Abstract
Background
There are limited contemporary data on the use of emergent coronary artery bypass grafting (CABG) in acute myocardial infarction.
Methods and Results
Adult (aged >18 years) acute myocardial infarction admissions were identified using the National (Nationwide) Inpatient Sample (2000–2017) and classified by tertiles of admission year. Outcomes of interest included temporal trends of CABG use; age‐, sex‐, and race‐stratified trends in CABG use; in‐hospital mortality; hospitalization costs; and hospital length of stay. Of the 11 622 528 acute myocardial infarction admissions, emergent CABG was performed in 1 071 156 (9.2%). CABG utilization decreased overall (10.5% [2000] to 8.7% [2017]; adjusted odds ratio [OR], 0.98 [95% CI, 0.98–0.98]; P<0.001), in ST‐segment–elevation myocardial infarction (10.2% [2000] to 5.2% [2017]; adjusted OR, 0.95 [95% CI, 0.95–0.95]; P<0.001) and non–ST‐segment–elevation myocardial infarction (10.8% [2000] to 10.0% [2017]; adjusted OR, 0.99 [95% CI, 0.99–0.99]; P<0.001), with consistent age, sex, and race trends. In 2012 to 2017, compared with 2000 to 2005, admissions receiving emergent CABG were more likely to have non–ST‐segment–elevation myocardial infarction (80.5% versus 56.1%), higher rates of noncardiac multiorgan failure (26.1% versus 8.4%), cardiogenic shock (11.5% versus 6.4%), and use of mechanical circulatory support (19.8% versus 18.7%). In‐hospital mortality in CABG admissions decreased from 5.3% (2000) to 3.6% (2017) (adjusted OR, 0.89; 95% CI, 0.88–0.89 [P<0.001]) in the overall cohort, with similar temporal trends in patients with ST‐segment–elevation myocardial infarction and non–ST‐segment–elevation myocardial infarction. An increase in lengths of hospital stay and hospitalization costs was seen over time.
Conclusions
Utilization of CABG has decreased substantially in acute myocardial infarction admissions, especially in patients with ST‐segment–elevation myocardial infarction. Despite an increase in acuity and multiorgan failure, in‐hospital mortality consistently decreased in this population.
Keywords: acute cardiovascular care, acute myocardial infarction, coronary artery bypass grafting, epidemiology, outcomes research
Subject Categories: Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- HCUP
Healthcare Cost and Utilization Project
- NIS
National (Nationwide) Inpatient Sample
Clinical Perspective
What Is New?
Utilization of emergent coronary artery bypass grafting (CABG) decreased among acute myocardial infarction hospitalizations between 2000 and 2017, with a more pronounced decrease in ST‐segment–elevation myocardial infarction compared with non–ST‐segment–elevation myocardial infarction hospitalizations.
Over time, admissions receiving CABG had increasing incidence of noncardiac acute organ failure and cardiogenic shock.
A steady and significant decline in in‐hospital mortality after CABG was identified over the 18‐year study period.
What Are the Clinical Implications?
Improvement in outcomes of percutaneous coronary intervention has resulted in the decline of emergent CABG use for acute myocardial infarction, especially for ST‐segment–elevation myocardial infarction.
In recent years, patients receiving emergent CABG are those with more advanced disease and those likely unsuitable for catheter‐based management.
In patients with acute myocardial infarction (AMI), especially in the subset of patients presenting with ST‐segment–elevation myocardial infarction (STEMI), prompt and timely reperfusion is essential, making fibrinolysis and/or primary percutaneous coronary intervention (PCI) the preferred revascularization strategy.1, 2 In the past 40 years, improvements in technology and pharmacotherapy in patients undergoing primary PCI for AMI have resulted in improved success rates and lower complication rates.3, 4 As a result, there have been notable reductions in the need for emergent coronary artery bypass grafting (CABG).4 The demographics of patients undergoing PCI for AMI have also changed, as patients are now older and have multiple comorbid conditions that place them at higher risk from CABG procedures.5 We previously reported a 90% reduction in the need for emergency CABG as compared with an earlier period where only percutaneous transluminal coronary angioplasty was performed.6
The need for CABG remains in patients with AMI, however, especially in patients with non–ST‐segment–elevation myocardial infarction (NSTEMI). Previous studies reporting on CABG utilization were derived from single‐center, regional registry data or do not include the contemporary era.3, 7, 8, 9 Moreover, the data are scant, especially relating to the temporal trends of the need for CABG as it relates to patients with AMI. This is relevant as the patterns of AMI have favored increases in the incidence of NSTEMI.10 The temporal trends and in‐hospital outcomes, therefore, need to be better defined from a contemporary, national sample stratified based on presentation with STEMI versus NSTEMI. Therefore, we sought to evaluate contemporary trends in CABG use and associated outcomes in the United States using a large nationally representative population.
