Abstract
Neuraminidase (NA) is the second most abundant glycoprotein on the surface of influenza A viruses (IAV). Neuraminidase type 1 (NA1) based virus-like particles (VLPs) have previously been shown to protect against challenge with H1N1 and H3N2 IAV. In this study, we produced neuraminidase type 2 (NA2) VLPs derived from the sequence of the seasonal IAV A/Perth/16/2009. Intramuscular vaccination of mice with NA2 VLPs induced high anti-NA serum IgG levels capable of inhibiting NA activity. NA2 VLP vaccination protected against mortality in a lethal A/Hong Kong/1/1968 (H3N2) virus challenge model, but not against lethal challenge with A/California/04/2009 (H1N1) virus. However, bivalent vaccination with NA1 and NA2 VLPs demonstrated no antigenic competition in anti-NA IgG responses and protected against lethal challenge with H1N1 and H3N2 viruses. Here we demonstrate that vaccination with NA VLPs is protective against influenza challenge and supports focusing on anti-NA responses in the development of future vaccination strategies.
Keywords: Neuraminidase (NA), Influenza virus vaccine, virus-like particle (VLP), Influenza virus
Introduction
Influenza A virus (IAV) is a causative agent of acute respiratory disease responsible for an estimated 290,000–645,000 deaths globally per annum during seasonal epidemics (Iuliano et al., 2018). While several small molecule inhibitors are licensed for treatment of IAV infection, current strategies to proactively mitigate the threat posed by IAV to the global population focus on seasonal vaccination (Grohskopf et al., 2013). However, recent seasonal influenza vaccines have demonstrated a suboptimal overall efficacy, ranging from 29%−40% from 2016–2019, with only 9% vaccine efficacy against circulating H3N2 strains and 5% efficacy against the emergent A(H3N2) clade 3C.3a viruses in the latter part of the influenza season in 2019 (Flannery et al., 2020, 2019; Rolfes et al., 2019). The efficacy of seasonal IAV vaccines has historically depended on sequence homology between the hemagglutinin (HA), the most abundant surface glycoprotein on the virion, of the vaccine strain and the circulating strain during the influenza season (Krammer and Palese, 2015).
HA and neuraminidase (NA) are the two most abundant glycoproteins on the surface of IAV and are divided into 18 and 11 subtypes, respectively. Currently licensed seasonal influenza vaccines only standardize the antigenic content of HA, with no standard potency requirement for NA, often leading to variable or nonexistent anti-NA responses after seasonal vaccination (Chen et al., 2018; Couch et al., 2012; Monto et al., 2015; Wohlbold and Krammer, 2014). The suboptimal anti-NA response post-vaccination is particularly problematic given that the antigenic evolution of NA has been suggested to be slower and discordant from that of HA (Kilbourne et al., 1990). Furthermore, cross-reactive anti-NA antibodies have been identified after infection with seasonal H1N1 influenza strains that inhibit NA activity of A/California/04/2009 H1N1 pandemic virus as well as against H5N1 viruses (Chen et al., 2012; Marcelin et al., 2011). However, previous work has suggested that anti-NA immune responses are dampened when both HA and NA are presented on the same viral particle, with HA being immunodominant over NA (Johannsson et al., 1987; Johansson and Kilbourne, 1993). Strategies attempting to overcome the immunodominance of HA over NA have included both supplementing seasonal vaccines with purified NA and more recently by extending the NA stalk to potentially expose additional epitopes on the NA molecule itself (Broecker et al., 2019; Johansson et al., 2002, 1998; Kim et al., 2017). Previous work has also shown that antigens delivered via VLPs in their native form within the context of a membrane demonstrated superior protection against heterologous IAV challenge compared with soluble recombinant antigen (Bright et al., 2008).
As anti-HA antibodies have been shown to be neutralizing and able to inhibit virus entry into cells (Krammer et al., 2015), IAV vaccine development has historically focused on HA responses. However, natural infection with IAV viruses induces a much more balanced immune response against both HA and NA (Chen et al., 2018). NA is present on the surface of virions at a ratio of 1 molecule of NA for every 4 molecules of HA (Harris et al., 2006). In the past 100 years, only N1 and N2 IAV subtypes have circulated in humans compared with H1, H2, and H3 IAV subtypes. Anti-NA based immunity and the contribution to protection against IAV infection has been appreciated since the 1960’s. Previous work has demonstrated that both natural infection (Murphy et al., 1972) and vaccination (Couch et al., 1974) with heterosubtypic HA but homosubtypic NA contributed to a reduction in illness and clinical signs upon infection with a homosubtypic NA. More recent work has demonstrated that anti-NA serum antibody titers were a stronger correlate of protection against mild to moderate influenza disease than anti-HA titers (Memoli et al., 2016). Historical work has shown that current IAV vaccination strategies targeting HA in an effort to prevent IAV infection could be augmented by targeting anti-NA immune responses as well.
The efficacy of NA1 VLPs produced using a Spodoptera frugiperda (Sf9) insect cell expression system has been previously demonstrated against influenza A virus in murine challenge models (Kim et al., 2019; Quan et al., 2012) as well as in a ferret challenge model (Smith et al., 2017). Additionally, Trichoplusia ni (Tni) insect cell expression systems have demonstrated an increased yield of influenza HA VLPs with a reduced amount recombinant baculovirus in VLP preparations compared to Sf9 cells (Krammer et al., 2010). However, production of NA VLPs in Tni cells and their subsequent use as a vaccine in a murine influenza A challenge model has not yet been reported, nor has the use of VLPs using NA2 only or the combined administration of NA1 and NA2 VLPs as a bivalent vaccine against influenza A viruses. In this study, we produced NA2 VLPs using the NA sequence derived from A/Perth/16/2009 in a Tni insect cell expression system. We compared different routes of NA2 VLP vaccination in a murine model in a lethal heterologous challenge model against A/Hong Kong/1/1968 (H3N2). Using the same expression system, we also produced NA1 VLPs using the NA sequence derived from A/California/04/2009 (H1N1). Vaccination with both NA1 and NA2 VLPs induced high levels of anti-NA serum IgG capable of inhibiting NA activity with no evidence of antigenic competition between NA1 and NA2. The bivalent administration of NA1 and NA2 VLPs was protective against mortality in both lethal H3N2 and H1N1 challenge models. This work demonstrates the efficacy of bivalent vaccination with NA1 and NA2 VLPs in mice against both lethal H1N1 and H3N2 virus challenge.
Materials and Methods
Cells and virus stocks
Spodoptera frugiperda (Sf9) cells (IPLB-Sf-21-AE, Expression Systems, Davis, CA, USA) were maintained at 27°C in suspension in shaker flasks using serum free SF900II media (Gibco/ThermoFisher Scientific, Waltham, MA, USA). Trichoplusia ni (Tni) cells (Expression Systems, Davis, CA, USA) were maintained at 27°C in suspension in shaker flasks using serum free ESF 921 media (Expression Systems, Davis, CA, USA). Madin-Darby canine kidney (MDCK) cells (ATCC CCL 34, American Type Culture Collection, Manassas, VA, USA) were maintained in Dulbecco’s modified Eagle’s media (DMEM) (Corning Life Sciences, Corning, NY, USA) supplemented with 10% fetal bovine serum (GE Healthcare Life Sciences, Westborough, MA, USA). A/California/04/2009 (H1N1) virus (BEI Resources, Manassas, VA, USA) was expanded one time in MDCK cells and then serially passaged in the lungs of BALB/c mice. Mice were anesthetized and thirty microliters of virus suspension was instilled intranasally. Three days post infection, mice were humanely euthanized. Lungs were harvested and homogenized in sterile PBS followed by intranasal (IN) instillation in subsequent mice for a total of 10 passages. A/Hong Kong/1/1968–1 Mouse-Adapted 12A (H3N2) virus was obtained from BEI Resources (Manassas, VA, USA) and expanded one passage in MDCK cells. Sequence identity of mouse adapted viruses was confirmed by sequencing on an Illumina iSeq 100 platform as previously described (Kyriakis et al., 2017; Maljkovic Berry et al., 2020).
Animals
Six to eight week old BALB/c mice (Charles River Laboratories, Wilmington, MA, USA) were housed in the Emory University Division of Animal Resources biosafety level 1 animal facility at Emory University Whitehead animal facility. Mice were transported to biosafety level 2 containment rooms in the same facility 1 day prior to infection. All animal experiments and procedures were conducted in accordance with protocols approved by Emory University’s Institutional Animal Care and Use Committee (IACUC) and in accordance with the United States Federal Animal Welfare Act (PL 89–544) and subsequent amendments.
VLP Production and Purification
The neuraminidase 1 (NA1) gene from A/California/04/2009, the neuraminidase 2 (NA2) gene from A/Perth/16/2009, and the matrix 1 gene from A/Michigan/73/2015 (M1) were synthesized with unique BamHI and HindIII restriction sites at the 5’ and 3’ ends, respectively (Integrated DNA Technologies, Coralville, IA, USA). Individual gene fragments were digested using BamHI and NotI restriction endonucleases and ligated into BamHI and NotI digested pFastBac1 vector from the Bac-to-Bac® Baculovirus Expression System kit (ThermoFisher Scientific, Waltham, MA, USA). Bacmid DNA was created and purified according to the Bac-to-Bac® manufacturer’s instructions. Recovered high molecular weight DNA was then transfected into SF9 cells in SF900II media. The resulting recombinant baculovirus (rBV) stocks were amplified in SF9 according to the Bac-to-Bac® manufacturer’s instructions.
Recombinant baculovirus stocks were used to coinfect Tni cells at a multiplicity of infection (MOI) of 5 for NA1 or NA2 rBVs and an MOI of 3 for M1 rBVs. VLPs recovered from the clarified supernatant were first pelleted at 136,000 x g at 4°C for 90 min. Resulting pellets were resuspended in 1xPBS overnight for approximately 12 hours at 4°C. Resuspended pellets were then subjected to purification via discontinuous sucrose density gradient centrifugation from 20%−60% sucrose at 190,000 x g for 16 hours. The resulting gradients were then fractionated followed by resolution via SDS-PAGE and western blotting.
VLP Characterization
Sucrose gradients were fractionated and analyzed via SDS-PAGE and Western Blot. Western Blots were probed with polyclonal anti-NA1 sera (NR-3136, BEI Resources, Manassas, VA, USA), polyclonal anti-NA2 sera (NR-3137, BEI Resources, Manassas, VA, USA), or polyclonal anti-M1 sera (NR-3134, BEI Resources, Manassas, VA, USA) where indicated. Blots were then probed with secondary rabbit anti-goat HRP conjugate antibody (Southern Biotech, Birmingham, AL, USA) and the resulting chemiluminescent signal was detected using SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher Scientific, Waltham, MA, USA). The signal was read using the Bio-Rad ChemiDoc Imaging System (Hercules, CA, USA). The three sucrose fractions containing the maximum respective NA and M1 signal were combined. In the case of M1 only VLPs, the three sucrose fractions containing the maximum M1 signal were combined. Combined fractions were then diluted in 1xPBS and pelleted via centrifugation at 136,000 x g for 90 minutes at 4°C. Pellets were resuspended in 1xPBS overnight for approximately 12 hours. Total protein VLP concentration was measured via BCA assay according to manufacturer’s instructions (Thermo Scientific, Waltham, MA, USA). Resuspended VLP preparations were then resolved via SDS-PAGE and followed by Coomassie staining or Western blotting. VLPs were visualized via transmission electron microscopy after negative staining with 1% phosphotungstic acid.
