Highlights
-
•
TDE and PFE can characterize the temporal organization of iBA in DOC patients.
-
•
DOC patients showed abnormal TDP and PFP mainly in DMN and SMN.
-
•
DOC patients had a shortened range of TD value relative to the healthy controls.
-
•
The temporal propagation structure of iBA can be used to predict clinical scores for DOC patients.
Keywords: Time delay estimation (TDE), Probabilistic flow estimation (PFE), Connectome-based predictive modeling (CPM), Propagation structure, Default mode network (DMN)
Abstract
Background
The detection of intrinsic brain activity (iBA) could assist clinical assessment for disorder of consciousness (DOC) patients. Previous studies have revealed the altered iBA in thalamocortical, frontoparietal, and default mode network in DOC patients using functional connectivity (FC) analysis. However, due to the assumption of synchronized iBA in FC, these studied may be inadequate for understanding the effect of severe brain injury on the temporal organization of iBA and the relationship between temporal organization and clinical feature in DOC patients. Recently, the time delay estimation (TDE) and probabilistic flow estimation (PFE) were proposed to analyze temporal organization, which could provide propagation structure and propagation probability at whole-brain level.
Methods
We applied voxel-wise TDE and PFE to assess propagation structure and propagation probability for the DOC patients and then applied the connectome-based predictive modeling (CPM) to predict clinical scores for patients based on the ROI-wise TDE and PFE.
Results
We found that: 1) the DOC patients showed abnormal voxel-wise time delay (TD) and probabilistic flow (PF) in the precentral gyrus, precuneus, middle cingulate cortex, and postcentral gyrus, 2) the range of TD value in the patients was shorter than that in the controls, and 3) the ROI-wise TD had a better predictive performance for clinical scores of the patients compared with that based on ROI-wise PF.
Conclusion
Our findings may suggest that the propagation structure of iBA could be used to predict clinical scores in DOC patients.
1. Introduction
Disorders of consciousness (DOC), including vegetative state/unresponsive wakefulness syndrome (VS/UWS) and minimally conscious state (MCS), refers a continuum of disruption in the wakefulness and awareness systems resulted from severe brain injury (Giacino et al., 2002, Laureys et al., 2010, Lee et al., 2020). As two components of consciousness, wakefulness refers to a physiological state of being awake and awareness is the perception of self and environment (Laureys, 2005). The VS/UWS patients could keep wakefulness but show no signs of awareness (Laureys, 2005). The MCS patients are characterized by inconsistent but reproducible signs of awareness (Giacino et al., 2002). However, it is difficult to determine the state of consciousness for DOC patients due to the lack of overt behavior response from patients (Schnakers et al., 2009). Recently, the functional magnetic resonance imaging (fMRI) technique is used to explore intrinsic brain activity (iBA) in DOC patients and the altered iBA is considered to assist the clinical assessment for DOC patients (Cao et al., 2019, Song et al., 2018). The iBA refers to the neural states that are produced spontaneously by the brain and not as responses to external stimulus (Boly et al., 2008). The iBA is believed to play a central role in human brain function and can be analyzed using resting-state fMRI (rs-fMRI) data (Raichle, 2015).
Previous studies analyzed inter-regional functional connectivity (FC) based on the rs-fMRI data and found abnormal iBA in the thalamocortical network (Schiff, 2008), frontoparietal network (Soddu et al., 2012), and default mode network (DMN) (Vanhaudenhuyse et al., 2010) in DOC patients. FC analyses assumed that the iBA among brain regions is synchronized or zero-time lag correlation (Preti et al., 2017). However, recent studies suggested that iBA exhibits dynamic fluctuation (Allen et al., 2014, Vidaurre et al., 2017). The abnormality of fluctuation of iBA has been identified in DOC patients (Cao et al., 2019, Demertzi et al., 2019, Huang et al., 2020, Luppi et al., 2019). For example, the reduced dynamic FC was found in the posterior DMN, sensory network and somatomotor network in DOC patients compared with healthy controls (Cao et al., 2019, Luppi et al., 2019).
In addition to the findings on fluctuation of iBA, accumulating evidence (Ferezou et al., 2007, Mitra et al., 2017b, Mohajerani et al., 2013, Mohajerani et al., 2010) showed an intrinsic brain propagation activity in the whole brain. Using electrodes, several animal studies found that the iBA is asynchronous in the rat brain (Ferezou et al., 2007, Mohajerani et al., 2013, Mohajerani et al., 2010) and inferred a time delay (TD) to describe the iBA propagation from one region to other regions. Based on the concept of TD, Mitra et al. (2014) introduced TD estimation (TDE) to assess propagation structure of iBA between a pair of regions in human brain. Different from dynamic FC analysis, TDE emphasizes the temporal ordering of iBA rather than the temporal fluctuation of iBA. The studies of TDE showed that iBA propagated across whole-brain brain networks on a time scale of ~1s in healthy controls (Mitra et al., 2015a, Mitra et al., 2014). Subsequently, based on TDE and correlation analysis, Mitra et al. (2020) introduced probabilistic flow estimation (PFE) to describe propagation probability of iBA by calculating the probabilistic flow (PF) between the timeseries of each voxel in whole brain. Totally, TDE and PFE offer the possibility to describe the features of intra- and inter-regional temporal organization of iBA. Previous TDE studies have reported the altered propagation structure in autism (King et al., 2018, Mitra et al., 2017a), post-traumatic stress disorder (PTSD) (Weng et al., 2018), and epilepsy patients (Shah et al., 2018, Shah et al., 2019). As for DOC patients, the impact of severe brain injury on the temporal organization of iBA is remain unclear.
Machine learning has been widely applied to neuroimaging data to aid the clinical assessment for various brain disorders (Edlow et al., 2021, Rehme et al., 2015, Shen et al., 2017). Several studies (Campbell et al., 2020, Cao et al., 2019, Song et al., 2018) used machine learning to explore the imaging indicator that can promote the diagnosis and/or prognosis of DOC patients. Song et al. (2018) proposed a prognostic model by taking the FC as features in a classification algorithm, and discriminated the DOC patients who would later recover consciousness from these not recover with an accuracy of 88%. However, we are still lacking the accurate and efficient neuroimaging indicator for accessing the clinical scores of DOC patients. In this study, we asked the question whether the TD or PF could be used as a neuroimaging indicator to predict clinical scores of DOC patients. Thus, we applied connectome-based predictive modeling (CPM), a data-driven machine-learning method (Shen et al., 2017), to assess the prediction performance of TD and PF for the clinical scores of patients. The CPM was selected because it is a validated approach for extracting and pooling the most relevant features from neuroimaging data to predict the clinical score (Finn et al., 2015, Shen et al., 2017).
Our aims were to reveal the altered propagation structure and propagation probability of iBA and then to evaluate the predictive performance of the temporal organization for clinical scores in DOC patients. We hypothesized that the severe brain injury may affect the propagation structure or propagation probability of iBA in several critical brain regions of DMN and FPN in DOC patients, and the temporal metrics (TD and PF) of iBA may be related to the clinical scores of DOC patients.
2. Methods
2.1. Subjects
A total of 45 DOC patients were recruited from the Liuhuaqiao Hospital in Guangzhou City, China. The patients were assessed by two medical doctors (QX and RY) according to the Coma Recovery Scale-Revised (CRS-R) scale, a 23-item scale for measuring the behavioral responses of DOC patients. The scale includes 6 subscales that measure the auditory, visual, motor, oromotor/verbal, communication and arousal levels of the patient, respectively. We also recruited 17 healthy volunteers who had no history of neurological or psychiatric illnesses as the control group. The study protocol was approved by the Ethics Committee of the Liuhuaqiao Hospital. Written informed consent was obtained from each patient’s guardian and healthy volunteers.
2.2. Data acquisition
The MRI data were acquired on a GE 3 T MR scanner with an eight-channel phased-array head coil. The rs-fMRI data were obtained using a single-shot multi-slices gradient-echo EPI sequence with the following parameters: repetition time (TR) = 2,000 ms, echo time (TE) = 26 ms, flip angle (FA) = 90°, field of view (FoV) = 240 × 240 mm2, data matrix = 64 × 64, 36 axial interleaved slices covering the whole brain, and 240 volumes obtained in about 8 min. Subjects were requested to keep their eyes open when subjects were in the rs-fMRI scanning. In practice, we had checked to make sure the patient eyes open before and after the scan. If a patient cannot keep eyes-opening, we stopped the scan procedure and resumed it later. The high-resolution brain structural images were also acquired using a T1-weighted 3D Fast Spoiled Gradient Recalled (FSPGR) sequence (TR = 8.86 ms, TE = 3.52 ms, FoV = 240 × 240 mm2, data matrix = 256 × 256, FA = 90°, voxel size = 0.94 × 0.94 × 1 mm3, and 176 sagittal slices). Additionally, the 2D T1-weighted and 2D T2-FLAIR images were also acquired for clinical diagnosis.
Fig. 1a shows the visual quality checking steps. All brain images under analysis were visually inspected and those showing an insufficient level of quality are excluded. The exclusion criteria for the patients were 1) focal brain damage, 2) artifacts, and 3) excessive head movement. In the calculation, for each subject, we have checked the head motion of the fMRI datasets and excluded the whole datasets from the further analysis if the mean framewise displacement mean (FD) > 0.2 (Power et al., 2012). We also excluded the patients who were diagnosed with locked-in syndrome (LIS), or emerged minimally conscious state (EMCS), or had disease course more than one year. According to the exclusion criteria, we excluded 28 patients’ data from the recruited 45 patients, and only retained 17 patients. We included 17 healthy subjects for the further analysis. The demographic and clinical profiles of the patients are described in Table 1.
Fig. 1.
