Abstract
Objective
To compare the effectiveness of initial treatment for infantile spasms.
Methods
The National Infantile Spasms Consortium prospectively followed up children with new-onset infantile spasms that began at age 2 to 24 months at 23 US centers (2012–2018). Freedom from treatment failure at 60 days required no second treatment for infantile spasms and no clinical spasms after 30 days of treatment initiation. We managed treatment selection bias with propensity score weighting and within-center correlation with generalized estimating equations.
Results
Freedom from treatment failure rates were as follows: adrenocorticotropic hormone (ACTH) 88 of 190 (46%), oral steroids 42 of 95 (44%), vigabatrin 32 of 87 (37%), and nonstandard therapy 4 of 51 (8%). Changing from oral steroids to ACTH was not estimated to affect response (observed 44% estimated to change to 44% [95% confidence interval 34%–54%]). Changing from nonstandard therapy to ACTH would improve response from 8% to 39% (17%–67%), and changing to oral steroids would improve response from 8% to 38% (15%–68%). There were large but not statistically significant estimated effects of changing from vigabatrin to ACTH (29% to 42% [15%–75%]), from vigabatrin to oral steroids (29% to 42% [28%–57%]), and from nonstandard therapy to vigabatrin (8% to 20% [6%–50%]). Among children treated with vigabatrin, those with tuberous sclerosis complex (TSC) responded more often than others (62% vs 29%; p < 0.05).
Discussion
Compared to nonstandard therapy, ACTH and oral steroids are superior for initial treatment of infantile spasms. The estimated effectiveness of vigabatrin is between that of ACTH/oral steroids and nonstandard therapy, although the sample was underpowered for statistical confidence. When used, vigabatrin worked best for TSC.
Classification of Evidence
This study provides Class III evidence that for children with new-onset infantile spasms, ACTH or oral steroids were superior to nonstandard therapies.
Infantile spasms (West syndrome) is an early-life epilepsy syndrome. Affected children often have developmental regression, intellectual disability, and lifelong epilepsy. Successful treatment may improve neurodevelopmental outcomes1-3 and, for some, lead to permanent remission of epilepsy.1,4-6
There are 3 recommended first treatments for infantile spasms: oral corticosteroids (typically prednisolone in the United States), adrenocorticotropic hormone (ACTH), and vigabatrin.7,8 Each medication has a different proposed mechanism of action. Oral steroids act on glucocorticoid and mineralocorticoid receptors in the brain, although the exact antiseizure and antiepilepsy effects are unknown.9,10 ACTH stimulates release of endogenous steroids and may have steroid-independent effects via melanocortin receptors and modulation of corticotropin-releasing hormone.11 Vigabatrin inhibits GABA transaminase, leading to increased brain concentrations of GABA.12
Our published analysis of a rigorous prospective multicentered observational study of infants with infantile spasms (the National Infantile Spasms Consortium [NISC]) suggested the superiority of ACTH over other treatments.13 However, our findings were based on a preliminary analysis of an active registry and did not fully account for treatment selection bias and center-to-center variations. Furthermore, we included resolution of hypsarrhythmia as a primary outcome, which has since been shown to have poor interrater reliability.14 Subsequent meta-analyses have suggested that response rates may not be appreciably different between ACTH (or tetracosactide) and oral steroids,15,16 further motivating a reanalysis. Here, we analyze the full NISC dataset to compare the effectiveness of ACTH, oral steroids, vigabatrin, and nonstandard therapies. We modified a “freedom from treatment failure” epilepsy outcome17,18 and adapted it for infantile spasms. We applied statistical techniques to account for selection bias and clustering of outcomes within centers. Hypsarrhythmia outcomes were compared as secondary analyses.
Methods
Study Design
NISC was a prospective multicenter observational cohort study of children with infantile spasms conducted primarily through chart review.13 The Institutional Review Board at each institution approved the study. Written informed consent was obtained from a parent or guardian for each enrolled child.
Data Source
From 2012 to 2018, 23 US pediatric epilepsy centers (all members of the Pediatric Epilepsy Research Consortium19) prospectively enrolled children in NISC. Data collected via chart review were entered into a Research Electronic Data Capture database (REDCap Consortium; Nashville, TN), supervised by site investigators (all pediatric epilepsy specialists).13
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board at all participating centers. Written informed consent was obtained for all participants (i.e., from parents or guardians). The study was observational only and not entered into a public trials registry. There are no recognizable persons in this publication.
Inclusion and Exclusion Criteria
NISC enrolled children with new-onset infantile spasms that began at age 2 to 24 months and recorded 1 follow-up (typically at 3 months). We excluded children with no treatment given for infantile spasms, <60 days of follow-up, or time from onset to first treatment >90 days (a strong predictor of poor outcomes).20,21 Children were also excluded for incomplete or contradictory data entry.
Exposure
We grouped children into 4 categories based on the first treatment for infantile spasms: ACTH, oral steroids, vigabatrin, and nonstandard. Target dose of medications, titration schedules, and serum levels were recorded as free text and not entered consistently for all participants.
Outcomes
Seizures
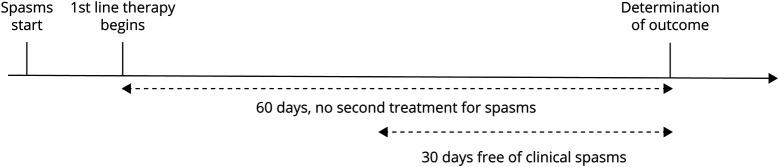
An infant was free from treatment failure at 60 days if (1) no second treatment was prescribed within 60 days and (2) the infant was free of infantile spasms beginning within 30 days of treatment initiation (Figure 1). We also report how often children needed a second treatment for infantile spasms and how often children were free of infantile spasms regardless of the use of additional treatments.
