Abstract
Background
Currently extended-spectrum β-lactamase (ESβL) and carbapenemase producing gram-negative bacteria are the greatest concern among the neonatal population with very limited therapeutic options. The aim of this study was to assess the prevalence of ESβL and carbapenemase producing gram-negative bacilli, associated factors and antimicrobial resistance patterns among neonates in intensive care units.
Methods
An institutional-based cross-sectional study was conducted from February to June 2021 on 212 neonates in intensive care units. Risk factors data were collected by using a well-designed questionnaire. A rectal swab sample was collected using a sterile cotton swab and inoculated on MacConkey agar. Bacterial isolates were identified using various biochemical tests. ESβL and carbapenemase were first screened by indicator cephalosporins (cefotaxime (30µg) and ceftazidine (30µg)) and carbapenem (meropenem and ertapenem), respectively. ESβL and carbapenemase were confirmed by a double-disk synergy test and modified carbapenem inactivation methods, respectively. SPSS version 21.0 was used for data analysis. A P-value ≤ 0.05 was considered as statistically significant.
Results
The overall prevalence of ESβL-producing gram-negative bacilli was 72/212 (34%). The predominant ESβL-producing isolate was Klebsiella pneumoniae 23/72 (31.9%) followed by Escherichia coli 17/72 (23.6%). Five (2.4%) carbapenemase-producing gram-negative bacilli were isolated. ESβL-producing isolates showed a high resistance against ampicillin 72/72 (100%), augmentin 69/72 (95.8%) and gentamycin 57/72 (79.2%). The majority 63/72 (87.5%) of isolated ESβL-producing gram-negative bacilli were multi-drug resistant (MDR). Rectal carriage of ESβL by neonates showed a statistically significant association with endotracheal intubation (p = 0.001; AOR = 4.2; 96% CI = (1.8–9.5)), treatment with ampicillin+gentamycin (p = 0.004; AOR = 3.3; 95% CI = (1.5–7.6)) and staying in a neonatal intensive care unit (NICU) between 11 and 20 days (p = 0.042; AOR = 2; 95% CI = (1.0–4.5)).
Conclusion
A high prevalence of ESβL-producing bacterial isolates was observed for commonly used antibiotics which needs further attention. Therefore, continuous and regular follow-ups of drug resistance patterns is important for the proper treatment and management of ESβL and carbapenemase producing gram-negative bacilli.
Keywords: extended-spectrum β-lactamase, carbapenemase, gram-negative bacilli
Introduction
Extended-spectrum β-lactamase producing gram-negative bacilli (ESβL-PGNB) and carbapenemase producing gram-negative bacilli (CP-PGNB) are a major public health problem in both health care-associated and community-acquired infections.1,2 The World Health Organization (WHO) has published a global priority pathogens list of antibiotic resistant gram-negative bacilli (GNB) including ESβL-PGNB and CP-PGNB. On the critically important list is carbapenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacteriaceae.3,4 These organisms are the most common causes of nosocomial infections and colonization especially in neonatal intensive care units (NICUs).5 Globally about one million newborns die annually due to bacterial infections during the first month of life and out of these infections, about 30% are estimated to be caused by antibiotic resistant bacteria. Mortality was increased in neonates with ESβL-PGNB infection.6
ESβLs are bacterial enzymes that can cleave the β-lactam ring of β-lactam antibiotics and confer increased resistance to commonly used and newer β-lactam antibiotics, including third- and fourth-generation cephalosporins and monobactams. Carbapenemases are enzymes that are able to hydrolyze nearly all β-lactamase antibiotics, including carbapenems.1,7
Neonates in the NICU are uniquely vulnerable to colonization and infection with pathogens such as ESβL-PGNB and CP-PGNB due to an immature immune system, administration of broad-spectrum antibiotics, contact with healthcare workers and exposure to invasive, life-sustaining procedures and surgical procedures.8 Healthcare-associated infections in neonates admitted to the NICU are associated with increased healthcare costs and length of stay, as well as significant morbidity and mortality to the patients.9,10 A high fecal carriage of ESβL-PGNB is a risk factor for subsequent infection and provides a reservoir of organisms for transmission within the hospital setting in NICU and nurseries, which leads to endemic and epidemic infections.11 Since high rectal carriage of ESβL-PGNB and CP-PGNB among neonates in ICUs leads to infections due to the immature immune system of neonates it is necessary to do microbiological analysis of ESβL-PGNB and CP-PGNB on rectal swabs taken from neonates in ICUs. Such kinds of data are scarce in the study area as well as nationally which is important for effective treatment and management of ESβL and carbapenemase producing gram-negative bacilli especially for neonates in the NICU. Therefore, this study was conducted with the aim to assess the prevalence of extended-spectrum β-lactamase and carbapenemase producing gram-negative bacilli, associated factors and antimicrobial resistance patterns among neonates in intensive care units.
Materials and Methods
Study Design, Period and Setting
The study was conducted in Arba Minch General Hospital (Arba Minch, Ethiopia) from February to June 2021. The hospital admits an average of 50 neonates per month and 600 neonates per year in their neonatal intensive care unit (NICU). The NICU has 32 beds in two partitioned rooms and has 24 staff. The inclusion criteria was all hospitalized neonates in the NICU for >48 hours during the study period. An exclusion criterion was neonates’ who carried ESβL-PGNB or CP-PGNB at admission.
Data Collection and Laboratory Processing
A pretested well designed questionnaire was used to collect risk factors data.
Isolation and Identification of Bacterial Isolates
Two rectal swabs were collected from each patient, one at admission within 48 hours and the second sample was collected at discharge from NICU. The swab was collected by using a sterile cotton swab by trained nurses. The collected swab specimens were placed in Cary-Blair transport media and packed in a cold box to prevent contamination and immediately transported to the Microbiology and Parasitology Laboratory of the College of Medicine and Health Sciences, Arba Minch University.
