Frequent consumption of nuts, an important component of plant-based diets, is associated with 15% lower total cardiovascular disease (CVD) and 23% lower CVD mortality rates.1 Small, short-term randomized controlled trials indicate that diets supplemented with nuts have a consistent cholesterol-lowering effect; however, no trials of nut-enriched diets for lipid changes focused on elderly individuals, recruited participants from diverse geographical locations, or lasted 2 years.2 Also, there is little information concerning the effects of nuts on lipoprotein subclasses.
We hypothesized that incorporating walnuts into the usual diet would improve the lipid profile irrespective of differences in geographical and dietary background. The WAHA study (Walnuts and Healthy Aging) is a 2-center (Barcelona, Spain and California, USA), 2-year, parallel-group randomized controlled trial testing the effects of walnut-supplemented diets in healthy elderly individuals (URL: https://www.clinicaltrials.gov; Unique identifier: NCT01634841).3 Lipoprotein changes were a prespecified secondary outcome. The study was approved by the Ethics Committee of each center. Data, analytic methods, and study materials will not be made available to other researchers because of Ethics Committees’ restrictions. Eligible candidates were cognitively healthy elders (63–79 years of age) without major comorbidities.3 After providing informed consent, participants (n=708) were allocated to either a walnut-free (control) or walnut-supplemented diet (≈15% of energy, 30–60 g/d). In 2-monthly visits, compliance, tolerance, medication changes, and body weight were recorded. At each visit, 8-week allotments of raw, pieced walnuts were delivered to the corresponding group. Throughout the study, participants were supervised by their primary physicians, who changed medications, including lipid-lowering drugs, according to their assessment of risk factor levels.
Baseline and 2-year fasting plasma glucose, cholesterol, triglycerides, and high-density lipoprotein cholesterol were determined by standard enzymatic methods; low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula. Advanced lipoprotein testing was performed with Liposcale, a validated 2-dimensional 1H-nuclear magnetic resonance spectroscopy, at Biosfer-Teslab (Reus, Spain).4 We analyzed 2-year differences in nutrient intake and body weight by 1-way ANOVA and changes in glucose and lipoproteins by multivariable-adjusted ANCOVA.
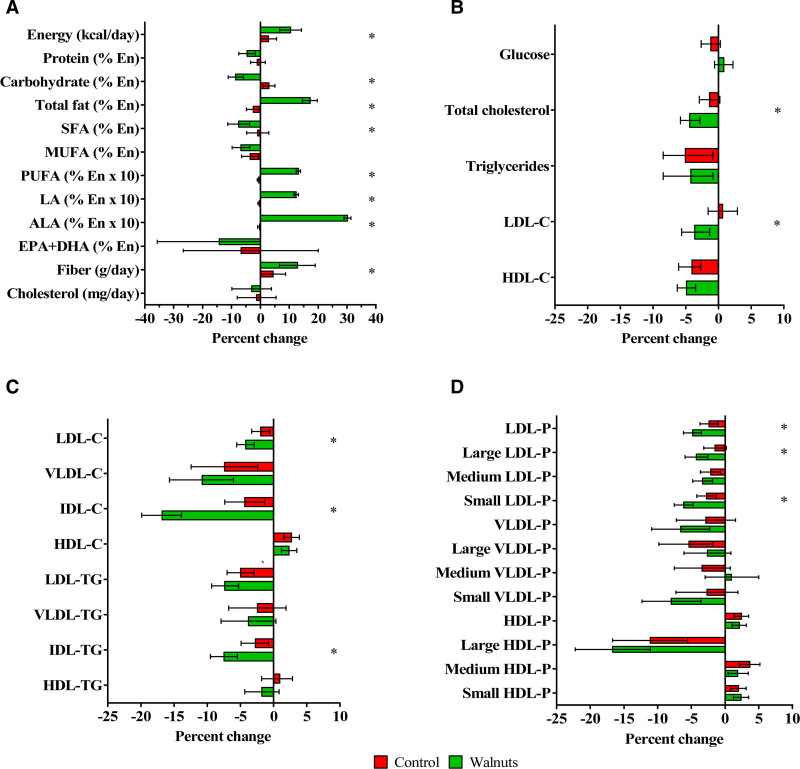
Results disclosed that 636 participants completed the study (90% retention rate) and 628 had full data for lipoprotein analyses (mean age 69 years, 67% women, 32% treated with statins). Their clinical characteristics did not differ from those of completers for the primary cognitive outcome.3 Mean baseline LDL-C and triglycerides were 117 and 105 mg/dL, respectively. In-trial statin changes were not different by treatment arm. Compliance with the walnut diet was good and body weight was stable, with mean 2-year changes of 0.06 kg (95% CI, –0.32 to 0.44) in the walnut diet and –0.51 kg (95% CI, –0.91 to –0.12) in controls. Reflecting the nutrient composition of walnuts, participants in the walnut group increased intake of energy, total fat, fiber, linoleic acid, and α-linolenic acid (Figure, A). No significant between-group changes in fasting glucose were observed (Figure, B). The