Abstract
Brain-derived neurotrophic factor (BDNF) belongs to the “neurotrophin” family of growth factors, and it has recently been associated to cardiovascular disease (CVD). We anticipated that BDNF Val66Met polymorphisms may alter CVD risk markers such as serum lipid profile differences, and interaction with total antioxidant capacity of diet (DTAC) could alter these clinical parameters. This cross-sectional study consisted of 667 diabetic patients (39.7% male and 60.3% female). DTAC was calculated by international databases. Biochemical markers including total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), superoxide dismutase (SOD), C-reactive protein (CRP), total antioxidant capacity (TAC), pentraxin-3 (PTX3), isoprostaneF2α (PGF2α). interleukin 18 (IL18), leptin and ghrelin were measured by standard protocol. Atherogenic indices (AIP, AC, CR-I, CR-II) were calculated. Genotyping of the BDNF Val66Met polymorphisms was conducted by the real-time PCR–RFLP method. The gene-diet interactions were evaluated using a generalized linear mode (GLMs). Carriers of the Val/Met genotype who were in the higher median intake of FRAP had lower HDL (P:0.04) and higher TG (P:0.005), AIP (P:0.02) and AC (P:0.02) index compared to Val/Val genotypes with lower median intake. Moreover, diabetic patients with Val/Met genotype who consumed higher ORAC intake had increased odds for anthropometric indices (BMI (P:0.01) and WC (P:0.03)), lipid profiles (TG) (P:0.01), and atherogenic index (AIP) (P:0.02), also decreased odds for HDL (P:0.03) concentration compared to reference group whit lower ORAC intake. Individuals with Val/Met genotype who consumed higher TRAP intake had increased odds for WC (P:0.04), TC (P:0.001), TG (P < 0.001), AIP (P < 0.001) and AC (P < 0.001). Finally, Val/Met patients with a higher median intake of TEAC had higher TG (P:0.02), AIP (P:0.009) and AC (P:0.03) compared to the reference group whit lower TEAC intake. Our study showed that Val/Met genotype had also the highest lipid profile and atherogenic indices even in the highest adherence to DTAC. While it seems that the presence of the Val/Val wild-type and BDNF Met/Met homozygotes in diabetic patients with a high DTAC is a protective factor.
Subject terms: Genetic interaction, Medical genetics, Cardiovascular diseases, Nutrition disorders, Genetics, Molecular medicine
Introduction
High lipid serum concentrations are involved in the development of CVD and metabolic disorders. As a result, lipid level detection and treatment are critical in preventing CVD and its implications in diabetes patients1,2. Significant evidence suggests that high TC and TG levels, as well as an inverse association with HDL-C, are directly or indirectly connected to the risk of CVD3,4. The TC/HDL ratio is a more specific and sensitive indicator of cardiovascular risk than TC, with a value more than 5.5 indicating significant atherogenic risk5. The atherogenic index of plasma, in addition to dyslipidemia, is one of the most powerful markers in predicting the risk of CVD. The atherogenic index of plasma (AIP) is a new index6–8, that has been used to quantify blood lipid levels and is often utilized as an optimum indication of dyslipidemia and related problems such as CVD9–11.
Environmental factors, such as dietary consumption, genetic variations, and their interactions, have an impact on blood lipid levels12–15. Dietary changes could impact the oxidation profile of patients with various clinical diseases including diabetes, dyslipidemia, and CVDs, which are affected by foods and diets including polyphenols, flavonoids, PUFAs, and the Mediterranean diet16,17. The influence of antioxidants alone in decreasing oxidative stress has been identified; however, combining antioxidants is shown to have a synergistic activity; the perfect way to demonstrate this is to estimate the cumulative function and complex effects of all antioxidants in body fluids; total antioxidant capacity (TAC) is characterized as the moles of a given free radical removed by a test solution16,18,19. According to studies, DTAC is inversely connected to cancer fatalities, cardiovascular disease deaths, and all other causes of death20. Given the impact of DTAC on the aforementioned parameters, it's not unreasonable to believe that DTAC is related to atherogenic and anthropometric indices.
Moreover, BDNF is a candidate gene among the genetic variables linked to serum lipid levels that have been reported. The human BDNF gene is found on chromosome 11, in the region p13–14, and is mainly expressed in brain tissue21,22. The valine (Val) to a methionine (Met) substitution at codon 66 of BDNF gene, which can disrupt construction of BDNF into secretory vesicles and lead to low secretion of BDNF23. Several studies have showed the possible association between Met allele and insulin resistance and obesity in patients with schizophrenia24. In study of Leipzig Childhood cohort on German obese children showed that the Met allele associated with lower body mass index (BMI), postprandial glucose and HbA1c levels, however had not significant association with serum insulin and lipid profile25. The effects of BDNF single-nucleotide polymorphisms (SNPs) on the risk of cardiovascular disease, type 2 diabetes, and metabolic syndrome have been investigated extensively26–28. Gene variations have been found to interact with nutritional intakes, eating preferences, and body composition in several investigations29,30. In some studies, met-allele carriers were directly correlated to the high intake of dietary total calorie, carbohydrate, and protein.
In Puerto Rican men, the BDNF rs6265 GG genotype has been related to increased, but in women, it has been related to a lower BMI. However the reasons for the gender disparities are unknown, they appeared to be related to variations in PUFA intake, and there was a significant interplay between the BDNF alleles and u-3 and u-6 PUFA consumption30. As a result, the interactions between the rs6265 BDNF variation and ethnic and nutritional factors appear to be essential, but they are still unknown. Although there are some nutrigenetic studies related to DTAC, to the best of our knowledge, no studies have investigated the interactions of BDNF variants with dietary TAC on atherogenic indices changes. Hence, this study aimed to find out the possible relationship between this dietary index and atherogenic indices and compare it among BDNF Val/Met polymorphism groups.
Result
Study population characteristics
In the current study, 667 patients with T2DM were evaluated, sex distribution of the population was 39.7% and 60.3% male and female, respectively, 17.5% were smoker and 81.8% had a family history of diabetes. The means and standard deviation (SD) of age, BMI, and WC of individuals were (54.03 ± 6.51 years, 29.42 ± 4.64 kg/m2, and 92.67 ± 10.72 cm), respectively. According to our findings, genotype distribution of BDNF Val66Met (rs6265) in type 2 diabetes population was 54.9%, 35.2% and 9.9% for Val/Val, Val/Met and Met/Met. Genotype frequencies were in Hardy Weinberg equilibrium (P > 0.05). Also, the median intake of DTAC was (FRAP (15.83) TRAP (8.19) TEAC (7.46), and ORAC (27,372.14)).
Association between population characteristics, biochemical parameters between DTAC and BDNF Val66Met polymorphism
We found that there was no significant association between lipid profiles and atherogenic indices among BDNF Val66Met genotypes (p > 0.05) (Table 1).
Table 1.
The association between BDNF Val/Met polymorphism with lipid profiles and atherogenic indices in T2DM patients.
| BDNF Val/Met polymorphism | P-value | |||
|---|---|---|---|---|
| Val/Val | Val/Met | Met/Met | ||
| Age (year) | 54.20 ± 6.28 | 53.91 ± 6.97 | 53.56 ± 6.19 | 0.71 |
| Sex (male) N% | 155(58.5%) | 81(30.6%) | 29(10.9%) | 0.11 |
| Cigarette smoking (yes) N% | 76 (58.5%) | 37 (28.5%) | 17 (13.1%) | 0.12 |
| Alcohol consumption (no) N% | 355 (55%) | 229 (35.4%) | 62 (9.6%) | 0.34 |
| Familial history of diabetes (yes) N% | 296 (54.9%) | 186 (34.5%) | 57 (10.6%) | 0.42 |
| Glucose-lowering medication |
297 (53.9%) 69 (59.5%) |
197 (35.8%) 38 (32.8%) |
57 (10.3%) 9 (7.8%) |
0.48 |
| Metformin and glybenclamid N% | ||||
| Other medications N% | ||||
| Supplementation use (yes) | 364 (55%) | 232 (35%) | 66 (10%) | 0.45 |
| Total energy intake, kcal/day | 2606.74 ± 1030.58 | 2627.05 ± 918.05 | 2537.43 ± 865.26 | 0.80 |
| BMI (kg/m2) | 29.19 ± 4.61 | 29.78 ± 4.86 | 29.38 ± 3.86 | 0.30 |
| WC (cm) | 92.67 ± 10.90 | 92.70 ± 10.61 | 92.62 ± 10.21 | 0.99 |
| HDL (mg/dl) | 54.95 ± 13.40 | 54.66 ± 12.11 | 55.83 ± 11.32 | 0.80 |
| LDL (mg/dl) | 109.43 ± 34.33 | 112.48 ± 37.13 | 116.50 ± 38.50 | 0.27 |
| LDL/HDL | 3.03 ± 13.16 | 2.11 ± 0.67 | 2.12 ± 0.65 | 0.48 |
| TC (mg/dl) | 193.06 ± 65.66 | 199.80 ± 75.98 | 199.72 ± 66.06 | 0.46 |
| TG (mg/dl) | 183.77 ± 106.1 | 179.10 ± 103.24 | 192.46 ± 138.80 | 0.67 |
| Leptin (ng/ml) | 24.28 ± 13.55 | 27.67 ± 16.54 | 23.01 ± 14.18 | 0.16 |
| Ghrelin (ng/ml) | 2.15 ± 1.18 | 2.37 ± 1.30 | 2.33 ± 1.21 | 0.41 |
| AIP | 0.47 ± 0.23 | 0.46 ± 0.24 | 0.45 ± 0.27 | 0.82 |
| AC | 2.68 ± 1.51 | 2.81 ± 1.62 | 2.64 ± 1.17 | 0.54 |
| CRI.II | 2.06 ± 0.69 | 2.11 ± 0.67 | 2.12 ± 0.65 | 0.63 |
| CRI-I | 3.80 ± 1.67 | 3.83 ± 1.46 | 3.51 ± 1.05 | 0.32 |
| FRAP (mmol Fe2+/100 g) | 18.17 ± 13.41 | 17.35 ± 7.72 | 16.95 ± 6.62 | 0.56 |
| TRAP (mmol TE/kg) | 9.68 ± 5.56 | 9.75 ± 5.62 | 8.65 ± 3.70 | 0.32 |
| TEAC (mmol TE/kg) | 8.63 ± 4.30 | 8.63 ± 4.14 | 7.98 ± 3.09 | 0.49 |
| ORAC (µmol TE/100 g) | 30,553.29 ± 16,659.94 | 29,889.21 ± 12,158.26 | 29,602.80 ± 10,295.74 | 0.81 |
Data are presented as mean ± standard deviation (SD).
BMI body mass index, HDL-c high density lipoprotein cholesterol, LDL-c low density lipoprotein cholesterol, TG triglyceride, CRP C-reactive protein, PTX3 pentraxin-3, IL18 interleukin 18, TAC total antioxidant capacity, SOD superoxide dismutase, PGF2α prostaglandinF2α, FRAP ferric reducing ability of plasma, TRAP total reactive antioxidant potential, TEAC trolox equivalent antioxidant capacity, ORAC oxygen radical absorbance capacity, AIP log (TG/HDL), AC (TC-HDL)/HDL, CRI.II (LDL/HDL), CRI-I (TC/HDL).
Lipid profiles and atherogenic indices among DTAC groups (ferric reducing-antioxidant power (FRAP), total radical-trapping antioxidant parameter (TRAP), total reactive antioxidant potential (TEAC), and oxygen radical absorbance capacity (ORAC)), is presented in Tables 2 and 3. An individual with higher adherence to FRAP (P = 0.02), TEAC (P = 0.04), and ORAC (P = 0.01) had lower TAC concentrations. Moreover, patients with a higher intake of TRAP (P = 0.03) were more likely to have higher IL-18 concentrations.