Neuraminidase Activity Assay
Purified VLPs preparations were tested for enzymatic neuraminidase activity using the NA-Star Influenza Neuraminidase Inhibitor Resistance Detection Kit (ThermoFisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Briefly, VLP solutions were diluted in NA-Star Assay Buffer and then incubated with substrate for 30 min at room temperature. NA-Star accelerator solution was then injected followed by measurement of the chemiluminescent signal in relative luminescent units (RLU) via Modulus II Microplate Luminometer (Promega, Madison, WI, USA). Inhibition of neuraminidase activity was measured similarly, with collected sera diluted in NA-Star Assay Buffer followed by addition of 40 ng or 25 ng of NA2 or NA1 VLPs, respectively. Sera and VLPS were incubated at 37°C for 30 minutes followed by addition of substrate and incubation at room temperature for 30 minutes. Plates were read after addition of accelerator using the Modulus II Microplate Luminometer. The highest sera dilution inhibiting at least 50% of NA activity was reported as the neuraminidase inhibition (NAI) titer.
Immunization and infection
Mice were immunized via either the IN or intramuscular (IM) route as indicated with the dose indicated for each experiment. Mice vaccinated via the IN route were lightly anesthetized via isoflurane prior to instillation of NA VLPs in 50 µl total volume. Mice vaccinated via the IM route were injected with a total volume of 50 µl of VLP solution. Mice were prime-boost vaccinated 28 days apart with sera collected 14 days post-prime vaccination and 14 days post-boost vaccination. Mice were challenged 28 days post-boost vaccination via IN instillation of 5xLD50 of the indicated virus under isoflurane anesthesia. The LD50 of viruses were determined via the Reed-Muench method (Reed and Muench, 1938). For measurement of viral replication in vaccinated mice, 3–5 mice per group were humanely euthanized at day 4 post-challenge and lungs were collected in sterile 1xPBS. Mice were monitored daily for morbidity via weight loss and were humanely euthanized upon reaching the experimental endpoint of 25% total weight loss.
Measurement of NA-specific antibody using ELISA
Total immunoglobulin G (IgG) levels were measured as previously described (Esser et al., 2016) with modifications. Nunc Maxisorb (ThermoFisher Scientific, Waltham, MA, USA) plates were coated with 100 ng / well of recombinant neuraminidase (rNA) derived from A/Wisconsin/67/2005 (rN2) (BEI Resources #NR-19237, Manassas, VA, USA) or A/California/04/2009 (rN1) (BEI Resources #NR-19234, Manassas, VA, USA) where indicated. Purified recombinant NA2 derived from the homologous A/Perth/16/2009 sequence was not commercially during the execution of the study. Total murine anti-NA IgG was detected against a standard curve of murine IgG with HRP conjugated goat anti-mouse secondary antibody (Southern Biotech, Birmingham, AL, USA).
Plaque Assay for virus titration
Whole lungs were homogenized as previously described (Littauer et al., 2017). Briefly, lungs were homogenized and passed through a 40 µm cell strainer. Virus containing supernatants were clarified by centrifugation at 800 x g for 10 minutes at 4°C. Clarified supernatants were added in 10-fold serial dilutions in DMEM media to confluent monolayers of MDCK cells in 6-well culture plates in a volume of 500 µl for 1 hour at room temperature with gentle rocking (Corning Life Sciences, Corning, NY, USA). Inoculations were aspirated followed by overlay with agar media as previously described (Littauer et al., 2018). Cells were incubated for 48–72 hours followed by visualization of plaques via crystal violet staining. The limit of detection of the assay was 20 plaque forming units (PFU) per 100 mg of lung tissue.
Statistics
Statistical comparisons between vaccinated groups were performed via one way ANOVA with post-hoc Bonferroni multiple comparisons using GraphPad Prism statistical software (GraphPad Software, San Diego, CA, USA) with an alpha of 0.05 (α ≤ 0.05).
Results
Characterization of NA VLPs
As production of NA2 VLPs had not been previously reported in a Tni insect cell expression system, we first set out to characterize NA2 VLPs produced by coinfection of Tni cells. Previous work with IAV HA VLPs indicated that a VLPs expressed in Tni cells migrated at a density of 40–50% sucrose in a sucrose gradient whereas HA VLPs expressed in an Sf9 expression system migrated at 35–45% sucrose (Krammer et al., 2010). To purify NA2 VLP preparations, NA2 VLPs produced by coinfection of Tni cells with NA2 and M1 rBVs were subjected to sucrose gradient ultracentrifugation followed by fractionation and Western blots probed with goat anti-NA2 and goat anti-M1 polyclonal sera. Similar to previous HA VLP work (Krammer et al., 2010), Western blots of sucrose fractions of NA2 VLPs expressed in Tni Cells revealed signals for NA2 and M1 that comigrated at an approximate density of 40–50% sucrose corresponding to fractions 5–7 after ultracentrifugation (Figure 1A). After fractions 5–7 were combined and washed, the VLP preparation revealed two defined bands upon SDS-PAGE electrophoresis followed by Coomassie staining that migrated at approximately 28 kDa and 55 kDa corresponding to M1 and NA2, respectively (Figure 1B). To confirm the identity of these bands, we performed a Western blot probing the membrane with polyclonal goat anti-NA2 sera and polyclonal goat anti-M1 sera. The resulting chemiluminescent signal revealed signals at the expected sizes of NA2 and M1 (Figure 1C). To examine both the size and morphology of the resulting VLPS, we visualized NA2 VLPs via electron microscopy. The resulting micrographs revealed spherical particles approximately 70–100 nm in size with morphology consistent with influenza VLPs and virions (Figure 1D)(Krammer et al., 2010). Lastly, we measured the neuraminidase activity of the NA2 VLPs to investigate the functional activity of NA2 incorporated into NA2 VLPs. The NA2 VLPs displayed increasing NA activity with increasing total protein concentration (Figure 1E). VLP preparations treated identically but created via infection with only M1 rBVs did not show any NA activity above background levels at any concentration examined (Figure 1E). These results suggest that coinfection of Tni cells with rBVs expressing the NA2 and M1 gene from IAV produce enzymatically active NA2 VLPs of the expected size and morphology.
Figure 1:
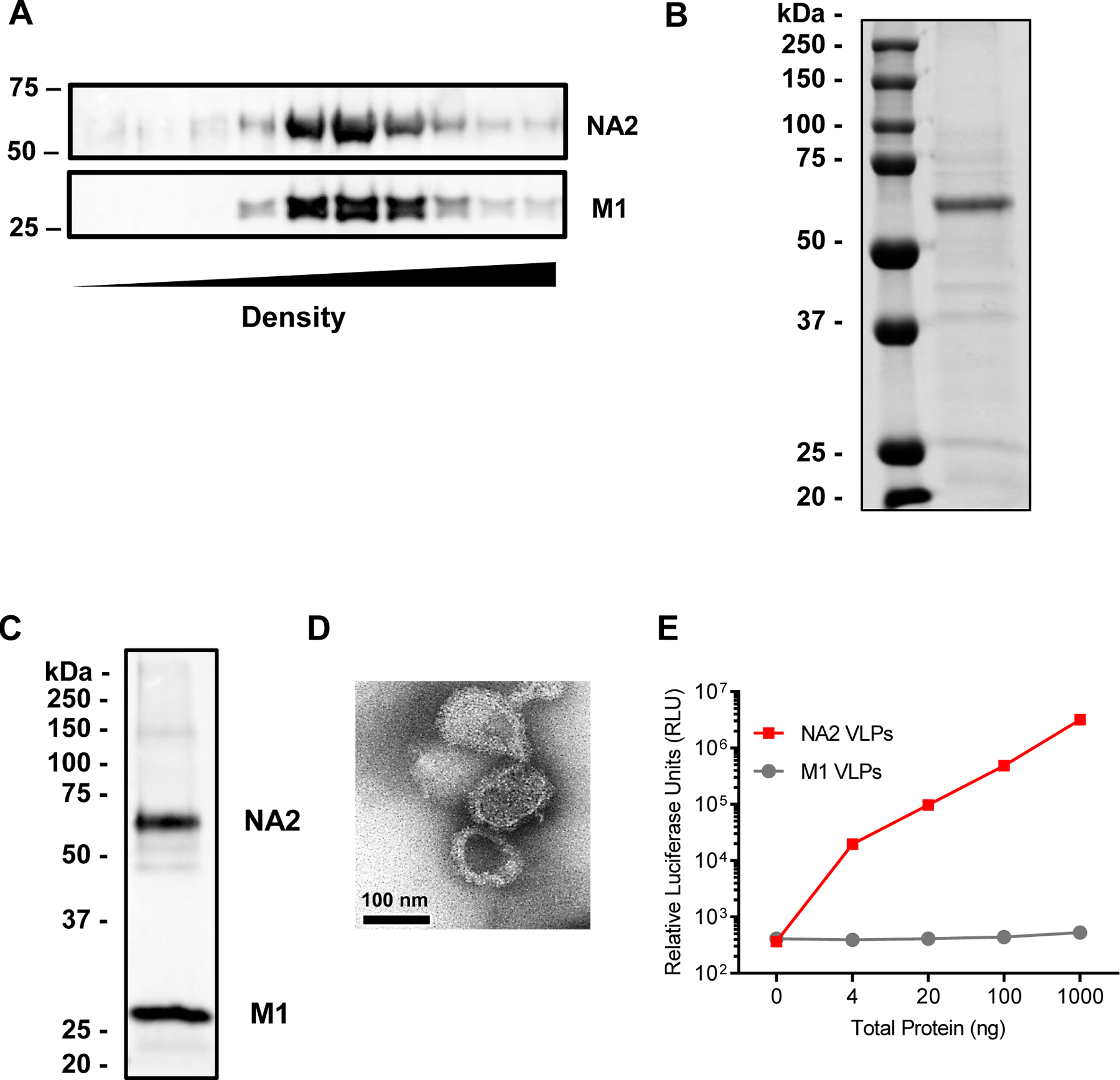
NA2 VLP purification and characterization. (A) Western blot analysis of the migration of NA2 VLPs in 20–60% sucrose gradients after ultracentrifugation. Lane 1 represents the fraction with the lowest density, lane 10 represents the fraction with the highest density. NA2 blots were probed with polyclonal goat anti-NA2 sera, while M1 blots were probed with polyclonal goat anti-M1 sera. (B) Coomassie stained SDS-PAGE gel loaded with 10 µg of NA2 VLP preparation. Molecular weight in kilodaltons (kDa) is indicated to the left. (C) Western blot of 10 µg of NA2 VLP preparation. Blot was probed with polyclonal goat anti-NA2 and anti-M1 sera. Molecular weight in kilodaltons (kDa) is indicated to the left. (D) Negative stain electron micrograph of NA2 VLP preparation. Scale bar represents 100 nm. (E) Neuraminidase activity by NA-STAR assay. The NA2 VLP preparation is indicated by the black line and symbols, while M1 only VLPs are indicated by the grey line and symbols. Individual symbols represent the mean of 3 replicates. Error bars represent the standard error of the mean (SEM).
