Abstract
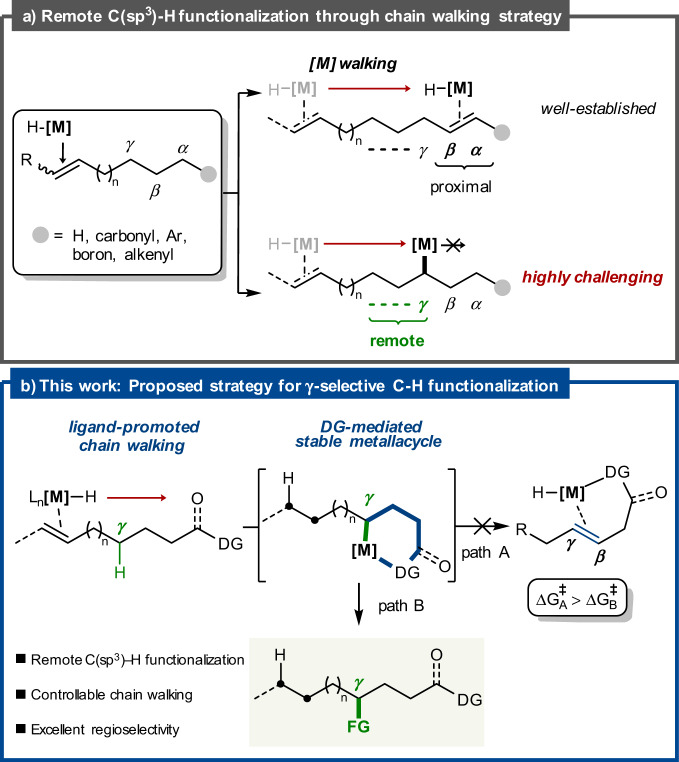
Remote functionalization of alkenes via chain walking has generally been limited to C(sp3)–H bonds α and β to polar-functional units, while γ-C(sp3)–H functionalization through controlled alkene transposition is a longstanding challenge. Herein, we describe NiH-catalyzed migratory formal hydroamination of alkenyl amides achieved via chelation-assisted control, whereby various amino groups are installed at the γ-position of aliphatic chains. By tuning olefin isomerization and migratory hydroamination through ligand and directing group optimization, γ-selective amination can be achieved via stabilization of a 6-membered nickellacycle by an 8-aminoquinoline directing group and subsequent interception by an aminating reagent. A range of amines can be installed at the γ-C(sp3)–H bond of unactivated alkenes with varying alkyl chain lengths, enabling late-stage access to value-added γ-aminated products. Moreover, by employing picolinamide-coupled alkene substrates, this approach is further extended to δ-selective amination. The chain-walking mechanism and pathway selectivity are investigated by experimental and computational methods.
Subject terms: Homogeneous catalysis, Synthetic chemistry methodology
Sequential alkene isomerization and cross-coupling enables remote functionalization, but coupling has been limited to positions at a carbon chain terminus or proximal to a functional group. Here the authors show a method to interrupt isomerization with a nickellacycle, which enables coupling at an atypical, unreactive position.
Introduction
The site-selective C–H functionalization of electronically and sterically similar aliphatic C–H bonds, ubiquitous in organic molecules, represents a powerful disconnection in chemical synthesis1,2. These methods typically require the presence of directing groups in close proximity to the reaction site to govern site selectivity. Recently, migratory cross-coupling reactions for remote C–H functionalization have attracted considerable attention as a powerful alternative strategy that does not require a local directing group3–8. For example, remote olefin functionalization via a combination of transition-metal-catalyzed alkene isomerization and cross-coupling chemistry allows for the direct installation of functionalities into inert sp3 C–H sites that are distant from the alkene initiation point, which would otherwise be difficult to achieve using conventional approaches. Because the driving force of alkene isomerization is the formation of the most thermodynamically favorable alkyl-metal intermediate generated upon chain walking, functionalization with coupling reagents generally occurs at terminal9–20 or the C(sp3)–H bonds α to functional groups such as aryl21–35, vinyl36, oxygen37–39, nitrogen40,41, boryl42,43, and nitrile motifs44. Recently, β-C(sp3)–H functionalization has been achieved by leveraging the haptotropic stability25,45 and electrophilic character of the β-carbon46 in α,β-unsaturated systems or using chelation-assisted strategies47. Despite significant advances in the field of alkene isomerization/functionalization, remote functionalization involving chain walking processes has been restricted to the installation of functional groups at C–H bonds α or β to polar-functional units in the substrate, as chain walking proceeds rapidly to form the most favorable α- or β-alkyl-metal intermediate prior to the cross-coupling process for product formation (Fig. 1a). In this regard, selective functionalization of remote γ-C(sp3)–H bonds through controllable alkene transposition remains a significant unexplored challenge.
Fig. 1. Regioselective migratory hydrofunctionalization of unactivated alkenes.
a Remote C(sp3)–H functionalization through chain walking strategy. b Proposed strategy for γ-selective C(sp3)–H functionalization.
With our objective being the efficient construction of a valuable class of γ-aminated building blocks48, we were intrigued by the possibility of achieving remote γ-selective C(sp3)–H functionalization of unactivated alkenes. The primary challenge in such an approach is the rapid and reversible chain walking process occurring along the alkyl chain to form the most stable conjugated system in such reactions. We reasoned that an alkyl-metal intermediate at the γ-methylene position generated in situ as a result of chain walking must be sufficiently stable to terminate further chain walking49. If successful, the versatility of this strategy would provide a synthetically flexible tool for the direct transformation of unactivated C(sp3)–H bonds at the γ-position to carbon-heteroatom bonds.
Recently, alkene functionalization reactions of alkenyl amides bearing the 8-aminoquinoline (AQ) directing group have been developed, in which stable AQ-chelated metallacycles were found to suppress competing β-H elimination48,50–58. Based on the features of the AQ directing group, we speculated that chain walking along the hydrocarbon chain could be controlled by a chelation-assisted strategy59,60 to dictate the regioselectivity of the olefin isomerization and functionalization site. Specifically, we considered whether NiH-catalyzed alkene isomerization61 induced by an appropriate external ligand could be interrupted by the thermodynamic stability of an AQ-chelated six-membered metallacycle in preference to α,β-unsaturated carbonyl intermediates. The resultant six-membered metallacycle could be subsequently intercepted by coupling partners to realize selective γ-C(sp3)–H functionalization (Fig. 1b).