METHODS
Study Population, Variables, and Outcomes
The National (Nationwide) Inpatient Sample (NIS) is the largest all‐payer database of hospital inpatient stays in the United States. NIS contains discharge data from a 20% stratified sample of community hospitals and is a part of the Healthcare Cost and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality (AHRQ).11 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 39 secondary diagnoses, and procedural diagnoses. Institutional review board approval was not sought because of the publicly available nature of this deidentified database. These data are available to other authors via the HCUP‐NIS database with the AHRQ.11
Using HCUP‐NIS data from 2000 to 2017, a retrospective cohort study of admissions with AMI in the primary diagnosis field (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] 410.x and International Classification of Diseases, Tenth Revision, Clinical Modification [ICD‐10‐CM] I21.x‐22.x) were identified. Admissions undergoing inpatient CABG were identified using ICD‐9‐CM 36.10–36.16, 36.19 and International Classification of Diseases, Tenth Revision, Procedure Coding System (ICD‐10‐PCS) 0210x–0213x. A CABG procedure at any time during the AMI hospitalization was considered emergent in our study because of the urgency in planning the procedure, the inability to perform it electively, and the mortality implications of not performing acute revascularization in AMI. This is consistent with the American College of Cardiology's National Cardiovascular Data Registry definition of emergent coronary revascularization.12 Longitudinal temporal trends were used to identify CABG utilization during this 18‐year study period. The study population was divided into tertiles of study period (2000–2005, 2006–2011, and 2012–2017) to compare their baseline, in‐hospital, and clinical characteristics. The burden of comorbid diseases was identified using Deyo modification of the Charlson Comorbidity Index.13 Demographic characteristics, hospital characteristics, acute organ failure, temporary mechanical circulatory support devices, cardiac procedures, and noncardiac procedures were identified for all admissions using previously used methodologies from our group (Table S1).14, 15, 16, 17, 18
The primary outcome was the temporal trends in the utilization of CABG among AMI hospitalizations. Secondary outcomes included temporal trends of: (1) CABG use stratified by patient (age, sex, and race) and hospital (location/teaching status, bed size, and region) characteristics; and (2) in‐hospital mortality. This study also analyzed hospital length of stay, hospitalization costs, and discharge disposition among tertiles of study period.
Statistical Analysis
As recommended by HCUP‐NIS, survey procedures using discharge weights provided with the HCUP‐NIS database were used to generate national estimates.19 Using the trend weights provided by the HCUP‐NIS, samples from 2000 to 2011 were reweighted to adjust for the 2012 HCUP‐NIS redesign.19 Chi‐square test and 1‐way ANOVA were used to compare categorical and continuous variables, respectively. Multivariable logistic regression was used to analyze trends over time (with 2000 being the reference year). Univariable analysis of temporal trends and outcomes was performed and is represented as odds ratio (OR) with 95% CI. Multivariable logistic regression for CABG utilization was performed, incorporating age, sex, race, primary payer, socioeconomic status, comorbidity, hospital location/teaching status, hospital bed size, and hospital region. Temporal trends of CABG utilization were evaluated among subgroups of age, sex, race, and hospital characteristics. Multivariable logistic regression analysis incorporating age, sex, race, socioeconomic stratum, hospital characteristics, weekend admission, comorbidities, acute organ failure, cardiogenic shock, cardiac arrest, type of AMI, cardiac procedures, noncardiac procedures, do‐not‐resuscitate status, and palliative care referral was performed for assessing temporal trends of in‐hospital mortality. For multivariable modeling, regression analysis with purposeful selection of statistically (liberal threshold of P<0.20 in univariate analysis) and clinically relevant variables was conducted.
The inherent restrictions of the HCUP‐NIS database related to research design, data interpretation, and data analysis were reviewed and addressed.19, 20 Pertinent considerations include not assessing individual hospital‐level volumes (because of changes to sampling design detailed above), treating each entry as an “admission” as opposed to individual patients, restricting the study details to inpatient factors since the HCUP‐NIS does not include outpatient data, and limiting administrative codes to those previously validated and used for similar studies. Two‐tailed P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 25.0 (IBM).
RESULTS
From January 1, 2000, to December 31, 2017, there were 11 622 528 admissions with a primary diagnosis of AMI, and among these, 1 071 156 (9.2%) received emergent inpatient CABG. Over the 18‐year study period, CABG utilization decreased among AMI admissions from 10.5% in 2000 to 8.7% in 2017, with a more pronounced decrease in STEMI compared NSTEMI (Figure 1A and 1B). There was a decrease in CABG utilization over the study period in several subgroups of patient age, sex, and race, and those admitted to urban and large hospitals (Figures 2A through 2F). Higher utilization of CABG was noted in admissions with age <75 years, men, those receiving care at urban teaching hospitals, and large hospitals. Over time, AMI admissions receiving CABG were more often men, of White race, and of lower socioeconomic status (all P<0.001) (Table 1). When stratified by tertiles of time period, AMI admissions more often received a CABG at urban teaching hospitals in recent years (57.7% in 2000–2005 to 71.6% in 2012–2017) (Table 1). When admissions receiving CABG were stratified by age and AMI presentation, there was a slight increase in CABG use for STEMI and NSTEMI in the 55‐ to 74‐year age group over the 18‐year period, while a slight decline was identified among other age subgroups (Figure S1).
Figure 1. Temporal trends in coronary artery bypass grafting (CABG) utilization and in‐hospital mortality (IHM) of acute myocardial infarction (AMI) admissions receiving CABG.
