Abstract
COVID-19 is a highly heterogeneous and complex medical disorder; indeed, severe COVID-19 is probably amongst the most complex of medical conditions known to medical science. While enormous strides have been made in understanding the molecular pathways involved in patients infected with coronaviruses an overarching and comprehensive understanding of the pathogenesis of COVID-19 is lacking. Such an understanding is essential in the formulation of effective prophylactic and treatment strategies. Based on clinical, proteomic, and genomic studies as well as autopsy data severe COVID-19 disease can be considered to be the connection of three basic pathologic processes, namely a pulmonary macrophage activation syndrome with uncontrolled inflammation, a complement-mediated endothelialitis together with a procoagulant state with a thrombotic microangiopathy. In addition, platelet activation with the release of serotonin and the activation and degranulation of mast cells contributes to the hyper-inflammatory state. Auto-antibodies have been demonstrated in a large number of hospitalized patients which adds to the end-organ damage and pro-thrombotic state. This paper provides a clinical overview of the major pathogenetic mechanism leading to severe COVID-19 disease.
Keywords: COVID-19, pathogenesis, autopsy, macrophage activation, micro-vasculitis, serotonin, complement, NETosis
Introduction
The COVID-19 pandemic has claimed over four million lives and shows no evidence of abating. While the majority of SARS-CoV-2 infections are self-limited, approximately 20% of patients are symptomatic, with many of these patients requiring hospitalization with approximately 3% symptomatic patients having a fatal outcome.1–3 Furthermore, in excess of 50% of patients who recover from symptomatic infections, independent of disease severity, develop the debilitating “long-haul syndrome”4 The human and economic toll of this disease is astronomical. In order to develop effective prophylactic and therapeutic strategies against COVID-19, an accurate understanding of its pathogenesis is required. While tens of thousands of publications have explored the clinical and basic science aspects of this disease, there is lack of an integrative, all-encompassing, and clinically focused review of the pathogenetic mechanisms of this disease.
Phases of COVID-19
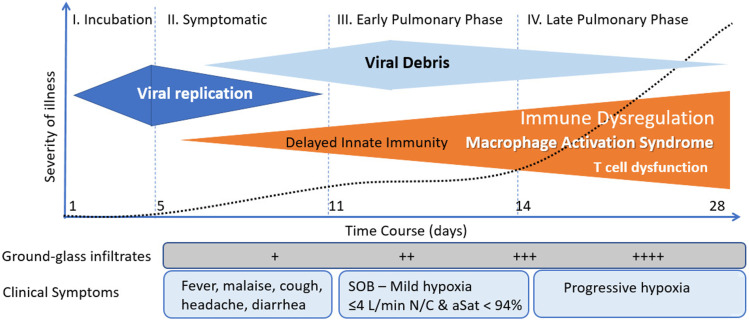
COVID-19 progresses through three distinct phases, namely, the incubation phase, the symptomatic phase, and the pulmonary phase (see Figure 1).5 SARS-CoV-2 is highly infectious being transmitted by droplet and aerosol spread.6–8 In distinction to SARS-CoV and Middle Eastern Respiratory Virus (MERS) patients infected with SARS-CoV-2 are most infectious during the late incubation/presymptomatic phase (highest viral load).9 SARS-CoV and SARS-CoV-2 infects human cells by binding to the cell-surface protein angiotensin-converting enzyme 2 (ACE-2) through the Receptor Binding Domain (RBD) of its spike protein.10,11 ACE-2 is expressed on ciliated epithelium of the nasopharynx and upper respiratory tract, bronchial epithelium, type II pneumocytes in addition to macrophages/monocytes, mast cells, and vascular endothelial cells.10 In the respiratory tract, there is a gradient of ACE-2 expression with greater expression in the upper than lower respiratory tract.12 ACE-2 receptor expression is highest in the microvasculature of the lung, fat, and brain with lower amounts in the liver, kidney, and heart.10,13 Once engaged with ACE-2, the ACE-2-bound viral spike protein undergoes proteolytic cleavage catalyzed by a host membrane-anchored protein, the transmembrane protease serine 2 (TMPRSS2).14 TMPRSS2 results in a conformational change in the spike protein that is required for host and virus membrane fusion. After this spike-mediated fusion process, the internalized virus particle releases its RNA genome and begins replication.15 More recently, a furin protease and the cellular receptor neuropilin-1 (NRP1) have been demonstrated to be involved in the infection process.16–18
Figure 1.
Clinical stages of COVID.
During the incubation and symptomatic phase SARS-CoV-2 infects the ciliated epithelium of the nasopharynx and upper airways.12 Control of viral spread depends on interactions between epithelial cells and immune cells, mediated by cytokine signaling and cell–cell contacts.19 Innate immunity is the first arm of the immune response to viral infections. After virus entry, the infected cell detects the presence of aberrant RNA structures through one of a number of pattern recognition receptors (PRRs).20 Engagement of virus-specific RNA structures culminates in oligomerization of the PRRs and activation of downstream transcription factors, the most important of which include interferon regulator factors (IRFs) and nuclear factor-κB (NF-κB).21,22 IRFs result in the induction of type I and III interferons (IFN) and the upregulation of IFN-stimulated genes which result in the transcription of various proteins that orchestrate the host’s primary antiviral defense.23 The expression of NF-κB results in the expression of pro-inflammatory cytokines and chemokines that coordinate the recruitment of specific subsets of leukocytes. In addition to triggering the expression of IRF’s and NF-κB, viruses are also able to induce activation of the pro-inflammatory cytokines IL-1β and IL-18 through triggering of inflammasomes.21 Inflammasomes are multiprotein complexes containing caspase-1 which when activated results in caspase mediated cleavage of precursor cytokine molecules and the release of IL-1β and IL-18.21,24
SARS-CoV-2 inhibits the synthesis of type I and III interferons.23,25,26 The SARS-CoV-2 gene products including non-structural protein-1 (NSP1), accessory proteins ORF6 and ORF3B as well as the nucleocapsid (N) gene products induce dysfunction of signal transducer and activator of transcription 1 (STAT1) leading to decreased interferon synthesis.25,27 It is likely that the balance between viral inoculum size, rate of viral replication, the host production of interferons, and pro-inflammatory mediators determines the outcome of infection with SARS-CoV-2.23,28,29 Those patients who develop a brisk interferon response with an effective innate immune response likely rapidly eliminate the virus. However, rapid viral replication leading to high viral concentrations in the upper airways occur in those who are infected with a large viral inoculum and those who have a poor or delayed interferon response.23 The delta variant replicates to achieve very high concentrations in the nasopharynx and this likely accounts for its increased transmissibility and virulence.30,31 Infected epithelial cells at the site of infection secrete chemokines that recruit and activate various immune cell populations. In patients with moderate–severe COVID-19, secretory cells show a significantly increased expression of the chemokines promoting the recruitment of macrophages, T cells and mast cells.32
Aspiration of the viral inoculum from the oropharynx into the lung likely occurs in those patients with a high viral load infecting type II pneumocytes and alveolar macrophages.12 This then sets the stage for progression into the pulmonary phase of the disease. The failure to develop a robust IFN-I and -III response, while simultaneously inducing high levels of chemokines, results in the recruitment of blood monocytes to the infected lung tissue. Macrophages express the ACE-2 receptor.33 In addition, macrophages express furin and TMPRSS2, two enzymes required for exposure of the SARS-CoV-2 binding site and fusion with the cell membrane.34 In the pulmonary phase of COVID-19, “macrophages may serve as a Trojan horse,” enabling viral anchoring specifically within the pulmonary parenchyma.34 Furthermore, the diverse expression of ACE-2 in macrophages among individuals might govern the severity of SARS-CoV-2 infection.34 Macrophages in the lower airways are reported to have greater expression of the genes encoding for inflammatory chemokines and cytokines than those within the upper airways.32 These macrophages express pro-inflammatory cytokines including IL-8, IL-1β, and TNF-α and various chemokines including CCL2, CCL3, CCL5 (RANTES), CXCL1, CXCL3, and CXCL10 and this macrophage subpopulation likely contributes to excessive lung inflammation by promoting further monocyte recruitment and macrophage differentiation.32 Activated platelets interact with circulating monocytes producing platelet–monocyte aggregates that likely potentiate pulmonary monocyte recruitment and activation.35
Pathogenetic pathways
