Abstract
Gonad development is a highly regulated process that coordinates cell specification and morphogenesis to produce sex-specific organ structures that are required for fertility, such as testicular seminiferous tubules and ovarian follicles. While sex determination occurs within specialized gonadal supporting cells, sexual differentiation is evident throughout the entire organ, including within the interstitial compartment, which contains immune cells and vasculature. While immune and vascular cells have been traditionally appreciated for their supporting roles during tissue growth and homeostasis, an increasing body of evidence supports the idea that these cell types are critical drivers of sexually dimorphic morphogenesis of the gonad. Myeloid immune cells, such as macrophages, are essential for multiple aspects of gonadogenesis and fertility, including for forming and maintaining gonadal vasculature in both sexes at varying stages of life. While vasculature is long known for supporting organ growth and serving as an export mechanism for gonadal sex steroids in utero, it is also an important component of fetal testicular morphogenesis and differentiation; additionally, it is vital for ovarian corpus luteal function and maintenance of pregnancy. These findings point toward a new paradigm in which immune cells and blood vessels are integral components of sexual differentiation and organogenesis. In this review we discuss the state of the field regarding the diverse roles of immune and vascular cells during organogenesis of the testis and ovary and highlight outstanding questions in the field that could stimulate new research into these previously underappreciated constituents of the gonad.
Keywords: macrophage, morphogenesis, vasculature, sexual differentiation, gonad
Graphical Abstract

Introduction
Sexually dimorphic development of the gonad is essential for successful gametogenesis and fertility. While a great deal of research has gone into studying the mechanisms of sex determination, i.e., identifying the genetic, molecular, or environmental trigger that launches testis- or ovary-specific developmental programs, we know less about sexual differentiation, the process by which male- and female-specific morphology is brought about. Arguably the process of sexual differentiation and its outputs, such as sex-specific gonadal architecture, are more vital to understand, since they are highly evolutionarily conserved among vertebrates (and to some extent invertebrates), despite divergent mechanisms of sex determination among different vertebrate classes [1].
Mammalian gonads (and generally most vertebrate gonads) consist of several major cell types (Fig. 1). Supporting cells, in which sex determination genes such as Sry are specifically expressed [2], comprise the first sex-specific cell type specified in the gonad; these cells are termed Sertoli cells in the testis and granulosa (or pre-granulosa) cells in the ovary. Supporting cells, as the name suggests, nurture gametogenesis upon primordial germ cell colonization of the fetal gonad until the final stages of gametogenesis in adulthood. These cells, in conjunction with germ cells and male-specific peritubular cells or female-specific theca cells, make up the basic functional units of the gonad, which are testicular seminiferous tubules (also called testis cords in fetal stages) and ovarian follicles.
Fig. 1. Architecture of the fetal and adult mammalian gonad.

Cartoon depicting the distribution of different cell types in the mouse E10.5 undifferentiated gonad (top), E13.5 fetal testis (middle left), E13.5 fetal ovary (middle right), adulttestis (bottom left) and adult ovary (bottom right). Cartoons also show fetal gonad morphology and adult gonadal architecture, focusing on seminiferous tubules in the testis and antral follicles and corpus luteum in the ovary, as well as their associated interstitial compartments. Note that structures and cell types are not drawn to scale.
Outside of these gametogenic compartments, there is also an interstitial compartment, whose major responsibility is the production of sex steroids, such as androgens (e.g., testosterone) in the male and estrogen and progesterone in the female. The interstitial compartment contains a wide variety of cell types in both sexes, including steroidogenic cells, smooth muscle, vascular cells, mesenchymal cells such as fibroblasts, and immune cells [3]. Recent studies have shown that the interstitial compartment is an active player in testicular spermatogenesis [4], suggesting that interstitial cells are not merely passive participants in gonadal development and homeostasis. While the interstitial compartment is traditionally appreciated for its steroidogenic function, testis-specific interstitial cell types, such as peritubular cells (also called peritubular myoid cells), vascular endothelial cells, and macrophages, may directly influence spermatogenesis within the seminiferous tubule [5–7]. Steroidogenesis in the testis and ovary is largely performed by Leydig cells in the testicular interstitium and theca cells surrounding ovarian follicles, respectively, although granulosa cells and corpus luteal cells (which are granulosa cells that luteinize after ovulation and produce progesterone) also contribute to sex steroid production in the ovary [8–10].
As compared to the supporting cell gametogenic compartment, less is known about how the interstitial compartment is sexually dimorphic and how the interstitium actively contributes to sex-specific morphogenesis of the gonad. In particular, the interactions between vasculature, immune cells, and the developing gonad are poorly understood, but are likely a fruitful area of research to understand more deeply the mechanisms driving testicular and ovarian differentiation. Here we will discuss the role of immune cells and vasculature in the development and function of the testis and ovary, highlighting previously underappreciated roles for these cell types in development and organogenesis.
Gonadal immune cells
The first immune cells to arise in the embryo are primitive macrophages and are detected in the vicinity of the nascent fetal gonad as early as E10.5 in mice, shortly after the initial specification of the gonadal primordium [11]. Therefore, during sex determination and the initial establishment of sexual dimorphism in the gonad, macrophages are the main immune cell population that can play a role in gonad morphogenesis and differentiation. Given their early appearance within the fetal gonad, together with their dominant presence as the most populous immune cell type in the adult testicular interstitium [12, 13], most research into gonadal immunology has focused on macrophages. However, over the course of fetal development until birth, other myeloid cells, such as monocytes and a small number of eosinophils, but not dendritic cells or neutrophils, are detected in the fetal gonad [14]. It is possible other myeloid cell types such as mast cells, and potentially also lymphoid cell types, play roles in later aspects of postnatal and adult gonad organogenesis. In general, relatively little is known about the role of these other (i.e., non-macrophage) immune cells in testicular and ovarian organogenesis and function.
Macrophages
Macrophage phenotype
Macrophages are the most versatile cells of the immune system, playing critical roles in tissue homeostasis by removing cellular debris and foreign pathogens via phagocytic mechanisms [15, 16]. Macrophages are also involved in innate and adaptive immune responses during organ developmental processes and chronic inflammatory diseases by interacting with other immune cells such as T cells [17–19]. Although macrophage phenotypes are heterogeneous and plastic, characterized by specific cellular surface markers and distinct cytokine secretion, they can be generally classified into two subsets: classically activated, or M1, macrophages (a pro-inflammatory profile) and alternatively activated, or M2, macrophages (an anti-inflammatory profile) [15, 16, 20], although recent trends are moving away from using such a binary system and moving more towards a spectrum of polarization phenotypes. In response to local microenvironmental stimuli and signals, macrophages can rapidly switch their functional phenotype between M1 and M2. The process is defined as macrophage polarization in which the dynamic change of M1/M2 ratio in the reproductive system is highly associated with physiological homeostasis of various tissues, such as the establishment of maternal-fetal tolerance and testicular immune privilege [21–23], as well as pathological changes such as pregnancy complications and ovarian cancer [21, 23–25].
M1 macrophages
M1 macrophages activated by Toll-like receptor (TLR) ligands from pathogens (e.g., lipopolysaccharide (LPS) or Th1 cell products such as interferon gamma, IFN-γ, and tumor necrosis factor alpha, TNFA), express CD80, CD86, inducible nitric oxide synthase (iNOS) and major histocompatibility complex class II receptor (MHCII) surface markers. They can induce the secretion of pro-inflammatory cytokines (interleukin-1α [IL1A], IL1B, IL6, IL12, and TNFA) and chemokines (chemokine [C-X-C motif] ligand 10 [CXCL10], and CXCL11) to further promote M1 polarization [20, 26–28]. In addition, granulocyte-macrophage colony-stimulating factor (GM-CSF), also known as colony-stimulating factor 2 (CSF2), increases the expression of monocyte and macrophage pro-inflammatory cytokines and induces macrophages towards an M1 state [29, 30]. LPS can stimulate CSF2 expression and, in turn, CSF2 priming also promotes LPS-induced release of proinflammatory mediators [31–33].
M2 macrophages
M2 macrophages induced by IL4, IL10, IL13, IL33, transforming growth factor beta (TGF-β) and macrophage colony-stimulating factor (CSF1, also called mCSF) can be identified by the expression of specific markers, such as mannose receptor (CD206/MRC1), CD163, CD36, found in inflammatory zone 1 (FIZZ1), arginase 1 (ARG1), and MAF [16, 23, 28, 34]. IL4 or IL13, as major inducers of M2 polarization, are produced by Th2 lymphocytes, eosinophils, basophils, and mast cells [35]. Activated M2 macrophages regulate immune and inflammatory reactions by secreting many cytokines and chemokines, such as IL10, TGF-β, CCL1, CCL17, CCL18, CCL22, and CCL24 [16, 23], as well as angiogenesis and lymphangiogenesis by producing vascular endothelial growth factor A/C/D (VEGFA/C/D), insulin-like growth factor 1/2 (IGF 1/2), platelet derived growth factor subunit B (PDGFB), and matrix metalloproteinase 2/9 (MMP2/9) [19, 36].
Given the modest overlap of some cell surface markers or secreted cytokines and transient phenotype shift in the process of M1/M2 polarization, it is difficult to discriminate between M1 or M2 macrophages in some cases. Therefore, in the future studies, it is important to find more differentiated macrophage markers in the development of monocytes under physiological and inflammatory conditions by epigenetic, transcriptomic, and proteomic analysis, as well as by using genetically modified mice. In addition, M1/M2 polarization may not fully correspond between in vitro experiments and in vivo situations due to the microenvironmental complexity of organogenesis and tissue homeostasis.
Testicular macrophage populations, from fetus to adult
Tissue-resident macrophages have complex and heterogeneous phenotypes in different tissues where they have both immune and non-immune activities [37]. In general, testicular macrophages (TM) are the most abundant leukocyte type in the testis and represent a large proportion of the interstitial cell population from embryogenesis to postnatal development [11, 13, 38]. In rats, TM were first reported at 19-20 days gestation, but in a subsequent study, they were detected in fetal testes 3 days earlier [39, 40]. A recent study in mice showed that macrophages arise in the urogenital ridge at embryonic day (E) 10.5, upon gonad specification, and increase in number near the gonad-mesonephros border and occasionally within the gonad at E11.5 and E12.5. Most fetal gonadal macrophages have a stellate morphology and strongly express M2 macrophage markers, such as CD206 (MRC1), MAF, and Arg1, suggesting that gonadal macrophages are M2-like in their polarization status [11]. Why fetal gonadal macrophages exhibit an M2-like phenotype is currently unclear. However, there are some possible clues: early gonadal macrophages are derived from primitive yolk sac macrophage progenitors [11], which progressively mature and develop an increased number of dendrites during their migration through bloodstream to infiltrate tissue and are often M2-like within developing organs [41]. Also, bone morphogenetic protein 4 (BMP4) and BMP7, TGF-β superfamily members, which can induce M2 macrophage differentiation [42, 43], are expressed in the fetal gonad before sexual differentiation [44, 45]. These gene expression patterns in the fetal gonad may contribute to a tissue microenvironment conducive to M2 macrophage activation. In addition, there are two macrophage populations from E14.5 until birth found in the testis: F4/80Hi macrophages and F4/80Int macrophages that highly express the M2 marker CD206 and monocyte marker LY6C, respectively [14]. M2 macrophages are generally considered to be immunosuppressive and are associated with vascular and tissue remodeling during development and cancer [37, 46]; therefore, M2 macrophages are well-suited to play an important role in fetal testis vascularization and development.
