Abstract
Purinergic signaling is a cell communication pathway mediated by extracellular nucleotides and nucleosides. Tri- and diphosphonucleotides are released in physiological and pathological circumstances activating purinergic type 2 receptors (P2 receptors): P2X ion channels and P2Y G protein-coupled receptors. The activation of these receptors triggers the production of reactive oxygen and nitrogen species and alters antioxidant defenses, modulating the redox biology of cells. The activation of P2 receptors is controlled by ecto-enzymes named ectonucleotidases, E-NTPDase1/CD39 and ecto-5’-nucleotidase/CD73) being the most relevant. The first enzyme hydrolyzes adenosine triphosphate (ATP) and adenosine diphosphate (ADP) into adenosine monophosphate (AMP), and the second catalyzes the hydrolysis of AMP to adenosine. The activity of these enzymes is diminished by oxidative stress. Adenosine actives P1 G-coupled receptors that, in general, promote the maintenance of redox hemostasis by decreasing reactive oxygen species (ROS) production and increase antioxidant enzymes. Intracellular purine metabolism can also contribute to ROS generation via xanthine oxidase activity, which converts hypoxanthine into xanthine, and finally, uric acid. In this review, we describe the mechanisms of redox biology modulated by purinergic signaling and how this signaling may be affected by disturbances in the redox homeostasis of cells.
Keywords: Oxidative stress, ROS, ATP, P2 receptors, Ectonucleotidases, Adenosine
Highlights
-
•
Purine metabolism contribute to oxidative stress via xanthine oxidase activity.
-
•
P2X7 receptor activation induces NADPH oxidase activation and superoxide production.
-
•
P2Y1 triggers the expression of antioxidant enzymes reducing oxidative stress.
-
•
Free radicals reduce CD39 and CD73 functionality.
1. Introduction
Purinergic signaling is an evolutionarily conserved cell communication pathway triggered by an increase of extracellular nucleotides surrounding cells, including adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), uridine triphosphate (UTP), uridine diphosphate (UDP), and the nucleoside adenosine (ADO). This signaling is involved in neurotransmission and other physiological processes; however, nucleotides can also act as alarmins or danger-associated molecular patterns in stress circumstances [1,2].
ATP was first described as a signaling transmitter in the non-adrenergic inhibitory innervation stimuli by Geoffrey Burnstock, considered the father of purinergic signaling [3,4]. Currently, it is well established that nucleotides and the nucleoside adenosine act as ligands of transmembrane purinergic receptors divided into two main families: P1 and P2 receptors. The P1 receptor family is composed of four G-coupled receptor subtypes (A1, A2A, A2B, and A3). In turn, P2 receptors are segregated into two subgroups: P2Y, which are metabotropic, and P2X, which are ionotropic proteins. The following physiological agonists activate P2 receptors: P2X and P2Y11–ATP; P2Y2,4–ATP and –UTP; P2Y1 P2Y12, and P2Y13–ADP; P2Y6–UDP; and P2Y14–UDP–glucose [[5], [6], [7], [8]]. These receptor activities are coordinated by ecto-enzymes known as ectonucleotidases, which metabolize their ligands through hydrolysis. The most prominent ones are E-NTPDase1/CD39, which hydrolyzes ATP and ADP into AMP, and ecto-5’-nucleotidase/CD73, which catalyzes AMP's hydrolysis to ADO, representing the final step of nucleotides degradation into nucleosides [[9], [10], [11]].
Purinergic receptors are expressed among various cell types and species [[12], [13], [14]]. They are critical for short-and long-term signaling, including neuromodulation processes, secretion, cell proliferation, differentiation, ossification, development, and regenerative episodes [15,16]. Purinoceptors encompass significant physiological specific phenomena in a variety of tissues and organs, including vasodilation and vasoconstriction, the release of hormones (e.g., insulin, glucagon, and others), bronchodilation, regulation of acid secretion in the gastrointestinal tract, control of reproductive systems, and other physiological functions [17].
Purinergic signaling has also been described in many pathological scenarios, including neurological, inflammatory, and infectious diseases [8,13,18,19]. In these situations of homeostatic disturbance, extracellular nucleotides can trigger the production and release of reactive oxygen species (ROS) and reactive nitrogen species (RNS). In addition, purinergic signaling can affect the activity of antioxidant enzymes promoting changes in the redox biology of cells [1,20,21]. In the present review, we aim to describe the mechanisms of redox biology modulated by purinergic signaling and the modulation of this signaling by disturbances in the redox biology of cells, primarily in neurological, inflammatory, and infectious diseases.
2. Free radical generation and antioxidant defenses
A radical is any molecule that contains one or more unpaired electrons generated from specific elements. In biological systems, oxygen and nitrogen are the most critical elements that generate radicals [22]; they are products of several metabolic pathways, including aerobic metabolism. Some perform essential functions, exerting physiological roles in cellular signaling, immunological responses, and other processes. However, radicals may also exist in free form as highly unstable molecules with unpaired electrons that interact with tissue components, causing oxidation and potentially damage DNA, proteins, and lipids [22,23].
Three of the most common and biologically important ROS are superoxide anions (O2-), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•). Regarding RNS, the best known are NO, nitrosonium cation (NO+), nitroxyl anion (NO−), and peroxynitrite (ONOO−) [24].
The superoxide anion (O2•−) is formed by the univalent reduction of molecular oxygen in a reaction mediated by NADPH oxidases and xanthine oxidase or nonenzymatically through redox-reactive compounds from the mitochondrial electron transport chain [25]. H2O2 is not a free radical but an ROS, whose cytotoxicity is likely due to the secondary production of singlet oxygen or hydroxyl radical (·OH) in redox reactions between transition-metal ions dismutations, the so-called Haber-Weiss reaction [26]. Therefore, H2O2 can be converted into a hydroxyl radical that is highly reactive and toxic to cells [27].
NO is a highly reactive, labile free radical that is short-lived. It has a vital role in the normal functioning of cells, mediating biological functions such as regulation of cardiac contractility and vasodilation [28,29]. Nitric oxide synthases (NOS) are isoforms responsible for NO synthesis by converting the amino acid l-arginine to l-citrulline. The NOS family contains three isoforms, neuronal NOS, endothelial NOS (eNOS), and the inducible form, iNOS. All NOS isoforms are similar concerning NO and other biological molecules such as l-citrulline [30].
Superoxide anion, H2O2, and NO can be dangerous when acting together and alone or with other molecules. In this context, the superoxide anion may react with NO to form peroxynitrite. H2O2 can be converted into hydroxyl radical (·OH) in the presence of reduced transition metals because it is highly reactive and toxic to the cell. H2O2 can also react with carbon dioxide, leading to the formation of the carbonate radical, which is a potent oxidant [27].
Reactive oxidants regulate physiological and pathophysiological conditions from prokaryotes to humans. ROS and RNS are constantly produced from internal metabolism and external exposure [[31], [32], [33]]. They act as critical signaling molecules to regulate cell division and programmed cell death [33]. However, uncontrolled production of oxidants results in oxidative stress that impairs cellular functions; this process contributes to the development of several diseases [22,[34], [35], [36]].
Oxidative stress can be characterized as ROS production exceeding the capacity of cellular antioxidant defenses to remove these toxic species, generating an imbalance between oxidizing and antioxidant agents [22,37,38]. Therefore, oxidation-reduction reactions (redox) in living cells are utilized to maintain homeostasis and are collectively referred to as redox signaling or redox control [39]. Redox homeostasis is maintained by redox signaling, which induces protective responses against oxidative stress.
The antioxidant enzyme system and the low-molecular-weight antioxidants have two protective mechanisms in cell scavenging ROS and maintaining redox homeostasis [22]. The low-molecular-weight antioxidants include glutathione, uric acid, ascorbic acid, alpha-tocopherol, and melatonin, which mediate neutralizing activities by causing transition metals' chelation preventing lipid peroxidation and others [40,41]. Ascorbic acid and alpha-tocopherol act in concert, with ascorbate being necessary to regenerate reduced alpha-tocopherol that provokes a reduction in lipid peroxidation. The peroxidation of lipids has deleterious consequences, disrupting the integrity of cell membranes through the oxidation of polyunsaturated fatty acids. This process may be initiated by highly reactive species such as hydroxyl and peroxyl radicals [42,43]. Thiol compounds such as thioredoxin can detoxify H2O2; however, they require conversion back to the reduced form by thioredoxin reductase [44]. This thioredoxin system, including thioredoxin reductase and NADPH, is a significant disulfide reductase system. It is a crucial antioxidant against oxidative stress mediated by its disulfide reductase activity, regulating protein dithiol/disulfide balance [45].
The enzymatic antioxidant defense system includes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) [46]. There are three metal-containing isoforms of SOD found in various cellular compartments [47,48]: SOD1 (copper-zinc SOD) is in the cytoplasm, nucleus, and plasma membrane; SOD2 (manganese SOD) is mainly in mitochondria [48,49]; and SOD3 (copper-zinc SOD) is unique in scavenging superoxide in the extracellular compartment, dismutating superoxides generated during the inflammatory cascade [48,50]. All isoforms of SOD catalyze the dismutation of superoxide radicals to generate H2O2, which is not a free radical and is less reactive than superoxide. However, it is called ROS because it is closely related to the generation and detoxification of free radicals. It can diffuse through cell and organelle membranes, where it acts as a second messenger in signal transduction pathways. H2O2 is in turn detoxified to water by CAT and GPx. Animal catalases are heme-containing enzymes that convert H2O2 to water and O2. They are localized mainly in subcellular organelles such as peroxisomes.
In contrast, GPx, a tetrameric selenoprotein, removes H2O2 by coupling its reduction with glutathione oxidation. GPx is present in the mitochondrial and cytoplasmic fractions [41,51,52]. GPx also reduces peroxides such as fatty acid hydroperoxides. Most animal tissues contain both CAT and GPx activity [47].
The antioxidant enzymes must act in concert since an imbalance in superoxide and H2O2 can result in the much more dangerous hydroxyl radical formation, as described previously. During the action of GPx in the detoxification of H2O2, glutathione (the primary cellular thiol redox buffer in cells, synthesized in the cytosol from l-glutamate, l-cysteine, and glycine) is oxidized (the glutathione disulfide form). It is an essential non-enzymatic endogenous antioxidant that can be regenerated by glutathione reductase and is responsible for the regeneration of reduced glutathione (glutathione form) with the consumption of NADPH. The latter is generated through the pentose phosphate pathway, of which glucose-6-phosphate dehydrogenase is the first enzyme [[53], [54], [55]]. In this manner, optimum levels of reduced glutathione are maintained [22]. The endogenous ratio of glutathione to glutathione disulfide indicates redox homeostasis within a cell. Higher glutathione levels also serve as a cofactor for other enzymes, including glyoxalase and peroxidase [56]. It has been suggested that the presence of GPx in the vicinity of folded catalase helps it remain functionally active [57].
The increase in antioxidant capacity removes excessive oxidants and prevents further severe oxidative injury. Therefore, the response of antioxidants to oxidative stress evolved as a critical defense mechanism to combat harmful effects of intrinsic and extrinsic oxidative insults and is preserved in all organisms [58]. Furthermore, in vivo studies in mammals suggest that organs and tissues contain distinct antioxidant systems that may form the basis for differential susceptibility to oxidative stress. Many advances have been made to understand the several antioxidant systems and their regulatory pathways [47].
