Abstract
Baroreceptors are mechanosensitive elements of the peripheral nervous system that maintain cardiovascular homeostasis by coordinating the responses to external and internal environmental stressors. While it is well-known that carotid and cardiopulmonary baroreceptors modulate sympathetic vasomotor and parasympathetic cardiac neural autonomic drive, to avoid excessive fluctuations in vascular tone and maintain intravascular volume, there is increasing recognition that baroreceptors also modulate a wide range of non-cardiovascular physiological responses via projections from the nucleus of the solitary tract to regions of the central nervous system, including the spinal cord. These projections regulate pain perception, sleep, consciousness, and cognition. In this review, we summarize the physiology of baroreceptor pathways and responses to baroreceptor activation with an emphasis on the mechanisms influencing cardiovascular function, pain perception, consciousness, and cognition. Understanding baroreceptor mediated effects on cardiac and extra-cardiac autonomic activities will further our understanding of the pathophysiology of multiple common clinical conditions, such as chronic pain, disorders of consciousness (e.g., abnormalities in sleep-wake), and cognitive impairment, which may result in the identification and implementation of novel treatment modalities.
Keywords: baroreceptors, baroreflex, pain, vagal afferents, sympathetic, parasympathetic, inflammation, pharmacology, cognition, sleep, arousal, consciousness, blood pressure, heart rate, nucleus tractus solitarious
Introduction
In the mid-19th century, it was understood that the natural or intrinsic oscillatory pattern of arterial pressure (AP) modulates sympathetic vascular tone (38, 185, 295). The anatomical substrates responsible for the associated cardiovascular oscillations in sympathetic tone were largely unknown until von Cyon and Ludwig reported in 1866 that stimulation of the proximal end of the cut depressor nerve, a nerve that innervates the aortic arch in rabbits, caused a vascular dilatation and a decrease in AP (564). In 1867, Stelling reported that transecting the spinal cord at the cervical level abolishes this response, indicating that this effect requires communication with supraspinal structures (509). Hering (218) in 1927 and Koch and Mies (271) in 1929 showed that the stimulation of a branch of the glossopharyngeal nerve (i.e., Hering’s nerve), which innervates high-pressure baroreceptors in the carotid sinuses, produces profound hypotension and bradycardia. Throughout the 1930s and 1940s, several studies showed that stimulation of carotid sinus baroreceptors evokes multiple non-cardiovascular effects, including, but not limited to, effects on arousal, consciousness, pain, and memory. In 1932, Koch demonstrated that mechanical stimulation of the carotid sinus with a surgically implanted balloon induces somnolence in dogs (270). This observation was extended to humans by Schlager and Meier (1947), who reported that carotid stimulation by neck massage elicits sleep in humans via evoking an inhibitory effect on CNS arousal (478). During the late 1970s and early 1980s, the observation that antihypertensive drugs induced analgesia led Dworkin et al. (154), Zamir et al. (604, 606), and Maixner et al. (323, 326) to independently demonstrate that there is a functional relationship between AP and central venous pressure on nociceptive behaviors and pain perception in rodents and humans that is mediated by the activation of the carotid sinus and cardiopulmonary baroreceptors. These early studies paved the way for more recent studies that have deepened our understanding of the anatomical and physiological basis underlying baroreceptor-mediated effects on cardiovascular and non-cardiovascular associated pathologies.
Baroreceptor Modulation of Cardiovascular System
Baroreceptors Afferents
Baroreceptor-mediated reflexes occur in response to stimuli that activate specialized stretch receptors (i.e., baroreceptors) following mechanical or chemical stimulation. High-pressure arterial baroreceptors are found in large arteries (e.g., carotid and aorta), and respond to resting levels and cardiac cycle-related changes in AP, whereas low-pressure (low-volume) cardiopulmonary baroreceptors reside in the heart and lungs where they detect changes in blood volume, ventricular and atrial stretch, and the dynamics of lung inflation and deflation. Nerve impulses generated by activation of high- and low-pressure baroreceptors convey information to the nucleus of the solitary tract (NTS) via autonomic afferents that travel in cranial nerves X and IX. Hypotension and hypovolemia result in the unloading of peripheral high-pressure baroreceptors and low-volume receptors, respectively. (439) Of note, some cardiopulmonary vagal afferents are chemoreceptors that can elicit a cardiovascular response that interacts with baroreflexes.
Carotid sinus and aortic arch autonomic afferents:
Slowly adapting myelinated Aδ and unmyelinated C-fibers, which branch and form loops within the inner adventitial layer of the arterial wall, serve as the peripheral transduction substrates associated with high-pressure baroreceptors (284). These carotid and aortic afferents respond to vascular wall stretch caused by transient changes in AP and invoke brainstem-mediated baroreflexes that maintain AP oscillation within a homeostatic range. The stabilization of AP within a homeostatic range is achieved by dynamically adjusting, on a beat-to-beat basis, the sympathetic and parasympathetic output to the heart, as well as the peripheral arterial and venous blood vessels. When AP rises, there is an increase in vagal output to the heart that lengthens the interbeat interval, as well as inhibition in sympathetic tone; these changes result in (a) reduced vascular α-adrenoceptor stimulation, which leads to vasodilatation and a drop in peripheral vascular resistance, and (b) reduced stimulation of myocardial β1-adrenoceptors resulting in depressed myocardial contraction and a reduction in cardiac stroke volume (35, 446). Opposite cardiovascular effects occur in response to a fall in AP (35, 446). There are two types of carotid baroreceptors, a) low-threshold type I baroreceptors mostly innervated by myelinated A-fibers that undergo acute resetting, and b) high-threshold type II baroreceptors innervated by both unmyelinated C-fiber and myelinated A-fibers axons that have a higher threshold and do not reset (480, 481). Based on their functional features, type I carotid baroreceptors might contribute to the stabilization of AP, whereas type II carotid baroreceptors encode absolute AP levels.
Two types of aortic baroreceptors have been identified in rats, the classical ‘quiescent’ baroreceptors that are silent below the AP threshold and the ‘autoactive’ baroreceptors that discharge continuously, even below the AP threshold. Subthreshold discharge is an intrinsic property of aortic ‘autoactive’ baroreceptors that is not affected by either resetting or changes in aortic vascular tone. Aortic ‘autoactive’ baroreceptors may extend the range of the baroreflex, but probably do not improve its sensitivity to transient fluctuations in AP or its ability to correct changes in mean pressure over extended periods (371). Two functionally different afferents, type A and C afferents, innervate aortic baroreceptors in rabbits and rats; Activation of C-afferents evokes a stronger and longer sympathetic inhibition and refractory period than A-afferents (390, 391). The operational features of these baroreceptors have been widely studied and are described in more detail in the sections below.
Cardiac vagal afferents:
Vagal Aδ- and C-type afferents innervate low-pressure baroreceptor stretch receptors located in large veins, atrium, as well as in the ventricles (22, 542). They respond to changes in blood volume and corresponding changes in central venous blood pressure and cardiac chamber pressures (4, 333). There are vagal Aδ afferent terminals at the junction of the vena cava and right atrium, and at the junction of the pulmonary vein and left atrium (22). Vagal Aδ afferents innervate two types of atrial receptors, type A receptors that are activated during the systolic upstroke of the cardiac cycle, and type B receptors that respond to atrial filling (402). Activation of type B afferents, by atrial pulsation or by an increase in blood volume, inhibits NTS neurons in cats (20). Like arterial high-pressure baroreceptors, cardiac low-pressure baroreceptors participate in a rapid negative feedback loop that regulates AP. Augmented central venous pressure and cardiac filling within the physiological range (e.g., by passive elevation of the legs in supine position) stimulate cardiac low-pressure baroreceptor activity that inhibits sympathetic tone and causes reflex vasodilation in skeletal muscles (451). Moreover, decreases in central venous pressure with lower body negative pressure or increases in venous pressure produced by leg elevation stimulate low-pressure baroreceptors without affecting arterial baroreceptor’s modulation of sinusal heart rate and AP in humans (527).
Under unique conditions, cardiac low-pressure baroreceptors located in the atria exert an excitatory rather than an inhibitory modulation of heart rate and AP, which functionally opposes the influence of arterial high-pressure baroreceptors. Specifically, non-physiological elevations in central venous pressure caused by either the fast intravenous injection of small volumes or the slow intravenous infusion of large volumes of saline or blood increase heart rate in dogs (22, 557), and to a lesser degree in humans (117). This phenomenon is known as the Bainbridge reflex. The elevation of central venous pressure increases venous atrial pressure, which initiates a chain of events that includes a) a rise in ventricular end-diastolic pressure, b) ventricular dilation, c) activation of cardiac low-pressure baroreceptors, d) reflex inhibition of vagal outflow and enhancement of sympathetic outflow to sinoatrial node resulting in sinus tachycardia (22). Conversely, reduction in central venous pressure (e.g., due to bleeding, dehydration) decreases atrial low-pressure baroreceptor firing, increases sympathetic outflow and vascular tone, leading to an increase in venous return, heart rate, cardiac output, and AP (408). Vagal deafferentation (22) and pharmacological autonomic blockade of the heart (557) abolish the Bainbridge reflex in dogs.
Vagal non-myelinated C-type afferents primarily innervate the endocardium of the left ventricle, especially, the inferoposterior wall (542). These afferents mediate the Bezold-Jarisch reflex (245, 246), described by Bezold and Hirt in 1876 (563). This cardiovascular reflex is represented by a triad of responses that includes bradycardia, hypotension, and apnea and occurs after intravenous infusions (563) of small intracoronary doses of veratrum alkaloids (245, 246), prostaglandin E2 (198), prostacyclin (405), and 5-HT3 receptor agonists (265), as well as after mechanical distension of the left heart ventricle (331, 542), or in response to myocardial ischemia or infarction (331). This reflex produces the inhibition of sympathetic outflow that results in a decrease in heart rate and peripheral vascular resistance (245, 246), and mediates hypotension-induced increases in plasma renin activity (198).
In addition to fast-acting neural reflexes, low-pressure baroreceptors produce slow dynamic adjustments in blood volume and AP via secretion of vasopressin, renin, angiotensin, and atrial natriuretic peptide. The pituitary secretion of vasopressin occurs in response to decreased plasma volume and hyperosmolarity (18). Vasopressin increases arteriole tone (increasing systemic vascular resistance) and augments renal water reabsorption (reducing diuresis), which acts in concert to reduce plasma osmolarity and increases both blood volume and AP (18, 439). Also, hypotension and hypovolemia elicit secretion of renin from the kidneys; renin, in turn, induces synthesis of angiotensin II, which further stimulates vasopressin’s release and vasopressor effects (18, 439). As a counterbalance, the atrial natriuretic peptide is released in response to volume expansion from atrial myocytes to promote natriuresis and diuresis (17). The subsequent activation of high-pressure baroreceptors evoked by an acute increase in AP selectively inhibits the spontaneous activity of vasopressin-containing magnocellular neurons located in the supraoptic nucleus and the paraventricular nucleus of the hypothalamus (202, 248).
Pulmonary vagal afferents:
The NTS receives input from vagal Aδ and C afferents originating in the lung and viscera that can influence baroreflex gain. Indeed, pulmonary vagal afferents exert a tonic inhibition on the vasomotor center (543). Four types of Aδ fibers have been functionally identified, which have been reviewed by Lee and Yu (297). These are (a) slowly-adapting stretch receptors, both low-threshold afferents that tonically discharge throughout the respiratory cycle and high-threshold afferents that display phasic firing during lung inflation; (b) rapidly-adapting receptors activated by stretch of respiratory mucosa, changes in lung compliance, and rate of lung inflation, (c) deflation receptors activated during expiration, and (d) high-threshold Aδ afferents, which are chemoreceptors that are stimulated by hypertonic saline, hydrogen peroxide, bradykinin, tumor necrosis factor alpha (TNFα), and interleukin 1 beta (IL-1β).
Non-myelinated C-fiber afferents innervate chemoreceptors that respond to inhaled irritants and inflammatory mediators (such as nicotine, ammonia, hydrogen ions, adenosine, reactive oxygen species, capsaicin, and phenyldiguanide), as well as changes in osmolarity and temperature. These chemical mediators can also act indirectly by changing the mechanical properties of airways and lung parenchyma (297). An increase in lung interstitial pressure or volume produced by a rise in pulmonary intra-capillary pressure stimulates vagal C-fiber endings (403). Several lines of evidence show that the activation of pulmonary vagal C-fibers during exercise and airway inflammatory diseases is associated with the sensation of dyspnea and cough (340). The injection of capsaicin into the pulmonary artery stimulates lung vagal C-fibers, which triggers a pulmonary chemoreflex that includes the triad of cardiovascular and respiratory responses similar to the Bezold-Jarisch reflex, i.e., initial apnea followed by rapid shallow breathing, hypotension, and bradycardia, as well as bronchoconstriction (514). Of note, species differences are of relevance since stimulation of lung vagal C-fibers by injection of capsaicin in the superior vena cava does not elicit the pulmonary chemoreflex but can produce cough in man (587).
There are functionally distinct pulmonary C-fibers exemplified by the selective activation of vagal C-fibers arising from the nodose ganglia that evokes only tachypnea; whereas, those arising from the jugular ganglia induce respiratory slowing and apnea in guinea pigs (94). Moreover, the breathing pattern observed during the classic pulmonary chemoreflex is a complex phenomenon that is initiated primarily by the stimulation of pulmonary C-fibers and which is significantly influenced by the stimulation of slowly adapting Aδ-fiber stretch receptors secondary to bronchoconstriction (514). The stimulation of pulmonary vagal C-fibers, evoked by increasing lung interstitial pressure or volume during moderate exercise, also leads to respiratory reflexes and inhibition of skeletal muscle reflexes (12, 403). Thus, pulmonary C-fiber-mediated chemoreflexes result in a complex summation of multiple processes (514).
Cranial and other vagal afferents:
The NTS also receives afferent sensory input from cranial vagal Aδ and C afferents arising from the ear’s concha, which can be activated by mechanical (216) and electrical stimuli (216, 602). Additionally, vagal afferents from different tissues and organs can be stimulated by the release of proinflammatory cytokines from neutrophils and monocytes during inflammation, which triggers a CNS-mediated reflex release of acetylcholine from vagal efferents, which counteract inflammation by inhibiting the secretion of pro-inflammatory cytokines from macrophages (549). This process is referred to as the ‘vagal inflammatory reflex.’ (for further details, see the parasympathetic modulation of inflammation section below)
Baroreceptor Central Pathways and Networks
Baroreceptor afferent stimulation activates two groups of pathways arising from the NTS (FIGURE 1). First, there are descending pathways that project to nearby brainstem nuclei, which send information to peripheral targets via sympathetic and parasympathetic autonomic efferents and represent the efferent limb of the baroreflex arc. Many of the brainstem regions that send projections to the intermedial lateral cell column of the spinal cord also send projections to the sensory spinal dorsal horn and contribute to baroreflex-mediated modulation of nociceptive processing (433, 545). Second, ascending pathways that project to several supraspinal CNS structures coordinate and integrate autonomic, somatosensory, motor, endocrine, affective, and immune responses to both external and internal environmental stimuli (317, 318, 431). Baroreflex descending and ascending branches support both a “bottom-up” and a “top-down” integration of physiological and behavioral responses to life-relevant environmental events by coordinating autonomic outflow, sensory awareness, cognitive-emotional states, consciousness, hormonal, and immune responses. TABLE 1 summarizes the major features of baroreceptor central pathways and networks.
FIGURE 1:
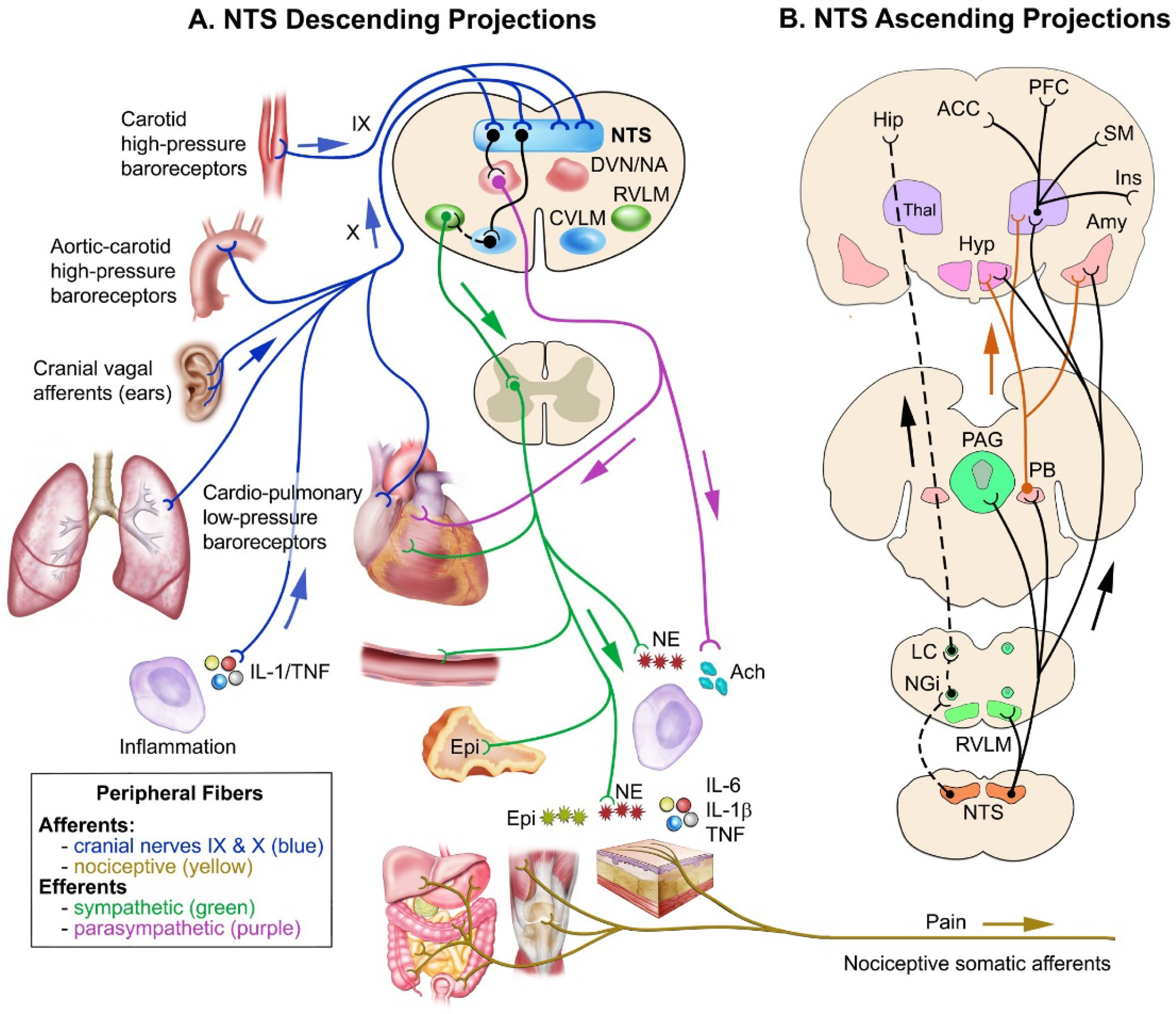
Schematic representation of key nuclei and pathways involved in the interactions between cardiovascular and pain modulatory systems. Panel A: The nucleus of the solitary tract (NTS), afferent input, and descending projections. NTS in the medulla oblongata received inputs from the glossopharyngeal (IX) nerve originating from carotid high-pressure baroreceptors and the vagus (X) nerve originating from the aortic high-pressure baroreceptors, cardiopulmonary low-pressure baroreceptors, concha, lung’s stretch-mechanoreceptors, lung’s chemoreceptors, and inflamed tissues. Afferents from the NTS excite the caudal ventrolateral medulla (CVLM), which in turn inhibits neurons in the rostral ventrolateral medulla (RVLM). Thus, there is a reduction in the RVLM tonic excitation of spinal intermediolateral neurons and a reduction in the sympathetic output to the heart and blood vessels. Afferents from the NTS also excite neurons in the dorsal vagal motor nucleus (DVN and nucleus ambiguous (NA), which enhances parasympathetic output. The resulting autonomic efferent balance diminishes heart rate and AP by a mechanism commonly referred to as the baroreflex. This change in autonomic balance can modulate inflammation, às a result, inflammatory pain. The baroreflex response is associated with increases in the release of acetylcholine (Ach) from vagal efferents, which reduces inflammation by inhibiting the secretion of pro-inflammatory cytokines from macrophages. Also, there is the sympathetic release of norepinephrine (NE) and adrenal epinephrine, which have both pro-inflammatory and anti-inflammatory effects depending on the stage of the inflammation. Panel B: NTS ascending afferent projections to rostral CNS sites. NTS afferents project directly to the parabrachial nucleus (PB), periaqueductal gray (PAG), amygdala (Amy), hypothalamus (Hyp), and thalamus (Thal), involved in autonomic and emotional responses to pain. PB provides a parallel ascending pathway to NTS afferents (continuous orange line). Also, the NTS indirectly projects to the: (1) insula (Ins), somatosensory cortex (SM), prefrontal cortex (PFC), anterior cingulate cortex (ACC) via the thalamus, (2) CA1 region of the dorsal hippocampus (Hip) and amygdala, through the nucleus paragigantocellularis (NGi) and locus coeruleus (LC) (broken black line). The ascending activation of these higher CNS structures by NTS afferents initiates a pattern of autonomic, sensory, and behavioral responses. Conversely, several CNS areas (e.g., Hyp and PAG) exert a descending modulation on NTS and RVLM mediated autonomic activity (not depicted). Similarly, respiratory centers, as well as from peripheral visceral and somatosensory afferents, exert an overall inhibitory influence on the baroreflex nuclei at the brainstem (not depicted).
TABLE 1:
Summary of baroreceptor central pathways and networks.
| • Baroreceptor afferent inputs activate the NTS, which project to key brainstem autonomic nuclei to adjust heart rate, peripheral resistance, and cardiac output. • NTS projects to the caudal ventrolateral medulla (CVLM), which converts baroreceptor input to a GABAergic inhibitory output to the rostral ventrolateral medulla (RVLM). The inhibition of the RVLM reduces the excitatory drive to the spinal intermediolateral cell column and sympathetic vasomotor outflow, and as a result, produces a reduction in cardiosympathetic tone and vascular resistance (peripheral vasodilation). • NTS also sends direct excitatory projections to the dorsal vagal motor nucleus and nucleus ambiguus, which enhances parasympathetic output and reduces heart rate. • Vagal efferent pathways arising from the dorsal vagal motor nucleus could mainly modulate heart rate, whereas those arising from the nucleus ambiguus could mediate the influence of respiration on heart rate. • Baroreflex brainstem centers receive modulatory inputs, generally inhibitory, from central respiratory structures, as well as peripheral renal and muscle afferents. • The baroreflex has three closed-loop components: the cardiac branch that modulates inter-beat-interval (chronotropic), the vascular branch that regulates sympathetic vasomotor tone, and the myocardial branch that influences stroke volume (inotropic). • NTS also conveys baroreceptor input to brain regions that influence non-cardiovascular functions, notably nociception, consciousness, and cognition, by ascending projections to the parabrachial nucleus, periaqueductal gray, hypothalamus, thalamus, prefrontal cortex, amygdala, and bed nucleus. • The parabrachial nucleus is a parallel system that additionally projects to the insular and entorhinal cortices either directly or indirectly through the thalamus. • Activation of the medial prefrontal cortex improves baroreflex sensitivity, selectively increases the parasympathetic (but not the sympathetic) component of the cardiac baroreflex, but it lacks tonic influence on brainstem vasomotor neurons. • Activation of the insula enhances the parasympathetic component of the cardiac baroreflex but shows a lack of tonic cardiovascular control. • The ‘defense areas’ produce an overall inhibitory (i.e., clamping) influence on baroreflexes. • Psychological stress resets baroreflex and sympathetic activity to a higher operating range by activating regions of the hypothalamus and PAG. • Baroreceptor influence on brain structures is asymmetric, with a stronger effect on the right hemisphere. |
Baroreceptor descending pathways:
Baroreceptor afferent input activates neuronal substrates in the NTS that project to key brainstem autonomic nuclei (parapyramidal region, rostral ventrolateral medulla, A5 cell group), which blunt AP oscillations by adjusting heart rate, peripheral resistance, and cardiac output (74). Specifically, the NTS sends excitatory glutamatergic projections to the caudal ventrolateral medulla (CVLM), which sends GABAergic inhibitory projections to the rostral ventrolateral medulla (RVLM; FIGURE 1A). This short neural circuit converts baroreceptor excitatory input that activated NTS neurons into an inhibitory output that reduces the descending excitatory tone originating in the RVLM via descending projects to sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord. Activation of this pathway produces a reduction in cardiosympathetic tone and peripheral vascular resistance. The NTS also sends direct excitatory projections to the dorsal vagal motor nucleus and to cardiovagal motor neurons in the periambigual field of the compact formation of the nucleus ambiguus, which enhances cardiac parasympathetic output and reduces heart rate. Based on phylogenetic, functional, and structural differences, Porges (416, 417) proposed two groups of vagal pathways that modulate heart rate, (a) unmyelinated efferent pathways arising from the dorsal vagal motor nucleus modulate heart rate, and (b) myelinated efferent pathways arising from the nucleus ambiguus mediate the effects of respiration on heart rate; in addition, there is evidence suggesting that the rapid vagal control of heart rate occurs only in mammals. However, Grossman and Taylor (203) have challenged this polyvagal hypothesis based on two premises, (a) the presence of cardiac aliasing – respiratory sinus arrhythmia occurring in a frequency range lower than the respiratory frequency when heart rate is at least twice that of respiration rate (457), which is seen in some newborns, and (b), the observation of fast vagally-mediated respiratory control systems of the heart that is seen in sub-mammalian species. In sum, baroreceptor input to the NTS shifts the autonomic balance towards the parasympathetic nervous system, exerting a tonic peripheral sympathetic inhibition and a cardiovagal activation, both of which increase during the systolic and decrease during the diastolic phase of the cardiac cycle under resting conditions (258, 416).
Baroreflex brainstem centers receive modulatory inputs from multiple central and peripheral sources. Brainstem respiratory neurons, which are thought to be localized in the preBötzinger complex (a respiratory rhythm generator) exert an oscillatory GABAergic inhibition of premotor parasympathetic cardioinhibitory neurons in the nucleus ambiguus during inspiration that increases heart rate during inspiration and can under certain conditions contribute to respiratory sinus arrhythmia (176). Furthermore, renal and muscle afferents exert inhibitory influences on baroreflex supraspinal centers during exercise and pathological conditions. Specifically, blockade of muscle afferents during exercise attenuates the baroreceptor-mediated resetting of AP levels to a higher level (504). Renal denervation, which interrupts the renal afferent input to the NTS, produces an improvement in arterial baroreflex function and heart rate variability and reduces resting cardiac sympathetic tone in animal models of congestive heart failure (477) and chronic kidney disease (85). Improvement in arterial baroreflex function in chronic kidney disease can be achieved by normalizing the exaggerated inhibitory GABAergic tone at the NTS (85). Thus, respiratory centers, as well as input provided by peripheral visceral and somatosensory afferents, exert an overall inhibitory influence on the baroreflex mechanisms in the brainstem.
Consistent with the baroreflex process listed above, Reyes del Paso et al. conceptualized three closed-loop control branches: (a) a cardiac branch that modulates interbeat interval; (b) a vascular branch that regulates vasomotor tone; and (c) a myocardial branch that influences stroke volume (441, 446). These branches are differentially affected by pathological conditions. For instance, in chronic hypertension, the inhibition of the baroreflex cardiac branch (i.e., heart rate) by transmural stimulation of the carotid baroreceptors is reduced, but this phenomenon is not observed in the vascular branch (177). Furthermore, differences in baroreflex responses can occur within the same functional branch depending on the location of stimulated specific baroreceptor afferents. For example, the stimulation of low-pressure cardiopulmonary baroreceptors has a predominant influence on forearm vessels, but only minor effects on splanchnic vessels, whereas stimulation of high-pressure carotid baroreceptors exhibits opposite modulatory effects (3).
Baroreceptor ascending pathways:
Afferent pathways originating in the NTS relay baroreceptor afferent signals to the midbrain, diencephalon, limbic forebrain, and cortical areas involved in integrating autonomic, sensory, interoceptive, and motor input-output responses to environmental demands to maintain homeostatic balance (FIGURE 1B) (74, 260). Complex higher level CNS processing is made possible because the NTS sends ascending projections to the parabrachial nucleus (PBN), periaqueductal gray (PAG), hypothalamus (paraventricular nucleus, dorsomedial and lateral areas), thalamus (paraventricular nucleus), central nucleus of the amygdala, medial prefrontal cortex (mPFC), and bed nucleus of the stria terminalis. The PBN also sends projects to these loci, as well as to the insular and entorhinal cortices either directly or indirectly through the thalamus (74, 260). Thus, the NTS conveys baroreceptor input to CNS regions that influence non-cardiovascular functions, notably nociception, consciousness, and cognition (FIGURE 1B).
Role of the prefrontal cortex in baroreflexes.
Several studies have demonstrated that discrete areas in the mPFC and insula send reciprocal afferent projections back to the NTS and other brainstem autonomic nuclei (537). The infralimbic area of the mPFC sends projections to the NTS, the RVLM (74), and the parasympathetic nuclei – dorsal motor nucleus and nucleus ambiguus (537). Excitotoxic lesions in the ventral mPFC (prelimbic and infralimbic areas) reduce baroreflex gain (sensitivity), and slightly lower the AP threshold for baroreceptor activation (resetting) without affecting resting mean arterial pressure (MAP) and heart rate (562). Reversible inhibition of neurons within the mPFC resets the threshold for the bradycardic response (parasympathetically mediated) to phenylephrine-evoked increase in AP to higher MAP values without affecting the tachycardiac response (sympathetically-mediated) evoked by nitroprusside administration (440). Electric or chemical stimulation of the mPFC lowers AP and enhances baroreflex bradycardia by activation of neural networks within NTS (400, 486). Excitation of infralimbic mPFC dampens the increase in heart rate and AP induced by environmental stress in rats (367). Overall, these findings suggest that the mPFC (a) improves BRS, (b) selectively augments the parasympathetic component of the cardiac baroreflex without significantly involving the sympathetic component, and (c) lacks tonic influence on brainstem vasomotor neurons. In addition, there is evidence that the activation of the mPFC (561) or the lateral PFC (518), with either electrical stimulation or glutamate microinjections, induces hypotensive responses and inhibits RVLM pre-sympathetic barosensitive neurons via a GABA receptor-mediated process (518).
Role of the insula in baroreflexes.
The insula is functionally more complex than the mPFC. Ischemic lesion of the insula and adjacent lateral frontoparietal cortices enhances reflex vagal bradycardia in response to a phenylephrine-induced increase in AP without altering resting MAP, heart rate, and sympathetic responses (461). In contrast, a reversible and selective blockade of the insula with lidocaine attenuates baroreflex gain in rats without altering resting AP, heart rate, or plasma norepinephrine levels (467). Focal stimulation reveals functional heterogeneity within the structure; for example, activation of the caudal and rostral areas of the posterior insula produces increases and decreases in heart rate and AP, respectively (398). Thus, like the mPFC, the insular cortex has a facilitatory influence on the parasympathetic component of the cardiac baroreflex and shows a lack of tonic cardiovascular control, as insula inhibition does not modify ongoing heart rate or AP. However, the insula is involved in more complex lateralized modulatory patterns, as reviewed by Oppenheimer and Cechetto (398).
Role of the autonomic cortical network in baroreflexes.
Gianaros et al. proposed a central network integrating autonomic and motor responses to stressors that involve the anterior cingulate cortex, mPFC, orbitofrontal cortex, amygdala, and insula (191). Neural networks within autonomic cortices integrate baroreceptor afferent input, as well as visceral, somatomotor, visual, cognitive, emotional, and interoceptive afferent information to generate responses that activate specific areas of the pontomedullary and hypothalamic-mesencephalic ‘defense areas’ (193). The ‘defense areas’ produce an overall inhibitory (i.e., clamping) influence on baroreflexes (108, 575). Thus, psychological stress resets baroreflex and sympathetic activity to a higher operating range by activating regions of the hypothalamus and PAG. These areas project excitatory neurons to (a) the medial NTS that inhibits the dorsolateral NTS, an area within the NTS area that receives baroreceptor input (121, 182), and (b) sympathetic premotor neurons in the RVLM (69, 575). In contrast, electrical and chemical (d,l-homocysteic acid) stimulation of the preoptic area of the hypothalamus elicits behavioral relaxation (511) and enhances baroreflex-mediated bradycardia (486). Thus, the hypothalamus generates an intricate modulatory pattern that depends on the triggering event, similar to the insula. Of note, the NTS conveys baroreceptor input through ascending projections to the supraoptic nucleus and the paraventricular nucleus of the hypothalamus to control the secretion of arginine vasopressin from the pituitary, which is relevant to the long-term regulation of vascular volume and AP (202, 248).
Functional neuroimaging studies have extended these findings to humans (266). Exercise and vasopressor activation of baroreceptors stimulate neuronal responses in the mPFC and the insular cortex in humans (80). Also, stimulation of cardiopulmonary baroreceptors with respiratory challenges (e.g., Valsalva maneuver) or lower body negative pressure alters neural activity in the insular cortex, anterior cingulate cortex, medial prefrontal cortex, amygdala, and cerebellum (266, 267).
Asymmetric baroreceptor modulation of cortical activity.
The outcomes of several studies reveal an asymmetric nature of baroreceptor influence on cortical function. In dexterous or right-hand subjects, baroreceptor activation during the systolic phase of the cardiac cycle produces a prolongation of sensorimotor task reaction time cued by a visual stimulus only if it is applied to the right eye field, suggesting that baroreceptor activation exerts a predominant influence on the left hemisphere (579). However, more recent electrophysiological and imaging studies have shown the right-hemisphere lateralization of baroreceptor modulation of cortical activity. Indeed, cardiac cycle and direct stimulation of carotid baroreceptors induce changes in event-evoked potential (569) and task-evoked cortical activation of BOLD signals (28) only or predominantly in the right hemisphere, even following bilateral baroreceptor stimulation (28). Basile et al. (28) suggested that this asymmetry in baroreceptor modulation of cortical activity reflects the prominent control of the right hemisphere on vagal function, which is consistent with the observed increased risk for developing complex arrhythmias in stroke patients with selective damage to the right insula (103).
Operational Features of the Baroreflex
TABLE 2 displays a summary of the relevant operational features of the baroreflex. Arterial baroreceptors are stimulated by changes in transmural pressure that are mainly mediated by systolic AP and intraluminal pulse pressure that stretches baroreceptor mechanoreceptors (137). Arterial baroreceptors are tonically active and exert a continuous restraining influence on heart rate and vasoconstrictor tone by modulating medullary vasomotor centers. This restraining activity increases when AP increases above resting levels, whereas it diminishes when AP is below the resting level (224). Indeed, baroreceptor modulation of sympathetic outflow correlates with cardiac cycle variations in AP rather than with static AP levels (519). The threshold for baroreceptor activation in normotensive humans is a mean carotid AP above 60 mmHg (329, 435). This threshold changes with age, 45 mmHg during the third decade and 80 mmHg during the sixth decade of life (172).
TABLE 2:
Summary of the most relevant operational features of the baroreceptor-mediated baroreflexes.
| • Changes in transmural pressure stimulate arterial baroreceptors. • Mechanosensitive ion-channels PIEZO1 and PIEZO2, and probably some types of voltage-gated calcium receptors, mediate AP-activation of arterial baroreceptors. • Baroreceptors exert a continuous restraining influence on heart rate and vasoconstrictor tone. • Arterial and cardiopulmonary baroreflexes influence short-term control AP mainly by reducing systemic vascular resistance rather than cardiac output. • Either arterial or cardiopulmonary baroreceptors could be sufficient for normal AP control, and both systems interact for a non-additive attenuation on cardiovascular centers. • Both arterial and cardiopulmonary baroreceptors inhibit sympathetically mediated vasoconstriction, causing vasodilatation; yet, only arterial baroreceptors parasympathetically influence the heart rate. • Chronic activation of arterial baroreceptors can contribute to long-term control of AP by diminishing sympathetic nerve renal activity (and circulating catecholamines), which reduces renin release, sodium reabsorption in the proximal tubule, vasopressin release, and sodium appetite; as a result, urine output increases. • Baroreceptors regulate either the occurrence or the strength of the sympathetic vasoconstrictor tone depending on the vascular bed (e.g., muscle vs. renal) and other moderating factors. • Baroreceptor activation produces a short-latency parasympathetic response on heart rate and a long-latency sympathetic response on vascular smooth muscle tone and myocardial contraction. • Baroreceptor activation exhibits laterality with respect to the side experiencing afferent stimulation. • Baroreceptors stimulation produces a hysteresis effect on vascular and heart rate responses to an increase in AP that is followed by a decrease in AP. • Baroreflex sensitivity (BRS) is the relation (slope) between variations of blood pressure and corresponding changes in cardiovascular effectors (e.g., heart rate, sympathetic vasoconstrictor, myocardial contractions) over time. • The baroreflex effectiveness index (BEI) is the ratio between the number of systolic AP ramps that evoke reflexive heart rate changes and the total number of systolic AP ramps. • Resetting of the baroreflex occurs when there is a change in the reflex operating point to adjust AP to a new level that meets environmental or internal demands (e.g., exercise) or during chronic hypertension. • Electrical field stimulation of the arterial baroreceptors can overcome their resetting and induce reductions in MAP and sympathetic outflow under chronic hypertension. • Baroreflex resetting has more influence on heart rate than on mean AP and systemic vascular resistance. • Changes in BRS generally occur in chronic baroreceptor resetting but usually not during acute resetting. • Baroreceptor resetting can be challenging to detect when vascular distensibility decreases with atherosclerosis or age. • Arterial baroreflexes are active during exercise but undergo resetting by central commands and the exercise pressor reflex. • The exercise pressor reflex begins with the activation of vagal mechanoreceptor (type III) and chemoreceptor (type IV) afferents. • Central commands initiate skeletal muscle contraction at the onset of exercise and inhibit NTS sensitivity to baroreceptor input, which results in a resetting of baroreflexes towards the prevailing pressure evoked by exercise. • The resetting of the arterial baroreflex to resting AP levels at the end of dynamic exercise occurs by the inactivation of central command and activation of cardiopulmonary reflexes. • Cardiopulmonary reflexes counteract exercise pressor reflex by inhibition of the sympathetic vasoconstrictor tone. • Cardiac baroreflexes override vascular baroreflexes to counter exercise evoked hypertensive stimuli. • Close-loop system studies have observed that exercise produces a baroreflex AP-heart rate stimulus-response curve where MAP resets to a higher AP set point with greater maximal response output (i.e., upward and rightward) without changes in the slope of the curves (i.e., constant BRS). • At the onset of exercise, there are dynamic changes in BRS that are effector-dependent in both animals and humans; thus, the gain of the baroreflex is lower for controlling heart rate (atrial sinus node), unchanged for regulating AP (vascular smooth muscle), and higher for modulating sympathetic nerve activity (post-ganglionic sympathetic nerve). • As workload exercise increases in humans, the gain of the baroreflex at the onset decreases for controlling heart rate and increases for modulating muscle sympathetic nerve activity during high intensity isometric or dynamic exercises, whereas it does not change for regulating AP at any intensity level. • The ‘exercise pressor reflex’ and central commands could mediate changes in BRS observed during the time course of exercise. • Open-loop system analysis reveals the differential dynamic properties of two subsystems responding to changes in pressure-load speeds. Baroreceptor input to the NTS activates the neural arc, which results in sympathetic output with fast changes in AP input, producing higher amplitude peripheral sympathetic nerve activity (e.g., high-pass filter). The peripheral arc is the chemical-mechanical coupling between sympathetic nerve endings and the innervated vascular smooth muscle, which determines the systemic AP. The peripheral arc exhibits low-pass filter dynamics such that faster changes in peripheral sympathetic activity have little effect on the systemic AP responses. • During orthostatic stress, the higher transfer function of the neural compensates for the lower transfer function associated with the peripheral arch. • BRS exhibits circadian variations; it is lower in the morning and higher in the evening. • Vagally mediated cardiovascular Bainbridge (tachycardia and hypertension following volume-induced atrial stimulation), and Bezold-Jarisch (bradycardia and hypotension after chemical ventricular stimulation) reflexes blunt arterial baroreflexes. • Cardiopulmonary reflexes suppress both the carotid baroreflexes and chemoreflexes. |
Vagal deafferentation or cold block increases mean AP (328, 394); this increment is much more significant when carotid sinus pressure is low compared to when it is high. Noteworthy, the rise in AP after aortic baroreceptor deafferentation is transient and does not cause chronic hypertension, suggesting that the arterial baroreflex is more relevant for short-term regulation of AP. Persson et al. (410) proposed that this lack of long-lasting hypertension could be due to either compensatory hormonal and renal mechanisms or the activity of cardiopulmonary receptors. Moreover, either arterial or cardiopulmonary baroreceptors are sufficient to maintain a normal AP, and both systems interact to produce non-additive effects on cardiovascular centers (410). However, the effectiveness of compensatory mechanisms in transient acute hypertension following baroreceptor denervation has been challenged (520). Finally, total sinoaortic and cardiopulmonary baroreceptor denervation in dogs does not produce a significant increase of MAP but does result in a greater variation in MAP values (253) as a result of reduced modulatory capacity; (for further discussion, see the section on Baroreceptor Long-term Regulation of Arterial Pressure below).
Under resting conditions, carotid baroreflex controls AP primarily by reducing systemic vascular resistance (165) rather than by decreasing heart rate and stroke volume and associated changes in cardiac output (396). Carotid baroreflex control of AP during exercise depends exclusively on modifications in systemic vascular resistance (396) with the sympathetically regulated vascular tone of arterioles and pre-capillaries, establishing the level of systemic vascular resistance (165). Changes in the vascular resistance across different vascular beds (e.g., skin, muscle, and viscera) show considerable variation and can change in opposite directions due to: a) differences in sympathetic vasoconstrictor outflow (81, 283); b) differences in the density and functional properties of α- and β-adrenoceptors in the vascular smooth muscle; c) local production of vasoactive metabolites; d) the presence of vasodilatory efferents (283). Like arterial baroreflexes, cardiopulmonary reflexes can also produce dilation of resistance vessels and a bradycardic response following low-frequency stimulation of otherwise silent vagal cardiac afferents, but additionally, they can also produce venous dilatation in dogs (394). Of note, carotid baroreceptor activity has a much more pronounced influence on vascular tone in the skeletal muscle than in the kidney, with a limited effect on heart rate, while cardiopulmonary baroreceptor activity has a more powerful modulation on both renal vessels and heart rate (394).
Baroreflex control of sympathetic vasoconstrictor response.
Baroreceptors produce a fine-tuning of vascular sympathetic discharges. Intraneural multi-unit recordings of skeletal muscle impulses in the median and the peroneal nerves at the level of the elbow and the fibula head are used to estimate baroreflex regulation of vasoconstrictor sympathetic efferents in man (124, 125). The sympathetic nature of these nerve recordings is supported by the correlation with maneuvers that elicit changes in vascular muscle resistance, such as changes in body position, Valsalva’s maneuver, muscle exercise (125), as well as with sympathetically-dependent noradrenaline release from the heart (570). FIGURE 2 shows that the activity pattern of muscle sympathetic efferents consists of bursts of discharges that follow the oscillation of AP waveform across the cardiac cycle that occurs predominantly during diastole rather than systole (124, 519). Kienbaum et al. (264) proposed that the baroreflex control of vasoconstriction is an on-off regulatory system that controls sympathetic outflow.
FIGURE 2.
