Abstract
Context
Chronic obesity is associated with several complications, including cognitive impairment and dementia. However, we have only piecemeal knowledge of the mechanisms linking obesity to central nervous system damage. Among candidate mechanisms are other elements of obesity-associated metabolic syndrome, such as hypertension, dyslipidemia, and diabetes, but also systemic inflammation. While there have been several neuroimaging studies linking adiposity to changes in brain morphometry, a comprehensive investigation of the relationship has so far not been done.
Objective
To identify links between adiposity and cognitive dysfunction.
Methods
This observational cohort study (UK Biobank), with an 8-year follow-up, included more than 20 000 participants from the general community, with a mean age of 63 years. Only participants with data available on both baseline and follow-up timepoints were included. The main outcome measures were cognitive performance and mediator variables: hypertension, diabetes, systemic inflammation, dyslipidemia, gray matter measures, and cerebrovascular disease (volume of white matter hyperintensities on magnetic resonance imaging).
Results
Using structural equation modeling, we found that body mass index, waist-to-hip ratio, and body fat percentage were positively related to higher plasma C-reactive protein, dyslipidemia, hypertension, and diabetes. In turn, hypertension and diabetes were related to cerebrovascular disease. Finally, cerebrovascular disease was associated with lower cortical thickness and volume and higher subcortical volumes, but also cognitive deficits (largest significant pcorrected = 0.02).
Conclusions
We show that adiposity is related to poor cognition, with metabolic consequences of obesity and cerebrovascular disease as potential mediators. The outcomes have clinical implications, supporting a role for the management of adiposity in the prevention of late-life dementia and cognitive decline.
Keywords: obesity, cerebrovascular disease, white matter hyperintensities, gray matter, cognition
Obesity is a significant human and financial burden (1, 2). Among its many adverse health outcomes, chronic obesity in midlife is associated with subsequent cognitive impairment and is a risk factor for vascular dementia and Alzheimer disease (3).
Obesity is defined as the excessive accumulation of adipose tissue. Its link with cognitive dysfunction has been attributed to the metabolic consequences of visceral adiposity, namely hypertension, dyslipidemia, and insulin resistance, a constellation referred to as the metabolic syndrome (4). Emerging evidence also points to the importance of systemic inflammation (5, 6). Adipose tissue releases proinflammatory cytokines, such as interleukin-6, and inflammation-related proteins, such as C-reactive protein (CRP), which, in obesity, lead to low-grade systemic inflammation (7-9). Endocrine activities of adipose tissue also increase the risk of diabetes, hypertension, and dyslipidemia (10-12). These comorbidities are causally related to cerebrovascular dysfunction (13-18). Vascular damage in white matter may lead to demyelination, loss of oligodendrocytes, and gliosis (13, 14, 19-23).
In magnetic resonance imaging (MRI), small vessel cerebrovascular disease manifests as white matter hyperintensities (WMH) on T2-weighted imaging and changes in white matter integrity measured by diffusion-weighted imaging (17, 24, 25). White matter disruption has been associated with gray matter atrophy in cross-sectional studies (23, 26, 27). There is evidence that WMH precede cortical thinning from a longitudinal study in Parkinson disease (28).
Previous work has demonstrated that obesity is associated with cortical and subcortical functional and volumetric alterations (29-38). These changes, together with white matter microstructure alterations, are associated with poorer cognitive performance (38-41). Cognitive domains affected are executive function, verbal memory, processing speed (38-41), fluid intelligence (42), and working memory (43). In sum, adiposity seems to affect white and gray matter integrity, and hence cognitive function, possibly via low-grade systemic inflammation, hypertension, diabetes, and dyslipidemia (40, 44-46).
While separate links between obesity and white matter damage, between obesity and gray matter atrophy, and between obesity and poor cognitive performance are established, no study has investigated the proposed pathway in full. In this study, we set out to investigate this mechanism in one model with the following hypotheses: (1) obesity measures, such as body mass index (BMI), waist-to-hip ratio (WHR), and body fat percentage, are positively related to inflammation, hypertension, diabetes, triglycerides levels, and negatively to high-density lipoprotein levels (dyslipidemia), which in turn affect WMH volume; (2) WMH load is related to gray matter morphometry; and (3) all these variables may be independently associated with cognitive performance. To test this, we first utilized structural equation modeling (SEM), creating a model in line with our hypotheses. Next, we followed up on all significant associations using mediation analyses to better understand precise relationships between single variables. We used BMI, WHR, and body fat percentage as obesity measures as they differentially predict obesity-related outcomes (47-49).
Here, we used data from the UK Biobank cohort study and available imaging-derived phenotypes (50). The large sample size (~20 000 participants) enabled us to generate a comprehensive SEM with latent factors combining measures of adiposity, hypertension, diabetes, dyslipidemia, inflammation, brain abnormalities, and cognitive dysfunction. Obesity and blood measures were collected 8 years prior to neurocognitive measures.
Methods
Sample Characteristics
Here, we used the UK Biobank dataset—a large-scale study with extensive phenotyping and brain imaging data (51, 52). Details of recruitment, measurement methodologies, and quality control are available at https://www.ukbiobank.ac.uk and in the cited publications. Our study was performed under UK Biobank application ID 35605. The number of participants with available imaging data was 21 333. Prior to all analyses, we excluded individuals with a diagnosis of neurological illness (n = 1123, Table S1; (65)). UK Biobank field IDs used in this study can be found in Table S2 (65). All participants signed informed consents prior to participating in the study, which was approved by the North-West Multi-Centre Research Ethics Committee. All UK Biobank actions are overseen by the UK Biobank Ethics Advisory Committee.
UK Biobank data are collected on different visits and in this study, we are using data collected at 2 different visits (timepoints): initial assessment and imaging visit (an average of 8 years apart).
Obesity Measures
We used BMI, WHR, and body fat percentage as measures of adiposity. BMI and body fat percentage data were provided by the UK Biobank. BMI was calculated as the ratio of weight to height squared. Weight and estimated body fat percentage were measured with the Tanita BC418ma bioimpedance device (Tanita, Tokyo, Japan). We calculated WHR by dividing waist circumference by hip circumference. Obesity measures used here were collected at the initial assessment visit.
Hypertension and Diabetes Measures
Presence of diabetes and hypertension in participants was determined based on existing diagnoses at the time of initial assessment visit. Diastolic and systolic blood pressure were calculated as the average of 2 automated measurements of resting blood pressure. These data were collected during the initial assessment visit.
Blood Markers
We used 5 blood markers in our analysis: serum CRP, a marker of low-grade systemic inflammation (7); serum triglyceride and high-density lipoprotein cholesterol (HDL) levels as indicators of dyslipidemia; and glycated hemoglobin A1c (HbA1c) and glucose as measures of diabetes. Detailed description of blood sampling and procedures can be found in (53). Blood samples were collected at the initial assessment visit. Participants were non-fasted.