3. Purine metabolism and redox biology
Nucleotides and nucleosides composing purinergic signaling can influence cell metabolism and fate. Thus, purines are involved with metabolic reprogramming and energy-dependent processes, in addition to composing genetic material and cofactors [59,60]. The availability of purines to bind purinergic receptors and nucleotidases is determined by purine metabolism, notoriously moderated by the purinosome, which is a purinergic metabolon. This temporary multimeric enzymatic complex efficiently binds to promote the physical proximity of the six enzymes involved in de novo purine pathway biosynthesis [61]. This dynamic association contains auxiliary proteins as well. It reverberates the concentration of purine molecules, as its shortage requests the de novo pathway upregulation, starting with the phosphoribosyl pyrophosphate (PRPP) as a substrate, originated from glycolysis and pentose phosphate pathway, and producing inosine 5’-monophosphate (IMP) to then increase nutrient demand with high energy consumption [62].
The purinosome colocalizes with mitochondria; this is a functional colocalization driven by microtubules that provides rapid substrates for this organelle to meet the high cellular demand for purines. This spatial interaction depends on mitochondria mTOR-related regular activity [63,64]. Notably, the activation of the P2X7 receptor downregulates the PI3K/AKT/mTOR pathway in tumor cell lines, impairing cell growth and, thus, cancer progression [65]. Various kinases may be involved in purinosome regulation, including the PI3/AKT cascade (directly related to mTOR) by managing PRPP availability and many more associated with G-protein-coupled receptors mitogenic signaling [66].
In addition to the de novo pathway, purine levels are controlled by the salvage pathway dependent on PRPP [67]. When purines are at homeostatic concentrations, most of the convertible purine pool (AMP, IMP, xanthosine monophosphate (XMP), and guanosine monophosphate [GMP]) is acquired by the salvage pathway through recycling of degraded nucleoside bases from precursor macromolecules, which requires less energy than the de novo pathway (Fig. 1) [59,66,68].
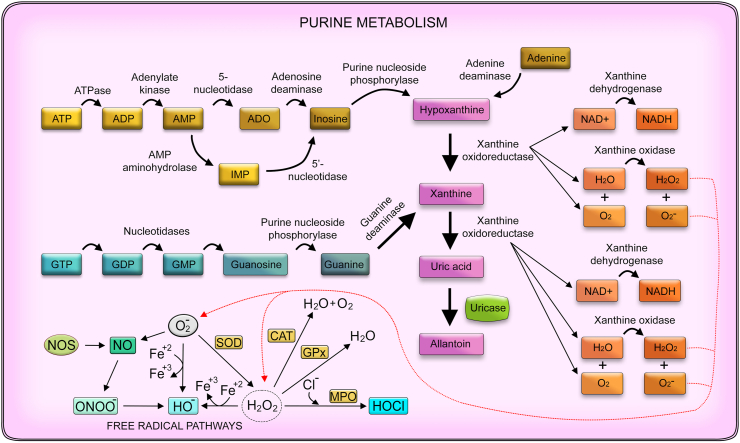
Fig. 1.
Purine metabolism and oxidative stress. Purine metabolism involves the hydrolysis of phosphate molecules such as ATP and GTP into nucleosides such as adenine, inosine, and guanine. Briefly, ATP is conversed into ADP by ATPase, then into ADP by adenylate kinase. Next, AMP can be transformed into ADO or IMP by 5’-nucleotidase and AMP aminohydrolase, respectively. Both can be converted into inosine by adenosine deaminase or 5’-nucleotidase as well. Finally, inosine can be metabolized into hypoxanthine, a purine derivative that compounds the salvage pathway that controls purine metabolism. Adenine can be converted into hypoxanthine as well by the action of the adenine deaminase enzyme. Hypoxanthine can be transformed into xanthine by xanthine oxidoreductase. Finally, GTP can be converted into GDP, GMP, and guanosine by different nucleotidases. Then, guanosine can be metabolized by purine nucleoside phosphorylase, generating guanine directly converted into xanthine by guanine deaminase. The purine-based xanthine, by the action of the xanthine oxidoreductase, can be altered to uric acid, representing the final stage of this enzymatic conversions in humans, as they lack the uricase enzyme that converts uric acid in allantoin, present in animals such as mice and rats. Thus, uric acid can accumulate and crystalize in the joints of humans and causes various conditions such as gout due to hyperuricemia. It is noteworthy that xanthine oxidoreductase possesses enzymatic forms, xanthine oxidase, and xanthine dehydrogenase, that in addition to altering purine metabolites, act in redox biology, as the former may produce reactive oxygen species such as superoxide and hydrogen peroxide as by-products and the latter may present NADH oxidase properties and thus, reduces NAD+ into NADH, contributing to the pro-oxidant system as well.
Purine metabolism is essential considering its association with diseases such as gout, an endocrine condition that occurs due to hyperuricemia, which increases blood urate (the final product of the salvage and de novo pathways). Accumulation and crystallization of urate in joints of patients are due to lack of uricase (urate oxidase), the enzyme that catalyzes the degradation of uric acid into allantoin [69]. Furthermore, monosodium urate crystals (MSU) can induce NLRP3 inflammasome active multimerization through potassium efflux [70]. This feature strongly correlates to P2X7 receptor activation, as the relationship between MSU and this purinergic receptor has already been described in regards to IL-1β and HMGB1 release [71].
The relationship between oxidative stress and neurodegenerative diseases has been widely described over the years, with increasing evidence of the complications being caused and worsened by oxygen-derived free radicals and nonradicals molecules, as well as flaws in the robust antioxidant defense [72]. Despite a strong link, antioxidant therapies have failed to prevent the advance of these pathological conditions. Urate has been described as an endogenous potent non-enzymatic antioxidant molecule. This compound is abundant in the cerebrospinal fluid and has presented a promising neuroprotective role for treating Parkinson's disease as it reduces dopamine oxidation rate, currently in a phase 3 clinical trial [73].
The antioxidant role of uric acid in oxidative stress is extensive per se or by synergy with other antioxidant systems, such as the interaction with the enzyme SOD as a mechanism to inhibit the conversion of superoxide anion to peroxynitrite after reacting with nitric oxide (NO) [74]. Urate is likewise a potent metallic antioxidant. It promotes stable chelation of ferric and ferrous ions, exerting its oxidative ability over ascorbate by forming urate-ion complexes without oxidizing itself and preventing liposomal lipid peroxidation by joining phospholipids within liposomal membranes [75,76].
The enzyme responsible for converting hypoxanthine into xanthine and then into uric acid is xanthine oxidoreductase (XOR), which takes two forms: xanthine dehydrogenase and xanthine oxidase (XO). The reversible interchange between these two forms occurs in numerous physiological and pathological situations, and it relies on an intermediary with both activities dependent on the C-terminal peptide [77]. Beyond urate production, xanthine oxidoreductase in a hypoxia context may also assist the antioxidant system via its nitrite reductase activity, generating NO, along with the withdrawal of electrons to reduce O2 at the FAD cavity within this enzyme [78].
In addition to antioxidant activity, xanthine oxidoreductase may produce ROS such as superoxide and hydrogen peroxide as subproducts of their oxygen via its XO and NADH oxidase proprieties enzymatic necessity, thereby significantly contributing to the pro-oxidant system (Fig. 1) [79,80]. Ives et al. found that ATP stimulation induces an increase in XO activity via PI3K–AKT signaling pathway forming uric acid and mitochondrial ROS, triggering NLRP3 inflammasome activation and IL-1β secretion [81]. These mechanisms can contribute to inflammation and disease. One of the pathological events that stimulate XO activity is contamination with the influenza virus, increasing free radicals synthesis correlated with the progression of the infection [82]. Hence, inhibition of XOR that attempts to decrease ROS assembly could substantially improve diseases such as hyperuricemia [83].
4. Mechanisms of ATP release
Adenosine triphosphate (ATP) is one of the most evolutionarily conserved nucleotides that acts as a signaling molecule under physiological or pathological contexts as a danger signaling molecule or alarmin [84]. This purine compound presents various features that classify it as an excellent alarmin: First, it can be quickly transported through exocytosis or pore channels; second, the substantial difference between its intra- and extracellular concentrations speeds transport and increases the signal-to-noise ratio nearly without background noise; and third, ATP is soluble in water, which facilitates its diffusion in the extracellular milieu that is predominantly aqueous [[85], [86], [87], [88]]. The receptors that recognize extracellular ATP, mainly P2 receptors, are expressed in almost every cell type, enabling this efficient danger signaling [87].
Hypoxia-mediated damage may induce cells to undergo necrotic death. ATP can be released into the extracellular environment due to cell membrane disruption, activating P2 receptors and NLRP3 inflammasome, creating a pro-inflammatory scenario [89]. Activation of pattern recognition receptors (PRRs) (i.e., Toll-like receptors—TLRs) also induces ATP secretion [90]. P2X7 receptor activation induces massive ATP release via its intrinsic ability to form a membrane pore or leading to secondary activation of pore-forming proteins [18]. Therefore, in addition to passive ATP release by cell rupture, this nucleotide can reach the extracellular medium mostly by active mechanisms such as vesicular exocytosis [91] or pore-forming channels [92]. Vesicular exocytosis is mediated by the vesicular nucleotide transporter VNUT, which controls ATP vesicular storage and consequently exocytosis [91,93]. The mechanism of pore-forming channels is composed of pannexin channels (e.g., pannexin-1), connexin hemichannels (connexin-43), and gasdermin pores [84,92], in addition to volume-regulated anion channels, calcium homeostasis modulator 1, and maxi-anion channels [94]. ATP non-lytic delivery may also occur utilizing transport vesicles or lysosomes [95].
Although they share some similarities, pannexin-dependent ATP release prevails under physiological conditions, while the role of connexin hemichannels in this context is not well defined [96]. Presumably, the action of connexins is more prominent during pathological circumstances, as their expression is closely associated with TLR activation and the ERK/AP1 signaling pathway [97]. Furthermore, pannexins cannot be classified as hemichannels. Their action does not rely on forming gap junctions between hemichannels from different cell membranes, connecting their cytoplasm directly, but instead on individual membrane channels of cells [98].
The phosphorylation of various connexin hemichannels residues induces conformational and functional changes during its life cycle, controlling its activity from synthesis until degradation, including its activation, permeability, and the open/close state [99,100]. Indeed, the phosphorylation of the serine residue Ser-368 reduces connexin-43 permeability [101]. Similarly, cell stress induced by metabolic inhibition in astrocytes increased the presence of connexin-43 (Cx43) in their membrane, accompanied by dephosphorylation and S-nitrosylation (a regular post-translational modification of proteins that usually occurs under oxidative stress). These alterations increase the permeability of the hemichannels, most likely because of inducing the open channel state [102,103]. During enteric neuroinflammation, glial cells stimulate enteric neurons death by producing NO, which in turn activates Cx43, corroborating its opening character, potentializing ATP release, and hence maintaining inflammation [104].
Hypoxic conditions in endothelial cells may reduce ATP release by reducing connexin-43 hemichannels expression concomitantly with an increase in Cx43 phosphorylation [105]. Moreover, the redox signaling could affect other properties within connexin hemichannels, including the oligomerization of the six connexin protein subunits to form the hemichannels and their electrical properties, cytoplasmatic transportation, and permeability [106].