Although patients may remain Polymerase Chain reaction (PCR) positive for up to 70 days, culturable virus is rarely detected after the 14th day of symptoms.36–39 A delayed interferon response together with the development of adaptive immunity (appearance of neutralizing antibodies) likely results in the cessation of viral replication (viral killing) (see Figure 2).40–42 After the cessation of viral replication, activated immune cells must be removed to prevent hyperactivation of the immune system and continuing tissue damage. The ongoing inflammatory response in patients with severe COVID-19 is a consequence of the hyperactivated immune system rather than of inadequate viral clearance. Transcriptional activation of macrophages with the robust production of cytokines continues beyond clearance of the virus.23,43 This may be related to the failure of natural killer (NK) and cytotoxic T cell to remove activated macrophage, as a consequence of the development of an exhausted cell phenotype.43,44 Furthermore, the high viral load leads to a high concentration of viral RNA fragments (a viral graveyard). Li et al.45 demonstrated that SARS-CoV ssRNA GU (guanosine, uridine) rich fragments had powerful immunostimulatory activity to induce considerable levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-12 via TLR7 and TLR8 pathways.45 Ongoing macrophage activation with the production of pro-inflammatory mediators despite viral clearance is likely responsible for the progressive pulmonary phase seen in patients with severe COVID-19 infection.46 Furthermore, the metabolism of SARS-CoV-2–infected macrophages becomes reprogrammed from mitochondrial oxidative phosphorylation to cytosolic glycolysis.47 This metabolic reprogramming causes SARS-CoV-2–infected macrophages to produce more cytokines leading to further exacerbation of the hyper-inflammatory condition. This is an important finding as simple therapeutic interventions may reverse this metabolic reprogramming.48,49 Patients infected with SARS-CoV-2 may have prolonged macrophage/monocyte activation. Indeed, Patterson and colleagues50 demonstrated the presence of activated monocytes containing spike protein in “long-haul patients” up to 15 months following infection.50 Furthermore, immune profiling has demonstrated additional abnormities in patients who have “recovered” from COVID-19. Orologas-Stavrou et al.51 demonstrated that at 2 months after recovery, convalescent plasma donors had reduced levels of CD4+ T and B cells.51 In this study, previously hospitalized convalescent plasma donors and very low levels of CD8+ regulatory cells together with a Th17 phenotype suggestive of a prolonged pro-inflammatory response. In a follow up study, these investigators demonstrated similar finding at eight months post COVID-19 infection.52
Figure 2.
Stages of COVID and time course of immune response.
While multiple biological pathways and processes underlie the pulmonary phase of COVID-19, we believe that two major pathogenetic processes cause severe COVID-19, namely i) the accumulation of activated macrophages in the lung (alveolar macrophage activation syndrome) with the resultant hyper-inflammatory state leading to multi-organ dysfunction, and ii) an endothelialitis with associated immunothrombosis involving the microvasculature of the lung as well as the brain and fatty tissue. This concept is based on autopsy studies, single-cell profiling of bronchoalveolar lavage (BAL) fluid obtained from critically ill patients as well as an evaluation of the clinical features of severe COVID-19. Consequently, the consensus of the current evidence suggests that a virus-independent immunopathology is the primary mechanism for severe COVID-19 disease.43,53 It is important to emphasize that severe COVID-19 is a multisystem disease affecting the brain, heart, gastrointestinal tract, liver, kidney, and skin in addition to the overwhelming involvement of the lung.46 A number of risk assessment models have been reported allowing for the early identification of hospitalized COVID-19 patients at risk of progressive organ failure, ICU admission, and death.54,55
The pathology of severe COVID-19 infection
Autopsy studies are helpful in determining the pathogenetic mechanisms of COVID-19 infection. In general, these studies revealed an extensive immune infiltrate consisting mainly of activated macrophages and monocytes as well as CD4+ and CD8+ lymphocytes, with features of diffuse alveolar damage (DAD) and an organizing pneumonia.56–63 Typically, the cellular infiltrate is most marked within lung parenchymal regions rather than within vascular/perivascular areas.56 Wang and colleagues64 demonstrated that the predominant cell type found in the alveoli at autopsy were activated macrophages expressing IL-6, IL-10, and TNF-α.64 Melms et al.65 performed single-nucleus RNA-sequencing on lung tissues from COVID-19 decedents.65 In this study, the lungs were highly inflamed with a dense infiltration of aberrantly activated monocyte-derived macrophages. The T cells demonstrated abnormal responsiveness. Alveolar type-2 cells adopted an inflammation-associated transient progenitor cell state and failed to undergo full transition into alveolar type 1 cells resulting in impaired lung regeneration. In addition, they identified expansion of CTHRC1+ pathological fibroblasts contributing to pulmonary fibrosis. Intranuclear inclusions suggestive of a viral cytopathic effect have rarely been reported in these studies.56–59,61,62 While macrophages/monocytes are the predominant immune infiltrate, neutrophils and neutrophil extracellular traps (NETs) have been reported in a number of autopsy studies.66–69 NETs are formed when neutrophils undergo a form of programmed cell death referred to as NETosis.68 NETs are extracellular webs of dsDNA, histones, antimicrobial peptides, and proteases that are released from apoptotic neutrophils.67 Oxidative stress and activated platelets in patients with COVID-19 have been suggested to trigger NETosis.66,68 NETS are important mediators in tissue inflammatory damage and NETs released by SARS-CoV-2–activated neutrophils likely promote lung epithelial and endothelial cell death.66–68,70,71 Furthermore NETosis promotes immunothrombosis which likely contributes to the pro-thrombotic state of COVID-19 patients.66,72 Macrophages play a key role in tissue repair by clearing apoptotic cells, debris and NET’s. Dysfunctional macrophages in COVID-19 may further promote NETosis. It is important to recognize that NETosis can be limited by treatment with anti-oxidants (e.g. vitamin C).73 Severe COVID-19 infection is typically associated with an endothelialitis with a microvascular thrombosis, involving predominantly the vasculature of the lung, brain, skin, and fatty tissue.56–59,61,62 A number of authors have reported complement-mediated microvascular injury with strong staining for C3d and C5b-9 complex deposition in lung tissues.59,74 Variations in this pattern of histologic findings are likely related to the duration of illness prior to death as well as clinical and immune phenotypes.74
Autopsy studies have demonstrated abundant viral RNA in the lung tissues where it localized to the alveolar macrophages and adjacent septal capillary’s endothelia.59 Rare viral RNA is evident in alveolar pneumocytes. Culturable virus is typically not detected in patients who have been symptomatic for greater than 2 weeks.57 Nuovo et al.75 demonstrated viral spike protein without viral RNA localized to ACE-2+ endothelial cells in microvessels in the subcutaneous fat and brain.75 These authors postulate that death of the endothelial cells of the pulmonary capillaries releases pseudovirions into the circulation, and that these pseudovirions dock on ACE-2+ endothelial cells activating the complement pathway/coagulation cascade resulting in a systemic endothelialitis and procoagulant state.59,75,76
Two reports have documented the histologic changes in the lung in the early stages of COVID-19 and provide further evidence demonstrating the predominant role of monocytes and macrophages in this disease.77,78 Tian et al.77 reported two cases of “accidental” lung sampling, in which surgeries were performed for tumors in the lungs at a time when superimposed infections with SARS-CoV-2 was not recognized.77 Histology of non-tumorous lung revealed extensive infiltration with alveolar macrophages, with minimal neutrophil infiltration. There was diffuse thickening of alveolar walls consisting of proliferating interstitial fibroblasts and type II pneumocyte hyperplasia. Focal fibroblast plugs and multinucleated giant cells were seen in the airspaces, indicating varying degrees of the proliferative phase of diffuse alveolar damage. Zeng et al.78 evaluated a biopsy specimen from the lung of a “pre-symptomatic” patient infected with SARS-Cov-2 with who underwent lobectomy for a benign pulmonary nodule reporting pulmonary infiltrates with macrophages being the predominant cell type.78
These histologic findings are strongly supported by single-cell RNA-sequencing of bronchoalveolar lavage (BAL) fluid collected from critically ill intubated COVID-19 patients.79,80 Analysis of BAL fluid demonstrates an abundance of macrophages. Further analysis revealed that the macrophages are primarily inflammatory monocyte-derived, with a relative paucity of resident alveolar macrophages. Consistent with the cytokine pattern in peripheral blood, macrophages have gene-expression signatures characteristic of classic M1 macrophages with increased expression of IL-1, IL-6, TNF-α, and genes encoding several chemokines, including CCL2, CCL3, CCL4, CCL5 (RANTES), and CCL9. Xiong et al. employing transcriptomic analysis of mononuclear/macrophage cells in BAL lavage and peripheral blood revealed increased production of CXCL10 and CCL2/MCP-1.81 These chemokines and cytokines are most likely orchestrating the movement of tissue derived and peripheral blood monocytes/macrophages to the site of infection and eventually replacing the alveolar macrophage as the unabated inflammatory response continues.
