Abstract
Diabetes is a multi-faceted disorder with increasing prevalence and rising healthcare costs. The burden of diabetes is increased because of associated complications affecting nearly all organs including the eye. The underlying pathophysiology for the onset of these ocular surface disorders is not well known. Enkephalins are endogenous opioids that originate in the brain and have numerous actions in the human body. Opioid growth factor (OGF), chemically termed [Met5]-enkephalin, binds to a novel, nuclear-associated receptor and mediates cellular homeostasis. Serum OGF levels are elevated in diabetic individuals and rodent models of diabetes. Sustained blockade of the OGF receptor (OGFr) with opioid receptor antagonists, such as naltrexone (NTX), reverses many complications of diabetes in the animal model, including delayed cutaneous wound healing, dry eye, altered corneal surface sensitivity, and keratopathy. The increased enkephalin levels observed in diabetes suggest a relationship between endogenous opioid peptides and the pathophysiology of diabetes. It is common for diabetic patients to undergo insulin therapy to restore normal blood glucose levels. However, this restoration does not alter OGF serum levels nor ameliorate ocular surface complications in the animal model of diabetes. Moreover, sex differences in the prevalence of diabetes, response to insulin therapy, and abnormalities in the OGF-OGFr axis have been reported. This review highlights current knowledge on the dysregulation of the OGF-OGFr pathway and possible relationships of insulin and enkephalins to the development of ocular surface defects in diabetes. It proposes that this dysregulation is a fundamental mechanism for the pathobiology of diabetic complications.
Keywords: Naltrexone, insulin, [Met5]-enkephalin, opioid growth factor, dry eye, diabetic keratopathy
Graphical Abstract
The insulin pathway and the OGF-OGFr axis word in parallel with regard to formation of ocular surface complications in diabetes.
1. Introduction
Diabetes mellitus is one of the leading public health concerns worldwide, with healthcare costs estimated at $760 billion [1–4]. It is a chronic disorder with multifactorial origins including genetic, environmental, and lifestyle factors [2,4]. Insulin deficiency and hyperglycemia are the primary indicators of diabetes and the leading targets for therapy [5,6]. Prolonged periods of hyperglycemia associated with pre-diabetes and diabetes result in various complications that impact nearly every organ system in individuals of both sexes and all ethnic backgrounds and include cardiovascular disease, stroke, diabetic nephropathy, neuropathy, retinopathy, and other visual system disorders [1,2,7–10].
Ocular surface complications, such as dry eye, corneal surface insensitivity, and delayed epithelialization following corneal abrasions occur in over 70% of individuals who are diagnosed with diabetes [3,4,9–12], and if untreated can lead to blindness. Diabetic individuals are approximately five times more at risk for cataract formation resulting from excess glucose conversion to sorbitol [11,12]. The incidence of diabetic retinopathy and associated microvascular damage increases during the course of disease progression. Other visual complications, including keratopathy and dry eye, also impact individuals with diabetes. Although these ocular surface disorders routinely do not lead to blindness, they can be chronic and result in substantial healthcare costs and decreased quality of life [12].
Treatments for visual system disorders vary, but are often related to the treatment of diabetes itself making insulin a prime therapeutic target. Insulin effectively regulates blood glucose levels that are either deficient as in type 1 diabetes (T1D) or resistant to circulating glucose levels as in type 2 diabetes (T2D). Despite clinical treatment with exogenous insulin from animals, insulin analogs, or human recombinant insulin, maintenance of serum glucose levels does not abrogate all ocular surface complications. Recent investigations have shown that systemic treatment of T1D rats with insulin (T1D-INS) did not prevent alterations in tear formation or corneal surface sensitivity [13–16], but may have played a role in re-epithelialization processes following corneal abrasion. Investigations utilizing controlled and uncontrolled diabetic rats revealed that the temporal onset and magnitude of ocular surface complications coincided with an elevation in serum and tissue levels of OGF [13, 15]. Although both sexes developed the same ocular surface defects, the onset and severity of the defect differed between male and female T1D rats [16]. Controlled T1D rats receiving systemic insulin also had elevated serum OGF levels, but expressed differences in some ocular surface complications from uncontrolled T1D animals. These data suggest that there are potential relationships between ocular surface complications in diabetes, enkephalins, and insulin therapy. This review examines preclinical research related to diabetic ocular surface complications and discusses differences between insulin-controlled and uncontrolled diabetes, sex hormone differences, and the dysregulation of the OGF-OGFr pathway in T1D.
1.1. Animal models of diabetes
The pathophysiology of diabetes is multifactorial and not fully understood [17,18]. Moreover, there is no perfect animal model [19]. T1D is considered an autoimmune disease with unknown etiology making it difficult to mimic in an animal model. Age of onset, the severity of disease, and the residual beta-cell mass are variables in determining the pathophysiology, as well as the type and severity of complications. The most prevalent animal model for T1D is the streptozotocin (STZ)-injected rat [19–23]. It may take ten years for chronic hyperglycemia to present in humans, whereas in the rat model, hyperglycemia presents within a few days of STZ injection. STZ has preferential toxicity towards insulin-producing beta islet cells in the pancreas and closely mimics the human T1D symptomatology by depleting pancreatic beta cells and downregulating insulin production. Elevated blood glucose levels and increased urine output are observed in the STZ-injected rat model, demonstrating symptomatology comparable to diabetic humans [20–23]. STZ-induced hyperglycemia can be reversed with insulin treatment in rats, providing another comparable aspect to human diabetes. Conversely, STZ is rapidly eliminated within 24–48 hours after intraperitoneal injection reducing acute toxicity. As a result, some animals exposed to STZ do not become hyperglycemic [23] making interpretation of results difficult. Humans and STZ-injected animals are similar in that diabetic complications are not entirely prevented or reversed by insulin [6,24]. Despite the similarities and differences, the STZ animal model provides a controlled paradigm to study type 1 diabetic complications including those related to the ocular surface [24]. Animal models of T2D are more heterogeneous and include diet induced obesity, hyperglycemia following high-fat diets, genetic models, or non-obese genetic models [25–27], making the study of T2D and related ocular surface complications less rigorous and reproducible.