Table 2.
The association between lipid profiles and atherogenic indices with DTAC (FRAP, TRAP) in T2DM patients.
| FRAP Mean ± SD |
P | TRAP Mean ± SD |
P | |||
|---|---|---|---|---|---|---|
| Low | High | Low | High | |||
| 327 | 321 | 335 | 325 | |||
| Age (year) | 53.83 ± 6.53 | 54.13 ± 6.36 | 0.55 | 54.06 ± 6.68 | 53.86 ± 6.28 | 0.68 |
| Sex (male) N% | 127 (49.2%) | 131 (50.8%) | 0.33 | 129 (49%) | 134 (51%) | 0.28 |
| BMI (kg/m2) | 29.42 ± 4.96 | 29.42 ± 4.36 | 0.98 | 29.43 ± 4.88 | 29.39 ± 4.40 | 0.90 |
| WC (cm) | 92.63 ± 10.64 | 92.89 ± 10.92 | 0.75 | 92.99 ± 11.20 | 92.38 ± 10.25 | 0.46 |
| Occupation unemployed | 168 (51.9%) | 156 (48.1%) | 0.26 | 158 (48.2%) | 170 (51.8%) | 0.27 |
| Education university educated | 67 (54%) | 57 (46%) | 0.21 | 73 (58.4%) | 52 (41.6%) | 0.03 |
| Cigarette smoking (yes) N% | 50 (40%) | 75 (60%) | 0.006 | 54 (42.2%) | 74(57.8%) | 0.02 |
| Alcohol consumption (NO) N% | 317 (50.6%) | 310 (49.4%) | 0.48 | 323 (50.5%) | 316 (49.5%) | 0.35 |
| Familial history of diabetes (yes) N% | 227 (50.3%) | 224 (49.7%) | 0.49 | 235 (51.2%) | 224 (48.8%) | 0.39 |
| Glucose-lowering medication |
279 (52.1%) 48 (42.58%) |
256 (47.9%) 65 (57.5%) |
0.03 |
283 (51.9%) 52 (45.2%) |
262 (48.1%) 63 (54.8%) |
0.11 |
| Metformin and glybenclamid N% | ||||||
| Other medications N% | ||||||
| Supplementation use (yes) | 326 (50.7%) | 317 (49.3%) | 0.18 | 334 (51%) | 321 (49%) | 0.17 |
| Total energy intake, kcal/day | 2594.64 ± 961.68 | 2639. ± 998.72 | 0.53 | 2569.34 ± 950.53 | 2654.10 ± 999.75 | 0.23 |
| Carbohydrate, g/day | 341.16 ± 141.08 | 352.74 ± 148.18 | 0.27 | 337.21 ± 131.41 | 355.31 ± 155.02 | 0.08 |
| Protein, g/day | 90.55 ± 35.87 | 91.73 ± 37 | 0.65 | 90.30 ± 35.60 | 91.72 ± 37.12 | 0.58 |
| Total fat, g/day | 104.83 ± 51.36 | 103.60 ± 47.07 | 0.73 | 104.06 ± 50.50 | 103.95 ± 47.60 | 0.97 |
| Saturated fatty acids, g/day | 26.70 ± 10.40 | 27.15 ± 12.19 | 0.58 | 26.49 ± 10.55 | 27.25 ± 11.98 | 0.35 |
| Monounsaturated fatty acids, g/day | 36.14 ± 19.79 | 35 ± 17.35 | 0.39 | 35.91 ± 19.28 | 35.29 ± 17.76 | 0.75 |
| Polyunsaturated fatty acids, g/day | 25.83 ± 16.29 | 25.43 ± 14.16 | 0.71 | 25.45 ± 15.55 | 25.73 ± 14.85 | 0.79 |
| Cholesterol, (mg/day) | 222.69 ± 143.12 | 221.49 ± 49 | 0.93 | 226.41 ± 160.26 | 221.60 ± 22.07 | 0.73 |
| Dietary fiber, g/day | 41.64 ± 21.86 | 44.28 ± 24.30 | 0.11 | 41.12 ± 12.34 | 44.58 ± 24.43 | 0.03 |
| HDL (mg/dl) | 54.48 ± 12.48 | 55.28 ± 13.05 | 0.43 | 54.93 ± 12.25 | 54.86 ± 13.24 | 0.94 |
| LDL (mg/dl) | 111.33 ± 35.51 | 110.50 ± 35.81 | 0.76 | 111.24 ± 35.60 | 110.69 ± 35.65 | 0.84 |
| Cholesterol (mg/dl) | 194.92 ± 69.25 | 195.40 ± 70.33 | 0.93 | 196.81 ± 68.23 | 194.15 ± 70.93 | 0.62 |
| TG (mg/dl) | 181.05 ± 110.92 | 182.38 ± 105.97 | 0.87 | 184.39 ± 116.49 | 180.72 ± 100.36 | 0.66 |
| Leptin (ng/ml) | 25.28 ± 13.58 | 25.45 ± 15.88 | 0.92 | 25.10 ± 13.70 | 25.29 ± 15.77 | 0.91 |
| Fat% | 36 ± 8.39 | 34.79 ± 7.63 | 0.05 | 35.85 ± 7.88 | 34.93 ± 8.19 | 0.14 |
| Ghrelin (ng/ml) | 2.19 ± 1.24 | 2.27 ± 1.25 | 0.61 | 2.22 ± 1.30 | 2.26 ± 1.65 | 0.77 |
| CRP (mg/l) | 2.06 ± 1.39 | 2.20 ± 1.57 | 0.56 | 2.12 ± 1.47 | 2.19 ± 1.50 | 0.76 |
| Pentrexin3 (ng/ml) | 2.63 ± 0.52 | 2.56 ± 0.39 | 0.36 | 2.62 ± 0.45 | 2.59 ± 0.46 | 0.70 |
| Interlukin18 (pg/ml) | 247.61 ± 32.24 | 248.68 ± 27.86 | 0.82 | 241.96 ± 32.05 | 252.24 ± 27.71 | 0.03 |
| TAC (g/dl) | 2.62 ± 0.58 | 2.40 ± 0.56 | 0.02 | 2.60 ± 0.59 | 2.44 ± 0.55 | 0.08 |
| SOD (U/ml) | 0.14 ± 0.04 | 0.15 ± 0.10 | 0.52 | 0.15 ± 0.04 | 0.16 ± 0. 08 | 0.65 |
| PGF2alpha (pg/ml) | 72.48 ± 6.46 | 72.52 ± 5.61 | 0.96 | 72.50 ± 6.39 | 72.38 ± 5.83 | 0.90 |
| AIP | 0.46 ± 0.25 | 0.47 ± 0.23 | 0.72 | 0.46 ± 0.25 | 0.47 ± 0.23 | 0.77 |
| AC | 2.73 ± 1.56 | 2.69 ± 1.49 | 0.74 | 2.72 ± 1.53 | 2.70 ± 1.51 | 0.86 |
| CRI.II | 2.10 ± 0.68 | 2.05 ± 0.66 | 0.36 | 2.08 ± 0.67 | 2.08 ± 0.68 | 0.93 |
| CRI-I | 3.83 ± 1.54 | 3.79 ± 1.57 | 0.74 | 3.76 ± 1.52 | 3.82 ± 1.59 | 0.61 |
Data are presented as mean ± standard deviation (SD). Bold values denote statistical signifcance at the P < 0.05 level.
BMI body mass index, HDL-c high density lipoprotein cholesterol, LDL-c low density lipoprotein cholesterol, TG triglyceride, CRP C-reactive protein, PTX3 pentraxin-3, IL18 interleukin 18, TAC total antioxidant capacity, SOD superoxide dismutase, PGF2α prostaglandinF2α, AIP log (TG/HDL), AC (TC-HDL)/HDL, CRI.II (LDL/HDL), CRI-I (TC/HDL).
Table 3.
The association between lipid profiles and atherogenic indices with DTAC (TEAC, ORAC) in T2DM patients.
| TEAC Mean ± SD |
P | ORAC Mean ± SD |
P | |||
|---|---|---|---|---|---|---|
| Low | High | Low | High | |||
| Age (year) | 53.95 ± 6.61 | 54.01 ± 6.34 | 0.89 | 54.02 ± 6.75 | 53.90 ± 6.22 | 0.80 |
| Sex (male) N% | 131 (49.8%) | 132 (50.2%) | 0.48 | 123 (46.9%) | 139 (53.1%) | 0.16 |
| BMI (kg/m2) | 29.29 ± 4.88 | 29.51 ± 4.41 | 0.54 | 29.36 ± 4.79 | 29.49 ± 4.54 | 0.72 |
| WC (cm) | 92.72 ± 11.19 | 92.68 ± 10.29 | 0.95 | 92.54 ± 10.68 | 92.93 ± 10.83 | 0.64 |
| Occupation unemployed N% | 166 (50.5%) | 163 (49.5%) | 0.45 | 158 (48.2%) | 170 (51.8%) | 0.27 |
| Education university educated N% | 64 (51.2%) | 61 (48.8%) | 0.42 | 70 (56.5%) | 54 (43.5%) | 0.05 |
| Cigarette smoking (yes) N% | 55 (40.3%) | 73 (57%) | 0.04 | 54 (41.9%) | 75 (58.1%) | 0.03 |
| Alcohol consumption (NO) N% | 321 (50.3%) | 317 (49.7%) | 0.32 | 316 (49.7%) | 320 (50.3%) | 0.34 |
| Familial history of diabetes (yes) N% | 267 (50%) | 267 (50%) | 0.44 | 224 (49.1%) | 232 (50.9%) | 0.42 |
| Glucose-lowering medication |
248 (52.2%) 46 (40%) |
260 (47.8%) 69 (60%) |
0.01 |
281 (51.8%) 44 (38.3%) |
261 (48.2%) 71 (61.7%) |
0.005 |
| Metformin and glybenclamid N% | ||||||
| Other medications N% | ||||||
| Supplementation use (yes) N% | 329 (50.3%) | 325 (49.95) | 0.18 | 325 (49.8%) | 327(50.2%) | 0.03 |
| Total energy intake, kcal/day | 2555.76 ± 946.78 | 2670.21 ± 1002.04 | 0.10 | 2572.94 ± 926.81 | 2654.90 ± 1023.31 | 0.24 |
| Carbohydrate, g/day | 336.14 ± 131.80 | 356.64 ± 154.67 | 0.04 | 330.73 ± 130.61 | 353.17 ± 154.60 | 0.03 |
| Protein, g/day | 90.16 ± 35.48 | 91.96 ± 37.23 | 0.49 | 90.05 ± 34.30 | 92.10 ± 38.35 | 0.43 |
| Total fat, g/day | 103.27 ± 50.12 | 104.86 ± 47.99 | 0.65 | 103.13 ± 49.99 | 105.15 ± 48.18 | 0.56 |
| Saturated fatty acids, g/day | 26.53 ± 10.82 | 27.22 ± 11.75 | 0.39 | 26.60 ± 10.35 | 27.16 ± 12.17 | 0.49 |
| Monounsaturated fatty acids, g/day | 35.52 ± 19.17 | 35.53 ± 19.17 | 0.99 | 35.38 ± 19.04 | 35.73 ± 18.06 | 0.79 |
| Polyunsaturated fatty acids, g/day | 25.31 ± 15.23 | 25.90 ± 15.18 | 0.59 | 25.11 ± 15.68 | 26.15 ± 14.73 | 0.34 |
| Cholesterol, (mg/day) | 222.44 ± 144.44 | 225.65 ± 232.93 | 0.81 | 218.93 ± 122.38 | 229.43 ± 245.65 | 0.45 |
| Dietary fiber, g/day | 41.18 ± 21.44 | 44.57 ± 24.37 | 0.04 | 42.06 ± 12.75 | 43.79 ± 24.24 | 0.29 |
| HDL (mg/dl) | 54.79 ± 12.33 | 55.02 ± 13.16 | 0.81 | 54.68 ± 12.50 | 55.07 ± 12.97 | 0.69 |
| LDL (mg/dl) | 111.91 ± 36.23 | 110.04 ± 35.03 | 0.50 | 110.52 ± 35.30 | 111.49 ± 36.08 | 0.72 |
| Cholesterol (mg/dl) | 196.97 ± 69.42 | 193.92 ± 69.79 | 0.57 | 194.26 ± 71.39 | 196.32 ± 67.68 | 0.70 |
| TG (mg/dl) | 185.42 ± 113.34 | 178.96 ± 103.33 | 0.45 | 184.29 ± 111.44 | 179.20 ± 105.06 | 0.55 |
| Leptin (ng/ml) | 25.19 ± 13.75 | 25.20 ± 15.57 | 0.99 | 24.82 ± 13.74 | 25.56 ± 15.62 | 0.68 |
| Fat% | 35.65 ± 7.81 | 35.15 ± 8.28 | 0.42 | 35.54 ± 8.08 | 35.30 ± 8.05 | 0.71 |
| Ghrelin (ng/ml) | 2.11 ± 1.12 | 2.35 ± 1.31 | 0.13 | 2.18 ± 1.19 | 2.29 ± 1.27 | 0.51 |
| CRP (mg/l) | 2.08 ± 1.47 | 2.23 ± 1.50 | 0.55 | 2.13 ± 1.36 | 2.15 ± 1.58 | 0.92 |
| Pentrexin3 (ng/ml) | 2.65 ± 0.50 | 2.56 ± 0.42 | 0.21 | 2.63 ± 0.52 | 2.57 ± 0.39 | 0.44 |
| Interlukin18 (pg/ml) | 247.35 ± 27.85 | 247.97 ± 32.16 | 0.89 | 245.56 ± 32.45 | 250.29 ± 27.33 | 0.33 |
| TAC (g/dl) | 2.60 ± .58 | 2.42 ± .55 | 0.04 | 2.62 ± 0.58 | 2.39 ± 0.55 | 0.01 |
| SOD (U/ml) | 0.15 ± 0.04 | 0.15 ± 0.09 | 0.85 | 0.14 ± 0.04 | 0.15 ± 0.10 | 0.33 |
| PGF2alpha (pg/ml) | 72.29 ± 6.22 | 72.56 ± 5.95 | 0.78 | 72.33 ± 6.49 | 72.50 ± 5.67 | 0.86 |
| AIP | 0.47 ± 0.24 | 0.46 ± 0.24 | 0.64 | 0.47 ± 0.25 | 0.46 ± 0.23 | 0.74 |
| AC | 2.73 ± 1.54 | 2.68 ± 1.49 | 0.66 | 2.69 ± 1.57 | 2.73 ± 1.47 | 0.77 |
| CRI.II | 2.09 ± .67 | 2.06 ± 0.67 | 0.56 | 2.07 ± 0.66 | 2.09 ± 0.68 | 0.80 |
| CRI-I | 3.81 ± 1.75 | 3.78 ± 1.32 | 0.80 | 3.80 ± 1.54 | 3.80 ± 1.56 | 0.98 |
Data are presented as mean ± standard deviation (SD). Bold values denote statistical signifcance at the P < 0.05 level.