Collectively, these data suggest that lung macrophages recruit inflammatory monocytes into the lung which produce cytokines and chemokines that further contribute to a vicious cycle of hyper-inflammation.82,83 The uncontrolled recruitment and activation of macrophages into the lung parenchyma appears to play a central role in the pathogenesis of severe COVID-19 infection. Cytotoxic CD8+ and natural killer (NK) T lymphocytes normally prevent the excessive accumulation of activated macrophages. In patients with severe COVID-19, there is a marked reduction in the number of CD8+ and NK T cells which have an exhausted phenotype.43,84–86 The excessive production of pro-inflammatory cytokines (particularly IL-6) has been linked to T cell dysfunction.44 Furthermore, the intracellular expression of the spike protein of SARS-CoV-2 in lung epithelial cells reduces the activation of NK cells and their ability to degranulate.87 The marked T cell dysfunction reported in patients with severe COVID-19 results in an increased risk of secondary bacterial and fungal infections.88–90
The clinical features of severe COVID-19 support the concept that extensive pulmonary infiltration with activated macrophages is a major pathogenetic factor in this disease. First, the distinctive pattern of progressive multifocal ground-glass opacities noted on CT scans of the chest strongly support a mononuclear cell alveolar infiltrate typical of organizing pneumonia (see Figure 3).91 Patients with COVID-19 pneumonitis almost universally have an increased serum ferritin level.92 An increased serum ferritin is typically associated with macrophage activation. And finally, the clinical features and multisystem organ involvement of severe COVID-19 closely overlaps with the macrophage activation syndrome/hemophagic lymphohistiocytosis syndrome. In the autopsy series of Bryce and colleagues,58’ conspicuous hemophagocytosis and a secondary hemophagocytic lymphohistiocytosis-like syndrome was present in many cases.58
Figure 3.
Progression of CT features of COVID-19 organizing pneumonia.
Other authors have reported features of hemophagocytosis on bone marrow aspirates.93 Indeed, severe COVID should be considered a subtype of the macrophage activation syndrome.
SARS-CoV-2 microangiopathy and complement activation
In addition, to the characteristic histologic changes in the lung as outlined above, severe COVID-19 infection is typically associated with an endothelialitis and microvascular thrombosis, involving predominantly the vasculature of the lung, brain, skin, and fatty tissue.56–59,61,94 The micro-vasculitis is associated with characteristic findings on light microscopy that includes endothelial degeneration and resultant basement membrane zone disruption and reduplication.59 Thrombi in medium-sized arteries, arterioles, and capillaries are typically present with widespread microthrombi and acute infarction in the brain of many descendents.58 The thrombi are noted to be platelet rich.57 It should be recognized that endothelial cells particularly in the lung, brain, and fatty tissue express high concentrations of the ACE-2 receptor. Magro et al. demonstrated complement-mediated microvascular injury affecting the septal capillaries of the lung, and the capillary, venous, and/or arterial microvasculature of the skin and brain in patients with severe COVID-19.59,60 The importance of complement activation is SARS-CoV-2 was demonstrated in an experimental SARS-CoV model where C3 knockout mice (C3 −/− ) demonstrated significantly less lung injury and inflammatory infiltrate than seen in wild-type mice.95
The complement system is part of the innate immune system and can be activated via three separate pathways: the antibody-dependent classical pathway, the mannose-binding lectin (MBL) pathway, and the alternative pathway.96 It is likely that complement is activated in COVID-19 via multiple pathways, both by SARS-CoV-2 itself and by damaged tissues and dying cells at later stages of the disease.60,96 Coronavirus spike glycoprotein binds with mannose-binding lectin (MBL) resulting in activation of MBL-associated serine protease-2 (MASP2).97 MASP2 cleaves complement proteins C2 and C4 activating C3 convertase resulting in the formation of C5b-9 complex. Further, it is important to note that MASP2 activates both the complement and the clotting pathways.98 The complement anaphylatoxins C3a and C5a activate platelets and increase the production of tissue factor further promoting a procoagulant state. In addition, as complement destroys the endothelium, the procoagulant von Willebrand factor and FVIII are released. Therefore, complement activation is closely tied to the development of a procoagulant state in patients with SARS-Co-V-2 infection.
Patients hospitalized with COVID-19 typically develop a hypercoagulable state characterized by increased levels of D-Dimer and a thrombocytopenia which may progress to life-threatening disseminated intravascular coagulation (DIC).99 In addition to the microvascular thrombosis as outlined above, patients are at increased risk of venous thromboembolism. Early and prolonged pharmacological thromboprophylaxis with low molecular weight heparin is therefore recommended.99
COVID-19 organizing pneumonia and NOT ARDS
It is widely, although incorrectly believed, that the pulmonary phase of COVID-19 is typical of ARDS.100,101 The pulmonary phase of COVID-19 has the features characteristic of an organizing pneumonia rather than that of classic ARDS.91 While COVID-19 organizing pneumonia meets the non-specific diagnostic criteria for the ARDS syndrome according to the Berlin Criteria,102 the clinical, radiographic, and histologic features of COVID-19 pneumonia differ significantly from classic ARDS,103–105 as well as the original description of ARDS by Asbaugh and colleagues.106 The radiographic features of COVID-19 are quite distinct and do not resemble the dependent air space consolidation (sponge/baby lung) seen with classic ARDS.107 The initial radiographic features of COVID-19 are peripheral, patchy, multilobar ground glass infiltrates. With disease progression, the radiographic features follow a stereotypic pattern (see Figure 3). ARDS is characterized by decreased pulmonary compliance; however, the lungs in patients with COVID-19 are quite compliant (at least initially).108,109 Most notably, ARDS is characterized by high extra-vascular lung water (non-cardiogenic pulmonary edema).110,111 This is an absolute requirement for the diagnosis of ARDS.112 We have measured the extra-vascular lung water index (EVLWI) in a cohort of ICU patients with COVID-19 organizing pneumonia; no patient had an elevated EVLWI (personal data on file). And lastly, the pathology of COVID-19 organizing pneumonia and classic ARDS are quite distinct. As reviewed above, COVID-19 lung disease is characterized by a massive infiltration of macrophages with few neutrophils. In contrast, ARDs is a neutrophil mediated disease.103–105 Neutropenia lessens the severity of ARDS,113 while in experimental models macrophage depletion reduces the severity of coronavirus lung disease.114 In addition, the complement-mediated microvascular endothelialitis found in the lungs and extra-pulmonary tissues are unique feature of COVID-19 pneumonia.60 While diffuse alveolar damage (DAD) is reported with both COVID-19 pneumonia and ARDS, DAD is a non-specific finding of advanced acute lung injury. The therapeutic implications of the distinction between COVID organizing pneumonia and ARDS is significant; it is likely that the standard treatment of ARDS (with incrementally increasing PEEP)115 will be injurious to the COVID lung and cause the disease one is trying to prevent. A curious finding in patients with COVID-19 (and not ARDS) is that of “silent hypoxia” with a blunted respiratory response.69,116 This phenomenon may be related to SARS-CoV-2 involvement of chemoreceptors of the carotid bodies and/or brain stem dysfunction (Figure 4).