Imaging-Derived Phenotypes
For brain analyses, we used an array of imaging-derived phenotypes available from UK Biobank. Imaging pipeline details can be found in (50). For cortical gray matter morphometry, we used cortical volume and thickness as derived by FreeSurfer (54) for each parcel of the Desikan-Killiany-Tourville (DKT) atlas (55). Subcortical volumes were derived using Functional Magnetic Resonance Imaging of the Brain (FMRIB)’s Integrated Registration and Segmentation Tool (FIRST; (56, 57)). Here, we included volumes of all the available subcortical structures: the thalamus, caudate nucleus, putamen, pallidum, hippocampus, amygdala, and nucleus accumbens. Volume of WMH was calculated using the Brain Intensity AbNormality Classification Algorithm (BIANCA; (58)).
Cognitive Measures
In the UK Biobank, cognitive tests were administered via a touchscreen interface. The tests, approximately 15 minutes in length (59), are described in detail online at http://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=100026. Overall, they correspond well with questionnaires measuring similar constructs and exhibit a good test-retest reliability (60). We investigated the following 6 cognitive abilities: (1) working memory, assessed by the digit span task; (2) fluid intelligence, assessed by a set of reasoning tasks; (3) executive functions, measured by the tower rearranging test; (4) prospective memory, assessed by a number of times an intention was forgotten on a prospective memory task; (5) visuospatial memory, assessed by a pairs matching task (here the measurement indicated the number of incorrect pair matchings); and (6) reaction time, a measure of processing speed. Some of the cognitive measures were not administered to all participants, hence sample sizes might be smaller. Because tests of visuospatial memory and reaction time were administered more than once, we used an average value of all trials. Note that for prospective memory, visuospatial memory, and reaction time, higher scores indicate worse performance. Cognitive measures were collected at the imaging visit.
Confounders
In all analyses we controlled for the following confounders: sex, age, average household income, Townsend Deprivation Index (a measure of socioeconomic status (61)), education, depression, frequency of drinking alcohol, physical activity, and smoking status. Since age, household income, physical activity and smoking status variables were collected at both visits (initial and imaging), when possible we used confounder measures collected at the same visit as the corresponding measure of interest. Confounders were controlled for either by residualizing variables of interest (SEM), or by adding them into analyses as covariates of no interest (post-SEM analyses).
Statistics
Structural equation model
We used an SEM to test the relation between obesity, other elements of metabolic syndrome, inflammation, and brain and cognitive dysfunction. We prepared the data for the analysis in the following ways: First, volumetric brain measures—volume of WMH and cortical and subcortical gray matter—were corrected for intracranial volume. Second, BMI, WMH, CRP, HDL, glucose, HbA1c, and triglycerides levels were log-transformed and all variables were z-scored prior to analysis. Further, we pairwise excluded outliers as defined by datapoints being 2.2 interquartile range below first or above third quartile (62-64), which resulted in a dataset containing 20 210 participants (Table S3; (65)) with missing values (which are allowed in the SEM strategy described below). Finally, we residualized all variables for confounders described in “Confounders.” Sample size for each variable used in the SEM can be found in Table S4 (65).
We used the lavaan package in R (version 0.6–5; (66)) to define an SEM consisting of 10 variables: obesity (consisting of BMI, WHR, and body fat percentage); 4 obesity-related metabolic and disease markers—inflammation (serum CRP), dyslipidemia (serum triglyceride and serum HDL), hypertension (hypertension diagnosis, systolic and diastolic blood pressure) and diabetes (diabetes diagnosis, serum glucose and HbA1c levels); cerebrovascular disease (WMH); cortical thickness (average cortical thickness over all DKT parcels weighted by surface area of each hemisphere); cortical volume (summary volume for all DKT parcels); subcortical volume (summary volume for all measured subcortical parcels); and cognition (consisting of 6 cognitive measures described in “Cognitive Measures”). In the model we allowed for residual correlations between gray matter brain measures, but also between WHR and diabetes, hypertension, and dyslipidemia, as they all belong to a cluster of metabolic syndrome (67). Because the latent variable dyslipidemia consisted only of 2 indicators (HDL and triglycerides), we fixed the loadings of the indicators on the latent variable to be the same for both indicators (68). HDL values were multiplied by −1 prior to the analysis, as lower values indicate better metabolic health. Scatterplots of associations between variables of interest can be found in Supplementary Materials (65).
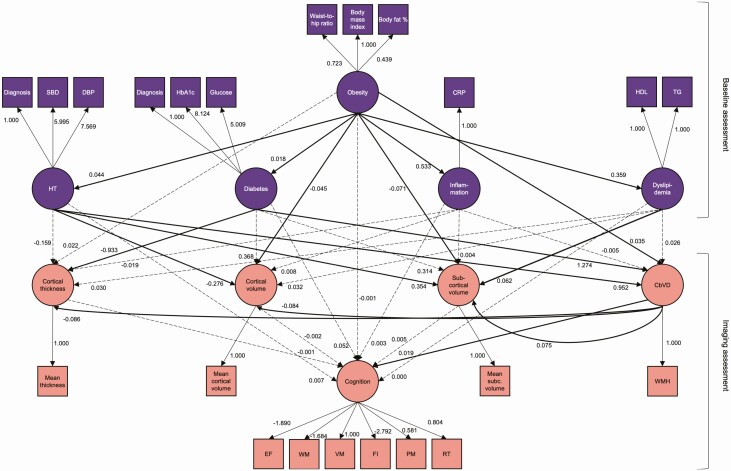
A representation of the model can be found in Fig. 1. In brief, in our model, obesity affected inflammation, dyslipidemia, hypertension, and diabetes, which all in turn affected brain measures—cortical thickness, volume, subcortical volume, and cerebrovascular disease. Furthermore, cerebrovascular disease also affected gray matter measures. Finally, all measures affected cognition. The model was tested using maximum likelihood estimation with pairwise missing values exclusions and robust standard errors. SEM P values for each parameter were corrected for multiple comparisons using an adjusted Bonferroni correction (69). Model fit was assessed using the following indices: root mean square of error approximation, standardized root mean square residual, and comparative fit index.
Figure 1.
Schematic representation of the structural equation model. Dashed lines represent nonsignificant associations. Note that not all measured variables contributing to cortical thickness, cortical volume, and subcortical volume are depicted in this graph. Age and sex were added to each of the associations between variables as covariates of no interest and are not depicted here. Education was added in each regression with cognition as outcome variable and is not depicted here. Abbreviations: CbVD, cerebrovascular disease; CRP, C-reactive protein; DBP, diastolic blood pressure; EF, executive function; FI, fluid intelligence; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HT, hypertension; PM, prospective memory; RT, reaction time; SBP, systolic blood pressure; subc., subcortical; TG, triglycerides, VM, visuospatial memory; WM, working memory; WMH, white matter hyperintensities.
In addition to the main model presented here, we investigated alternative models with fewer variables to determine whether they would provide a better description of the relations between obesity and cognition. We also investigated a model in which, in contrast to our hypotheses, gray matter measures influenced cerebrovascular disease. However, given our a priori hypotheses based on previous research, we decided to present those models in the supplemental data to this article (section “Alternative structural equation models” (65)).
