Abstract
Objectives:
Limited cohort studies have assessed the association between uncontrolled pain and risk for behavioral and psychological symptoms of dementia (BPSD). We conducted a longitudinal cohort study to examine whether associations exist between uncontrolled pain and risk for two common BPSD—depression and behavioral symptoms—among long-term care (LTC) residents with Alzheimer disease and related dementia (ADRD).
Design:
This retrospective cohort study analyzed quarterly data from the 5% Medicare sample linked to Minimum Data Set (MDS) 3.0 between January 1, 2011, and December 31, 2015.
Setting and Participants:
LTC residents aged 50 years or older with ADRD who had chronic pain and at least two quarterly MDS 3.0 assessments.
Methods:
LTC residents were followed up quarterly from first observed quarterly MDS 3.0 until first outcome event or last observed quarterly MDS 3.0. Uncontrolled pain was defined as numerical rating scale >4, verbal descriptor scale of moderate or severe pain, or ≥1 pain indicators on the Checklist of Nonverbal Pain Indicators. Depression was defined as ≥10 on the Patient Health Questionnaire 9; behavioral symptoms were defined as the presence of psychotic (delusions or hallucinations) or disruptive behaviors (rejection of care, or physical, verbal, or other aggressive behaviors). Generalized linear models (GLMs) with marginal structural modeling (MSM) stabilized weights were used to examine uncontrolled pain and outcome risk.
Results:
The incidence rate of depression and behavioral symptoms during follow-up was 9.4 and 23.1 per 100 resident-years, respectively. Results from the MSM-GLMs showed that LTC residents with uncontrolled pain had a higher risk than those with controlled pain for developing depression (hazard ratio=1.67, 95% CI=1.54–1.81) and behavioral symptoms (hazard ratio=1.28, 95 CI=1.19–1.37).
Conclusions and Implications:
Uncontrolled pain was associated with elevated risk for depressive and behavioral symptoms in dementia, underscoring the importance of pain assessment and control among LTC residents with ADRD.
Keywords: Uncontrolled Pain, Behavioral symptoms, Depression, ADRD, Nursing Home
Brief Summary:
This cohort study demonstrated that uncontrolled pain is associated with increased risk for depressive and behavioral symptoms, underscoring the importance of pain control in residents with dementia.
Introduction
Behavioral and psychological symptoms of dementia (BPSD) affect 97% of people with Alzheimer disease and related dementia (ADRD)1 at some point in time during the disease course and is one of the main reasons for nursing home admission.2 Common behavioral symptoms of dementia include agitation and aggression, while psychological symptoms include depression and anxiety.3,4 BPSD adversely affects individuals’ quality of life and physical and cognitive functioning and increases caregiver distress.4 Treatment of BPSD remains challenging, largely owing to the lack of effective targeted therapies and concerns about the safety of psychopharmacological medications.4 Current clinical guidelines highly recommend identifying risk factors that precipitate BPSD before initiation of any suggested pharmacological treatment.5,6
Although pain has been implicated as an important risk factor for BPSD,5,6 the magnitude of risk conferred by uncontrolled pain remains unclear. Available effect estimates have been inferred from cross-sectional studies that show a higher prevalence of depression, agitated and aggressive behaviors, and rejection of care among individuals with ADRD with versus without pain.7–10 To date, limited cohort studies have assessed the association between uncontrolled pain and risk for BPSD,11–14 and findings regarding pain control and risk for aggression and agitation are inconsistent.11–13 These inconsistencies may be due to small sample sizes, outdated data, and most importantly, failure to account for the time-varying feature of pain and confounders (e.g., use of pain medications), which can result in biased estimations of pain control on BPSD outcomes.15
Owing to serious adverse consequences of BPSD in persons with ADRD,4 the association between pain and BPSD deserves further investigation through a longitudinal cohort study design that addresses the aforementioned study limitations. Using a marginal structural modeling (MSM) approach15 to account for time-varying pain control exposure and time-varying confounders, the present study examined the associations between uncontrolled pain and risk of two common BPSD—depression and behavioral symptoms—among LTC residents with ADRD. We hypothesized that residents with (versus without) uncontrolled pain had a higher risk of developing depressive and behavioral symptoms in dementia.
Methods
Study design and data source
We conducted a retrospective cohort study of a 5% Medicare sample linked to the Minimum Data Set, version 3.0 (MDS 3.0), from 2011 to 2015. The Medicare data contain enrollees’ medical billing records for Parts A (inpatient), B (outpatient), and D (prescription drugs) as well as beneficiary-level sociodemographic characteristics and enrollment status. The MDS 3.0 is the latest version of a federal clinical assessment required for all residents of nursing homes certified by the Centers For Medicare and Medicaid Services (CMS).16 We leveraged the MDS 3.0 data to measure a key exposure (pain intensity) and two BPSD outcomes while accounting for important medication-related confounders, including the use of prescription pain medications, the use of psychotropic medications, and polypharmacy, all of which were ascertained from the Medicare Part D data. An Institutional Review Board approved the study and waived informed patient consent.
