ABSTRACT
β-Hydroxy-α-amino acids are useful compounds for pharmaceutical development. Enzymatic synthesis of β-hydroxy-α-amino acids has attracted considerable interest as a selective, sustainable, and environmentally benign process. In this study, we identified a novel amino acid hydroxylase, AEP14369, from Sulfobacillus thermotolerans Y0017, which is included in a previously constructed CAS-like superfamily protein library, to widen the variety of amino acid hydroxylases. The detailed structures determined by nuclear magnetic resonance and X-ray crystallography analysis of the enzymatically produced compounds revealed that AEP14369 catalyzed threo-β-selective hydroxylation of l-His and l-Gln in a 2-oxoglutarate-dependent manner. Furthermore, the production of l-threo-β-hydroxy-His and l-threo-β-hydroxy-Gln was achieved using Escherichia coli expressing the gene encoding AEP14369 as a whole-cell biocatalyst. Under optimized reaction conditions, 137 mM (23.4 g liter−1) l-threo-β-hydroxy-His and 150 mM l-threo-β-hydroxy-Gln (24.3 g liter−1) were obtained, indicating that the enzyme is applicable for preparative-scale production. AEP14369, an l-His/l-Gln threo-β-hydroxylase, increases the availability of 2-oxoglutarate-dependent hydroxylase and opens the way for the practical production of β-hydroxy-α-amino acids in the future. The amino acids produced in this study would also contribute to the structural diversification of pharmaceuticals that affect important bioactivities.
IMPORTANCE Owing to an increasing concern for sustainability, enzymatic approaches for producing industrially useful compounds have attracted considerable attention as a powerful complement to chemical synthesis for environment-friendly synthesis. In this study, we developed a bioproduction method for β-hydroxy-α-amino acid synthesis using a newly discovered enzyme. AEP14369 from the moderate thermophilic bacterium Sulfobacillus thermotolerans Y0017 catalyzed the hydroxylation of l-His and l-Gln in a regioselective and stereoselective fashion. Furthermore, we biotechnologically synthesized both l-threo-β-hydroxy-His and l-threo-β-hydroxy-Gln with a titer of over 20 g liter−1 through whole-cell bioconversion using recombinant Escherichia coli cells. As β-hydroxy-α-amino acids are important compounds for pharmaceutical development, this achievement would facilitate future sustainable and economical industrial applications.
KEYWORDS: β-hydroxy-α-amino acid, asymmetric hydroxylation, l-threo-β-hydroxy-His, l-threo-β-hydroxy-Gln, 2-oxoglutarate-dependent hydroxylase, CAS-like superfamily, dioxygenases
INTRODUCTION
β-Hydroxy-α-amino acids, which occur in several natural products, are regarded as an important class of industrially useful compounds, especially for pharmaceutical development (1). Moreover, β-hydroxy amino acids are applicable chiral building blocks and, thus, can be used to synthesize optically active β-lactam antibiotics (2). Although many attempts have been made to chemically synthesize β-hydroxy-α-amino acids, their selective synthesis remains highly challenging. For this purpose, enzyme catalysis is recognized as an alternative tool that can overcome the drawbacks of commonly used synthesis procedures by enabling the development of selective, economical, and environmentally benign processes (3, 4).
In the enzymatic synthesis of β-hydroxy-α-amino acids, two possible methods are considered: an aldolase process and a hydroxymethyltransferase process. Microbial Thr aldolase has attracted much attention because it catalyzes the retro-aldol reaction as well as the aldol reaction. For the retro-aldol reaction, the enzymatic resolution of either d- or l-isomers can be achieved from β-hydroxy-dl-amino acids with a maximum molar yield of 50% (5). In contrast, aldolase catalyzes the aldol reaction using Gly (donor) with various aldehydes (acceptors), including aliphatic and aromatic structures, to form natural and unnatural β-hydroxy-α-amino acids. Aldolases, which are classified as l-Thr aldolase (EC 4.1.2.5), l-allo-Thr aldolase (EC 4.1.2.48), and d-Thr aldolase (EC 4.1.2.42), catalyze C-C bond formation to produce various β-hydroxy-α-amino acids (6, 7). Although Thr aldolases catalyze the highly selective formation of d- or l-isomers, they exhibit remarkably broad substrate specificity for aldehydes. These properties are favorable for producing various useful compounds in a stereoselective manner. However, some drawbacks have been elucidated. First, an excess amount of Gly is required for the aldol reaction to produce β-hydroxy-α-amino acids because of the reverse reaction. Further, an aldehyde is often harmful to enzyme activity, and it leads to a low product yield caused by substrate inhibition or enzyme inactivation (8). In addition, Thr aldolase generally has low diastereoselectivity at the β-position in an aldol reaction.
Ser hydroxymethyltransferase (EC 2.1.2.1) is also a promising enzyme to produce β-hydroxy-α-amino acids, as it catalyzes the aldol reaction using Gly and some aldehydes (9). The substrate specificity of hydroxymethyltransferase is relatively limited in both donors and acceptors compared with that of aldolases. To enhance diastereoselectivity and broaden substrate specificity, protein engineering was performed by rational design or random mutagenesis coupled with a high-throughput assay. Although partial improvement has been demonstrated, the problems of diastereoselectivity and equilibrium have not been addressed sufficiently (10–12).
The recently reported microbial 2-oxoglutarate (2-OG)-dependent amino acid hydroxylase provides a critical solution to overcoming the disadvantages of the enzymes described above (13, 14). The hydroxylase catalyzes the hydroxylation of amino acids in a highly regioselective and stereoselective manner with an irreversible reaction; thus, it could be used as an alternative tool for genuine diastereoselective β-hydroxy-α-amino acid synthesis. Hydroxylases are highly attractive enzymes; however, the available enzymes are relatively limited compared with the well-established Thr aldolases or hydroxymethyltransferases. Thus, further enzyme screening and engineering of hydroxylases could facilitate the practical production of various β-hydroxy-α-amino acids.
