Abstract
In recent years, immune checkpoint inhibitors (ICIs) targeting CTLA-4 or PD1/PDL1 have achieved remarkable success in the treatment of bladder cancer (BLCA), but only a few patients have shown durable clinical benefits. The prognostic role of a mutant form of the tumor suppressor gene TP53 (TP53-MT) in predicting the efficacy of ICIs is highly controversial; therefore, in this study, we obtained data for 210 patients from an immunotherapy cohort, 412 patients from The Cancer Genome Atlas (TCGA)-BLCA cohort and 18 BLCA cell lines from Genomics of Drug Sensitivity in Cancer (GDSC), and we performed integrated bioinformatic analysis to explore the relationships between TP53-MT and clinical benefits derived from ICI treatment and the underlying mechanisms. We conclude that TP53-MT is a potential indicator of a relatively good response to ICIs and associated with prolonged overall survival (OS) (log-rank test, hazard ratio (HR) = 0.65 [95% confidence interval (CI), 0.44-0.99], p = 0.041). Through integrated analysis with several platforms, we found that TP53-MT patients were more likely to benefit from ICIs than wild-type P53 (TP53-WT) patients, which may be the result of 2 major mechanisms. First, the patients with TP53-MT showed stronger tumor antigenicity and tumor antigen presentation, as indicated by a higher tumor mutational load, a higher neoantigen load and increased expression of MHC; second, the antitumor immunity preexisting in tumors was stronger in samples with TP53-MT than in those with TP53-WT, including enrichment of interferon-gamma, positive regulation of TNF secretion pathways and increased expression of some immunostimulatory molecules, such as CXCL9 and CXCL10. This study provided some clues for identifying patients who would potentially benefit from ICIs at the somatic genomic level, developing new indications for targeted second-generation sequencing and promoting the development of precision medicine.
Keywords: TP53, mutant form of TP53 (TP53-MT), immune checkpoint inhibitors (ICIs), bladder cancer (BLCA), prognostic factor, bioinformatic analysis
Introduction
Worldwide, bladder cancer (BLCA) ranks as the second most frequently diagnosed cancer of the urinary tract after prostate cancer, with an estimated 429,793 new cases and 165,084 deaths in 20121. Approximately 25% of newly diagnosed patients have muscle-invasive (MIBC) or metastatic disease,1 which limits the benefits achieved with platinum-based traditional chemotherapy,2 and the relative 5-year overall survival (OS) rate of metastatic disease is approximately 15%.3 Meanwhile, the remaining 75% of non-muscular invasive bladder cancer (NMIBC) refers to papillary malignant tumors that are confined to the mucosa (Tis, Ta) or lamina propria (T1), and can be classified into papillary urothelial neoplasms of low malignant potential, noninvasive low-grade (LG) and high-grade (HG) papillary urothelial carcinoma (The 2016 WHO classification system). Because HG NMIBC are more atypia and have higher incidence of recurrence or progression, belonging to high-risk NMIBC, it is of most importance in grading of noninvasive disease4 and management of HG NMIBC should be more radical.
High-risk NMIBC includes high grade (grade 2004)/grade 2-3(grade 1973) or presence of carcinoma in situ. Bacillus Calmette-Guérin (BCG) is a standard strategy to prevent recurrence of high-risk NMIBC as results of stimulating the inherent and adaptive immune response, but 30%-50% of patients develop resistance to BCG.5 The failure may be explained by some factors, like immune escape, particularly due to CTLA4/B27 and PD-1/PD-L1 axis.6 In addition, according studies in a large cohort including more than 1,000 BCG-treated T1G3 NMIBC patients, Ferro et al. found that the increased number of absolute basophil before Transurethral Resection of bladder tumor (TURBT) may be related to the recurrence.7 Besides, they found that Overweight and obesity were significantly associated with an increased risk of recurrence and progression.8
Following BCG, enormous clinical investigations have led to the accelerated development of other kind of immunotherapies, including immune checkpoint inhibitors (ICIs). The use of ICIs directed against cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1) or its ligand PD-L1 has led to unprecedented success in the treatment of numerous types of solid tumors in recent years, but the challenges are the same as that in BCG, only a minority of patients obtaining clinical benefit.9 Similarly, multiple clinical trials have suggested that the overall response rate of high-grade or metastatic bladder tumors to ICIs fluctuates between 15% and 29%.10
There is a clinical need for biomarkers to identify optimal candidates for ICIs. Early studies have suggested that the tumor mutational burden (TMB),11 PD-L1 expression level on the tumor cell surface,12 and CD8+ lymphocyte infiltration level13 are associated with the response to ICIs, but most of these markers are from quantitation of protein or mRNA levels. Driver alterations in some genes leading to tumorigenesis and cancer progression are 1 of the 10 major characteristics of neoplasms,14 and prior studies have demonstrated that tumor microsatellite instability (MSI)15,16 and mutations in the DNA damage response and repair (DDR) pathways17-19 can be potentially applicable for the clinical selection of patients for ICI treatment. In addition, mutations in some other genes may be related to the efficacy of ICIs.20-23 For example, a previous report indicated that somatic mutations in SERPINB3 or SERPINB4 were correlated with survival following treatment with ICIs targeting CTLA4 in 2 independent cohorts of melanoma patients,24 suggesting that mutations in a hub gene may also be a prognostic marker for ICI treatment. Similarly, a recent study found that recurrent mutations in TET1 (TET1-MT) were predictive of relatively good durable clinical benefit from ICIs and improvement in OS across multiple cancer types,25 which also indicates that TET1-MT is a novel predictive biomarker for ICI treatment. However, it is worth noting that the above mutations in a single gene were not predictive of the prognosis of patients receiving non-ICI therapy.
The frequency of the mutant form of the tumor suppressor gene TP53 (TP53-MT) in bladder tumors is close to 50%,26 with TP53 functionally inactivated in 76% of samples,27 but the prognostic role of TP53-MT is not clear. Some studies have confirmed that TP53-MT may be related to a poor prognosis. For example, TP53-MT is more common in extravesical and lymph node-positive conditions and associated with poor outcomes in BLCA patients treated with radical cystectomy.28 Moreover, another study indicated that TP53-MT was associated with the progression of non-muscle-invasive bladder cancer (NMIBC).29 As expected, TP53-MT is associated with the same characteristics in other types of tumors, such as mantle cell lymphoma (MCL)30,31 and breast cancer (BC).32 Specifically, TP53-MT is significantly associated with Ki67 positivity >30%, a high-risk MCL International Prognostic Index (MIPI) score and inferior responses to both induction and high-dose chemotherapy in young patients with MCL. In the case of BC, TP53-MT was found to be significantly associated with a high-risk IHC4 group and an independent prognostic factor for shortened BC-specific survival, enhancing the prognostic accuracy of IHC4 and PAM50 assays.
To elucidate some biomarkers for identifying responders to ICI treatment, some studies have focused on exploring the correlation between TP53-MT and the efficacy of ICIs, but the predictive role of TP53-MT in immunotherapy remains debatable. An early study suggested that TP53-MT was associated with a trend toward a relatively poor response, short PFS and short OS in 110 patients receiving CTLA-4 blockade.33 However, inconsistent results were observed in another cohort of patients with non-small cell lung cancer treated with PD-1 plus CTLA-4 blockade.34 The authors found that TP53 mutations were enriched in responders, suggesting that TP53 may be associated with an enhanced response to combination therapy. In addition, Dong et al. demonstrated that lung adenocarcinoma patients with TP53-MT responded relatively well when receiving PD-1 inhibitors, suggesting that TP53 may help to inform clinical decisions on the use of ICIs.35 These observations could be attributed to the fact that patients with TP53-MT tend to exhibit increased genomic instability and some pathway-specific activation,26 as well as increased PD-L1 expression and a higher TMB.35
While clinical trials of immunotherapy for BLCA incorporating molecular biomarkers are increasingly common, there is still a lack of research on the relationship between the mutation status of TP53 and the efficacy of ICIs and the possible mechanisms. Therefore, in this study, we obtained BLCA data sets from The Cancer Genome Atlas (TCGA), Memorial Sloan Kettering Cancer Center (MSKCC) and Genomics of Drug Sensitivity in Cancer (GDSC) and performed integrated bioinformatic analyses of genomic, transcriptomic, and clinical data for molecules and pathways to explore whether TP53-MT is related to the efficacy of ICIs and elucidate the underlying mechanism.