Rectal swabs were inoculated into MacConkey agar and incubated overnight at 37 °C. Biochemical tests like the indole test, methyl red/Voges Proskauer test, oxidase test, triple sugar iron, motility, citrate utilization, urease, gas production and hydrogen sulfide production were done for species identifications.12
Screening of ESβL and Carbapenemase Producing Gram-Negative Bacilli
ESβL-producing gram-negative bacilli were first screened for ESβL production by indicator cephalosporins (cefotaxime (30µg) and ceftazidine (30µg)). Isolates having a zone of inhibition ≤22 mm for ceftazidine and ≤27 mm for cefotaxime were considered a potential ESβL producer. Entrapenem non-susceptibility was used as the indicator of carbapenemase production. Tests resistant to one or more agents of third generation cephalosporin usually are indicators of Carbapenemase production.13
Double-Disk Synergy Test (DDST)
Disks containing cephalosporins (cefotaxime and ceftazidine) were applied to plates next to a disk with clavulanic acid (amoxicillin-clavulanic acid). A positive result was indicated when the inhibition zones around any of the cephalosporin disks were enhanced or there was a “keyhole” in the direction of the disk containing clavulanic acid. The distance between the disks, 20 mm from center-to-center, is optimal for cephalosporin 30 μg disks. However, the distance between the disks will be reduced (15 mm) or expanded (30 mm) for strains with very high or low levels of resistance, respectively.13
Modified Carbapenem Inactivation Method (mCIM)
One milliliter loop-full of Enterobacteriaceae or 10 mL loop-full of P. aeruginosa from blood agar plates were emulsified in 2 mL trypticase soy broth (TSB). Meropenem disc was immersed in the suspension and incubated for a minimum of 4 hours at 35 °C. A 0.5 McFarland suspension of E. coli ATCC 25922 was prepared in saline using the direct colony suspension method. A Mueller Hinton agar (MHA) plate was inoculated with E. coli ATCC 25922 using the routine disk diffusion procedure. The meropenem disk was removed from the TSB and placed on an MHA plate previously inoculated with the E. coli ATCC 25922 indicator strains. Plates were incubated at 35 °C in ambient air for 18–24 hours. An inhibition zone diameter of 6–15 mm or colonies within a 16–18 mm zone was considered a positive result and a zone of inhibition ≥19 mm was considered negative result.13
Antibiotic Susceptibility Testing
Antibiotic susceptibility patterns of bacterial isolates were done by the Kirby Bauer disk diffusion method using the following antibiotics: ampicillin (10 µg), amoxicillin/clavulanic acid (20/10 µg), cefuroxime (30 µg), ceftazidine (30 µg), ceftriaxone (30 µg), cefotaxime (30 µg), meropenem (10 µg), amikacin (30 µg), gentamicin (10 µg), nalidixic acid (30 µg), ciprofloxacin (5 µg), cotrimoxazole (25 µg), chloramphenicol (30 µg) and tetracycline (30 µg). The diameter of the zone of inhibition around the disc was measured in millimeters using a ruler or caliper. The results were recorded as resistant, intermediate and sensitive based on CLSI 2019.13
Data Quality Assurance
A pre-test was done on 5% of the sample size before actual work. Reference strains E. coli ATCC 25922 and K. pneumoniae ATCC 700603 were used as controls. Standard Operating Procedures (SOP) were prepared and implemented strictly. All culture media were prepared following the manufacturer’s instruction and sterility of the culture media was tested by incubating 5% of the batch at 35–37 °C overnight for evaluation of possible contamination.
Statistical Analysis
Data was analyzed using IBM Statistical Package for Social Sciences (SPSS) software Version 21.0. Binary logistic regression analysis was used to determine the association between carriage rate and associated factors of ESβLs and carbapenemase producing gram-negative bacilli. All variables with P-value < 0.25 in the bivariate analysis was included in the final model/multivariate analysis. A P-value ≤ 0.05 was considered statistically significant.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from the Institutional Review Board of Arba Minch University, College of Medicine and Health Sciences. The parental consent was obtained from family/guardians of the neonates.
The purpose of the study was clearly described to the parent/guardian of study participants including the benefits and risk. Parents of the neonates were clearly informed that participation was voluntarily; and if they were not willing their baby to be involved in the study, they could withdraw at any time without any reason. Any information concerning the patients was kept confidential and the specimens collected from the patients were analyzed for the intended purpose only. For each confirmed case the responsible pediatrician of the patient was informed for appropriate interventions.
This study was conducted in accordance with the Declaration of Helsinki.
Results
Socio-Demographic Characteristics of Study Participants
A total of 212 neonates participated in this study with a 98.6% response rate. The majority of the study participants, 129 (60.8%), were male. The mean gestational age of the neonates was 36.9 weeks with SD of ±2.8 weeks and their mean birth weight was 2715 g with SD of ±858.6 g. The mean age of the neonate at admission was 3.7 days with SD of ±5.3 days. The majority of the neonates were admitted to the NICU at an age less than two days, 152 (71.7%). More than half of them were born in hospital by normal vaginal delivery (Table 1).
Table 1.
Socio-Demographic Characteristic of Study Participant in the NICU
| Variables | Category | ESβL-PGNB Carrier | Total N = 212 (%) | |
|---|---|---|---|---|
| -ve N (%) | +ve N (%) | |||
| Sex | Male | 87(67.4) | 42(32.6) | 129(60.8) |
| Female | 53(63.9) | 30(36.1) | 83(39.2) | |
| Gestational age group (weeks) | <32 | 6(35.3) | 11(64.7) | 17(8.0) |
| 32–36 | 28 (58.3) | 20(41.7) | 48(22.6) | |
| >36 | 106(72.1) | 41(27.9) | 147(69.3) | |
| Birth weight (g) | <2500 | 47(56) | 37(44) | 84(39.6) |
| >2500 | 93(72.7) | 35(27.3) | 128(60.4) | |
| Age at admission (days) | 0–2 | 98(64.5) | 54(35.5) | 152(71.7) |
| 3–28 | 42(69.1) | 18(30.5) | 60(28.3) | |
| Birth place | Hospital | 99(63.5) | 57(36.5) | 156(73.6) |
| Health center | 41(73.2) | 15(26.8) | 56(26.4) | |
| Type of delivery | NVD | 101(64.3) | 56(35.7) | 157(74.0) |
| CSD | 39(70.9) | 16(29.1) | 55(26) | |
| Types of feeding at admission | Breast feeding | 72(70.6) | 30(29.4) | 102(48.1) |
| Formula milk | 25(74.1) | 10(28.6) | 35(16.5) | |
| No feeding | 43(57.3) | 32(42.7) | 75(35.4) | |
Abbreviations: NVD, normal vaginal delivery; CSD, cesarean section delivery; -ve, negative; +ve, positive; ESβL-PGNB, extended spectrum β-lactamase producing gram negative bacilli; NICU, neonatal intensive care unit.