During the screening of 2-OG-dependent hydroxylases, we constructed a clavaminic acid synthase (CAS)-like superfamily library using genome data mining and then discovered six novel l-Lys hydroxylases with two hydroxylation methods: 3S-hydroxylation and 4R-hydroxylation of l-Lys (15). In the previous study, our interest was primarily focused on finding l-Lys hydroxylase; thus, the substrate specificity of other CAS-like superfamily enzymes remains unclear.
Here, we assessed the substrate specificity of 36 CAS-like superfamily proteins using proteinogenic amino acids as their substrates for producing diverse hydroxy-amino acids, including β-hydroxy-α-amino acids. Among these, we found a novel amino acid hydroxylase that catalyzes the hydroxylation of l-His and l-Gln and developed a process for their production.
RESULTS
Substrate and reaction specificity of CAS-like superfamily proteins for various amino acids.
We assessed the substrate specificity of the CAS-like superfamily proteins for all proteinogenic amino acids. Among the 36 proteins tested using an Escherichia coli whole-cell reaction, only AEP14369 converted l-His and l-Gln in a 2-OG-dependent manner. Among the other 35 proteins, we previously reported that six have l-Lys hydroxylation activity (15). However, these six hydroxylases did not convert other amino acids, and the remaining 29 proteins investigated did not have hydroxylation activity for any proteinogenic amino acid. To evaluate the conversions of l-His and l-Gln in further detail, we purified AEP14369 by Ni2+ affinity chromatography (see Fig. S1 in the supplemental material), followed by l-His and l-Gln conversion.
Omission tests, where the reaction mixture lacked either 2-OG, l-ascorbic acid, FeSO4, or AEP14369, are summarized in Table 1. The results indicated a stringent requirement of 2-OG for the hydroxylation of l-His and l-Gln as the electron donor, which was not replaceable by NAD(P)H. Although not indispensable, l-ascorbic acid stimulated the l-Gln hydroxylation reaction. Fe2+ was essential for maximum activity; however, slight activity was detected in both hydroxylation reactions even in the absence of Fe2+, possibly because a minor amount of host-derived Fe2+ remained in the active center of the enzyme after protein purification. This endogenous Fe2+ was captured by ethylenediaminetetraacetic acid (EDTA), resulting in diminished activity. These results provide conclusive evidence that AEP14369 is a member of the Fe2+/2-OG-dependent dioxygenase family enzyme.
TABLE 1.
Reaction specificity of AEP14349
| Componenta | Sp actd (μmol min−1 mg–1) |
|
|---|---|---|
| l-His | l-Gln | |
| 2-OG,b VC,c Fe2+, enzyme | 0.208 ± 0.024 | 0.262 ± 0.023 |
| VC, Fe2+, enzyme | 0 | 0 |
| 2-OG, Fe2+, enzyme | 0.206 ± 0.020 | 0.051 ± 0.003 |
| 2-OG, VC, enzyme | 0.025 ± 0.002 | 0.030 ± 0.002 |
| 2-OG, VC, Fe2+ | 0 | 0 |
| 2-OG, VC, Fe2+, EDTA, enzyme | 0 | 0 |
| NADH, VC, Fe2+, enzyme | 0 | 0 |
| NADPH, VC, Fe2+, enzyme | 0 | 0 |
l-His or l-Gln was included in each reaction.
2-Oxoglutarate.
cl-Ascorbic acid.
Each concentration is described in Materials and Methods. Data are presented as the means ± SD from the results of three independent experiments.
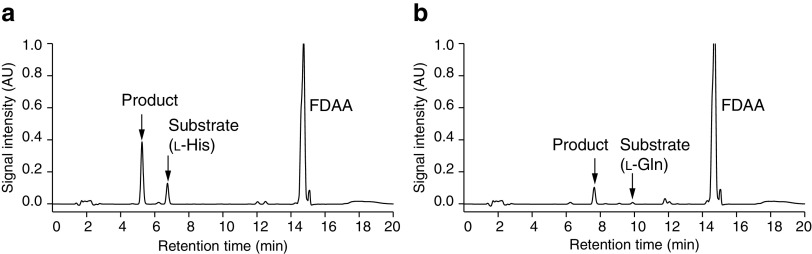
The reaction mixtures were subjected to high-performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) analyses following hydroxylation. The HPLC chromatograms of each mixture after enzymatic conversion showed that the peaks at the retention times of 5.25 min (Fig. 1a) and 7.65 min (Fig. 1b) corresponded to possible hydroxy-l-His and hydroxy-l-Gln, respectively. In the LC-MS analysis of each mixture, 1-fluoro-2,4-dinitrophenyl-5-l-alaninamide (FDAA)-derivatized protonated ions at m/z = 423.72 from the l-His hydroxylation product and m/z = 414.71 from the l-Gln hydroxylation product indicated the presence of hydroxy-l-His and hydroxy-l-Gln, respectively, because these m/z values both were greater than those of the respective substrate by 16. However, the enzyme did not accept any d-amino acids, including d-His and d-Gln, as substrates.
FIG 1.
HPLC chromatograms of reaction mixtures with AEP14369. (a) l-His conversion; (b) l-Gln conversion.
Amino acid sequence analysis.
We identified l-His/l-Gln hydroxylase activity in AEP14369 from the previously constructed CAS-like protein library (15). The corresponding gene (orf Y53) resides on the pY0017 plasmid of S. thermotolerans Y0017 (16), whereas its related strains, including S. thermotolerans L15 (16) and Kr1T (17, 18), lack this gene. BLAST search using the amino acid sequence of AEP14369 revealed that two bacterial strains, Sulfobacillus sp. strain DSM 109850 and Sulfobacillus sp. strain hg2, had related proteins with 95.0% and 94.5% identity, respectively, suggesting the presence of similar l-His/l-Gln hydroxylases. AEP14369 and these proteins possessed CAS-like domain structures (conserved domain family cd00250).
Structures of enzymatically hydroxylated l-His and l-Gln.