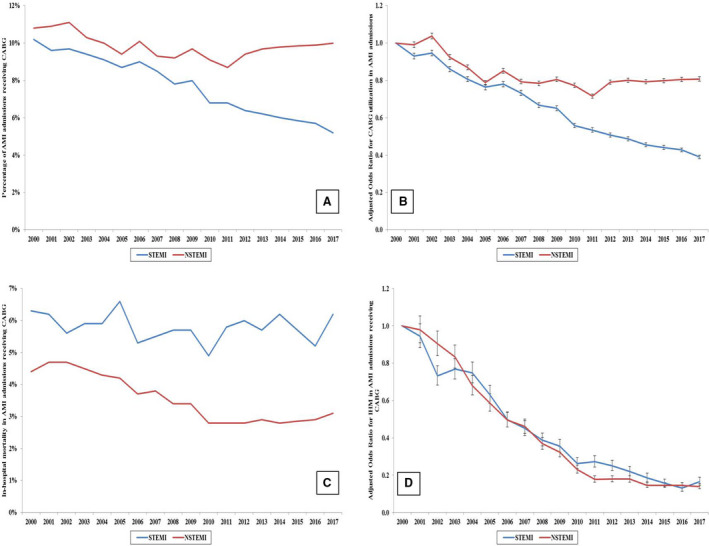
A, Unadjusted temporal trends in CABG utilization stratified by type of AMI (P<0.001 for trend over time). B, Adjusted temporal trends in CABG utilization (with 2000 as the referent year); adjusted for age, sex, race, primary payer, socioeconomic status, comorbidity, hospital location/teaching status, hospital bed size, and hospital region (all P<0.001 for trend over time). C, Unadjusted temporal trends in in‐hospital mortality of AMI admissions receiving CABG (P<0.001 for trend over time). D, Adjusted temporal trends in in‐hospital mortality of AMI admissions receiving CABG (2000 as referent year); adjusted for age, sex, race, primary payer, socioeconomic status, comorbidity, hospital location/teaching status, hospital bed size, and hospital region, weekend admission, cardiogenic shock, cardiac arrest, acute organ failure, coronary angiography, percutaneous coronary intervention, pulmonary artery catheterization, mechanical circulatory support, invasive mechanical ventilation, noninvasive ventilation, acute hemodialysis, palliative care referral, and do‐not‐resuscitate status (all P<0.001 for trend over time). NSTEMI indicates non–ST‐segment–elevation myocardial infarction; and STEMI, ST‐segment–elevation myocardial infarction.
Figure 2. Temporal trends of coronary artery bypass grafting (CABG) utilization among various subgroups of patient and hospital characteristics.
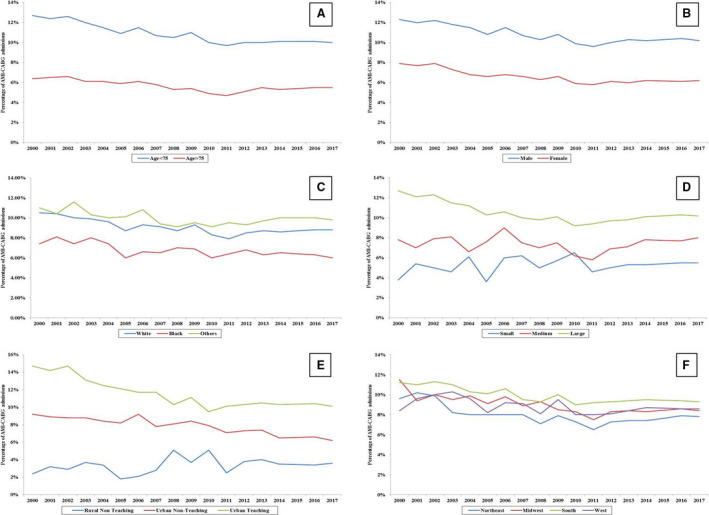
Eighteen‐year temporal trends of acute myocardial infarction (AMI) admissions receiving CABG stratified by age (A), sex (B), race (C), hospital location and teaching status (D), hospital region (E), and hospital bed size (F) (all P<0.001 for trend). Other indicates Hispanic, Asian, and Native American.
Table 1.
Baseline Characteristics of AMI Admissions Receiving CABG
| Baseline Characteristics | 2000–2005 (n=427 825) | 2006–2011 (n=322 620) | 2012–2017 (n=320 711) | P Value |
|---|---|---|---|---|
| Age, y | 65.4±11.5 | 64.8±11.4 | 65.1±10.9 | <0.001* |
| Women | 30.3 | 28.5 | 27.3 | <0.001† |
| Race/ethnicity | ||||
| White | 58.0 | 61.3 | 71.2 | <0.001† |
| Black | 4.4 | 5.8 | 7.7 | |
| Other‡ | 37.6 | 32.9 | 21.1 | |
| Quartile of median household income for zip code | ||||
| 0–25th | 15.3 | 28.2 | 30.7 | <0.001† |
| 26th–50th | 26.6 | 27.9 | 27.8 | |
| 51st–75th | 26.0 | 24.4 | 23.6 | |
| 75th–100th | 32.0 | 19.6 | 18.0 | |
| Primary payer | ||||
| Medicare | 52.4 | 49.5 | 52.2 | <0.001† |
| Medicaid | 5.1 | 6.4 | 8.8 | |
| Private | 35.0 | 33.1 | 29.3 | |
| Other§ | 7.5 | 11.0 | 9.7 | |
| Charlson Comorbidity Index | ||||
| 0–3 | 39.4 | 36.9 | 38.8 | <0.001† |
| 4–6 | 54.0 | 49.8 | 43.0 | |
| ≥7 | 6.7 | 13.3 | 18.2 | |
| Comorbidities | ||||
| Hypertension | 58.5 | 69.6 | 66.8 | <0.001† |
| Hyperlipidemia | 39.4 | 57.0 | 61.1 | <0.001† |
| Cancer | 5.3 | 6.4 | 4.8 | <0.001† |
| Heart failure | 29.4 | 30.7 | 28.1 | <0.001† |
| Chronic lung disease | 22.3 | 23.6 | 19.0 | <0.001† |
| Weekend admission | 21.5 | 22.8 | 23.1 | <0.001† |
| Hospital teaching status and location | ||||
| Rural | 4.0 | 4.4 | 3.6 | <0.001† |
| Urban nonteaching | 38.3 | 39.3 | 24.8 | |
| Urban teaching | 57.7 | 56.3 | 71.6 | |
| Hospital bed size | ||||
| Small | 4.6 | 6.4 | 8.9 | <0.001† |
| Medium | 18.4 | 19.0 | 25.0 | |
| Large | 76.9 | 74.6 | 66.1 | |
| Hospital region | ||||
| Northeast | 19.3 | 16.3 | 15.7 | <0.001† |
| Midwest | 22.2 | 23.3 | 22.2 | |
| South | 43.2 | 43.4 | 44.1 | |
| West | 15.4 | 17.0 | 18.0 | |
Values are represented as percentage or mean±SD. AMI indicates acute myocardial infarction; and CABG, coronary artery bypass grafting.