1.2. Ocular surface complications in diabetes
Diabetic keratopathy, dry eye, and abnormal corneal surface sensitivity are the three most prevalent ocular surface complications associated with diabetes [28–34], as well as other non-visual system-related diseases [35]. Circumstances of sustained hyperglycemia result in loss of corneal nerve fibers leading to diminished innervation and sensation [28,29]. The decreased corneal surface sensitivity contributes to dry eye, keratopathy, and even somatic neuropathy.
Dry eye is a progressive condition often with an unknown cause. Risk factors for dry eye include age, gender, physical health, impaired corneal nerve sensitivity, and environment [28,33]. The environment can cause increased tear evaporation which leads to dry eye if not offset by tear production. Diabetes exacerbates dry eye which may cause additional reduced corneal surface sensitivity. The lacrimal glands supply most of the tears, but produce less when no longer stimulated by the corneal surface nerves [33]. Dry eye can be remedied with additional tears using artificial drops or conservation of tears through surgical blockage of tear drainage ducts [36].
Diabetic keratopathy relates to abnormal mechanisms of wound healing leading to persistent corneal epithelial defects associated with hyperglycemia [11]. Epithelial wound healing can be delayed or incomplete in diabetes leading to persistent and recurrent ulcers and potentially severe visual defects [11]. The mechanisms underlying keratopathy are not fully understood, but many pathways are implicated including insulin-like growth factor, nerve growth factor, platelet-derived growth factor, and opioid growth factor (OGF) [28,29]. These growth factors are important for the regulation of replication, migration, and differentiation of epithelial cells. In diabetes, the accumulation of advanced glycation end products (AGEs) deters cellular migration leading to delayed wound healing [28,37,38].
1.3. Insulin and signaling pathways in diabetes
Insulin is an endogenous hormone produced by pancreatic beta cells in response to elevated blood glucose [39,40]. Insulin plays a role in the metabolism of lipids, proteins and carbohydrates by absorbing circulating glucose and increasing storage in skeletal muscle cells, fat and the liver [41–47]. Insulin-like growth factors (IGFs) are specific proteins with a similar sequence to that of insulin. IGF-2 is commonly associated with growth during early development, and IGF-1is a growth regulating hormone in adults [43]. Both IGF pathways are considered potential targets for the development of treatments for diabetic complications.
Briefly, the signaling pathway of insulin is dependent on the presence of the insulin receptor substrate (IRS) resulting in either IRS-non-mediated or IRS-mediated signal transduction between the insulin receptor (IR) on the cell membrane and insulin [44,45]. In the IRS-mediated insulin signaling pathway the Rasmitogen-activated protein kinase (MAPK) pathway, also known as the growth signaling pathway, or the phosphatidylinositol-3-kinase(PI3K)-protein kinase B (Akt or PKB) pathway, which carries out the metabolic functions of insulin is activated [46]. This latter pathway utilized by insulin may invoke growth regulatory properties that coincide or compete with those related to the OGF-OGFr axis. The non-IRS-mediated insulin signaling pathway utilizes insulin receptor substrates to act on the GLUT4 transporter to transverse through the cell membrane and enabling glucose uptake [47,48], and is less likely to be involved in elevated OGF levels and resulting diabetic-related complications.
Insulin stimulates protein synthesis and amino acid metabolism. In diabetes, oxidative stress and hyperglycemia result in the production of AGEs resulting from excess glucose and protein [38,49,50]. Accumulation of AGEs modifies protein structure and sets off the cascade of events activating pro-inflammatory cytokines that lead to cellular damage associated with complications of diabetes in the retina, cornea, lens, and lacrimal gland [38]. The identification of AGEs on ocular tissues has suggested their association with diabetes. The development of AGEs and the resulting oxidative damage and tissue dysfunction contribute to the lacrimal gland and ocular surface alterations [38]. Clinically, patients with definitive T1D require life-long insulin therapy to limit the advancement of macrovascular and microvascular complications and to survive.
1.5. Endogenous opioids and the OGF-OGFr regulatory pathway
Endogenous opioids and their receptors are located throughout the central and peripheral nervous systems and play an essential role in stress response, drug addiction, motivation and pain regulation [51–53]. The endogenous opioid system is comprised of two endogenous opioids, termed [Met5]-enkephalin and [Leu5]-enkephalin, that are derived from the precursor genes pre-proenkephalin A (PPE) or pro-opiomelanocortin (POMC), and three canonical G protein-coupled receptors termed mu, delta, and kappa. The pentapeptide [Met5]-enkephalin is widely distributed and detected in the visual, cardiovascular, and gastrointestinal systems, as well as within the placenta and the adrenal medulla [53–56]. Although the receptors and peptides have some regional selectively, the peptides have been shown to bind to all three receptors [53] and comparable levels of affinity enabling a single receptor antagonist to block binding at all receptors.
OGF, chemically termed [Met5]-enkephalin, inhibits cell replication [54]. The distinct identification emerged from the need to distinguish the peptide role in growth regulation from that of neurotransmission, and its pharmacological opioid properties. OGF is a potent, reversible, tissue-nonspecific negative growth regulator that is conserved across species [57]. The specific opioid receptor for OGF is a nuclear-associated receptor with little or no proteomic or genomic similarity to classical opioid receptors. However, it can be pharmacologically blocked by naloxone and NTX antagonists of classical opioid receptors [58].