Nuclear magnetic resonance (NMR) and X-ray crystallography analyses were performed to determine the detailed structure and absolute configuration of the hydroxylated products. The NMR spectra for β-hydroxy-His were as follows. 1H NMR (D2O, 600 MHz) obtained the following data: δ 4.27 (1H, d, J = 3.6 Hz), 5.15 (1H, d, J = 3.6 Hz), 7.09 (1H, s), and 7.70 (1H, s). 13C NMR (D2O, 150 MHz) obtained the following data: δ 63.96, 71.78, 118.82, 119.44, 138.73, and 166.84. The spectra for β-hydroxy-Gln were as follows. 1H NMR (D2O, 600 MHz) obtained the following data: δ 2.57 (1H, dd, J = 12.0 and 18.0), 2.70 (1H, dd, J = 3.0 and 8.0), 3.72 (1H, d, J = 6.0 Hz), and 4.47 (1H, ddd, J = 3.0, 6.0, and 12.0 Hz). 13C NMR (D2O, 150 MHz) obtained the following data: δ 42.74, 62.23, 69.54, 174.93, and 178.21.
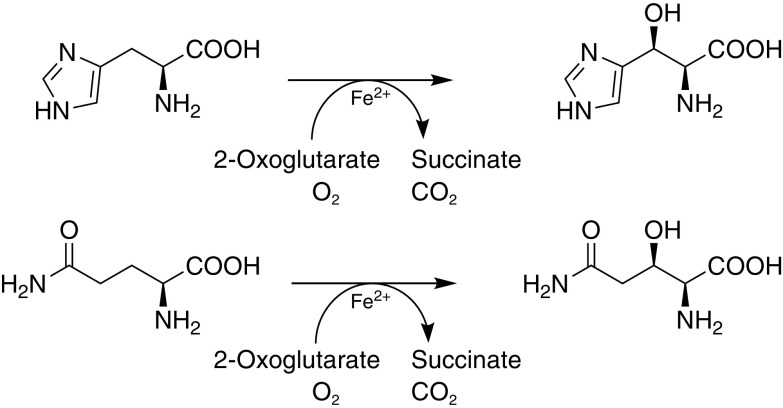
ORTEP diagrams of hydroxy-l-His and hydroxy-l-Gln (Fig. 2) were constructed based on the X-ray crystallography data. Their absolute configurations indicated (2S,3S)-β-hydroxy-His and (2S,3R)-β-hydroxy-Gln. These data revealed that AEP14369 catalyzed the threo-β-selective hydroxylation of l-His and l-Gln with no other isomers (Fig. 3). We consequently termed AEP14369 the l-His/l-Gln threo-β-hydroxylase.
FIG 2.

ORTEP diagrams of l-threo-β-hydroxy-His (a) and l-threo-β-hydroxy-Gln (b).
FIG 3.
Regioselective and stereoselective hydroxylation of l-His and l-Gln using AEP14369.
Enzymatic characterization of AEP14369.
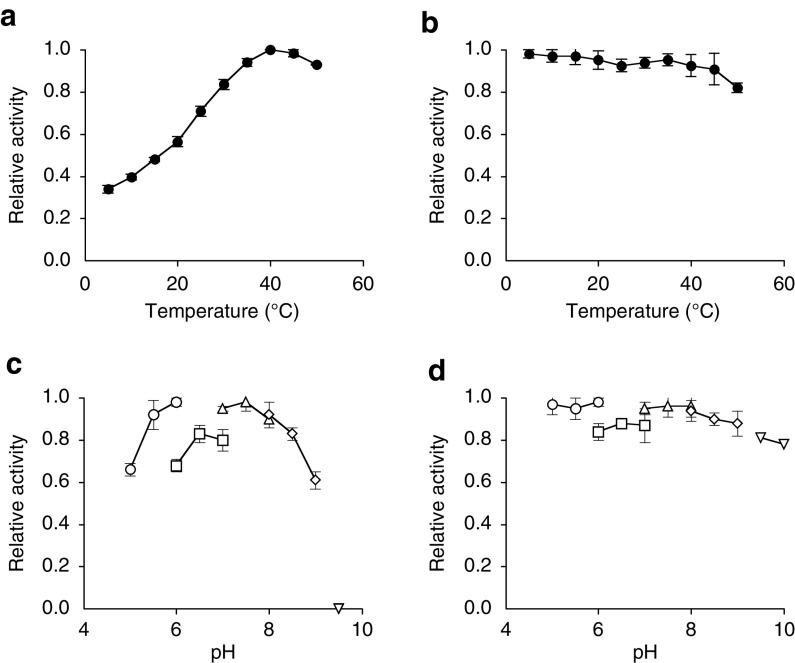
The effect of temperature on the β-hydroxylation activity of l-His was determined. AEP14369 had an optimum temperature of 40°C, and its activity gradually decreased at temperatures above 45°C (Fig. 4a). After a 1-h incubation at 5 to 55°C, at least 80% activity was retained below 50°C (Fig. 4b).
FIG 4.
Effects of temperature and pH on enzyme activity. Temperature dependence (a) and tolerance (b) as well as pH dependence (c) and tolerance (d) are shown. Buffers tested were the following: open circles, sodium acetate (pH 5.0 to 6.0); open squares, potassium phosphate (pH 6.0 to 7.0); open triangles, HEPES-NaOH (pH 7.0 to 8.0); open diamonds, Tris-HCl (pH 8.0 to 9.0); open inverted triangles, sodium carbonate (pH 9.5 to 10.0). Data are expressed as the mean ± standard deviation (SD) results from three independent experiments.
The effect of pH on AEP14369 activity was also determined. The enzyme had an optimum pH of 7.5 (Fig. 4c) and was stable in the pH range of 5.0 to 10.0 (Fig. 4d). Although the enzyme maintained its activity over a broad range, activity decreased at pH > 9.
Steady-state kinetic parameters (Km and kcat) based on the Michaelis-Menten plot were determined at 35°C (pH 7.5) (Fig. S2). Kinetic analysis revealed that the Km and kcat values for l-His and l-Gln were similar (Table 2). Thus, these kinetic parameters alone do not explain the physiological roles of the enzyme.
TABLE 2.
