Abstract
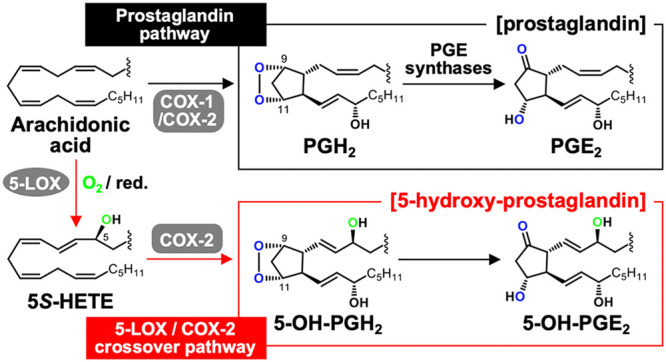
The biosynthetic crossover of 5-lipoxygenase (5-LOX) and cyclooxygenase-2 (COX-2) enzymatic activities is a productive pathway to convert arachidonic acid into unique eicosanoids. Here, we show that COX-2 catalysis with 5-LOX derived 5-hydroxy-eicosatetraenoic acid yields the endoperoxide 5-hydroxy-PGH2 that spontaneously rearranges to 5-OH-PGE2 and 5-OH-PGD2, the 5-hydroxy analogs of arachidonic acid derived PGE2 and PGD2. The endoperoxide was identified via its predicted degradation product, 5,12-dihydroxy-heptadecatri-6E,8E,10E-enoic acid, and by SnCl2-mediated reduction to 5-OH-PGF2α. Both 5-OH-PGE2 and 5-OH-PGD2 were unstable and degraded rapidly upon treatment with weak base. This instability hampered detection in biologic samples which was overcome by in situ reduction using NaBH4 to yield the corresponding stable 5-OH-PGF2 diastereomers and enabled detection of 5-OH-PGF2α in activated primary human leukocytes. 5-OH-PGE2 and 5-OH-PGD2 were unable to activate EP and DP prostanoid receptors, suggesting their bioactivity is distinct from PGE2 and PGD2.
Keywords: arachidonic acid, prostaglandin, eicosanoid, bioactive lipid, catalysis, endoperoxide, leukocyte
1. Introduction
The inducible form of cyclooxygenase (COX), COX-2, has a larger active site1 resulting in a wider substrate specificity than that of COX-1, the isoform that reacts preferably with arachidonic acid.2,3 Among the unique substrates for COX-2 is 5-hydroxy-eicosatetraenoic acid (5-HETE), a product of the reaction of 5-lipoxygenase (5-LOX) with arachidonic acid.4 The reaction of COX-2 with 5-HETE yields the highly oxygenated hemiketal (HK) eicosanoids HKE2 and HKD2 that result from triple oxygenation of the substrate,5 while arachidonic acid is reacted with two molecules of oxygen when forming prostaglandins (Scheme 1).6,7 The reaction of COX-2 with 5-HETE and other isoform-specific substrates like fatty acid amides (anandamide)8 and esters (2-arachidonyl-glycerol)9 suggests that the resulting products serve specific biologic functions that are distinct from those of traditional prostaglandins.3,10
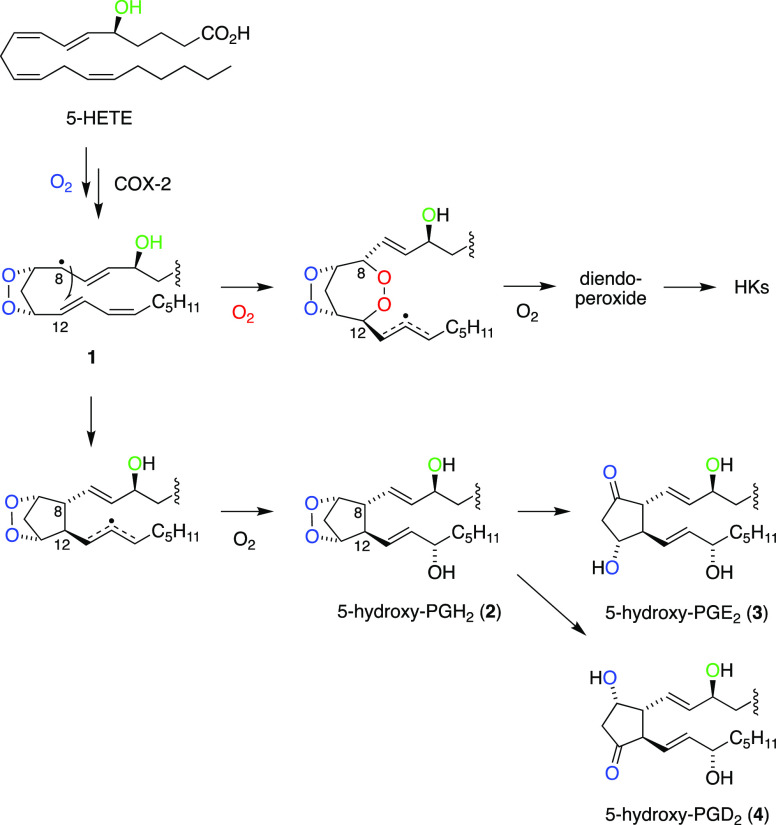
Scheme 1. Biosynthesis and Transformation of Endoperoxides in the Reaction of Cyclooxygenases with Arachidonic Acid11 and 5-HETE5,12.