Figure 4.
Pathogenetic mechanism of severe COVID-19 disease.
While macrophage activation and an immune mediated endothelialitis underlie the major pathogenetic mechanism in severe COVID-19 infection, it is likely that other interacting pathways may also play an important role. These include (but are not limited to) platelet activation with high circulating serotonin levels, mast cell activation, auto-antibodies, and a dysregulated renin-angiotenin system.
Platelet activation and increased circulating serotonin
Infection of endothelial cells with SARS-CoV-2 and pseudovirons as well as the dysregulated immune system damages the endothelium and activates blood clotting, causing a severe endothelialitis with the formation of micro and macro blood clots. Clotting activation may occur directly due to increased expression of Factor Xa and tissue factor as well as endothelial injury with the release of large aggregates of von Willebrand factor.117 Furthermore, ACE-2 receptors are present on platelets and this may contribute to the massive platelet aggregation characteristic of severe COVID-19 disease.35,118,119 Platelet activation contributes to the pro-thrombotic state and increases the inflammatory response.35,118,120,121 Activated platelets interact with circulating monocytes forming platelet monocyte aggregates. The aggregates are associated with tissue factor expression by monocytes.35 In addition, platelets from severe COVID-19 patients have been demonstrated to induce tissue factor expression ex vivo in monocytes from healthy volunteers.35 Not only are platelets hyperactivated in COVID-19 but the degree of platelet activation appears to correlate with disease severity. Patients with severe COVID-19 have been shown to harbor a higher degree of platelet activation and platelet–monocyte aggregation compared with patients with COVID-19 that was less severe.35,122 Furthermore, it has been demonstrated that platelets from patients with COVID-19 are activated much more efficiently than platelets from patients with ARDS of non-COVID-19 etiologies in response to thrombin.123
Patients with COVID-19 have increased circulating levels of serotonin (5-hydroxytryptamine, 5HT) likely the result of increased platelet activation and decreased removal by the pulmonary circulation.122–125 Among the mediators released from the granules of activated platelets in COVID-19, serotonin is unique in that 95% of the total body serotonin pool is stored within the platelet granules, and a healthy pulmonary endothelium in required for the clearance of the released serotonin.126–128 Increased circulating serotonin is associated with pulmonary, renal, and cerebral vasoconstriction, and may partly explain the ventilation/perfusion (V/Q) mismatch and reduced renal blood flow noted in patients with severe COVID-19 infection.129–132 Serotonin is a well-established mediator of pulmonary vascular tone and of hypoxic pulmonary vasoconstriction. It exerts its effect on the pulmonary vessels by constricting smooth muscle of both arterioles and postcapillary venules.129,132 Furthermore, serotonin itself enhances platelet aggregation creating a propagating immuno-thrombotic cycle.133 Serotonin promotes pulmonary fibrosis and may contribute to the progressive fibrosis which develops in patients with severe COVID.134 Increased circulating levels of serotonin may explain a number of unique clinical observations noted with COVID-19 infection, these include the unexplained presence of severe hyperventilation (inappropriate rapid breathing),135 the high incidence of ankle clonus and hyperreflexia,136 severe diarrhea, and myocardial ischemia due to coronary vasospasm.137–139 Furthermore, based on high-resolution CT analysis, diffuse vasoconstriction of the pulmonary microvascular bed appears to be one of the earliest abnormalities in the COVID-19 lung injury, preceding the appearance of significant lung infiltrates. Increased serotonin may be responsible for this finding. Elevated plasma serotonin may play a role in the breakdown of the blood brain barrier and contribute to cerebral macrovascular vasoconstriction contributing to the neurological findings reported in COVID-19.140 It is likely that effective serotonin receptor (5HT-2) antagonism may reverse serotonin-mediated pulmonary vasoconstriction, lessen pulmonary platelet trapping, inhibit platelet activation and aggregation, normalize increased respiratory drive, mitigate risk of pulmonary fibrosis, and counteract adverse renal, neurologic, and cardiovascular phenomena in severe COVID-19.122
Antidepressant medications (SSRI) deplete platelet serotonin content, thereby diminishing the release of serotonin following platelet aggregation.141–143 The use of antidepressants has been associated with a lower risk of intubation and death in patients hospitalized with COVID-19.144,145 Similarly, fluvoxamine has been demonstrated to improve the outcome of those with COVID-19.146,147 Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) that activates sigma-1 receptors decreasing cytokine production. These studies support the concept that increased circulating serotonin plays a role in the pathogenesis of COVID-19 disease.
Mast cell activation
Mast cells (MC) are specialized innate immune cells that are strategically localized within the subendothelium.148 MCs are equipped with TLRs and receptors for inflammatory mediators, allowing them to act as sentinels for tissue damage and pathogen exposure.149 Viruses can activate MCs directly or indirectly through viral or inflammatory products such as ssRNA or dsRNA replication intermediates, complement, and cytokines. Mast cells are typically activated by allergic triggers, but they can also be triggered by PAMPS via activation of Toll-like receptors.148 In addition, mast cells express ACE-2 required for SARS-CoV-2 binding, and TMPRSS2, required for priming of the spike protein. Activated MC release vasoactive mediators, including histamine, leukotriene B4 and LTC4, prostaglandin D2, vascular endothelial growth factor, and serine proteases, such as tryptase and chymase.148 MCs also contribute to cytokine networking by releasing the type-2 cytokine IL-4 and IL-6. MC may be an additional source of cytokines and chemokines is patients with COVID-19.150,151 Activated mast cells have been detected in the lungs of deceased patients with COVID-19.152 Furthermore, MC-derived proteases are elevated in COVID-19 patients’ sera and lung tissues.153 However, the pathogenetic role of MC’s in severe COVID-19 disease requires further evaluation.
COVID-19 as an auto-immune disease
As a consequence of molecular mimicry (probably with spike protein), infection with SARS-CoV-2 results in the production of a broad spectrum of auto-antibodies which contributes to the pathophysiology of COVID-19 infection.154–156 In a cohort of 172 hospitalized patients with COVID-19, Wang et al.157 demonstrated a high prevalence of auto-antibodies against immunomodulatory proteins, including cytokines, chemokines, complement components, and cell-surface proteins.157 In a mouse model of SARS-CoV-2 infection, these auto-antibodies increased disease severity. Zuo et al.158 reported that 52% of patients hospitalized with COVID-19 had anti-phospholipid antibodies.158 These antibodies contribute to the profound pro-thrombotic state of severe COVID-19 infection. Both COVID-19 infection as well as spike protein–producing vaccines are associated with anti-platelet antibodies, thrombocytopenia, and a profound procoagulant state.159–161
Pascolini et al.162 reported that 15 of 33 patients (45%) with COVID-19 pneumonia tested positive for at least one autoantibody, including 11 who tested positive for anti-nuclear antibodies (ANAs).162 Four of the patients had a nucleolar ANA pattern while four had a speckled pattern. It should be noted that the nucleolar pattern of ANA is often associated with the interstitial pneumonia that characterizes the clinical course of systemic sclerosis.163 None of the patients had antineutrophil cytoplasmic antibodies (ANCAs). Furthermore, those patients who had auto-antibodies had a worse prognosis. Other authors have similarly reported the presence of ANAs in patients with COVID-19 with nucleolar reactivity being the most frequent pattern detected.164,165 Auto-antibodies against type I interferon are associated with severe life threatening infection.166 Cross reactive neuronal antibodies have been associated with the diverse neurological complications associated with COVID-19 disease.167,168 Type I diabetes and the presence of GAD65 A antibodies have been reported following infection with SARS-CoV-2.169
Altered expression of ACE-2, an unbalanced RAAS and increased bradykinin
ACE-2 is an integral membrane protein that cleaves the carboxyl-terminal amino acid phenylalanine from angiotensin II to produce the vasodilator angiotensin 1–7.170 SARS-CoV-2-induced ACE-2 downregulation and its subsequent deficiency blocks the conversion of angiotensin II into angiotensin 1–7.171 Studies in mice infected by SARS-CoV have demonstrated that internalization of ACE-2 following virus entry in epithelial cells worsens lung inflammation by down-modulating the surface expression of ACE-2.172 SARS-CoV-2–infected patients showed a significant increase in angiotensin II plasma levels. These enhanced angiotensin II plasma levels were inversely correlated with viral load.173 Increased circulating angiotensin I and angiotensin II have been associated with inflammation, oxidative stress and fibrosis. Angiotensin 1–7 also exhibits anti-inflammatory activities in the vascular system by decreasing levels of pro-inflammatory proteins. In addition, ACE-2 degrades bradykinin with ACE-2 deficiency leading to excessive circulating bradykinin.174 In animal models, recombinant ACE-2 has been demonstrated to protect mice from severe acute lung injury.175
Limitations of this study
COVID-19 is an extremely complex and dynamic disease. While we have attempted to be as current as possible, it is likely that important new pathogenetic mechanisms will be reported that were not included in this review. Furthermore, SARS-CoV-2 is a rapidly mutating virus and much of the data presented applies to the original “Wuhan variant”; the pathophysiology of this disease may therefore be dynamically changing with each new variant. Finally, while we believe that macrophage activation is central to the pathogenesis of severe COVID-19, the role of mast cells, endothelial cells, neutrophils, and lymphocyte sub-populations requires further elucidation.