BMI body mass index, HDL-c high density lipoprotein cholesterol, LDL-c low density lipoprotein cholesterol, TG triglyceride, CRP C-reactive protein, PTX3 pentraxin-3, IL18 interleukin 18, TAC total antioxidant capacity, SOD superoxide dismutase, PGF2α prostaglandinF2α, AIP log (TG/HDL), AC (TC-HDL)/HDL, CRI.II (LDL/HDL), CRI-I (TC/HDL).
Interaction between the BDNF Val66Met polymorphism and DTAC
Tables 4, 5, 6, 7 show the interactions between BDNF Val66Met polymorphism and DTAC (FRAP, TRAP, TEAC, and ORAC) on anthropometric indices (BMI and WC), lipid profiles (HDL, LDL, TC, and TG), and atherogenic indices (AIP, AC, CRI.II and CRI).
Table 4.
The interaction of BDNF Val/Met polymorphism and FRAP on anthropometric indices, lipid profile and atherogenic indices.
| Variable | Allele | High adherence of FRAP | |||
|---|---|---|---|---|---|
| β | 95% CI | P | |||
| BMI | Crud | Met/Met | 0.55 | − 1.94 to 3.04 | 0.66 |
| Val/Met | 0.66 | − 0.88 to 2.21 | 0.40 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.64 | − 1.85 to3.12 | 0.61 | |
| Val/Met | 0.72 | − 0.81 to 2.26 | 0.35 | ||
| Val/Val | Reference | ||||
| WC | Crud | Met/Met | − 0.15 | − 5.92 to 5.60 | 0.86 |
| Val/Met | 0.48 | − 3.11 to 4.07 | 0.79 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.48 | − 6.09 to 5.11 | 0.86 | |
| Val/Met | 0.23 | − 3.25 to 3.73 | 0.89 | ||
| Val/Val | Reference | ||||
| HDL | Crud | Met/Met | − 7.25 | − 14.04 to − 0.47 | 0.03 |
| Val/Met | − 4.39 | − 8.63 to − 0.16 | 0.04 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 6.10 | − 12.69 to 0.49 | 0.07 | |
| Val/Met | − 4.18 | − 8.29 to − 0.68 | 0.04 | ||
| Val/Val | Reference | ||||
| TG | Crud | Met/Met | 25.52 | − 32.43 to 83.48 | 0.86 |
| Val/Met | 49.98 | 13.86 to 86.11 | 0.007 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 24.16 | − 34.37 to 82.71 | 0.41 | |
| Val/Met | 51.84 | 15.39 to 88.30 | 0.005 | ||
| Val/Val | Reference | ||||
| LDL | Crud | Met/Met | − 1.66 | − 20.67 to 17.33 | 0.43 |
| Val/Met | − 0.43 | − 12.30 to 11.43 | 0.07 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 3.37 | − 15.55 to 22.31 | 0.72 | |
| Val/Met | 0.16 | − 11.64 to 11.97 | 0.97 | ||
| Val/Val | Reference | ||||
| TC | Crud | Met/Met | − 25.42 | − 62.49 to 11.64 | 0.17 |
| Val/Met | 19.56 | − 3.64 to 42.77 | 0.09 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 22.09 | − 59.24 to 15.06 | 0.24 | |
| Val/Met | 21.79 | − 1.44 to 45.03 | 0.06 | ||
| Val/Val | Reference | ||||
| AIP | Crud | Met/Met | 0.07 | − 0.05 to 0.20 | 0.23 |
| Val/Met | 0.12 | 0.03 to 0.20 | 0.004 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.06 | − 0.06 to 0.20 | 0.30 | |
| Val/Met | 0.12 | 0.04 to 0.20 | 0.003 | ||
| Val/Val | Reference | ||||
| AC | Crud | Met/Met | − 0.02 | − 0.84 to 0.78 | 0.94 |
| Val/Met | 0.57 | 0.05 to 1.08 | 0.02 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.06 | − 0.87 to 0.75 | 0.87 | |
| Val/Met | 0.59 | 0.08 to 1.10 | 0.02 | ||
| Val/Val | Reference | ||||
| LDL/HDL.CRI.II | Crud | Met/Met | 0.23 | − 0.12 to 0.59 | 0.21 |
| Val/Met | 0.05 | − 0.16 to 0.28 | 0.62 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.27 | − 0.08 to 0.62 | 0.13 | |
| Val/Met | 0.05 | − 0.16 to 0.27 | 0.60 | ||
| Val/Val | Reference | ||||
| TC/HDL.CRI.I | Crud | Met/Met | − 0.25 | − 1.10 to 0.59 | 0.55 |
| Val/Met | 0.20 | − 0.72 to 0.31 | 0.43 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.28 | − 1.13 to 0.56 | 0.51 | |
| Val/Met | − 0.18 | − 0.74 to 0.34 | 0.49 | ||
| Val/Val | Reference | ||||
Val/Val genotype is considered as a reference. Low adherence of DTAC is considered as a reference. Generalized linear model; crude model and adjusted model Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes, as covariates. Bold values denote statistical signifcance at the P < 0.05 level.
FRAP ferric reducing ability of plasma, CI confidence interval, AIP (Atherogenic index of plasma): log(TG/HDL), AC (Atherogenic coefficient): (TC-HDL/HDL), CRI-II (Castelli s risk index): LDL/HDL, CRI-I (Castelli s risk index): TC/HDL, TC total cholesterol, TG triglyceride, HDL high density lipoprotein, LDL low density lipoprotein, WC waist circumference.
Table 5.
The interaction of BDNF Val/Met polymorphism and ORAC on anthropometric indices, lipid profile and atherogenic indices.
| Variable | Allele | High adherence of ORAC | |||
|---|---|---|---|---|---|
| β | 95% CI | P | |||
| BMI | Crud | Met/Met | 1.57 | − 0.88 to − 4.02 | 0.20 |
| Val/Met | 1.69 | 0.16 to 3.23 | 0.03 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 1.62 | − 0.82 to 4.06 | 0.19 | |
| Val/Met | 1.84 | 0.31 to 3.37 | 0.01 | ||
| Val/Val | Reference | ||||
| WC | Crud | Met/Met | 1.75 | − 3.91 to 7.43 | 0.54 |
| Val/Met | 3.60 | 0.04 to 7.16 | 0.04 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 1.50 | − 4.17 to 7.19 | 0.60 | |
| Val/Met | 3.75 | 0.19 to 7.31 | 0.03 | ||
| Val/Val | Reference | ||||
| HDL | Crud | Met/Met | − 5.50 | − 12.20 to 1.19 | 0.10 |
| Val/Met | − 4.71 | − 8.92 to − 0.51 | 0.02 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 5.09 | − 11.82 to 1.62 | 0.13 | |
| Val/Met | − 4.62 | − 8.83 to − 0.41 | 0.03 | ||
| Val/Val | Reference | ||||
| TG | Crud | Met/Met | − 0.23 | − 57.60 to 57.13 | 0.99 |
| Val/Met | 44.95 | 9.09 to 80.82 | 0.01 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 1.18 | − 59.34 to 56.98 | 0.96 | |
| Val/Met | 45.52 | 9.30 to 81.73 | 0.01 | ||
| Val/Val | Reference | ||||
| LDL | Crud | Met/Met | − 6.57 | − 19.06 to 5.91 | 0.30 |
| Val/Met | 6.78 | − 0.77 to 14.33 | 0.07 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 3.46 | − 22.29 to 15.37 | 0.71 | |
| Val/Met | 2.44 | − 9.33 to 14.22 | 0.68 | ||
| Val/Val | Reference | ||||
| TC | Crud | Met/Met | − 27.31 | − 63.90 to 9.28 | 0.14 |
| Val/Met | 13.20 | − 9.81 to36.22 | 0.26 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 22.16 | − 58.97 to 14.63 | 0.23 | |
| Val/Met | 15.15 | − 7.92 to 38.22 | 0.19 | ||
| Val/Val | Reference | ||||
| AIP | Crud | Met/Met | − 0.03 | − 0.16 to 0.09 | 0.63 |
| Val/Met | 0.09 | 0.01 to 0.18 | 0.01 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.04 | − 0.17 to 0.08 | 0.50 | |
| Val/Met | 0.09 | 0.01 to 0.17 | 0.02 | ||
| Val/Val | Reference | ||||
| AC | Crud | Met/Met | − 0.23 | − 1.04 to 0.56 | 0.56 |
| Val/Met | 0.47 | − 0.02 to 0.98 | 0.06 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.26 | − 1.07 to 0.53 | 0.51 | |
| Val/Met | 0.43 | − 0.07 to 0.93 | 0.09 | ||
| Val/Val | Reference | ||||
| LDL/HDL.CRI.II | Crud | Met/Met | 0.01 | − 0.34 to 0.37 | 0.94 |
| Val/Met | 0.13 | − 0.08 to 0.36 | 0.23 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.02 | − 0.32 to 0.37 | 0.88 | |
| Val/Met | 0.10 | − 0.11 to 0.32 | 0.36 | ||
| Val/Val | |||||
| TC/HDL.CRI.I | Crud | Met/Met | 0.05 | − 0.77 to 0.88 | 0.90 |
| Val/Met | − 0.17 | − 0.69 to 0.33 | 0.49 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.01 | − 0.82 to 0.85 | 0.96 | |
| Val/Met | − 0.16 | − 0.68 to 0.35 | 0.53 | ||
| Val/Val | Reference | ||||
Val/Val genotype is considered as a reference. Low adherence of DTAC is considered as a reference. Generalized linear model; crude model and adjusted model Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes, as covariates. Bold values denote statistical signifcance at the P < 0.05 level.