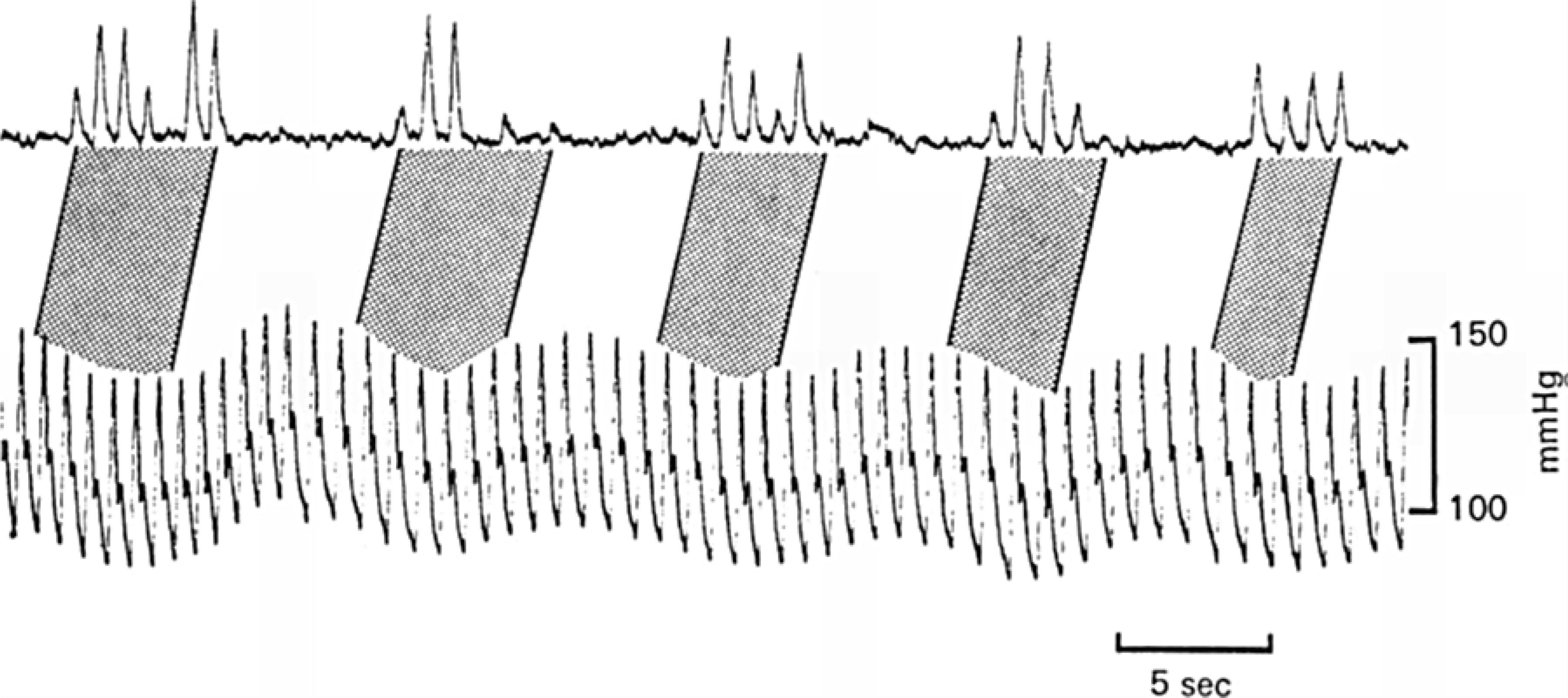
The relationship between variations in AP pressure and muscle sympathetic activity from recordings made from the right peroneal nerve. Record showing more bursts occurring during decreasing than during increasing AP. Dotted areas indicate corresponding sequences of bursts and heartbeats. There is compensation made for a reflex delay of 1.3 sec between blood pressure and neural events. Reproduced with permission (519).
Baroreceptor processes also regulate the strength (amplitude) of sympathetic effector responses in the kidney. Acute cutaneous heat pain and volume-induced stimulation of low-pressure cardiopulmonary baroreceptors decrease renal sympathetic burst amplitude without altering burst frequency in the rat (129). Similarly, stimulation of high-pressure arterial baroreceptors mainly diminishes the burst amplitude of renal sympathetic discharges in conscious Wistar-Kyoto and spontaneously hypertensive rat strains (130). In patients with congestive heart failure, the amplitude of sympathetic bursts in multiunit mean voltage recordings is related to the firing frequency of individual vasoconstrictor sympathetic fibers (523).
It is not clear how this differential modulation of specific components of the sympathetic outflow occurs, but central inputs contribute to and even override peripheral baroreceptor afferent influences (264). For example, mental stress increases the amplitude of muscle sympathetic bursts, but it does not change the occurrence of the discharges (221). In contrast, the frequency of muscle sympathetic burst increases with age in healthy subjects without modifying sympathetic burst amplitudes (523). Kienbaum et al. (264) proposed that baroreceptor modulation of sympathetic outflow occurs at two CNS locations in a manner that is dependent on the strength of the respective input (FIGURE 3). In one region, arterial baroreceptor input acts in concert with other CNS influences to mediate graded effects on the amplitude of the sympathetic impulses (264). In another region, impulses from the arterial baroreceptors exert a “gate control” that regulates sympathetic discharge (264). This dual regulatory system is involved in distinct populations of arterial baroreceptor afferents, which differ in modulatory sensitivity, AP operational ranges (371), and effects on the strength and duration of sympathetic inhibition and refractory period (390, 391). The clinical relevance of these physiological features remains to be established.
FIGURE 3.
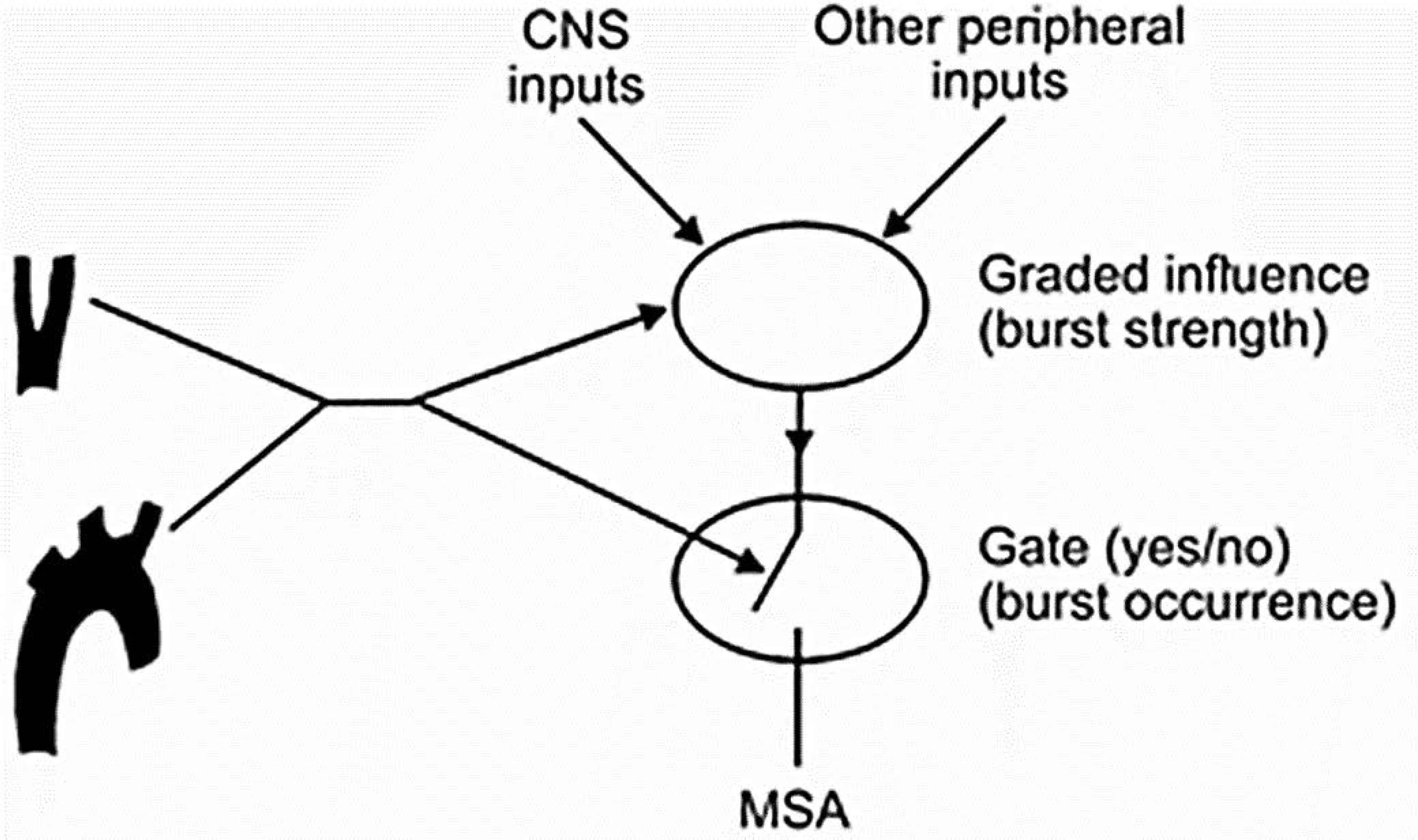
Hypothetical model for arterial baroreceptor influence on muscle sympathetic activity at two CNS synapses proposed by Kienbaum et al. Baroreceptors modulate the strength and occurrence of sympathetic outflow at two CNS locations depending on the strength of the respective input. Arterial baroreceptor input and other CNS influences have graded effects on the amplitude of the sympathetic impulses on one site, whereas they exert a gate control on the occurrence of a sympathetic discharge on the other site. Reproduced with permission (264).
Baroreflex response latency.
There are differences in the response latencies between the autonomic branches associated with the arterial baroreflex. The cardiac component of the baroreflex is largely parasympathetically-mediated and produces a latency response of less than a second (50). In contrast, the vasomotor and myocardial component of the baroreflex is sympathetically mediated, resulting in a slower resulting from a longer latency of onset, duration, and offset (50, 455). In humans, the baroreflex delay from an AP pulse wave to the corresponding inhibition of sympathetic activity in the skeletal muscle ranges from 0.9–1.4 seconds, depending on the recording site (124). This is accompanied by a corresponding lag of 1–2 heartbeats between a change in systolic AP and the subsequent effect on sympathetic discharge (264, 330, 519). Reyes del Paso et al. (441) attribute these dynamic differences to the relatively slow release and diffusion of norepinephrine from the nerve ending effectors and the slower conduction velocities of sympathetic fibers compared to the rapid release and action of acetylcholine released from postganglionic vagal fibers.
Laterality and asymmetry of the baroreceptor function.
There is a functional asymmetry in the baroreceptor modulation of the heart. Mechanical stimulation of the right carotid sinus slows heart rate more than stimulation of the left, whereas the stimulation of the left carotid sinus reduces cardiac contractility more than the stimulation of the right vagus (526). These differences result from the asymmetric cardiac innervation and ipsilateral projections of carotid sinus afferents to the NTS (526). Also, there is hemispheric laterality with respect to the side experiencing afferent stimulation (as discussed in the previous section Asymmetric Baroreceptor Modulation of Cortical Activity above). Within the CNS, the baroreceptor delaying effect on a visuospatial attention task is associated with positive modulation of the right brain (28). (Also, see the section Influence of Cognitive Demands on Baroreceptor Function, below).
Baroreceptor sensitivity (BRS).
BRS is a measure of the gain of the baroreflex and is determined by examining the relationship between an afferent stimulus input (e.g., resting AP oscillations, acute phasic AP changes) and an efferent output (e.g., heart rate, vasoconstrictor sympathetic discharges) over time. This index is commonly measured by deriving a slope from a baroreflex-generated stimulus-response (SR) curve, where the slope of the curve is an index for gain. BRS is an index of reflex efficacy that can be estimated for each functional branch of the autonomic nervous system (i.e., the cardiac, vascular, and myocardial branches of the autonomic nervous system). The cardiac BRS can be measured from the slope derived from the inter-beat interval (R-R interval) versus the level of AP, whereas vascular BRS can be measured from the slope derived between the change in AP versus the resulting change in peripheral vascular resistance (441, 446). High cardiac BRS results in a broader variation of heart rate and a narrower oscillation of AP, and vice versa for low cardiac BRS (106). The cardiac BRS can be further analyzed by evaluating the mechanical and neural transduction mechanisms of the baroreflex (231, 533). Specifically, the mechanical transduction component, i.e., vascular compliance to blood pressure changes, is the relationship between ultrasonographic systolic carotid diameter and AP changes. The neural transduction, i.e., the conversion of vessel stretching into baroreceptor afferent discharges, central integration, and efferent autonomic activity on the heart, is the relationship between systolic carotid diameter and interbeat interval (R-R interval). Within the normotensive range of resting AP, there is an inverse relationship between BRS and resting AP (79, 148). Acute changes in AP do not generally change BRS (373); however, BRS decreases in chronic hypertension (79) and increases in persistent hypotension (144). BRS values display an inverted U-shaped distribution, being maximal in the mid-portion of the stimulus-response curve (around resting AP) and decrease with APs lower or higher than the resting AP (224).
Cardiac BRS exhibits a functional hysteresis. Under resting conditions, BRS is greater during spontaneous ascending systolic AP ramps associate with a bradycardic response than during descending AP ramps associated with a tachycardiac response (44, 446). BRS assessed during rising AP correlates more closely with heart rate than with BRS assessed during decreasing (446). In cats, the muscarinic receptor antagonist atropine blocks both the decrease in heart rate and the high-frequency heart rate variability induced by an increase in AP, whereas the β-adrenergic antagonist practolol blocks the increase in heart rate evoked by a decrease in AP (341). Under resting conditions, the parasympathetic mediates the bradycardic response to rising AP without changing the peripheral vascular resistance, whereas sympathetic activation and vagal withdrawal provoke the tachycardiac response to falling AP (446, 475). Vascular BRS also displays a functional hysteresis, yet unlike cardiac BRS, vascular BRS is mainly sympathetically mediated. In humans, vascular BRS also displays a functional hysteresis such that spontaneous decreases in AP produce a greater increase in systemic vascular resistance than the decrease in systemic vascular resistance produced by an increase in spontaneous AP, suggesting a more efficient baroreflex control of the systemic vascular resistance during decreases in AP compared to increases in AP (519). This differential efficiency permits the maintenance of perfusion to vital organs, e.g., brain and viscera.
Baroreflex effectiveness index.
Progressive beat-to-beat increases or decreases in AP do not always trigger homeostatic baroreflex responses. Di Rienzo et al. (127) proposed a baroreflex effectiveness index (BEI), which is the ratio between the number of systolic AP ramps followed by reflex changes in heart rate and the total number of systolic AP ramps. This ratio is near zero following the denervation of arterial baroreceptors in cats (127). BEI and BRS are differentially affected by physiological variables; BEI decreases, whereas BRS increases during sleep (127), and it is differentially affected by distinct types of cognitive demands in humans (443). Like BRS, BEI is asymmetric since it was more substantial for increasing systolic AP ramps – “up” sequences – than for increasing systolic AP ramps – “down” sequences – (443). Thus, BEI and BRS provide complementary information, and as a result, they give a more comprehensive assessment of the baroreceptor regulation of the heart (443).
Baroreceptor resetting.
Under acute external environmental or internal demand, the AP homeostatic operating point is centrally reset to a new level (e.g., it increases during exercise), and arterial baroreflex acts as a negative feedback loop to adjust AP to a new homeostatic level (131). After the external stressor subsides, the arterial baroreflex readjusts AP back to a lower operating point (131). Acute resetting of the aortic arch afferents (< 20 min) occurs following rapid changes in AP and is quickly reversed in about an hour, whereas the BRS remains unchanged (373). Moreover, very high AP levels acutely evoke a constant tonic firing of baroreceptors, but eventually, they adapt and begin reflecting once again the cardiac-cycle entrained oscillations of the AP (152).
Resetting also occurs as an adaptation to chronic hypertension, in which there is an adjustment of the AP threshold for baroreceptor activation to higher values, where the baroreflex stimulus-response function shifts to the right (280). In spontaneously hypertensive rats, there is a resetting of the baroreflex inhibition of renal sympathetic nerve activity arising from stimulation of either high-pressure or low-pressure baroreceptors, located respectively in the carotid sinus and right atrium (130). Patients with renal hypertension, in addition to resetting, display smaller slopes in the baroreflex stimulus-response function (i.e., reduced BRS, compared to normotensive controls) (503). Persistent hypotension produces baroreceptor resetting, but unlike chronic hypertension, without altering the gain of the baroreflex (274). Baroreceptor resetting can be challenging to detect when vascular distensibility decreases with atherosclerosis or age; this lessened distensibility results in an increased systolic AP and a compensatory change in the setpoint for vagally mediated cardiopulmonary reflexes (326).
Since AP is the product of cardiac output (set by heart rate and stroke volume) and total peripheral vascular resistance (set by sympathetic vasoconstrictor activity), resetting of resting AP occurs by the heart rate (165, 167, 458) and/or total peripheral vascular resistance (130, 132, 356). The resetting of the baroreflex stimulation-response curve for the heart rate is greater than that for mean AP, the latter being more influenced by total peripheral resistance (165, 396). Baroreflex resetting can take place at several levels of the baroreflex pathway (412), including but not limited to baroreceptor afferents, the sinoatrial node, vascular adrenoceptors, the NTS, and central regulatory structures (329, 412). Most of the peripheral baroreflex resetting is at the level of baroreceptor afferent mechanotransduction (311). Moreover, carotid type I baroreceptors (mainly myelinated A-fibers) undergo acute resetting, whereas carotid type II baroreceptors (unmyelinated C-fibers and myelinated A-fibers) have do not reset (480). Aortic baroreceptor afferents (unmyelinated C-fibers) do not reset during hypertension in rabbits, and instead, they exhibit an increased tonic firing (32). Finally, there are two neural mechanisms involved in baroreceptor resetting during exercise, (a) central command arising from CNS centers that set basic patterns of cardiovascular activity and (b) the exercise pressor reflex that provides homeostatic feedback from contracting skeletal muscles (458).
Baroreceptors and exercise.
Arterial baroreflexes are active during both rest and exercise but reset in a magnitude related to the intensity of exercise (165). There are two basic types of physical exercises, isometric/static exercise (e.g., muscle contraction without a noticeable change in muscle length, which can be produced by handgrip exercise) and dynamic exercise (e.g., active shortening and lengthening of large muscles, which can be produced by treadmill running). Both types of exercises evoke an ‘exercise pressor reflex’ that increases systolic AP, and to a lesser extent, diastolic AP, heart rate, and cardiac output (10). Muscle contraction activates mechanoreceptors and chemoreceptors (vagal thinly myelinated type III and unmyelinated IV afferents, respectively), which reflexly increase mean AP and cardiac output to meet the energetic demands of the organism (167, 342, 458). Although the simultaneous increase in heart rate and AP during exercise suggest at first glance baroreflexes may not be active. The arterial baroreflex indeed remains functional to stabilize AP (165, 396). As such, functional blockade of carotid baroreceptors, by reversible vascular isolation of carotid sinus plus deafferentation of the aortic arch, causes exaggerated increases in AP in response to dynamic running exercise in dogs (568). Similarly, preventing the activation of baroreceptors evoked by the rise of AP during handgrip exercise with the vasodepressor nitroprusside enhances exercise-induced increases in heart rate and sympathetic muscle activity in humans (476). These findings suggest that arterial baroreceptor pathways are functional during exercise.
Rowel and O’Leary (458) proposed that isometric exercise produces metabolites that stimulate muscle chemoreflexes that increase AP sympathetically-mediated vasoconstriction. During dynamic exercise, there is an initial activation of central commands that produce a vagal withdrawal resulting in an increase in heart rate and baroreflex resetting of resting AP to a higher value (458). If the rise of AP meets tissue perfusion needs, there is no sympathetic activation; if it does not meet the metabolic demands, AP level rises further due to a sympathetically-mediated increase in cardiac output, with an additional contribution of sympathetically-mediated vasoconstriction resulting in increased systemic vascular resistance (458). Hence, exercise-related vasoconstriction may be related to an amelioration of baroreceptor inhibitory modulation on sympathetic outflow due to baroreflex resetting. Indeed, there is an upward resetting of the arterial baroreflex control of renal sympathetic outflow in rabbits (132) and rats (356) during dynamic exercise. At the end of dynamic exercise, the central command is no longer active (131), and cardiopulmonary reflexes reset the operating point of the arterial baroreflex to a lower pressure (366, 397) by decreasing sympathetic vasoconstrictor tone resulting in a lower systemic vascular resistance.
Cardiopulmonary baroreflexes can also influence the sympathetic response to dynamic exercises (e.g., treadmill). Muscle pumping during dynamic exercise increases central blood volume and pressure (599), which activates the cardiopulmonary baroreceptors. Activation of cardiopulmonary baroreceptors with blood volume expansion reduces the muscle chemoreflex-mediated increase in mean AP and heart rate elicited by mild dynamic exercise in rats (104). Conversely, procaine-block of cardiac vagal afferents augments the increase in renal sympathetic activity induced by dynamic exercise in rabbits (393). In humans, increasing central blood volume via postural changes that stimulate cardiopulmonary baroreceptors reduces the magnitude of exercise-induced increases in mean AP and resets carotid baroreflex function to lower AP levels (397). Cardiopulmonary baroreflex influence on the exercise pressor reflex takes place through the inhibition of the sympathetic vasoconstrictor tone. Indeed, the postural elevation of central venous pressure decreases muscle sympathetic nerve activity during dynamic exercises (436). Of note, light-intensity dynamic exercise inhibits muscle sympathetic nerve activity, presumably by a mechanism mediated by cardiopulmonary baroreceptors (466). However, high-intensity dynamic exercise increases muscle sympathetic nerve activity, and in this case, by activation of muscle chemoreceptors due to increased metabolic demands (466). Together, cardiopulmonary baroreceptors exert a tonic inhibitory influence on the pressor response to mild-intensity dynamic exercise, which may be overcome by the facilitatory modulation of muscle chemoreflexes at high-intensity levels (366). Redistribution of the cardiac output to contracting skeletal muscle occurs due to the generation of vasodilatory metabolites (e.g., nitric oxide), which produce a reduction in vascular resistance in active muscle relative to inactive muscles (366). Finally, cardiopulmonary baroreflexes exert a limited modulation on sympathetic response to isometric exercises (e.g., handgrip) in humans (469, 482), perhaps due to the limited impact of this type of exercise on central blood volume and central venous pressure.
Central commands arising from higher brain structures reset the baroreflex during exercise in animals. The electrical stimulation of the mesencephalic locomotor region resets carotid baroreflex upwardly in paralyzed cats (346). Non-human exercise studies have provided evidence that pre-sympathetic neurons on the hypothalamic paraventricular nucleus contribute to baroreflex resetting via vasopressin inhibitory projections to the NTS; see Michelinin et al. for a review (355). The exercise pressor reflex produces a GABA-mediated inhibition of the NTS (120). Similarly, there might be a reduction in the tonic GABAergic inhibitory input to the hypothalamic paraventricular nucleus, which leads to further inhibition of the NTS function. These modulatory changes could be relevant during long-term sympathetic challenges but negligible for short-term sympathetic challenges (120). Also, deep-brain stimulation in humans has revealed two central structures engaged in autonomic changes of the circulation during exercise, the periaqueductal gray and the subthalamic nucleus. Electrical stimulation of the dorsal subthalamic nucleus and ventrolateral periaqueductal gray enhances vasomotor BRS and reduces the fall in AP following an acute orthostatic challenge (522).
Several studies have provided evidence that central command pathways reset baroreflexes during exercise in humans; (see Fadel and Raven for a review (167)). Central commands engage several motor centers to initiate skeletal muscle contraction at the onset of exercise while simultaneously inhibiting the NTS sensitivity to baroreceptor input; thus, the arterial baroreflex resets the operating setpoint to a higher AP level around the prevailing exercising pressure (131, 366). Baroreflex resetting makes baroreceptors less responsive to increases in AP elicited by the exercise pressor reflex and reduces their inhibitory influence, allowing heart rate and AP to rise to meet exercise hemodynamic demands. FIGURE 4 shows that under steady-state exercise conditions, the baroreflex resetting for the control of heart rate and mean AP is different at different levels of exercise intensity.
FIGURE 4.
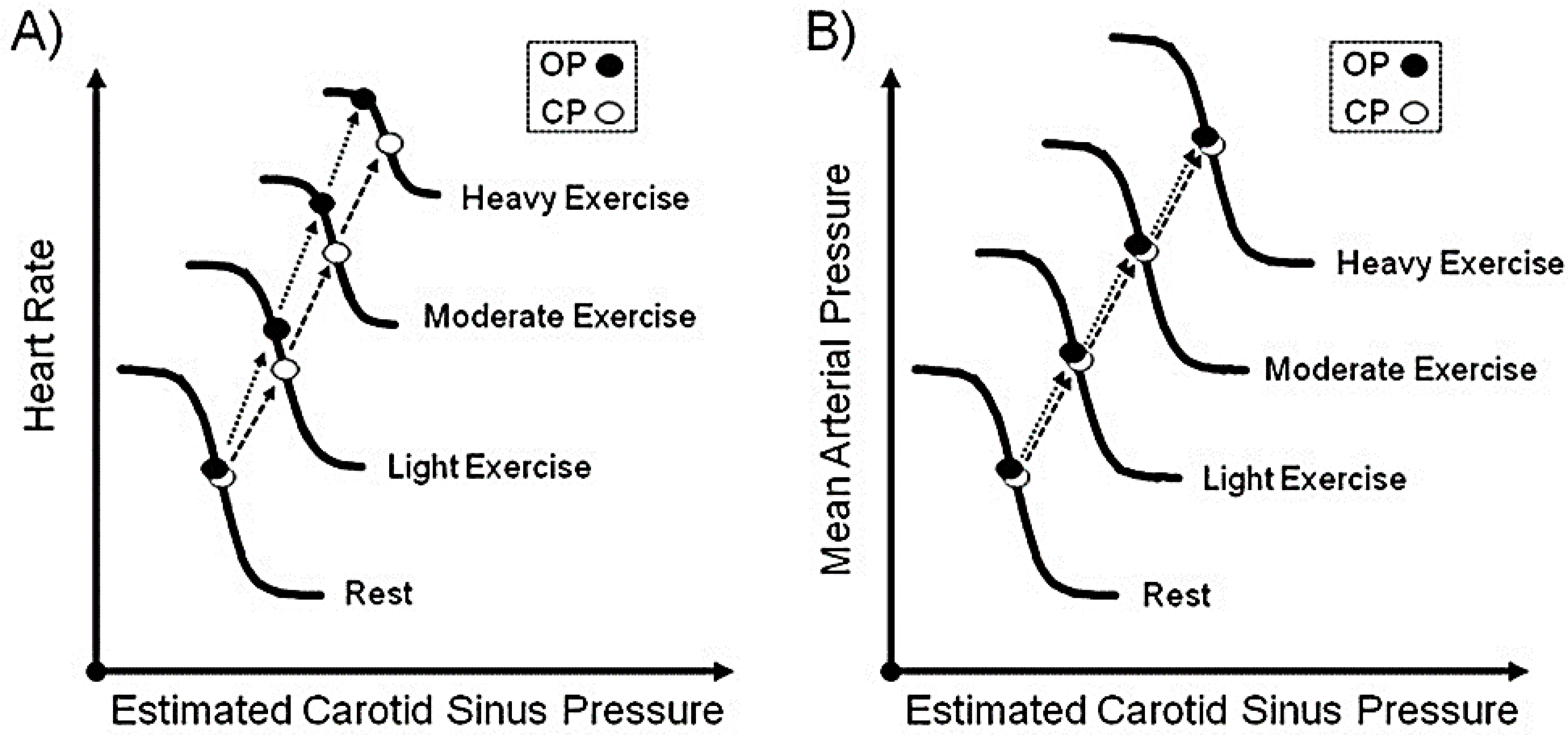
Intensity-dependent resetting of the carotid baroreflex that controls heart rate and AP during steady-state dynamic exercise. The operating point (OP) is the ongoing, either resting or exercising, prevailing AP before carotid sinus stimulation. The centering point (CP) is the carotid sinus pressure that, when applied to the baroreceptor, can equally evoke either increases or decreases in heart rate or mean AP, and at which the maximal gain of the baroreflex is estimated. The threshold is the point in the stimulus-response curve, where no further increase in mean AP or heart rate occurs despite reductions in the estimated carotid sinus pressure. The stimulus-response curve for heart rate (panel A) and MAP (panel B) gradually resets upward and rightward with increasing levels of exercise intensity without changes in the slope of the curve, i.e., the gain of the baroreflex (BRS) remains constant during steady-state dynamic exercise. Reproduced with permission (167)
The stimulus-response curves shown in FIGURE 4 display three parameters that are routinely analyzed in response to exercise. First, the operating point, which is the ongoing resting or exercising associated heart rate or AP (167). Second, the centering point (CP), which is the point at which there is a depressor or pressor response following a change in AP. Changes in CP produce either increases or decreases in heart rate or mean AP, where the maximal gain of the baroreflex is estimated (172, 437). Third, the threshold is the point in the stimulus-response curve, where no further increase in mean AP or heart rate occurs despite reductions in the estimated carotid sinus pressure (172). Measurements done under steady-state exercise conditions reveal that the AP-heart rate baroreceptor mediated stimulus-response curve resets during dynamic exercise, resulting in higher carotid sinus pressure, higher maximal heart rate, and mean AP output responses (i.e., upward and rightward shift of the stimulus-response curve) as exercise intensity increases, without a change in the curve’s slope of the stimulus-response curves (i.e., a constant BRS value) (167). The heart rate operating point resets to a higher value away from the stimulus-response curve’s centering point and closer to the estimated carotid sinus pressure threshold, resulting in a narrower stimulus-response range (165, 167). In contrast to the baroreflex associated mean AP stimulus-response curve, the operating point also resets to a higher value but remains at the centering point of the stimulus-response curve resulting in a constant operating range (165, 167). The relocation of the operating setpoint for heart rate control (but not for mean AP control) provides feedforward central command signals during exercise that produce a greater modulation of heart rate relative to AP (428). Moreover, the displacement of the operating point away from the centering point of the curve, and toward the baroreflex threshold, during exercise reduces the ability to respond to hypotension while enhancing the capacity to buffer against hypertension in response to escalating exercise intensity (172).
Isometric and dynamic exercises increase the renal sympathetic activity in rats (356), rabbits (132), and cats (337, 338) that results from the rapid resetting of baroreflexes (FIGURE 5). Matsukawa et al. (338) proposed that the initial increase in renal sympathetic activity during sustained electrically-evoked muscle contraction in anesthetized cats is due to the activation of muscle mechanoreceptors. In addition, post-exercise inhibition of sympathetic nerve activity can contribute to post-exercise hypotension (356).
FIGURE 5.
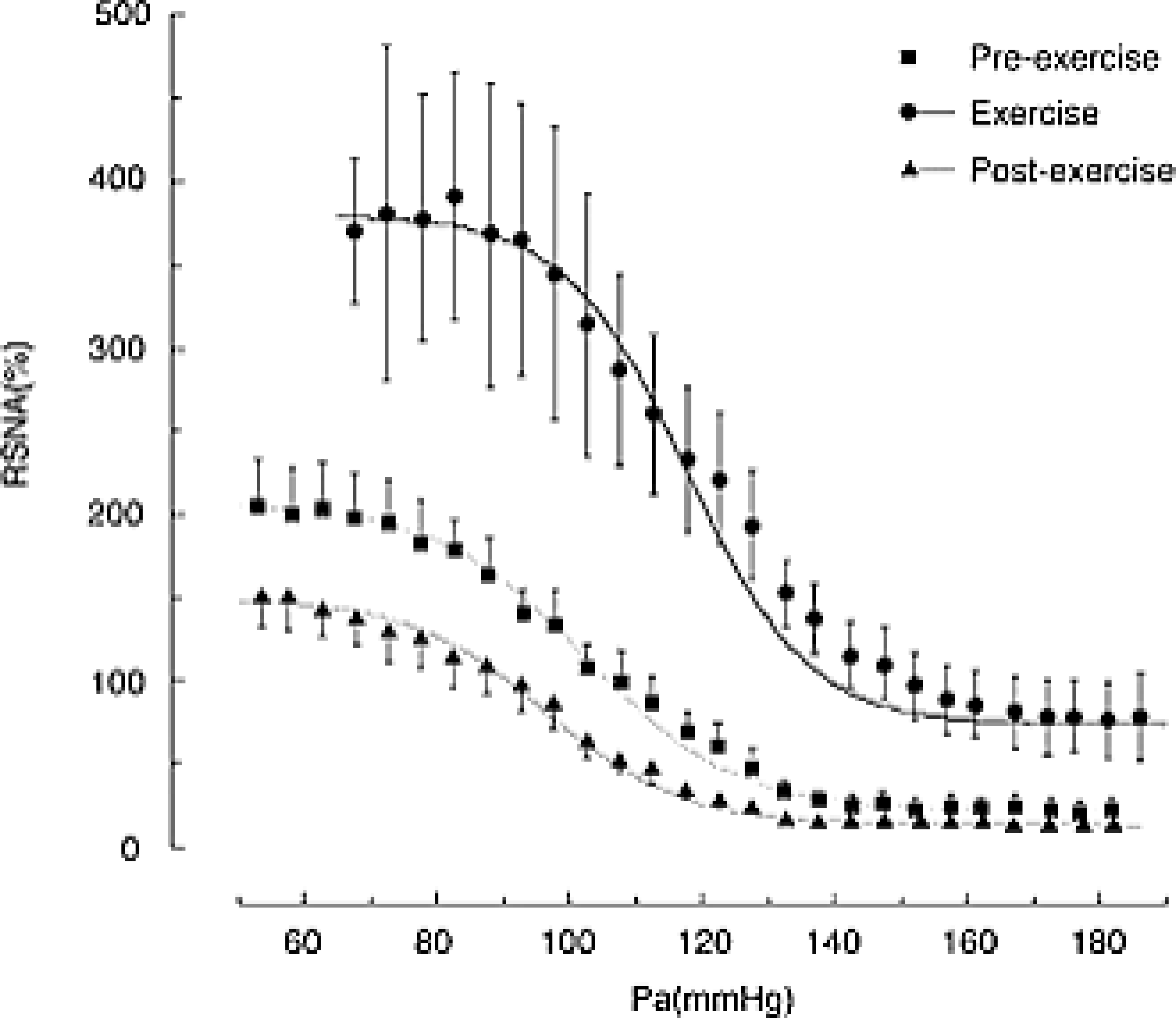
Shifts in the baroreflex curve for renal sympathetic nerve activity (RSNA) obtained during pre-exercise (resting), treadmill exercise, and the post-exercise periods. Curves reflect data averaged from 11 animals, whereas symbols and bars indicate means ± s.e.m., respectively, estimated over each 2.5 mmHg bin of arterial pressure (Pa). Unlike the baroreflex controlling heart rate and mean blood, notice an increase in the slope of the curve during the exercise, which indicates a higher gain of the baroreflex. Reproduced with permission (356).
Dynamic features of the baroreflex during exercise.
Dynamic exercise produces changes in cardiac-derived and vascular-derived BRS values (165). Temporal changes in BRS, either at the onset of exercise or during incremental workload exercise, are effector-dependent. At the onset of exercise, cardiac and vascular BRS is diminished, producing an increase in both heart rate and sympathetic nerve activity. In decerebrate cat preparations, the initial skeletal muscle contraction evoked by electrical stimulation of ventral roots activates muscle afferents triggering a central command mediated ‘exercise pressor reflex’ that attenuates cardiac BRS (349). In conscious cats, central command systems also blunt cardiac BRS at the onset of voluntary isometric exercise (275, 336, 376). Noteworthy, the gain of the baroreflex on AP remains unchanged at the onset of exercise (275, 336, 376).
In humans, baroreflex control of heart rate, AP, and peripheral sympathetic activity exhibit different dynamic responses during exercise. While the gain of the baroreflex on AP does not change across a range of exercise intensities, there is a transient blunting of the gain of the baroreflex on heart rate at the onset of high-intensity isometric hand-grip exercise – but not at lower intensities (173). In contrast, the effect of BRS on muscle sympathetic nerve activity increases at the onset of isometric handgrip exercise with a delay of ~ 60 seconds (483). This delayed increase in sympathetic nerve activity is likely due to the time needed for the accumulation of metabolites around the metaboreceptor afferents and subsequent activation of the muscle metaboreflex (118, 234). Also, the BRS for controlling muscle sympathetic nerve activity increases during the time course of the incremental workload of dynamic exercise – leg cycling (233). Specifically, compared with resting values, the slope of the relationship between spontaneous beat-to-beat diastolic AP and muscle sympathetic nerve activity (vascular BRS) is smaller during very mild exercise, is unchanged during mild and moderate exercise, and is higher during heavy and exhausting exercise (233). Likewise, moderate dynamic exercise does not affect vascular BRS as estimated by the reduction in both muscle sympathetic activity (166) and vascular conductance (261) induced by mechanical activation of carotid baroceptors when compared with resting values.
The evidence presented above indicates that the vascular branch of the baroreflex differentially modulates peripheral sympathetic nerve activity and AP. Moreover, the increase in the amplitude of the muscle sympathetic nerve activity evoked by orthostatic stress is smaller when baroreceptor loading with head-up tilt is applied at a slow or very slow speed – compared with higher speed – in healthy humans (FIGURE 6A), whereas no significant difference is observed for heart rate and AP (FIGURE 6B and 6C) (255). To understand the mechanisms contributing to these regulatory differences, baroreflex activity cannot be analyzed as a closed-loop feedback system because effector responses depend not only on the instantaneous input but also on the history of the input change (517, 598). Open-loop models are required. In open-loop animals (236) and humans (256) studies, experiments have unveiled the dynamic properties of two baroreflex subsystems, the neural and peripheral arcs, which differentially respond to pressure loading speed experienced by the terminal endings on baroreceptors (236, 256). Baroreceptor input is quickly conveyed through the neural reflex arc, whereas the resulting baroreflex changes in AP occur at a slower rate by way of peripheral processes. Baroreflex changes in AP depend on a slow chemical-mechanical coupling between sympathetic nerve endings and the innervated end-organs, i.e., vascular smooth muscle, sinus node, and the myocardium, which determines the dynamics of baroreflex responses associated with the peripheral vascular resistance (316, 558), heart rate (406, 558), and cardiac contractility (282). Thus, the neural arc, which is activated by AP input, produces a change in sympathetic nerve activity that exhibits high-pass filter dynamics (i.e., faster AP changes) that produce higher amplitudes of peripheral sympathetic nerve activity (FIGURE 6A). In contrast, the peripheral arc regulates AP in a manner that exhibits low-pass filter features (FIGURE 6B and 6C), i.e., faster AP loading has little effect on baroreflex responses of end-organs (e.g., systemic AP and heart rate) (255).
FIGURE 6:
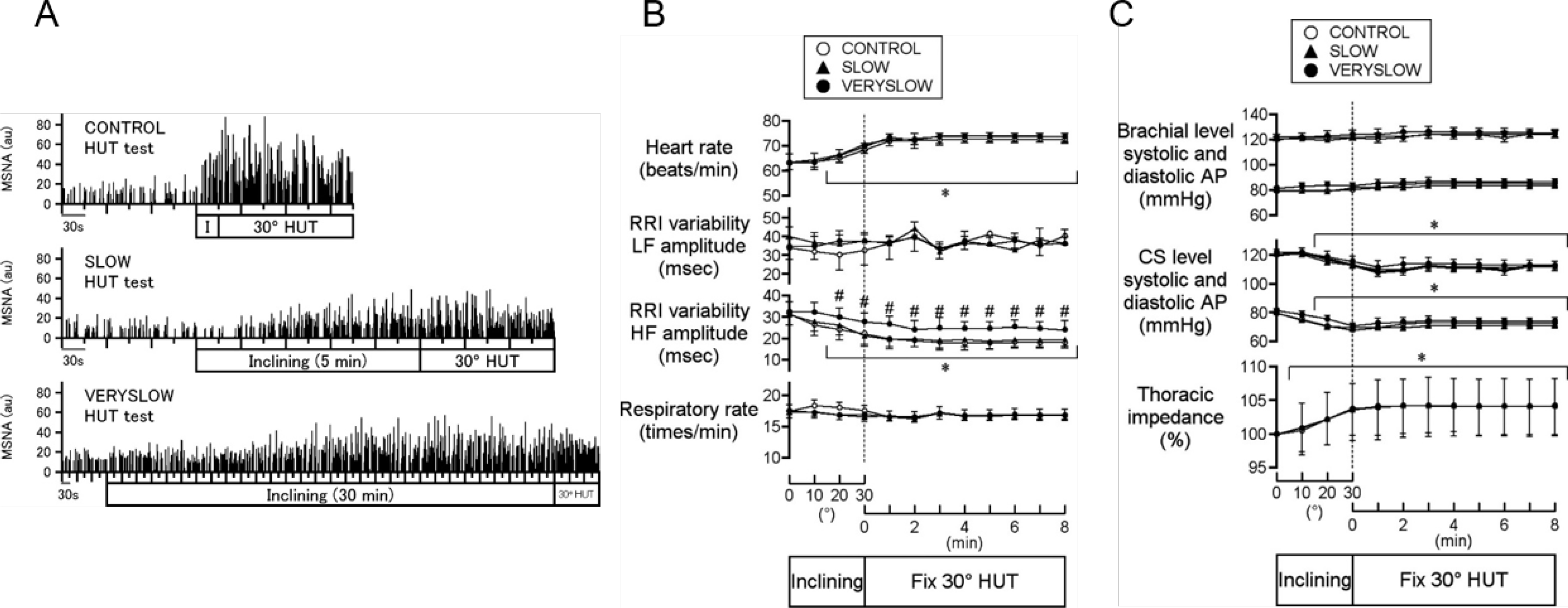
A. Representative muscle sympathetic nerve activity (MSNA; integrated signals) data during control (top), slow (middle), and very slow (bottom) head-up tilt (HUT) tests in a human subject. I (top), a period of inclination of the tilt bed from 0° supine to 30° HUT posture at an inclining speed of 1°/s. Inclining (middle and bottom), a period of inclination of the tilt bed at speeds of 0.1 and 0.0167°/s, respectively. au, Arbitrary units. B. Heart rate, the amplitude of low frequency (LF) and high frequency (HF) component of R-R interval (RRI) variability, and respiratory rate during control (○), slow (▴), and very slow (•) HUT tests. The x-axis to the left of the vertical dotted line indicates that data are averaged over every 10° tilt angle during inclination from 0° supine to 30° HUT, and the x-axis to the right of the dotted line indicates that data are averaged over every 1 min after reaching 30° HUT. #P < 0.05 vs. control and slow tests; *P < 0.05 vs. 0° supine posture. C. Systolic and diastolic arterial pressure (AP) measured at the height of brachial level and predicted at the height of carotid sinus (CS) level, and thoracic impedance (percentage of baseline value at 0° supine) during control (○), slow (▴), and very slow (•) HUT tests. The x-axis to the left of the vertical dotted line indicates that data are averaged over every 10° tilt angle during inclination from 0° supine to 30° HUT, and the x-axis to the right of the dotted line indicates that data are averaged over every 1 min after reaching 30° HUT. *P < 0.05 vs. 0° supine posture. Error bars denote SE. Modified and reproduced with permission (255).
The higher transfer function of the neural arc depends on the change in speed (i.e., acceleration) of the pressure load, which compensates for the lower transfer function observed in the peripheral arc observed during orthostasis (256). Ikeda et al. (236) assessed the physiological relevance of the open-loop system dynamic properties of the neural arc by performing a closed-loop system simulation in response to exogenous perturbations (e.g., orthostasis) under varying levels of baroreflex gain. They found that the neural arc is involved in optimizing the AP response to attain quick adjustments and stable AP levels. In FIGURE 7A, the simulation under neural arc acceleration shows that the attenuation of pressure changes in response to exogenous perturbation becomes larger when baroreflex gain changes from 1 to 3 but produces increases in oscillatory response and instability in the system (236). However, at a gain of 2 (bold line on FIGURE 7A,) a quick response is achieved that is stable with minimal undershooting. In the absence of neural arc acceleration (FIGURE 7B), the step pressure change results in a slow, undershooting response, indicating system instability. Of note, anesthetics reduce BRS in dogs (26–28), which implies that baroreflex regulation is more unstable in the conscious state (with higher BRS) than in anesthetized states. Ikeda et al. (236) concluded that the accelerating properties of the neural arc are more crucial in stabilizing AP in conscious compared to anesthetized conditions.