In the adult testis, most TM express the common tissue-resident macrophage markers F4/80, AIF1 (IBA1), and (C-X3-C motif) receptor 1 (CX3CR1). The heterogeneity of TM in adult mice has been demonstrated based on distinct surface marker expression patterns, anatomical localization, gene expression profiles, and lineage of origin. There are at least 2 TM populations: 1) macrophages located in the interstitium (also called interstitial TM), which highly express CD64 and CSF1R; and 2) macrophages located on the surface of the seminiferous tubules (also called peritubular TM), which express MHCII [7, 14, 47]. A recent study using high-dimensional mass cytometric analyses revealed the presence of a CD206−MHCII− macrophage population, which declines in number during postnatal testis development, with the concurrent emergence of a CD206−MHCII+ macrophage population [14], indicating that CD206−MHCII− macrophages may be the precursor of peritubular TM. TM are implicated in the regulation of spermatogonial development and Leydig cell differentiation and steroidogenesis [7, 11, 48–50]. They also contribute to the establishment of an immunoprivileged niche by exhibiting a M2 macrophage phenotype, which is characterized by the absence of proinflammatory gene expression and high expression levels of many immunosuppressive and M2-type activation factors, such as CD163 in both populations [47]. In the human testis, CD14+CD163+ resident macrophages represent the largest myeloid cell subset and also highly express the M2 macrophage marker CD163 [51]. Although it is well-known that the phenotypes of TM are dynamic and diverse throughout the entire lifecycle, more details of macrophage heterogeneity likely need to be examined.
M2 phenotype of testicular macrophages
Once macrophages colonize organs, specific signals from the local tissue microenvironment control the functional polarization of resident macrophages. One potential major source of organ-specific signals is the testicular interstitial fluid. Testicular interstitial fluid contains numerous immunomodulatory molecules, such as prostaglandins (PGs), steroid hormones (testosterone and corticosterone), and activin and inhibin (which belong to the TGF-β protein superfamily), all of which may contribute to TM phenotype and function [52–54]. Recent data show that prostaglandins PGE2 and PGI2, testosterone, and corticosterone present in rat testicular interstitial fluid can shift the polarization of blood-derived or CSF2-derived M1 macrophages: interstitial-fluid-exposed macrophages polarize toward the M2 macrophage phenotype, likely to perform immunosuppressive functions by inducing CD163 expression and secretion of the anti-inflammatory cytokine IL10, with concomitant low production of TNFA [53, 54]. The production of activin and inhibin by the testis also could be a potent inducer of M2 macrophage activation [16]. Testes from adult mice with elevated activin A levels exhibit an increased number of CD206+MHCII− cells, indicating that activin A does have an effect on M2 macrophage activation [55]. In addition, IL13 produced in the testis induces the expression of the M2 macrophage marker YM1 (official name CHIL3), dependent on IL13R signaling [56]. However, whether the testis can secrete other molecules to govern TM phenotype and function needs to be further explored.
Macrophage populations in the ovary
During mouse embryonic development, ovarian macrophages at E14.5 were comprised of a dominant CD11bIntF4/80Hi population [57], consistent with yolk-sac-derived primitive macrophages that are also observed in the fetal testis and rest of the embryo at that stage [11, 58]. In contrast, CD11bHiF4/80Int cells, a phenotype consistent with definitive macrophages, were dramatically increased in number in the E16.5 ovary to similar levels as CD11bIntF4/80Hi cells [57], indicating a dramatic shift in macrophage populations as was reported in the fetal testis [59]. In addition, CD11bHiF4/80Int macrophages strongly expressed monocyte markers, such LY6C and CCR2, as compared to CD11bIntF4/80Hi macrophages that expressed the M2 macrophage marker CD206. But MHCII, a M1 marker, was not expressed in either macrophage population [57], indicating that fetal ovarian macrophages exhibit an M2-like phenotype. In addition to immunophenotyping, single-cell RNA sequencing has become a widely used technique to uncover cell identity and heterogeneity and to elucidate dynamic processes during tissue development. Single-cell RNA sequencing has revealed clear macrophage populations in fetal mouse (E14.5), monkey (E84 and E116), and human (20-26 weeks) ovaries [60–62]. CD 14+ primitive monocytes and activated MHCII+ monocyte-derived cells were present in human fetal ovaries at 24 weeks of gestation [63], supporting reports of ovarian macrophages in the fetal ovary. However, the immune phenotype of fetal ovarian macrophages in mammals is not well-defined, and their roles in ovarian developmental processes including vascularization, germ cell meiosis, and pre-granulosa cell differentiation and maturation are also unclear.
In the adult mouse ovary, macrophages are also the most abundant immune cell population and widely distributed in the thecal, interstitial, and luteal regions, as well as in the granulosa cell layer of atretic follicles [64–66]. In human ovaries, CD68-positive macrophages are mainly localized near vascular cells and the theca-lutein cell layer of the corpus luteum, while MHCII-positive macrophages reside within corpora lutea [63, 67, 68]. Several studies showed that MHCIIHiCD11b+F4/80+ macrophages are the most common cells among ovarian phagocytes and commonly display flattened cell bodies with dendritic morphology, which is consistent with an activated macrophage phenotype [57, 66]. A small MHCIILoCD206+ macrophage population arises at birth and maintains a stable frequency during postnatal development [57]. However, there is still no direct evidence addressing whether the ovarian macrophage population can be classified as M1 or M2. In a recent study, monocyte recruitment and macrophage alternative activation (M2) have a distinct increase in aged ovaries as compared to young ovaries, suggesting that aging-associated chronic inflammation may cause a shift in ovarian macrophage ontogeny and polarization [69].
Macrophage origins
Tissue-resident macrophages arise embryonically from distinct and successive hematopoietic waves, including yolk sac (YS)-derived primitive macrophages at E7.0-7.5 (in mice), erythro-myeloid progenitors (EMPs) at E8.0-8.5, and aorta-gonad-mesonephros (AGM)-derived early hematopoietic stem cells (HSCs) at E10.5 that migrate to the fetal liver (FL) and generate myeloid progenitors and monocyte-like cells [37, 70–73]. Starting from E16.5, the presumptive bone marrow (BM) gives rise to all lineages of definitive hematopoiesis, including blood monocytes contributing to tissue macrophages [74]. For example, brain microglia and a subset of liver Langerhans cells are exclusively derived from early YS progenitors [70, 75], while large proportions of resident macrophages in the liver, lung, skin, and mammary gland have embryonic origins either from EMPs or FL-derived monocytes [76–79]. Macrophages in the intestine, dermis, and pancreas are maintained by progressive replacement of embryonic macrophages by BM-derived monocytes [80–82]. Therefore, each organ appears to have distinct ontogeny (or ontogenies) for their specific tissue-resident macrophages and have unique kinetics of how macrophages colonize tissues over the lifespan.
Likewise, the origins of resident macrophages in the reproductive organs have been investigated (Fig. 2), albeit in less detail than other organs. In the fetal testis, primitive YS hematopoietic progenitors give rise to the first macrophages that colonize the gonadal-mesonephric region [11], although recent single-cell analyses have revealed that multiple myeloid cell types, likely of different ontogeny, reside in the fetal testis [14]. In the adult testis, interstitial macrophages, in close proximity to Leydig cells, were thought to arise from embryonic progenitors, whereas peritubular macrophages were proposed to be exclusively seeded in the testis starting from postnatal stages, seemingly from BM-derived progenitors [47]. However, two recent studies showed that postnatal and adult BM-derived cells likely contribute minimally to adult testicular macrophage populations [14, 59]; specifically, FL-derived progenitors, rather than YS-derived or BM-derived cells, likely have a dominant contribution to both interstitial macrophages and peritubular macrophages [14]. In adult ovaries, both embryonic and BM-derived macrophages have the capacity to give rise to distinct macrophage subtypes [57]. However, the exact contribution of each of these hematopoietic waves to tissue-resident macrophages in adult life is still debated due to a lack of clarity from lineage-tracing mouse models, such as lack of specific tools to label FL-derived monocytes.
Fig. 2. Model of testicular macrophage origin.
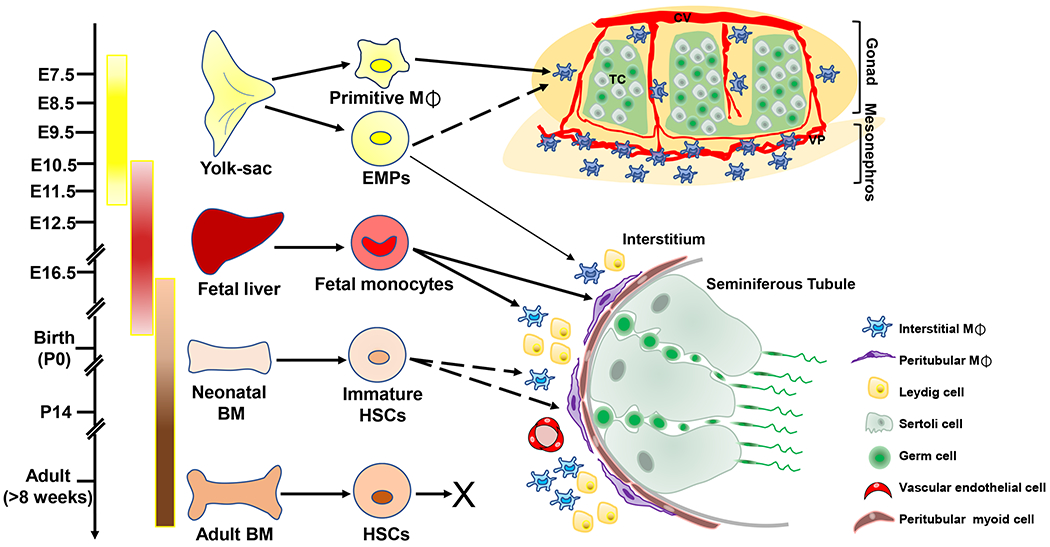
Primitive macrophages generated in the yolk sac (E7.5) give rise to the majority of gonadal–mesonephric macrophages localized near vasculature. EMPs emerge in the yolk sac (E8.5) and exclusively contribute to a small subset of adult interstitial macrophages. Fetal liver-derived monocytes at mid-late fetal stages (E11.5-E14.5) can give rise to two testicular macrophage populations including interstitial macrophages, which are localized in the interstitial compartment in close contact with Leydig cells and blood vessels, and peritubular macrophages, which are localized to the peritubular myoid cell layer. However, adult BM-derived monocytes do not substantially contribute to testicular macrophages. Solid arrows indicate a positive contribution and dotted arrows indicate a currently unclear relationship. BM, bone marrow; EMPs, erythro-myeloid progenitors; HSCs, hematopoietic stem cells; M⌽, macrophage; CV, coelomic vessel; TC, testis cord; VP, vascular plexus.
The roles of macrophages during gonadal angiogenesis and lymphangiogenesis
The complex vascular system composed of blood vessels and lymphatic vessels play essential roles in the transport of oxygen, metabolites, fluids, and circulating leukocytes between tissues and organs [83]. Angiogenesis and lymphangiogenesis are not only associated with many pathological processes such as tissue repair, inflammation, and tumor growth, but are also involved in organogenesis and tissue homeostasis [83, 84]. In these microenvironments, recruited or tissue-resident macrophages are an important source of angiogenic/lymphangiogenic mediators to regulate vessel sprouting and function [19, 85]. Here, we will highlight the roles of macrophages in angiogenesis and lymphangiogenesis during testis and ovarian development.
Role of macrophages during fetal testis vascularization
Macrophages derived from primitive yolk-sac hematopoietic progenitors circulate in the blood and colonize the developing mouse embryo by as early as E9.5 in mice [58, 86], and yolk-sac-derived primitive macrophages are detected in the fetal gonad primordium during initial gonad formation as early as E10.5. Macrophages are localized near developing vasculature in the nascent testis, gradually accumulating from E10.5 to E13.5, specifically near the gonad-mesonephric vascular plexus region at the peak of de novo vascularization and vascular remodeling of the testis (E11.5-E13.5), as well as along the coelomic surface artery and its associated side branches [11]. Fetal gonadal macrophages are in an M2-like polarization status, a state that is linked to tissue remodeling and angiogenesis [11, 87].