ORAC oxygen radical absorbance capacity, CI confidence interval, AIP (Atherogenic index of plasma): log(TG/HDL), AC (Atherogenic coefficient): (TC-HDL/HDL), CRI-II (Castelli s risk index): LDL/HDL, CRI-I (Castelli s risk index): TC/HDL, TC total cholesterol, TG triglyceride, HDL high density lipoprotein, LDL low density lipoprotein, WC waist circumference.
Table 6.
The interaction of BDNF Val/Met polymorphism and TEAC on anthropometric indices, lipid profile and atherogenic indices.
| Variable | Allele | High adherence of TEAC | P | ||
|---|---|---|---|---|---|
| β | 95% CI | ||||
| BMI | Crud | Met/Met | − 7.09 | − 13.77 to − 0.24 | 0.04 |
| Val/Met | − 1.82 | − 6.02 to 2.38 | 0.39 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 1.60 | − 0.85 to 4.05 | 0.20 | |
| Val/Met | 1.31 | − 0.19 to 2.83 | 0.08 | ||
| Val/Val | Reference | ||||
| WC | Crud | Met/Met | − 20.43 | − 57.26 to 16.40 | 0.27 |
| Val/Met | 22.44 | − 0.52 to 45.42 | 0.05 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 3.15 | − 2.40 to 8.70 | 0.26 | |
| Val/Met | 2.31 | − 1.13 to 5.76 | 0.18 | ||
| Val/Val | Reference | ||||
| HDL | Crud | Met/Met | − 7.09 | − 13.77 to − 0.24 | 0.04 |
| Val/Met | − 1.82 | − 6.02 to 2.38 | 0.39 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 6.03 | − 12.64 to 0.56 | 0.07 | |
| Val/Met | − 5.67 | − 5.79 to 2.41 | 0.41 | ||
| Val/Val | Reference | ||||
| LDL | Crud | Met/Met | − 5.99 | − 24.91 to 12.93 | 0.53 |
| Val/Met | − 1.65 | − 13.43 to 10.11 | 0.78 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 1.42 | − 20.29 to 17.44 | 0.88 | |
| Val/Met | − 1.30 | − 13.02 to 10.41 | 0.82 | ||
| Val/Val | Reference | ||||
| TC | Crud | Met/Met | − 20.43 | − 57.26 to 16.40 | 0.27 |
| Val/Met | 22.44 | − 0.52 to 45.42 | 0.05 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 17.15 | − 54.06 to 19.75 | 0.36 | |
| Val/Met | 26.39 | 3.40 to 49.38 | 0.02 | ||
| Val/Val | Reference | ||||
| TG | Crud | Met/Met | − 30.82 | − 88.36 to 26.71 | 0.29 |
| Val/Met | 53.51 | 17.80 to 89.22 | 0.003 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 32.04 | − 90.16 to 26.08 | 0.28 | |
| Val/Met | 55.85 | 19.85 to 91.84 | 0.002 | ||
| Val/Val | Reference | ||||
| AIP | Crud | Met/Met | 0.005 | − 0.12 to 0.13 | 0.94 |
| Val/Met | 0.105 | 0.02 to 0.18 | 0.01 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.002 | − 0.13 to 0.12 | 0.98 | |
| Val/Met | 0.10 | 0.02 to 0.18 | 0.009 | ||
| Val/Val | Reference | ||||
| AC | Crud | Met/Met | 0.03 | − 0.77 to 0.83 | 0.93 |
| Val/Met | 0.51 | 0.01 to 1.02 | 0.04 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.02 | − 0.78 to 0.82 | 0.96 | |
| Val/Met | 0.55 | 0.05 to 1.06 | 0.03 | ||
| Val/Val | Reference | ||||
| LDL/HDL.CRI.II | Crud | Met/Met | 0.10 | − 0.25 to 0.47 | 0.55 |
| Val/Met | − 0.04 | − 0.26 to 0.17 | 0.69 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.15 | − 0.20 to 0.50 | 0.39 | |
| Val/Met | − 0.05 | − 0.27 to 0.16 | 0.64 | ||
| Val/Val | Reference | ||||
| TC/HDL.CRI.I | Crud | Met/Met | 0.25 | − 0.58 to 1.09 | 0.55 |
| Val/Met | − 0.04 | − 0.56 to 0.46 | 0.86 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.22 | − 0.61 to 1.06 | 0.60 | |
| Val/Met | − 0.03 | − 0.54 to 0.48 | 0.91 | ||
| Val/Val | Reference | ||||
Val/Val genotype is considered as a reference. Low adherence of DTAC is considered as a reference. Generalized linear model; crude model and adjusted model Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes, as covariates. Bold values denote statistical signifcance at the P < 0.05 level.
TEAC trolox equivalent antioxidant capacity, CI confidence interval, AIP (Atherogenic index of plasma): log(TG/HDL), AC (Atherogenic coefficient): (TC-HDL/HDL), CRI-II (Castelli s risk index): LDL/HDL, CRI-I (Castelli s risk index): TC/HDL, TC total cholesterol, TG triglyceride, HDL high density lipoprotein, LDL low density lipoprotein, WC waist circumference.
Table 7.
The interaction of BDNF Val/Met polymorphism and TRAP on anthropometric indices, lipid profile and atherogenic indices.
| Variable | Allele | High adherence of TRAP | |||
|---|---|---|---|---|---|
| β | 95% CI | P | |||
| BMI | Crud | Met/Met | 0.87 | − 1.61 to 3.36 | 0.49 |
| Val/Met | 1.20 | − 0.32 to 2.73 | 0.12 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.81 | − 1.66 to 3.29 | 0.52 | |
| Val/Met | 1.81 | − 0.33 to 2.69 | 0.12 | ||
| Val/Val | Reference | ||||
| WC | Crud | Met/Met | 0.88 | − 4.86 to 6.63 | 0.76 |
| Val/Met | 3.75 | 0.20 to 7.29 | 0.03 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.93 | − 4.66 to 6.53 | 0.74 | |
| Val/Met | 3.36 | − 0.07 to 6.80 | 0.05 | ||
| Val/Val | Reference | ||||
| HDL | Crud | Met/Met | − 3.58 | − 10.40 to 3.23 | 0.30 |
| Val/Met | − 3.86 | − 8.06 to 0.34 | 0.07 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 3.16 | − 9.83 to 3.49 | 0.35 | |
| Val/Met | − 3.71 | − 7.80 to 0.38 | 0.07 | ||
| Val/Val | Reference | ||||
| TC | Crud | Met/Met | − 18.56 | − 55.53 to 18.40 | 0.32 |
| Val/Met | 34.93 | 12.10 to 57.76 | 0.003 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 18.29 | − 55.35 to 18.76 | 0.33 | |
| Val/Met | 37.20 | 14.38 to 60.03 | 0.001 | ||
| Val/Val | Reference | ||||
| TG | Crud | Met/Met | − 33.52 | − 91.54 to 24.48 | 0.25 |
| Val/Met | 63.78 | 28.09 to 99.46 | < 0.001 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 37.97 | − 96.61 to 20.67 | ||
| Val/Met | 65.28 | 29.36 to 101.20 | < 0.001 | ||
| Val/Val | Reference | ||||
| LDL | Crud | Met/Met | 1.37 | − 17.70 to 20.46 | 0.88 |
| Val/Met | 1.39 | − 10.36 to 13.14 | 0.81 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 5.53 | − 13.50 to 24.56 | 0.56 | |
| Val/Met | 1.21 | − 10.46 to 12.90 | 0.83 | ||
| Val/Val | Reference | ||||
| AIP | Crud | Met/Met | − 0.01 | − 0.14 to 0.11 | 0.85 |
| Val/Met | 0.16 | 0.07 to 0.24 | < 0.001 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.02 | − 0.15 to 0.11 | 0.76 | |
| Val/Met | 0.16 | 0.08 to 0.24 | < 0.001 | ||
| Val/Val | Reference | ||||
| AC | Crud | Met/Met | − 0.12 | − 0.93 to 0.68 | 0.76 |
| Val/Met | 0.88 | 0.38 to 1.38 | 0.001 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.15 | − 0.96 to 0.65 | 0.71 | |
| Val/Met | 0.89 | 0.39 to 1.39 | < 0.001 | ||
| Val/Val | Reference | ||||
| LDL/HDL.CRI.II | Crud | Met/Met | 0.14 | − 0.21 to 0.51 | 0.42 |
| Val/Met | 0.09 | − 0.13 to 0.31 | 0.41 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | 0.19 | − 0.15 to 0.55 | 0.27 | |
| Val/Met | 0.07 | − 0.14 to 0.29 | 0.48 | ||
| Val/Val | Reference | ||||
| TC/HDL.CRI.I | Crud | Met/Met | − 0.25 | − 1.10 to 0.60 | 0.56 |
| Val/Met | − 0.21 | − 0.72 to 0.30 | 0.41 | ||
| Val/Val | Reference | ||||
| Adjusted | Met/Met | − 0.23 | − 1.09 to 0.61 | 0.58 | |
| Val/Met | − 0.21 | − 0.73 to 0.30 | 0.41 | ||
| Val/Val | Reference | ||||
Val/Val genotype is considered as a reference. Low adherence of DTAC is considered as a reference. Generalized linear model; crude model and adjusted model Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes, as covariates. Bold values denote statistical signifcance at the P < 0.05 level.
TRAP total reactive antioxidant potential, CI confidence interval, AIP (Atherogenic index of plasma): log(TG/HDL), AC (Atherogenic coefficient): (TC-HDL/HDL), CRI-II (Castelli s risk index): LDL/HDL, CRI-I (Castelli s risk index): TC/HDL, TC total cholesterol, TG triglyceride, HDL high density lipoprotein, LDL low density lipoprotein, WC waist circumference.