Materials and Methods
Clinical Cohorts and GDSC-BLCA Cell Lines
An immunotherapy cohort including annotated clinical data and mutational data for 215 patients treated with ICIs (a PD-1/PD-L1 inhibitor or CTLA-4 inhibitors+PD-(L)1 inhibitors) was obtained from a previously published study by Samstein et al.,36 and samples in this cohort were sequenced by targeted next-generation sequencing (MSK-IMPACT). Updated somatic mutation, gene expression (Illumina HiSeq and RNA-Seq) and clinical data (including OS) for BLCA, composing the TCGA-BLCA cohort, were obtained from the Genomic Data Commons (https://portal.gdc.cancer.gov/) using the R package TCGAbiolinks,37 and additional survival data (disease-free survival, DFS; progression-free survival, PFS) were downloaded from cBioportal.38 In the immunotherapy cohort, 210 patients with with nonsynonymously mutational data were included, including TP53-MT(99) and TP53-WT(111).In addition, 18 BLCA cell lines with median inhibitory concentration (IC50) data for chemotherapeutic agents (4 of which expressed TP53-WT, and 14 of which expressed TP53-MT) were obtained from GDSC (https://www.cancerrxgene.org/), and the cell lines with TP53-MT were compared with those without TP53-MT.
Analysis of Gene Mutation Characteristics and Tumor Immunogenicity
Corresponding whole-exome sequencing (WES) data for BLCA cell lines were obtained from GDSC,39 and neoantigen load (NAL) data for the TCGA-BLCA cohort were obtained from a previously published article.40 The TMB was calculated by dividing the number of nonsynonymous mutations by 38 MB.41 Visualizing the genetic mutations and clinical characteristics of the top 20 most mutated genes in the immunotherapy cohort and TCGA-BLCA cohort were performed by using the R package ComplexHeatmap,42 and visualizing the TP53 gene mutation sites was performed by using the R package Maftools.43
Infiltration of Immune-Related Cells and Expression of Immune-Related Molecules
We inferred the infiltrating cells in the tumor microenvironment (TME) by uploading tissue expression profiles to the CIBERSORT web portal (http://cibersort.stanford.edu/),44 running the algorithm using the LM22 signature and 1,000 permutations, and comparing the fractions of 22 immune cell types in the samples with TP53-MT to those in the samples without TP53-MT. In addition, we obtained 2 lists of immune marker genes classified by cell type45 and immunomodulators with their functional classifications40 from published reports. The expression levels of these genes were quantified as log2(FPKM + 1) and compared between TP53-MT and TP53-WT samples, and genes with an absolute value of logFC ≥ 0.58 and p ≤ 0.05 were considered to be significantly differentially expressed.
Copy Number Variation (CNV) Analysis
We downloaded level 3 CNV (hg19) data without germline CNV for BLCA from the Broad GDAC Firehose (http://firebrowse.org/). Then, we used GenePattern (http://cloud.Genepattern.org/gp/pages/index.jsf) to perform GISTIC 2.0 analysis based on the downloaded Segment file without resetting parameters except the confidence interval (CI) equaling 0.99 and the X chromosome included.46 Next, we used the R package Maftools to visualize the results for somatic CNVs.43
Gene Set Enrichment Analysis (GSEA) and Genomic Profiles of DDR-Related Mutations
The R package edgeR was used to normalize RNA-Seq data in the TCGA-BLCA cohort,47 and the R package clusterProfiler48 was used to complete the enrichment analysis of gene clusters whose results satisfied a nominal P-value cutoff of 0.05 and were therefore considered statistically significant. Gene sets were downloaded from the MSigDB database of Broad Institute (http://software.broadinstitute.org/gsea/msigdb/index.jsp).49 A DDR pathway gene set was also obtained from the MSigDB database of Broad Institute (Supplemental Table S1). Based on the DDR-related gene set, we evaluated the number of synonymous somatic mutations in DDR-related pathways in the immunotherapy cohort, TCGA-BLCA cohort, and GDSC-BLCA cell lines and investigated whether there were significant differences between the group with TP53-MT and the group without TP53-MT.
Statistical Analysis
The differences in the TMB, the NAL, age, MSI scores, tumor-infiltrating lymphocytes (TILs), immune-related molecule expression and the number of DDR-related pathway gene mutations between TP53-WT and TP53-MT tumors were examined using the Mann-Whitney U test. The associations between the TP53 status and the top 20 currently mutated genes in the TCGA-BLCA cohort were examined using the chi-square test, while those in the immunotherapy cohort were examined using Fisher’s exact test. The associations between the TP53 status and sex, race, ethnicity and tumor stage in the TCGA-BLCA cohort were examined using Fisher’s exact test. Survival curves for the subgroups defined by the TP53-MT status in each data set were generated using the Kaplan-Meier method, and the log-rank test was used to determine the statistical significance of differences. A value of P <0.05 was considered statistically significant, and all statistical tests were 2 sided. In addition, the R package ggpurb was used to create boxplots.50 All statistical tests and visualizations were performed with R (version 3.6.1).
Results
TP53-MT Is a Protective Factor in BLCA Patients Receiving ICIs
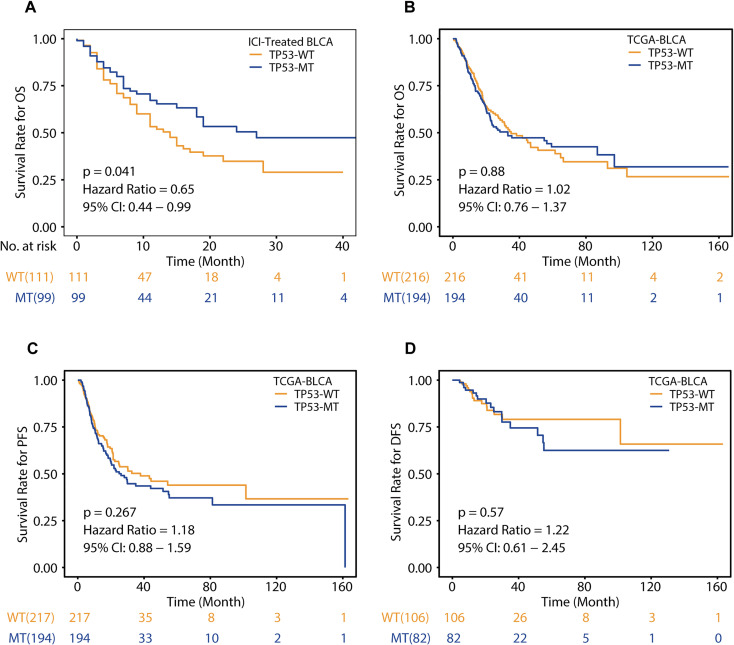
In the immunotherapy cohort, survival analysis based on the mutation status of TP53 showed that patients with TP53-MT were significantly associated with prolonged OS (Figure 1A, log-rank test, hazard ratio (HR) = 0.65 [95% CI 0.44-0.99], p = 0.041), suggesting that TP53-MT may be associated with a relatively good prognosis in patients treated with ICIs. To verify whether TP53-MT is related to only the prognosis of ICI treatment, we performed the same analysis on the TCGA-BLCA cohort. We found that compared with TP53-WT patients, patients with TP53-MT had no significant difference in OS (Figure 1B, HR = 1.02 [95% CI, 0.76-1.37], p = 0.88). Consistent with the results for OS benefit, there were also no significant differences in PFS or DFS (Figure 1C, HR = 1.18 [95% CI, 0.88-1.59], p = 0.267; Figure 1D, HR = 1.22 [95% CI, 0.61-2.45], p = 0.57), indicating that the efficacy of non-ICIs may not be related to the TP53 mutation status.