Likewise, the phosphorylation of serine residue Ser-206 in pannexin channels can be modulated by NO in the cell lineage HEK-293 cells, reducing the pore-forming channel activity probably as a defensive mechanism to prevent uncontrolled cell death [107]. The post-translational changes on cysteine residues previously mentioned as S-nitrosylation take place in pannexins as well; however, unlike connexins, this modification prompts channel closing [108].
5. P2 receptors signaling in redox biology
In 1989, Kuroki and Minamaki published a seminal article showing that extracellular ATP could modulate redox biology. They found that human neutrophils stimulated by ATP in the presence of cytochalasin B could generate superoxide (O2-) [109]. Subsequent studies identified how ATP can induce the generation of free radicals. Nakanishi and colleagues found that peritoneal macrophages from guinea pigs did not require a priming stimulus as human neutrophils to generate O2- in response to ATP treatment [110]. ATP and UTP were implicated in O2- generation via NADPH oxidase activation [111]. Furthermore, lipopolysaccharide (LPS)-primed macrophages stimulated by extracellular ATP showed an increase in iNOS (nitric oxide synthase) mRNA levels [112,113]. Thus, purinergic signaling began to gain prominence as a signaling pathway capable of interfering with redox biology.
The first evidence linking purinergic receptors and redox signaling came from Shen and colleagues. The authors sought to understand the mechanism by which H2O2 was able to trigger contraction in the rat aorta. They found that this effect was mediated by activating ATP receptors [114]. Following this idea, they demonstrated for the first time the possible involvement of P2 receptors in rat aorta contraction activated by H2O2 using two non-selective P2 antagonists (suramin and RB-2) that completely abolished aorta contraction [115].
A close relation was established between changes in cytosolic [Ca+2] and ROS generation through the activation of signaling pathways composed by calcium-dependent proteins – isoforms of protein kinase C (PKC), MAP kinase members, and phospholipase A2 [116,117]. These mechanisms promoted the NADPH oxidase activation, the main enzyme involved in O2- generation [117]. Motivated by these reports about the relation of intracellular signaling pathways involved in ROS generation and also by the ability of extracellular ATP in triggering these pathways, Cruz and colleagues stimulated primary alveolar macrophages with ATP and other extracellular nucleotides (i.e., ADP, UTP, and UDP) and they measured increased ROS levels [118]. Despite a transient response, high ROS levels activated the phosphatidylinositol-3 kinase (PI3K) pathway, leading to AKT and ERK1/2 phosphorylation contributing to caspase-1 activation and IL-1β and IL-18 secretion (Fig. 2) [119]. Following the elucidative processes behind ATP-induced ROS generation, several reports found that P2 agonists or antagonists modulated ROS production [117].
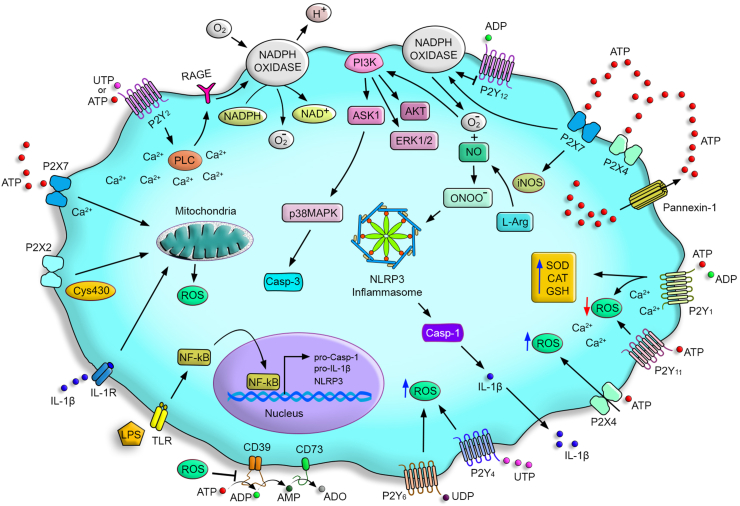
Fig. 2.
P2 receptors signaling pathways in the modulation of redox status. Pathogens can release pathogen-associated molecular patterns), such as lipopolysaccharides (LPS), which can activate pattern recognition receptors (PRRs) (i.g, TLRs). This activation leads to the transcription of genes involved in NLRP3 inflammasome activation (NLRP3, pro-casp 1, and pro-IL1β) and ATP release. ATP in high concentrations activates the P2X7 receptor inducing ROS production via NADPH oxidase activation and peroxynitrite. ROS is responsible for activating the phosphatidylinositol-3 kinase (PI3K) pathway, ASK1, AKT, ERK1/2, and p38 MAPK. These mechanisms can induce apoptosis via caspase-3 activation and NLRP3 inflammasome assembly, leading to caspase-1 activation and pro-IL-1β processing. Low concentrations of ATP or UTP can activate the P2Y2 receptor inducing PLC activation and Ca+2 mobilization; these components activate NADPH oxidase, responsible for producing ROS in a mechanism dependent on RAGE expression. The ATP also activates the P2X4 receptor that promotes ROS production and potentiates the activity of the P2X7 receptor for activating NADPH oxidase. The P2X7 also induces GSH release, increasing intracellular ROS. P2Y1 receptor increases the expression of antioxidant genes and activity of SOD, CAT, and GSH via intracellular Ca+2 signals. The P2Y1 receptor also induces the phosphorylation of endothelial nitric oxide synthase (eNOS). The sensor Cys430 allows the P2X2 receptor to sense the cell redox state in front of ROS production. P2Y4 and P2Y6 receptors promote ROS production, while the activation of the P2Y11 and P2Y12 receptor activation can inhibit ROS generation. ROS inhibits CD39 and CD73 activities.
5.1. P2X receptors
P2X receptors have been implicated in the modulation of redox biology. The P2X7 receptor is the most relevant in this context [13,18,120]. In the early 2000s, P2X7 receptor activation was described as an inductor of H2O2 production in primary rat microglia through a mechanism dependent on Ca+2 release from intracellular stores. These results were confirmed by using PPDAS and oxidized-ATP (P2X7 inhibitors) to block H2O2 production or BzATP (P2X7 agonist) to stimulate ROS release via activation of NADPH oxidase [121]. Pfeiffer and colleagues reported the same effect in RAW 264.7 murine macrophages. These effects were potentiated in LPS-primed cells, whereas P2X7 absence reduced the cell capacity to produce ROS. The P2X7 agonist BzATP also stimulated MAP kinase phosphorylation [122]. In the same line of evidence, ATP-P2X7 receptor signaling promoted ROS generation via NOX2 in murine macrophages [123,124]. The P2X7 receptor-induced ROS production leads to caspase-3 activation through the ASK1-p38 MAPK pathway resulting in apoptosis [123]. In addition to the relevance of the ATP-P2X7 axis for the MAPK pathway, NADPH oxidase activation and peroxynitrite generation are also crucial for IL-1β processing and release by endotoxin primed-human monocytes (Fig. 2) [125]. Recently, Zhang et al. described that P2X7 receptor activation induces GSH efflux, promoting an accumulation of intracellular ROS and subsequent NLRP3 inflammasome activation in the J774.1 murine macrophage cell line [126]. They also found that GSH and GSSG treatment inhibited NLRP3 inflammasome assembly and IL-1β secretion.
ROS generation via P2X7 receptor activation is not only related to immune cells. Mouse and rat submandibular glands produced ROS via P2X7 receptor activation; however, this occurred by different mechanisms. Rat submandibular glands produced ROS via NADPH oxidase in a calcium-dependent manner after treatment with P2X7 receptor agonists, whereas mice submandibular glands demonstrated a calcium-independent mechanism [127,128]. Primary gingival epithelial cells showed ROS production in response to eATP-P2X7 receptor signaling and NADPH oxidase activation [129]. P2X7 receptor can induce the production of reactive species through NADPH oxidase by increasing the expression of the p47phox subunit that binds to membrane subunit gp91phox producing reactive species [130].
Murine erythroleukemia cells (an erythroid cell line) also produced ROS following P2X7 receptor activation [131]. However, cortical neurons stimulated by P2X7 agonists presented mitochondrial dysfunction and cell death [132]. ATP treatment leads to mitochondrial toxicity and oxidative stress in HEK–hP2X7 cells culminating in apoptosis [133], suggesting some cell types are more susceptible to ATP-induced free radical production.
Interestingly, the P2X7 receptor is not the only P2X receptor that triggers the generation of free radicals. P2X4 receptor also mediates ROS production and autophagy in macrophages, and the mechanism appears to depend on both P2X4 and P2X7 receptors [134]. Regarding other P2X receptors, one that is also involved in the redox processes is the P2X2 receptor, which demonstrated increased activity in front of mitochondrial stress inducers, mercury, and H2O2. It is mediated by an intracellular redox sensor Cys430 that allows this receptor to sense the cell redox state [135].
Rat synoviocytes with collagen-induced arthritis presented predominant expression of P2X4 and P2Y2 receptors. When treated with ATP or BzATP, these cells produced ROS. However, treatment with BBG or rhein (a natural bioactive anthraquinone) suppressed ATP/BzATP-induced ROS production, which has been suggested to be controlled by P2X4-mediated extracellular Ca+2 entry [136]. In addition, breast cell cancer treated with ivermectin demonstrated a regulated ROS generation mechanism. The authors suggested that ivermectin induces P2X4/P2X7-dependent activation and then ATP release via pannexin-1. This mechanism potentiated P2X7 receptor signaling to activate NADPH oxidase and ROS generation, leading to cancer cell death [137].
Alterations in redox biology triggered by P2X receptors are pivotal to host defense against pathogens [1,138]. Toxoplasma gondii-infected macrophages control the parasite load via P2X7 receptor-induced ROS production [139]. P2X7 receptor induces ROS production via NADPH oxidase, while the NLRP3 inflammasome activation and IL-1β secretion induce mitochondrial ROS production via IL-1R activation [140]. Interestingly, in cerebral toxoplasmosis, the P2X7 receptor appears to be relevant for ROS production as its absence resulted in a reduced ROS production and increased number of cysts in the brain [141]. In bacterial sepsis, P2X7 and P2X4-mediated ROS production may be necessary to pathogen control in phagocytes [142]. However, excessive ROS and NO production in sepsis result in oxidative stress, tissue damage, organ dysfunction, and poor outcomes [8,143,144]. In this context, P2X7 receptor deletion attenuated ROS and RNS production in the mice liver and brain, promoting increased SOD and catalase (CAT) activity, which diminishes oxidative stress and protection from organ injury [143,144].
5.2. P2Y receptors
P2Y are G-coupled receptors also identified as modulators of cell redox status. The Gq/11-coupled P2Y1 receptor activated by ADP protected astrocytes from H2O2-induced cell death, upregulating the expression of oxidoreductase genes. This effect was observed by treating cells with P2Y1 agonist 2-methylthio-adenosine-5’-diphosphate (2MeSADP). The mechanism was dependent on phospholipase C activation and Ca+2 release. When cells were treated with P2Y1 antagonist MRS2179, the protective effect in astrocytes was abolished [145]. Likewise, 2MeSADP treatment reduced ROS generation, while UDP (P2Y6 agonist) increased ROS production in hippocampal astrocyte cultures (Fig. 2) [146].