Figure 1. Freedom From Treatment Failure at 60-Day Outcome.
The epilepsy outcome was freedom from treatment failure at 60 days, which required that the child did not require a second medication for infantile spasms and was free of clinical spasms for 30 days.
Electroencephalogram
Hypsarrhythmia commonly (but not always) accompanies infantile spasms. Its resolution is often a required indicator of successful treatment,13,22 although not in large recent trials.20,21 However, the NISC dataset did not include central review of EEGs. Furthermore, assessment of hypsarrhythmia had poor interrater reliability14 before the recent development of the Burden of Amplitudes and Epileptiform Discharges score, which was not included in data collection.23 We included resolution of hypsarrhythmia in secondary analyses.
Covariates
Demographic covariates included age at infantile spasms onset, sex, ethnicity, race, gestational age at birth, distance from home to the medical center, and insurance. Clinical covariates included etiology, prior antiseizure medication (ASM), and days from infantile spasms onset to treatment. We also included neurologic covariates: head size, developmental delay at infantile spasms onset, developmental regression before treatment, brain imaging, and EEG. The treating child neurologist determined clinical and neurologic factors. MRI and EEG findings were based on the clinical report.
Selection Bias
We examined selection bias via bivariate analyses among the 4 groups. As an omnibus test, we used the χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. For post hoc analysis of χ2 tests, we compared the standard Pearson residuals of each cell to a χ2 distribution with 1 df, adjusting p values with Bonferroni correction.24
Multivariable Analyses
We performed 6 pairwise analyses using multivariable logistic regression: ACTH vs oral steroids, ACTH vs vigabatrin, oral steroids vs vigabatrin, and nonstandard vs each standard treatment. We accounted for selection bias among observed characteristics with inverse probability of treatment weighting (weighting by odds,25 also called SMR weighting26 or average treatment effect on the treated27). We adjusted standard errors for correlation of outcomes within each center via generalized estimating equations, with medical center as a clustering variable. We did not further adjust the weighted generalized estimating equations with covariates.25 In each analysis, we selected a reference medication and a comparison medication for the counterfactual estimate. For example, when comparing nonstandard (reference) to ACTH (comparison), we estimated the counterfactual question: “what would be the effect of using ACTH (comparison) instead of nonstandard (reference) among the children who received nonstandard (reference)?”
We developed the propensity score using logistic regression to estimate the probability that an infant would receive the reference medication, including all covariates. We assigned a weight of 1.0 to individuals treated with the reference medication and used the propensity score to weight those treated with the comparison medication: ps/(1 – ps), where ps is propensity score. We assessed the balance of covariates by examining the standard difference (Cohen d) between the groups.18 In a randomized controlled trial (RCT), d is expected to be 0 for each covariate, with a standard error of 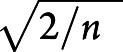 (n = size of each group).28 We calculated |d| for each covariate. If |d| was <1 standard error, we called the balance excellent; if d was between 1 and 2 standard errors, good; and if d was >2 standard errors, poor. Roughly, then, we expected the balance to be excellent for 68% of the covariates, good for 27%, and poor for 5% (with 45 covariates: 31 excellent, 12 good, and 2 poor). We thus qualified the overall balance as excellent if ≤2 covariates had poor balance, good if 3 to 5 had poor balance, and poor if ≥6 had poor balance. This approach is statistically conservative; it treats a k-level categorical variable as k-independent measurements for k ≥ 3 rather than as a single measurement.
(n = size of each group).28 We calculated |d| for each covariate. If |d| was <1 standard error, we called the balance excellent; if d was between 1 and 2 standard errors, good; and if d was >2 standard errors, poor. Roughly, then, we expected the balance to be excellent for 68% of the covariates, good for 27%, and poor for 5% (with 45 covariates: 31 excellent, 12 good, and 2 poor). We thus qualified the overall balance as excellent if ≤2 covariates had poor balance, good if 3 to 5 had poor balance, and poor if ≥6 had poor balance. This approach is statistically conservative; it treats a k-level categorical variable as k-independent measurements for k ≥ 3 rather than as a single measurement.
Missing Data
We treated unknown as its own category. We excluded cases with incomplete or contradictory data (i.e., complete case analysis).
Sensitivity Analyses
We performed several sensitivity analyses. First, we examined different outcomes including spasms free at 60 days (regardless of second treatment) and no second treatment for infantile spasms (independently of clinical spasms resolution). Second, we examined a shorter time outcome (spasms free for 2 weeks after 1 month). Third, we analyzed the subpopulation of children with hypsarrhythmia at presentation via 3 outcomes: hypsarrhythmia resolved, hypsarrhythmia resolved and the infant was spasms free at 60 days, and hypsarrhythmia resolved and the infant was spasms free at 60 days and no second medication was required (i.e., hypsarrhythmia resolved and free from failure). We report analyses only if the regression converged and the overall balance was good or excellent.
Unmeasured Confounding
To estimate the amount of unmeasured confounding required to explain away any significant effects, we calculate the E-value.29 The E-value is the minimum association of an unmeasured set of confounders with the treatment and the outcome required to explain away the finding. To do so, we calculated the ratio of the estimated and observed response rate, which we interpreted on the risk ratio scale. We then used the formula E-value = RR + sqrt (RR × [RR – 1]), where RR is the risk ratio. To obtain a lower limit, we repeated the calculation using the lower limit of the estimated response rate.