Summary and conclusions
Severe COVID-19 infection is the consequence of the overlapping effects of macrophage activation with uncontrolled inflammation, a complement-mediated endothelialitis and a thrombotic microangiopathy with platelet activation and high circulating serotonin. In addition, mast cell activation, auto-antibodies, and an imbalanced RAAS contribute to the pathogenesis of severe COVID-19 disease. During the first 6 months of the pandemic, the World Health Organization (WHO) and almost all national guidelines recommended a “supportive care only” strategy for the management of severe COVID-19.176 Based on our increased understanding of this disease, such therapeutic nihilism is no longer acceptable. Patients’ transition through a number of different phases (clinical stages) and treatment must be tailored to each specific phase. Antiviral therapy is likely to be effective only during the viral replicative symptomatic phase. As patients progress into the pulmonary phase, they require treatment with multiple therapeutic agents that target the major pathogenetic mechanisms; these include anti-inflammatory agents (methylprednisolone, ivermectin, and fluvoxamine, etc), anticoagulants (heparin and ASA), and anti-serotonin agents (cyproheptadine).5,177,178 And finally, there is no one-size-fits-all protocol, and it is essential that the treatment strategy must be individualized according to the clinical phenotype of each patient.
Footnotes
Author Contributions: PM, concept of paper, review of literature, first draft of paper, reviewed and approves final paper.JI, concept of paper, review of literature, revised manuscript, reviewed and approves final paper.JV, review of literature, revised manuscript, reviewed and approves final paper.PK, review of literature, revised manuscript, reviewed and approves final paper
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor Statement: Paul Marik, MD takes responsibility for the content of the manuscript
ORCID iD
Paul E Marik https://orcid.org/0000-0001-5024-3949
References
- 1.Wu Z, McGoogan JM. (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323: 1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal N, Zhun Cao Z, Gundrum J, et al. (2020) Risk factors associated with in-hospital mortality in a US National Sample of patients with COVID-19. JAMA Network Open 3: e2029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Huang DQ, Zou B, et al. (2020) Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors and outcomes. Journal of Medical Virology 93: 1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalbandian A, Sehgal K, Gupta A, et al. (2021) Post-acute COVID-19 syndrome. Nature Medicine 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kory P, GU M, Iglesias J, et al. (2020) Clinical and scientific rationale for the “MATH+” hospital treatment protocol for COVID-19. Journal of Intensive Care Medicine 36: 135–156. [DOI] [PubMed] [Google Scholar]
- 6.Johansson MA, Kada S, Prasad PV, et al. (2021) SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Network Open 4: e2035057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon KS, Park JI, Park YJ, et al. (2020) Evidence of long-distance droplet transmission of SARS-CoV-2 by direct air flow in a restaurant in Korea. Journal of Korean Medical Science 35: e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh WC, Naing L, Chaw L, et al. (2020) What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. Public Library of Science One 15: e0240205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benefield AE, Skrip LA, Clement A, et al. (2020) SARS-CoV-2 viral load peaks prior to symptom onset: a systematic review and individual-pooled analysis of coronavirus viral load from 66 studies. medRxiv. [Google Scholar]
- 10.Li MY, Li L, Zhang Y, et al. (2020) Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infectious Diseases of Poverty 9: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J, Ge J, Yu J, et al. (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581: 215–220. [DOI] [PubMed] [Google Scholar]
- 12.Hou YJ, Okuda K, Edwards CE, et al. (2020) SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182: 429–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kr S, Becari C, et al. (2018) Comparative expression of renin-angiotensin pathway proteins in visceral versus subcutaneous fat. Front Physiol 9: 1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glowacka I, Bertram S, Muller MA, et al. (2011) Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol 85: 4122–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaduganathan M, Vardeny O, Michel T, et al. (2020) Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 382: 1653–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyrou I, Randeva HS, Spandidos DA, et al. (2021) Not only ACE2 - the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduction and Targeted Therapy 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Mann M, Syed Z, et al. (2021) Furin cleavage of the SARS-CoV-2 spike is modulated by O-glycosylation. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayi BS, Leibowitz JA, Woods AT, et al. (2021) The role of neuropilin-1 in COVID-19. PLos Pathog 17: e1009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachin JM. (2004) The role of measurement reliability in clinical trials. Clinical Trials 1: 553–566. [DOI] [PubMed] [Google Scholar]
- 20.Bowie ASG, Unterholzner L. (2008) Viral evasion and subversion of pattern-recognition receptor signalling. Nature Reviews 8: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farag NS, Breitinger U, Breitinger HG, et al. (2020) Viroporins and inflammasomes: A key to understand virus-induced inflammation. International Journal of Biochemistry and Cell Biology 122: 105738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkins C, Gale M. (2010) Recognition of viruses by cytoplasmic sensors. Current Opinion in Immunology 22: 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. (2020) Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues TS, de Sa KS, Ishimoto AY, et al. (2020) Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. The Journal of Experimental Medicine 218: e20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuyama T, Kubli SP, Yoshinaga SK, et al. (2020) An aberrant STAT pathway is central to COVID-19. Cell Death & Differentation 27: 3209–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia H, Cao Z, Xie X, et al. (2020) Evasion of type I interferon by SARS-CoV-2. Cell Reports 33: 108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frieman MB, Chen J, Morrison TE, et al. (2010) SARS-CoV pathogenesis is regulated by a STAT1 dependent but type I,II and III interferon receptor independnet mechanism. PLos Pathogens 6: e1000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sa Ribero M, Jouvenet N, Dreux M, et al. (2020) Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathogens 16: e1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadjadj J, Yatim N, Barnabel L, et al. (2020) Impaired type I interferon activity and inflammatory respnses in severe COVID-19 patients. Science 369: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B, Deng A, Li K, et al. (2021) Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 delta variant. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheikh A, McMenamin J, Taylor B, et al. (2021) SARS-C-V-2 delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 397: 2461–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua RL, Lukassen S, Trump S, et al. (2020) COVID-19 severity correlates with airway epithelial-immune cell interactions identified by single-cell analysis. Nature Biotechnology 38: 970–979. [DOI] [PubMed] [Google Scholar]
- 33.Nie W, Yan H, Li S, et al. (2009) Angiotensin-(1-7) enhances angiotensin II induced phosphorylation of ERK1/2 in mouse bone marrow-derived dendritic cells. Molecular Immunology 46: 355–361. [DOI] [PubMed] [Google Scholar]
- 34.Abassi Z, Knaney Y, Karram T, et al. (2020) The lung macrophage in SARS-CoV-2 infection: a friend or a foe? Frontiers in Immunology 11: 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hottz ED, Azevedo-Quintanilha I, Palhinha L, et al. (2020) Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 136: 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perera RA, Tso E, Tsang OT, et al. (2020) SARS-CoV-2 virus culture from the upper respiratory tract: Correlation with viral load, subgenomic viral RNA and duration of illness. medRxiv. [Google Scholar]
- 37.van Kampen JJ, van de Vijver DA, Fraaij PL, et al. (2020) Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfel R, Corman VM, Guggemos W, et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465–469. [DOI] [PubMed] [Google Scholar]
- 39.Young BE, Ong S, Ng LF, et al. (2020) Viral dynamics and immune correlates of COVID-19 disease severity. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poland GA, Ovsyannikova IG, Kennedy RB. (2020) SARS-CoV-2 immunity: a review and applications to phase 3 vaccine candidates. Lancet 396: 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan W, Lu Y, Zhang J, et al. (2020) Viral kinetics and antibody responses in patients with COVID-19. medRxiv. [Google Scholar]