Study sample
The study sample included LTC residents aged 50 years or older who (1) entered a cohort on the date of their first observed quarterly MDS 3.0 pain assessment (i.e., index date), with at least 6 months of continuous Medicare enrollment prior to that date; (2) were diagnosed with ADRD and not comatose17 before the index date between January 1, 2011, and December 31, 2015. To study a homogeneous population regarding pain conditions, we further restricted the sample to those with a diagnosis of chronic pain but without cancer, palliative, or hospice care during the 6-month pre-index period (baseline). Supplementary Table 1 lists the diagnoses of diseases and service care considered in sample selection.
We created two independent cohorts to detect the risk of depression and behavioral symptoms outcomes. For each cohort, we included only residents who had no clinically diagnosed or MDS-assessed outcome of interest during the 6-month baseline or on the index date. Residents were followed up from the index date until the first outcome event or the last observed quarterly MDS 3.0 prior to death, nursing home discharge, Medicare Part D disenrollment, or study end, whichever came first. We excluded residents who had no quarterly MDS 3.0 assessment during follow-up for outcome ascertainment. Figure 1 shows the procedures of sample selection for each cohort.
Fig. 1. Flow chart of the retrospective cohort study samples for depression and for behavioral symptom outcomes.
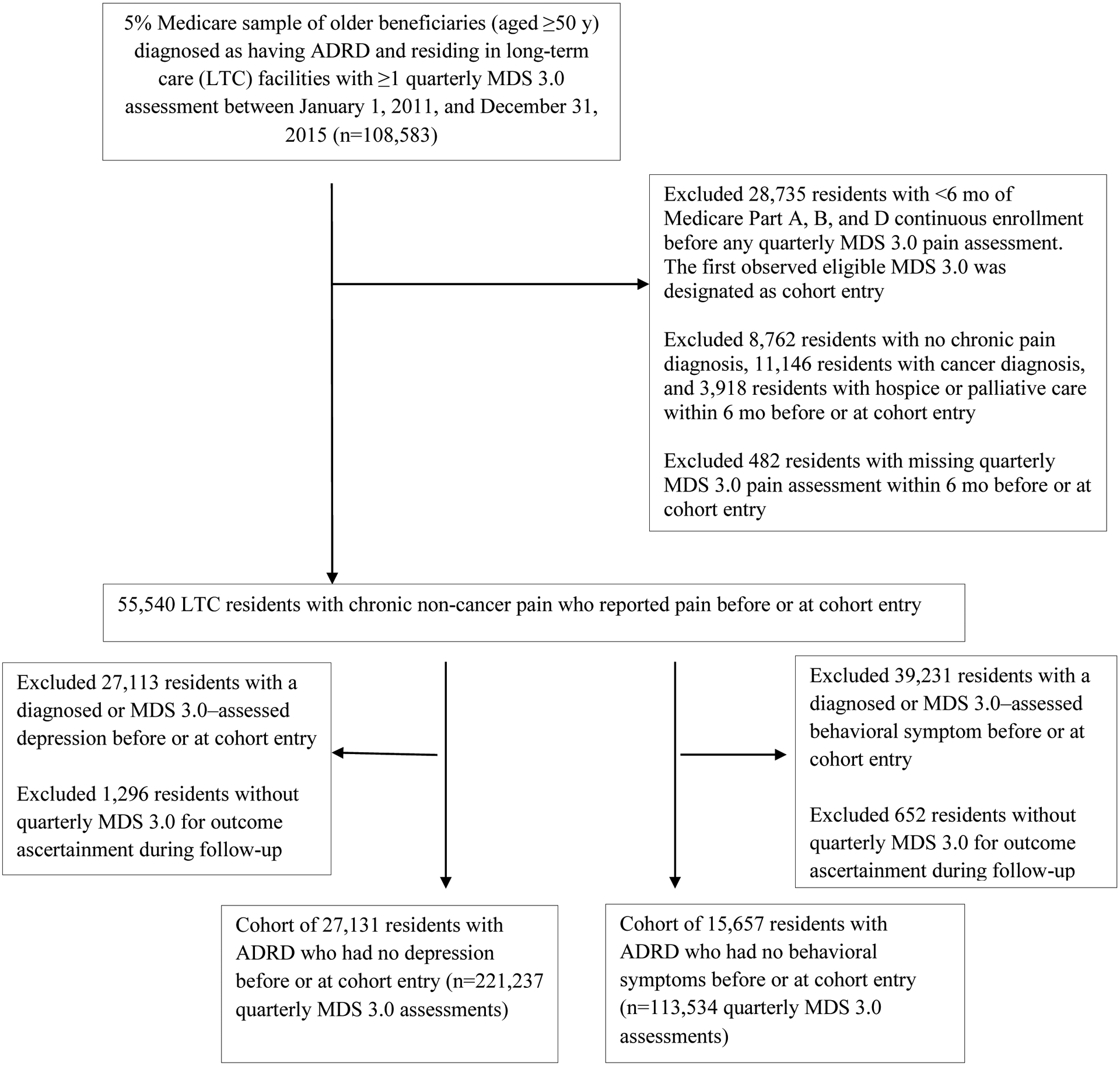
ADRD represents Alzheimer disease and related dementia; and MDS, Minimum Data Set.