Figure 1.
Associations of the TP53 mutation status and clinical outcomes. A, Kaplan-Meier survival curves of overall survival (OS) in the immunotherapy cohort comparing patients with TP53-MT and patients with TP53-WT. B-D, Kaplan-Meier survival curves of overall survival (OS), progression-free survival (PFS) and disease-free survival (DFS) in the TCGA-BLCA cohort comparing patients with TP53-MT and patients with TP53-WT. A-D, Differences between the TP53-MT and TP53-WT groups were tested using the log-rank test.
In order to explore whether TP53 mutation is the best predictor of ICIs therapy, we added Supplemental Table 4 to show the clinical and molecular characteristics of patients with or without TP53-MT in the immunotherapy cohort (Supplemental Table 4).We found that only the TMB (TP53-MT, 10.822 (0.878-209.547); TP53-WT, 7.807 (0.878-70.217); P = 0.020) between the groups was significantly different, indicating that the status of TP53 or TMB may be prognostic factors. In addition, the univariate and multivariate Cox regression analysis of the above factors were performed (Supplemental Figure S3). In the univariate Cox regression analysis, we found that in addition to the status of TP53 (TP53-MT vs TP53-WT, HR = 0.65 95%CI: 0.42-0.98, p = 0.04), sample type (primary vs metastatic, HR = 0.63[95%CI: 0.42-0.95], p = 0.03) and TMB score (HR = 0.98[95%CI: 0.96-1.00], p = 0.03) are also prognostic factors. In further multivariate Cox regression analysis, sample type (primary vs metastatic, HR = 0.64[95%CI: 0.54-0.96], p = 0.03) and TMB score (HR = 0.98[95%CI: 0.96-1.00], P = 0.03) are still independent prognostic factors, and the status of TP53 (HR = 0.68[95%CI: 0.45-1.04], p = 0.07) shows trend to be prognostic, with the p value being slightly greater than 0.05 and 95% confidence interval(CI) being 0.45-1.04, which may be due to the small sample size. Based on these observations, it is necessary to explore the potential mechanisms involving different types of TP53 mutants and the efficacy of ICIs.
Patients With TP53-MT BLCA Show Increased Tumor Immunogenicity
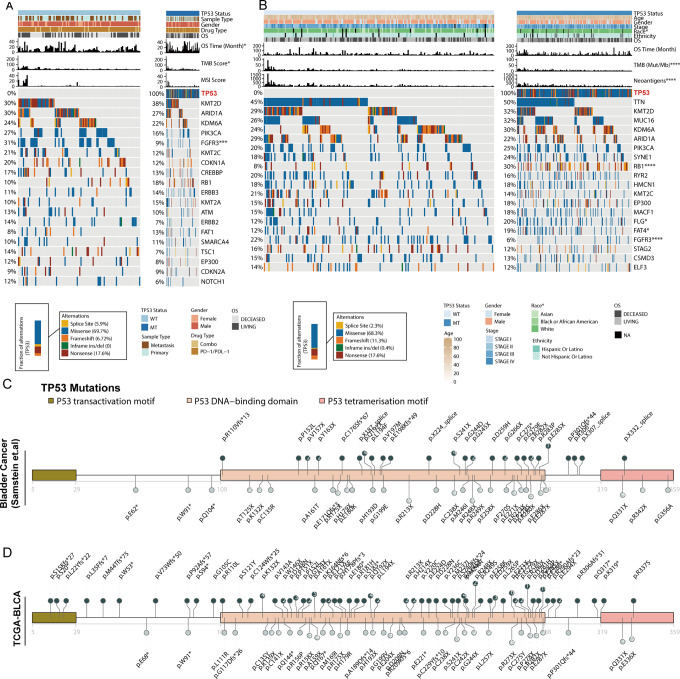
To clarify the underlying factors that allow TP53-MT to predict the efficacy of ICIs, we grouped patients from an immunotherapy cohort based on their TP53 status to explore the relationships of this status with patient clinical and genomic characteristics (Figure 2A). The results agreed with the results of the analysis shown in Figure 1A, showing that the OS of patients with TP53-MT was significantly longer than that of patients with TP53-WT. Recurrent gene mutations, including KMT2D, ARID1A, KDM6A, etc. were found in the immunotherapy cohorts. Alterations in some genes, such as PIK3CA, FGFR3, KMT2C, and CDKN1A, were mainly enriched in the TP53-WT group. However, only the FGFR3 mutation status was significantly different between the TP53-WT and TP53-MT patients (Fisher’s exact test, P <0.001). Then, we performed the same analysis with the TCGA-BLCA cohort (Figure 2B). As expected, the OS benefit was comparable between the patients with TP53-MT and those with TP53-WT (log-rank test, p > 0.05). In addition, we found a significant difference in ethnic distribution between TP53-MT and TP53-WT (Supplemental Figure S1). The fraction of Caucasian patients with TP53-MT was higher than that of Asian patients, suggesting that the frequency of TP53 mutations in Caucasians is higher (chi-square test, p = 0.003). Due to the large number of samples in this cohort, we were able to find mutant genes with significantly different distributions in addition to FGFR3, such as RB1, FLG and FAT4 (chi-square test, all p <0.05). However, these newly identified gene mutations were enriched in patients carrying TP53-MT, suggesting that they may occur with TP53-MT.
Figure 2.
Landscape of genomic alterations and lollipop charts of TP53 mutation sites. A, Two hundred ten BLCA samples from the immunotherapy cohort with mutation data grouped by the TP53 mutation status and mutation profile, with clinical and molecular features annotated above. B, Four hundred twelve BLCA samples from the TCGA-BLCA cohort with mutation data grouped by the TP53 mutation status and mutation profile, with clinical and molecular features annotated above. C-D, Amino acid positions of TP53 mutations in the immunotherapy cohort (C) and TCGA-BLCA cohort (D). Number in circles represent the frequency of the corresponding site mutation. Asterisks indicate the relationship with the TP53 mutation status (the TMB, the NAL, age and the MSI score were tested using the Mann-Whitney U test; the top 20 currently mutated genes in the TCGA-BLCA cohort were tested using the chi-Square test, and those in the immunotherapy cohort were tested using Fisher’s exact test; and sex, race, ethnicity and disease stage in the TCGA-BLCA cohort were tested using Fisher’s exact test. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05). TMB: tumor mutational burden; NAL: neoantigen load; MSI: microsatellite instability.
Then, we used the R package Maftools to visualize different mutations in TP53. In the immunotherapy cohort, TP53 mutations were mainly enriched in the core region that binds to DNA, with residues 213, 248, 280 and 285 as the most common hotpots, and there were few mutations in the N and C termini (Figure 2C). In the TCGA-BLCA cohort, TP53 mutations were also mainly in the DNA-binding domain, but there were also a small number of mutations in the transcription activation domain (Figure 2D).