The workflow to illustrate the exclusion criteria for the patients with disorders of consciousness (DOC) and the estimation of time delay (TD) and probabilistic flow (PF). (a) Exclusion criteria for the fMRI datasets of DOC patients. A total of 28 patients’ fMRI datasets were excluded because of the following reasons: 1) excessive brain lesion or atrophy, 2) heavy artifact, 3) overlarge head movement, 4) diagnosed as patients with locked-in syndrome (LIS) or emerged minimally conscious state (EMCS), and 5) other types of imaging quality problems. This study retained the resting-state fMRI datasets of 17 patients, including 11 patients with vegetative state/unresponsive wakefulness syndrome (VS/UWS) and 6 patients in a minimally conscious state (MCS), for the further analysis. (b) Illustration of time delay estimation (TDE). We computed the lagged cross-covariate function (CCF) between pairs of timeseries in two different voxels (i and j) and determined the maximum of CCF by using a parabolic interpolation. The τi,j was defined as TD value when CCF reached maximum. (c) Illustration of probabilistic flow estimation (PFE). The values of correlation coefficient (r) and the time delay (TD) were normalized to [-1, 1]. The PF value was computed by combining the normalized r and the normalized TD. (d) Calculation of the lag projection of TD and PF (TDP and PFP). We obtained the TDP (PFP) by averaging each column of the TD (PF) matrix for each subject. (e) Illustration of connectome-based predictive modeling (CPM). After acquiring the ROI-wise TD/PF matrix based on Shen-268 atlas, we first calculated Pearson’s correlation coefficient between the edges (TD/PF value) and the clinical scores, and then summed the significant edges as the feature for each subject. Using the LOOCV, we split the all patients into the training set to build the linear regression model and the test set to predict the clinical score of the remaining patient. At last, the significance of this model was evaluated by 1,000 nonparametric permutation tests.
Table 1.
The demographic and clinical information of the patients with disorders of consciousness (DOC).
| Patient | Gender (M/F) | Months to the scan | Age (years old) | VS/MCS | Etiology | CRS-R score |
|---|---|---|---|---|---|---|
| Au/V/M/O/C/Ar/T | ||||||
| P01 | M | 1 | 21 | VS | HIE | 1/0/2/1/0/1/5 |
| P02 | M | 2 | 39 | VS | TBI | 0/0/2/1/0/2/5 |
| P03 | M | 2 | 16 | VS | HIE | 0/0/1/0/0/2/3 |
| P04 | M | 2 | 64 | VS | TBI | 0/0/1/1/0/2/4 |
| P05 | M | 1 | 62 | VS | HIE | 0/0/1/0/0/2/3 |
| P06 | M | 1 | 43 | VS | HIE | 0/0/1/1/0/2/4 |
| P07 | M | 1 | 48 | VS | HIE | 0/0/1/0/0/2/3 |
| P08 | M | 9 | 32 | VS | HIE | 1/0/1/1/0/2/5 |
| P09 | M | 1 | 39 | VS | HIE | 0/0/2/1/0/2/5 |
| P10 | M | 1 | 62 | VS | HIE | 0/0/1/0/2/2/3 |
| P11 | F | 1 | 52 | VS | HIE | 0/0/0/1/0/2/3 |
| P12 | M | 1 | 30 | MCS | TBI | 1/1/3/2/0/2/9 |
| P13 | F | 1 | 59 | MCS | TBI | 1/0/5/1/0/2/9 |
| P14 | F | 1 | 15 | MCS | HIE | 1/3/5/1/0/2/12 |
| P15 | F | 2 | 20 | MCS | TBI | 1/1/2/1/0/2/7 |
| P16 | M | 9 | 41 | MCS | HIE | 2/3/2/1/0/1/9 |
| P17 | M | 3 | 47 | MCS | HIE | 1/3/0/1/0/2/7 |
Abbreviations: M, male; F, female; VS, vegetable state; MCS, minimally conscious state; TBI, traumatic brain injury; HIE, hypoxic ischemic encephalopathy; CRS-R, Coma Recovery Scale-Revised; Au, auditory; V, visual; M, motor; O, oromotor; C, communication; Ar, arousal; T, total CRS-R score.
2.3. Data preprocessing
The functional images were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and DPABI (Yan et al., 2016). For each individual’s rs-fMRI data, we removed the first ten volumes, performed slice-timing correction, corrected head movement. We co-registered functional images to the individual structural images and normalized them in the MNI standard space. Finally, the functional images were smoothed with a 6 mm full-width-at-half-maximum (FWHM) Gaussian kernel and filtered to 0.01–0.1 Hz. In the calculations, the nuisance variables, including 24 head movement parameters (Friston et al., 1996) and the mean signals of brain white matter (WM), cerebrospinal fluid, and whole brain, were regressed out. To reduce the computational load, we resampled the functional data to (6 mm)3 isotropic voxels and acquired n = 8,434 voxels in the MNI space for each individual brain.
2.4. Relationship between FC, TD, and PF
FC and TD are independent metrics to describe the spatial and temporal relationship between a pair of blood oxygen level dependent (BOLD) signals, respectively. Both these metrics could be used to describe systems-level organization of iBA in large-scale network level (Mitra et al., 2020). The conventional FC, by measuring the zero-lag Pearson’s correlation coefficient between pairs of timeseries, represents the synchronization between spatially distinct neurophysiological events (Friston et al., 1993). However, the FC may be unsuitable to reveal the directionality of inter-regional FC in the whole brain. In fact, there are several approaches can be taken to infer the directionality of BOLD signal propagation, including dynamic causal modelling (DCM) and Granger causality analysis (GCA). However, DCM is limited in the number of ROIs and specific hypotheses, and thus is unsuited to an exploratory analysis at the whole-brain level (Stephan et al., 2010). GCA could be used in whole-brain analysis, but the theoretical assumption of GCA may not fit the properties of fMRI data (a low sampling rate and a long delay between the BOLD signal and neural activity) (Friston et al., 2013). Thus, we took TDE and PFE to estimate the direction of inter-regional interactions.
2.5. Time delay estimation
We performed TDE by calculating the lagged CCF between BOLD signals at two voxels (Mitra et al., 2014). The lagged CCF represents the correlation between two signals with all possible lags (Mitra et al., 2014) and is given by
| (1) |
where τ represents the lag between the two BOLD signals xi and xj at two spatial indexes i and j, and the τ value is defined as TD when reaches the maximum. The zero-lag (τ = 0) CCF is the conventional inter-regional FC. As shown in Fig. 1b, the maximum of was obtained using a parabolic interpolation. The space index n represents the total voxels of the whole brain. In this study, the window size is the total scan time t = 230 TRs (TR = 2 s) of the functional data. The τ value was set not to exceed 6 s since BOLD response is typically modeled as the convolution of a stimulus and the hemodynamic response function (HRF) which typically peaks at 5–6 s after human brain respond to the stimulus delivery (Aguirre et al., 1998, Saad et al., 2003). Due to the aperiodicity of BOLD timeseries (He et al., 2010), the TD value is single and well-defined. The TD between any pairs of timeseries was estimated across all voxels (n = 8,434) in whole brain according to Eq.1, and the resulting set of τ which represents TD value was assembled into a matrix as follows:
| (2) |
where TD matrix is a square anti-symmetric matrix. The off-diagonal elements τi,j = -τi,j, and the diagonal elements are zeros.
For each subject, we computed the lag projection of time delay (TDP) by the following formula:
| (3) |
| (4) |
where the voxel-wise TDP is calculated through averaging the elements in each column of the TD matrix, which indicates whether the BOLD signal in a given voxel is either earlier or later than the rest of the brain. Fig. 1d illustrates the steps for calculating the voxel-wise TDP for each subject. In each group, the group-level TDP map was obtained by averaging voxel-wise TDP across all DOC patients or healthy controls, indicating the propagation structure of iBA at the group-level. In this way, we obtained the voxel-wise TDP for each subject and obtained the group-level TDP map.
2.6. Probabilistic flow estimation
The probabilistic flow (PF) was calculated according to Mitra et al. (2020). For a pair of voxel-wise timeseries, we first estimated the TD (τ) value by using lagged CCF and a parabolic interpolation. Meanwhile, the correlation value (r) at τ was acquired using lagged CCF and was termed the peak correlation (Fig. 1c). This calculation was repeated for all pairs of voxels. To diminish the influence of noise signals on PF, we set the threshold |r| = 0.10 in calculating the peak correlation (Raut et al., 2019b) and set PF = 0 when |r| < 0.10. Next, both r and TD values were separately normalized to [-1, 1] to make sure their magnitudes on the same scale. The PF value, propagation probability for a pair of timeseries at voxels, was given by (Mitra et al., 2020):
| (5) |
where r represents the peak correlation coefficient, and τ represents the TD value.
For each subject, we first acquired the asymmetric PF matrix from all pairs of timeseries at voxels, which is given by Mitra et al. (2020)
| (6) |
Then we calculated the voxel-wise lag projection of probabilistic flow (PFP) according to the following equation:
| (7) |
where PFip = i/nnj=1PFi,j shown in Fig. 1d, n = 8,434 is the number of voxels. The group-level PFP map was obtained by averaging the voxel-wise PFP values across all the DOC patients or the healthy controls. To build probabilistic distributions for the PF, we normalized the cumulative sum of all positive and negative prob to one, separately. The detailed theory and MATLAB code of TDE and PFE can be found in (Mitra et al., 2020, Raut et al., 2019b).
2.7. Statistical analysis for TDP and PFP between groups
A nonparametric permutation t-test with 5,000 iterations was applied based on FSL Randomize tool (Chen et al., 2018b, Winkler et al., 2016) to test between-group differences in whole-brain TDP and PFP. The significance threshold p-values were set to 0.05 and was corrected for multiple comparisons using threshold-free cluster enhancement (TFCE). In the calculations, the mean FD, gender, and age were taken as nuisance covariates.
2.8. Connectome-based predictive modeling for the CRS-R scores
Fig. 1e shows the procedures of the CPM analysis. Consistent with previous studies on CPM (Finn et al., 2015, Rosenberg et al., 2016), we selected Shen-268 atlas (Shen et al., 2013) to extract the timeseries of each ROI by averaging all voxels’ signals. Next, we estimated the values of TD and PF for all pairs of ROIs by using TDE and PFE, resulting in a 268 × 268 TD matrix and a 268 × 268 PF matrix for each subject. Since the TD and PF matrices are anti-symmetric, we only selected the upper triangle elements as the edges (i.e., TD or PF) for the following analysis.