Outcome measures
Outcomes included the presence of depression and the presence of behavioral symptoms (including psychotic and disruptive behaviors) extracted from the MDS 3.0 data. In the MDS 3.0, depression was measured by residents’ self-assessment of mood status in the previous 2 weeks using the Patient Health Questionnaire (PHQ)-9, a validated tool to detect major depressive disorder with high sensitivity and specificity (both >85%).18,19 The depression status of residents who were nonverbal was measured by a staff-assessed PHQ-9. Each of the PHQ-9 items scored symptoms from 0 (not at all) to 3 (nearly every day), resulting in a total score ranging from 0 to 27. Residents whose PHQ-9 score was ≥10 were classified as having moderate-to-severe depression; otherwise, they were classified as having no or mild depression.18,19
In the MDS 3.0, nursing staff assessed the presence of two common behavioral symptoms during the previous 7 days, including (1) psychotic behaviors (i.e., delusions and hallucinations) and (2) disruptive behaviors, including physical or behavioral symptoms directed toward others, verbal behavioral symptoms directed toward others, other behavioral symptoms not directed toward others, and rejection of care.20,21 Wandering was not included because this behavior is not commonly displayed among residents with pain.10 Residents were only considered to have a behavioral symptom if they exhibited any of the five behaviors.
Both the MDS 3.0-assessed depression and behavioral items have been psychometrically tested in residents who are verbal and nonverbal and have shown excellent nurse-to-nurse interrater reliability (kappa >0.90).22 We relied on MDS 3.0 assessments rather than on diagnostic codes for the ascertainment of depression and behavioral symptoms during follow-up because these symptoms are often delayed or underdiagnosed in older adults.23
Pain control
Pain intensity was extracted from quarterly MDS 3.0 assessments.24 At each assessment, residents were asked to rate their worst pain intensity in the previous 5 days using a numeric rating scale (NRS; from 0 to 10) or verbal descriptor scale (VDS; no, mild, moderate, or severe) for residents who were verbal. For nonverbal residents, nurses evaluated their pain intensity using the Checklist of Nonverbal Pain Indicators (CNPI) to assess the presence (1) or absence (0) of four pain behaviors (i.e., nonverbal sounds, vocal complaints of pain, facial expressions, and body postures) in the previous 5 days. Residents were classified as having uncontrolled pain if they had an NRS of >4, a VDS indicating moderate or severe pain, or ≥1 pain indicators in the CNPI; otherwise, they were considered to have controlled pain. Missing pain value data during baseline or cohort entry were low (<1%; n=652 residents), and these residents were excluded.
Statistical analysis
We measured pain control, outcomes of depression and behavioral symptoms, and confounders (Supplementary Method 1) at baseline and updated at each quarterly MDS 3.0 assessment during follow-up. Thus, resident assessment was the unit of analysis. Because we censored dichotomized depression and behavioral symptoms at quarterly intervals, to model interval-censored outcomes, we used a generalized linear model (GLM) with a complementary log-log link function to examine the association of prior uncontrolled pain (exposure) with the subsequent risk of the outcome of interest.25
To account for time-varying pain control and confounders, we used an MSM approach.26 Unlike conventional covariate adjustments, MSMs adjust for time-varying confounders by assigning weights to individuals, and thus create a pseudo sample in which all observed potential confounders are equally distributed between the pain controlled and pain uncontrolled groups, yielding results that approximate causal relationships.26,27 The use of an MSM involves two steps: (1) calculating a stabilized weight by multiplying the inverse probability of treatment (or exposure) weights (IPTWs) and inverse probability of censoring weights (IPCWs) of each resident assessment; and (2) incorporating the calculated stabilized weights into the GLMs to estimate the weighted associations between uncontrolled pain with outcomes of interests.28 To estimate IPTWs and IPCWs, we fit two separate pooled multivariable logistic regression models, with uncontrolled pain and censoring (due to death or Medicare Part D disenrollment) as the dependent variable, respectively, and the time-fixed and time-varying variables as the independent variables. Weights were truncated at the 1st and 99th percentiles to reduce the influence of outliers on estimates. In the second step, we reported hazard ratios (HRs) and 95% confidence intervals (CIs) derived from the MSM-weighted GLM for each outcome. Generalized estimating equations were included in the final weighted models to account for within-resident correlations from quarterly repeated measures of pain control.29
To evaluate the robustness of our findings, we conducted several subgroup and sensitivity analyses. For sensitivity analyses, we compared estimates from MSM-GLMs with those from conventional unweighted models that adjusted for baseline covariates as well as with estimates from GLMs with IPTW. We also truncated weights at the 0.5th and 99.5th percentiles and at the 2nd and 98th percentiles as a sensitivity analysis.28 To test the positivity assumption (i.e., any individual has a positive, nonzero probability of experiencing exposure at any given combination of covariates), we examined the distribution of propensity scores by baseline pain control. In subgroup analysis, we stratified the MSM-GLM analysis by dementia severity, use of prescription pain medications, and use of pain management including pharmacological and non-pharmacological approaches at baseline to explore their potential effect modification. Non-pharmacological pain management approaches documented in MDS 3.0 included but not limited to comfort therapy (e.g., heat/cold application), physical therapy (e.g., exercises), neurostimulation (e.g., electrical nerve stimulation), and alternative therapy (massage, acupuncture, and chiropractic).30 We chose these three effect modifiers because prior studies have reported that the association between pain and BPSD differed according to use of pain interventions31,32 and severity of cognitive impairment.7,14 All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was set at P < 0.05, and all tests were 2-sided.