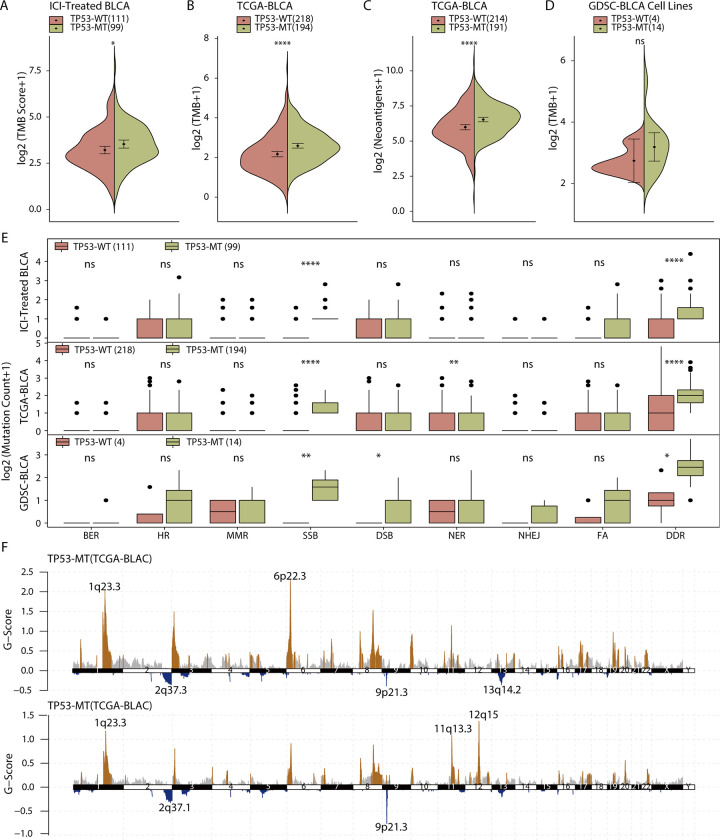
Many studies have shown that the TMB and NAL are related to the efficacy of ICIs, so we next explored the relationship between the TMB or NAL and TP53. BLCA patients treated with ICIs or non-ICIs all showed a higher TMB in the patients with TP53-MT than in those with TP53-WT (Figure 3A-B, Mann-Whitney U test, p < 0.05 and p < 0.001). Moreover, in the TCGA-BLCA cohort where NAL data were available, we found that both the TMB and the NAL were higher in the patients with TP53-MT than in those with TP53-WT (Figure 3C). However, although the same trend was observed in GDSC cell lines, it was not statistically significant (Figure 3D, Mann-Whitney U test, p > 0.05)
Figure 3.
Patients with TP53-MT show increased genomic instability. A, In the immunotherapy cohort, the TMB in the patients with TP53-MT was significantly higher than that in the patients with TP53-WT. The values of the TMB are plotted on a log scale. B, In the TCGA-BLCA cohort, the TMB in the patients with TP53-MT was significantly higher than that in the patients with TP53-WT. The values of the TMB are plotted on a log scale. C, In the TCGA-BLCA cohort, the NAL in the patients with TP53-MT was significantly higher than that in the patients with TP53-WT. D, In the GDSC-BLCA cell lines, there was no significant difference in the TMB between the TP53-MT group and TP53-WT group. E, In the 3 included data sets, TP53-MT was associated with an increased number of nonsense mutations in the overall DDR-related pathway. F, The distributions of CNVs in the TP53-WT and TP53-MT groups were visualized. The differences in the TMB, NAL and quantity of nonsense mutations in DDR-related pathways were tested using the Mann-Whitney U test. ns: not significant; * p < 0.05; ** p < 0.01; *** p < 0.001, ****p < 0.0001. TMB: tumor mutational burden; NAL: neoantigen load; DDR: DNA damage response and repair. CNV: copy number variation.
Given that genomic instability is one of the hallmarks of cancer, DDR-related genes play an important role in maintaining the stability of the genome. In addition, TP53 is one of the genes in the single_strand_DNA_binding (GO: 0003697) pathway of the DDR pathway-related gene sets. Therefore, in this study, we obtained 8 gene sets for DDR-related pathways (Supplemental Table S1) from MSigDB and explored whether they are related to the TP53 mutation status. Although only SSB consistently exhibited a significantly difference across the 3 database datasets evaluated, NER was significantly different in the TCGA-BLCA cohort, the overall number of DDR-related gene mutations in the TP53-MT group was significantly higher than that in the TP53-WT group (Figure 3E, Mann-Whitney U test; immunotherapy cohort: p < 0.0001, TCGA-BLCA cohort: p < 0.0001, GDSC cell lines: p < 0.05)
Next, to identify recurrently significant CNVs between patients with TP53-MT and those with TP53-WT that may explain the different efficacies of ICIs, we used GISTIC 2.0 to analyze the segment file from the TCGA-BLCA cohort. Regardless of the status of TP53, there were a large number of CNVs in BLCA patients (Supplemental Figure S2), and by comparing groups defined by the TP53 mutation status, we found that there were more CNVs in the patients with TP53-MT (Figure 3F). A significant deletion in TP53-MT patients included 9p21.3, which spans the tumor suppressor genes CDKN2A and CDKN2B. The significantly amplified regions in TP53-MT samples included 11q13.2-13.3, 1q23.3 and 6p22.3, of which 11q13.2-13.3 span the CCND1 (cyclin D1) gene.
Patients With TP53-MT BLCA Show Relatively Strong Antitumor Immunity
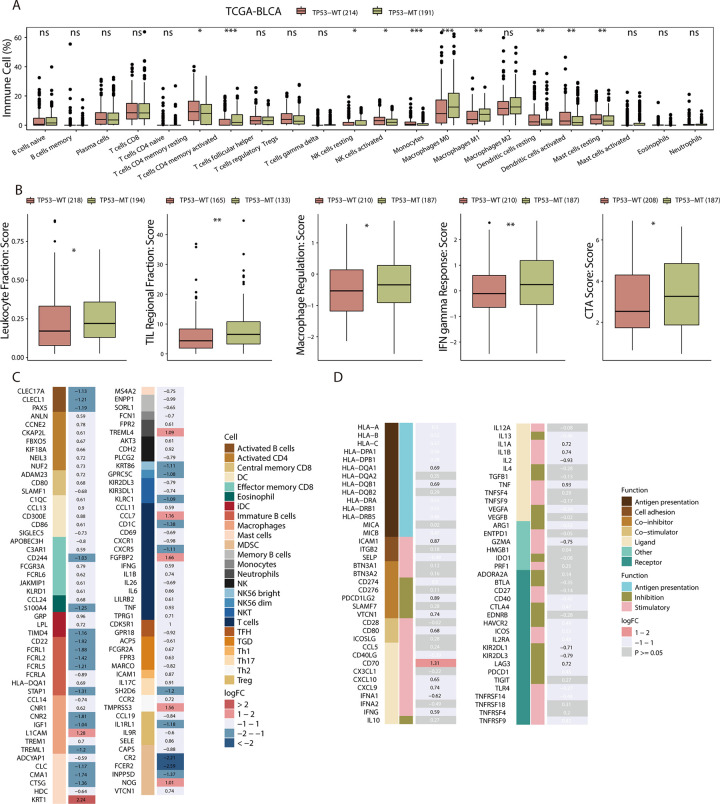
The tumor immune microenvironment (TIME) is essential to resisting tumors, making it vital in predicting the efficacy of ICIs. Therefore, we used 3 methods to further explore the relationships among TILs, immune-related molecules and the TP53 mutation status. First, we used CIBERSORT to analyze mRNA expression data for the TCGA-BLCA cohort to estimate the fractions of 22 immune cell types and compared the results between the patients with TP53-WT and those with TP53-MT. We observed that the infiltration of M0 and M1 macrophages was higher in the TP53-MT patients but there was no significant difference in M2 macrophages between the 2 groups (Figure 4A). In addition, resting and activated dendritic cells, resting mast cells, and resting memory CD4+ T cells were more abundant in the TP53-WT patients.
Figure 4.
BLCA patients in the TCGA cohort with TP53-MT show relatively strong preexisting antitumor immunity. A, The fractions of 22 immune cell types in the TP53-MT group and TP53-WT group based on RNA-Seq data. B, The immune-related scores of the TP53-WT group and the TP53-MT group based on the expression of a marker gene set. C-D, Heatmap showing average changes in the expression levels of immune-related molecules between the TP53-MT and TP53-WT groups. The molecules corresponding to the same lymphocyte or function are identified by the same color on the left side of the squares, and each square with an exact number represents the logFC of a molecule, filled with different back colors, i.e. from red to blue (C) or gray (D). The logFC values marked in black font indicate that the absolute value of logFC is ≥0.58 with statistical significance, while the logFC values in white font are nonsignificant. The differences in tumor-infiltrating lymphocytes and immune-related molecule expression between TP53-WT and TP53-MT tumors were tested using the Mann-Whitney U test. A-B, ns: not significant; *p < 0.05; **p < 0.01; ***p < 0.001. D, Dark gray corresponds to a nonsignificant difference (p > 0.05).
