for Healthcare Research and Quality. The NIS is the largest all‐payer database of hospitalized patients in the United States and includes ≈8 million hospitalizations annually, representing a 20% stratified sample of all non‐federal US hospitals drawn from participating states. Each record within the NIS data set describes a single hospitalization encounter and includes deidentified patient demographics, comorbidities, hospitalization outcomes, and admission diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM). The NIS does not include patient admission data from long‐term acute care facilities, chemical dependency units, psychiatric hospitals, or observational‐status admissions. The NIS does not include information on outpatient encounters or emergency room visits unless those encounters are converted into inpatient admissions.
In 2012, the NIS was redesigned to improve sampling methodology, thereby increasing the precision of national discharge estimates and providing regional census division stratification. To account for these changes in sampling methodology, this analysis used a revised set of discharge weights, referred to as trend weights, in all patient‐level analyses before 2012. It is important to note that each NIS data point represents one unique hospitalization encounter and a rehospitalized patient may be represented by multiple data points within the same year. Individual patients cannot be tracked longitudinally by the NIS. The NIS is not designed for individual state analysis. The design of the NIS data set and analytic guidelines have been previously described.21, 22, 23, 24, 25, 26, 27 The Institutional Review Board at University of California San Diego waived the need for an Institutional Review Board application because the NIS is a deidentified, publicly available data source.
Study Population and Variables
The current study included all NIS hospital admissions data from 2002, 2006, 2010, and 2014 to identify US hospitalization encounters of patients aged ≥18 years with a primary hospitalization ICD‐9 diagnosis of HF using the Clinical Classifications Software code number 108. These hospitalization encounters were then further analyzed for secondary ICD‐9 diagnoses of amphetamine abuse (304.4x, 305.7x, 969.72), cocaine abuse (304.2x, 305.6x, 970.81), and alcohol abuse (291.2, 291.4, 291.81, 303.0x, 305.0x, 425.5, 980.8, 980.9). Combined drug use was not independently assessed in this study. Diagnostic codes do not discriminate between type of amphetamine abuse, but previous evidence has shown that such acute care coding usually correlates with clinical and/or toxicology evidence of methamphetamine abuse, especially in patients with HF.9, 28, 29, 30 A primary hospitalization diagnosis of HF and a secondary diagnosis of amphetamine abuse was considered as a MethHF hospitalization; all other primary HF hospitalizations without a secondary diagnosis of amphetamine abuse were identified as non‐Meth HF hospitalizations. The annual number of substance abuse‐associated HF hospitalizations was then divided by the total annual number of HF hospitalizations to determine substance abuse‐associated HF hospitalization prevalence rates for each year. Only hospitalizations with a primary discharge diagnosis of HF were included in this analysis.
To better understand the unique characteristics of MethHF hospitalizations, demographics, and characteristics for 2014 MethHF hospitalizations were compared with all other primary HF hospitalizations without amphetamine abuse (non‐Meth HF). NIS data from 2014 were used as it was the most contemporary NIS data available before the transition from ICD‐9 to ICD‐10 diagnostic coding. Outcomes during hospitalization encounters were compared between the 2 groups. General population substance abuse trends regardless of HF were also analyzed. Using 2014 regional census division stratification data, national geographic heat maps were generated to show prevalence of MethHF per total HF hospitalizations and prevalence of methamphetamine abuse per total hospitalizations. The 9 regional census divisions were divided as following: New England (ME, NH, VT, MA, RI, CT), Mid‐Atlantic (NY, NJ, PA), East North Central (WI, MI, IL, IN, OH), West North Central (MO, KS, NE, IA, MN, SD, ND), South Atlantic (DE, MD, DC, VA, WV, NC, SC, GA, FL), East South Central (KY, TN, MS, AL), West South Central (OK, AR, LA, TX), Mountain (ID, MT, WY, CO, UT, NV, AZ, NM), and Pacific (CA, OR, WA, HI, AK). All states were included except for Alabama, Alaska, Delaware, New Hampshire, Idaho, and Mississippi because these states did not participate in the 2014 NIS database.
Statistical Analysis
All statistical analyses were performed using statistical methods for survey data by considering sampling weights from the stratified sample design of the NIS. Analysis was completed by following the specific methodology described by the Healthcare Cost and Utilization Project.31 To account for the 2012 change in NIS design, trend weight analysis was applied to all years before 2012. Descriptive statistics were reported as means and standard deviations for continuous, and percents for categorical variables, while groups are compared using t‐test for continuous and Chi‐Squared test for categorical variables. Prevalence estimates for each year were calculated using weighted averages based on inverse probability weighting and compared over time using Wald test statistics.32, 33, 34 Data were analyzed using SPSS (SPSS, Version 9.6.0.0, Armonk, NY: IBM Corp) and R (R Core Team, 2013). As previously described, data values shown are weighted to reflect national estimates. All diagnostic coding string variables were converted to numeric variables to query ICD‐9 coding. Data from 2014 was divided into 9 different US census divisions to show regional hospitalization prevalence. When applicable, data analysis was validated against publicly available analysis on HCUPnet.35 Given the large sample sizes, effect sizes were reported using Cohen d for continuous and Cohen h for binary outcomes, in addition to P values. Effect sizes are regarded as small, medium and large, if they are around 0.2, 0.5, and 0.8, respectively.