Clinical Characteristics of Hospitalized Neonates
Neonates were admitted and hospitalized to the NICU due to different reasons. The most common reason was neonatal infections 196 (92.5%). Hospitalized neonates were exposed to different invasive medical devices as a lifesaving procedure. The majority 204 (96.2%) were exposed to venous catheter. Two hundred and six (97.2%) neonates were treated with antibiotics during their NICU stay and the most commonly, 131 (61.8%), used antibiotic was an ampicillin and gentamycin combination therapy as empirical treatment for suspected neonatal sepsis. The mean duration of antibiotic use was 9.4 days with SD of ±4.8 days and the mean length of hospitalization in the NICU was 12 days with SD of ±5.7 days. About half of them were hospitalized for 1–10 days (Table 2).
Table 2.
Clinical Characteristics of Neonates Hospitalized in the NICU
| Variables and Category | ESβL-PGNB Carrier | Total N = 212(%) | |||
|---|---|---|---|---|---|
| -ve: N (%) | +ve: N (%) | ||||
| Reason for hospitalization | Prematurity | Yes | 30(48.4) | 32(51.6) | 62(29.2) |
| No | 110(73.3 | 40(26.7) | 150(70.8) | ||
| Respiratory distress | Ye | 65(62.5) | 39(37.5) | 104(49.1) | |
| No | 75(69.4) | 33(30.6) | 108(50.9) | ||
| Neonatal infections | Ye | 130(66.3) | 66(33.7) | 196(92.5) | |
| No | 10(62.5) | 6(37.5) | 16(7.5) | ||
| Surgical pathology | Ye | 4(44.4) | 5(55.6) | 9(4.2) | |
| No | 136(67.0) | 67(33.0) | 203(95.8) | ||
| Birth defect | Yes | 7(50) | 7(50) | 14(6.6) | |
| No | 133(67.2) | 65(32.8) | 198(93.4) | ||
| Hypothermia | Yes | 26(49.1) | 27(50.9) | 53(25) | |
| No | 114(71.7) | 45(28.3) | 159(75) | ||
| Exposure to invasive device | Parenteral nutrition | Yes | 63(54.8) | 52(44.3) | 115(54.2) |
| No | 77(79.4) | 20(20.6) | 97(45.8) | ||
| Venous catheter | Yes | 135(66.2) | 69(33.8) | 204(96.2) | |
| No | 5(62.5) | 3(37.5) | 8(3.8) | ||
| CPAP | Yes | 79(62.7) | 47(37.3) | 126(59.4) | |
| No | 61(70.9) | 25(29.1) | 86(40.6) | ||
| Endotracheal intubation | Yes | 27(42.9) | 36(57.1 | 63(32) | |
| No | 113(75.8) | 36(24.5) | 149(70.3) | ||
| Antibiotic treatment status after admitted | Treated with antibiotics | Yes | 136(66.0) | 70(34.0) | 206(97.2) |
| No | 4(66.7) | 2(33.3) | 6(2.8) | ||
| Ampicillin +Gentamycin | Yes | 74(56.5) | 57(43.5) | 131(61.8) | |
| No | 66(81.5) | 15(18.5) | 81(38.2) | ||
| 3rd-G Cephalosporin + Gentamycin | Yes | 16(66.7) | 8(33.3) | 24(11.3) | |
| No | 124 (66) | 64(34) | 188(88.7) | ||
| Length of NICU stay and duration of antibiotic use | Duration of antibiotic use (days) during hospitalization | 0 | 4(66.7) | 2(33.3) | 6(2.8) |
| 1–5 | 29(76.3) | 9(23.7) | 38(18) | ||
| 6–10 | 70(71.4) | 28(28.6) | 98(46.2) | ||
| >10 | 37(52.9) | 33(47.1) | 70(33) | ||
| Length of stay in NICU | 1–10 | 85(77.3) | 25(22.7) | 110(51.9) | |
| 11–20 | 48(56.5) | 37(43.5) | 85(40.1) | ||
| >20 | 7(41.2) | 10(58.8) | 17(8) | ||
Abbreviations: CPAP, continuous positive airway pressure; CSD, cesarean section delivery; -ve, negative; +ve, positive; ESβL-PGNB, extended spectrum β-lactamase producing gram negative bacilli; NICU, neonatal intensive care unit.
Prevalence of ESβL and Carbapenemase Producing Gram-Negative Bacilli
The overall prevalence of ESβL and carbapenemase producing gram-negative bacilli among hospitalized neonates was 72/212 (34%) and 5/212 (2.4%), respectively. No neonate was found to have both ESβL- and carbapenemase-producer organisms at the same time.
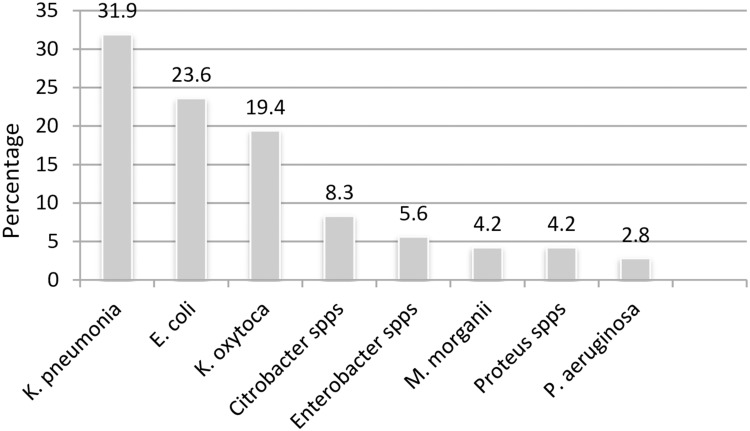
Seventy-two (35.1%) bacterial isolates were phenotypically confirmed as ESβL producers. The predominant ESβL producing organism was K. pneumonia, 23 (31.9%), followed by E. coli, 17 (23.6%), and K. oxytoca, 14 (19.4%) (Figure 1).
Figure 1.