We used the leave-one-out cross validation (LOOCV) approach to construct linear regression model in the training set and to predict the clinical score of the remaining patient in the test set. A total of 16 of 17 DOC patients were taken as the training set, and the remaining one patient was selected as the test set. We calculated Pearson’s correlation coefficient between CRS-R score (the sum of subscale score) and each edge in the TD (PF) matrix for each patient in the training set. Then, edges that significantly positively and negatively correlated with the clinical score (threshold pedge < 0.001) were retained. These edges will make up the positive (negative) network (Fig. 5c). Next, we summed the strength of edges within the positive (negative) network for each subject as the positive (negative) features.
Fig. 5.
The CPM results derived from TD matrix. (a) Model performance (rperf) at different edge selection thresholds (pedge). The model performance reached the maximum (rperf = 0.662, p = 0.006) at the threshold pedge = 0.001. (b) Scatterplots between the predicted and observed CRS-R scores at the threshold pedge = 0.001, standardized for visualization. (c) Circle plots for the positive and negative predictive networks. All 42 edges showing significantly correlations with CRS-R scores were classified in ten macroscale brain regions, of which 21 edges constituted of the positive network and 21 negative edges constituted of the negative network. The ten macroscale brain regions were organized around the circle according to their anatomical locations and each semicircle represents a hemisphere of the brain. (d) The nodes and its propagation direction with other nodes. These 11 nodes are in the cerebellum (CB), prefrontal cortex (PFC), and other regions (parietal, limbic, and subcortical region). The detailed information of the nodes is labeled in Table 3. The blue line indicates the propagation direction of iBA from other nodes to the labelled node and the red line indicates the propagation direction of iBA from the labelled node to other nodes. (e) Glass brain plot. The nodal size is proportional to the number of edges connected with the node. Abbreviations: L (R), left (right) hemisphere; CB, cerebellum; PFC, prefrontal cortex. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the training set, we took the positive and negative features and the CRS-R scores of 16 subjects to build a linear regression model by:
| (8) |
where Y is the CRS-R scores, Xpos is the positive feature, Xneg is the negative feature, w1 and w2 are the weight for Xpos and Xneg. Then the predicted score was calculated using the linear regression model based on the features of the remaining patient in test set. The procedure was repeated 17 times (because of 17 patients) and the predicted CRS-R score of each patient was acquired at last. In the calculation, LOOCV can control the influence of false positives in feature selection because when there was a high proportion of false positives at the feature selection step in the training set, the model cannot generalize well in the new data in the test set (Finn et al., 2015, Rosenberg et al., 2016).
We acquired the model performance (rperf) by calculating the Pearson's correlation coefficient between the predicted and observed CRS-R scores. The p-value was calculated by using 1,000 nonparametric permutation tests. The CPM scripts are available from (https://www.nitrc.org/projects/bioimagesuite/) and visualization tool for the results presentation is available from (http://bisweb.yale.edu/connviewer/).
To test the effect of different thresholds on the accuracy and generalizability of the CPM result (Shen et al., 2017), we set the edges with additional thresholds to select features for each subject. In total, we set five thresholds (threshold pedge = 0.01, 0.005, 0.001, 0.0005, and 0.0001), which include the default threshold pedge = 0.001, to determine the optimal threshold. The predictive procedures were repeated for each threshold.
3. Results
3.1. Demographical and clinical information
The rs-fMRI data of 17 DOC (13 M/4F, aged 40.6 ± 16.4 years old, 11VS/6MCS) and 17 health controls (9 M/8F, aged 32.9 ± 8.6 years old) was analyzed in this study. No significant between-group differences were found in age (t = 1.43, p = 0.09), gender (χ2 = 2.69, p = 0.15), and mean FD (t = 0.05, p = 0.27).
3.2. Voxel-wise time delay estimation and probabilistic flow estimation
Fig. 2a and 2c show TDP map for the DOC patients and the healthy controls, respectively. The voxel color-coded in cool (warm) indicate that the BOLD signal in the voxel is earlier (later) than that of the rest voxels of the whole brain. Fig. 2e shows the t-map in TDP between the DOC patients and the healthy controls. Fig. 2b and 2d show PFP map for the DOC patients and the healthy controls, respectively. The cool hue represents that a voxel receives BOLD signals from the rest voxels while the warm hue represented that a voxel sends out BOLD signals to the rest voxels. The deeper the hue is, the more likely it is that the voxel will act as the sender/receiver. The sum of all the cool (or warm) hues equals one. Fig. 2f shows the t-map in PFP between the DOC patients and the healthy controls.
Fig. 2.
TDP, PFP, and t-map on brain surface for the disorders of consciousness (DOC) patients and the healthy controls. (a) TDP map of the DOC patients (averaged TDP across all DOC patients). The clock-like color bar signifies that the propagated intrinsic brain activity (iBA) is along the clockwise direction. The hour hand and minute hand represent the bottom and top quartile in the range of propagated iBA in the DOC patients or healthy controls. The cool (warm) hues represent that the lag structure of this region is early (late) relative to the rest of the brain. (b) PFP map of the DOC patients (averaged PFP across all DOC patients). The cool hues represent region that send out signals to the other region and the warm hues represent region that receive signals in the whole brain. The sum of all the cool (or warm) hues equals one. (c) TDP map of the healthy controls (averaged TDP across all healthy controls). (d) PFP map of the healthy controls (averaged PFP across all healthy controls). (e) Between-group comparison in TDP. (f) Between-group comparison in PFP.
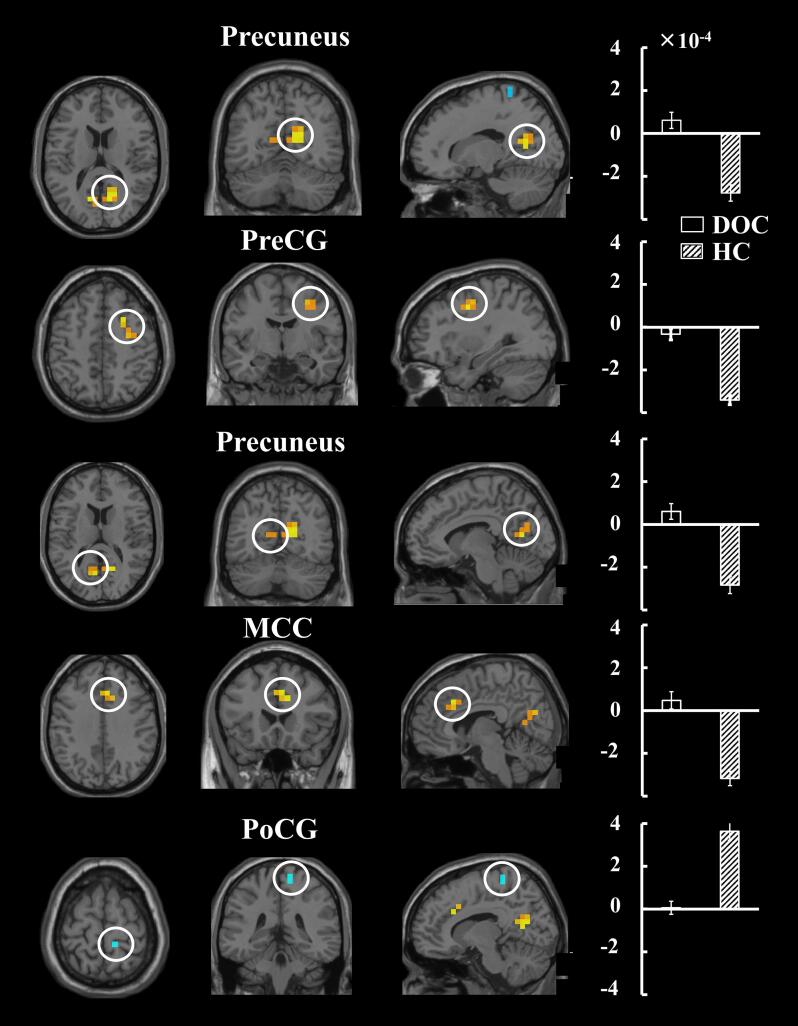
Fig. 3 shows each cluster with significant TDP difference between the two groups. Relative to the TDP in the healthy controls, the DOC patients showed significant differences in 1) the right precentral gyrus (PreCG), 2) right precuneus, 3) right middle cingulate cortex (MCC), 4) right inferior frontal gyrus (IFG), 5) left PreCG, and 6) right postcentral gyrus (PoCG). Specifically, the right PreCG, right precuneus, right MCC, right IFG, left PreCG show early iBA in the healthy controls but not in the DOC patients. In addition, the right PoCG shows late iBA in the healthy controls but not in the DOC patients.
Fig. 3.
Clusters with significant between-group differences in TDP. Each cluster is indicated with a circle in the t-map. Bar plot represents the group-level TDP of the DOC patients and the healthy controls for the corresponding cluster. Abbreviations: IFG, inferior frontal gyrus; PoCG, postcentral gyrus; PreCG, precentral gyrus; MCC, middle cingulate cortex; R (L), right (left) hemisphere.
Fig. 4shows the clusters with significant between-group differences in PFP. Compared with the healthy controls, the DOC patients showed significantly different PFP in five clusters, which were located in 1) the right precuneus, 2) right PreCG gyrus, 3) left precuneus, 4) right MCC, and 5) right PoCG. Specifically, the right precuneus, right PreCG gyrus, left precuneus, and right MCC are high probability sender of iBA in the healthy controls but not in the DOC patients, whereas the right PoCG is a high probability receiver in the healthy controls but not in the DOC patients.
Fig. 4.
Clusters with significant between-group differences in PFP. Each cluster is indicated with a circle in the t-map. Bar plot indicates the group-level PFP of the DOC patients and the healthy controls for the corresponding cluster. The PFP > 0 (or < 0) represents that this voxel is a receiver (or sender) relative to the rest voxels. The higher the value of |PFP|, the higher probability the voxel as a sender or receiver. Abbreviations: PoCG, postcentral gyrus; PreCG, precentral gyrus; MCC, middle cingulate cortex; R (L), right (left) hemisphere.