Steady-state kinetic parameters for the β-hydroxylation of l-His and l-Glna
| Substrate | Km (mM) | kcat (min–1) | kcat/Km (min–1 mM–1) |
|---|---|---|---|
| l-His | 0.83 ± 0.06 | 35.1 ± 3.9 | 42.7 ± 5.8 |
| l-Gln | 1.17 ± 0.27 | 43.8 ± 4.1 | 38.2 ± 5.0 |
Data are expressed as the mean ± SD results from three independent experiments.
Preparative-scale production of l-threo-β-hydroxy-His and l-threo-β-hydroxy-Gln using E. coli whole cells.
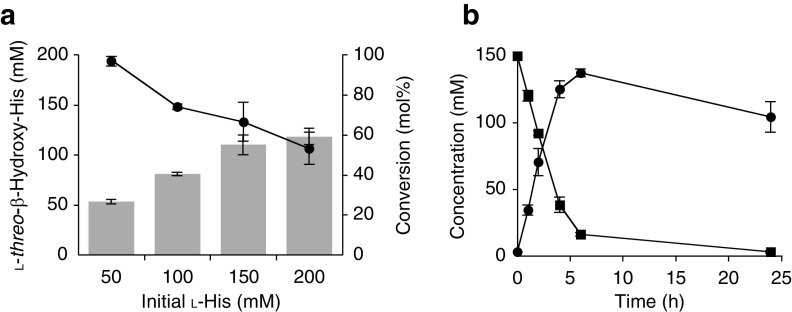
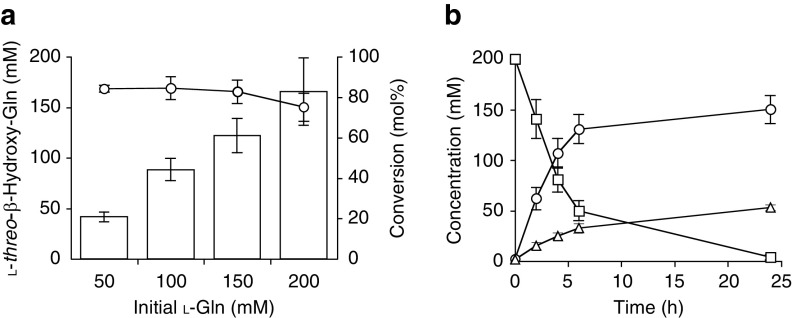
To produce hydroxy amino acids at a preparative scale, substrates l-His and l-Gln were converted through a whole-cell reaction with E. coli expressing the gene encoding AEP14369. We first varied the concentration of the substrate and E. coli whole cells. The substrate concentration was increased from 50 mM to 200 mM in a stepwise manner for each reaction. Conversion of l-His gradually decreased as the substrate concentration increased (Fig. 5a); therefore, we increased the cell concentration from an optical density at 600 nm (OD600) of 30 to 80 for better conversion efficiency. Under these conditions, we obtained 137 mM l-threo-β-hydroxy-His from 150 mM l-His after 6 h, which gradually degraded in a prolonged reaction for 24 h (Fig. 5b). However, an increased concentration of l-Gln did not affect the conversion efficiency at a maximum of 200 mM at an OD600 of 30 (Fig. 6a). We obtained 150 mM l-threo-β-hydroxy-Gln from 200 mM l-Gln at 24 h, and 53 mM l-Glu was coproduced through the whole-cell reaction (Fig. 6b).
FIG 5.
Production of l-threo-β-hydroxy-His using whole-cell reaction. (a) Effect of initial l-His concentration on production efficiency. Symbols: bars, concentration of l-threo-β-hydroxy-His; circles, conversion ratio. (b) Time course under the optimized conditions. Symbols: circles, l-threo-β-hydroxy-His; squares, l-His. Data are expressed as the mean ± SD results from three independent experiments.
FIG 6.
Production of l-threo-β-hydroxy-Gln using whole-cell reaction. (a) Effect of initial l-Gln concentration on production efficiency. Symbols: bars, concentration of l-threo-β-hydroxy-Gln; circles, conversion ratio. (b) Time course under the optimized conditions. Symbols: circles, l-threo-β-hydroxy-Gln; squares, l-Gln; triangles, l-Glu. Data are expressed as the mean ± SD results from three independent experiments.
DISCUSSION
In this study, we demonstrated that the CAS-like superfamily protein library (15) comprises a novel 2-OG-dependent hydroxylase, which catalyzes the hydroxylation of l-His and l-Glu to form l-threo-β-hydroxy-His and l-threo-β-hydroxy-Gln, respectively. The substrate/product specificity of AEP14369 is unique because no such 2-OG-dependent hydroxylase has been reported previously.
Widely known amino acid hydroxylases, such as l-Pro hydroxylases (19), l-Arg β-hydroxylase VioC (20), l-Asn β-hydroxylase AsnO (21), and l-Leu δ-hydroxylase (22), are responsible for the biosynthesis of secondary metabolites, such as etamycin, viomycin, daptomycin-like lipopeptide, and nostopeptide, respectively. GLOXY3 from Glarea lozoyensis, which is involved in pneumocandin biosynthesis, has also been found to catalyze l-Gln β-hydroxylation in an in vitro assay (23). Based on the biosynthetic analysis of pneumocandin, the hydroxylated product is hypothesized to be l-erythro-β-hydroxy-Gln [(3S)-3-hydroxy-l-Gln] (24, 25), which differs from the AEP14369 reaction product in diastereoselectivity. No significant sequence similarity was detected between AEP14369 and GLOXY3 (19% identity). The recently reported l-Glu β-hydroxylase IboH (26) also shared poor amino acid sequence similarity with AEP14369 (16% identity), although these enzymes act on substrates with similar structures.
l-His β-hydroxylation has been reported in some secondary metabolite biosynthesis pathways. For example, in nikkomycin biosynthesis, l-His anchored in the NikP1 carrier domain is hydroxylated by NikQ hydroxylase, which requires NADPH with an electron transfer protein, such as a bacterial cytochrome P450 oxygenase (27). Furthermore, β-hydroxy-His is also found in several secondary metabolites, including bleomycin (28, 29), pyoverdine-type siderophores (30–32), and exochelin MN (33). These hydroxylation systems differ from AEP14369 in enzymatic functions and physiological roles. AEP14369-catalyzed hydroxylation occurs in the presence of 2-OG (Table 1), whereas in some systems, l-His hydroxylation requires NAD(P)H as an electron donor. The gene responsible for amino acid hydroxylation is frequently involved as a member of a biosynthetic gene cluster; however, no such protein, including a nonribosomal peptide synthetase, was found in the flanking region of the gene locus of AEP14369. The physiological roles of l-threo-β-hydroxy-His and l-threo-β-hydroxy-Gln remain unclear; thus, further investigation will be necessary to understand the functions of these activities in S. thermotolerans Y0017 and its related species.