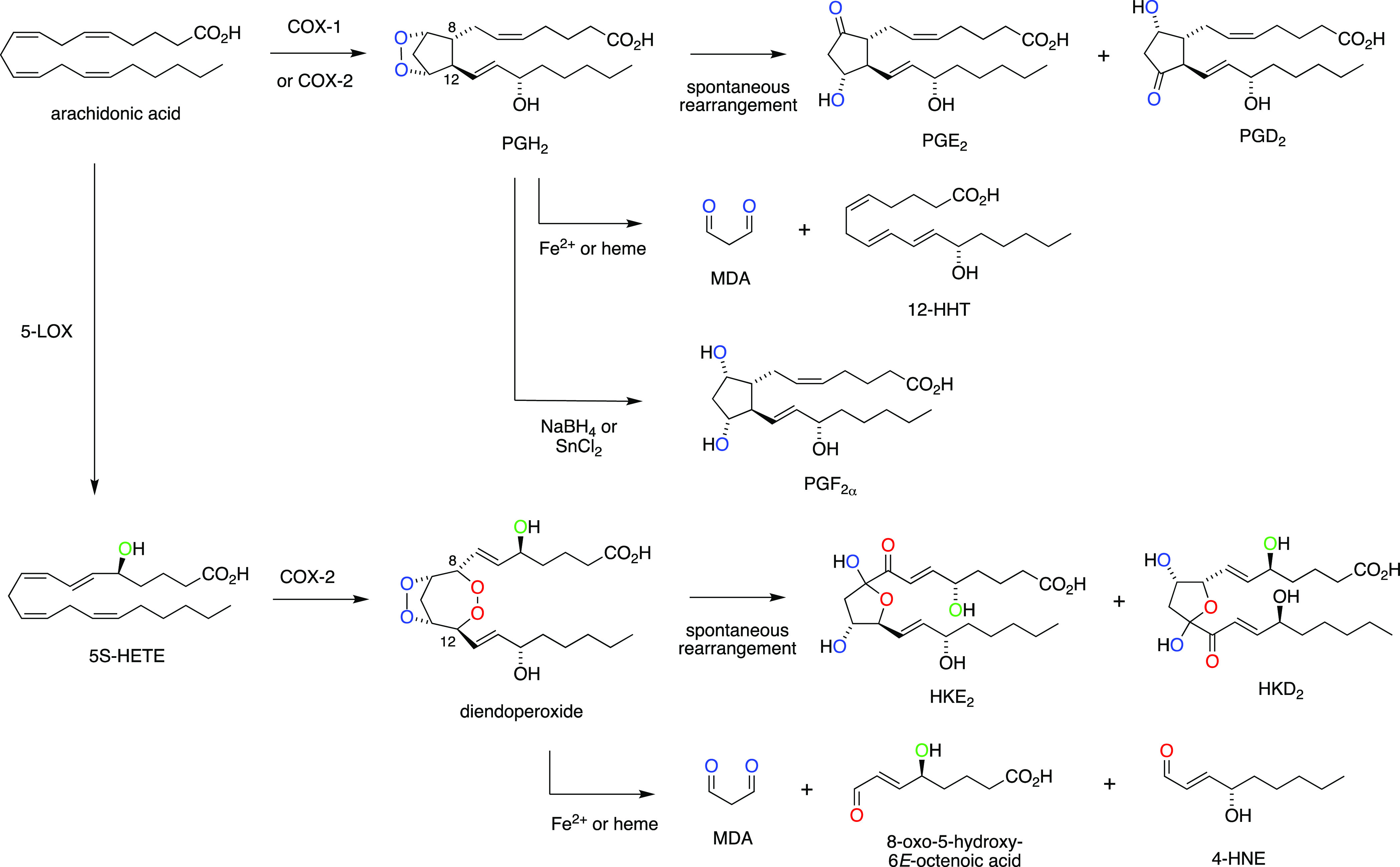
The main catalytic product of the reaction of COX-2 with 5-HETE is a diendoperoxide as a precursor to the two HK eicosanoids (Scheme 1).4,5 The diendoperoxide features two fused 5- and 7-membered rings with peroxides linking carbons 9 and 11 as well as 8 and 12, respectively. The spontaneous opening of both peroxides, in a reaction equivalent to the nonenzymatic rearrangement of PGH2 to PGE2 and PGD211 followed by formation of the hemiketal ring moiety, yields HKE2 and HKD2 (Scheme 1).5 Alternatively, the diendoperoxide degrades to the aldehyde fragments malondialdehyde (MDA), 4-hydroxy-2E-nonenal (4-HNE), and 8-oxo-5-hydroxy-6E-octenoic acid in the presence of ferrous iron or excess heme,12 again in a reaction equivalent to the degradation of PGH2 to MDA and 12-hydroxy-heptadecatrienoic acid (12-HHT).11 Other, less abundant products resulting from the COX-2 reaction with 5-HETE are 5,11- and 5,15-diHETE,13 the 5-hydroxy analogues of the minor AA/COX products, 11- and 15-HETE.11,14
A critical intermediate in the COX-2 transformation of 5-HETE is the C-8 carbon radical of the 9,11-endoperoxide 1 (Scheme 2). During prostaglandin formation from arachidonic acid, the C-8 radical reacts with C-12 to yield the five-membered prostanoid ring. During formation of the diendoperoxide from 5-HETE, the C-8 radical reacts with O2 followed by the reaction of the peroxyl radical with C-12, thus expanding the prostanoid ring to include peroxide linking carbons 8 and 12.4 The final product in either case is formed by stereospecific oxygenation and reduction at C-15. Direct addition of C-8 to C-12 would predict the formation of 5-hydroxy-analogue 2 of PGH2 as a precursor to novel prostanoids (Scheme 2). Here, we describe the conversion of 5-HETE to 5-OH-PGH2 by COX-2 yielding 5-OH-PGE2 and -PGD2 as novel products of the biosynthetic crossover of 5-LOX and COX-2 and their formation in human leukocytes that have been activated to express both enzymes.
Scheme 2. Reaction of the C-8 Carbinyl Radical Intermediate 1 in COX-2 Catalysis with 5-HETE Determines the Formation of Hemiketals (HK)5 or 5-Hydroxy-Prostaglandins as Enzymatic Products.
2. Results and Discussion
Novel Products in the Reaction of COX-2 with 5-HETE
Negative ion LC-MS analysis of products formed by the reaction of recombinant human COX-2 with 5-HETE revealed two products 3 and 4 with m/z 367.3, equivalent to a molecular weight of 368.5 g/mol (Figure 1). The increase of 16 mass units compared to prostaglandins like PGD2 and PGE2 (MW 352.5 g/mol) suggested that the novel products represent 5-hydroxy analogs of PGD2 and PGE2. Products 3 and 4 eluted at a retention time similar to the hemiketal products HKD2 and HKE2 (m/z 399.3; MW 400.5 g/mol), while unreacted 5-HETE substrate eluted at 5.1 min retention time (Figure 1).
Figure 1.
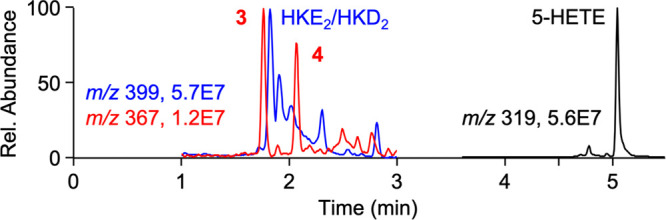
LC-MS detection of HKE2 and HKD2 (m/z 399 ion trace) and novel products 3 and 4 (m/z 367) in a reaction of recombinant human COX-2 with 5-HETE substrate (m/z 319).
A comparison of the ion intensities in negative ion mode MS1 analyses, albeit an imperfect measure of relative intensities, suggested that 3 and 4 were about 20% of the abundance of HKE2 and HKD2, the major products formed in the reaction of COX-2 with 5-HETE in vitro as determined using radiolabeled [1-14C]5-HETE substrate.5
Identification of 5-OH-PGD2 and 5-OH-PGE2
Product 4 was isolated from a large-scale reaction of 5-HETE with COX-2 conducted in the presence of hematopoietic prostaglandin D synthase (H-PGDS). H-PGDS was included in the enzymatic reaction to facilitate transformation of the predicted COX-2 catalytic product, 5-OH-PGH22, to 5-OH-PGD2. Homo- and heteronuclear 1D- and 2D-NMR analyses of product 4 showed signals for a 5-membered prostanoid ring with 9-hydroxyl and 11-keto moieties and the side chains at C-8 and C-12 in trans configuration (SI, Table S1). The presence of two allylic hydroxy groups, representing the 5-OH-6E-ene and 15-OH-13E-ene moieties, confirmed the identification of 4 as 5-OH-PGD2. Incubations of 5-HETE with COX-2 did not afford a sufficient amount for NMR analysis of 3 which was presumed to be 5-OH-PGE2. As an alternative approach, a standard of 5-OH-PGE2 was independently prepared by chemical synthesis starting from PGE2 as described below. Comparison to the synthetic standard confirmed identification of 3 as 5-OH-PGE2.