Results
We identified a cohort of 27,131 eligible LTC residents with ADRD who had no depression outcome (contributing 221,237 resident MDS assessments) and a cohort of 15,657 LTC residents with ADRD who had no behavioral symptoms outcome (contributing 113,534 resident MDS assessments), 6 months before or at cohort entry. (Figure 1). Baseline summary statistics for the cohort of depression and behavioral sample are presented in Supplementary Table 2. The mean (SD) length of follow-up was 1.6 (1.3) years (median, 1.2 years; interquartile range [IQR], 0.5–2.4 years) for the depression cohort and 1.4 (1.2) years (median, 1.2 years; IQR, 0.5–2.1 years) for the behavioral cohort. During the follow-up period, 8.9% of residents in the depression cohort and 6.5% in the behavioral cohort died and were censored at the time of death.
Table 1 gives the characteristics of LTC residents with or without pain control before or at cohort entry in the depression and behavioral cohorts. At baseline, 15.1% (4087 of 27,131) of residents with ADRD in the depression cohort and 20.4% (3192 of 15,657) of residents in the behavioral cohort experienced uncontrolled pain. In both cohorts, residents whose pain was uncontrolled (vs controlled) were more likely to be younger (50–64 years old), female, White, and have five or more comorbidities, but were less likely to have moderate or severe dementia. The residents with uncontrolled pain were also more likely to receive prescription pain medications, use pharmacological or non-pharmacological pain interventions, and experience any hospitalization and emergency department visit at baseline.
Table 1.
Baseline* Demographic and Clinical Characteristics of Long-term Care Residents with or without Pain Control† in the Depression and Behavioral Symptoms Cohorts
| Depression cohort (n=27,131) | Behavioral symptoms cohort (n=15,657) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline characteristic* | Residents with uncontrolled pain, %* | Residents with controlled pain, %* | Adjusted OR (95% CI) | P-value | Residents with uncontrolled pain, %* | Residents with controlled pain, %* | Adjusted OR (95% CI) | P-value |
| Total, No. | 4087 | 23,044 | 3192 | 12,465 | ||||
| Age group, y | ||||||||
| 50–64 | 6.0 | 5.2 | Reference | 8.2 | 6.2 | Reference | ||
| 65–74 | 12.4 | 10.2 | 0.91 (0.75–1.11) | .34 | 14.9 | 11.5 | 0.92 (0.74–1.13) | .42 |
| 75–84 | 28.0 | 24.8 | 0.86 (0.72–1.03) | .10 | 28.4 | 24.3 | 0.84 (0.70–1.03) | .09 |
| ≥85 | 53.7 | 59.7 | 0.72 (0.60–0.86) | <.001 | 48.4 | 58.0 | 0.71 (0.59–0.86) | <.001 |
| Sex | ||||||||
| Male | 20.6 | 26.5 | Reference | 19.1 | 24.2 | Reference | ||
| Female | 79.4 | 73.5 | 1.23 (1.12–1.37) | <.001 | 80.9 | 75.8 | 1.24 (1.10–1.40) | <.001 |
| Race/ethnicity | ||||||||
| White | 82.2 | 75.4 | Reference | 84.5 | 77.8 | Reference | ||
| Black | 12.0 | 16.0 | 0.75 (0.66–0.84) | <.001 | 9.6 | 13.4 | 0.74 (0.64–0.87) | <.001 |
| Other‡ | 5.8 | 8.6 | 0.77 (0.65–0.90) | .002 | 5.9 | 8.8 | 0.76 (0.63–0.93) | .006 |
| Region of United States | ||||||||
| Northeast | 17.6 | 24.0 | Reference | 17.0 | 22.8 | Reference | ||
| Northcentral | 30.7 | 25.6 | 1.27 (1.13–1.42) | <.001 | 30.8 | 26.0 | 1.30 (1.13–1.50) | <.001 |
| West | 11.6 | 11.0 | 1.41 (1.22–1.63) | <.001 | 11.6 | 11.2 | 1.41 (1.18–1.67) | <.001 |
| South | 40.1 | 39.4 | 1.28 (1.14–1.42) | <.001 | 40.6 | 40.0 | 1.26 (1.10–1.43) | <.001 |
| Receipt of low-income subsidy | ||||||||
| No | 18.5 | 16.8 | Reference | 16.5 | 16.2 | Reference | ||
| Yes | 81.5 | 83.2 | 0.88 (0.79–0.97) | .01 | 83.5 | 83.8 | 0.94 (0.83–1.07) | 0.37 |