Rat hippocampal cultures treated with eATP showed neuroprotective effects against oxidative stress and cell death in a mechanism dependent on the P2Y1 receptor. The suppression of the P2Y1 receptor by selective siRNAs inhibited the protection against cell death. A critical molecule involved in this process is the cytokine IL-6 because 2MeSADP treatment of astrocytes cultures showed increased IL-6 levels, and the neuroprotection was suppressed in the presence of anti-IL-6 antibody [147]. Protective effects of P2Y1 receptor activation also involve intracellular Ca+2 release and subsequent expression of antioxidant genes such as CAT, SOD2, SOD3, and genes involved in glutathione (GSH) metabolism (Fig. 2) [148].
Other cell types express P2Y1 receptors, such as cultured endothelial cells. ADP promoted the phosphorylation of endothelial signaling proteins in these cell cultures, such as eNOS. However, this phosphorylation was not observed when these cultures were preincubated with PEG-catalase, which degrades H2O2. In this way, endogenous H2O2 mediates ADP signaling responses in vascular walls [149]. Skeletal muscle cells with electrical stimulation produce ROS in a mechanism dependent on ATP release and P2Y1 receptor activation. This response is possibly mediated by NOX2 and PKC signaling [150].
Human endothelial cells treated with oxidized-LDL and ATP/UTP showed increased ROS production, NADPH oxidase activation and induced the receptor for advanced glycation end-products (RAGE) expression. This effect was inhibited by the P2Y2 receptor and RAGE siRNA-transfected endothelial cells (ECs), demonstrating that ROS production depended on this receptor and RAGE expression [151]. Similarly, osteoblast-like cells express only the P2Y2 receptor, and in the presence of H2O2, these cells release ATP and modify Ca+2 metabolism, possibly via P2Y2 [152]. Leishmania amazonensis-infected macrophages treated with UTP produced ROS, which might be attributed to P2Y2 and P2Y4 receptors upregulation. The same effect has been demonstrated in vivo, once UTP treatment promoted ROS production and phagocytic cells recruitment, limiting parasite replication [153,154].
The P2Y11 receptor stimulation attenuated ROS production in aortas isolated from LPS-treated rats [155]. The ATP also exerts an inhibitory action in frog neuromuscular junction, and the P2Y12 receptor-mediated this effect. This was demonstrated using the P2Y12 receptor-specific antagonist 2-methylthioadenosine-5’-monophosphate (2-MeSAMP), which did not reduce end-plate currents [156,157]. ATP induces H2O2 increases with lipid peroxidation [156]; the authors found that NADPH oxidases (NOX) are probably the primary source of ROS responsible for the inhibitory action of ATP.
Interestingly, a nucleoside 5′-thiophosphate analog, the 2-SMe-ADP(α-S), showed antioxidant/neuroprotective activities in pC12 cells stimulated with FeSO4. This compound acted via P2Y1 and P2Y12 receptor activation, inhibiting ROS production [158]. The P2Y12 receptor is also involved in diabetic wounding once the ADP can reduce ROS production, mitigating the inflammatory response. Meanwhile, a specific P2Y12 antagonist, clopidogrel, restored ROS production [159].
6. Ectonucleotidase functionality and oxidative stress
Ectonucleotidases are nucleotide-metabolizing enzymes critical for the sophisticated regulation of purinergic receptors ligands availability. The ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases), ecto-nucleotide pyrophosphatase/phosphodiesterases (E-NPPs), and ecto-5′-nucleotidase are the leading ectonucleotidases families. E-NTPDases hydrolyze extracellular tri- and diphosphonucleosides to monophosphonucleosides, while E-NPPs hydrolyze pyrophosphate and phosphodiester bonds in a wide range of substrates, including tri- and diphosphonucleosides [11,160]. Among the ectonucleotidases, E-NTPDase1/CD39 and ecto-5’-nucleotidase/CD73 are the most relevant enzymes [11].
In the 1990s, a seminal paper characterized the biochemical activity of vascular CD39. The authors found that CD39 enzymatic activity and immunocontent were significantly decreased by oxidative stress [161]. Further studies found that saturated fatty acids and antioxidants can restore CD39 activity by reducing lipid peroxidation [161,162]. CD73 activity is also inhibited by oxidative stress, and antioxidants reversed this inhibition (Fig. 2) [163].
CD39 and CD73 are critical enzymes of the immunoregulatory machinery of regulatory T cells (Treg) by scavenging ATP generating adenosine [164,165]. Recently, Gerner et al., 2020 found that the TGF-β-mediated CD39 upregulation in Tregs is impaired by ROS [166]. Furthermore, interferon gamma-producing CD8+ T cells generate ROS, which increases CD39 expression as a regulatory mechanism via JNK and NFκB signaling [167]. The increase of CD39 expression favors adenosine generation and A2A activation with immunoregulatory effects [167].
Although the activity of these enzymes is affected by free radicals, the overexpression of human CD39 and CD73 protects EC against H2O2-induced oxidative stress and damage [168]. Intracellular pathogens have shown the ability to manipulate the host cell, upregulating CD73 expression for decreasing ROS generation [169]. P. gingivalis, a gram-negative oral bacterium, significantly boosts CD73 activity and expression in host cells to overcome the microbicidal effects of ROS [169]. CD73 expression in the mouse brain also promotes parasite spreading in a model of cerebral toxoplasmosis [170].
7. P1 receptors signaling in redox biology
As has been extensively discussed elsewhere, adenosine is a powerful vasoactive nucleotide derivative component in hearts submitted with hypoxic stress [171]. Hypoxia may occur because of obstruction or insufficient blood frow, namely ischemia; in these circumstances, adenosine concentration increases substantially and may act through A1 and A3 receptors to promote myocardial ischemic preconditioning, a preventive mechanism after ischemic lesions [172] that aids cytoprotection by a variety of responses, such as inhibited production of ROS by activated neutrophils [173,174].
Adenosine has a close relationship with hypoxia-inducible factor-1 (HIF-1), enhancing its expression in human melanoma cells at the translation level in a hypoxic framework mediated through p44/42 MAP and p38 MAP kinases pathways stimulated by A3 receptor activation [175]. However, HIF-1 might play dual roles in ischemia-induced by hypoxia, being protective or toxic depending on several inducible factors of this illness, as well as the cell type [176]. In this context, agonism of A1 and A3 receptors, together with LPS incitement, suppresses HIF-1 expression and aggregation in astrocytes by inhibiting phosphorylation of MAP kinases such as p44 and p38 MAPK, hence preventing inflammation and aggressive hypoxic injury, for example, by reducing iNOS inflammatory influence in these cells [177]. In high-altitude hypoxia, resolution can be achieved by augmenting CD73 activity, which in turn expands adenosine plasma concentration, instigating A2B receptors in erythrocytes and promoting AMPK-mediated 2,3-bisphosphoglycerate production, a molecule generated in glycolysis that increases these cell's ability to deliver O2 from hemoglobin into urgent sites [178].
The brain tumor glioblastoma possesses a cellular subpopulation of stem-like cells responsible for its high invasiveness capacity stimulated by the hypoxic extracellular adenosine boost conducted by heightening CD73 activity [179]. Still, concerning cerebral ischemia, the upregulation of the A1 receptor diminishes neuronal damage, protecting the brain against the disturbance, unlike the damaging effects produced by the A2A receptor [180,181]. The eventual damage caused after restoring average blood flux following cardiac ischemia is known as ischemia-reperfusion injury. This involves various pathological conditions that oxygen radicals can worsen. Thus, it can be partly attenuated at a long-term period by antioxidants components [182,183].
Activation of the A3 receptor increased the activity of numerous antioxidant enzymes that maintain the cellular redox homeostasis by decreasing ROS concentration, such as SOD, catalase, and glutathione peroxidase [184]. In addition, the use of adenosine analog in mobilizing A2A and A2B receptors after ischemia-reperfusion injury showed the cardioprotective impact of mitochondria in vivo and in vitro when dampening mitochondrial superoxide generation per electron transport chain [185]. Furthermore, oxygen-derived radicals such as H2O2 elicit a negative feedback loop in cytotoxicity by increasing the expression of cytoprotective A1 receptors at smooth muscle cells in an NF-kB-dependent manner [186]. Once again, the outcome is reversed in A2A receptors, as ROS reduced their NF-kB-dependent expression [187].
P1 receptors can be liable for a variety of other diseases. ROS was accountable for apoptosis in Treg cells in human and mouse tumor cases, a type of programmed cell death that enables these immune structures to create an immunosuppressive microenvironment via active A2A receptors [188]. The A2A receptors are also related to increased intraocular pressure by upregulating inflammatory markers, such as ROS that elicit the release of NO, IL-1β, and TNF, heightening oxidative stress present in other pathological circumstances [189]. The main effects of adenosine receptors on redox biology are summarized in Table 1.
Table 1.
Modulation of redox status by adenosine receptors.
| Adenosine receptor | G-coupled protein | Cell type | Effect redox status of cell |
|---|---|---|---|
| A2A | Gs → ↑Adenylate cyclase | Microglial cells | ↑ROS, NO, IL-1β, TNF [175] |
| Cardiac myoblast cells | ↓ROS [171] | ||
| A2B | Gs → ↑Adenylate cyclase | Erythrocytes | ↑2,3-BPG, ↑O2 [164] |
| Gq → ↑ PLCγ | |||
| A1 | Gi → Ø Adenylate cyclase Go → ↑PLCβ |
Neutrophils | ↓ROS [159,160] |
| Astrocytes | ↓HIF-1, iNOS [163] | ||
| Smooth muscle cells | ↓ROS, ↑NF-kB [172] | ||
| A3 | Gi → Ø Adenylate cyclase Gq → ↑PLCγ |
Neutrophils | ↓ROS [159,160] |
| Melanoma cells | ↑HIF-1 [161] | ||
| Astrocytes | ↓HIF-1, iNOS [163] | ||
| Basophilic leukemia cell | ↑SOD, CAT, GPx [170] |
PLC: Phospholipase C; ROS: Reactive oxygen species, NO: Nitrogen oxide, IL-1β: Interleukin 1 beta, TNF: Tumor necrosis factor, 2,3-BPG: 2,3-Bisphosphoglyceric acid, HIF-1: Hypoxia inducible factor-1α, iNOS: inducible nitrogen oxide synthase, SOD: superoxide dismutase, CAT: catalase, GPx: glutathione peroxidase.
8. Conclusion
Purinergic signaling and purine metabolism are modulators of redox biology in certain circumstances. The final product of purine metabolism in humans, uric acid, has antioxidant effects per se or synergy with antioxidant systems such as the interaction with the enzyme SOD as a mechanism to inhibit the conversion of superoxide anion to peroxynitrite. In addition, the enzyme responsible for the conversion of hypoxanthine into xanthine and then into uric acid in humans XO produces ROS such as superoxide and hydrogen peroxide as subproducts of their oxygen enzymatic necessity, thereby contributing to ROS-mediated NLRP3 inflammasome activation and IL-1β secretion.
P2 signaling activated by tri -and diphosphonucleotides can modulate the redox status of cells. In general, P2X receptors, mainly P2X7 and P2X4, have a prominent role in mediating ROS production during inflammation and infection. P2Y receptors can also induce ROS and NO production. However, P2Y1 and P2Y11 can exert antioxidant effects, causing protection against oxidative stress due to inflammation or cell damage.