Data Analysis
We used SAS (SAS Institute, Inc, Cary, NC) and R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Classification of Evidence
For the research question “what is the comparative effectiveness among 4 therapeutic choices for infantile spasms (ACTH, oral steroids, vigabatrin, or nonstandard)?”, the journal classified this study design as providing Class III evidence.
Results
Study Sample
The NISC database includes 629 children with infantile spasms. We excluded 206: 35 because there was no treatment specific for infantile spasms, 82 had <60 days of follow up, 35 did not have a recorded date for the last spasm, 10 received the first drug after the last infantile spasm, 1 had several dates recorded after the last known follow-up, and 43 did not receive the first medication until 90 days after spasms onset. This left 423 in the analytical cohort: 190 treated with ACTH, 95 with oral steroids (73 prednisolone, 22 prednisone), 87 with vigabatrin, and 51 with nonstandard therapies. The 51 nonstandard treatments included 29 with topiramate, 8 with levetiracetam, 5 with clobazam, 3 with zonisamide, 3 with dietary therapies, 2 with rufinamide, and 1 with oxcarbazepine (Figure 2 and Table 1).
Figure 2. Flow Diagram of Participants.
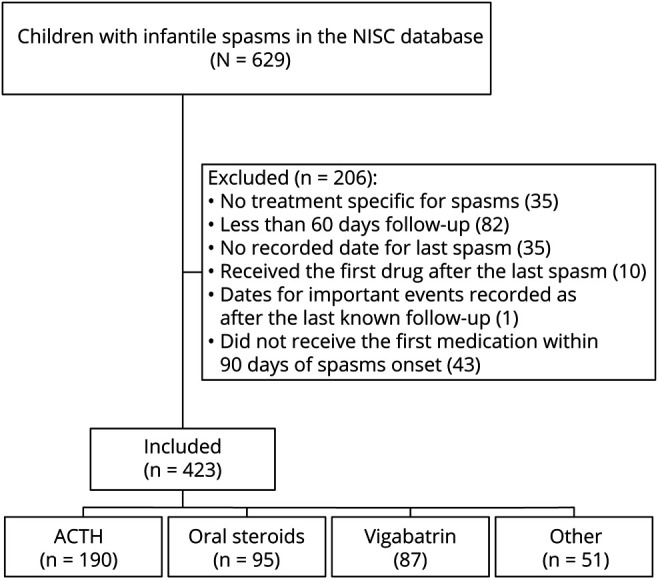
Flow diagram indicating which participants in the National Infantile Spasms Consortium (NISC) were included in the analysis. ACTH = adrenocorticotropic hormone.
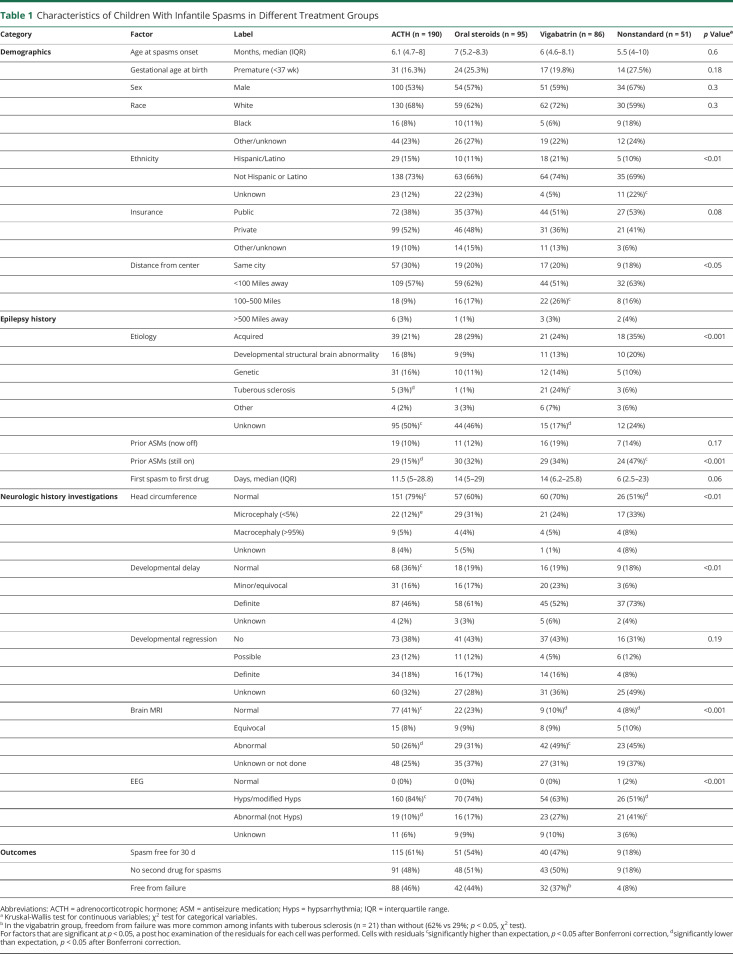
Table 1.
Characteristics of Children With Infantile Spasms in Different Treatment Groups
Selection Bias
Several factors were significantly associated with treatment selection, including demographics (ethnicity, distance from center), epilepsy history (etiology, ongoing use of ASMs when the infantile spasms begin), neurologic history (head circumference, developmental delay), and findings from brain MRI and EEG. There was also a suggestion of an association with insurance and time to treatment (Table 1).