Post-SEM analyses
Having established a relationship between obesity, inflammation, dyslipidemia, hypertension, diabetes, brain measures, and cognition, we also analyzed the variables separately, instead of pooling them into latent variables. This allowed us to investigate different aspects of obesity—BMI, body fat percentage, and WHR—as well as separate brain structures and parcels, and cognitive measures to look at associations identified in the SEM in more depth. Here, we investigated the following: (1) the association between obesity and cerebrovascular disease and its mediation by hypertension, diabetes, dyslipidemia and inflammation; (2) the relationship between obesity and gray matter measures, and its mediation by cerebrovascular disease; and (3) the association between obesity and cognition, and its mediation by cerebrovascular disease. Note that the relationships between obesity and cortical thickness and obesity and cognition were not significant in the SEM. However, as cerebrovascular disease is a hypothesized mediator in both of those relationships and was included in the SEM regressions, the lack of significance could mean a complete mediation of those relationships. We therefore decided to include these 2 analyses in our investigation.
From the sample described in “Sample Characteristics” we excluded BMI, WHR, body fat percentage, and WMH outliers listwise from analysis (2.2 interquartile range below/above first and third quartile, respectively; (62-64)). The resulting sample consisted of 18 778 individuals (Table S3; (65)). Next, we excluded outliers from our measures of interest. This was done separately for measures of cortical thickness and cortical and subcortical gray matter volume. However, for the sake of sample size consistency within each of those modalities, any outlier identified in one cortical parcel/subcortical structure was removed in all other parcels/structures. For cognitive measures, due to sample size differences, we decided to exclude outliers separately for each of the measures. Next, volumetric brain measures were corrected for total intracranial volume. Finally, we log-transformed WMH volume, BMI, and all serum/blood level variables to achieve a normal distribution of these variables. Prior to all analyses, all numerical variables were z-scored. In every analysis step we corrected for confounders described in “Confounders.” Initially, we also included age squared in our models, however, it did not significantly alter the results and we decided to exclude it from analyses. To avoid exclusions due to missing values in the confounding variables, we imputed those values using the Multivariate Imputation by Chained Equations (MICE) package in R with the predictive mean matching method. All analyses were performed in R (v. 3.6.0). Figures were prepared in MATLAB R2019b and python (v. 3.7.4) with the nilearn (v. 0.5.1) library (70). Scripts used in the study can be found at https://github.com/FilipMorys/WMHOB.
Obesity associations with gray matter morphometry and cognition.
We used a general linear model to assess the associations between obesity measures—BMI and WHR—and gray matter morphometry, but also obesity measures and cognition. P values were corrected for multiple comparisons using false discovery rate (FDR) Benjamini-Hochberg correction and were considered significant at α = 0.05 (71). The correction was applied separately for each gray matter morphometry modality, and collectively for all cognitive measures.
Sample sizes for these analyses and the following mediation analyses using the same measures of interest were: (1) cortical thickness: 17 813 (965 excluded); (2) cortical volume: 17 353 (1425 excluded); (3) subcortical volume: 16 979 (1799 excluded); (4) working memory: 8267 (10 511 excluded); (5) fluid intelligence: 16 693 (2085 excluded); (6) executive function: 6803 (11 975 excluded); (7) prospective memory: 17 094 (1684 excluded); (8) visuospatial memory: 17 094 (1684 excluded); and (9) reaction time: 17 094 (1684 excluded). Large sample sizes differences resulted from the fact that some cognitive measures were not administered to all participants.
Mediation analyses.
Based on our SEM, we tested whether inflammation, hypertension, diabetes, serum HDL, triglycerides, glucose, HbA1c levels, and systolic and diastolic blood pressure mediated the relationship between obesity and WMH volume. The sample size for these analyses after outlier exclusion was 12 193. This parallel mediation model was calculated in lavaan package for R (v. 0.6–5). We also tested whether WMH volume mediated the relationship between obesity measures and gray matter morphometry, but also obesity measures and cognition.
In each analysis, criteria for a significant mediation were: (1) significant total effect; (2) significant mediation effect; and (3) total and mediation effects of the same sign (72). Unless otherwise specified, mediation estimates and P values were assessed using 1000 quasi-Bayesian Monte Carlo simulations using “mediation” package for R (73). P values were corrected for multiple comparisons using FDR Benjamini-Hochberg correction and were considered significant at α = 0.05 (71). The correction was applied separately for each gray matter morphometry modality and collectively for all cognitive measures.
Results
Structural Equation Model
With this model we tested overall associations between obesity, inflammation, dyslipidemia, hypertension, diabetes, cerebrovascular disease, gray matter, and cognition. In line with the literature, we found that obesity is related to inflammation, hypertension, diabetes, and dyslipidemia. Obesity, hypertension, and diabetes measured 8 years prior, in turn, were associated with increased cerebrovascular disease, which was significantly related to cortical volume and thickness (negatively) and subcortical volumes (positively). We also found that diabetes was negatively related to cortical thickness, obesity and hypertension were negatively related to cortical volume, obesity was negatively related to subcortical volume, and hypertension and dyslipidemia were positively related to subcortical volume. Finally, we found that only cerebrovascular disease was directly associated with impaired cognitive function (Fig. 1, Table S4 and S5; (65)). Note that increased scores on the latent variable “cognition” mean worse cognitive performance. The SEM estimation produced the following fit measures: Χ 2(231) = 85417.770, P < 0.0001; standardized root mean square residual = 0.026, root mean square of error approximation = 0.031, comparative fit index = 0.959.
Relationship Between Obesity and Cerebrovascular Disease Is Mediated by Hypertension, Diabetes, Inflammation, and Dyslipidemia
We found that the relationship between obesity (BMI, WHR, body fat percentage) and cerebrovascular disease (WMH) was mediated by hypertension and diabetes diagnoses, serum glucose levels, and also systolic and diastolic blood pressure (Table 1). Additionally, serum HDL levels mediated the association between body fat percentage/WHR and WMH, and serum CRP levels mediated the association between waist-to-hip ratio and WMH.
Table 1.