Herein, we present an γ-C(sp3)–H amination strategy achieved via controlled migratory hydroamination of various δ,ε-, ε,ζ-, and ζ,η-alkene substrates under mild conditions, whereby a range of amino groups are installed at the γ-position with excellent levels of site selectivity. Moreover, unusual δ-selective amination is achieved by employing picolinamide (PA)-coupled alkene substrates to afford synthetically challenging and important chemical building blocks.
Results
Reaction discovery and optimization
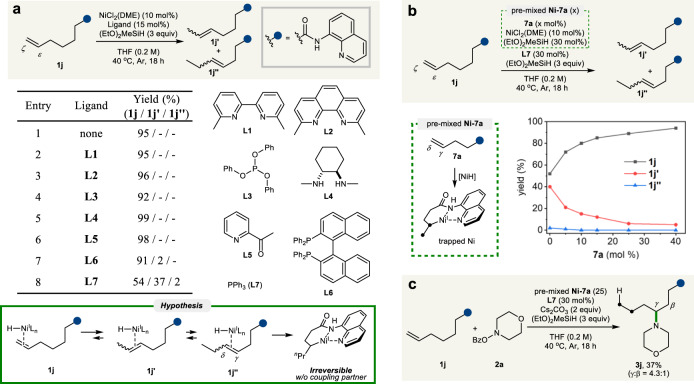
At the outset, we anticipated challenges with the proposed approach because the initial formation of the Ni-bound bidentate directing complex could retard the kinetics of the chain walking process. Therefore, we first explored the feasibility of alkene isomerization using eight-AQ-coupled alkenamide 1j by investigating possible ligand candidates (Fig. 2a). Substituted 2,2′-bipyridine (L1)21–24, 1,10-phenanthroline (L2)34, triphenylphosphite (L3)59, and cyclohexanediamine (L4)43, all of which have previously been employed in nickel-catalyzed migratory functionalization, failed to promote alkene isomerization in the current system. 2-Acetylpyridine (L5)26, used previously in palladium-catalyzed 1,n-arylamination was also tested, but proved to be ineffective. When bisphosphine ligand L6 was used, only a trace amount of the isomerization product was detected. The Zhu group reported that remote C–H amination can proceed at either terminal or benzylic site with excellent levels of regioselectivity based on ligand control, where the use of monophosphine ligand enabled benzylic-selective C–H amination24. Interestingly, the use of triphenylphosphine (L7) led to an improved yield of olefin isomer mixtures arising from olefin isomerization, and 1j′ was obtained in 37% yield, accompanied by the formation of 1j″ in only 2% of yield (Fig. 2a, entry 8).
Fig. 2. Preliminary results.
a Ligand screening for alkene isomerization with 1j; All reactions were performed on a 0.1 mmol scale; Yields were determined by 1H NMR spectroscopy; 30 mol% of L3, L5, L7 were used; THF: tetrahydrofuran. b Sequestration of a nickel catalyst. c Migratory hydroamination with a sequestered nickel catalyst.
Despite extensive optimization using L7, however, our attempts to fully isomerize 1j to the γ,δ-alkene 1j″ were unsuccessful, and only partial conversion was observed at a certain point. Considering this unfavorable outcome, we assumed that the thermodynamically stable Ni-bound six-membered complex formed from in situ generated 1j″ possibly led to the sequestration of the nickel catalyst, thus resulting in cessation of the isomerization process. This consideration prompted us to examine whether the isomerization process could be influenced by the addition of γ,δ-alkenyl amide 7a, which readily forms a chelated six-membered metallacycle (Fig. 2b). Upon testing the hypothesis, we found that the addition of only 5% of 7a significantly inhibited alkene isomerization, and the isomerization process deteriorated in accordance to increasing amounts of 7a. Considering this process, we speculated that the catalytic cycle could be restored by the participation of the coupling partners to deliver the γ-functionalized product and release the free Ni catalyst. Indeed, when O-benzoylhydroxylamine 2a was present as an aminating reagent62–65, we were pleased to find that γ-aminated product 3j was readily obtained as a major product along with about 60% of aminated product formed from 7a in the presence of Ni premixed with 25 mol% of 7a, thus confirming the validity of our conceptual design (Fig. 2c).
Based on these encouraging preliminary results, we next set out to optimize the migratory hydroamination using N-(quinolin-8-yl)-hex-5-enamide (1a), and morpholino benzoate (2a) as the electrophile in the presence of (EtO)2MeSiH as the hydride source (Table 1). After careful evaluation of the reaction parameters, we observed that amination occurred predominantly at the γ-position to deliver the desired γ-aminated product 3a with minimal formation of the β-aminated product (78%, >20:1; entry 1) by employing NiCl2(DME), tris(4-methoxyphenyl)phosphine (L8), and Cs2CO3 (see Supplementary Table 1). Among the solvents screened, the THF/DMA cosolvent system was found to be optimal, with inferior results being obtained with other tested solvents or with the use of only THF or DMA (entries 9 and 10). Of the various nickel precatalysts tested, NiCl2(DME) gave rise to optimal results in terms of both reactivity and selectivity (entry 2). As expected, the presence of the ligand influenced the reaction outcome substantially, and a significantly lower yield was obtained in the absence thereof (entry 3). Our evaluation of the electronic effects of substituted phosphine ligands revealed that electron-poor phosphine ligands, such as tris(4-trifluoromethyl)phosphine (L9), were less effective in this system, resulting in a decrease in reactivity (entries 4 and 5). The use of bisphosphine ligand L6 led to a significantly diminished yield of 3a (entry 6). NiCl2(PPh3)2 was also applicable, being second in order of efficacy (entry 7). Replacement of (EtO)2MeSiH with Ph2SiH2 resulted in a diminished yield (entry 8). Notably, we found that the transformation gave the best results in the presence of Cs2CO3, with other carbonate counter-cations resulting in decreased reactivity and selectivity (entries 11 and 12). Control experiments revealed that the reaction did not proceed in the absence of either catalyst or silane (entry 13).
Table 1.