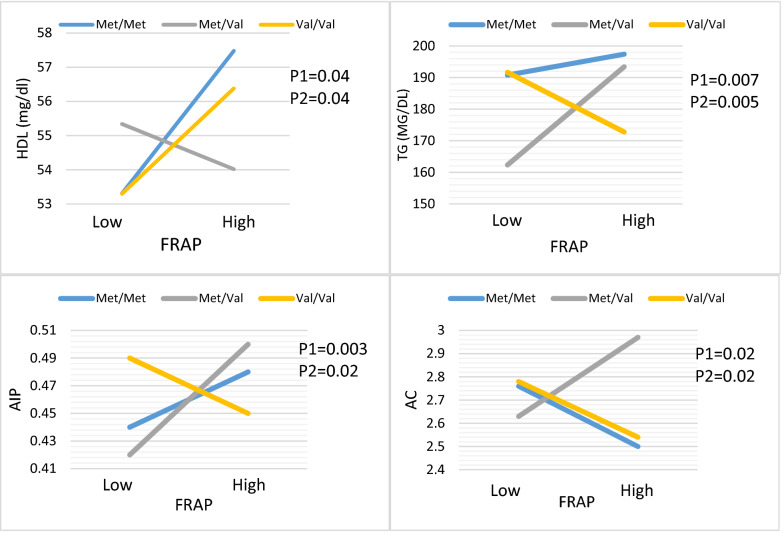
In both crud and adjusted model for potential confounders, BDNF Val66Met and FRAP interactions were significant in terms of HDL, TG, AIP and AC index. Carriers of the Val/Met genotype who were in the higher median intake of FRAP had lower HDL (β: − 4.18, 95%CI − 8.29 to − 0.68, P:0.04) and higher TG concentration (β: 51.84, 95%CI 15.39 to 88.30, P:0.005), AIP (β: 0.12, 95%CI 0.04 to 0.20, P:0.003), and AC (β: 0.59, 95%CI 0.08 to 1.10, P:0.02) index compared to Val/Val genotypes whit lower median intake (Table 4, Fig. 1).
Figure 1.
Interaction between the BDNF Val66Met polymorphism and DTAC (FRAP) intake with regard to TG, HDL, AIP and AC according to the median DTAC, the participants were dichotomized into low and high categories. P 1 = P value with unadjusted (crude) model, P 2 = P value with adjustments for potential confounding factors including (Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes).
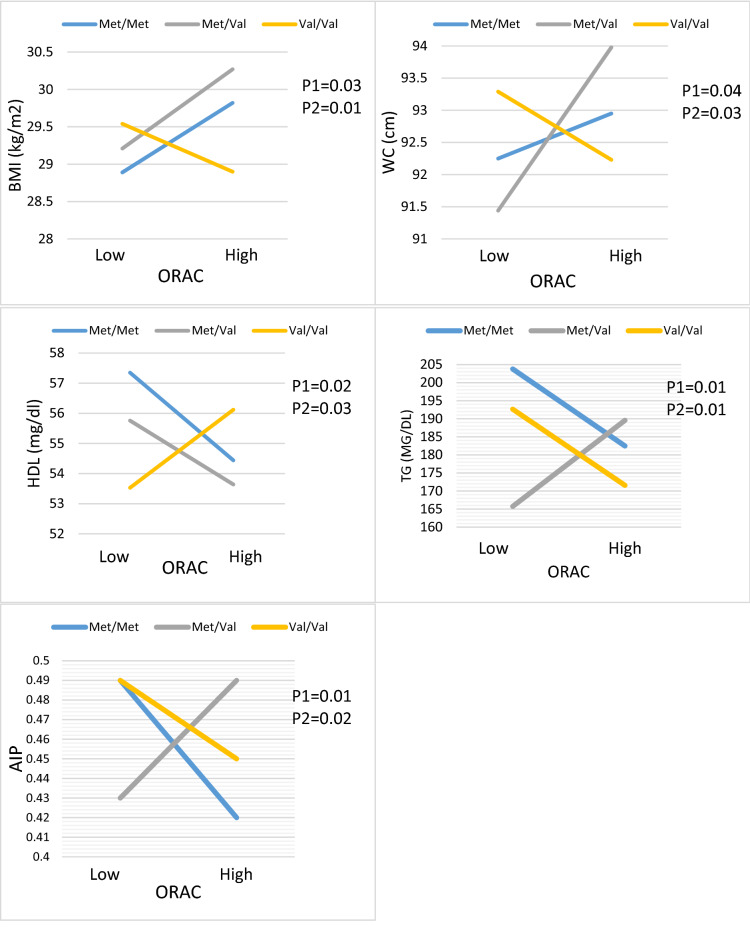
Besides, BDNF Val66Met and ORAC interactions were significant in terms of anthropometric indices (BMI and WC), lipid profiles (HDL and TG), and atherogenic index (AIP) in crude model and after adjusted to cofounding factors. Diabetic patients with Val/Met genotype who consumed higher ORAC intake had increased odds for BMI (β: 1.84, 95%CI 0.31 to 3.37, P:0.01), WC (β: 3.75, 95%CI 0.19 to 7.31, P:0.03), TG (β: 45.52, 95%CI 9.30 to 81.73, P:0.01) and AIP (β: 0.09, 95%CI 0.01 to 0.17, P:0.02), also decreased odds for HDL concentration (β: − 4.62, 95%CI − 8.83 to − 0.41, P:0.03) compared to reference group whit lower ORAC intake (Table 5, Fig. 2).
Figure 2.
Interaction between the BDNF Val66Met polymorphism and DTAC (ORAC) intake with regard to TG, HDL, AIP and AC according to the median DTAC, the participants were dichotomized into low and high categories. P 1 = P value with unadjusted (crude) model, P 2 = P value with adjustments for potential confounding factors including (Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes).
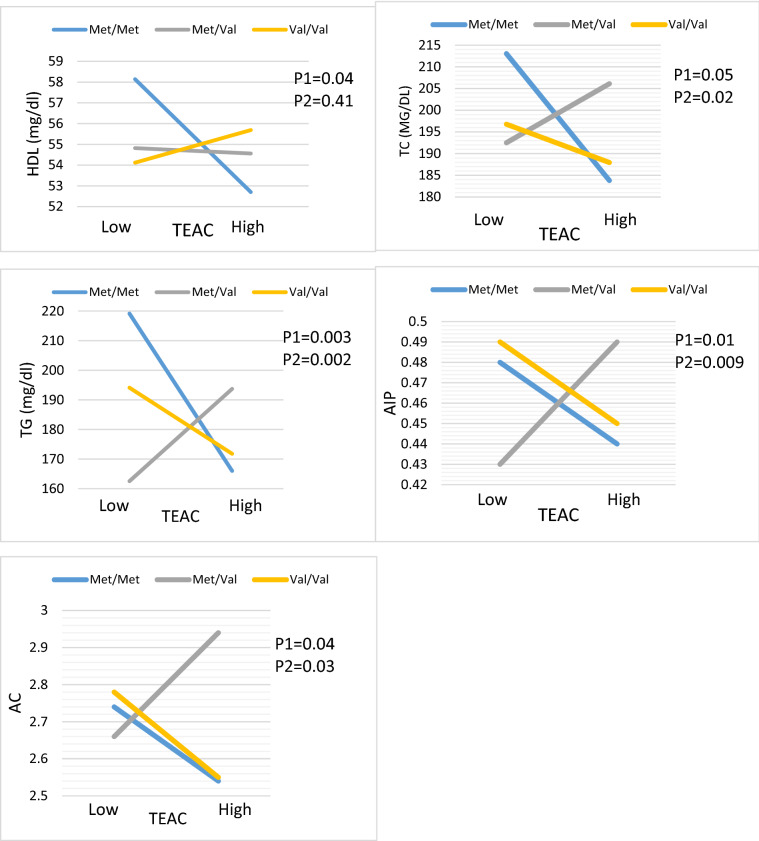
Moreover, Val/Met patients with a higher median intake of TEAC had higher TG (β: 55.85, 95%CI 19.85 to 91.84, P = 0.002), AIP (β: 0.10, 95%CI 0.02 to 0.18, P = 0.009) and AC (β: 0.55, 95%CI 0.05 to 1.06, P = 0.03) compared to reference group whit lower TEAC intake in both crud and adjusted model. Moreover, in the crude model, there was no significant interaction between Val/Met group and TEAC intake on TC (β: 22.44, 95% CI − 0.52 to 45.42, P:0.05) however, after controlling for confounders, a significant interaction was found on TC (β: 26.39, 95% CI 3.40 to 49.38, P:0.02). In crude model, Met/Met group with higher TEAC intake had lower HDL (β: − 7.09, 95% CI − 13.77 to − 0.24, P:0.04), also after adjustment for cofounders this association was disappeared (β: − 6.03, 95% CI − 12.64 to 0.56, P:0.07) (Table 6, Fig. 3).
Figure 3.
Interaction between the BDNF Val66Met polymorphism and DTAC (TEAC) intake with regard to TG, HDL, AIP and AC according to the median DTAC, the participants were dichotomized into low and high categories. P 1 = P value with unadjusted (crude) model, P 2 = P value with adjustments for potential confounding factors including (Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes).
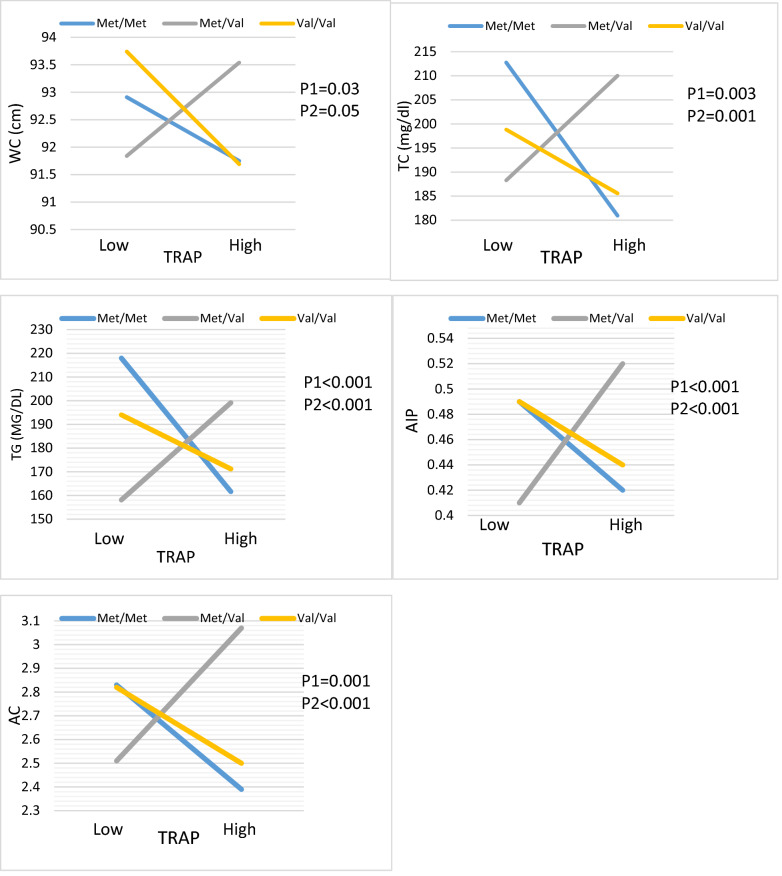
Finally, BDNF Val66Met and TRAP interactions were significant in terms of the anthropometric index (WC), lipid profiles (TC and TG), and atherogenic indices (AIP and AC). Diabetic patients with Val/Met genotype who consumed higher TRAP intake had increased odds for TC (β: 37.20, 95%CI 14.38 to 60.03, P:0.001), TG (β: 65.28, 95%CI 29.36 to 101.20, P < 0.001), AIP (β: 0.16, 95%CI 0.08 to 0.24, P < 0.001) and AC (β: 0.89, 95%CI 0.39 to 1.39, P < 0.001) compared to reference group whit lower TRAP intake in both crud and adjusted model (Table). Moreover, in the crude model, there was a significant interaction between the Val/Met group in comparison with the reference group (Val/Val) on WC (β: 3.75, 95%CI 0.20 to 7.29, P:0.03), however, after controlling for confounders, a significant interaction was disappeared WC (β: 3.36, 95%CI − 0.07 to 6.80, P:0.05) (Table 7, Fig. 4).
Figure 4.