Mediation analysis between obesity measures and white matter hyperintensities with the following measures: C-reactive protein levels, hypertension, diabetes, HDL levels, TG levels, glucose levels, HbA1c levels, DBP, and SBP
| Mediation | Total effect estimate | Total effect P value | ACME estimate | ACME P value | ADE estimate | ADE P value | Proportion mediated |
|---|---|---|---|---|---|---|---|
| BMI—CRP—WMH | 0.0980 | <0.0001 | 0.0061 | 0.1019 | 0.0470 | <0.0001 | 0.0625 |
| BMI—hypertension—WMH | 0.0118 | <0.0001 | 0.1208 | ||||
| BMI—diabetes—WMH | 0.0032 | 0.0098 | 0.0331 | ||||
| BMI—HDL—WMH | 0.0054 | 0.0674 | 0.0548 | ||||
| BMI—TG—WMH | -0.0052 | 0.0713 | -0.0531 | ||||
| BMI—glucose—WMH | 0.0020 | 0.0249 | 0.0210 | ||||
| BMI—HbA1c—WMH | 0.0005 | 0.5987 | 0.0054 | ||||
| BMI—DBP—WMH | 0.0217 | <0.0001 | 0.2224 | ||||
| BMI—SBP—WMH | 0.0050 | 0.0060 | 0.0516 | ||||
| WHR—CRP—WMH | 0.1210 | <0.0001 | 0.0074 | 0.0292 | 0.0720 | <0.0001 | 0.0607 |
| WHR—hypertension—WMH | 0.0120 | <0.0001 | 0.0993 | ||||
| WHR—diabetes—WMH | 0.0037 | 0.0092 | 0.0306 | ||||
| WHR—HDL—WMH | 0.0065 | 0.0385 | 0.0538 | ||||
| WHR—TG—WMH | -0.0090 | 0.0228 | -0.0741 | ||||
| WHR—glucose—WMH | 0.0023 | 0.0228 | 0.0190 | ||||
| WHR—HbA1c—WMH | 0.0005 | 0.6856 | 0.0044 | ||||
| WHR—DBP—WMH | 0.0211 | <0.0001 | 0.1740 | ||||
| WHR—SBP—WMH | 0.0046 | 0.0086 | 0.0382 | ||||
| BF% – CRP—WMH | 0.1090 | <0.0001 | 0.0106 | 0.0491 | 0.0420 | 0.0002 | 0.0980 |
| BF% – hypertension—WMH | 0.0134 | <0.0001 | 0.1231 | ||||
| BF% – diabetes—WMH | 0.0037 | 0.0057 | 0.0338 | ||||
| BF% – HDL—WMH | 0.0078 | 0.0167 | 0.0722 | ||||
| BF% – TG—WMH | -0.0059 | 0.0988 | -0.0546 | ||||
| BF% – glucose—WMH | 0.0024 | 0.0167 | 0.0217 | ||||
| BF% – HbA1c—WMH | 0.0007 | 0.4952 | 0.0065 | ||||
| BF% – DBP—WMH | 0.0285 | <0.0001 | 0.2627 | ||||
| BF% – SBP—WMH | 0.0058 | 0.0057 | 0.0536 |
Abbreviations: ACME, average causal mediation effect; ADE, average direct effect; BF%, body fat percentage; BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; SBP, systolic blood pressure; TG, triglycerides, WHR, waist-to-hip ratio.
Overall, those significant factors mediated approximately 45%, 46%, and 54% of the relationships between WMH and BMI, WHR, and body fat percentage, respectively.
Obesity Is Associated With Gray Matter Alterations and Cognition
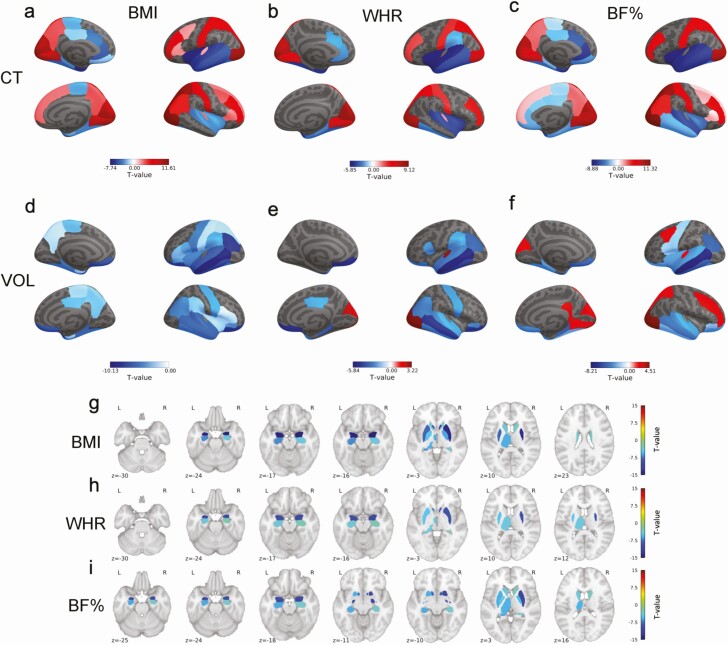
We observed widespread associations between obesity measures, cortical thickness, and gray matter volumes. Regarding cortical thickness, we found negative correlations with BMI, WHR, and body fat percentage in the bilateral temporal, entorhinal, orbitofrontal, and cingulate cortices. Positive correlations were also observed with the frontal, parietal, and occipital cortex (Table S6 (65), Fig. 2). Similarly, BMI, WHR, and body fat percentage correlated negatively with gray matter volume in the bilateral temporal, temporoparietal, parietal, and lateral frontal cortices, with less pronounced positive correlations in the occipital and posterior cingulate cortex for WHR and body fat percentage (Table S6 (65), Fig. 2). With regard to subcortical structures, BMI and body fat percentage correlated negatively with volume of the left thalamus and bilateral caudate, putamen, pallidum, hippocampus, amygdala, and nucleus accumbens (Table S7 (65), Fig. 2). WHR correlated negatively with volume of the left thalamus and bilateral putamen, hippocampus, amygdala, and nucleus accumbens (Table S7 (65), Fig. 2).
Figure 2.
Associations between: a, body mass index and cortical thickness; b, waist-to-hip ratio and cortical thickness; c, body fat percentage and cortical thickness; d, body mass index and cortical volume; e, waist-to-hip ratio and cortical volume; f, body fat percentage and cortical volume; g, body mass index and subcortical volume; h, waist-to-hip ratio and subcortical volume; i, body fat percentage and subcortical volume. Figures depict T-values of significant associations. Warm colors depict positive associations, while cold colors depict negative associations. T-value cutoff for a significant association: +-2.1294. Associations were corrected for multiple comparisons using Benjamini-Hochberg correction. Abbreviations: BF%, body fat percentage; BMI, body mass index; CT, cortical thickness; VOL, cortical volume; WHR, waist-to-hip-ratio.
Regarding cognition, BMI was related to lower working memory and better executive functions and visuospatial memory (Table S8; (65)), WHR was related to lower working memory and fluid intelligence (Table S8; (65)), while body fat percentage was related to lower working memory and better visuospatial memory (Table S8; (65)).
Cerebrovascular disease mediates the relationship between obesity and cognition, and obesity and gray matter morphometry
The association between BMI and working memory was mediated 9% by WMH load (Table 2). The relationships between WHR and working memory and fluid intelligence were mediated by WMH load 7% and 21%, respectively (Table 2). Finally, the association between body fat percentage and working memory was mediated by WMH 9%.
Table 2.
Mediation effects of white matter hyperintensities on the associations between body mass index and cognitive abilities
| Measure of interest | Total effect estimate | Total effect P value | ACME estimate | ACME P value | ADE estimate | ADE P value | Proportion mediated | Proportion P value |
|---|---|---|---|---|---|---|---|---|
| Body mass index | ||||||||
| Working memory | −0.0522 | 0.0020 | −0.0046 | 0.0060 | −0.0476 | 0.0020 | 0.0875 | 0.0080 |
| Executive functions | 0.1161 | <0.0001 | −0.0159 | 0.0030 | 0.1320 | <0.0001 | −0.1364 | 0.0030 |
| Visuospatial memory | −0.0381 | <0.0001 | 0.0069 | <0.0001 | −0.0450 | <0.0001 | −0.1819 | <0.0001 |
| Waist-to-hip ratio | ||||||||
| Working memory | −0.0661 | <0.0001 | −0.0046 | 0.0200 | −0.0615 | <0.0001 | 0.0698 | 0.0200 |
| Fluid intelligence | −0.0274 | 0.0080 | −0.0057 | <0.0001 | −0.0216 | 0.0340 | 0.2086 | 0.0160 |
| Body fat percentage | ||||||||
| Working memory | −0.0487 | <0.0001 | −0.0043 | 0.0140 | −0.0444 | <0.0001 | 0.0888 | 0.0140 |
| Visuospatial memory | −0.0391 | <0.0001 | 0.0057 | <0.0001 | −0.0448 | <0.0001 | −0.1438 | <0.0001 |
Abbreviations: ACME, average causal mediation effect; ADE, average direct effect.