Post hoc analysis suggested that the following observations accounted for differences. Compared to the expected proportions in the contingency tables (Table 1), children who received ACTH were more likely to have unknown etiology, normal head circumference, normal development, normal brain MRI, and hypsarrhythmia (or variant) on EEG. They were less likely to have tuberous sclerosis complex (TSC) or prior ongoing treatment with an ASM. Infants who received vigabatrin were more likely to live 100 to 500 miles from the hospital, to have TSC, and to have an abnormal MRI. They were less likely to have an unknown etiology. Infants who received nonstandard therapy were more likely to have unknown ethnicity, prior ongoing treatment with an ASM, or an abnormal EEG that was not hypsarrhythmia. They were also less likely to have a normal head circumference or a normal brain MRI.
In the unadjusted analysis, freedom from treatment failure was achieved in 46% of those treated with ACTH, 44% treated with oral steroids, 37% treated with vigabatrin, and 8% treated with nonstandard therapies. About half the infants in each of the 3 standard treatment groups received a second medication within 60 days for infantile spasms. In the nonstandard therapy group, most (82%) received a second medication for infantile spasms. Clinical response rate (free from infantile spasms for 30 days at 2 months) was better than the freedom from failure outcome: 61% for ACTH, 54% for oral steroids, 47% for vigabatrin, and 18% for nonstandard. Within the vigabatrin group, freedom from failure among infants with TSC (n = 21) was more likely than for other etiologies (13 of 21 infants with TSC [62%] free from failure vs 19 of 65 other infants [29%]; p = 0.01) (Table 1). Six infants with TSC received hormonal therapy first (5 ACTH, 1 oral steroids); half responded (2 ACTH, 1 oral steroids).
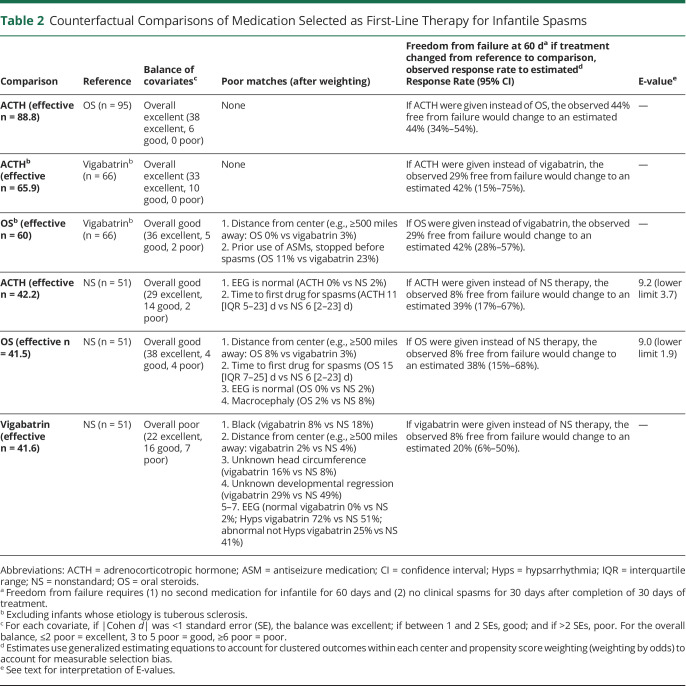
In the comparative-effectiveness analysis, the overall balance of the covariates was excellent for 2 comparisons, good for 3, and poor for 1 (Table 2). Among infants treated with oral steroids, changing to ACTH would have little effect: freedom from treatment failure would change from the observed 44% to an estimated 44% (95% confidence interval [CI] 34–54). Among infants treated with vigabatrin (not including infants with TSC), changing from vigabatrin to ACTH would improve freedom from treatment failure from the observed 29% to an estimated 42% (95% CI 15%–75%); changing from vigabatrin to oral steroids, from 29% to an estimated 42% (95% CI 28%–57%).
Table 2.
Counterfactual Comparisons of Medication Selected as First-Line Therapy for Infantile Spasms
Comparison of standard therapies to nonstandard therapies showed the potential for large improvements in response rates. Changing from nonstandard therapy would significantly improve freedom from failure from the observed 8% to an estimated 39% (95% CI 17%–67%) for ACTH and to 38% (95% CI 15%–68%) for oral steroids. The number needed to treat for 1 additional infant free from failure was 3.2 (95% CI 1.7–11) for ACTH and 3.3 (95% CI 1.7–14) for oral steroids. Changing from nonstandard therapy to vigabatrin would improve freedom from failure from the observed 8% to an estimated 20% (95% CI 6–50) (Table 2).
To explain away the estimated superiority of ACTH over nonstandard therapy, an unmeasured set of confounders that were 9.2 (lower limit 3.7) times more common among those who received ACTH and associated with a 9.2-fold (lower limit 3.7-fold) increased chance of treatment response would be sufficient. To explain away the estimated superiority of oral steroids over nonstandard therapies, an unmeasured set of confounders that were 9.0 (lower limit 3.2) times more common among those who received oral steroids and associated with a 9.0-fold (lower limit 3.2-fold) increased chance of treatment response would be sufficient. In both cases, a decrease in the relative prevalence of the unmeasured confounders could be offset by an increase in the association with treatment response, or vice versa.