We also calculated the top and bottom quartile of TDP to measure the range of TD value for the DOC patients and the healthy controls, separately. In the calculations, we took the mean FD, age, and gender as covariates. We found that the top quartile of TDP was smaller (t = -3.33, p = 0.002) in the DOC patients (0.147 ± 0.025 s) than that in the healthy controls (0.184 ± 0.026 s), and the bottom quartile of TDP was larger (t = 3.92, p < 0.001) in the DOC patients (−0.147 ± 0.024 s) than that in the healthy controls (−0.187 ± 0.027 s). Meanwhile, we also repeated the TDE calculation without regressing out the global signal and found the range of TD in DOC patients was still shorter than that in the healthy controls. Specifically, the top quartile of TDP was significantly smaller (t = -1.97, p = 0.028) in the DOC patients (0.202 ± 0.050 s) than that in the healthy controls (0.242 ± 0.049 s), and the bottom quartile of TDP was significantly larger (t = 2.45, p = 0.009) in the patients (-0.206 ± 0.070 s) than that in the controls (-0.258 ± 0.068 s). Thus, the shortened range of TD in DOC patients was not associated with the global signal.
3.3. CPM for predicting CRS-R scores
Table 2 lists the detailed model performance (rperf value) among five thresholds based on whole-brain ROI-wise TD matrix and PF matrix, respectively. Specifically, for default threshold pedge = 0.001, we found that the features based on TD matrix (rperf = 0.662, p = 0.006) could be used for significantly predicting the clinical scores of DOC patients. However, the model performance based on TD matrix reduces when the pedge is too large (pedge = 0.005 and 0.01) or too small (pedge = 0.0005 and 0.0001, see Fig. 5a), which indicated that additional/insufficient edges may do affect the models’ predictive performance (Ren et al., 2021). In addition, the model performance based on PF matrix is poor (rperf from −0.639 to −0.053) compared with based on TD matrix. Because the model performance based on the TD matrix is better than that based on the PF matrix and the best performance based on the TD matrix is acquired when threshold pedge = 0.001, we selected the result based on TD matrix in default threshold pedge = 0.001 to perform the further analysis. For a better visualization, we converted the CRS-R scores by (Beaty et al., 2018), where × is the raw observed (predicted) clinical score of each patient, μ is the mean of the observed (predicted) clinical scores of all patients, δ is the standard deviation of the observed (predicted) clinical scores of all patients. Fig. 5b shows the scatterplots of standardized observed and predicted clinical scores. In addition, to test the robustness of CPM result based on TD matrix, we also repeated the calculations by taking the Brainnetome atlas (BN-274) (Fan et al., 2016) to construct TD/PF matrices at the optimal threshold (pedge = 0.001, Fig. 5a) that determined by Shen-268 atlas. We found that the TD matrix (rperf = 0.505, p = 0.031) rather than the PF matrix (rperf = 0.05) can accurately predict the clinical scores of DOC patients. This result is consistent with that derived from Shen-268 atlas.
Table 2.
The model performance (rperf) and its significant level (p) in CPM predicting CRS-R scores for DOC patients under different thresholds of the edge selection.
| Threshold pedge | CPM performance based on
TD |
CPM performance based on
PF |
||
|---|---|---|---|---|
| rperf | p | rperf | p | |
| 0.0001 | 0.452 | 0.060 | −0.639 | / |
| 0.0005 | 0.226 | 0.004* | −0.378 | / |
| 0.001 | 0.662 | 0.006* | −0.053 | / |
| 0.005 | 0.631 | 0.001* | −0.236 | / |
| 0.01 | 0.515 | 0.006* | −0.433 | / |
The permutation test will be not calculated when the model performance rperf < 0.2. *, p < 0.01 (Bonferroni correction, p < 0.05/5 = 0.01).
When using pedge = 0.001, the CPM results shows that a total of 42 edges in TD matrix are significantly correlated with clinical scores. Fig. 5c shows vertical and left view of the glass brain on the distribution of 42 edges. To characterize the functional roles of predictive networks which consist of the 42 significant edges, we distributed the all 268 ROIs into ten macroscale brain resting state networks (RSNs) according to previous studies (Beaty et al., 2018, Shen et al., 2017) and checked the number of significant edges between RSNs. Fig. 5d shows the distribution of 42 edges in predictive networks. Due to not all brain regions equally involved in the prediction, we only display the nodes connected with more than one edge, including 11 nodes with total 24 edges. Table 3 lists detailed information on the 11 nodes. Fig. 5e shows the propagation direction of these 11 nodes with other nodes in whole brain. Among these 11 nodes in the predictive networks, 5 nodes in the prefrontal cortex (PFC) joins 8 significant edges; 3 nodes in the cerebellum joins 7 significant edges; 1 node in the limbic region joins 3 significant edges; 1 node in the subcortical region joins 2 significant edges; 1 node in the parietal region joins 2 significant edges. Specifically, we found 8 of 11 nodes are distributed in the cerebellum and PFC, and dense edges are the connections between the cerebellum and PFC.
Table 3.
The 11 nodes connected with more than one edge in CPM according to the TD matrix.
| Label of node | Number of edges | Brodmann’s Area | MNI coordinate (x, y, z) | L/R | Network location | ||
|---|---|---|---|---|---|---|---|
| 1 | 3 | / | –22.7 | −57.9 | −48.8 | L | Cerebellum |
| 2 | 3 | 24 | 5.3 | −1.0 | 35.6 | R | Limbic |
| 3 | 2 | / | 6.1 | −50.7 | −12.3 | R | Cerebellum |
| 4 | 2 | / | −34.6 | −50.3 | −54.0 | L | Cerebellum |
| 5 | 2 | 46 | −43.0 | 42.0 | 11.0 | L | Prefrontal |
| 6 | 2 | 10 | −18.2 | 57.0 | −14.3 | L | Prefrontal |
| 7 | 2 | 8 | 14.3 | 36.9 | 48.9 | R | Prefrontal |
| 8 | 2 | 44 | 40.0 | 17.6 | 29.2 | R | Prefrontal |
| 9 | 2 | 11 | −18.2 | 19.0 | −21.0 | L | Prefrontal |
| 10 | 2 | 39 | −53.4 | −43.5 | 38.8 | L | Parietal |
| 11 | 2 | / | 12.6 | 20.2 | −0.7 | R | Subcortical |
Abbreviations: MNI, Montreal Neurological Institute; R (L), right (left) hemisphere.
4. Discussion
In the current study, we first analyzed the altered propagation structure of intrinsic brain activity (iBA) by using the time delay estimation (TDE), and then detected abnormal propagation probability of iBA by using the probabilistic flow estimation (PFE) for the patients with disorders of consciousness (DOC). With the connectome-based predictive modeling (CPM), we also predicted the clinical scores for the patients according to the ROI-wise time delay (TD) and probabilistic flow (PF) matrix of iBA.
4.1. Clusters with altered TDP and PFP in DOC patients
After calculating TDE and PFE, we compared the spatial distribution of the clusters with significant between-group differences in TDP and PFP (Fig. 2e and 2f). Specifically, we found four shared clusters with abnormal TDP and abnormal PFP, including the precentral gyrus, precuneus, middle cingulate cortex, and postcentral gyrus. However, the range of spatial distribution for each shared cluster with difference in PFP is relatively smaller than that of the cluster with difference in TDP.
We observed abnormal TDP and PFP in the precuneus (or PCC/precuneus) between groups (Fig. 3, Fig. 4), which showed the altered temporal ordering of iBA in precuneus in the DOC patients. The precuneus, a hub region of the DMN (Buckner et al., 2008), plays a vital role in maintaining self-referential processing and introspection (Cavanna and Trimble, 2006). Previous studies reported the abnormal functional interactions of precuneus in DOC patients by using either static FC (Qin et al., 2015, Stender et al., 2014, Wu et al., 2019) or dynamic FC (Cao et al., 2019, Luppi et al., 2019). Several recent dynamic causal modelling (DCM) studies (Chen et al., 2018a, Crone et al., 2017, Crone et al., 2015) also found the disrupted effective connectivity between PCC/precuneus and other regions (e.g., medial frontal cortex, inferior parietal lobule, and globus pallidus) in DOC patients, reflecting the abnormal information flow related to precuneus at ROI level.
Moreover, our studies found that the precuneus possibly acted as a source in propagation structure of iBA in healthy controls, which was in line with previous voxel-wise TDE studies (Mitra et al., 2015a, Mitra et al., 2014). However, the role of precuneus in propagation structure was lost in the DOC patients (Fig. 3), which may reflect that a steady propagation activity of iBA in precuneus is associated with the maintenance of consciousness. We inferred that the alteration in precuneus may be related to the disrupted brain WM connection in DOC patients (Fernández-Espejo et al., 2012, Lant et al., 2016, Zheng et al., 2017). Using the diffusion tensor imaging (DTI) technique, previous studies revealed the disrupted WM tracts between the precuneus and other regions (e.g., thalamus, hippocampus, and temporoparietal junctions) in DOC patients (Fernández-Espejo et al., 2012, Tan et al., 2019).
The DOC patients showed the altered TDP and PFP in both the precentral gyrus (PreCG) and postcentral gyrus (PoCG) compared with the controls (Fig. 3, Fig. 4). In the healthy controls, we found that the timing of PreCG was earlier than other regions, indicating that it possibly acted as a source for the information transmitting, while the timing of PoCG was later than other regions, indicating that it possibly acted as a destination for the information reception and integration (Mitra, Kraft, et al., 2018; Mitra & Raichle, 2018). Their roles in the brain information flow may reflect the brain physiological function. Specifically, both the PreCG and PoCG belong to the sensorimotor network (SMN), the PreCG is responsible for the body movement (Lemon, 2008), which may need to transmit the information to other regions. The PoCG is responsible for the processing of peripheral sensory information (Kropf et al., 2019), which may correspond to the information reception and integration in propagation structure. The altered temporal ordering in PreCG and PoCG may be related to the impaired sensorimotor function in clinical performance for the DOC patients. Previous fMRI studies also reported abnormal brain activity in the PreCG and PoCG in DOC patients (Demertzi et al., 2015, Ovadia-Caro et al., 2012, Yao et al., 2015). Yao et al. (2015) found a decreased amplitude of low-frequency fluctuations (ALFF) in the bilateral PreCG in DOC patients. Ovadia-Caro et al. (2012) found a decreased FC between the bilateral PoCG in DOC patients compared with healthy controls.