The OGF-OGFr signaling pathway has not been fully defined and evidence of interactions with other pathways is just beginning to be investigated. OGF utilizes the p16INK4a and p21WAF1/CIP1 cyclin-dependent inhibitory kinase pathways to delay the G1-S phase of the cell cycle [59]. Investigations utilizing cultures of human umbilical vein endothelial cells and human epidermal keratinocytes revealed marked cell proliferation following targeted expression of p16 and p21, with little or no response on p15, p18, p19, or p22 protein expression. The inhibitory action of OGF requires internalization of the peptide [60]. The mechanism of action is dependent on clathrin-mediated endocytosis [60]. Rho-tagged OGF added to cell culture was visualized within 30 minutes inside the nucleus and remained visible for 5 hours. It can inhibit DNA synthesis and be blocked by NTX. Golgi or endosomal mediation did not play a role in OGF internalization. For inhibitory action, OGF must bind to OGFr and be transported into the cell’s nucleus by way of nuclear localization signals (NLS). OGFr has three NLS; NLS383–386 and NLS456–460 are required for entry into the nucleus. [61]. Using eGFP-OGFr fusion protein, nucleocytoplasmic transport of the receptor required karyopherin β1 and Ran, suggesting controlled entry into the nucleus [61]. Export of OGFr is CRM1 dependent with C-terminal tandem repeats that seem to be required for inhibitory activity by OGF [62].
1.6. The OGF-OGFr Pathway and diabetes
The relationship between OGF and diabetes was initially noted in the mid 1990’s by Falluca et al. [63,64]. Elevated levels of OGF were recorded in the plasma of T1D patients, as well as patients who were pregnant. These investigators were the first to observe a relationship between insulin and enkephalin, and postulated that insulin remaining in the pancreas may contribute to sufficient insulin levels that appear to provide a protective effect during pregnancy [64]. Protein and gene expression of OGF and OGFr have been identified on the ocular surface tissues of normal and diabetic animals [57,65,66]. Elevated OGF levels were reported in genetically obese and diabetic mice simulating the T2D model [67–69]. Thus, dysregulation of the OGF-OGFr axis may be a factor in the onset of complications associated with diabetes. Further support for this hypothesis is based on studies showing that blockade of the OGF-OGFr axis with NTX reverses many of the diabetic-associated ocular surface complications such as dry eye, altered surface sensitivity, and delayed corneal repair [14–16, 70].
2. Ocular surface complications and insulin treatment in the diabetic animal model
Only a few animal and human studies have investigated the direct application of insulin as a therapy for corneal surface defects [71–74]. Mudolo and others reported the influence of insulin on the lacrimal gland and ocular surface of diabetic rats, suggesting that after five weeks of diabetes, animals treated with insulin were comparable to normal controls based on a histological assessment of the lacrimal gland [71]. Their study further suggested that insulin replacement therapy can directly influence hormonal action on the ocular surface and related glands. Cruz-Cazarim and colleagues developed a microparticulate release system for topical administration of insulin [72]. Their innovation enabled maintaining physical and chemical properties that would be sufficient for ocular administration, and were favorable in pre-clinical studies to treat corneal lesions and dry eye [72]. A clinical study using 160 participants in a double blind trial evaluated the administration of topical insulin over four weeks to treat dry eyes in individuals with diabetes. The authors reported improvement of the ocular surface disease index (OSDI) [73]. Using a model of T1D, Zagon and colleagues reported that systemic insulin treatment ameliorated impaired corneal re-epithelialization relative to that monitored in vehicle-treated diabetic rats [74].
3. Ocular surface complications and naltrexone treatment in the diabetic animal model
The initial observation suggesting that the OGF-OGFr axis played a role in the homeostasis of the cornea came from tissue culture experiments where corneal explants from rats [75] and human donor corneas [76] had delayed growth when treated with OGF. In contrast, explant outgrowth was rapid and more organized in cultures treated with NTX. The manipulation of the OGF-OGFr pathway has been studied extensively in vivo using normal mice and rats [61,77,78]. OGF inhibits normal homeostasis of the corneal epithelium, and NTX accelerates re-epithelialization without exuberant granulation tissue formation, neovascularization, or damage to adhesion complexes [79]. In type 1 and type 2 diabetic animal models these observations have been reproduced with experiments documenting that systemic or topical applications of NTX to the ocular surface accelerated corneal epithelial repair without side effects or pathology, and restored tear production and corneal surface sensitivity [61,78].
3.1. Sex differences in ocular surface complications and naltrexone treatment in the diabetic animal model
Sex differences are evident in the prevalence of diabetes, as well as the underlying pathophysiology. Protection against severe cardiovascular complications exists for premenopausal women, suggesting that moderate estrogen levels are a factor [79]. Meta-analyses of clinical studies that included pre- and post-menopausal women, women on hormonal replacement, and men revealed that prevalence levels of T1D and T2D are lower for pre-menopausal women relative to men, and that estrogen may play a role in protection against the onset of disease and its complications [79, 80]
Although there are limited data reporting sex differences in the onset of ocular surface complications in T1D, an animal study that performed parallel investigations on male and female rats rendered hyperglycemic by STZ revealed sex differences in nearly all parameters supporting the role of estrogen as a potential protective factor [16]. Female rats with T1D had more severe complications than male T1D rats. Normal female rats had higher tear production values than males, and diabetic animals of both sexes had a substantial loss in tear volume relative to their respective baseline or normal counterparts. Tear production was negatively correlated with estrogen and with OGF. Insulin did not protect either sex from experiencing low tear production. Serum OGF levels differed between sexes, with normal females recording a 2-fold higher baseline value of OGF than normal male rats. Within the groups of T1D rats, serum levels for female rats were more than 3-fold greater than the 2-fold increase for male T1D rats relative to their respective normal counterparts. Insulin did not protect against the elevation in serum OGF for either sex but appeared to reduce serum OGF in females T1D rats more than in male diabetic animals. There were strong correlations between testosterone and OGF serum levels, with simple linear regression analyses showing significant deviations for each group.
4. Interrelationships of the OGF-OGFr axis and ocular surface complications in diabetes
The interrelationship of AGEs increasing because of altered insulin regulation of hyperglycemia along with elevated OGF levels that inhibit cell replication begin to define potential biological mechanisms underlying the onset of ocular surface complications.