Intramuscular vaccination with NA2 VLPs is immunogenic and protective in a heterologous challenge model
Previous work with NA VLPs in a murine model has demonstrated that NA1 VLPs produced in Sf9 insect cells were immunogenic and effective against homologous challenge when administered via the IN route (Quan et al., 2012) and the IM route (Kim et al., 2019). To determine if NA2 VLPs produced in Tni cells were immunogenic, we prime-boost vaccinated groups of BALB/c mice with 5 µg NA2 VLPs via the IN route and the IM route 28 days apart. A separate group of mice were mock vaccinated IM with 1xPBS to serve as controls. Sera were collected 14 days post-prime and 14 days post-boost vaccination. Mice were challenged with 5xLD50 of homosubtypic but heterologous A/Hong Kong/1/1968 (H3N2) (85.9% neuraminidase amino acid homology between the NA2 VLP antigen A/Perth/16/2009 and A/Hong Kong/1/1968) virus 28 days post-boost vaccination with lung homogenates collected 4 days post-infection to evaluate viral lung titers. To evaluate the immunogenicity of NA2 VLPs, we measured total serum anti-NA2 VLP IgG levels via ELISA. In sera collected 14 days post-prime vaccination, IM vaccinated mice showed an approximate 4-fold increase over IN vaccination in total serum IgG levels (Figure 2A). The magnitude of this difference increased 14 days post-boost vaccination, with IM vaccinated mice showing an approximate 9-fold increase over IN vaccinated mice in total serum IgG levels (Figure 2B). Higher total serum IgG levels correlated with an increased functional anti-NA2 immune response. Sera collected from mice post-boost vaccination via the IM route demonstrated a 2-fold increase in neuraminidase inhibition (NAI) titer compared to mice vaccinated IN (Figure 2C). Mice vaccinated via the IM route survived lethal infection with heterologous H3N2 virus despite a peak mean weight loss of 14.9% at day 5 post-challenge (Figure 2D). Fifty percent of mice vaccinated IN reached the experimental endpoint in the challenge experiment and were humanely euthanized (Figure 2E). All mock vaccinated mice reached the experimental endpoint by day 6 post-challenge and were humanely euthanized (Figure 2E). IM vaccinated mice demonstrated an approximate 2-log reduction in viral titers measured in lung lysates harvested 4 days post-infection (dpi) compared to mock vaccinated control mice (Figure 2F). Alternatively, the mean viral lung titer of IN vaccinated mice was reduced compared to mock vaccinated mice however this reduction was not statistically significant (p = 0.063) (Figure 2F). Taken together, these data suggest that IM vaccination with NA2 VLPs produced in Tni cells affords increased protection compared to IN vaccination in a lethal H3N2 IAV challenge model.
Figure 2:
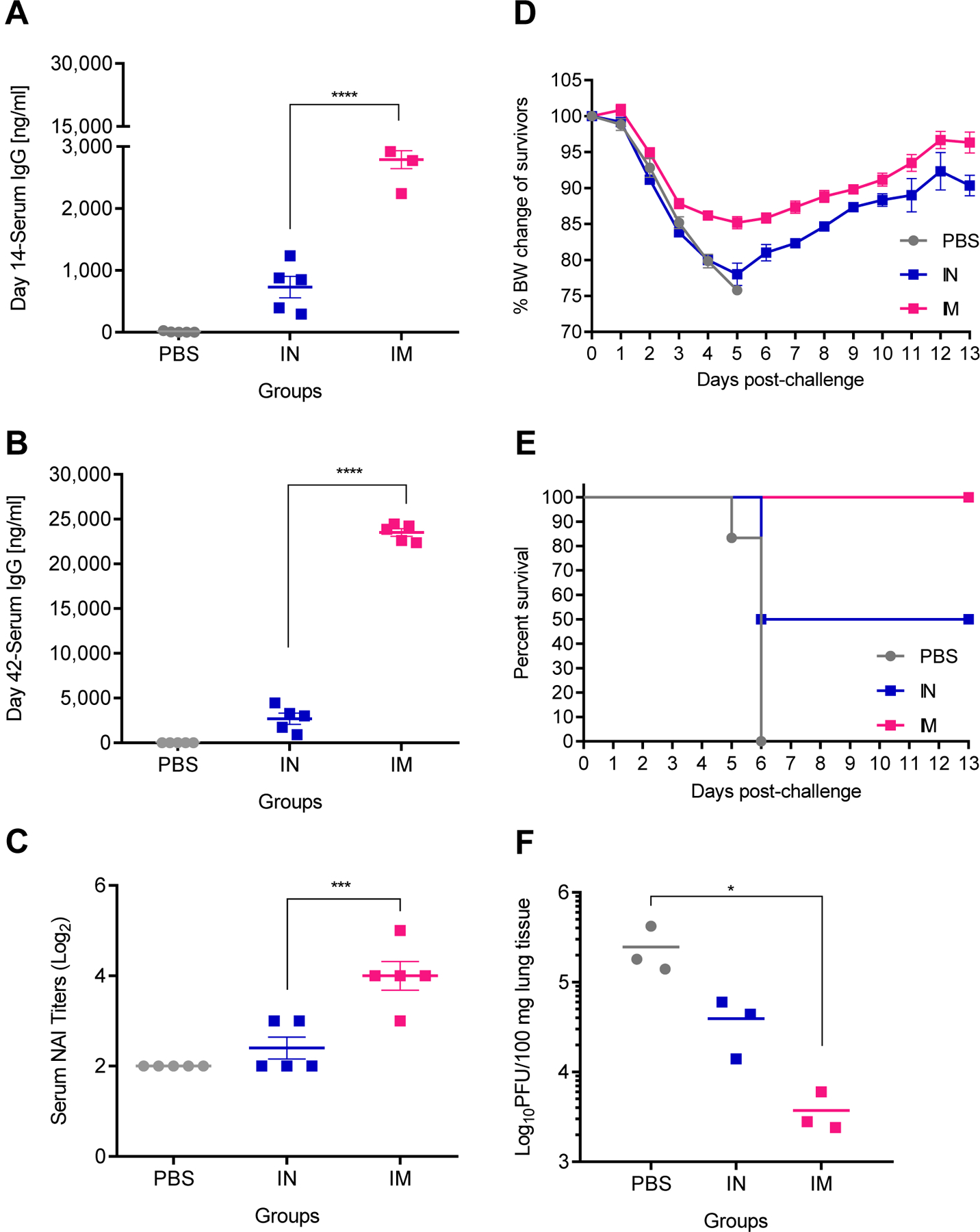
The route of administration affects immune responses to NA2 VLPs. Groups of BALB/c mice were vaccinated in a prime-boost regimen 28 days apart with 5 µg total protein of NA2 VLP preparation intramuscularly (IM), intranasally (IN), or mock vaccinated IM with PBS. Mice were then challenged 28 days post-boost vaccination with 5xLD50 mouse adapted A/Hong Kong/1/1968 (H3N2) virus. (A) Total serum IgG responses measured by ELISA at 14 days post-prime vaccination (N=5). (B) Total serum IgG responses measured by ELISA at 14 days post-boost vaccination (N=5) (C) Neuraminidase inhibition (NAI) titers measured 14 days post-boost vaccination (N=5). (D) Body weight changes of mice after challenge with heterologous H3N2 virus (N=5). (E) Mortality of mice after challenge with heterologous H3N2 virus (N=5). (F) Viral titers of lung homogenates harvested at 4 days post infection (N=3). Error bars represent SEM. *= p<0.05; ***=p<0.001; ****=p<0.0001.
Vaccination with NA2 VLPs has a dose dependent effect on serum IgG levels and morbidity after challenge
Previous work has used varying doses of NA VLPs produced using Sf9 insect cell expression systems ranging from 5 µg – 10 µg per dose in murine models(Kim et al., 2019; Quan et al., 2012). In our IM model of vaccination, all mice displayed signs of morbidity in the form of weight loss at a dose of 5 µg of total protein (Figure 2D). In an effort to improve upon the observed morbidity after challenge, we next sought to optimize the dose of NA VLPs in our IM vaccination regimen. Mice were prime-boost vaccinated 28 days apart followed by lethal challenge using 5xLD50 H3N2 virus 28 days after boost. Sera was collected 14 days post each vaccination and lung tissues were harvested 4 days post-infection. Four groups of mice were vaccinated IM with 1 µg, 5 µg, 10 µg, or 15 µg of total protein of NA2 VLPs. One group was mock vaccinated with 1xPBS to serve as controls.
Post-prime vaccination, all mice vaccinated with NA2 VLPs showed elevated anti-NA2 serum IgG levels compared to mock vaccinated control mice, with the 15 µg dose yielding a statistically significant total serum IgG concentration compared to both the 1 µg and 5 µg dose (Figure 3A). However, post-boost vaccination, serum IgG levels of mice vaccinated with 10 µg and 15 µg of NA2 VLPs were approximately 1.6 fold and 2.9 fold higher than the groups vaccinated with 5 µg and 1 µg of NA2 VLPs, respectively (Figure 3B). Interestingly, increasing the dose to 15 µg from 10 µg of NA2 VLPs did not result in a significant increase in mean serum anti-NA2 IgG levels (p = 0.98). Similar to the previous experiment investigating the route of administration, functional anti-NA2 antibody responses followed the same trend as the total serum anti-NA2 IgG levels. NAI titers increased with increasing doses of NA2 VLPs, however a plateau was observed at the 10 µg dose with no significant increase observed in total NAI titer at a 15 µg dose (p = 0.8272) (Figure 3C). Along these lines, observed morbidity decreased with an increasing dose of NA2 VLPs with a plateau observed when increasing from a 10 µg dose to a 15 µg dose of NA2 VLPs (Figure 3D). All mice experienced morbidity at all doses examined, however increasing the dose from 5 µg to 10 µg decreased the mean peak weight loss from 15.2% to 9.6%, respectively. No decrease in mean peak weight loss was observed when increasing the dose from 10 µg to 15 µg of total NA2 VLPs. While all groups experienced morbidity, mice vaccinated with a dose of 5 µg of total NA2 VLPs or higher were completely protected from mortality (Figure 3E). One individual animal vaccinated with a 1 µg dose did reach the experimental endpoint and was humanely euthanized 7 dpi, while all mice receiving the mock PBS vaccination reached the experimental endpoint and were humanely euthanized by 6 dpi (Figure 3E). Similar to the serology and challenge data, the 10 µg and 15 µg dose groups showed over a 1.5 log reduction in mean viral lung titers compared to mock vaccinated control mice, with no statistically significant difference between these groups, whereas the 5 µg dose group demonstrated an approximate 1 log reduction in viral titers (Figure 3F). While increasing the dose of NA2 VLPs resulted in a dose dependent reduction in mean viral titers from 1 µg up to a 10 µg dose, no statistical difference was observed between the vaccinated groups given the small sample size.