Optimization of the reaction conditions.
| Entry | Deviation from standard conditions | Yield (%)a | γ:β |
|---|---|---|---|
| 1 | None | 78 (74)b | >20:1 |
| 2 | NiBr2(DME), Ni(acac)2, Ni(COD)2 | 67–77 | 9:1–20:1 |
| 3 | w/o ligand | 24 | >20:1 |
| 4 | L7, instead of L8 | 66 | 10:1 |
| 5 | L9, instead of L8 | 49 | >20:1 |
| 6c | L6, instead of L8 | 34 | >20:1 |
| 7 | NiCl2(PPh3)2, instead of NiCl2(DME), L8 | 66 | 10:1 |
| 8 | Ph2SiH2, instead of (EtO)2MeSiH | 43 | >20:1 |
| 9 | THF only | 72 | 12:1 |
| 10 | DMA only | 59 | 5.7:1 |
| 11 | K2CO3, instead of Cs2CO3 | 22 | 1.8:1 |
| 12 | Na2CO3, Li2CO3, no base | trace | - |
| 13 | w/o catalyst or silane | 0 | - |
Reaction conditions: 1a (0.1 mmol), 2a (0.2 mmol), NiCl2(DME) (10 mol%), L8 (30 mol%), base (0.2 mmol), hydride source (0.3 mmol), and solvent (0.5 mL) at 40 °C under Ar for 18 h.
DMA:N,N-dimethylacetamide.
aYields and product isomer ratios (γ:β) were determined by 1H NMR spectroscopy.
b1 mmol scale.
cLigand (15 mol%) was used.
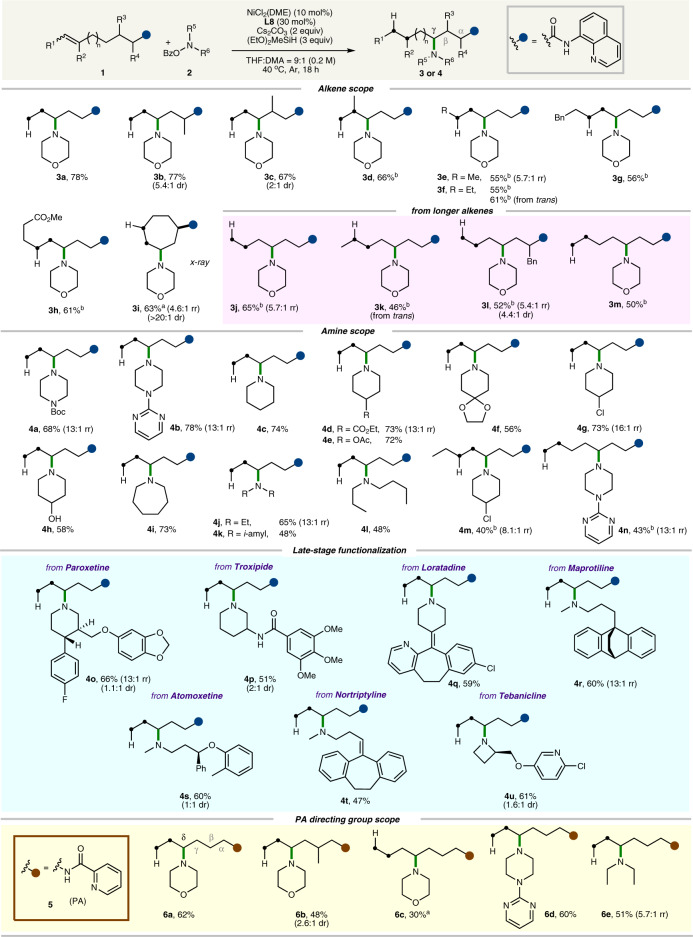
Substrate scope studies
With the optimized conditions in hand, we investigated the generality of the Ni-catalyzed migratory hydroamination. Gratifyingly, a diverse range of alkene structures were found to be compatible with this protocol, providing excellent site selectivity (>20:1 rr if not denoted otherwise) and reactivity, as summarized in Fig. 3. First, δ,ε-unsaturated alkene substrates with diverse methyl substitution patterns afforded the desired γ-selective products with excellent regioselectivity (3b–3e). Importantly, exploration of the alkene scope revealed that not only terminal olefins but also internal olefins engaged in the reaction successfully to afford corresponding products 3e and 3f. In addition, various functional groups on the internal alkene, such as benzyl and ester groups, were well tolerated under the optimized conditions (3g and 3h). The intriguing synthetic value of this protocol was further demonstrated by the distinct preference for the γ-position over the benzylic position in the alkyl chain (3g) without the occurrence of olefin migration into the benzylic position, as previously reported21–35. Employing a δ,ε-cyclic alkene with a cycloheptene motif resulted in the formation of a synthetically useful building block (3i) (confirmed by X-ray crystallographic analysis, see the Supplementary information, Appendix II). The preparation of cyclohept-4-enecarboxylic acid is more efficient than the corresponding γ,δ-unsaturated alkene substrate66. Therefore, the synthetic utility of this approach is highlighted to construct target molecule 3i by employing cyclohept-4-enecarboxylic acid as a substrate. Moreover, expanding the scope from δ,ε-alkenes to a variety of alkenes with an increasingly distal amide functionality broadened the synthetic utility of the current method. For example, the reaction with ε,ζ-alkenes furnished the desired γ-selective products (3j) with excellent regiocontrol. Similarly, ε,ζ-alkenes bearing methyl (3k) and benzyl (3l) groups in the alkane chain also readily participated in this reaction. Importantly, beyond the ε,ζ-alkenes, the current strategy could be extended to ζ,η-alkene substrates with an excellent degree of selectivity for the γ position (3m). We next explored the scope of amine electrophiles and established that the protocol could be successfully applied to various amine sources. Piperazine derivatives bearing a Boc (4a) or pyrimidine (4b) group, often found in biologically active compounds, could be well accommodated in this reaction. Amine sources bearing an ester (4d and 4e) or acetal (4f) group were successfully employed to furnish the desired products. Notably, we observed that amine sources containing a chloride (4g) and hydroxyl (4h) group were well tolerated, providing a handle for further synthetic transformations. In addition, our method could be expanded to substrates containing seven-membered cyclic amine (4i) and acyclic amines (4j–4l) moieties. When primary amine electrophile such as O-benzoyl-N-(tert-butyl)hydroxylamine was used, less than 20% of the desired products were obtained. To further extend the versatility of our reaction, we evaluated the reactivity of an internal δ,ε-alkene, which enabled the incorporation of 4-chloropiperidine (4m) at the γ-position. Furthermore, pyrimidine-bearing piperazine could also be incorporated into the γ-position when an ζ,η-alkene was employed to furnish the corresponding desired product 4n. To further highlight the utility of the present method, we investigated the late-stage modification of complex and biologically active compounds, as outlined in Fig. 3. The amine moiety present in several complex substances could be successfully coupled with δ,ε-alkenyl amides to afford the desired γ-selective products with excellent site selectivity. As this method enables rapid access to various amine-substituted γ-acid derivatives, it provides ready access to medicinally relevant complex γ-amino acid derivatives. Specifically, a series of amine-based pharmaceuticals, including paroxetine (4o), troxipide (4p), loratadine (4q), maprotiline (4r), atomoxetine (4s), and nortriptyline (4t), were efficiently employed to afford the corresponding products with excellent regiocontrol and functional group tolerance. Notably, both aryl fluoride and chloride were found to be compatible during the reactions. Furthermore, the structurally strained azetidine in the analgesic tebanicline could be effectively installed at the γ-position to afford the desired product 4u.