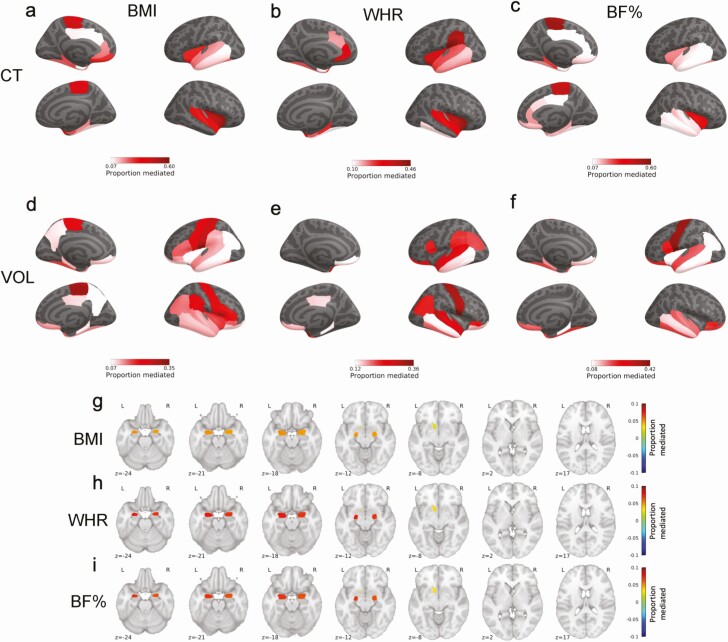
Further, WMH mediated the relationship between obesity measures (both BMI and WHR) and cortical thickness and volume in almost all DKT parcels that were negatively related to obesity measures (Fig. 3, Tables S9-S14). These parcels were predominantly located in the bilateral temporal lobes, entorhinal cortex, temporoparietal junction, cingulate cortex, and orbitofrontal cortex. The magnitude of mediation varied between 7% and 60%, with the largest mediating effect in the paracentral lobule and insula. With regard to subcortical structures, WMH mediated the relationship between all obesity measures and subcortical volumes in the bilateral amygdala and the left nucleus accumbens (Fig. 3, Table S15 (65)). The mediations were modest ranging from 2% to 8%.
Figure 3.
Proportion of relationships mediated by white matter hyperintensities between a, body mass index and cortical thickness; b, waist-to-hip ratio and cortical thickness; c, body fat percentage and cortical thickness; d, body mass index and cortical volume; e, waist-to-hip ratio and cortical volume; f, body fat percentage and cortical volume; g, body mass index and subcortical volume; h, waist-to-hip ratio and subcortical volume; i, body fat percentage and subcortical volume. Figures depict only significant consistent mediations. Associations were corrected for multiple comparisons using Benjamini-Hochberg correction. Abbreviations: BF%, body fat percentage; BMI, body mass index; CT, cortical thickness; VOL, cortical volume; WHR, waist-to-hip-ratio.
Discussion
We investigated the relationship between obesity, gray and white matter disruption, and cognition, and the possible mechanisms that link them. Using structural equation modeling, we find that obesity is related to systemic inflammation, hypertension, diabetes, and dyslipidemia. In turn, inflammation, hypertension, and diabetes are associated with cerebrovascular disease, which is further related to gray matter alterations and impaired cognition. We describe specific spatial patterns of cortical atrophy that are associated with obesity and white matter hyperintensities. We also find that certain cognitive domains are related to obesity and white matter hyperintensities.
The associations between obesity, especially visceral adiposity, and systemic inflammation, dyslipidemia, hypertension, and diabetes, are consistent with previous reports (74). Our mediation analyses are in line with those findings and show that inflammation, hypertension, and diabetes mediate the relationship between obesity and WMH independent of each other. Consequently, they might separately constitute potential targets for therapeutic interventions aimed at improving brain and cognitive outcomes in obesity.
Further, we showed that WMH are related to cortical gray matter loss. In our SEM, cerebrovascular disease was related to lower cortical thickness and volume. Our follow-up analyses show that WMH mediate the relationship between obesity and reduced gray matter volume and cortical thickness, but also that obesity is negatively related to gray matter morphometry measures independent of WMH. This suggests that the metabolic abnormalities due to adiposity may damage gray matter both directly and via white matter lesions. The spatial pattern of reductions in cortical volume is in line with previous reports. In their meta-analysis, Garcia-Garcia and colleagues showed that higher BMI was related to lower cortical volume in the medial prefrontal cortex, precentral gyrus, temporal pole, and cerebellum (33), which resembles our findings. With regard to cortical thickness, our results are also consistent with previous reports showing lower thickness in the temporal and occipital lobes in obesity (32, 75-78). Contrary to our hypotheses, in the SEM, we showed that the volume of WMH was related to higher volume of subcortical structures. This may represent true hypertrophy of these structures, but it may also be explained by subcortical segmentation inaccuracies, where periventricular WMH (which appear hypointense on T1-weighted MRI, with similar intensities to the neighboring subcortical gray matter structures) are misclassified as gray matter, leading to inflated estimates of subcortical volumes. This phenomenon, in fact, was demonstrated in a recent study (79). It could also explain the positive association between hypertension and dyslipidemia and volume of subcortical structures. Irrespective of this, our analyses of the direct relationship between obesity and subcortical volumes produced results consistent with the previous literature (31, 78, 80, 81), which shows that that obesity is related to lower volume of subcortical structures.
While some studies using the UK Biobank dataset report associations between obesity and global cortical thickness/gray matter volume (80-83), our analysis allowed a more fine-grained spatial differentiation. Thus, we also identified areas with greater cortical thickness and volume associated with higher BMI and body fat percentage predominantly in the occipital and frontal lobes. Similar findings were present in some previous studies regarding cortical volumes in young adults and using smaller sample sizes (37, 84, 85), but we are aware of only one study that showed similar patterns in cortical thickness, in this case using the Human Connectome Project dataset (32). One explanation for higher cortical thickness and volume in obesity might be neuroinflammation-related astrocytosis and microgliosis which could result in higher volume and thickness of gray matter regions (86-91). Alternatively, it is possible that juxtacortical white matter damage in obesity might be incorrectly classified as gray matter and therefore gray matter would appear thicker (92,93). Further, given the possible bidirectional relationship between brain and obesity (31), it is also possible that higher cortical thickness and volume in obesity in certain areas reflects a brain-based trait that leads to overeating and obesity, while lower volumes in other areas may reflect an effect of metabolic syndrome, as suggested by our SEM. Higher cortical thickness in frontal and parietal regions is in line with findings showing obesity-related functional and anatomical alterations in the same regions that are ultimately linked to eating behavior (94-97). In keeping with the theory that certain cortical alterations represent an underlying phenotype related to overeating, Vainik et al showed that cortical thickness associations with BMI in young adults were largely heritable (32). Nonetheless, the main direction of associations here are consistent with tissue damage, as demonstrated by the mediating effect of certain obesity-associated metabolic anomalies and cerebrovascular lesions.