Figure 3:
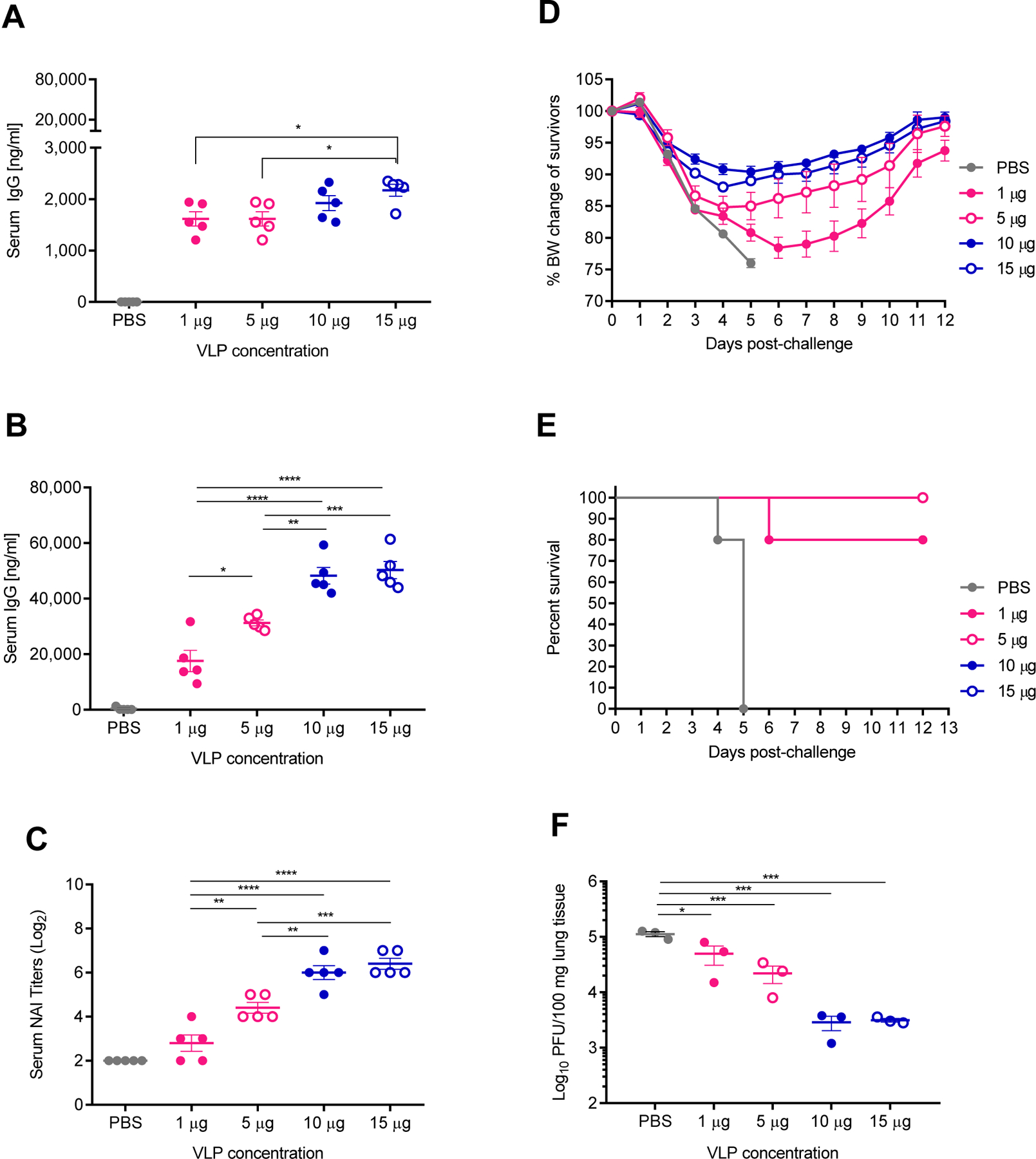
Dose response to intramuscular (IM) vaccination with differing amounts of NA2 VLPs. Groups of BALB/c mice were vaccinated IM in a prime-boost regimen 28 days apart with 1 µg, 5 µg, 10 µg, or 15 µg total protein of NA2 VLP preparation or mock vaccinated with PBS. Mice were then challenged 28 days post-boost vaccination with 5xLD50 mouse adapted A/Hong Kong/1/1968 (H3N2) virus. (A) Total serum IgG responses measured by ELISA at 14 days post-prime vaccination (N=5). (B) Total serum IgG responses measured by ELISA at 14 days post-boost vaccination (N=5) (C) Neuraminidase inhibition (NAI) titers measured 14 days post-boost vaccination (N=5). (D) Body weight changes of mice after challenge with heterologous H3N2 virus (N=5). (E) Mortality of mice after challenge with heterologous H3N2 virus (N=5). (F) Viral titers of lung homogenates harvested at 4 days post infection (N=3). Error bars represent SEM. *= p<0.05; **=p<0.01; ***=p<0.001; ****=p<0.0001.
These data suggest that increasing the dose from 5 µg to 10 µg of total protein of NA2 VLP preparation reduces viral replication in lung tissues and reduces morbidity observed in our lethal H3N2 challenge model. Conversely, reducing the dose to 1 µg of NA2 VLPs resulted in a 0.5 log reduction in viral lung titers but only 80% survival after lethal challenge. Lastly, while positive effects were observed increasing the dose from 5 µg to 10 µg, no additional statistical benefit was observed by increasing the dose further to 15 µg of NA2 VLPs. These results suggest that a 10 µg dose of total protein of NA2 VLP preparation is the optimal dose in this challenge model.
Vaccination with NA2 VLPs was not protective in a heterosubtypic H1N1 lethal challenge model
It has been previously reported that vaccination with NA1 VLPs produced in an Sf9 insect cell expression system protected against mortality but not morbidity in a heterosubtypic, lethal H3N2 murine challenge model (Kim et al., 2019; Quan et al., 2012). However, the efficacy of NA2 VLPs against H1N1 viruses has not been previously investigated. To this end, we sought to investigate the ability of NA2 VLPs to protect mice at various doses against a lethal challenge using 5xLD50 of A/California/04/2009 (H1N1).
In this experiment, all mice were prime-boost vaccinated with 1 µg, 5 µg, 10 µg, 15 µg of NA2 VLPs or mock vaccinated with 1xPBS. All mice experienced morbidity in the form of rapid weight loss following lethal H1N1 virus infection (Figure 4A). Furthermore, all mice in all groups reached experimental endpoints by day 9 post infection and were humanely euthanized (Figure 4B). Based on these data, vaccination with NA2 VLPs did not provide protection against morbidity or mortality at any dose when challenged with a lethal dose of heterosubtypic H1N1 virus.
Figure 4:
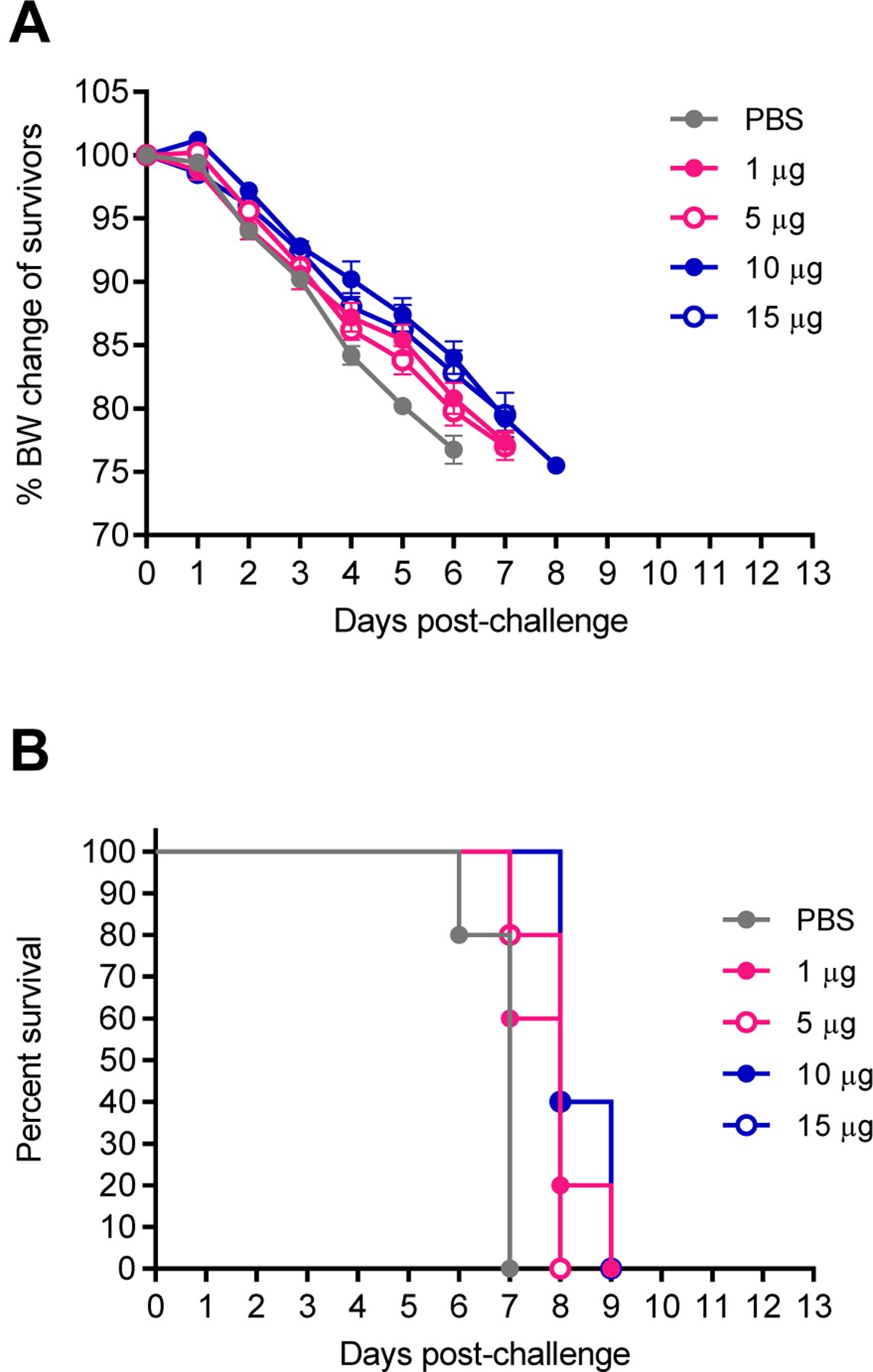
Intramuscular vaccination with differing amounts of NA2 VLPs in a heterosubtypic H1N1 challenge model. Groups of BALB/c mice were intramuscularly (IM) vaccinated in a prime-boost regimen 28 days apart with 1 µg, 5 µg, 10 µg, or 15 µg total protein of NA2 VLP preparation or mock vaccinated with PBS. Mice were then challenged 28 days post-boost vaccination with 5xLD50 mouse adapted A/California/04/2009 (H1N1) virus. (A) Body weight changes of mice after challenge with heterosubtypic H1N1 virus. (B) Mortality of mice after challenge with heterosubtypic H1N1 virus. N=5 mice per group. Error bars represent SEM.