Results
Temporal Trends
Between 2002 and 2014, the total weighted number of annual NIS hospital discharges analyzed were 36 523 831; 38 076 556; 37 352 014; and 35 358 818 for the years 2002, 2006, 2010, and 2014 respectively. The weighted number of annual adult primary HF hospitalizations decreased from 1 019 515 in 2002 to 900 000 in 2014 (Figure 1, Bar Graph). Despite this decline in primary HF hospitalizations, there was a 12‐fold increase in the number of annual MethHF hospitalizations from 2002 to 2014 (547 to 6625, +1111%; P<0.0001). There was a lesser increase in the number of annual cocaine‐associated HF hospitalizations (5556 to 10 295, +85%; P<0.0001) and alcohol‐associated HF hospitalizations (8948 to 11 655, +47%; P<0.0001) over the same interval. From 2010 to 2014, the prevalence of MethHF rose from 2.5 cases per 1000 HF cases in 2010 to 7.4 cases per 1000 HF cases in 2014 (P<0.0001) while there was not a significant rise in the prevalence of cocaine‐associated HF (11.5 to 11.4; P=0.952) or alcohol‐associated HF (13.0 to 13.0; P=0.943) over the same interval (Figure 1, Line graph).
Figure 1. Prevalence of adult cardiotoxic substance abuse‐associated heart failure hospitalizations shown as prevalence per 1000 total heart failure hospitalizations, weighted National Inpatient Sample data 2002 through 2014.
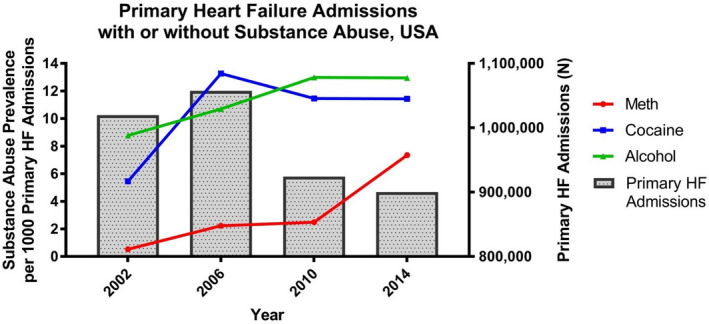
Total number of annual weighted heart failure hospitalizations shown in bar graphs. HF indicates heart failure.
Geographic Differences
After stratifying the 2014 HF hospitalization data by national census division, there was a heavy concentration of MethHF hospitalizations along the Pacific coast, Figure 2 (CA, HI, OR, WA; prevalence=47.0 MethHF cases per 1000 HF hospitalizations), with the prevalence of MethHF hospitalizations nearly 500 times that of the Middle Atlantic region (NJ, NY, PA; prevalence=0.1 per 1000 HF hospitalizations; Cohen h=0.42; P<0.0001). The West Mountain region also had a high prevalence of methamphetamine abuse among HF hospitalizations (MT, WY, CO, UT, NV, AZ, NM; prevalence=18.8 MethHF cases per 1000 HF hospitalizations). To further investigate the association between methamphetamine and HF on a national scale, prevalence of methamphetamine abuse per 2014 all‐cause hospitalizations was analyzed and compared with methamphetamine abuse per 2014 HF hospitalizations, Table S1. Methamphetamine abuse was slightly more common among HF hospitalizations (7.4 MethHF cases per 1000 HF hospitalizations) than among all‐cause hospitalizations (6.4 meth abuse cases per 1000 all‐cause hospitalizations; Cohen h=0.012; P<0.001), particularly in the Pacific region (47.0 MethHF per 1000 HF versus 22.9 meth per 1000 all‐cause; Cohen h=0.13; P<0.0001), although effect sizes are small in both cases. The geographic distribution of MethHF hospitalizations was proportionate to the distribution of hospitalizations with the primary diagnosis of methamphetamine abuse, Figure S1. Statistical comparisons of MethHF prevalence between different regions are shown in Table S2.
Figure 2. Nationwide prevalence of 2014 adult methamphetamine‐associated heart failure hospitalizations separated by the 9 different US census divisions, shown as prevalence per 1000 primary heart failure hospitalizations.