- 42.Chen Y, Zuisani A, Fischinger S, et al. (2020) Quick COVID-19 Healers Sustain Anti-SARS-CoV-2 Antibody Production. Cell 183: 1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giamarellos-Bouboulis EJ, Netea MG, Rovina N, et al. (2020) Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host & Microbe 27: 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzoni A, Salvati L, Maggi L, et al. (2020) Impaired immune cell cytotoxicity in severe COVID-10 is IL-6 dependent. The Journal of Clinical Investigation 130: 4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Chen M, Cao H, et al. (2013) Extraordinary GU-rich singlestrand RNA identified from SARS coronavirus contributes an excessive innate immune response. Microbes and Infection 15: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavriatopoulou M, Korompoki E, Fotiou D, et al. (2020) Organ-specific manifestations of COVID-19 infection. Clinical and Experimental Medicine 20: 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campos Coda A, Davanzo GG, de Brito Monteiro L, et al. (2020) Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1 alpha/glycolysis-dependnet axis. Cell Metabolism 32: 498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiter RR, Sharma R, Castillo R, et al. (2021) Coronavirus-19, Monocyte/Macrophage glycolysis and inhibition by melatonin. Journal SARS-CoV2 COVID 2: 29–31. [Google Scholar]
- 49.Reiter RJ, Sharma R, Ma Q, et al. (2020) Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: a mechanistic analysis. Medicine in Drug Discovery 6: 100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patterson BK, Francisco EB, Yogendra R, et al. (2021) Persistence of SARS CoV-2 S1 protein in CD16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orologas-Stavrou N, Politou M, Rousakis P, et al. (2020) Peripheral blood immune profiling of convalescent plasma donors revelas alterations in specific immune subpopulations even at 2 months post SARS-CoV-2 infection. Viruses 13: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kostopoulos IV, Orologas-Stavrou N, Rousakis P, et al. (2021) Recovery of innate immune cells and persisting alternations in adaptive immunity in the peripheral blood of convalescent donors at eight months post SARS-CoV-2 infection. Microorganisms 9: 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorward DA, Russell CD, Um IH, et al. (2020) Tissue-specific immunopathology in fatal COVID-19. American Journal Respiratory and Critical Care Medicine 203: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerotziafas GT, Sergentanis TN, Voiriot G, et al. (2020) Derivation and validation of a predictive score for disease worsening in patients with COVID-19. Thrombosis and Haemostasis 120: 1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmad Q, DePerrior SE, Dodani S, et al. (2020) Role of inflammatory biomarkers in the prediction of ICU admission and mortality in patients with COVID-19. Medical Research Archives 8: 1–10. [Google Scholar]
- 56.Dorward DA, Russell CD, Um IH, et al. (2021) Tissue-specific immunopathology in fatal COVID-19. American Journal Respiratory and Critical Care Medicine 203: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borczuk AC, Salvatore SP, Seshan SV, et al. (2020) COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Modern Pathology 33: 2156–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryce C, Grimes Z, Pujadas E, et al. (2020) The Mount Sinai COVID-19 Autopsy Experience. Pathopysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. medRxiv. [Google Scholar]
- 59.Magro CM, Mulvey J, Kubiak J, et al. (2021) Severe COVID-19: a multifaceted viral vasculopathy syndrome. Annals of Diagnostic Pathology 50: 151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magro C, Mulvey JJ, Berlin D, et al. (2020) Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Translational Research 220: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carsana L, Sonzogni A, Nasr A, et al. (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infectious Disease 20: 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Felix JC, Sheinin YM, Suster D, et al. (2021) Diffuse interstitial pneumonia-like/macrophage activation syndrome-like changes in patients with COVID-19 correlate with lenght of illness. Annals of Diagnostic Pathology 53: 151744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parra-Medina R, Herrera S, Mejia J. (2020) Comments to: A Systematic Review of Pathological Findings in COVID-19: a Pathophysiological Timeline and Possible Mechanisms of Disease Progression. Modern Pathology 34: 1608–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C, Xie J, Zhao L, et al. (2020) Alveolar macrophage activation and cytokine storm in the pathogenesis of two severe severe COVID-19 patients. EBioMedicine 57: 102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melms JC, Biermann J, Huang H, et al. (2021) A molecular single-cell lung atlas of lethal COVID-19. Nature 595: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Middleton EA, He XY, Denorme F, et al. (2020) Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136: 1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo Y, Yalavarthi S, Shi H, et al. (2020) Neutrophil extracellular traps in COVID-19. JCI Insight 5: e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schonrich G, Raftery MJ, Samstag Y. (2020) Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs) and T cell suppression. Advances in Biological Regulation 77: 100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schurink B, Roos E, Radonic T, et al. (2020) Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe 1: e290–e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dupont A, Rauch A, Staessens S, et al. (2021) Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arteriosclerosis, Thrombosis, and Vascular Biology 41: 1760–1773. [DOI] [PubMed] [Google Scholar]
- 71.Veras FR, Pontelli MC, Silva CM, et al. (2020) SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. The Journal Experimental Medicine 217: e20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arcanjo A, Logullo J, Menezes CC, et al. (2020) Emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Scientific Reports 10: 19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohammed BM, Fisher BJ, Kraskauskas D, et al. (2013) Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 5: 3131–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nienhold R, Ciani Y, Koelzer VH, et al. (2020) Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nature Communications 11: 5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nuovo GJ, Magro C, Shaffer T, et al. (2021) Endothelial cell damage is the central part of COVID-19 and a mouse model of injection by the S1 spike subunit of the spike protein. Annals of Diagnostic Pathology 51: 151682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Magro CM, Mulvey JJ, Laurence J, et al. (2020) Docked severe acute respiratory syndrome coronavirus 2 proteins within the cutaneous and subcutaneous microvasculature and their role in the pathogenesis of severe coronavirus disease 2019. Human Pathology 106: 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian S, Hu W, Niu L, et al. (2020) Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. Journal of Thoracic Oncology 15: 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng Z, Xu L, Xie XY, et al. (2020) Pulmonary pathology of early-phase COVID-19 pneumonia in a patient with a benign lung lesion. Histopathology 77: 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liao M, Liu Y, Yuan J, et al. (2020) Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nature Medicine 26: 842–844. [DOI] [PubMed] [Google Scholar]
- 80.Wauters E, Van Mol P, Garg AD, et al. (2021) Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalvelar lavages. Cell Research 31: 272–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong Y, Liu Y, Cao L, et al. (2020) Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cels in COVID-19 patients. Emerging Microbes & Infection 9: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scala S, Pacelli R. (2020) Fighting the host reaction to SARS-COv-2 in critically ill patients: the possible contribution of off-label drugs. Front Immunology 11: 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J, Subbarao K. (2007) The immunobiology of SARS. Annual Review Immunology 25: 443–472. [DOI] [PubMed] [Google Scholar]