The use of whole cells avoids complex and expensive protein purification and makes the process amenable to industrial application (34–36). Given the practical use of this enzyme, we demonstrate that AEP14369 is useful for producing both threo-β-hydroxy-l-His and threo-β-hydroxy-l-Gln on a preparative scale. Using E. coli expressing the gene encoding AEP14369 as a whole-cell biocatalyst, 137 mM (23.4 g liter−1) l-threo-β-hydroxy-His was produced from 150 mM l-His with a yield of 91%. In this case, a prolonged reaction time of up to 24 h lowered the l-threo-β-hydroxy-His accumulation, suggesting its degradation by the E. coli-endogenous enzymes. Using the same strain, 150 mM (24.3 g liter−1) l-threo-β-hydroxy-Gln was produced from 200 mM l-Gln with a yield of 75%. Unlike the case of l-His hydroxylation, degradation of the substrate l-Gln occurred, probably owing to E. coli endogenous glutaminase that competed with l-Gln hydroxylation. Glutaminase, a major l-Gln-degrading enzyme, catabolizes l-Gln to l-Glu and releases ammonia, which leads to l-Glu accumulation (Fig. 6b). To increase the efficiency of l-threo-β-hydroxy-Gln, the use of glutaminase-deficient E. coli would allow the avoiding of the glutaminase pathway. In both cases, the product concentration exceeded 20 g liter−1, suggesting the potential for future practical production process development similar to other bioprocesses, including l-threo-β-hydroxy-Asp (37), (2S,3S)-β-hydroxy-Lys, and (2S,4R)-γ-hydroxy-Lys (15). 2-OG, an essential cosubstrate for amino acid hydroxylation, can be supplied from industrially inexpensive materials, such as glucose and glycerol, via the E. coli metabolic pathway. This aspect appears to be beneficial for industrial applications. Hydroxyproline and hydroxyisoleucine have been produced previously with 2-OG supplied through the E. coli metabolic pathway (38, 39).
In conclusion, we revealed that the novel 2-OG-dependent hydroxylase from S. thermotolerans Y0017 catalyzed the β-hydroxylation of l-His and l-Gln in a threo-selective manner. To assess the potential of the enzyme for industrial application, we produced l-threo-β-hydroxy-His and l-threo-β-hydroxy-Gln through the bioconversion of recombinant E. coli. Only a few β-hydroxy-α-amino acids are currently available for enzymatic asymmetric hydroxylation because of the strict substrate specificity of the 2-OG-dependent hydroxylase. Although the accessibility of 2-OG-dependent hydroxylases is relatively limited compared to that of aldolases, these hydroxylases show excellent diastereoselectivity. The findings of this study indicate the feasibility of enzymatic asymmetric β-hydroxy-α-amino acid production. Further extensive searches for enzymes homologous to AEP14369 could expand the variety of 2-OG-dependent hydroxylases available for producing diverse hydroxy-amino acids.
MATERIALS AND METHODS
Materials.
All chemicals were of analytical grade and were obtained from Wako Pure Chemical Industries (Osaka, Japan) and Tokyo Chemical Industry (Tokyo, Japan). The cultivation methods for recombinant E. coli carrying each plasmid for the expression of CAS-like superfamily proteins have been described previously (15). This article does not contain any studies involving human participants or animals performed by any of the authors.
Screening of amino acid hydroxylase in CAS-like library.
For initial screening, l-amino acids (5 mM) were individually converted by whole cells of E. coli expressing CAS-like protein (OD600 of 10) in the presence of 10 mM 2-OG, 5 mM l-ascorbic acid, and 1 mM FeSO4 in a total volume of 1 ml. The reaction was performed with vigorous shaking at 30°C for 3 h.
Enzyme assay.
AEP14369 purified by Ni2+ affinity chromatography was used to determine reaction specificity, optimum pH, and temperature. To determine reaction specificity, the standard reaction mixture containing 5 mM l-His or l-Gln, 6 mM 2-OG, 1 mM l-ascorbic acid, 0.5 mM FeSO4, 0.1 mg ml−1 AEP14369, and 20 mM HEPES-NaOH buffer (pH 7.5) in a total volume of 0.1 ml was incubated at 35°C for 30 min. An omission test was carried out by removing each component. In addition, cofactor preference [5 mM NAD(P)H instead of 2-OG] and the effects of chelating reagent (2 mM EDTA) were assessed.
To determine the optimum conditions for enzyme activity, the reaction mixture contained 5 mM l-His, 10 mM 2-OG, 0.5 mM FeSO4, 0.1 mg ml−1 AEP14369, and 50 mM HEPES-NaOH buffer (pH 7.5) in a total volume of 0.2 ml and was initiated by adding the purified enzyme under varied pH (5 to 10) or temperature (5 to 50°C). To determine heat stability, after a 1-h incubation at various temperatures (5 to 50°C), the treated enzyme was applied to the standard reaction conditions (35°C, pH 7.5). To determine pH stability, the enzyme was incubated at various pH values (5 to 10) in an ice bath for 1 h and then applied to the standard reaction conditions.