This review was stimulated by observing that topical NTX and insulin are independently effective but not additive in the acceleration of corneal epithelial healing in T1D rats [81, 82]. A recent study by the same group reported that ocular surface defects such as dry eye and altered corneal surface sensitivity were not prevented in rats with controlled hyperglycemia through insulin implants [13–15]. However, it was noted that wound healing, and possibly the process of re-epithelialization, was protected by systemic insulin in that T1D rats receiving insulin implants did not display delayed corneal wound closure to the same extent as T1D animals. Given that distinct mechanisms are involved – cell replication related to wound closure versus a neural response for tear production and sensitivity, the relationship between insulin and peptides begins to take shape. Specific processes such as DNA synthesis were not measured in this study. Nonetheless, a comparison of systemic treatment of insulin with systemic NTX to determine which treatment results in a more vibrant “protective” effect against diabetic complications is warranted. Understanding that both insulin and NTX treatments are effective independently suggests that there may be two pathways functioning simultaneously in response to hyperglycemia (Figure 1).
Figure 1.
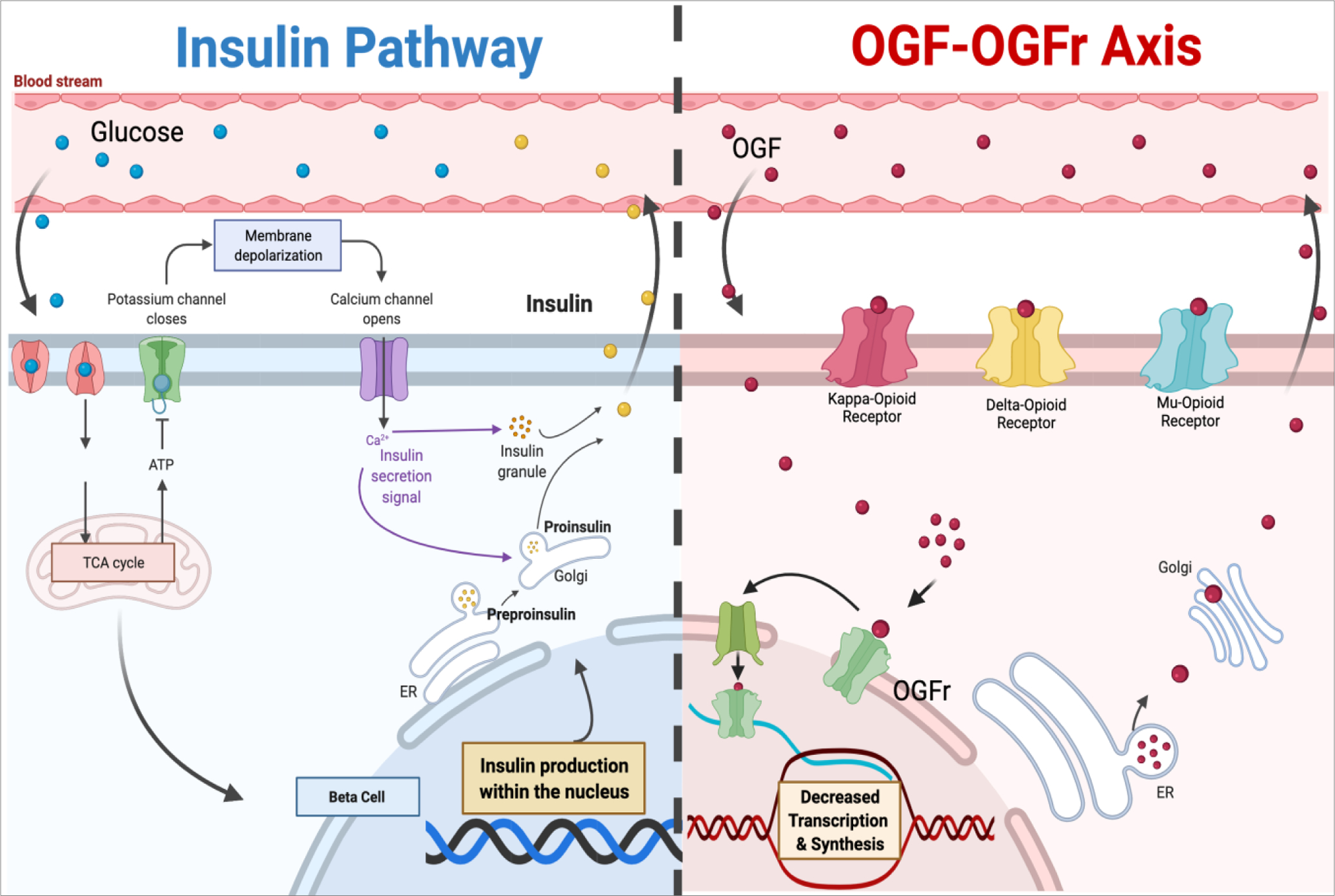
Parallel pathways illustrating potential interrelationships between insulin and the OGF-OGFr axis. Created by adaptation of templates from Biorender.com.
The elevated expression levels of OGF and OGFr measured in corneal tissue in the T1D and T1D-INS rats, coupled with higher serum OGF values in controlled and uncontrolled T1D, may indicate that dysregulation of the OGF-OGFr pathway supersedes the control mechanisms of insulin. Moreover, systemic treatment with NTX significantly reduced OGF serum levels, whereas the topical NTX had no effect. Interestingly, systemic NTX, as well as topical NTX, did not affect OGFr levels in serum. As may be expected, systemic treatment with NTX had no effect on corneal tissue levels of OGF or OGFr. In contrast, topical NTX applied twice daily restored tissue OGF and OGFr expression in the cornea to that recorded in normal animals. The combination of NTX treatment applied to insulin-controlled T1D animals has not been thoroughly investigated, such that the synergy between systemic insulin and topical opioid receptor antagonism is unclear. There is limited knowledge explaining the potential interplay between insulin, OGF, and diabetes. The pathways associated with hyperglycemia are often discussed with insulin signaling, while studies are starting to seek the interplay between the OGF-OGFr axis. Insulin is required to establish homeostasis in the body due to glucose imbalance. It has been shown in an animal model of T1D that dysregulation of the OGF-OGFr axis occurs at weeks 4 and 5 following the establishment of hyperglycemia in female and male rats, respectively. A subset of these animals was also treated with systemic insulin. Despite treatment with systemic insulin, ocular surface complications occurred and progressed during the experiment, coincident with the onset of OGF-OGFr axis dysregulation. This finding suggests that despite the ability of insulin to regulate blood glucose levels, insulin is not enough to prevent OGF-OGFr axis dysregulation. There may not be an overlap between the OGF and insulin pathways individually; however, there may be a domino effect resulting in insulin being unable to prevent OGF-OGFr dysregulation.