ANOVA test.
χ2 test.
Hispanic, Asian, Native American, other.
Uninsured, no charge, other.
Over time, admissions receiving CABG were more likely to have been admitted with NSTEMI (56.1% in 2000–2005 to 80.5% in 2012–2017, P<0.001) and had increasing incidence of noncardiac acute organ failure (8.4% in 2000–2005 to 26.1% in 2012–2017) and cardiogenic shock (6.5% in 2000–2005 to 11.5% in 2012–2017) (Table 2). Concomitant PCI use was seen in 9% of 12% of the population receiving CABG. An increase in the use of mechanical circulatory support and invasive and noninvasive mechanical ventilation was identified, whereas use of pulmonary artery catheterization decreased (Table 2). A slight increase in both palliative care referral and do‐not‐resuscitate status use was seen in the latter third of the study period (Table 2).
Table 2.
In‐Hospital Course and Management of AMI Admissions Receiving CABG
| Characteristics | 2000–2005 (n=427 825) | 2006–2011 (n=322 620) | 2012–2017 (n=320 711) | P Value |
|---|---|---|---|---|
| AMI type | ||||
| STEMI | 43.9 | 31.6 | 19.5 | <0.001* |
| NSTEMI | 56.1 | 68.4 | 80.5 | <0.001* |
| Acute organ failure | ||||
| Multiorgan | 8.4 | 16.8 | 26.1 | <0.001* |
| Respiratory | 7.3 | 11.3 | 17.0 | <0.001* |
| Renal | 9.8 | 17.4 | 24.7 | <0.001* |
| Hepatic | 0.6 | 1.7 | 2.4 | <0.001* |
| Hematologic | 7.2 | 11.8 | 19.0 | <0.001* |
| Neurologic | 2.1 | 4.7 | 6.6 | <0.001* |
| Cardiac arrest | 5.2 | 5.6 | 5.6 | <0.001* |
| Cardiogenic shock | 6.4 | 10.0 | 11.5 | <0.001* |
| Coronary angiography | 83.8 | 83.2 | 77.8 | <0.001* |
| PCI | 9.9 | 12.1 | 9.4 | <0.001* |
| Pulmonary artery catheterization | 5.7 | 5.6 | 4.8 | <0.001* |
| Mechanical circulatory support | ||||
| Total | 18.7 | 22.9 | 19.8 | <0.001* |
| IABP | 18.5 | 22.8 | 19.2 | <0.001* |
| pLVAD | 0.0 | 0.1 | 0.7 | <0.001* |
| ECMO | 0.0 | 0.1 | 0.4 | <0.001* |
| Invasive mechanical ventilation | 7.9 | 11.6 | 12.2 | <0.001* |
| Noninvasive ventilation | 0.5 | 1.9 | 3.4 | <0.001* |
| Acute hemodialysis | 1.0 | 1.7 | 1.0 | <0.001* |
| Do‐not‐resuscitate status | 0.0 | 0.1 | 1.3 | <0.001* |
| Palliative care referral | 0.0 | 0.3 | 0.7 | <0.001* |
Values are represented as percentage. AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; ECMO, extracorporeal membrane oxygenation; IABP, intra‐aortic balloon pump; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; pLVAD, percutaneous left ventricular assist device; and STEMI, ST‐segment–elevation myocardial infarction.
χ2 test.
In‐hospital mortality in AMI admissions receiving CABG decreased from 5.2% in 2000 to 2005 to 3.5% in 2012 to 2017 (Table 3). While unadjusted temporal trends demonstrated a relatively stable percentage of in‐hospital mortality among STEMI admissions, a decline was seen among those with NSTEMI (Figure 1C). Patient‐, hospital‐, and severity‐adjusted analysis, however, showed a steady decline in in‐hospital mortality among both STEMI and NSTEMI admissions receiving CABG (Figure 1D). A similar decline in in‐hospital mortality was seen in subgroups of age, sex, race, hospital bed size, hospital location/teaching status, and hospital region (Figures 3A through 3F). In multivariable logistic regression analysis, advanced age, female sex, lower socioeconomic status, admission to small hospitals, STEMI, cardiogenic shock, cardiac arrest, acute noncardiac organ failure, use of mechanical circulatory support, invasive mechanical ventilation, and acute hemodialysis were all independently predictive of in‐hospital mortality (Table 4). During the study period, there was an increase in lengths of hospital stay (median length of 9 days in 2000–2005 to 10 days in 2012–2017) and hospitalization costs. In recent years, admissions receiving CABG were less frequently discharged home (53.8% in 2000–2005 to 39.5% in 2012–2017) and more often to skilled nursing facilities (18.7% in 2000–2005 to 26.1% in 2012–2017) (Table 3).
Table 3.