Kinetic analysis of AEP14369 was performed at 35°C in a reaction mixture with a total volume of 0.2 ml, containing 0.5 to 5 mM l-His or 0.5 to 5 mM l-Gln, 20 mM 2-OG, 0.5 mM FeSO4, 0.1 mg ml−1 enzyme, and 50 mM HEPES-NaOH buffer (pH 7.5). The reaction was initiated by adding the enzyme and was performed at 35°C for 10 min with reciprocal shaking. After the reaction was terminated by heat treatment at 90°C for 10 min, the amount of synthesized l-threo-β-hydroxy-His or l-threo-β-hydroxy-Gln was determined by HPLC. The kinetic parameters were calculated using a Michaelis-Menten plot.
Whole-cell reaction.
To produce l-threo-β-hydroxy-His or l-threo-β-hydroxy-Gln by a whole-cell reaction, the concentrations of the substrate and E. coli cells were considered. The reaction mixture containing 50 to 200 mM l-His or l-Gln, 60 to 400 mM 2-OG, 10 mM FeSO4, 50 mM HEPES (pH 7.5), and E. coli whole cells (OD600 of 30) in a total volume of 50 ml was incubated at 30°C for 24 h with shaking at 150 rpm in a 500-ml Erlenmeyer flask. For optimized l-His hydroxylation, the reaction mixture contained 150 mM l-His, 180 mM 2-OG, 10 mM FeSO4, and whole cells (OD600 of 80). For l-Gln hydroxylation, the reaction mixture contained 200 mM l-Gln, 400 mM 2-OG, 10 mM FeSO4, and whole cells (OD600 of 30). At each interval, 100 μl of the reaction mixture was withdrawn, and the supernatant was collected by centrifugation at 20,000 × g for 10 min at 4°C.
Analytical methods.
All amino acids were determined by precolumn derivatization with FDAA using a Chromaster HPLC system (Hitachi High-Tech, Tokyo, Japan). The system was equipped with a LaChrom II C18 column (4.6-mm inner diameter [i.d.] by 150-mm length; Hitachi High-Tech) maintained at 40°C in a column oven. The following mobile phases were used: eluent A (45 mM phosphate buffer [pH 2.7], 5% [vol/vol] methanol, and 5% [vol/vol] acetonitrile) and eluent B (30 mM phosphate buffer [pH 2.7], 5% methanol [vol/vol], and 35% [vol/vol] acetonitrile). Gradient elution was performed using the following program with a flow rate of 1 ml min−1: 30% to 80% B (0 to 12 min) and 80% B (12 to 15 min). Eluted amino acids were detected by UV absorption at 340 nm.
To determine the molecular masses, FDAA-derivatized amino acids were determined using an LCQ Fleet system (Thermo Fisher Scientific, Waltham, MA, USA). The LC conditions were the following: eluent A (0.1% formic acid-acetonitrile, 98:2 [vol/vol]); eluent B (0.1% formic acid-acetonitrile, 2:98 [vol/vol]); column, SUPERIOREX ODS (2.0-mm i.d. by 150-mm length; Osaka Soda, Osaka, Japan); column temperature, 40°C; gradient program, 30% to 80% B (0 to 10 min), 80% B (10.1 to 12 min); and a flow rate of 0.2 ml min−1. The electrospray ionization mass spectrometry conditions were the following: sheath gas flow rate, 30 arbitrary units (AU); auxiliary gas flow rate, 30 AU; spray voltage, 5 kV; capillary temperature, 350°C; capillary voltage, 17 V; and tube lens offset, 5 V.
NMR spectra were obtained using an AVANCE 600 spectrometer (Bruker, Billerica, MA, USA). l-threo-β-hydroxy-His and l-threo-β-hydroxy-Gln were dissolved in D2O containing 0.05% (wt/vol) 3-(trimethylsilyl)-propionic-2,2,3,3-d4 acid sodium salt (Sigma, St. Louis, MO, USA), which was used as the internal standard.
The absolute configuration was determined by single-crystal X-ray structures. For l-threo-β-hydroxy-His, a colorless needle crystal (approximate dimensions of 0.8 by 0.1 by 0.1 mm) was formed using the hanging-drop method and mounted on a glass fiber. For l-threo-β-hydroxy-Gln, a colorless platelet crystal (approximate dimensions of 0.4 by 0.4 by 0.1 mm) was generated by cooling after concentration and was mounted on a glass fiber. All measurements were made on an R-AXIS RAPID diffractometer (Rigaku, Tokyo, Japan) using graphite-monochromated Cu-Kα radiation. The structures were validated and illustrated as ORTEP diagrams by the crystallographic tool PLATON (40).
Data availability.
The nucleotide sequence of codon-modified AEP14369 for E. coli expression has been deposited in the DDBJ/EMBL/GenBank databases under the accession number LC638500. The crystallographic data obtained were deposited in the Cambridge Crystallographic Data Centre (CCDC deposition numbers 2092468 for l-threo-β-hydroxy-His and 2092469 for l-threo-β-hydroxy-Gln).
ACKNOWLEDGMENTS
R.H. received funding from the Japan Society for the Promotion of Science KAKENHI (grant no. 18K05400). We thank the Materials Characterization Central Laboratory of Waseda University and S. Suzuki for technical assistance. We thank Editage for English language editing.
R.H. and K.K. designed the experiments and supervised the project. R.H. wrote the manuscript draft. R.H. and K.K. revised the manuscript. R.H., Y.N., H.Y., and R.G. performed the general experiments. I.H. performed X-ray crystallography analysis.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Kuniki Kino, Email: kkino@waseda.jp.
Haruyuki Atomi, Kyoto University.