Testis vasculature depletion in a whole-organ culture system results in a robust reduction of fetal gonadal macrophages, suggesting that vascularization of the fetal testis may be critical for macrophage migration and/or maintenance. In addition, the depletion of macrophages using a genetic cell-ablation technique leads to a disorganized vasculature and aberrant testis cord formation [11]. The mRNA expression of the proangiogenic factor Vegfa within sorted gonadal macrophages was significantly lower than that of the non-macrophage cell populations of the gonad/mesonephros, suggesting that macrophage-produced VEGFA is unlikely a major source of VEGFA in the gonad [11]. Similarly, during renal organogenesis most F4/80+CD206+ kidney macrophages are perivascular. Macrophage-depleted kidneys at E12.5 exhibit higher numbers of isolated and unconnected endothelial structures and a reduction in the average size of CD31+ (PECAM1+) structures, indicating that macrophages can positively shape the structure of blood vessels in fetal kidneys [88]. However, whether fetal renal macrophages produce proangiogenic factors, such as VEGFA, has not been investigated. Conversely, the CD64HiCD206+ population of cardiac tissue macrophages at E14 and E17 expressing the highest levels of Vegfa mRNA seems to have a potential function in regulating cardiac angiogenesis [89]. Macrophages are also involved in fetal brain angiogenesis independently of VEGF [90]. Together, these studies indicate that macrophages, especially those that exhibit an M2 phenotype during embryogenesis, can regulate the remodeling of vasculature during organogenesis in a VEGFA-dependent or -independent fashion. In terms of fetal gonad development, macrophages play an important role in fetal testis neovascularization.
Effect of macrophages on blood vessels of the adult testis
Although adult interstitial TM are clearly associated with CD31+ interstitial vasculature, the morphology and number of endothelial cells are normal after short-term macrophage depletion [7, 14], suggesting that TM do not seem to have any direct effects on the structure of adult testis vasculature. In other organs, for example, long-term depletion of resident macrophages in the young choroid results in choroidal vascular atrophy and is accompanied by the reduction of Vegfa mRNA expression [91]. Therefore, we speculate that short-term and long-term macrophage depletion may have different outcomes for vascular development. Under pathological conditions, such as in germ cell tumors and Klinefelter syndrome, infiltrating TM play an important role in mediating neovascularization during tumor growth [92–95]. However, whether resident TM in the adult testis have a specific function for testis vasculature needs to be further addressed, and sustained TM depletion is likely necessary to assess their role in vascular development and homeostasis. In addition, resident TM express high levels of Il10 and Il1b [47], which are thought to promote vascular permeability and angiogenesis [96, 97], Therefore, whether IL10 and IL1B are involved in vascular permeability of the adult testis is also unclear.
Role of macrophages on angiogenesis of the ovarian corpus luteum
Angiogenesis is highly associated with ovarian follicular growth and development, as endothelial cells are recruited to the theca layer during latter stages of folliculogenesis [98]. However, other aspects of female reproduction are also reliant on angiogenesis, such as the corpus luteum, which is a transient endocrine organ in the mammalian adult ovary required for the establishment and maintenance of pregnancy by producing progesterone [99]. Similar to developing tumors, the sprouting of preexisting vessels from the follicular theca layer is accompanied by leukocytes. The disruption of angiogenesis inhibits corpus luteum formation, steroidogenesis, and ovulation [98, 100]. Thus, angiogenesis has critical roles in normal luteal function.
Macrophages are the most abundant immune cells within the ovary and significantly increase in number in the developing corpus luteum [101, 102]. Progressive macrophage ablation increases the amount of ovarian hemorrhage in luteal and thecal tissue due to significant endothelial cell depletion and erythrocyte accumulation [103]. Conditional depletion of macrophages during embryo implantation leads to the breakdown of the luteal vasculature and the decrease of ovarian expression of genes that encode vascular endothelial growth factors [104]. Therefore, macrophages play essential roles in the formation and maintenance of luteal vasculature. In epithelial ovarian cancer, tumor-associated macrophages promote angiogenesis and metastasis by inducing the production of IL8 or IGF [105–107]. However, the mechanism underlying how macrophages regulate angiogenesis in normal corpora lutea is still not well-defined. The VEGF, EGF (epidermal growth factor), IGF, or MMP signaling pathways may be considered a bridge between luteal macrophages and angiogenesis. Earlier studies have shown that EGF and IGF can be produced by ovarian macrophages [108, 109]; therefore, macrophages could be considered another potential source of these growth factors in the ovary.
Role of macrophages in testicular and ovarian lymphangiogenesis during development
During embryonic development, lymphatic endothelial cells, a distinct endothelial cell lineage, bud off the embryonic cardinal vein at E9.5-E10.5 in mice [110]. Lymphatic vascular structures are present within many organs after E14.5 through the processes of primary lymphangiogenesis, sprouting, maturation, and terminal differentiation [111, 112]. Lymphatic endothelial cells are characterized by expression of FMS-like tyrosine kinase 4 (FLT4; also called VEGFR3), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), prospero homeobox 1 (PROX1), and the membrane glycoprotein podoplanin (PDPN), which distinguishes them from blood endothelial cells [113]. A recent study using a lymphatic-specific Prox1-EGFP reporter mouse model showed that lymphangiogenesis in the testes begins during late gestation. However, in contrast to other organs, lymphatic vessels are restricted to the testis sheath, or tunica albuginea, from fetus to adult, and unlike blood vessels, do not invade the testicular interstitium [114]. Conversely, ovarian lymphangiogenesis is rare through fetal stages until well after birth. Adult ovaries develop a rich lymphatic network, mainly located in the medulla and in the theca layer of developing follicles [114]. In addition, lymphatic vessels are also abundant in the corpora lutea of mice, rats, and primates [115–117].
VEGFR3 binding its secreted ligand, namely VEGFC or VEGFD, is the main driver of lymphangiogenesis in developmental and pathological contexts [118, 119]. The numbers of LYVE1+ lymphatic endothelial cells in the primate corpus luteum increase from early to mid-luteal phase, associated with the increase of both VEGFC and VEGFR3 mRNA levels [115]. The similar expression trends of VEGFC, VEGFD, VEGFR3, and LYVE1 are also observed in the corpus luteum of buffalo and cow [120, 121]. These data suggest that VEGFC/VEGFD–VEGFR3 interactions may regulate the function of the corpus luteum via lymphangiogenesis. VEGFR3 neutralization inhibits ovarian lymphangiogenesis, leading to the decrease of normal follicular number and disruption of hormone levels during pregnancy [122]. However, the expression and function of lymphangiogenic-related genes within the testis have not been thoroughly investigated to date. It is possible that lymphangiogenesis may be not important for testis development because of the limited lymphatic vessels in testis throughout life. Therefore, fewer studies in the field have focused on testicular lymphangiogenesis.
In addition to angiogenesis, macrophages may produce additional important signals to stimulate developmental and inflammation-induced lymphangiogenesis [19, 123]. Activated macrophages regulate the proliferation of pre-existing lymphatic endothelial cells by secreting lymphangiogenic factors, such as VEGFC and VEGFD [36]. For example, embryonic dermal macrophages expressing VEGFC and VEGFD have the capacity to mediate developmental lymphangiogenesis and lymphatic remodeling [124]. In macrophage-deficient embryos, dermal lymphatic vessels are hyperplastic, but the phenotype in jugular lymph sacs is hypoplastic [125]. Csf1op/op mice, which exhibit a severe reduction in macrophages, exhibit reduced lymphatic branching in postnatal tracheas and ears; however, embryonic lymphangiogenesis seems to be normal [126]. These findings indicate that macrophages may play tissue- or time-dependent roles during developmental lymphangiogenesis. In reproductive organs, the relationship between macrophages and lymphangiogenesis has not yet been explored in depth during development, especially in the ovary, which has extensive lymphatic vessels from birth throughout adulthood. Macrophage depletion or conditional knockout of macrophage-derived genes that are associated with lymphangiogenesis in the ovary will help address this question.
Other immune cell types
Evidence for CD11b+ myeloid cell differentiation into vascular cells
Emerging evidence suggests that macrophages contribute to growing vasculature by transdifferentiating into pericytes (a vascular wall cell type) and vascular and lymphatic endothelial cells. A recent study showed that 30% of pericytes were labeled in E15.5 skin by using a pan-hematopoietic Vav-iCre driver to permanently label hematopoietic-derived cells with a fluorescent reporter. When using a monocyte/macrophage myeloid-specific CD11b-Cre driver to lineage-trace CD11b-derived cells, 15% of pericytes were labeled in E15.5 skin, suggesting that some pericytes are derived from tissue-localized myeloid progenitors in embryonic skin vasculature [127]. Myeloid angiogenic cells (MACs), a subset of monocytes expressing endothelial markers CD31, VEGFR2, and Tie-2 (also called TEK, an angiopoietin receptor), are widely described as endothelial progenitor cells [19, 128]. Although MACs do not differentiate into endothelial cells, they can significantly induce angiogenesis through IL8 secretion [129]. However, recent studies have provided indirect evidence that a large number of endothelial cells in adult blood vessels originate from YS-derived EMPs, which are also a source of tissue-resident macrophages [130], suggesting that endothelial cells of blood vessels have an embryonic hematopoietic origin. In many inflammatory models and tumor environments, macrophages also can transdifferentiate into lymphatic endothelial cells to promote lymphangiogenesis [131–134]. CD11b+ monocytes are considered the main source of lymphatic endothelial cell progenitors in the majority of studies [36]. Although whether macrophages differentiate or transdifferentiate into endothelial cells has not been clearly defined until now, it remains an interesting question. In the ovary and testis, it is also worthy to study whether CD11b+ myeloid cells contribute to pericytes and vascular endothelial cells.
Monocytes
Other than serving as the precursor cell type for definitive macrophages, the exact function of monocytes themselves in normal biology is somewhat unclear. Given their relative abundance in circulating blood, they are proposed to be important sentinels in both pathological and homeostatic states by patrolling vascular and tissue microenvironments [135, 136]. Monocytes have been implicated in regulating angiogenic processes during cancer [137], but in normal contexts, in particular within reproductive tissues, very little is known about monocytes. Monocytes have only recently been identified as a definitive cell population in the fetal and adult testis [14, 59]. Specifically, it was shown that self-renewing monocyte populations, derived during fetal stages independently of circulating blood progenitors, are critical precursors for testicular macrophages [59]; additionally, two recent studies have demonstrated that monocytes from definitive bone-marrow-derived hematopoiesis do not appreciably contribute to testicular macrophages [14, 59], in contrast to a previous study that claimed peritubular macrophages were exclusively derived from bone-marrow-derived circulating progenitors (i.e., monocytes) [47]. Despite classical dogma proposing that monocytes are short-lived circulating cells, there is a precedent for long-lived monocytes in organogenesis: in the fetal and perinatal lung, tissue-resident alveolar macrophages are derived from a stable fetal monocyte population that is long-lived and self-maintains throughout life [77]. In light of the recent discovery of testicular monocytes, more studies should be undertaken to determine any specific functions of monocytes versus macrophages in the testis and the ovary.
Mast cells
Another myeloid cell that has been implicated in testicular function, and more specifically male infertility, is the mast cell. Historically, mast cells are known for their roles in allergy and inflammation [138], but their function within the testis is unclear. Human studies have revealed an increase of mast cells within the testis of infertility patients and a shift of mast cell localization from the interstitium to the seminiferous tubule peritubular wall [139–141], suggesting that inflammation mediated by mast cells, as well as promotion of testicular fibrosis [142], may be an important underlying factor in male infertility. Few studies have focused on the role of mast cells in normal gonadal function, but it has been proposed that specific mast-cell-derived secreted factors, such as tryptase, chymase, and histamine, play roles in multiple aspects of testicular and ovarian function [143–147]. In particular, mast cells have been implicated in testicular steroidogenesis in mice and hamsters [148, 149]; however, molecular mechanisms of this process remain to be elucidated. Further complicating dissection of mast cell function in the testis is the finding that there is great variation in testicular mast cell number among mammalian species [150].
Much less is known about mast cells in the ovary [145]. However, several pieces of evidence indicate that there is a link between hormones and ovarian mast cells, such as hormone-mediated mast cell activation and recruitment, dynamic mast cell number in the ovary at various points of estrus cycle in diverse animal species, and a loss of mast cells in the postmenopausal ovary [151–155]. Of note is the observation that rodent ovaries rarely contain mast cells, unlike human and bovine ovaries, and instead mast cells are mostly limited to the hilum of the rodent ovary [154], indicating potential species-specific functions for ovarian mast cells. Overall, future studies are needed to uncover the specific roles of mast cells in reproductive biology, both in normal and pathological conditions.