4.2. Decreased range of TD in DOC patients
The current study found that the range of TD value for the patients was shorter than that of the healthy controls by comparing the quartile between groups (Fig. 2c). We observed that the TDP was in a range of 1 s across the controls (Fig. 2c), consisting with most previous TDE studies listed in Table 4. A possible explanation was that the impaired propagation structure of iBA in critical regions lead to the shortened TDP range in the patients. The precuneus, PreCG, and PoCG were a source or destination of the propagation activity in the controls but not in the DOC patients (Fig. 3). Another reason for the shortened TDP range may be the low level of brain activity in DOC patients (Demertzi et al., 2019, Fridman et al., 2014, Sinitsyn et al., 2018). Previous studies found a global decreased metabolic regulation in DOC patients compared with healthy controls (Di Perri et al., 2016, Fridman et al., 2014).
Table 4.
Summary of human brain resting-state fMRI studies based on the time delay estimation conducted with lagged cross-covariance function (Jan. 2014-Dec. 2020).
| No. | Aims | Subjects | Sequence parameters | TD span | Findings | Refs. |
|---|---|---|---|---|---|---|
| 1 | Specific propagation structure of iBA in amygdala subdivisions with cortical cortex in HC | HC: 10 | TR = 2.2 s 3 T |
/ | HC showed a consistent propagation structure of iBA in the cortex relative to amygdala subdivisions. | (Sylvester et al., 2020) |
| 2 | Specific propagation structure of iBA in DMN subnetworks of HC | HC: 10 | TR = 2.2 s 3 T |
/ | DMN subnetworks exhibited a systematic TDP. | (Gordon et al., 2020) |
| 3 | Detecting epileptic activation and propagation of iBA and estimating the effects of Levetiracetam on RE | HC: 27 RE: 43 |
TR = 2 s 3 T |
/ | 1) There were earlier intrinsic activations in bilateral Rolandic
areas after central-temporal spike in RE. 2) Patients with therapy showed a lagged iBA in Rolandic regions compared with drug-naïve patients. |
(Xu et al., 2020) |
| 4 | Altered propagation structure of iBA in DOC patients | DOC: 48 HC: 27 |
TR = 2 s 3 T |
±0.2 s | Significant altered TDP was found in critical regions (e.g., middle cingulate cortex, precuneus, inferior frontal gyrus) are found in DOC patients. | (Rudas et al., 2020) |
| 5 | Specific propagation structure of iBA at the individual level | HC: 11 |
TR = 1.16/2.2 s 3 T |
±0.5 s | Propagation structure of iBA was stable at individual level and similar to that of the group level. | (Raut et al., 2019a) |
| 6 | Possible sources of propagation structure of iBA in visual cortex in HC | HC: 1,250 | TR = 0.72/0.65 s 1.5 T |
± 0.3 s | The iBA in early visual cortex propagated from the central to peripheral visual fields in HC. | (Park et al., 2019) |
| 7 | Relationship between preoperative temporal latency and postoperative seizure foci lateralization | PE: 38 HC: 259 |
TR = 2.07 s 1.5 T |
/ | There is a relationship between preoperative fMRI propagation structure and postoperative seizure foci lateralization. | (Shah et al., 2019) |
| 8 | Altered propagation structure of iBA in PTSD patients | PTSD: 27 HC: 30 |
TR = 2 s 3 T |
±0.5 s | 1) Significant altered TDP was found in
PCC/precuneus, middle prefrontal cortex, right angular, and left pre-
and post-central cortex in PTSD patients. 2) There was a correlation between clinical scores and TDP in PTSD. |
(Weng et al., 2018) |
| 9 | Specific propagation structure of iBA between the DMN and the DAN in HC | HC: 1,376 | TR = 3 s 3 T |
±1 s | 1) The iBA in DMN propagated from the retrosplenial cortex to
prefrontal cortex. 2) The iBA in DAN propagates from the frontal eye fields to the parietal cortex. |
(Mitra et al., 2018) |
| 10 | Relationship between propagation structure of iBA in PE patients and laterality | PE: 26 | TR = 2.07 s 3 T |
±1 s | The TD of PE patients was correlated with seizure foci lateralization. | (Shah et al., 2018) |
| 11 | Propagation structure of iBA between the cerebellum and cerebral cortex in HC | HC: 10 | TR = 2.2 s 3 T |
±0.8 s | The iBA in cerebellum lagged the brain cortex about 0.1–0.4 s in HC. | (Marek et al., 2018) |
| 12 | Potential propagation structure of iBA in ASD patients | ASD: 23 HC: 23 |
TR = 2.2 s 3 T |
±0.5 s | 1) Significant changed TDP was found in frontopolar
cortex, occipital cortex, and putamen in ASD patients. 2) There was a correlation between clinical scores and TDP in ASD. |
(Mitra et al., 2017a) |
| 13 | Similarity in propagation structure of iBA between sleeping infants and awake/sleeping adults | Infant: 45 Adult: 63 |
TR = 2/2.5 s 3 T |
±0.75 s | The propagation structure of iBA for infants was more similar to sleeping adults rather than awake adults. | (Mitra et al., 2017b) |
| 14 | Differences in propagation structure of iBA between the hippocampus and brain cortex under awake and SWS | HC: 38 | TR = 2.08 s 3 T |
±1.5 s | The iBA propagated in opposite directions between the hippocampus and cerebral cortex under awake and SWS. | (Mitra et al., 2016) |
| 15 | Propagation structure of iBA in HC under wake and SWS | HC: 63 | TR = 2.08 s 3 T |
±0.75 s | Significant differences in propagation structure between cerebral and subcortical cortex were found under wake and SWS. | (Mitra et al., 2015b) |
| 16 | Describing lag processes of iBA in HC | HC: 1,376 | TR = 3 s 3 T |
±1 s | Multiple, highly reproducible, temporal sequences of iBA were reported in HC, which termed lag thread. | (Mitra et al., 2015a) |
| 17 | Temporal features of propagation structure of iBA in HC | HC: 692 | TR = 3 s 3 T |
±0.5 s | 1) The iBA propagated across the whole brain on a timescale of about
1 s. 2) Propagation activity of iBA reflected neuronal processes rather than hemodynamic delay. |
(Mitra et al., 2014) |
Abbreviations: ASD, Autism spectrum disorder; BOLD, blood oxygen level-dependent; CCF, cross-correlation function; DAN, dorsal attention network; DMN, default mode network; DOC, disorders of consciousness; HC, healthy controls; iBA, intrinsic brain activity; PE, pediatric epilepsy; PTSD, Posttraumatic stress disorder; TDP, lag projection of time delay; RE, Rolandic epilepsy; SWS, slow wave sleep; TLE, Temporal lobe epilepsy; TR, repetition time; PCC, posterior cingulate cortex.
4.3. CPM predicting clinical scores based on TD and PF matrices
Using CPM, we found that the ROI-wise TD matrix can be used to predict clinical scores for DOC patients (Fig. 5a). The predictive results expanded the application of CPM in predicting the clinical scores through using the inter-regional temporal information (e.g., TD) in addition to the inter-regional spatial information (e.g., FC). However, the ROI-wise PF matrix showed a worse predictive performance compared with the TD matrix in predicting the clinical scores for DOC patients. This may be associated with the distinct properties of TD and PF. The PF was built on the TD and the peak correlation. To reduce the influence of low correlations from noise signals on the PF, we set the PF between pairs of regions as zero when the peak correlation value r < 0.1 by following Mitra et al., (2020). These zero values in PF matrix may distort Pearson's correlation coefficient between the PF value and clinical scores at feature selection, which may further hinder linear model construction in training set and the model prediction in the test set.
For the results derived from TD matrix, we found that the nodes playing a predictive role were mainly located in the cerebellum and PFC (Fig. 5e). In fact, the cerebellum is believed involving in many cognitive functions in addition to maintaining movement, such as working memory, task switching, and language processing (Kansal et al., 2017). Cao et al. (2019) found that the dynamic FC between the cerebellum and somatomotor network could be used to predict clinical scores of DOC patients. In addition, the PFC is believed subserving for decision making, planning complex behavior, and moderating social behavior (Carlen, 2017, Miller, 2000). Previous studies indicated that WM tracts between the PFC and thalamus was critical in sustaining consciousness (Schiff, 2010) and the WM tracts have been found to be correlated with clinical scores (Lant et al., 2016).
In addition, previous studies showed that the cerebellum and PFC play key roles in propagation structure of iBA (Marek et al., 2018, Mitra et al., 2015a). For example, Marek et al. (2018) showed that the BOLD signals of cerebellum were lagged behind the entire cerebral cortex at both individual-level and group-level in healthy controls based on the Midnight Scan Club (MSC) data. Mitra et al. (2014) analyzed 692 healthy subjects’ fMRI data and reported that the cerebellum and PFC were located at the late stage in the propagation structure. Based on the above description, our conjecture is that the cerebellum and PFC are the receiver of the iBA, and the impeded degree of propagation structure in these regions may be helpful for accessing the clinical scores of DOC patients. Further studies are needed to uncover the mechanism behind the relationship between the CRS-R scores and the identified predictive networks before the findings can be translated into clinical practice.
5. Limitations
Several limitations should be addressed. First, the small sample size may affect the generality of the results. This study included only 17 DOC patients as the trade-off between the sample size and imaging quality, although we obtained the rs-fMRI and high-resolution brain structural images from 45 DOC patients. Further studies could test the results of TDP, PFP, and CPM in a larger DOC patients sample.
Second, this study revealed that the precuneus acts as a source of propagation structure in the healthy controls, which was in line with previous TDE (Mitra et al., 2015a, Mitra et al., 2020) and GCA studies (Deshpande et al., 2011, Garg et al., 2011). However, we also noticed that the precuneus was a receiver of information in several studies (Crone et al., 2015, Deco et al., 2021, Seguin et al., 2019). This discrepancy may be caused by the different analysis level including voxel level or ROI level. Our result derived from a voxel-wise TDE, while the other results were mainly estimated on the ROI-wise analysis. Crone et al. (2015) estimate the interaction relationship between 4 ROIs, including the medial frontal cortex (MFC), PCC, left inferior parietal lobule (IPL), and right IPL by using DCM and found that the PCC acts as the main driven hub in healthy controls but not in DOC patients. Based on the voxel-wise TDE, however, Mitra et al. (2014) analyzed the directionality of BOLD signals in whole-brain voxels and showed the precuneus was a source of the propagation structure in healthy controls. Thus, we also estimated propagation structure of iBA for either the DOC patients or healthy controls at a ROI level to analyze the temporal ordering of BOLD signal between the PCC/precuneus and the other regions. The results showed that the iBA in PCC/precuneus was not earlier than all the rest ROIs in the healthy controls (see Supplemental Materials).