Clinical Outcomes of AMI Admissions Receiving CABG
| Outcomes | 2000–2005 (n=427 825) | 2006–2011 (n=322 620) | 2012–2017 (n=320 711) | P Value |
|---|---|---|---|---|
| In‐hospital mortality | 5.2 | 4.0 | 3.5 | <0.001† |
| Length of stay, d | 9 (7–13) | 9 (7–13) | 10 (7–13) | <0.001* |
| Hospitalization costs (×1000 US$) | 74 (53–113) | 123 (86–187) | 172 (121–262) | <0.001* |
| Disposition | ||||
| Home | 53.8 | 43.7 | 39.5 | <0.001† |
| Transfer | 1.1 | 1.2 | 1.2 | |
| Skilled nursing facility | 18.7 | 22.8 | 26.1 | |
| Home with home health care | 26.3 | 32.2 | 33.0 | |
| Against medical advice | 0.1 | 0.1 | 0.1 | |
Values are represented as percentage or median (interquartile range). AMI indicates acute myocardial infarction; and CABG, coronary artery bypass grafting.
ANOVA.
χ2 test.
Figure 3. Temporal trends of in‐hospital mortality in coronary artery bypass grafting (CABG) recipients among various subgroups of patient and hospital characteristics.
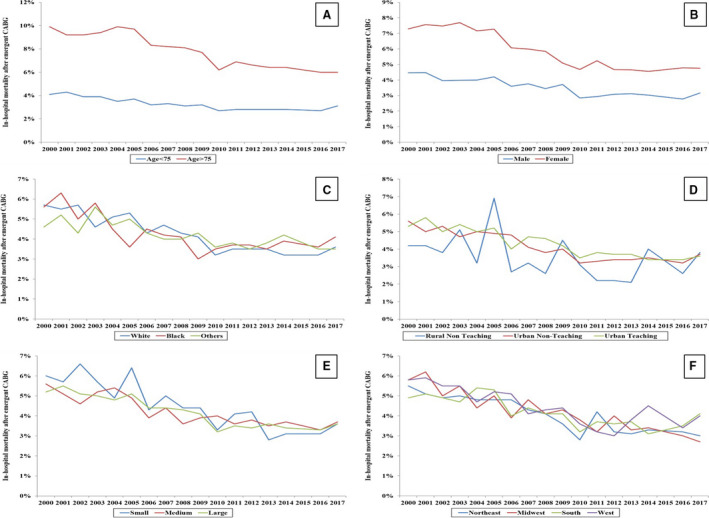
Eighteen‐year temporal trends of in‐hospital mortality in CABG recipients stratified by age (A), sex (B), race (C), hospital location and teaching status (D), hospital bed size (E), and hospital region (F) (all P<0.001 for trend). Other indicates Hispanic, Asian, and Native American.
Table 4.
Multivariable Logistic Regression Analysis for In‐Hospital Mortality in AMI Admissions Receiving CABG
| AMI‐CABG Admissions (N=1 071 156) | OR | 95% CI | P Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Age groups, y | ||||
| 19–49 | Reference category | |||
| 50–59 | 1.23 | 1.16 | 1.31 | <0.001 |
| 60–69 | 1.56 | 1.46 | 1.66 | <0.001 |
| 70–79 | 2.27 | 2.12 | 2.44 | <0.001 |
| ≥80 | 3.22 | 3.00 | 3.46 | <0.001 |
| Women | 1.50 | 1.46 | 1.53 | <0.001 |
| Race/ethnicity | ||||
| White | Reference category | |||
| Black | 1.04 | 0.99 | 1.10 | 0.08 |
| Other* | 1.03 | 1.01 | 1.06 | 0.01 |
| Primary payer | ||||
| Medicare | Reference category | |||
| Medicaid | 0.93 | 0.88 | 0.98 | 0.01 |
| Private | 0.76 | 0.74 | 0.79 | <0.001 |
| Other† | 0.97 | 0.93 | 1.02 | 0.31 |
| Quartile of median household income for zip code | ||||
| 0–25th | Reference category | |||
| 26th–50th | 0.97 | 0.94 | 1.00 | 0.03 |
| 51st–75th | 0.90 | 0.87 | 0.93 | 0.000 |
| 75th–100th | 0.90 | 0.87 | 0.93 | 0.000 |
| Study period | ||||
| 2000–2005 | Reference category | |||
| 2006–2011 | 0.44 | 0.43 | 0.45 | <0.001 |
| 2012–2017 | 0.23 | 0.22 | 0.24 | <0.001 |
| Weekend admissions | 0.97 | 0.94 | 0.99 | 0.02 |
| Charlson Comorbidity Index | ||||
| 0–3 | Reference category | |||
| 4–6 | 1.32 | 1.28 | 1.37 | <0.001 |
| ≥ 7 | 1.40 | 1.33 | 1.46 | <0.001 |
| Hospital teaching status and location | ||||
| Rural | Reference category | |||
| Urban nonteaching | 0.99 | 0.93 | 1.05 | 0.70 |
| Urban teaching | 1.00 | 0.94 | 1.06 | 0.96 |
| Hospital bed size | ||||
| Small | Reference category | |||
| Medium | 0.92 | 0.88 | 0.97 | 0.001 |
| Large | 0.85 | 0.81 | 0.89 | <0.001 |
| Hospital region | ||||
| Northeast | Reference category | |||
| Midwest | 1.04 | 1.00 | 1.08 | 0.030 |
| South | 1.21 | 1.17 | 1.25 | <0.001 |
| West | 0.90 | 0.87 | 0.94 | <0.001 |
| AMI type | ||||
| STEMI | Reference category | |||
| NSTEMI | 0.92 | 0.90 | 0.94 | <0.001 |
| Cardiac arrest | 5.98 | 5.82 | 6.15 | <0.001 |
| Cardiogenic shock | 2.32 | 2.26 | 2.39 | <0.001 |
| Acute organ dysfunction | ||||
| Respiratory | 1.47 | 1.43 | 1.51 | <0.001 |
| Renal | 2.93 | 2.86 | 3.01 | <0.001 |
| Hepatic | 3.25 | 3.10 | 3.40 | <0.001 |
| Hematologic | 1.26 | 1.23 | 1.30 | <0.001 |
| Neurological | 1.02 | 0.98 | 1.06 | 0.35 |
| Coronary angiography | 0.76 | 0.74 | 0.78 | <0.001 |
| Percutaneous coronary intervention | 0.96 | 0.93 | 0.99 | 0.01 |
| Pulmonary artery catheterization | 0.92 | 0.88 | 0.96 | <0.001 |
| Mechanical circulatory support | 2.65 | 2.59 | 2.72 | <0.001 |
| Invasive mechanical ventilation | 1.76 | 1.71 | 1.81 | <0.001 |
| Noninvasive mechanical ventilation | 0.59 | 0.54 | 0.64 | <0.001 |
| Hemodialysis | 1.95 | 1.85 | 2.05 | <0.001 |
| Palliative care referral | 28.17 | 25.38 | 31.26 | <0.001 |
| Do‐not‐resuscitate status | 10.77 | 9.87 | 11.76 | <0.001 |
AMI indicates acute myocardial infarction; CABG, coronary artery bypass grafting; NSTEMI, non–ST‐segment–elevation myocardial infarction; OR, odds ratio; and STEMI, ST‐segment–elevation myocardial infarction.