Frequency of ESβL-producing gram negative bacilli (K. pneumonia is the leading ESβL producing gram-negative bacteria).
Five (5) bacterial isolates were phenotypically confirmed as carbapenemase producers including 3 K. pneumonia and 2 K. oxytoca.
A total of 240 gram-negative bacilli were isolated from 212 hospitalized neonates. The predominant organism was Klebsiella pneumonia, 74 (30.8%), followed by Escherichia coli, 66 (27.5%), and Klebsiella oxytoca, 46 (19.2%) (Table 3). Mono-bacteria were isolated from 184 (76.7%) neonates and double bacteria were isolated from 28 neonates 28 (23.3%).
Table 3.
Types of Gram-Negative Bacilli Isolated from Hospitalized Neonates
| S. No | Types of Isolated Bacteria | Frequency | Percentage |
|---|---|---|---|
| 1 | Klebsiella pneumonia | 74 | 30.8 |
| 2 | Escherichia coli | 66 | 27.5 |
| 3 | Klebsiella oxytoca | 46 | 19.2 |
| 4 | Morganella morganii | 15 | 6.3 |
| 5 | Pseudomonas aeruginosa | 6 | 2.5 |
| 6 | Citrobacter species | 17 | 7.1 |
| 7 | Enterobacter species | 10 | 4.1 |
| 8 | Proteus species | 6 | 2.5 |
| Total | 240 | 100 |
Factors Associated with Carriage of ESβL-Producing Gram-Negative Bacilli
Bivariate analysis showed that the following variables were associated with a carriage rate of ESβL-producing gram-negative bacilli during hospitalization in the NICU: gestational age (weeks) less than 32 (p = 0.004; OR = 4.7; 95% CI = (1.6–13.7)), Birth weight <2500 g (p = 0.003; OR = 2.1; 95% CI = (1.2–3.7)), parenteral nutrition (p = 0.000; OR = 3.2; 9% CI = (1.7–5.9)), hypothermia (p = 0.003; OR = 2.6; 95% CI = (1.4–5.0)), endotracheal intubation (p = 0.000; OR = 4.2; 95% CI = (2.2–7.8)). However in multivariate analysis only three variables were independently associated with rectal carriage rate by ESβL-producing gram-negative bacilli: endotracheal intubation (p = 0.001; AOR = 4.2; 96% CI = (1.8–9.5)), treatment with ampicillin+gentamicin (p = 0.004; AOR = 3.3; 95% CI = (1.5–7.6)) and staying in the NICU between (11–20) days (p = 0.042; AOR = 2; 95% CI = (1.0–4.5)) (Table 4).
Table 4.
Prevalence and Association of ESβL-Producing Gram-Negative Bacilli Carriage Rate with Demographic and Clinical Factors Among Hospitalized Neonates
| Variables and Category | ESβL + N(%) | Bivariate | Multivariate | ||
|---|---|---|---|---|---|
| OR (95%, CI) | P-value | AOR (95%, CI) | P-value | ||
| Gestational age (weeks) | |||||
| <32 | 11(64.7) | 4.7(1.6–13.7) | 0.004 | 0.8(0.9–6.5) | 0.805 |
| 32–36 | 20(41.7) | 1.8(0.938–3.6) | 0.076 | 0.6(0.9–4.2) | 0.621 |
| >36 | 41(27.9) | 1 | 1 | ||
| Birth weight (g) | |||||
| <2500 | 37(44) | 2.1(1.2–3.7) | 0.013 | 1.8(0.66.0) | 0.308 |
| >2500 | 35(27.3) | 1 | 1 | ||
| Birth place | |||||
| Hospital | 57(36.5) | 1.6(0.8–3.1) | 0.188 | 0.8(0.35–1.9) | 0.686 |
| Health center | 15(26.8) | 1 | 1 | ||
| Types of feeding | |||||
| Breast feeding | 30(29.4) | 0.6(0.3–1.0) | 0.069 | 2(0.7–5.6) | 0.173 |
| Formula milk | 10(28.6) | 0.5(0.23–1.3) | 0.159 | 1.7(0.5–5.9) | 0.399 |
| No feeding | 32(42.7) | 1 | 1 | ||
| Prematurity | |||||
| Yes | 32(51.6) | 2.9(1.6–5.4) | 0.000 | 1(0.2–5.9) | 0.933 |
| No | 40(26.7) | 1 | 1 | ||
| Surgical intervention | |||||
| Yes | 5(55.6) | 2.5(0.7–9.7) | 0.175 | 4(0.7–33.2) | 0.101 |
| No | 67(33.0) | 1 | 1 | ||
| Birth defect | |||||
| Yes | 7(50) | 2(0.7–6.0) | 0.197 | 0.8(0.2–4.6) | 0.887 |
| No | 65(32.8) | 1 | 1 | ||
| Hypothermia | |||||
| Yes | 27(50.9) | 2.6(1.4–5.0) | 0.003 | 1.5(0.6–3.7) | 0.293 |
| No | 45(28.3) | 1 | 1 | ||
| Parenteral nutrition | |||||
| Yes | 52(44.3) | 3.2(1.7–5.9) | 0.000 | 2(0.8–5.5) | 0.138 |
| No | 20(20.6) | 1 | 1 | ||
| CPAP | |||||
| Yes | 47(37.3) | 1.5(0.8–2.6) | 0.215 | 0.6(0.3–1.4) | 0.283 |
| No | 25(29.1) | 1 | 1 | ||
| Endotracheal intubation | |||||
| Yes | 36(57.1 | 4.2(2.2–7.8) | 0.000 | 4.2(1.8–9.5) | 0.001 |
| No | 36(24.5) | 1 | |||
| Treated with AMP+GENT | |||||
| Yes | 57(43.5) | 3.4(1.6–6.5) | 0.000 | 3.3(1.5–7.6) | 0.004 |
| No | 15(18.5) | 1 | |||
| Length of stay in NICU | |||||
| 1–10 | 25(22.7) | 1 | 1 | ||
| 11–20 | 37(43.5) | 2.6(1.4–4.8) | 0.002 | 2(1.0–4.5) | 0.042 |
| >20 | 10(58.8) | 4.9(1.7–14.0) | 0. 004 | 1.9(0.5–6.8) | 0.350 |
Abbreviations: CPAP, continuous positive airway pressure; AMP, ampicillin; GENT, gentamicin; OR, odds ratio; AOR, adjusted odds ratio; NICU, neonatal intensive care unit.