Synthesis of 5-OH-PGD2 and 5-OH-PGE2
5-OH-PGD2 and 5-OH-PGE2 were synthesized by singlet oxygen oxidation of PGD2 and PGE2, respectively. Reaction of PGD2 in methanol with methylene blue and visible light followed by reduction (PPh3) yielded 5S–OH-PGD24 and 5R–OH-PGD25 as well as the 6-OH-4E-isomers (6, 7) (SI, Figure S1A). Singlet oxygen oxidation of PGE2 gave two major products 3 and 8, identified as 5S- and 5R-OH-PGE2, respectively, with insignificant formation of the 6-OH isomers (SI, Figure S1B and Tables S1–S4). The absolute configuration of C-5 of the synthesized 5-OH-PGs (3, 4, 5, and 8) was determined by LC-MS analysis of diastereomeric cleavage products formed by methylation (CH2N2), derivatization with (−)-menthyl chloroformate, and oxidative ozonolysis15 (SI, Figure S2). Authentic standards for the assignment of the configuration of (−)-menthoxycarbonyl 2S- and 2R-hydroxy-1,6-hexanedioic acid-6-methyl ester diastereomers were obtained starting with synthetic 5S- and 5R-HETE, respectively, for which the absolute configuration was known.16
Reduction and degradation of 5-OH-PGH2
The structural analogy of 5-OH-PGD2 and 5-OH-PGE2 with PGD2 and PGE2 suggested that the former were formed by the spontaneous rearrangement of an endoperoxide precursor, predicted to be 5-OH-PGH22, and formed as the actual product of the COX-2 reaction with 5-HETE. 5-OH-PGH2 was predicted to be too unstable to attempt isolation and direct structural identification. In order to test whether endoperoxide 2 was the product of COX-2 catalysis with 5-HETE, the enzymatic reaction was treated with SnCl2 as a mild reducing agent for the endoperoxide group.17 LC-MS analysis showed formation of 5-OH-PGF2α9 as a reduction product with a molecular ion at m/z 369 that was absent without SnCl2 treatment (Figure 2A and Table S4). Product 9 coeluted with 5-OH-PGF2α that was formed by NaBH4 reduction of synthetic 5-OH-PGD2 as described below.
Figure 2.
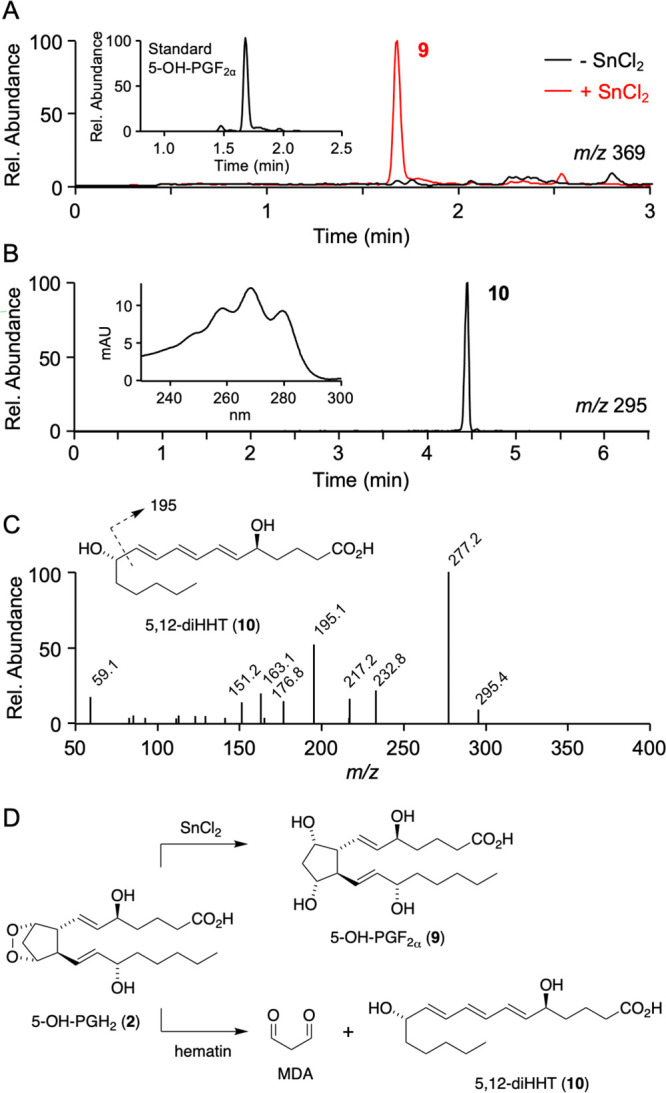
Indirect identification of 5-OH-PGH2. (A) Treatment with SnCl2 of a 5 min reaction of COX-2 with 5-HETE generated product 9 with m/z 369 that had the same retention time as a standard of 5-OH-PGF2α formed by reduction (NaBH4) of 5-OH-PGD2 (inset). (B) Treatment of the enzymatic reaction with hematin yielded product 10 identified as 5,12-dihydroxy-heptadecatri-6E,8E,10E-enoic acid (5,12-diHHT) due to its UV spectrum characteristic of a conjugated triene in E,E,E configuration (inset) and (C) fragment ions derived from m/z 295.4 (10) in the MS2 spectrum. (D) Products of the reaction of 5-OH-PGH2 (2) with SnCl2 and hematin.
Further evidence for 5-OH-PGH2 as the product of COX-2 catalysis with 5-HETE was obtained from its degradation in the presence of hematin, similar to the transformation of PGH2 to 12-HHT.18 Recombinant human COX-2 was incubated with 5-HETE for 5 min followed by addition of hematin to induce degradation. The products were extracted and analyzed by RP-HPLC with diode array detection and by LC-MS. The RP-HPLC chromatogram showed elution of product 10 with a UV chromophore typical of a conjugated triene with λmax at 268 nm and diagnostic shoulders at 260 and 280 nm, with the deeply defined bathochromic shoulder indicating E,E,E-configuration of the conjugated triene (Figure 2B).19,20 Product 10 was isolated using RP-HPLC and analyzed by LC-MS operated in the negative ion mode which gave a molecular ion at m/z 295.4, indicating an MW of 296.4 g/mol (Figure 2C). UV spectrum, MW, and the MS fragment ions indicated that product 10 was 5,12-dihydroxy-heptadecatri-6E,8E,10E-enoic acid (5,12-diHHT). The transformation reactions with SnCl2 and hematin identified the immediate product of COX-2 catalysis with 5-HETE as 5-OH-PGH22 (Figure 2D).