Third, this study only applied rs-fMRI to analyze the BOLD signals of DOC patients, which lacked the verification of multimodal imaging techniques. In the future, the more reliable results about propagation structure of iBA could be obtained by combining multimodal non-invasive techniques (Peterson et al., 2020), such as electroencephalogram (EEG) (Claassen et al., 2019, Gui et al., 2020, Pan et al., 2020) or magnetoencephalography (MEG) (Boto et al., 2018) or transcranial magnetic stimulation (TMS) (Rosanova et al., 2012) or transcranial direct-current stimulation (tDCS) (Bourzac, 2016).
TDE has been used in the studies of healthy subjects (Mitra and Raichle, 2016, Mitra et al., 2016) and patients (Mitra et al., 2017a, Shah et al., 2019) for characterizing temporal organization of iBA (Mitra et al., 2017a, Raut et al., 2019a). To better understand the application of TDE, we summarized the last 7 years (Jan. 2014- Dec. 2020) healthy controls and patient’s fMRI studies on TD in Table 4. The PFE could be used to reflect the probability of a given region in receiving or sending out information with other regions. In addition to constructing a PF matrix by following Mitra et al., (2020), we suggested that the TD and FC matrix may be used to build a directional FC network for reflecting the inter-regional interaction directionality across the brain for patients with DOC or other brain diseases in the future work.
6. Conclusion
In the current study, we estimated time delay (TD) and probabilistic flow (PF) of BOLD signals to infer the propagation structure and propagation probability of intrinsic brain activity in DOC patients. We found that the DOC patients showed not only the altered TD and PF in DMN and SMN but also a shortened range of TD compared with the healthy controls. The findings of the aberrant temporal properties of intrinsic brain activity in unconsciousness may contribute to understanding the pathophysiology of DOC patients. With CPM, we predicted CRS-R scores of DOC patients by using the whole-brain ROI-wise TD and PF matrices. The CPM analysis showed that the TD rather than the PF had a superior predictive performance to the CRS-R scores, in which the cerebellum and prefrontal cortex were the main predictive regions. The results suggested that the propagation structure of iBA in DOC patients may provide a potential neuroimaging indicator to aid clinical applications.
Funding
The study was supported by grants from the National Natural Science Foundation of China (Grant numbers: 82171914, 81871338, 81371535, 82171174, and 81974154) the National Key R&D Program of China (Grant number: 2018YFC1705006), and Key Realm R&D Program of Guangzhou (Grant number: 202007030005). The funding organizations played no further role in study design, data collection, analysis and interpretation, or paper writing.
CRediT authorship contribution statement
Bolin Cao: Conceptualization, Methodology, Software, Writing – original draft, Writing - review & editing, Visualization. Yu Guo: Conceptualization, Methodology, Validation, Writing - review & editing, Visualization. Yequn Guo: Investigation, Data curation, Resources, Writing - review & editing. Qiuyou Xie: Investigation, Writing - review & editing, Supervision, Data curation, Resources. Lixiang Chen: Methodology, Writing - review & editing. Huiyuan Huang: Methodology, Writing - review & editing. Ronghao Yu: Investigation, Data curation, Resources. Ruiwang Huang: Conceptualization, Writing - review & editing, Supervision, Project administration.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102797.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aguirre G.K., Zarahn E., D'Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8(4):360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Allen E.A., Damaraju E., Plis S.M., Erhardt E.B., Eichele T., Calhoun V.D. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex. 2014;24(3):663–676. doi: 10.1093/cercor/bhs352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty R.E., Kenett Y.N., Christensen A.P., Rosenberg M.D., Benedek M., Chen Q., Fink A., Qiu J., Kwapil T.R., Kane M.J., Silvia P.J. Robust prediction of individual creative ability from brain functional connectivity. Proc Natl Acad Sci U S A. 2018;115(5):1087–1092. doi: 10.1073/pnas.1713532115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M., Phillips C., Tshibanda L., Vanhaudenhuyse A., Schabus M., Dang-Vu T.T., Moonen G., Hustinx R., Maquet P., Laureys S. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boto E., Holmes N., Leggett J., Roberts G., Shah V., Meyer S.S., Munoz L.D., Mullinger K.J., Tierney T.M., Bestmann S., Barnes G.R., Bowtell R., Brookes M.J. Moving magnetoencephalography towards real-world applications with a wearable system. Nature. 2018;555(7698):657–661. doi: 10.1038/nature26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourzac K. Neurostimulation: Bright sparks. Nature. 2016;531(7592):S6–8. doi: 10.1038/531S6a. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Campbell J.M., Huang Z., Zhang J., Wu X., Qin P., Northoff G., Mashour G.A., Hudetz A.G. Pharmacologically informed machine learning approach for identifying pathological states of unconsciousness via resting-state fMRI. Neuroimage. 2020;206:116316. doi: 10.1016/j.neuroimage.2019.116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Chen Y., Yu R., Chen L., Chen P., Weng Y., Chen Q., Song J., Xie Q., Huang R. Abnormal dynamic properties of functional connectivity in disorders of consciousness. Neuroimage Clin. 2019;24:102071. doi: 10.1016/j.nicl.2019.102071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M. What constitutes the prefrontal cortex? Science. 2017;358(6362):478–482. doi: 10.1126/science.aan8868. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen P., Xie Q., Wu X., Huang H., Lv W., Chen L., Guo Y., Zhang S., Hu H., Wang Y., Nie Y., Yu R., Huang R. Abnormal Effective Connectivity of the Anterior Forebrain Regions in Disorders of Consciousness. Neurosci Bull. 2018;34(4):647–658. doi: 10.1007/s12264-018-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Lu B., Yan C.G. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Human Brain Mapping. 2018;39(1):300–318. doi: 10.1002/hbm.23843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen J., Doyle K., Matory A., Couch C., Burger K.M., Velazquez A., Okonkwo J.U., King J.R., Park S., Agarwal S., Roh D., Megjhani M., Eliseyev A., Connolly E.S., Rohaut B. Detection of Brain Activation in Unresponsive Patients with Acute Brain Injury. N Engl J Med. 2019;380(26):2497–2505. doi: 10.1056/NEJMoa1812757. [DOI] [PubMed] [Google Scholar]
- Crone J.S., Lutkenhoff E.S., Bio B.J., Laureys S., Monti M.M. Testing Proposed Neuronal Models of Effective Connectivity Within the Cortico-basal Ganglia-thalamo-cortical Loop During Loss of Consciousness. Cereb Cortex. 2017;27(4):2727–2738. doi: 10.1093/cercor/bhw112. [DOI] [PubMed] [Google Scholar]
- Crone J.S., Schurz M., Holler Y., Bergmann J., Monti M., Schmid E., Trinka E., Kronbichler M. Impaired consciousness is linked to changes in effective connectivity of the posterior cingulate cortex within the default mode network. Neuroimage. 2015;110:101–109. doi: 10.1016/j.neuroimage.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Vidaurre D., Kringelbach M.L. Revisiting the global workspace orchestrating the hierarchical organization of the human brain. Nat Hum Behav. 2021;5(4):497–511. doi: 10.1038/s41562-020-01003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demertzi A., Antonopoulos G., Heine L., Voss H.U., Crone J.S., de Los Angeles C., Bahri M.A., Di Perri C., Vanhaudenhuyse A., Charland-Verville V., Kronbichler M., Trinka E., Phillips C., Gomez F., Tshibanda L., Soddu A., Schiff N.D., Whitfield-Gabrieli S., Laureys S. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain. 2015;138(9):2619–2631. doi: 10.1093/brain/awv169. [DOI] [PubMed] [Google Scholar]
- Demertzi A., Tagliazucchi E., Dehaene S., Deco G., Barttfeld P., Raimondo F., Martial C., Fernandez-Espejo D., Rohaut B., Voss H.U., Schiff N.D., Owen A.M., Laureys S., Naccache L., Sitt J.D. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci Adv. 2019;5(2):eaat7603. doi: 10.1126/sciadv.aat7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G., Santhanam P., Hu X. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage. 2011;54(2):1043–1052. doi: 10.1016/j.neuroimage.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Perri C., Bahri M.A., Amico E., Thibaut A., Heine L., Antonopoulos G., Charland-Verville V., Wannez S., Gomez F., Hustinx R., Tshibanda L., Demertzi A., Soddu A., Laureys S. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: a cross-sectional multimodal imaging study. Lancet Neurol. 2016;15(8):830–842. doi: 10.1016/S1474-4422(16)00111-3. [DOI] [PubMed] [Google Scholar]