Lymphocytes
Lymphocytes are comprised of T cells, B cells, and natural killer (NK) cells, which play vital roles in immune function mainly within the adaptive immune response. While hematopoietic progenitors with lymphoid potential are detected in the mouse yolk sac and embryo even before sex determination takes place [156, 157], definitively committed or differentiated lymphoid cells are not appreciably present in the embryo until around E14.5 [158, 159]. Relatively little is known about the origins and dynamics of lymphocyte colonization in the gonad. Consistent with the kinetics and timing of their differentiation in the embryo, lymphocytes were not detected in the E13.5 fetal mouse gonad [11], and it is not as of yet clear when, or even if, lymphocytes colonize the fetal gonad. Testicular studies of human and other species have reported that, under normal conditions, there are relatively few T cells (as compared to macrophages) and virtually no B cells in the adult mammalian testis [160, 161], likely a result of the immunosuppressed testicular environment.
The function of lymphoid populations in gonad morphogenesis and differentiation has not been definitively addressed. Most research into gonadal lymphocytes has focused on the role of T cells in adult testicular autoimmune pathologies [162, 163] and in ovarian cancer [164]. However, lymphocyte-deficient mice, such as Rag1-mutant males and females that lack mature B and T cells, were reported to be fertile [165, 166], indicating that mature lymphocytes, under normal conditions, are not required for fertility or gonad organogenesis. One caveat is that in-depth studies of reproductive physiology and development of lymphocyte-deficient mice have not been performed, so subtle defects in gonad development or fertility may occur but have not yet been reported.
Roles of vasculature and perivascular mesenchymal stem cells in gonadogenesis
Sex-specific development of vasculature during gonadogenesis
Testes and ovaries arise from a common precursor, the bipotential gonad, which possesses similar primitive vasculature before sex determination in both XX and XY gonads. Despite this common beginning, gonadal vascular formation occurs in a sex-specific pattern. At E11.0, blood vessels are apparent at the gonad-mesonephric border of both XX and XY gonads, but only a few endothelial cells have crossed the border into the gonad [167]. At E11.5, shortly after Sry is expressed in Sertoli cells in the XY gonad, individual endothelial cells released from the breakdown of mesonephric vasculature migrate into the XY gonad and surround developing testis cords [167–171]. Between E11.5-E12.5, mesonephros-derived endothelial cells reaggregate and remodel to form the new testis-specific coelomic artery, concurrent with the formation of testis cords. The establishment of a new testicular arterial network is critical for the export of androgens produced by Leydig cells, as well as for partitioning Sertoli cells into distinct cord structures (see below). At the same time that new testicular surface vasculature is forming, no remnant vascular plexus or large vessels can be observed near the gonad-mesonephric border [169–171]. In contrast, mesonephric vasculature in the XX gonad remains intact, undergoing minimal local reorganization, and mesonephric cells do not migrate into the gonad as observed in the testis. These less dramatic vascular events do not result in any obvious vascular pattern in the early fetal ovary as compared to the fetal testis [171].
Fetal testis vascularization
Expression of Sry in pre-Sertoli cells triggers organogenesis of the testis, which includes de novo vascularization resulting from endothelial cells released from breakdown of mesonephric vasculature and remodeling of these cells into a testis-specific vascular network. There are a number of molecular pathways and cellular players that have been implicated in vascular development of the testis (Fig. 3):
Fig. 3. Different pathways associated with fetal testis vascularization.

This diagram represents the six key players involved in vascularization during development of the fetal testis. Dashed line indicates areas needing further study. Arrows indicate a positive influence and T-shaped lines indicate an inhibitory effect. WNT, wingless-type MMTV integration site family, member 4; FST, follistatin; SRY, sex determining region on Y chromosome; FGF9; fibroblast growth factor 9; SOX9, SRY (sex determining region Y)-box 9; AMH, anti-Mullerian hormone; VEGFA, vascular endothelial growth factor a; FLT1, VEGFR1; KDR, VEGFR2; PDGFB, platelet-derived growth factor b; PDGFRA/B, platelet-derived growth factor receptor a/b; PROKR2, Prokineticin receptor 2; CDH5, cadherin 5/VE-cadherin.
Vascular endothelial growth factor (VEGF)
VEGF is a likely candidate for the regulation of angiogenesis by stimulation of endothelial cell proliferation and migration through the activation of its receptors, tyrosine kinases FLT1 (VEGFR1) and KDR (FLK/VEGFR2) [172]. In XY gonads, Vegfa, encoding a VEGF ligand, is expressed throughout the XY gonad, especially in the coelomic surface domain, whereas it is absent in the coelomic domain of XX gonads. Both VEGF receptors, FLT1 and KDR, are expressed within microvasculature and the male-specific coelomic artery. Systemic inhibition of VEGF signaling is embryonic lethal, as systemic mutations of either VEGF receptor are lethal prior to testis differentiation (i.e., well before E11.5 in mice) [173, 174]. BV13, a monoclonal blocking antibody directed to the extracellular region of CDH5 [175], or VEGF-Trap (aflibercept), a specific reagent that contains critical domains of FLT1 and KDR that bind and inhibit secreted VEGFA [176, 177], are used in ex vivo organ culture methods to overcome the early embryonic lethality and/or rapidly induced lethality in vivo of systemic or conditional mutations in genes encoding VEGF receptors. A Vegfa-lacZ reporter allele and cell-type-specific gene expression profiling indicated that VEGFA is likely mostly secreted from the interstitial mesenchyme in the fetal testis, suggesting an indirect influence of vasculature on cord formation. Inhibiting VEGFA activity (via VEGF-Trap) blocks the migration of mesonephros-derived endothelial cells, resulting in a strong blockade of male-specific vascular development. Real-time live imaging of whole XY gonads showed that vascular migration triggers the expansion of interstitial mesenchymal cells at the coelomic surface. Following BV13 treatment in XY gonads at E11.5, interstitial cells failed to proliferate, which is required for the expansion of the coelomic domain [176]. It is now clear that endothelial cells influence interstitial mesenchymal cell proliferation, though the molecular mechanism requires further clarification.
Platelet-derived growth factor (PDGF)
Vessel-derived PDGFs and their receptors PDGFRA and PGFRB (encoded by Pdgfra and Pdgfrb) have important roles in migration, proliferation, and differentiation of cells during embryonic development [178]. Generally, PDGFA, PDGFB, and PDGFC, are expressed in Sertoli cells, endothelial cells, and mesonephros/gonad boundary in XY gonads, respectively [179]. Although PDGFA is strongly expressed in Sertoli cells within testis cords, Pdgfa-null embryos show normal fetal testis morphology [180], indicating that Pdgfa is either dispensable or is redundant with other PDGF ligands. Both Pdgfra and Pdgfrb are weakly expressed in the mesenchyme of the mesonephros and are expressed in the coelomic region of E11.5 XY gonads. Later, they are strongly expressed in the mesenchyme of the gonadal interstitium [179]. Pdgfra null mutation leads to defects in testis cord formation and vascular development in XY gonads, whereas knockdown of Pdgfrb does not result in overt defects in early XY or XX gonad development [179]. BV13 treatment in XY gonads results in a slight reduction of Pdgfb expression instead of Pdgfra. The addition of recombinant PDGF-BB rescued mesenchymal cell proliferation after inhibition of VEGFA or blockade of endothelial cells, although testis cord aggregation was not evident [176]. Taken together, these reports demonstrate the failure of interstitial proliferation in the absence of vasculature might be due to defects in PDGF signaling.
Macrophages
Macrophages are among the most prevalent cells within the testicular interstitial compartment. Various cell ablation models targeting macrophages have revealed two important functions of testicular macrophages: testicular Leydig cell steroidogenesis [181] and the self-renewal or differentiation of spermatogonial stem/progenitor cells (SSCs) [7]. Initial studies revealing critical roles for macrophages utilized Csf1 mutant mice, which have reduced macrophage number in most tissues. Csf1 mutant mice showed reductions in mating capability, low sperm count, and 90% lower serum testosterone levels, suggesting that testicular macrophages are vitally important for male reproductive function [182, 183]; additionally, CSF1 has been implicated as an extrinsic factor that promotes self-renewal of spermatogonial stem cells in vitro [184]. However, given the widespread loss of macrophages throughout the body in most targeting strategies, it is still somewhat unclear how much of the deleterious effects of macrophage depletion (and Csf1 loss) upon reproductive function is due to local (i.e., testis-specific) versus systemic loss of macrophages.
Macrophages are initially localized near the developing vascular plexus in the bipotential gonad and have been implicated in vascular remodeling. Depletion of fetal testis macrophages via a macrophage-specific Cx3cr1-mediated cell ablation method, instead of Csf1 mutation, thus avoiding compensation of maternal circulating CSF1 [185], resulted in disorganized surface vasculature and aberrant testis cord architecture (see also above in “Role of macrophages during fetal testis vascularization” section) [11]. These findings indicate that macrophages play an important role in testis-specific vascularization and morphogenesis.
Activins
Activins, consisting of either homodimers or heterodimers of inhibin beta A (INHB A) and beta B (INHBB) subunits (activin A=beta A:beta A, activin B=beta B:beta B, and activin AB=beta A:beta B), are involved in the patterning and organogenesis of vertebrate embryos, including within the reproductive organs. Inhbb expression is similar in XY and XX gonads at E11.5, at the onset of testis-specific vascularization, but is down-regulated by the WNT/CTNNB1 (β-catenin)/follistatin (FST) pathway in the XX gonad at E12.5 [186, 187], Knockout of Inhbb shows defects of testis-specific vasculature in the XY gonad, with varying degrees of vascular malformation and decreased vascular branching. Inhbb knockout in XX gonads, in the context of XX Wnt4- and Fst-knockout gonads that normally exhibit ectopic male-like vasculature, leads to reduced ectopic vasculature, indicating that Inhbb function is required in XX Wnt4- and Fst-knockout gonads for the ectopic formation of testis-like vasculature. Furthermore, exogenously administered activin B is able to induce ectopic testis-like vasculature in the ex vivo cultured wild-type fetal ovary, indicating that activin B contributes to coelomic vessel formation independent of the SRY pathway [187]. Ctnnb1 conditional knockout or Wnt4 mutant XX gonads, as well as fibroblast growth factor 9 (FGF9)-treated cultured XX gonads (which stabilizes SOX9 expression in XX gonads), also show ectopic male-like vasculature, indicating that vascular remodeling is part of normal testicular development and must be actively repressed by the ovarian developmental program [188–191].
Prokr2
Prokineticin receptor 2 (Prokr2), which represents 1 of the 2 receptors of the prokineticin signaling pathway [192], is associated with gonadotropin releasing hormone (GnRH)-neuron migration [193] and endothelial vessel integrity and permeability [194]. Prokr2 displays male-specific expression in interstitial cells of the developing testis, but not in ovaries. Prokr2 is significantly up-regulated starting at E11.5, and its expression peaks at E12.5, corresponding with the critical time of vascular remodeling in fetal testis. Loss of Prokr2 specifically compromises the integrity of the testis vasculature at E16.5 [195], suggesting that Prokr2 promotes fetal testis vascularization.
Significance of vasculature for testis development
Between E11.5-12.5, Sertoli cells surround germs cells to form testis cords [196]. Several studies revealed that vascular endothelial cells drive testis cord formation [171, 175, 176]. Time-lapse organ culture experiments demonstrated that mesonephros-derived endothelial cells migrate into the gonad within restricted tracts, thus subdividing Sertoli cells into presumptive testis cords before they are morphologically distinct [171]. Vegfa is expressed specifically by the undifferentiated mesenchyme, and its receptor tyrosine kinases, KDR and neuropilin 1 (NRP1), are expressed in the male-specific coelomic vessel and microvasculature of the gonad [176]. VEGF pathway inhibitor (VEGF-Trap) and CDH5 (VE-cadherin) blocking antibody (BV13) experiments, which disrupted initial vascular remodeling starting at E11.5, revealed that vascular-mesenchymal interactions initiate testis cord morphogenesis [175, 176]. However, later vascular ablation at E12.5 did not affect existing testis cord structure [197]. Taken together, these results indicate that vasculature is essential for the initiation of testis cord formation but is dispensable for the maintenance of testis cord architecture (Fig. 4).