Stability of 5-OH-PGD2 and 5-OH-PGE2
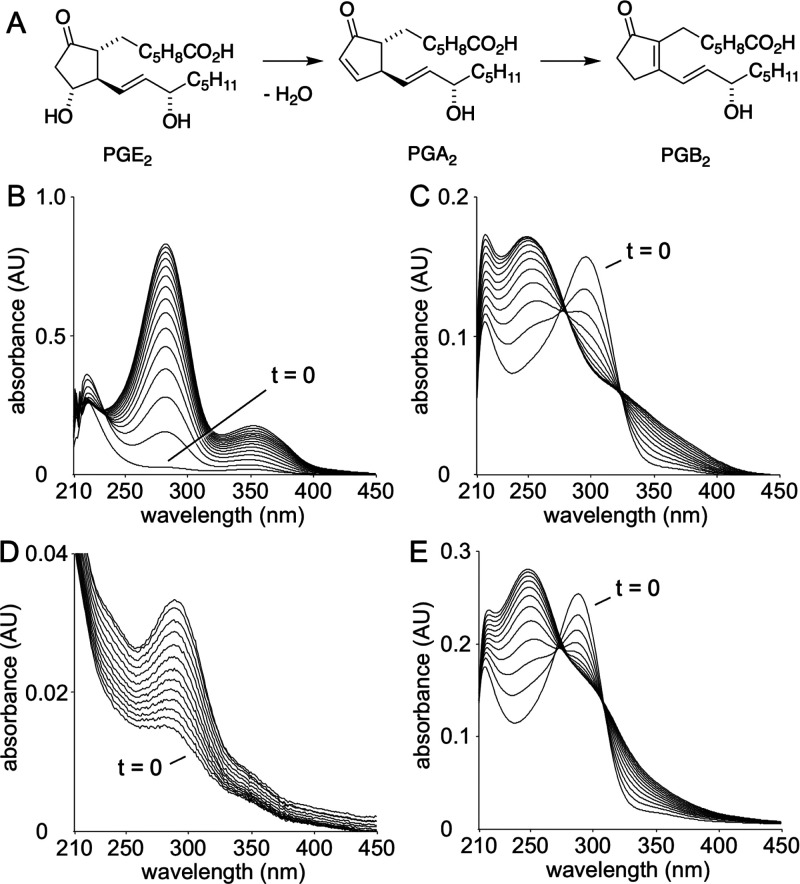
The treatment of PGE2 and PGD2 with base induces dehydration of the hydroxyl groups at C-11 or C-9, respectively, resulting in the formation of the respective enone prostanoids.21,22 Base treatment dehydrates PGE2 (λmax 205 nm) to the cyclopentenone PGA2 (λmax 217 nm)23 that further rearranges to the dienone PGB2 with λmax at 278 nm (Figure 3A).24 Likewise, PGD2 dehydrates to PGJ2 that rearranges to Δ12-PGJ2 and undergoes a second dehydration to yield 15-deoxy-Δ12,14-PGJ2.22,25
Figure 3.
Base-catalyzed dehydration of prostaglandins. (A) Dehydration of PGE2 yields PGA2 that rearranges to PGB2. (B) Time-course of dehydration of PGE2 in NaOH (200 mM) illustrates formation of PGA2 (λmax 217 nm) and PGB2 (λmax at 278 nm). UV/vis spectra were acquired every 2 min. (C) Degradation of 5-OH-PGE2 in 10-times dilute NaOH (20 mM) indicating complete conversion to a product with λmax 295 nm at the time of the first scan. (D) Degradation of 5-OH-PGE2 in Tris-HCl (50 mM, pH 8). (E) Degradation of 5-OH-PGD2 in NaOH (20 mM), with t = 0 min indicating conversion to a product with λmax 295 nm. The UV/vis scanning rate was 1/min in panels C–E.
When base treatment is conducted in a UV/vis cuvette, the dehydration can be followed by observing the change of the chromophores over time. For example, treatment of PGE2 with NaOH (200 mM) in Figure 3B illustrates the time course of formation of PGB2. To test the stability of 5-OH-PGE2 a 10-fold lower concentration of base was used (20 mM NaOH) and the scan frequency was doubled to 1/min. Under these conditions, the first scan recorded immediately after addition of 5-OH-PGE2 to the cuvette indicated complete conversion to a product with a dienone-like chromophore at UV295 nm (Figure 3C). The dienone-like chromophore decreased rapidly to yield products with less distinct absorbance. This indicated that 5-OH-PGE2 was much more sensitive to base-induced dehydration than PGE2, and that further transformation yielded several undefined products. When 5-OH-PGE2 was incubated in a buffer of pH 8 the time course of formation of the UV295 nm chromophore, taken as a measure of the initial dehydration, indicated a half-life of ≈12 min (Figure 3D). When 5-OH-PGD2 was treated with base (20 mM NaOH, scan rate 1/min) a similar change of chromophores was observed (Figure 3E), suggesting a comparable half-life.
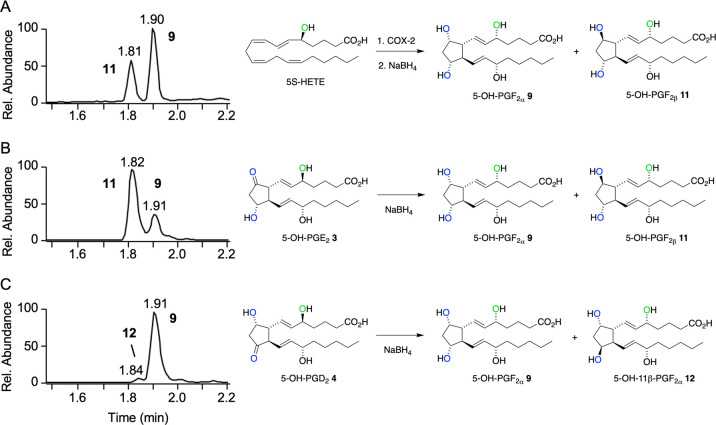
In Situ Reduction of 5-OH-PGs
The chemical instability was predicted to impede analysis of 5-OH-PG biosynthesis in a biological environment due to rapid transformation to uncharacterized products. To minimize the loss of 5-OH-PGs during cell incubations, extraction, and analysis, a method was developed for in situ reduction to the 5-OH-PGF2 analogues. Reduction of the dehydration-prone cyclopentanones and remaining endoperoxide was predicted to increase stability as is observed for PGF2α and F2-isoprostanes relative to cyclopentanone PGs.26 Recombinant COX-2 was incubated with 5-HETE for 5 min followed by addition of NaBH4 in order to achieve reduction of the immediate products to 5-OH-PGF2 diastereomers. Analysis using LC-MS gave two products with the expected m/z 369 molecular ion, with 11 eluting at 1.81 min and 9 at 1.90 min retention time, respectively (Figure 4A). It was assumed that 11 and 9 were diastereomers of 5-OH-PGF2, but the configuration of the hydroxy groups on the prostanoid ring was unclear, i.e., whether it was α or β. Reduction of synthetic 5-OH-PGE2 gave 11 and 9 in a ratio of ≈75:25 (Figure 4B). Reduction of synthetic 5-OH-PGD2 gave 9 as the overwhelming product together with isomer 12 (Figure 4C).27 These analyses suggested that the α and β diastereomers were not formed in a 1:1 ratio in the reduction of the carbonyl by NaBH4. It should be noted that reduction of 5-OH-PGE2 and 5-OH-PGD2 will only yield three diastereomers since both precursors will form the F2α diastereomer while the other diastereomer is unique. Comparison to the PGF2 diastereomers formed by reduction of PGD2 and PGE2 (SI, Figure S3) enabled identification of product 11 as 5-OH-PGF2β and 9 as 5-OH-PGF2α. The third diastereomer, 5-OH-11β-PGF2α12, was formed only at low abundance by reduction of 5-OH-PGD2.