- 84.Diao B, Wang C, Tan Y, et al. (2021) Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19). medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng M, Gao Y, Wang G, et al. (2020) Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cellular & Molecular Immunology 17: 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jewett A. (2020) The potential effect of novel Coronavirus SARS-CoV-2 on NK cells; A perspective on potential therapeutic intervensions. Front Immunology 11: 1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bortolotti D, Gentili V, Rizzo S, et al. (2020) SARS-CoV-2 Spike 1 protein controls natural killer cell activation via the HLA-E/NKG2A pathway. Cell 9: 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Musuuza JS, Watson L, Parmasad V, et al. (2021) Prevalence and outcomes of co-infections and superinfections with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS One 16: e0251770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verroken A, Scohy A, Gerard L, et al. (2020) Co-infections in COVID-19 critically ill and antibiotic management: a prospective cohort analysis. Critical Care 24: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fekkar A, Lampros A, Mayaux J, et al. (2021) Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. American Journal Respiratory and Critical Care Medicine 203: 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kory P, Kanne JP. (2020) SARS-CoV-2 organizing pneumonia:”Has there been a widespread failure to identify and treat this prevalent condition in COVID-19?”. BMJ Open Respiratory Research 7: e000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng L, Li H, Li L, et al. (2020) Ferritin in the coronavirus disease 2019 (COVID-19) A systematic review and meta-analysis. Journal of Clinical Laboratory Analysis 34: e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Debliquis A, Harzallah I, Mootien JY, et al. (2020) Haemophagocytosis in bone marrow aspirates in patients with COVID-19. British Journal Haematology 190: e70–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ackermann M, Verleden SE, Kuehnel M, et al. (2020) Pulmonary vascular endothelialitis, Thrombosis, and Angiogenesis in COVID-19. New England Journal Medicine 383: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gralinski LE, Sheahan TP, Morrison TE, et al. (2018) Complemenat activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 9: e01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song WC, FitzGerald GA. (2020) COVID-19, microangiopathy, hemostatic activation, and complement. Journal of Clinical Investigation 130: 3950–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou Y, Lu K, Pfefferle S, et al. (2010) A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. Journal of Virology 84: 8753–8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krarup A, Wallis R, Presanis JS, et al. (2007) Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One 2: e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. (2020) Hematological findings and complications of COVID-19. American Journal Hematology 95: 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grasselli G, Tonetti T, Protti A, et al. (2020) Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respiratory Medicine 8: 1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiumello D, Cressoni M, Gattinoni L. (2020) Covid-19 does not lead to a “typical” acute respiratory distress syndrome. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.ARDS Definition Task Force . Acute respiratory distress syndrome; The Berlin definition. JAMA 2012; 307: 2526–2533. [DOI] [PubMed] [Google Scholar]
- 103.Matthay MA, Ware LB, Zimmerman GA. (2012) The acute respiratory distress syndrome. Journal Clinical Investigation 122: 2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mac Sweeney R, McAuley DF. (2016) Acute respiratory distress syndrome. Lancet 388: 2416–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ware LB, Matthay MA. (2000) The acute respiratory distress syndrome. New England Journal Medicine 342: 1334–1349. [DOI] [PubMed] [Google Scholar]
- 106.Ashbaugh DG, Bigelow DB, Petty TL, et al. (1967) Acute respiratory distress in adults. Lancet 1: 319–323. [DOI] [PubMed] [Google Scholar]
- 107.Gattinoni L, Pesenti A. (2005) The concept of "baby lung. Intensive Care Medicine 31: 776–784. [DOI] [PubMed] [Google Scholar]
- 108.Gattinoni L, Chiumello D, Caironi P, et al. (2020) COVID-19 pneumonia: different respiratory treatment for different phenotypes? Intensive Care Medicine 46: 1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gattinoni L, Chiumello D, Rossi S. (2020) COVID-19 pneumonia: ARDS or not? Critical Care 24: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tagami T, Sawabe M, Kushimoto S, et al. (2013) Quantative diagnosis of diffuse alveolar damage using extravascular lung water. Critical Care Medicine 41: 2144–2150. [DOI] [PubMed] [Google Scholar]
- 111.Tagami T, Kushimoto S, Yamamoto Y, et al. (2010) Validation of extravascular lung water measurement by single transpulmonary thermodilution: human autopsy study. Criical Care 14: R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Phillips CR. (2013) The Berlin definition: real change or the emperor's new clothes? Critical Care 2013: 17–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Azoulay E, Darmon M, Delclaux C, et al. (2021) Deterioration of previous acute lung injury during neutropenia recovery. Critical aare Medicine 30: 781–786. [DOI] [PubMed] [Google Scholar]
- 114.Channappanavar R, Fett C, Mack M, et al. (2017) Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. Journal Immunology 198: 4046–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New English Journal Medicine 2000; 342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 116.Tobin MJ, Laghi F, Jubran A. (2020) Why COVID-19 silent hypoxemia is baffling to physicians. American Journal Respiratory Critical Care Medicine 202: 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Varatharajah N. (2020) COVID-19 CLOT: What is it? Why in the lungs? Extracellular histone, “auto-activation” of prothrombin, emperipolesis, megakaryocytes, “self-association” of Von Willebrand factor and beyond. Preprints. [Google Scholar]
- 118.Zhang S, Liu Y, Wang X, et al. (2020) SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. Journal of hematology & oncology 13: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barrett TJ, Lee AH, Xia Y, et al. (2020) Platelet and vascular biomarkers associated with thrombosis and death in coronavirus disease. Circulation Research 127: 945–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barrett TJ, Lee AH, Xia Y, et al. (2020) Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease. Circulation Research 127: 945–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cloutier N, Allaeys I, Marcoux G, et al. (2018) Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration 115: PNAS: E1550–E1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jalali F, Rezaie S, Rola P, et al. (2021) COVID-19 Pathophysiology: Are Platelets and Serotonin Hiding in Plain Sight? Ssrn. [Google Scholar]
- 123.Zaid Y, Guessous F, Puhm F, et al. (2021) Platelet reactivity to thrombin differs between patients with COVID-19 and those with ARDS unrelated to COVID-19. Blood Advances 5: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zaid Y, Puhm F, Allaeys I, et al. (2020) Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circulation Research 127: 1404–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dawson C, Christensen CW, Rickaby DA, et al. (1985) Lung damage and pulmonary uptake of serotonin in intact dogs. Journal of Applied Physiology 58: 1761–1766. [DOI] [PubMed] [Google Scholar]
- 126.Adnot S, Houssaini A, Abid S, et al. (2013) Serotonin transporters and serotonin receptors. Handbook of Experimental Pharmacology 218: 365–389. [DOI] [PubMed] [Google Scholar]
- 127.Cloutier N, Pare A, Farndale RW, et al. (2012) Platelets can enhance vascular permability. Blood 120: 1334–1343. [DOI] [PubMed] [Google Scholar]
- 128.Dawson CA, Christensen CW, Rickaby DA, et al. (1985) Lung damage and pulmonary uptake of serotonin in intact dogs. Journal of Applied Physiology 58: 1761–1766. [DOI] [PubMed] [Google Scholar]
- 129.MacLean MR, Herve P, Eddahibi S, et al. (2000) 5-hydroxytryptamine and the pulmonary circulation: receptors, transporters and the relevance to pulmonary arterial hypertension. British Journal of Pharmacology 131: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blackshear JL, Orlandi C, Hollenberg NK. (1991) Constrictive effect of serotonin on visible renal arteries: a pharmacoangiographic study in anesthetized dogs. Journal of Cardiovascular Pharmacology 17: 68–73. [DOI] [PubMed] [Google Scholar]
- 131.Watchorn J, Hang DY, Joslin J, et al. (2021) Critically ill COVID-19 patients with acute kidney injury have reduced renal blood flow and perfusion despite preserved cardiac function: A case-control study using contrast enhanced ultrasound. Shock 55: 479–487. [DOI] [PubMed] [Google Scholar]
- 132.McGoon MD, Vanhoutte PM. (1984) Aggregating platelets contract isolated canine pulmonary arteries by releasing 5-hydroxytryptamine. Journal of Clinical Investigation 74: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Almqvist P, Skudder P, Kuenzig M, et al. (1984) Effect of cyproheptadine on endotoxin-induced pulmonary platelet trapping. American Surgeon 50: 503–505. [PubMed] [Google Scholar]