The paradox of insulin efficacy on some, but not all, ocular surface complications is not unique to this model. Santoleri and Titchenell reported that intrahepatic and extrahepatic pathways mediate insulin control of glucose, with the confounding results of insulin signaling leading to hepatic insulin resistance [83]. In the field of cancer, research has shown that insulin-like growth factor-1 (IGF-1) regulates cell growth by binding to its cell surface receptor IGF-1R. A dichotomy of decorin activation of IGF-1 enhances cell proliferation in normal cells, and in some cases, maintains cancer cell line transformation [84]. Insulin resistance also can be paradoxical. In the central nervous system, it was shown in animal and human studies that dysregulated insulin resistance pathways may converge to be a patho-mechanism in both T2D and neurodegenerative diseases such as Alzheimer’s. These observations are in contrast to the protective role of insulin commonly attributed to IGF-1 signaling pathways. The divergence of pathways following either the MAP kinase or PI3 kinase regulatory pathway provides two individual outcomes associated with either cognitive regulation in neurodegenerative disorders or metabolic regulations such as hepatic glucose protection and glucose-insulin balance.
5. Conclusions and future research
A novel regulatory pathway comprised of OGF, chemically termed [Met5]-enkephalin, and its nuclear-associated receptor, OGFr, mediates cellular homeostasis. This pathway becomes dysregulated in diabetes leading to elevated tissue and serum levels of OGF, and subsequent repressed cell replication and delayed re-epithelialization of the corneal surface, and other complications such as dry eye and abnormal ocular surface sensitivity. Preclinical studies demonstrated that sustained blockade of the OGFr with opioid receptor antagonists, such as NTX, reverses many complications of diabetes, including delayed corneal wound healing, dry eye, and altered corneal sensitivity in the animal model. These diabetic complications were observed in both insulin-controlled and uncontrolled models of T1D, suggesting that, although insulin mediates the elevated blood glucose and body weight deficits normally observed with T1D, it had little or no regulatory effects on other biological processes and animals receiving systemic insulin presented with dry eye, delayed wound healing, or altered corneal surface sensitivity. Whether these pathways are parallel or interrelated in other ways is unknown. Investigations of NTX treatment for diabetic-associated complications have proven to be successful. It remains unknown whether patients with insulin-controlled diabetes will benefit more than uncontrolled diabetics from this combined therapeutic approach.
With the increase in individualized medicine and targeted therapeutics, knowledge about the pathophysiology of diabetes and the dysregulation of the OGF-OGFr pathway should be evaluated for select treatment for diabetic ocular complications. Whether the elevated serum OGF levels result from too much secretion or too little metabolism is under investigation. Studies investigating changes in the OGF-OGFr pathway and the detection of pre-diabetes are also warranted as early treatment may prevent the onset of dry eye or corneal surface insensitivity. The interrelationships between insulin and OGF should also be studied from the perspective of preventing diabetes and thus deterring the development of ocular surface complications.
Acknowledgements
These studies were funded by grants from NIH and the Pennsylvania Department of Health, and currently by NIH grant 5R01 EY029223.
Abbreviations:
- OGF
opioid growth factor
- OGFr
opioid growth factor receptor
- NTX
naltrexone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
I.P: None
PJM, ISZ, and JSW have intellectual property owned by Penn State Research Foundation that is related to treatment of dry eye, but receive no financial compensation or royalties.
References
- 1.National Center for Chronic Disease Prevention and Health Promotion. Diabetes and prediabetes (2020) https://www.cdc.gov/chronicdisease/resources/publications/factsheets/diabetes-prediabetes.htm
- 2.International Diabetes Federation. IDF Diabetes Atlas, 9th Edition2019. https://www.diabetesatlas.org/en/
- 3.Cho NH, Shaw JE, Karuranga S. Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract (2018) 138:271–281. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Chronic Disease Prevention and Health Promotion (CDC). National diabetes statistics report, 2020. Estimates of diabetes and its burden in the United States.https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- 5.Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, Richika MR, Gorain B, Kesharwani P. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J Tradit Complement Med (2017) 8:361–376. doi: 10.1016/j.jtcme.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor SI, Yazdi ZS, Beitelshees Al. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest (2021)1307:193–212. doi: 10.1172/JCI14224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Qiao S, Shi C, Wang S, Ji G. Metabolomics window into diabetic complications. J Diabetes Investig (2018) 9:244–255. doi: 10.1111/jdi.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavin DP, White MF, Brazil DP. IRS proteins and diabetic complications. Diabetologia (2016) 59:2280–2291. doi: 10.1007/s00125-016-4072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Eye complications. https://www.diabetes.org/diabetes/complications/eye-complications