| Body mass index | ||||||||
| Normal | 34.7 | 41.0 | Reference | 31.3 | 37.1 | Reference | ||
| Underweight | 8.4 | 8.6 | 1.02 (0.88–1.18) | .77 | 6.0 | 7.6 | 0.86 (0.71–1.04) | 0.13 |
| Overweight | 27.7 | 28.8 | 1.05 (0.96–1.16) | .26 | 26.4 | 28.8 | 1.06 (0.94–1.19) | 0.34 |
| Obese | 29.2 | 21.72 | 1.09 (0.99–1.21) | .09 | 36.4 | 26.5 | 1.13 (1.00–1.27) | 0.04 |
| Pain reporting | ||||||||
| Staff-observed | 8.5 | 16.1 | Reference | 3.5 | 11.8 | Reference | ||
| Self-reported | 91.5 | 83.9 | 1.01 (0.87–1.18) | .89 | 96.5 | 88.2 | 1.44 (1.12–1.85) | 0.005 |
| ADL dependence | ||||||||
| No | 28.4 | 25.6 | Reference | 33.2 | 28.8 | Reference | ||
| Mild | 32.6 | 32.5 | 0.92 (0.83–1.01) | .09 | 33.5 | 32.9 | 0.87 (0.78–0.98) | .02 |
| Moderate | 26.6 | 26.7 | 0.98 (0.88–1.10) | .75 | 24.4 | 25.8 | 0.86 (0.76–0.98) | .02 |
| Severe | 12.4 | 15.2 | 1.00 (0.87–1.15) | .99 | 9.0 | 12.5 | 0.90 (0.75–1.08) | .27 |
| Comorbidity | ||||||||
| 0–2 | 21.6 | 29.6 | Reference | 17.2 | 24.9 | Reference | ||
| 3–4 | 34.4 | 36.5 | 1.10 (0.99–1.21) | .09 | 33.0 | 36.9 | 1.01 (0.89–1.15) | .88 |
| 5–6 | 25.2 | 21.7 | 1.18 (1.05–1.31) | .006 | 28.8 | 23.9 | 1.13 (0.99–1.31) | .07 |
| ≥7 | 18.9 | 12.2 | 1.30 (1.15–1.48) | <.001 | 21.0 | 12.3 | 1.14 (0.98–1.34) | .09 |
| Dementia severity | ||||||||
| Mild | 67.7 | 41.0 | Reference | 82.7 | 57.1 | Reference | ||
| Moderate | 26.3 | 44.8 | 0.50 (0.45–0.54) | <.001 | 14.9 | 32.8 | 0.55 (0.49–0.62) | <.001 |
| Severe | 6.0 | 14.3 | 0.43 (0.36–0.52) | <.001 | 2.4 | 10.1 | 0.47 (0.35–0.64) | <.001 |
| Pain management (yes vs. no as reference) | ||||||||
| Receipt of prescription pain medication | 78.8 | 46.4 | 1.94 (1.76–2.13) | <.001 | 83.8 | 52.5 | 1.87 (1.65–2.11) | <.001 |
| Use of PRN pain medication | 76.4 | 23.4 | 4.98 (4.52–5.49) | <.001 | 78.0 | 26.6 | 4.70 (4.19–5.26) | <.001 |
| Use of scheduled pain medication | 61.4 | 35.6 | 1.47 (1.34–1.62) | <.001 | 67.6 | 39.8 | 1.69 (1.51–1.89) | <.001 |
| Use of pain management | 94.9 | 51.5 | 3.55 (2.97–4.24) | <.001 | 96.4 | 56.3 | 3.73 (2.96–4.70) | <.001 |
| Use of psychotropic medication | 62.0 | 58.2 | 0.93 (0.86–1.02) | .11 | 71.2 | 61.3 | 0.96 (0.86–1.07) | .42 |
| Polypharmacy | 91.7 | 84.3 | 0.97 (0.84–1.12) | .70 | 93.6 | 86.2 | 0.97 (0.80–1.17) | 0.75 |
| Healthcare utilization | ||||||||
| Any hospitalization | 43.3 | 34.4 | 1.19 (1.06–1.33) | .003 | 39.3 | 24.5 | 1.43 (1.24–1.65) | <.001 |
| Any ED visit | 55.7 | 44.8 | 1.34 (1.20–1.50) | <.001 | 49.6 | 33.0 | 1.36 (1.19–1.65) | <.001 |
| Depression (PHQ-9 ≥10) | N/A | N/A | N/A | N/A | 9.4 | 5.5 | 1.56 (1.31–1.84) | <.001 |
| Behavioral symptoms | 23.8 | 27.4 | 0.98 (0.90–1.08) | .74 | N/A | N/A | N/A | N/A |
Abbreviations: ADL, activities of daily living; ED, emergency department; N/A, not available; OR, odds ratio; PHQ-9, Patient Health Questionnaire (PHQ)-9; PRN, pro re nata.
Baseline was defined as 6 months before the index Minimum Data Set (MDS) 3.0 assessment;
Uncontrolled pain was defined based on pain assessment of the index MDS 3.0.
Included Hispanic, Asian, Pacific Islander, and Native American individuals.
Table 2 gives the unadjusted incidence estimate of depression and behavioral symptoms among eligible LTC residents with ADRD. The overall incidence rate of depression symptoms and of behavioral symptoms during follow-up was 9.4 and 23.1 per 100 resident-years, respectively. The rates were higher among residents with rather than without uncontrolled pain (12.2 vs. 8.9 per 100 resident-years for risk of depression; 25.9 vs. 22.4 per 100 resident-years for risk of behavioral symptoms).
Table 2.