- Edlow B.L., Claassen J., Schiff N.D., Greer D.M. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol. 2021;17(3):135–156. doi: 10.1038/s41582-020-00428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Li H., Zhuo J., Zhang Y., Wang J., Chen L., Yang Z., Chu C., Xie S., Laird A.R., Fox P.T., Eickhoff S.B., Yu C., Jiang T. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016;26(8):3508–3526. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I., Haiss F., Gentet L.J., Aronoff R., Weber B., Petersen C.C. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56(5):907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Fernández-Espejo D., Soddu A., Cruse D., Palacios E.M., Junque C., Vanhaudenhuyse A., Rivas E., Newcombe V., Menon D.K., Pickard J.D., Laureys S., Owen A.M. A role for the default mode network in the bases of disorders of consciousness. Annals of neurology. 2012;72(3):335–343. doi: 10.1002/ana.v72.310.1002/ana.23635. [DOI] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Scheinost D., Rosenberg M.D., Huang J., Chun M.M., Papademetris X., Constable R.T. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E.A., Beattie B.J., Broft A., Laureys S., Schiff N.D. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc Natl Acad Sci U S A. 2014;111(17):6473–6478. doi: 10.1073/pnas.1320969111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S.J., Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D., Liddle P.F., Frackowiak R.S. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13(1):5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Moran R., Seth A.K. Analysing connectivity with Granger causality and dynamic causal modelling. Curr Opin Neurobiol. 2013;23(2):172–178. doi: 10.1016/j.conb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg R., Cecchi G.A., Rao A.R. Full-brain auto-regressive modeling (FARM) using fMRI. Neuroimage. 2011;58(2):416–441. doi: 10.1016/j.neuroimage.2011.02.074. [DOI] [PubMed] [Google Scholar]
- Giacino J.T., Ashwal S., Childs N., Cranford R., Jennett B., Katz D.I., Kelly J.P., Rosenberg J.H., Whyte J., Zafonte R.D., Zasler N.D. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349–353. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Marek S., Raut R.V., Gratton C., Newbold D.J., Greene D.J., Coalson R.S., Snyder A.Z., Schlaggar B.L., Petersen S.E., Dosenbach N.U.F., Nelson S.M. Default-mode network streams for coupling to language and control systems. Proc Natl Acad Sci U S A. 2020;117(29):17308–17319. doi: 10.1073/pnas.2005238117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui P., Jiang Y., Zang D., Qi Z., Tan J., Tanigawa H., Jiang J., Wen Y., Xu L., Zhao J., Mao Y., Poo M.M., Ding N., Dehaene S., Wu X., Wang L. Assessing the depth of language processing in patients with disorders of consciousness. Nat Neurosci. 2020;23(6):761–770. doi: 10.1038/s41593-020-0639-1. [DOI] [PubMed] [Google Scholar]
- He B.J., Zempel J.M., Snyder A.Z., Raichle M.E. The Temporal Structures and Functional Significance of Scale-free Brain Activity. Neuron. 2010;66(3):353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Zhang J., Wu J., Mashour G.A., Hudetz A.G. Temporal circuit of macroscale dynamic brain activity supports human consciousness. Sci Adv. 2020;6(11):eaaz0087. doi: 10.1126/sciadv.aaz0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansal K., Yang Z., Fishman A.M., Sair H.I., Ying S.H., Jedynak B.M., Prince J.L., Onyike C.U. Structural cerebellar correlates of cognitive and motor dysfunctions in cerebellar degeneration. Brain. 2017;140(3):707–720. doi: 10.1093/brain/aww327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.B., Prigge M.B.D., King C.K., Morgan J., Dean D.C., Freeman A., Villaruz J.A.M., Kane K.L., Bigler E.D., Alexander A.L., Lange N., Zielinski B.A., Lainhart J.E., Anderson J.S. Evaluation of Differences in Temporal Synchrony Between Brain Regions in Individuals With Autism and Typical Development. JAMA Netw Open. 2018;1(7):e184777. doi: 10.1001/jamanetworkopen.2018.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf E., Syan S.K., Minuzzi L., Frey B.N. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz J Psychiatry. 2019;41(3):261–269. doi: 10.1590/1516-4446-2018-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lant N.D., Gonzalez-Lara L.E., Owen A.M., Fernandez-Espejo D. Relationship between the anterior forebrain mesocircuit and the default mode network in the structural bases of disorders of consciousness. Neuroimage-Clinical. 2016;10:27–35. doi: 10.1016/j.nicl.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005;9(12):556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Laureys, S., Celesia, G. G., Cohadon, F., Lavrijsen, J., Leon-Carrion, J., Sannita, W. G., Sazbon, L., Schmutzhard, E., von Wild, K. R., Zeman, A., Dolce, G., & European Task Force on Disorders of, C. (2010). Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med, 8, 68. 10.1186/1741-7015-8-68. [DOI] [PMC free article] [PubMed]
- Lee H.Y., Park J.H., Kim A.R., Park M., Kim T.W. Neurobehavioral recovery in patients who emerged from prolonged disorder of consciousness: a retrospective study. BMC Neurol. 2020;20(1):198. doi: 10.1186/s12883-020-01758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon Roger N. Descending pathways in motor control. Annu Rev Neurosci. 2008;31(1):195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Luppi A.I., Craig M.M., Pappas I., Finoia P., Williams G.B., Allanson J., Pickard J.D., Owen A.M., Naci L., Menon D.K., Stamatakis E.A. Consciousness-specific dynamic interactions of brain integration and functional diversity. Nat Commun. 2019;10(1):4616. doi: 10.1038/s41467-019-12658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Siegel J.S., Gordon E.M., Raut R.V., Gratton C., Newbold D.J., Ortega M., Laumann T.O., Adeyemo B., Miller D.B., Zheng A., Lopez K.C., Berg J.J., Coalson R.S., Nguyen A.L., Dierker D., Van A.N., Hoyt C.R., McDermott K.B., Norris S.A., Shimony J.S., Snyder A.Z., Nelson S.M., Barch D.M., Schlaggar B.L., Raichle M.E., Petersen S.E., Greene D.J., Dosenbach N.U.F. Spatial and Temporal Organization of the Individual Human Cerebellum. Neuron. 2018;100(4):977–993 e977. doi: 10.1016/j.neuron.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Mitra A., Raichle M.E. How networks communicate: propagation patterns in spontaneous brain activity. Philos Trans R Soc Lond B Biol Sci. 2016;371(1705) doi: 10.1098/rstb.2015.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra Anish, Raichle Marcus E. Principles of cross-network communication in human resting state fMRI. Scand J Psychol. 2018;59(1):83–90. doi: 10.1111/sjop.2018.59.issue-110.1111/sjop.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Snyder A.Z., Blazey T., Raichle M.E. Lag threads organize the brain's intrinsic activity. Proc Natl Acad Sci U S A. 2015;112(17):E2235–2244. doi: 10.1073/pnas.1503960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Snyder A.Z., Constantino J.N., Raichle M.E. The Lag Structure of Intrinsic Activity is Focally Altered in High Functioning Adults with Autism. Cerebral Cortex. 2017;27(2):1083–1093. doi: 10.1093/cercor/bhv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Snyder A.Z., Hacker C.D., Pahwa M., Tagliazucchi E., Laufs H., Leuthardt E.C., Raichle M.E. Human cortical-hippocampal dialogue in wake and slow-wave sleep. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(44):E6868–E6876. doi: 10.1073/pnas.1607289113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Snyder A.Z., Hacker C.D., Raichle M.E. Lag structure in resting-state fMRI. Journal of Neurophysiology. 2014;111(11):2374–2391. doi: 10.1152/jn.00804.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Kraft A., Wright P., Acland B., Snyder A.Z., Rosenthal Z., Czerniewski L., Bauer A., Snyder L., Culver J., Lee J.M., Raichle M.E. Spontaneous infra-slow brain activity has unique spatiotemporal dynamics and laminar structure. Neuron. 2018;98(2):297–305 e296. doi: 10.1016/j.neuron.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra Anish, Snyder Abraham Z., Raichle Marcus E. Probabilistic flow in brain-wide activity. Neuroimage. 2020;223:117321. doi: 10.1016/j.neuroimage.2020.117321. [DOI] [PubMed] [Google Scholar]
- Mitra Anish, Snyder Abraham Z., Tagliazucchi Enzo, Laufs Helmut, Elison Jed, Emerson Robert W., Shen Mark D., Wolff Jason J., Botteron Kelly N., Dager Stephen, Estes Annette M., Evans Alan, Gerig Guido, Hazlett Heather C., Paterson Sarah J., Schultz Robert T., Styner Martin A., Zwaigenbaum Lonnie, Schlaggar Bradley L., Piven Joseph, Pruett John R., Raichle Marcus, Hayasaka Satoru. Resting-state fMRI in sleeping infants more closely resembles adult sleep than adult wakefulness. PLoS One. 2017;12(11):e0188122. doi: 10.1371/journal.pone.0188122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., Snyder A.Z., Tagliazucchi E., Laufs H., Raichle M.E. Propagated infra-slow intrinsic brain activity reorganizes across wake and slow wave sleep. Elife. 2015;4 doi: 10.7554/eLife.10781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajerani M.H., Chan A.W., Mohsenvand M., LeDue J., Liu R., McVea D.A., Boyd J.D., Wang Y.T., Reimers M., Murphy T.H. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat Neurosci. 2013;16(10):1426–1435. doi: 10.1038/nn.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajerani M.H., McVea D.A., Fingas M., Murphy T.H. Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J Neurosci. 2010;30(10):3745–3751. doi: 10.1523/JNEUROSCI.6437-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadia-Caro Smadar, Nir Yuval, Soddu Andrea, Ramot Michal, Hesselmann Guido, Vanhaudenhuyse Audrey, Dinstein Ilan, Tshibanda Jean-Flory L., Boly Melanie, Harel Michal, Laureys Steven, Malach Rafael, Valdes-Sosa Pedro Antonio. Reduction in inter-hemispheric connectivity in disorders of consciousness. PLoS One. 2012;7(5):e37238. doi: 10.1371/journal.pone.0037238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Xie Q., Qin P., Chen Y., He Y., Huang H., Wang F., Ni X., Cichocki A., Yu R., Li Y. Prognosis for patients with cognitive motor dissociation identified by brain-computer interface. Brain. 2020;143(4):1177–1189. doi: 10.1093/brain/awaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B.Y., Shim W.M., James O., Park H. Possible links between the lag structure in visual cortex and visual streams using fMRI. Sci Rep. 2019;9(1):4283. doi: 10.1038/s41598-019-40728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A., Owen A.M., Karlawish J. Translating the Discovery of Covert Consciousness Into Clinical Practice. JAMA Neurol. 2020;77(5):541–542. doi: 10.1001/jamaneurol.2020.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preti M.G., Bolton T.A., Van De Ville D. The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage. 2017;160:41–54. doi: 10.1016/j.neuroimage.2016.12.061. [DOI] [PubMed] [Google Scholar]