Fig. 4. Roles of vasculature in fetal testis morphogenesis and differentiation.

Model of sex-specific vascular development during gonadogenesis and illustration of the mechanisms through which vasculature influences fetal testis development. Note that little is known about: vasculature’s role in fetal ovarian organogenesis; the role of perivascular cells in the fetal ovary; and whether active Notch signaling is involved in ovarian organogenesis, tc, testis cord.
Even though vascular disruption in ex vivo culture experiments did not induce hypoxia and did not affect testis cord morphology and Sertoli cell differentiation, it did result in excessive Leydig cell differentiation within the E12.5 XY gonad [197]. These interactions are likely mediated by cell-cell contact between endothelium and mesenchymal cells, which are required for Notch signaling. In mammals, the Notch signaling pathway mainly consists of Notch receptors (NOTCH1, NOTCH2, NOTCH3, and NOTCH4), Notch ligands (Delta like (DLL) 1 (DLL1), DLL3, DLL4, Jagged (JAG) 1 (JAG1), and JAG2) and intracellular effector molecule (CBF1, officially named RBPJ (recombination signal binding protein-j)) [198, 199]. Notch family member genes normally associated with angiogenesis [198], such as Dll4, Jagged2, Notch1, and Notch4, are specifically enriched in endothelial cells of the XY gonad [200, 201], Overexpression of the NOTCH1 intracellular domain in the XY gonad results in a loss of Leydig cells but does not affect Sertoli cells [202]. Notch2 is enriched in Sertoli cells and interstitial cells at E12.5 and E13.5, while Notch3 is only slightly expressed in interstitial cells. In addition, Jagged1 is specifically expressed in vasculature-associated cells in the interstitium of the fetal and postnatal testis. However, Dll1 and Dll3 are not detectable in the fetal gonad [200].
Active Notch signaling (via the receptor NOTCH2) in perivascular mesenchymal cells, driven by endothelial Notch ligands (likely DLL4), maintains interstitial mesenchymal progenitor cells, and restricts their differentiation into fetal Leydig cells [197, 202, 203]. Blocking Notch signaling via the γ-secretase inhibitor DAPT results in a failure of progenitors to either self-renew or maintain themselves in an undifferentiated state in XY gonads [197, 202]. Disruption of vasculature results in loss of active Notch signaling in the interstitium but does not affect active Notch signaling in Sertoli cells [197], indicating that Notch signaling between vasculature and mesenchyme regulates the Leydig progenitor niche. While Notch signaling has been well-characterized as a regulator of endothelial cell differentiation and angiogenesis [198], studies of ex vivo inhibition of Notch signaling in the fetal testis have not reported any defects in testis-specific vascular development [197, 202]. These findings indicate that during early testicular differentiation, communication via Notch is largely unidirectional from the endothelium to the mesenchyme. However, it is possible, and perhaps likely, that Notch signaling is required for gonadal vascular development prior to or after stages examined (E11.5-E13.5) in previous studies.
Nestin, an intermediate filament protein that marks many stem/progenitor populations [204], is expressed adjacent to endothelial cells in both fetal and adult testes [197, 205], indicating an interaction between vasculature and perivascular cells. A recent study revealed an established function of fetal testicular vasculature in providing a specialized niche that controls the maintenance and differentiation of perivascular Nestin+ stem cells, which in the fetal testis are a multipotent progenitor population that gives rise to Leydig cells, smooth muscle cells, and pericytes. Following vascular disruption in E12.5 XY gonads, which does not have an influence on testis cord morphogenesis at that stage, Nestin+ cells, which were regulated via active Notch signaling, failed to be maintained and excessively differentiated into Leydig cells [197]. In all, blood vessels play a critical role in regulating specialized microenvironments that are directly associated with testis development. Therefore, it appears that vasculature regulates the balance between maintenance and differentiation of Leydig cell progenitors in the fetal testis (Fig. 4).
Perspectives
Blood vessels and immune cells have been classically defined by their supportive roles during organ development and homeostasis. However, an increasing body of evidence supports the emerging idea that blood vessels and immune cells are important developmental regulators of tissue differentiation and cell specification. Here we have outlined some examples of how these constituents of the gonadal interstitial compartment contribute to sex-specific organ morphogenesis and function. To gain a deeper understanding of the role of these cells in organogenesis of the reproductive organs, several areas of investigation will likely lead to new discoveries revealing the diverse functions of vascular and immune cells.
With regard to immune cells, it is critical to address the mechanism underlying how macrophages promote vascular remodeling during fetal testis morphogenesis: is it due to a co-opting of angiogenic pathways, similar to what it is seen during tumorigenesis? Do macrophages degrade existing vasculature via their well-known phagocytic capacity? Is the extracellular matrix modified in a sex-specific manner by macrophages either physically or biochemically? The diversity of macrophage phenotypes uncovered by developmental biologists and immunologists suggest that macrophages can act in a multitude of ways. This diversity in macrophage phenotype may be due to their various potential cellular ontogenies, such as yolk sac versus fetal liver versus bone-marrow-derived origins. These distinct origins may impart unique characteristics to cells, but more likely the tissue microenvironment may drive specific macrophage identities. For example, do different adult testicular macrophage populations have distinct origins, and how are their cellular functions unique, for example, regulating other interstitial cells (e.g., vasculature, Leydig cells) versus spermatogenic cells?
Another outstanding question that needs to be addressed is the sex-specific nature of immune cells in the gonad. Do they have similar origins and kinetics of tissue colonization and turnover in the testis versus the ovary? What cytokines and chemokines do testis and ovarian macrophages release (or respond to?) to influence angiogenesis and lymphangiogenesis in the gonad? Do macrophages regulate ovarian blood and lymphatic vessels to support follicular growth and development; given the synchronous increase of all three cell types from birth to adulthood, how are they linked to one another? Addressing all these questions will likely require in-depth high-throughput techniques, such as single-cell studies of the transcriptome, epigenome, and proteome. Overall, this review has highlighted a particular lack of knowledge regarding fetal ovarian macrophages and their contributions to ovarian organogenesis and function relative to their testicular counterparts.
Regarding vasculature’s role in the fetal testis and its contributions to testis morphogenesis and differentiation, there are several outstanding questions in the field have not been addressed, e.g., identifying disrupted genes and pathways in mesenchymal and other cell types after vascular depletion in the fetal testis, or the role of endothelial-derived cytokines and growth factors in these processes. In contrast to the testis, our knowledge of the role of vasculature and perivascular cells in ovarian development is relatively limited, representing a major knowledge gap in the field. There are several areas of future research that will likely be productive, including examining the molecular mechanism of vascularization in the fetal ovary, elucidating interactions between endothelium and mesenchymal cells during ovarian organogenesis, and identifying how vasculature influences breakdown of oocyte cysts and follicle formation during ovarian development.
All of the above questions will help us understand the previously underappreciated roles of immune and vascular cells in gonadogenesis and fertility. Given that no organ exists in a vacuum, complex cellular interactions in the gonad likely exist on both local and systemic axes. Knowledge that we can gain about various gonadal cell types will likely prove useful to understand and treat medical conditions of the reproductive system such as gonad dysgenesis and infertility.
Acknowledgements
Work from the DeFalco lab is supported by the National Institutes of Health (R35GM119458 and R01HD094698 to TD) and Lalor Foundation (postdoctoral fellowship to SL).
Abbreviations:
- AGM
aorta-gonad-mesonephros region
- ARG1
arginase 1
- BM
bone marrow
- BMP
bone morphogenetic protein
- CD
cluster of differentiation
- CDH5
cadherin 5 (VE-cadherin)
- CSF1
macrophage colony-stimulating factor
- CTNNB1
catenin (cadherin associated protein), beta 1 (β-catenin)
- CX3CR1
chemokine (C-X3-C motif) receptor 1
- CXCL
chemokine (C-X-C motif) ligand
- DLL
delta like canonical Notch ligand
- EDS
ethane dimethanesulphonate
- EGF
epidermal growth factor
- EMPs
erythro-myeloid progenitors
- FGF9
fibroblast growth factor 9
- FL
fetal liver
- FLT1
FMS-like tyrosine kinase 1
- FST
follistatin
- GM-CSF
granulocyte-macrophage colony-stimulating factor (CSF2)
- GnRH
gonadotropin releasing hormone
- HSCs
hematopoietic stem cells
- IGF
insulin-like growth factor
- IL
interleukin
- INHBA
inhibin beta A
- INHBB
inhibin beta B
- KDR
kinase insert domain protein receptor (VEGFR2)
- LPS
lipopolysaccharide
- LYVE1
lymphatic vessel endothelial hyaluronan receptor 1
- MACs
myeloid angiogenic cells
- M1
classically activated macrophage
- M2
alternatively activated macrophage
- MHCII
major histocompatibility complex class II receptor
- MMP
matrix metalloproteinase
- PDGF
platelet-derived growth factor
- PG
prostaglandin
- PROKR2
prokineticin receptor 2
- PROX1
prospero homeobox 1
- RBPJ
recombination signal binding protein for immunoglobulin kappa J region
- SOX9
SRY (sex determining region Y)-box 9
- SRY
sex determining region of chromosome Y
- SSCs
spermatogonial stem cells
- TGF-β
transforming growth factor beta
- TLR
Toll-like receptor
- TM
testicular macrophages
- TNFA
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- WNT4
wingless-type MMTV integration site family, member 4
- YS
yolk sac
Footnotes
Conflicts of interest
None to report.