Fig. 3. Substrate scope.
Reaction conditions: 1 or 5 (0.1 mmol), 2 (0.2 mmol), NiCl2(DME) (10 mol%), L8 (30 mol%), Cs2CO3 (0.2 mmol) and (EtO)2MeSiH (0.3 mmol) in THF/DMA = 9:1 (0.5 mL) at 40 °C under Ar for 18 h. cis-Alkenes were used if not denoted otherwise. Yields of isolated γ-product. Isomeric ratios (γ:β) were determined by 1H NMR analysis of the crude mixture (>20:1 rr if not denoted otherwise). a2 (2.5 equiv) was used at 60 °C. bNiCl2(DME), 2 and (EtO)2MeSiH were added at 60 °C in two batches of 5 mol%, 1.25 equiv and 1.5 equiv, respectively.
As amines are ubiquitous in organic molecules, we next sought to extend the scope with respect to amine-derived PA alkene substrates to broaden the utility of the unified protocol. We observed that the current strategy could be successfully applied to ε,ζ-unsaturated PA alkenes to access δ-amination products (6a and 6b) with excellent site selectivity (>20:1) under the standard conditions. Furthermore, a longer ζ,η-alkene substrate bearing a PA group proved suitable, enabling δ-selective amination (6c). In addition, the use of various amine electrophiles, such as pyrimidine-bearing piperazine (6d) and acyclic amine (6e) afforded the corresponding products.
Control experiments and mechanistic studies
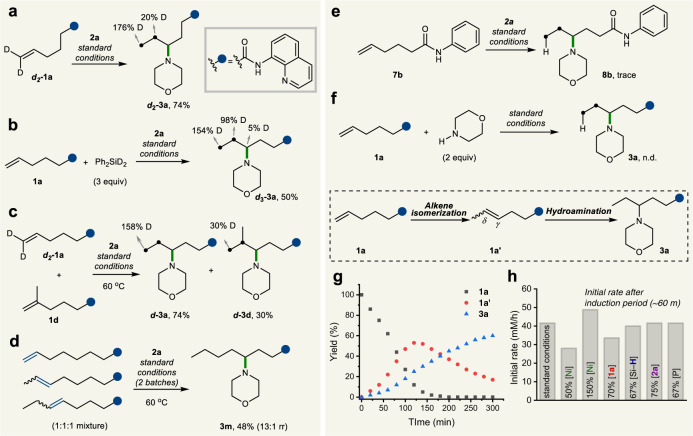
To acquire further insights into the migratory process, a series of deuterium labeling experiments were performed, as shown in Fig. 4a–c. First, when geminally dideuterated alkene d2-1a was subjected to the standard conditions, the incorporation of deuterium at the δ (0.20D) and ε (1.76D) positions was observed (Fig. 4a). Moreover, when an isotope labeling experiment was performed with Ph2SiD2 instead of (EtO)2MeSiH, the corresponding product d3-3a was obtained in 50% yield with deuterium incorporation at the γ (0.05D), δ (0.98D), and ε (1.54D) positions (Fig. 4b). These results collectively support that the NiH catalyst migrates along the alkyl chain via iterative β-H elimination and reinsertion processes. In particular, the sum of deuterium incorporation in d3-3a was above 1.0, giving a strong indication of rapid dissociation and reassociation processes of free NiH/NiD. Subsequently, we conducted a crossover experiment with d2-1a and 1d as alkene substrates to afford deuterium-incorporated products d-3a and d-3d, respectively (Fig. 4c), further supporting the dissociation of NiH/NiD species from the NiH/NiD-alkene complex throughout the relay process in this transformation. The method is highly regioconvergent, and the γ- aminated product 3m (γ:β = 13:1) was obtained from isomeric mixtures of three olefins, as shown in Fig. 4d. When the reaction was performed with a substrate bearing an aniline-derived directing group (7b), only trace amounts of the product could be detected, demonstrating the importance of the bidentate directing group (Fig. 4e). We next confirmed that the free amine species generated via direct reduction under metal hydride conditions67 did not serve as the amine source (Fig. 4f). We next performed kinetic analysis to gain further information about the reaction mechanism (Fig. 4g, h). The time course of the reaction showed alkene isomerization to proceed at a faster rate than the hydroamination process, suggesting that the hydroamination step contributes significantly to the overall reaction rate. Next, we measured the fractional order of NiCl2(DME) and 1a by conducting different-excess experiments68, thus suggesting the off-cycle formation of NiH dimers48 as the catalyst resting state (Supplementary Fig. 11).
Fig. 4. Deuterium labeling studies and control experiments.
a Deuterium migration experiment. b Deuteration with Ph2SiD2. c Crossover experiment. d Regioconvergent experiment. e Monodentate directing group. f Free amine as an coupling partner. g Time profiling experiment. h Summary of different-excess experiment.