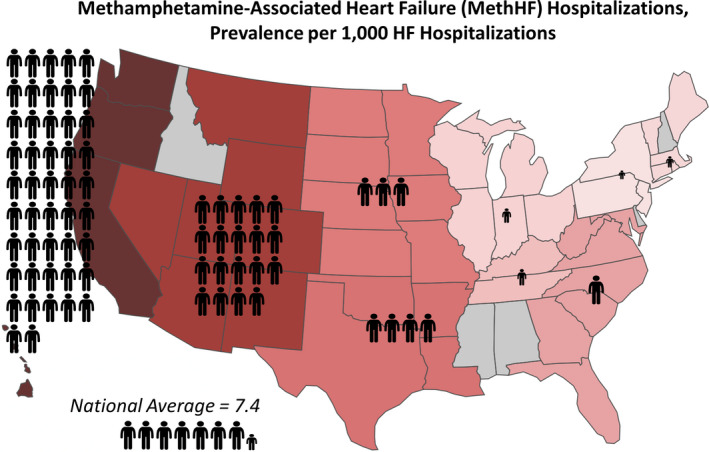
Note that Alabama, Alaska, Delaware, New Hampshire, Idaho, and Mississippi are not included in the 2014 National Inpatient Sample data. HF indicates heart failure.
Characteristics of MethHF
All of the weighted 2014 HF hospitalization encounters were divided into either MethHF (6625) or non‐Meth HF (894 795), Table 1. Patients with MethHF were 26 years younger on average compared with patients with non‐Meth HF (median age, 49 [interquartile range, 42–57] versus 75 [interquartile range, 64–84] years; mean age, 48.9 (SD, 9.97) versus 72.4 (SD, 14.02) years; Cohen d=1.93; P<0.0001) and were more commonly men (79.2% versus 51.1%; Cohen h=0.6; P<0.0001). Patients with MethHF were more often of Hispanic (18.1% versus 7.1%; Cohen h=0.34; P<0.001) or Asian/Pacific Islander (9.1% versus 1.8%; Cohen h=0.34; P<0.001) descent. It is important to note that Hispanic, Asian, and Pacific Islander populations were more densely concentrated in geographical census regions with higher prevalence of methamphetamine abuse. Patients with MethHF were most commonly admitted to hospitals in largely populated central metropolitan areas (43.8% versus 28.3%; Cohen h=0.32; P<0.001). Health insurance coverage differed between the groups with patients with MethHF more often uninsured (12.0% versus 2.6%; Cohen h=0.36; P<0.0001) or insured through Medicaid (59.4% versus 8.9%; Cohen h=1.16; P<0.0001) and were less often covered by Medicare (18.2% versus 74.8%; Cohen h=1.21; P<0.0001). The clinical characteristics of MethHF and non‐Meth HF hospitalization encounters are shown in Table 2. Compared with the older cohort of patients with non‐Meth HF, the relatively younger patients with MethHF had less cardiac comorbidities such as hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, and atrial fibrillation. Mood and psychotic disorders were more common among patients with MethHF as was homelessness. Longitudinal analysis of the demographic and clinical characteristics of MethHF admissions from 2002 to 2014 can be found in Table S3.
Table 1.
Demographic Characteristics of Adult HF Hospitalizations in Patients With MethHF Compared With Patients With Non‐Meth HF
| Demographic characteristics | HF hospitalizations, MethHF (n=6625) | HF hospitalizations, Non‐Meth HF (n=893 375) |
Cohen h (P value) |
|---|---|---|---|
| Age, y, mean | 48.9 | 72.4 | 1.93 (<0.001) |
| 18–24 | 30 (0.5) | 1550 (0.2) | 0.05 (<0.001) |
| 25–34 | 610 (9.2) | 8565 (1.0) | 0.42 (<0.001) |
| 35–44 | 1380 (20.1) | 24 070 (2.7) | 0.62 (<0.001) |
| 45–54 | 2690 (40.1) | 72 700 (8.1) | 1.20 (<0.001) |
| 55–64 | 1615 (24.4) | 139 955 (15.7) | 0.22 (<0.001) |
| 65–74 | 280 (4.2) | 195 155 (21.8) | 0.56 (<0.001) |
| 75–84 | 15 (0.2) | 236 085 (26.4) | 0.98 (<0.001) |
| 85+ | 5 (0.1) | 215 295 (24.1) | 0.98 (<0.001) |
| Male sex | 5245 (79.2) | 456 160 (51.1) | 0.60 (<0.001) |
| Payer | |||
| Medicare | 1205 (18.2) | 669 690 (75.0) | 1.21 (<0.001) |
| Medicaid | 3935 (59.4) | 79 010 (8.8) | 1.16 (<0.001) |
| Private insurance | 480 (7.3) | 102 985 (11.5) | 0.15 (<0.001) |
| Uninsured (self‐pay) | 795 (12.0) | 23 575 (2.6) | 0.38 (<0.001) |
| Other | 210 (3.2) | 18 115 (2.1) | 0.07 (<0.001) |
| Location | |||
| Large central metro (1m+) | 2900 (43.8) | 252 730 (28.3) | 0.32 (<0.001) |
| Large fringe metro (suburbs; 1m+) | 1050 (15.8) | 208 650 (23.4) | 0.19 (<0.001) |
| Medium/small metro (50k‐1m) | 1995 (30.1) | 262 620 (29.4) | 0.02 (0.207) |
| Rural (<50k) | 555 (8.4) | 166 965 (18.7) | 0.31 (<0.001) |
| Missing | 125 (1.9) | 2410 (0.3) | 0.17 (<0.001) |
| Race/ethnicity | |||