Then, based on the transcriptional data of samples and markers on the surface of lymphocytes, we found that the intratumoral lymphocyte fraction, regulatory TIL fraction, macrophage regulation, and INF-gamma marker scores were significantly higher in the TP53-MT patients (Figure 4B, Mann-Whitney U test, all p < 0.05). Moreover, we found that myeloid-derived suppressor cells (MDSCs) were mainly enriched in the TP53-WT expression, according to molecular marker expression on the cell surface (Figure 4C).
Finally, we evaluated the expression of immune-related molecules classified by function in patients carrying TP53-WT or TP53-MT (Figure 4D). CD70, CD80, CXCL9/10, IL1A/1B, ICAM1, IFNG, TNF and other immunomodulators were significantly overexpressed in the TP53-MT patients. For antigen-presenting MHC molecules, we found that most genes had higher expression levels in the tumors with TP53-MT than in those with TP53-WT, and the results for HLA-DQA1 and HLA-DQB1 were statistically significant. Immunosuppressive molecules, such as KIR2DL1 and KIR2DL3, were highly expressed in the TP53-WT group, while LAG3, PDCD1LG2 and VTCN1 were more highly expressed in the TP53-MT group; one of them is an ICI target. The expression levels of PDCD1, CD274 (PD-L1), CTLA-4, IDO1, TGFB1, IL4 and IL10 were similar between the 2 groups. In addition, compared with that in the TP53-MT group, GZMA expression in the TP53-WT group was higher.
Enrichment of Cell Cycle and Inflammatory Pathways in TP53-MT Patients
Pathway enrichment analysis representing the expression of several function-related genes is more convincing than analysis of individual genes, so we ran GSEA to explore the pathways that were upregulated in samples with different TP53 mutation statuses. Gene ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome terms were identified with a cutoff of p < 0.05 (Supplemental Table S2 and S3).
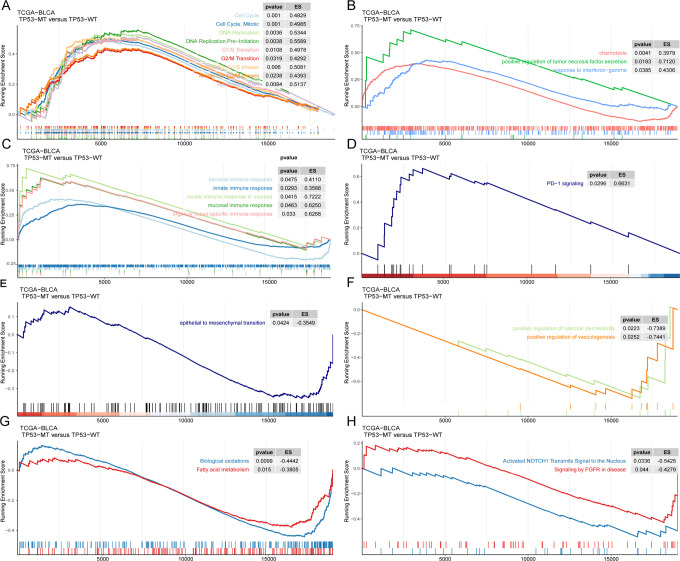
We found that cell cycle-related pathways were enriched in cancers with TP53-MT both in the TCGA-BLCA cohort and the GDSC-BLCA cell lines (Figure 5A), showing accelerated cell cycling and DNA replication. In addition, we found enrichment of other pathways, including the INF-gamma pathway, the pathway positively regulating TNF secretion, and natural immune response-related pathways (such as the innate immune response, mucosal immune response, organ- or tissue-specific immune response, etc.) and humoral immune pathways, in the patients with TP53-MT in the TCGA-BLCA cohort (Figure 5B-C). Interestingly, we also found that the PD-1 signaling pathway was significantly upregulated in the group with TP53-MT (Figure 5D). In contrast, epithelial-mesenchymal transition (EMT) and the pathways positively regulating angiogenesis and vascular permeability were downregulated in the samples with TP53-MT (Figure 5E-F). Fatty acid metabolism- and biological oxidation-related pathways, NOTCH1 activation pathways and FGFR pathways were more abundant in the tumors without TP53-MT (Figure 5H-G).
Figure 5.
GSEA results for the patients with TP53-MT in the TCGA-BLCA cohort. Significantly enriched pathways in patients with TP53-MT, including cell cycle-related pathways (A), cytokine-related pathways (B), immune response-related pathways (C), and the PD-1 signaling pathway (D). Significantly enriched pathways in patients with TP53-WT, including epithelial-mesenchymal transition (E), angiogenesis and increased vascular permeability (F), fatty acid metabolism and biological oxidation pathways (G), and the NOTCH1 and FGFR signaling pathway (H).
BLCA Cells With TP53-MT were Sensitive to Some Chemotherapeutic Agents
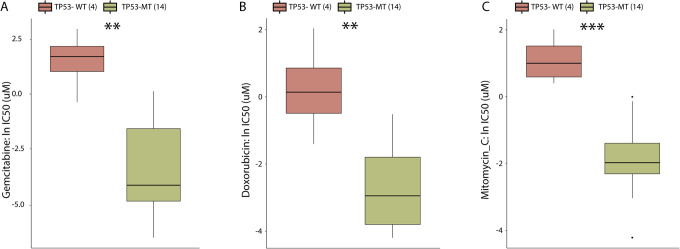
Chemotherapy is still the standard treatment for BLCA, so we explored the GDSC database to investigate whether the alteration in TP53 was correlated with chemotherapeutic agent sensitivity in BLCA. The results suggested that BLCA cancer cells with TP53-MT are significantly more sensitive to 3 chemotherapeutics, gemcitabine, mitomycin C and doxorubicin, than BLCA cancer cells with TP53-WT (Figure 6A-C, all p < 0.01), making them possibly favorable selections for treatment of BLCA with TP53-MT.
Figure 6.
TP53-MT affects the efficacy of common BLCA chemotherapeutic agents in GDSC-BLCA cell lines. Ln(IC50) values of gemcitabine (A), doxorubicin (B) and mitomycin C (C) between TP53-MT and TP53-WT cell lines. IC50 s reported in GDSC were loge transformed. The differences in the ln(IC50) values of different drugs between the TP53-MT and TP53-WT cell lines were tested using the Mann-Whitney U test. ns: not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
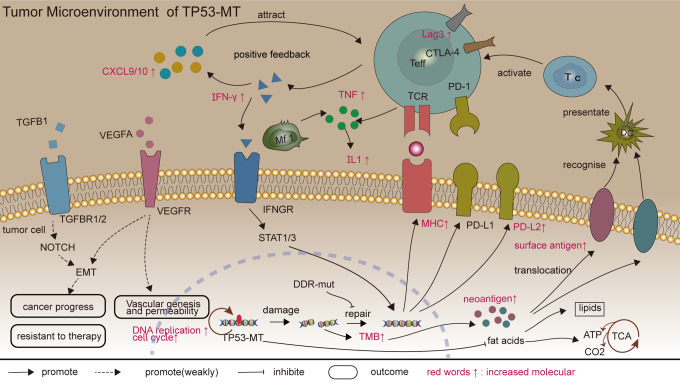
Here, based on careful evaluation of clinical data and somatic mutation data from an immunotherapy cohort consisting of BLCA patients, we observed that TP53-MT is a potential indicator of clinical benefit derived from ICI treatment and associated with prolonged OS (Figure 1A). We did not find a significant difference in prognosis between the patients with TP53-MT and those with TP53-WT (Figure 1B-C) in the TCGA-BLCA cohort, suggesting that the efficacy of non-ICI treatment is not related to the TP53 status. By performing integrated analysis of genes, mRNAs and proteins, as well as some functional molecules, we found that the mechanisms by which TP53-MT patients are more likely to benefit from ICIs may include an increased TMB and NAL and relatively strong preexisting antitumor immunity.51 There are some clues to the main mechanisms mentioned above in Figure 7.