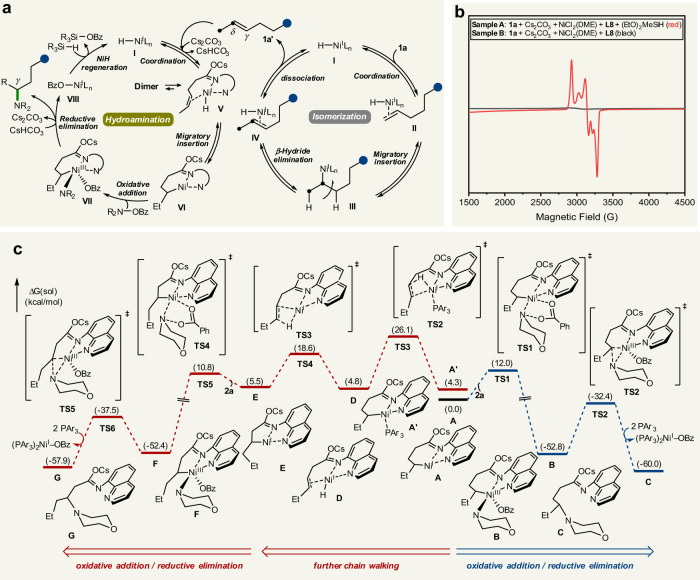
Based on the aforementioned mechanistic studies, a plausible pathway for this remote C–H amination is illustrated in Fig. 5a. Active nickel(I) hydride species I, formed from the combination between the nickel precatalyst and hydrosilane69,70, inserts into the olefin to generate alkyl-nickel intermediate III, which undergoes reversible iterative chain walking along the alkyl chain to eventually form isomeric γ,δ-alkene intermediate IV. At this stage, ligand exchange from the phosphine ligand to the AQ directing group, followed by a migratory insertion, leads to nickellacycle V in preference to other alkyl-nickel species, providing a driving force for chain walking. Controlled by the stability of the AQ-chelated six-membered complex, selective cross-coupling with an aminating reagent readily proceeds through oxidative addition and reductive elimination processes to afford the desired γ-aminated product along with LnNi(I)–OBz (VIII). Active catalyst I is then regenerated by hydrosilane to complete the catalytic cycle for the migratory hydroamination reaction. Our DFT calculations revealed that the oxidative addition of complex A with 2a is a reasonable reaction pathway with a moderate barrier of 12.0 kcal/mol and is exergonic by −52.8 kcal/mol to give rise to thermodynamically stable intermediate B (Fig. 5c). On the other hand, β-hydride elimination of A leading to intermediate D occurs with a higher energy barrier of 26.1 kcal/mol. These results were consistent with the experimentally observed site selectivity.
Fig. 5. Proposed reaction mechanism and DFT calculation result.
a Proposed reaction mechanism. b EPR spectra of paramagnetic Ni(I) complex. c Free energy profile from nickellacyle. Geometry, vibrational frequency, and solvation energy were calculated using the B3LYP-D3 and LACVP**, and B3LYP-D3 and cc-pVTZ(-f)/LACV3P** were employed to determine single-point energy.
In addition to our DFT studies, we conducted EPR experiments to support that Ni(I) species participate in this catalytic system. First, the reaction mixtures in the absence of an electrophile (sample A) resulted in a new EPR signal while this signal disappeared when hydrosilane was not added (sample B), as shown in Fig. 5b. To observe the ligand environment of the Ni(I) species, we further conducted EPR measurements with control sample C (1a', base, NiCl2(DME), and hydrosilane) and sample D (L8, NiCl2, and hydrosilane) (Supplementary Figs. 7 and 8). As expected, the ligand employed influenced the outcome of the EPR spectra. The EPR spectrum of sample C (Supplementary Fig. 8) exhibited a similar signal to that of sample A, suggesting the intermediacy of the Ni(I) complex with 8-AQ under the standard reaction conditions.
Discussion
In summary, we have developed a NiH-catalyzed remote γ-C(sp3)–H amination protocol that proceeds via a controlled migratory hydroamination relay process. A wide range of amines can be selectively installed at the γ-position of various aliphatic chains of alkenes under mild reaction conditions with good functional group tolerance, providing efficient access to a series of value-added γ-aminated products in a late-stage fashion. Chain walking occurs to translocate the alkyl-nickel complex to the γ-position, enabled by AQ directing group stabilization of the six-membered nickellacycle, subsequently intercepted by an aminating reagent. The current method constitutes a unique approach for achieving various γ-C(sp3)–H amination disconnections in organic synthesis. We believe that this study has potential application in the further development of site-selective remote C–H functionalization of significant synthetic utility.
Methods
Representative procedure for the migratory hydroamination of unactivated alkenes
To a flame-dried 12 mL test tube equipped with a Teflon-coated magnetic bar were added N-(quinolin-8-yl)hex-5-enamide (1a) (24.0 mg, 0.10 mmol), morpholino benzoate (2a) (41.4 mg, 0.20 mmol), and tris(4-methoxyphenyl)phosphine (10.6 mg, 0.030 mmol). The test tube was sealed with a PTFE/silicone septa cap, which was pierced by a 22-gauge needle. The sealed test tube was placed into an argon-filled glovebox. In glovebox, Cs2CO3 (65.2 mg, 0.20 mmol) and NiCl2(DME) (2.2 mg, 0.010 mmol) were added to the test tube. The reaction mixture was diluted with THF:DMA = 9:1 (0.5 mL, 0.2 M) and stirred at room temperature for 5 min. After the addition of (EtO)2MeSiH (48.1 μL, 0.30 mmol), the reaction test tube was sealed with a septa cap, and removed from the glovebox. The reaction mixture was stirred at 40 °C for 18 h. The reaction mixture was monitored by TLC using EA:Hx = 1:1 (Rf = 0.3) as the mobile phase. After the disappearance of starting material, the reaction mixture was diluted with 50 mL of ethyl acetate and washed with aqueous NaHCO3 (2 × 25 mL) and brine (25 mL). The combined organic layer was dried over Na2SO4. After removal of solvent, the residue was purified by flash column chromatography on silica gel (EA:Hx = 1:1 to MeOH:EA:Hx = 1:9:10) to give a desired product compound 3a (25.6 mg, 78%) as yellowish oil.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This research was supported financially by Institute for Basic Science (IBS-R010-A2). We thank Dr. Dongwook Kim (IBS) for XRD analysis, and Dr. Jinwoo Kim (IBS), Mr. Jeonguk Kweon (KAIST) for EPR analysis.
Author contributions
C.L., H.S., and J.J. performed the experiments. C.L. conducted computational studies. S.H. directed the project. All authors contributed to the preparation of the manuscript.
Data availability
Experimental procedure and characterization data of new compounds are available within Supplementary Information. Computational details, optimized Cartesian coordinates of all structures, vibrational frequencies, and energy components. This material is available free of charge via the Internet. The X-ray crystallographic coordinate for structure 3i has been deposited at the Cambridge Crystallographic Data Centre (CCDC) under the deposition number CCDC 2071903, and can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other requests for materials and information are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-25696-z.