CD39/CD73 axis functionality is impaired during oxidative stress aggravating pathological conditions of inflammation, infection, and tissue injury. By contrast, the upregulation of these enzymes can decrease ATP-induced oxidative stress and minimizes ROS-induced cell damage but favors adenosine generation and pathogen survival during infection. Finally, in general, adenosine receptors have been shown to activate the mechanisms for maintaining redox homeostasis in pathological situations.
Therefore, elucidating mechanisms and signaling pathways by which purinergic signaling and purine metabolism can affect the redox biology of the cell may suggest new therapeutic approaches for pathologies caused by oxidative stress, such as P2 receptor antagonists or soluble apyrases for nucleotide degradation. In addition, in infectious diseases where P2 receptor activation contributes to reactive species production and pathogen elimination, the administration of agonists and ectonucleotidase neutralizing antibodies are feasible strategies.
Declaration of competing interest
The authors declare no competing or financial interests.
Acknowledgments
This work was supported by funds from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil – CNPq (306839/2019-9) RCS and (305857/2020-7) LEBS. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro - FAPERJ (E-26/202.701/2019 and E-26/010.002260/2019 to LEBS; E-26/010.002985/2014, E-26/010.101036/2018 and E-26/202.774/2018 to RCS), and Fundação e Amparo à Pesquisa do Rio Grande do Sul (FAPERGS/19/2551-0001684-0 2019 – PqG to ATSW).
References
- 1.Coutinho-Silva R., Savio L.E.B. Purinergic signalling in host innate immune defence against intracellular pathogens. Biochem. Pharmacol. 2021 doi: 10.1016/j.bcp.2021.114405. [DOI] [PubMed] [Google Scholar]
- 2.Idzko M., Ferrari D., Eltzschig H.K. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non‐adrenergic inhibitory nerves in the gut. Br. J. Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- 5.Ralevic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 6.Fredholm B.B., Abbracchio M.P., Burnstock G., Daly J.W., Harden K.T., Jacobson K.A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. doi: 10.1016/j.physbeh.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson K.A., Delicado E.G., Gachet C., Kennedy C., von Kügelgen I., Li B., Miras-Portugal M.T., Novak I., Schöneberg T., Perez-Sen R., Thor D., Wu B., Yang Z., Müller C.E. Update of P2Y receptor pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020;177:2413–2433. doi: 10.1111/bph.15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leite-Aguiar R., Alves V.S., Savio L.E.B., Coutinho-Silva R. Targeting purinergic signaling in the dynamics of disease progression in sepsis. Front. Pharmacol. 2021;11:1–6. doi: 10.3389/fphar.2020.626484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann H., Zebisch M., Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robson S.C., Sévigny J., Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G., Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol. 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 13.Alves V.S., Leite-Aguiar R., da Silva J.P., Coutinho-Silva R., Savio L.E.B. Purinergic signaling in infectious diseases of the central nervous system. Brain Behav. Immun. 2020:1–11. doi: 10.1016/j.bbi.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnstock G., Knight G.E. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 15.Burnstock G., Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9–10. doi: 10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burnstock G. Short- and long-term (trophic) purinergic signalling. Phil. Trans. B. 2016;371 doi: 10.1098/rstb.2015.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnstock G. 2020. Purinergic Signaling: Methods and Protocols. [DOI] [PubMed] [Google Scholar]
- 18.Savio L.E.B., Mello P. de A., da Silva C.G., Coutinho-Silva R. The P2X7 receptor in inflammatory diseases: angel or demon? Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutinho-Silva R., Ojcius D.M. Role of extracellular nucleotides in the immune response against intracellular bacteria and protozoan parasites. Microb. Infect. 2012;14:1271–1277. doi: 10.1016/j.micinf.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naviaux R.K. Metabolic features of the cell danger response. Mitochondrion. 2014;16:7–17. doi: 10.1016/j.mito.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Piacenza L., Trujillo M., Radi R. Reactive species and pathogen antioxidant networks during phagocytosis. J. Exp. Med. 2019;216:501–516. doi: 10.1084/jem.20181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliwell B. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gadalla M.M., Snyder S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halliwell B., Zentella A., Gomez E.O., Kershenobich D. Antioxidants and human disease: a general introduction. Nutr. Rev. 1997;55:S44–S49. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H., Wallace D.C., Epstein C.J. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 26.Fridovich I. Superoxide dismutases. Adv. Enzymol. Relat. Area Mol. Biol. 1974;41:35–97. doi: 10.1080/09553008314551231. [DOI] [PubMed] [Google Scholar]
- 27.Illés E., Mizrahi A., Marks V., Meyerstein D. Carbonate-radical-anions, and not hydroxyl radicals, are the products of the Fenton reaction in neutral solutions containing bicarbonate. Free Radic. Biol. Med. 2019;131:1–6. doi: 10.1016/j.freeradbiomed.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Cannon R.O. Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin. Chem. 1998;44:1809–1819. [PubMed] [Google Scholar]
- 29.Lind M., Hayes A., Caprnda M., Petrovic D., Rodrigo L., Kruzliak P., Zulli A. Inducible nitric oxide synthase: good or bad? Biomed. Pharmacother. 2017;93:370–375. doi: 10.1016/j.biopha.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres M.A. ROS in biotic interactions. Physiol. Plantarum. 2010;138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 33.Torres M.A., Morales J., Sánchez-Rodríguez C., Molina A., Dangl J.L. Functional interplay between arabidopsis NADPH oxidases and heterotrimeric G protein. Mol. Plant Microbe Interact. 2013;26:686–694. doi: 10.1094/MPMI-10-12-0236-R. [DOI] [PubMed] [Google Scholar]
- 34.Harman D. Aging: a theory on free radical radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 35.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 36.Harman D. The aging process. Proc. Natl. Acad. Sci. U. S. A. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sies H. On the history of oxidative stress: concept and some aspects of current development. Curr. Opin. Toxicol. 2018;7:122–126. doi: 10.1016/j.cotox.2018.01.002. [DOI] [Google Scholar]
- 38.Leopold J.A., Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic. Biol. Med. 2009;47:1673–1706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elias R.J., Kellerby S.S., Decker E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008;48:430–441. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- 41.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 42.McCay P.B. Vitamin E: interactions with free radicals and ascorbate. Annu. Rev. Nutr. 1985;5:323–340. doi: 10.1146/annurev.nutr.5.1.323. [DOI] [PubMed] [Google Scholar]
- 43.Wyse A.T.S., Netto C.A. Behavioral and neurochemical effects of proline. Metab. Brain Dis. 2011;26:159–172. doi: 10.1007/s11011-011-9246-x. [DOI] [PubMed] [Google Scholar]
- 44.Argyrou A., Blanchard J.S. Flavoprotein disulfide reductases: advances in chemistry and function progress in nucleic acid research and molecular biology. Prog. Nucleic Acid Res. Mol. Biol. 2004;78:89–142. doi: 10.1016/S0079-6603(04)78003-4. http://www.sciencedirect.com/science/article/B7CV7-4CPDVJF-4/2/38153ef1f733038948db65015442e065 The flavoprotein disulfide reductases represent a family of enzymes that show high sequence and structural homology. They catalyze the pyridine-nucleotide-depe [DOI] [PubMed] [Google Scholar]
- 45.Lu J., Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 46.Griendling K.K., Sorescu D., Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.RES.86.5.494. [DOI] [PubMed] [Google Scholar]
- 47.Limón-Pacheco J., Gonsebatt M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009;674:137–147. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Ye Z.W., Zhang J., Townsend D.M., Tew K.D. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta Gen. Subj. 2015;1850:1607–1621. doi: 10.1016/j.bbagen.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trachootham D., Lu W., Ogasawara M.A., Del Valle N.R., Huang P. Redox regulation of cell survival. Antioxidants Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nightingale H., Kemp K., Gray E., Hares K., Mallam E., Scolding N., Wilkins A. Changes in expression of the antioxidant enzyme sod3 occur upon differentiation of human bone marrow-derived mesenchymal stem cells in vitro. Stem Cell. Dev. 2012;21:2026–2035. doi: 10.1089/scd.2011.0516. [DOI] [PubMed] [Google Scholar]
- 51.Friedmann Angeli J.P., Conrad M. Selenium and GPX4, a vital symbiosis. Free Radic. Biol. Med. 2018;127:153–159. doi: 10.1016/j.freeradbiomed.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Tappel M.E., Chaudiere J., Tappel A.L. Glutathione peroxidase activities of animal tissues. Comp. Biochem. Physiol. B. 1982;73:945–949. doi: 10.1016/0305-0491(82)90341-8. [DOI] [PubMed] [Google Scholar]
- 53.Gul M., Kutay F.Z., Temocin S., Hanninen O. Cellular and clinical implications of glutathione. Indian J. Exp. Biol. 2000;38:625–634. [PubMed] [Google Scholar]
- 54.Dringen R., Gutterer J.M., Hirrlinger J. Glutathione metabolism in brain: metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000;267:4912–4916. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 55.Moosavi F., Hosseini R., Saso L., Firuzi O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2015;10:23–42. doi: 10.2147/DDDT.S96936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohen R., Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 57.Furkan M., Rizvi A., Alam M.T., Naeem A. Peroxidase improves the activity of catalase by preventing aggregation during TFE-induced denaturation. J. Biomol. Struct. Dyn. 2018;36:551–560. doi: 10.1080/07391102.2017.1287007. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H., Davies K.J.A., Forman H.J. Oxidative stress response and Nrf 2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036.Oxidative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang Z., Xie N., Illes P., Di Virgilio F., Ulrich H., Semyanov A., Verkhratsky A. From purines to purinergic signalling: molecular functions and human diseases. Signal Transduct. Target. Ther. 2021;6 doi: 10.1038/s41392-021-00553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang Z., Ye W., Chen H., Kuang X., Guo J., Xiang M., Peng C., Chen X., Liu H. Role of purines in regulation of metabolic reprogramming. Purinergic Signal. 2019;15:423–438. doi: 10.1007/s11302-019-09676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.An S., Kumar R., Sheets E.D., Benkovic S.J. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. doi: 10.1126/science.1152241. [DOI] [PubMed] [Google Scholar]