Interaction between the BDNF Val66Met polymorphism and DTAC (TRAP) intake with regard to TG, HDL, AIP and AC according to the median DTAC, the participants were dichotomized into low and high categories. P 1 = P value with unadjusted (crude) model, P 2 = P value with adjustments for potential confounding factors including (Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes).
Discussion
The key findings of the current study were the significant interaction result of BDNF Val66Met polymorphism with DTAC on lipid profile and atherogenic indices in T2DM patients. High DTAC intake modified the association of the BDNF Val66Met genotypes with the odds of higher lipid profile and atherogenic indices. Particularly, we revealed that increased DTAC did not influence the negative consequences of the Val/Met genotype. While it seems that the presence of the Val/Val wildtype and BDNF Met/Met homozygotes in diabetic patients with a high DTAC is a protective factor.
In this present study, we revealed that an individual with higher adherence to DTAC had lower TAC concentrations. Moreover, patients with a higher intake of TRAP were more likely to have higher IL-18 concentrations. There have been few investigations on the relationship between DTAC and metabolic indicators such as lipid profiles and inflammatory markers; the findings of numerous research have contradicted the findings of the current study20,31–35. In line with our findings, Mozaffari et al.36, have shown subjects in the highest tertile of dietary TRAP had higher BMI than those in the lowest tertile. Numerous findings have reported obesity has been linked to a persistent low-grade inflammatory disease, which has been linked to the development of T2DM and CVD37,38. In these situations, human adipose tissue secretes a high amount of inflammatory markers, including IL-18, in these circumstances39.
We did not find significant association between BDNF rs6267 groups and biochemical markers. BDNF play important role in etiology of diabetes and obesity by probably biological mechanism including controlling food behaviour, energy homeostasis and anorexigenic effects40. Some experimental studies have also suggested that hyperphagia, hyperinsulinemia, and higher levels of serum leptin and body weight following a decrease in BDNF levels, among BDNF-knockout mice41. In terms of human study, association between Val66Met polymorphism with obesity, dyslipidaemia and diabetes are controversial. For instance, several studies have shown that Met/Met genotype have higher risk for insulin resistance, obesity and dyslipidaemia24,42,43. Bonaccorso et al. revealed, Met-allele of BDNF Val66Met polymorphism was to be positively associated with serum levels TG and TG/HDL-C ratio24. However, some authors have suggested the Met-allele carriers have lower risk for obesity, postprandial glucose and HbA1c levels25,44,45. Additionally, also some studies have observed no association with anthropometric indices and lipid profile46–48. The inconsistent results have been revealed on the association between the Val66Met polymorphism and obesity and lipid profile, proposing that environmental factors like dietary intake may be modify this association.
In this study, we discovered that a diet high in overall antioxidant capacity is more beneficial in homozygotes carriers than in Val/Met carriers. This is the first research to investigate at how dietary antioxidants interact with the BDNF polymorphism. In addition, only a few studies have examined the relationship between BDNF rs6267 and dietary patterns, as well as food and nutrient intakes29,30. According to a pervious study, individuals with BDNF Val/Met and Met/Met had a lower risk for T2DM in low energy intake and especially BDNF Val/Met had a negative association with low-protein, high-carbohydrate, and low-fat diet. In comparison to BDNF Val/Val, BDNF Val/Met reduced the risk of HOMA-IR in low-energy intake but raised the risk of HOMA-B in high-energy intake. HOMA-B is an insulin secretion capacity index that could aim to decrease the incidence of T2DM. When high-energy consumption is combined with BDNF Val/Met, insulin secretion is increased in terms of maintaining normoglycemia condition. As a result, individuals with BDNF Val/Met may also have a higher potential to compensate for the development of T2DM29. In this term, another study has shown, total food intake, total caloric intake, and protein intake were not related to the BDNF rs6265 variation. Regarding obesity indicators, although, this variation interacted with PUFA and total food intake. Met allele carriers in men exhibited a higher BMI as their PUFA intake increased, and a smaller waist as their n-3: n-6 PUFA ratio increased. In contrast to heterozygotes, Val/Val homozygous men showed the opposite trend in BMI: BMI dropped with increased PUFA intake and higher n-3:n-6 PUFA ratio increased waist circumference30. Furthermore, another study indicated that when Met allele carriers were exposed to a high-CHO diet, their chance of developing carbohydrate-induced hypertriglyceridemia enhanced49. In contrast, another study has shown, Val66Met polymorphism did not appear to affect the link between food quality and BDNF serum in terms of depression prediction50. Previous research has revealed that dietary intakes alter the relationship between BDNF genotype and obesity-related behaviors, which is corroborated by findings in rats51–53. As a result, food consumption may influence the relationship between BDNF polymorphism and cardiovascular disease indicators via BDNF expression and serum protein modulation.
On the effect of diet on the BDNF serum, some nutrigenomic studies have done based on diet or other macronutrient induced obesity and healthy diet. For example, a previous study found that high glucose concentrations decreased BDNF release54. As a result, blood BDNF levels in T2DM patients were shown to be considerably higher than in healthy controls in humans and were found to be strongly associated with triglyceride levels55. In diabetic mice, subcutaneous injections of BDNF were found to considerably improve lipid and glucose profiles56. In this regard, experimental studies have revealed that diet-induced obese mice include high-fat and high-sugar diets53,57,58 or n-3 PUFA deficient diets51 and chronic high-fat DIO mice53 lowered BDNF expression in the hippocampus by more than 30%, which has been associated to weaker inhibitory regulation of food consumption and, as a result, promoted obesity-related phenotype, while low-fat mice showed no difference.
We observed that the presence of the Val/Val wild-type and BDNF Met/Met homozygotes in diabetic patients with a high DTAC is a protective factor. Antioxidant-rich diets may affect previously unknown biological processes, altering vulnerability to cardiovascular disease. Antioxidant-rich diets have been demonstrated to have a positive influence on metabolic syndrome components, cardiovascular disease risk factors, and obesity-related aspects in several epidemiological investigations36,59,60. Reduced inflammation and oxidative stress, increased leptin gene expression, appetite regulation, adipocyte metabolism regulation, and suppression of nuclear factor-B factor are all probable mechanisms36,61,62.
We investigated the nutrigenomic research on BDNF serum because there was no nutrigenetics study in this term. In adult rats, fish oil treatment resulted in a considerable increase in BDNF expression52. Furthermore, administration of whole-grain (WG) rye has been demonstrated to upregulate BDNF levels. When compared to white wheat flour-based bread meals raised BDNF levels by 27% after fasting63. Furthermore, prebiotic feeding elevated the expression of BDNF and peptide YY64. Furthermore, there was a ‘very probable' rise in BDNF levels with protein supplementation65. A Mediterranean-style diet66, omega-3 fatty acids67,68, and even vitamin E and refined flavonoids consumption have all been associated with increased concentrations of brain BDNF. There are some possible mechanisms for these favourable effects in our study and nutrigenomic studies include similar components. Endothelial dysfunction is prevented by a high antioxidant diet, which is linked to reduced levels of pro-inflammatory cytokines in the plasma69–72 Moreover, pro-inflammatory cytokines like IL-6 and TNF- may also suppress BDNF expression73. Compliance to these interventions would be predicted to be related with greater plasma BDNF concentrations under this mechanism. These antioxidant components increase BDNF levels and phosphorylation of the CREB pathway74–76. Although we observed being in BDNF Val/Val wild-type and BDNF Met/Met homozygotes reduced lipid markers and atherogenic indices between homozygotes participant, heterozygotes have shown increased these factors even in high DTAC. These results go beyond previous reports, showing the negative effect of heterozygotes BDNF mic opposite of homozygotes. The heterozygotes BDNF val66met genotype is correlated with cortical morphology that differs from that of BDNF val66met homozygotes. The BDNF Val/Met genotype, in particular, may affect brain tissue volumes and neurodevelopment, resulting in phenotypic differences between BDNF Val/Val wildtype and BDNF Met/Met homozygotes77–79. Interestingly, 5-HT turnover was impaired in heterozygous BDNF+/− mice with lowered BDNF expression and resulting in increased food intake and obese phenotypes80. Kernie et al. found that heterozygous BDNF/– mice with low BDNF mRNA expression gain 300% more body fat and develop obesity than homozygous BDNF/– mice with high BDNF mRNA expression81. Consequently, diet-induced dyslipidemia may be exacerbated by the downregulation of BDNF in heterozygotes individuals. Besides, these discrepancies may be attributed to variations in food choices, fortification, and preferences, which could also impact antioxidant intake through various dietary sources, as well as some changes in antioxidant activity and availability that occur during food processing and preparation82–85. The unfavorable impact found in our study between Val/Met genotypes could be related to a higher intake of high-calorie antioxidant-rich foods and a higher energy intake, since we discovered in our study that greater DTAC consumption was associated with higher carbohydrate intake in diabetic patients86. We did not separate different sources of TAC, also based on previous studies source of TAC may have significant effect on biochemical markers87.
Limitations and strengths
limitations of the present study including the cross-sectional design, so any causality cannot be argued; the use of FFQ for dietary assessing. Due to financial limitations, it was not possible to perform a western blot analysis to determine whether rs6265 SNP alters the expression of BDNF. markers. Furthermore, our participants were from the Iranian country which may not be generalized due to racial and regional differences. Despite the limitations mentioned above, this is the first effort to study the interaction between BDNF Val66Met polymorphism and DTAC on lipid profiles and atherogenic indices. Recognition of these gene-diet interactions could be determining in prescribe personalized nutritional recommendations for the improvement and management of CVD risk in T2DM patients. Finally, these results can be used in combination with a patient’s genetic history to provide more applicable and tailored nutritional advice for preventing or attenuating cardiovascular disease in T2DM patients.
Conclusion
However, this study has several strengths among which it should be emphasized that is the first study of the gene-environment interaction in diabetic patients exploring how BDNF polymorphism (Val66Met) affects the diet in correlation with lipid profiles and atherogenic indices, adding important information to previous studies that assessed dietary habits and lipid markers in diabetic patient’s groups without considering genetic implications. Further functional studies are necessary to confirm the exact mechanism through which this SNP influences food intake regulation.
Method
Study population
The current cross-sectional study was carried out on 667 T2DM patients who were referred from diabetes referral clinics in Tehran, Iran. Our study comprised diabetic patients with fasting blood sugar levels of > 126 mg/dl or who were on glucose-lowering medicines. The complete inclusion and exclusion criteria, demographic, physical activity (METs) information, and anthropometric measurements (body mass index (BMI) and WC), were taken based on our previous larger investigation88. The International Physical Activity Questionnaire (IPAQ) short form was used to assess physical activity. The reliability and validity of the IPAQ has previously been evaluated in Iranian adolescents89. All of the patients were asked to give their informed permission. The study was conducted based on the Declaration of Helsinki, and Ethics Committee of the Tehran University of Medical Sciences approved the protocol (no. 15060).
Biochemical assessments
The study participants' venous blood samples were taken after they had fasted for 12 h. The levels of HDL-C and LDL-C in the blood were measured using a Roche Hitachi analyzer using turbidimetry (Roche, Germany). The ELISA approach was also used to determine the serum levels of leptin and ghrelin (Bioassay Technology Co, China and Mediagnost, Germany, respectively). The number of inflammatory markers in the blood, such as IL-18 and PTX3, was measured using the ELISA method (Shanghai Crystal Day Biotech Co., Ltd). The intra-assay and interassay coefficients of variation (CV) were less than 10% and 12%, respectively, for the IL-18 ELISA kit, which had a sensitivity of 28 ng/l. The intra-assay and interassay CVs were less than 10% and 12%, respectively, for the PTX3 ELISA kit, which has a sensitivity of 0.05 ng/ml. The levels of hs-CRP in the blood were measured using an ELISA kit (Diagnostic Biochem Canada Inc., London, Ontario, Canada). The intra-assay and interassay CVs were both less than 5% and 9.5%, respectively. Both the intra- and inter-assay CVs were less than 5% and 9.5%, respectively. The total antioxidant capacity of the serum was determined using specttrophometry (TAC). The serum enzymatic activity of SOD was measured using a colorimetric method (Cayman Chemical Company, USA). The concentration of 8-isoprostane F2 in the blood was measured using an ELISA (Shanghai Crystal Day Biot). The Nutrition and Genomics Laboratory at TUMS was used to conduct all of the tests.