Unadjusted Absolute Rate of Depression and Behavioral Symptoms among Long-term Care Residents with ADRD, Overall and by Status of Pain Control at Baseline
| Variable | Depression cohort | Behavioral symptoms cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of residents | No. of events | Resident-years | Incidence rate per 100 resident years | Follow-up, mean ±SD; median (IQR), years | No. of residents | No. of events | Resident-years | Incidence rate per 100 resident years | Follow-up, mean ±SD; median (IQR), years | |
| Overall | 27,131 | 4187 | 44,454 | 9.4 | 1.64±1.28; 1.25 (0.54–2.45) | 15,657 | 5220 | 22,565 | 23.1 | 1.24±1.20; 1.10 (0.49–2.06) |
| Pain status | ||||||||||
| Uncontrolled | 4087 | 793 | 6502 | 12.2 | 1.59±1.28; 1.23 (0.50–2.42) | 3192 | 1204 | 4643 | 25.9 | 1.45±1.22; 1.10 (0.48–2.14) |
| Controlled | 23,044 | 3394 | 37952 | 8.9 | 1.64±1.28; 1.25 (0.57–2.46) | 12,465 | 4016 | 17,922 | 22.4 | 1.44±1.20; 1.11 (0.49–2.04) |
Abbreviations: ADRD, Alzheimer disease and related dementia; IQR, interquartile range; SD, standard deviation.
Table 3 gives the associations between uncontrolled pain and risk for depression and behavioral symptoms. The crude estimate without confounding adjustment (conventional model) indicated that uncontrolled pain was associated with 35% increased risk for depression (95% CI, 1.25–1.46) and 22% increased risk for behavioral symptoms (95% CI, 1.14–1.30). Compared with the crude estimates, both conventional baseline adjustment and IPTW models yielded lower effect estimates for depression (HR, 1.30; 95% CI, 1.18–1.42 and HR, 1.25; 95% CI, 1.17–1.43) and for behavioral symptoms (HR, 1.17; 95% CI, 1.09–1.27 and HR, 1.20; 95% CI, 1.14–1.27). The weighted MSM that accounted for time-varying confounders yielded the largest estimate, with a 67% increased risk for depression (95% CI, 1.54–1.81) and 28% for behavioral symptoms (95% CI, 1.19–1.37).
Table 3.
Adjusted Associations between Uncontrolled Pain and Risk of Depression and Behavioral Symptoms among Long-term Care Residents with ADRD
| Analysis | Risk for depression | Risk for behavioral symptoms | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Main analysis | P-value | |||
| Conventional models | ||||
| Without adjustment | 1.35 (1.25–1.46) | <.001 | 1.22 (1.14–1.30) | <.001 |
| Adjusted for baseline variables | 1.30 (1.18–1.42) | <.001 | 1.17 (1.09–1.27) | <.001 |
| Weighted models | ||||
| IPTW | 1.25 (1.17–1.43) | <.001 | 1.20 (1.14–1.27) | <.001 |
| MSM estimates | 1.67 (1.54–1.81) | <.001 | 1.28 (1.19–1.37) | <.001 |
| Subgroup analysis | P-value for interaction | P-value for interaction | ||
| Dementia severity | ||||
| Mild | 1.71 (1.53–1.90) | 0.40 | 1.31 (1.21–143) | 0.21 |
| Moderate | 1.91 (1.67–2.21) | 1.36 (1.16–1.60) | ||
| Severe | 1.42 (1.03–1.95) | 1.53 (0.97–2.40) | ||
| Use of prescription pain medication | ||||
| Yes | 1.56 (1.41–1.72) | 0.03 | 1.26 (1.16–1.37) | 0.30 |
| No | 1.90 (1.64–2.12) | 1.38 (1.18–1.62) | ||
| Use of pain management | ||||
| Yes | 1.54 (1.40–1.69) | 0.02 | 1.20 (1.11–1.30) | <.001 |
| No | 1.98 (1.65–2.37) | 1.76 (1.48–2.10) | ||
Abbreviations: ADRD, Alzheimer disease and related dementia; CI, confidence interval; IPTW, inverse probability of treatment (or exposure) weight; MSM, marginal structural modeling.
Subgroup and sensitivity analyses
Uncontrolled pain had statistically significant and large effects on depression or behavioral symptom outcomes for LTC residents with ADRD who had no baseline use of any prescription pain medications (vs. use; P-value for interaction=0.03 for depression only) and who had no baseline use of any pain management (vs. use; P=0.02 for depression and P<.001 for behavioral symptoms) (Table 3). We did not observe a statistically significant modification effect of dementia severity on the association between pain control and risk for depression (P=0.40) and behavioral symptoms (P=0.21). Sensitivity analyses that truncated weights at different percentiles did not alter our findings (Supplementary Table 3). We did not find evidence of violation of the positivity assumption (Supplementary Figure 1).
Discussion
The present study using MDS 3.0 assessments linked to Medicare claims data is among the first to provide population-based data on pain control and risk for depression and behavioral symptoms among LTC residents with ADRD. Using an MSM approach to account for time-varying confounders, we found that uncontrolled pain increased the risk of developing depression by 1.67-fold and of developing behavioral symptoms by 1.28-fold. The direction of association was generally consistent across different models, with smaller magnitudes found in conventional adjusted and IPTW models compared with weighted MSMs. Findings were also consistent across sensitivity and subgroup analyses.
Associations between pain and depression and behavioral symptoms are well documented among older adults with intact cognition,33 but less well documented among those with cognitive impairment. Limited longitudinal cohort studies have assessed the association between uncontrolled pain and risk for BPSD.11–14 Prior findings have been consistent regarding pain and risk of depression,11–13 but inconsistent regarding pain and risk of aggression and agitation, with two studies showing a positive association11,12 whereas another study indicating no association.13 Our study utilizing the most recent version of MDS data in a large sample of LTC residents found positive associations between poor pain control and depression or behavioral symptoms for residents with ADRD after accounting for time-varying pain control and time-varying variables that could act as confounders and intermediate variables simultaneously.