Antimicrobial Susceptibility Patterns of ESβL and Carbapenemase Producing Gram-Negative Bacilli
ESβL-producing gram-negative bacilli isolate showed high resistance against ampicillin 72 (100%) and augmentin 69 (95.8%), but less resistance against amikacin 5 (6.9%). Klebsiella pneumonia isolates showed higher resistance to ampicillin 23 (100%), gentamycin 23 (100%), and augmentin 23 (100%). Escherichia coli isolates showed higher resistance against ampicillin 17 (100%) and augmentin 16 (94%), but less resistance against amikacin 2 (11.8%), nalidixic acid 2 (11.8%) and meropenem 2 (11.8%) (Table 5). On the other hand the carbapenemase positive isolates showed resistance against all tested antibiotics except amikacin.
Table 5.
Antimicrobial Resistance Patterns of ESβL-Producing Gram-Negative Bacilli Among Hospitalized Neonates
| Bacteria | No. (%) of Resistant Isolates Respective to Each Antibiotics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMC | AUG | GEN | AMK | TE | NAL | CIP | SXT | CRN | MER | |
| K. pneumoniae (n=23) | 23(100) | 23(100) | 23(100) | 0 (0) | 20(87) | 6(26.1) | 15(65.2) | 19(82.6) | 14(60.6) | 2(8.7%) |
| E. coli (n=17) | 17(100) | 16(94) | 11(64.7) | 2(11.8) | 11(64.7) | 2(11.8) | 7(41.2) | 7(41.2) | 8(47.1) | 2(11.8) |
| K. oxytoca (n=14) | 14(100) | 12(85.7) | 13(93) | 0 (0) | 13(93) | 3(21.4) | 8(57.1) | 8(57.1) | 10(71.4) | 2(14.3) |
| Enterobacter spps (n=6) | 6(100) | 4(100) | 4(66.7) | 1(16.7) | 4(66.7) | 3(50) | 2(33.3) | 5(83.3) | 2(33.3) | 0(0) |
| Citrobacter spps (n=4) | 4(100) | 4(100) | 1(25) | 1(25) | 1(25) | 1(25) | 2 (50) | 1(25) | 2 (50) | 1(25) |
| M. morganii (n=3) | 3(100) | 3(100) | 2(66.7) | 0 (0) | 1(33.3) | 1(33.3) | 2(66.7) | 2(66.7) | 1(33.3) | 1(33.3) |
| Proteus spps (n=3) | 3(100) | 3(100) | 1(33.3) | 0 (0) | 2(66.7) | 0 (0) | 0 (0) | 1(33.3) | 2(66.7) | 0(0) |
| P. aeruginosa (n=2) | 2(100) | 2(100) | 2(100) | 1(50) | 1(50) | 0 (0) | 1(50) | 2(100) | 2(100) | 0(0) |
| Total (n=72) | 72(100) | 69(95.8) | 57(79.2) | 5(6.9) | 53(73.6) | 16(22) | 37(51.4) | 45(62.5) | 41(56.9) | 8(11) |
Abbreviations: AMC, ampicillin; AUG, augmentin; GEN, gentamycin; AMK, amikacin; TE, tetracycline; NAL, nalidixic acid; CIP, ciprofloxacin; SXT, trimethoprim- sulfamethoxazole; CR, chloramphenicol; MER, meropenem.
In this study, the overall prevalence of MDR extended-spectrum β-lactamase producing gram-negative bacilli was 63/72 (87.5%). K. pneumonia, 22/72 (30.6%), and E. coli, 16/72 (22.2%), were the commonest MDR bacterial isolates. Most, 66.7% (48/72), MDR ESβL producing gram-negative bacilli showed resistance to more than five classes of antibiotics (Table 6).
Table 6.
Multidrug Resistance Patterns of ESβL Producing Gram-Negative Bacilli Among Hospitalized Neonates
| Bacteria | Multiple Antibiotics Resistance | Total | |||||
|---|---|---|---|---|---|---|---|
| R0 | R1 | R2 | R3 | R4 | R5 and Above | ||
| K. pneumonia | 0 | 0 | 1 | 2 | 1 | 19 | 23 |
| E. coli | 0 | 1 | 0 | 3 | 3 | 10 | 17 |
| K. oxytoca | 0 | 1 | 2 | 1 | 1 | 9 | 14 |
| M. morganii | 0 | 0 | 0 | 1 | 0 | 2 | 3 |
| P. aeruginosa | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Citrobacter spp | 0 | 2 | 2 | 0 | 0 | 3 | 6 |
| Enterobacter spp | 0 | 0 | 1 | 0 | 2 | 1 | 4 |
| Proteus spps | 0 | 0 | 0 | 1 | 0 | 2 | 3 |
| Total | 0 | 3 | 6 | 8 | 7 | 48 | 72 |
Notes: R0, bacterial isolates sensitive to all antibiotics; R1, resistant against one class of antibiotics; R2, resistant against two classes; R3, resistant against three classes; R4, resistant against four classes and R5; resistant against five classes and above of antibiotics.
Discussion
The incidence of hospital-acquired gram-negative bacilli that produce ESβLs had increased worldwide. The widespread presence of ESβL-producing bacteria not only affects the choice of antibiotics but also causes excessive morbidity, mortality and economic crisis on a global scale.14
In our study the overall prevalence of ESβL-producing gram-negative bacilli was high among hospitalized neonates (34%). This finding is comparable with studies done in Brazil (27%15 and 30.5%16). Our result showed a higher prevalence rate compared to studies conducted in Germany 5.7%,17 Italy 6.6–20.1%,18 Israel 13.6%,8 India 5.3%,19 Turkey 18.6%,20 Saudi Arabia 6.8%21 and Tanzania 25.4%.22 The reasons for a lower prevalence rate in those countries may be attributed to the quality of health care, high level of socioeconomic standard, good healthcare-associated infection prevention strategies, aseptic technique practice and standard diagnostic setup. However our finding was lower than studies conducted in Ethiopia 74%,23 Morocco 77%,24 Ghana 46.1%,25 Kenya 55%,26 Mexico 67.2%,28 USA 87.7%,27 Ecuador 56%29 and Austria 51%.30 The possible reasons for the variability could be attributed to sample size, methodology and antibiotic use policy variations.