Hispanic, Asian, Native American, other.
Uninsured, no charge, other.
DISCUSSION
During the 18‐year study period, there was a decrease in the number of AMI hospitalizations receiving CABG, with a more significant decline among those with STEMI. Higher rates of CABG were seen among younger patients, men, and those receiving care at urban, teaching, and large hospitals. Over the study period, AMI admissions receiving CABG had higher rates of acute noncardiac organ failure, cardiogenic shock, and mechanical circulatory support use. There was a steady decrease in in‐hospital mortality, and an increase in hospital lengths of stay and hospitalization costs over the study period.
Consistent with prior data on AMI,10 temporal trends for CABG utilization in patients in our study presenting with AMI clearly demonstrated a decline in STEMI with a corresponding increase in NSTEMI (80% of all AMI) in the past 2 decades. Frequent changes in the diagnostic biomarker criteria of AMI poses significant challenges in the interpretation of the observed trends.21, 22 However, changes in demographics, especially as cardiovascular disease and AMI now disproportionately affect sicker, older adults with higher comorbidity burden, improvement in adherence to evidence‐based medications and advances in therapeutic approaches might have contributed to this shift.5 Although we did not observe a meaningful increase in age in the contemporary era, there was an increase in CABG use for both STEMI and NSTEMI among patients in the 56‐ to 74‐year age subgroup over the study period compared with younger age groups. Further, CABG use increased significantly in those with the highest comorbidity burden and doubled in high‐risk groups such as admissions from lowest income quartile. The rates of coronary angiography and PCI observed in our study were lower than those reported in the contemporary data. It is important to note that a PCI during the same hospitalization is unlikely in patients receiving CABG and this is reflected in our results (<10% of patients receiving CABG have PCI during the same hospitalization). The lower rates of coronary angiography are possibly the result of these patients having angiography performed during a different hospitalization. Since the NIS captures only one specific hospitalization, we are unable to establish this temporal relationship to evaluate this hypothesis.
In patients receiving CABG, the rates of concomitant PCI (either before or after CABG), cardiac arrest, invasive monitoring, and mechanical circulatory support have changed relatively slightly over the course of the present study. There has, however, been a significant increase in shock and multiorgan failure in patients receiving CABG with longer lengths of stay, higher costs, and more frequent disposition to a skilled care facility. This is likely because of the treatment of sicker patients presenting with higher comorbidity burden and shock and willingness of cardiac surgeons to perform emergent CABG in sicker patients presenting with AMI. Importantly success of catheter‐based management has reduced the need for emergent CABG in the majority of AMI cases with PCI being increasingly used in high‐risk and acute clinical presentations.3 As such, patients not suitable for PCI such as those with advanced disease or greater comorbidity are likely to receive CABG. Similar findings of an increase in comorbidity burden in patients undergoing CABG were seen in reports from Medicare and Veterans Affairs databases.23, 24 These studies also documented a decline in in‐hospital mortality in recent years.23, 24 Indeed, in our study, we observed that the in‐hospital mortality continued to decline, with the most notable 77% reduction displayed in the most recent era despite the increase in acuity.25 Further, the improvements in medical care, refinement in supportive therapies along with improved surgical revascularization techniques, preoperative and postoperative management, quality metrics, and an increasing proportion of patients receiving PCI before CABG have improved patients' ability to survive the AMI and postcardiac surgical course during the hospitalization. The in‐hospital mortality in the last tertile declined only slightly and this was consistent with observations from the Society of Thoracic Surgeons Adult Cardiac Surgery Database updates on outcomes and quality over the past few years.26 The increasing acuity in patients receiving CABG and standardization of care may be the cause for the observed plateauing.