FIGURE 7:
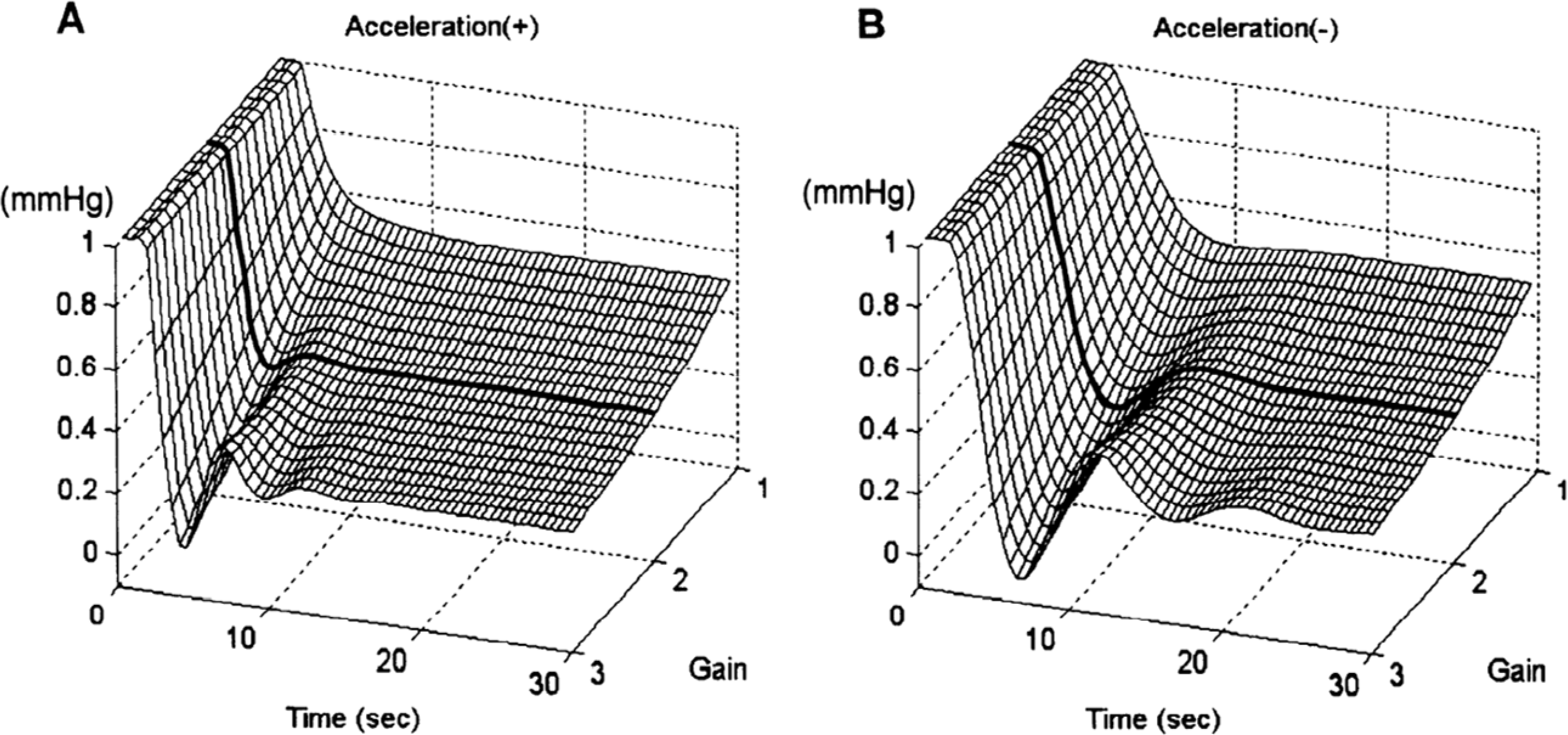
Simulation of a closed-loop step-change in response to exogenous perturbation with (A) or without (B) neural acceleration while the gain of baroreflex is changed from one to three. The magnitude of exogenous step perturbation is one mmHg. Attenuation of pressure changes in response to exogenous perturbation becomes larger with an increased gain of baroreflex, whereas response becomes increasingly oscillatory at a higher gain, indicating instability of the system. Step response at gain 2.0 with the neural acceleration revealed a quick and stable response (bold line in A). In contrast, step response without acceleration showed a slow and undershooting response in arterial pressure (bold line in B), indicating instability of the system. Reproduced with permission (236).
Circadian variability of the baroreflex.
Physiologically, baroreflexes are involved not only in the well-established short-term modulation of AP but also in the regulation of AP throughout the day (107). BRS exhibits a very complex circadian rhythm. Hossmann et al. (227) were the first to report circadian variations of the cardiac BRS on norepinephrine-evoked increases in AP; they found that BRS reaches its highest levels at 3:00 am and 12:00 pm and lowest values at 3:00 pm and 9:00 am. The variation in the pattern of cardiac BRS on resting AP oscillations is different, highest during sleep (11:00 pm - 1:00 am) and lowest in the morning (9:00 am - 11:00 am) and afternoon (4:00 pm - 6:00 pm) (407). Furthermore, spontaneous cardiac BRS shows a bimodal pattern during the evening with a peak at 7:00 pm and trough at 11:00 pm (548). These circadian patterns influence baroreflex activity in such a way that baroreflexes exert greater control of heart rate and AP during sleep compared to restful wakefulness (500). The diminished BRS observed in the morning results from (a) a decrease in carotid sinus mechanical sensitivity in response to a rise in AP and (b) a reduction in neural sensitivity in response to falling in AP (534).
The mechanisms underlying these patterns are not fully understood. However, Clock genes (Cry) have been reported to regulate BRS circadian rhythm in part by altered α1-adrenergic receptor-mediated vasoconstriction of peripheral vessels (335). Cry-null mice do not show a diurnal variation in BRS but exhibit an enhanced cardiac baroreflex where resting AP is controlled by cardiac output rather than by peripheral vascular resistance (335).
Clinically, baroreflex circadian variability leads to cardiovascular circadian rhythm generation (127, 327), which expresses as diurnal changes in AP, heart rate, endothelial function, platelet aggregation, and thrombus formation (89). Noteworthy, the day-night variations in AP, heart rate, and cardiac BRS coincide with diurnal variations in arrhythmias (418), myocardial infarction (369), and sudden cardiac death (368, 593). The disruption of circadian rhythm predicts the onset of the aforementioned cardiovascular events (89). Taylor et al. proposed that interventions that reverse diminished cardiac BRS in the morning should be considered when managing hypertension (534).
Baroreceptors interactions with other vagally mediated cardiovascular reflexes.
The Bainbridge reflex (tachycardia and hypertension following volume atrial stimulation) and the Bezold-Jarisch (bradycardia and hypotension after chemical ventricular stimulation) are two vagally mediated cardiovascular reflexes that are functionally opposed. These reflexes do not show the expected homeostatic inverse relationship between heart rate and AP (86, 557). Indeed, these vagally mediated cardiovascular reflexes evoke a response in which both heart rate and AP change in the same direction (22, 245, 246). Given the antagonistic nature of these cardiovascular reflexes, they should mutually interfere with each other. In this regard, baroreflexes are either disable or ineffective during the Bainbridge reflex since denervation of the arterial baroreceptors does not enhance the volume-loading reflex tachycardia in conscious dogs (557). Furthermore, the Bainbridge reflex diminishes the cardiac BRS since the elevation of atrial pressure with volume loading decreases heart rate in response to rises in AP induced by intravenous injection methoxamine in conscious dogs (557).
Similarly, Bainbridge’s reflex evoked by increasing right atrial pressure in response to volume expansion reduces baroreflex responses evoked by changes in isolated carotid sinuses pressure (87). Specifically, the Bainbridge reflex diminishes both the bradycardic/depressor response to carotid sinuses pressure rise and the tachycardiac/pressor response to carotid pressure fall (87). Transecting the aortic nerve prevents Bainbridge reflex inhibition of the carotid baroreflex depressor/bradycardic response, whereas sectioning the cervical vagus prevents Bainbridge reflex inhibition on the carotid baroreflex pressor responses (87). Thus, the Bainbridge reflex inhibits baroreflex pressor response through cardiopulmonary vagal afferents, whereas it reduces the baroreflex depressor and bradycardic responses via aortic vagal afferents. Finally, the intensity of stimulation determines the activation and predominance of some vagally-mediated cardiovascular reflexes. For example, a modest increase in central blood volume induces a cardiopulmonary baroreflex that produces bradycardia, whereas a sustained volume expansion elicits the Bainbridge reflex that caused tachycardia in humans (24).
Activation of the Bezold-Jarisch reflex with intravenous veratridine attenuates the carotid baroreflex tachycardia in anesthetized rabbits (86). Also, stimulation of left ventricular vagal afferents dramatically attenuates the arterial baroreflex control of heart rate in awaked dogs (613). FIGURE 8 shows the interaction between the Bezold-Jarisch reflex evoked by intracoronary infusion of veratrine (that mainly acts on the left ventricle) and the carotid sinus baroreflex elicited by AP manipulations with the hydraulic occlusion of either the thoracic inferior vena cava (AP fall) or the descending thoracic aorta (AP rise) in dogs (224). The Bezold-Jarisch reflex substantially inhibits the carotid baroreflex tachycardia to AP fall, whereas there is a slight or no alteration in the carotid baroreflex bradycardia to AP rise. The Bezold-Jarisch reflex resets the carotid baroreflex control of heart rate to lower AP values (left shift of the heart rate-AP curve), as well as a reduction in BRS (224). The Bezold-Jarisch reflex induces a downward resetting of the cardiac baroreflex curve by an atropine-resistant sympathetic mechanism, whereas it elicits a reduction of BRS by decreasing vagal withdrawal during baroreceptor unloading (224).
FIGURE 8.
Interaction between the Bezold-Jarisch reflex (evoked by intracoronary veratrine) and the carotid sinus baroreflex. Mean curves representing the mean arterial blood pressure (MABP) – heart rate relationship (top) and MABP – baroreflex sensitivity relationship (bottom) during control and intracoronary veratrine infusion (Ver.-L.C.) in 10 dogs. Note that the Bezold-Jarisch reflex resets mean AP optimal level for maximal BRS to lower levels and diminishes BRS. Control mean baseline values: MABP = 93 mmHg and heart rate = 91 beat/min. Reproduced with permission (613).
Finally, the selective blockade of cardiopulmonary baroreflexes potentiates carotid chemoreflex (muscle vasoconstriction, paw vasodilation, and increased ventilation), and augments the gain of the carotid baroreflex at low carotid pressures in anesthetized, ventilated dogs (273). Thus, cardiopulmonary reflexes suppress both the carotid baroreflex and chemoreflex via different central autonomic mechanisms (273).
Baroreceptor long-term regulation of arterial pressure.
Besides its crucial role in short-term regulation, the arterial baroreflex contributes to long-term control of AP modulating hormonal and renal processes (311). Studies using acute preparation for short recordings have observed that the baroreflex rapidly resets during sustained increases in AP and that baroreceptor deafferentation does not produce long-lasting hypertension or an increase in the AP levels in ongoing hypertension (112, 113, 544). The extrapolation of these findings to chronic states has led to the viewpoint that arterial baroreflexes did not contribute to the long-term regulation of AP (311). However, studies using telemetry-based implantable amplifiers for prolonged recordings (up to one week) have shown that the relationship between MAP-renal sympathetic nerve activity, i.e., the vascular baroreflex branch, does not reset in rabbits with angiotensin II-induced chronic hypertension; in contrast, there is a resetting of the association between MAP and heart rate, i.e., cardiac baroreflex branch (27).
Moreover, there is a sustained reduction in renal sympathetic nerve activity throughout the acute and chronic phases of angiotensin II-induced hypertension in rabbits (27). A reduction in efferent renal sympathetic nerve activity elevates renal blood flow and glomerular filtration rate (due to vasodilation), decreases the renal tubular reabsorption of sodium and water (leading to natriuresis and increased urine output), and increases renin release (250). Thus, Lohmeier and Iliescus (311) recently proposed that baroreflexes have indeed a role in the long-term regulation of AP by exerting a sustained suppression of renal sympathetic nerve activity and increasing renal sodium excretion in chronic hypertension.
The disruption of the arterial baroreflex afferent pathways by sinoaortic denervation abolishes the inhibition of renal sympathetic nerve activity under chronic hypertension (26). Brainstem c-fos expression during angiotensin II-induced chronic hypertension reveals an increased neural activation of NTS (primary recipient of baroreceptor afferent input) and CVLM but without activation of the RVLM (314). Baroreceptor-mediated chronic suppression of sympathoexcitatory cells in the RVLM exerts a descending excitatory influence on basal sympathetic outflow at the spinal level, which diminishes renal sympathetic nerve activity and augments sodium excretion (311). Together, chronic activation of arterial baroreceptors can contribute to the long-term control of AP by regulating sympathetically mediated natriuresis.
Electrical field stimulation of the carotid sinuses induces reductions in MAP and plasma norepinephrine concentration for up to 3 weeks, overcoming central and peripheral baroreceptor resetting (312, 313). Thus, electrical stimulation of carotid sinuses to control baroreceptor afferent input into the central nervous system could allow a device-based approach for the treatment of pharmacological-resistant hypertension in humans (312).
Pharmacology of Baroreceptors
Numerous pharmacological agents modify the baroreceptor function. These agents include clinically used drugs, exogenous irritants, and endogenous molecules used at physiological and pharmacological concentrations. These agents exert their actions at peripheral and central sites to modify baroreceptor function. This section describes the pharmacological effects of agents known to alter baroreceptor function and distinguishes direct actions on baroreceptor pathways from indirect processes that result from secondary actions mediated by the cardiovascular system. TABLE 3 lists pharmacological agents and endogenous substances that affect isolated arterial and cardiopulmonary baroreceptors preparations.
TABLE 3:
Physiology and pharmacology of baroreceptor afferents
| Location Afferent nerves | Physiologic Stimulus | Pharmacologic Stimulants | Pharmacologic Inhibitors | Systemic Responses to Stimulations |
|---|---|---|---|---|
| Carotid sinus / Glossopharyngeal nerve (Cranial nerve IX) | ↑ AP | Adenosine (A1 receptor) (92) Arachidonic acid (345) Capsaicin (611) Endothelin-1 (low dose) (301) Nifedipine (Ca-dependent) (217) Prostacyclin – PGI2 (88, 345) Serotonin (5-HT3 receptors) (600) Simvastatin (362) Veratrum alkaloids (211) |
Agmatine (Ca-dependent, α2AR-mediated) (425)
Amiloride (stretch DEG/ENaC channels) (139) Benzamil (amiloride analog)(139) Bradykinin (PGs-independent, NO-mediated) (588) Doxapram (399) Endothelin-1 (ETAR, high dose) (301) 17beta-estradiol (574) Gadolinium (211) Ginkgolide B (diterpenoid trilactone) (572) Moxonidine (α2AR-mediated) (591) NSAIDs (aspirin, indomethacin) (345) Streptomycin (426) Verapamil (Na-dependent) (217) |
↓ Sympathetic activity ↑ Parasympathetic activity ∆ EEG asynchronization (179) ↓ “Sham rage” behavior (608) ↓ Somatomotor reflexes (e.g., analgesia) (56) ↓ Angina pectoris (57) |
|
Aortic arch / Vagal nerve (Cranial nerve X) |
↑ AP | Acetylcholine (mAchR/NO-mediated, low conc.) (372) Acetylcholine (direct, nAchR) (372) Prostacyclin – PGI2 (305) Norepinephrine (under distension) (1) |
Acetylcholine (mAChR/VSM-mediated, high conc.) (372) Amiloride (140) Angiotensin II (VSM contraction-mediated) (374) Norepinephrine (under contraction) (1) |
↓ Sympathetic activity ↑ Parasympathetic activity |
|
Cardiopulmonary structures / Vagal nerve (Cranial nerve X) |
Atrial and ventricular stretch ↑ Vascular volume ↑ Central venous pressure ↑ Lung inflation rate ↑ Lung stiffness Airways light touch |
Ammonia (230, 297) Bradykinin (37) Cigarette smoke, Nicotine (279, 534) Endotoxin (292) Histamine (37) Opiates (430) (systemic administration) Ozone (102) PBG/Serotonin (559) SP/capsaicin (37) Tumor necrosis factor-alpha (306) Veratrum alkaloids (339) |
↓ Sympathetic activity ↑ Parasympathetic activity ∆ EEG asynchronization (179) ↓ “Sham rage” behavior (608) ↓ Somatomotor reflexes, e.g., analgesia (56) |
|
|
Auricle (cymba conchae) / Vagal nerve (Cranial nerve X) |
Mechanical(216) (acupuncture) Electrical (TENS)(602) |
↓ Sympathetic activity (95) ↑ HRV (95) ↑ Mood (602) ↓ Major Depression (105) ↓ Seizure (216, 602) ↓ Chronic pain: migraine, pelvic pain, diabetic neuropathy (303, 370, 382) |
Note: Data obtained from the local administration of drugs in isolated afferents recording preparations except for opioids. AP: arterial blood pressure. α2AR: α2-adrenoceptors. EEG: electroencephalogram. ETAR: endothelin type-A receptor. HRV: heart rate variability. mAchR: muscarinic acetylcholine receptors. mAchR/NO: the mAchR-mediated release of NO in NE-pre-constricted artery. mAchR/VSM: mAchR-induced relaxation of vascular smooth muscle. nAchR: direct activation of nicotinic AchR (few baroreceptors). NE: norepinephrine. NO: nitric oxide. PBG: PGs: Prostaglandins. Phenylbiguanide, 5-HT3 receptor agonist. SP: Substance P. TENS: transcutaneous electrical nerve stimulation. VSM: vascular smooth muscle.
Isolated afferent preparations and signal transduction:
Electrophysiological studies examining the effects of direct application of various substances to isolated carotid sinus or aortic arch preparations have contributed to the elucidation of the transduction mechanisms by which mechanical pressure stimuli induce baroreceptor afferent discharges. The use of these isolated tissue preparations has also permitted the characterizing of the pharmacological actions on baroreceptor afferents. TABLE 4 provides a summary of the effects of several pharmacological agents and endogenous mediators on baroreflex stimulus-response curves obtained using isolated carotid and aortic preparations.
TABLE 4:
Changes in the baroreflex stimulus-response functions following perfusion of selected pharmacological agents into isolated carotid and aortic preparations.
| Pharmacologic agent Overall effect on baroreflex | Pressure threshold | Saturation pressure | Slope (reflex gain) | Curve horizontal shift | Curve vertical shift | Reflex Responses: Effect of systemic administration |
|---|---|---|---|---|---|---|
| Adenosine (92) / Facilitation | Decrease | Decrease | Increase | Leftward | Upward | Increased pressure-evoked carotid sinus nerve activity / Hypotension (222) |
| Angiotensin II (374)/ Inhibition | Increase | No change | No change | Rightward | Downward | Reduced pressure-evoked carotid sinus nerve activity / Hypertension (18, 439) |
| Agmatine (425) / Inhibition | Increase | No change | Decrease | Rightward | Downward | Reduced pressure-evoked carotid sinus nerve activity / Hypotension (by vasodilation) (360) |
| Bradykinin (588) / Inhibition | Increase | Increase | Decrease | Rightward | Upward | Reduced inhibition on mean AP / Hypotension (by vasodilation) |
| Cholecystokinin octapeptide (CCK-8) / Inhibition | Increase | Increase | Decrease | Rightward | Upward | Reduced inhibition of mean AP / Hypertension |
| Doxapram (399) / Inhibition | Increase | Increase | Decrease | Rightward | Downward | Reduced pressure-evoked carotid sinus nerve activity/ Hypertension |
| Endothelin-1 low conc. (301)/ Facilitation | Decrease | Decrease | Increase | Leftward | Downward | Enhanced inhibition of mean AP / Hypotension (6, 93) |
| Endothelin-1 high conc. (301)/ Inhibition | Increase | Increase | Decrease | Rightward | Upward | Reduced inhibition of mean AP / Hypertension |
| 17-β Estradiol (574) / Inhibition | Increase | Increase | Decrease | Rightward | Upward | Reduced inhibition of mean AP / Hypotension (by vasodilation) (309) |
| Ginkgolide B (572) / Inhibition | Increase | Increase | Decrease | Rightward | Upward | Reduced inhibition of mean AP / Hypotension |
| Indomethacin Aspirin (345)/ Inhibition | Increase | Increase | Decrease | Leftward | Downward | Reduced pressure-evoked inhibition of lumbar sympathetic nerve activity / Hypertension |
| Moxonidine (591) / Inhibition | Increase | Increase | Reduction | Rightward | Upward | Reduced inhibition of mean AP / Hypotension |
| Prostacyclin (PGI2) (88, 345) / Facilitation | Decrease | Decrease | Increase | Rightward | Upward | Increased pressure-evoked inhibition of lumbar sympathetic nerve activity / Hypotension (pulmonary vascular bed) (123) |
| Simvastatin (51, 362, 507)/Facilitation | No change | Decrease | Increase | Leftward | Upward | Increased pressure-evoked aortic depressor nerve activity / Hypotension (51, 507) |
| Streptomycin (426) / Inhibition | Increase | Increase | Decrease | Rightward | Upward | Reduced inhibition of mean AP / Hypotension (toxic effect) (97) |
Note 1: Stimulus is the intraluminal arterial pressure in the carotid sinus or aortic arc. Responses are neural (baroreceptors neural activity, sympathetic outflow) or vascular (systemic mean AP).
Note 2: The vertical displacement of the baroreflex function for the neural responses are generally equivalent to opposite shifts in vascular responses.
Note 3: Pressure threshold is the lowest intraluminal arterial pressure that elicits a baroreflex response. Saturation pressure is the intraluminal arterial pressure level beyond which there is no further increase in baroreflex response.
Note 4: Although agmatine, bradykinin, and 17-β estradiol inhibit the carotid baroreflex, systemic administration of these agents induce hypotension by peripheral vasodilation.
Baroreceptor mechanotransduction of AP levels.
The exposure of the isolated carotid sinus to veratrine (a veratrum alkaloid) evokes discharges from baroreceptor fibers in rabbits by triggering action potentials at the spike initiation zone and prevents voltage-gated sodium channel inactivation (211). Small decreases in extracellular sodium concentrations substantially diminish the firing of baroreceptive C-fibers both at constant intraluminal pressure and during a pressure ramp in aortic arch/aortic nerve preparations in rats (541). The rise in transmural AP stretches the arterial wall producing a mechanical deformation, which is transduced into an electrical depolarization of the baroreceptor afferents by stretch-activated ion channels that are sensitive to gadolinium (211). The molecular components of these stretch-activated mechanisms are related to the degenerin/epithelial sodium channel, which is non-voltage gated and initially characterized as a touch-sensitive mechanoreceptor in nematode Caenorhabditis elegans (229). Earlier studies found that the blockade of a specific degenerin protein, β epithelial sodium channel, with low concentrations of amiloride or its analog benzamil, inhibits baroreceptor activity in mouse carotid and aortic preparations (139, 140). Later studies identified PIEZO1 and PIEZO2 channels as the mammalian homologous of degenerins (111). Genetic ablation of both PIEZO1 and PIEZO2 in baroreceptive primary neurons located in the nodose and petrosal sensory ganglia abolishes both drug-induced baroreflex and aortic depressor nerve activity in mice (610). Optogenetic activation of Piezo2+ sensory afferents is sufficient to initiate a baroreflex response in mice (610). These mice exhibit labile hypertension and increased AP variability, similar to baroreceptor-denervated animals and humans with baroreflex failure (610).
The role of calcium channels on baroreceptor mechanotransduction of AP is not clear. Voltage-activated T-type calcium channels Cav3.2 contribute to the function of tactile C-low threshold mechanoreceptors in humans (379) and D-hair mechanoreceptors in mice (141). Mibefradil, a selective T-type calcium channel antagonist, inhibits venoconstrictor reflex in response to tilt and causes postural hypotension in the rabbit, suggesting a role for this channel in baroreceptor afferent mechanotransduction (141). In contrast, the L-type, voltage-dependent calcium channel agonist Bay K 8644, and the inorganic calcium channel blocker cobalt, do not alter aortic arch or atrium baroreceptor activity in cats (13). Similarly, the L-type, voltage-dependent calcium channel antagonists nitrendipine, diltiazem, and verapamil do not alter single baroreceptor fiber discharges in rat aortic arch preparations in rats (287). These findings suggest that calcium entry does not contribute to baroreceptor transduction in rats and cats (13, 287). However, L-type calcium channel antagonists alter the firing of carotid sinus nerves in dogs; nifedipine increases activity by a calcium-dependent mechanism, whereas verapamil decreases activity by a sodium-dependent mechanism (217). Together, these findings suggest that PIEZO1 and PIEZO2 channels mediate the conversion of pressure-induced stretch stimuli into electrical signals at the terminal fields of baroreceptor afferents, whereas the contribution of voltage-gated calcium channels to afferent activation is species-dependent.
Several exogenous agents and endogenous substances modify ongoing stretch-evoked baroreceptor activity. Capsaicin increases pressure-evoked nerve afferent discharges from perfused isolated carotid sinus preparations in anesthetized rats by stimulating the vanilloid receptor TRPV1, which mediates the opening of K+(ATP) channels (611). Intravenous capsaicin produces a triphasic arterial pressure response that consists of an initial fall, recovery, and a delayed progressive fall in rats (7). The involvement of TRPV1 receptors, which are stimulated by acidity, temperature, and several irritants, indicates that a wide range of diverse stimuli influences the baroreceptor function.
Pharmacology of the carotid baroreceptors:
Endogenous substances and pharmacologic agents can modify carotid sinus glossopharyngeal afferent results in either acute changes or chronic dysregulation of AP. Serotonin (5-HT) and endothelin-1 are early markers of endothelial dysfunction that increase in plasma before the development of essential arterial hypertension. Young humans (6) and spontaneously hypertensive rats (93) have elevated plasma endothelin-1 and serum 5-HT. The administration of a low concentration (1 nM) of endothelin-1 into isolated carotid sinus preparations facilitates the baroreflex in anesthetized rats by shifting the stimulus-response curve to the left and downward with an increase in the curve’s slope and baroreflex mediated inhibition of the mean AP (301). Conversely, a higher concentration (10 nM) of endothelin-1 inhibits the carotid baroreflex by shifting the stimulus-response curve to the right and upward with a decrease in the curve’s slope and baroreflex mediated inhibition of the mean AP (301). 5-HT displays a complex systemic cardiovascular effect. It produces a triphasic AP response in anesthetized rats characterized by a short-lasting depressor phase with intense bradycardia, a pressor phase, followed by prolonged hypotension (254). The 5-HT pressor response is reversed by blockade of 5-HT2 receptors with ketanserin (254) without affecting carotid sinus activity induced by intravenous 5-HT (600). Serotonin-evoked stimulation of carotid sinus nerve activity in anesthetized rats is mediated by 5-HT3 receptors because the selective and competitive 5-HT3 receptor antagonist GR38032F produces a parallel rightward shift of the stimulus-response curve (600). Of note, intravenous administration of the neuropeptide cholecystokinin octapeptide (CCK-8), which stimulates CCK-A receptors, evokes a pressor response by shifting the baroreflex stimulus-response curve to the right and upward (308). Young spontaneously hypertensive rats display an increased density of CCK-8 binding sites in the nucleus accumbens that precedes the onset of hypertension (268). These findings suggest that the local actions of endothelin-1, 5-HT, and CCK-8 on arterial baroreceptors contribute to the development of essential hypertension.
Some endogenous vasoactive mediators, such as adenosine, prostaglandins, agmatine, bradykinin, and 17-β estradiol, enhance their hypotensive effects by altering the carotid baroreceptor stimulus-response properties. Adenosine stimulates adenosine A1 receptors and can induce hypotension by an endothelial-dependent relaxation of smooth muscle (222). Adenosine can also enhance pressure-evoked sinus nerve activity in perfused isolated carotid sinus preparations of rats, which is associated with a leftward and upward shift in the stimulus-response curve and an increase in the curve’s slope (92). Administration of prostacyclin (PGI2) and arachidonic acid to isolated carotid sinus preparations displaces the stimulus (AP) – response (lumbar sympathetic nerve activity) curve leftwards and upwards with an associated increase in the curve’s slope (88, 345). This PGI2 effect is selective in cats because prostaglandins PGE2, PGA2, and PGF2α do not affect carotid baroreceptor and chemoreceptor activity (348). Conversely, the COX inhibitors aspirin and indomethacin (345) with the mechanical removal of the carotid endothelium (88) decrease the relationship of intrasinus pressure, sinus nerve activity, and lumbar sympathetic nerve activity. These findings suggest that the endothelium releases prostaglandins in response to pressure-induced stretching of the carotid sinus, which in turn augments the activation of baroreceptors (88, 345).
Several agents inhibit action baroreceptor function by producing shifts in baroceptor stimulus-response curves. Agmatine, an endogenous agonist of α2-adrenergic and imidazoline receptors (302), lowers AP and sympathetic nerve activity (360) by acting centrally on brainstem nuclei (594) and the peripheral vasculature by releasing nitric oxide (215). In addition, agmatine induces vasodilation by the direct stimulation of imidazoline I-2-receptors (90). Agmatine shifts the intrasinus pressure–carotid sinus nerve activity curve to the right and downward, with a reduction in the stimulus-response slope via an α-adrenergic receptor-mediated inhibition of calcium influx (425). Bradykinin, an endogenous hypotensive and vasodilator peptide, shifts the baroreflex stimulus-response curve to the right and upward, resulting in a reduction of the slope of the stimulus-response curve and a decrease in resting mean AP. These effects have been attributed to the activation of nitric oxide synthase and cyclooxygenases (588). The sex hormone 17-β estradiol has anti-hypertensive activity (309) and shifts the baroreflex stimulus-response curve to the right and upwards. It also produces a decrease in the curve’s slope by a mechanism sensitive to blockade with the nitric oxide synthase inhibitor L-NAME (574). Thus, bradykinin, 17-β estradiol, and agmatine share in common an inhibitory effect on the baroreflex sensitivity; these three endogenous molecules diminish AP by inducing peripheral vasodilation that is independent of an effect on baroreceptor function.
The cardiovascular effects of some clinically used drugs such as doxapram, streptomycin, simvastatin, and moxonidine are due, at least in part, to actions on arterial baroreceptor pathways. For example, doxapram, a central respiratory stimulant that increases AP and heart rate, inhibits the baroreflex by shifting downwards the intrasinus pressure–nerve activity curve recorded from perfused isolated carotid sinus resulting in a reduction in baroreflex activation thresholds and an increase in the maximal reflex responses in rabbits (399). Streptomycin, an antibiotic with hypotensive and hypoventilatory toxic effects (97), shifts the carotid reflex stimulus-response curve to the right and upward, with an associated decrease in the curve’s slope, which blunts the baroreflex reduction in AP (426). Streptomycin’s effects have been linked to a reduction in calcium influx in unidentified structures within the carotid sinus (426). Oral administration of simvastatin, a lipid-lowering HMG-CoA reductase inhibitor used to prevent atherosclerosis-related complications, increases aortic depressor nerve activity and baroreflex gain in male spontaneously hypertensive rats (362). The simvastatin-elicited enhancement of baroreceptor function contributes to the pleiotropic effects of statins, which include blood pressure reduction (51, 507), reduced muscle sympathetic nerve activity (196), and the production of anti-inflammatory mediators (68). The antihypertensive moxonidine (α2-adrenergic/imidazoline receptor agonist) displaces the carotid baroreflex stimulus-response curve to the right and downward, with a reduction in the curve’s slope and the reflex inhibition of mean AP (591). Although moxonidine inhibits the carotid baroreflex, it systemically lowers AP by reducing sympathetic outflow and evoking peripheral vasodilation. Finally, the diterpenoid trilactone Ginkgolide B, a biologically active component of the traditional medicine plant Ginkgo biloba, displaces the baroreflex functional curve to the right and upward, decreases the curve’s slope and reflex decrease in mean AP (572). Ginkgolide B inhibitory effect on baroreceptor reflexes results from decreasing calcium influx and increasing potassium efflux in carotid baroreceptor nerve endings (572). Thus, the hypotensive effect of Ginkgolide B is mediated by producing vasodilation secondary to blockade of L-type calcium channels (424, 473). Of note, there is no conclusive clinical evidence of the efficacy of Ginkgo biloba in the treatment of essential hypertension (590).
Pharmacology of the aortic arch baroreceptors:
Compared to carotid baroreceptors, there is less known about the pharmacology of aortic baroreceptors, which is mostly limited to the effects of endogenous substances. Like the carotid baroreceptors, prostaglandins also have an excitatory effect on aortic baroreceptors. Indeed, the PGI2 analog carbacyclin diminishes the activity of inhibitory calcium-activated potassium channels in cultured aortic baroreceptor neurons from rat nodose ganglia, which facilitates baroreceptor activity (305). Acetylcholine has complex pharmacological effects on aortic baroreceptor activity. The perfusion of relatively low concentrations of acetylcholine into the rabbit aortic arch/aortic nerve preparation indirectly increases the discharges of baroreceptor afferents by producing a relaxation of aortic smooth muscle by stimulating the muscarinic receptor-mediated synthesis of nitric oxide (372). Of note, acetylcholine can also increase the activity of a subset of aortic baroreceptor afferents by stimulating nicotinic receptors (372). In contrast, higher concentrations of acetylcholine indirectly diminish the discharges of aortic baroreceptor afferents by inducing a muscarinic receptor-mediated contraction of smooth muscle (372). The intravenous infusion of norepinephrine increases aortic baroreceptor sensitivity to hemodynamic aortic distension in rabbits, whereas it reduces baroreceptor discharges as the aortic diameter diminishes due to contraction (1).
Electrical stimulation of cervical sympathetic efferent fibers increases the firing rate of carotid sinus afferent nerves independent of changes in AP (468). Together, this finding suggests that sympathetic efferents innervating the carotid sinus and the aortic arch sensitize baroreceptors by either a direct action of norepinephrine on afferent endings or changing vascular wall tension maintained at a constant intrasinus pressure (468). Of note, angiotensin II improves aortic wall tension by locally inducing vasoconstriction. As a result, it inhibits the baroreflex, which is reflected by displacement of the pressure-discharge curve to the right and downward and reduces baroreceptor afferent firing frequency recorded from a rabbit aortic arch–aortic nerve preparation (374).
Pharmacology of the cardiopulmonary afferents:
Several pharmacologic agents can stimulate cardiopulmonary afferents either by a direct action on afferent nerve endings or by the indirect induction of mechanical changes in respiratory tissues. Inflammatory mediators can activate pulmonary sensory afferents and generate clinical signs and symptoms (e.g., cough, bronchoconstriction, neurogenic inflammation) of airway inflammatory (306). For example, tumor necrosis factor-alpha (TNFα) increases the sensitivity of vagal pulmonary C-fibers and silent, rapidly adapting receptors to capsaicin through a direct action on TNFα receptors located in pulmonary sensory neurons (306). Histamine, capsaicin, and bradykinin, released in response to the lung defense reaction, activate pulmonary rapidly adapting receptors by indirectly increasing airway smooth muscle tone in guinea pigs (37). Capsaicin and bradykinin, but not histamine, activate lung C-fiber endings (37). Furthermore, the pulmonary arterial injection of capsaicin induces rapid shallow breathing (tachypnea) in response to activating pulmonary C-fibers (201).
Systemic infection can release endotoxins into the circulation, inducing respiratory distress characterized by increased respiratory rate and tidal volume in both humans (39, 54) and non-humans (530). Bilateral cervical vagotomy prevents tachypnea induced by the intravenous administration of B4 lipopolysaccharide (LPS) in the rat (530). The intravenous administration of E. coli LPS indirectly activates rapidly adapting receptors in rats by inducing bronchoconstriction (292). Endotoxins can also stimulate vagal pulmonary C fibers in rats, which is blocked by COX inhibitors and hydroxyl radical scavengers (292). However, treatment with ibuprofen does not prevent acute respiratory distress syndrome and does not improve survival in patients with sepsis (39).
Injury of the endothelial cells by pulmonary and systemic hypertension reduces their capacity to remove serotonin from the lungs before the expression of observable functional and anatomical abnormalities (214). Indeed, the lungs of spontaneously hypertensive rats uptake and metabolize less 5-HT (456). In rats, intravenous infusion of the 5-HT3 receptor agonist phenylbiguanide blunts the vagal C fiber mediated reduction in renal sympathetic nerve activity in response to volume loading (559). This effect of phenylbiguanide has been proposed to be mediated by activation of lung chemoreceptors because the intravenous administration of this 5-HT3 receptor agonist increases the firing of single vagal pulmonary afferent C fibers (101, 560). Of note, pulmonary C-type afferents contribute to respiratory symptoms (e.g., dyspnea, tachypnea) seen in pulmonary hypertension and edema (201, 450).
The exposure of lungs to exogenous irritants directly activates cardiopulmonary afferents. Ammonia exerts an excitatory influence on lung spinal sensory afferents by a mechanism that is drastically reduced after vagal nerve transection (230). High-nicotine cigarette smoke increases vagal afferent activity evoked from rapidly-adapting afferents (279), and nicotine aerosol stimulates slowly-adapting stretch receptors in the dog’s lungs, indirectly by producing nicotine-induced bronchoconstriction (534). Similarly, intravenous administration of veratridine stimulates slowly adapting stretch receptors in the lung (339).
There are opioid receptors on subpopulations of pulmonary vagal afferents that contribute to the generation of cough and the antitussive activity of opioid codeine (34). Vagotomy or vagal block suppresses cough in humans and animals (34). Activation of pulmonary C-fiber afferents inhibits cough reflexes, whereas slowly-adapting pulmonary stretch receptors strengthen the reflex cough (532). Intravenous administration of the opioid agonist [D-Ala 2]-methionine enkephalinamide, as well as volume expansion, induces analgesia in rats by a mechanism that is blocked by bilateral cervical vagotomy that further supports a role for cardiopulmonary afferents activation in modulating pain (324, 430). Of note, cough-related sensations are commonly described as “irritation, “rawness,” and even “pain” (581).
Factors moderating drug effects on baroreceptors:
Dose, route of administration, mechanism of action, functional tissue status, and type of baroreceptor are factors that determine the effects of various agents on baroreceptor pathways. Dose-dependent biphasic effects occur following the exposure of carotid and aortic baroreceptors to endothelin-1 and acetylcholine, respectively; in both cases, low doses stimulate, whereas high doses inhibit baroreceptor afferent activity (301, 372). However, high concentrations of acetylcholine can stimulate, rather than inhibit, a small subset of aortic baroreceptor afferents (33% of the firing units) by directly activating nicotinic receptors, whereas it inhibits most baroreceptor afferents (66% of the units) by indirectly contracting vascular smooth muscle via stimulation of muscarinic receptors (372). Vascular muscle tone is an important factor that determines the direction of drug effects on baroreceptor responses. The facilitatory effect of a low concentration of acetylcholine on baroreceptor activity secondary to vasorelaxation is prevented or augmented in the pre-relaxed or pre-contracted aortic arch, respectively (372). Similarly, intravenous norepinephrine excites or inhibits aortic baroreceptor afferents under pre-distension or pre-constriction of the aortic arch, respectively (1). Finally, the pharmacological effects of many agents can vary depending on the type of baroreceptor afferent stimulated. Angiotensin II inhibits aortic baroreceptor afferents by inducing aortic arch vasoconstriction (374), but it does not affect carotid baroreceptor afferents (344). PGI2 inhibits high-pressure carotid baroreceptor activity but does not act on cardiopulmonary low-pressure baroreceptors (508).
Pharmacology of baroreceptor-modulation of nociception:
Pharmacologic studies using systemic administration or microinjections of drugs in selected CNS structures have shown that opioid receptors and adrenoceptors modulate baroreceptor-mediated physiological responses, including nociception. The opioid receptor antagonist naloxone blocks the hypoalgesia observed in both genetically- and experimentally-induced hypertensive rats (326, 383, 462, 463, 501, 604, 607). In hypertensive rats that show elevated opioid activity in the spinal cord and CNS structures (605, 607), the systemic administration of naloxone blocks pressor-induced baroreflex bradycardia evoked by the stimulation baroreceptor afferents in rabbits (578). The intravenous administration of opioids in normotensive rats produces a profound hypoalgesia, which is dependent on intact vagal afferents (429–431). Hypertensive patients who are less sensitive to experimentally administered noxious stimuli have increased concentrations of circulating endorphins (494), but naloxone does not block this association (343, 479).
Several animal studies support the involvement of adrenoceptors in baroreceptor-mediated physiological processes. Systemic administration of the β-adrenergic receptor antagonist propranolol lowers AP and reverses the hypoalgesia observed in spontaneously hypertensive rats, whereas the α2-adrenergic agonist clonidine does not (463). However, the direct administration of the α2-adrenergic receptor agonist clonidine into the NTS results in naloxone-sensitive hypotension, bradycardia, and analgesia in spontaneously hypertensive rats and some normotensive rat strains (286). Finally, the NTS, RVLM, and CVLM, which are relay nuclei for baroreflexes, contain noradrenergic and adrenergic neurons (414, 521). Although there is no direct evidence in human subjects, elevated AP within the normotensive range is associated with increased pain tolerance along with higher circulating levels of norepinephrine (453).
Baroreceptor Modulation of Non-Cardiovascular Functions
In the late 1970s and early ‘80s, Lacey and Lacey (288, 289) postulated that natural variations in arterial baroreceptor activation produce changes in cortical inhibition, which creates differences in sensorimotor performance across the cardiac cycle. More recently, investigations have further characterized and described the complexity of this baroreceptor-mediated process. The following sections review the three more extensively studied non-cardiovascular functions modulated by baroreflexes: pain perception, consciousness, and cognition.
Baroreceptor Regulation of Pain and other Sensory Perceptions
In this section, we describe the evidence supporting a role for baroreceptors in modulating the perception of pain and its physiological mechanisms. TABLE 5 summarizes the content of this section.
TABLE 5:
Summary of baroreceptor regulation of pain and other sensory perceptions.
| • Hypoalgesia occurs in rats following an acute rise in AP and chronic hypertension. • Low BRS allows the development of neuropathic pain in rats. • Acute baroreceptor stimulation during systole or neck suction reduces experimental pain ratings and pain-evoked EEG potentials in humans. • Chronic hypertension and hypotension are associated with hypoalgesia and hyperalgesia, respectively. • Chronic pain patients have low BRS. • The inverse relationship between baroreceptor function and pain is altered by resting systolic and diastolic AP levels, pain modality, type of vagal afferent activated, and the presence of a chronic pain condition. • Parasympathetic efferents exert an anti-inflammatory influence, whereas the sympathetic activity is mostly pro-inflammatory. • Baroreceptors modulate parasympathetic and sympathetic outflow to regulate inflammation and associated pain. • Orthostatic reduction in AP increases pain perception by hormonal (non-baroreceptor) mechanisms, such as activation of the renin-angiotensin-aldosterone system, and subsequently, stimulation of angiotensin II type-2 receptors. • Baroreceptors exert a gating of nociception at brainstem nuclei and cortical/subcortical structures. • Both AP levels and pain are under the influence of variations in the gene encoding catechol-O-methyl-transferase (COMT). • Although pain is not always associated with an increase in AP, nociceptive stimuli can increase in AP and heart rate, and the subsequent baroreceptor activation is a compensatory negative feedback to avoid an exaggerated cardiovascular response to pain. • Baroreceptors modulate other sensory inputs, including touch, cold, heat, or non-cutaneous senses, vision, and hearing. • The physiological mechanisms underlying the association between hypoalgesia and increased arterial blood pressure or baroreceptor activation have not been elucidated. However, current evidence suggests central and peripheral mechanisms. • At the level of the brainstem, baroreflex input to the NTS inhibits pronociceptive ON-cells and excites antinociceptive OFF-cells in the rostroventral medial medulla via projection to the lateral parabrachial nucleus, which leads to enhanced descending control on pain transmission within the dorsal spinal cord. • Baroreceptor input also modulates the neural activity within cortical and subcortical structures to modulate the different dimensions of pain, such as the cognitive aspects of pain perception, by influencing the neural activity of the anterior insula. • Peripherally, baroreflexes indirectly regulate tissue inflammation by modulating parasympathetic and sympathetic outflow. For example, inflammatory mediators activate vagal afferents and trigger the vagal anti-inflammatory reflex that attenuates the production of pro-nociceptive molecules. |
Blood pressure influence on pain.
Numerous studies have reported that increases in either arterial or venous blood pressure and BRS are associated with hypoalgesia in laboratory animals and humans; see review by Suarez-Roca et al. (515). Briefly, hypoalgesia has been observed in rats in the following conditions, (a) acute increase in AP in response to vasopressor agents (154) or abdominal aortic occlusion (545), (b) experimentally-induced chronic hypertension by renal artery clipping or increasing dietary salt in salt-sensitive animals (607), (c) genetically induced spontaneous hypertension in rats (326), (d) elevation of venous pressure by volume expansion with activation of low-pressure cardiopulmonary baroreceptors (324, 363, 431), and (e) pharmacological activation of baroreceptor afferents with intravenously administered morphine, met-enkephalimamide, or other vagal afferent stimulants (429–431). Moreover, only those rats that exhibit reduced baroreceptor function develop neuropathic pain 2 – 3 weeks after nerve ligation (188). Finally, like baroreceptor afferents, the activation of cranial vagal afferents with long-term auricular electrical stimulation excites the NTS and prevents the development of thermal hyperalgesia and mechanical allodynia in Zucker diabetic fatty (fa/fa) rats by enhancing central serotoninergic activity (303).
In humans, several experimental findings support an inverse relationship between baroreceptor activity and pain. Pain sensitivity decreases as resting AP increases in healthy normotensive individuals (63, 64, 170, 171, 207, 343, 411). Pain perception diminishes during systole, i.e., maximal baroreceptor load, compared to diastole (153, 156, 157, 163). The direct activation of carotid baroreceptors reduces electrically-induced pain ratings and somatosensory-evoked cortical potentials (N150-P260) in normotensive subjects (15). In agreement, activation of carotid baroreceptors with phase-related external neck suction (systole simulation) decreases electrically induced cutaneous pain ratings (138, 153). Clinical evidence also supports an inhibitory influence of baroreceptors on pain perception in humans. Chronic hypertensive patients experience hypoalgesia (189, 190, 606), whereas subjects with chronic hypotension display thermal hyperalgesia (142). Finally, it has been recently reported that chronic pain patients display significantly lower BRS (66).