In the present study the predominant ESβL-producing gram-negative bacilli was K. pneumonia (31.9%) followed by E. coli (23.6%). This is in line with previous studies conducted in Tanzania,22 Ghana,25 Morocco,24 India,19 Saudi Arabia,21 Turkey,20 Israel,8 Italy18 and Ethiopia.23 However in studies conducted in German17 and Ecuador29 E. coli isolates were found to be the predominant ESβL producer followed by K. pneumoniae among hospitalized neonates; whereas in a study conducted in Austria30 K. oxytoca was the predominant ESβL-producing isolate followed by K. pneumoniae. These variations may be due to the difference in geographical area, hospital setup, sample size and methodological variations.
The prevalence rate of carbapenemase-producing gram-negative bacilli among hospitalized neonates was 2.4%. This result is comparable with studies conducted in Ethiopia 2%,23 Morocco 1.8%24 and Algeria 1.6%.31 However, a study conducted in Ghana reported higher rates (7.9%) of carbapenemase producing gram-negative bacilli. These variations may be due to the low utilization of carbapenems in our study area and this antibiotic is not available over the counter.
In our study ESβL producing gram-negative bacilli showed a higher resistance against ampicillin (100%), gentamycin (79.2%), tetracycline (73.6%), cotrimoxazole (62.5%), chloramphenicol (57%). These findings are in line with a study done in Kenya that ESβL isolates from hospitalized neonates showed higher resistance to chloramphenicol, trimethoprim-sulfamethoxazole, quinolones, and gentamicin.26 Another study conducted in Morocco reported similar results with our study that ESβL producers isolated from hospitalized neonates were 99% resistant to gentamicin, 60.2% to trimethoprim-sulfamethoxazole and 58.2% to ciprofloxacin.24 This indicates the increasing rate of ESβL producing gram-negative bacilli which needs great attention towards prevention and control of transmission of such organisms especially among hospitalized neonates.
ESβL-producing K. pneumoniae and E. coli showed a higher level of resistance against ampicillin, gentamycin, tetracycline, cotrimoxazole, chloramphenicol and ciprofloxacin. These findings are comparable with studies conducted in Ethiopia,23 Kenya,26 Morocco,24 Mexico,28 Ecuador29 and Egypt.32 The higher level of resistance shown by these bacterial isolates may be due to over-the-counter availability, use of antibiotics without prescription, misuse of antibiotics or due to the isolated organisms being hospital acquired and may be attributed to prolonged use of antibiotics in the NICU. This makes the treatment of infection caused by ESβL-producing bacteria a very challenging task.
In our study the overall prevalence of MDR extended-spectrum β-lactamase producing gram-negative bacilli was 87.5%. This finding is comparable to studies conducted in Ethiopia 71%33 and Morocco 91%.24 However it is higher than a study conducted in Ghana 49.6%.25 The reason for the increment of drug resistance in our study may be due to frequent use of broad spectrum antibiotics, over-the-counter availability of antibiotics and the inappropriate prescription of antibiotics.
This study indicated that the rectal carriage rate of hospitalized neonates with ESβL producing gram-negative bacilli in NICU was increased by four times in neonates exposed to endotracheal intubation (p = 0.001; AOR = 4.2; 96% CI = (1.8–9.5)). However a study conducted in Turkey showed that urinary catheterization and surgical procedures were associated with the colonization of ESβL in hospitalized neonates.20 These variations may be due to the small surgical case in our study and no catheterization procedure was done.
Our study indicated that staying in the NICU for about two to three weeks (11–20 days) was strongly associated with the carriage rate of ESβL producing gram-negative bacilli in the NICU, (p = 0.042; AOR = 2; 95% CI = (1.0–4.5)). Similar studies done in Brazil,26 Turkey,2 India19 and Ecuador29 reported that staying in the NICU for a long time was independently associated with ESβL colonization in the NICU. A prolonged stay in the NICU could contribute to the transmission and/or dissemination of resistant bacteria among neonates in the NICU.
ESβL producing gram-negative bacilli carriage was significantly associated with being treated with ampicillin+gentamycin (p = 0.004; AOR = 3.3; 95% CI = (1.5–7.6)). A study conducted in Italy indicated that total length of hospital stay and duration of combined ampicillin/gentamicin therapy was independently associated with acquisition and colonization of ESβL producing gram-negative bacilli among hospitalized neonates in the NICU.18
Since safe and effective therapeutic options in carbapenem-resistant Gram-negative infections are severely limited, the characterization of these isolates by phenotypic and molecular methods is important to provide information on the epidemiological characteristics of these pathogens.34–39
Limitations of the Study
Molecular characterization and minimum inhibitory concentration were not done due to resource limitation and budget constraints.
Conclusions
The overall prevalence rate of ESβL producing gram-negative bacilli among hospitalized neonates was high (34%). The predominant isolates of ESβL-producing gram-negative bacteria were K. pneumoniae and E. coli. ESβL-producing gram-negative bacteria isolates showed a higher degree of resistance against the commonly used antibiotics such as ampicillin, gentamycin, tetracycline, cotrimoxazole, ciprofloxacin and chloramphenicol. On the other hand the prevalence of carbapenemase producing bacteria (2.4%) is an alarming situation, because the bacteria developed the resistant gene to carbapenem prior to use in our setup, which needs critical attention from all responsible bodies.
Therefore continuous and regular follow-ups of drug resistance patterns is important for proper treatment and management of extended-spectrum β-lactamase and carbapenemase producing gram-negative bacilli. Implementing infection prevention protocols may reduce colonization by extended-spectrum β-lactamase and carbapenemase producing gram-negative bacilli.
Acknowledgments
The authors would like to thank those who were involved in this research.
Abbreviations
AOR, adjusted odd ratio; AST, antimicrobial susceptibility test; CI, confidence interval; CLSI, Clinical Laboratory Standards Institute guidelines; CP-PGNB, carbapenemase-producing gram-negative bacilli; DDS, double-disk synergy test; ESβL, extended spectrum β-lactamase; ESBL-PGNB, extended spectrum β-lactamase producing gram-negative bacilli; GNB, gram-negative bacilli; mCIM, modified carbapenem inactivation method; MDR, multi drug resistant; MHA, Mueller Hinton agar; NICU, neonatal intensive care unit; SOP, standard operating procedures; TSB, trypticase soy broth; TSI, triple sugar iron; WHO, World Health Organization; Spp, species.