| White | 3640 (54.9) | 585 875 (65.6) | 0.22 (<0.001) |
| Black | 890 (13.4) | 166 445 (18.6) | 0.14 (<0.001) |
| Hispanic | 1200 (18.1) | 62 880 (7.0) | 0.34 (<0.001) |
| Asian/Pacific Islander | 600 (9.1) | 16 005 (1.8) | 0.34 (<0.001) |
| Native American | 70 (1.1) | 4070 (0.5) | 0.07 (<0.001) |
| Other | 130 (2.0) | 19 840 (2.2) | 0.02 (0.167) |
| Missing | 95 (1.4) | 38 260 (4.3) | 0.18 (<0.001) |
2014 weighted National Inpatient Sample data, specific for the 6625 methamphetamine‐associated heart failure hospitalizations from the 894 795 heart failure without associated methamphetamine abuse hospitalizations. Data are presented as counts (percentage) unless stated otherwise. HF indicates heart failure; MethHF, methamphetamine‐associated heart failure; and Non‐Meth HF, heart failure without associated methamphetamine abuse.
Table 2.
Clinical Characteristics Based on ICD‐9 Diagnoses of Methamphetamine Abusers Hospitalized for HF Compared With Non‐Methamphetamine Abusers Hospitalized for HF
| Clinical characteristics | HF hospitalizations, MethHF (n=6625) | HF hospitalizations, Non‐Meth HF (n=893 375) | Cohen h | P value |
|---|---|---|---|---|
| Hypertension | 4330 (65.4) | 715 965 (80.1) | 0.33 | <0.001 |
| Diabetes mellitus | 2050 (30.9) | 425 850 (47.7) | 0.34 | <0.001 |
| Renal dysfunction | 2310 (34.9) | 469 805 (52.6) | 0.36 | <0.001 |
| Hyperlipidemia | 1775 (26.8) | 437 115 (48.9) | 0.46 | <0.001 |
| Coronary artery disease | 2090 (31.5) | 496 340 (55.6) | 0.49 | <0.001 |
| Atrial fibrillation | 990 (14.9) | 377 335 (42.2) | 0.62 | <0.001 |
| Atrial flutter | 285 (4.3) | 35 805 (4.0) | 0.01 | 0.236 |
| HIV | 85 (1.3) | 4110 (0.5) | 0.09 | <0.001 |
| Acute stroke | 40 (0.6) | 4430 (0.5) | 0.01 | 0.247 |
| Pulmonary heart disease | 1355 (20.5) | 196 670 (22.0) | 0.04 | 0.002 |
| Mood disorders | 1045 (15.8) | 112 120 (12.6) | 0.09 | <0.001 |
| Schizophrenia/psychotic disorders | 160 (2.4) | 12 415 (1.4) | 0.08 | <0.001 |
| Homelessness | 610 (9.2) | 2710 (0.3) | 0.51 | <0.001 |
| Left against medical advice | 485 (7.3) | 9870 (1.1) | 0.34 | <0.001 |
2014 weighted National Inpatient Sample data, specific for the 6625 MethHF hospitalizations from the 894 795 non‐Meth HF hospitalizations. Data are presented as counts (percentage). HF indicates heart failure; ICD‐9, International Classification of Diseases, Ninth Revision, MethHF, methamphetamine‐associated heart failure; and Non‐Meth HF, heart failure without associated methamphetamine abuse.
Discussion
Through catecholamine excess and increased sympathetic response, methamphetamine abuse can acutely cause hypertension, tachycardia, coronary vasospasm, and/or direct cardiac myotoxicity, eventually leading to dilated cardiomyopathy or pulmonary hypertension in some instances; many of these patients present with clinical HF.4, 5, 10, 11, 12 In recent years, HF hospitalizations for patients with MethHF have been increasing as evidenced in case series and regional studies.3, 4, 5, 6, 7, 8, 9, 10 The national inpatient data presented in this study show a striking increase in MethHF hospitalizations from 2002 to 2014, especially when compared with recent HF hospitalization trends associated with other cardiotoxic substances of abuse such as cocaine or alcohol. While previous studies have brought attention to the rising methamphetamine epidemic both domestically and worldwide,13, 28, 36, 37 this is the first study to demonstrate the worsening MethHF public health crisis across the United States, highlight differences in geographical prevalence, and present some key clinical characteristics of those hospitalized with MethHF.