The data on utilization of CABG during index hospital admissions following AMI are largely derived from older patients or from single‐center registries.8, 9 The rate of in‐patient CABG decreased from 14.4% to 10.2% over a 20‐year period among Medicare beneficiaries, similar to our study.27 One major limitation of our study is our inability to delineate the indications of CABG in patients with AMI. The need for emergency CABG has plummeted following failed PCI to <0.5%.6 PCI and/or pharmacoinvasive approaches are preferred in patients with STEMI as time‐to‐perfusion is critical2, 28; however, a minority of patients with mechanical complications or with untreated left main/3‐vessel disease may still be candidates for urgent CABG if their symptoms or ischemia continue. In our study, we observed a 56% reduction in CABG utilization in patients who had STEMI, with significant increased need in patients with NSTEMI. These trends correspond to general trends in AMI with an increase in NSTEMI and a decline in STEMI. It is conceivable that most patients in our study with NSTEMI waited longer for clinical/hemodynamic stabilization or antiplatelet therapy–free interval before they underwent CABG.
Limitations
The HCUP‐NIS attempts to mitigate potential errors by using several internal and external quality‐control measures. However, this study has several limitations that are inherent to using a large administrative database. The use of previously validated administrative codes reduces the inherent errors in the study. Important clinical data including angiographic findings such as lesion classification, presence of multivessel disease, degree of stenosis, revascularization failure, and other operative characteristics that may significantly influence outcomes were not available in this database. There are limited data on patient‐ and family‐specific limitations to therapeutic options that may influence the clinical outcomes in this population. Other factors such as the delay in presentation from time of onset of AMI symptoms, timing of cardiogenic shock and/or cardiac arrest, timing of multiorgan failure, and treatment‐limiting decisions of organ support could not be reliably identified in this database. The inability to identify the timing of CABG in relation to these events may have influenced the observed results and trends. Despite accounting for confounding using extensive covariate adjustment and analyses appropriate for survey data, it is possible that observed results are influenced by residual confounding from unmeasured covariates which may, in part, be related to the changes in hospitalization inclusion criteria in the HCUP‐NIS samples over time. Our data are reflective of in‐hospital outcomes and we are unable to comment on the postdischarge or long‐term outcomes of these patients. Despite these limitations, this study provides important information as the largest contemporary epidemiological study of CABG utilization, characteristics of patients receiving CABG, other concomitant management strategies, and outcomes in AMI admissions.
CONCLUSIONS
CABG was utilized in 9.2% of all AMI admissions, with a significant decrease for STEMI admissions over the study period. An increase in rates of concomitant noncardiac organ failure and cardiogenic shock was noted in AMI admissions treated with CABG. A steady and significant decline in in‐hospital mortality with a concomitant increase in hospital lengths of stay and hospitalization costs was identified.
Sources of Funding
None.
Disclosures
None.
Supporting information
Table S1
Figure S1
Acknowledgments
Author contributions: Study design, literature review, statistical analysis: S.H.P., A.K., W.C., R.P.D., and S.V.; data management, data analysis, article drafting: S.H.P., A.K., W.C., R.P.D., and S.V.; access to data: S.H.P., A.K., W.C., R.P.D., J.M.S., D.R.H., M.R.B., M.S., and S.V.; article revision, intellectual revisions, mentorship: J.M.S., D.R.H., M.R.B., M.S., and S.V.; final approval: S.H.P., A.K., W.C., R.P.D., J.M.S., D.R.H., M.R.B., M.S., and S.V.
(J Am Heart Assoc. 2021;10:e020517. DOI: 10.1161/JAHA.120.020517.)
A portion of these results were presented at the American College of Cardiology Scientific Sessions, May 15–17, 2021.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020517
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, CF/AHA Task Force , et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College Of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;127:529–555. DOI: 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 2.Ibánez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Rev Esp Cardiol (Engl Ed). 2017;70:1082. DOI: 10.1016/j.rec.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 3.Kataruka A, Maynard CC, Kearney KE, Mahmoud A, Bell S, Doll JA, McCabe JM, Bryson C, Gurm HS, Jneid H, et al. Temporal trends in percutaneous coronary intervention and coronary artery bypass grafting: Insights from the Washington cardiac care outcomes assessment program. J Am Heart Assoc. 2020;9:e015317. DOI: 10.1161/JAHA.119.015317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh M, Rihal CS, Gersh BJ, Lennon RJ, Prasad A, Sorajja P, Gullerud RE, Holmes DR Jr. Twenty‐five‐year trends in in‐hospital and long‐term outcome after percutaneous coronary intervention: a single‐institution experience. Circulation. 2007;115:2835–2841. DOI: 10.1161/CIRCULATIONAHA.106.632679 [DOI] [PubMed] [Google Scholar]
- 5.Singh M, Spertus JA, Gharacholou SM, Arora RC, Widmer RJ, Kanwar A, Sanjanwala RM, Welle GA, Al‐Hijji MA. Comprehensive geriatric assessment in the management of older patients with cardiovascular disease. Mayo Clin Proc. 2020;95:1231–1252. DOI: 10.1016/j.mayocp.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 6.Yang EH, Gumina RJ, Lennon RJ, Holmes DR Jr, Rihal CS, Singh M. Emergency coronary artery bypass surgery for percutaneous coronary interventions: changes in the incidence, clinical characteristics, and indications from 1979 to 2003. J Am Coll Cardiol. 2005;46:2004–2009. DOI: 10.1016/j.jacc.2005.06.083 [DOI] [PubMed] [Google Scholar]
- 7.Tran DT, Welsh RC, Ohinmaa A, Thanh NX, Bagai A, Kaul P. Quality of acute myocardial infarction care in Canada: a 10‐year review of 30‐day in‐hospital mortality and 30‐day hospital readmission. Can J Cardiol. 2017;33:1319–1326. DOI: 10.1016/j.cjca.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 8.Gu YL, van der Horst IC, Douglas YL, Svilaas T, Mariani MA, Zijlstra F. Role of coronary artery bypass grafting during the acute and subacute phase of ST‐elevation myocardial infarction. Neth Heart J. 2010;18:348–354. DOI: 10.1007/BF03091790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone GW, Brodie BR, Griffin JJ, Grines L, Boura J, O'Neill WW, Grines CL. Role of cardiac surgery in the hospital phase management of patients treated with primary angioplasty for acute myocardial infarction. Am J Cardiol. 2000;85:1292–1296. DOI: 10.1016/S0002-9149(00)00758-X [DOI] [PubMed] [Google Scholar]
- 10.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP, Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. DOI: 10.1161/CIRCULATIONAHA.109.897249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HCUP . Introduction to HCUP National Inpatient Sample (NIS) 2012. 2012. https://www.hcup‐us.ahrq.gov/. Accessed February 15, 2021.