REFERENCES
- 1.Qian Y, Jing C, Liu S, Hu W. 2013. A highly enantioselective four-component reaction for the efficient construction of chiral β-hydroxy-α-amino acid derivatives. Chem Commun 49:2700–2702. 10.1039/c3cc40546j. [DOI] [PubMed] [Google Scholar]
- 2.Kimura T, Vassilev VP, Shen G-J, Wong C-H. 1997. Enzymatic synthesis of β-hydroxy-α-amino acids based on recombinant d- and l-threonine aldolases. J Am Chem Soc 119:11734–11742. 10.1021/ja9720422. [DOI] [Google Scholar]
- 3.Steinreiber J, Fesko K, Reisinger C, Schürmann M, van Assema F, Wolberg M, Mink D, Griengl H. 2007. Threonine aldolases—an emerging tool for organic synthesis. Tetrahedron 63:918–926. 10.1016/j.tet.2006.11.035. [DOI] [Google Scholar]
- 4.Vassilev VP, Uchiyama T, Kajimoto T, Wong C-H. 1995. l-Threonine aldolase in organic synthesis: preparation of novel β-hydroxy-α-amino acids. Tetrahedron Lett 36:4081–4084. 10.1016/0040-4039(95)00720-W. [DOI] [Google Scholar]
- 5.Liu J-Q, Dairi T, Itoh N, Kataoka M, Shimizu S, Yamada H. 2000. Diversity of microbial threonine aldolases and their application. J Mol Catal B Enzym 10:107–115. 10.1016/S1381-1177(00)00118-1. [DOI] [Google Scholar]
- 6.Fesko K. 2016. Threonine aldolases: perspectives in engineering and screening the enzymes with enhanced substrate and stereo specificities. Appl Microbiol Biotechnol 100:2579–2590. 10.1007/s00253-015-7218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dückers N, Baer K, Simon S, Gröger H, Hummel W. 2010. Threonine aldolases-screening, properties and applications in the synthesis of non-proteinogenic β-hydroxy-α-amino acids. Appl Microbiol Biotechnol 88:409–424. 10.1007/s00253-010-2751-8. [DOI] [PubMed] [Google Scholar]
- 8.Nozaki H, Kuroda S, Watanabe K, Yokozeki K. 2008. Purification and gene cloning of α-methylserine aldolase from Ralstonia sp. strain AJ110405 and application of the enzyme in the synthesis of α-methyl-l-serine. Appl Environ Microbiol 74:7596–7599. 10.1128/AEM.00677-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeed A, Young DW. 1992. Synthesis of l-β-hydroxyaminoacids using serine hydroxymethyltransferase. Tetrahedron 48:2507–2514. 10.1016/S0040-4020(01)88770-6. [DOI] [Google Scholar]
- 10.Chen Q, Chen X, Feng J, Wu Q, Zhu D, Ma Y. 2019. Improving and inverting Cβ-stereoselectivity of threonine aldolase via substrate-binding-guided mutagenesis and a stepwise visual screening. ACS Catal 9:4462–4469. 10.1021/acscatal.9b00859. [DOI] [Google Scholar]
- 11.Zheng W, Chen K, Wang Z, Cheng X, Xu G, Yang L, Wu J. 2020. Construction of a highly diastereoselective aldol reaction system with l-threonine aldolase by computer-assisted rational molecular modification and medium engineering. Org Lett 22:5763–5767. 10.1021/acs.orglett.0c01792. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, Yu H, Fang S, Chen K, Wang Z, Cheng X, Xu G, Yang L, Wu J. 2021. Directed evolution of l-threonine aldolase for the diastereoselective synthesis of β-hydroxy-α-amino acids. ACS Catal 11:3198–3205. 10.1021/acscatal.0c04949. [DOI] [Google Scholar]
- 13.Islam MS, Leissing TM, Chowdhury R, Hopkinson RJ, Schofield CJ. 2018. 2-Oxoglutarate-dependent oxygenases. Annu Rev Biochem 87:585–620. 10.1146/annurev-biochem-061516-044724. [DOI] [PubMed] [Google Scholar]
- 14.Peters C, Buller RM. 2019. Industrial application of 2-oxoglutarate-dependent oxygenases. Catalysts 9:221. 10.3390/catal9030221. [DOI] [Google Scholar]
- 15.Hara R, Yamagata K, Miyake R, Kawabata H, Uehara H, Kino K. 2017. Discovery of lysine hydroxylases in the clavaminic acid synthase-like superfamily for efficient hydroxylysine bioproduction. Appl Environ Microbiol 83:e00693-17. 10.1128/AEM.00693-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deane SM, Rawlings DE. 2011. Two large, related, cryptic plasmids from geographically distinct isolates of Sulfobacillus thermotolerans. Appl Environ Microbiol 77:8175–8180. 10.1128/AEM.06118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panyushkina AE, Babenko VV, Nikitina AS, Selezneva OV, Tsaplina IA, Letarova MA, Kostryukova ES, Letarov AV. 2019. Sulfobacillus thermotolerans: new insights into resistance and metabolic capacities of acidophilic chemolithotrophs. Sci Rep 9:15069. 10.1038/s41598-019-51486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdanova TI, Tsaplina IA, Kondrat'eva TF, Duda VI, Suzina NE, Melamud VS, Tourova TP, Karavaiko GI. 2006. Sulfobacillus thermotolerans sp. nov., a thermotolerant, chemolithotrophic bacterium. Int J Syst Evol Microbiol 56:1039–1042. 10.1099/ijs.0.64106-0. [DOI] [PubMed] [Google Scholar]
- 19.Hara R, Kino K. 2020. Enzymatic reactions and microorganisms producing the various isomers of hydroxyproline. Appl Microbiol Biotechnol 104:4771–4779. 10.1007/s00253-020-10603-1. [DOI] [PubMed] [Google Scholar]
- 20.Yin X, Zabriskie TM. 2004. VioC is a non-heme iron, α-ketoglutarate-dependent oxygenase that catalyzes the formation of 3S-hydroxy-l-arginine during viomycin biosynthesis. Chembiochem 5:1274–1277. 10.1002/cbic.200400082. [DOI] [PubMed] [Google Scholar]
- 21.Strieker M, Kopp F, Mahlert C, Essen LO, Marahiel MA. 2007. Mechanistic and structural basis of stereospecific Cβ-hydroxylation in calcium-dependent antibiotic, a daptomycin-type lipopeptide. ACS Chem Biol 2:187–196. 10.1021/cb700012y. [DOI] [PubMed] [Google Scholar]