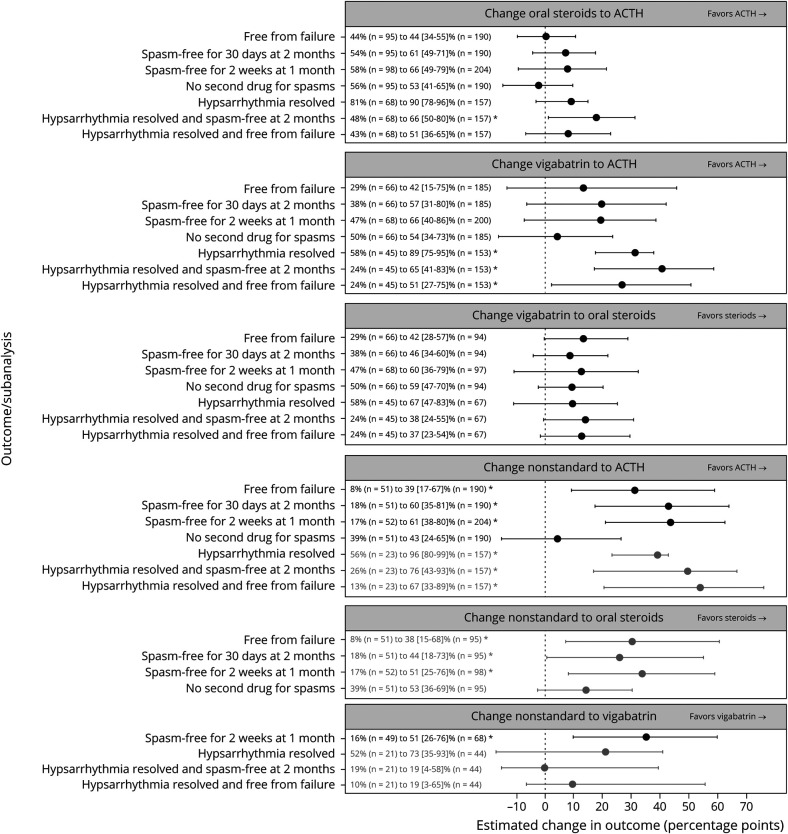
In the sensitivity analyses, 36 of the planned 42 analyses converged and had good or excellent balance of covariates. Among infants who presented with hypsarrhythmia, changing from oral steroids to ACTH would improve the combined outcome of resolution of both hypsarrhythmia and clinical spasms from the observed rate of 48% to an expected rate of 66 (95% CI 50%–80%). Similarly, changing from vigabatrin to ACTH would improve 3 outcomes: resolution of hypsarrhythmia (58%–89% [95% CI 75%–95%]), the combined outcome of resolution of hypsarrhythmia and clinical spasms (24%–65% [95% CI 41%–83%]), and the combined outcome of resolution of hypsarrhythmia and freedom from treatment failure (24%–51% [95% CI 27%–75%]). Changing from nonstandard treatment to any of the 3 standard treatments would significantly outcomes, including 6 of 7 outcomes for ACTH, 3 of 4 for oral steroids, and 1 of 4 for vigabatrin (Figure 3).
Figure 3. Sensitivity Analyses for 6 Comparisons.
Each of the 6 comparisons (panels) was performed for the primary endpoint (free from failure) and the 2 subendpoints (spasms free for 30 days at 2 months and no second drug for spasms). Comparisons were also conducted in a larger group with at least 28 days of follow-up (204 treated with adrenocorticotropic hormone [ACTH], 98 with oral steroids, 68 with vigabatrin, 52 with nonstandard treatment) using a 1-month outcome (spasms free for 2 weeks at 1 month) and in a subgroup who had hypsarrhythmia at baseline (157 treated with ACTH, 68 with oral steroids, 45 with vigabatrin, 23 with nonstandard treatment) for 3 outcomes (hypsarrhythmia resolved, hypsarrhythmia resolved and spasms free at 2 months, and hypsarrhythmia resolved and free from failure). Comparisons with vigabatrin excluded infants with tuberous sclerosis complex. Observed outcome rate and estimated counterfactual outcome rate (with 95% confidence intervals [CIs]) are provided as text, including the sample size for each group. Estimated difference in outcome rate (in percentage points) is plotted (x-axis) with 95% CIs. If the overall quality of the match was excellent, the result appears in black; if good, gray. Analyses that had poor overall match or did not converge are not shown. *Statistically significant differences (p < 0.05).
Discussion
We provide real-world head-to-head comparisons of different treatments for infantile spams. Among children with infantile spasms, treatment with anything other than the 3 recommended therapies resulted in a dismal response: only 4 of 51 were free from treatment failure. By our estimates, ACTH would have led to freedom from failure in 20 of the 51, and oral steroids would have led to freedom from failure in 19 of the 51. The E-values were high (9.2 and 9), suggesting that unmeasured confounding is unlikely to explain away these findings.
Comparing the 3 standard therapies showed that ACTH and oral steroids were similarly effective for infantile spasms, although the study was underpowered to rule out a clinically important effect. For infants without TSC, the point estimates suggest that vigabatrin may have a clinically important lower response rate compared to hormonal therapy; however, the sample size was underpowered to confirm this observation. For infants with TSC, vigabatrin was particularly effective.
The sensitivity analyses suggested that ACTH performed better than oral steroids or vigabatrin when resolution of hypsarrhythmia was included as an outcome. However, interpretation of this finding must be tempered. The NISC dataset did not include standardized or centralized assessment of hypsarrhythmia, which is known to have poor interrater reliability.14 In addition, although time to resolution of clinical spasms is directly correlated with 18-month developmental outcomes,30 the effect of timing of resolution of hypsarrhythmia is less clear.