Figure 4.
5-OH-PGF2 diastereomers formed by NaBH4 reduction of (A) a 5 min reaction of 5-HETE with COX-2, (B) synthetic 5-OH-PGD2, and (C) synthetic 5-OH-PGE2. The ion trace for m/z 369 from LC-MS analysis of the reactions is shown. Products in panel A are expected to be mainly derived from reduction of 5-OH-PGH2.
Reduction of the diendoperoxide and hemiketals in a crude reaction of 5-HETE with COX-2 as well as reduction of HKE2 gave a single peak (RT 1.69 min) with m/z 403, representing 5,8,9,11,12,15-hexahydroxy-eicosadienoic acid 13 (SI, Figure S4). Detection of a single peak indicated that fewer than the expected diastereomers were formed and/or that the diastereomers were not resolved chromatographically. This matter was not further investigated.
Formation of 5-OH-PGs by Human Leukocytes
The biosynthetic crossover of 5-LOX and COX-2 activities, in leukocyte mixtures isolated from normal human volunteers and stimulated ex vivo with lipopolysaccharide (LPS) and calcium ionophore A23187, yielded HKE2, HKD2, as well as 5,11- and 5,15-diHETEs.28,29 The use of inhibitors confirmed the role of the two enzymes in biosynthesis.28,29 Thus, activated human leukocytes appeared to be a suitable model to analyze the biosynthesis of 5-OH-PGs in a cellular system. In order to overcome issues related to the instability of 5-OH-PGs, leukocytes were activated and then treated in situ with NaBH4 to reduce unstable 5-OH-PGs to the 5-OH-PGF2 diastereomers prior to extraction and LC-MS analysis.
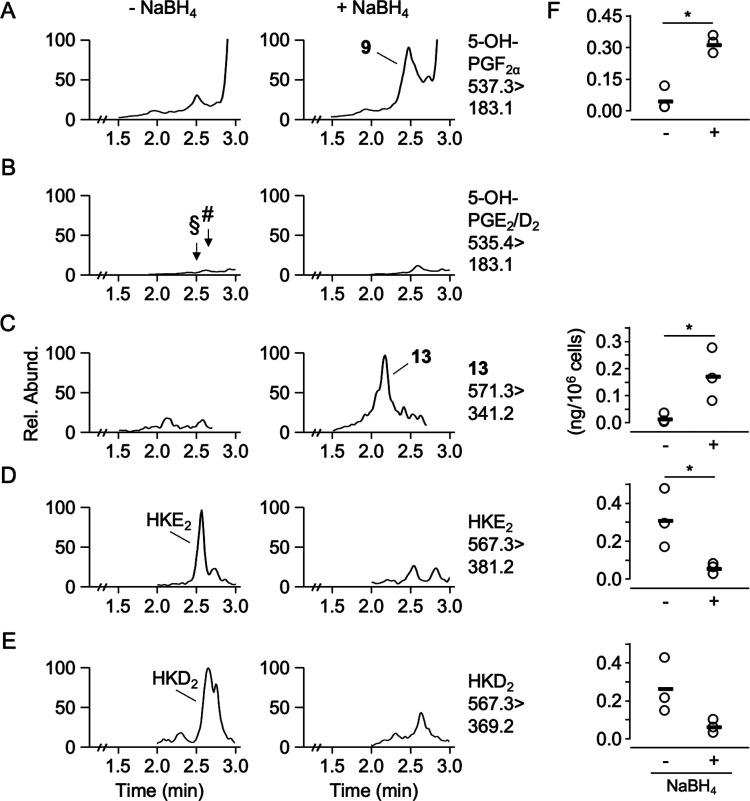
Freshly isolated human leukocytes stimulated with LPS and A23187 and treated with NaBH4 showed formation of 5-OH-PGF2α, indicating successful detection of the biosynthesis of 5-OH-PGs (Figure 5A). Without NaBH4 treatment, 5-OH-PGF2α was negligible. 5-OH-PGD2 and 5-OH-PGE2 were not detected irrespective of NaBH4 treatment (Figure 5B). Their absence was attributed to their chemical and/or metabolic instability, and in situ reduction was confirmed as a suitable approach to detect their biosynthesis via analysis of the reduction products. The absence of the 5-OH-PGF2β diastereomer indicated that the leukocyte mixtures preferentially formed 5-OH-PGD2 over 5-OH-PGE2 or that 5-OH-PGH2 was the most abundant product at the time when NaBH4 was added. HKE2 and HKD2 were detected in the nonreduced samples, and treatment with NaBH4 resulted in the formation of 5,8,9,11,12,15-hexahydroxy-eicosadienoic acid 13, the reduction product of HKs and their diendoperoxide precursor, respectively (Figure 5C–E).
Figure 5.
Formation of 5-OH-PGs and HKs by activated human leukocytes. Cells were treated with LPS for 45 min and then with A23187 for 15 min, followed by reduction or not with NaBH4, extracted, derivatized with AMPP, and analyzed using LC-MS in the positive ion SRM mode. Ion traces for the detection of (A) 5-OH-PGF2α, (B) 5-OH-PGD2 and 5-OH-PGE2, (C) 5,8,9,11,12,15-hexahydroxy-eicosadienoic acid 13, (D) HKE2, and (E) HKD2 are shown. (F) Quantification of the eicosanoids shown to the left in human leukocyte samples (n = 3) treated with or without NaBH4 (*, p < 0.05). The arrows in panel B indicate the retention times of 5-OH-PGE2 (§) and 5-OH-PGD2 (#), respectively.