Figure 7.
Potential mechanisms of TP53-MT patients to benefit from ICIs. Abbreviation: ICIs, immune checkpoint inhibition.
Previous studies have suggested that a high TMB (TMB-H) is associated with improved survival after ICI treatment across different types of tumors, such as melanoma52 and non-small cell lung cancer.34,53 Although there is currently no universal definition for a TMB-H, the TMB has been clinically validated as a biological indicator that can reliably predict the efficacy of ICIs.12,36 We observed that patients with TP53-MT had a significantly higher TMB than patients with TP53-WT in both the immune cohort and TCGA-BLCA cohort, and the patients with TP53-MT in the immune cohort responded better to ICIs, consistent with the above studies. However, we did not draw the same conclusion with GDSC cell lines because while the TMB in the TP53-MT group showed a higher trend than that in the TP53-WT group, the difference was not statistically significant. We thought the reason might be that the number of cell lines included was fairly low.
Although the median TMB of patients benefitting from ICIs is higher than that of patients who do not derive benefit, there was considerable overlap between TP53-MT and TP53-WT patients in this study, indicating that there may be other factors that affect the efficacy of ICIs. Defects in DDR-related pathways lead to increased genomic instability, and previous studies have suggested that mutations in DDR-related pathways are associated with a TMB-H and may be a screening indicator for potential responders to ICIs in solid tumors.17,54 In our study, the results for the immunotherapy cohort, TCGA-BLCA cohort, and GDSC cell datasets all suggested that TP53-MT corresponded with more nonsynonymous mutations in DDR-related pathways than TP53-WT and supported that there is increased instability in the genome in the TP53-MT group, which is attributed to the increased accumulation of missense mutations. In the results of GSEA, we found that the pathways related to the cell cycle and DNA replication were mainly enriched in TP53-MT, which showed that cell cycling and DNA replication were accelerated, and a previous study showed similar enrichment in TP53-MT lung adenocarcinoma.35 Thus, the body does not have enough time to process unrepaired DNA-damaged cancer cells, which may also explain the accumulation of errors in DNA replication and missense mutations.
In the 2 cohorts, TP53 alteration was mainly concentrated in the core region of the DNA-binding domain, reflecting the importance of the DNA-binding ability and transcriptional activation function in the role of a tumor suppressor gene. In addition, there were also a small number of mutations in the transcription activation domain in the TCGA-BLCA cohort, so missense mutations in this domain can also cause TP53 dysfunction. The results of the CNV analysis suggest that TP53-MT is associated with increased amplification of oncogenes and deletion of tumor suppressor genes, validating that TP53-MT increases genomic instability.26 Specifically, we found that the most common deletion in TP53-MT was 9p21.3, which spans the tumor suppressor genes CDKN2A and CDKN2B, and the most significantly amplified regions were 11q13.2-13.3, 1q23.3 and 6p22.3, of which 11q13.2-13.3 spans the CCND1 (cyclin D1) gene.55
Describing the landscape of genomic alterations helped us find that some commonly mutated genes were enriched in the TP53-WT or TP53-MT group. Interestingly, we observed significant enrichment of FGFR3-MT in the TP53-WT group, consistent with reported results in BLCA. Spruck et al. discoveried that TP53 mutations were more frequent in carcinoma in situ (CIS) and invasive tumors, and they highlighted that urothelial cell carcinomas progress via 2 distinct pathways.56 Later, Bakkar et al. reported that mutations in FGFR3 and TP53 were almost mutually exclusive. TP53-MT was associated with high-stage, high-grade tumors (eg, CIS), whereas FGFR3-MT was associated with low-stage, low-grade tumors (eg, pTa), which define separate pathways at initial diagnosis of BLCA.57 In the immunotherapy cohort, only the sample type (primary or metastatic sample) of the tumor can be obtained, and the distribution of them was not significantly different between TP53-MT and TP53-WT patients (p = 0.70, Supplemental Table 3). However, FGFR3-MT and TP53-MT were mutually exclusive regardless of tumor stage and grade, The FGFR3-MT was found in 31% of TP53-WT and 9% of TP53-MT patients. In addition, a previous study suggested that FGFR3-MT could become activated through increased ligand-independent dimerization and phosphorylation and was associated with low-level lymphocyte infiltration into tumors.58 Therefore, ICIs plus FGFR3-targeted inhibitors may be more effective than monotherapy in patients with FGFR3-MT. In addition, due to the larger number of samples in the TCGA-BLCA cohort, we found genes that are susceptible to comutation with TP53, such as RB1, FLG, and FAT4. Robertson et al.2 reported that simultaneous mutations in the above genes and TP53 could lead to increased genomic instability, which can result in the accumulation of more tumor neoantigens and increased immunogenicity.
The alterations in TP53 could increase tumor immunogenicity as mentioned above, stimulating the body’s antitumor immunity effectively. Marker scores for the leukocyte fraction and regulatory TIL fraction were higher in TP53-MT samples than in TP53-WT samples, and the levels of infiltration by M0 and M1 macrophages were higher in TP53-MT, but MDSCs were enriched more in TP53-WT, suggesting that an antitumor TIME was formed in the TP53-MT tumors. In addition, higher expression of IFNG, TNF, and MHCI/II and enrichment of the interferon-gamma pathway and the pathway positively regulating TNF secretion were observed in TP53-MT patients compared with TP53-WT patients. Increased levels of TNF can stimulate IL1 expression,59 and IFN-gamma secreted by effector T cells can promote tumor antigen recognition by activating STAT1 to upregulate the expression of MHC-I in tumor cells,60 which can also promote the expression of PD-L1 and PD-L2 on the surface of tumor cells through STAT1/3,61 increasing patient benefit derived from ICIs. Moreover, CD70/80, CXCL9/10, IL1A/B, ICAM1, IFNG, TNF and other immunostimulatory molecules were significantly overexpressed in TP53-MT disease, suggesting that immune signaling pathways were enriched. After interacting with tumor cells, effector T cells induce increased secretion of CXCL9 and CXCL10 through the IFN-γ pathway, which attracts more effector T cells via a positive feedback loop.51 Collectively, these results suggested that there was strong preexisting antitumor immunity in samples with TP53-MT.
The expression of immunosuppressive molecules suggested that patients with TP53-MT may be more likely to benefit from ICIs than those with TP53-WT. In our study, some immunosuppressive molecules, such as LAG3, PDCD1LG2 (PD-L2) and VTCN1, were more highly expressed in the TP53-MT group. Although there was a trend toward relatively high expression of PDCD1 and PD-L1, the GSEA results indicated that the PD-1 signaling pathway was enriched in TP53-MT samples, suggesting that TP53-MT patients are more likely to benefit from PD1/PD-L1 inhibitor treatment than are TP53-WT patients. Additionally, the expression of PDCD1LG2 in TP53-MT tumors was higher than that in WT tumors. Previous studies suggested that high expression of PD-L2 is related to the clinical benefit derived from ICIs across various tumors,62,63 so we hypothesize that patients with elevated expression of PD-L1 and DP-L2 are more likely to benefit from ICIs than patients with low expression. The metabolites of IDO1 can inhibit antitumor immunity,64 but their expression levels were close between the groups, suggesting PD1/CTLA-4 inhibitors combined with IDO1 inhibitors may have limited efficacy.