A controversial issue is the hypoalgesia observed in four cases, (a) borderline hypertension (189), (b) normotensives with a family history of hypertension (8, 9, 135, 136, 174, 175), (c) after resetting of baroreceptors during hypertension, and (d) before the expression of arterial hypertension in spontaneously hypertensive rats (326). In these cases, cardiopulmonary baroreflex mechanisms could also explain hypoalgesia in normotensives, such as an early increase in venous pressure preceding the onset of chronic hypertension, which stimulates low-pressure cardiopulmonary baroreceptors and induces hypoalgesia before the development of hypertension (326, 332, 603).
Physiological factors moderating baroreceptor modulation of pain.
Several physiological factors moderate the magnitude and the direction of baroreceptor modulation of pain perception, including (a) resting systolic and diastolic AP, (b) pain modality and dimension, (d) type of activated vagal afferent, and (e) the presence of a chronic pain condition. Resting AP levels modifies baroreceptor modulation on pain in a complex manner. Several studies have shown that systolic AP has a stronger influence on pain perception than diastolic AP (63, 205, 207, 343, 377, 490). Moreover, resting systolic AP and spontaneous BRS (under resting conditions) correlate inversely with the inhibition of suprathreshold cold-pressor pain (146) and threshold electrical pain in normotensive (15).
Conversely, resting DAP shows a positive correlation with threshold electrical pain in normotensive (15). Resting AP levels also influence the effect of direct mechanical baroreceptor stimulation on pain. FIGURE 9 shows that neck suction activation of carotid baroreceptor reduces pain-evoked cortical potentials and subjective pain scores in subjects with higher (above group mean) resting systolic AP; in contrast, individuals with lower (below the group mean) systolic AP exhibit either opposite effects (59) or are not affected at all (15). Also, carotid baroreceptor stimulation exerts an inhibitory influence on pain perception in borderline hypertensives (systolic AP, 130 – 160 mmHg) by prolonging the latency for electrical pain detection, whereas it has opposite effects on normotensive subjects with relatively lower AP (163).
FIGURE 9.
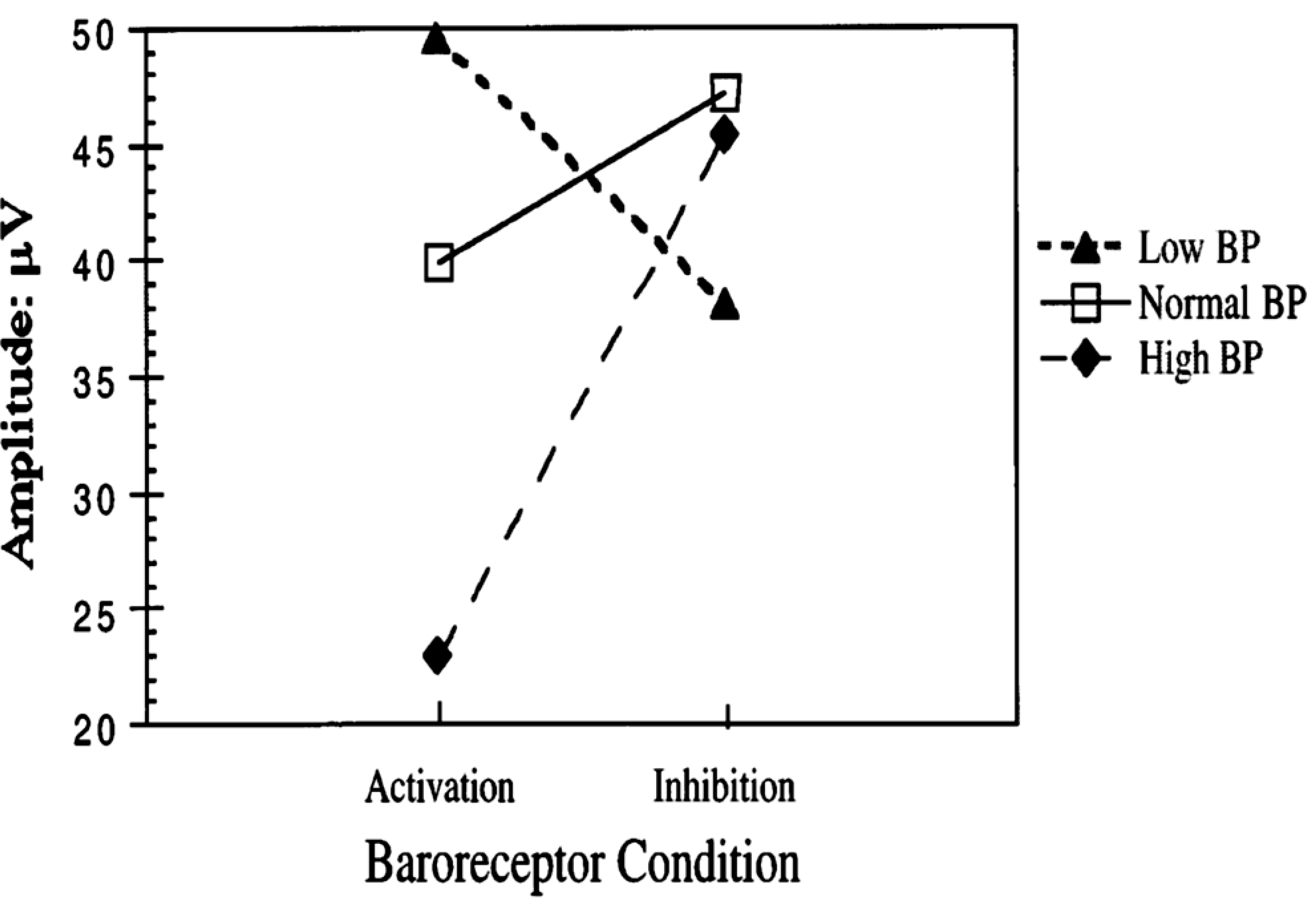
Baroreceptor activation and inhibition by external mechanical manipulations of the neck have an opposite influence on pain-evoked potentials in the three groups of normotensive subjects with relatively low, normal, and high resting AP levels. Compared with the inhibitory condition, baroreceptor activation decreases and increases the amplitude of pain-evoked potentials in normotensive subjects with relatively high and low AP, respectively (59).
Baroreceptor modulation of experimentally evoked pain also depends on the sensory modality, i.e., thermal, electrical, mechanical, or ischemic. Neck suction baroreceptor activation decreases the sensitivity to threshold mechanical pain, but it does not alter the threshold for thermal pain detection in normotensive and non-treated labile hypertensive subjects (434). Similarly, stimulation of baroreceptors by neck phase-related external suction reduces subjective pain ratings to electrical stimuli (138), but it does not affect electrical detection thresholds (138, 257). This modality-dependent baroreceptor modulation of pain perception could be due to the type of baroreceptor afferent being stimulated. Generally, anti-nociceptive effects take place after high-intensity electrical stimulation that mainly activates non-myelinated vagal afferent C-type fibers, whereas pro-nociceptive effects occur following low-intensity electrical stimulation that likely excites A-type fibers (55, 387, 438).
Finally, ongoing chronic pain impairs the relationship between AP levels, presumably through impairments in baroreceptor function and pain perception. Chronic low back pain patients report greater electrically-evoked cutaneous pain during neck suction stimulation of carotid baroreceptors than pain-free patients (59). Similarly, there is a positive relationship between resting diastolic AP and ischemic pain in patients with chronic low back pain (65). Maixner et al. reported impairment in the relationship between resting AP and the perception of ischemic and thermal pain perception in patients with temporomandibular disorders (321). Conditioned pain modulation values, a marker of the strength of endogenous pain regulatory systems, are inversely correlated with reports of acute and chronic postoperative pain (596) and are inversely associated with persistent post-endodontic pain (386). Thus, altered baroreceptor inhibitory modulation of pain in chronic pain conditions may contribute to the general dysregulation of pain perception observed in acute and chronic pain conditions.
Parasympathetic modulation of inflammation.
Acute inflammation results from the production of diverse local mediators, including but not limited to cytokines, chemokines, and essential fatty acids derivatives that promote leukocyte recruitment to the site of injury (249). In healthy subjects, acute inflammation is self-limited through an active resolution mechanism that requires the synthesis of specialized pro-resolving mediators (SPMs) and other molecules (484). The autonomic nervous system can influence the initiation and resolution of acute inflammation by changing the balance and tone of the parasympathetic (484) and sympathetic (415) branches. A review by Serhan et al. (484) summarizes the current evidence supporting the anti-inflammatory effect of parasympathetic vagal activation. Right vagotomy increases the magnitude of acute infectious peritonitis in mice by causing amplified neutrophil infiltration, reduces bacteria phagocytosis by leukocytes, and increases exudate bacterial counts (119, 358). Right vagotomy delays the resolution phase of both acute sterile (zymosan) and infectious (Escherichia coli) peritoneal inflammation in rodents (119, 358). Vagotomy-induced delay in resolution occurs by reducing the local expression of netrin-1, an axonal guidance molecule and resolution activator (358), and the secretion of pro-resolving mediators, including the protectin conjugate in tissue regeneration 1 (PCTR1) from Group 3 innate lymphoid cells, (119) which are involved in regulating both innate and adaptive immune responses (364).
Conversely, the pharmacological and electrical stimulation of the vagus nerve diminishes the production of TNFα serum and heart, but not lungs, in rats (41). In the rat, electrical vagal stimulation attenuates endotoxin-induced production of TNFα in the spleen and liver but not in the lung (232). In humans, repeated electrical stimulation of the vagus reduces LPS-induced release of pro-inflammatory cytokines from whole-blood in vitro and significantly attenuates the disease’s severity in rheumatoid arthritis patients (276). Experimental evidence indicates that acetylcholine mediates vagal modulation of inflammation. Acetylcholine and nicotine attenuate the release of TNFα, IL-1β, IL-6, and IL-8 from LPS-stimulated human macrophage cultures, whereas it does not alter the activity of anti-inflammatory cytokines IL-10 and transforming growth factor-β (52). These cholinergic agonists inhibit the synthesis of proinflammatory cytokines post-transcriptionally (52). Acetylcholine released from vagal efferents binds and stimulates the nicotinic acetylcholine receptor subunit α7 (α7nAChR) to inhibit the intracellular cascade that is involved in the release of proinflammatory cytokines (549, 550). Mice deficient in the gene encoding α7nAChR (Chrna7) exhibit a blunted cytokine response to endotoxemia, suggesting that an inhibitory cholinergic tone on innate immune responses (573). Clinical evidence supports the translational value of this observation as observed in a randomized, double-blind study where transdermal nicotine reduced the severity of ulcerative colitis (423), and nicotine enemas diminished the Crohn’s disease activity index (238).
The activation of innate immune cells by tissue injury and pathogenic agents induces the release of cytokines and other pro-inflammatory molecules, which stimulate sensory vagal afferents (549, 550) that are capable of sensing low threshold levels of inflammatory mediators at the site of tissue injury even before a rise in circulating concentrations (549). Subdiaphragmatic vagotomy (212) or administration of selective IL-1 antagonist (194) prevents the onset of fever induced by the intraperitoneal injection of IL-1. Of note, vagal afferent discharges activate neuronal pathways in the NTS, which results in stimulation of vagal efferents and parasympathetic suppression of the release of pro-inflammatory cytokines (549, 550), prostaglandins, leukotrienes (485), and increases the local production of SPM at the site of injury (485). In addition to the SPM produced by inflamed tissue, the vagus nerve provides another source of SPM involved in an anti-inflammatory reflex. Electrical stimulation of postmortem of human vagus nerves produces SPMs (RvE1, NPD1/PD1, MaR1, RvD5, and LXA4), whereas freshly dissected mouse vagus nerves release a different SPM profile (RvD3, RvD6, and RvE3), demonstrating a species-selectivity (485). On the contrary, electrical vagus stimulation decreases pro-inflammatory prostaglandins (PGD2, PGE2, and PGF2α), as well as leukotrienes, chemoattractant LTB4, and slow-reacting substances LTC4, LTD4, and LTE4 (485). Similar to electrical stimulation, the incubation of human and murine vagus nerve with Escherichia coli increases the production of SPMs (485).
Alteration in autonomic tone precedes the onset of chronic inflammatory diseases. Among individuals at risk of developing rheumatoid arthritis (i.e., asymptomatic carriers of autoimmune antibodies), those with relatively elevated resting heart rates subsequently developed arthritis, which suggests an early imbalance in parasympathetic/sympathetic tone (277). Alterations in peripheral nicotinic receptors (α7nAChR) contribute to autonomic parasympathetic dysfunction because individuals at risk for rheumatoid arthritis, who have elevated resting heart rates, show a reduction in the expression of α7nAChR on circulation monocytes (277), which impairs the cholinergic anti-inflammatory influence of parasympathetic efferents. Stimulation of α7nAChR attenuates proinflammatory cytokine (e.g., TNFα, IL-1β) production in whole human blood and human monocytes (62, 454). Also, the activation of parasympathetic cholinergic efferents by electrically stimulating the vagus nerve or by direct pharmacological activation of peripheral α7nAChR improves arthritis, reduces cytokine production, and protects against progressive joint destruction (278). Alpha7nAChR knockout mice display an increase in arthritis disease activity (554). These findings suggest that augmenting cholinergic parasympathetic activity may prove effective in treating rheumatoid arthritis and other inflammatory pain conditions. It is plausible that inflammatory mediators stimulate vagal afferents and, thus, trigger functionally opposing sympathetic reflex responses at local and systemic levels (415).
Sympathetic modulation of inflammation.
Indeed, the sympathetic system promotes inflammatory responses through the peripheral release of norepinephrine (415). Sympathetic efferents directly influence inflammation through the modulation of immune cells expressing adrenoceptors (381). Norepinephrine increases the LPS-elicited rise in plasma TNFα by activation of α2-adrenoceptors on murine macrophages (506). Stress-induced sympathetic activation increases the production of immature, proinflammatory monocytes in the bone marrow by stimulation of β-adrenoceptors (420). β2- and β3-adrenoceptors blockade reduces the secretion of pro-inflammatory and pro-nociceptive cytokines in rodents (378, 529, 535). In humans, there is a positive correlation between sympathetic tone and circulating levels of proinflammatory TNFα (421) and IL-6 (42). Sympathetic efferent activation indirectly enhance inflammation by decreasing lymphatic flow to immune organs in complex regional pain syndrome patients (228).
There is also evidence that sympathetic activation can produce complex effects on inflammatory responses that are tissue and stimulus-dependent. Bilateral cervical vagotomy does not affect the LPS-induced rise in circulating TNFα in the rat, whereas sympathectomy (by cutting greater splanchnic nerves) enhances it, which suggests a substantial anti-inflammatory sympathetic mediated effect (334). Selective sympathetic denervation of the intestine, but not vagotomy, augments the clinical signs of chemically-induced colitis in mice, whereas stimulation of the sympathetic intestinal nerves reduces the clinical manifestations of colitis (169, 582). Pharmacological sympathectomy (by pre-treatment with reserpine) enhances LPS-evoked elevation of TNFα plasma levels (525). The non-selective adrenergic receptor antagonist phentolamine increases neurogenic microvascular response to antidromic stimulation (353), suggesting a tonic sympathetic inhibitory influence on cutaneous inflammation.
The complexity of the sympathetic modulation likely results from the ability of norepinephrine to activate functionally opposing mechanisms in a concentration-dependent manner. Norepinephrine produces a dose-dependent biphasic modulation of cytokine production in immune cells; at low norepinephrine concentrations (0.1 μM) increases LPS-induced mRNA expression levels of pro-inflammatory cytokines IL-1β, IL-6, IL-12, and TNF-α in murine bone marrow-derived macrophages (583). In contrast, higher norepinephrine concentrations significantly decrease the expression and release of cytokines in mice, whereas an opposite effect is exerted on the anti-inflammatory cytokine IL-10 (583). High concentrations of the β2-adrenoceptor agonist salbutamol mimic the inhibitory effect of high norepinephrine concentrations (583). Similarly, the non-selective β-adrenoceptor agonist isoproterenol inhibits LPS-evoked rise in TNFα plasma levels by a mechanism sensitive to β-adrenoceptor antagonist propranolol (525).
Loss of sympathetic innervation also plays a role in chronic inflammation. In humans, there is a reduced density or a dysfunctional sympathetic innervation in some chronic visceral inflammatory conditions, such as Crohn’s disease and intestinal areas adjacent to endometrial lesions, which correlates with the severity of pain and other clinical features (47, 169, 512). Similarly, sympathetic innervation density is lower in fresh synovial tissue from rheumatoid arthritis patients compared with osteoarthritis patients and other controls (577). In animal models of arthritis, the loss of sympathetic innervation is due to macrophage production of the nerve repellent factors of sympathetic nerve fibers semaphorin 3C, and SEMA3F and TNFα (285).
The effects of the sympathetic nervous system on inflammatory responses depend on the stage of the inflammation. Sympathectomy performed at different time points during the development of type II collagen-induced arthritis in mice provides evidence that early sympathetic ablation lowers arthritis scores and increases tissue anti-inflammatory cytokines, whereas late sympathectomy increases both arthritis scores and tissue pro-inflammatory cytokines (213). This biphasic effect of sympathectomy suggests that sympathetic efferents exert an early pro-inflammatory and late anti-inflammatory modulation (415). Moreover, Pongratz and Strauban suggested that the early pro-inflammatory sympathetic activity takes place in lymphoid organs (e.g., spleen and lymph nodes), whereas the late anti-inflammatory sympathetic influence occurs at the site of local inflammation (415). The mechanisms involved in this complex sympathetic modulation of the inflammatory responses depend on changes in the pattern of sympathetic discharge across time that result in different local and systemic norepinephrine levels, e.g., initial low pro-inflammatory concentrations followed by late high anti-inflammatory concentrations. Also, a reduction in the local sympathetic innervation density at late stages of inflammation, with intact sensory innervation (i.e., preserved neurogenic inflammation), can cause an uncoupling from central nervous inhibitory regulation and an autonomic-sensory imbalance that marks the turning point from acute to chronic inflammation.
Baroreceptor modulation of inflammation.
Very few studies have directly evaluated whether baroreceptors can influence host inflammatory response to injury and infection. Animal studies have found that activation of aortic baroreceptors by electrical stimulation of aortic depressor nerve reduces neuroinflammation in response to LPS challenge by a mechanism that is independent of hemodynamic changes (60). Similarly, electrical stimulation of the carotid sinus nerve attenuates circulating markers of systemic inflammation in conscious rats, suggesting a modulation of the innate immune response to an endotoxemic challenge (470). Conversely, several clinical studies show that patients with carotid atherosclerosis (553), subclinical hypothyroidism (524), and pregnancy-induced hypertension (516) exhibit impaired baroreceptors function (reduced BRS) along with elevated low-grade inflammation markers. Baroreceptors modulate both the parasympathetic and sympathetic autonomic systems, and thus, they exert a dual control on inflammation. Thus, manipulation of baroreceptor activity could be a therapeutic strategy for the management of chronic inflammatory pain conditions.
AP modulation of pain by hormonal mechanisms:
Hormonal mechanisms also contribute to the modulation of chronic pain by changes in AP, although it could be different in normotensive vs. hypertensive subjects. In healthy young males, a 70o tilt-up postural maneuver increases the perception of electrically-evoked pain, as shown by reduced thresholds for nociceptive C/Aδ-type fibers stimulation (496). Long-lasting upright posture produces two primary physiological responses. First, there is a decrease in plasma volume (due to lower extremities blood pooling), venous blood return, cardiac output, and systolic AP, which leads to cardiopulmonary and arterial baroreceptor unloading that is accompanied by increases in heart rate and plasma catecholamines. (155, 243) Second, there is an activation of the renin-angiotensin-aldosterone system reflected by elevated plasma renin activity, aldosterone, and vasopressin (155, 243). Thus, it is plausible that, in addition to baroreceptor-mediated mechanisms, the activation of the renin-angiotensin-aldosterone system (with increased levels of angiotensin II) can contribute to the hyperalgesia observed in upright posture-evoked changes in AP levels.
The blockade of the renin-angiotensin-aldosterone pathways with angiotensin II type-2 receptor antagonists elicits analgesic effects on reflexive measures of mechanical pain hypersensitivity in animal models of neuropathic and cancer pain (4, 375). Of note, angiotensin II type-2 receptor antagonists diminish ongoing neuropathy pain-like behaviors in mice (493) and thermal and mechanical hypersensitivity under conditions of chronic inflammation (82), although they do not alter responses to acute inflammatory induced mechanical and cold pain (493). Angiotensin II concentration increases at the site of sciatic nerve injury, which leads to the activation of angiotensin II type-2 receptors located on infiltrating macrophages (492). The blockade of angiotensin II receptors, the chemogenetic depletion of the macrophages, or the transplantation of bone marrow tissue lacking angiotensin II receptors attenuates the neuropathic mechanical and cold pain hypersensitivity in mice (492). Similarly, the pharmacological blockade of angiotensin II type-2 receptors attenuates both thermal and mechanical hypersensitivity in chronic inflammation in rats (82). A phase II clinical trial provides supporting translational evidence because the angiotensin II type-2 receptor antagonist EMA401 ameliorates pain in post-herpetic neuralgia patients (448). Finally, a human exome sequencing study has shown that the angiotensin pathway is enriched in individuals responsive to noxious stimuli (585), providing further evidence that angiotensin pathways contribute to pain perception.
In chronic hypertensive subjects, there is an increased threshold (hypoalgesia) and tolerance to dental pain, which is reversed by 4–8 month antihypertensive treatment with the angiotensin-converting enzyme inhibitor enalapril (206). However, the enalapril’s anti-hypoalgesic effect is not related to the normalization of AP (i.e., unloading arterial baroreceptors) since the reduction of AP for 3-months with diuretics or β-blockers does not restore dental pain sensitivity in hypoalgesic hypertensive patients (189). Similarly, the inhibition of angiotensin II synthesis or its receptors abolishes the analgesia observed in spontaneously hypertensive rats, and these anti-hypoalgesic effects are independent of changes in AP (240). Shimoda et al. (496) suggested that the activation of the renin-angiotensin-aldosterone system can contribute to the regulation of pain perception when moving to an upright posture. Angiotensin II might evoke a pro-algesic effect in normotensives and analgesic action in chronic hypertension. The current lack of explanation for this difference warrants further investigation.
Role of brainstem mechanisms in baroreceptor modulation of pain.
Although the neural mechanisms by which baroreceptors modulate pain have not yet been elucidated, it is well-known that it requires baroreceptor afferent input. The hypoalgesia observed under hypertensive conditions (154, 324, 350, 432) and following circulatory volume expansion (324, 363, 431) is attenuated or abolished by impairing the vagal afferent limb of the cardiopulmonary baroreflex system. Activation of arterial and cardiopulmonary baroreceptors initiates a cascade of physiologic events, which generate signals that travel to the NTS (FIGURE 10). Afferent neurons from the NTS activate or inhibit neuronal brainstem networks originating in the PAG, rostral ventromedial medulla (RVMM), and reticular formation, which integrates the descending modulation of spinal pain transmission and autonomic output (23, 239, 429, 431, 471). For example, the natural or experimentally induced changes in AP correlate with spontaneous fluctuations of pro-nociceptive ON-cells and anti-nociceptive OFF-cells in the RVM (546, 547). Resting MAP negatively and positively correlates with the spontaneous fluctuations in ON-cells and OFF-cells activities, respectively, and this relationship is not affected by cardiopulmonary vagal deafferentation (547). In contrast, intact cardiopulmonary vagal innervation is required for experimentally-induced increases in MAP that diminishes the activity of pro-nociceptive ON-cells and augments the activity of antinociceptive OFF-cells in rats (547). These findings suggest that baroreceptor activity evoked by phasic changes in AP (but not by resting AP levels) modulates ON-cell and OFF-cell activity (547). Of note, baroreceptor stimulation activates neurons in the mediocaudal NTS (mcNTS) that send inhibitory projections to a subset of neurons in the lateral parabrachial nucleus (lateral PBN) (247), which exerts a net GABAergic inhibition on anti-nociceptive OFF-cells and a net glutamatergic excitation on pro-nociceptive ON-cells in the rostral ventromedial medulla (91)(FIGURE 10A). Thus, inhibition of the lateral PBN by baroreceptor-activation of the mcNTS disinhibits OFF-cells and diminishes the excitation of ON-cells in RVMM, resulting in a reduction of nociceptive reflexes at the spinal level. Of note, the activity of wide-dynamic-range and high-threshold nociceptive neurons is lower in chronically hypertensive rats (433), and descending inhibition is higher during acute increases in AP in rats (545). In addition, the stimulation of cervical vagal afferents results in inhibition of both the spontaneous and somatically evoked firing in thoracic spinoreticular neurons in cats (539, 540). Electrical stimulation of cardiac vagal afferents blunts the digastric sensorimotor reflex evoked by noxious tooth-pulp stimulation in cats by inhibiting nociceptive neurons in the trigeminal nucleus (55, 319).
FIGURE 10.
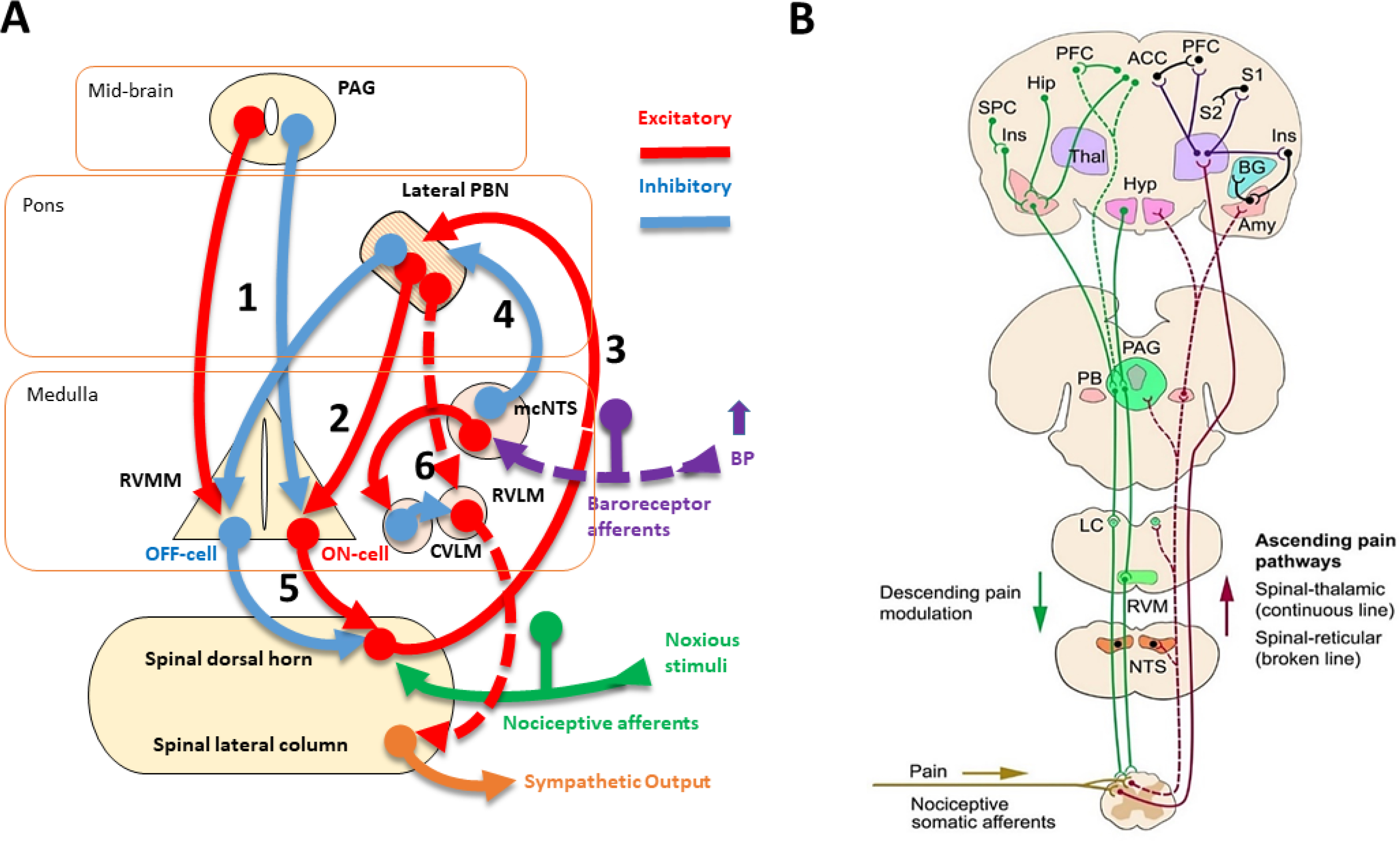
Interactions between baroreceptors and pain pathways. Panel A. Rostral ventromedial medulla (RVMM) receives two major modulatory inputs from 1) the periaqueductal grey (PAG) that inhibits ON-cells (pro-nociceptive) and excites OFF-cells (anti-nociceptive), underlying stress- and placebo-induced analgesia, and 2) the lateral parabrachial nucleus (lateral PBN) that excites ON-cells (pronociceptive) and inhibits OFF-cells (anti-nociceptive) in the RVMM, facilitating pain reflexes. 3) The superficial lamina of the spinal cord projects to the lateral PBN, which facilitates pain by acting on the RVMM. 4) The mediocaudal nucleus of the solitary tract (mcNTS) projects to the lateral PBN, which inhibits a subset of neurons in the lateral PBN following inputs from vagal baroreceptor afferents. 5) Inhibition of lateral PBN neurons reduces and increases OFF-cell and ON-cell activities in the RVMM, respectively, facilitating C-fiber-driven second-order spinal neurons. 6) The rostral ventrolateral medulla (RVLM) neurons receive a dual input: inhibitory from the mcNTS via caudal ventrolateral medulla (CVLM) that leads to baroreflex-mediated bradycardia and excitatory input from the Lateral PBN that leads to pain-induced tachycardia. Panel B. Ascending and descending pain pathways in the CNS. Noxious stimuli applied to somatic structures and inflamed viscera activate primary afferents, which stimulate second-order spinal neurons of lamina I and V, which give rise to the ascending spinothalamic (continuous red line) and spinoreticular/spinobrachial (broken red line) pathways that reach PAG, parabrachial nucleus (PB), locus coeruleus (LC), cerebellum, and thalamus in humans. The dorsal horn also sends direct nociceptive information to the NTS. PB and PAG afferents project to the amygdala (Amy) and nucleus accumbens in the basal ganglia (BG), whereas thalamic afferents project to the primary somatosensory (SM) cortex S1, secondary SM cortex S2, anterior cingulate cortex (ACC), prefrontal cortex (PFC), and insula (Ins). Multiple descending pathways from brain structures to PAG and rostroventral medulla (RVM) modulate different components of pain perception, such as the ACC-PFC-PAG circuitry related to placebo analgesia and unpleasantness (broken green line) and the superior parietal cortex (SPC)-insula-amygdala-PAG pathway related to modulation of pain by attention (continuous green line). BG, basal ganglia; Hip, hippocampus; Hyp, hypothalamus; Thal, thalamus; PB, parabrachial nucleus.
Other brainstem structures are also involved in baroreceptor-mediated antinociception. The periaqueductal gray (PAG) receives dual input from nociceptors and baroreceptors (69, 74, 260). The PAG, along with RVMM, integrates outputs from the anterior cingulate cortex to modulate the emotional features of pain (69). Although the PAG’s role in baroreceptor-mediated analgesia has not been extensively examined, activation of hypothalamic-brainstem PAG circuits during the defense reaction “clamp” brainstem-mediated baroreceptor reflexes resulting in a behavioral hypoalgesia with a concomitant increase in AP and heart rate (i.e., cardiovascular defense reaction).
Cortical and subcortical mechanisms and baroreflex modulation of pain.
In addition to brainstem mechanisms, baroreceptors can modulate pain via ascending pathways that project to higher CNS structures involved in pain processing (FIGURE 10B). It is well-established that nociceptive input is conveyed, either directly or indirectly through the thalamus, to the amygdala, prefrontal cortex, and insula (69), which receive baroreceptor input and integrate cardiovascular responses to internal and external environmental events (74, 260). Descending modulatory pathways originating from the prefrontal cortex mediated placebo analgesia, whereas pathways arising from the superior parietal cortex, insula, amygdala contribute to attentional influences on pain perception (69). It is not clear whether baroreceptor activity affects nociceptive and pain processing through these higher CNS structures. Behavioral studies also suggest that higher CNS processing of baroreceptor input has effects on pain perception. Specifically, the cue-induced expectancy of the painfulness before an electrical stimulus results in the activation of higher-order CNS processes indexed by enhanced pain-evoked event-related P2 potentials, which is blunted by baroreceptor activation during systole (200). Thus, the baroreceptor gating of nociception is not only located at the brainstem nuclei but also manifests in cortico-limbic structures such as the anterior insula (200).
Cardiorespiratory vagal input reaches the anterior insula via polysynaptic projection from the NTS (33) to the parabrachial nucleus and the thalamus (281, 422). The anterior insula is a region within the primary interoceptive cortex that integrates visceral and somatic sensory signals with other brain regions and generates emotional states (115, 116, 513). Besides, functional MRI studies in humans have found that the anterior insula integrates pain intensity and the expectation of painful experience (168). Thus, baroreceptor input may either inhibit or enhance the cognitive aspects of pain perception by influencing the neural activity within the anterior insula.
Finally, baroreceptors may influence the emotional aspects of pain, especially in the presence of clinical depression. There is a strong association between chronic pain and clinical depression (491). Preliminary clinical trials have found improvement of clinical depression following vagal stimulation, and animal studies indicate this nerve stimulation affects many of the same brain areas, neurotransmitters, and signal transduction mechanisms as those found with traditional antidepressants (76). Thus, it is plausible that carotid baroreceptor stimulation can influence the emotional dimension of pain (e.g., unpleasantness).
Gene regulation of baroreceptor modulation of pain.
Twin studies point out that genetic factors mediate around 40% of BRS variability (178, 531). Similarly, twin studies show genetic factors contribute to approximately 30% of the variability in heat and cold pressor pain thresholds (16, 388); the only study examining BRS measurements and heat and cold pain tolerance assessments in twins did not find a correlation between BRS and pain perception (178). In humans, both AP levels and pain are under the influence of variations in the gene encoding the enzyme catechol-o-methyl-transferase (COMT). Individuals with the single nucleotide polymorphism of COMT Met158Met have diminished μ-opioid system responses to pain in several brain structures and increased pain perception (612), as well as lower COMT enzyme activity (290). On the other hand, human subjects who harbor high-activity COMT haplotypes display reduced sensitivity to acute experimental pain (128). Regarding chronic pain conditions, homozygotes for a variant in the COMT gene that codes for high COMT enzyme activity (290) show a lower prevalence of non-migrainous headache (210) and fibromyalgia (208). Similarly, individuals who harbor polymorphisms that code for high-activity COMT enzyme are less sensitive to noxious stimuli and have a lower risk of developing myogenous temporomandibular joint disorder (128).
COMT genetic variations also affect AP values. Individuals who are homozygotes for the variant Val158Val resulting in high COMT enzyme activity (290) have a higher prevalence of increased systolic AP compared to individuals without this variant (209). Although systemic pharmacological inhibition of COMT does not change AP and heart rate at rest nor during exercise in healthy volunteers with intact baroreflexes (237), it elicits a pressor response under impaired baroreflex conditions (252). Thus, it is plausible that COMT polymorphism can influence the relationship between baroreceptor function and cardiovascular dynamics.
Effects of nociceptive input on cardiac baroreceptor pathways.
Reciprocally, nociceptive stimulation influences cardiovascular function and can induce an increase in AP and heart rate. The anatomical basis of this reciprocal relationship results from the activation of ascending spinal-reticular projections from nociceptive spinal neurons to key brainstem autonomic nuclei, remarkably the NTS (53, 114, 184) and lateral PBN (40), which modify cardiovascular function (FIGURE 10B). The lateral PBN receives direct nociceptive input from the contralateral spinal superficial lamina (40); in turn, the lateral PBN sends direct excitatory projections to RVLM that counteract the indirect inhibitory influence of the NTS (via the CVLM), which disinhibits the RVLM, and as a result, impedes baroreceptor-mediated bradycardia (299, 300), increases sympathetic output, and elicits a nociceptive-evoked tachycardia (53). Although pain is not always associated with an increase in AP, the inhibitory effect of baroreceptor stimulation on pain perception may occur in parallel with the classical compensatory negative feedback process that restores excessively elevated AP and heart rate to baseline (153).
Baroreceptor modulation of other sensory systems.
It is not clear whether baroreceptors modulate other somatic sensations, such as touch, cold, heat, as well as vision, and hearing. Sensory detection thresholds, sensory evoked potentials, and stimulus-response reaction times have been measured under externally evoked stimulation of the baroreceptor and during the cardiac cycle where the systole and diastole phases of the cardiac cycle generate natural alternative increases and reductions in baroreceptor activity. Visual-evoked potentials have a lower amplitude either during systole (569) or following direct carotid sinus stimulation with serotonin (272). Also, slower reaction time responses to visual stimuli occur during systole compared to diastole (158, 579). Similarly, auditory sensitivity is reduced (474), and reaction times are slower to auditory stimuli delivered 300 ms after the R-wave, i.e., during systole, compared to stimuli presented 600 ms post-R-wave, i.e., during diastole (46, 158).
In cats, electrical stimulation of vagal afferents arising from the aortic arch diminishes neuronal activity evoked by light touch and non-noxious electrical skin stimulation in the cuneate nucleus and medial lemniscus, which is thought to be mediated by presynaptic inhibition of primary sensory afferents (183). However, the influence of baroreceptors on tactile (non-painful) perception in humans is controversial. Healthy humans subjected to a 70o tilt-up postural change, which physiologically decreases BRS, reduces electrical perception thresholds for non-nociceptive Aβ-fibers, i.e., cutaneous hypersensitivity (496). However, the externally evoked activation of carotid baroreceptors with phase-related external neck suction (systole simulation) does not affect non-painful sensory thresholds (138, 153). Moreover, the examination of cutaneous sensory detection thresholds to electrical stimulation time-locked to the cardiac cycle results in higher cutaneous sensibility (i.e., lower threshold) to non-painful electrical stimuli during the systolic phase of the cardiac cycle (159). These diverse findings on cutaneous sensibility are likely due to methodological differences in baroreceptor or test stimulation procedures, as well as confounding effects of baroreceptor influence on motor function. Indeed, behavioral reaction times to tactile stimulation during systole (at 300 ms post-R-wave of the ECG) are slower than during the diastolic phase of the cardiac cycle (158), and externally evoked baroreceptor activation attenuates EMG-measured motor reflexes in humans (138, 153).
Baroreceptor Influences on Consciousness
Similar to pain perception, baroreflexes can profoundly modify consciousness and sleep-wake status by influencing cortical activity. Both experimentally induced changes in AP and direct activation of baroreceptors by manipulating carotid sinus and aortic arc baroreceptors influence arousal in humans and animals. In 1932, Koch reported for the first time that a rise of AP within an isolated carotid sinus rapidly induced behavioral signs of profound sleep (270). Carotid sinus stimulation-evoked sleep was later found to be associated with inhibition of global cortical activity in cats. In 1941, Gellhorn et al. (187) reported that increasing AP by head-down tilt, which distends the carotid sinus and activates baroreceptors at the cervical level, partially or entirely inhibits chemically-induced convulsions. Bonvallet et al. (49) reported in 1954 that direct pressure distension of the carotid sinus produces cortical electroencephalogram (EEG) synchronization (high amplitude, low-frequency EEG with sleep spindles) that resembles non-REM sleep independent of any cardiovascular effect. In 1965, Padel and Dell (401) reported that, like the carotid sinus, direct baroreceptor activation of the aortic arch elicits sleep-like EEG synchronization.
In humans, Bridgers et al. in 1985 (58) reported the first evidence of baroreceptor modulation of cortical activity, observing that stimulation of the carotid sinus induced an EEG delta-wave slowing that was sensitive to lidocaine blockade of the carotid sinus and independent of cardiovascular dynamics. This inhibitory baroreceptor modulation of the cortex can explain how stimulation of the carotid sinus with manual massage can abort seizures in epilepsy (293). Reduction in AP with hypotensive drugs leads to cortical disinhibition in cats (380) and arousal in lambs (225).
Cortical inhibition is substantially reduced or even abolished by sinoaortic denervation, implying the involvement of baroreceptors in these findings (225, 380). Moreover, cutting the carotid sinus and depressor nerves diminishes the total duration of synchronized sleep in cats (30), which suggests that, in addition to a phasic influence, there is a background baroreceptor activity driven by resting oscillatory AP that inhibits cortical activity to maintain sleep. Similarly, decreasing baroreceptor stimulation by an upright postural change that lowers AP induces EEG arousal in humans (98). However, it should be noted that some studies have found that increases in AP stimulate arousal. For example, brief rises in AP following aortic occlusion or rapid saline injections and more sustained rises in AP produced by the administration of vasopressin in cats (30, 31) or with phenylephrine in humans (262), produces behavioral arousal. The behavioral arousal induced by brief AP rises in lambs is associated with changes in cortical EEG activity in a stimulus strength-dependent manner (226). Furthermore, sinoaortic denervation prevents arousal induced by transient changes in AP (225, 226), suggesting that phasic baroreceptor activation exerts an excitatory influence on cortical activity. In support of this view, direct stimulation of the cervical vagal nerve induces a complete desynchronization of cortical, i.e., low amplitude, high-frequency EEG that is associated with awake state (609).
Physiological parameters associated with baroreceptor modulation of consciousness.
The controversial effects of baroreceptor activation on the brain cortex can be explained by methodological factors such as frequency, intensity, duration, and pattern (e.g., phasic or sustained) of peripheral afferent stimulation, as well as physiological or pathological conditions of the involved neural and vascular structures. Chase et al. (84) found that high-frequency and low voltage stimulation of the cervical vagal nerve results in EEG cortical synchronization (e.g., sleep behavior) while cortical EEG cortical desynchronization (e.g., cortical activation and arousal behavior) occurs at lower frequencies and higher voltages of stimulation. The bidirectional modulation of cortical activity may depend on activation of functionally and structurally discrete, fast, and slow conducting vagal afferents, which synchronizes (sleep) and desynchronizes (arousal) the cortical EEG, respectively (83). Silvani et al. (500) proposed that AP can exert a bidirectional modulation of cortical arousal depending on stimulus intensity: (a) very mild stimulation of arterial baroreceptors (e.g., external massage of the carotid region) decreases cortical arousal, and (b) greater stimulation or inhibition of baroreceptor activity causes arousal under physiological conditions. Additionally, brain functional status and medical conditions moderate the baroreceptor modulation of cortical arousal. Under anesthesia, which depresses brain activity, baroreceptor stimulation decreases cortical arousal (500). Also, aging and atherosclerosis prevent baroreflex modulation of cortical activity by impairing the pressure-distension transduction within the carotid sinus and aortic arch (58).
Neural mechanisms mediating baroreceptor modulation of sleep.
The neuronal pathways mediating the baroreceptor modulation of consciousness have not been elucidated. The ascending arousal system arises from multiple nuclei that project from the medulla to the midbrain (472, 576). Saper et al. (472) separated ascending arousal system in two major branches; the first branch arises from the pedunculopontine and laterodorsal tegmental nuclei (cholinergic), several brainstem monoaminergic nuclei, and the parabrachial nucleus and influences the cerebral cortex by relaying nuclei in the thalamus (reticular, midline, and intralaminar nuclei). The second branch originates in the LC (noradrenergic), raphe nuclei (serotoninergic), PAG (dopaminergic), and tuberomammillary nucleus (histaminergic) and reaches the cortex evading the thalamus, and is reinforced by projections from the lateral hypothalamus (peptidergic, orexin/hypocretin) and basal forebrain areas (cholinergic, GABAergic). Current evidence supports the role of these structures in baroreceptor modulation of arousal (FIGURE 11).
FIGURE 11.