Quantification of activated and NaBH4-reduced leukocytes showed that 5-OH-PGF2α was about twice as abundant as 5,8,9,11,12,15-hexahydroxy-eicosadienoic acid 13 (Figure 5F). The discrepancy with the in vitro incubations that showed that the higher formation of HKs than 5-OH-PGs can be explained by the propensity of HKs to form adducts with nucleophilic cysteines (e.g., glutathione)5 and the about 3-fold lower yield of 13 (6%) compared to 9 (16%) upon NaBH4-reduction of their respective precursors in vitro. Both events result in a decrease of HK-derived 13 relative to 5-OH-PG-derived 9. NaBH4 treatment of stimulated leukocytes did not affect levels of 5-HETE (1.4 ± 0.4 ng/106 cells) and LTB4 (44 ± 24 ng/106 cells).
Receptor Activation
The understanding of the biological role of the 5-LOX/COX-2 crossover eicosanoids is still in its infancy. A factor that holds back progress is the difficulty in preparing HKs by chemical or enzymatic synthesis.30,31 The straightforward synthesis, albeit on a small scale, of 5-OH-PGE2 and 5-OH-PGD2, starting from PGE2 and PGD2, respectively, enabled us to test whether they activate EP and DP prostanoid receptors. Prostanoid receptors appeared to be a logical target due to the close structural similarity between prostaglandins and their 5-OH-analogues. However, neither 5-OH-PGE2 nor 5-OH-PGD2 activated the EP (EP1-EP4), DP1, FP, IP, or TP prostanoid receptors (SI, Figures S5 and S6). This outcome was consistent with the absence of a 5-hydroxy modification in the structures of the known prostanoid analogues that are agonists of these receptors (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=58; accessed March 2021). The inability of the 5-OH-PGs to activate traditional prostanoid receptors is reminiscent of the so-called specialized proresolving eicosanoid mediators that act on G-protein coupled receptors related to chemokine and formyl peptide receptors rather than prototypical eicosanoid receptors and are likewise biosynthesized through the action of multiple oxygenases, including 5-LOX.32,33
3. Conclusion
The biosynthesis of 5-OH-PGH2 from 5-HETE follows the known radical and oxygenation reactions catalyzed in the COX reaction with arachidonic acid.6 The 9,11-endoperoxide-8-carbinyl radical is a crucial catalytic intermediate (Scheme 2). Reaction of the carbon radical with oxygen or not prior to addition to the 12E double bond determines the outcome of the enzymatic reaction, i.e., formation of the diendoperoxide versus 5-OH-PGH2 as the precursors to HKs and 5-OH-PGs, respectively. Using the purified enzyme in vitro, HKs are about 5-fold more abundant than 5-OH-PGs, indicating that oxygenation of the C-8 radical is favored over a direct reaction with C-12. Nevertheless, it is interesting to speculate on the mechanism of how the enzyme might control the ratio between the products. Control may be accomplished, for example, via regulating the concentration of O2 or through an allosteric effect of the noncatalytic subunit on the catalytic subunit of the functional COX-2 heterodimer.34,35 In the latter scenario, the binding of nonsubstrate fatty acids like palmitate to the noncatalytic (allosteric) subunit of COX-2 increases the efficiency of the catalytic subunit with arachidonic acid by more than 2-fold.36,37 Subunit allosteric effects also account for substrate-selective inhibitor potency. For example, the NSAIDs ibuprofen and mefenamic acid inhibited the COX-2 oxygenation of 2-AG with orders of magnitude greater potency than the oxygenation of arachidonic acid.38,39 This testifies to regulation of catalysis through the interaction between the subunits of the COX dimer, and similar regulatory events may control the reactivity of the C-8 radical and thus the ratio of HKs versus 5-OH-PGs formed in COX-2 catalysis with 5-HETE.
The formation of 5-OH-PGs is biologically relevant beyond the mechanistic insight into COX-2 catalysis since it occurred in response to the ex vivo stimulation of primary leukocytes isolated from normal human volunteers. The biological effects of the novel prostanoids await to be uncovered, but the lack of activation of EP and DP prostanoid receptors by 5-OH-PGs suggests they exhibit bioactivity that is unique and different from traditional prostaglandins.
4. Experimental Section
Reaction of Recombinant COX-2 with 5S-HETE
Samples for product analysis by LC-MS were conducted in 100 μL of volume using buffer (100 mM Tris-HCl, pH 8), recombinant COX-2 (1 μM), hematin (1 μM), and phenol (0.5 mM). The reaction was started by the addition of 5S-HETE (30 μM), conducted for 5 min, and terminated by addition of 0.1% HOAc (900 μL) and methanol (50 μL). Products were extracted using a preconditioned Waters HLB cartridge (30 mg), washed with water, and eluted with ethyl acetate (500 μL) and methanol (500 μL). The combined eluates were concentrated under a stream of N2 and dissolved in acetonitrile/water 1:1 (50 μL).
For the isolation of 5-OH-PGD24, the reaction volume was 2 mL of buffer (100 mM Tris-HCl, pH 8) containing recombinant COX-2 (1 μM), hematin (1 μM), phenol (0.5 mM), glutathione (10 μM), MgCl2 (10 mM), and recombinant human H-PGDS (1 μM). The reaction was started by the addition of 5-HETE (30 μg), conducted for 5 min, and terminated by acidification to pH 5 (1 N HCl) and addition of methanol (50 μL). Typically, 20 reactions were conducted in parallel and two 2 mL-reactions each were combined and extracted using a preconditioned Waters HLB cartridge (30 mg) as described above. The product was isolated using RP-HPLC and a gradient of water/acetonitrile/HOAc 80/20/0.01, by vol., to water/acetonitrile/HOAc 30/70/0.01, by vol., in 10 min at 0.4 mL/min and held for 10 min with UV detection at 205 nm. Product 4 was further purified using an Agilent Zorbax RX-SIL 5-μm column (4.6 × 250 mm) eluted with a solvent of hexane/i-PrOH/HOAc 80/20/0.1, by vol., at 1 mL/min flow rate.
Reduction and Degradation of 5-OH-PGH2
COX-2 (final concentration 370 nM) was reconstituted with hematin (1 μM) in 100 mM Tris-HCl buffer, pH 8.0, containing 500 μM phenol and incubated at 37 °C for 5 min. After adding 13.6 μg of 5S-HETE, incubation was carried out for 5 min at room temperature and terminated by addition of 140 μL of 200 mM SnCl2 as an aqueous suspension. After acidification to pH 3–4 with acetic acid, the products were recovered by extraction using a 30-mg Waters HLB cartridge.
For the degradation of 5-OH-PGH2, the COX-2 reaction with 5-HETE was conducted for 5 min and then 1 mL of a 500 μM solution of hematin was added and the reaction was continued for 1 h. The reaction mixture was acidified with acetic acid to pH 4, and the products were recovered by extraction using a 30-mg Waters HLB cartridge. RP-HPLC analysis of the reaction products used a Waters Symmetry C18 column (5 μm, 250 × 4.6 mm) eluted with a gradient of 20% acetonitrile to 80% acetonitrile in 0.01% aqueous acetic acid in 20 min at a flow rate of 1 mL/min. Elution of the products was monitored using an Agilent 1200 diode array detector.