In addition to the GSEA results discussed above, we found some enrichment of pathways related to primary or acquired resistance to ICIs and cancer progression, such as EMT, angiogenesis and positive regulation of vessel permeability pathways, in TP53-WT samples.65 The expression of VEGFA/VEGFB, which are molecules related to angiogenesis and the regulation of permeability, was not significantly different between the 2 groups, suggesting that other regulatory factors may exist to achieve the observed effects. In addition, upregulation of NOTCH1 pathway activity can induce the progression of malignant tumors by promoting EMT,66 so the enrichment of NOTCH1 and EMT pathways in TP53-WT samples may indicate a relatively poor prognosis. Fatty acid metabolism and biological oxidation pathways are also enriched in TP53-WT disease to meet the needs related to increased lipid membrane and signal molecule synthesis, facilitating the growth and proliferation of malignant cells. In recent decades, although the role of fatty acid oxidation in macrophages and T cells has been controversial, the key role of substance metabolism in determining tumor progression or regression has gradually been shown.67
Chemotherapy is still the standard therapy for controlling BLCA tumorigenesis and development, and detection of mutations in TP53 can also provide guidance for personalized chemotherapy in BLCA patients. The analysis of GDSC data showed that cell lines containing TP53-MT were more sensitive to gemcitabine, mitomycin c, and doxorubicin, which are all drugs used in first-line chemotherapy for BLCA68 that can reduce tumor cell proliferation and metastasis, reduce tumor recurrence and prolong relapse-free survival through a variety of mechanisms, than those containing TP53-WT.
In addition to the content discussed above suggesting that patients with TP53-MT show stronger tumor antigenicity, tumor antigen presentation and antitumor immunity preexisting in tumors, some more available and cheaper clinical biomarkers could also be considered to reflect systemic inflammation, which indirectly reflects the immunogenicity of the tumor, such as CRP, neutrophils, white blood cells, and neutrophil-to-lymphocyte ratio (NLR). For example, the increased modified Glasgow Prognostic Score (mGPS), which is calculated based on the levels of albumin and CRP in circulating blood from BLCA patients, is related to the longer recurrence-free survival period after radical bladder resection.69 Decreased NLR before treatment may be an indicator of a longer disease-specific and overall survival for MIBC patients receiving neoadjuvant chemotherapy and radical surgery.70
Immunotherapy may also play an important role in the field of neoadjuvant therapy. Neoadjuvant chemotherapy (NAC) is the standard treatment for MIBC, which is helpful to Pathological complete responses and tumor downstaging to NMIBC. However NAC is less effective in patients with predominant variant histology (VH), the clinical trial results of PURE-01 and ABACUS all indicated that neoadjuvant Pembrolizumab or Atezolizumab could be beneficial to patients with MINBC(inlcuding patients presented with VH), especially for those with high PD-L1 expression.71,72 In PURE-01 trial, it was also found that people with high TMB responded better to neoadjuvant Pembrolizumab. In the field of neoadjuvant therapy, the discovery of predictive biomarkers is also of great significance for identification of these responding tumors.
There are some limitations to this retrospective study. First, the patients we included were from an immunotherapy cohort (MSKCC_IMPACT) that was analyzed with targeted next-generation sequencing and the TCGA-BLCA cohort analyzed by full-exon sequencing, which may have caused some patient selection bias. Second, we defined any type of mutation in TP53 as TP53-MT, which may introduce some variability. There are many types of TP53 mutations, and their functions may be different. Therefore, the potential mechanisms involving different types of TP53 mutants and the efficacy of ICIs need to be further explored. Third, although, in the included immunotherapy cohort, we found that TP53-MT is associated with a relatively good prognosis for ICI treatment, whether TP53-MT can predict the efficacy of ICIs and the related mechanism need more clinical data to be validated.
In summary, our study suggested that BLCA patients with TP53-MT are more likely to benefit from ICIs at the genomic level than are those with TP53-WT and explored possible mechanisms. Our research provides some clues for identifying patients who would potentially benefit from ICIs at the DNA level, increasing indications for targeted second-generation sequencing (i.e., MSKCC_IMPACT), and this indicator can be coordinated with other indicators to promote the development of precision medicine. In the near future, a larger cohort in prospective studies will be needed to validate the prognostic value of TP53-MT for predicting ICI treatment outcomes and to explore the possible role of TP53-MT in immunotherapy.
Supplemental Material
Supplemental Material, sj-csv-1-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-csv-2-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-docx-1-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-tif-1-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-tif-2-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-tif-3-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-xlsx-1-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Abbreviations
BLCA: bladder cancer; MIBC: muscle-invasive bladder cancer; NMIBC: non-muscle-invasive bladder cancer; ICIs: immune checkpoint inhibitors; TP53-MT: mutant TP53; TP53-WT: wild-type TP53; TMB: tumor mutational burden; NAL: neoantigen load; CNVs: copy number variations; OS: overall survival; DFS: disease-free survival; PFS: progression-free survival; MSI: microsatellite instability; DDR: DNA damage response and repair; TCGA: The Cancer Genome Atlas; GDSC: Genomics of Drug Sensitivity in Cancer; MCL: mantle cell lymphoma; BC: breast cancer; FPKM: reads per kilobase of exon model per million mapped reads.
Footnotes
Authors’ Note: JZ and PL contributed conception and design of the study; AL performed the statistical analysis; QL wrote the first draft of the manuscript; QL, AL, MC, and AX wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version. Publicly available datasets were analyzed in this study. The data can be found in the TCGA [https://portal.gdc.cancer.gov/], cBioportal[http://www.cbioportal.org/] and GDSC[https://www.cancerrxgene.org/] database. There was no ethics statement in this study. In the study, we obtained data for 210 patients from an immunotherapy cohort, 412 patients from The Cancer Genome Atlas (TCGA)-BLCA cohort and 18 BLCA cell lines from Genomics of Drug Sensitivity in Cancer (GDSC), and we performed integrated bioinformatic analysis to explore the relationships between TP53-MT and clinical benefits derived from ICI treatment and the underlying mechanisms. So, there were no animal and human studies in our study, and we did not provide an ethics statement.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Peng Luo, MD  https://orcid.org/0000-0002-8215-2045
https://orcid.org/0000-0002-8215-2045
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71(1):96–108. [DOI] [PubMed] [Google Scholar]
- 2.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadal R, Bellmunt J. Management of metastatic bladder cancer. Cancer Treat Rev. 2019;76:10–21. [DOI] [PubMed] [Google Scholar]
- 4.Rice-Stitt T, Valencia-Guerrero A, Cornejo KM, Wu CL. Updates in histologic grading of urologic neoplasms. Arch Pathol Lab Med. 2020;144(3):335–343. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Liu J, Dai R, Wu S. Current status and future perspectives of immunotherapy in bladder cancer treatment [published online August 26, 2020]. Sci China Life Sci. 2020. [DOI] [PubMed] [Google Scholar]
- 6.Roumiguié M, Compérat E, Chaltiel L, et al. PD-L1 expression and pattern of immune cells in pre-treatment specimens are associated with disease-free survival for HR-NMIBC undergoing BCG treatment [published online July 14, 2020]. World J Urol. 2020. [DOI] [PubMed] [Google Scholar]
- 7.Ferro M, Di Lorenzo G, Vartolomei MD, et al. Absolute basophil count is associated with time to recurrence in patients with high-grade T1 bladder cancer receiving bacillus Calmette-Guérin after transurethral resection of the bladder tumor. World J Urol. 2020;38(1):143–150. [DOI] [PubMed] [Google Scholar]
- 8.Ferro M, Vartolomei MD, Russo GI, et al. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol. 2019;37(3):507–514. [DOI] [PubMed] [Google Scholar]
- 9.Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenehjem DD, Tran D, Nkrumah MA, Gupta S. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco Targets Ther. 2018;11:5973–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 15.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site—when a biomarker defines the indication. N Engl J Med. 2017;377(15):1409–1412. [DOI] [PubMed] [Google Scholar]