References
- 1.Bergman RG. C–H activation. Nature. 2007;446:391–393. doi: 10.1038/446391a. [DOI] [PubMed] [Google Scholar]
- 2.Newhouse T, Baran PS. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhungana RK, Sapkota RR, Niroula D, Giri R. Walking metals: catalytic difunctionalization of alkenes at nonclassical sites. Chem. Sci. 2020;11:9757–9774. doi: 10.1039/D0SC03634J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer H, Julia-Hernandez F, Martin R, Marek I. Walking metals for remote functionalization. ACS Cent. Sci. 2018;4:153–165. doi: 10.1021/acscentsci.8b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen‐Müller D, Sahoo B, Sun SZ, Martin R. Tackling remote sp3 C−H functionalization via Ni‐catalyzed “chain‐walking” reactions. Isr. J. Chem. 2019;60:195–206. doi: 10.1002/ijch.201900072. [DOI] [Google Scholar]
- 6.Vasseur A, Bruffaerts J, Marek I. Remote functionalization through alkene isomerization. Nat. Chem. 2016;8:209–219. doi: 10.1038/nchem.2445. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Wu D, Cheng HG, Yin G. Difunctionalization of alkenes involving metal. Migr. Angew. Chem. Int. Ed. 2020;59:7990–8003. doi: 10.1002/anie.201913382. [DOI] [PubMed] [Google Scholar]
- 8.Massad I, Marek I. Alkene isomerization through allylmetals as a strategic tool in stereoselective synthesis. ACS Catal. 2020;10:5793–5804. doi: 10.1021/acscatal.0c01174. [DOI] [Google Scholar]
- 9.Gao Y, et al. Visible-light-induced nickel-catalyzed cross-coupling with alkylzirconocenes from unactivated alkenes. Chem. 2020;6:675–688. doi: 10.1016/j.chempr.2019.12.010. [DOI] [Google Scholar]
- 10.Sun SZ, Borjesson M, Martin-Montero R, Martin R. Site-selective Ni-catalyzed reductive coupling of α-haloboranes with unactivated olefins. J. Am. Chem. Soc. 2018;140:12765–12769. doi: 10.1021/jacs.8b09425. [DOI] [PubMed] [Google Scholar]
- 11.Sun SZ, Romano C, Martin R. Site-selective catalytic deaminative alkylation of unactivated olefins. J. Am. Chem. Soc. 2019;141:16197–16201. doi: 10.1021/jacs.9b07489. [DOI] [PubMed] [Google Scholar]
- 12.Borah AJ, Shi Z. Rhodium-catalyzed, remote terminal hydroarylation of activated olefins through a long-range deconjugative isomerization. J. Am. Chem. Soc. 2018;140:6062–6066. doi: 10.1021/jacs.8b03560. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Qu S, Zhao W. Rhodium-catalyzed remote C(sp3)-H borylation of silyl enol ethers. Angew. Chem. Int. Ed. 2020;59:2360–2364. doi: 10.1002/anie.201913281. [DOI] [PubMed] [Google Scholar]
- 14.Zhou F, Zhu J, Zhang Y, Zhu S. NiH-catalyzed reductive relay hydroalkylation: a strategy for the remote C(sp3)-H alkylation of alkenes. Angew. Chem. Int. Ed. 2018;57:4058–4062. doi: 10.1002/anie.201712731. [DOI] [PubMed] [Google Scholar]
- 15.Gaide T, Bianga J, Schlipköter K, Behr A, Vorholt AJ. Linear selective isomerization/hydroformylation of unsaturated fatty acid methyl esters: a bimetallic approach. ACS Catal. 2017;7:4163–4171. doi: 10.1021/acscatal.7b00249. [DOI] [Google Scholar]
- 16.Buslov I, Song F, Hu X. An easily accessed nickel nanoparticle catalyst for alkene hydrosilylation with tertiary silanes. Angew. Chem. Int. Ed. 2016;55:12295–12299. doi: 10.1002/anie.201606832. [DOI] [PubMed] [Google Scholar]
- 17.Buslov I, Becouse J, Mazza S, Montandon-Clerc M, Hu X. Chemoselective alkene hydrosilylation catalyzed by nickel pincer complexes. Angew. Chem. Int. Ed. 2015;54:14523–14526. doi: 10.1002/anie.201507829. [DOI] [PubMed] [Google Scholar]
- 18.Obligacion JV, Chirik PJ. Bis(imino)pyridine cobalt-catalyzed alkene isomerization-hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 2013;135:19107–19110. doi: 10.1021/ja4108148. [DOI] [PubMed] [Google Scholar]
- 19.Dupuy S, Zhang KF, Goutierre AS, Baudoin O. Terminal-selective functionalization of alkyl chains by regioconvergent cross-coupling. Angew. Chem. Int. Ed. 2016;55:14793–14797. doi: 10.1002/anie.201608535. [DOI] [PubMed] [Google Scholar]
- 20.Julia-Hernandez F, Moragas T, Cornella J, Martin R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature. 2017;545:84–88. doi: 10.1038/nature22316. [DOI] [PubMed] [Google Scholar]
- 21.Xiao J, He Y, Ye F, Zhu S. Remote sp3 C–H amination of alkenes with nitroarenes. Chem. 2018;4:1645–1657. doi: 10.1016/j.chempr.2018.04.008. [DOI] [Google Scholar]
- 22.Zhang Y, Xu X, Zhu S. Nickel-catalysed selective migratory hydrothiolation of alkenes and alkynes with thiols. Nat. Commun. 2019;10:1752. doi: 10.1038/s41467-019-09783-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Cai Y, Zhu S. Mild and regioselective benzylic C–H functionalization: Ni-catalyzed reductive arylation of remote and proximal olefins. J. Am. Chem. Soc. 2017;139:1061–1064. doi: 10.1021/jacs.6b11962. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, He J, Song P, Wang Y, Zhu S. Ligand-enabled NiH-catalyzed migratory hydroamination: chain walking as a strategy for regiodivergent/regioconvergent remote sp3 C–H amination. CCS Chem. 2020;2:2259–2268. [Google Scholar]
- 25.Romano C, Fiorito D, Mazet C. Remote functionalization of α,β-unsaturated carbonyls by multimetallic sequential catalysis. J. Am. Chem. Soc. 2019;141:16983–16990. doi: 10.1021/jacs.9b09373. [DOI] [PubMed] [Google Scholar]
- 26.Han C, et al. Palladium-catalyzed remote 1,n-arylamination of unactivated terminal alkenes. ACS Catal. 2019;9:4196–4202. doi: 10.1021/acscatal.9b00688. [DOI] [Google Scholar]