References
- 1.DeFalco T & Capel B (2009) Gonad morphogenesis in vertebrates: divergent means to a convergent end. Annu Rev Cell Dev Biol 25, 457–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht KH & Eicher EM (2001) Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol 240, 92–107. [DOI] [PubMed] [Google Scholar]
- 3.Svingen T & Koopman P (2013) Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev 27, 2409–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potter SJ & DeFalco T (2017) Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction 153, R151–R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhang DH, Kim BJ, Kim BG, Schadler K, Baek KH, Kim YH, Hsiao W, Ding B S, Rafii S, Weiss MJ, Chou ST, Kolon TF, Ginsberg JP, Ryu BY & Ryeom S (2018) Testicular endothelial cells are a critical population in the germline stem cell niche. Nat Commun 9, 4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LY, Willis WD & Eddy EM (2016) Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc Natl Acad Sci U S A 113, 1829–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFalco T, Potter SJ, Williams AV, Waller B, Kan MJ & Capel B (2015) Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep 12, 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edson MA, Nagaraja AK & Matzuk MM (2009) The mammalian ovary from genesis to revelation. Endocr Rev 30, 624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zirkin BR & Papadopoulos V (2018) Leydig cells: formation, function, and regulation. Biol Reprod 99, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards JS, Ren YA, Candelaria N, Adams JE & Rajkovic A (2018) Ovarian Follicular Theca Cell Recruitment, Differentiation, and Impact on Fertility: 2017 Update. Endocr Rev 39, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFalco T, Bhattacharya I, Williams AV, Sams DM & Capel B (2014) Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A 111, E2384–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hume DA, Halpin D, Charlton H & Gordon S (1984) The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of endocrine organs. Proc Natl Acad Sci U S A 81, 4174–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niemi M, Sharpe RM & Brown WR (1986) Macrophages in the interstitial tissue of the rat testis. Cell Tissue Res 243, 337–344. [DOI] [PubMed] [Google Scholar]
- 14.Lokka E, Lintukorpi L, Cisneros-Montalvo S, Mäkelä JA, Tyystjärvi S, Ojasalo V, Gerke H, Toppari J, Rantakari P & Salmi M (2020) Generation, localization and functions of macrophages during the development of testis. Nat Commun 11, 4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray PJ & Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11, 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viola A, Munari F, Sanchez-Rodriguez R, Scolaro T & Castegna A (2019) The Metabolic Signature of Macrophage Responses. Front Immunol 10, 1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilhorst M, Shirai T, Berry G, Goronzy JJ & Weyand CM (2014) T cell-macrophage interactions and granuloma formation in vasculitis. Front Immunol 5, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S & Amit I (2014) Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corliss BA, Azimi MS, Munson JM, Peirce SM & Murfee WL (2016) Macrophages: An Inflammatory Link Between Angiogenesis and Lymphangiogenesis. Microcirculation 23, 95–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FO & Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown MB, von Chamier M, Allam AB & Reyes L (2014) M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol 5, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meinhardt A, Wang M, Schulz C & Bhushan S (2018) Microenvironmental signals govern the cellular identity of testicular macrophages. J Leukoc Biol 104, 757–766. [DOI] [PubMed] [Google Scholar]
- 23.Yao Y, Xu XH & Jin L (2019) Macrophage Polarization in Physiological and Pathological Pregnancy. Front Immunol 10, 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A & Di W (2014) A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maccio A, Gramignano G, Cherchi MC, Tanca L, Melis L & Madeddu C (2020) Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci Rep 10, 6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A & Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25, 677–686. [DOI] [PubMed] [Google Scholar]
- 27.Murray PJ (2017) Macrophage Polarization. Annu Rev Physiol 79, 541–566. [DOI] [PubMed] [Google Scholar]
- 28.Orecchioni M, Ghosheh Y, Pramod AB & Ley K (2019) Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front Immunol 10, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleetwood AJ, Lawrence T, Hamilton JA & Cook AD (2007) Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol 178, 5245–5252. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton JA (2019) GM-CSF-Dependent Inflammatory Pathways. Front Immunol 10, 2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorens B, Mermod JJ & Vassalli P (1987) Phagocytosis and inflammatory stimuli induce GM-CSF mRNAin macrophages through posttranscriptional regulation. Cell 48, 671–679. [DOI] [PubMed] [Google Scholar]
- 32.Eggesbo JB, Hjermann I, Joo GB, Ovstebo R & Kierulf P (1995) LPS-induced release of EGF, GM-CSF, GRO alpha, LIF, MIP-1 alpha and PDGF-AB in PBMC from persons with high or low levels of HDL lipoprotein. Cytokine 7, 562–567. [DOI] [PubMed] [Google Scholar]
- 33.Parajuli B, Sonobe Y, Kawanokuchi J, Doi Y, Noda M, Takeuchi H, Mizuno T & Suzumura A (2012) GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J Neuroinflammation 9, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu M, Tong Z, Ding C, Luo F, Wu S, Wu C, Albeituni S, He L, Hu X, Tieri D, Rouchka EC, Hamada M, Takahashi S, Gibb AA, Kloecker G, Zhang HG, Bousamra M 2nd, Hill BG, Zhang X & Yan J (2020) Transcription factor c-Maf is a checkpoint that programs macrophages in lung cancer. J Clin Invest 130, 2081–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Junttila IS (2018) Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front Immunol 9, 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ran S & Montgomery KE (2012) Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 4, 618–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bleriot C, Chakarov S & Ginhoux F (2020) Determinants of Resident Tissue Macrophage Identity and Function. Immunity 52, 957–970. [DOI] [PubMed] [Google Scholar]
- 38.Hutson JC (1992) Development of cytoplasmic digitations between Leydig cells and testicular macrophages of the rat. Cell Tissue Res 267, 385–389. [DOI] [PubMed] [Google Scholar]
- 39.Hutson JC (1990) Changes in the Concentration and Size of Testicular Macrophages during Development. Biol Reprod 43, 885–890. [DOI] [PubMed] [Google Scholar]
- 40.Livera G, Delbes G, Pairault C, Rouiller-Fabre V & Habert R (2006) Organotypic culture, a powerful model for studying rat and mouse fetal testis development. Cell Tissue Res 324, 507–521. [DOI] [PubMed] [Google Scholar]
- 41.Stremmel C, Schuchert R, Wagner F, Thaler R, Weinberger T, Pick R, Mass E, Ishikawa-Ankerhold HC, Margraf A, Hutter S, Vagnozzi R, Klapproth S, Frampton J, Yona S, Scheiermann C, Molkentin JD, Jeschke U, Moser M, Sperandio M, Massberg S, Geissmann F & Schulz C (2018) Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat Commun 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singla DK, Singla R & Wang J (2016) BMP-7 Treatment Increases M2 Macrophage Differentiation and Reduces Inflammation and Plaque Formation in Apo E−/− Mice. PLoS One 11, e0147897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez VG, Rubio C, Martinez-Fernandez M, Segovia C, Lopez-Calderon F, Garin MI, Teijeira A, Munera-Maravilla E, Varas A, Sacedon R, Guerrero F, Villacampa F, de la Rosa F, Castellano D, Lopez-Collazo E, Paramio JM, Vicente A & Duenas M (2017) BMP4 Induces M2 Macrophage Polarization and Favors Tumor Progression in Bladder Cancer. Clin Cancer Res 23, 7388–7399. [DOI] [PubMed] [Google Scholar]
- 44.Ross A, Munger S & Capel B (2007) Bmp7 regulates germ cell proliferation in mouse fetal gonads. Sex Dev 1, 127–137. [DOI] [PubMed] [Google Scholar]
- 45.Ohinata Y, Ohta H, Shigeta M, Yamanaka K, Wakayama T & Saitou M (2009) A signaling principle for the specification of the germ cell lineage in mice. Cell 137, 571–584. [DOI] [PubMed] [Google Scholar]
- 46.Nucera S, Biziato D & De Palma M (2011) The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol 55, 495–503. [DOI] [PubMed] [Google Scholar]
- 47.Mossadegh-Keller N, Gentek R, Gimenez G, Bigot S, Mailfert S & Sieweke MH (2017) Developmental origin and maintenance of distinct testicular macrophage populations. J Exp Med 214, 2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhushan S & Meinhardt A (2017) The macrophages in testis function. J Reprod Immunol 119, 107–112. [DOI] [PubMed] [Google Scholar]
- 49.Hales DB (2002) Testicular macrophage modulation of Leydig cell steroidogenesis. J Reprod Immunol 57, 3–18. [DOI] [PubMed] [Google Scholar]
- 50.Heinrich A & DeFalco T (2020) Essential roles of interstitial cells in testicular development and function. Andrology 8, 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ponte R, Dupuy FP, Brimo F, Mehraj V, Brassard P, Belanger M, Yurchenko E, Jenabian MA, Bernard NF, Routy JP & group Os (2018) Characterization of myeloid cell populations in human testes collected after sex reassignment surgery. J Reprod Immunol 125, 16–24. [DOI] [PubMed] [Google Scholar]
- 52.Bhushan S, Theas MS, Guazzone VA, Jacobo P, Wang M, Fijak M, Meinhardt A & Lustig L (2020) Immune Cell Subtypes and Their Function in the Testis. Front Immunol 11, 583304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang M, Fijak M, Hossain H, Markmann M, Nüsing RM, Lochnit G, Hartmann MF, Wudy SA, Zhang L, Gu H, Konrad L, Chakraborty T, Meinhardt A & Bhushan S (2017) Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages. J Immunol 198, 4327–4340. [DOI] [PubMed] [Google Scholar]
- 54.Barakat B, O’Connor AE, Gold E, de Kretser DM & Loveland KL (2008) Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction 136, 345–359. [DOI] [PubMed] [Google Scholar]
- 55.Indumathy S, Pueschl D, Klein B, Fietz D, Bergmann M, Schuppe HC, Da Silva N, Loveland BE, Hickey MJ, Hedger MP & Loveland KL (2020) Testicular immune cell populations and macrophage polarisation in adult male mice and the influence of altered activin A levels. J Reprod Immunol 142, 103204. [DOI] [PubMed] [Google Scholar]
- 56.Maresz K, Ponomarev ED, Barteneva N, Tan Y, Mann MK & Dittel BN (2008) IL-13 induces the expression of the alternative activation marker YM1 in a subset of testicular macrophages. J Reprod Immunol 78, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jokela H, Lokka E, Kiviranta M, Tyystjarvi S, Gerke H, Elima K, Salmi M & Rantakari P (2020) Fetal-derived macrophages persist and sequentially maturate in ovaries after birth in mice. Eur J Immunol 50, 1500–1514. [DOI] [PubMed] [Google Scholar]
- 58.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ & Geissmann F (2012) A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90. [DOI] [PubMed] [Google Scholar]
- 59.Wang M, Yang Y, Cansever D, Wang Y, Kantores C, Messiaen S, Moison D, Livera G, Chakarov S, Weinberger T, Stremmel C, Fijak M, Klein B, Pleuger C, Lian Z, Ma W, Liu Q, Klee K, Handler K, Ulas T, Schlitzer A, Schultze JL, Becher B, Greter M, Liu Z, Ginhoux F, Epelman S, Schulz C, Meinhardt A & Bhushan S (2021) Two populations of self-maintaining monocyte-independent macrophages exist in adult epididymis and testis. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Dong J, Yan L, Yong J, Liu X, Hu Y, Fan X, Wu X, Guo H, Wang X, Zhu X, Li R, Yan J, Wei Y, Zhao Y, Wang W, Ren Y, Yuan P, Yan Z, Hu B, Guo F, Wen L, Tang F & Qiao J (2017) Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell Stem Cell 20, 858–873 e854. [DOI] [PubMed] [Google Scholar]
- 61.Niu W & Spradling AC (2020) Two distinct pathways of pregranulosa cell differentiation support follicle formation in the mouse ovary. Proc Natl Acad Sci U S A 117, 20015–20026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao ZH, Li CY, Meng TG, Wang Y, Liu WB, Li A, Cai YJ, Hou Y, Schatten H, Wang ZB, Sun QY & Sun Q (2020) Single-cell RNA sequencing reveals regulation of fetal ovary development in the monkey (Macaca fascicularis). Cell Discov 6, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bukovsky A, Caudle MR, Keenan JA, Wimalasena J, Upadhyaya NB & Van Meter SE (1995) Is corpus luteum regression an immune-mediated event? Localization of immune system components and luteinizing hormone receptor in human corpora lutea. Biol Reprod 53, 1373–1384. [DOI] [PubMed] [Google Scholar]
- 64.Van der Hoek KH, Maddocks S, Woodhouse CM, van Rooijen N, Robertson SA & Norman RJ (2000) Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary. Biol Reprod 62, 1059–1066. [DOI] [PubMed] [Google Scholar]
- 65.Li XQ, Itoh M, Yano A, Xie Q, Miyamoto K & Takeuchi Y (1998) Distribution of F4/80-positive cells in developing ovaries in the mouse. Arch Histol Cytol 61, 353–360. [DOI] [PubMed] [Google Scholar]
- 66.Carlock C, Wu J, Zhou C, Ross A, Adams H & Lou Y (2013) Ovarian phagocyte subsets and their distinct tissue distribution patterns. Reproduction 146, 491–500. [DOI] [PubMed] [Google Scholar]