- 12.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College Of Cardiology‐National Cardiovascular Data Registry (ACC‐NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–2245. DOI: 10.1016/S0735-1097(01)01372-9 [DOI] [PubMed] [Google Scholar]
- 13.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. DOI: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 14.Vallabhajosyula S, Kumar V, Vallabhajosyula S, Subramaniam AV, Patlolla SH, Verghese D, Ya'Qoub L, Stulak JM, Sandhu GS, Prasad A, et al. Acute myocardial infarction‐cardiogenic shock in patients with prior coronary artery bypass grafting: a 16‐year national cohort analysis of temporal trends, management and outcomes. Int J Cardiol. 2020;310:9–15. DOI: 10.1016/j.ijcard.2020.02.033 [DOI] [PubMed] [Google Scholar]
- 15.Vallabhajosyula S, Patlolla SH, Dunlay SM, Prasad A, Bell MR, Jaffe AS, Gersh BJ, Rihal CS, Holmes DR Jr, Barsness GW. Regional variation in the management and outcomes of acute myocardial infarction with cardiogenic shock in the United States. Circ Heart Fail. 2020;13:e006661. DOI: 10.1161/CIRCHEARTFAILURE.119.006661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallabhajosyula S, Dunlay SM, Prasad A, Kashani K, Sakhuja A, Gersh BJ, Jaffe AS, Holmes DR Jr, Barsness GW. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73:1781–1791. DOI: 10.1016/j.jacc.2019.01.053 [DOI] [PubMed] [Google Scholar]
- 17.Vallabhajosyula S, Patlolla SH, Miller PE, Cheungpasitporn W, Jaffe AS, Gersh BJ, Holmes DR Jr, Bell MR, Barsness GW. Weekend effect in the management and outcomes of acute myocardial infarction in the United States, 2000–2016. Mayo Clin Proc Innov Qual Outcomes. 2020;4:362–372. DOI: 10.1016/j.mayocpiqo.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallabhajosyula S, Patlolla SH, Cheungpasitporn W, Holmes DR Jr, Gersh BJ. Influence of seasons on the management and outcomes acute myocardial infarction: an 18‐year US study. Clin Cardiol. 2020;43:1175–1185. DOI: 10.1002/clc.23428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khera R, Krumholz HM. With great power comes great responsibility: Big data research from the national inpatient sample. Circ Cardiovasc Qual Outcomes. 2017;10:e003846. DOI: 10.1161/CIRCOUTCOMES.117.003846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the national inpatient sample. JAMA. 2017;318:2011–2018. DOI: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandoval Y, Thygesen K, Jaffe AS. The universal definition of myocardial infarction: present and future. Circulation. 2020;141:1434–1436. DOI: 10.1161/CIRCULATIONAHA.120.045708 [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Executive Group on behalf of the Joint European Society of Cardiology /American College of Cardiology /American Heart Association /World Heart Federation Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72:2231–2264. DOI: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 23.Cornwell LD, Omer S, Rosengart T, Holman WL, Bakaeen FG. Changes over time in risk profiles of patients who undergo coronary artery bypass graft surgery: the veterans affairs surgical quality improvement program (VASQIP). JAMA Surgery. 2015;150:308–315. DOI: 10.1001/jamasurg.2014.1700 [DOI] [PubMed] [Google Scholar]
- 24.McNeely C, Markwell S, Vassileva C. Trends in patient characteristics and outcomes of coronary artery bypass grafting in the 2000 to 2012 medicare population. Ann Thorac Surg. 2016;102:132–138. DOI: 10.1016/j.athoracsur.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 25.Brieger DB, Ng AC, Chow V, D'Souza M, Hyun K, Bannon PG, Kritharides L. Falling hospital and postdischarge mortality following CABG in new south wales from 2000 to 2013. Open Heart. 2019;6:e000959. DOI: 10.1136/openhrt-2018-000959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, Shahian DM. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2019 update on outcomes and quality. Ann Thorac Surg. 2019;107:24–32. DOI: 10.1016/j.athoracsur.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 27.Krumholz HM, Normand ST, Wang Y. Twenty‐year trends in outcomes for older adults with acute myocardial infarction in the united states. JAMA Netw Open. 2019;2:e191938. DOI: 10.1001/jamanetworkopen.2019.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallabhajosyula S, Verghese D, Subramaniam AV, Kumar V, Ya'Qoub L, Patlolla SH, Cheungpasitporn W, Sundaragiri PR, Singh M, Jaffe AS, et al. Management and outcomes of uncomplicated ST‐segment elevation myocardial infarction patients transferred after fibrinolytic therapy. Int J Cardiol. 2020;321:54–60. DOI: 10.1016/j.ijcard.2020.08.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