In sum, our findings relating obesity to gray matter atrophy in the temporoparietal regions support previous theories that obesity accelerates aging and is a risk factor for dementia (98, 99). Indeed, aging is associated with loss of cortical gray matter in the temporal lobes and temporoparietal regions first, while other areas are affected at a later time (100). Additionally, brain atrophy in Alzheimer disease also seems to follow this pattern (101). Specifically, reductions in cortical thickness in individuals with mild cognitive impairment have been found in the temporal, parietal, and frontal cortices, while individuals with Alzheimer disease showed further cortical thinning in the lateral temporal lobe. This resembles the obesity-related atrophy patterns found here in the temporal, parietal, and frontal cortices, and provides a mechanism for the known association between adiposity in midlife and later Alzheimer disease (3, 102).
Finally, we show that obesity affects cognition via WMH. Consistent with the idea that it is visceral adipose tissue that is most related to inflammation, hypertension, and cardiovascular disease—factors that influence WMH (74)—we find that WHR, rather than BMI or body fat percentage, is a better correlate of poor cognition, as it was associated with worse outcomes in multiple cognitive domains while BMI and body fat percentage were only related to lower working memory.
Our study uses obesity and blood measures collected 8 years prior to imaging and cognitive assessments. A fully longitudinal design of all variables would allow for causal inferences on the origin of brain changes and impaired cognitive performance in obesity. Currently, it is not possible to infer causality from the presented associations. This is because the neurocognitive changes could have existed already at the first measurement timepoint or even preceded the obesity and blood measures. Even the link between inflammation and subsequent cerebrovascular disease could result from a reverse association, as cerebrovascular events may acutely raise CRP levels (103). Causality should be investigated in future studies and could be achieved with a fully longitudinal design.
In the same vein, with the design of our study, it is impossible to determine the causality between brain measures, especially regarding the question whether gray matter changes lead to WMH, or whether WMH lead to gray matter changes. This, however, has been studied previously (28, 104).
Next, only CRP blood levels were used to quantify inflammation. Extending the methodology to include more inflammatory factors is warranted and recommended for future studies on the topic. Finally, as in all UK Biobank studies, there is a possibility of a collider bias and attrition bias affecting our findings (105, 106). This is because the UK Biobank is not a sample representative of a population, but an overall healthier sample (107). Participation in the imaging follow-up might also have been negatively influenced by developing symptoms of diabetes, cerebrovascular disease, or other measurements used in our study, which could lead to collider bias. In addition, the imaging sample has a lower BMI than their age-matched counterparts from the baseline assessment. This is indicative of a possible attrition bias, which could also alter the results, if data from multiple timepoints are analyzed. So far, we did not use any strategies to correct for such biases as they mostly rely on value imputation or assumptions that are not testable (105, 106). Therefore, our results should be interpreted with caution. Collider bias due to recruitment or attrition effects may either cause spurious associations or obscure true ones. Overall, due to the design of our study—cross-sectional data collected on different timepoints—and possible biases, it is impossible to compare the strength of our associations with other true cross-sectional studies.
In sum, based on our results, a model of obesity-related brain and cognitive changes emerges. Greater adiposity is related to a low-grade systemic inflammation, dyslipidemia, hypertension, and diabetes, which in turn are associated with small vessel disease occurring as white matter hyperintensities. Together, they are related to subcortical and cortical alterations and cognitive deficits in obesity. Our results suggest that obesity can lead to accelerated brain aging and act as a risk factor for dementia. This has clinical implications for the management of obesity and prevention of dementia. In fact, studies show that lifestyle interventions including dietary changes and anti-hypertensive medication might be effective in decreasing the pace of cognitive deficits in older adults (108-113).
Acknowledgments
This work was supported by a Foundation Scheme award to A.D. from the Canadian Institutes of Health Research, and by computing resources from Calcul Québec (www.calculquebec.ca) and Compute Canada (www.computecanada.ca).
Financial Support: This work was supported by a Foundation Scheme award to A.D. from the Canadian Institutes of Health Research.
Glossary
Abbreviations
- BMI
body mass index
- CRP
C-reactive protein
- DKT
Desikan-Killiany-Tourville
- HbA1c
glycated hemoglobin A1c
- HDL
high-density lipoprotein
- MRI
magnetic resonance imaging
- SEM
structural equation modeling
- WHR
waist-to-hip ratio
- WMH
white matter hyperintensities
Additional Information
Disclosures: F.M., M.D., and A.D. have nothing to declare.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Hammond RA, Levine R. The economic impact of obesity in the United States. Diabetes Metab Syndr Obes. 2010;3:285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176-s185. [PubMed] [Google Scholar]
- 3. Whitmer RA, Gunderson EP, Quesenberry CP Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4(2):103-109. [DOI] [PubMed] [Google Scholar]
- 4. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004;292(18):2237-2242. [DOI] [PubMed] [Google Scholar]
- 6. Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab. 2013;2(4):356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107(1):E13. [DOI] [PubMed] [Google Scholar]
- 10. Zhou J, Qin G. Adipocyte dysfunction and hypertension. Am J Cardiovasc Dis. 2012;2(2):143-149. [PMC free article] [PubMed] [Google Scholar]
- 11. Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2010;10(4):306-315. [DOI] [PubMed] [Google Scholar]
- 12. Hwang YC, Fujimoto WY, Hayashi T, Kahn SE, Leonetti DL, Boyko EJ. Increased Visceral Adipose Tissue Is an Independent Predictor for Future Development of Atherogenic Dyslipidemia. J Clin Endocrinol Metab. 2016;101(2):678-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsuchou H, Kastin AJ, Mishra PK, Pan W. C-reactive protein increases BBB permeability: Implications for obesity and neuroinfammation. Cell Physiol Biochem. 2012;30(5):1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wardlaw JM, Makin SJ, Valdés Hernández MC, et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimer’s Dement. 2017;13(6):634-643. [Google Scholar]
- 15. Dufouil C, de Kersaint-Gilly A, Besançon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56(7):921-926. [DOI] [PubMed] [Google Scholar]
- 16. Tamura Y, Araki A. Diabetes mellitus and white matter hyperintensity. Geriatr Gerontol Int. 2015;15 Suppl 1:34-42. [DOI] [PubMed] [Google Scholar]
- 17. Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4(6):001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bowman GL, Kaye JA, Quinn JF. Dyslipidemia and blood-brain barrier integrity in Alzheimer’s disease. Curr Gerontol Geriatr Res. 2012;2012:184042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689-701. [DOI] [PubMed] [Google Scholar]