- 27.Kohler DG, Gockel SN, Kennemur JL, Waller PJ, Hull KL. Palladium-catalysed anti-Markovnikov selective oxidative amination. Nat. Chem. 2018;10:333–340. doi: 10.1038/nchem.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, et al. Nickel-catalyzed remote and proximal Wacker-type oxidation. Commun. Chem. 2019;2:5. doi: 10.1038/s42004-018-0107-y. [DOI] [Google Scholar]
- 29.Lee WC, et al. Nickel-catalysed para-C–H activation of pyridine with switchable regioselective hydroheteroarylation of allylarenes. Chem. Commun. 2015;51:17104–17107. doi: 10.1039/C5CC07455J. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, et al. Migratory arylboration of unactivated alkenes enabled by nickel catalysis. Angew. Chem. Int. Ed. 2019;58:4612–4616. doi: 10.1002/anie.201814572. [DOI] [PubMed] [Google Scholar]
- 31.Meng QY, Schirmer TE, Katou K, Konig B. Controllable isomerization of alkenes by dual visible-light-cobalt catalysis. Angew. Chem. Int. Ed. 2019;58:5723–5728. doi: 10.1002/anie.201900849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Cheng Z, Guo J, Lu Z. Asymmetric remote C–H borylation of internal alkenes via alkene isomerization. Nat. Commun. 2018;9:3939. doi: 10.1038/s41467-018-06240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheuermann ML, Johnson EJ, Chirik PJ. Alkene isomerization-hydroboration promoted by phosphine-ligated cobalt catalysts. Org. Lett. 2015;17:2716–2719. doi: 10.1021/acs.orglett.5b01135. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, et al. Reaction scope and mechanistic insights of nickel-catalyzed migratory Suzuki-Miyaura cross-coupling. Nat. Commun. 2020;11:417. doi: 10.1038/s41467-019-14016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou L, Zhu C, Bi P, Feng C. Ni-catalyzed migratory fluoro-alkenylation of unactivated alkyl bromides with gem-difluoroalkenes. Chem. Sci. 2019;10:1144–1149. doi: 10.1039/C8SC04162H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu R, Rajasekar S, Fang X. Enantioselective nickel-catalyzed migratory hydrocyanation of nonconjugated dienes. Angew. Chem. Int. Ed. 2020;59:21436–21441. doi: 10.1002/anie.202008854. [DOI] [PubMed] [Google Scholar]
- 37.Matt C, Kern C, Streuff J. Zirconium-catalyzed remote defunctionalization of alkenes. ACS Catal. 2020;10:6409–6413. doi: 10.1021/acscatal.0c01605. [DOI] [Google Scholar]
- 38.Romano C, Mazet C. Multicatalytic stereoselective synthesis of highly substituted alkenes by sequential isomerization/cross-coupling reactions. J. Am. Chem. Soc. 2018;140:4743–4750. doi: 10.1021/jacs.8b02134. [DOI] [PubMed] [Google Scholar]
- 39.Ebe Y, Onoda M, Nishimura T, Yorimitsu H. Iridium-catalyzed regio- and enantioselective hydroarylation of alkenyl ethers by olefin isomerization. Angew. Chem. Int. Ed. 2017;56:5607–5611. doi: 10.1002/anie.201702286. [DOI] [PubMed] [Google Scholar]
- 40.Yuan Q, Sigman MS. Palladium-catalyzed enantioselective relay heck arylation of enelactams: accessing α,β-unsaturated delta-lactams. J. Am. Chem. Soc. 2018;140:6527–6530. doi: 10.1021/jacs.8b02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian D, Hu X. Ligand-controlled regiodivergent hydroalkylation of pyrrolines. Angew. Chem. Int. Ed. 2019;58:18519–18523. doi: 10.1002/anie.201912629. [DOI] [PubMed] [Google Scholar]
- 42.Hu M, Ge S. Versatile cobalt-catalyzed regioselective chain-walking double hydroboration of 1,n-dienes to access gem-bis(boryl)alkanes. Nat. Commun. 2020;11:765. doi: 10.1038/s41467-020-14543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Ding C, Yin G. Catalyst-controlled enantioselective 1,1-arylboration of unactivated olefins. Nat. Catal. 2020;3:951–958. doi: 10.1038/s41929-020-00523-8. [DOI] [Google Scholar]
- 44.Zhang C, Santiago CB, Kou L, Sigman MS. Alkenyl carbonyl derivatives in enantioselective redox relay Heck reactions: accessing α,β-unsaturated systems. J. Am. Chem. Soc. 2015;137:7290–7293. doi: 10.1021/jacs.5b04289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohlmann DM, Goossen LJ, Dierker M. Regioselective synthesis of β-aryl- and β-amino-substituted aliphatic esters by rhodium-catalyzed tandem double-bond migration/conjugate addition. Chem. Eur. J. 2011;17:9508–9519. doi: 10.1002/chem.201100654. [DOI] [PubMed] [Google Scholar]
- 46.Yu X, et al. Site-selective alkene borylation enabled by synergistic hydrometallation and borometallation. Nat. Catal. 2020;3:585–592. doi: 10.1038/s41929-020-0470-9. [DOI] [Google Scholar]
- 47.Chen X, Rao W, Yang T, Koh MJ. Alkyl halides as both hydride and alkyl sources in catalytic regioselective reductive olefin hydroalkylation. Nat. Commun. 2020;11:5857. doi: 10.1038/s41467-020-19717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon J, Lee C, Seo H, Hong S. NiH-catalyzed proximal-selective hydroamination of unactivated alkenes. J. Am. Chem. Soc. 2020;142:20470–20480. doi: 10.1021/jacs.0c10333. [DOI] [PubMed] [Google Scholar]
- 49.Shrestha B, et al. Ni-catalyzed regioselective 1,2-dicarbofunctionalization of olefins by intercepting heck intermediates as imine-stabilized transient metallacycles. J. Am. Chem. Soc. 2017;139:10653–10656. doi: 10.1021/jacs.7b06340. [DOI] [PubMed] [Google Scholar]