Several factors may explain the difference between this and our preliminary work,13 based on the first 2 years of data from the NISC registry, which suggested that the response to ACTH was superior to the response to other treatments (55% positive response to ACTH compared to 39% for oral steroids, 36% for vigabatrin, and 9% for nonstandard treatment). First, in the present analysis, the primary outcome did not include hypsarrhythmia. In addition, the sample size of the current work is larger, adjusts more fully for biases that may influence treatment selection, and used a 60-day outcome rather than a 3-month outcome. It is also possible that dosing regimens evolved over the 7 years of study enrollment. For example, work from the 1990s reported prednisone and prednisolone dosing similar to that of asthma treatment (2 mg/kg/d),31,32 whereas studies from the 2000s used higher doses (4–6 mg/kg/d),20,33 and contemporary publications report higher still doses (8 mg/kg/d).34 ACTH is often dosed at 150 U/m2,31 although good results may be achievable with less.35
We found biases that influence neurologists toward or away from certain medications for infantile spasms. ACTH is more often selected for children who fit the (now retired) concept of cryptogenic infantile spasms, that is, unknown epilepsy etiology, normal head circumference, no developmental delay, normal brain MRI, and hypsarrhythmia on EEG. However, these are not necessarily rational factors to select a medication. The absence of hypsarrhythmia does not affect the likelihood of response to medication, nor do imaging findings (with the caveat that findings suggestive of TSC suggest a higher response rate if treated with vigabatrin).22 The findings that nonmedical factors (race, ethnicity, and insurance status) may influence medication selection suggest disparities in care and merit further investigation.
As has been shown previously, we found that vigabatrin works particularly well for children with TSC36 but less well than hormonal therapy (oral steroids or ACTH) for other etiologies of infantile spasms.13,20,33 Our results do not definitively resolve the comparative effectiveness of oral steroids vs ACTH (natural or synthetic), which remains uncertain. In both large UK-based randomized studies of treatment for infantile spasms, the response rates to tetracosactide (synthetic ACTH) were somewhat higher than the response to oral steroids; however, the selection of these 2 hormonal therapies was not randomized, leaving open the possibility of selection bias (i.e., that children more likely to respond were also more likely to be prescribed ACTH).20,21
Nonstandard medications are a poor choice for infantile spasms and are rarely successful (4 in 51 free from failure). Topiramate, in particular, is commonly used for infantile spasms13,37 due to early reports suggesting its efficacy38,39 and was used in about half of the nonstandard therapy group in our data. However, recent reports have increasingly found it ineffective.40-42 We recognize, however, that there are other potential explanations for the poor response to nonstandard medications; that is, selection of nonstandard medication could be a marker of unobserved predictors of poor response. However, E-values >9 indicate that these predictors would need to have large differences in prevalence and an enormous effect size.
Our analysis does not contribute to understanding the role of the ketogenic diet in infantile spasms treatment because only 3 children received it as first-line treatment. We included these 3 cases in the nonstandard group. Response rates to ketogenic diet as first-line therapy for infantile spasms are 28 to 33 percentage points lower than in comparison groups treated with ACTH.43,44
Our findings further support the American Academy of Neurology quality measure recommending ACTH, prednisolone, or vigabatrin as first-line therapy for infantile spasms.45 Quality measures are needed when, despite strong evidence, effective interventions are not delivered consistently. Our findings also support the crisis standard of care to prefer prednisolone for first-line therapy for infantile spasms when health care resources are acutely limited.46
Several limitations merit discussion. First, for observational studies, statistical techniques can effectively account for observed factors that may lead to selection bias but cannot account for unobserved factors. Although observational data can support causal inference for comparative effectiveness,18 the findings are not as robust as in an RCT. An RCT may be justified when there is equipoise (e.g., ACTH vs oral steroids); however, the low rate of response in the nonstandard therapy group suggests that an RCT that includes a nonstandard therapy arm would be unethical. Second, we have done a complete case analysis by removing cases that had clear data inconsistencies or irregularities, which can also introduce bias.47 Third, we did not have reliable dosage data. Fourth, recurrence may occur months after initial response and may not have been captured by our 60-day follow-up period. Fifth, we did not further adjust the effect estimates in Table 2 for multiple comparisons; the optimal statistical approach to observational comparative effectiveness of multiple treatments is emerging but not established.48,49
Recent trial data suggest that combination therapy (vigabatrin plus hormonal therapy) may improve short-term response rate compared to hormonal therapy alone,21 although with uncertain effect on developmental outcomes.30 Additional studies are needed to clarify the role of combination therapy. The observation that ACTH may resolve hypsarrhythmia more quickly than oral steroids also merits additional study.
These data reaffirm that infantile spasms should be treated with 1 of the 3 recommended therapies, with a preference for hormonal therapies unless the infant has TSC. Use of nonstandard therapies as first-line treatment for infantile spasms should be strongly discouraged.
Glossary
- ACTH
adrenocorticotropic hormone
- ASM
antiseizure medication
- CI
confidence interval
- NISC
National Infantile Spasms Consortium
- RCT
randomized controlled trial
- TSC
tuberous sclerosis complex
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Podcast: NPub.org/08xsdf
Study Funding
Funding provided by the Pediatric Epilepsy Research Foundation.