Multivalent vaccination with NA1 and NA2 VLPs efficiently induce both anti-N1 and anti-N2 antibodies
As vaccination with NA2 VLPs alone did not confer protection from morbidity or mortality in a heterosubtypic H1N1 challenge model, we next asked if simultaneous vaccination with NA1 and NA2 VLPs in a multivalent vaccine would afford to protection against both an H1N1 and H3N2 IAV. While attempting to co-express both NA1 and NA2 antigens in a single host cell to create multivalent VLPs is an attractive approach capable of potentially enriching cross-reactive B cell epitopes in germinal center reactions, controlling for equivalent expression of both NA1 and NA2 antigens in a single preparation presents unique technical challenges. Along these lines, we blended NA1 and NA2 VLPs together after production to create an equal mixture of both VLP preparations. Previous work has identified potential intramolecular dominance of specific antigens and epitopes in multivalent vaccines with a biased response observed towards specific antigens in vaccine formulations (Schutze et al., 1989). To determine if inclusion of both NA1 and NA2 VLPs administered concomitantly in a multivalent form induced a biased immune response towards either group 1 or group 2 neuraminidases, we next examined sera from mice vaccinated with a multivalent NA1 + NA2 VLP vaccine. Groups of BALB/c mice were prime-boost vaccinated 28 days apart with 10 µg of a multivalent NA1 + NA2 vaccine (5 µg NA1 VLPs plus 5 µg NA2 VLPS), 10 µg of monovalent NA1 VLPs, 10 µg of monovalent NA2 VLPs, or mock vaccinated with 1xPBS. Mice were bled 14 days post-prime vaccination and 14 days post-boost vaccination to assess serological responses to both NA1 and NA2 antigen.
As expected, mock vaccination with 1xPBS produced minimal or undetectable serum IgG responses against rNA2 post-prime and post-boost vaccination (Figures 5A and 5B). While mean anti rNA2 serum IgG levels were slightly elevated post-boost vaccination with NA1 VLPs, this difference was not statistically significant (p = 0.4245) (Figure 5B). Furthermore, the commercially sourced rNA2 coating antigen was produced using a baculovirus expression system and purified via metal affinity chromatography, potentially leading to contaminating insect cell and baculovirus proteins contributing to background non-NA2 IgG reactivity in serum samples. Multivalent vaccination with NA1 + NA2 VLPs afforded an equivalent response against rNA2 to monovalent vaccination with NA2 VLPs alone (Figure 5A). Post-boost, vaccination with NA1 + NA2 VLPs demonstrated an increased anti-NA2 IgG response compared to monovalent vaccination with NA2 VLPs (Figure 5B). Similar to previous experiments, total IgG levels correlated with an increased functional anti-NA titer. Specifically, mice receiving multivalent NA1 + NA2 VLPs had higher functional NAI titers against NA2 VLPs than mice vaccinated with monovalent NA2 VLPS (Figure 5C). Based on these data, multivalent vaccination with NA1 + NA2 VLPs induced a superior IgG response against group 2 NA.
Figure 5:
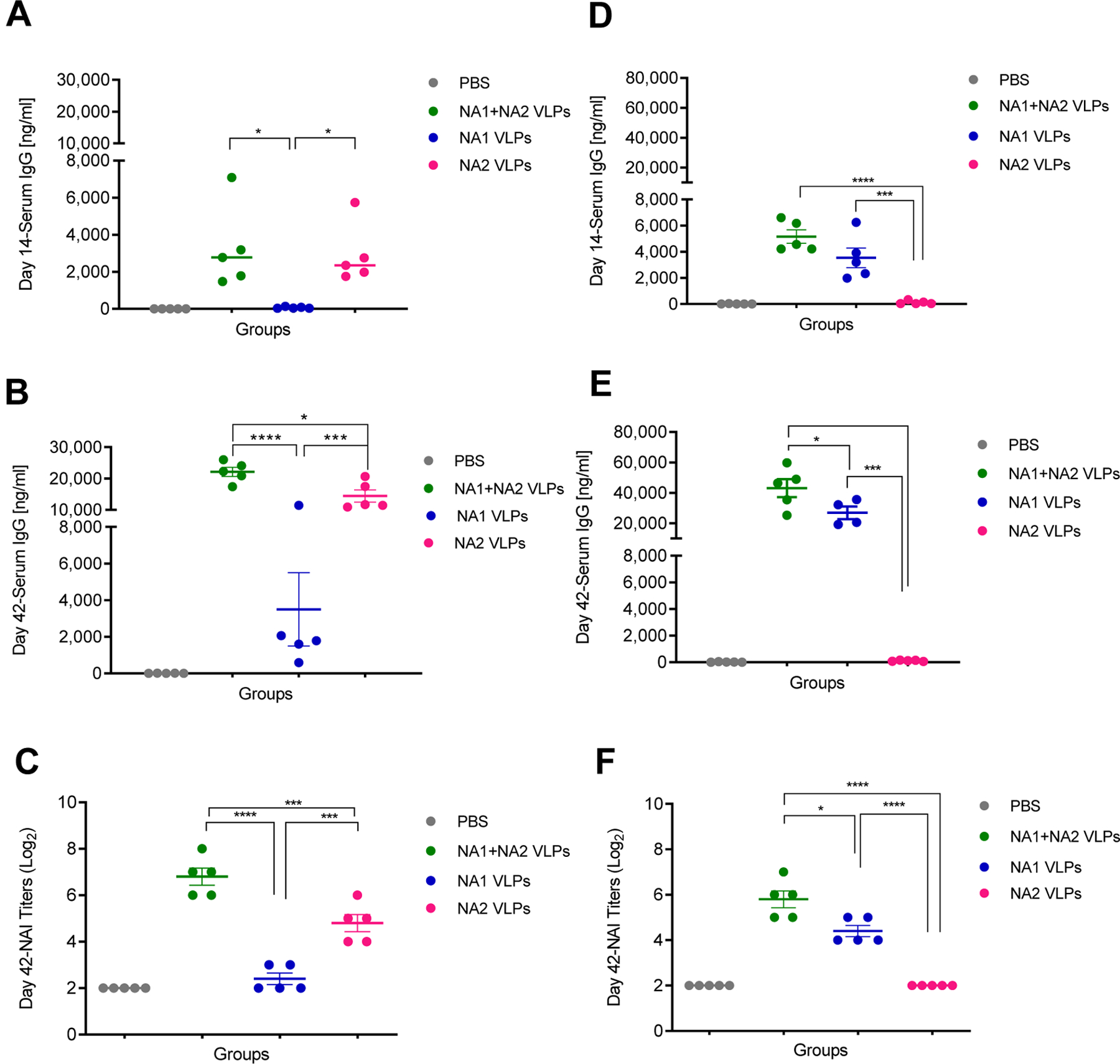
Serological responses to the combined administration of NA1 + NA2 VLPs. Groups of BALB/c mice were vaccinated IM in a prime-boost regimen 28 days apart with 10 µg NA1 + NA2 VLPs (5 µg NA1 VLPs + 5 µg NA2 VLPs), 10 µg NA1 VLPs, 10 µg NA2 VLPs or mock vaccinated with PBS. (A) Total serum anti NA2 IgG responses measured by ELISA at 14 days post-prime vaccination. (B) Total serum anti NA2 IgG responses measured by ELISA at 14 days post-boost vaccination. (C) Neuraminidase inhibition (NAI) titers against NA2 VLPs measured 14 days post-boost vaccination. (D) Total serum anti NA1 IgG responses measured by ELISA at 14 days post-prime vaccination. (E) Total serum anti NA1 IgG responses measured by ELISA at 14 days post-boost vaccination. (F) NAI titers against NA1 VLPs measured 14 days post-boost vaccination. N=5 mice per group. Error bars represent SEM. *= p<0.05; ***=p<0.001; ****=p<0.0001.
Similarly, vaccination with 1xPBS or NA2 VLPs did not produce an appreciable serum IgG response against rNA1 post-prime or post-boost vaccination (Figures 5D and 5E). However, mean serum anti-N1 IgG levels were elevated post-boost vaccination in mice vaccinated with NA1+NA2 VLPs compared to vaccination with NA1 VLPs alone (Figure 5E). Likewise, sera collected post-boost vaccination from mice receiving either 1xPBS or NA2 VLPs did not exhibit any inhibition of NA1 neuraminidase (Figure 5F). Conversely, sera from mice vaccinated with NA1 + NA2 VLPs exhibited a significant increase in mean NAI titers compared to mice vaccinated with NA1 VLPs in a monovalent form. Similar to the serology results against group 2 NA, these data suggest that multivalent vaccination with NA1 + NA2 VLPs induced a superior IgG response against group 1 NA.
Multivalent vaccination with NA1 and NA2 VLPs is protective against lethal challenge with IAVs containing group 1 or group 2 neuraminidases
As vaccination with multivalent NA1 + NA2 VLPs appeared to have a positive effect on anti-NA IgG responses (Figure 5), we next sought to investigate the protection after lethal challenge with H1N1 and H3N2 IAVs. Mice were IM vaccinated in a prime-boost regimen with 10 µg total protein of NA1 VLPs, NA2 VLPs, a combination of NA1 (5 µg) + NA2 (5 µg) VLPs, or mock vaccinated with 1xPBS. All groups were challenged 28 days post-boost vaccination with either 5xLD50 of H1N1 or H3N2 virus and monitored for morbidity in the form of weight loss, mortality, and for viral replication in lung tissues.
After challenge with H3N2 virus, all mice mock vaccinated with 1xPBS or vaccinated with NA1 VLPs showed severe morbidity (Figure 6A) and were humanely euthanized after reaching experimental endpoints by day 7 and day 8 post infection, respectively (Figure 6B). Similar to previous experiments, all mice vaccinated with either the monovalent NA2 VLPs or the multivalent NA1 + NA2 VLPs experienced minor morbidity with peak mean weight loss of approximately 8% and 5%, respectively (Figure 6A). However, all mice were completely protected from mortality when administered NA2 VLPs or NA1 + NA2 VLPs (Figure 6B). Notably, vaccination with NA1 + NA2 VLPs or NA2 VLPs alone resulted in an approximate 2.5 log and 1.5 log reduction in viral lung titers compared to mock PBS vaccination, respectively (Figure 6C). Vaccination with NA1 VLPs did not lead to a reduction in viral lung titers compared to the mock vaccinated group (Figure 6C). Vaccination with NA1 + NA2 VLPs did reduce the mean viral lung titer compared to monovalent vaccination with NA2 VLPs, although this reduction was not statistically significant (p = 0.0828). Taken together, these results demonstrate that multivalent vaccination with NA1 + NA2 VLPs protected mice against mortality after lethal challenge with heterologous H3N2 IAV.