Data Sharing Statement
Data cannot be shared publicly because of ethical issues. However the data underlying the results presented in the study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2017. Stockholm: ECDC; 2017. Available from:https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017. Accessed January1, 2021. [Google Scholar]
- 2.WHO. Antimicrobial resistance global report on surveillance: 2014 summary. World Health Organization; 2014. Available from:https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf. Accessed January1, 2021. [Google Scholar]
- 3.WHO. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. World Health Organization; 2017. Available from:https://www.who.int/medicines/areas/rational_use/PPLreport_2017_09_19.pdf. Accessed January1, 2021. [Google Scholar]
- 4.Shrivastava SR, Shrivastava PS, Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J Med Soc. 2018;32(1):76–77. doi: 10.4103/jms.jms_25_17 [DOI] [Google Scholar]
- 5.Flores-Carrero A, Labrador I, Paniz-Mondolfi A, Peaper DR, Towle D, Araque M. Nosocomial outbreak of extended-spectrum β-lactamase-producing Enterobacter ludwigii co-harbouring CTX-M-8, SHV-12 and TEM-15 in a neonatal intensive care unit in Venezuela. J Glob Antimicrob Resist. 2016;7:114–118. PMID: 27750157. doi: 10.1016/j.jgar.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 6.Johnson J, Quach C. Outbreaks in the neonatal ICU: a review of the literature. Curr Opin Infect Dis. 2017;30(4):395–403. PMID: 28582313. doi: 10.1097/QCO.0000000000000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez-Villodres Á, Gil-Marqués ML, Álvarez-Marín R, et al. Extended-spectrum resistance to β-lactams/β-lactamase inhibitors (ESRI) evolved from low-level resistant Escherichia coli. J Antimicrob Chemother. 2020;75:77–85. PMID: 31613964. doi: 10.1093/jac/dkz393 [DOI] [PubMed] [Google Scholar]
- 8.Leikin-Zach V, Eilon Shany M, Tali Shafat M, Borer A, Melamed R. Neonatal risk factors for colonization with extended-spectrum beta-lactamase-producing bacteria in the neonatal intensive care unit. Isr Med Assoc J. 2018;20:286–290. PMID: 29761673. [PubMed] [Google Scholar]
- 9.Hooven TA, Polin RA. Healthcare-associated infections in the hospitalized neonate: a review. Early Hum Dev. 2014;90:S4–S6. PMID: 24709456. doi: 10.1016/S0378-3782(14)70002-7 [DOI] [PubMed] [Google Scholar]
- 10.Curtis C, Shetty N. Recent trends and prevention of infection in the neonatal intensive care unit. Curr Opin Infect Dis. 2008;21(4):350–356. PMID: 18594285. doi: 10.1097/QCO.0b013e3283013af4 [DOI] [PubMed] [Google Scholar]
- 11.Li X, Xu X, Yang X, et al. Risk factors for infection and/or colonisation with extended-spectrum β-lactamase-producing bacteria in the neonatal intensive care unit: a meta-analysis. Int J Antimicrob Agents. 2017;50(5):622–628. PMID: 28733213. doi: 10.1016/j.ijantimicag.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 12.Giuliano C, Patel CR, Kale-Pradhan PB. A guide to bacterial culture identification and results interpretation. P T. 2019;44:192–200. PMC6428495. [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. PA, USA: Clinical and Laboratory Standards Institute; 2019. [Google Scholar]
- 14.Villar HE, Baserni MN, Jugo J. Faecal carriage of ESBL-producing Enterobacteriaceae and carbapenems resistant gram negative bacilli in community settings. J Infect Dev Ctries. 2013;7:630–634. PMID: 23949299. doi: 10.3855/jidc.2900 [DOI] [PubMed] [Google Scholar]
- 15.Cassettari V, Da Silveira I, Dropa M, et al. Risk factors for colonisation of newborn infants during an outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae in an intermediate-risk neonatal unit. J Hosp Infect. 2009;71:340–347. PMID: 19147256. doi: 10.1016/j.jhin.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 16.Sakai AM, Iensue TN, Pereira KO, et al. Colonization by multidrug-resistant microorganisms of hospitalized newborns and their mothers in the neonatal unit context. J Infect Dev Ctries. 2020;14:765–771. PMID: 32794468. doi: 10.3855/jidc.12091 [DOI] [PubMed] [Google Scholar]
- 17.Denkel LA, Schwab F, Kola A, et al. The mother as most important risk factor for colonization of very low birth weight (VLBW) infants with extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E). J Antimicrob Chemother. 2014;69:2230–2237. PMID: 24729603. doi: 10.1093/jac/dku097 [DOI] [PubMed] [Google Scholar]
- 18.Giuffrè M, Geraci DM, Bonura C, et al. The increasing challenge of multidrug-resistant gram-negative bacilli: results of a 5-year active surveillance program in a neonatal intensive care unit. Medicine. 2016;95:e3016. PMID: 26962817. doi: 10.1097/MD.0000000000003016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayakanthi N, Bahl D, Kaur N, Maria A, Dubey NK. Frequency and characteristics of infections caused by extended-spectrum beta-lactamase-producing organisms in neonates: a prospective cohort study. Biomed Res Int. 2013;2013:756209. PMCID: PMC3794505. doi: 10.1155/2013/756209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiremitçi A, Dinleyici EÇ, Yargıç ZA, et al. Prevalence and risk factors of fecal carriage of Extended-Spectrum β-lactamase (ESBL)-producing Enterobacteriaceae in hospitalized and ambulatory children. J Pediatr Infect. 2011;5:54–58. doi: 10.5152/ced.2011.22 [DOI] [Google Scholar]
- 21.Somily AM, Alsubaie SS, BinSaeed AA, et al. Extended-spectrum β-lactamase-producing Klebsiella pneumoniae in the neonatal intensive care unit: does vancomycin play a role? Am J Infect Control. 2014;42:277–282. PMID: 24581016. doi: 10.1016/j.ajic.2013.09.028 [DOI] [PubMed] [Google Scholar]