- 62.Pedley A.M., Benkovic S.J. A new view into the regulation of purine metabolism: the purinosome. Trends Biochem. Sci. 2017;42:141–154. doi: 10.1016/j.tibs.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.French J.B., Jones S.A., Deng H., Pedley A.M., Kim D., Chan C.Y., Hu H., Pugh R.J., Zhao H., Zhang Y., Huang T.J., Fang Y., Zhuang X., Benkovic S.J. Spatial colocalization and functional link of purinosomes with mitochondria. Science. 2016;351:733–737. doi: 10.1126/science.aac6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan C.Y., Pedley A.M., Kim D., Xia C., Zhuang X., Benkovic S.J. Microtubule-directed transport of purine metabolons drives their cytosolic transit to mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2018;115:13009–13014. doi: 10.1073/pnas.1814042115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bian S., Sun X., Bai A., Zhang C., Li L., Enjyoji K., Junger W.G., Robson S.C., Wu Y. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PloS One. 2013;8 doi: 10.1371/journal.pone.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang Y., French J., Zhao H., Benkovic S. G-protein-coupled receptor regulation of de novo purine biosynthesis: a novel druggable mechanism. Biotechnol. Genet. Eng. Rev. 2013;29:31–48. doi: 10.1080/02648725.2013.801237. [DOI] [PubMed] [Google Scholar]
- 67.Camici M., Garcia-gil M., Pesi R., Allegrini S., Tozzi M.G. Purine-metabolising enzymes and apoptosis in cancer. Cancers. 2019;11:1–27. doi: 10.3390/cancers11091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murray A.W. The biological significance of purine salvage. Annu. Rev. Biochem. 1971;40:811–826. doi: 10.1146/annurev.bi.40.070171.004115. [DOI] [PubMed] [Google Scholar]
- 69.Richette P., Bardin T. Gout, Lancet. 2010;375:318–328. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- 70.Hari A., Zhang Y., Tu Z., Detampel P., Stenner M., Ganguly A., Shi Y. Activation of NLRP3 inflammasome by crystalline structures via cell surface contact. Sci. Rep. 2014;4:1–8. doi: 10.1038/srep07281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marinho Y., Marques-da-Silva C., Santana P.T., Chaves M.M., Tamura A.S., Rangel T.P., Gomes-e-Silva I.V., Guimarães M.Z.P., Coutinho-Silva R. MSU Crystals induce sterile IL-1β secretion via P2X7 receptor activation and HMGB1 release. Biochim. Biophys. Acta Gen. Subj. 2019;1864 doi: 10.1016/j.bbagen.2019.129461. [DOI] [PubMed] [Google Scholar]
- 72.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.1007/s00330-016-4419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crotty G.F., Ascherio A., Schwarzschild M.A. Targeting urate to reduce oxidative stress in Parkinson disease. Exp. Neurol. 2017;298:210–224. doi: 10.1016/j.expneurol.2017.06.017.Targeting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. http://www.ncbi.nlm.nih.gov/pubmed/17237348%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/17237348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies K.J.A., Sevanian A., Muakkassah-Kelly S.F., Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem. J. 1986;235:747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sevanian A., Davies K.J.A., Hochstein P. Serum urate as an antioxidant for ascorbic acid. Am. J. Clin. Nutr. 1991;54:1129S–1134S. doi: 10.1007/BF02545280. [DOI] [PubMed] [Google Scholar]
- 77.Nishino T., Okamoto K., Kawaguchi Y., Matsumura T., Eger B.T., Pai E.F., Nishino T. The C-terminal peptide plays a role in the formation of an intermediate form during the transition between xanthine dehydrogenase and xanthine oxidase. FEBS J. 2015;282:3075–3090. doi: 10.1111/febs.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cantu-Medellin N., Kelley E.E. Xanthine oxidoreductase-catalyzed reduction of nitrite to nitric oxide: insights regarding where, when and how, Nitric Oxide - Biol. Chem. 2013;34:19–26. doi: 10.1016/j.niox.2013.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bortolotti M., Polito L., Battelli M.G., Bolognesi A. Xanthine oxidoreductase: one enzyme for multiple physiological tasks. Redox Biol. 2021;41 doi: 10.1016/j.redox.2021.101882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Furuhashi M. 2020. New Insights into Purine Metabolism in Metabolic Diseases: Role of Xanthine Oxidoreductase Activity. [DOI] [PubMed] [Google Scholar]
- 81.Ives A., Nomura J., Martinon F., Roger T., LeRoy D., Miner J.N., Simon G., Busso N., So A. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat. Commun. 2015;6 doi: 10.1038/ncomms7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Akaike T., Ando M., Oda T., Doi T., Ijiri S., Araki S., Maeda H. Dependence on O2- generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J. Clin. Invest. 1990;85:739–745. doi: 10.1172/JCI114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu N., Xu H., Sun Q., Yu X., Chen W., Wei H., Jiang J., Xu Y., Lu W. The role of oxidative stress in hyperuricemia and xanthine oxidoreductase (XOR) inhibitors. Oxid. Med. Cell. Longev. 2021;2021:1–15. doi: 10.1155/2021/1470380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Junger W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yegutkin G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014;49:473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 86.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 87.Di Virgilio F., Vuerich M. Purinergic signaling in the immune system. Auton. Neurosci. Basic Clin. 2015;191:117–123. doi: 10.1016/j.autneu.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Dosch M., Gerber J., Jebbawi F., Beldi G. Mechanisms of ATP release by inflammatory cells. Int. J. Mol. Sci. 2018;19:1–16. doi: 10.3390/ijms19041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iyer S.S., Pulskens W.P., Sadler J.J., Butter L.M., Teske G.J., Ulland T.K., Eisenbarth S.C., Florquin S., Flavell R.A., Leemans J.C., Sutterwala F.S. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen H.B., Briggs K.T., Marino J.P., Ravid K., Robson S.C., Mosser D.M. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood. 2013;122:1935–1945. doi: 10.1182/blood-2013-04-496216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sawada K., Echigo N., Juge N., Miyaji T., Otsuka M., Omote H., Yamamoto A., Moriyama Y. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Praetorius H.A., Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moriyama Y., Hiasa M., Sakamoto S., Omote H., Nomura M. Vesicular nucleotide transporter (VNUT): appearance of an actress on the stage of purinergic signaling. Purinergic Signal. 2017;13:387–404. doi: 10.1007/s11302-017-9568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taruno A. ATP release channels. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antonioli L., Pacher P., Vizi E.S., Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lohman A.W., Isakson B.E. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014;588:1379–1388. doi: 10.1016/j.febslet.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin J., Zhang G., Zhang X., Tan B., Lv Z., Liu M., Ren H., Qian M., Du B. TLR-activated gap junction channels protect mice against bacterial infection through extracellular UDP release. J. Immunol. 2016;196:1790–1798. doi: 10.4049/jimmunol.1501629. [DOI] [PubMed] [Google Scholar]
- 98.Sosinsky G.E., Boassa D., Dermietzel R., Duffy H.S., Laird D.W., MacVicar B.A., Naus C.C., Penuela S., Scemes E., Spray D.C., Thompson R.J., Zhao H.B., Dahl G. Pannexin channels are not gap junction hemichannels. Channels. 2011;5:193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solan J.L., Lampe P.D. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J. Membr. Biol. 2007;217:35–41. doi: 10.1007/s00232-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solan J.L., Lampe P.D. Connexin 43 phosphorylation: structural changes and biological effects. Biochem. J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bao X., Reuss L., Altenberg G.A. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of serine 368. J. Biol. Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- 102.Retamal M.A., Cortés C.J., Reuss L., Bennett M.V.L., Sáez J.C. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Retamal M.A. Connexin and Pannexin hemichannels are regulated by redox potential. Front. Physiol. 2014;5 FEB:1–9. doi: 10.3389/fphys.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown I.A.M., McClain J.L., Watson R.E., Patel B.A., Gulbransen B.D. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol. 2016;2:77–91. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faigle M., Seessle J., Zug S., El Kasmi K.C., Eltzschig H.K. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PloS One. 2008;3 doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.García I.E., Sánchez H.A., Martínez A.D., Retamal M.A. Redox-mediated regulation of connexin proteins; focus on nitric oxide. Biochim. Biophys. Acta Biomembr. 2018;1860:91–95. doi: 10.1016/j.bbamem.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 107.Poornima V., Vallabhaneni S., Mukhopadhyay M., Bera A.K. Nitric oxide inhibits the pannexin 1 channel through a cGMP-PKG dependent pathway. Nitric Oxide - Biol. Chem. 2015;47:77–84. doi: 10.1016/j.niox.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Lohman A.W., Weaver J.L., Billaud M., Sandilos J.K., Griffiths R., Straub A.C., Penuela S., Leitinger N., Laird D.W., Bayliss D.A., Isakson B.E. S-nitrosylation inhibits pannexin 1 channel function. J. Biol. Chem. 2012;287:39602–39612. doi: 10.1074/jbc.M112.397976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuroki M., Minakami S. Extracellular ATP triggers superoxide production in human neutrophils. Biochem. Biophys. Res. Commun. 1989;162:377–380. doi: 10.1016/0006-291x(89)92007-x. https://www.unhcr.org/publications/manuals/4d9352319/unhcr-protection-training-manual-european-border-entry-officials-2-legal.html?query=excom 1989 [DOI] [PubMed] [Google Scholar]
- 110.Nakanishi M., Takihara H., Minoru Y., Yagawa K. Extracellular ATP itself elicits superoxide generation in Guinea pig peritoneal macrophages. FEBS Lett. 1991;282:91–94. doi: 10.1016/0014-5793(91)80451-8. [DOI] [PubMed] [Google Scholar]
- 111.Seifert R., Burde R., Schultz G. Activation of NADPH oxidase by purine and pyrimidine nucleotides involves G proteins and is potentiated by chemotactic peptides. Biochem. J. 1989;259:813–819. doi: 10.1042/bj2590813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tonetti M., Sturla L., Bistolfi T., Benatti U., Deflora A. Extracellular ATP potentiates nitric oxide synthase expression induced by lipopolysaccharide in RAW-264.7 murine macrophages. Biochem. Biophys. Res. Commun. 1994;203:430–435. doi: 10.1006/bbrc.1994.2200. [DOI] [PubMed] [Google Scholar]
- 113.Tonetti M., Sturla L., Giovine M., Benatti U., Deflora A. Extracellular ATP enhances mRNA levels of nitric oxide synthase and TNF-α in lipopolysaccharide-treated raw 264.7 murine macrophages. Biochem. Biophys. Res. Commun. 1995;214:125–130. doi: 10.1006/bbrc.1995.2265. [DOI] [PubMed] [Google Scholar]