Disclosure
Dr. Zachary Grinspan receives research support from the Pediatric Epilepsy Research Foundation, Weill Cornell Medicine, and the Morris and Alma Schapiro Fund. He serves as a paid consultant for Alpha Insights and Bio-Pharm Solutions (South Korea). Dr. Chellamani Harini reports no disclosures. Dr. Catherine Chu has received research support from NIH and Biogen Inc and/or has served as a paid consultant for Biogen Inc and SleepMed Inc. Dr. Courtney Wusthoff was supported by NIH K02NS102598. Dr. John Mytinger reports no disclosures. Dr. Renee Shellhaas is supported by NIH, Patient-Centered Outcomes Research Institute, the Pediatric Epilepsy Research Foundation, and the University of Michigan. She serves as associate editor for Neurology® and is a consultant for the Epilepsy Study Consortium. Dr. William Gaillard reports no disclosures. Dr. Kelly Knupp has received support from West Therapeutics, Zogenix, and the Pediatric Epilepsy Research Fund and has served as paid consultant for Zogenix, Epygenix, Biomarin, Biocodex, GW Pharmaceuticals, Stoke Therapeutics, and Encoded. Dr. Jason Coryell, Dr. Cynthia Keator, and Dr. Ivan Fernandez report no disclosures. Dr. Anup Patel receives research support from Pediatric Epilepsy Research Foundation. Dr. Douglos Nordli, Dr. Elissa Yozawitz, Dr. Elaine Wirrell, Dr. Ignacio Valencia, Dr. Nilika Singhal, Dr. Wendy Mitchell, Dr. Tobias Loddenkemper, Dr. Shaun Hussain, and Dr. Anne T. Berg report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Kivity S, Lerman P, Ariel R, Danziger Y, Mimouni M, Shinnar S. Long-term cognitive outcomes of a cohort of children with cryptogenic infantile spasms treated with high-dose adrenocorticotropic hormone. Epilepsia 2004;45(3):255-262. [DOI] [PubMed] [Google Scholar]
- 2.Eisermann MM, DeLaRaillere A, Dellatolas G, et al. . Infantile spasms in Down syndrome: effects of delayed anticonvulsive treatment. Epilepsy Res 2003;55(1-2):21-27. [DOI] [PubMed] [Google Scholar]
- 3.Goh S, Kwiatkowski DJ, Dorer DJ, Thiele EA. Infantile spasms and intellectual outcomes in children with tuberous sclerosis complex. Neurology. 2005;65(2):235-238. [DOI] [PubMed] [Google Scholar]
- 4.Riikonen R. Long-term outcome in children with infantile spasms treated with vigabatrin: a cohort of 180 patients. Epilepsia. 2015;56(5):807-809. [DOI] [PubMed] [Google Scholar]
- 5.Riikonen R. A long-term follow-up study of 214 children with the syndrome of infantile spasms. Neuropediatrics. 1982;13(1):14-23. [DOI] [PubMed] [Google Scholar]
- 6.Riikonen R. Long-term outcome of West syndrome: a study of adults with a history of infantile spasms. Epilepsia. 1996;37(4):367-372. [DOI] [PubMed] [Google Scholar]
- 7.Pellock JM, Hrachovy R, Shinnar S, et al. . Infantile spasms: a U.S. consensus report. Epilepsia. 2010;51(10):2175-2189. [DOI] [PubMed] [Google Scholar]
- 8.Wilmshurst JM, Gaillard WD, Vinayan KP, et al. . Summary of recommendations for the management of infantile seizures: task force report for the ILAE Commission of Pediatrics. Epilepsia. 2015;56(8):1185-1197. [DOI] [PubMed] [Google Scholar]
- 9.Mehta V, Ferrie CD, Cross JH, Vadlamani G. Corticosteroids including ACTH for childhood epilepsy other than epileptic spasms. Cochrane Database Syst Rev. 2015;2015(6):CD005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watzka M, Bidlingmaier F, Beyenburg S, et al. . Corticosteroid receptor mRNA expression in the brains of patients with epilepsy. Steroids. 2000;65(12):895-901. [DOI] [PubMed] [Google Scholar]
- 11.Brunson KL, Avishai-Eliner S, Baram TZ. ACTH treatment of infantile spasms: mechanisms of its effects in modulation of neuronal excitability. Int Rev Neurobiol. 2002;49:185-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Menachem E. Mechanism of action of vigabatrin: correcting misperceptions. Acta Neurol Scand Suppl. 2011(192):5-15. [DOI] [PubMed] [Google Scholar]
- 13.Knupp KG, Coryell J, Nickels KC, et al. . Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016;79(3):475-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain SA, Kwong G, Millichap JJ, et al. . Hypsarrhythmia assessment exhibits poor interrater reliability: a threat to clinical trial validity. Epilepsia. 2015;56(1):77-81. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Zhong X, Hong S, Li T, Jiang L. Prednisolone/prednisone as adrenocorticotropic hormone alternative for infantile spasms: a meta-analysis of randomized controlled trials. Dev Med Child Neurol. 2020;62(5):575-580. [DOI] [PubMed] [Google Scholar]
- 16.Chang YH, Chen C, Chen SH, Shen YC, Kuo YT. Effectiveness of corticosteroids versus adrenocorticotropic hormone for infantile spasms: a systematic review and meta-analysis. Ann Clin Transl Neurol. 2019;6(11):2270-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glauser TA, Cnaan A, Shinnar S, et al. . Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362(9):790-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grinspan ZM, Shellhaas RA, Coryell J, et al. . Comparative effectiveness of levetiracetam vs phenobarbital for infantile epilepsy. JAMA Pediatr. 2018;172(4):352-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry MS. Meaningful results in a jiffy: a PERC of multicenter collaborations. Epilepsy Curr. 2016;16(5):299-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lux AL, Edwards SW, Hancock E, et al. . The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364(9447):1773-1778. [DOI] [PubMed] [Google Scholar]