- 20. Bailey EL, Smith C, Sudlow CL, Wardlaw JM. Pathology of lacunar ischemic stroke in humans–a systematic review. Brain Pathol. 2012;22(5):583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aribisala BS, Valdés Hernández MC, Royle NA, et al. Brain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936. Eur Radiol. 2013;23(4):1084-1092. [DOI] [PubMed] [Google Scholar]
- 22. Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82(2):126-135. [DOI] [PubMed] [Google Scholar]
- 23. Lambert C, Sam Narean J, Benjamin P, Zeestraten E, Barrick TR, Markus HS. Characterising the grey matter correlates of leukoaraiosis in cerebral small vessel disease. Neuroimage Clin. 2015;9:194-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hakim AM. Small Vessel Disease. Front Neurol. 2019;10:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker KA, Power MC, Hoogeveen RC, et al. Midlife systemic inflammation, late-life white matter integrity, and cerebral small vessel disease: the atherosclerosis risk in communities study. Stroke. 2017;48(12):3196-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pareek V, Rallabandi VS, Roy PK. A Correlational study between microstructural white matter properties and macrostructural gray matter volume across normal ageing: conjoint DTI and VBM analysis. Magn Reson Insights 2018;11:1178623X1879992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tuladhar AM, Reid AT, Shumskaya E, et al. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke. 2015;46(2):425-432. [DOI] [PubMed] [Google Scholar]
- 28. Dadar M, Zeighami Y, Yau Y, et al. White matter hyperintensities are linked to future cognitive decline in de novo Parkinson’s disease patients. Neuroimage Clin. 2018;20:892-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horstmann A, Busse FP, Mathar D, et al. Obesity-related differences between women and men in brain structure and goal-directed behavior. Front Hum Neurosci. 2011;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Garcia I, Neseliler S, Morys F, et al. Relationship between impulsivity, uncontrolled eating and body mass index: a hierarchical model. bioRxiv 2020:348821. 10.1101/348821. Accessed February 20, 2021. [DOI] [PubMed] [Google Scholar]
- 31. García-García I, Morys F, Dagher A. Nucleus accumbens volume is related to obesity measures in an age-dependent fashion. J Neuroendocrinol. Published online December 11, 2019. doi:. doi: 10.1111/jne.12812. [DOI] [PubMed] [Google Scholar]
- 32. Vainik U, Baker TE, Dadar M, et al. Neurobehavioral correlates of obesity are largely heritable. Proc Natl Acad Sci. 2018;115(37):9312-9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. García-García I, Michaud A, Dadar M, et al. Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. Int J Obes. 2018;43(5):943-951. [DOI] [PubMed] [Google Scholar]
- 34. Rapuano KM, Zieselman AL, Kelley WM, Sargent JD, Heatherton TF, Gilbert-Diamond D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proc Natl Acad Sci U S A. 2017;114(1):160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horstmann A, Fenske WK, Hankir MK. Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obes Rev. 2015;16(10):821-830. [DOI] [PubMed] [Google Scholar]
- 36. Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3191-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31(4):1419-1425. [DOI] [PubMed] [Google Scholar]
- 38. Beyer F, Kharabian Masouleh S, Kratzsch J, et al. A metabolic obesity profile is associated with decreased gray matter volume in cognitively healthy older adults. Front Aging Neurosci. 2019;11:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kharabian Masouleh S, Arélin K, Horstmann A, et al. Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol Aging. 2016;40:1-10. [DOI] [PubMed] [Google Scholar]
- 40. Zhang R, Beyer F, Lampe L, et al. White matter microstructural variability mediates the relation between obesity and cognition in healthy adults. Neuroimage. 2018;172:239-249. [DOI] [PubMed] [Google Scholar]
- 41. Samara A, Murphy T, Strain J, et al. Neuroinflammation and white matter alterations in obesity assessed by diffusion basis spectrum imaging. Front Hum Neurosci. 2019;13:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spyridaki EC, Simos P, Avgoustinaki PD, et al. The association between obesity and fluid intelligence impairment is mediated by chronic low-grade inflammation. Br J Nutr. 2014;112(10):1724-1734. [DOI] [PubMed] [Google Scholar]
- 43. Alarcón G, Ray S, Nagel BJ. Lower Working Memory Performance in Overweight and Obese Adolescents Is Mediated by White Matter Microstructure. J Int Neuropsychol Soc. 2016;22(3):281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10-21. [DOI] [PubMed] [Google Scholar]
- 45. Lampe L, Zhang R, Beyer F, et al. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol. 2018;85(2):ana.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68(2):136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Noble RE. Waist-to-hip ratio versus BMI as predictors of cardiac risk in obese adult women. West J Med. 2001;174(4):240-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G. A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord. 2001;25(5):652-661. [DOI] [PubMed] [Google Scholar]
- 50. Alfaro-Almagro F, Jenkinson M, Bangerter NK, et al. Image processing and quality control for the first 10 000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. Plos Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Miller KL, Alfaro-Almagro F, Bangerter NK, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19(11):1523-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elliott P, Peakman TC; UK Biobank . The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37(2):234-244. [DOI] [PubMed] [Google Scholar]
- 54. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Klein A, Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci. 2012;6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790. [DOI] [PubMed] [Google Scholar]
- 57. Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Griffanti L, Zamboni G, Khan A, et al. BIANCA (Brain Intensity AbNormality Classification Algorithm): a new tool for automated segmentation of white matter hyperintensities. Neuroimage. 2016;141:191-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cullen B, Nicholl BI, Mackay DF, et al. Cognitive function and lifetime features of depression and bipolar disorder in a large population sample: Cross-sectional study of 143 828 UK Biobank participants. Eur Psychiatry. 2015;30(8):950-958. [DOI] [PubMed] [Google Scholar]
- 60. Fawns-Ritchie C, Deary IJ. Reliability and validity of the UK Biobank cognitive tests. Plos One. 2020;15(4):e0231627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. London: Croom Helm; 1988. [Google Scholar]
- 62. Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. J Am Stat Assoc. 1987;82(400):1147-1149. [Google Scholar]
- 63. Hoaglin DC, Iglewicz B, Tukey JW. Performance of some resistant rules for outlier labeling. J Am Stat Assoc. 1986;81(396):991-999. [Google Scholar]
- 64. Tukey JW. Exploratory Data Analysis. 1st ed. Reading, Mass: Pearson; 1977. [Google Scholar]
- 65. Morys F, Dadar M, Dagher A. Association between mid-life obesity, its metabolic consequences, cerebrovascular disease and cognitive decline: Supplementary Materials. Posted December 27, 2020. 10.6084/m9.figshare.13490319.v1 [DOI]
- 66. Rosseel Y. lavaan: an R package for structural equation modelinge human forearm during rythmic exercise. J Stat Softw. 2012;48(2):1-36. [Google Scholar]
- 67. Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-1645. [DOI] [PubMed] [Google Scholar]