- 114.Shen J.Z., Zheng X.F., Kwan C.Y. Differential contractile actions of reactive oxygen species on rat aorta: selective activation of ATP receptor by H2O2. Life Sci. 2000;66:291–296. doi: 10.1016/S0024-3205(00)00539-7. [DOI] [PubMed] [Google Scholar]
- 115.Shen J.Z., Zheng X.F., Kwan C.Y. Evidence for P2-purinoceptors contribution in H2O2-induced contraction of rat aorta in the absence of endothelium. Cardiovasc. Res. 2000;47:574–585. doi: 10.1016/S0008-6363(00)00123-1. [DOI] [PubMed] [Google Scholar]
- 116.Gordeeva A.V., Zvyagilskaya R.A., Labas Y.A. Review: cross-talk between reactive oxygen species and calcium in living cells. Biokhimiya. 2003;68:1318–1322. doi: 10.1023/a:1026398310003. [DOI] [PubMed] [Google Scholar]
- 117.Guerra A.N., Gavala M.L., Chung H.S., Bertics P.J. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signal. 2007;3:39–51. doi: 10.1007/s11302-006-9035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Da Cruz C.M., Marques Ventura A.L., Schachter J., Costa H.M., Da Silva Souza H.A., Gomes F.R., Coutinho-Silva R., Ojcius D.M., Persechini P.M. Activation of ERK1/2 by extracellular nucleotides in macrophages is mediated by multiple P2 receptors independently of P2X 7-associated pore or channel formation. Br. J. Pharmacol. 2006;147:324–334. doi: 10.1038/sj.bjp.0706559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cruz C.M., Rinna A., Forman H.J., Ventura A.L.M., Persechini P.M., Ojcius D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parvathenani L.K., Tertyshnikova S., Greco C.R., Roberts S.B., Robertson B., Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J. Biol. Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 122.Pfeiffer Z.A., Guerra A.N., Hill L.M., Gavala M.L., Prabhu U., Aga M., Hall D.J., Bertics P.J. Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic. Biol. Med. 2007;42:1506–1516. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Noguchi T., Ishii K., Fukutomi H., Naguro I., Matsuzawa A., Takeda K., Ichijo H. Requirement of reactive oxygen species-dependent activation of ASK1-p38 MAPK pathway for extracellular ATP-induced apoptosis in macrophage. J. Biol. Chem. 2008;283:7657–7665. doi: 10.1074/jbc.M708402200. [DOI] [PubMed] [Google Scholar]
- 124.Moore S.F., MacKenzie A.B. NADPH oxidase NOX2 mediates rapid cellular oxidation following ATP stimulation of endotoxin-primed macrophages. J. Immunol. 2009;183:3302–3308. doi: 10.4049/jimmunol.0900394. [DOI] [PubMed] [Google Scholar]
- 125.Hewinson J., Moore S.F., Glover C., Watts A.G., MacKenzie A.B. A key role for redox signaling in rapid P2X 7 receptor-induced IL-1β processing in human monocytes. J. Immunol. 2008;180:8410–8420. doi: 10.4049/jimmunol.180.12.8410. [DOI] [PubMed] [Google Scholar]
- 126.Zhang T., Tsutsuki H., Islam W., Ono K., Takeda K., Akaike T., Sawa T. ATP exposure stimulates glutathione efflux as a necessary switch for NLRP3 inflammasome activation. Redox Biol. 2021;41:101930. doi: 10.1016/j.redox.2021.101930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seil M., Fontanils U., Etxebarria I.G., Pochet S., Garcia-Marcos M., Marino A., Dehaye J.P. Pharmacological evidence for the stimulation of NADPH oxidase by P2X7 receptors in mouse submandibular glands. Purinergic Signal. 2008;4:347–355. doi: 10.1007/s11302-008-9118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fontanils U., Seil M., Pochet S., El Ouaaliti M., Garcia-Marcos M., Dehaye J.P., Marino A. Stimulation by P2X7 receptors of calcium-dependent production of reactive oxygen species (ROS) in rat submandibular glands. Biochim. Biophys. Acta Gen. Subj. 2010;1800:1183–1191. doi: 10.1016/j.bbagen.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 129.Choi C.H., Spooner R., Deguzman J., Koutouzis T., Ojcius D.M., Yilmaz Ö. Porphyromonas gingivalis-nucleoside-diphosphate-kinase inhibits ATP-induced reactive-oxygen-species via P2X7 receptor/NADPH-oxidase signalling and contributes to persistence. Cell Microbiol. 2013;15:961–976. doi: 10.1111/cmi.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chatterjee S., Rana R., Corbett J., Kadiiska M.B., Goldstein J., Mason R.P. P2X7 receptor-NADPH oxidase axis mediates protein radical formation and Kupffer cell activation in carbon tetrachloride-mediated steatohepatitis in obese mice. Free Radic. Biol. Med. 2012;52:1666–1679. doi: 10.1016/j.freeradbiomed.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang B., Sluyter R. P2X7 receptor activation induces reactive oxygen species formation in erythroid cells. Purinergic Signal. 2013;9:101–112. doi: 10.1007/s11302-012-9335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nishida K., Nakatani T., Ohishi A., Okuda H., Higashi Y., Matsuo T., Fujimoto S., Nagasawa K. Mitochondrial dysfunction is involved in P2X7 receptor-mediated neuronal cell death. J. Neurochem. 2012;122:1118–1128. doi: 10.1111/j.1471-4159.2012.07868.x. [DOI] [PubMed] [Google Scholar]
- 133.Seeland S., Kettiger H., Murphy M., Treiber A., Giller J., Kiss A., Sube R., Krähenbühl S., Hafner M., Huwyler J. ATP-induced cellular stress and mitochondrial toxicity in cells expressing purinergic P2X7 receptor. Pharmacol. Res. Perspect. 2015;3:1–13. doi: 10.1002/prp2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kawano A., Tsukimoto M., Mori D., Noguchi T., Harada H., Takenouchi T., Kitani H., Kojima S. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem. Biophys. Res. Commun. 2012;420:102–107. doi: 10.1016/j.bbrc.2012.02.122. [DOI] [PubMed] [Google Scholar]
- 135.Coddou C., Codocedo J.F., Li S., Lillo J.G., Acuña-Castillo C., Bull P., Stojilkovic S.S., Huidobro-Toro J.P. Reactive oxygen species potentiate the P2X2 receptor activity through intracellular Cys430. J. Neurosci. 2009;29:12284–12291. doi: 10.1523/JNEUROSCI.2096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu F., Zhu D., Pei W., Lee I., Zhang X., Pan L., Xu J. Rhein inhibits ATP-triggered inflammatory responses in rheumatoid rat fibroblast-like synoviocytes. Int. Immunopharm. 2019;75 doi: 10.1016/j.intimp.2019.105780. [DOI] [PubMed] [Google Scholar]
- 137.Draganov D., Gopalakrishna-Pillai S., Chen Y.R., Zuckerman N., Moeller S., Wang C., Ann D., Lee P.P. Modulation of P2X4/P2X7/Pannexin-1 sensitivity to extracellular ATP via Ivermectin induces a non-apoptotic and inflammatory form of cancer cell death. Sci. Rep. 2015;5:1–17. doi: 10.1038/srep16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Savio L.E.B., Coutinho-Silva R. Immunomodulatory effects of P2X7 receptor in intracellular parasite infections. Curr. Opin. Pharmacol. 2019;47:53–58. doi: 10.1016/j.coph.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 139.Corrêa G., Marques da Silva C., de Abreu Moreira-Souza A.C., Vommaro R.C., Coutinho-Silva R. Activation of the P2X7 receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microb. Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 140.Moreira-Souza A.C.A., Almeida-da-Silva C.L.C., Rangel T.P., Rocha G. da C., Bellio M., Zamboni D.S., Vommaro R.C., Coutinho-Silva R. The P2X7 receptor mediates Toxoplasma gondii Control in Macrophages through canonical NLRP3 inflammasome activation and reactive oxygen species production. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.01257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moreira-Souza A.C.A., Rangel T.P., da Silva S.R.B., Figliuolo V.R., Savio L.E.B., Schmitz F., Takiya C.M., Wyse A.T.S., Vommaro R.C., Coutinho-Silva R. Disruption of purinergic receptor P2X7 signaling increases susceptibility to cerebral toxoplasmosis. Am. J. Pathol. 2019;189:730–738. doi: 10.1016/j.ajpath.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 142.Csóka B., Németh Z.H., Szabó I., Davies D.L., Varga Z.V., Pálóczi J., Falzoni S., Di Virgilio F., Muramatsu R., Yamashita T., Pacher P., Haskó G. Macrophage P2X4 receptors augment bacterial killing and protect against sepsis. JCI Insight. 2018;3:1–18. doi: 10.1172/jci.insight.99431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larrouyet-Sarto M.L., Tamura A.S., Alves V.S., Santana P.T., Ciarlini-magalhães R., Rangel T.P., Siebert C., Hartwig J.R., Marcon T., Wyse A.T.S., Takiya C.M., Coutinho-silva R., Eduardo L., Savio B. P2X7 receptor deletion attenuates oxidative stress and liver damage in sepsis. Purinergic Signal. 2020 doi: 10.1007/s11302-020-09746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Savio L.E.B., Andrade M.G.J., de Andrade Mello P., Santana P.T., Moreira-Souza A.C.A., Kolling J., Longoni A., Feldbrügge L., Wu Y., Wyse A.T.S., Robson S.C., Coutinho-Silva R. P2X7 receptor signaling contributes to sepsis-associated brain dysfunction. Mol. Neurobiol. 2017;54:6459–6470. doi: 10.1007/s12035-016-0168-9. [DOI] [PubMed] [Google Scholar]
- 145.Shevozaki Y., Koizumi S., Ishida S., Sawada J.I., Ohno Y., Inoue K. Cytoprotection against oxidative stress-induced damage of astrocytes by extracellular ATP via P2Y1 receptors. Glia. 2005;49:288–300. doi: 10.1002/glia.20118. [DOI] [PubMed] [Google Scholar]
- 146.Kahlert S., Blaser T., Tulapurkar M., Reiser G. P2Y receptor-activating nucleotides modulate cellular reactive oxygen species production in dissociated hippocampal astrocytes and neurons in culture independent of parallel cytosolic Ca2+ rise and change in mitochondrial potential. J. Neurosci. Res. 2007;85:3443–3456. doi: 10.1002/jnr.21316. [DOI] [PubMed] [Google Scholar]
- 147.Fujita T., Tozaki-Saitoh H., Inoue K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia. 2009;57:244–257. doi: 10.1002/glia.20749. [DOI] [PubMed] [Google Scholar]