- 21.O'Callaghan FJ, Edwards SW, Alber FD, et al. . Safety and effectiveness of hormonal treatment versus hormonal treatment with vigabatrin for infantile spasms (ICISS): a randomised, multicentre, open-label trial. Lancet Neurol. 2017;16(1):33-42. [DOI] [PubMed] [Google Scholar]
- 22.Demarest ST, Shellhaas RA, Gaillard WD, et al. . The impact of hypsarrhythmia on infantile spasms treatment response: observational cohort study from the National Infantile Spasms Consortium. Epilepsia. 2017;58(12):2098-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mytinger JR, Hussain SA, Islam MP, et al. . Improving the inter-rater agreement of hypsarrhythmia using a simplified EEG grading scale for children with infantile spasms. Epilepsy Res. 2015;116:93-98. [DOI] [PubMed] [Google Scholar]
- 24.Agresti A. An Introduction to Categorical Data Analysis, 2nd ed. Wiley-Interscience; 2007. [Google Scholar]
- 25.Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS One. 2011;6(3):e18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. [DOI] [PubMed] [Google Scholar]
- 30.O'Callaghan FJK, Edwards SW, Alber FD, et al. . Vigabatrin with hormonal treatment versus hormonal treatment alone (ICISS) for infantile spasms: 18-month outcomes of an open-label, randomised controlled trial. Lancet Child Adolescent Health. 2018;2(10):715-725. [DOI] [PubMed] [Google Scholar]
- 31.Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97(3):375-379. [PMC free article] [PubMed] [Google Scholar]
- 32.Hrachovy RA, Frost JD Jr, Kellaway P, Zion TE. Double-blind study of ACTH vs prednisone therapy in infantile spasms. J Pediatr. 1983;103(4):641-645. [DOI] [PubMed] [Google Scholar]
- 33.Lux AL, Edwards SW, Hancock E, et al. . The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4(11):712-717. [DOI] [PubMed] [Google Scholar]
- 34.Eliyan Y, Heesch J, Alayari A, Rajaraman RR, Sankar R, Hussain SA. Very-high-dose prednisolone before ACTH for treatment of infantile spasms: evaluation of a standardized protocol. Pediatr Neurol. 2019;99:16-22. [DOI] [PubMed] [Google Scholar]
- 35.Hrachovy RA, Frost JD Jr, Glaze DG. High-dose, long-duration versus low-dose, short-duration corticotropin therapy for infantile spasms. J Pediatr. 1994;124(5 pt 1):803-806. [DOI] [PubMed] [Google Scholar]
- 36.Aicardi J, Mumford JP, Dumas C, Wood S. Vigabatrin as initial therapy for infantile spasms: a European retrospective survey: Sabril IS Investigator and Peer Review Groups. Epilepsia. 1996;37(7)638-642. [DOI] [PubMed] [Google Scholar]
- 37.Mytinger JR, Joshi S; Pediatric Epilepsy Research Consortium, Section on Infantile Spasms. The current evaluation and treatment of infantile spasms among members of the Child Neurology Society. J Child Neurol. 2012;27(10):1289-1294. [DOI] [PubMed] [Google Scholar]
- 38.Glauser TA, Clark PO, Strawsburg R. A pilot study of topiramate in the treatment of infantile spasms. Epilepsia. 1998;39(12):1324-1328. [DOI] [PubMed] [Google Scholar]
- 39.Hosain SA, Merchant S, Solomon GE, Chutorian A. Topiramate for the treatment of infantile spasms. J child Neurol. 2006;21(1):17-19. [DOI] [PubMed] [Google Scholar]
- 40.Weber A, Cole JW, Mytinger JR. Infantile spasms respond poorly to topiramate. Pediatr Neurol. 2015;53(2):130-134. [DOI] [PubMed] [Google Scholar]
- 41.Rajaraman RR, Lay J, Alayari A, Anderson K, Sankar R, Hussain SA. Prevention of infantile spasms relapse: zonisamide and topiramate provide no benefit. Epilepsia. 2016;57(8):1280-1287. [DOI] [PubMed] [Google Scholar]
- 42.Mahmoud AA, Rizk TM, Mansy AA, Ali JA, Al-Tannir MA. Ineffectiveness of topiramate and levetiracetam in infantile spasms non-responsive to steroids: open labeled randomized prospective study. Neurosciences. 2013;18(2):143-146. [PubMed] [Google Scholar]
- 43.Dressler A, Benninger F, Trimmel-Schwahofer P, et al. . Efficacy and tolerability of the ketogenic diet versus high-dose adrenocorticotropic hormone for infantile spasms: a single-center parallel-cohort randomized controlled trial. Epilepsia. 2019;60(3):441-451. [DOI] [PubMed] [Google Scholar]
- 44.Kossoff EH, Hedderick EF, Turner Z, Freeman JM. A case-control evaluation of the ketogenic diet versus ACTH for new-onset infantile spasms. Epilepsia. 2008;49(9):1504-1509. [DOI] [PubMed] [Google Scholar]
- 45.Patel AD, Berg AT, Billinghurst L, et al. . Quality improvement in neurology: child neurology quality measure set: executive summary. Neurology. 2018;90(2):67-73. [DOI] [PubMed] [Google Scholar]
- 46.Grinspan ZM, Mytinger JR, Baumer FM, et al. . Crisis standard of care: management of infantile spasms during COVID-19. Ann Neurol. 2020;88(2):215-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pedersen AB, Mikkelsen EM, Cronin-Fenton D, et al. . Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida K, Hernandez-Diaz S, Solomon DH, et al. . Matching weights to simultaneously compare three treatment groups: comparison to three-way matching. Epidemiology. 2017;28(3):387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu L, Gu C, Lopez M, Ji J, Wisnivesky J. Estimation of causal effects of multiple treatments in observational studies with a binary outcome. Stat Methods Med Res. 2020;29:3218-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.