- 134.Skurikhin EG, Andreeva TV, Khnelevskaya ES, et al. (2012) Effect of antiserotonin drug on the development of lung fibrosis and blood system reactions after intratracheal administration of bleomycin. Bulletin of Experimental Biology and Medicine 152: 519–523. [DOI] [PubMed] [Google Scholar]
- 135.Pamenter ME, Powell F. (2016) Time domains of the hypoxic ventilatory response and their molecular basis. Comprehensive Physiology 6: 1345–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Helms J, Kremer S, Merdji H, et al. (2020) Neurologic features in severe SARS-CoV-2 infection. New England Journal of Medicine 382: 2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rivero F, Antuna P, Cuesta J, et al. (2021) Severe coronary spasm in a COVID-19 patient. Cathet Cardiovascular Inverventions 97: e670–e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bangalore S, Sharma A, Slotwiner A, et al. (2020) ST-Segment elevation in patients with Covid-19: a case series. New England Journal of Medicine 382: 2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.El-Bialy A, Shenoda M, Caraang C. (2006) Refractory coronary vasospasm following drug-eluting stent placement treated with cyproheptadine. Journal of Invasive Cardiology 18: E95–E98. [PubMed] [Google Scholar]
- 140.Lins M, Vandevenne J, Thillai M, et al. (2020) Asessment of small pulmonary blood vessels in COVID-19 patients using HRCT. Academic Radiology 27: 1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maurer-Spurej E, Pittendreigh C, Solomons K. (2004) The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thrombosis Haemostasis 91: 119–128. [DOI] [PubMed] [Google Scholar]
- 142.Bismuth-Evenzal Y, Gonopolsky Y, Gurwitz D, et al. (2012) Decreased serotonin content and reduced agonist-induced aggregation in platelets of chronically medicated with SSRI drugs. Journal of Affective Disorders 136: 99–103. [DOI] [PubMed] [Google Scholar]
- 143.Javors MA, Houston JP, Tekell JL, et al. (2000) Reduction of platelet serotonin content in depressed patients treated with either paroxetine or desipramine. International Journal of Neuropsychopharmacology 3: 229–235. [DOI] [PubMed] [Google Scholar]
- 144.Hoertel N, Sanchez-Rico M, Vernet R, et al. (2021) Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVId-19: Results from an observational study. Molecular Psychiatry. [DOI] [PubMed] [Google Scholar]
- 145.Zimering MB, Razzaki T, Tsang T, et al. (2020) Inverse association between serotonin 2A receptor antagonist medication use and mortality in severe COVID-19 infection. Endocrinology Diabetes Metabolism Journal 4: 1–5. [PMC free article] [PubMed] [Google Scholar]
- 146.Lenze EJ, Mattar C, Zorumski CF, et al. (2020) Fluvoxamine vs placebo and clinical deterioration in outpatietns with symptomatic COVID-19. A randomized clinical trial. JAMA 324: 2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seftel D, Boulware DR. (2021) Prospective cohort of fluvoxamine for early treatment of COVID-19. Open Forum Infectious Diseases 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Theoharides TC, Valent P, Akin C. (2015) Mast cells, mastocytosis, and related disorders. New England Journal Medicine 373: 163–172. [DOI] [PubMed] [Google Scholar]
- 149.St John AL, Abraham SN. (2021) Innate immunity and its regulation by Mast cells. Journal of Immunology 190: 4458–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Theoharides TC. (2021) Potential association of mast cells with coronavirus 2019. Annals of Allergy Asthma Immunology 126: 217–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Afrin LB, Weinstock LB, Molderings GJ. (2020) Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. International Journal of Infectious Diseases 100: 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.da Silva Motta Junior J, dos Santos Miggiolaro AF, Nagashima S, et al. (2020) Mast cells in alveolar septa of COVID-19 patients: A pathogenic pathway that may link interstitial edema to immunothrombosis. Frontiers in Immunology 11: 574862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gebremeskel S, Schanin J, Coyle KM, et al. (2021) Mast cell and eosinophil activation are associated with COVID-19 and TLR-mediated viral inflammation: Implications for an anti-siglec-8 antibody. Frontiers in Immunology 12: 650331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schiaffino MT, Di Natale M, Garcia-Martinez E, et al. (2020) Immunoserologic detection and diagnostic relevance of cross-reactive autoantibodies in Coronavirus disease 2019 patients. Journal of Infectious Diseases 222: 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trahtemberg U, Fritzler MJ. (2021) COVID-19-associated autoimmunity as a feature of acute respiratory failure. Intensive Care Medicine 47: 801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Woodruff MC, Ramoneli RP, Lee FE, et al. (2020) Broadly-targeted autoreactivity is common in severe SARS-CoV-2 infection. medRxiv. [Google Scholar]
- 157.Wang EY, Mao T, Klein J, et al. (2021) Diverse functional autoantibodies in patients with COVID-19. Nature. [DOI] [PubMed] [Google Scholar]
- 158.Zuo Y, Estes SK, Ali RA, et al. (2020) Prothrombotic autoantibodies in the serum from patients hospitalized with COVID-19. Science Translation Medicine 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen S, Zhang J, Fang Y, et al. (2021) SARS-CoV-2 interacts with platelets and megakarocytes via ACE-2 independent mechanism. Journal of Hematology and Oncology 14: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.See I, Su JR, Lale A, et al. (2021) US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination. JAMA 325: 2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pascolini S, Granito A, Muratori K, et al. (2021) Coronavirus disease associated immune thrombocytopenia: Causation or correlation? Journal of Microbiol Immunology and Infection 54: 531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pascolini S, Vannini A, Deleonardi G, et al. (2021) COVID-19 and immunological dysregulation: Can autoantibodies be useful? Clinical and Translation Science 14: 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Betteridge ZE, Woodhead F, Lu H, et al. (2016) Anti-eukaryotic initiation factor 2B autoantibodies are assocaited with interstitial lung disease in patients with systemic sclerosis. Arthritis & Rheumatology 68: 2778–2783. [DOI] [PubMed] [Google Scholar]
- 164.Muratori P, Lenzi M, Muratori L, et al. (2021) Antinuclear antibodies in COVID-19. Clinical Translation Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang SH, Minn D, Kim YK. (2021) Autoantibodies in moderate and critical cases of COVID-19. Clinical Translation Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bastard P, Rosen LB, Zhang Q, et al. (2020) Auto-antibodies against type I IFNs in patients with life-treatening COVID-19. Science 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kreye J, Reincke SM, Pruss H. (2020) Do cross-reactive antibodies cause neuropathology in COVID-19? Nature Reviews 20: 645–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Franke C, Ferse C, Kreye J, et al. (2021) High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain, Behavior, and Immunity 93: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marchand L, Pecquet M, Luyton C. (2020) Type 1 diabetes onset triggered by COVID-19. Acta Diabetologica 57: 1265–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Donoghue M, Hsieh F, Baronas E, et al. (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) convets angiotensin I to angiotensin 1-9. Circulation Research 87: e1–e9. [DOI] [PubMed] [Google Scholar]
- 171.Lee C, Choi WJ. (2021) Overview of COVID-19 inflammatory pathogenesis from the therapeutic perspective. Archivesof Pharmacal Research 44: 99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kuba K, Imai Y, Rao S, et al. (2005) A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nature Medicine 11: 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Y, Yang Y, Zhang C, et al. (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Science China Life Science 63: 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, et al. (2018) Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. American Journal of Physiology Lung Cellular Molecular Physiology 314: L17–L31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Imai Y, Kuba K, Rao S, et al. (2005) Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.World Health Organization (2020) Clinical Management of COVID-19. Interim Guidance. 27th May 2020. https://www.who.int/publications/i/item/clinical-management-of-covid-19 WHO/2019-nCoV/clinical/2020.5. [Google Scholar]
- 177.Marik PE, Kory P, Varon J, et al. (2020) MATH+ protocol for the treatment of SARS-CoV-2 infection: the scientific rationale. Expert Review of Anti Infective Therapy 19: 129–135. [DOI] [PubMed] [Google Scholar]
- 178.Kory P, GU M, Iglesias J, et al. (2020) Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. American Journal of Therapeutics 28: e299–e318. [DOI] [PMC free article] [PubMed] [Google Scholar]