- 148.Förster D., Reiser G. Nucleotides protect rat brain astrocytes against hydrogen peroxide toxicity and induce antioxidant defense via P2Y receptors. Neurochem. Int. 2016;94:57–66. doi: 10.1016/j.neuint.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 149.Kalwa H., Sartoretto J.L., Martinelli R., Romero N., Steinhorn B.S., Tao M., Ozaki C.K., Carman C.V., Michel T. Central role for hydrogen peroxide in P2Y1 ADP receptor-mediated cellular responses in vascular endothelium. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3383–3388. doi: 10.1073/pnas.1320854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Díaz-Vegas A., Campos C.A., Contreras-Ferrat A., Casas M., Buvinic S., Jaimovich E., Espinosa A. ROS production via P2Y1-PKC-NOX2 is triggered by extracellular ATP after electrical stimulation of skeletal muscle cells. PloS One. 2015;10:1–14. doi: 10.1371/journal.pone.0129882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eun S.Y., Park S.W., Lee J.H., Chang K.C., Kim H.J. P2Y2R activation by nucleotides released from oxLDL-treated endothelial cells (ECs) mediates the interaction between ECs and immune cells through RAGE expression and reactive oxygen species production. Free Radic. Biol. Med. 2014;69:157–166. doi: 10.1016/j.freeradbiomed.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 152.D'Andrea P., Romanello M., Bicego M., Steinberg T.H., Tell G. H2O2 modulates purinergic-dependent calcium signalling in osteoblast-like cells. Cell Calcium. 2008;43:457–468. doi: 10.1016/j.ceca.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 153.Marques-da-Silva C., Chaves M.M., Chaves S.P., Figliuolo V.R., Meyer-Fernandes J.R., Corte-Real S., Lameu C., Ulrich H., Ojcius D.M., Rossi-Bergmann B., Coutinho-Silva R. Infection with Leishmania amazonensis upregulates purinergic receptor expression and induces host-cell susceptibility to UTP-mediated apoptosis. Cell Microbiol. 2011;13:1410–1428. doi: 10.1111/j.1462-5822.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- 154.Marques-da-Silva C., Chaves M.M., Thorstenberg M.L., Figliuolo V.R., Vieira F.S., Chaves S.P., Meyer-Fernandes J.R., Rossi-Bergmann B., Savio L.E.B., Coutinho-Silva R. Intralesional uridine-5′-triphosphate (UTP) treatment induced resistance to Leishmania amazonensis infection by boosting Th1 immune responses and reactive oxygen species production. Purinergic Signal. 2018;14:201–211. doi: 10.1007/s11302-018-9606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dănilă M.D., Privistirescu A., Duicu O.M., Rațiu C.D., Angoulvant D., Muntean D.M., Sturza A. The effect of purinergic signaling via the P2Y11 receptor on vascular function in a rat model of acute inflammation. Mol. Cell. Biochem. 2017;431:37–44. doi: 10.1007/s11010-017-2973-5. [DOI] [PubMed] [Google Scholar]
- 156.Giniatullin A., Petrov A., Giniatullin R. The involvement of P2Y12 receptors, NADPH oxidase, and lipid rafts in the action of extracellular ATP on synaptic transmission at the frog neuromuscular junction. Neuroscience. 2015;285:324–332. doi: 10.1016/j.neuroscience.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 157.Giniatullin A.R., Grishin S.N., Sharifullina E.R., Petrov A.M., Zefirov A.L., Giniatullin R.A. Reactive oxygen species contribute to the presynaptic action of extracellular ATP at the frog neuromuscular junction. J. Physiol. (Camb.) 2005;565:229–242. doi: 10.1113/jphysiol.2005.084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Azran S., Danino O., Förster D., Kenigsberg S., Reiser G., Dixit M., Singh V., Major D.T., Fischer B. Identification of highly promising antioxidants/neuroprotectants based on nucleoside 5′-phosphorothioate scaffold. Synthesis, activity, and mechanisms of action. J. Med. Chem. 2015;58:8427–8443. doi: 10.1021/acs.jmedchem.5b00575. [DOI] [PubMed] [Google Scholar]
- 159.Borges P.A., Waclawiak I., Georgii J.L., Fraga-Junior V. da S., Barros J.F., Lemos F.S., Russo-Abrahão T., Saraiva E.M., Takiya C.M., Coutinho-Silva R., Penido C., Mermelstein C., Meyer-Fernandes J.R., Canto F.B., Neves J.S., Melo P.A., Canetti C., Benjamim C.F. Adenosine diphosphate improves wound healing in diabetic mice through P2Y12 receptor activation. Front. Immunol. 2021;12:1–18. doi: 10.3389/fimmu.2021.651740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Savio L.E.B., De Giorgi M., Robson S.C. Ectonucleotidases in immunobiology. Encycl. Immunobiol. 2016;2:15–25. [Google Scholar]
- 161.Kaczmarek E., Koziak K., Sévigny J., Siegel J.B., Anrather J., Beaudoin A.R., Bach F.H., Robson S.C. Identification and characterization of CD39/vascular ATP diphosphohydrolase. J. Biol. Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 162.Robson S.C., Daoud S., Bégin M., Côté Y.P., Siegel J.B., Bach F.H., Beaudoin A.R. Modulation of vascular ATP diphosphohydrolase by fatty acids. Blood Coagul. Fibrinolysis. 1997;8:21–27. doi: 10.1097/00001721-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 163.Chalmers A.H., Blake-Mortimer J.S., Winefield A.H. Lymphocyte 5’-ectonucleotidase: an indicator of oxidative stress in humans? Redox Rep. 2000;5:89–91. doi: 10.1179/135100000101535618. [DOI] [PubMed] [Google Scholar]
- 164.Deaglio S., Dwyer K.M., Gao W., Friedman D., Usheva A., Erat A., Chen J.F., Enjyoji K., Linden J., Oukka M., Kuchroo V.K., Strom T.B., Robson S.C. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Savio L.E.B., Robson S.C., Longhi M.S. Ectonucleotidase modulation of lymphocyte function in gut and liver. Front. Cell Dev. Biol. 2021;8:1–9. doi: 10.3389/fcell.2020.621760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gerner M.C., Ziegler L.S., Schmidt R.L.J., Krenn M., Zimprich F., Uyanik-Ünal K., Konstantopoulou V., Derdak S., Del Favero G., Schwarzinger I., Boztug K., Schmetterer K.G. The TGF-b/SOX4 axis and ROS-driven autophagy co-mediate CD39 expression in regulatory T-cells. FASEB J. 2020;34:8367–8384. doi: 10.1096/fj.201902664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bai A., Moss A., Rothweiler S., Serena Longhi M., Wu Y., Junger W.G., Robson S.C. NADH oxidase-dependent CD39 expression by CD8 + T cells modulates interferon gamma responses via generation of adenosine. Nat. Commun. 2015;6:1–12. doi: 10.1038/ncomms9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chisci E., De Giorgi M., Zanfrini E., Testasecca A., Brambilla E., Cinti A., Farina L., Kutryb-Zajac B., Bugarin C., Villa C., Grassilli E., Combi R., Gaipa G., Cerrito M.G., Rivolta I., Smolenski R.T., Lavitrano M., Giovannoni R. Simultaneous overexpression of human E5NT and ENTPD1 protects porcine endothelial cells against H2O2-induced oxidative stress and cytotoxicity in vitro. Free Radic. Biol. Med. 2017;108:320–333. doi: 10.1016/j.freeradbiomed.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 169.Lee J.S., Chowdhury N., Roberts J.A.S., Yilmaz Ö. Host surface ectonucleotidase-CD73 and the opportunistic pathogen, Porphyromonas gingivalis, cross-modulation underlies a new homeostatic mechanism for chronic bacterial survival in human epithelial cells. Virulence. 2020;11:414–429. doi: 10.1080/21505594.2020.1763061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mahamed D.A., Mills J.H., Egan C.E., Denkers E.Y., Bynoe M.S. CD73-generated adenosine facilitates Toxoplasma gondii differentiation to long-lived tissue cysts in the central nervous system. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16312–16317. doi: 10.1073/pnas.1205589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Berne R.M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am. J. Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 172.De Jong J.W., De Jonge R., Keijzer E., Bradamante S. The role of adenosine in preconditioning. Pharmacol. Ther. 2000;87:141–149. doi: 10.1016/S0163-7258(00)00044-9. [DOI] [PubMed] [Google Scholar]
- 173.Ramkumar V., Hallam D.M., Nie Z. Adenosine, oxidative stress and cytoprotection. Jpn. J. Pharmacol. 2001;86:265–274. doi: 10.1254/jjp.86.265. [DOI] [PubMed] [Google Scholar]
- 174.Cronstein B.N., Kramer S.B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J. Exp. Med. 1983;158:1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Merighi S., Benini A., Mirandola P., Gessi S., Varani K., Leung E., MacLennan S., Baraldi P.G., Borea P.A. A3 adenosine receptors modulate hypoxia-inducible factor-1α expression in human A375 melanoma cells. Neoplasia. 2005;7:894–903. doi: 10.1593/neo.05334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fan X., Heijnen C.J., van der Kooij M.A., Groenendaal F., van Bel F. The role and regulation of hypoxia-inducible factor-1α expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res. Rev. 2009;62:99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 177.Gessi S., Merighi S., Stefanelli A., Fazzi D., Varani K., Borea P.A. A1 and A3 adenosine receptors inhibit LPS-induced hypoxia-inducible factor-1 accumulation in murine astrocytes, Pharmacol. Res. 2013;76:157–170. doi: 10.1016/j.phrs.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 178.Liu H., Zhang Y., Wu H., D'Alessandro A., Yegutkin G.G., Song A., Sun K., Li J., Cheng N.Y., Huang A., Wen Y.E., Weng T.T., Luo F., Nemkov T., Sun H., Kellems R.E., Karmouty-Quintana H., Hansen K.C., Zhao B., Subudhi A.W., Jameson-Van Houten S., Julian C.G., Lovering A.T., Eltzschig H.K., Blackburn M.R., Roach R.C., Xia Y. Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation. 2016;134:405–421. doi: 10.1161/CIRCULATIONAHA.116.021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Torres Á., Erices J.I., Sanchez F., Ehrenfeld P., Turchi L., Virolle T., Uribe D., Niechi I., Spichiger C., Rocha J.D., Ramirez M., Salazar-Onfray F., San Martín R., Quezada C. Extracellular adenosine promotes cell migration/invasion of Glioblastoma Stem-like Cells through A3 Adenosine Receptor activation under hypoxia. Canc. Lett. 2019;446:112–122. doi: 10.1016/j.canlet.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 180.Rudolphi K.A., Keil M., Fastbom J., Fredholm B.B. Ischaemic damage in gerbil hippocampus is reduced following upregulation of adenosine (A1) receptors by caffeine treatment. Neurosci. Lett. 1989;103:275–280. doi: 10.1016/0304-3940(89)90112-2. [DOI] [PubMed] [Google Scholar]
- 181.Chen J.F., Huang Z., Ma J., Zhu J.M., Moratalla R., Standaert D., Moskowitz M.A., Fink J.S., Schwarzschild M.A. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/jneurosci.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bolli R. Oxygen-derived free radicals and myocardial reperfusion injury: an overview. Cardiovasc. Drugs Ther. 1991;5:249–268. doi: 10.1007/BF00054747. [DOI] [PubMed] [Google Scholar]
- 183.Ramkumar V., Nie Z., Rybak L.P., Maggirwar S.B. Adenosine, antioxidant enzymes and cytoprotection. Trends Pharmacol. Sci. 1995;16:283–285. doi: 10.1016/S0165-6147(00)89051-3. [DOI] [PubMed] [Google Scholar]
- 184.Maggirwar S.B., Dhanraj D.N., Somani S.M., Ramkumar V. Adenosine acts as an endogenous activator of the cellular antioxidant defense system. Biochem. Biophys. Res. Commun. 1994;201:508–515. doi: 10.1006/bbrc.1994.1731. [DOI] [PubMed] [Google Scholar]
- 185.Xu J., Bian X., Liu Y., Hong L., Teng T., Sun Y., Xu Z. Adenosine A2 receptor activation ameliorates mitochondrial oxidative stress upon reperfusion through the posttranslational modification of NDUFV2 subunit of complex I in the heart. Free Radic. Biol. Med. 2017;106:208–218. doi: 10.1016/j.freeradbiomed.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 186.Nie Z., Mei Y., Ford M., Rybak L., Marcuzzi A., Ren H., Stiles G.L., Ramkumar V. Oxidative stress increases A1 adenosine receptor expression by activating nuclear factor κB. Mol. Pharmacol. 1998;53:663–669. doi: 10.1124/mol.53.4.663. [DOI] [PubMed] [Google Scholar]
- 187.Nie Z., Mei Y., Malek R.L., Marcuzzi A., Lee N.H., Ramkumar V. A role of p75 in NGF-mediated down-regulation of the A2A adenosine receptors in PC12 cells, mol. Pharma. 1999;56:947–954. doi: 10.1124/mol.56.5.947. [DOI] [PubMed] [Google Scholar]
- 188.Maj T., Wang W., Crespo J., Zhang H., Wang W., Wei S., Zhao L., Vatan L., Shao I., Szeliga W., Lyssiotis C., Liu J.R., Kryczek I., Zou W. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aires I.D., Boia R., Rodrigues-Neves A.C., Madeira M.H., Marques C., Ambrósio A.F., Santiago A.R. Blockade of microglial adenosine A 2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells. Glia. 2019;67:896–914. doi: 10.1002/glia.23579. [DOI] [PMC free article] [PubMed] [Google Scholar]