Figure 6:
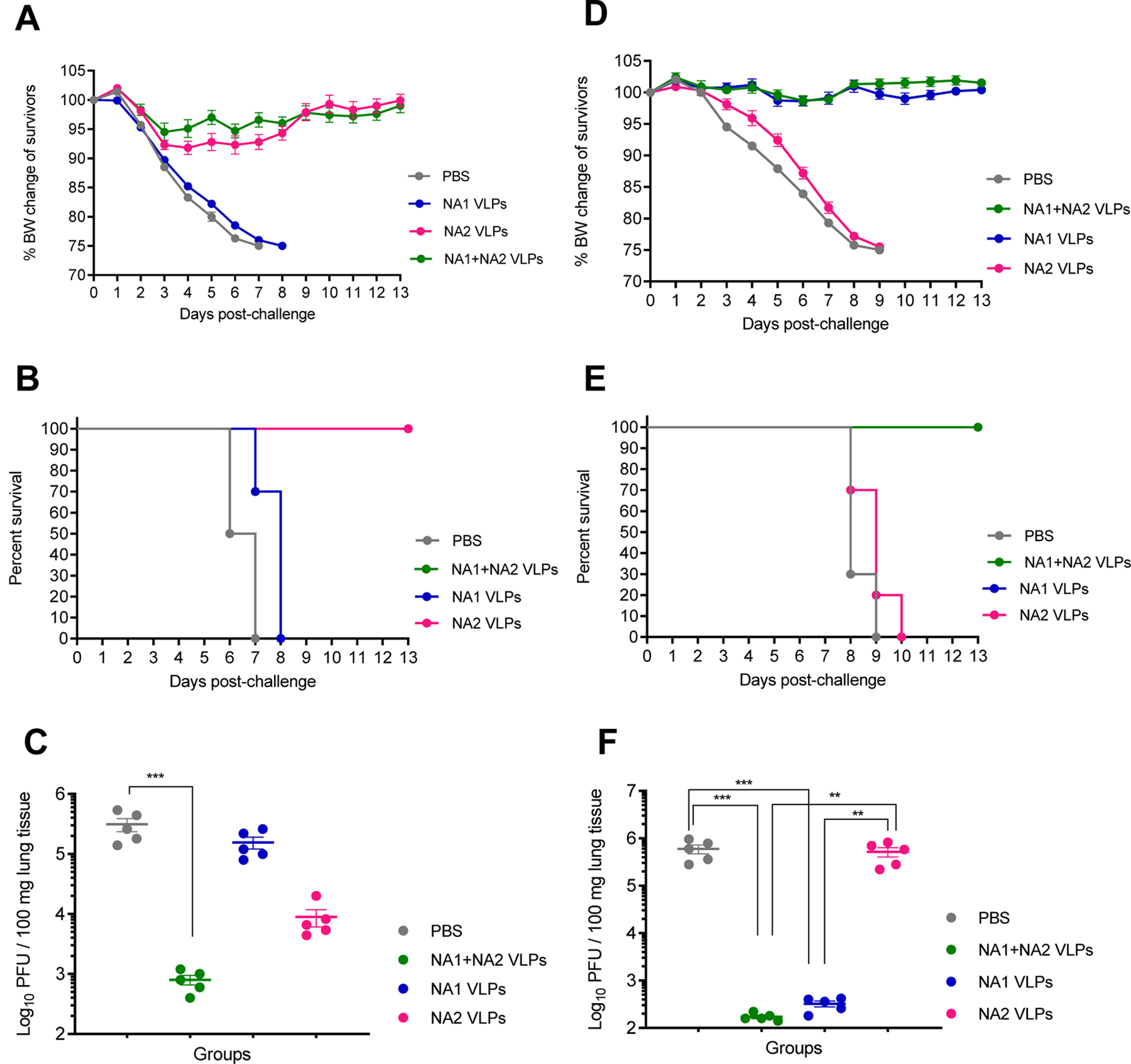
Lethal challenge of mice vaccinated with combined administration of NA1 + NA2 VLPs using H3N2 and H1N1 viruses. Groups of BALB/c mice were vaccinated IM in a prime-boost regimen 28 days apart with 10 µg NA1 + NA2 VLPs (5 µg NA1 VLPs + 5 µg NA2 VLPs), 10 µg NA1 VLPs, 10 µg NA2 VLPs or mock vaccinated with PBS. Mice were then challenged 28 days post-boost vaccination with either 5xLD50 mouse adapted A/Hong Kong/1/1968 (H3N2) virus or 5xLD50 mouse adapted A/California/04/2009 (H1N1) virus. (A) Body weight changes of mice after challenge with H3N2 virus (N=10). (B) Mortality of mice after challenge with H3N2 virus (N=10). (C) Viral titers of lung homogenates harvested at 4 days post infection with H3N2 virus (N=5). (D) Body weight changes of mice after challenge with H1N1 virus (N=10). (E) Mortality of mice after challenge with H1N1 virus (N=10). (F) Viral titers of lung homogenates harvested at 4 days post-infection with H1N1 virus (N=5). Error bars represent SEM. **=p<0.01; ***=p<0.001.
All mice vaccinated with 1xPBS or NA2 VLPs experienced weight loss (Figure 6D) and were humanely euthanized after reaching experimental endpoints after 9 and 10 days post infection with H1N1 virus, respectively (Figure 6E). Conversely, all mice vaccinated with monovalent NA1 VLPs or multivalent NA1 + NA2 VLPs were fully protected against homologous H1N1 IAV, with no signs of morbidity (Figure 6D) and no mortality (Figure 6E) throughout the observation period. Similarly, administration of both NA1 VLPs and NA1 + NA2 VLPs led to an equivalent approximate 3.5 log reduction in viral lung titers compared to mock vaccination with 1xPBS after homologous challenge (Figure 6F). Expectedly, viral lung titers in mice vaccinated with NA2 VLPs did not differ significantly from mice mock vaccinated with 1xPBS after a heterosubtypic challenge with H1N1 IAV (Figure 6F). Based on these data, the multivalent administration of NA1 + NA2 VLPs is protective against lethal challenge with homologous H1N1 IAV and equivalent to monovalent NA1 VLPs alone.
Discussion
Currently licensed influenza vaccines both target and predominantly induce antibody responses against HA glycoproteins with highly variable or non-existent anti-NA responses (Chen et al., 2018; Couch et al., 2012; Laguio-Vila et al., 2015; Powers et al., 1996). However, anti-NA2 responses have been shown to contribute to protection after both infection and immunization in humans (Beutner et al., 1979; Monto and Kendal, 1973; Murphy et al., 1972). Previous works investigating the role of NA VLPs as candidate IAV vaccines have focused on using NA1 as the immunizing antigen (Easterbrook et al., 2012; Kim et al., 2019; Quan et al., 2012; Smith et al., 2017), however none have investigated the role of only NA2 as an immunizing antigen in an insect cell based NA VLP expression system. Here, we used a murine model to demonstrate that IM vaccination with NA2 VLPs containing the NA2 antigen from A/Perth/16/2009 provided protection against a distant heterologous mouse adapted H3N2 virus (A/Hong Kong/1/1968) but failed to protect mice in a lethal heterosubtypic challenge with H1N1 virus (A/California/04/2009). However, bivalent vaccination with NA1 and NA2 VLPs induced stronger humoral immune responses against both NA1 and NA2 antigens as well as an overall decrease in morbidity and viral lung titers in our H3N2 challenge model. High anti-NA serum levels measured by ELISA and NAI have correlated with protection in a ferret and murine models of infection (Quan et al., 2012; Smith et al., 2017; Walz et al., 2018) and as an independent correlate of protection in humans (Monto et al., 2015). Additionally, passive transfer of anti-NA immune sera to naïve animals has been demonstrated to be protective in murine challenge models suggesting that humoral immunity is sufficient to confer protection (Walz et al., 2018; Wohlbold et al., 2015a). In this study, total IgG levels and NAI titers correlated with protection of mice against morbidity and mortality as well as reducing viral replication after lethal challenge with IAV.
The majority of trivalent and quadrivalent inactivated split virus vaccines and recombinant purified HA protein vaccines currently licensed for use are administered intramuscularly. Additionally, a live attenuated influenza vaccine is licensed for IN administration. In this study, we compared both routes of administration for NA2 VLPs. Previous work has demonstrated protection against both homologous and heterosubtypic challenge using NA1 VLPs via both the IM and IN routes (Kim et al., 2019; Quan et al., 2012). However, IN vaccination did not offer complete protection against mortality in our heterologous challenge model. Of note, NA VLPs used in this study were produced in Tni insect cells where previous work with NA VLPs in insect cell expression systems used Sf9 expression systems (Kim et al., 2019; Quan et al., 2012; Smith et al., 2017). Vaccine production platforms require the ability to scale to produce adequate supply and Tni cells offer an attractive expression system for influenza VLPs with their increased VLP protein yields over Sf9 cells while minimizing contaminating baculovirus production (Krammer et al., 2010). However, local activation of the innate immune system by residual, contaminating baculovirus has been shown to increase the immunogenicity and subsequent survival after vaccination with influenza VLPs in a murine model (Heinimäki et al., 2017; Margine et al., 2012). Previous work has also shown that IN vaccination with a purified recombinant NA combined with an adjuvant afforded superior protection compared with IM vaccination using the same NA protein in a murine model (Wohlbold et al., 2015a), and that the incorporation of membrane bound adjuvants into VLPs has demonstrated increased immunogenicity after vaccination against influenza and other viruses (Liu et al., 2018; Skountzou et al., 2007). Further studies are needed to determine if the addition of adjuvants could improve the immunogenicity of both IN and IM administered NA VLPs produced in Tni cells.
Licensed influenza vaccines contain both an H1N1 and H3N2 IAV strain in addition to one or two Influenza B strains and, therefore, do not need to rely on heterosubtypic cross protection for efficacy. Multiple previous studies have shown that vaccination with NA1 VLPs was completely protective against mortality, but not morbidity, after challenge with a heterosubtypic H3N2 virus (Kim et al., 2019; Quan et al., 2012). Conversely, other studies vaccinating with purified recombinant NA have demonstrated homosubtypic but not heterosubtypic protection (Deroo et al., 1996; Wohlbold et al., 2015b). While the NA2 VLPs produced in the current study were protective against a heterologous homosubtypic H3N2 virus challenge, the NA2 VLPs afforded no protection against morbidity or mortality in a lethal heterosubtypic H1N1 virus challenge (Figure 4A and 4B). However, vaccination with a bivalent NA1 and NA2 VLPs preparation did offer protection against morbidity and mortality in both H3N2 and H1N1 challenge models. Similar to previous work examining the administration of a bivalent mixture of NA1 and NA2 purified proteins, we did not observe any antigenic competition between the NA1 or NA2 subtypes when both antigens were administered concomitantly (Johansson and Kilbourne, 1994). Conversely, we observed an increase in total mean IgG levels and NAI titers against each individual antigen similar to that observed by Johansson and Kilbourne, suggesting possible common B cell or T cell epitopes between subtypes. Indeed, previous work has identified shared B cell epitopes between NA subtypes and multiple monoclonal antibodies specific for epitopes that bind to and inhibit multiple NA subtypes (Doyle et al., 2013; Gravel et al., 2010; Rijal et al., 2019). Specifically, Doyle et al. identified a monoclonal antibody specific for the universally conserved influenza A NA active site epitope “ILRTQESEC” capable of inhibiting NA activity in 9 different subtypes. It is also possible that shared T cell epitopes between NA subtypes contribute to increased positive selection during B cell germinal center affinity maturation leading to increased IgG levels observed in this study. Studies aimed at generating broadly neutralizing antibodies targeting the conserved HA stalk have taken the approach of repeatedly vaccinating with the same HA stalk with different chimeric irrelevant globular HA heads included in each subsequent vaccination (Krammer et al., 2013). This approach has yielded an increase in antibodies targeting the shared epitopes presented in each subsequent vaccination and afforded protection independent of CD8 T cells. The specific mechanisms responsible for enrichment of both total IgG and functional anti-NA antibodies warrant further investigation. Investigation of broadly reactive anti-NA antibodies capable of inhibiting multiple subtypes will require further research investigating antibody responses at a monoclonal level while the contribution of potential shared T cell epitopes will require further studies to map T cell epitopes for individual NA molecules. Nonetheless, functional anti-NA immunity and its subsequent contribution to protection against morbidity caused by influenza virus infection appears to be a useful and underappreciated correlate of protection.