- 68. Little TD, Lindenberger U, Nesselroade JR. On selecting indicators for multivariate measurement and modeling with latent variables: When “good” indicators are bad and “bad” indicators are good. Psychol Methods 1999;4(2):192-211. [Google Scholar]
- 69. Smith CE, Cribbie RA. Multiplicity control in structural equation modeling: incorporating parameter dependencies. Struct Equ Model A Multidiscip J. 2013;20(1):79-85. [Google Scholar]
- 70. Abraham A, Pedregosa F, Eickenberg M, et al. Machine learning for neuroimaging with scikit-learn. Front Neuroinform. 2014;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57(1):289-300. [Google Scholar]
- 72. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173-1182. [DOI] [PubMed] [Google Scholar]
- 73. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1-38.26917999 [Google Scholar]
- 74. Mathieu P, Poirier P, Pibarot P, Lemieux I, Després JP. Visceral obesity: the link among inflammation, hypertension, and cardiovascular disease. Hypertension. 2009;53(4):577-584. [DOI] [PubMed] [Google Scholar]
- 75. Veit R, Kullmann S, Heni M, et al. Reduced cortical thickness associated with visceral fat and BMI. Neuroimage Clin. 2014;6:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shaw ME, Sachdev PS, Abhayaratna W, Anstey KJ, Cherbuin N. Body mass index is associated with cortical thinning with different patterns in mid- and late-life. Int J Obes (Lond). 2018;42(3):455-461. [DOI] [PubMed] [Google Scholar]
- 77. Medic N, Ziauddeen H, Ersche KD, et al. Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes (Lond). 2016;40(7):1177-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Beyer F, García-García I, Heinrich M, et al. Neuroanatomical correlates of food addiction symptoms and body mass index in the general population. Hum Brain Mapp. 2019;40(9):2747-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dadar M, Potvin O, Camicioli R, Duchesne S. Beware of white matter hyperintensities causing systematic errors in grey matter segmentations! Hum Brain Mapp. 2021. doi: 10.1002/hbm.25398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dekkers IA, Jansen PR, Lamb HJ. Obesity, brain volume, and white matter microstructure at MRI: a cross-sectional UK biobank study. Radiology. 2019:291(3):763-771. [DOI] [PubMed] [Google Scholar]
- 81. Hamer M, Batty GD. Association of body mass index and waist-to-hip ratio with brain structure: UK Biobank study. Neurology. 2019;92(6):e594-e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ward MA, Carlsson CM, Trivedi MA, Sager MA, Johnson SC. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurol. 2005;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gustafson D, Lissner L, Bengtsson C, Björkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63(10):1876-1881. [DOI] [PubMed] [Google Scholar]
- 84. Herrmann MJ, Tesar AK, Beier J, Berg M, Warrings B. Grey matter alterations in obesity: a meta-analysis of whole-brain studies. Obes Rev. 2019;20(3):464-471. [DOI] [PubMed] [Google Scholar]
- 85. Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1428 healthy individuals. Obesity (Silver Spring). 2008;16(1):119-124. [DOI] [PubMed] [Google Scholar]
- 86. DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139 Suppl 2:136-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hsuchou H, Kastin AJ, Pan W. Blood-borne metabolic factors in obesity exacerbate injury-induced gliosis. J Mol Neurosci. 2012;47(2):267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maldonado-Ruiz R, Montalvo-Martínez L, Fuentes-Mera L, Camacho A. Microglia activation due to obesity programs metabolic failure leading to type two diabetes. Nutr Diabetes. 2017;7(3):e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57(6):563-581. [DOI] [PubMed] [Google Scholar]
- 91. Guillemot-Legris O, Muccioli GG. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 2017;40(4):237-253. [DOI] [PubMed] [Google Scholar]
- 92. Chard DT, Parker GJ, Griffin CM, Thompson AJ, Miller DH. The reproducibility and sensitivity of brain tissue volume measurements derived from an SPM-based segmentation methodology. J Magn Reson Imaging. 2002;15(3):259-267. [DOI] [PubMed] [Google Scholar]
- 93. Chard DT, Jackson JS, Miller DH, Wheeler-Kingshott CA. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J Magn Reson Imaging. 2010;32(1):223-228. [DOI] [PubMed] [Google Scholar]
- 94. Han JE, Boachie N, Garcia-Garcia I, Michaud A, Dagher A. Neural correlates of dietary self-control in healthy adults: A meta-analysis of functional brain imaging studies. Physiol Behav. 2018;192:98-108. [DOI] [PubMed] [Google Scholar]
- 95. Kakoschke N, Lorenzetti V, Caeyenberghs K, Verdejo-García A. Impulsivity and body fat accumulation are linked to cortical and subcortical brain volumes among adolescents and adults. Sci Rep. 2019;9(1). doi: 10.1038/s41598-019-38846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang H, Wen B, Cheng J, Li H. Brain structural differences between normal and obese adults and their links with lack of perseverance, negative urgency, and sensation seeking. Sci Rep. 2017;7. doi:. doi: 10.1038/srep40595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mehl N, Morys F, Villringer A, Horstmann A. Unhealthy yet avoidable — how cognitive bias modification alters behavioral and brain responses. Nutrients 2019;11(874). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Alford S, Patel D, Perakakis N, Mantzoros CS. Obesity as a risk factor for Alzheimer’s disease: weighing the evidence. Obes Rev. 2018;19(2):269-280. [DOI] [PubMed] [Google Scholar]
- 99. Ronan L, Alexander-Bloch AF, Wagstyl K, et al. ; Cam-CAN . Obesity associated with increased brain age from midlife. Neurobiol Aging. 2016;47:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29(48):15223-15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain. 2006;129(Pt 11):2885-2893. [DOI] [PubMed] [Google Scholar]
- 102. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10): 1556-1560. [DOI] [PubMed] [Google Scholar]
- 103. den Hertog HM, van Rossum JA, van der Worp HB, et al. ; PAIS investigators . C-reactive protein in the very early phase of acute ischemic stroke: association with poor outcome and death. J Neurol. 2009;256(12):2003-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dadar M, Camicioli R, Duchesne S, Collins DL; Alzheimer’s Disease Neuroimaging Initiative . The temporal relationships between white matter hyperintensities, neurodegeneration, amyloid beta, and cognition. Alzheimers Dement (Amst). 2020;12(1):e12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Munafò MR, Tilling K, Taylor AE, Evans DM, Davey Smith G. Collider scope: when selection bias can substantially influence observed associations. Int J Epidemiol. 2018;47(1): 226-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11(1):5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186(9):1026-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-2263. [DOI] [PubMed] [Google Scholar]
- 109. Lee KS, Lee Y, Back JH, et al. Effects of a multidomain lifestyle modification on cognitive function in older adults: an eighteen-month community-based cluster randomized controlled trial. Psychother Psychosom. 2014;83(5): 270-278. [DOI] [PubMed] [Google Scholar]
- 110. Dufouil C, Chalmers J, Coskun O, et al. ; PROGRESS MRI Substudy Investigators . Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112(11):1644-1650. [DOI] [PubMed] [Google Scholar]
- 111. Lam LCW, Chan WC, Leung T, Fung AWT, Leung EMF. Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: a cluster randomized controlled trial. PLoS One 2015;10(3). doi: 10.1371/journal.pone.0118173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. Bmj. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(Pt 4):765-772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.