Atherogenic indices of plasma (AIP) and lipid ratio assessment
The atherogenic indices of plasma were calculated using the logarithmic ratio of (TG to HDL-C) (AIP). Furthermore, Olamoyegun et al. invented the lipid ratio, which is calculated using the following formula: CRI-I = TC/HDL-C, CRI-II = LDL-C/HDL-C, AC = (TC − HDL-C)/HDL-C.
Dietary assessment and DTAC calculation
Dietary data were analyzed using a standard semi-quantitative FFQ that included 147 food categories and was specifically prepared for usage in Iran90. Face-to-face personal interviews with professional dietitians were used to complete the FFQ. Participants were asked to rate the frequency with which they consumed each food item throughout the previous year. Using household measures, the portion sizes of ingested food products were translated to grams per day. The Total Antioxidant Capacity (TAC) is an indicator of overall plasma antioxidant status consisting of four indices: TEAC and TRAP relying on Italian food databases91, FRAP based on Norwegian antioxidant table and reduce ferric iron to ferrous iron92, and ORAC based on United States Department of Agriculture (USDA) databases, that also demonstrates the sample's capacity to exchange hydrogen to stabilize a free radical93.
DNA extraction and gene sequencing
For DNA extraction, a salting-out approach was applied20. The PCR–RFLP method was used to genotype the Val66Met polymorphism. The following primers were used to amplify rs6265: Forward: 5′-CACTAGCCCAGAGAGAGGAGTG-3′, Revers:50-TGAGCCCAGCCGCACACTAAC. 75 ng genomic DNA, 0.6 mM of each primer, and 2X Taq DNA Polymerase Master Mix were included in the final volume of the PCR result (Amplicon; Germany). Denaturation at 95 °C for the 30 s (40 cycles), annealing at 8 °C for 30 s, and 40 s of extension at 72 °C were used in the PCR cycles, with a final extension at 65 °C for 30 min. Finally, the products were electrophoresed on 2% agarose gels. Importantly, 15% of the samples were directly sequenced for confirmation of the PCR–RFLP results. The sequencing process performed using the ABI PRISM 3730 automated sequencer (Applied Biosystems, Foster City, Calif, USA).
Statistical analyses
The Kolmogorov–Smirnov test was used to determine the data's normality. In this study, the sample size was calculated according to following formula: N = (([(Z1 − α + Z1 − β) × √1 − r 2]/r) 2 + 2), whit considering r = 0.15, β = 0.95 and α = 0.05. The Hardy–Weinberg equilibrium (HWE) was assessed with the χ2 test. Based on their FRAP, TRAP, TEAC, and ORAC scores, the subjects were separated into two groups: low and high intakes. Qualitative variables were compared with one-way ANOVA and analysis of covariance (ANCOVA) in crude and adjusted models respectively. Potential interactions between the rs6265 genotype and DTAC on lipid profiles and atherogenic indices were investigated using the generalized linear models (GLMs) model. Age, physical activity, sex, smoking, alcohol, energy intake, lipid, and glucose-lowering medicines, and family history of diabetes were all used as cofounder factors in adjusted analyses. All stages of our research's analysis were conducted using SPSS software (SPSS Inc., Chicago, IL, USA, version 25). A p-value of less than 0.05 was also considered significant.
Ethics approval and consent to participate
The protocol of the study was approved by the ethics committee of TUMS. All participants completed a written informed consent.
Acknowledgements
We would like to thank all participants who gave their time to carry out this project.
Author contributions
F.A.: conceptualization, investigation, methodology, validation, formal analysis, visualization, writing—original draft. F.A. and M.R.: editing. F.K.: funding acquisition, supervision.
Funding
This study was supported by a grant from Tehran University of Medical Sciences (15060).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care. 2013;40:195–211. doi: 10.1016/j.pop.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linton, M. R. F. et al. In Endotext. The Role of Lipids and Lipoproteins in Atherosclerosis (eds Feingold, K. R.) (MDText.com, Inc.; 2000). [PubMed]
- 3.Mahdy Ali K, Wonnerth A, Huber K, et al. Cardiovascular disease risk reduction by raising HDL cholesterol–current therapies and future opportunities. Br. J. Pharmacol. 2012;167:1177–1194. doi: 10.1111/j.1476-5381.2012.02081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upadhyay RK. Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids. 2015;2015:971453. doi: 10.1155/2015/971453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhary MK, Eräranta A, Koskela J, et al. Atherogenic index of plasma is related to arterial stiffness but not to blood pressure in normotensive and never-treated hypertensive subjects. Blood Press. 2019;28:157–167. doi: 10.1080/08037051.2019.1583060. [DOI] [PubMed] [Google Scholar]
- 7.Dobiásová M. AIP–atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr. Lek. 2006;52:64–71. [PubMed] [Google Scholar]
- 8.Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin. Biochem. 2001;34:583–588. doi: 10.1016/S0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 9.Bora K, Pathak MS, Borah P, et al. Association of the apolipoprotein A-I gene polymorphisms with cardiovascular disease risk factors and atherogenic indices in patients from Assam, Northeast India. Balkan J. Med. Genet. 2017;20:59–70. doi: 10.1515/bjmg-2017-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai G, Shi G, Xue S, et al. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine. 2017;96:e8058. doi: 10.1097/MD.0000000000008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang SH, Du Y, Li XL, et al. Triglyceride to high-density lipoprotein cholesterol ratio and cardiovascular events in diabetics with coronary artery disease. Am. J. Med. Sci. 2017;354:117–124. doi: 10.1016/j.amjms.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Abaj F, Rafiee M, Koohdani F. Interaction between CETP polymorphism and dietary insulin index and load in relation to cardiovascular risk factors in diabetic adults. Sci. Rep. 2021;11:15906. doi: 10.1038/s41598-021-95359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abaj F, Sotoudeh G, Karimi E, Rafiee M, Koohdani F. Interaction between the dietary indices and PPAR-γ Pro12Ala gene variants on cardiovascular risk factors in patients with type 2 diabetes mellitus. Int. J. Clin. Pract. 2021;75(8):e14307. doi: 10.1111/ijcp.14307. [DOI] [PubMed] [Google Scholar]
- 14.Hannon BA, Khan NA, Teran-Garcia M. Nutrigenetic contributions to dyslipidemia: A focus on physiologically relevant pathways of lipid and lipoprotein metabolism. Nutrients. 2018;10:1404. doi: 10.3390/nu10101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ordovas JM, Lopez-Miranda J, Mata P, et al. Gene-diet interaction in determining plasma lipid response to dietary intervention. Atherosclerosis. 1995;118:S11–S27. doi: 10.1016/0021-9150(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 16.Ávila-Escalante ML, Coop-Gamas F, Cervantes-Rodríguez M, et al. The effect of diet on oxidative stress and metabolic diseases-clinically controlled trials. J. Food Biochem. 2020;44:e13191. doi: 10.1111/jfbc.13191. [DOI] [PubMed] [Google Scholar]
- 17.Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients. 2019;11:2090. doi: 10.3390/nu11092090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puchau B, Zulet MA, de Echávarri AG, et al. Dietary total antioxidant capacity: A novel indicator of diet quality in healthy young adults. J. Am. Coll. Nutr. 2009;28:648–656. doi: 10.1080/07315724.2009.10719797. [DOI] [PubMed] [Google Scholar]
- 19.Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: Is the Total Antioxidant Capacity the right tool? Redox Rep. Commun. Free Radic. Res. 2004;9:145–152. doi: 10.1179/135100004225004814. [DOI] [PubMed] [Google Scholar]
- 20.Parohan M, Anjom-Shoae J, Nasiri M, et al. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2019;58:2175–2189. doi: 10.1007/s00394-019-01922-9. [DOI] [PubMed] [Google Scholar]
- 21.Cattaneo A, Cattane N, Begni V, et al. The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl. Psychiatry. 2016;6:e958–e958. doi: 10.1038/tp.2016.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 24.Bonaccorso S, Sodhi M, Li J, et al. The brain-derived neurotrophic factor (BDNF) Val66Met polymorphism is associated with increased body mass index and insulin resistance measures in bipolar disorder and schizophrenia. Bipolar Disord. 2015;17:528–535. doi: 10.1111/bdi.12294. [DOI] [PubMed] [Google Scholar]
- 25.Kalenda A, Landgraf K, Löffler D, et al. The BDNF Val66Met polymorphism is associated with lower BMI, lower postprandial glucose levels and elevated carbohydrate intake in children and adolescents. Pediatr. Obes. 2018;13:159–167. doi: 10.1111/ijpo.12238. [DOI] [PubMed] [Google Scholar]
- 26.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen NH, Tarp J, Andersen LB, et al. The association between serum brain-derived neurotrophic factor and a cluster of cardiovascular risk factors in adolescents: The CHAMPS-study DK. PLoS ONE. 2017;12:e0186384. doi: 10.1371/journal.pone.0186384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozanska O, Uruska A, Zozulinska-Ziolkiewicz D. Brain-derived neurotrophic factor and diabetes. Int. J. Mol. Sci. 2020;21:841. doi: 10.3390/ijms21030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daily JW, Park S. Interaction of BDNF rs6265 variants and energy and protein intake in the risk for glucose intolerance and type 2 diabetes in middle-aged adults. Nutrition. 2017;33:187–194. doi: 10.1016/j.nut.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Ma XY, Qiu WQ, Smith CE, et al. Association between BDNF rs6265 and obesity in the Boston Puerto Rican Health Study. J. Obes. 2012;2012:102942. doi: 10.1155/2012/102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farhangi, M. A., Vajdi, M. & Fathollahi, P. Dietary total antioxidant capacity (TAC), general and central obesity indices and serum lipids among adults: An updated systematic review and meta-analysis. Int. J. Vitam. Nutr. Res. 1–17 (2020). [DOI] [PubMed]
- 32.Franzini L, Ardigò D, Zavaroni I. Dietary antioxidants and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:471–476. doi: 10.1097/MCO.0b013e328303be79. [DOI] [PubMed] [Google Scholar]
- 33.Hermsdorff HHM, Puchau B, Volp ACP, et al. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr. Metab. 2011;8:59. doi: 10.1186/1743-7075-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffari H, Daneshzad E, Surkan P, et al. Dietary total antioxidant capacity and cardiovascular disease risk factors: A systematic review of observational studies. J. Am. Coll. Nutr. 2018;37:1–13. doi: 10.1080/07315724.2018.1441079. [DOI] [PubMed] [Google Scholar]
- 35.Puchau B, Zulet M, Echávarri A, et al. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young men. Nutrition. 2009;26:534–541. doi: 10.1016/j.nut.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Mozaffari H, Daneshzad E, Surkan PJ, et al. Dietary total antioxidant capacity and cardiovascular disease risk factors: A systematic review of observational studies. J. Am. Coll. Nutr. 2018;37:533–545. doi: 10.1080/07315724.2018.1441079. [DOI] [PubMed] [Google Scholar]
- 37.Ellulu MS, Patimah I, Khaza'ai H, et al. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarvottam K, Yadav RK. Obesity-related inflammation & cardiovascular disease: Efficacy of a yoga-based lifestyle intervention. Indian J. Med. Res. 2014;139:822–834. [PMC free article] [PubMed] [Google Scholar]
- 39.Bruun J, Stallknecht B, Helge J, et al. Interleukin-18 in plasma and adipose tissue: Effects of obesity, insulin resistance, and weight loss. Eur. J. Endocrinol. 2007;157:465–471. doi: 10.1530/EJE-07-0206. [DOI] [PubMed] [Google Scholar]