- Qin P.M., Wu X.H., Huang Z.R., Duncan N.W., Tang W.J., Wolff A., Hu J., Gao L., Jin Y., Wu X., Zhang J.F., Lu L., Wu C.P., Qu X.Y., Mao Y., Weng X.C., Zhang J., Northoff G. How Are Different Neural Networks Related to Consciousness? Annals of neurology. 2015;78(4):594–605. doi: 10.1002/ana.24479. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci. 2015;370(1668) doi: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut R.V., Mitra A., Marek S., Ortega M., Snyder A.Z., Tanenbaum A., Laumann T.O., Dosenbach N.U.F., Raichle M.E. Organization of Propagated Intrinsic Brain Activity in Individual Humans. Cereb Cortex. 2019 doi: 10.1093/cercor/bhz198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut R.V., Mitra A., Snyder A.Z., Raichle M.E. On time delay estimation and sampling error in resting-state fMRI. Neuroimage. 2019;194:211–227. doi: 10.1016/j.neuroimage.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme A.K., Volz L.J., Feis D.L., Bomilcar-Focke I., Liebig T., Eickhoff S.B., Fink G.R., Grefkes C. Identifying Neuroimaging Markers of Motor Disability in Acute Stroke by Machine Learning Techniques. Cereb Cortex. 2015;25(9):3046–3056. doi: 10.1093/cercor/bhu100. [DOI] [PubMed] [Google Scholar]
- Ren Zhiting, Daker Richard J., Shi Liang, Sun Jiangzhou, Beaty Roger E., Wu Xinran, Chen Qunlin, Yang Wenjing, Lyons Ian M., Green Adam E., Qiu Jiang. Connectome-Based Predictive Modeling of Creativity Anxiety. Neuroimage. 2021;225:117469. doi: 10.1016/j.neuroimage.2020.117469. [DOI] [PubMed] [Google Scholar]
- Rosanova M., Gosseries O., Casarotto S., Boly M., Casali A.G., Bruno M.A., Mariotti M., Boveroux P., Tononi G., Laureys S., Massimini M. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain. 2012;135:1308–1320. doi: 10.1093/brain/awr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.D., Finn E.S., Scheinost D., Papademetris X., Shen X., Constable R.T., Chun M.M. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19(1):165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudas J., Martinez D., Castellanos G., Demertzi A., Martial C., Carriere M., Aubinet C., Soddu A., Laureys S., Gomez F. Time-Delay Latency of Resting-State Blood Oxygen Level-Dependent Signal Related to the Level of Consciousness in Patients with Severe Consciousness Impairment. Brain Connect. 2020;10(2):83–94. doi: 10.1089/brain.2019.0716. [DOI] [PubMed] [Google Scholar]
- Saad Z.S., DeYoe E.A., Ropella K.M. Estimation of FMRI response delays. Neuroimage. 2003;18(2):494–504. doi: 10.1016/S1053-8119(02)00024-1. [DOI] [PubMed] [Google Scholar]
- Schiff N.D. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Molecular and Biophysical Mechanisms of Arousal, Alertness, and Attention. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Schiff N.D. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33(1):1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnakers C., Vanhaudenhuyse A., Giacino J., Ventura M., Boly M., Majerus S., Moonen G., Laureys S. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin C., Razi A., Zalesky A. Inferring neural signalling directionality from undirected structural connectomes. Nat Commun. 2019;10(1):4289. doi: 10.1038/s41467-019-12201-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M.N., Mitra A., Goyal M.S., Snyder A.Z., Zhang J., Shimony J.S., Limbrick D.D., Raichle M.E., Smyth M.D. Resting state signal latency predicts laterality in pediatric medically refractory temporal lobe epilepsy. Childs Nerv Syst. 2018;34(5):901–910. doi: 10.1007/s00381-018-3770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah Manish N., Nguyen Ryan D., Pao Ludovic P., Zhu Liang, CreveCoeur Travis S., Mitra Anish, Smyth Matthew D. Role of resting state MRI temporal latency in refractory pediatric extratemporal epilepsy lateralization. J Magn Reson Imaging. 2019;49(5):1347–1355. doi: 10.1002/jmri.v49.510.1002/jmri.26320. [DOI] [PubMed] [Google Scholar]
- Shen X., Finn E.S., Scheinost D., Rosenberg M.D., Chun M.M., Papademetris X., Constable R.T. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017;12(3):506–518. doi: 10.1038/nprot.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Tokoglu F., Papademetris X., Constable R.T. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403–415. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinitsyn D.O., Legostaeva L.A., Kremneva E.I., Morozova S.N., Poydasheva A.G., Mochalova E.G., Chervyakova O.G., Ryabinkina J.V., Suponeva N.A., Piradov M.A. Degrees of functional connectome abnormality in disorders of consciousness. Hum Brain Mapp. 2018;39(7):2929–2940. doi: 10.1002/hbm.24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soddu Andrea, Vanhaudenhuyse Audrey, Bahri Mohamed Ali, Bruno Marie-Aurelie, Boly Mélanie, Demertzi Athena, Tshibanda Jean-Flory, Phillips Christophe, Stanziano Mario, Ovadia-Caro Smadar, Nir Yuval, Maquet Pierre, Papa Michele, Malach Rafael, Laureys Steven, Noirhomme Quentin. Identifying the default-mode component in spatial IC analyses of patients with disorders of consciousness. Human Brain Mapping. 2012;33(4):778–796. doi: 10.1002/hbm.v33.410.1002/hbm.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, M., Yang, Y., He, J. H., Yang, Z. Y., Yu, S., Xie, Q. Y., Xia, X. Y., Dang, Y. Y., Zhang, Q., Wu, X. H., Cui, Y., Hou, B., Yu, R. H., Xu, R. X., & Jiang, T. Z. (2018). Prognostication of chronic disorders of consciousness using brain functional networks and clinical characteristics. Elife, 7. doi:ARTN e3617310.7554/eLife.36173. [DOI] [PMC free article] [PubMed]
- Stender J., Gosseries O., Bruno M.-A., Charland-Verville V., Vanhaudenhuyse A., Demertzi A., Chatelle C., Thonnard M., Thibaut A., Heine L., Soddu A., Boly M., Schnakers C., Gjedde A., Laureys S. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. The Lancet. 2014;384(9942):514–522. doi: 10.1016/s0140-6736(14)60042-8. [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Moran R.J., den Ouden H.E., Daunizeau J., Friston K.J. Ten simple rules for dynamic causal modeling. Neuroimage. 2010;49(4):3099–3109. doi: 10.1016/j.neuroimage.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Yu Q., Srivastava A.B., Marek S., Zheng A., Alexopoulos D., Smyser C.D., Shimony J.S., Ortega M., Dierker D.L., Patel G.H., Nelson S.M., Gilmore A.W., McDermott K.B., Berg J.J., Drysdale A.T., Perino M.T., Snyder A.Z., Raut R.V., Laumann T.O., Gordon E.M., Barch D.M., Rogers C.E., Greene D.J., Raichle M.E., Dosenbach N.U.F. Individual-specific functional connectivity of the amygdala: A substrate for precision psychiatry. Proc Natl Acad Sci U S A. 2020;117(7):3808–3818. doi: 10.1073/pnas.1910842117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Xufei, Zhou Zhen, Gao Jian, Meng Fanxia, Yu Yamei, Zhang Jie, He Fangping, Wei Ruili, Wang Junyang, Peng Guoping, Zhang Xiaotong, Pan Gang, Luo Benyan. Structural connectome alterations in patients with disorders of consciousness revealed by 7-tesla magnetic resonance imaging. Neuroimage Clin. 2019;22:101702. doi: 10.1016/j.nicl.2019.101702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A., Noirhomme Q., Tshibanda L.J., Bruno M.A., Boveroux P., Schnakers C., Soddu A., Perlbarg V., Ledoux D., Brichant J.F., Moonen G., Maquet P., Greicius M.D., Laureys S., Boly M. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133(Pt 1):161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre D., Smith S.M., Woolrich M.W. Brain network dynamics are hierarchically organized in time. Proc Natl Acad Sci U S A. 2017;114(48):12827–12832. doi: 10.1073/pnas.1705120114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y., Qi R., Chen F., Ke J., Xu Q., Zhong Y., Chen L., Li J., Zhang Z., Zhang L., Lu G. The Temporal Propagation of Intrinsic Brain Activity Associate With the Occurrence of PTSD. Front Psychiatry. 2018;9:218. doi: 10.3389/fpsyt.2018.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Douaud G., Nichols T.E., Smith S.M. Faster permutation inference in brain imaging. Neuroimage. 2016;141:502–516. doi: 10.1016/j.neuroimage.2016.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Xiaoyan, Xie Qiuyou, Liu Xiaojin, Huang Huiyuan, Ma Qing, Wang Junjing, Zhong Miao, He Yanbin, Niu Chen, Chen Yan, Deng Feng, Ni Xiaoxiao, He Yuan, Guo Yequn, Yu Ronghao, Huang Ruiwang. Spatially Overlapping Regions Show Abnormal Thalamo-frontal Circuit and Abnormal Precuneus in Disorders of Consciousness. Brain Topography. 2019;32(3):445–460. doi: 10.1007/s10548-018-0693-0. [DOI] [PubMed] [Google Scholar]
- Xu Q., Hu Z., Yang F., Bernhardt B.C., Zhang Q., Stufflebeaskm S.M., Zhang Z., Lu G. Resting state signal latency assesses the propagation of intrinsic activations and estimates anti-epileptic effect of levetiracetam in Rolandic epilepsy. Brain Res Bull. 2020;162:125–131. doi: 10.1016/j.brainresbull.2020.05.016. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Wang X.D., Zuo X.N., Zang Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics. 2016;14(3):339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yao S., Song J., Gao L., Yan Y., Huang C., Ding H., Huang H., He Y., Sun R., Xu G. Thalamocortical Sensorimotor Circuit Damage Associated with Disorders of Consciousness for Diffuse Axonal Injury Patients. J Neurol Sci. 2015;356(1–2):168–174. doi: 10.1016/j.jns.2015.06.044. [DOI] [PubMed] [Google Scholar]
- Zheng Zhong S., Reggente Nicco, Lutkenhoff Evan, Owen Adrian M., Monti Martin M. Disentangling disorders of consciousness: Insights from diffusion tensor imaging and machine learning. Hum Brain Mapp. 2017;38(1):431–443. doi: 10.1002/hbm.v38.110.1002/hbm.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.