There is evidence that when HA and NA are presented together in the same virus particle, the immune response to HA is immunodominant over the NA response in priming both B and T lymphocytes (Johannsson et al., 1987). More recent work has improved the immunogenicity of the NA molecule by extending the length of the NA stalk by as few as 15 or 30 amino acids to potentially improve recognition by B cell receptors of infected hosts (Broecker et al., 2019). Vaccination with NA VLPs where NA is presented on an individual particle in the absence of HA molecules has the potential to overcome the limitation of HA immunodominance. However, other work has shown that while both N1 VLPs and H5N1 VLPs protected against mortality in a homologous lethal H5N1 ferret challenge model, the experimental group vaccinated with H5N1 VLPs containing both the homologous HA and NA showed a reduction in morbidity and nasal wash titers after challenge compared to the group vaccinated with N1 VLPs alone (Smith et al., 2017). While the role of anti-HA immunity and its ability to prevent virus infection has long been appreciated in homologous challenge models, the additional supplementation of seasonal influenza vaccines with purified NA has been shown augment the anti-NA immune response and increase the breadth of immunity against potentially drifted influenza viruses (Johansson et al., 2002, 1998; Kim et al., 2017). Indeed, individuals with higher anti-NA2 antibody titers developed after infection with circulating H2N2 viruses were less likely to be infected upon emergence of the 1968 Hong Kong H3N2 virus into the human population (Monto and Kendal, 1973; Murphy et al., 1972). Furthermore, anti-NA1 antibody titers have been previously credited with lessening the severity of the 2009 H1N1 pandemic (Marcelin et al., 2011). However, more recent work has shown that current seasonal vaccine responses are dominated by anti-HA recall responses with very poor anti-NA responses (Chen et al., 2018). NA VLPs offer a platform to supplement existing strategies already effective at eliciting anti-HA immune responses while presenting the NA molecule to the host immune system in its native, membrane bound conformation.
Taken together, the data from this study suggest that bivalent vaccination with NA1 and NA2 VLPs results in a balanced immune response against both group 1 and group 2 neuraminidases capable of protecting mice from mortality and reducing viral replication in both an H1N1 and H3N2 challenge model. Furthermore, these data show that there is no deleterious effect from antigenic competition between the two different NA subtypes in our murine model. While murine models of influenza infection are inexpensive and well established, mice are not natural hosts for IAVs. Expanding these studies to a ferret or swine model of IAV infection would be a logical next step and provide valuable insight into the applicability of these data in an outbred animal model.
Research Highlights.
Neuraminidase 2 virus-like particle vaccination protected mice against H3N2
Vaccinating mice with neuraminidase 1 and 2 together improved responses in mice
Influenza vaccines could benefit from a balanced neuraminidase response
Acknowledgements
Special thank you to Hong Yi of the Robert P. Apkarian Integrated Electron Microscopy Core of Emory University for technical assistance with electron microscopy in this publication.
Funding Support
This work was supported by the National Institutes of Health (1R01 AI110680–04) and BAA-NIAID-DMID-NIH (HHSN272201400004C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL, 1979. Evaluation of a neuraminidase-specific influenza a virus vaccine in children: Antibody responses and effects on two successive outbreaks of natural infection. J. Infect. Dis 140, 844–850. 10.1093/infdis/140.6.844 [DOI] [PubMed] [Google Scholar]
- Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, Kumar NM, Pushko P, Smith G, Tumpey TM, Ross TM, 2008. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One 3. 10.1371/journal.pone.0001501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broecker F, Zheng A, Suntronwong N, Sun W, Bailey MJ, Krammer F, Palese P, 2019. Extending the Stalk Enhances Immunogenicity of the Influenza Virus Neuraminidase. J. Virol 93, 4–9. 10.1128/jvi.00840-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Wohlbold TJ, Zheng NY, Huang M, Huang Y, Neu KE, Lee J, Wan H, Rojas KT, Kirkpatrick E, Henry C, Palm AKE, Stamper CT, Lan LYL, Topham DJ, Treanor J, Wrammert J, Ahmed R, Eichelberger MC, Georgiou G, Krammer F, Wilson PC, 2018. Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 173, 417–429.e10. 10.1016/j.cell.2018.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kim L, Subbarao K, Jin H, 2012. The 2009 pandemic H1N1 virus induces anti-neuraminidase (NA) antibodies that cross-react with the NA of H5N1 viruses in ferrets. Vaccine 30, 2516–22. 10.1016/j.vaccine.2012.01.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RB, Atmar RL, Keitel WA, Quarles JM, Wells J, Arden N, Niño D, 2012. Randomized comparative study of the serum antihemagglutinin and antineuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine 31, 190–195. 10.1016/j.vaccine.2012.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch RB, Kasel JA, Gerin JL, Schulman JL, Kilbourne ED, 1974. Induction of partial immunity to influenza by a neuraminidase specific vaccine. J. Infect. Dis 129, 411–420. 10.1093/infdis/129.4.411 [DOI] [PubMed] [Google Scholar]
- Deroo T, Min Jou W, Fiers W, 1996. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine 14, 561–569. 10.1016/0264-410X(95)00157-V [DOI] [PubMed] [Google Scholar]
- Doyle TM, Hashem AM, Li C, Van Domselaar G, Larocque L, Wang J, Smith D, Cyr T, Farnsworth A, He R, Hurt AC, Brown EG, Li X, 2013. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antiviral Res 100, 567–574. 10.1016/j.antiviral.2013.09.018 [DOI] [PubMed] [Google Scholar]
- Easterbrook JD, Schwartzman LM, Gao J, Kash JC, Morens DM, Couzens L, Wan H, Eichelberger MC, Taubenberger JK, 2012. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 432, 39–44. 10.1016/j.virol.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser ES, Romanyuk AA, Vassilieva EV, Jacob J, Prausnitz MR, Compans RW, Skountzou I, 2016. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy. J. Control. Release 236, 47–56. 10.1016/j.jconrel.2016.06.026 [DOI] [PubMed] [Google Scholar]
- Flannery B, Chung JR, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Rolfes MA, Spencer S, Fry AM, 2019. Influenza Vaccine Effectiveness in the United States during the 2016–2017 Season. Clin. Infect. Dis 68, 1798–1806. 10.1093/cid/ciy775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery B, Kondor RJG, Chung JR, Gaglani M, Reis M, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Martin ET, Belongia EA, McLean HQ, Kim SS, Blanton L, Kniss K, Budd AP, Brammer L, Stark TJ, Barnes JR, Wentworth DE, Fry AM, Patel M, 2020. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J. Infect. Dis 221, 8–15. 10.1093/INFDIS/JIZ543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel C, Li C, Wang J, Hashem AM, Jaentschke B, Xu K. wei, Lorbetskie B, Gingras G, Aubin Y, Van Domselaar G, Girard M, He R, Li X, 2010. Qualitative and quantitative analyses of virtually all subtypes of influenza A and B viral neuraminidases using antibodies targeting the universally conserved sequences. Vaccine 28, 5774–5784. 10.1016/j.vaccine.2010.06.075 [DOI] [PubMed] [Google Scholar]
- Grohskopf LA, Shay DK, Shimabukuro TT, Sokolow LZ, Keitel WA, Bresee JS, Cox NJ, 2013. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013–2014. MMWR. Morb. Mortal. Wkly. Rep 62, 1–48. [PMC free article] [PubMed] [Google Scholar]
- Harris A, Cardone G, Winkler DC, Heymann JB, Brecher M, White JM, Steven AC, 2006. Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc. Natl. Acad. Sci 103, 19123–19127. 10.1073/pnas.0607614103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinimäki S, Tamminen K, Malm M, Vesikari T, Blazevic V, 2017. Live baculovirus acts as a strong B and T cell adjuvant for monomeric and oligomeric protein antigens. Virology 511, 114–122. 10.1016/j.virol.2017.08.023 [DOI] [PubMed] [Google Scholar]
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson MA, Bresee JS, Azziz-Baumgartner E, Cheng PY, Dawood F, Foppa I, Olsen S, Haber M, Jeffers C, MacIntyre CR, Newall AT, Wood JG, Kundi M, Popow-Kraupp T, Ahmed M, Rahman M, Marinho F, Sotomayor Proschle CV, Vergara Mallegas N, Luzhao F, Sa L, Barbosa-Ramírez J, Sanchez DM, Gomez LA, Vargas XB, Acosta Herrera a. B., Llanés MJ, Fischer TK, Krause TG, Mølbak K, Nielsen J, Trebbien R, Bruno A, Ojeda J, Ramos H, an der Heiden M, del Carmen Castillo Signor L, Serrano CE, Bhardwaj R, Chadha M, Narayan V, Kosen S, Bromberg M, Glatman-Freedman A, Kaufman Z, Arima Y, Oishi K, Chaves S, Nyawanda B, Al-Jarallah RA, Kuri-Morales PA, Matus CR, Corona MEJ, Burmaa A, Darmaa O, Obtel M, Cherkaoui I, van den Wijngaard CC, van der Hoek W, Baker M, Bandaranayake D, Bissielo A, Huang S, Lopez L, Newbern C, Flem E, Grøneng GM, Hauge S, de Cosío FG, de Moltó Y, Castillo LM, Cabello MA, von Horoch M, Medina Osis J, Machado A, Nunes B, Rodrigues AP, Rodrigues E, Calomfirescu C, Lupulescu E, Popescu R, Popovici O, Bogdanovic D, Kostic M, Lazarevic K, Milosevic Z, Tiodorovic B, Chen M, Cutter J, Lee V, Lin R, Ma S, Cohen AL, Treurnicht F, Kim WJ, Delgado-Sanz C, de mateo Ontañón S, Larrauri A, León IL, Vallejo F, Born R, Junker C, Koch D, Chuang JH, Huang WT, Kuo HW, Tsai YC, Bundhamcharoen K, Chittaganpitch M, Green HK, Pebody R, Goñi N, Chiparelli H, Brammer L, Mustaquim D, 2018. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391, 1285–1300. 10.1016/S0140-6736(17)33293-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsson BE, Moran TM, Kilbourne ED, 1987. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc. Natl. Acad. Sci. U. S. A 84, 6869–6873. 10.1073/pnas.84.19.6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BE, Kilbourne ED, 1994. Immunization with purified N1 and N2 influenza virus neuraminidases demonstrates cross-reactivity without antigenic competition. Proc. Natl. Acad. Sci. U. S. A 91, 2358–2361. 10.1073/pnas.91.6.2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BE, Kilbourne ED, 1993. Dissociation of influenza virus hemagglutinin and neuraminidase eliminates their intravirionic antigenic competition. J. Virol 67, 5721–5723. 10.1128/jvi.67.10.5721-5723.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BE, Matthews JT, Kilbourne ED, 1998. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine 16, 1009–15. 10.1016/S0264-410X(97)00279-X [DOI] [PubMed] [Google Scholar]