The presented national census division data show that the prevalence of MethHF hospitalizations is much more heavily concentrated west of the Mississippi River, particularly along the Pacific coast. Figure 2 demonstrates that MethHF hospitalizations were nearly 500 times as prevalent in the Pacific region as compared with the Middle Atlantic region in 2014. While regional differences in diagnostic practice and medical documentation of methamphetamine abuse should be considered, a significant component of this geographical divide likely relates to both the increased availability of methamphetamine and the increased purity of methamphetamine supply in the Western United States. Since the Combat Methamphetamine Epidemic Act of 2006, the Drug Enforcement Agency reports a consistent decline in domestic methamphetamine production with 2017 domestic production at the lowest levels since 2000.14, 15, 16, 17 A large proportion of methamphetamine available in the United States is produced clandestinely in Mexico and smuggled across the southwestern border, predominantly via legal ports of entry with an emphasis across the San Diego corridor.17 From 2016 to 2017, seizures of methamphetamine across the California‐Mexico and Texas‐Mexico borders increased 61% and 18%, respectively (California‐Mexico, 19 320 kg; Texas‐Mexico, 4908 kg). With increased drug trafficking, the average price of methamphetamine has fallen from $189/gram (2006) to $80/gram (2012) to $62/gram (2016), making it a relatively inexpensive substance of abuse when compared with cocaine or even alcohol as users report up to 24 hours of euphoria with doses of only 0.05 to 0.1 grams.12, 13, 14, 15, 16, 17 Additionally, the purity of methamphetamine seized from Mexico is higher compared with most domestically produced methamphetamine.14, 15, 16, 17 Given that a dose‐dependent relationship may exist between methamphetamine abuse and the development of cardiomyopathy, this could help explain the diagnostic divide in MethHF prevalence. Indeed the present study highlighted a proportionate national prevalence of methamphetamine abuse in HF admissions compared with all‐cause admissions. As methamphetamine abuse becomes more affordable, MethHF prevalence trends are unlikely to decline, especially as more potent methamphetamine becomes available across the Eastern United States. Unfortunately, from 2016 to 2017 the Drug Enforcement Agency reported increased availability of methamphetamine in the field divisions of Atlanta, Chicago, Detroit, Houston, Miami, Philadelphia, San Diego, and Washington DC.17
Previous data have suggested that methamphetamine abuse is widely prevalent in rural areas.13, 38 While the current study shows a high rural incidence of MethHF, the study cohort suggests that MethHF hospitalizations are more frequently located in large, central metropolitan areas of >1 million people. This could be partially attributable to an increase in methamphetamine toxicology testing, diagnostic coding, and diagnostic vigilance for substance abuse in urban centers, in addition to the increased potency of urban methamphetamine, which is often imported, as compared with the domestically produced clandestine methamphetamine more often found in rural locations.14, 15, 16, 17 Since most large urban areas rely on multiple different medical systems to supply care, the development of MethHF treatment strategies will require collaborative care coordination among distinct medical systems to help track HF readmissions and provide continuity of care. While additional studies are needed to further explore exactly why these geographical discrepancies exist, greater efforts should be made by all healthcare providers to diagnose and document methamphetamine abuse in patients regardless of HF suspicion, especially considering methamphetamine’s potent euphoric and physiologic effects can sometimes mask traditional HF symptoms in acutely intoxicated individuals and prolong the time to HF diagnosis.10, 11, 12
In contrast to patients with non‐Meth HF, patients with MethHF were more likely to be insured by Medicaid or without medical insurance altogether. Among other socioeconomic barriers, Medicaid and uninsured patients often have limited access to affordable outpatient medications, low sodium diets, transportation to clinic, and home monitoring equipment such as blood pressure cuffs or weight scales thus preventing optimal outpatient HF care. Supply and uptitration of guideline‐directed HF medications is difficult in these patients. Although this study does not distinguish HF with reduced versus preserved ejection fraction, methamphetamine‐associated cardiomyopathy is typically associated with depressed left ventricular function and demands pharmacologic beta blockade as essential long‐term medical therapy.5, 6 Patients with MethHF may be more sensitive to the side effects of pharmacologic treatments including beta‐blockade as these patients function in a high catecholaminergic state during times of abuse.10, 11, 12 Furthermore, hospitalized patients with MethHF were more likely to be discharged prematurely against medical advice. As a result, management of HF in methamphetamine abusers may rely disproportionately on costly and sporadic recurrent HF readmissions that may be otherwise preventable. As mentioned above, these readmissions can be difficult to longitudinally track in urban centers where care may be spread over multiple different healthcare provider networks. Additionally, patients with MethHF were more likely to have diagnoses of psychiatric disorders or homelessness, 2 conditions that are often underappreciated in administrative data among substance abusers and present challenges to the delivery of medical care, particularly outpatient care.39 As compared with non‐Meth HF, MethHF had a higher relative prevalence among Hispanic and Asian/Pacific Islander patients and a lower relative prevalence among White and Black patients. While the aforementioned geographical distribution of MethHF may contribute to some of these differences, it is important to note that MethHF was present across all racial groups and White patients accounted for 55% of MethHF cases.