- 10.Shen Y, Shi L, Nauman E, et al. Race and sex differences in rates of diabetic complications. J Diabetes (2019) 11:449–456. doi: 10.1111/1753-0407.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Priyadarsini S, Whelchel A, Nicholas S, Sharif R, Riaz K, Karamichos D. Diabetic keratopathy: Insights and challenges. Surv Ophthalmol (2020) 65:513–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaji Y Diabetic eye disease. In: Diabetes and Aging-Related Complications (2017) doi: 10.1007/978-981-10-4376-5_2 [DOI] [Google Scholar]
- 13.Zagon IS, Sassani JW, Purushothaman I, McLaughlin PJ. Dysregulation of the OGF–OGFr pathway correlates with elevated serum OGF and ocular surface complications in the diabetic rat. Exp Biol Med (2020) 245:1414–1421. doi: 10.1177/1535370220940273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zagon IS, Sassani JW, Purushothaman I, McLaughlin PJ. Blockade of OGFr delays the onset and reduces the severity of diabetic ocular surface complications. Exp Biol Med (2021) 246:629–636. doi: 10.1177/1535370220972060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purushothaman I, Sassani JW, Zagon IS, , McLaughlin PJ. Ocular surface complications result from dysregulation of the OGF-OGFr signaling pathway in female diabetic rats. Exp Ther Med (2021) 22:687. doi: 10.3892/etm.2021.10119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purushothaman I, Zagon IS, Sassani JW, Zhou S, McLaughlin PJ. Sex differences in the magnitude of diabetic ocular surface complications: Role of serum OGF. Physiol Behav (2021) 237:113436. doi: 10.1016/j.physbeh.2021.113436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol (2020) 16:377–390. doi: 10.1038/s41581-020-0278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharroubi AT. Diabetes mellitus: The epidemic of the century. World J Diabetes (2015) 6:850–867. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet (2018) 391:2449–2462. doi: 10.1016/S0140-6736(18)31320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goyal SN, Reddy NM, Patil KR, Nakhate KT, Ojha S, Patil CR, Agrawal YO. Challenges and issues with streptozotocin-induced diabetes - A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem Biol Interact (2016) 244:49–63. doi: 10.1016/j.cbi.2015.11.032 [DOI] [PubMed] [Google Scholar]
- 21.Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr Protoc Pharmacol (2015) 70:5.47.1–5.47.20. doi: 10.1002/0471141755.ph0547s70 [DOI] [PubMed] [Google Scholar]
- 22.Wei M, Ong L, Smith MT, Ross FB, Schmid K, Hoey AJ, Burstow D, Brown L. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ (2003) 12:44–50. doi: 10.1046/j.1444-2892.2003.00160.x [DOI] [PubMed] [Google Scholar]
- 23.King AJF. The use of animal models in diabetes research. Brit J Pharmacol (2012) 166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dewi PAS, Sitompul R, Pawitan J, Naroeni A. Animal model of diabetic keratopathy. ARSHI Vet Lett (2019) 3(3). doi: 10.29244/avl.3.3.57-58 [DOI] [Google Scholar]
- 25.Lindström P The physiology of obese-hyperglycemic mice [ob/ob mice]. ScientificWorld J (2007) 7:666–685. doi: 10.1100/tsw.2007.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee MY, Shim MS, Kim BH, Hong SW, Choi R, Lee EY, Man SM, Kim GW, Shin JY, Shin YG, Chung CH. Effects of spironolactone and losartan on diabetic nephropathy in a type 2 diabetic rat model. Diabetes Metab J (2011) 35L130–137. doi: 10.4093/dmj.2011.35.2.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giroix MH, Irminger JC, Lacraz G, Noll C, Calderari S, Ehses JA, Coulaud J, Cornut M, Kassis N, schmidlin F, Paul JL, Kergoat M, Janel N, Halban PA, Homo-Delarche F. Hypercholesterolaemia, signs of islet microangiopathy and altered angiogenesis precede onset of type 2 diabetes in the Goto-Kakizaki (GK) rat. Diabetologia (2011) 54:2451–2462. doi: 10.1007/s00125-011-2223-4 [DOI] [PubMed] [Google Scholar]
- 28.Lbujimov AV. Diabetic complications in the cornea. Vision Res 2017;139:138–152. doi: 10.1016/j.visres.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L, Titone R, Robertson DM. The impact of hyperglycemia on the corneal epithelium: molecular mechanisms and insight. Ocul Surf 2019: 17:644–654. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Otake H, Hiramatsu N, Yamamoto N, Taga A, Nagai N. A proteomic approach for understanding the mechanisms of delayed corneal wound healing in diabetic keratopathy using diabetic model rat. Int J Mol Sci 2018November18;19(11):3635. doi: 10.3390/ijms19113635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Buey MA, Casas P, Caramello C, Lopez N, de la Rica M, Subiron AB, Lanchares E, Huerva V, Grzybowski A, Ascaso FJ. An update on corneal biomechanics and architecture in diabetes. J. Ophthalmol (2019) 2019:7645352. J Ophthalmol. 2019 Jun 2;2019:7645352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Zhao L, Deng S. Dry eye syndrome in patients with diabetes mellitus: prevalence, etiology, and clinical characteristics. J Ophthalmol (2016) 2016:8201053. doi: 10.1155/2016/8201053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinic Mayo. Dry eyes. www.mayoclinic.org/diseases-conditions/dry-eyes/symptoms-causes
- 34.Sanchez-Thorin JC. The cornea in diabetes mellitus. Int Ophthalmol Clin (1998) 38:19–36. [PubMed] [Google Scholar]