- 22.Hibi M, Kawashima T, Sokolov PM, Smirnov SV, Kodera T, Sugiyama M, Shimizu S, Yokozeki K, Ogawa J. 2013. l-Leucine 5-hydroxylase of Nostoc punctiforme is a novel type of Fe(II)/α-ketoglutarate-dependent dioxygenase that is useful as a biocatalyst. Appl Microbiol Biotechnol 97:2467–2472. 10.1007/s00253-012-4136-7. [DOI] [PubMed] [Google Scholar]
- 23.Renata H, Shimizu E, Zwick CR. 2021. Regiodivergent biocatalytic hydroxylation of l-glutamine facilitated by characterization of non-heme dioxygenases from non-ribosomal peptide biosyntheses. Tetrahedron 90:132190. 10.1016/j.tet.2021.132190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Lan N, Xu L, Yue Q. 2018. Biosynthesis of pneumocandin lipopeptides and perspectives for its production and related echinocandins. Appl Microbiol Biotechnol 102:9881–9891. 10.1007/s00253-018-9382-x. [DOI] [PubMed] [Google Scholar]
- 25.Hüttel W. 2017. Structural diversity in echinocandin biosynthesis: the impact of oxidation steps and approaches toward an evolutionary explanation. Z Naturforsch C J Biosci 72:1–20. 10.1515/znc-2016-0156. [DOI] [PubMed] [Google Scholar]
- 26.Obermaier S, Muller M. 2020. Ibotenic acid biosynthesis in the fly agaric is initiated by glutamate hydroxylation. Angew Chem Int Ed Engl 59:12432–12435. 10.1002/anie.202001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Hubbard BK, O'Connor SE, Walsh CT. 2002. Formation of β-hydroxy histidine in the biosynthesis of nikkomycin antibiotics. Chem Biol 9:103–112. 10.1016/S1074-5521(02)00090-X. [DOI] [PubMed] [Google Scholar]
- 28.Gu J, Codd R. 2012. Copper(II)-based metal affinity chromatography for the isolation of the anticancer agent bleomycin from Streptomyces verticillus culture. J Inorg Biochem 115:198–203. 10.1016/j.jinorgbio.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Owa T, Otsuka M, Ohno M. 1988. Enantioselective synthesis of erythro-β-hydroxy-l-histidine, the pivotal amino acid of bleomycin–Fe(II)−O2 complex. Chem Lett 17:83–86. 10.1246/cl.1988.83. [DOI] [Google Scholar]
- 30.Budzikiewicz H, Kilz S, Taraz K, Meyer J-M. 1997. Identical pyoverdines from Pseudomonas fluorescens 9AW and from Pseudomonas putida 9BW. Z Naturforsch C J Biosci 52:721–728. 10.1515/znc-1997-11-1202. [DOI] [Google Scholar]
- 31.Hancock DK, Reeder DJ. 1993. Analysis and configuration assignments of the amino acids in a pyoverdine-type siderophore by reversed-phase high-performance liquid chromatography. J Chromatogr A 646:335–343. 10.1016/0021-9673(93)83346-T. [DOI] [Google Scholar]
- 32.Hancock DK, Coxon B, Wang S-Y, White VE, Reeder DJ, Bellama JM. 1993. l-Threo-β-hydroxyhistidine, an unprecedented iron(III) ion-binding amino acid in a pyoverdine-type siderophore from Pseudomonas fluorescens 244. J Chem Soc Chem Commun 5:468–470. 10.1039/C39930000468. [DOI] [Google Scholar]
- 33.Sharman GJ, Williams DH, Ewing DF, Ratledge C. 1995. Determination of the structure of exochelin MN, the extracellular siderophore from Mycobacterium neoaurum. Chem Biol 2:553–561. 10.1016/1074-5521(95)90189-2. [DOI] [PubMed] [Google Scholar]
- 34.Ishige T, Honda K, Shimizu S. 2005. Whole organism biocatalysis. Curr Opin Chem Biol 9:174–180. 10.1016/j.cbpa.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Schrewe M, Julsing MK, Buhler B, Schmid A. 2013. Whole-cell biocatalysis for selective and productive C-O functional group introduction and modification. Chem Soc Rev 42:6346–6377. 10.1039/c3cs60011d. [DOI] [PubMed] [Google Scholar]
- 36.de Carvalho CC. 2017. Whole cell biocatalysts: essential workers from nature to the industry. Microb Biotechnol 10:250–263. 10.1111/1751-7915.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara R, Nakano M, Kino K. 2015. One-pot production of l-threo-3-hydroxyaspartic acid using asparaginase-deficient Escherichia coli expressing asparagine hydroxylase of Streptomyces coelicolor A3(2). Appl Environ Microbiol 81:3648–3654. 10.1128/AEM.03963-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibasaki T, Mori H, Ozaki A. 2000. Enzymatic production of trans-4-hydroxy-l-proline by regio- and stereospecific hydroxylation of l-proline. Biosci Biotechnol Biochem 64:746–750. 10.1271/bbb.64.746. [DOI] [PubMed] [Google Scholar]
- 39.Smirnov SV, Kodera T, Samsonova NN, Kotlyarova VA, Rushkevich NY, Kivero AD, Sokolov PM, Hibi M, Ogawa J, Shimizu S. 2010. Metabolic engineering of Escherichia coli to produce (2S,3R,4S)-4-hydroxyisoleucine. Appl Microbiol Biotechnol 88:719–726. 10.1007/s00253-010-2772-3. [DOI] [PubMed] [Google Scholar]
- 40.Spek A. 2003. Single-crystal structure validation with the program PLATON. J Appl Crystallogr 36:7–13. 10.1107/S0021889802022112. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2. Download AEM.01335-21-s0001.pdf, PDF file, 1.9 MB (1.9MB, pdf)
Data Availability Statement
The nucleotide sequence of codon-modified AEP14369 for E. coli expression has been deposited in the DDBJ/EMBL/GenBank databases under the accession number LC638500. The crystallographic data obtained were deposited in the Cambridge Crystallographic Data Centre (CCDC deposition numbers 2092468 for l-threo-β-hydroxy-His and 2092469 for l-threo-β-hydroxy-Gln).