walnut diet significantly decreased (mg/dL) total cholesterol (mean –8.5 [95% CI, –11.2 to –5.4]), LDL-C (mean –4.3 [–6.6 to –1.6]), and intermediate-density lipoprotein cholesterol (–1.3 [–1.5 to –1.0]), corresponding to reductions of 4.4%, 3.6%, and 16.8%, respectively, whereas triglycerides and high-density lipoprotein cholesterol were unaffected (Figure, B and C). Total LDL particles and small LDL particle number decreased by 4.3% and 6.1%, respectively (Figure, D). Results were not different by study site. Lipid responses to the walnut diet differed by sex: LDL-C was reduced by 7.9% in men and by 2.6% in women (P-interaction=0.007).
Figure.
Mean percent changes at 2 years in dietary variables and lipid and lipoprotein subclasses by intervention group. A, Energy and nutrient intake. B, Measured lipid and lipoprotein cholesterol concentrations. C, Estimated lipoprotein lipid concentrations by nuclear magnetic resonance spectroscopy. D, Estimated lipoprotein particle number by nuclear magnetic resonance spectroscopy. Error bars represent 95% CIs. ALA indicates α-linolenic acid; C, cholesterol; DHA, docosahexaenoic acid; En, energy; EPA, eicosapentaenoic acid; HDL, high-density lipoprotein; LA, linoleic acid; LDL, low-density lipoprotein; MUFA, monounsaturated fatty acids; P, particle number; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; TG, triglyceride; and VLDL, very-low-density lipoprotein. *P<0.05. P values for differences in nutrient intake were obtained by 1-way ANOVA. P values for differences in lipids and lipoproteins obtained by ANCOVA adjusted by center, age, sex, body mass index, smoking status (ever smoker/never smoker), APOE ε4 carriership (yes/no), physical activity changes, diabetes (yes/no), dyslipidemia (yes/no), hypertension (yes/no), statin treatment (yes/no), changes in statin doses standardized to simvastatin, and the baseline value of each variable. The estimated marginal means of the changes were used to calculate percent changes from baseline in both groups.
The results of this 2-year randomized controlled trial demonstrate that incorporating daily doses of walnuts (≈15% of energy) to the habitual diet of free-living elderly individuals with an essentially normal lipid profile resulted in a mean 4.3 mg/dL LDL-C reduction, which is modest, although greater responses have been observed among individuals with hypercholesterolemia.2 Our data also support a beneficial effect of the walnut diet on nuclear magnetic resonance–assessed lipoprotein subfractions, with reductions of intermediate-density lipoprotein cholesterol (a sizable contributor to remnant cholesterol) and total LDL particles. Prospective studies have reported that LDL particle number consistently outperforms LDL-C in CVD risk prediction and that remnant cholesterol causally relates to CVD independent of LDL-C.5 That lipid responses were not different in the 2 cohorts consuming diverse diets strengthens the generalization of our results. WAHA is the largest and longest nut trial to date, overcoming the limitations of prior smaller and shorter nut studies. The novel finding of sexual dimorphism in LDL-C response to walnut supplementation needs confirmation. WAHA was conducted in free-living individuals, who chose their daily foods, which may be viewed as desirable because it is closer to real life than the situation in controlled feeding studies.