The present study also explored the effect modification of the association between uncontrolled pain and depression or behavioral symptoms by dementia severity and by use of pain treatment and management at baseline. We observed statistically significant and stronger associations for subgroups of LTC residents with ADRD who had no prescription pain treatment or no pain management at baseline, compared with their counterparts who had intervention(s). Our finding is analogous to results from published randomized clinical trials showing that pain treatment (vs. no treatment) is associated with decreased pain and subsequent risk for agitation in patients with moderate-to-severe dementia.31,32 We did not find evidence of an effect modification by dementia severity. Our null finding is consistent with the result of a prior study of residents with dementia14 but inconsistent with that of a study of community-dwelling persons with dementia whose pain was primarily assessed by their caregivers.7
Our findings re-emphasized the importance of pain assessment in LTC residents with ADRD for early detection and intervention of BPSD given the lack of effective treatment and potential harms of psychotropic medications for BPSD. For individuals with ADRD who reside in LTC facilities, the MDS 3.0 could serve as a useful data source because it regularly assesses and documents the pain status of residents, most of whom are diagnosed as having ADRD. Our incidence estimate of depression ascertained from the MDS 3.0 is consistent with prior data.34 Overall, our findings may assist healthcare professionals in distinguishing LTC residents with ADRD who have a higher predisposition to depression or behavioral symptoms. It is particularly important to focus on residents with ADRD who are younger, female, white, and have multiple comorbidities, all of which are important risk factors associated with uncontrolled pain demonstrated in the present study.
A strength of our study is that we adjusted for time-varying pain control and time-varying confounders using an MSM approach. Causality may be inferred when the MSM assumptions of positivity, consistency, exchangeability, and correctness of model specifications are fulfilled.28,35 In our study, the positivity assumption was satisfied, as the probability of any resident experiencing the exposure was positive within each stratum of covariate combination. The consistency assumption was also satisfied, as our results remain unchanged after truncation of weights at various percentiles. However, it is challenging to test the other MSM assumptions; thus, the interpretation of our study findings in light of causality remains limited.
There are several additional limitations to this study. First, the validity of pain intensity, PHQ-9 depression, and behavioral symptom assessment in the MDS 3.0 is uncertain, particularly for residents with ADRD. Our previous pilot study found a moderate-to-high agreement for these three MDS 3.0 measures against medical records of a local Medicare- and Medicaid-certified LTC facilities.36,37 Studies using a nationally representative sample of LTC residents are warranted to better understand the validity of MDS 3.0-based measures. Second, while studies of cognitively intact populations show sex and racial differences in pain perception and report,38 limited evidence exists, with only one pilot examining sex differences in pain response among patients with ADRD.39 More studies that understand biopsychosocial mechanisms underlying sex and racial differences among ADRD may help explain our finding on being female and white as risk factors of uncontrolled pain. Third, although we accounted for many potential confounders measured from the MDS 3.0 data and Medicare claims, unmeasured confounders are possible and could influence our estimates. Finally, our results could only be generalized to Medicare older adults with ADRD who resided in LTC facilities.
Conclusions and Implications
In this study of Medicare LTC residents with ADRD, uncontrolled pain is associated with increased risk for two common BPSD— depressive and behavioral symptoms. Our findings re-emphasized the importance of pain assessment in LTC residents with ADRD, particularly those with identified risk factors associated with uncontrolled pain. Given that there is no cure for ADRD and the potential harms of psychotropic medication administered for treatment of BPSD, it is important to regularly assess, prevent, and manage pain in LTC residents with ADRD to prevent BPSD.
Supplementary Material
Acknowledgements:
Sponsor’s role:
The National Institute on Aging had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Funding sources:
This project was funded by the National Institute on Aging (Mentored Research Scientist Award K01AG054764, Dr. Wei).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of interest: The authors have no conflicts of interest.