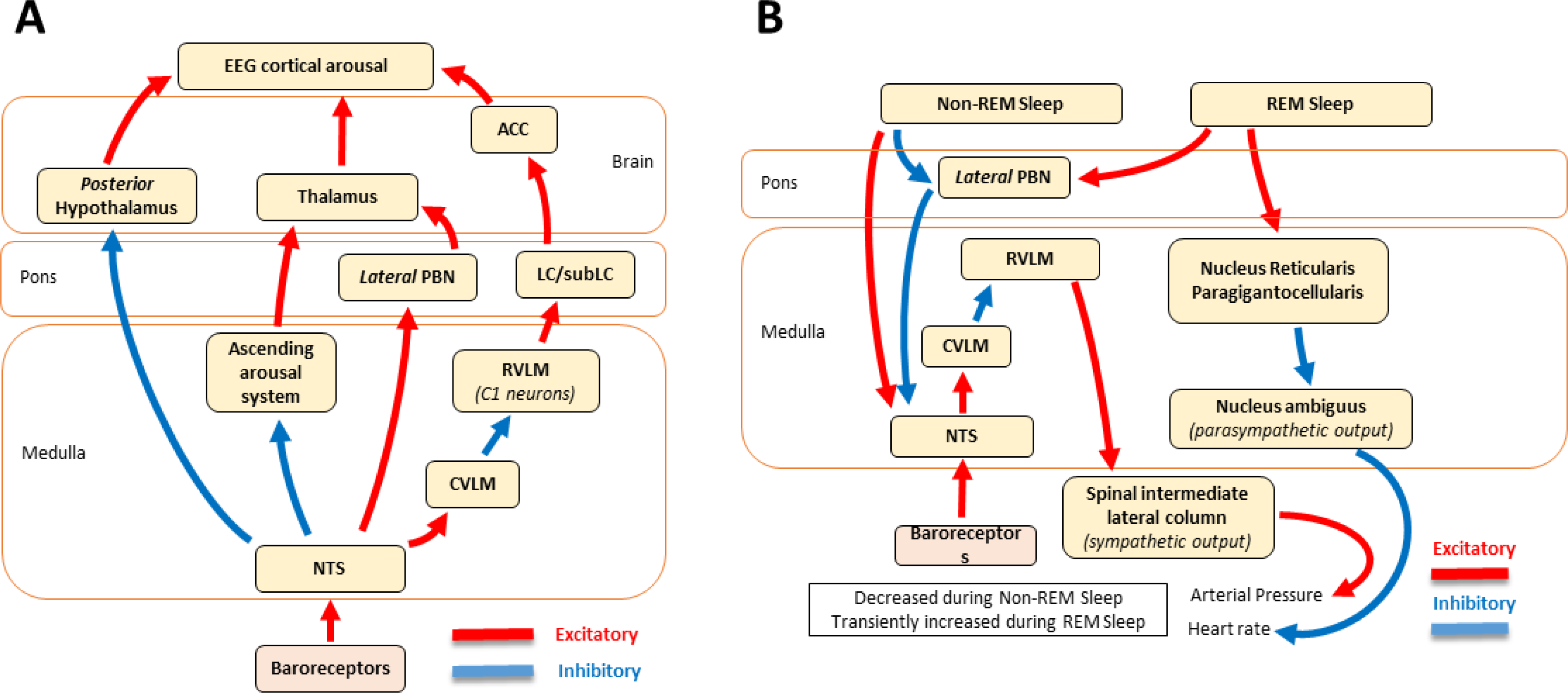
The reciprocal influence between cardiovascular function and sleep. Arrows represent excitatory (red) and inhibitory (blue) functional influences that occur through monosynaptic or polysynaptic anatomical connections and relay nuclei (e.g., thalamus). Panel A: Oversimplified view of major neural structures involved in baroreceptor modulation of arousal. NTS projections to the ascending arousal system, posterior hypothalamus, and CVLM/RVLM reduce arousal and prompt sleep, whereas NTS projections to the PBN pathways promote arousal. Panel B: Sleep influences cardiovascular function. Non-REM sleep disinhibits the NTS, and as a result, decreases sympathetic output at the spinal lateral column, which enhances baroreflexes and reduces arterial pressure. In contrast, REM sleep reduces the parasympathetic output by inhibiting the nucleus ambiguus, which dampens baroreflexes and leads to a transient increase in AP and heart rate. ACC, anterior cingulate cortex; LC/subLC, locus coeruleus/locus subcoeruleus; Lateral PBN, lateral parabrachial nucleus; RVLM, rostral ventrolateral medulla; CVLM, caudal ventrolateral medulla; NTS, nucleus tractus solitarious. Lateral PBN and LC are part of the ascending reticular activating system.
Nucleus of the solitary tract.
Early studies found that medullary transection prevents sleep and EEG changes evoked by carotid sinus stimulation in dogs, suggesting the requirement for an intact connection between the NTS and supramedullary CNS structures (49, 270). The integrity of the NTS is necessary to maintain sleep. Indeed, discrete bulbar lesions in the rostral NTS produce a pronounced arousal-like desynchronization of EEG along with the elevation of AP in cats (48). This effect is anatomically selective because lesions in caudal NTS do not affect EEG activity but increases AP by affecting baroreflexes (48). Also, microinjection of serotonin (291) or electrical stimulation (195) of the NTS synchronizes EEG and produces a baroreflex-induced transient fall in AP in anesthetized rats. Of note, the NTS neuronal activity increases during non-REM sleep, independently of AP changes, suggesting that this structure maintains the stability of non-REM sleep (160). The electrical stimulation of the NTS induces EEG synchronization (increases the power of 4–6 Hz wave) in spinalized rats (195). Taken together, the NTS plays an essential role in baroreceptor modulation of cortical arousal and sleep.
Hypothalamus.
The hypothalamus is another CNS structure that contributes to baroreceptor modulation of sleep due to its dual capability to regulate cardiovascular tone and arousal. Indeed, lesions of the posterior hypothalamus elicit a fall in AP and heart rate, whereas lesions of the anterior hypothalamus produce an opposite effect, which suggests that the posterior and anterior hypothalamus mediate sympathetic and parasympathetic cardiovascular tone, respectively (186). Of note, orexin neurons in the posterior (perifornical and dorsomedial) hypothalamus modulate arousal (219, 251), and GABAergic neurons in the anterior hypothalamus (ventrolateral preoptic region) promote sleep. Furthermore, a selective lesion of this area produces profound and prolonged insomnia (472). A unilateral lesion in the posterior hypothalamus reduces or abolishes arousal-resembling EEG desynchronization (i.e., decreases the amplitude of slow potentials and increases the amplitude of fast potentials) evoked by intravenously administrated acetylcholine (122, 380). Direct activation of baroreceptor afferents by vagal nerve stimulation inhibits externally unprovoked attacks of aggressive and hostile behaviors in decorticate cats, (i.e., sham rage) (608), behaviors that are mediated by the posterior (caudal) hypothalamus (25, 219, 404). Collectively, these findings suggest that baroreceptor activation facilitates sleep by inhibiting neural substrates in the posterior hypothalamus via projections from the NTS (FIGURE 11A).
Ascending brain stem arousal system.
Earlier electrophysiological studies, using focal electrical stimulation and site-specific lesions, showed the central brainstem core, extending from the medulla to the pontile and mesencephalic tegmentum into the caudal diencephalon regulates the sleep-wakefulness cycle in anesthetized cats (365). Vagal stimulation (expected to activate neural pathways in the NTS) elicits a generalized reduction in cortical activity, which was thought to be mediated by inhibition of an ascending brainstem arousal system (608). Direct stimulation of the NTS induces EEG synchronization (sleep pattern), which does not occur under a permanent lesion or reversible inhibition (muscimol microinjection) of the medullary cerebral vasodilator area located in the lateral tegmental field just caudal to the RVLM (195). While tonic inhibition of the ascending brain stem arousal system by baroreceptor activation is substantial in cats, it does not appear to elicit sleep-like slow delta EEG activity in humans (409), perhaps due to a more limited role of the ascending brain stem-thalamic-cortical pathway in arousal in humans than previously thought (180). These findings suggest an NTS-ventral medullary pathway that modulates sleep and arousal.
The RVLM, locus coeruleus, and lateral PBN receive baroreceptor input via the NTS (471), and these loci participate in modulating the arousal-sleep cycle (FIGURE 11A). Optogenetic stimulation of epinephrine-containing C1 neurons of the RVLM, which respond to both hypoxia and a reduction in AP, evokes arousal in rats by a mechanism that is independent of cardiorespiratory effects (2). Lesions of the locus coeruleus and anterior cingulate cortex prevent EEG arousal in response to exposure to novel environmental stimuli (197). The locus coeruleus, which receives monosynaptic glutamatergic excitatory projections from C1 neurons of the RVLM (223), is activated by a fall in AP (304, 419), and its optogenetic activation produces cortical arousal during sleep (78), which is thought to be mediated by the anterior cingulate cortex (197). Besides, the lateral PBN receives direct baroreceptor input from the NTS and is activated by an increase in AP (471). Lesion studies have found that PBN is necessary to maintain arousal in rats (180), especially in response to hypercapnia (259). Thus, Silvani et al. (500) proposed two brainstem circuitries that mediated sleep and arousal. First, the NTS–RVLM (C1 group) circuit that decreases arousal (facilitates sleep) in response to very mild baroreflex activation or in non-physiological conditions such as anesthesia. Second, the NTS-PBN circuit promotes arousal in response to substantial baroreflex activation in conscious conditions (FIGURE 11A). The predominance of a given pathway appears to depend on the strength of baroreceptor afferent input, e.g., a substantial input (large changes in AP) activate the lateral PBN pathway to induce arousal, whereas mild input (carotid massage) activates the CVLM/RVLM pathway to reduce arousal and prompt sleep.
Baroreceptor modulation of motor function and implications for narcolepsy-cataplexy.
In addition to inhibition of cortical arousal, baroreceptor activation can indirectly facilitate sleep by diminishing skeletal muscle tone (153) and attenuating startle motor reflexes (392). Baroreceptor activation can also dampen voluntary movements by modulating descending corticospinal tract activity. In anesthetized cats, the mechanical stimulation of carotid baroreceptors diminishes the discharge of cortical pyramidal tract cells evoked by orthodromic stimulation of the thalamus (100). This dual inhibition of arousal and skeletal muscle tone by baroreceptors has been associated with the pathogenesis of narcolepsy and cataplexy. Wilson and Watson (1934) were the first to report the observation that narcoleptic patients have higher sleep responses induced by carotid sinus stimulation (586). Case reports have noted the reversal of narcolepsy-cataplexy after removing a salivary tumor (29) or a thyroid tumor (361) compressing the carotid sinus. Of note, and perhaps relatedly, there is an increased risk of obstructive sleep apnea observed with vagal nerve stimulation treatments (452). These findings suggest that altered baroreflex mechanisms contribute to common sleep disorders (269). Indeed, abnormalities in the NTS function may be involved in the orexin deficiency observed in cataleptic patients who exhibit reduced cardio-vagal regulation (43).
Reciprocal influence of sleep and arousal on baroreflexes.
The relationship between baroreceptors and consciousness is bidirectional (FIGURE 11B). Cortical arousal reciprocally modulates baroreflexes (500), although the effects of sleep on BRS are controversial because 24-hour sleep deprivation resets the baroreflex to higher AP levels (77, 395). Central autonomic commands override brainstem-mediated baroreflexes during REM sleep (499) and the transition from non-REM sleep to arousal (199, 487), which leads to a transient increase in heart rate and AP. The PBN mediates the central autonomic override of baroreflexes. Indeed, activation of the PBN by direct electrical and glutamatergic stimulation induces inhibition of cardiac baroreflexes, which leads to tachycardia and hypertension (296). PNB activity decreases during non-REM sleep and increases during REM sleep (465). Furthermore, the microinjection of orexins into the NTS decreases heart rate at lower concentrations and increases it at higher concentrations by a mechanism mediated by bidirectional modulation of brainstem parasympathetic tone (495). Silvani et al. (500) proposed that non-REM sleep disinhibits the NTS, which enhances BRS and reduces AP, whereas REM sleep inhibits the NTS, which lowers BRS and leads to a transient increase in AP and heart rate. Indeed, there is an increase in heart rate during REM sleep (36), which likely results from inhibition of the nucleus ambiguus by GABAergic projections from the nucleus reticularis paragigantocellularis (126). Of note, dysregulation of the interaction between the sleep-wake cycle and baroreceptor function is present in several sleep and cardiovascular disorders (500).
Baroreceptor Regulation of Cognition
Cognition is the process of “knowing” and requires attention, remembering, and reasoning. The reaction times and response accuracy during a cognitive task are indices commonly used to quantify cognitive performance. Baroreceptor activation and high AP levels slow and reduce these cognitive indices, respectively, in tasks designed to evaluate attention, memory, executive, and intellectual functions. Thus, like pain perception, baroreceptors can exert an overall inhibitory influence on several cognitive processes. TABLE 6 displays a summary of the most relevant characteristics of baroreceptor regulation of cognition described in the sections below in detail.
TABLE 6:
Summary of the inhibitory influence of baroreceptors and resting AP with cognition.
| • Acute mechanical stimulation of baroreceptor diminishes reaction times and accuracy (performance) during cognitive tasks assessing executive function. • Natural cardiac cycle stimulation of baroreceptors (during systole) reduces cognitive performance in arithmetic and executive tasks. • Healthy subjects or patients with high baroreflex gain (BRS) trait have a poorer executive function. • Untreated chronically hypertensive subjects have a lessened performance in executive function, learning, and memory (nonverbal and working) tests. • Untreated chronically hypotensive subjects display diminished performance in attentional alertness, focusing, and flexibility, as well as in memory tasks. • Pharmacological treatment of essential hypertension and hypotension that normalizes resting AP levels reverses the cognitive deficits. • Neural mechanisms (central baroreceptor pathways and autonomic outflow) are involved in cognitive modulation by short-lasting changes in AP and baroreceptor activation. • Secondary hemodynamic and vascular changes (e.g., atherosclerosis, disturbed blood flow), mediate cognition deficits in patients with chronic hypertension or hypotension. • Aging and resting arterial pressure levels can moderate the relationship between baroreceptor function and cognitive performance. • The relationship between resting AP level and cognitive performance seems to be an inverted U-shaped, and socio-demographic factors, remarkably aging moderate it. • Reciprocally, cognitive demands influence baroreceptor function. Visual outward attention enhances BRS (heart rate deceleration), whereas arithmetic and executive tasks reduce BRS (heart rate acceleration). This bidirectional modulation of BRS could shunt and allocate neural processing resources towards either the externally-oriented or internally-focused cognitive task being performed. • Baroreceptor-mediated changes in cognitive testing under experimental conditions may not be reflected in usual everyday activities, but it could impact cognitive performance under unusual stress or adverse events. |
Effect of baroreceptor activation and baroreflex sensitivity on cognition.
Several studies have reported that acute baroreceptor activation in normotensive conditions can impair executive function. Also, subjects with high BRS traits, i.e., stronger baroreflexes, exhibit poorer performance in several executive tasks. Conceptually, the executive function involves three latent variables, (a) shifting between different tasks and mental processes, (b) updating the working memory with new relevant information by directing attention to incoming information and evaluation of the ongoing task, and (c) inhibition of other task-irrelevant behaviors (359). Executive function is commonly assessed by measuring simple reaction times and accuracy during perceptual-motor tasks. Mechanical stimulation of carotid baroreceptors with neck suction during visual-spatial attention tasks slows simple reaction times without affecting performance accuracy (28). Reaction times for motor responses to tactile (158), visual (158, 579), and auditory stimuli (46, 158) are slower during cardiac cycle triggered systolic baroreceptor activation compared to responses occurring during the diastolic phase of the cardiac cycle, with responses to tactile and auditory stimuli showing higher sensitivity to cardiac cycle modulation than visual stimuli (158). Similarly, children with ADHD who exhibit higher resting BRS have diminished executive function performance as compared with those with lower BRS (134).
Baroreceptor activation with neck suction reduces skeletal muscle tone (153) and inhibits motor reflexes (392), which could be responsible for the decreased simple reaction times observed during executive tasks. However, the involvement of motor impairment in baroreflex inhibition of reaction time can be rule out since (a) baroreceptor activation influences the time elapsed from stimulus onset (visual or sound cues) to electromyographic activation, i.e., premotor reaction time, an index of central processing speed, and (b) it does not affect the time elapsed from electromyographic activation to performance of the movement, i.e., motor reaction time, an index of motor speed (158). Besides its influence on executive function, BRS is associated with the performance of intellectual tasks. Several studies have found that resting BRS correlates inversely with performance accuracy of intellectual, cognitive task, e.g., level of correct responses in a difficult mental arithmetic task (147, 445, 447, 597).
Factors moderating baroreceptor modulation of cognition.
Resting AP levels and aging are factors that moderate the relationship between BRS and cognitive processes. At relatively high resting systolic AP (i.e., above the normotensive group mean), there is the expected negative correlation between BRS (at the task) and cognitive performance, whereas this correlation is positive at relatively low systolic AP, i.e., below the group mean (445). Of note, aging reverses the reciprocal relationship between BRS and cognitive performance and memory observed in young normotensive subjects (459, 488, 556). Also, lower resting values of BRS independently predict poor memory performance in healthy older individuals without affecting executive and attentional domains (464).
Effect of resting arterial pressure on cognition.
The influence of AP levels on several aspects of cognition has been mostly studied under conditions of essential hypertension or hypotension. Compared to normotensives, untreated young subjects with essential hypertension (especially females) have reduced performance in cognitive tasks requiring speed, and psychomotor coordination, when the behaviors observed were self-initiated (489). Similarly, factory workers with untreated high AP perform poorly in learning and memory tests compared to normotensive control subjects (567). A longitudinal study reported that nonverbal memory and confrontation naming decline more pronouncedly in individuals with higher systolic AP, especially at older ages (566). Furthermore, higher systolic AP correlates with poorer nonverbal memory in nondrinkers, whereas higher diastolic BP is associated with lesser working memory among less-educated individuals (566).
Treatment with antihypertensive drugs to lower AP improves cognitive performance toward normotensive scores in young hypertensive patients, whereas those who stay untreated remain deficient as compared with controls (357). After statistical adjustment for age and education, older patients with poorly controlled hypertension display the worst performance on nonverbal memory, perceptual-motor speed, executive function, and manual dexterity tasks among normotensive and well-controlled hypertensive groups (565). These findings suggest that behavioral deficits in hypertension can be attributable to elevated AP and are reversible (357). It is not known if these cognitive deficits are innate and present in individuals prior to the development of hypertensive (489).
Like chronic hypertension, chronic low resting AP levels can also influence cognitive processes, especially attention and executive functions. Operationally, attention has four primary dimensions: general alertness or arousal, selective attention, divided attention, and sustained attention (555). After controlling for motor function and mood, subjects with chronic low resting AP show prolonged reaction times in all attentional tasks examined. Hypotension is also associated with decreased accuracy in tasks assessing sustained attention and working memory (145). After adjusting by age, hypotensive patients show prolonged reaction times, reduced performance speed in attentional tasks, and lower concentration capacity (150). These findings imply a reduced vigilance and readiness to respond to significant environmental stimuli, as well as decreased selective attention in hypotensives (150). Furthermore, hypotensives display diminished attentional flexibility, as reflected by reduced speed and longer reaction times during the cognitive task (580). Finally, hypotensive show a decreased accuracy in several classical executive function tasks compared to normotensives (144).
Both hemodynamic and neural mechanisms can mediate the cognitive deficit observed in chronic hypertension and hypotension. The elevation of AP in hypotensive subjects with the administration of etilefrine shortens reaction times in the cognitive tasks (143). Thus, cognitive deficits are related to AP levels. Etilefrine is a peripherally-acting vasoconstrictive α- and β-adrenergic agonist that induces increases in heart rate, cardiac output, stroke volume, central venous pressure, mean arterial pressure in healthy subjects (99). Low cardiac output and stroke volume are associated with reduced mental performance (146). Therefore, a cognitive improvement may result from an enhancement in brain blood flow linked to etilefrine-elicited hemodynamic changes (143). Attentional impairment in hypotension is associated with blunted blood flow responses in both middle cerebral arteries measured with transcranial Doppler sonography (149).
The autonomic nervous system and central mechanisms also participate in the cognitive deficit of hypotensives. Although hypotensives have low resting systolic AP, stroke volume, and cardiac output, they show a pathologically increased cardiac BRS that does not decline during cognitive tasks as it does in normotensives (144). Indeed, the execution of an inward attention task inhibits baroreflexes in healthy normotensive subjects, and as a result, they have enhanced sympathetic and reduced vagal cardiovascular influences (147). These cognitive tasks evoked inhibition of baroreflexes is physiological and can lead to improved cognitive functioning in healthy individuals. In support, modulations of sympathetic arousal by adrenergic stimulation with epinephrine improves memory (70), whereas β-adrenergic blockade with propranolol impairs memory (71). The autonomic influence on different components of cognition can be bimodal. Specifically, systemic sympathetic activation during moderate aerobic exercise enhances cognitive information processing, but it decreases both attention and task accuracy (592). Finally, it is not clear whether the known inhibitory influence of baroreceptors on cortical activity mediates the cognitive deficits under conditions of chronically altered resting AP.
In summary, cognitive alterations are present in both essential hypertension and hypotension. Patients with essential hypertension have deficits in nonverbal memory, confrontation naming, perceptual-motor speed, and manual dexterity. In contrast, individuals with chronically hypotensive conditions show impairments of some specific components in attentional processes, i.e., attentional alertness, selective or focused attention, and attentional flexibility. The cognitive effects in chronic hypertension and hypotension are mediated by overlapping mechanisms, predominantly baroreceptor neural inhibitory input to the CNS and altered blood perfusion of the brain.
Factors moderating arterial pressure modulation of cognition.
The relationship between systolic AP and cognitive function is described by an inverted U-shaped function rather than a linear function(566). As noted previously, subjects with either essential chronic hypotension (145, 150) or hypertension (357, 489, 565, 567) display lower cognitive performance compared with matched normotensive controls, with the best performance at the normotensive level (110). Waldstein et al. (566) found that the functional relationship between diastolic AP and specific cognitive tasks is a U-shaped function moderated by age, education, and antihypertensive medications. Specifically, both high and low diastolic AP are associated with worse performance on tests of executive function and confrontation.
Similar to cognitive deficits, some cross-sectional studies show that the risk for dementia and Alzheimer’s disease has an age-dependent inverted U-shaped distribution with respect to resting AP; that is, the highest risk is in mid-life subjects with highest resting AP and late-life (>80 years) subject with the lowest resting AP (427). In general, lowering resting AP with antihypertensive therapy provides a protective effect against dementia, Alzheimer’s disease, and stroke-related cognitive decline, as reviewed previously (427). Atherosclerosis associated with long-lasting hypertension results in reduced cerebral blow flow and ischemia, which contributes to cognitive disturbances (427); however, the role of a chronically impaired baroreceptor function remains to be examined.
Influence of cognitive demands on baroreceptor function.
The link between baroreceptor function and cognitive processes is bidirectional. Cognitive-attentional processes influence cardiovascular function through changes in the baroreceptor function that are moderated by the type of cognitive process. For example, visual attention to an external cue decreases heart rate (288, 289) by enhancing BRS (443). Visual attention enhancement of baroreceptor function inhibits cognitive processing related to intellectual tasks and allocates processing resources to carry out externally-oriented sensorimotor responses, resulting in improve sensorimotor performance (443). In contrast, a difficult arithmetic task increases heart rate and AP by reducing BRS, which leads to a rise in cerebral blood flow velocities during the task (11). The internal cognitive load inhibition of baroreceptor function disinhibits and releases cognitive processing resources to perform internally-oriented difficult intellectual tasks, reducing the processing of perceptual input from distracting environmental stimuli, leading to an improvement in the performance accuracy (151, 597). Thus, the bidirectional cognitive modulation of the cardiovascular function shifts neural processing resources towards the ongoing task to improve its efficiency. Of note, baroreflex effectiveness (number of times baroreflexes drive heart rate following AP variation ramps) is more sensitive to external attention conditions, whereas BRS (gain of the baroreflex) is more sensitive to internal cognitive elaboration conditions (443). It is unknown if the baroreceptor’s laterality (28) and asymmetry (579) features explain the differential influence on the distinct components of cognition. The practical significance of these physiological findings in learning and behavior has not been established.
It is important to note that alterations in the performance in tests that evaluate simple reaction times, attention, memory, and complex executive intellectual functions do not seem to interfere substantially with everyday behavior, ordinary work, and social activities (489). This apparent mismatch can be explained by the fact that neuropsychological testing demands a mental response capacity beyond that needed to function within a natural, real-life environment. However, deficits in these neuropsychological tests might be predictive of poor cognitive performance under stress conditions, age, disease, or other substantial adverse events.
Neural mechanisms mediating baroreceptor modulation of cognition.
The neural mechanisms by which baroreceptors modulate cognitive processes have not been elucidated. TABLE 7 summarizes available findings. Current evidence supports the role of high-pressure baroreceptors in modulating cognitive processes. Stimulation of aortic and carotid baroreceptors slows executive reaction times, whereas cardiopulmonary baroreceptor activation is ineffective (347). Functional magnetic resonance imaging (i.e., blood-oxygen-level-dependent, BOLD) has allowed the identification of CNS structures activate by the execution of cognitive tasks and influenced by baroreceptor input. In the absence of any cognitive tasks, neck suction stimulation of carotid sinus produces BOLD responses in known autonomic and emotion centers, such as insula and amygdala, as well as cognitive-related structures, such as putamen, caudate nucleus, temporal cortex, and parahippocampal gyrus (28). When carotid sinus baroreceptor stimulation takes place during visual-spatial attention tasks, there is a prolongation of the task reaction times along with activation of attention-related areas, including the prefrontal, posterior parietal, and associative occipital cortices, as well as the thalamus and cerebellum (28). There is also activation of other areas that are not part of the known visual-attentional network, including medial temporal poles, periaqueductal gray, and nuclei of the brainstem that integrates autonomic input and cognitive processing (28). Thus, the expansion of task-induced BOLD signaling may reflect neural compensation for the considerable effort required to correctly accomplish the visuospatial attention task under the inhibitory influence of arterial baroreceptor stimulation on cortex processing (28).
TABLE 7:
Neural mechanisms mediating the baroreceptor modulation of cognition.
| • Activation of high-pressure aortic and carotid baroreceptors modulate cognition, whereas low-pressure cardiopulmonary baroreceptors seem to be ineffective. • Baroreceptor modulation of cognition is associated with the expansion of fMRI BOLD signal from forebrain structures to more posterior and caudal structures (brainstem) to compensate for cortical inhibition by baroreceptors. • EEG contingent negative variation (CNV) is a slow cortical potential associated with cognitive cortical activation. • The relationship between CNV amplitude and cognitive arousal is U-shaped, with the lowest CNV values occur at the lowest and highest level of arousal. • CNV decreases with acute increases in AP or external baroreceptor stimulation, but its association with baroreceptor modulation of cognitive performance has not yet been established. • CNV is smaller in chronically low AP (hypotension) and is associated with reduced cognitive performance. • Reduced cognitive performance in hypotensives could be due to reduced brain blood flow rather than a baroreceptor influence on cortical activation. • Baroreceptor stimulation indirectly improves aspects of cognition by facilitating slow-wave sleep, which is known to facilitate memory recognition and consolidation. • The neural pathway ascends from the NTS to the hippocampus through the nucleus paragigantocellularis, and locus coeruleus is involved in enhancing word-recognition memory following vagal nerve stimulation in humans. • Cognitive modulation of baroreceptor function occurs centrally within CNS autonomic circuits. • Interoceptive input from baroreceptor vagal afferents can modulate the anterior insula and cingulate cortices via ascending NTS and parabrachial nucleus pathways. • The anterior insula integrates cardiovascular, respiratory, and emotional signals to deliver an integrated internal emotional experience and awareness. • The anterior insula is involved in the detection of salient environmental stimuli, whereas the anterior cingulate cortex plays a role in action selection. Thus, baroreceptor input can modulate the performance of cognitive tasks by influencing these cortical areas. |
Since BOLD signals underestimate the extent of cortical activation in humans (310), EEG contingent negative variation (CNV), has provided additional information on the influence of baroreceptors on cognition (162). The CNV is an EEG slow cortical potential evoked during the interval between a first warning signal and a second imperative stimulus demanding a motor, verbal, or cognitive response (536, 571). Early CNV occurs during the processing of information arising from the warning stimulus and the selection of the response (45). Late CNV correlates with anticipatory attention towards the stimulus and preparation (both cognitive and motor) for delivering the required response (45). Acute increases in AP induced by either behavioral or pharmacological manipulations, or with carotid sinus mechanical distension (with external neck suction), elicits a reduction in the CNV amplitude in frontal-central areas (161, 435). Although CNV changes following acute increases in AP, it is not evident that this is a causal association that influences cognition.
Like the acute rises in AP, hypotensive subjects with chronic low resting AP also display smaller CNV amplitudes compared with normotensives, which directly correlates with their systolic AP levels (580). This reduced CNV occurs along with diminished attentional flexibility in hypotensive subjects, reflected as longer reaction times resulting from reduced speed for routine switching during the cognitive task (580). Of note, acute pharmacological elevation of AP in chronically hypotensive subjects reduces rather than increases CNV amplitude, while it improves rather than worsens cognitive performance, as indicated by briefer reaction times during the cognitive tasks (143). This dissociation between CNV amplitude and cognitive performance in chronically hypotensive subjects suggests that cognitive deficits could be related to hemodynamic changes associated with AP levels (143), such as brain blood flow(99), rather than a baroreceptor influence on cortical activation. Furthermore, this dissociation could be related to the fact that the relationship between CNV amplitude and cognitive arousal is U-shaped rather than linear; that is, the lowest CNV values occur at the lowest and highest levels of arousal (220).
In contrast to the overall inhibitory influence on many cognitive processes, baroreceptors have a positive impact on memory formation by influencing the level of alertness or arousal. Baroreceptor input facilitates slow-wave sleep (49, 270), which is known to consolidate episodic and procedural memories in humans (133). Some animal studies have provided an anatomical basis for baroreceptor influences on recognition memory and consolidation. The NTS projects to the nucleus paragigantocellularis (14, 315), which in turn provides significant glutamatergic excitatory input to the locus coeruleus (164). Indeed, peripheral stimulation of cervical vagal afferents elicits an inhibition-excitation sequence in the locus coeruleus (528), and glutamate activation of the locus coeruleus that potentiates evoked neuronal activity in the dorsal hippocampus through β-adrenoceptors (19). This functional anatomic evidence suggests that an NTS/nucleus paragigantocellularis/locus coeruleus/hippocampus pathway is involved in noradrenergic-mediated object recognition and memory consolidation in rats (351, 352, 584). A similar pathway likely contributes to the enhancement of word-recognition memory in humans following vagal nerve stimulation (96), a procedure that activates the NTS (32). Moreover, short- and long-term vagal nerve stimulation can improve cognitive and memory function in both healthy volunteers and patients with epilepsy and Alzheimer’s disease (61). Vagal nerve stimulation improves early postoperative learning and memory dysfunction by inhibiting systemic and neuro-inflammation (589).
Cognitive-attentional demands significantly influence cardiovascular function through modulation of baroreflex gain by affecting the activity of central autonomic structures. In support of this view, a functional magnetic resonance imaging study has revealed using a multi-source interference task, to assess the cingulate-frontal-parietal cognitive/attention network, evokes higher activity in CNS autonomic centers (mainly the cingulate cortex, insula, amygdala, and PAG), which covaries with a reduction in BRS (192). In addition, this cognitive interference task increases connectivity between the anterior insula, cingulate, and amygdala with the PAG and pons (192).
The role of insula and cingulate cortex in baroreceptor modulation of emotions, awareness, and attention.
The CNS receives inputs from two major systems, the somatosensory (exteroceptive) and the interoceptive systems; the first deals with discriminating environmentally evoked sensations (e.g., pain, touch, temperature), whereas the latter conveys vague internal sensations arising from the cardiovascular, digestive, respiratory, genitourinary, and musculoskeletal structures. The interoceptive system is a hierarchically organized homeostatic system that dynamically maintains the integrity of the body (116). Current evidence supports a role for the insula and cingulate cortex in processing interoceptive input, which regulates autonomic homeostasis by asymmetrically setting the parasympathetic and sympathetic balance; for a detailed review, see Craig (115, 116) and Strigo and Craig (513). The insula processes interoceptive input by integrating cardiovascular, respiratory, and emotionally arousing neural signals in a manner that results in an internal emotional experience (513). The primary interoceptive representation is generated in the dorsal posterior insula in monkeys and the right anterior insula in humans, which contributes to emotional awareness and consciousness (116).
Baroreceptor activity can influence the insula functions and contribute to modulate the generation of homeostatic emotions and cognition (513). Current evidence supports the direct influence of cardiorespiratory vagal activity on the primary interoceptive cortex in monkeys (115, 116, 513). These cardiorespiratory vagal influences are exerted in the monkey through two polysynaptic projections from the NTS (33) and parabrachial nucleus (422) to the basal ventromedial nucleus of the thalamus. In the monkey, Ito and Craig (242) found that stimulation of the right vagus nerve evokes the most robust bilateral neural activity, not in the basal ventromedial nucleus of the thalamus, but in the thalamic parafascicular nucleus, which projects to the rostral striatum. This observation suggests the involvement of the rostral striatum in processing homeostatic afferent input and regulation of autonomic function (241). The anterior insula is involved in the detection of salient environmental stimuli, whereas the anterior cingulate cortex plays a role in action selection (460, 513), which are associated with attention switching and biasing of sensory input, allowing for scrutinizing ongoing environmental stimuli and adjusting for task-relevant stimuli (595). Thus, baroreceptor input can modulate awareness, emotions, and performance of cognitive tasks by influencing the dynamic integration of neural signals within insular and cingulate areas.
Clinical implications of baroreceptor dysfunction
The impairment of the baroreceptor function is associated with a wide range of pathological conditions, recently reviewed by Kaufmann et al. (258). Complete baroreflex dysfunction can occur following surgery, radiation, accidental trauma, neurodegenerative lesions, or congenital autonomic disorders that affect baroreceptor pathways. These lesions can occur at any level, including peripheral sensors (thyroid and larynx surgery, carotid surgery, carotid dissection), autonomic afferents (neck radiation, neuropathic degeneration in Guillain-Barre and Groll-Hischowitz syndromes), central circuits (Syringobulbia, brainstem stroke affecting the NTS, Leigh syndrome), or autonomic efferents (familial dysautonomia) (35, 263). Baroreceptor dysfunction produces chronic labile hypertension, which is characterized by exaggerated oscillations in AP (towards hypertensive and hypotensive levels) and heart rate; these oscillations in AP and heart rate are triggered by stress, drowsiness, and posture (263). Moreover, patients with baroreflex dysfunction have increased levels of circulating norepinephrine, epinephrine, and vasopressin following postural and emotional challenges, corroborating the inhibitory influence of baroreceptors on these neurohumoral responses (258).
Moderate baroreceptor dysfunction alters the stimulus-response curve of a still active baroreflex, affecting the reflex gain, stimulus threshold, and maximal response, which causes milder clinical cardiovascular changes. Since the baroreceptor function modulates sensory perception, consciousness, and cognitive processes, baroreceptor dysfunction is likely to contribute to the risk, onset, and maintenance of chronic pain, sleep disorders, and cognitive impairments. Indeed, patients with chronic musculoskeletal pain, such as fibromyalgia, temporomandibular disorders, and chronic back pain (64, 65, 181, 320, 442), as well as chronic perioperative (389), inflammatory (5, 277), and visceral pain (505) have diminished baroreflexes. Impairments in baroreflexes can also mediate the autonomic dysfunction – decreased parasympathetic and increased sympathetic activities – observed in chronic pain patients (551). Moreover, there is evidence that dysfunction in baroreceptor function may precede the onset of clinical features associated with chronic inflammatory pain syndromes, such as rheumatoid arthritis (277). Vagal nerve stimulation, which activates vagal afferents as well as efferents (601), has been used to treat headaches (497, 552), rheumatoid arthritis(276), fibromyalgia (294), and chronic pelvic pain (382). Evidence supporting the role of an altered baroreceptor activity in sleep disorders and cognitive dysfunction is still scarce. However, patients with obstructive sleep apnea have attenuated BRS (75), which is thought to contribute to hemodynamic alterations (204, 384, 385) and an increased risk for cardiovascular morbidity (21). However, it remains an open question if baroreceptor dysfunction contributes to the clinical features of obstructive sleep apnea.
Lower resting BRS predicts poor memory performance in the healthy older population (464). Although it is unclear if baroreceptor dysfunction correlates with the development of cognitive dysfunction, such correlation is suspected because one-year treatment with vagal nerve stimulation has beneficial effects for Alzheimer’s patients (354, 502) and diminishes cognitive decline in older individuals (244). We have recently examined other medical conditions that exhibit reduced BRS (515). Several therapeutic interventions can improve baroreceptor function; for example, systolic extinction training reduces pain in fibromyalgia patients with elevated AP response to stress by improving BRS (538). Finally, it is imperative to note that all of these medical conditions impose a physical and psychological stress burden on patients that further contribute to impair baroreceptor function. It is known that exposure to psychological stressors reduces BRS (449, 510). Fortunately, behavioral conditioning procedures, such as relaxation (510) and biofeedback-assisted self-control (444), have proved to be capable of enhancing BRS in healthy subjects. Respiratory training also improves BRS in subjects with chronic spinal cord injury (298). Moreover, early studies found that exercise training increases BRS in normal and hypertensive rats (67, 498). Later, several clinical studies have demonstrated the efficacy of exercise training in increasing BRS and reducing AP in healthy adult males (413), hypertensive postmenopausal women (307), and patients with stable post-infarction congestive heart failure (235). Finally, intraoral orthotics that increase heart rate variability (109) might also improve BRS (322).
Conclusions
Baroreceptor pathways represent bidirectional neural conduits between the periphery and the central nervous system, enabling both “bottom-up” (ala the James Lange model) (72, 73) and “top-down” (ala William Canon model) (72) regulation of complex physiological and psychological processes (325). While it is generally recognized that baroreceptor stimulation promotes inhibitory sympathetic and excitatory parasympathetic tone, less acknowledged is the contribution of baroreceptor pathways to pain perception, arousal, and cognition, three bodily functions. Physiological, demographic, and pathological factors (e.g., resting AP, age, health status) alter the relationship between baroreceptor input and the physiological functions described in this review. Baroreceptor-mediated effects on non-cardiovascular functions are not due to subsequent baroreflex-mediated hemodynamic changes or motor impairment, but instead, they are related to activation of neuronal networks within and between several CNS loci. Baroreceptor dysfunction occurs with the onset and maintenance of several medical conditions: chronic pain syndromes, sleep disorders (insomnia, obstructive sleep apnea), and cognitive dysfunctions (memory deficit, learning disabilities). The possibility of manipulating and reinforcing baroreflex function opens a window of opportunity to design novel therapies for these medical conditions. Future research should continue to deepen our understanding of these complex physiological mechanisms, their clinical implications, with the goal of developing non-pharmacological and pharmacological methods to restore and maintain baroreceptor function.
FIGURE 1. Teaching Points:
Baroreceptors have a pivotal role in the integration of physiological and behavioral responses to life-relevant environmental events by coordinating autonomic outflow, sensory awareness, cognitive-emotional states, consciousness, hormonal, and immune responses. Autonomic afferents sense the functional status of peripheral tissues and convey the information to the nucleus of the solitary tract in the brainstem. The nucleus of the solitary tract processes this information and initiates “top-down” mechanisms that set the autonomic outflow to several organs and systems, as well as “bottom-up” mechanisms that influence central structures involved in perception, emotions, cognition, arousal, and autonomic activity. Thus, alteration in baroreceptor function can exert changes in multiple physiological processes beyond the cardiovascular realm.
FIGURE 2. Teaching Points:
Under resting conditions, carotid baroreflex controls AP primarily by reducing systemic vascular resistance rather than decreasing heart rate and stroke volume and associated changes in cardiac output. Notice in the recording that burst of vasoconstrictive sympathetic activity mainly occurs during the decrease in arterial pressure. Thus, baroreflex control of vasoconstrictor sympathetic outflow is an on-off regulatory system.
FIGURE 3. Teaching Points:
The strength (graded component) and occurrence (gated component) of sympathetic outflow is subject to different controls. Baroreceptor input, along with other CNS influences, exerts graded effects on the strength of the sympathetic activity. Impulses from the arterial baroreceptors exert a gate control that determines the occurrence of a sympathetic discharge. The clinical relevance of these physiological features remains to be established.
FIGURE 4. Teaching Points:
During dynamic exercise, central commands arising from higher brain structures can reset the baroreflex that controls heart rate and mean blood pressure to higher arterial pressure levels. As a result, baroreceptors are less responsive to lower pressure stimulation at the carotid baroreceptors, which reduces their inhibitory influence and allows the rise of heart rate and arterial pressure to higher maximal levels to meet exercise hemodynamic demands. Baroreflex resetting is revealed by an upward displacement of the stimulus (estimated carotid sinus pressure) – response (heart rate and mean arterial pressure) curve as exercise intensity increases. Notice that the slopes of the curves remain unchanged as exercise intensity increases, which indicates the efficiency (gain) of the baroreflex (change in heart rate or AP by a given unit of change in pressure at the baroreceptor) is constant.
FIGURE 5. Teaching Points:
Exercise activation of central commands also reset the baroreflex that controls sympathetic nerve activity to higher levels of arterial pressure. Baroreceptors are less responsive to lower pressure stimulation, which reduces their inhibitory influence, allowing sympathetic nerve activity (RSNA) to rise to higher maximal levels, the subsequent increase in peripheral vasoconstriction and arterial pressures. Unlike the baroreflex controlling heart rate and mean blood, notice that the slope of the curve is steeper during the exercise, which indicates that the efficiency (gain) of the baroreflex (change in sympathetic nerve activity by a given unit of change in pressure at the baroreceptor) is higher during the exercise.
FIGURE 6: Teaching Points:
The vascular branch of the baroreflex consists of two subsystems (neural and peripheral – vascular arcs) with different dynamic properties. Baroreceptor input is quickly conveyed through the neural reflex arc so that the sympathetic nerve activity output closely follows the speed of AP changes (orthostatic challenge). In contrast, these changes in the sympathetic nerve activity cause negligible effects on arterial pressure (and heart rate) due to the slow chemical-mechanical coupling between sympathetic nerve endings and effectors, such as vascular smooth muscle, the sinus node, and myocardium, to increase peripheral vascular resistance, heart rate, and cardiac contractility, respectively. Panel A. muscle sympathetic nerve activity (MSNA) reaches a higher amplitude during fast control (top) than during slow (middle) and very slow (bottom) changes in pressure loading of the baroreceptors (angular speed during head-up tilt test) in a human subject. Panel B. Heart rate and the variability of the interbeat interval (an index of heart rate autonomic control) are not altered by the speed of pressure loading on the baroreceptors. Panel C. Similarly, systolic and diastolic arterial pressure (AP) reflex changes do not depend on the speed of pressure loading during the orthostatic challenge (head-up tilt test).
FIGURE 7: Teaching Points:
The neural arc transfers the sympathetic output at a relatively high speed, which provides acceleration to the baroreflex and compensates for the slower response time of the peripheral arc (i.e., sympathetic-evoked vasocontraction) during orthostasis. The interplay of these two subsystems is subject to a trade-off between the modulation strength and the instability of the arterial pressure. Panel A: A mathematical modeling of the system shows that, as the efficiency of the baroreflex (i.e., gain) increases from 1 to 3, the attenuation of pressure changes is higher, but the oscillation in AP (instability) is more significant (compare Gain of 2 – bold line – with Gain of 3). Panel B: In the absence of the neural arc acceleration, there is a slower and deeper attenuation of pressure changes, followed by an increased oscillatory behavior in pressure levels; note that this instability is now present at lower baroreflex efficiency, i.e., at Gain of 2.
FIGURE 8. Teaching Points:
Baroreceptors reciprocally interact with other vagally-mediated cardiovascular reflexes. For example, the Bezold-Jarisch reflex (bradycardia and hypotension after chemical stimulation of heart ventricles) attenuates the carotid baroreflex tachycardia elicited by acute changes in arterial pressure. Activation of the Bezold-Jarisch reflex by ventricle stimulation causes a downward displacement of the baroreflex stimulus-response curve (mean arterial blood pressure, MABP – heart rate), as well as a reduction of the baroreflex sensitivity to pressure (shallower slope).
FIGURE 9. Teaching Points:
Baroreceptor function can either inhibit or decrease pain depending on resting levels of systolic and diastolic AP. Notice that arterial baroreceptor activation in subjects with high resting systolic AP diminishes pain-evoked brain cortical activity (and as a result, pain perception) when compared with conditions associated with the inhibition of baroreceptors. In contrast, baroreceptor activation increases pain-evoked brain cortical activity and pain perception in individuals with low resting systolic AP. This physiological pattern indicates that regulatory mechanisms are not necessarily linear; instead, they exhibit more complex non-linear behaviors that are under the influence of the organism and environmental factors.
FIGURE 10. Teaching Points:
Baroreceptor modulation of pain responses, and presumably pain perception, occurs through mechanisms that involved the brainstem (panel A) and upper brain structures (panel B). In both cases, the nucleus of the solitary tract (NTS) in the brainstem plays a pivotal role as a relay station that receives, processes, and distributes signals originating from baroreceptors. The NTS, via lateral parabrachial nucleus (lateral PBN) in the pons, regulates the activity of neurons located in the rostral ventromedial medulla (RVMM), which either inhibit or enhance pain transmission via descending projections to the spinal cord. Also, the NTS sends ascending projections to the brain that modulates the activity of multiples structures involved not only in pain perception but also in cognition, emotions, and sleep.