- 67.Petrovska M, Sedlak R, Nouza K, Presl J & Kinsky R (1992) Development and distribution of the white blood cells within various structures of the human menstrual corpus luteum examined using an image analysis system. Am J Reprod Immunol 28, 77–80. [DOI] [PubMed] [Google Scholar]
- 68.Gaytan F, Morales C, Garcia-Pardo L, Reymundo C, Bellido C & Sanchez-Criado JE (1998) Macrophages, cell proliferation, and cell death in the human menstrual corpus luteum. Biol Reprod 59, 417–425. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Z, Schlamp F, Huang L, Clark H & Brayboy L (2020) Inflammaging is associated with shifted macrophage ontogeny and polarization in the aging mouse ovary. Reproduction 159, 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM & Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Epelman S, Lavine KJ & Randolph GJ (2014) Origin and functions of tissue macrophages. Immunity 41, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F & Rodewald HR (2015) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perdiguero EG & Geissmann F (2016) The development and maintenance of resident macrophages. Nat Immunol 17, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coskun S, Chao H, Vasavada H, Heydari K, Gonzales N, Zhou X, de Crombrugghe B & Hirschi KK (2014) Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep 9, 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sheng J, Ruedl C & Karjalainen K (2015) Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity 43, 382–393. [DOI] [PubMed] [Google Scholar]
- 76.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M & Ginhoux F (2012) Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 209, 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H & Lambrecht BN (2013) Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210, 1977–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, Beaudin AE, Lum J, Low I, Forsberg EC, Poidinger M, Zolezzi F, Larbi A, Ng LG, Chan JK, Greter M, Becher B, Samokhvalov IM, Merad M & Ginhoux F (2015) C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jappinen N, Felix I, Lokka E, Tyystjarvi S, Pynttari A, Lahtela T, Gerke H, Elima K, Rantakari P & Salmi M (2019) Fetal-derived macrophages dominate in adult mammary glands. Nat Commun 10, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B & Henri S (2013) Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938. [DOI] [PubMed] [Google Scholar]
- 81.Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D & Mowat AM (2014) Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15, 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, Murphy KM, Yokoyama WM, Randolph GJ & Unanue ER (2015) The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med 212, 1497–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams RH & Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8, 464–478. [DOI] [PubMed] [Google Scholar]
- 84.Potente M, Gerhardt H & Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887. [DOI] [PubMed] [Google Scholar]
- 85.Varricchi G, Loffredo S, Galdiero MR, Marone G, Cristinziano L, Granata F & Marone G (2018) Innate effector cells in angiogenesis and lymphangiogenesis. Curr Opin Immunol 53, 152–160. [DOI] [PubMed] [Google Scholar]
- 86.Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L, Jacome-Galarza CE, Handler K, Klughammer J, Kobayashi Y, Gomez-Perdiguero E, Schultze JL, Beyer M, Bock C & Geissmann F (2016) Specification of tissue-resident macrophages during organogenesis. Science 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, Roncalli M, Palumbo GA, Introna M & Golay J (2009) M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than M1 cells in vitro. J Immunol 182, 4415–4422. [DOI] [PubMed] [Google Scholar]
- 88.Munro DAD, Wineberg Y, Tarnick J, Vink CS, Li Z, Pridans C, Dzierzak E, Kalisky T, Hohenstein P & Davies JA (2019) Macrophages restrict the nephrogenic field and promote endothelial connections during kidney development. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gula G, Ruminski S, Niderla-Bielinska J, Jasinska A, Kiernozek E, Jankowska-Steifer E, Flaht-Zabost A & Ratajska A (2020) Potential functions of embryonic cardiac macrophages in angiogenesis, lymphangiogenesis and extracellular matrix remodeling. Histochem Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW & Ruhrberg C (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang X, Zhao L, Campos MM, Abu-Asab M, Ortolan D, Hotaling N, Bharti K & Wong WT (2020) CSF1R blockade induces macrophage ablation and results in mouse choroidal vascular atrophy and RPE disorganization. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones A, Fujiyama C, Turner K, Fuggle S, Cranston D, Turley H, Valtola R, Bicknell R & Harris AL (2000) Angiogenesis and lymphangiogenesis in stage 1 germ cell tumours of the testis. BJU Int 86, 80–86. [DOI] [PubMed] [Google Scholar]
- 93.Diez-Torre A, Silvan U, Diaz-Nunez M & Arechaga J (2010) The role of microenvironment in testicular germ cell tumors. Cancer Biol Ther 10, 529–536. [DOI] [PubMed] [Google Scholar]
- 94.Batool A, Wang YQ, Hao XX, Chen SR & Liu YX (2018) A miR-125b/CSF1-CX3CL1/tumor-associated macrophage recruitment axis controls testicular germ cell tumor growth. Cell Death Dis 9, 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Willems M, Vloeberghs V, Gies I, De Schepper J, Tournaye H, Goossens E & Van Saen D (2020) Testicular immune cells and vasculature in Klinefelter syndrome from childhood up to adulthood. Hum Reprod 35, 1753–1764. [DOI] [PubMed] [Google Scholar]
- 96.Dace DS, Khan AA, Kelly J & Apte RS (2008) Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PLoS One 3, e3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fahey E & Doyle SL (2019) IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front Immunol 10, 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fraser HM (2006) Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reynolds LP & Redmer DA(1999) Growth and development of the corpus luteum. J Reprod Fertil Suppl 54, 181–191. [PubMed] [Google Scholar]
- 100.Woad KJ & Robinson RS (2016) Luteal angiogenesis and its control. Theriogenology 86, 221–228. [DOI] [PubMed] [Google Scholar]
- 101.Wu R, Van der Hoek KH, Ryan NK, Norman RJ & Robker RL (2004) Macrophage contributions to ovarian function. Hum Reprod Update 10, 119–133. [DOI] [PubMed] [Google Scholar]
- 102.Abdulrahman N & Fair T (2019) Contribution of the immune system to follicle differentiation, ovulation and early corpus luteum formation. Anim Reprod 16, 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turner EC, Hughes J, Wilson H, Clay M, Mylonas KJ, Kipari T, Duncan WC & Fraser HM (2011) Conditional ablation of macrophages disrupts ovarian vasculature. Reproduction 141, 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV & Robertson SA (2013) Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest 123, 3472–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Zhu Q, Lin Y, Wu L, Wu X, Wang K, He Q, Xu C, Wan X & Wang X (2017) Crosstalk between TEMs and endothelial cells modulates angiogenesis and metastasis via IGF1-IGF1R signalling in epithelial ovarian cancer. Br J Cancer 117, 1371–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moughon DL, He H, Schokrpur S, Jiang ZK, Yaqoob M, David J, Lin C, Iruela-Arispe ML, Dorigo O & Wu L (2015) Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer. Cancer Res 75, 4742–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X, Zhao X, Wang K, Wu L & Duan T (2013) Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesis in vitro. Cancer Sci 104, 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Katabuchi H, Fukumatsu Y, Araki M, Suenaga Y, Ohtake H & Okamura H (1996) Role of macrophages in ovarian follicular development. Horm Res 46 Suppl 1, 45–51. [DOI] [PubMed] [Google Scholar]
- 109.Nagaoka I, Trapnell BC & Crystal RG (1990) Regulation of insulin-like growth factor I gene expression in the human macrophage-like cell line U937. J Clin Invest 85, 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tammela T & Alitalo K (2010) Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140, 460–476. [DOI] [PubMed] [Google Scholar]
- 111.Kazenwadel J & Harvey NL (2018) Lymphatic endothelial progenitor cells: origins and roles in lymphangiogenesis. Curr Opin Immunol 53, 81–87. [DOI] [PubMed] [Google Scholar]
- 112.Brown HM & Russell DL (2014) Blood and lymphatic vasculature in the ovary: development, function and disease. Hum Reprod Update 20, 29–39. [DOI] [PubMed] [Google Scholar]
- 113.Petrova TV & Koh GY (2020) Biological functions of lymphatic vessels. Science 369. [DOI] [PubMed] [Google Scholar]
- 114.Svingen T, François M, Wilhelm D & Koopman P (2012) Three-dimensional imaging of Prox1-EGFP transgenic mouse gonads reveals divergent modes of lymphangiogenesis in the testis and ovary. PLoS One 7, e52620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu F & Stouffer RL (2009) Existence of the lymphatic system in the primate corpus luteum. Lymphat Res Biol 7, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ichikawa S, Uchino S & Hirata Y (1987) Lymphatic and blood vasculature of the forming corpus luteum. Lymphology 20, 73–83. [PubMed] [Google Scholar]
- 117.Brown HM, Robker RL & Russell DL (2010) Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology 151, 5446–5455. [DOI] [PubMed] [Google Scholar]
- 118.Alitalo K (2011) The lymphatic vasculature in disease. Nat Med 17, 1371–1380. [DOI] [PubMed] [Google Scholar]
- 119.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C & Alitalo K (2004) Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5, 74–80. [DOI] [PubMed] [Google Scholar]
- 120.Ali I, Chouhan VS, Dangi SS, Gupta M, Tandiya U, Hyder I, Yadav VP, Panda RP, Babitha V, Nagar V, Sonwane A, Khan FA, Das BC, Singh G, Bag S & Sarkar M (2014) Expression and localization of locally produced growth factors regulating lymphangiogenesis during different stages of the estrous cycle in corpus luteum of buffalo (Bubalus bubalis). Theriogenology 81, 428–436. [DOI] [PubMed] [Google Scholar]
- 121.Nitta A, Shirasuna K, Haneda S, Matsui M, Shimizu T, Matsuyama S, Kimura K, Bollwein H & Miyamoto A (2011) Possible involvement of IFNT in lymphangiogenesis in the corpus luteum during the maternal recognition period in the cow. Reproduction 142, 879–892. [DOI] [PubMed] [Google Scholar]
- 122.Rutkowski JM, Ihm JE, Lee ST, Kilarski WW, Greenwood VI, Pasquier MC, Quazzola A, Trono D, Hubbell JA & Swartz MA (2013) VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy. Am J Pathol 183, 1596–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harvey NL & Gordon EJ (2012) Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis. Vase Cell 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bohmer R, Neuhaus B, Buhren S, Zhang D, Stehling M, Bock B & Kiefer F (2010) Regulation of developmental lymphangiogenesis by Syk(+) leukocytes. Dev Cell 18, 437–449. [DOI] [PubMed] [Google Scholar]
- 125.Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA & Harvey NL (2010) Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development 137, 3899–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H & Suda T (2009) M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 206, 1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamazaki T, Nalbandian A, Uchida Y, Li W, Arnold TD, Kubota Y, Yamamoto S, Ema M & Mukouyama YS (2017) Tissue Myeloid Progenitors Differentiate into Pericytes through TGF-beta Signaling in Developing Skin Vasculature. Cell Rep 18, 2991–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chambers SE, O’Neill CL, O’Doherty TM, Medina RJ & Stitt AW (2013) The role of immune-related myeloid cells in angiogenesis. Immunobiology 218, 1370–1375. [DOI] [PubMed] [Google Scholar]
- 129.Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA & Stitt AW (2011) Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med 17, 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Plein A, Fantin A, Denti L, Pollard JW & Ruhrberg C (2018) Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 562, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hall KL, Volk-Draper LD, Flister MJ & Ran S (2012) New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One 7, e31794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C & Christofori G (2009) Myeloid cells contribute to tumor lymphangiogenesis. PLoS One 4, e7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, Wu HP, Nathan SD, Cuttitta F, McCoy JP, Gochuico BR & Moss J (2009) Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A 106, 3958–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, Losordo DW & Streilein JW (2005) Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 115, 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Robinson A, Han CZ, Glass CK & Pollard JW (2021) Monocyte Regulation in Homeostasis and Malignancy. Trends Immunol 42, 104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stansfield BK & Ingram DA (2015) Clinical significance of monocyte heterogeneity. Clin Transl Med 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.De Palma M, Murdoch C, Venneri MA, Naldini L & Lewis CE (2007) Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol 28, 519–524. [DOI] [PubMed] [Google Scholar]