- 16.Lin A, Zhang J, Luo P. Crosstalk between the MSI status and tumor microenvironment in colorectal cancer. Front Immunol. 2020;11:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Zhao J, Wang G, et al. Comutations in DNA damage response pathways serve as potential biomarkers for immune checkpoint blockade. Cancer Res. 2018;78(22):6486. [DOI] [PubMed] [Google Scholar]
- 18.Yi R, Lin A, Cao M, Xu A, Luo P, Zhang J. ATM mutations benefit bladder cancer patients treated with immune checkpoint inhibitors by acting on the tumor immune microenvironment. Front Genet. 2020;11:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin A, Zhang H, Hu X, et al. Age, sex, and specific gene mutations affect the effects of immune checkpoint inhibitors in colorectal cancer. Pharmacol Res. 2020;159:105028. [DOI] [PubMed] [Google Scholar]
- 20.Lin W, Lin A, Li Z, et al. Potential predictive value of SCN4A mutation status for immune checkpoint inhibitors in melanoma. Biomed Pharmacother. 2020;131:110633. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Zhou N, Lin A, et al. ZFHX3 mutation as a protective biomarker for immune checkpoint blockade in non-small cell lung cancer. Cancer Immunol Immunother. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, Lin A, Luo P, et al. EPHA5 mutation predicts the durable clinical benefit of immune checkpoint inhibitors in patients with lung adenocarcinoma. Cancer Gene Ther. 2020. [DOI] [PubMed] [Google Scholar]
- 23.Niu Y, Lin A, Luo P, et al. Prognosis of lung adenocarcinoma patients with NTRK3 mutations to immune checkpoint inhibitors. Front Pharmacol. 2020;11:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riaz N, Havel JJ, Kendall SM, et al. Recurrent SERPINB3 and SERPINB4 mutations in patients who respond to anti-CTLA4 immunotherapy. Nat Genet. 2016;48(11):1327–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu HX, Chen YX, Wang ZX, et al. Alteration in TET1 as potential biomarker for immune checkpoint blockade in multiple cancers. J Immunother Cancer. 2019;7(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donehower LA, Soussi T, Korkut A, et al. Integrated analysis of TP53 gene and pathway alterations in the cancer genome atlas. Cell Rep. 2019;28(5):1370–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim PH, Cha EK, Sfakianos JP, et al. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. Eur Urol. 2015;67(2):198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu G, Wang F, Li K, et al. Significance of TP53 mutation in bladder cancer disease progression and drug selection. PeerJ. 2019;7:e8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eskelund CW, Dahl C, Hansen JW, et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood. 2017;130(17):1903–1910. [DOI] [PubMed] [Google Scholar]
- 31.Aukema SM, Hoster E, Rosenwald A, et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood. 2018;131(4):417–420. [DOI] [PubMed] [Google Scholar]
- 32.Lin CH, Chen IC, Huang CS, et al. TP53 Mutational analysis enhances the prognostic accuracy of IHC4 and PAM50 assays. Sci Rep. 2015;5:17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao W, Du N, Huang T, et al. TP53 mutation as potential negative predictor for response of anti-CTLA-4 therapy in metastatic melanoma. Ebiomedicine. 2018;32:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellmann MD, Nathanson T, Rizvi H. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33(5):843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong ZY, Zhong WZ, Zhang XC, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012–3024. [DOI] [PubMed] [Google Scholar]
- 36.Samstein RM, Lee C, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colaprico A, Silva TC, Olsen C, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8):e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W, Soares J, Greninger P, et al. Genomics of drug sensitivity in cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41(Database issue):D955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity. 2018;48(4):812–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–2849. [DOI] [PubMed] [Google Scholar]
- 43.Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28(11):1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao D, Liu J, Chen M, et al. Immunogenomic analyses of advanced serous ovarian cancer reveal immune score is a strong prognostic factor and an indicator of chemosensitivity. Clin Cancer Res. 2018;24(15):3560–3571. [DOI] [PubMed] [Google Scholar]
- 46.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38(5):500–501. [DOI] [PubMed] [Google Scholar]
- 47.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kassambara A. (2018): ggplot2: Elegant Graphics for Data Analysis. R package version 0.1.7. https://CRAN.R-project.org/package=ggpubr
- 51.Blank CU, Haanen JB, Ribas A, Schumacher TN. Cancer Immunology. The ‘cancer immunogram". Science. 2016;352(6286):658–660. [DOI] [PubMed] [Google Scholar]
- 52.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teo MY, Seier K, Ostrovnaya I, et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol. 2018;36(17):1685–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol. 2013;31(25):3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spruck CR, Ohneseit PF, Gonzalez-Zulueta M, et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 1994;54(3):784–788. [PubMed] [Google Scholar]
- 57.Bakkar AA, Wallerand H, Radvanyi F, et al. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res. 2003;63(23):8108–8112. [PubMed] [Google Scholar]
- 58.Sweis RF, Spranger S, Bao R, et al. Molecular drivers of the non-T-cell-inflamed tumor microenvironment in urothelial bladder cancer. Cancer Immunol Res. 2016;4(7):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braun T, Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res Ther. 2011;13(4):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi KS, van den Elsen PJ. NLRC5: a key regulator of MHC class I-dependent immune responses. Nat Rev Immunol. 2012;12(12):813–820. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Diaz A, Shin DS, Moreno BH, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19(6):1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmid P, Hegde PS, Zou W, et al. Association of PD-L2 expression in human tumors with atezolizumab activity. J Clin Oncol. 2016;34(15_suppl):11506. [Google Scholar]
- 63.Yearley JH, Gibson C, Yu N, et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin Cancer Res. 2017;23(12):3158–3167. [DOI] [PubMed] [Google Scholar]
- 64.Li H, Bullock K, Gurjao C, et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat Commun. 2019;10(1):4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma. Cell. 2017;168(3):542. [DOI] [PubMed] [Google Scholar]
- 66.Pal D, Kolluru V, Chandrasekaran B, et al. Targeting aberrant expression of Notch-1 in ALDH+ cancer stem cells in breast cancer. Mol Carcinogen. 2017;56(3):1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van den Bossche J, van der Windt GJW. Fatty acid oxidation in macrophages and T cells: time for reassessment? Cell Metab. 2018;28(4):538–540. [DOI] [PubMed] [Google Scholar]
- 68.Yu C, Hequn C, Jinbo C, Feng Z, Xiongbing Z, Jian D.Gemcitabine/cisplatin versus methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive bladder cancer: a systematic review and meta-analysis. J Cancer Res Ther. 2018;14(6):1260–1265. [DOI] [PubMed] [Google Scholar]
- 69.Ferro M, De Cobelli O, Buonerba C, et al. Modified Glasgow prognostic score is associated with risk of recurrence in bladder cancer patients after radical cystectomy: a multicenter experience. Medicine (Baltimore). 2015;94(42):e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Black AJ, Zargar H, Zargar-Shoshtari K, et al. The prognostic value of the neutrophil-to-lymphocyte ratio in patients with muscle-invasive bladder cancer treated with neoadjuvant chemotherapy and radical cystectomy. Urol Oncol. 2020;38(1):3–17. [DOI] [PubMed] [Google Scholar]
- 71.Necchi A, Raggi D, Gallina A, et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol. 2020;77(4):439–446. [DOI] [PubMed] [Google Scholar]
- 72.Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019;25(11):1706–1714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-csv-1-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-csv-2-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-docx-1-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-tif-1-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-tif-2-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-tif-3-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control
Supplemental Material, sj-xlsx-1-ccx-10.1177_1073274820976665 for Alterations in TP53 Are a Potential Biomarker of Bladder Cancer Patients Who Benefit From Immune Checkpoint Inhibition by Qiong Lyu, Anqi Lin, Manming Cao, Abai Xu, Peng Luo and Jian Zhang in Cancer Control