FIGURE 11. Teaching Points:
There is a reciprocal influence between baroreceptors and sleep mechanisms. The NTS influences the activity of crucial cortical areas involved in sleep and arousal via ascending reticular activating pathways that project to the thalamus and the other that engages other subcortical nuclei (e.g., hypothalamus, locus coeruleus – LC). On the other hand, sleep activates descending central commands that can override baroreceptor control of cardiovascular function. The resting AP and HR decrease throughout non-REM sleep due to a dampening of sympathetic output, whereas transient increases in AP and HR occur during paradoxical sleep (rapid eye movement – REM sleep).
Further Reading.
Hierarchical organization of autonomic pathways
Mechanisms of autonomic integration (legacy)
Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system
Hypothalamic integration of autonomic nervous system
Cross-references.
Arterial baroreflexes in humans (legacy)
Cardiopulmonary baroreflexes in humans (legacy)
Baroreflex control of systemic arterial pressure and vascular bed (legacy)
Autonomic adjustments to exercise in humans
Autonomic changes related to sleep-wake states
Autonomic nervous system and immune system interactions
Didactic Synopsis.
Major Teaching Points:
Baroreceptors are specialized stretch receptors activated by increases in blood pressure or vascular volume.
High-pressure baroreceptors are located in the carotid sinus and aortic arch. Low-pressure baroreceptors are found in the heart and lungs.
Baroreceptor stimulation triggers a reflex that reduces arterial blood pressure and heart rate by decreasing and increasing sympathetic vasomotor and parasympathetic cardiomotor outflows, respectively.
In addition to its cardiovascular effects, baroreceptor reflexes exert a substantial influence on nociception, consciousness, and cognition.
Baroreceptor influences on cardiovascular and non-cardiovascular functions are mediated by descending and ascending central projections arising from the nucleus of the solitary tract.
Baroreceptor stimulation predominantly diminishes nociception, although an opposite influence is present under certain conditions.
Baroreceptor stimulation promotes sleep.
There is an overall inverse association between baroreceptor function and cognitive processes that is influenced by low resting systolic AP, aging, and sleep.
Baroreceptor dysfunction can lead to altered pain perception, sleep, consciousness, and cognition.
Acknowledgments
The senior author (WM) would like to dedicate this review in memory of his graduate school advisors Dr. John Paul Long (thesis chair), Dr. Michael J. Brody (graduate school advisor). Studies and conversations with Dr. Alan Randich (colleague and collaborator), Dr. Jerry Gebhart (graduate school advisor), and his postdoctoral advisor, Dr. Ronald Dubner, are also greatly appreciated. Supported by grant Nos. R21NS112912 (WM), U01DE017018 (WM), and P01NS045685 (WM) from the National Institutes of Health (Bethesda, Maryland) and institutional support from the Department of Anesthesiology, Duke University, Durham, North Carolina.
References
- 1.Aars H. Effects of noradrenaline on activity in single aortic baroreceptor fibres. Acta Physiol (Oxf) 83: 335–343, 1971. [DOI] [PubMed] [Google Scholar]
- 2.Abbott SB, Coates MB, Stornetta RL, and Guyenet PG. Optogenetic stimulation of c1 and retrotrapezoid nucleus neurons causes sleep state-dependent cardiorespiratory stimulation and arousal in rats. Hypertension 61: 835–841, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abboud FM, Eckberg DL, Johannsen UJ, and Mark AL. Carotid and cardiopulmonary baroreceptor control of splanchnic and forearm vascular resistance during venous pooling in man. J Physiol 286: 173–184, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abboud FM, and Thames MD. Interaction of cardiovascular reflexes in circulatory control. In: Handbook of Physiology - The Cardiovascular System, edited by Shepherd JT, and Abboud FM. Bethesda, MD: American Physiological Society, 1984, p. 675–753. [Google Scholar]
- 5.Adlan AM, Paton JF, Lip GY, Kitas GD, and Fisher JP. Increased sympathetic nerve activity and reduced cardiac baroreflex sensitivity in rheumatoid arthritis. J Physiol 595: 967–981, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aflyatumova GN, Nigmatullina RR, Sadykova DI, Chibireva MD, Fugetto F, and Serra R. Endothelin-1, nitric oxide, serotonin and high blood pressure in male adolescents. Vascular Health Risk Manag 14: 213–223, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akella A, and Deshpande SB. Reflex hypertensive response induced by capsaicin involves endothelin-dependent mechanisms. Indian J Physiol Pharmacol 59: 23–29, 2015. [PubMed] [Google Scholar]
- 8.al’Absi M, Buchanan T, and Lovallo WR. Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiology 33: 655–661, 1996. [DOI] [PubMed] [Google Scholar]
- 9.al’Absi M, Petersen KL, and Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain 96: 197–204, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Alam M, and Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aletti F, Gambarotta N, Penn AH, Ferrario M, and Schmid-Schonbein GW. Heart period and blood pressure characteristics in splanchnic arterial occlusion shock-induced collapse. J Clin Monit Comput 31: 167–175, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand A, and Paintal AS. Reflex effects following selective stimulation of J receptors in the cat. J Physiol 299: 553–572, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andresen MC, and Kunze DL. Ionic sensitivity of baroreceptors. Circ Res 61: I66–71, 1987. [PubMed] [Google Scholar]
- 14.Andrezik JA, Chan-Palay V, and Palay SL. The nucleus paragigantocellularis lateralis in the rat. Demonstration of afferents by the retrograde transport of horseradish peroxidase. Anat Embryol (Berl) 161: 373–390, 1981. [DOI] [PubMed] [Google Scholar]
- 15.Angrilli A, Mini A, Mucha RF, and Rau H. The influence of low blood pressure and baroreceptor activity on pain responses. Physiol Behav 62: 391–397, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Angst MS, Phillips NG, Drover DR, Tingle M, Ray A, Swan GE, Lazzeroni LC, and Clark JD. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain 153: 1397–1409, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, and McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev 84: 169–208, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Aoyagi T, Koshimizu TA, and Tanoue A. Vasopressin regulation of blood pressure and volume: findings from V1a receptor-deficient mice. Kidney Int 76: 1035–1039, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Babstock DM, and Harley CW. Paragigantocellularis stimulation induces beta-adrenergic hippocampal potentiation. Brain Res Bull 28: 709–714, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Baertschi AJ, Munzner RF, Ward DG, Johnson RN, and Gann DS. Right and left atrial B-fiber input to the medulla of the cat. Brain Res 98: 189–193, 1975. [DOI] [PubMed] [Google Scholar]
- 21.Baguet JP, Barone-Rochette G, Tamisier R, Levy P, and Pepin JL. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol 9: 679–688, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Bainbridge FA. The influence of venous filling upon the rate of the heart. J Physiol 50: 65–84, 1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bandler R, Carrive P, and Zhang SP. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res 87: 269–305, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Barbieri R, Triedman JK, and Saul JP. Heart rate control and mechanical cardiopulmonary coupling to assess central volume: a systems analysis. Am J Physiol Regul Integr Comp Physiol 283: R1210–1220, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Bard P A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. American Journal of Physiology-Legacy Content 84: 490–515, 1928. [Google Scholar]
- 26.Barrett CJ, Guild S-J, Ramchandra R, and Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension (Dallas, Tex : 1979) 46: 168–172, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Barrett CJ, Ramchandra R, Guild S-J, Lala A, Budgett DM, and Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res 92: 1330–1336, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Basile B, Bassi A, Calcagnini G, Strano S, Caltagirone C, Macaluso E, Cortelli P, and Bozzali M. Direct stimulation of the autonomic nervous system modulates activity of the brain at rest and when engaged in a cognitive task. Hum Brain Mapp 34: 1605–1614, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates CE. Mixed salivary tumor in the right tonsil fossa with narcolepsy and cataplexy. Ann Otol Rhinol Laryngol 54: 812–817, 1945. [DOI] [PubMed] [Google Scholar]
- 30.Baust W, and Heinemann H. The role of the baroreceptors and of blood pressure in the regulation of sleep and wakefulness. Exp Brain Res 3: 12–24, 1967. [DOI] [PubMed] [Google Scholar]
- 31.Baust W, Niemczyk H, and Vieth J. The action of blood pressure on the ascending reticular activating system with special reference to adrenaline-induced EEG arousal. Electroencephalogr Clin Neurophysiol 15: 63–72, 1963. [DOI] [PubMed] [Google Scholar]
- 32.Beaumont E, Campbell RP, Andresen MC, Scofield S, Singh K, Libbus I, KenKnight BH, Snyder L, and Cantrell N. Cervical vagus nerve stimulation augments spontaneous discharge in second- and higher-order sensory neurons in the rat nucleus of the solitary tract. Am j Physiol Heart Cir Physiol 313: H354–H367, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckstead RM, Morse JR, and Norgren R. The nucleus of the solitary tract in the monkey: projections to the thalamus and brain stem nuclei. J Comp Neurol 190: 259–282, 1980. [DOI] [PubMed] [Google Scholar]
- 34.Belvisi MG, and Hele DJ. Cough sensors. III. Opioid and cannabinoid receptors on vagal sensory nerves. Handb Exp Pharmacol 187: 63–76, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Benarroch EE. The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology 71: 1733–1738, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Benarroch EE. Brainstem integration of arousal, sleep, cardiovascular, and respiratory control. Neurology 91: 958–966, 2018. [DOI] [PubMed] [Google Scholar]
- 37.Bergren DR. Sensory receptor activation by mediators of defense reflexes in guinea-pig lungs. Respir Physiol 108: 195–204, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Bernard C. Sur les effects de la section de la portion encéphalique du grand sympatique. Mem Soc de Biol (CR) IV: 168, 1852. [Google Scholar]
- 39.Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD, Higgins SB, and Swindell BB. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N Engl J Med 336: 912–918, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Bernard JF, Dallel R, Raboisson P, Villanueva L, and Le Bars D. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol 353: 480–505, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, and Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med 195: 781–788, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein IM, Damron D, Schonberg AL, Sallam RM, and Shapiro R. The relationship of plasma volume, sympathetic tone, and proinflammatory cytokines in young healthy nonpregnant women. Reprod Sci 16: 980–985, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berteotti C, and Silvani A. The link between narcolepsy and autonomic cardiovascular dysfunction: a translational perspective. Clin Auton Res 28: 545–555, 2018. [DOI] [PubMed] [Google Scholar]
- 44.Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, and Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–81, 1985. [PubMed] [Google Scholar]
- 45.Birbaumer N, Elbert T, Canavan AG, and Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev 70: 1–41, 1990. [DOI] [PubMed] [Google Scholar]
- 46.Birren JE, Cardon PV, Jr., and Phillips SL. Reaction time as a function of the cardiac cycle in young adults. Science 140: 195–196, 1963. [DOI] [PubMed] [Google Scholar]
- 47.Boisse L, Chisholm SP, Lukewich MK, and Lomax AE. Clinical and experimental evidence of sympathetic neural dysfunction during inflammatory bowel disease. Clin Exp Pharmacol Physiol 36: 1026–1033, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Bonvallet M, and Allen MB Jr. Prolonged spontaneous and evoked reticular activation following discrete bulbar lesions. Electroencephalogr Clin Neurophysiol 15: 969–988, 1963. [DOI] [PubMed] [Google Scholar]
- 49.Bonvallet M, Dell P, and Hiebel G. Tonus sympathique et activite electrique corticale. Electroencephalogr Clin Neurophysiol 6: 119–144, 1954. [DOI] [PubMed] [Google Scholar]
- 50.Borgers AJ, van den Born BJ, Alkemade A, Eeftinck Schattenkerk DW, van Lieshout JJ, Wesseling KH, Bisschop PH, and Westerhof BE. Determinants of vascular and cardiac baroreflex sensitivity values in a random population sample. Med Biol Eng Comput 52: 65–73, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Borghi C, Dormi A, Veronesi M, Immordino V, and Ambrosioni E. Use of lipid-lowering drugs and blood pressure control in patients with arterial hypertension. J Clin Hypertens (Greenwich) 4: 277–285, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, and Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Boscan P, Pickering AE, and Paton JF. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol 87: 259–266, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Bossink AW, Groeneveld J, Hack CE, and Thijs LG. Prediction of mortality in febrile medical patients: How useful are systemic inflammatory response syndrome and sepsis criteria? Chest 113: 1533–1541, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Bossut DF, and Maixner W. Effects of cardiac vagal afferent electrostimulation on the responses of trigeminal and trigeminothalamic neurons to noxious orofacial stimulation. Pain 65: 101–109, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Bossut DF, Whitsel EA, and Maixner W. A parametric analysis of the effects of cardiopulmonary vagal electrostimulation on the digastric reflex in cats. Brain Res 579: 253–260, 1992. [DOI] [PubMed] [Google Scholar]
- 57.Braunwald E, Epstein SE, Glick G, Wechsler AS, and Braunwald NS. Relief of angina pectoris by electrical stimulation of the carotid-sinus nerves. N Engl J Med 277: 1278–1283, 1967. [DOI] [PubMed] [Google Scholar]
- 58.Bridgers SL, Spencer SS, Spencer DD, and Sasaki CT. A cerebral effect of carotid sinus stimulation. Observation during intraoperative electroencephalographic monitoring. Arch Neurol 42: 574–577, 1985. [DOI] [PubMed] [Google Scholar]
- 59.Brody S, Angrilli A, Weiss U, Birbaumer N, Mini A, Veit R, and Rau H. Somatotosensory evoked potentials during baroreceptor stimulation in chronic low back pain patients and normal controls. Int J Psychophysiol 25: 201–210, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Brognara F, Castania JA, Dias DPM, Lopes AH, Fazan R Jr., Kanashiro A, Ulloa L, and Salgado HC. Baroreflex stimulation attenuates central but not peripheral inflammation in conscious endotoxemic rats. Brain Res 1682: 54–60, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broncel A, Bocian R, Kłos-Wojtczak P, Kulbat-Warycha K, and Konopacki J. Vagal nerve stimulation as a promising tool in the improvement of cognitive disorders. Brain Res Bull 155: 37–47, 2020. [DOI] [PubMed] [Google Scholar]
- 62.Bruchfeld A, Goldstein RS, Chavan S, Patel NB, Rosas-Ballina M, Kohn N, Qureshi AR, and Tracey KJ. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J Intern Med 268: 94–101, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruehl S, Carlson CR, and McCubbin JA. The relationship between pain sensitivity and blood pressure in normotensives. Pain 48: 463–467, 1992. [DOI] [PubMed] [Google Scholar]
- 64.Bruehl S, and Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev 28: 395–414, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Bruehl S, Chung OY, Ward P, Johnson B, and McCubbin JA. The relationship between resting blood pressure and acute pain sensitivity in healthy normotensives and chronic back pain sufferers: the effects of opioid blockade. Pain 100: 191–201, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Bruehl S, Olsen RB, Tronstad C, Sevre K, Burns JW, Schirmer H, Nielsen CS, Stubhaug A, and Rosseland LA. Chronic pain-related changes in cardiovascular regulation and impact on comorbid hypertension in a general population: the Tromso study. Pain 159: 119–127, 2018. [DOI] [PubMed] [Google Scholar]
- 67.Brum PC, Da Silva GJ, Moreira ED, Ida F, Negrão CE, and Krieger EM. Exercise training increases baroreceptor gain sensitivity in normal and hypertensive rats. Hypertension (Dallas, Tex : 1979) 36: 1018–1022, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Bu DX, Griffin G, and Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol 22: 165–170, 2011. [DOI] [PubMed] [Google Scholar]
- 69.Bushnell MC, Ceko M, and Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14: 502–511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cahill L, and Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiol Learn Mem 79: 194–198, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Cahill L, Prins B, Weber M, and McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature 371: 702–704, 1994. [DOI] [PubMed] [Google Scholar]
- 72.Cannon WB. The James-Lange Theory of Emotions: A Critical Examination and an Alternative Theory. Am J Physiol 100: 567–586, 1987. [PubMed] [Google Scholar]
- 73.Cannon WB. The wisdom of the body. New York, NY, US: W W Norton & Co, 1932, p. 312–312. [Google Scholar]
- 74.Card JP, and Sved AF. Central Autonomic Pathways. In: Central Regulation of Autonomic Functions, edited by Llewellyn-Smith IJ, and Verberne AJM. New York, USA: Oxford University Press, 2011, p. 3–22. [Google Scholar]
- 75.Carlson JT, Hedner JA, Sellgren J, Elam M, and Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 154: 1490–1496, 1996. [DOI] [PubMed] [Google Scholar]
- 76.Carreno FR, and Frazer A. Vagal Nerve Stimulation for Treatment-Resistant Depression. Neurotherapeutics 14: 716–727, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carter JR, Durocher JJ, Larson RA, DellaValla JP, and Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol 302: H1991–1997, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, and de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13: 1526–1533, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carthy ER. Autonomic dysfunction in essential hypertension: A systematic review. Ann Med Surg 3: 2–7, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cechetto DF. Cortical control of the autonomic nervous system. Exp Physiol 99: 326–331, 2014. [DOI] [PubMed] [Google Scholar]
- 81.Celander O, and Folkow B. A comparison of the sympathetic vasomotor fibre control of the vessels within the skin and the muscles. Acta Physiol Scand 29: 241–250, 1953. [DOI] [PubMed] [Google Scholar]
- 82.Chakrabarty A, Liao Z, and Smith PG. Angiotensin II receptor type 2 activation is required for cutaneous sensory hyperinnervation and hypersensitivity in a rat hind paw model of inflammatory pain. J Pain 14: 1053–1065, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chase MH, Nakamura Y, Clemente CD, and Sterman MB. Afferent vagal stimulation: neurographic correlates of induced EEG synchronization and desynchronization. Brain Res 5: 236–249, 1967. [DOI] [PubMed] [Google Scholar]
- 84.Chase MH, Sterman MB, and Clemente CD. Cortical and subcortical patterns of response to afferent vagal stimulation. Exp Neurol 16: 36–49, 1966. [DOI] [PubMed] [Google Scholar]
- 85.Chen H-H, Cheng P-W, Ho W-Y, Lu P-J, Lai C-C, Tseng Y-M, Fang H-C, Sun G-C, Hsiao M, Liu C-P, and Tseng C-J. Renal Denervation Improves the Baroreflex and GABA System in Chronic Kidney Disease-induced Hypertension. Sci Rep 6: 38447–38447, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen HI. Interaction between the baroreceptor and Bezold-Jarisch reflexes. Am J Physiol 237: H655–661, 1979. [DOI] [PubMed] [Google Scholar]
- 87.Chen HI. Mechanism of alteration in baroreflex cardiovascular responses due to volume loading. Jpn J Physiol 28: 749–756, 1978. [DOI] [PubMed] [Google Scholar]
- 88.Chen HI, Chapleau MW, McDowell TS, and Abboud FM. Prostaglandins contribute to activation of baroreceptors in rabbits. Possible paracrine influence of endothelium. Circ Res 67: 1394–1404, 1990. [DOI] [PubMed] [Google Scholar]
- 89.Chen L, and Yang G. Recent advances in circadian rhythms in cardiovascular system. Front Pharmacol 6: 71, 2015. DOI: 10.3389/fphar.2015.00071 (eCollection 2015. electronic publication, 16 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen MF, Tsai JT, Chen LJ, Wu TP, Yang JJ, Yin LT, Yang YL, Chiang TA, Lu HL, and Wu MC. Characterization of imidazoline receptors in blood vessels for the development of antihypertensive agents. BioMed Res Int 2014: 182846, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen Q, Roeder Z, Li MH, Zhang Y, Ingram SL, and Heinricher MM. Optogenetic Evidence for a Direct Circuit Linking Nociceptive Transmission through the Parabrachial Complex with Pain-Modulating Neurons of the Rostral Ventromedial Medulla (RVM). eNeuro 4(3): ENEURO.0202-17.2017, 2017. DOI: 10.1523/ENEURO.0202-17.2017 (eCollection May–Jun 2017. electronic publication, 16 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S, Fan ZZ, and He RR. [Adenosine facilitates carotid baroreceptor activity in rats]. Sheng Li Xue Bao 50: 525–531, 1998. [PubMed] [Google Scholar]
- 93.Chibireva MD, Aflyatumova GN, Matveeva VL, Bilalova DF, Kuz’mina OI, Sadykova DI, and Nigmatullina RR. Nitrogen Oxide, Endothelin-1, and Serotonin in the Blood of Immature Spontaneously Hypertensive Rats. Bull Exp Biol Med 162: 310–312, 2017. [DOI] [PubMed] [Google Scholar]
- 94.Chou YL, Scarupa MD, Mori N, and Canning BJ. Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 295: R1572–1584, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, and Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 7: 871–877, 2014. [DOI] [PubMed] [Google Scholar]
- 96.Clark KB, Naritoku DK, Smith DC, Browning RA, and Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 2: 94–98, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Cohen LS, Wechsler AS, Mitchell JH, and Glick G. Depression of cardiac function by streptomycin and other antimicrobial agents. Am J Cardiol 26: 505–511, 1970. [DOI] [PubMed] [Google Scholar]
- 98.Cole RJ. Postural baroreflex stimuli may affect EEG arousal and sleep in humans. J App Physiol (Bethesda, Md : 1985) 67: 2369–2375, 1989. [DOI] [PubMed] [Google Scholar]
- 99.Coleman AJ, Leary WP, and Asmal AC. The cardiovascular effects of etilefrine. Eur J Clin Pharmacol 8: 41–45, 1975. [DOI] [PubMed] [Google Scholar]
- 100.Coleridge HM, Coleridge JC, and Rosenthal F. Prolonged inactivation of cortical pyramidal tract neurones in cats by distension of the carotid sinus. J Physiol 256: 635–649, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coleridge JC, and Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. [DOI] [PubMed] [Google Scholar]
- 102.Coleridge JC, Coleridge HM, Schelegle ES, and Green JF. Acute inhalation of ozone stimulates bronchial C-fibers and rapidly adapting receptors in dogs. J App Physiol (Bethesda, Md : 1985) 74: 2345–2352, 1993. [DOI] [PubMed] [Google Scholar]
- 103.Colivicchi F, Bassi A, Santini M, and Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke 35: 2094–2098, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Collins HL, and DiCarlo SE. Cardiac afferents attenuate the muscle metaboreflex in the rat. J App Physiol (Bethesda, Md : 1985) 75: 114–120, 1993. [DOI] [PubMed] [Google Scholar]
- 105.Conway CR, Kumar A, Xiong W, Bunker M, Aaronson ST, and Rush AJ. Chronic Vagus Nerve Stimulation Significantly Improves Quality of Life in Treatment-Resistant Major Depression. J Clin Psychiatry 79(5): 18m12178, 2018. DOI: 10.4088/JCP.18m12178 (electronic publication, 7 pages). [DOI] [PubMed] [Google Scholar]
- 106.Conway J, Boon N, Davies C, Jones JV, and Sleight P. Neural and humoral mechanisms involved in blood pressure variability. J Hypertens 2: 203–208, 1984. [DOI] [PubMed] [Google Scholar]
- 107.Conway J, Boon N, Jones JV, and Sleight P. Involvement of the baroreceptor reflexes in the changes in blood pressure with sleep and mental arousal. Hypertension 5: 746–748, 1983. [DOI] [PubMed] [Google Scholar]
- 108.Coote JH, Hilton SM, and Zbrozyna AW. The ponto-medullary area integrating the defence reaction in the cat and its influence on muscle blood flow. J Physiol 229: 257–274, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coruzzi P, Gualerzi M, Bernkopf E, Brambilla L, Brambilla V, Broia V, Lombardi C, and Parati G. Autonomic cardiac modulation in obstructive sleep apnea: effect of an oral jaw-positioning appliance. Chest 130: 1362–1368, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Costa M, Stegagno L, Schandry R, and Bitti PE. Contingent negative variation and cognitive performance in hypotension. Psychophysiology 35: 737–744, 1998. [PubMed] [Google Scholar]
- 111.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, and Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science (New York, NY) 330: 55–60, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cowley AW, and DeClue JW. Quantification of baroreceptor influence on arterial pressure changes seen in primary angiotension-induced hypertension in dogs. Circ Res 39: 779–787, 1976. [DOI] [PubMed] [Google Scholar]
- 113.Cowley AW Jr. Long-term control of arterial blood pressure. Physiol Rev 72: 231–300, 1992. [DOI] [PubMed] [Google Scholar]
- 114.Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361: 225–248, 1995. [DOI] [PubMed] [Google Scholar]
- 115.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70, 2009. [DOI] [PubMed] [Google Scholar]
- 116.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13: 500–505, 2003. [DOI] [PubMed] [Google Scholar]
- 117.Crystal GJ, and Salem MR. The Bainbridge and the “reverse” Bainbridge reflexes: history, physiology, and clinical relevance. Anesth Analg 114: 520–532, 2012. [DOI] [PubMed] [Google Scholar]
- 118.Cui J, Wilson TE, Shibasaki M, Hodges NA, and Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during posthandgrip muscle ischemia in humans. J App Physiol (Bethesda, Md : 1985) 91: 1679–1686, 2001. [DOI] [PubMed] [Google Scholar]
- 119.Dalli J, Colas RA, Arnardottir H, and Serhan CN. Vagal Regulation of Group 3 Innate Lymphoid Cells and the Immunoresolvent PCTR1 Controls Infection Resolution. Immunity 46: 92–105, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dampney RA, Michelini LC, Li DP, and Pan HL. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol 315: H1200–h1214, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dampney RA, Polson JW, Potts PD, Hirooka Y, and Horiuchi J. Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell Mol Neurobiol 23: 597–616, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Darrow C, and Gellhorn E. The effects of adrenalin on the reflex excitability of the autonomic nervous system. Am J Physiol 127: 243–251, 1939. [Google Scholar]
- 123.Del Pozo R, Hernandez Gonzalez I, and Escribano-Subias P. The prostacyclin pathway in pulmonary arterial hypertension: a clinical review. Expert Rev Respir Med 11: 491–503, 2017. [DOI] [PubMed] [Google Scholar]
- 124.Delius W, Hagbarth KE, Hongell A, and Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972. [DOI] [PubMed] [Google Scholar]
- 125.Delius W, Hagbarth KE, Hongell A, and Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972. [DOI] [PubMed] [Google Scholar]
- 126.Dergacheva O, Wang X, Lovett-Barr MR, Jameson H, and Mendelowitz D. The lateral paragigantocellular nucleus modulates parasympathetic cardiac neurons: a mechanism for rapid eye movement sleep-dependent changes in heart rate. J Neurophysiol 104: 685–694, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Di Rienzo M, Parati G, Castiglioni P, Tordi R, Mancia G, and Pedotti A. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am J Physiol Regul Integr Comp Physiol 280: R744–751, 2001. [DOI] [PubMed] [Google Scholar]
- 128.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, and Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet 14: 135–143, 2005. [DOI] [PubMed] [Google Scholar]
- 129.DiBona GF, and Jones SY. Reflex effects on components of synchronized renal sympathetic nerve activity. Am J Physiol 275: F441–446, 1998. [DOI] [PubMed] [Google Scholar]
- 130.DiBona GF, Jones SY, and Sawin LL. Reflex effects on renal nerve activity characteristics in spontaneously hypertensive rats. Hypertension 30: 1089–1096, 1997. [DOI] [PubMed] [Google Scholar]
- 131.DiCarlo SE, and Bishop VS. Central baroreflex resetting as a means of increasing and decreasing sympathetic outflow and arterial pressure. Ann N Y Acad Sci 940: 324–337, 2001. [DOI] [PubMed] [Google Scholar]
- 132.DiCarlo SE, and Bishop VS. Onset of exercise shifts operating point of arterial baroreflex to higher pressures. Am J Physiol 262: H303–307, 1992. [DOI] [PubMed] [Google Scholar]
- 133.Diekelmann S, and Born J. The memory function of sleep. Nat Rev Neurosci 11: 114–126, 2010. [DOI] [PubMed] [Google Scholar]
- 134.Dietrich A, Althaus M, Hartman CA, Buitelaar JK, Mindera RB, van den Hoofdakker BJ, and Hoekstra PJ. Baroreflex sensitivity during rest and executive functioning in attention-deficit/hyperactivity disorder. The TRAILS study. Biol Psychol 90: 249–257, 2012. [DOI] [PubMed] [Google Scholar]
- 135.Ditto B, France J, and France CR. Risk for hypertension and pain sensitivity in women. Int J Behav Med 4: 117–130, 1997. [DOI] [PubMed] [Google Scholar]
- 136.Ditto B, Seguin JR, Boulerice B, Pihl RO, and Tremblay RE. Risk for hypertension and pain sensitivity in adolescent boys. Health Psychol 17: 249–254, 1998. [DOI] [PubMed] [Google Scholar]
- 137.Dorward PK, Andresen MC, Burke SL, Oliver JR, and Korner PI. Rapid resetting of the aortic baroreceptors in the rabbit and its implications for short-term and longer term reflex control. Circ Res 50: 428–439, 1982. [DOI] [PubMed] [Google Scholar]
- 138.Droste C, Kardos A, Brody S, Greenlee MW, Roskamm H, and Rau H. Baroreceptor stimulation: pain perception and sensory thresholds. Biol Psychol 37: 101–113, 1994. [DOI] [PubMed] [Google Scholar]
- 139.Drummond HA. betaENaC is a molecular component of a VSMC mechanotransducer that contributes to renal blood flow regulation, protection from renal injury, and hypertension. Front Physiol 3: 341, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Drummond HA, Price MP, Welsh MJ, and Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron 21: 1435–1441, 1998. [DOI] [PubMed] [Google Scholar]
- 141.Dubreuil A-S, Boukhaddaoui H, Desmadryl G, Martinez-Salgado C, Moshourab R, Lewin GR, Carroll P, Valmier J, and Scamps F. Role of T-type calcium current in identified D-hair mechanoreceptor neurons studied in vitro. J Neurosci 24: 8480–8484, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Duschek S, Dietel A, Schandry R, and del Paso GA. Increased sensitivity to heat pain in chronic low blood pressure. Eur J Pain 13: 28–34, 2009. [DOI] [PubMed] [Google Scholar]
- 143.Duschek S, Hadjamu M, and Schandry R. Dissociation between cortical activation and cognitive performance under pharmacological blood pressure elevation in chronic hypotension. Biol Psychol 75: 277–285, 2007. [DOI] [PubMed] [Google Scholar]
- 144.Duschek S, Hoffmann A, Reyes Del Paso GA, and Ettinger U. Autonomic Cardiovascular Control and Executive Function in Chronic Hypotension. Ann Behav Med 51: 442–453, 2017. [DOI] [PubMed] [Google Scholar]
- 145.Duschek S, Matthias E, and Schandry R. Essential hypotension is accompanied by deficits in attention and working memory. Behav Med 30: 149–158, 2005. [DOI] [PubMed] [Google Scholar]
- 146.Duschek S, Mück I, and Del Paso GR. Relationship between baroreceptor cardiac reflex sensitivity and pain experience in normotensive individuals. Int J Psychophysiol 65: 193–200, 2007. [DOI] [PubMed] [Google Scholar]
- 147.Duschek S, Muckenthaler M, Werner N, and del Paso GAR. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol Psychol 81: 110–117, 2009. [DOI] [PubMed] [Google Scholar]
- 148.Duschek S, and Reyes del Paso GA. Quantification of cardiac baroreflex function at rest and during autonomic stimulation. J Physiol Sci 57: 259–268, 2007. [DOI] [PubMed] [Google Scholar]
- 149.Duschek S, and Schandry R. Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology 41: 905–913, 2004. [DOI] [PubMed] [Google Scholar]
- 150.Duschek S, Weisz N, and Schandry R. Reduced cognitive performance and prolonged reaction time accompany moderate hypotension. Clin Auton Res 13: 427–432, 2003. [DOI] [PubMed] [Google Scholar]
- 151.Duschek S, Werner N, Kapan N, and Reyes Del Paso GA. Patterns of cerebral blood flow and systemic hemodynamics during arithmetic processing. J Psychophysiol 22: 81–90, 2008. [Google Scholar]
- 152.Dworkin B. Hypertension as a learned response: the baroreceptor reinforcement hypothesis. In: Behavioral Medicine in Cardiovascular Disorders, edited by Elbert T, Langosch W, Steptoe A, and Vaiti D. New York: Jonh Wiley and Sons Ltd., 1988, p. 17–49. [Google Scholar]
- 153.Dworkin BR, Elbert T, Rau H, Birbaumer N, Pauli P, Droste C, and Brunia CH. Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proc Natl Acad Sci U S A 91: 6329–6333, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dworkin BR, Filewich RJ, Miller NE, Craigmyle N, and Pickering TG. Baroreceptor activation reduces reactivity to noxious stimulation: implications for hypertension. Science 205: 1299–1301, 1979. [DOI] [PubMed] [Google Scholar]
- 155.Eckberg DL. Parasympathetic cardiovascular control in human disease: a critical review of methods and results. Am J Physiol 239: H581–593, 1980. [DOI] [PubMed] [Google Scholar]
- 156.Edwards L, McIntyre D, Carroll D, Ring C, France CR, and Martin U. Effects of artificial and natural baroreceptor stimulation on nociceptive responding and pain. Psychophysiology 40: 762–769, 2003. [DOI] [PubMed] [Google Scholar]
- 157.Edwards L, Ring C, McIntyre D, and Carroll D. Modulation of the human nociceptive flexion reflex. Psychophysiology 38: S38–S38, 2001. [PubMed] [Google Scholar]
- 158.Edwards L, Ring C, McIntyre D, Carroll D, and Martin U. Psychomotor speed in hypertension: effects of reaction time components, stimulus modality, and phase of the cardiac cycle. Psychophysiology 44: 459–468, 2007. [DOI] [PubMed] [Google Scholar]
- 159.Edwards L, Ring C, McIntyre D, Winer JB, and Martin U. Sensory detection thresholds are modulated across the cardiac cycle: evidence that cutaneous sensibility is greatest for systolic stimulation. Psychophysiology 46: 252–256, 2009. [DOI] [PubMed] [Google Scholar]
- 160.Eguchi K, and Satoh T. Convergence of sleep-wakefulness subsystems onto single neurons in the region of cat’s solitary tract nucleus. Arch Ital Biol 118: 331–345, 1980. [PubMed] [Google Scholar]
- 161.Elbert T, Roberts LE, Lutzenberger W, and Birbaumer N. Modulation of slow cortical potentials by instrumentally learned blood pressure responses. Psychophysiology 29: 154–164, 1992. [DOI] [PubMed] [Google Scholar]
- 162.Elbert T, Rockstroh B, Canavan A, Birbaumer N, Lutzenberger W, von Bülow I, and Linden A. Self-Regulation of Slow Cortical Potentials and Its Role in Epileptogenesis. In: International Perspectives on Self-Regulation and Health, edited by Carlson JG, and Seifert AR. Boston, MA: Springer US, 1991, p. 65–94. [Google Scholar]
- 163.Elbert T, Rockstroh B, Lutzenberger W, Kessler M, and Pietrowsky R. Baroreceptor stimulation alters pain sensation depending on tonic blood pressure. Psychophysiology 25: 25–29, 1988. [DOI] [PubMed] [Google Scholar]
- 164.Ennis M, and Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci 8: 3644–3657, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fadel PJ. Arterial baroreflex control of the peripheral vasculature in humans: rest and exercise. Med Sci Sports Exerc 40: 2055–2062, 2008. [DOI] [PubMed] [Google Scholar]
- 166.Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, and Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 280: H1383–1390, 2001. [DOI] [PubMed] [Google Scholar]
- 167.Fadel PJ, and Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fazeli S, and Buchel C. Pain-Related Expectation and Prediction Error Signals in the Anterior Insula Are Not Related to Aversiveness. J Neurosci 38: 6461–6474, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ferrero S, Haas S, Remorgida V, Camerini G, Fulcheri E, Ragni N, Straub RH, and Capellino S. Loss of sympathetic nerve fibers in intestinal endometriosis. Fertil Steril 94: 2817–2819, 2010. [DOI] [PubMed] [Google Scholar]
- 170.Fillingim RB, Maixner W, Bunting S, and Silva S. Resting blood pressure and thermal pain responses among females: effects on pain unpleasantness but not pain intensity. Int J Psychophysiol 30: 313–318, 1998. [DOI] [PubMed] [Google Scholar]
- 171.Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, and Harris MB. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain 12: 260–269, 1996. [DOI] [PubMed] [Google Scholar]
- 172.Fisher JP, Kim A, Young CN, and Fadel PJ. Carotid baroreflex control of arterial blood pressure at rest and during dynamic exercise in aging humans. Am J Physiol Regul Integr Comp Physiol 299: R1241–1247, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fisher JP, Ogoh S, Young CN, Keller DM, and Fadel PJ. Exercise intensity influences cardiac baroreflex function at the onset of isometric exercise in humans. J Applied Physiol (Bethesda, Md : 1985) 103: 941–947, 2007. [DOI] [PubMed] [Google Scholar]
- 174.France CR, and Stewart KM. Parental history of hypertension and enhanced cardiovascular reactivity are associated with decreased pain ratings. Psychophysiology 32: 571–578, 1995. [DOI] [PubMed] [Google Scholar]
- 175.France CR, and Suchowiecki S. Assessing supraspinal modulation of pain perception in individuals at risk for hypertension. Psychophysiology 38: 107–113, 2001. [PubMed] [Google Scholar]
- 176.Frank JG, and Mendelowitz D. Synaptic and intrinsic activation of GABAergic neurons in the cardiorespiratory brainstem network. PLoS One 7: e36459–e36459, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Freitas IMG, de Almeida LB, Pereira NP, Mira PAC, de Paula RB, Martinez DG, Toschi-Dias E, and Laterza MC. Baroreflex gain and vasomotor sympathetic modulation in resistant hypertension. Clin Auton Res 27: 175–184, 2017. [DOI] [PubMed] [Google Scholar]
- 178.Frenzel H, Bohlender J, Pinsker K, Wohlleben B, Tank J, Lechner SG, Schiska D, Jaijo T, Ruschendorf F, Saar K, Jordan J, Millan JM, Gross M, and Lewin GR. A genetic basis for mechanosensory traits in humans. PLoS Biol 10: e1001318, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Freye EAJ. Perfusion of the fourth cerebral ventricle with fentanyl induces naloxone-reversible bradycardia, hypotension, and EEG synchronisation in conscious dogs. Naunyn Schmiedebergs Arch Pharmacol 307: 123–128, 1979. [DOI] [PubMed] [Google Scholar]
- 180.Fuller PM, Sherman D, Pedersen NP, Saper CB, and Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519: 933–956, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Furlan R, Colombo S, Perego F, Atzeni F, Diana A, Barbic F, Porta A, Pace F, Malliani A, and Sarzi-Puttini P. Abnormalities of cardiovascular neural control and reduced orthostatic tolerance in patients with primary fibromyalgia. J Rheumatol 32: 1787–1793, 2005. [PubMed] [Google Scholar]
- 182.Furlong TM, McDowall LM, Horiuchi J, Polson JW, and Dampney RA. The effect of air puff stress on c-Fos expression in rat hypothalamus and brainstem: central circuitry mediating sympathoexcitation and baroreflex resetting. Eur J Neurosci 39: 1429–1438, 2014. [DOI] [PubMed] [Google Scholar]
- 183.Gahery Y, and Vigier D. Inhibitory effects in the cuneate nucleus produced by vago-aortic afferent fibers. Brain Res 75: 241–259, 1974. [DOI] [PubMed] [Google Scholar]
- 184.Gamboa-Esteves FO, Tavares I, Almeida A, Batten TF, McWilliam PN, and Lima D. Projection sites of superficial and deep spinal dorsal horn cells in the nucleus tractus solitarii of the rat. Brain Res 921: 195–205, 2001. [DOI] [PubMed] [Google Scholar]
- 185.Gaskell WH. On the Tonicity of the Heart and Blood Vessels. J Physiol 3: 48–92.16, 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gellhorn E, Nakao H, and Redgate ES. The influence of lesions in the anterior and posterior hypothalamus on tonic and phasic autonomic reactions. J Physiol 131: 402–423, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gellhorn E, Yesinick L, Kessler M, and Hailman H. Carotid sinus reflexes and convulsions. Am J Physiol 137: 396–403, 1942. [Google Scholar]
- 188.Gemes G, Rigaud M, Dean C, Hopp FA, Hogan QH, and Seagard J. Baroreceptor reflex is suppressed in rats that develop hyperalgesia behavior after nerve injury. Pain 146: 293–300, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ghione S, Rosa C, Mezzasalma L, and Panattoni E. Arterial hypertension is associated with hypalgesia in humans. Hypertension 12: 491–497, 1988. [DOI] [PubMed] [Google Scholar]
- 190.Ghione S, Rosa C, Panattoni E, Nuti M, Mezzasalma L, and Giuliano G. Comparison of sensory and pain threshold in tooth pulp stimulation in normotensive man and essential hypertension. J Hypertens Suppl 3: S113–115, 1985. [PubMed] [Google Scholar]
- 191.Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, and Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology 42: 627–635, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, and Critchley HD. Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp 33: 1700–1716, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gianaros PJ, and Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 47: 922–936, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goehler LE, Relton JK, Dripps D, Kiechle R, Tartaglia N, Maier SF, and Watkins LR. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull 43: 357–364, 1997. [DOI] [PubMed] [Google Scholar]
- 195.Golanov EV, and Reis DJ. Neurons of nucleus of the solitary tract synchronize the EEG and elevate cerebral blood flow via a novel medullary area. Brain Res 892: 1–12, 2001. [DOI] [PubMed] [Google Scholar]
- 196.Gomes ME, Tack CJ, Verheugt FW, Smits P, and Lenders JW. Sympathoinhibition by atorvastatin in hypertensive patients. Circ J 74: 2622–2626, 2010. [DOI] [PubMed] [Google Scholar]
- 197.Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, and Lu J. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci 30: 14543–14551, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gorman AJ, Chen JS, Foster S, Snyder RG, and Iams SG. Intracoronary PGE2 and veratrine inhibits renin release in conscious dogs via chemosensitive ventricular afferents. Clin Exp Pharmacol Physiol 19: 645–655, 1992. [DOI] [PubMed] [Google Scholar]