- 138.Galli SJ & Tsai M (2012) IgE and mast cells in allergic disease. Nat Med 18, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maseki Y, Miyake K, Mitsuya H, Kitamura H & Yamada K (1981) Mastocytosis occurring in the testes from patients with idiopathic male infertility. Fertil Steril 36, 814–817. [DOI] [PubMed] [Google Scholar]
- 140.Meineke V, Frungieri MB, Jessberger B, Vogt H & Mayerhofer A (2000) Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil Steril 74, 239–244. [DOI] [PubMed] [Google Scholar]
- 141.Nagai T, Takaba H, Miyake K, Hirabayashi Y & Yamada K (1992) Testicular mast cell heterogeneity in idiopathic male infertility. Fertil Steril 57, 1331–1336. [PubMed] [Google Scholar]
- 142.Frungieri MB, Weidinger S, Meineke V, Kohn FM & Mayerhofer A (2002) Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARgamma : Possible relevance to human fibrotic disorders. Proc Natl Acad Sci U S A 99, 15072–15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Adam M, Schwarzer JU, Kohn FM, Strauss L, Poutanen M & Mayerhofer A (2011) Mast cell tryptase stimulates production of decorin by human testicular peritubular cells: possible role of decorin in male infertility by interfering with growth factor signaling. Hum Reprod 26, 2613–2625. [DOI] [PubMed] [Google Scholar]
- 144.Albrecht M, Frungieri MB, Gonzalez-Calvar S, Meineke V, Kohn FM & Mayerhofer A (2005) Evidence for a histaminergic system in the human testis. Fertil Steril 83, 1060–1063. [DOI] [PubMed] [Google Scholar]
- 145.Elieh Ali Komi D, Shafaghat F & Haidl G (2020) Significance of mast cells in spermatogenesis, implantation, pregnancy, and abortion: Cross talk and molecular mechanisms. Am J Reprod Immunol 83, e13228. [DOI] [PubMed] [Google Scholar]
- 146.Mayerhofer A, Walenta L, Mayer C, Eubler K & Welter H (2018) Human testicular peritubular cells, mast cells and testicular inflammation. Andrologia 50, e13055. [DOI] [PubMed] [Google Scholar]
- 147.Windschuttl S, Nettersheim D, Schlatt S, Huber A, Welter H, Schwarzer JU, Kohn FM, Schorle H & Mayerhofer A (2014) Are testicular mast cells involved in the regulation of germ cells in man? Andrology 2, 615–622. [DOI] [PubMed] [Google Scholar]
- 148.Mayerhofer A, Bartke A, Amador AG & Began T (1989) Histamine affects testicular steroid production in the golden hamster. Endocrinology 125, 560–562. [DOI] [PubMed] [Google Scholar]
- 149.Pap E, Racz K, Kovacs JK, Varga I, Buzas E, Madarasz B, Foldes C, Szalai C, Watanabe T, Ohtsu H, Ichikawa A, Nagy A & Falus A (2002) Histidine decarboxylase deficiency in gene knockout mice elevates male sex steroid production. J Endocrinol 175, 193–199. [DOI] [PubMed] [Google Scholar]
- 150.Anton F, Morales C, Aguilar R, Bellido C, Aguilar E & Gaytan F (1998) A comparative study of mast cells and eosinophil leukocytes in the mammalian testis. Zentralbl Veterinarmed A 45, 209–218. [DOI] [PubMed] [Google Scholar]
- 151.Gaytan F, Aceitero J, Bellido C, Sanchez-Criado JE & Aguilar E (1991) Estrous cycle-related changes in mast cell numbers in several ovarian compartments in the rat. Biol Reprod 45, 27–33. [DOI] [PubMed] [Google Scholar]
- 152.Heider U, Pedal I & Spanel-Borowski K (2001) Increase in nerve fibers and loss of mast cells in polycystic and postmenopausal ovaries. Fertil Steril 75, 1141–1147. [DOI] [PubMed] [Google Scholar]
- 153.Jones RE, Duvall D, Guillette LJ Jr. & Lopez KH (1994) Number and state of rat ovarian mast cells after exogenous administration of luteinizing hormone. Comp Biochem Physiol Comp Physiol 108, 555–559. [DOI] [PubMed] [Google Scholar]
- 154.Krishna A, Beesley K & Terranova PF (1989) Histamine, mast cells and ovarian function. J Endocrinol 120, 363–371. [DOI] [PubMed] [Google Scholar]
- 155.Zhu TH, Ding SJ, Li TT, Zhu LB, Huang XF & Zhang XM (2018) Estrogen is an important mediator of mast cell activation in ovarian endometriomas. Reproduction 155, 73–83. [DOI] [PubMed] [Google Scholar]
- 156.Boiers C, Carrelha J, Lutteropp M, Luc S, Green JC, Azzoni E, Woll PS, Mead AJ, Hultquist A, Swiers G, Perdiguero EG, Macaulay IC, Melchiori L, Luis TC, Kharazi S, Bouriez-Jones T, Deng Q, Ponten A, Atkinson D, Jensen CT, Sitnicka E, Geissmann F, Godin I, Sandberg R, de Bruijn MF & Jacobsen SE (2013) Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell 13, 535–548. [DOI] [PubMed] [Google Scholar]
- 157.Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH & Yoder MC (2012) Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood 119, 5706–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yokota T, Huang J, Tavian M, Nagai Y, Hirose J, Zuniga-Pflucker JC, Peault B & Kincade PW (2006) Tracing the first waves of lymphopoiesis in mice. Development 133, 2041–2051. [DOI] [PubMed] [Google Scholar]
- 159.Yokota T, Kouro T, Hirose J, Igarashi H, Garrett KP, Gregory SC, Sakaguchi N, Owen JJ & Kincade PW (2003) Unique properties of fetal lymphoid progenitors identified according to RAG1 gene expression. Immunity 19, 365–375. [DOI] [PubMed] [Google Scholar]
- 160.Pollanen P & Maddocks S (1988) Macrophages, lymphocytes and MHC II antigen in the ram and the rat testis. J Reprod Fertil 82, 437–445. [DOI] [PubMed] [Google Scholar]
- 161.Pollanen P & Niemi M (1987) Immunohistochemical identification of macrophages, lymphoid cells and HLA antigens in the human testis. Int J Androl 10, 37–42. [DOI] [PubMed] [Google Scholar]
- 162.Gong J, Zeng Q, Yu D & Duan YG (2020) T Lymphocytes and Testicular Immunity: A New Insight into Immune Regulation in Testes. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jacobo P, Guazzone VA, Theas MS & Lustig L (2011) Testicular autoimmunity. Autoimmun Rev 10, 201–204. [DOI] [PubMed] [Google Scholar]
- 164.Santoiemma PP & Powell DJ Jr. (2015) Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther 16, 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kekalainen E, Pontynen N, Meri S, Arstila TP & Jarva H (2015) Autoimmunity, Not a Developmental Defect, is the Cause for Subfertility of Autoimmune Regulator (Aire) Deficient Mice. Scand J Immunol 81, 298–304. [DOI] [PubMed] [Google Scholar]
- 166.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S & Papaioannou VE (1992) RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877. [DOI] [PubMed] [Google Scholar]
- 167.Bott RC, Clopton DT, Fuller AM, McFee RM, Lu N, McFee RM & Cupp AS (2010) KDR-LacZ-expressing cells are involved in ovarian and testis-specific vascular development, suggesting a role for VEGFA in the regulation of this vasculature. Cell Tissue Res 342, 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R & Capel B (1997) Male-specific cell migration into the developing gonad. Curr Biol 7, 958–968. [DOI] [PubMed] [Google Scholar]
- 169.Brennan J, Karl J & Capel B (2002) Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev Biol 244, 418–428. [DOI] [PubMed] [Google Scholar]
- 170.Buehr M, Gu S & McLaren A (1993) Mesonephric contribution to testis differentiation in the fetal mouse. Development 117, 273–281. [DOI] [PubMed] [Google Scholar]
- 171.Coveney D, Cool J, Oliver T & Capel B (2008) Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A 105, 7212–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25, 581–611. [DOI] [PubMed] [Google Scholar]
- 173.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman M1 & Schuh AC (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376, 62–66. [DOI] [PubMed] [Google Scholar]
- 174.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ & Moore MW (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442. [DOI] [PubMed] [Google Scholar]
- 175.Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A & Koopman P (2009) Endothelial cell migration directs testis cord formation. Dev Biol 326, 112–120. [DOI] [PubMed] [Google Scholar]
- 176.Cool J, DeFalco TJ & Capel B (2011) Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci U S A 108, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD & Rudge JS (2002) VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A 99, 11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alvarez RH, Kantarjian HM & Cortes JE (2006) Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc 81, 1241–1257. [DOI] [PubMed] [Google Scholar]
- 179.Brennan J, Tilmann C & Capel B (2003) Pdgfr-alpha mediates testis cord organization and fetal Ley dig cell development in the XY gonad. Genes Dev 17, 800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gnessi L, Basciani S, Mariani S, Arizzi M, Spera G, Wang C, Bondjers C, Karlsson L & Betsholtz C (2000) Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (PDGF)-A-deficient mice. J Cell Biol 149, 1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bergh A, Damber JE & van Rooijen N (1993) Liposome-mediated macrophage depletion: an experimental approach to study the role of testicular macrophages in the rat. J Endocrinol 136, 407–413. [DOI] [PubMed] [Google Scholar]
- 182.Cohen PE, Chisholm O, Arceci RJ, Stanley ER & Pollard JW (1996) Absence of colony-stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice results in male fertility defects. Biol Reprod 55, 310–317. [DOI] [PubMed] [Google Scholar]
- 183.Cohen PE, Hardy MP & Pollard JW (1997) Colony-stimulating factor-1 plays a major role in the development of reproductive function in male mice. Mol Endocrinol 11, 1636–1650. [DOI] [PubMed] [Google Scholar]
- 184.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW & Brinster RL (2009) Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 136, 1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roth P, Dominguez MG & Stanley ER (1998) The effects of colony-stimulating factor-1 on the distribution of mononuclear phagocytes in the developing osteopetrotic mouse. Blood 91, 3773–3783. [PubMed] [Google Scholar]
- 186.Matzuk MM & Lamb DJ (2008) The biology of infertility: research advances and clinical challenges. Nat Med 14, 1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yao HH, Aardema J & Holthusen K (2006) Sexually dimorphic regulation of inhibin beta B in establishing gonadal vasculature in mice. Biol Reprod 74, 978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.De Falco T, Takahashi S & Capel B (2011) Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352, 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu CF, Bingham N, Parker K & Yao HH (2009) Sex-specific roles of beta-catenin in mouse gonadal development. Hum Mol Genet 18, 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B & Swain A (2003) Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130, 3663–3670. [DOI] [PubMed] [Google Scholar]
- 191.DiNapoli L, Batchvarov J & Capel B (2006) FGF9 promotes survival of germ cells in the fetal testis. Development 133, 1519–1527. [DOI] [PubMed] [Google Scholar]
- 192.Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H & Zhou QY (2002) Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem 277, 19276–19280. [DOI] [PubMed] [Google Scholar]
- 193.Prosser HM, Bradley A & Caldwell MA (2007) Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation. Eur J Neurosci 26, 3339–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Urayama K, Dedeoglu DB, Guilini C, Frantz S, Ertl G, Messaddeq N & Nebigil CG (2009) Transgenic myocardial overexpression of prokineticin receptor-2 (GPR73b) induces hypertrophy and capillary vessel leakage. Cardiovasc Res 81, 28–37. [DOI] [PubMed] [Google Scholar]
- 195.Svingen T, McClelland KS, Masumoto K, Sujino M, Nagano M, Shigeyoshi Y & Koopman P (2011) Prokr2-deficient mice display vascular dysmorphology of the fetal testes: potential implications for Kallmann syndrome aetiology. Sex Dev 5, 294–303. [DOI] [PubMed] [Google Scholar]
- 196.Cool J, DeFalco T & Capel B (2012) Testis formation in the fetal mouse: dynamic and complex de novo tubulogenesis. Wiley Interdiscip Rev Dev Biol 1, 847–859. [DOI] [PubMed] [Google Scholar]
- 197.Kumar DL & DeFalco T (2018) A perivascular niche for multipotent progenitors in the fetal testis. Nat Commun 9, 4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Phng LK & Gerhardt H (2009) Angiogenesis: a team effort coordinated by notch. Dev Cell 16, 196–208. [DOI] [PubMed] [Google Scholar]
- 199.Rehman AO & Wang CY (2006) Notch signaling in the regulation of tumor angiogenesis. Trends Cell Biol 16, 293–300. [DOI] [PubMed] [Google Scholar]
- 200.Defalco T, Saraswathula A, Briot A, Iruela-Arispe ML & Capel B (2013) Testosterone levels influence mouse fetal Leydig cell progenitors through notch signaling. Biol Reprod 88, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jameson SA, Natarajan A, Cool J, DeFalco T, Maatouk DM, Mork L, Munger SC & Capel B (2012) Temporal transcriptional profiling of somatic and germ cells reveals biased lineage priming of sexual fate in the fetal mouse gonad. PLoS Genet 8, e1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tang H, Brennan J, Karl J, Hamada Y, Raetzman L & Capel B (2008) Notch signaling maintains Leydig progenitor cells in the mouse testis. Development 135, 3745–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liu C, Rodriguez K & Yao HH (2016) Mapping lineage progression of somatic progenitor cells in the mouse fetal testis. Development 143, 3700–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR & Wobus AM (2004) Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci 61, 2510–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF & Muller D (2004) Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol 167, 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]