- Johansson BE, Pokorny BA, Tiso VA, 2002. Supplementation of conventional trivalent influenza vaccine with purified viral N1 and N2 neuraminidases induces a balanced immune response without antigenic competition. Vaccine 20, 1670–1674. 10.1016/S0264-410X(01)00490-X [DOI] [PubMed] [Google Scholar]
- Kilbourne ED, Johansson BE, Grajower B, 1990. Independent and disparate evolution in nature of influenza A virus hemagglutinin and neuraminidase glycoproteins. Proc. Natl. Acad. Sci 87, 786–790. 10.1073/pnas.87.2.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Lee YT, Park S, Jung YJ, Lee Y, Ko EJ, Kim YJ, Li X, Kang SM, 2019. Neuraminidase expressing virus-like particle vaccine provides effective cross protection against influenza virus. Virology 535, 179–188. 10.1016/j.virol.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Ko EJ, Kim MC, Lee YN, Kim KH, Jung YJ, Kang SM, 2017. Roles of antibodies to influenza A virus hemagglutinin, neuraminidase, and M2e in conferring cross protection. Biochem. Biophys. Res. Commun 493, 393–398. 10.1016/j.bbrc.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Palese P, 2015. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov 14, 167–182. 10.1038/nrd4529 [DOI] [PubMed] [Google Scholar]
- Krammer F, Palese P, Steel J, 2015. Advances in Universal Influenza Virus Vaccine Design and Antibody Mediated Therapies Based on Conserved Regions of the Hemagglutinin. Curr. Top. Microbiol. Immunol 286, 301–321. 10.1007/82_2014_408 [DOI] [PubMed] [Google Scholar]
- Krammer F, Pica N, Hai R, Margine I, Palese P, 2013. Chimeric Hemagglutinin Influenza Virus Vaccine Constructs Elicit Broadly Protective Stalk-Specific Antibodies. J. Virol 87, 6542–6550. 10.1128/jvi.00641-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Schinko T, Palmberger D, Tauer C, Messner P, Grabherr R, 2010. Trichoplusia ni cells (High FiveTM) are highly efficient for the production of influenza A virus-like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol. Biotechnol 45, 226–234. 10.1007/s12033-010-9268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis CS, Zhang M, Wolf S, Jones LP, Shim BS, Chocallo AH, Hanson JM, Jia MR, Liu D, Tripp RA, 2017. Molecular epidemiology of swine influenza A viruses in the Southeastern United States, highlights regional differences in circulating strains. Vet. Microbiol 211, 174–179. 10.1016/j.vetmic.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguio-Vila MR, Thompson MG, Reynolds S, Spencer SM, Gaglani M, Naleway A, Ball S, Bozeman S, Baker S, Martínez-Sobrido L, Levine M, Katz J, Fry AM, Treanor JJ, 2015. Comparison of serum hemagglutinin and neuraminidase inhibition antibodies after 2010–2011 trivalent inactivated influenza vaccination in healthcare personnel. Open forum Infect. Dis 2, ofu115. 10.1093/ofid/ofu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littauer EQ, Esser ES, Antao OQ, Vassilieva EV, Compans RW, Skountzou I, 2017. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS Pathog 13, e1006757. 10.1371/journal.ppat.1006757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littauer EQ, Mills LK, Brock N, Esser ES, Romanyuk A, Pulit-Penaloza JA, Vassilieva EV, Beaver JT, Antao O, Krammer F, Compans RW, Prausnitz MR, Skountzou I, 2018. Stable incorporation of GM-CSF into dissolvable microneedle patch improves skin vaccination against influenza. J. Control. Release 276, 1–16. 10.1016/j.jconrel.2018.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ren Z, Wang Hongmei, Zhao Y, Wilker PR, Yu Z, Sun W, Wang T, Feng N, Li Y, Wang Hualei, Ji X, Li N, Yang S, He H, Qin C, Gao Y, Xia X, 2018. Influenza virus-like particles composed of conserved influenza proteins and GPI-anchored CCL28/GM-CSF fusion proteins enhance protective immunity against homologous and heterologous viruses. Int. Immunopharmacol 63, 119–128. 10.1016/j.intimp.2018.07.011 [DOI] [PubMed] [Google Scholar]
- Maljkovic Berry I, Melendrez MC, Bishop-Lilly KA, Rutvisuttinunt W, Pollett S, Talundzic E, Morton L, Jarman RG, 2020. Next Generation Sequencing and Bioinformatics Methodologies for Infectious Disease Research and Public Health: Approaches, Applications, and Considerations for Development of Laboratory Capacity. J. Infect. Dis 221, S292–S307. 10.1093/infdis/jiz286 [DOI] [PubMed] [Google Scholar]
- Marcelin G, DuBois R, Rubrum A, Russell CJ, McElhaney JE, Webby RJ, 2011. A contributing role for anti-neuraminidase antibodies on immunity to pandemic h1n1 2009 influenza a virus. PLoS One 6, 14–16. 10.1371/journal.pone.0026335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margine I, Martinez-Gil L, Chou Y. ying, Krammer F, 2012. Residual Baculovirus in Insect Cell-Derived Influenza Virus-Like Particle Preparations Enhances Immunogenicity. PLoS One 7. 10.1371/journal.pone.0051559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT, Taubenberger JK, 2016. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. MBio 7, 1–12. 10.1128/mBio.00417-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Kendal AP, 1973. Effect of Neuraminidase Antibody on Hong Kong Influenza. Lancet 301, 623–625. 10.1016/S0140-6736(73)92196-X [DOI] [PubMed] [Google Scholar]
- Monto AS, Petrie JG, Cross RT, Johnson E, Liu M, Zhong W, Levine M, Katz JM, Ohmit SE, 2015. Antibody to influenza virus neuraminidase: An independent correlate of protection. J. Infect. Dis 212, 1191–1199. 10.1093/infdis/jiv195 [DOI] [PubMed] [Google Scholar]
- Murphy BR, Kasel JA, Chanock RM, 1972. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N. Engl. J. Med 286, 1329–32. 10.1056/NEJM197206222862502 [DOI] [PubMed] [Google Scholar]
- Powers DC, Kilbourne ED, Johansson BE, 1996. Neuraminidase-specific antibody responses to inactivated influenza virus vaccine in young and elderly adults. Clin. Diagn. Lab. Immunol 3, 511–516. 10.1128/cdli.3.5.511-516.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Kim MC, Lee BJ, Song JM, Compans RW, Kang SM, 2012. Influenza M1 VLPs containing neuraminidase induce heterosubtypic cross-protection. Virology 430, 127–135. 10.1016/j.virol.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H, 1938. A Simple Method Of Estimating Fifty Percent Endpoints. Am. J. Epidemiol 27, 493–497. 10.1093/oxfordjournals.aje.a118408 [DOI] [Google Scholar]
- Rijal P, Wang BB, Tan TK, Schimanski L, Janesch P, Dong T, McCauley JW, Daniels RS, Townsend AR, Huang KYA, 2019. Broadly inhibiting anti-neuraminidase monoclonal antibodies induced by trivalent influenza vaccine and H7N9 infection in humans. bioRxiv 94, 1–17. 10.1101/682450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes MA, Flannery B, Chung JR, O’Halloran A, Garg S, Belongia EA, Gaglani M, Zimmerman RK, Jackson ML, Monto AS, Alden NB, Anderson E, Bennett NM, Billing L, Eckel S, Kirley PD, Lynfield R, Monroe ML, Spencer M, Spina N, Talbot HK, Thomas A, Torres SM, Yousey-Hindes K, Singleton JA, Patel M, Reed C, Fry AM, McLean HQ, King JP, Nowalk MP, Balasubramani GK, Bear TM, Hickey R, Williams JV, Reis EC, Moehling KK, Eng H, Jackson LA, Smith Michael, Raiyani C, Clipper L, Murthy K, Chen W, Reis M, Petrie JG, Malosh RE, McSpadden EJ, Segaloff HE, Cheng CK, Truscon R, Johnson E, Lamerato LE, Rosenblum B, Ford S, Johnson M, Raviotta JM, Sax T, Steele J, Susick M, Chabra R, Garofolo E, Iozzi P, Kevish B, Middleton DB, Urbanski L, Ponder T, Crumbaker T, Iosefo I, Sleeth P, Gandy V, Bounds K, Kylberg M, Rao A, Fader R, Walker K, Volz M, Ray J, Price D, Thomas J, Wehbe-Janek H, Beeram M, Boyd J, Walkowiak J, Probe R, Couchman G, Motakef S, Arroliga A, Kaniclides A, Bouldin E, Baker C, Berke K, Smith Mackenzie, Rajesh N, Alleman E, Bauer S, Groesbeck M, Brundidge K, Hafeez N, Jackson J, Anastasia I, Kadoo G, Petnic S, Ryan A, Maslar A, Meek J, Chen R, Stephens S, Thomas S, Segler S, Openo K, Fawcett E, Farley M, Martin A, Ryan P, Sunkel R, Lutich T, Perlmutter R, Grace B, Blood T, Zerrlaut C, McMahon M, Strain A, Christensen J, Angeles K, Butler L, Khanlian S, Mansmann R, McMullen C, Pradhan E, Manzi K, Felsen C, Gaitan M, Long K, Fisher N, Hawley E, O’Shaughnessy R, Scott M, Crawford C, Schaffner W, Markus T, Leib K, Dyer K, Santibanez T, Zhai Y, Lu P, Srivastav A, Hung MC, 2019. Effects of Influenza Vaccination in the United States during the 2017–2018 Influenza Season. Clin. Infect. Dis 69, 1845–1853. 10.1093/cid/ciz075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze MP, Deriaud E, Przewlocki G, LeClerc C, 1989. Carrier-induced epitopic suppression is initiated through clonal dominance. J. Immunol 142, 2635–40. https://doi.org/http://www.jimmunol.org/content/142/8/2635 [PubMed] [Google Scholar]
- Skountzou I, Quan F-S, Gangadhara S, Ye L, Vzorov A, Selvaraj P, Jacob J, Compans RW, Kang S-M, 2007. Incorporation of glycosylphosphatidylinositol-anchored granulocyte-macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J. Virol 81, 1083–94. 10.1128/JVI.01692-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Sun X, Bai Y, Liu YV, Massare MJ, Pearce MB, Belser JA, Maines TR, Creager HM, Glenn GM, Flyer D, Pushko P, Levine MZ, Tumpey TM, 2017. Neuraminidase-based recombinant virus-like particles protect against lethal avian influenza A(H5N1) virus infection in ferrets. Virology 509, 90–97. 10.1016/j.virol.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz L, Kays S-K, Zimmer G, von Messling V, 2018. Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype. J. Virol 92. 10.1128/jvi.01006-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Krammer F, 2014. In the shadow of hemagglutinin: A growing interest in influenza viral neuraminidase and its role as a vaccine antigen. Viruses 6, 2465–2494. 10.3390/v6062465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F, 2015a. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio 6, e02556. 10.1128/mBio.02556-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbold TJ, Nachbagauer R, Xu H, Tan GS, Hirsh A, Brokstad KA, Cox RJ, Palese P, Krammer F, 2015b. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio 6, 1–13. 10.1128/mBio.02556-14 [DOI] [PMC free article] [PubMed] [Google Scholar]