- 199.Gosselin N, Michaud M, Carrier J, Lavigne G, and Montplaisir J. Age difference in heart rate changes associated with micro-arousals in humans. Clin Neurophysiol 113: 1517–1521, 2002. [DOI] [PubMed] [Google Scholar]
- 200.Gray MA, Minati L, Paoletti G, and Critchley HD. Baroreceptor activation attenuates attentional effects on pain-evoked potentials. Pain 151: 853–861, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Green JF, Schmidt ND, Schultz HD, Roberts AM, Coleridge HM, and Coleridge JC. Pulmonary C-fibers evoke both apnea and tachypnea of pulmonary chemoreflex. J Appl Physiol Respir Environ Exerc Physiol 57: 562–567, 1984. [DOI] [PubMed] [Google Scholar]
- 202.Grindstaff RJ, Grindstaff RR, Sullivan MJ, and Cunningham JT. Role of the locus ceruleus in baroreceptor regulation of supraoptic vasopressin neurons in the rat. Am J Physiol Regul Integr Comp Physiol 279: R306–319, 2000. [DOI] [PubMed] [Google Scholar]
- 203.Grossman P, and Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol 74: 263–285, 2007. [DOI] [PubMed] [Google Scholar]
- 204.Grote L, Kraiczi H, and Hedner J. Reduced alpha- and beta(2)-adrenergic vascular response in patients with obstructive sleep apnea. Am J Respir Crit Care Med 162: 1480–1487, 2000. [DOI] [PubMed] [Google Scholar]
- 205.Guasti L, Gaudio G, Zanotta D, Grimoldi P, Petrozzino MR, Tanzi F, Bertolini A, Grandi AM, and Venco A. Relationship between a genetic predisposition to hypertension, blood pressure levels and pain sensitivity. Pain 82: 311–317, 1999. [DOI] [PubMed] [Google Scholar]
- 206.Guasti L, Grimoldi P, Diolisi A, Petrozzino MR, Gaudio G, Grandi AM, Rossi MG, and Venco A. Treatment with enalapril modifies the pain perception pattern in hypertensive patients. Hypertension 31: 1146–1150, 1998. [DOI] [PubMed] [Google Scholar]
- 207.Guasti L, Zanotta D, Petrozzino MR, Grimoldi P, Diolisi A, Garganico D, Gaudio G, Grandi AM, Bertolini A, and Venco A. Relationship between dental pain perception and 24 hour ambulatory blood pressure: a study on 181 subjects. J Hypertens 17: 1799–1804, 1999. [DOI] [PubMed] [Google Scholar]
- 208.Gursoy S, Erdal E, Herken H, Madenci E, Alasehirli B, and Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int 23: 104–107, 2003. [DOI] [PubMed] [Google Scholar]
- 209.Hagen K, Pettersen E, Stovner LJ, Skorpen F, Holmen J, and Zwart JA. High systolic blood pressure is associated with Val/Val genotype in the catechol-o-methyltransferase gene. The Nord-Trondelag Health Study (HUNT). Am J Hypertens 20: 21–26, 2007. [DOI] [PubMed] [Google Scholar]
- 210.Hagen K, Pettersen E, Stovner LJ, Skorpen F, and Zwart JA. The association between headache and Val158Met polymorphism in the catechol-O-methyltransferase gene: the HUNT Study. J Headache Pain 7: 70–74, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hajduczok G, Chapleau MW, Ferlic RJ, Mao HZ, and Abboud FM. Gadolinium inhibits mechanoelectrical transduction in rabbit carotid baroreceptors. Implication of stretch-activated channels. J Clin Invest 94: 2392–2396, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hansen MK, O’Connor KA, Goehler LE, Watkins LR, and Maier SF. The contribution of the vagus nerve in interleukin-1beta-induced fever is dependent on dose. Am J Physiol Regul Integr Comp Physiol 280: R929–934, 2001. [DOI] [PubMed] [Google Scholar]
- 213.Harle P, Mobius D, Carr DJ, Scholmerich J, and Straub RH. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum 52: 1305–1313, 2005. [DOI] [PubMed] [Google Scholar]
- 214.Hart CM, and Block ER. Lung serotonin metabolism. Clin Chest Med 10: 59–70, 1989. [PubMed] [Google Scholar]
- 215.Haulica I, Bild W, Iliescu R, Georgescu R, and Frunza F. Preliminary research on possible relationship of NO with agmatine at the vascular level. Rom J Physiol 36: 67–79, 1999. [PubMed] [Google Scholar]
- 216.He W, Wang X, Shi H, Shang H, Li L, Jing X, and Zhu B. Auricular acupuncture and vagal regulation. Evid Based Complement Alternat Med 2012: 786839, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Heesch CM, Miller BM, Thames MD, and Abboud FM. Effects of calcium channel blockers on isolated carotid baroreceptors and baroreflex. Am J Physiol 245: H653–661, 1983. [DOI] [PubMed] [Google Scholar]
- 218.Hering HE. Die Karotissinusreflexe auf Herz und Gefässe vom normalphysiologischen, pathologisch-physiologischen und klinischen Standpunkt. Dresden: Steinkopff, 1927. [Google Scholar]
- 219.Hess WR, and Brügger M. Das subkortikale Zentrum der affektiven Abwehr [The subcortical center of affective defense]. Helv Physiol Pharmacol Acta 1: 33–52, 1943. [Google Scholar]
- 220.Higuchi S, Watanuki S, and Yasukouchi A. Effects of reduction in arousal level caused by long-lasting task on CNV. Appl Human Sci 16: 29–34, 1997. [DOI] [PubMed] [Google Scholar]
- 221.Hjemdahl P, Fagius J, Freyschuss U, Wallin BG, Daleskog M, Bohlin G, and Perski A. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. Am J Physiol 257: E654–664, 1989. [DOI] [PubMed] [Google Scholar]
- 222.Ho MF, and Rose’Meyer RB. Vascular adenosine receptors; potential clinical applications. Curr Vasc Pharmacol 11: 327–337, 2013. [DOI] [PubMed] [Google Scholar]
- 223.Holloway BB, Stornetta RL, Bochorishvili G, Erisir A, Viar KE, and Guyenet PG. Monosynaptic Glutamatergic Activation of Locus Coeruleus and Other Lower Brainstem Noradrenergic Neurons by the C1 Cells in Mice. J Neurosci 33: 18792–18805, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Holmberg MJ, Gorman AJ, Cornish KG, and Zucker IH. Attenuation of arterial baroreflex control of heart rate by left ventricular receptor stimulation in the conscious dog. Circ Res 52: 597–607, 1983. [DOI] [PubMed] [Google Scholar]
- 225.Horne RS, Berger PJ, Bowes G, and Walker AM. Effect of sinoaortic denervation on arousal responses to hypotension in newborn lambs. Am J Physiol 256: H434–440, 1989. [DOI] [PubMed] [Google Scholar]
- 226.Horne RS, De Preu ND, Berger PJ, and Walker AM. Arousal responses to hypertension in lambs: effect of sinoaortic denervation. Am J Physiol 260: H1283–1289, 1991. [DOI] [PubMed] [Google Scholar]
- 227.Hossmann V, Fitzgerald GA, and Dollery CT. Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovasc Res 14: 125–129, 1980. [DOI] [PubMed] [Google Scholar]
- 228.Howarth D, Burstal R, Hayes C, Lan L, and Lantry G. Autonomic regulation of lymphatic flow in the lower extremity demonstrated on lymphoscintigraphy in patients with reflex sympathetic dystrophy. Clin Nucl Med 24: 383–387, 1999. [DOI] [PubMed] [Google Scholar]
- 229.Huang M, and Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 367: 467–470, 1994. [DOI] [PubMed] [Google Scholar]
- 230.Hummel T, Sengupta JN, Meller ST, and Gebhart GF. Responses of T2–4 spinal cord neurons to irritation of the lower airways in the rat. Am J Physiol 273: R1147–1157, 1997. [DOI] [PubMed] [Google Scholar]
- 231.Hunt BE, Fahy L, Farquhar WB, and Taylor JA. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension 37: 1362–1368, 2001. [DOI] [PubMed] [Google Scholar]
- 232.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, and Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, and Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol 586: 2753–2766, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ichinose M, Saito M, Kondo N, and Nishiyasu T. Time-dependent modulation of arterial baroreflex control of muscle sympathetic nerve activity during isometric exercise in humans. Am J Physiol Heart Circ Physiol 290: H1419–1426, 2006. [DOI] [PubMed] [Google Scholar]
- 235.Iellamo F, Manzi V, Caminiti G, Sposato B, Massaro M, Cerrito A, Rosano G, and Volterrani M. Dose-response relationship of baroreflex sensitivity and heart rate variability to individually-tailored exercise training in patients with heart failure. Int J Cardiol 166: 334–339, 2013. [DOI] [PubMed] [Google Scholar]
- 236.Ikeda Y, Kawada T, Sugimachi M, Kawaguchi O, Shishido T, Sato T, Miyano H, Matsuura W, Alexander J, Jr., and Sunagawa K. Neural arc of baroreflex optimizes dynamic pressure regulation in achieving both stability and quickness. Am J Physiol 271: H882–890, 1996. [DOI] [PubMed] [Google Scholar]
- 237.Illi A, Sundberg S, Ojala-Karlsson P, Scheinin M, and Gordin A. Simultaneous inhibition of catechol-O-methyltransferase and monoamine oxidase A: effects on hemodynamics and catecholamine metabolism in healthy volunteers. Clin Pharmacol Ther 59: 450–457, 1996. [DOI] [PubMed] [Google Scholar]
- 238.Ingram JR, Rhodes J, Evans BK, and Thomas GA. Nicotine enemas for active Crohn’s colitis: an open pilot study. Gastroenterol Res Pract 2008: 237185, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Inui K, Murase S, and Nosaka S. Facilitation of the arterial baroreflex by the ventrolateral part of the midbrain periaqueductal grey matter in rats. J Physiol 477: 89–101, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Irvine RJ, White JM, and Head RJ. The renin angiotensin system and nociception in spontaneously hypertensive rats. Life Sci 56: 1073–1078, 1995. [DOI] [PubMed] [Google Scholar]
- 241.Ito S, and Craig AD. Striatal projections of the vagal-responsive region of the thalamic parafascicular nucleus in macaque monkeys. J Comp Neurol 506: 301–327, 2008. [DOI] [PubMed] [Google Scholar]
- 242.Ito S, and Craig AD. Vagal-evoked activity in the parafascicular nucleus of the primate thalamus. J Neurophysiol 94: 2976–2982, 2005. [DOI] [PubMed] [Google Scholar]
- 243.Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, and Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J App Physiol (Bethesda, Md : 1985) 84: 914–921, 1998. [DOI] [PubMed] [Google Scholar]
- 244.Jacobs HI, Riphagen JM, Razat CM, Wiese S, and Sack AT. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol Aging 36: 1860–1867, 2015. [DOI] [PubMed] [Google Scholar]
- 245.Jarisch A, and Richter H. Die afferenten bahnen des veratrineeffektes in den herznerven. Arch Exp Pathol Pharmacol 193: 355–371, 1939. [Google Scholar]
- 246.Jarisch A, and Richter H. Die kreislauf des veratrins. Arch Exp Pathol Pharmacol 193: 347–354, 1939. [Google Scholar]
- 247.Jhamandas JH, and Harris KH. Influence of nucleus tractus solitarius stimulation and baroreceptor activation on rat parabrachial neurons. Brain Res Bull 28: 565–571, 1992. [DOI] [PubMed] [Google Scholar]
- 248.Jhamandas JH, and Renaud LP. A gamma-aminobutyric-acid-mediated baroreceptor input to supraoptic vasopressin neurones in the rat. J Physiol 381: 595–606, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ji RR, Nackley A, Huh Y, Terrando N, and Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 129: 343–366, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Johns EJ, Kopp UC, and DiBona GF. Neural control of renal function. Compr Physiol 1: 731–767, 2011. [DOI] [PubMed] [Google Scholar]
- 251.Johnson PL, Molosh A, Fitz SD, Truitt WA, and Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res 198: 133–161, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jordan J, Lipp A, Tank J, Schroder C, Stoffels M, Franke G, Diedrich A, Arnold G, Goldstein DS, Sharma AM, and Luft FC. Catechol-o-methyltransferase and blood pressure in humans. Circulation 106: 460–465, 2002. [DOI] [PubMed] [Google Scholar]
- 253.Just A, Wittmann U, Nafz B, Wagner CD, Ehmke H, Kirchheim HR, and Persson PB. The blood pressure buffering capacity of nitric oxide by comparison to the baroreceptor reflex. The American journal of physiology 267: H521–H527, 1994. [DOI] [PubMed] [Google Scholar]
- 254.Kalkman HO, Engel G, and Hoyer D. Three distinct subtypes of serotonergic receptors mediate the triphasic blood pressure response to serotonin in rats. J Hypertens Suppl 2: S143–145, 1984. [PubMed] [Google Scholar]
- 255.Kamiya A, Kawada T, Shimizu S, Iwase S, Sugimachi M, and Mano T. Slow head-up tilt causes lower activation of muscle sympathetic nerve activity: loading speed dependence of orthostatic sympathetic activation in humans. Am J Physiol Heart Circ Physiol 297: H53–58, 2009. [DOI] [PubMed] [Google Scholar]
- 256.Kamiya A, Kawada T, and Sugimachi M. Systems physiology of the baroreflex during orthostatic stress: from animals to humans. Front Physiol 5: 256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kardos A, Rau H, Greenlee MW, Droste C, Brody S, and Roskamm H. Reduced pain during baroreceptor stimulation in patients with symptomatic and silent myocardial ischaemia. Cardiovasc Res 28: 515–518, 1994. [DOI] [PubMed] [Google Scholar]
- 258.Kaufmann H, Norcliffe-Kaufmann L, and Palma J-A. Baroreflex Dysfunction. New Eng J Med 382: 163–178, 2020. [DOI] [PubMed] [Google Scholar]
- 259.Kaur S, Pedersen NP, Yokota S, Hur EE, Fuller PM, Lazarus M, Chamberlin NL, and Saper CB. Glutamatergic signaling from the parabrachial nucleus plays a critical role in hypercapnic arousal. J Neurosci 33: 7627–7640, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kawai Y. Differential Ascending Projections From the Male Rat Caudal Nucleus of the Tractus Solitarius: An Interface Between Local Microcircuits and Global Macrocircuits. Front Neuroanat 12: 63, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Keller DM, Fadel PJ, Ogoh S, Brothers RM, Hawkins M, Olivencia-Yurvati A, and Raven PB. Carotid baroreflex control of leg vasculature in exercising and non-exercising skeletal muscle in humans. J Physiol 561: 283–293, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kesler B, Anand A, Launois SH, and Weiss JW. Drug-induced arterial pressure elevation is associated with arousal from NREM sleep in normal volunteers. J Applied Physiol (Bethesda, Md : 1985) 87: 897–901, 1999. [DOI] [PubMed] [Google Scholar]
- 263.Ketch T, Biaggioni I, Robertson R, and Robertson D. Four faces of baroreflex failure: hypertensive crisis, volatile hypertension, orthostatic tachycardia, and malignant vagotonia. Circulation 105: 2518–2523, 2002. [DOI] [PubMed] [Google Scholar]
- 264.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, and Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kilpatrick GJ, Butler A, Burridge J, and Oxford AW. 1-(m-chlorophenyl)-biguanide, a potent high affinity 5-HT3 receptor agonist. Eur J Pharmacol 182: 193–197, 1990. [DOI] [PubMed] [Google Scholar]
- 266.Kimmerly DS. A review of human neuroimaging investigations involved with central autonomic regulation of baroreflex-mediated cardiovascular control. Auton Neurosci 207: 10–21, 2017. [DOI] [PubMed] [Google Scholar]
- 267.Kimmerly DS, O’Leary DD, Menon RS, Gati JS, and Shoemaker JK. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569: 331–345, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kirouac GJ, and Ganguly PK. Up-regulation of cholecystokinin receptors in the nucleus accumbens of the young prehypertensive spontaneously hypertensive rat. Neurosci Lett 191: 197–199, 1995. [DOI] [PubMed] [Google Scholar]
- 269.Kleitman N. Narcolepsy, Cataplexy, and Sleep Paralysis. In: Sleep and wakefulness. Chicago: The University of Chicago Press, 1963, p. 233–242. [Google Scholar]
- 270.Koch E. Die Irradiation der pressoreceptorischen Kreislaufreflexe. J Mol Med 11: 225–227, 1932. [Google Scholar]
- 271.Koch E, and Mies H. Chronischer arterieller Hochdruck durch experimentelle Dauerasschaltung der Blutdruckzugler. Krankheitsforschung 7: 241–256, 1929. [Google Scholar]
- 272.Koella WP, Smythies JR, Levy CK, and Czicman JS. Modulatory influence on cerebral cortical optic response form the carotid sinus area. Am J Physiol 199: 381–386, 1960. [DOI] [PubMed] [Google Scholar]
- 273.Koike H, Mark AL, Heistad DD, and Schmid PG. Influence of cardiopulmonary vagal afferent activity on carotid chemoreceptor and baroreceptor reflexes in the dog. Circ Res 37: 422–429, 1975. [DOI] [PubMed] [Google Scholar]
- 274.Koike MK, Moreira ED, da Silva GJ, Consolim-Colombo FM, Ida F, Irigoyen MC, and Krieger EM. Resetting of aortic baroreceptors in response to hypotension does not alter gain sensitivity. Clin Exp Pharmacol Physiol 33: 679–684, 2006. [DOI] [PubMed] [Google Scholar]
- 275.Komine H, Matsukawa K, Tsuchimochi H, and Murata J. Central command blunts the baroreflex bradycardia to aortic nerve stimulation at the onset of voluntary static exercise in cats. Am J Physiol Heart Circ Physiol 285: H516–526, 2003. [DOI] [PubMed] [Google Scholar]
- 276.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, and Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA 113(29): 8284–8289, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Koopman FA, Tang MW, Vermeij J, de Hair MJ, Choi IY, Vervoordeldonk MJ, Gerlag DM, Karemaker JM, and Tak PP. Autonomic Dysfunction Precedes Development of Rheumatoid Arthritis: A Prospective Cohort Study. EBioMedicine 6: 231–237, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Koopman FA, van Maanen MA, Vervoordeldonk MJ, and Tak PP. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J Intern Med 282: 64–75, 2017. [DOI] [PubMed] [Google Scholar]
- 279.Kou YR, and Lee LY. Stimulation of rapidly adapting receptors in canine lungs by a single breath of cigarette smoke. J App Physiol (Bethesda, Md : 1985) 68: 1203–1210, 1990. [DOI] [PubMed] [Google Scholar]
- 280.Krieger EM. Mechanisms of complete baroreceptor resetting in hypertension. Drugs 35Suppl 6: 98–103, 1988. [DOI] [PubMed] [Google Scholar]
- 281.Krout KE, and Loewy AD. Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol 428: 475–494, 2000. [DOI] [PubMed] [Google Scholar]
- 282.Kubota T, Alexander J Jr., Itaya R, Todaka K, Sugimachi M, Sunagawa K, Nose Y, and Takeshita A. Dynamic effects of carotid sinus baroreflex on ventriculoarterial coupling studied in anesthetized dogs. Circ Res 70: 1044–1053, 1992. [DOI] [PubMed] [Google Scholar]
- 283.Kullmann R, Schonung W, and Simon E. Antagonistic changes of blood flow and sympathetic activity in different vascular beds following central thermal stimulation. I. Blood flow in skin, muscle and intestine during spinal cord heating and cooling in anesthetized dogs. Pflugers Arch 319: 146–161, 1970. [DOI] [PubMed] [Google Scholar]
- 284.Kumada M, Terui N, and Kuwaki T. Arterial baroreceptor reflex: its central and peripheral neural mechanisms. Prog Neurobiol 35: 331–361, 1990. [DOI] [PubMed] [Google Scholar]
- 285.Kunath J, Delaroque N, Szardenings M, Neundorf I, and Straub RH. Sympathetic nerve repulsion inhibited by designer molecules in vitro and role in experimental arthritis. Life Sci 168: 47–53, 2017. [DOI] [PubMed] [Google Scholar]
- 286.Kunos G, Mosqueda-Garcia R, Mastrianni JA, and Abbott FV. Endorphinergic mechanism in the central cardiovascular and analgesic effects of clonidine. Can J Physiol Pharmacol 65: 1624–1632, 1987. [DOI] [PubMed] [Google Scholar]
- 287.Kunze DL, Andresen MC, and Torres LA. Do calcium antagonists act directly on calcium channels to alter baroreceptor function? J Pharmacol Exp Ther 239: 303–310, 1986. [PubMed] [Google Scholar]
- 288.Lacey BC, and Lacey JI. Cognitive modulation of time-dependent primary bradycardia. Psychophysiology 17: 209–221, 1980. [DOI] [PubMed] [Google Scholar]
- 289.Lacey BC, and Lacey JI. Two-way communication between the heart and the brain. Significance of time within the cardiac cycle. Am Psychol 33: 99–113, 1978. [DOI] [PubMed] [Google Scholar]
- 290.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, and Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6: 243–250, 1996. [DOI] [PubMed] [Google Scholar]
- 291.Laguzzi R, Reis DJ, and Talman WT. Modulation of cardiovascular and electrocortical activity through serotonergic mechanisms in the nucleus tractus solitarius of the rat. Brain Res 304: 321–328, 1984. [DOI] [PubMed] [Google Scholar]
- 292.Lai CJ, Ruan T, and Kou YR. The involvement of hydroxyl radical and cyclooxygenase metabolites in the activation of lung vagal sensory receptors by circulatory endotoxin in rats. J App Physiol (Bethesda, Md : 1985) 98: 620–628, 2005. [DOI] [PubMed] [Google Scholar]
- 293.Laine Green A, and Weaver DF. Vagal stimulation by manual carotid sinus massage to acutely suppress seizures. J Clin Neurosci 21: 179–180, 2014. [DOI] [PubMed] [Google Scholar]
- 294.Lange G, Janal MN, Maniker A, Fitzgibbons J, Fobler M, Cook D, and Natelson BH. Safety and efficacy of vagus nerve stimulation in fibromyalgia: a phase I/II proof of concept trial. Pain Med 12: 1406–1413, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Langley JN. Sketch of the progress of discovery in the eighteenth century as regards the autonomic nervous system. J Physiol 50: 225–258, 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Lara JP, Parkes MJ, Silva-Carvhalo L, Izzo P, Dawid-Milner MS, and Spyer KM. Cardiovascular and respiratory effects of stimulation of cell bodies of the parabrachial nuclei in the anaesthetized rat. J Physiol 477: 321–329, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lee LY, and Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. [DOI] [PubMed] [Google Scholar]
- 298.Legg Ditterline BE, Aslan SC, Randall DC, Harkema SJ, Castillo C, and Ovechkin AV. Effects of Respiratory Training on Heart Rate Variability and Baroreflex Sensitivity in Individuals With Chronic Spinal Cord Injury. Arch Phys Med Rehabil 99: 423–432, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Len W, Chan SH, and Chan JY. Parabrachial nucleus induces suppression of baroreflex bradycardia by the release of glutamate in the rostral ventrolateral medulla of the rat. J Biomed Sci 7: 401–411, 2000. [DOI] [PubMed] [Google Scholar]
- 300.Len WB, and Chan JY. Glutamatergic projection to RVLM mediates suppression of reflex bradycardia by parabrachial nucleus. Am J Physiol 276: H1482–1492, 1999. [DOI] [PubMed] [Google Scholar]
- 301.Li DP, Fan ZZ, and He RR. [Modulatory effects of endothelin on carotid baroreflex in anesthetized rats]. Sheng Li Xue Bao 50: 169–175, 1998. [PubMed] [Google Scholar]
- 302.Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, and Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science 263: 966–969, 1994. [DOI] [PubMed] [Google Scholar]
- 303.Li S, Sun C, Rong P, Zhai X, Zhang J, Baker M, and Wang S. Auricular vagus nerve stimulation enhances central serotonergic function and inhibits diabetic neuropathy development in Zucker fatty rats. Mol Pain 14: 1744806918787368, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Li YW, and Dampney RA. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience 61: 613–634, 1994. [DOI] [PubMed] [Google Scholar]
- 305.Li Z, Lee HC, Bielefeldt K, Chapleau MW, and Abboud FM. The prostacyclin analogue carbacyclin inhibits Ca(2+)-activated K+ current in aortic baroreceptor neurones of rats. J Physiol 501 ( Pt 2): 275–287, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lin RL, Gu Q, Khosravi M, and Lee LY. Sustained sensitizing effects of tumor necrosis factor alpha on sensory nerves in lung and airways. Pulm Pharmacol Ther 47: 29–37, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lin Y-Y, and Lee S-D. Cardiovascular Benefits of Exercise Training in Postmenopausal Hypertension. Int J Mol Sci 19: 2523, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Liu YX, Zhang H, Ma HJ, and He RR. Cholecystokinin octapeptide inhibits carotid sinus baroreflex in anesthetized rats. Sheng Li Xue Bao 56: 25–30, 2004. [PubMed] [Google Scholar]
- 309.Liu Z, Duan YL, Ge SL, Zhang CX, Gong WH, and Xu JJ. Effect of estrogen on right ventricular remodeling of monocrotaline-induced pulmonary arterial hypertension in rats and its mechanism. Eur Rev Med Pharmacol Sci 23: 1742–1750, 2019. [DOI] [PubMed] [Google Scholar]
- 310.Logothetis NK, Pauls J, Augath M, Trinath T, and Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001. [DOI] [PubMed] [Google Scholar]
- 311.Lohmeier TE, and Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology (Bethesda) 30: 148–158, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Lohmeier TE, and Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation: mechanisms and potential for hypertension therapy. Hypertension (Dallas, Tex : 1979) 57: 880–886, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, and Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Cir Physiol 299: H402–H409, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lohmeier TE, Lohmeier JR, Warren S, May PJ, and Cunningham JT. Sustained activation of the central baroreceptor pathway in angiotensin hypertension. Hypertension (Dallas, Tex : 1979) 39: 550–556, 2002. [DOI] [PubMed] [Google Scholar]
- 315.Lovick TA. Projections from brainstem nuclei to the nucleus paragigantocellularis lateralis in the cat. J Auton Nerv Syst 16: 1–11, 1986. [DOI] [PubMed] [Google Scholar]
- 316.Lundvall J, and Edfeldt H. Very large range of baroreflex sympathetic control of vascular resistance in human skeletal muscle and skin. J App Physiol (Bethesda, Md : 1985) 76: 204–211, 1994. [DOI] [PubMed] [Google Scholar]
- 317.Maixner W. Autonomic and somatosensory interactions: physiological and pathophysiological implications. Proc Fin Dental Soc 85: 395–407, 1989. [PubMed] [Google Scholar]
- 318.Maixner W. Interaction between cardiovascular and pain modulatory systems: physiological and pathophysiological implications. J Cardiovasc Electrophysiol 2: S3–S12, 1991. [Google Scholar]
- 319.Maixner W, Bossut DF, and Whitsel EA. Evaluation of vagal afferent modulation of the digastric reflex in cats. Brain Res 560: 55–62, 1991. [DOI] [PubMed] [Google Scholar]
- 320.Maixner W, Fillingim R, Kincaid S, Sigurdsson A, and Harris MB. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med 59: 503–511, 1997. [DOI] [PubMed] [Google Scholar]
- 321.Maixner W, Fillingim R, Kincaid S, Sigurdsson A, and Harris MB. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med 59: 503–511, 1997. [DOI] [PubMed] [Google Scholar]
- 322.Maixner W, Greenspan JD, Dubner R, Bair E, Mulkey F, Miller V, Knott C, Slade GD, Ohrbach R, and Diatchenko L. Potential autonomic risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 12: T75–T91, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Maixner W, Long JP, and Gebhart GF. Factors influencing the altered pain perception in the spontaneously hypertensive rat. Brain Res 237(1): 137–145, 1982. [DOI] [PubMed] [Google Scholar]
- 324.Maixner W, and Randich A. Role of the right vagal nerve trunk in antinociception. Brain Res 298: 374–377, 1984. [DOI] [PubMed] [Google Scholar]
- 325.Maixner W, Sigurdsson A, Fillingim R, Lundeen T, and Booker D. Regulation of acute and chronic orofacial pain. In: Orofacial Pain and Temporomandibular Disorders, edited by Fricton JR, and Dubner RB. New York: Raven Press, Ltd, 1995, p. 85–102. [Google Scholar]
- 326.Maixner W, Touw KB, Brody MJ, Gebhart GF, and Long JP. Factors influencing the altered pain perception in the spontaneously hypertensive rat. Brain Res 237: 137–145, 1982. [DOI] [PubMed] [Google Scholar]
- 327.Makino M, Hayashi H, Takezawa H, Hirai M, Saito H, and Ebihara S. Circadian rhythms of cardiovascular functions are modulated by the baroreflex and the autonomic nervous system in the rat. Circulation 96: 1667–1674, 1997. [DOI] [PubMed] [Google Scholar]
- 328.Mancia G, Donald DE, and Shepherd JT. Inhibition of adrenergic outflow to peripheral blood vessels by vagal afferents from the cardiopulmonary region in the dog. Circ Res 33: 713–721, 1973. [DOI] [PubMed] [Google Scholar]
- 329.Mancia G, Ferrari A, Leonetti G, Pomidossi G, and Zanchetti A. Carotid sinus baroreceptor control of arterial pressure in renovascular hypertensive subjects. Hypertension 4: 47–50, 1982. [DOI] [PubMed] [Google Scholar]
- 330.Marchi A, Bari V, De Maria B, Esler M, Lambert E, Baumert M, and Porta A. Simultaneous Characterization of Sympathetic and Cardiac Arms of the Baroreflex through Sequence Techniques during Incremental Head-Up Tilt. Front Physiol 7: 438, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol 1: 90–102, 1983. [DOI] [PubMed] [Google Scholar]
- 332.Mark AL, and Kerber RE. Augmentation of cardiopulmonary baroreflex control of forearm vascular resistance in borderline hypertension. Hypertension 4: 39–46, 1982. [DOI] [PubMed] [Google Scholar]
- 333.Mark AL, and Mancia G. Cardiopulmonary baroreflexes in humans. Compr Physiol (Suppl 8): 2011. 10.1002/cphy.cp020321. [DOI] [Google Scholar]
- 334.Martelli D, Yao ST, McKinley MJ, and McAllen RM. Reflex control of inflammation by sympathetic nerves, not the vagus. J Physiol 592: 1677–1686, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Masuki S, Todo T, Nakano Y, Okamura H, and Nose H. Reduced alpha-adrenoceptor responsiveness and enhanced baroreflex sensitivity in Cry-deficient mice lacking a biological clock. J Physiol 566: 213–224, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Matsukawa K, Komine H, Nakamoto T, and Murata J. Central command blunts sensitivity of arterial baroreceptor-heart rate reflex at onset of voluntary static exercise. Am J Physiol Heart Circ Physiol 290: H200–208, 2006. [DOI] [PubMed] [Google Scholar]
- 337.Matsukawa K, Mitchell JH, Wall PT, and Wilson LB. The effect of static exercise on renal sympathetic nerve activity in conscious cats. J Physiol 434: 453–467, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Matsukawa K, Wall PT, Wilson LB, and Mitchell JH. Reflex responses of renal nerve activity during isometric muscle contraction in cats. Am J Physiol 259: H1380–1388, 1990. [DOI] [PubMed] [Google Scholar]
- 339.Matsumoto S, Ikeda M, Nishikawa T, Yoshida S, Tanimoto T, Ito M, Saiki C, and Takeda M. Excitatory mechanism of deflationary slowly adapting pulmonary stretch receptors in the rat lung. J Pharmacol Exp Ther 300: 597–604, 2002. [DOI] [PubMed] [Google Scholar]
- 340.Mazzone SB, and Undem BJ. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol Rev 96: 975–1024, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.McCabe PM, Yongue BG, Ackles PK, and Porges SW. Changes in heart period, heart-period variability, and a spectral analysis estimate of respiratory sinus arrhythmia in response to pharmacological manipulations of the baroreceptor reflex in cats. Psychophysiology 22: 195–203, 1985. [DOI] [PubMed] [Google Scholar]
- 342.McCloskey DI, and Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.McCubbin JA, and Bruehl S. Do endogenous opioids mediate the relationship between blood pressure and pain sensitivity in normotensives? Pain 57: 63–67, 1994. [DOI] [PubMed] [Google Scholar]
- 344.McCubbin JW, Page IH, and Bumpus FM. Effect of synthetic angiotonin on the carotid sinus. Circ Res 5: 458–460, 1957. [DOI] [PubMed] [Google Scholar]
- 345.McDowell TS, Axtelle TS, Chapleau MW, and Abboud FM. Prostaglandins in carotid sinus enhance baroreflex in rabbits. Am J Physiol 257: R445–450, 1989. [DOI] [PubMed] [Google Scholar]
- 346.McIlveen SA, Hayes SG, and Kaufman MP. Both central command and exercise pressor reflex reset carotid sinus baroreflex. Am J Physiol Heart Circ Physiol 280: H1454–1463, 2001. [DOI] [PubMed] [Google Scholar]
- 347.McIntyre D, Ring C, Hamer M, and Carroll D. Effects of arterial and cardiopulmonary baroreceptor activation on simple and choice reaction times. Psychophysiology 44: 874–879, 2007. [DOI] [PubMed] [Google Scholar]
- 348.McQueen DS, and Belmonte C. The effects of prostaglandins E2, A2 and F2 alpha on carotid baroreceptors and chemoreceptors. Q J Exp Physiol Cogn Med Sci 59: 63–71, 1974. [DOI] [PubMed] [Google Scholar]
- 349.McWilliam PN, Yang T, and Chen LX. Changes in the baroreceptor reflex at the start of muscle contraction in the decerebrate cat. J Physiol 436: 549–558, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Meller ST, Lewis SJ, Brody MJ, and Gebhart GF. Nociceptive afferent vagal input is enhanced after transection of the aortic depressor nerve. Hypertension 15: 797–802, 1990. [DOI] [PubMed] [Google Scholar]
- 351.Mello-Carpes PB, da Silva de Vargas L, Gayer MC, Roehrs R, and Izquierdo I. Hippocampal noradrenergic activation is necessary for object recognition memory consolidation and can promote BDNF increase and memory persistence. Neurobiol Learn Mem 127: 84–92, 2016. [DOI] [PubMed] [Google Scholar]
- 352.Mello-Carpes PB, and Izquierdo I. The Nucleus of the Solitary Tract --> Nucleus Paragigantocellularis --> Locus Coeruleus --> CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem 100: 56–63, 2013. [DOI] [PubMed] [Google Scholar]
- 353.Merhi M, Helme RD, and Khalil Z. Age-related changes in sympathetic modulation of sensory nerve activity in rat skin. Inflamm Res 47: 239–244, 1998. [DOI] [PubMed] [Google Scholar]
- 354.Merrill CA, Jonsson MA, Minthon L, Ejnell H, son Silander HC, Blennow K, Karlsson M, Nordlund A, Rolstad S, Warkentin S, Ben-Menachem E, and Sjogren MJ. Vagus nerve stimulation in patients with Alzheimer’s disease: Additional follow-up results of a pilot study through 1 year. J Clin Psychiatry 67: 1171–1178, 2006. [DOI] [PubMed] [Google Scholar]
- 355.Michelini LC, O’Leary DS, Raven PB, and Nobrega AC. Neural control of circulation and exercise: a translational approach disclosing interactions between central command, arterial baroreflex, and muscle metaboreflex. Am J Physiol Heart Circ Physiol 309: H381–392, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Miki K, Yoshimoto M, and Tanimizu M. Acute shifts of baroreflex control of renal sympathetic nerve activity induced by treadmill exercise in rats. J Physiol 548: 313–322, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Miller RE, Shapiro AP, King HE, Ginchereau EH, and Hosutt JA. Effect of antihypertensive treatment on the behavioral consequences of elevated blood pressure. Hypertension 6: 202–208, 1984. [PubMed] [Google Scholar]
- 358.Mirakaj V, Dalli J, Granja T, Rosenberger P, and Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med 211: 1037–1048, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, and Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol 41: 49–100, 2000. [DOI] [PubMed] [Google Scholar]
- 360.Molderings GJ, and Haenisch B. Agmatine (decarboxylated l-arginine): Physiological role and therapeutic potential. Pharmacol Ther 133: 351–365, 2012. [DOI] [PubMed] [Google Scholar]
- 361.Moller E, and Ostenfeld I. Studies on the cerebral carotid sinus syndrome and the physiological basis of consciousness. Acta Psychiatr Scand 24: 59–80, 1949. [Google Scholar]
- 362.Moreira ED, Mostarda CT, Moraes-Silva IC, Ferreira JB, Dos Santos F, Lacchini S, De Angelis K, Rodrigues B, and Irigoyen MC. Effect of simvastatin in the autonomic system is dependent on the increased gain/sensitivity of the baroreceptors. Physiol Rep 1: e00045, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Morgan MM, and Fields HL. Activity of nociceptive modulatory neurons in the rostral ventromedial medulla associated with volume expansion-induced antinociception. Pain 52: 1–9, 1993. [DOI] [PubMed] [Google Scholar]
- 364.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, and Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343: 1249288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Moruzzi G, and Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1: 455–473, 1949. [PubMed] [Google Scholar]
- 366.Mueller PJ, Clifford PS, Crandall CG, Smith SA, and Fadel PJ. Integration of Central and Peripheral Regulation of the Circulation during Exercise: Acute and Chronic Adaptations. Compr Physiol 8: 103–151, 2017. [DOI] [PubMed] [Google Scholar]
- 367.Muller-Ribeiro FC, Zaretsky DV, Zaretskaia MV, Santos RA, DiMicco JA, and Fontes MA. Contribution of infralimbic cortex in the cardiovascular response to acute stress. Am J Physiol Regul Integr Comp Physiol 303: R639–650, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, and Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation 75: 131–138, 1987. [DOI] [PubMed] [Google Scholar]
- 369.Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, and et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 313: 1315–1322, 1985. [DOI] [PubMed] [Google Scholar]
- 370.Multon S, and Schoenen J. Pain control by vagus nerve stimulation: from animal to man...and back. Acta Neurol Belg 105: 62–67, 2005. [PubMed] [Google Scholar]
- 371.Munch PA. Discharge characteristics and rapid resetting of autoactive aortic baroreceptors in rats. J Physiol 458: 501–517, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Munch PA. Endothelium-mediated and direct actions of acetylcholine on rabbit aortic baroreceptors. Circ Res 74: 422–433, 1994. [DOI] [PubMed] [Google Scholar]
- 373.Munch PA, Andresen MC, and Brown AM. Rapid resetting of aortic baroreceptors in vitro. Am J Physiol 244: H672–680, 1983. [DOI] [PubMed] [Google Scholar]
- 374.Munch PA, and Longhurst JC. Contrasting effects of vasopressin and angiotensin II on rabbit aortic baroreceptors. Am J Physiol 260: H811–820, 1991. [DOI] [PubMed] [Google Scholar]
- 375.Muralidharan A, Wyse BD, and Smith MT. Analgesic efficacy and mode of action of a selective small molecule angiotensin II type 2 receptor antagonist in a rat model of prostate cancer-induced bone pain. Pain Med 15: 93–110, 2014. [DOI] [PubMed] [Google Scholar]
- 376.Murata J, Matsukawa K, Komine H, Tsuchimochi H, and Nakamoto T. Central inhibition of the aortic baroreceptors-heart rate reflex at the onset of spontaneous muscle contraction. J Applied Physiol (Bethesda, Md : 1985) 97: 1371–1378, 2004. [DOI] [PubMed] [Google Scholar]
- 377.Myers CD, Robinson ME, Riley JL 3rd, and Sheffield D. Sex, gender, and blood pressure: contributions to experimental pain report. Psychosom Med 63: 545–550, 2001. [DOI] [PubMed] [Google Scholar]
- 378.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, and Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain 128: 199–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Nagi SS, Dunn JS, Birznieks I, Vickery RM, and Mahns DA. The effects of preferential A- and C-fibre blocks and T-type calcium channel antagonist on detection of low-force monofilaments in healthy human participants. BMC Neurosci 16: 52–52, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Nakao H, Ballim HM, and Gellhorn E. The role of the sino-aortic receptors in the action of adrenaline, nor-adrenaline and acetylcholine on the cerebral cortex. Electroencephalogr Clin Neurophysiol 8: 413–420, 1956. [DOI] [PubMed] [Google Scholar]
- 381.Nance DM, and Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21: 736–745, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Napadow V, Edwards RR, Cahalan CM, Mensing G, Greenbaum S, Valovska A, Li A, Kim J, Maeda Y, Park K, and Wasan AD. Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med 13: 777–789, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Naranjo JR, and Fuentes JA. Association between hypoalgesia and hypertension in rats after short-term isolation. Neuropharmacology 24: 167–171, 1985. [DOI] [PubMed] [Google Scholar]
- 384.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, and Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation 98: 1071–1077, 1998. [DOI] [PubMed] [Google Scholar]
- 385.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, and Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 98: 772–776, 1998. [DOI] [PubMed] [Google Scholar]
- 386.Nasri-Heir C, Khan J, Benoliel R, Feng C, Yarnitsky D, Kuo F, Hirschberg C, Hartwell G, Huang CY, Heir G, Korczeniewska O, Diehl SR, and Eliav E. Altered pain modulation in patients with persistent postendodontic pain. Pain 156: 2032–2041, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Ness TJ, Fillingim RB, Randich A, Backensto EM, and Faught E. Low intensity vagal nerve stimulation lowers human thermal pain thresholds. Pain 86: 81–85, 2000. [DOI] [PubMed] [Google Scholar]
- 388.Nielsen CS, Knudsen GP, and Steingrimsdottir OA. Twin studies of pain. Clin Genet 82: 331–340, 2012. [DOI] [PubMed] [Google Scholar]
- 389.Nielsen R, Nikolajsen L, Kroner K, Molgaard H, Vase L, Jensen TS, and Terkelsen AJ. Pre-operative baroreflex sensitivity and efferent cardiac parasympathetic activity are correlated with post-operative pain. Acta Anaesthesiol Scand 59: 475–485, 2015. [DOI] [PubMed] [Google Scholar]
- 390.Numao Y, Saito M, Terui N, and Kumada M. Physiological and pharmacological properties of the three subsystems constituting the aortic nerve-renal sympathetic reflex in rabbits. J Auton Nerv Syst 9: 361–380, 1983. [DOI] [PubMed] [Google Scholar]
- 391.Numao Y, Siato M, Terui N, and Kumada M. The aortic nerve-sympathetic reflex in the rat. J Auton Nerv Syst 13: 65–79, 1985. [DOI] [PubMed] [Google Scholar]
- 392.Nyklicek I, Wijnen V, and Rau H. Effects of baroreceptor stimulation and opioids on the auditory startle reflex. Psychophysiology 42: 213–222, 2005. [DOI] [PubMed] [Google Scholar]
- 393.O’Hagan KP, Bell LB, Mittelstadt SW, and Clifford PS. Cardiac receptors modulate the renal sympathetic response to dynamic exercise in rabbits. J App Physiol (Bethesda, Md : 1985) 76: 507–515, 1994. [DOI] [PubMed] [Google Scholar]
- 394.Oberg B, and White S. Circulatory effects of interruption and stimulation of cardiac vagal afferents. Acta Physiol Scand 80: 383–394, 1970. [DOI] [PubMed] [Google Scholar]
- 395.Ogawa Y, Kanbayashi T, Saito Y, Takahashi Y, Kitajima T, Takahashi K, Hishikawa Y, and Shimizu T. Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique. Sleep 26: 986–989, 2003. [DOI] [PubMed] [Google Scholar]
- 396.Ogoh S, Fadel PJ, Nissen P, Jans O, Selmer C, Secher NH, and Raven PB. Baroreflex-mediated changes in cardiac output and vascular conductance in response to alterations in carotid sinus pressure during exercise in humans. J Physiol 550: 317–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ogoh S, Fisher JP, Fadel PJ, and Raven PB. Increases in central blood volume modulate carotid baroreflex resetting during dynamic exercise in humans. J Physiol 581: 405–418, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Oppenheimer S, and Cechetto D. The insular cortex and the regulation of cardiac function. Compr Physiol 6: 1081–1133, 2016. [DOI] [PubMed] [Google Scholar]
- 399.Osanai S, Takahashi T, Nakano H, and Kikuchi K. Doxapram inhibits carotid sinus baroreceptors in rabbits. Auton Autacoid Pharmacol 25: 79–84, 2005. [DOI] [PubMed] [Google Scholar]
- 400.Owens NC, Sartor DM, and Verberne AJ. Medial prefrontal cortex depressor response: role of the solitary tract nucleus in the rat. Neuroscience 89: 1331–1346, 1999. [DOI] [PubMed] [Google Scholar]
- 401.Padel Y, and Dell P. [Bulbar and Reticular Effects of Soporific Stimulation of the Vago-Aortic Trunk]. J Physiol (Paris) 57: 269–270, 1965. [PubMed] [Google Scholar]
- 402.Paintal A. A study of right and left atrial receptors. J Physiol 120: 596, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Paintal AS. Mechanism of stimulation of type J pulmonary receptors. J Physiol 203: 511–532, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Panksepp J. Aggression elicited by electrical stimulation of the hypothalamus in albino rats. Physiol Behav 6: 321–329, 1971. [DOI] [PubMed] [Google Scholar]
- 405.Panzenbeck MJ, Tan W, Hajdu MA, and Zucker IH. Intracoronary infusion of prostaglandin I2 attenuates arterial baroreflex control of heart rate in conscious dogs. Circ Res 63: 860–868, 1988. [DOI] [PubMed] [Google Scholar]