- 35.Shah R, Amador C, Tormanen K, Ghiam S, Sehghizadeh M, Arumugaswami V, Kumar A, Kramerov AA, Ljubimov AV. Systemic diseases and the cornea. Exp Eye Res (2021) 204:108455 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peponis V, Bonovas S, Kapranou A, Peponi E, Filioussi K, Magkou C, Sitaras NM. Conjunctival and tear film changes after vitamin C and E administration in non-insulin dependent diabetes mellitus. Med Sci Monit (2004) 10:CR213–CR217. [PubMed] [Google Scholar]
- 37.Zieske JD. Extracellular matrix and wound healing. Curr Opin Ophthalmol (2001) 12:237–241. [DOI] [PubMed] [Google Scholar]
- 38.Kaji Y, Usui T, Oshika T. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci (2000) 41:362–368. [PubMed] [Google Scholar]
- 39.Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. J Endocrinol (2004) 181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 40.Liu M, Weiss MA, Arunagiri A, Yong J, Rege N, Sun J, Haataja L, Kaufman RJ, Arvan P. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes, Obes Metab (2018) Suppl 2:28–50. doi: 10.1111/dom.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karakose E, Ackeifi C, Wang P, Stewart AF. Advances in drug discovery for human beta cell regeneration. Diabetologia (2018) 61:1693–1699. doi: 10.1007/s00125-018-4639-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerman C, Forlenza G, Schatz D. The discovery and structure of human insulin. Pediatr Endocrinol Rev (2020) 17(Suppl 1); 131–137. doi: 10.17458/per.vol17.2020.zfs.discoverystructureinsulin. [DOI] [PubMed] [Google Scholar]
- 43.Zhou S, Tang X, Chen HZ. Sirtuins and insulin resistance. Front Endocrinol (Lausanne). (2018) 9:748. doi: 10.3389/fendo.2018.00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CJ, Sears CL, Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann N Y Acad Sci (2020) 1461:37–52. doi: 10.1111/nyas.14107 [DOI] [PubMed] [Google Scholar]
- 45.Hall C, Yu H, Choi E. Insulin receptor endocytosis in the pathophysiology of insulin resistance. Exp Mol Med (2020) 52:911–920. doi: 10.1038/s12276-020-0456-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ono H Molecular mechanisms of hypothalamic insulin resistance. Int J Mol Sci (2019) 20:1317. doi: 10.3390/ijms20061317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampath Kumar A, Maiya AG, Shastry BA, Vaishali K, Ravishankar N, Hazani A, Gundmi S, Jadhav R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann Phys Rehabil Med. (2019) 62:98–103. . doi: 10.1016/j.rehab.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Clemmons DR. Role of igf-binding proteins in regulating igf responses to changes in metabolism. J Mol Endocrinol (2018) 61:T139–T169. doi: 10.1530/JME-18-0016 [DOI] [PubMed] [Google Scholar]
- 49.Trommelen J, Groen BBL, Hamer HM, De Groot LCPGM, Van Loon LJC. Mechanisms in endocrinology: Exogenous insulin does not increase muscle protein synthesis rate when administered systemically: A systematic review. Eur J Endocrinol (2015) 173:R25–R34. doi: 10.1530/EJE-14-0902 [DOI] [PubMed] [Google Scholar]
- 50.Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans (2006) 34(pt 2):213–216. doi: 10.1042/bst0340213 [DOI] [PubMed] [Google Scholar]
- 51.Emery MA, Akil H. Endogenous opioids at the intersection of opioid addiction, pain, and depression: The search for a precision medicine approach. Annu Rev Neurosci (2020) 43:355–374. Doi: 10.1146/annurev-neuro-110719-095912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLaughlin PJ, Zagon IS. POMC-derived opioid peptides. In: Handbook of Biologically Active Peptides (2013). doi: 10.1016/B978-0-12-385095-9.00217-7 [DOI] [Google Scholar]
- 53.Kaur J, Kumar V, Sharma K, Kaur S, Gat Y, Goyal A, Tanwar B. Opioid peptides: An overview of functional significance. Int J Pept Res Ther (2020) 26:33–41. doi: 10.1007/s10989-019-09813-7 [DOI] [Google Scholar]
- 54.Zagon IS, McLaughlin PJ. Identification of opioid peptides regulating proliferation of neurons and glia in the developing nervous system. Brain Res (1991) 542:318–323. doi: 10.1016/0006-8993(91)91585-O [DOI] [PubMed] [Google Scholar]
- 55.Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre- proenkephalin. Nature (1996) 383: 535–538. doi: 10.1038/383535a0 [DOI] [PubMed] [Google Scholar]
- 56.Harno E, Ramamoorthy TG, Coll AP, White A. POMC: The physiological power of hormone processing. Physiol Rev (2018) 98:2381–2430. doi: 10.1152/physrev.00024.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zagon IS, Sassani JW, Allison G, McLaughlin PJ. Conserved expression of the opioid growth factor, [Met5]enkephalin, and the zeta (ζ) opioid receptor in vertebrate cornea. Brain Res. (1995) 671: 105–111. doi: 10.1016/0006-8993(94)01314-8 [DOI] [PubMed] [Google Scholar]
- 58.Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr). Brain Res Rev (2002) 38:351–376. doi: 10.1016/S0165-0173(01)00160-6 [DOI] [PubMed] [Google Scholar]
- 59.Cheng F, McLaughlin PJ, Verderame MF, Zagon IS. The OGF-OGFr axis utilizes the p16INK4a and p21WAF1/CIP1 pathways to restrict normal cell proliferation. Mol Biol Cell (2009) 20:319–327 doi: 10.1091/mbc.e08-07-0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng F, McLaughlin PJ, Banks WA, Zagon IS. Internalization of the opioid growth factor, [Met5]-enkephalin, is dependent on clathrin-mediated endocytosis for downregulation of cell proliferation. Am J Physiol Reg Integr Comp Physiol (2010) 299:R774–R785. doi: 10.1152/ajpregu.00318.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng F, McLaughlin PJ, and Zagon IS. 2010.Regulation of cell proliferation by the opioid growth factor is dependent on karyopherin β and Ran for nucleocytoplasmic trafficking. Exp. Biol. Med (2010) 235:1093–1101. doi: 10.1258/ebm.2010.010139 [DOI] [PubMed] [Google Scholar]