- 40.Bariohay B, Lebrun B, Moyse E, et al. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–5620. doi: 10.1210/en.2005-0419. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchida A, Nonomura T, Nakagawa T, Itakura Y, Ono-Kishino M, Yamanaka M, Sugaru E, Taiji M, Noguchi H. Brain-derived neurotrophic factor ameliorates lipid metabolism in diabetic mice. Diabetes Obes. Metab. 2002;4(4):262–269. doi: 10.1046/j.1463-1326.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Ezquerro JD, Rendón-Macías ME, Zamora-Mendoza G, et al. Association between the brain-derived neurotrophic factor Val66Met polymorphism and overweight/obesity in pediatric population. Arch. Med. Res. 2017;48:599–608. doi: 10.1016/j.arcmed.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Morales-Marín ME, Genis-Mendoza AD, Tovilla-Zarate CA, Lanzagorta N, Escamilla M, Nicolini H. Association between obesity and the brain-derived neurotrophic factor gene polymorphism Val66Met in individuals with bipolar disorder in Mexican population. Neuropsychiatr. Dis. Treat. 2016;12:1843. doi: 10.2147/NDT.S104654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skledar M, Nikolac M, Dodig-Curkovic K, et al. Association between brain-derived neurotrophic factor Val66Met and obesity in children and adolescents. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36:136–140. doi: 10.1016/j.pnpbp.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Sustar A, Nikolac Perkovic M, Nedic Erjavec G, et al. A protective effect of the BDNF Met/Met genotype in obesity in healthy Caucasian subjects but not in patients with coronary heart disease. Eur. Rev. Med. Pharmacol. Sci. 2016;20:3417–3426. [PubMed] [Google Scholar]
- 46.Tudor L, Konjevod M, Nikolac Perkovic M, et al. Genetic variants of the brain-derived neurotrophic factor and metabolic indices in veterans with posttraumatic stress disorder. Front. Psychiatry. 2018;9:637. doi: 10.3389/fpsyt.2018.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng JH, Liu CW, Pan SL, et al. Potential unfavorable impacts of BDNF Val66Met polymorphisms on metabolic risks in average population in a longevous area. BMC Geriatr. 2017;17:4. doi: 10.1186/s12877-016-0393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidović V, Maksimović N, Novaković I, et al. Association of the brain-derived neurotrophic factor val66met polymorphism with body mass index, fasting glucose levels and lipid status in adolescents. Balkan J. Med. Genet. 2020;23:77–82. doi: 10.2478/bjmg-2020-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu M, Li R, Zhang Z, et al. Abstract P107: Interactions of the BDNF Val66Met polymorphism, gender, and high-carbohydrate diets on risk factors for cardiovascular diseases. Circ. Res. 2011;109:AP107. doi: 10.1161/res.109.suppl_1.AP107. [DOI] [Google Scholar]
- 50.Froud A, Murphy J, Cribb L, et al. The relationship between dietary quality, serum brain-derived neurotrophic factor (BDNF) level, and the Val66met polymorphism in predicting depression. Nutr. Neurosci. 2019;22:513–521. doi: 10.1080/1028415X.2017.1415281. [DOI] [PubMed] [Google Scholar]
- 51.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vines A, Delattre AM, Lima MM, et al. The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism. Neuropharmacology. 2012;62:184–191. doi: 10.1016/j.neuropharm.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Yu Y, Wang Q, Huang XF. Energy-restricted pair-feeding normalizes low levels of brain-derived neurotrophic factor/tyrosine kinase B mRNA expression in the hippocampus, but not ventromedial hypothalamic nucleus, in diet-induced obese mice. Neuroscience. 2009;160:295–306. doi: 10.1016/j.neuroscience.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 54.Passaro A, Dalla Nora E, Morieri ML, et al. Brain-derived neurotrophic factor plasma levels: Relationship with dementia and diabetes in the elderly population. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:294–302. doi: 10.1093/gerona/glu028. [DOI] [PubMed] [Google Scholar]
- 55.Eyileten C, Kaplon-Cieslicka A, Mirowska-Guzel D, et al. Antidiabetic effect of brain-derived neurotrophic factor and its association with inflammation in type 2 diabetes mellitus. J. Diabetes Res. 2017;2017:2823671. doi: 10.1155/2017/2823671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuchida A, Nonomura T, Nakagawa T, et al. Brain-derived neurotrophic factor ameliorates lipid metabolism in diabetic mice. Diabetes Obes. Metab. 2002;4:262–269. doi: 10.1046/j.1463-1326.2002.00206.x. [DOI] [PubMed] [Google Scholar]
- 57.Molteni R, Barnard RJ, Ying Z, et al. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/S0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 58.Molteni R, Wu A, Vaynman S, et al. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, et al. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puchau B, Zulet MA, de Echávarri AG, et al. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young adults. Nutrition. 2010;26:534–541. doi: 10.1016/j.nut.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Bahadoran Z, Golzarand M, Mirmiran P, et al. Dietary total antioxidant capacity and the occurrence of metabolic syndrome and its components after a 3-year follow-up in adults: Tehran Lipid and Glucose Study. Nutr. Metab. 2012;9:70. doi: 10.1186/1743-7075-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sepidarkish M, Morvaridzadeh M, Akbari-Fakhrabadi M, et al. Effect of omega-3 fatty acid plus vitamin E Co-Supplementation on lipid profile: A systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13:1649–1656. doi: 10.1016/j.dsx.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 63.Sandberg JC, Bjorck IME, Nilsson AC. Increased plasma brain-derived neurotrophic factor 10.5 h after intake of whole grain rye-based products in healthy subjects. Nutrients. 2018;10:1097. doi: 10.3390/nu10081097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savignac HM, Corona G, Mills H, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem. Int. 2013;63:756–764. doi: 10.1016/j.neuint.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brain derived neurotrophic factor response during endurance cycle training Impact of carbohydrate and protein supplementation.pdf.
- 66.Sanchez-Villegas A, Galbete C, Martinez-Gonzalez MA, et al. The effect of the Mediterranean diet on plasma brain-derived neurotrophic factor (BDNF) levels: The PREDIMED-NAVARRA randomized trial. Nutr. Neurosci. 2011;14:195–201. doi: 10.1179/1476830511Y.0000000011. [DOI] [PubMed] [Google Scholar]
- 67.Cysneiros RM, Ferrari D, Arida RM, et al. Qualitative analysis of hippocampal plastic changes in rats with epilepsy supplemented with oral omega-3 fatty acids. Epilepsy Behav. 2010;17:33–38. doi: 10.1016/j.yebeh.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arthur AE, Peterson KE, Shen J, et al. Diet and proinflammatory cytokine levels in head and neck squamous cell carcinoma. Cancer. 2014;120:2704–2712. doi: 10.1002/cncr.28778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di Pietro N, Baldassarre MPA, Cichelli A, et al. Role of polyphenols and carotenoids in endothelial dysfunction: An overview from classic to innovative biomarkers. Oxid. Med. Cell. Longev. 2020;2020:6381380. doi: 10.1155/2020/6381380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J, Lee J, Oh JH, et al. Circulating interleukin-6 level, dietary antioxidant capacity, and risk of colorectal cancer. Antioxidants. 2019;8:595. doi: 10.3390/antiox8120595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Varadharaj S, Kelly OJ, Khayat RN, et al. Role of dietary antioxidants in the preservation of vascular function and the modulation of health and disease. Front. Cardiovasc. Med. 2017;4:64. doi: 10.3389/fcvm.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anisman H. Cascading effects of stressors and inflammatory immune system activation: Implications for major depressive disorder. J. Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 74.Li Q, Zhao HF, Zhang ZF, et al. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159:1208–1215. doi: 10.1016/j.neuroscience.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma. 2004;21:1457–1467. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 76.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 77.Forde NJ, Ronan L, Suckling J, et al. Structural neuroimaging correlates of allelic variation of the BDNF val66met polymorphism. Neuroimage. 2014;90:280–289. doi: 10.1016/j.neuroimage.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 78.Hashimoto T, Fukui K, Takeuchi H, et al. Effects of the BDNF Val66Met polymorphism on gray matter volume in typically developing children and adolescents. Cereb. Cortex. 2016;26:1795–1803. doi: 10.1093/cercor/bhw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu ME, Huang CC, Chen MH, et al. Effect of the BDNF Val66Met polymorphism on regional gray matter volumes and cognitive function in the Chinese population. NeuroMol. Med. 2014;16:127–136. doi: 10.1007/s12017-013-8265-7. [DOI] [PubMed] [Google Scholar]
- 80.Lyons WE, Mamounas LA, Ricaurte GA, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Doherty Jensen K, Holm L. Preferences, quantities and concerns: Socio-cultural perspectives on the gendered consumption of foods. Eur. J. Clin. Nutr. 1999;53:351–359. doi: 10.1038/sj.ejcn.1600767. [DOI] [PubMed] [Google Scholar]
- 83.Wardle J, Haase AM, Steptoe A, et al. Gender differences in food choice: The contribution of health beliefs and dieting. Ann. Behav. Med. 2004;27:107–116. doi: 10.1207/s15324796abm2702_5. [DOI] [PubMed] [Google Scholar]
- 84.Yang M, Chung SJ, Floegel A, et al. Dietary antioxidant capacity is associated with improved serum antioxidant status and decreased serum C-reactive protein and plasma homocysteine concentrations. Eur. J. Nutr. 2013;52:1901–1911. doi: 10.1007/s00394-012-0491-5. [DOI] [PubMed] [Google Scholar]
- 85.Yu L, Gao B, Li Y, et al. Home food preparation techniques impacted the availability of natural antioxidants and bioactivities in kale and broccoli. Food Funct. 2018;9:585–593. doi: 10.1039/C7FO00948H. [DOI] [PubMed] [Google Scholar]
- 86.Gaeini Z, Bahadoran Z, Mirmiran P, et al. Tea, coffee, caffeine intake and the risk of cardio-metabolic outcomes: Findings from a population with low coffee and high tea consumption. Nutr. Metab. 2019;16:28. doi: 10.1186/s12986-019-0355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villaverde P, Lajous M, MacDonald CJ, et al. High dietary total antioxidant capacity is associated with a reduced risk of hypertension in French women. Nutr. J. 2019;18:31. doi: 10.1186/s12937-019-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rafiee M, Sotoudeh G, Djalali M, et al. The interaction between apolipoprotein B insertion/deletion polymorphism and macronutrient intake on lipid profile and serum leptin and ghrelin levels in type 2 diabetes mellitus patients. Eur. J. Nutr. 2019;58:1055–1065. doi: 10.1007/s00394-018-1621-5. [DOI] [PubMed] [Google Scholar]
- 89.Moghaddam MHB, Aghdam F, Asghari Jafarabadi M, et al. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: Content and construct validity, factor structure, internal consistency and stability. World Appl. Sci. J. 2012;18:1073–1080. [Google Scholar]
- 90.Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain intake and the prevalence of hypertriglyceridemic waist phenotype in Tehranian adults. Am. J. Clin. Nutr. 2005;81:55–63. doi: 10.1093/ajcn/81.1.55. [DOI] [PubMed] [Google Scholar]
- 91.Pellegrini N, Serafini M, Colombi B, et al. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003;133:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- 92.Carlsen MH, Halvorsen BL, Holte K, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010;9:3. doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.ORAC ref.pdf.