On the basis of associations ascertained in cohort studies,5 the observed shift of the lipoprotein subclass phenotype suggests a reduction of lipoprotein-related CVD risk by long-term consumption of walnuts, which provides novel mechanistic insight for their potential cardiovascular benefit beyond effects on the standard lipid panel. Our data reinforce the notion that regular walnut consumption may be a useful part of a multicomponent dietary intervention or dietary pattern to lower atherogenic lipids and improve CVD risk.
Acknowledgments
The authors thank the participants in the trial for their enthusiastic collaboration and E. Corbella for expert statistical assistance. CIBEROBN is an initiative of Instituto de Salud Carlos III, Spain.
Sources of Funding
This work was supported by a grant from the California Walnut Commission, Folsom, CA. The funding agency had no involvement in the study design, data collection, analyses, and interpretation of the data or writing of the manuscript. Dr Sala-Vila holds a Miguel Servet fellowship (CP17/00029) and is supported by Fondo de Investigación Sanitaria-FEDER (PI15/01014 grant), Instituto de Salud Carlos III, Spain.
Disclosures
Drs Rajaram, Sala-Vila, Sabaté, and Ros have received research funding through their institutions from the California Walnut Commission (CWC), Folsom, CA. Dr Sala-Vila has also received support from CWC to attend professional meetings. Drs Sabaté and Ros were nonpaid members of the Scientific Advisory Council of the CWC. Dr Ros was a paid member of the CWC Health Research Advisory Group and has received personal money from the CWC for presentations. All other authors declare no competing interests.
Nonstandard Abbreviations and Acronyms
- CVD
- cardiovascular disease
- LDL-C
- low-density lipoprotein cholesterol
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01634841.
For Sources of Funding and Disclosures, see page 1085.
Contributor Information
Sujatha Rajaram, Email: srajaram@llu.edu.
Montserrat Cofán, Email: mcofan@clinic.cat.
Aleix Sala-Vila, Email: asala3@imim.es.
Ella Haddad, Email: ehaddad@llu.edu.
Edward Bitok, Email: ebitok@llu.edu.
Irene Roth, Email: roth@clinic.cat.
Tania M. Freitas-Simoes, Email: freita@clinic.cat.
Amandeep Kaur, Email: akaur1@llu.edu.
Cinta Valls-Pedret, Email: cintavalls@gmail.com.
Mónica Doménech, Email: mdomen@clinic.cat.
Keiji Oda, Email: koda@llu.edu.
Dolores Corella, Email: dolores.corella@uv.es.
Joan Sabaté, Email: jsabate@llu.edu.
References
- 1.Becerra-Tomás N, Paz-Graniel I, Kendall CWC, Kahleova H, Rahelić D, Sievenpiper JL, Salas-Salvadó J. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Nutr Rev. 2019; 77:691–709. doi: 10.1093/nutrit/nuz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010; 170:821–827. doi: 10.1001/archinternmed.2010.79 [DOI] [PubMed] [Google Scholar]
- 3.Sala-Vila A, Valls-Pedret C, Rajaram S, Coll-Padrós N, Cofán M, Serra-Mir M, Pérez-Heras AM, Roth I, Freitas-Simoes TM, Doménech M, et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: a randomized controlled trial. Am J Clin Nutr. 2020; 111:590–600. doi: 10.1093/ajcn/nqz328 [DOI] [PubMed] [Google Scholar]
- 4.Mallol R, Amigó N, Rodríguez MA, Heras M, Vinaixa M, Plana N, Rock E, Ribalta J, Yanes O, Masana L, et al. Liposcale: a novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy. J Lipid Res. 2015; 56:737–746. doi: 10.1194/jlr.D050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langlois MR, Chapman MJ, Cobbaert C, Mora S, Remaley AT, Ros E, Watts GF, Borén J, Baum H, Bruckert E, et al. ; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and very low concentrations of LDL cholesterol. A consensus statement from EAS and EFLM. Clin Chem. 2018; 64:1006–1033. doi: 10.1373/clinchem.2018.287037 [DOI] [PubMed] [Google Scholar]