- 22.Nelson E, Kayega J, Seni J, et al. Evaluation of existence and transmission of extended spectrum beta lactamase producing bacteria from post-delivery women to neonates at Bugando Medical Center, Mwanza-Tanzania. BMC Res Notes. 2014;7:279. PMID: 24886506. doi: 10.1186/1756-0500-7-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desta K, Woldeamanuel Y, Azazh A, et al. High gastrointestinal colonization rate with extended-spectrum β-lactamase-producing Enterobacteriaceae in hospitalized patients: emergence of carbapenemase-producing K. pneumoniae in Ethiopia. PLoS One. 2016;11(8):e0161685. PMID: 27574974. doi: 10.1371/journal.pone.0161685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arhoune B, Oumokhtar B, Hmami F, et al. Rectal carriage of extended-spectrum β-lactamase-and carbapenemase-producing Enterobacteriaceae among hospitalised neonates in a neonatal intensive care unit in Fez, Morocco. J Glob Antimicrob Resist. 2017;8:90–96. PMID: 28039104. doi: 10.1016/j.jgar.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 25.Labi AK, Bjerrum S, Enweronu-Laryea CC, et al. editors. High carriage rates of multidrug-resistant gram-negative bacteria in neonatal intensive care units from Ghana. Open Forum Infect Dis. 2020;7:ofaa109. PMID: 32373647. doi: 10.1093/ofid/ofaa109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagia N, Kosgei P, Ooko M, et al. Carriage and acquisition of extended-spectrum β-lactamase–producing Enterobacterales among neonates admitted to hospital in Kilifi, Kenya. CID. 2019;69:751–759. doi: 10.1093/cid/ciy976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song X, Soghier L, Floyd TT, Harris TR, Short BL, DeBiasi RL. Reassessing the need for active surveillance of extended-spectrum beta-lactamase–producing Enterobacteriaceae in the neonatal intensive care population. Infect Control Hosp Epidemiol. 2018;39:1436–1441. PMID: 30345942. doi: 10.1017/ice.2018.260 [DOI] [PubMed] [Google Scholar]
- 28.Huerta-García GC, Miranda-Novales G, Díaz-Ramos R, Vázquez-Rosales G, Solórzano-Santos F. Intestinal colonization by extended-spectrum beta-lactamase-producing Enterobacteriaceae in infants. Rev Invest Clin. 2015;67:313–317. PMID: 26696335. [PubMed] [Google Scholar]
- 29.Nordberg V, Peralta AQ, Galindo T, et al. High proportion of intestinal colonization with successful epidemic clones of ESBL-producing Enterobacteriaceae in a neonatal intensive care unit in Ecuador. PLoS One. 2013;8(10):e76597. PMID: 24146896. doi: 10.1371/journal.pone.0076597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberhart M, Grisold A, Lavorato M, Resch E, Trobisch A, Resch B. Extended-spectrum Beta-lactamase (ESBL) producing Enterobacterales in stool surveillance cultures of preterm infants are no risk factor for necrotizing enterocolitis: a retrospective case–control study over 12 years. Infection. 2020;48:853–860. PMID: 32462287. doi: 10.1007/s15010-020-01453-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mairi A, Touati A, Bessai SA, et al. Carbapenemase-producing Enterobacteriaceae among pregnant women and newborns in Algeria: prevalence, molecular characterization, maternal-neonatal transmission, and risk factors for carriage. Am J Infect Control. 2019;47:105–108. PMID: 30220617. doi: 10.1016/j.ajic.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Wahab F, Ghoneim M, Khashaba M, El-Gilany A-H, Abdel-Hady D. Nosocomial infection surveillance in an Egyptian neonatal intensive care unit. J Hosp Infect. 2013;83:196–199. PMID: 23374289. doi: 10.1016/j.jhin.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 33.Aklilu A, Manilal A, Ameya G, Woldemariam M, Siraj M. Gastrointestinal tract colonization rate of extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae and associated factors among hospitalized patients in Arba Minch general hospital, Arba Minch, Ethiopia. Infect Drug Resist. 2020;13:1517–1526. PMID: 32547121. doi: 10.2147/IDR.S239092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gajdács M, Kárpáti K, Stájer A, Zanetti S, Donadu MG. Insights on carbapenem-resistant Pseudomonas aeruginosa: phenotypic characterization of relevant isolates. Acta Biol Szeged. 2021;65:105–112. doi: 10.14232/abs.2021.1.105-112 [DOI] [Google Scholar]
- 35.Donadu MG, Zanetti S, Nagy AL, Barrak I, Gajdács M. Insights on carbapenem-resistant Acinetobacter baumannii: phenotypic characterization of relevant isolates. Acta Biol Szeged. 2021;65:85–92. doi: 10.14232/abs.2021.1.85-92 [DOI] [Google Scholar]
- 36.Montazeri EA, Khosravi AD, Saki M, Sirous M, Keikhaei B, Seyed-Mohammadi S. Prevalence of extended-spectrum betalactamase-producing Enterobacteriaceae causing bloodstream infections in cancer patients from southwest of Iran. Infect Drug Resist. 2020;13:1319. doi: 10.2147/IDR.S254357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheikh AF, Bandbal MM, Saki M. Emergence of multidrug-resistant Shigella species harboring extended-spectrum betalactamase genes in pediatric patients with diarrhea from Southwest of Iran. Mol Biol Rep. 2020;47(9):7097–7106. doi: 10.1007/s11033-020-05776-x [DOI] [PubMed] [Google Scholar]
- 38.Mansour Amin MS, Javaherizadeh H, Motamedifar M, et al. Antibiotic resistance pattern and molecular characterization of extended-spectrum β-lactamase producing enteroaggregative Escherichia coli isolates in children from southwest Iran. Infect Drug Resist. 2018;11:1097. doi: 10.2147/IDR.S167271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yazdansetad S, Alkhudhairy MK, Najafpour R, et al. Preliminary survey of extended-spectrum β-lactamases (ESBLs) in nosocomial uropathogen Klebsiella pneumoniae in northcentral Iran. Heliyon. 2019;5(9):e02349. doi: 10.1016/j.heliyon.2019.e02349 [DOI] [PMC free article] [PubMed] [Google Scholar]