Stability of PGE2 and 5-OH-PGs
PGE2, 5-OH-PGE2, and 5-OH-PGD2 were placed in 500 μL quartz cuvettes containing NaOH (200 or 20 mM) or Tris-HCl buffer pH 8 (50 mM). The solutions were scanned from 450 to 210 nm every one or 2 min for 10–15 cycles.
Prostanoid Receptor Activation
A TGFα shedding assay was performed as previously described.40 HEK cells (2 × 105 cells/well) were seeded into 12-well plates and incubated for 24 h. The cells were then transfected with the GPCR expression vector, Gα expression vector and pSS-AP-TGFα vector [pcDNA3-EP1, pcDNA3-EP2 with Gαq/s, pcDNA3-EP3 with Gαq/i1, pcDNA3-EP4 with Gαq/s, pcDNA3-DP1 with Gαq/s, pcDNA3-TP, pcDNA3-FP with Gαq/s, pcDNA3-IP with Gαq/s, or pcDNA3 with Gαq/s]. At 24 h post-transfection, the cells were detached and resuspended in Hank’s balanced salt solution (HBSS) containing 5 mM HEPES (pH 7.4) and then seeded in a 96-well plate. After 30 min incubation for 37 °C, cells were stimulated with the ligand for 1 h. The conditioned medium (CM) was transferred into another 96-well plate, and p-nitrophenyl phosphate (p-NPP) solution (10 mM p-NPP, 40 mM Tris-HCl (pH 9.5), 40 mM NaCl and 10 mM MgCl2) was added to both a CM plate and a cell plate. Absorbance at 405 nm of the plates was read before and after 2 h incubation at 37 °C using a microplate reader (Biorad). The TGFα shedding AP activity was calculated as follows: ΔODCM = OD405CM (2 h) – OD405CM (0 h). ΔODcell = OD405cell (2 h) – OD405cell (0 h). The AP activity = ΔODCM/(ΔODCM + ΔODcell).
Preparation and Incubation of Human Leukocytes
The study protocol was approved by the Vanderbilt University IRB, protocol #091243. Healthy volunteers gave informed consent by signing a consent form prior to enrolling in the study. Venous blood (45 mL) was collected into a plastic syringe containing sodium citrate (136 mM; 4.5 mL) and 10 mL of 6% (w/v) dextran (250 kDa, average molecular mass). After blood collection, the syringe was kept upright for 1 h to allow red blood cells to settle into the dextran phase while leukocytes occupied the top phase (ca. 30 mL). The opaque top phase containing leukocytes was removed from the syringe and cells were collected by centrifugation. The pelleted cells were washed with PBS, and remaining red cells were removed by hypotonic lysis by treatment with a 10-fold excess of deionized water for 30 s followed by addition of 10x PBS to restore tonicity. The leukocytes were centrifuged, washed, and diluted in PBS containing 5 mM glucose. Cells (7.5 × 106) were diluted to1 mL in PBS containing Ca2+ and Mg2+, LPS (10 μg/mL) was added, and the cells were incubated at 37 °C for 45 min. Calcium ionophore A23187 (5 μM) was added, and the cells were incubated for additional 15 min for a total of 1 h of LPS treatment. For termination, the samples were centrifuged at 3500 x g for 5 min, and the supernatant was transferred to a new vial, acidified by acetic acid (pH 3–4) and extracted using a 30 mg Waters HLB cartridge.
Acknowledgments
This work was supported by award R01GM076592 from the National Institute of General Medical Sciences to C.S. and in part by the Vanderbilt CTSA (UL1 RR024975 from NCRR/NIH), and MEXT/JSPS KAKENHI Grant Numbers, 19K07357 to T.O., 18H02627, 19KK0199 to T.Y. J.A.G.-B. was supported by a postdoctoral fellowship award from the American Heart Association (16POST30690001). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Glossary
Abbreviations
- COX
cyclooxygenase
- ESI
electrospray ionization
- HETE
hydroxy-eicosatetraenoic acid
- HHT
hydroxy-heptadecatrienoic acid
- 4-HNE
4-hydroxy-nonenal
- H-PGDS
hematopoietic prostaglandin D synthase
- LC-MS
liquid chromatography–mass spectrometry
- LOX
lipoxygenase
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- PG
prostaglandin
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.1c00177.
Experimental procedures, SI figures, and NMR data for new compounds (PDF)
Author Present Address
$ Harvard Medical School, Division of Nephrology and Center for Vascular Biology Research, Beth Israel Deaconess Medical Center, Boston, MA, U.S.A
Author Present Address
# Laboratory of Food and Health, Dept. Food Science and Technology, CEBAS-CSIC, Murcia, Spain.
The authors declare no competing financial interest.
Supplementary Material
References
- Blobaum A. L.; Marnett L. J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]
- Smith W. L.; DeWitt D. L.; Garavito R. M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Rouzer C. A.; Marnett L. J. Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids. J. Biol. Chem. 2008, 283, 8065–8069. 10.1074/jbc.R800005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.; Boeglin W. E.; Yin H.; Stec D. F.; Voehler M. Convergent oxygenation of arachidonic acid by 5-lipoxygenase and cyclooxygenase-2. J. Am. Chem. Soc. 2006, 128, 720–721. 10.1021/ja056517y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesser M.; Suzuki T.; Tejera N.; Mont S.; Boeglin W. E.; Pozzi A.; Schneider C. Biosynthesis of hemiketal eicosanoids by cross-over of the 5-lipoxygenase and cyclooxygenase-2 pathways. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6945–6950. 10.1073/pnas.1019473108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Donk W. A.; Tsai A. L.; Kulmacz R. J. The cyclooxygenase reaction mechanism. Biochemistry 2002, 41, 15451–15458. 10.1021/bi026938h. [DOI] [PubMed] [Google Scholar]
- Schneider C.; Pratt D. A.; Porter N. A.; Brash A. R. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 2007, 14, 473–488. 10.1016/j.chembiol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; Ives D.; Ramesha C. S. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J. Biol. Chem. 1997, 272, 21181–21186. 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- Kozak K. R.; Rowlinson S. W.; Marnett L. J. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J. Biol. Chem. 2000, 275, 33744–33749. 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Hermanson D. J.; Gamble-George J. C.; Marnett L. J.; Patel S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol. Sci. 2014, 35, 358–367. 10.1016/j.tips.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M.; Samuelsson B. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 1967, 242, 5344–5354. 10.1016/S0021-9258(18)99434-2. [DOI] [PubMed] [Google Scholar]