- 406.Papelier Y, Escourrou P, Gauthier JP, and Rowell LB. Carotid baroreflex control of blood pressure and heart rate in men during dynamic exercise. J App Physiol (Bethesda, Md : 1985) 77: 502–506, 1994. [DOI] [PubMed] [Google Scholar]
- 407.Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, and Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension 12: 214–222, 1988. [DOI] [PubMed] [Google Scholar]
- 408.Parati G, Di Rienzo M, and Mancia G. How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hypertens 18: 7–19, 2000. [PubMed] [Google Scholar]
- 409.Perez-Borja C, and Meyer JS. Electroencephalographic monitoring during reconstructive surgery of the neck vessels. Electroencephalogr Clin Neurophysiol 18: 162–169, 1965. [DOI] [PubMed] [Google Scholar]
- 410.Persson P, Ehmke H, Kirchheim H, and Seller H. Effect of sino-aortic denervation in comparison to cardiopulmonary deafferentiation on long-term blood pressure in conscious dogs. Pflugers Archiv 411: 160–166, 1988. [DOI] [PubMed] [Google Scholar]
- 411.Pfleeger M, Straneva PA, Fillingim RB, Maixner W, and Girdler SS. Menstrual cycle, blood pressure and ischemic pain sensitivity in women: a preliminary investigation. Int J Psychophysiol 27: 161–166, 1997. [DOI] [PubMed] [Google Scholar]
- 412.Pickering TG, Gribbin B, Petersen ES, Cunningham DJ, and Sleight P. Effects of autonomic blockade on the baroreflex in man at rest and during exercise. Circ Res 30: 177–185, 1972. [DOI] [PubMed] [Google Scholar]
- 413.Pierce GL, Harris SA, Seals DR, Casey DP, Barlow PB, and Stauss HM. Estimated aortic stiffness is independently associated with cardiac baroreflex sensitivity in humans: role of ageing and habitual endurance exercise. J Hum Hypertens 30: 513–520, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Pilowsky PM, and Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 20: 1675–1688, 2002. [DOI] [PubMed] [Google Scholar]
- 415.Pongratz G, and Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther 16: 504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology 32: 301–318, 1995. [DOI] [PubMed] [Google Scholar]
- 417.Porges SW. The polyvagal perspective. Biol Psychol 74: 116–143, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE, and Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med Rev 16: 151–166, 2012. [DOI] [PubMed] [Google Scholar]
- 419.Potts PD, Polson JW, Hirooka Y, and Dampney RA. Effects of sinoaortic denervation on Fos expression in the brain evoked by hypertension and hypotension in conscious rabbits. Neuroscience 77: 503–520, 1997. [DOI] [PubMed] [Google Scholar]
- 420.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, and Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 110: 16574–16579, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Poyhonen-Alho MK, Manhem K, Katzman P, Kibarskis A, Antikainen RL, Erkkola RU, Tuomilehto JO, Ebeling PE, and Kaaja RJ. Central sympatholytic therapy has anti-inflammatory properties in hypertensive postmenopausal women. J Hypertens 26: 2445–2449, 2008. [DOI] [PubMed] [Google Scholar]
- 422.Pritchard TC, Hamilton RB, and Norgren R. Projections of the parabrachial nucleus in the old world monkey. Exp Neurol 165: 101–117, 2000. [DOI] [PubMed] [Google Scholar]
- 423.Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, and et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med 330: 811–815, 1994. [DOI] [PubMed] [Google Scholar]
- 424.Qi XY, Zhang ZX, Cui QQ, Shi WB, and Xu YQ. [The effect of ginkgolide B on action potential, L-type calcium current and delayed rectifier potassium current in ischemic guinea pig ventricular myocytes]. Chinese J Applied Physiol 20: 24–28, 2004. [PubMed] [Google Scholar]
- 425.Qin XM, Fan ZZ, and He RR. [Agmatine inhibits the afferent activity of carotid baroreceptor in rats]. Sheng Li Xue Bao 53: 137–141, 2001. [PubMed] [Google Scholar]
- 426.Qin XM, and He RR. [Role of calcium in the mechanism underlying the inhibitory effect of streptomycin on carotid sinus baroreflex in rats]. Sheng Li Xue Bao 52: 463–467, 2000. [PubMed] [Google Scholar]
- 427.Qiu C, Winblad B, and Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol 4: 487–499, 2005. [DOI] [PubMed] [Google Scholar]
- 428.Querry RG, Smith SA, Strømstad M, Ide K, Raven PB, and Secher NH. Neural blockade during exercise augments central command’s contribution to carotid baroreflex resetting. Am J Physiol Heart Cir Physiol 280: H1635–H1644, 2001. [DOI] [PubMed] [Google Scholar]
- 429.Randich A, and Gebhart GF. Vagal afferent modulation of nociception. Brain Res Brain Res Rev 17: 77–99, 1992. [DOI] [PubMed] [Google Scholar]
- 430.Randich A, and Maixner W. [D-Ala2]-methionine enkephalinamide reflexively induces antinociception by activating vagal afferents. Pharmacol Biochem Behav 21: 441–448, 1984. [DOI] [PubMed] [Google Scholar]
- 431.Randich A, and Maixner W. Interactions between cardiovascular and pain regulatory systems. Neurosci Biobehav Rev 8: 343–367, 1984. [DOI] [PubMed] [Google Scholar]
- 432.Randich A, and Maixner W. The role of sinoaortic and cardiopulmonary baroreceptor reflex arcs in nociception and stress-induced analgesia. Ann N Y Acad Sci 467: 385–401, 1986. [DOI] [PubMed] [Google Scholar]
- 433.Randich A, and Robertson JD. Spinal nociceptive transmission in the spontaneously hypertensive and Wistar-Kyoto normotensive rat. Pain 58: 169–183, 1994. [DOI] [PubMed] [Google Scholar]
- 434.Rau H, Brody S, Larbig W, Pauli P, Vohringer M, Harsch B, Kroling P, and Birbaumer N. Effects of PRES baroreceptor stimulation on thermal and mechanical pain threshold in borderline hypertensives and normotensives. Psychophysiology 31: 480–485, 1994. [DOI] [PubMed] [Google Scholar]
- 435.Rau H, and Elbert T. Psychophysiology of arterial baroreceptors and the etiology of hypertension. Biol Psychol 57: 179–201, 2001. [DOI] [PubMed] [Google Scholar]
- 436.Ray CA, Rea RF, Clary MP, and Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol 264: H1–7, 1993. [DOI] [PubMed] [Google Scholar]
- 437.Raymond J, Davis GM, van Der Plas MN, Groeller H, and Simcox S. Carotid baroreflex control of heart rate and blood pressure during ES leg cycling in paraplegics. J Applied Physiol (Bethesda, Md : 1985) 88: 957–965, 2000. [DOI] [PubMed] [Google Scholar]
- 438.Ren K, Randich A, and Gebhart GF. Modulation of spinal nociceptive transmission from nuclei tractus solitarii: a relay for effects of vagal afferent stimulation. J Neurophysiol 63: 971–986, 1990. [DOI] [PubMed] [Google Scholar]
- 439.Renaud LP. CNS pathways mediating cardiovascular regulation of vasopressin. Clin Exp Pharmacol Physiol 23: 157–160, 1996. [DOI] [PubMed] [Google Scholar]
- 440.Resstel LB, Fernandes KB, and Correa FM. Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Res 1015: 136–144, 2004. [DOI] [PubMed] [Google Scholar]
- 441.Reyes Del Paso GA, de la Coba P, Martin-Vazquez M, and Thayer JF. Time domain measurement of the vascular and myocardial branches of the baroreflex: A study in physically active versus sedentary individuals. Psychophysiology 54: 1528–1540, 2017. [DOI] [PubMed] [Google Scholar]
- 442.Reyes del Paso GA, Garrido S, Pulgar A, and Duschek S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J Psychosom Res 70: 125–134, 2011. [DOI] [PubMed] [Google Scholar]
- 443.Reyes del Paso GA, González I, and Hernández JA. Baroreceptor sensitivity and effectiveness varies differentially as a function of cognitive-attentional demands. Biol Psychol 67: 385–395, 2004. [DOI] [PubMed] [Google Scholar]
- 444.Reyes del Paso GA, and Gonzalez MI. Modification of baroreceptor cardiac reflex function by biofeedback. Appl Psychophysiol Biofeedback 29: 197–211, 2004. [DOI] [PubMed] [Google Scholar]
- 445.Reyes Del Paso GA, Gonzalez MI, Hernandez JA, Duschek S, and Gutierrez N. Tonic blood pressure modulates the relationship between baroreceptor cardiac reflex sensitivity and cognitive performance. Psychophysiology 46: 932–938, 2009. [DOI] [PubMed] [Google Scholar]
- 446.Reyes Del Paso GA, Langewitz W, Robles H, and Perez N. A between-subjects comparison of respiratory sinus arrhythmia and baroreceptor cardiac reflex sensitivity as non-invasive measures of tonic parasympathetic cardiac control. J Psychophysiol 22: 163–171, 1996. [DOI] [PubMed] [Google Scholar]
- 447.Reyes Del Paso GA, Mata JL, and Martin-Vazquez M. Relationships between baroreceptor cardiac reflex sensitivity and cognitive performance: Modulations by gender and blood pressure. Psychophysiology 49: 138–144, 2012. [DOI] [PubMed] [Google Scholar]
- 448.Rice ASC, Dworkin RH, McCarthy TD, Anand P, Bountra C, McCloud PI, Hill J, Cutter G, Kitson G, Desem N, Raff M, and group EMAs. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet (London, England) 383: 1637–1647, 2014. [DOI] [PubMed] [Google Scholar]
- 449.Robbe HW, Mulder LJ, Ruddel H, Langewitz WA, Veldman JB, and Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension 10: 538–543, 1987. [DOI] [PubMed] [Google Scholar]
- 450.Roberts AM, Bhattacharya J, Schultz HD, Coleridge HM, and Coleridge JC. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res 58: 512–522, 1986. [DOI] [PubMed] [Google Scholar]
- 451.Roddie IC, Shepherd JT, and Whelan RF. Reflex changes in vasoconstrictor tone in human skeletal muscle in response to stimulation of receptors in a low-pressure area of the intrathoracic vascular bed. J Physiol 139: 369–376, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Romero-Osorio O, Gil-Tamayo S, Narino D, and Rosselli D. Changes in sleep patterns after vagus nerve stimulation, deep brain stimulation or epilepsy surgery: Systematic review of the literature. Seizure 56: 4–8, 2018. [DOI] [PubMed] [Google Scholar]
- 453.Rosa C, Ghione S, Mezzasalma L, Pellegrini M, Basile Fasolo C, Giaconi S, Gazzetti P, and Ferdeghini M. Relationship between pain sensitivity, cardiovascular reactivity to cold pressor test and indexes of activity of the adrenergic and opioid system. Clin Exp Hypertens A 10Suppl 1: 383–390, 1988. [DOI] [PubMed] [Google Scholar]
- 454.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, and Tracey KJ. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 15: 195–202, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Rosenbaum M, and Race D. Frequency-response characteristics of vascular resistance vessels. Am J Physiol 215: 1397–1402, 1968. [DOI] [PubMed] [Google Scholar]
- 456.Roth RA, and Wallace KB. Disposition of biogenic amines and angiotensin I by lungs of spontaneously hypertensive rats. Am J Physiol 239: H736–741, 1980. [DOI] [PubMed] [Google Scholar]
- 457.Rother M, Witte H, Zwiener U, Eiselt M, and Fischer P. Cardiac aliasing--a possible cause for the misinterpretation of cardiorespirographic data in neonates. Early Hum Dev 20: 1–12, 1989. [DOI] [PubMed] [Google Scholar]
- 458.Rowell LB, and O’Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Applied Physiol (Bethesda, Md : 1985) 69: 407–418, 1990. [DOI] [PubMed] [Google Scholar]
- 459.Royall DR, Gao JH, and Kellogg DL Jr. Insular Alzheimer’s disease pathology as a cause of “age-related” autonomic dysfunction and mortality in the non-demented elderly. Med Hypotheses 67: 747–758, 2006. [DOI] [PubMed] [Google Scholar]
- 460.Rushworth MF. Intention, choice, and the medial frontal cortex. Ann N Y Acad Sci 1124: 181–207, 2008. [DOI] [PubMed] [Google Scholar]
- 461.Saad MA, Huerta F, Trancard J, and Elghozi JL. Effects of middle cerebral artery occlusion on baroreceptor reflex control of heart rate in the rat. J Auton Nerv Syst 27: 165–172, 1989. [DOI] [PubMed] [Google Scholar]
- 462.Saavedra JM. Naloxone reversible decrease in pain sensitivity in young and adult spontaneously hypertensive rats. Brain Res 209: 245–249, 1981. [DOI] [PubMed] [Google Scholar]
- 463.Saavedra JM. Spontaneously (genetic) hypertensive rats: naloxone-reversible and propranolol-reversible decrease in pain sensitivity. Experientia 37: 1002–1003, 1981. [DOI] [PubMed] [Google Scholar]
- 464.Saint Martin M, Sforza E, Thomas-Anterion C, Barthelemy JC, Roche F, and Group PS. Baroreflex sensitivity, vascular risk factors, and cognitive function in a healthy elderly population: the PROOF cohort. J Am Geriatr Soc 61: 2096–2102, 2013. [DOI] [PubMed] [Google Scholar]
- 465.Saito H, Sakai K, and Jouvet M. Discharge patterns of the nucleus parabrachialis lateralis neurons of the cat during sleep and waking. Brain Res 134: 59–72, 1977. [DOI] [PubMed] [Google Scholar]
- 466.Saito M, Tsukanaka A, Yanagihara D, and Mano T. Muscle sympathetic nerve responses to graded leg cycling. J App Physiol (Bethesda, Md : 1985) 75: 663–667, 1993. [DOI] [PubMed] [Google Scholar]
- 467.Saleh TM, and Connell BJ. Role of the insular cortex in the modulation of baroreflex sensitivity. Am J Physiol 274: R1417–1424, 1998. [DOI] [PubMed] [Google Scholar]
- 468.Sampson SR, and Mills E. Effects of sympathetic stimulation on discharges of carotid sinus baroreceptors. Am J Physiol 218: 1650–1653, 1970. [DOI] [PubMed] [Google Scholar]
- 469.Sanders JS, and Ferguson DW. Cardiopulmonary baroreflexes fail to modulate sympathetic responses during isometric exercise in humans: direct evidence from microneurographic studies. J Am Coll Cardiol 12: 1241–1251, 1988. [DOI] [PubMed] [Google Scholar]
- 470.Santos-Almeida FM, Domingos-Souza G, Meschiari CA, Favaro LC, Becari C, Castania JA, Lopes A, Cunha TM, Moraes DJA, Cunha FQ, Ulloa L, Kanashiro A, Tezini G, and Salgado HC. Carotid sinus nerve electrical stimulation in conscious rats attenuates systemic inflammation via chemoreceptor activation. Sci Rep 7: 6265, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25: 433–469, 2002. [DOI] [PubMed] [Google Scholar]
- 472.Saper CB, Scammell TE, and Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257–1263, 2005. [DOI] [PubMed] [Google Scholar]
- 473.Satoh H, and Nishida S. Electropharmacological actions of Ginkgo biloba extract on vascular smooth and heart muscles. Clin Chim Acta 342: 13–22, 2004. [DOI] [PubMed] [Google Scholar]
- 474.Saxon SA. Detection of near threshold signals during four phases of cardiac cycle. Ala J Med Sci 7: 427–430, 1970. [PubMed] [Google Scholar]
- 475.Scher AM, and Young AC. Reflex control of heart rate in the unanesthetized dog. Am J Physiol 218: 780–789, 1970. [DOI] [PubMed] [Google Scholar]
- 476.Scherrer U, Pryor SL, Bertocci LA, and Victor RG. Arterial baroreflex buffering of sympathetic activation during exercise-induced elevations in arterial pressure. J Clin Invest 86: 1855–1861, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Schiller AM, Pellegrino PR, and Zucker IH. The renal nerves in chronic heart failure: efferent and afferent mechanisms. Front Physiol 6: 224–224, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Schlager E, and Meier T. A strange Balinese method of inducing sleep with some notes about balyans. Acta Trop 4: 127–134, 1947. [PubMed] [Google Scholar]
- 479.Schobel HP, Handwerker HO, Schmieder RE, Heusser K, Dominiak P, and Luft FC. Effects of naloxone on hemodynamic and sympathetic nerve responses to pain in normotensive vs. borderline hypertensive men. J Auton Nerv Syst 69: 49–55, 1998. [DOI] [PubMed] [Google Scholar]
- 480.Seagard JL, Gallenberg LA, Hopp FA, and Dean C. Acute resetting in two functionally different types of carotid baroreceptors. Circ Res 70: 559–565, 1992. [DOI] [PubMed] [Google Scholar]
- 481.Seagard JL, Hopp FA, Drummond HA, and Van Wynsberghe DM. Selective contribution of two types of carotid sinus baroreceptors to the control of blood pressure. Circ Res 72: 1011–1022, 1993. [DOI] [PubMed] [Google Scholar]
- 482.Seals DR. Cardiopulmonary baroreflexes do not modulate exercise-induced sympathoexcitation. J App Physiol (Bethesda, Md : 1985) 64: 2197–2203, 1988. [DOI] [PubMed] [Google Scholar]
- 483.Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Applied Physiol (Bethesda, Md : 1985) 66: 2472–2478, 1989. [DOI] [PubMed] [Google Scholar]
- 484.Serhan CN, de la Rosa X, and Jouvene C. Novel mediators and mechanisms in the resolution of infectious inflammation: evidence for vagus regulation. J Intern Med 286(3): 240–258, 2019. [DOI] [PubMed] [Google Scholar]
- 485.Serhan CN, de la Rosa X, and Jouvene CC. Cutting edge: Human vagus produces specialized proresolving mediators of inflammation with electrical stimulation reducing proinflammatory eicosanoids. J Immunol 201: 3161–3165, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Sevoz-Couche C, Comet MA, Bernard JF, Hamon M, and Laguzzi R. Cardiac baroreflex facilitation evoked by hypothalamus and prefrontal cortex stimulation: role of the nucleus tractus solitarius 5-HT2A receptors. Am J Physiol Regul Integr Comp Physiol 291: R1007–1015, 2006. [DOI] [PubMed] [Google Scholar]
- 487.Sforza E, Jouny C, and Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol 111: 1611–1619, 2000. [DOI] [PubMed] [Google Scholar]
- 488.Shah AJ, Su S, Veledar E, Bremner JD, Goldstein FC, Lampert R, Goldberg J, and Vaccarino V. Is heart rate variability related to memory performance in middle-aged men? Psychosom Med 73: 475–482, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Shapiro AP, Miller RE, King HE, Ginchereau EH, and Fitzgibbon K. Behavioral consequences of mild hypertension. Hypertension 4: 355–360, 1982. [DOI] [PubMed] [Google Scholar]
- 490.Sheffield D, Biles PL, Orom H, Maixner W, and Sheps DS. Race and sex differences in cutaneous pain perception. Psychosom Med 62: 517–523, 2000. [DOI] [PubMed] [Google Scholar]
- 491.Sheng J, Liu S, Wang Y, Cui R, and Zhang X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast 2017: 9724371–9724371, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Shepherd AJ, Mickle AD, Golden JP, Mack MR, Halabi CM, de Kloet AD, Samineni VK, Kim BS, Krause EG, Gereau RWt, and Mohapatra DP. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci U S A 115: E8057–e8066, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Shepherd AJ, and Mohapatra DP. Attenuation of Unevoked Mechanical and Cold Pain Hypersensitivities Associated With Experimental Neuropathy in Mice by Angiotensin II Type-2 Receptor Antagonism. Anesth Analg 128: e84–e87, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Sheps DS, Bragdon EE, Gray TF 3rd, Ballenger M Usedom JE, and Maixner W. Relation between systemic hypertension and pain perception. Am J Cardiol 70: 3F–5F, 1992. [DOI] [PubMed] [Google Scholar]
- 495.Shih CD, and Chuang YC. Nitric oxide and GABA mediate bi-directional cardiovascular effects of orexin in the nucleus tractus solitarii of rats. Neuroscience 149: 625–635, 2007. [DOI] [PubMed] [Google Scholar]
- 496.Shimoda O, and Ikuta Y. The current perception thresholds vary between horizontal and 70 degrees tilt-up positions. Anesth Analg 91: 398–402, 2000. [DOI] [PubMed] [Google Scholar]
- 497.Silberstein SD, Calhoun AH, Lipton RB, Grosberg BM, Cady RK, Dorlas S, Simmons KA, Mullin C, Liebler EJ, Goadsby PJ, and Saper JR. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology 87: 529–538, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Silva GJ, Brum PC, Negrão CE, and Krieger EM. Acute and chronic effects of exercise on baroreflexes in spontaneously hypertensive rats. Hypertension (Dallas, Tex : 1979) 30: 714–719, 1997. [DOI] [PubMed] [Google Scholar]
- 499.Silvani A. Physiological sleep-dependent changes in arterial blood pressure: central autonomic commands and baroreflex control. Clin Exp Pharmacol Physiol 35: 987–994, 2008. [DOI] [PubMed] [Google Scholar]
- 500.Silvani A, Calandra-Buonaura G, Benarroch EE, Dampney RA, and Cortelli P. Bidirectional interactions between the baroreceptor reflex and arousal: an update. Sleep Med 16: 210–216, 2015. [DOI] [PubMed] [Google Scholar]
- 501.Sitsen JM, and de Jong W. Hypoalgesia in genetically hypertensive rats (SHR) is absent in rats with experimental hypertension. Hypertension 5: 185–190, 1983. [DOI] [PubMed] [Google Scholar]
- 502.Sjogren MJ, Hellstrom PT, Jonsson MA, Runnerstam M, Silander HC, and Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J Clin Psychiatry 63: 972–980, 2002. [DOI] [PubMed] [Google Scholar]
- 503.Sleight P, Robinson JL, Brooks DE, and Rees PM. Characteristics of single carotid sinus baroreceptor fibers and whole nerve activity in the normotensive and the renal hypertensive dog. Circ Res 41: 750–758, 1977. [DOI] [PubMed] [Google Scholar]
- 504.Smith SA, Querry RG, Fadel PJ, Gallagher KM, Strømstad M, Ide K, Raven PB, and Secher NH. Partial blockade of skeletal muscle somatosensory afferents attenuates baroreflex resetting during exercise in humans. J Physiol 551: 1013–1021, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Spaziani R, Bayati A, Redmond K, Bajaj H, Mazzadi S, Bienenstock J, Collins SM, and Kamath MV. Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterol and Motility 20: 336–342, 2008. [DOI] [PubMed] [Google Scholar]
- 506.Spengler RN, Allen RM, Remick DG, Strieter RM, and Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol 145: 1430–1434, 1990. [PubMed] [Google Scholar]
- 507.Sposito AC, Mansur AP, Coelho OR, Nicolau JC, and Ramires JA. Additional reduction in blood pressure after cholesterol-lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin-converting enzyme inhibitors (enalapril or lisinopril). Am J Cardiol 83: 1497–1499, a1498, 1999. [DOI] [PubMed] [Google Scholar]
- 508.Staszewska-Barczak J. Prostanoids and cardiac reflexes of sympathetic and vagal origin. Am J Cardiol 52: 36a–45a, 1983. [DOI] [PubMed] [Google Scholar]
- 509.Stelling C. Experimentelle Untersuchungen über den Einfluss des Nervus depressor auf die Herztätigkeit und den Blutdruck. In: Dissertation Dorpat 1867, p. 38–41. [Google Scholar]
- 510.Steptoe A, and Sawada Y. Assessment of baroreceptor reflex function during mental stress and relaxation. Psychophysiology 26: 140–147, 1989. [DOI] [PubMed] [Google Scholar]
- 511.Sterman MB, and Clemente CD. Forebrain inhibitory mechanisms: cortical synchronization induced by basal forebrain stimulation. Exp Neurol 6: 91–102, 1962. [DOI] [PubMed] [Google Scholar]
- 512.Straub RH, Grum F, Strauch U, Capellino S, Bataille F, Bleich A, Falk W, Scholmerich J, and Obermeier F. Anti-inflammatory role of sympathetic nerves in chronic intestinal inflammation. Gut 57: 911–921, 2008. [DOI] [PubMed] [Google Scholar]
- 513.Strigo IA, and Craig AD. Interoception, homeostatic emotions and sympathovagal balance. Philos Trans R Soc Lond B Biol Sci 371(1708): 20160010, 2016. (electronic publication, 9 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Strong EB, and Green JF. Multireceptor activation of the pulmonary chemoreflex. J Applied Physiol (Bethesda, Md : 1985) 70: 368–370, 1991. [DOI] [PubMed] [Google Scholar]
- 515.Suarez-Roca H, Klinger RY, Podgoreanu MV, Ji RR, Sigurdsson MI, Waldron N, Mathew JP, and Maixner W. Contribution of Baroreceptor Function to Pain Perception and Perioperative Outcomes. Anesthesiology 130: 634–650, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Subha M, Pal P, Pal GK, Habeebullah S, Adithan C, and Sridhar MG. Decreased baroreflex sensitivity is linked to sympathovagal imbalance, low-grade inflammation, and oxidative stress in pregnancy-induced hypertension. Clin Exp Hypertens 38: 666–672, 2016. [DOI] [PubMed] [Google Scholar]
- 517.Sugimachi M, Imaizumi T, Sunagawa K, Hirooka Y, Todaka K, Takeshita A, and Nakamura M. A new method to identify dynamic transduction properties of aortic baroreceptors. Am J Physiol 258: H887–895, 1990. [DOI] [PubMed] [Google Scholar]
- 518.Sun MK. Medullospinal vasomotor neurones mediate hypotension from stimulation of prefrontal cortex. J Auton Nerv Syst 38: 209–217, 1992. [DOI] [PubMed] [Google Scholar]
- 519.Sundlof G, and Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Sved AF, Schreihofer AM, and Kost CK, Jr. Blood pressure regulation in baroreceptor-denervated rats. Clin Exp Pharmacol Physiol 24: 77–82, 1997. [DOI] [PubMed] [Google Scholar]
- 521.Sved AF, Tsukamoto K, and Schreihofer AM. Stimulation of alpha 2-adrenergic receptors in nucleus tractus solitarius is required for the baroreceptor reflex. Brain Res 576: 297–303, 1992. [DOI] [PubMed] [Google Scholar]
- 522.Sverrisdottir YB, Green AL, Aziz TZ, Bahuri NF, Hyam J, Basnayake SD, and Paterson DJ. Differentiated baroreflex modulation of sympathetic nerve activity during deep brain stimulation in humans. Hypertension 63: 1000–1010, 2014. [DOI] [PubMed] [Google Scholar]
- 523.Sverrisdottir YB, Rundqvist B, Johannsson G, and Elam M. Sympathetic neural burst amplitude distribution: A more specific indicator of sympathoexcitation in human heart failure. Circulation 102: 2076–2081, 2000. [DOI] [PubMed] [Google Scholar]
- 524.Syamsunder AN, Pal P, Pal GK, Kamalanathan CS, Parija SC, Nanda N, and Sirisha A. Decreased baroreflex sensitivity is linked to the atherogenic index, retrograde inflammation, and oxidative stress in subclinical hypothyroidism. Endocr Res 42: 49–58, 2017. [DOI] [PubMed] [Google Scholar]
- 525.Szelenyi J, Kiss JP, and Vizi ES. Differential involvement of sympathetic nervous system and immune system in the modulation of TNF-alpha production by alpha2- and beta-adrenoceptors in mice. J Neuroimmunol 103: 34–40, 2000. [DOI] [PubMed] [Google Scholar]
- 526.Tafil-Klawe M, Raschke F, and Hildebrandt G. Functional asymmetry in carotid sinus cardiac reflexes in humans. Eur J Appl Physiol Occup Physiol 60: 402–405, 1990. [DOI] [PubMed] [Google Scholar]
- 527.Takeshita A, Mark AL, Eckberg DL, and Abboud FM. Effect of central venous pressure on arterial baroreflex control of heart rate. Am J Physiol 236: H42–47, 1979. [DOI] [PubMed] [Google Scholar]
- 528.Takigawa M, and Mogenson GJ. A study of inputs to antidromically identified neurons of the locus coeruleus. Brain Res 135: 217–230, 1977. [DOI] [PubMed] [Google Scholar]
- 529.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, and Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal 19: 251–260, 2007. [DOI] [PubMed] [Google Scholar]
- 530.Tang GJ, Kou YR, and Lin YS. Peripheral neural modulation of endotoxin-induced hyperventilation. Crit Care Med 26: 1558–1563, 1998. [DOI] [PubMed] [Google Scholar]
- 531.Tank J, Jordan J, Diedrich A, Stoffels M, Franke G, Faulhaber HD, Luft FC, and Busjahn A. Genetic influences on baroreflex function in normal twins. Hypertension 37: 907–910, 2001. [DOI] [PubMed] [Google Scholar]
- 532.Tatar M, Webber SE, and Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol 402: 411–420, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Taylor CE, Atkinson G, Willie CK, Jones H, Ainslie PN, and Tzeng YC. Diurnal variation in the mechanical and neural components of the baroreflex. Hypertension (Dallas, Tex : 1979) 58: 51–56, 2011. [DOI] [PubMed] [Google Scholar]
- 534.Taylor RF, Lee LY, Jewell LA, and Frazier DT. Effect of nicotine aerosol on slowly adapting receptors in the airways of the dog. J Neurosci Res 15: 583–593, 1986. [DOI] [PubMed] [Google Scholar]
- 535.Tchivileva IE, Tan KS, Gambarian M, Nackley AG, Medvedev AV, Romanov S, Flood PM, Maixner W, Makarov SS, and Diatchenko L. Signaling pathways mediating beta3-adrenergic receptor-induced production of interleukin-6 in adipocytes. Mol Immunol 46: 2256–2266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Tecce JJ. Contingent negative variation (CNV) and psychological processes in man. Psychol Bull 77: 73–108, 1972. [DOI] [PubMed] [Google Scholar]
- 537.Terreberry RR, and Neafsey EJ. Rat medial frontal cortex: a visceral motor region with a direct projection to the solitary nucleus. Brain Res 278: 245–249, 1983. [DOI] [PubMed] [Google Scholar]
- 538.Thieme K, Meller T, Evermann U, Malinowski R, Mathys M, Gracely RH, Maixner W, and Turk DC. Efficacy of Systolic Extinction Training (SET) in Fibromyalgia Patients with elevated Blood Pressure Response to Stress - A Tailored RCT Study. Arthritis Care Res (Hoboken) doi: 10.1002/acr.23615., 2018. [DOI] [PubMed] [Google Scholar]
- 539.Thies R, and Foreman RD. Descending inhibition of spinal neurons in the cardiopulmonary region by electrical stimulation of vagal afferent nerves. Brain Res 207: 178–183, 1981. [DOI] [PubMed] [Google Scholar]
- 540.Thies R, and Foreman RD. Inhibition and excitation of thoracic spinoreticular neurons by electrical stimulation of vagal afferent nerves. Exp Neurol 82: 1–16, 1983. [DOI] [PubMed] [Google Scholar]
- 541.Thoren P, Andresen MC, and Brown AM. Effects of changes in extracellular ionic concentrations on aortic baroreceptors with nonmyelinated afferent fibers. Circ Res 50: 413–418, 1982. [DOI] [PubMed] [Google Scholar]
- 542.Thoren PN. Characteristics of left ventricular receptors with nonmedullated vagal afferents in cats. Circ Res 40: 415–421, 1977. [DOI] [PubMed] [Google Scholar]
- 543.Thoren PN, Donald DE, and Shepherd JT. Role of heart and lung receptors with nonmedullated vagal afferents in circulatory control. Circ Res 38: 2–9, 1976. [DOI] [PubMed] [Google Scholar]
- 544.Thrasher TN. Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am J Physiol Reg Integ Comp Physiol 288: R819–R827, 2005. [DOI] [PubMed] [Google Scholar]
- 545.Thurston CL, and Randich A. Acute increases in arterial blood pressure produced by occlusion of the abdominal aorta induces antinociception: peripheral and central substrates. Brain Res 519: 12–22, 1990. [DOI] [PubMed] [Google Scholar]
- 546.Thurston CL, and Randich A. Effects of vagal afferent stimulation on ON and OFF cells in the rostroventral medulla: relationships to nociception and arterial blood pressure. J Neurophysiol 67: 180–196, 1992. [DOI] [PubMed] [Google Scholar]
- 547.Thurston CL, and Randich A. Responses of on and off cells in the rostral ventral medulla to stimulation of vagal afferents and changes in mean arterial blood pressure in intact and cardiopulmonary deafferented rats. Pain 62: 19–38, 1995. [DOI] [PubMed] [Google Scholar]
- 548.Tochikubo O, Kawano Y, Miyajima E, Toshihiro N, and Ishii M. Circadian variation of hemodynamics and baroreflex functions in patients with essential hypertension. Hypertens Res 20: 157–166, 1997. [DOI] [PubMed] [Google Scholar]
- 549.Tracey KJ. The inflammatory reflex. Nature 420: 853–859, 2002. [DOI] [PubMed] [Google Scholar]
- 550.Tracey KJ. Reflex control of immunity. Nat Rev Immunol 9: 418–428, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Tracy LM, Ioannou L, Baker KS, Gibson SJ, Georgiou-Karistianis N, and Giummarra MJ. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 157: 7–29, 2016. [DOI] [PubMed] [Google Scholar]
- 552.Trimboli M, Al-Kaisy A, Andreou AP, Murphy M, and Lambru G. Non-invasive vagus nerve stimulation for the management of refractory primary chronic headaches: A real-world experience. Cephalalgia 333102417731349, 2017. [DOI] [PubMed] [Google Scholar]
- 553.Ulleryd MA, Prahl U, Borsbo J, Schmidt C, Nilsson S, Bergstrom G, and Johansson ME. The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS One 12: e0174974, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, and Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor alpha7 subunit gene knockout mice. Ann Rheum Dis 69: 1717–1723, 2010. [DOI] [PubMed] [Google Scholar]
- 555.Van Zomeren AH, and Brouwer WH. Clinical Neuropsychology of Attention. New York: Oxford University Press, 1994. [Google Scholar]
- 556.Vasudev A, Saxby BK, O’Brien JT, Colloby SJ, Firbank MJ, Brooker H, Wesnes K, and Thomas AJ. Relationship between cognition, magnetic resonance white matter hyperintensities, and cardiovascular autonomic changes in late-life depression. Am J Geriatr Psychiatry 20: 691–699, 2012. [DOI] [PubMed] [Google Scholar]
- 557.Vatner SF, Boettcher DH, Heyndrickx GR, and McRitchie RJ. Reduced baroreflex sensitivity with volume loading in conscious dogs. Circ Res 37: 236–242, 1975. [DOI] [PubMed] [Google Scholar]
- 558.Vatner SF, Franklin D, Van Citters RL, and Braunwald E. Effects of carotid sinus nerve stimulation on blood-flow distribution in conscious dogs at rest and during exercise. Circ Res 27: 495–503, 1970. [DOI] [PubMed] [Google Scholar]
- 559.Veelken R, Hilgers KF, Ditting T, Fierlbeck W, Geiger H, and Schmieder RE. Subthreshold stimulation of a serotonin 5-HT3 reflex attenuates cardiovascular reflexes. Am J Physiol 271: R1500–1506, 1996. [DOI] [PubMed] [Google Scholar]
- 560.Veelken R, Leonard M, Stetter A, Hilgers KF, Mann JF, Reeh PW, Geiger H, and Luft FC. Pulmonary serotonin 5-HT3-sensitive afferent fibers modulate renal sympathetic nerve activity in rats. Am J Physiol 272: H979–986, 1997. [DOI] [PubMed] [Google Scholar]
- 561.Verberne AJ. Medullary sympathoexcitatory neurons are inhibited by activation of the medial prefrontal cortex in the rat. Am J Physiol 270: R713–719, 1996. [DOI] [PubMed] [Google Scholar]
- 562.Verberne AJ, Lewis SJ, Worland PJ, Beart PM, Jarrott B, Christie MJ, and Louis WJ. Medial prefrontal cortical lesions modulate baroreflex sensitivity in the rat. Brain Res 426: 243–249, 1987. [DOI] [PubMed] [Google Scholar]
- 563.von Bezold A, and Hirt L. Uber die physiologischen wirkungen des essigsauren veratrins. Untersuchungen aus dem physiologischen laboratorium Wurzburg 1: 75–156, 1867. [Google Scholar]
- 564.von Cyon E, and Ludwig C. Die Reflexe eines der sensiblen Nerven des Herzens auf die motorischen der Blutgefässe. Arbeiten aus der Physiologischen Anstalt zu Leipzig 1: 128–149, 1866. [Google Scholar]
- 565.Waldstein SR, Brown JR, Maier KJ, and Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med 29: 174–180, 2005. [DOI] [PubMed] [Google Scholar]
- 566.Waldstein SR, Giggey PP, Thayer JF, and Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension 45: 374–379, 2005. [DOI] [PubMed] [Google Scholar]
- 567.Waldstein SR, Ryan CM, Manuck SB, Parkinson DK, and Bromet EJ. Learning and memory function in men with untreated blood pressure elevation. J Consult Clin Psychol 59: 513–517, 1991. [DOI] [PubMed] [Google Scholar]
- 568.Walgenbach SC, and Donald DE. Inhibition by carotid baroreflex of exercise-induced increases in arterial pressure. Circ Res 52: 253–262, 1983. [DOI] [PubMed] [Google Scholar]
- 569.Walker BB, and Sandman CA. Visual evoked potentials change as heart rate and carotid pressure change. Psychophysiology 19: 520–527, 1982. [DOI] [PubMed] [Google Scholar]
- 570.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, and Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol 453: 45–58, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Walter WG, Cooper R, Aldridge VJ, McCallum WC, and Winter AL. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature 203: 380–384, 1964. [DOI] [PubMed] [Google Scholar]
- 572.Wang CY, Wu YM, Xiao L, Xue HM, Wang R, Wang FW, and He RR. Ginkgolide B inhibits carotid sinus baroreflex in anesthetized male rats. Sheng Li Xue Bao 60: 17–22, 2008. [PubMed] [Google Scholar]
- 573.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, and Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. [DOI] [PubMed] [Google Scholar]
- 574.Wang S, Fan ZZ, and He RR. [17beta -estradiol inhibits carotid sinus baroreflex in male rats]. Sheng Li Xue Bao 52: 445–449, 2000. [PubMed] [Google Scholar]
- 575.Watkins L, Sherwood A, Goldstein DS, and Maixner W. Mechanisms underlying cardiovascular defense reaction evoked by dorsal periaqueductal gray stimulation. Am J Physiol 265: R1155–1161, 1993. [DOI] [PubMed] [Google Scholar]
- 576.Weber F, and Dan Y. Circuit-based interrogation of sleep control. Nature 538: 51–59, 2016. [DOI] [PubMed] [Google Scholar]
- 577.Weidler C, Holzer C, Harbuz M, Hofbauer R, Angele P, Scholmerich J, and Straub RH. Low density of sympathetic nerve fibres and increased density of brain derived neurotrophic factor positive cells in RA synovium. Ann Rheum Dis 64: 13–20, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Weinstock M, Schorer-Apelbaum D, and Rosin AJ. Endogenous opiates mediate cardiac sympathetic inhibition in response to a pressor stimulus in rabbits. J Hypertens 2: 639–646, 1984. [DOI] [PubMed] [Google Scholar]
- 579.Weisz J, and Ádám G. The influence of cardiac phase on reaction time depending on heart period length and on stimulus and response laterality. Psychobiology 24: 169–175, 1996. [Google Scholar]
- 580.Weisz N, Schandry R, Jacobs AM, Mialet JP, and Duschek S. Early contingent negative variation of the EEG and attentional flexibility are reduced in hypotension. Int J Psychophysiol 45: 253–260, 2002. [DOI] [PubMed] [Google Scholar]
- 581.Widdicombe J. Lung afferent activity: implications for respiratory sensation. Respir Physiol Neurobiol 167: 2–8, 2009. [DOI] [PubMed] [Google Scholar]
- 582.Willemze RA, Welting O, van Hamersveld HP, Meijer SL, Folgering JHA, Darwinkel H, Witherington J, Sridhar A, Vervoordeldonk MJ, Seppen J, and de Jonge WJ. Neuronal control of experimental colitis occurs via sympathetic intestinal innervation. Neurogastroenterol Motil 30(3), 2018. DOI: 10.1111/nmo.13163 (Epub 2017 Jul 26. electronic publication, 13 pages). [DOI] [PubMed] [Google Scholar]
- 583.Willemze RA, Welting O, van Hamersveld P, Verseijden C, Nijhuis LE, Hilbers FW, Meijer SL, Heesters BA, Folgering JHA, Darwinkel H, Blancou P, Vervoordeldonk MJ, Seppen J, Heinsbroek SEM, and de Jonge WJ. Loss of intestinal sympathetic innervation elicits an innate immune driven colitis. Mol Med 25: 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Williams CL, and McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav Neurosci 107: 955–962, 1993. [PubMed] [Google Scholar]
- 585.Williams FMK, Scollen S, Cao D, Memari Y, Hyde CL, Zhang B, Sidders B, Ziemek D, Shi Y, Harris J, Harrow I, Dougherty B, Malarstig A, McEwen R, Stephens JC, Patel K, Menni C, Shin S-Y, Hodgkiss D, Surdulescu G, He W, Jin X, McMahon SB, Soranzo N, John S, Wang J, and Spector TD. Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study. PLoS Genet 8: e1003095, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Wilson DC, and Watson RF. Narcolepsy: With special reference to the carotid sinus reflex. South Med J 27: 754–759, 1934. [Google Scholar]
- 587.Winning AJ, Hamilton RD, Shea SA, and Guz A. Respiratory and cardiovascular effects of central and peripheral intravenous injections of capsaicin in man: evidence for pulmonary chemosensitivity. Clin Sci (Lond) 71: 519–526, 1986. [DOI] [PubMed] [Google Scholar]
- 588.Wu YM, and He RR. [Bradykinin inhibits carotid sinus baroreflex in anesthetized rats]. Sheng Li Xue Bao 51: 303–309, 1999. [PubMed] [Google Scholar]
- 589.Xiong J, Wang H, Bao Y, Guo Y, and Sun Y. Electric vagal nerve stimulation inhibits inflammation and improves early postoperation cognitive dysfunction in aged rats. BMC Anesthesiol 19: 217–217, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Xiong XJ, Liu W, Yang XC, Feng B, Zhang YQ, Li SJ, Li XK, and Wang J. Ginkgo biloba extract for essential hypertension: a systemic review. Phytomedicine 21: 1131–1136, 2014. [DOI] [PubMed] [Google Scholar]
- 591.Xu YF, and He RR. Effect of moxonidine on carotid sinus baroreflex in anesthetized rats. Zhongguo Yao Li Xue Bao 20: 604–608, 1999. [PubMed] [Google Scholar]
- 592.Yagi Y, Coburn KL, Estes KM, and Arruda JE. Effects of aerobic exercise and gender on visual and auditory P300, reaction time, and accuracy. Eur J Appl Physiol Occup Physiol 80: 402–408, 1999. [DOI] [PubMed] [Google Scholar]
- 593.Yang G, Paschos G, Curtis AM, Musiek ES, McLoughlin SC, and FitzGerald GA. Knitting up the raveled sleave of care. Sci Transl Med 5: 212rv213, 2013. [DOI] [PubMed] [Google Scholar]
- 594.Yang J, Wang WZ, Shen FM, and Su DF. Cardiovascular effects of agmatine within the rostral ventrolateral medulla are similar to those of clonidine in anesthetized rats. Exp Brain Res 160: 467–472, 2005. [DOI] [PubMed] [Google Scholar]
- 595.Yantis S. The Neural Basis of Selective Attention: Cortical Sources and Targets of Attentional Modulation. Curr Dir Psychol Sci 17: 86–90, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 23: 611–615, 2010. [DOI] [PubMed] [Google Scholar]
- 597.Yasumasu T, Reyes Del Paso GA, Takahara K, and Nakashima Y. Reduced baroreflex cardiac sensitivity predicts increased cognitive performance. Psychophysiology 43: 41–45, 2006. [DOI] [PubMed] [Google Scholar]
- 598.Yoshida T, Harasawa Y, Kubota T, Chishaki H, Kubo T, Sunagawa K, and Takeshita A. Role of carotid sinus baroreflex in attenuating systemic arterial pressure variability studied in anesthetized dogs. Am J Physiol 266: H720–729, 1994. [DOI] [PubMed] [Google Scholar]
- 599.Yoshiga C, Dawson EA, Volianitis S, Warberg J, and Secher NH. Cardiac output during exercise is related to plasma atrial natriuretic peptide but not to central venous pressure in humans. Exp Physiol 104: 379–384, 2019. [DOI] [PubMed] [Google Scholar]
- 600.Yoshioka M. Effect of a novel 5-hydroxytryptamine3-antagonist, GR38032F, on the 5-hydroxytryptamine-induced increase in carotid sinus nerve activity in rats. J Pharmacol Exp Ther 250: 637–641, 1989. [PubMed] [Google Scholar]
- 601.Yuan H, and Silberstein SD. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache 56: 71–78, 2016. [DOI] [PubMed] [Google Scholar]
- 602.Yuan H, and Silberstein SD. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part III. Headache 56: 479–490, 2016. [DOI] [PubMed] [Google Scholar]
- 603.Zamir N, and Maixner W. The relationship between cardiovascular and pain regulatory systems. Ann N Y Acad Sci 467: 371–384, 1986. [DOI] [PubMed] [Google Scholar]
- 604.Zamir N, and Segal M. Hypertension-induced analgesia: changes in pain sensitivity in experimental hypertensive rats. Brain Res 160: 170–173, 1979. [DOI] [PubMed] [Google Scholar]
- 605.Zamir N, Segal M, and Simantov R. Opiate receptor binding in the brain of the hypertensive rat. Brain Res 213: 217–222, 1981. [DOI] [PubMed] [Google Scholar]
- 606.Zamir N, and Shuber E. Altered pain perception in hypertensive humans. Brain Res 201: 471–474, 1980. [DOI] [PubMed] [Google Scholar]
- 607.Zamir N, Simantov R, and Segal M. Pain sensitivity and opioid activity in genetically and experimentally hypertensive rats. Brain Res 184: 299–310, 1980. [DOI] [PubMed] [Google Scholar]
- 608.Zanchetti A. Reflex and brain stem inhibition of sham rage behaviour. Prog Brain Res 22: 195–205, 1968. [DOI] [PubMed] [Google Scholar]
- 609.Zanchetti A, Wang SC, and Moruzzi G. The effect of vagal afferent stimulation on the EEG pattern of the cat. Electroencephalogr Clin Neurophysiol 4: 357–361, 1952. [DOI] [PubMed] [Google Scholar]
- 610.Zeng W-Z, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, and Patapoutian A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science (New York, NY) 362: 464–467, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Zhang H, Liu YX, Wu YM, Wang ZM, and He RR. Capsaicin facilitates carotid sinus baroreceptor activity in anesthetized rats. Acta Pharmacol Sin 25: 1439–1443, 2004. [PubMed] [Google Scholar]
- 612.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, and Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 299: 1240–1243, 2003. [DOI] [PubMed] [Google Scholar]
- 613.Zucker IH. Left ventricular receptors: physiological controllers or pathological curiosities? Basic Res Cardiol 81: 539–557, 1986. [DOI] [PubMed] [Google Scholar]