Patients with MethHF were much younger than patients with non‐Meth HF, with a 25‐year median age gap between the 2 groups. This relative youth is consistent with previous MethHF studies7, 8 and emphasizes the rapid, destructive effects of methamphetamine abuse on the cardiovascular system. Our study did show a lower prevalence of cardiovascular comorbidities such as hypertension, coronary artery disease, and atrial fibrillation in the MethHF cohort compared with the older non‐Meth HF cohort, however, the prevalence of these comorbidities was still higher than in age‐matched all‐cause hospitalizations of the general US population. It is difficult to expand further on any causative relationships about methamphetamine abuse and these cardiovascular comorbidities using this large event‐level data set. This study addresses a potentially preventable public health issue and traditional metrics for clinical significance may miss findings that have population‐level implications. Future prospective studies aimed at better understanding the exact causative relationship between methamphetamines and cardiovascular disease are necessary to better understand why some methamphetamine abusers develop HF while others do not. Previous literature has also suggested a high propensity of patients with MethHF towards tobacco and other substances of abuse such as marijuana, cocaine, and alcohol which may provide future insight.29 Regardless, methamphetamine is associated with HF in younger adults which is particularly relevant given a recent increase in HF‐related cardiovascular mortality rates in the United States from 2012 to 2017, especially among younger adults.20, 40 Timely identification of methamphetamine abuse in patients presenting with HF is imperative as previous studies have shown that cessation of methamphetamine abuse can lead to positive cardiac remodeling and improvement in clinical outcomes.4, 5 Decreasing methamphetamine abuse is no simple feat, for many of the reasons mentioned above, and will require coordinated public health policies and systems of care. Most importantly, the first step is recognizing the rising prevalence of methamphetamine‐associated HF.
Study Limitations
To estimate the prevalence of MethHF hospitalizations among US adults, this cross‐sectional study used a nationally weighted, all‐payer inpatient database. Although the large size of the NIS is a strength of the study, there were limitations. The NIS is exclusively an inpatient national database and does not include data from emergency department visits. The Nationwide Emergency Department Sample could provide further insight, however, previous studies have discussed HF and methamphetamine in the emergency department3, 9 and this study focused on the HF hospitalization burden of MethHF. Second, the NIS is an event‐level database so patients are not tracked after discharge and patient history does not carry over to future inpatient encounters, thus these data cannot distinguish between incident MethHF and recurrent MethHF. To assess MethHF readmission data from 2010 onward, the Nationwide Readmissions Database could be used in future studies. Third, this study relied on accurate ICD‐9 coding of substance abuse, HF, and other comorbidities of interest. There is no mechanism within the NIS data set to discern diagnostic motivations or, more specific to this investigation, determine how a diagnosis of substance abuse is made (ie, clinical history, urine toxicology, blood toxicology). While urine toxicology assays are the most common methamphetamine screening test used in the United States, use of these tests vary by region and cases of false positives and false negatives have been reported.38, 39 Diagnoses of substance abuse are often omitted from the medical record for various reasons; thus this study likely underestimates the national burden of methamphetamine abuse in patients admitted for HF. As awareness of the methamphetamine epidemic became more apparent over time, it is possible that sites were more likely to code for substance abuse thus earlier timepoints may underestimate prevalence. Also, since this analysis deliberately focused on hospitalizations with a primary diagnosis of HF and excluded hospitalizations with a secondary diagnosis of HF, these data are likely an underestimation of the nationwide MethHF inpatient burden. Other limitations of the NIS data set have been previously described.21, 22
This analysis maintains a focus on clinical HF and does not discriminate based upon HF with reduced ejection fraction, pulmonary hypertension, coronary artery disease, or atrial fibrillation. This study was designed broadly to evaluate trends in MethHF hospitalizations. Furthermore, this study is limited in defining the risk related to MethHF as clinical outcomes were not assessed. Regarding substance abuse, these data do not allow for identification for the route of amphetamine abuse (ie, smoking, injection, oral ingestion, or nasal administration) which may have important implications in the type of resultant HF or pulmonary hypertension.30 While previous literature has suggested a high propensity of patients with MethHF towards tobacco and other substances of abuse such as marijuana, cocaine, and alcohol,29 this study did not analyze combination drug abuse. Additional studies with granular data such as route of amphetamine abuse, echocardiography measurements, therapeutic strategies, and clinical outcomes are ongoing and may provide greater insight.