- Griesser M.; Boeglin W. E.; Suzuki T.; Schneider C. Convergence of the 5-LOX and COX-2 pathways. Heme-catalyzed cleavage of the 5S-HETE-derived di-endoperoxide into aldehyde fragments. J. Lipid Res. 2009, 50, 2455–2462. 10.1194/jlr.M900181-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta S.; Suzuki T.; Tejera Hernandez N.; Griesser M.; Boeglin W. E.; Schneider C. Identification and absolute configuration of dihydroxy-arachidonic acids formed by oxygenation of 5S-HETE by native and aspirin-acetylated COX-2. J. Lipid Res. 2010, 51, 575–585. 10.1194/jlr.M001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer A. N.; Costello P. B.; Green F. A. Stereospecificity of the products of the fatty acid oxygenases derived from psoriatic scales. J. Lipid Res. 1991, 32, 341–347. 10.1016/S0022-2275(20)42094-2. [DOI] [PubMed] [Google Scholar]
- Hamberg M. A novel transformation of 13-LS-hydroperoxy-9,11-octadecadienoic acid. Biochim. Biophys. Acta, Lipids Lipid Metab. 1983, 752, 191–197. 10.1016/0005-2760(83)90112-1. [DOI] [PubMed] [Google Scholar]
- Schneider C.; Yu Z.; Boeglin W. E.; Zheng Y.; Brash A. R. Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. Methods Enzymol. 2007, 433, 145–157. 10.1016/S0076-6879(07)33008-5. [DOI] [PubMed] [Google Scholar]
- Valmsen K.; Järving I.; Boeglin W. E.; Varvas K.; Koljak R.; Pehk T.; Brash A. R.; Samel N. The origin of 15R-prostaglandins in the Caribbean coral Plexaura homomalla: Molecular cloning and expression of a novel cyclooxygenase. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 7700–7705. 10.1073/pnas.131022398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M.; Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc. Natl. Acad. Sci. U. S. A. 1974, 71, 3400–3404. 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeat P.; Samuelsson B. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J. Biol. Chem. 1979, 254, 7865–7869. 10.1016/S0021-9258(18)36026-5. [DOI] [PubMed] [Google Scholar]
- Maas R. L.; Brash A. R.; Oates J. A. A second pathway of leukotriene biosynthesis in porcine leukocytes. Proc. Natl. Acad. Sci. U. S. A. 1981, 78, 5523–5527. 10.1073/pnas.78.9.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polet H.; Levine L. Metabolism of prostaglandins E, A, and C in serum. J. Biol. Chem. 1975, 250, 351–357. 10.1016/S0021-9258(19)41907-8. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A.; Wynalda M. A. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J. Biol. Chem. 1983, 258, 11713–11718. 10.1016/S0021-9258(17)44287-6. [DOI] [PubMed] [Google Scholar]
- Pike J. E.; Lincoln F. H.; Schneide Wp. Prostanoic acid chemistry. J. Org. Chem. 1969, 34, 3552.-& 10.1021/jo01263a071. [DOI] [PubMed] [Google Scholar]
- Floyd M. B.; Schaub R. E.; Siuta G. J.; Skotnicki J. S.; Grudzinskas C. V.; Weiss M. J.; Dessy F.; VanHumbeeck L. Prostaglandins and congeners. 22. Synthesis of 11-substituted derivatives of 11-deoxyprostaglandins E1 and E2. Potential bronchodilators. J. Med. Chem. 1980, 23, 903–913. 10.1021/jm00182a018. [DOI] [PubMed] [Google Scholar]
- Kikawa Y.; Narumiya S.; Fukushima M.; Wakatsuka H.; Hayaishi O. 9-Deoxy-delta 9, delta 12–13,14-dihydroprostaglandin D2, a metabolite of prostaglandin D2 formed in human plasma. Proc. Natl. Acad. Sci. U. S. A. 1984, 81, 1317–1321. 10.1073/pnas.81.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab. Rev. 2000, 32, 377–385. 10.1081/DMR-100102340. [DOI] [PubMed] [Google Scholar]
- Hamberg M.; Samuelsson B. Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 1973, 70, 899–903. 10.1073/pnas.70.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejera N.; Boeglin W. E.; Suzuki T.; Schneider C. COX-2-dependent and -independent biosynthesis of dihydroxy-arachidonic acids in activated human leukocytes. J. Lipid Res. 2012, 53, 87–94. 10.1194/jlr.M017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Bastida J. A.; Shibata T.; Uchida K.; Schneider C. Roles of 5-lipoxygenase and cyclooxygenase-2 in the biosynthesis of hemiketals E2 and D2 by activated human leukocytes. FASEB J. 2017, 31, 1867–1878. 10.1096/fj.201601136R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Bastida J. A.; Suzuki T.; Sprinkel K. C.; Boeglin W. E.; Schneider C. Biomimetic synthesis of hemiketal eicosanoids for biological testing. Prostaglandins Other Lipid Mediators 2017, 132, 41–46. 10.1016/j.prostaglandins.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer R. E.; Gimenez-Bastida J. A.; Boutaud O.; Jana S.; Schneider C.; Sulikowski G. A. Total synthesis and biological activity of the arachidonic acid metabolite Hemiketal E2. Org. Lett. 2018, 20, 4020–4022. 10.1021/acs.orglett.8b01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirault J.; Back M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front. Pharmacol. 2018, 9, 1273. 10.3389/fphar.2018.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N.; Serhan C. N. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020, 64, 443–462. 10.1042/EBC20200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C.; Rieke C. J.; Rimon G.; Wingerd B. A.; Smith W. L. Partnering between monomers of cyclooxygenase-2 homodimers. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 6142–6147. 10.1073/pnas.0601805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L.; Malkowski M. G. Interactions of fatty acids, nonsteroidal anti-inflammatory drugs, and coxibs with the catalytic and allosteric subunits of cyclooxygenases-1 and −2. J. Biol. Chem. 2019, 294, 1697–1705. 10.1074/jbc.TM118.006295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L.; Vecchio A. J.; Sharma N. P.; Jurban B. J.; Malkowski M. G.; Smith W. L. Human cyclooxygenase-2 is a sequence homodimer that functions as a conformational heterodimer. J. Biol. Chem. 2011, 286, 19035–19046. 10.1074/jbc.M111.231969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L.; Zou H.; Yuan C.; Hong Y. H.; Kuklev D. V.; Smith W. L. Different fatty acids compete with arachidonic acid for binding to the allosteric or catalytic subunits of cyclooxygenases to regulate prostanoid synthesis. J. Biol. Chem. 2016, 291, 4069–4078. 10.1074/jbc.M115.698001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusakiewicz J. J.; Duggan K. C.; Rouzer C. A.; Marnett L. J. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry 2009, 48, 7353–7355. 10.1021/bi900999z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan K. C.; Hermanson D. J.; Musee J.; Prusakiewicz J. J.; Scheib J. L.; Carter B. D.; Banerjee S.; Oates J. A.; Marnett L. J. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat. Chem. Biol. 2011, 7, 803–809. 10.1038/nchembio.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T.; Ishitani T.; Yokomizo T. Biochemical characterization of three BLT receptors in zebrafish. PLoS One 2015, 10, e0117888 10.1371/journal.pone.0117888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.