References
- 1.Malara A, De Biase GA, Bettarini F, et al. Pain Assessment in Elderly with Behavioral and Psychological Symptoms of Dementia. J Alzheimers Dis. 2016;50(4):1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaugler JE, Yu F, Krichbaum K, et al. Predictors of nursing home admission for persons with dementia. Med Care. 2009;47(2):191–198. [DOI] [PubMed] [Google Scholar]
- 3.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. [DOI] [PubMed] [Google Scholar]
- 4.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kales HC, Gitlin LN, Lyketsos CG, et al. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 7.Hodgson N, Gitlin LN, Winter L, et al. Caregiver’s perceptions of the relationship of pain to behavioral and psychiatric symptoms in older community-residing adults with dementia. Clin J Pain. 2014;30(5):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tosato M, Lukas A, van der Roest HG, et al. Association of pain with behavioral and psychiatric symptoms among nursing home residents with cognitive impairment: results from the SHELTER study. Pain. 2012;153(2):305–310. [DOI] [PubMed] [Google Scholar]
- 9.Ishii S, Streim JE, Saliba D. A conceptual framework for rejection of care behaviors: review of literature and analysis of role of dementia severity. J Am Med Dir Assoc. 2012;13(1):11–23e11–12. [DOI] [PubMed] [Google Scholar]
- 10.Ahn H, Horgas A. The relationship between pain and disruptive behaviors in nursing home residents with dementia. BMC Geriatr. 2013;13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunik ME, Snow AL, Davila JA, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry. 2010;71(9):1145–1152. [DOI] [PubMed] [Google Scholar]
- 12.Sampson EL, White N, Lord K, et al. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: a longitudinal cohort study. Pain. 2015;156(4):675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volicer L, Frijters DH, Van der Steen JT. Relationship between symptoms of depression and agitation in nursing home residents with dementia. Int J Geriatr Psychiatry. 2012;27(7):749–754. [DOI] [PubMed] [Google Scholar]
- 14.Erdal A, Flo E, Selbaek G, et al. Associations between pain and depression in nursing home patients at different stages of dementia. J Affect Disord. 2017;218:8–14. [DOI] [PubMed] [Google Scholar]
- 15.Mansournia MA, Etminan M, Danaei G, et al. Handling time varying confounding in observational research. BMJ. 2017;359:j4587. [DOI] [PubMed] [Google Scholar]
- 16.Rahman AN, Applebaum RA. The nursing home Minimum Data Set assessment instrument: manifest functions and unintended consequences--past, present, and future. Gerontologist. 2009;49(6):727–735. [DOI] [PubMed] [Google Scholar]
- 17.The Centers for Medicare & Medicaid Services (CMS). Chronic Conditions Data Warehouse. 2020; https://www2.ccwdata.org/web/guest/condition-categories.AccessedJanuary 3, 2021.
- 18.Levis B, Benedetti A, Thombs BD, et al. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365:l1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber-Baldini AL, Boustani M, Sloane PD, et al. Behavioral symptoms in residential care/assisted living facilities: prevalence, risk factors, and medication management. J Am Geriatr Soc. 2004;52(10):1610–1617. [DOI] [PubMed] [Google Scholar]
- 21.Galik E, Resnick B, Vigne E, et al. Reliability and Validity of the Resistiveness to Care Scale Among Cognitively Impaired Older Adults. J Am Med Dir Assoc. 2017;18(1):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saliba D, Buchanan J. Rand Corporation Health: Development & validation of a revised nursing home assessment tool: MDS 3.0 health. Accessed on December 11, 2015 from https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/downloads/mds30finalreport.pdf.2008; AccessedJanuary 1, 2021.
- 23.Allan CE, Valkanova V, Ebmeier KP. Depression in older people is underdiagnosed. Practitioner. 2014;258(1771):19-22, 12-13. [PubMed] [Google Scholar]
- 24.Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13(7):602–610. [DOI] [PubMed] [Google Scholar]
- 25.Farrington CP. Interval censored survival data: a generalized linear modelling approach. Stat Med. 1996;15(3):283–292. [DOI] [PubMed] [Google Scholar]
- 26.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 27.Williamson T, Ravani P. Marginal structural models in clinical research: when and how to use them? Nephrol Dial Transplant. 2017;32(suppl_2):ii84–ii90. [DOI] [PubMed] [Google Scholar]
- 28.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 30.The Centers for Medicare & Medicaid Services (CMS). Long-Term Care Facility Resident Assessment Instrument 3.0 User’s Manual, Version 1.17.1. 2019; https://downloads.cms.gov/files/mds-3.0-rai-manual-v1.17.1_october_2019.pdf.AccessedJanuary 1, 2021. [Google Scholar]
- 31.Husebo BS, Ballard C, Sandvik R, et al. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Husebo BS, Ballard C, Fritze F, et al. Efficacy of pain treatment on mood syndrome in patients with dementia: a randomized clinical trial. Int J Geriatr Psychiatry. 2014;29(8):828–836. [DOI] [PubMed] [Google Scholar]
- 33.IsHak WW, Wen RY, Naghdechi L, et al. Pain and Depression: A Systematic Review. Harv Rev Psychiatry. 2018;26(6):352–363. [DOI] [PubMed] [Google Scholar]
- 34.Payne JL, Sheppard JM, Steinberg M, et al. Incidence, prevalence, and outcomes of depression in residents of a long-term care facility with dementia. Int J Geriatr Psychiatry. 2002;17(3):247–253. [DOI] [PubMed] [Google Scholar]
- 35.Platt RW, Delaney JA, Suissa S. The positivity assumption and marginal structural models: the example of warfarin use and risk of bleeding. Eur J Epidemiol. 2012;27(2):77–83. [DOI] [PubMed] [Google Scholar]
- 36.Wei YJ, Solberg L, Chen C, et al. Pain Assessments in MDS 3.0: Agreement with Vital Sign Pain Records of Nursing Home Residents. J Am Geriatr Soc. 2019;67(11):2421–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei YJ, Solberg L, Chen C, et al. Agreement of Minimum Data Set 3.0 depression and behavioral symptoms with clinical diagnosis in a nursing home. Aging Ment Health. 2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cowan RL, Beach PA, Atalla SW, et al. Sex Differences in the Psychophysical Response to Contact Heat in Moderate Cognitive Impairment Alzheimer’s Disease: A Cross-Sectional Brief Report. J Alzheimers Dis. 2017;60(4):1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