Conclusions
Heart failure hospitalizations associated with methamphetamine abuse are increasing in the United States, especially in comparison with HF hospitalizations associated with cocaine or alcohol. The Pacific region is currently the most affected. Recognizing the severity of the growing MethHF epidemic is a critical first step before effectively addressing it through coordinated public health policies and systems of care.
Sources of Funding
None.
Disclosures
None.
Supporting information
Table S1–S3
Figure S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018370
For Sources of Funding and Disclosures, see page 9.
References
- 1.Havakuk O, Rezkalla SH, Kloner RA. The cardiovascular effects of cocaine. J Am Coll Cardiol. 2017;70:101–113. DOI: 10.1016/j.jacc.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 2.George A, Figueredo VM. Alcoholic cardiomyopathy: a review. J Card Fail. 2011;17:844–849. DOI: 10.1016/j.cardfail.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Diercks DB, Fonarow GC, Kirk JD, Jois‐Bilowich P, Hollander JE, Weber JE, Wynne J, Mills RM, Yancy C, Peacock WF IVth, et al. Illicit stimulant use in a United States heart failure population presenting to the emergency department (from the Acute Decompensated Heart Failure National Registry Emergency Module). Am J Cardiol. 2008;102:1216–1219. DOI: 10.1016/j.amjcard.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 4.Zhao SX, Kwong C, Swaminathan A, Gohil A, Crawford MH. Clinical characteristics and outcome of methamphetamine‐associated pulmonary arterial hypertension and dilated cardiomyopathy. JACC Heart Fail. 2018;6:209–218. DOI: 10.1016/j.jchf.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Schürer S, Klingel K, Sandri M, Majunke N, Besler C, Kandolf R, Lurz P, Luck M, Hertel P, Schuler G, et al. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine‐associated cardiomyopathy. JACC Heart Fail. 2017;5:435–445. DOI: 10.1016/j.jchf.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Sliman S, Waalen J, Shaw D. Methamphetamine‐associated congestive heart failure: increasing prevalence and relationship of clinical outcomes to continued use or abstinence. Cardiovasc Toxicol. 2016;16:381–389. DOI: 10.1007/s12012-015-9350-y. [DOI] [PubMed] [Google Scholar]
- 7.Won S, Hong RA, Shohet RV, Seto TB, Parikh NI. Methamphetamine‐associated cardiomyopathy. Clin Cardiol. 2013;36:737–742. DOI: 10.1002/clc.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, Kudelko K, Liu J, Hsi A, Rupp A, et al. Features and outcomes of methamphetamine‐associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:788–800. DOI: 10.1164/rccm.201705-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards JR, Harms BN, Kelly A, Turnipseed SD. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am J Emerg Med. 2018;36:1423–1428. DOI: 10.1016/j.ajem.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Hassan SF, Wearne TA, Cornish JL, Goodchild AK. Effects of acute and chronic systemic methamphetamine on respiratory, cardiovascular and metabolic function, and cardiorespiratory reflexes. J Physiol. 2016;594:763–780. DOI: 10.1113/JP271257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paratz ED, Cunningham NJ, MacIsaac AI. The cardiac complications of methamphetamines. Heart Lung Circ. 2016;25:325–332. DOI: 10.1016/j.hlc.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Kevil CG, Goeders NE, Woolard MD, Bhuiyan MS, Dominic P, Kolluru GK, Arnold CL, Traylor JG, Orr AW. Methamphetamine use and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2019;39:1739–1746. DOI: 10.1161/ATVBAHA.119.312461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkelman TNA, Admon LK, Jennings L, Shippee ND, Richardson CR, Bart G. Evaluation of amphetamine‐related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018;1:e183758. DOI: 10.1001/jamanetworkopen.2018.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Justice . National Drug Threat Assessment 2011. Johnstown, PA: National Drug Intelligence Center; 2011. [Google Scholar]
- 15.US Department of Justice . National Drug Threat Assessment 2012. Johnstown, PA: National Drug Intelligence Center; 2012. [Google Scholar]
- 16.US Department of Justice . National Drug Threat Assessment 2017. Johnstown, PA: National Drug Intelligence Center; 2017. [Google Scholar]
- 17.US Department of Justice . National Drug Threat Assessment 2018. Johnstown, PA: National Drug Intelligence Center; 2018. [Google Scholar]