- 62.Kren NP, Zagon IS, McLaughlin PJ. Nuclear export of opioid growth factor receptor is CRM1 dependent. Exp Biol Med (2016) 241:273–281. doi: 10.1177/1535370215605585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fallucca F, Tonnarini G, Di Biase N, D’Alessandro M, Negri M. Plasma met-enkephalin levels in diabetic patients: Influence of autonomic neuropathy. Metabolism (1996) 45:1065–1068. doi: 10.1016/S0026-0495(96)90004-9 [DOI] [PubMed] [Google Scholar]
- 64.Negri M, Fallucca F, Tonnarini G, Mariani P, D’alessandro M, Pachí A. High levels of circulating met-enkephalin in pregnant and menstruating type 1 diabetic women. Gynecol Endocrinol (1990) 4:25–31. doi: 10.3109/09513599009030688 [DOI] [PubMed] [Google Scholar]
- 65.Isayama T, McLaughlin PJ, and Zagon IS. Ontogeny of preproenkephalin mRNA expression in the rat retina. Visual Neurosci (1996) 13:695–704. doi: 10.1017/s0952523800008580 [DOI] [PubMed] [Google Scholar]
- 66.Isayama T, Hurst WJ, McLaughlin PJ, and Zagon IS. Ontogeny of the opioid growth factor, [Met5]-enkephalin, and its binding activity in the rat retina. Visual Neurosci (1995) 12:939–950. doi: 10.1017/s0952523800009494 [DOI] [PubMed] [Google Scholar]
- 67.Timmers K, Voyles NR, Zalenski C, Wilkins S, Recant L. Altered β-endorphin, met- and leuenkephalins, and enkephalin-containing peptides in pancreas and pituitary of genetically obese diabetic (db/db) mice during development of diabetic syndrome. Diabetes (1986)35:1143–1151. doi: 10.2337/diab.35.10.1143 [DOI] [PubMed] [Google Scholar]
- 68.Timmers K, Coleman DL, Voyles NR, Powell AM, Rokaeus A, Recant L. Neuropeptide content in pancreas and pituitary of obese and diabetes mutant mice: Strain and sex difference. Metabolism (1990) 39:378–383. doi: 10.1016/0026-0495(90)90252-8. [DOI] [PubMed] [Google Scholar]
- 69.Kolta MG, Pierzchala K, Houdi AA, Van Loon GR. Effect of diabetes on the levels of two forms of met-enkephalin in plasma and peripheral tissues of the rat. Neuropeptides (1992) 21:55–63. doi: 10.1016/0143-4179(92)90152-m [DOI] [PubMed] [Google Scholar]
- 70.Sassani JW, Mc Laughlin PJ, Zagon IS. The yin and yang of the opioid growth regulatory system: Focus on diabetes - The Lorenz E. Zimmerman tribute lecture. J Diabetes Res. (2016) 2016:9703729. doi: 10.1155/2016/9703729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Módulo CM, Jorge AG, Dias AC, Braz AM, Bertazolli-Filho R, Jordao AA, Marchini JS, Rocha EM. Influence of insulin treatment on the lacrimal gland and ocular surface of diabetic rats. Endocrine (2009) 36:161–168. doi: 10.1007/s12020-009-9208-9 [DOI] [PubMed] [Google Scholar]
- 72.Cruz-Cazarim ELC, Cazarim MS, Ogunjimi AT, Petrilli R, Rocha EM, Lopez RFV. Prospective insulin-based ophthalmic delivery systems for the treatment of dry eye syndrome and corneal injuries. Eur J Pharm Biopharm (2019) 140:1–20. doi: 10.1016/j.ejpb.2019.04.014 [DOI] [PubMed] [Google Scholar]
- 73.Aniah Azmi N, Bastion MLC. Short-term results of trial of topical insulin for treatment of dry eyes in diabetics. Eye Contact Lens (2020) 46 Supp1 1: S25–S32. doi: 10.1097/ICL.0000000000000623 [DOI] [PubMed] [Google Scholar]
- 74.Zagon IS, Klocek MS, Griffith JW, Sassani JW, Komaromy AM, and McLaughlin PJ. Prevention of exuberant granulation tissue and neovascularization in the rat cornea by naltrexone. Arch. Ophthalmol (2008) 126:501–506. doi: 10.1001/archopht.126.4.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zagon IS, Sassani JW, McLaughlin PJ. Opioid growth factor modulates corneal epithelial outgrowth in tissue culture. Am J Physiol (1995) 268:R942–R950. doi: 10.1152/ajpregu.1995.268.4.R942 [DOI] [PubMed] [Google Scholar]
- 76.Zagon IS, Sassani JW and McLaughlin PJ. Reepithelialization of the human cornea is regulated by endogenous opioid. Invest Ophthalmol Vis Sci (2000) 41:73–81. [PubMed] [Google Scholar]
- 77.McLaughlin PJ, Sassani JW, Zagon IS. Opioid growth factor receptor blockade by naltrexone as a treatment for diabetic complications. Adv Medicine Biology (2019) 138:109–145. [Google Scholar]
- 78.McLaughlin PJ, Sassani JW, Zagon IS. Naltrexone as a novel therapeutic for diabetic corneal complications. J Cell Immunol. (2020) 2:42–46. doi: 10.33696/immunology.1.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Paoli M, Werstuck GH. Role of estrogen in Type 1 and Type 2 diabetes mellitus: A review of clinical and preclinical data. Canad J Diabetes 44 (2020) 448–452. doi: 10.1016/j.jcjd.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Gale EAM, Gillespie KM. Diabetes and gender. Diabetologia 44:3–15, 2001. [DOI] [PubMed] [Google Scholar]
- 81.Klocek MS, Sassani JW, McLaughlin PJ, and Zagon IS. Naltrexone and insulin are independently effective but not additive in accelerating corneal epithelial healing in type 1 diabetic rats. Exp Eye Res (2009) 89:686–692. doi: 10.1016/j.exer.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zagon IS, Sassani JW and McLaughlin PJ. Insulin treatment ameliorates impaired corneal re-epithelialization in diabetic rats. Diabetes (2006) 55:1141–1147. doi: 10.2337/diabetes.55.04.06.db05-1581. [DOI] [PubMed] [Google Scholar]
- 83.Santoleri D, Titchenell PM. Resolving the paradox of hepatic ionsulin resistance. Cell Mol Gastroenterol Hepatol (2019) 7:447–456. doi: 10.1016/j.jcmgh.2018.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morrione A, Neill T, Iozzo RV. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J (2013) 280:2138–2149. doi: 10.1111/febs.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]