- 50.Liu Z, et al. Palladium(0)-catalyzed directed syn-1,2-carboboration and -silylation: alkene scope, applications in dearomatization, and stereocontrol by a chiral auxiliary. Angew. Chem. Int. Ed. 2019;58:17068–17073. doi: 10.1002/anie.201910304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Puyl VA, Derosa J, Engle KM. Directed, nickel-catalyzed umpolung 1,2-carboamination of alkenyl carbonyl compounds. ACS Catal. 2018;9:224–229. doi: 10.1021/acscatal.8b04516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang KS, Gurak JA, Jr, Liu Z, Engle KM. Catalytic, regioselective hydrocarbofunctionalization of unactivated alkenes with diverse C–H nucleophiles. J. Am. Chem. Soc. 2016;138:14705–14712. doi: 10.1021/jacs.6b08850. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, et al. Palladium-catalyzed amide-directed enantioselective hydrocarbofunctionalization of unactivated alkenes using a chiral monodentate oxazoline ligand. J. Am. Chem. Soc. 2018;140:3542–3546. doi: 10.1021/jacs.8b00641. [DOI] [PubMed] [Google Scholar]
- 54.Lv H, Xiao LJ, Zhao D, Zhou QL. Nickel(0)-catalyzed linear-selective hydroarylation of unactivated alkenes and styrenes with aryl boronic acids. Chem. Sci. 2018;9:6839–6843. doi: 10.1039/C8SC02101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y, et al. Directed copper-catalyzed intermolecular aminative difunctionalization of unactivated alkenes. J. Am. Chem. Soc. 2019;141:18475–18485. doi: 10.1021/jacs.9b07607. [DOI] [PubMed] [Google Scholar]
- 56.Gurak JA, Yang KS, Liu Z, Engle KM. Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation. J. Am. Chem. Soc. 2016;138:5805–5808. doi: 10.1021/jacs.6b02718. [DOI] [PubMed] [Google Scholar]
- 57.Li M, Kwong FY. Cobalt-catalyzed tandem C–H activation/C–C cleavage/C–H cyclization of aromatic amides with alkylidenecyclopropanes. Angew. Chem. Int. Ed. 2018;57:6512–6516. doi: 10.1002/anie.201801706. [DOI] [PubMed] [Google Scholar]
- 58.Rouquet G, Chatani N. Catalytic functionalization of C(sp2)–H and C(sp3)–H bonds by using bidentate directing groups. Angew. Chem. Int. Ed. 2013;52:11726–11743. doi: 10.1002/anie.201301451. [DOI] [PubMed] [Google Scholar]
- 59.Basnet P, et al. Ni-catalyzed regioselective β,δ-diarylation of unactivated olefins in ketimines via ligand-enabled contraction of transient nickellacycles: rapid access to remotely diarylated ketones. J. Am. Chem. Soc. 2018;140:7782–7786. doi: 10.1021/jacs.8b03163. [DOI] [PubMed] [Google Scholar]
- 60.Li W, Boon JK, Zhao Y. Nickel-catalyzed difunctionalization of allyl moieties using organoboronic acids and halides with divergent regioselectivities. Chem. Sci. 2018;9:600–607. doi: 10.1039/C7SC03149A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lv H, et al. Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation. Nat. Commun. 2019;10:5025. doi: 10.1038/s41467-019-12949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoo EJ, Ma S, Mei T-S, Chan KSL, Yu J-Q. Pd-catalyzed intermolecular C–H amination with alkylamines. J. Am. Chem. Soc. 2011;133:7652–7655. doi: 10.1021/ja202563w. [DOI] [PubMed] [Google Scholar]
- 63.Zhu S, Niljianskul N, Buchwald SL. Enantio- and regioselective CuH-catalyzed hydroamination of alkenes. J. Am. Chem. Soc. 2013;135:15746–15749. doi: 10.1021/ja4092819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miki Y, Hirano K, Satoh T, Miura M. Copper-catalyzed intermolecular regioselective hydroamination of styrenes with polymethylhydrosiloxane and hydroxylamines. Angew. Chem. Int. Ed. 2013;52:10830–10834. doi: 10.1002/anie.201304365. [DOI] [PubMed] [Google Scholar]
- 65.Berman AM, Johnson JS. Copper-catalyzed electrophilic amination of diorganozinc reagents. J. Am. Chem. Soc. 2004;126:5680–5681. doi: 10.1021/ja049474e. [DOI] [PubMed] [Google Scholar]
- 66.Xie C, et al. Catalytic Aza-Wacker annulation: tuning mechanism by the activation mode of amide and enantioselective syntheses of melinonine‑E and strychnoxanthine. Org. Lett. 2018;20:2386–2390. doi: 10.1021/acs.orglett.8b00725. [DOI] [PubMed] [Google Scholar]
- 67.Niu D, Buchwald SL. Design of modified amine transfer reagents allows the synthesis of α-chiral secondary amines via CuH-catalyzed hydroamination. J. Am. Chem. Soc. 2015;137:9716–9721. doi: 10.1021/jacs.5b05446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blackmond DG. Kinetic profiling of catalytic organic reactions as a mechanistic tool. J. Am. Chem. Soc. 2015;137:10852–10866. doi: 10.1021/jacs.5b05841. [DOI] [PubMed] [Google Scholar]
- 69.Cornella J, Gómez-Bengoa E, Martin R. Combined experimental and theoretical study on the reductive cleavage of inert C−O bonds with silanes: ruling out a classical Ni(0)/Ni(II) catalytic couple and evidence for Ni(I) intermediates. J. Am. Chem. Soc. 2013;135:1997–2009. doi: 10.1021/ja311940s. [DOI] [PubMed] [Google Scholar]
- 70.Saper NI, Hartwig JF. Mechanistic investigations of the hydrogenolysis of diaryl ethers catalyzed by nickel complexes of N-heterocyclic carbene ligands. J. Am. Chem. Soc. 2017;139:17667–17676. doi: 10.1021/jacs.7b10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Experimental procedure and characterization data of new compounds are available within Supplementary Information. Computational details, optimized Cartesian coordinates of all structures, vibrational frequencies, and energy components. This material is available free of charge via the Internet. The X-ray crystallographic coordinate for structure 3i has been deposited at the Cambridge Crystallographic Data Centre (CCDC) under the deposition number CCDC 2071903, and can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other requests for materials and information are available from the corresponding author upon reasonable request.