- 18.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJV, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. DOI: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 19.Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail. 2014;7:590–595. DOI: 10.1161/CIRCHEARTFAILURE.113.001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glynn P, Lloyd‐Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol. 2019;73:2354–2355. DOI: 10.1016/j.jacc.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 21.Khera R, Krumholz HM. With great power comes great responsibility: big data research from the national inpatient sample. Circ Cardiovasc Qual Outcomes. 2017;10:e003846. DOI: 10.1161/CIRCOUTCOMES.117.003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318:2011–2018. DOI: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khera R, Pandey A, Kumar N, Singh R, Bano S, Golwala H, Kumbhani DJ, Girotra S, Fonarow GC. Variation in hospital use and outcomes associated with pulmonary artery catheterization in heart failure in the United States. Circ Heart Fail. 2016;9:e003226. DOI: 10.1161/CIRCHEARTFAILURE.116.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal SK, Wruck L, Quibrera M, Matsushita K, Loehr LR, Chang PP, Rosamond WD, Wright J, Heiss G, Coresh J. Temporal trends in hospitalization for acute decompensated heart failure in the United States, 1998–2011. Am J Epidemiol. 2016;183:462–470. DOI: 10.1093/aje/kwv455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal P, Almarzooq ZI, Horn EM, Karas MG, Sobol I, Swaminathan RV, Feldman DN, Minutello RM, Singh HS, Bergman GW, et al. Characteristics of hospitalizations for heart failure with preserved ejection fraction. Am J Med. 2016;129:e15–e26. DOI: 10.1016/j.amjmed.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure–associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259–1267. DOI: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;61:1078–1088. DOI: 10.1016/j.jacc.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura M, Ma J, Fox S, Toomu A, Mojaver S, Juang DK, Maisel AS, Thomas IC. Characteristics and outcomes of methamphetamine abuse among veterans with Heart Failure. Am J Cardiol. 2019;124:907–911. DOI: 10.1016/j.amjcard.2019.05.068. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura M, Bhatia H, Ma J, Dickson SD, Alshawabkeh L, Adler E, Maisel A, Criqui MH, Greenberg B, Thomas IC. The impact of substance abuse on heart failure Hospitalizations. Am J Med. 2020;133:207–213.e1. DOI: 10.1016/j.amjmed.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben‐Yehuda O, Siecke N. Crystal methamphetamine: a drug and cardiovascular epidemic. JACC Heart Fail. 2018;6:219–221. DOI: 10.1016/j.jchf.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Overview of the National (Nationwide) Inpatient Sample (NIS) . Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed on February 1, 2020.
- 32.Horvitz DG, Thompson DJ. A generalization of sampling without replacement from a finite universe. J American Stat Assoc. 1952;47:663–685. DOI: 10.1080/01621459.1952.10483446. [DOI] [Google Scholar]
- 33.Richardson S, Lin T, Li Y, Niu X, Xu M, Stander V, Tu X. Guidance for use of weights: an analysis of different types of weights and their implications when using SAS PROCs. General Psychiatry. 2019;32:e100038. DOI: 10.1136/gpsych-2018-100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang W, He H, Tu XM. Applied categorical and count data analysis. FL, USA: Chapman & Hall/CRC; 2012. [Google Scholar]
- 35.Healthcare Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project (HCUP). Available at: https://www.ahrq.gov/research/data/hcup/index.html. Accessed on June 1, 2019. [PubMed]
- 36.Kueh SA, Gabriel RS, Lund M, Sutton T, Bradley J, Kerr AJ, Looi JL. Clinical characteristics and outcomes of patients with amphetamine‐associated cardiomyopathy in South Auckland, New Zealand. Heart Lung Circ. 2016;25:1087–1093. DOI: 10.1016/j.hlc.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Mau MK, Asao K, Efird J, Saito E, Ratner R, Hafi M, Seto T. Risk factors associated with methamphetamine use and heart failure among native Hawaiians and other Pacific Island peoples. Vasc Health Risk Manag. 2009;5:45–52. [PMC free article] [PubMed] [Google Scholar]
- 38.Begeman A, Franssen EJF. Lack of detection of new amphetamine‐like drugs using conventional urinary immunoassays. Ther Drug Monit. 2018;40:135–139. DOI: 10.1097/FTD.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 39.Sethi R, Din A, McAllister R, Lester A. I have never used methamphetamine, but my urinalysis says i do. Prim CARE Companion CNS Disord. 2018;20:17l02129. DOI: 10.4088/PCC.17l02129. [DOI] [PubMed] [Google Scholar]
- 40.Wong CM, Hawkins NM, Jhund PS, MacDonald MR, Solomon SD, Granger CB, Yusuf S, Pfeffer MA, Swedberg K, Petrie MC, et al. Clinical characteristics and outcomes of young and very young adults with heart failure: the CHARM programme (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity). J Am Coll Cardiol. 2013;62:1845–1854. DOI: 10.1016/j.jacc.2013.05.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3
Figure S1


