Abstract
Anxiety is recognized as a major health issue and is quite prevalent among older adults. An efficient way to manage anxiety is abdominal breathing. Breathing exercises seem to reduce anxiety and to increase parasympathetic activity assessed by HRV indexes. Yet, the effect of abdominal breathing on physiological stress (HRV) and anxiety in older adults remains poorly understood. Therefore, the aim of this study is to test the effects of deep and slow breathing (DSB, low inhale/exhale ratio) on physiological stress and anxiety in older adults (n = 22) in comparison with younger ones (n = 25). DSB increased significantly HFpower and reduced state anxiety in both younger and older adults. Interestingly, the increased in HF power was significantly higher among older adults than younger ones. As expected, the ratio inhale/exhale being not equal, RMSSD did not increase following DSB. Thus, we provide evidence suggesting that DSB is more beneficial to older adults than younger ones to restore vagal outflow. Despite future work being required, those results provide relevant clinical application leads to manage state anxiety among older adults and to promote successfull aging.
Subject terms: Public health, Therapeutics
Introduction
Anxiety and its associated physiological stress response have became ubiquitous in our modern societies1,2. Anxiety is one of the most common complaints3 frequently associated with poor mental health4 at a psychological level and increased all-cause mortality5 at a medical level. Anxiety can be defined as a temporally diffused emotional state, in anticipation of a potential threat, associated with physiological stress responses6. The consequences of anxiety might even worsen with age as physiological changes reduce the body’s ability to adapt to it. More precisely, healthy older adults exhibit higher mean diurnal levels of stress biomarkers than younger individuals7. Short-term physiological stress responses are healthy regulations8; however, more prolonged physiological responses to anxiety can lead to an impaired ability to recover from anxiogenic events9, increased medical diseases (e.g., increased risk of chronic diseases10), cognitive disorders (e.g., memory impairments11) and degraded mental health (e.g., depressive symptoms12 and notably anxiety disorders13). Elderly adults are aware of these effects as anxiety is quite prevalent in this population14. These data highlight the need to develop an efficient treatment for anxiety. Even though several pharmacological treatments exist, they are sometimes ineffective and associated with harmful iatrogenic effects15,16. By contrast, psychotherapeutic interventions have gained in popularity17 and some of them, in particular abdominal breathing, are efficient, cheap and simple ways to reduce anxiety18.
Breathing can indeed directly affect the activity of the autonomic nervous system, including the heart rate19. Heart rate is regulated by a dynamic balance between the sympathetic nervous system (mainly associated with physiological “flight or fight” responses) and parasympathetic nervous system (depending on vagal activity, mainly related to energy conservation, rest, relaxation, etc.20). During inhalation, the cardiovascular center inhibits vagal outflow, thus resulting in sympathetic predominance which speeds up the heart rate21. Conversely, during exhalation, the vagal outflow is restored and results in a slowing-down of the heart rate22,23. The balance between sympathetic and parasympathetic influences is reflected by heart rate variability (HRV), which is the fluctuation of instantaneous heart period over time24,25. Since HRV reflects the activity of the autonomic nervous system, it is often used to reliably assess the physiological stress response (as a low parasympathetic activity is considered as a marker of stress)26 and can be a powerful tool for testing the effect of relaxation techniques based on breathing. The high frequency (HF) of the HRV power spectrum coincides with respiration (typically 0.15 to 0.4 Hz in adults) under conditions of parasympathetic activity27, while lower frequencies (LF, 0.04 to 0.15 Hz) seem to be associated with sympathetic activity28, although this is still a subject of debate. Therefore, a breathing exercise (even as short as 5 min) proposing an equal inhalation/exhalation ratio29 promotes a balance between sympathetic and parasympathetic activity30,31, increases HRV and promotes psychophysiological coherence (for a review on the link between HRV and self-regulation, see18).
In the case of anxiety, it might be preferable to reinforce the parasympathetic activity beyond the point of equilibrium in order to maximize relaxation32. This aim could be achieved by using deep and slow breathing (DSB), a method characterized by a longer exhale than inhale duration. Accordingly, 5 min of DSB efficiently increases parasympathetic activity, as assessed by HF power (i.e., % of HF in the total HRV power) and decreased perceived anxiety level among young adults33–35. Therefore, DSB should constitute a reliable method for promoting vagal tone and reducing anxiety. Nevertheless, and surprisingly, no study to date has tested the effects of DSB (i.e., exhalation longer than inhalation) on physiological stress and perceived state anxiety among older adults. Even though aging is associated with endothelial dysfunction and arterial stiffness36 and reduced autonomic reactivity37, vagal modulation of the heart rate appears preserved38. As a consequence, older adults should still benefit from DSB, albeit to a lesser extent than younger adults.
Hypotheses
The purpose of this study was therefore to compare the effects of a 5-min DSB exercise (pre vs post-DSB) on subjective (i.e., self-reported) state anxiety and physiological stress (i.e., measured by HRV) in young and older adults. It was expected that anxiety should decrease following the DSB exercise. Moreover, DSB should specifically increase HF power (and not another parasympathetic HRV index less affected by respiration), as exhalation is longer than inhalation, among both young and older adults. However, this increase should be greater in young than in older adults due to their healthier cardiovascular systems. Finally, state anxiety should be negatively correlated with HF power.
Methods
The experimental protocols were approved by the French South-West and Overseas Regional Ethics Committee for Medical and Health Research Ethics (ID-RCB: 2020-A02193-36). The procedures were carried out in accordance with the approved recommended guidelines for assessing HRV variables in psychophysiological research39. Informed consent was obtained from all participants before the experimental session started. Sample size was determined by using the “pwr”40 package in R with the parameters found in Chinagudi et al. (2014)41. The data obtained from the sample made it possible to compute an effect size of *Cohen’s d = .85.* which was associated with a within-study design, with .95 power and .05 as significance level. A sample size of 20 participants per group was then determined as the minimum necessary to conduct the study.
Participants
In total, 71 participants (including 30 older adults and 41 young adults) were enrolled in this study. In exchange for their participation, participants could win one of four gift vouchers worth 50 each. Participants were eligible to participate if they did not present prior cardiovascular illnesses (e.g. arrhythmia, heart failure), severe inflammation (e.g. arthritis), or a neurological (head trauma, epilepsy, etc.), physiological (hypo- or hyperthyroidism, type 2 diabetes) or neurodevelopmental disorder (autism, dyslexia, etc.). Furthermore, they did not take any medication influencing the cardiovascular system (e.g. antidepressant, antipsychotic, antihypertensive, psychotropic) and they met certain sociodemographic criteria (age, sex, fluency in French etc.). Beyond medical history, lifestyle habits which might affect the functioning of the autonomic nervous system were also collected. These included smoking, drinking alcohol or the body mass index (BMI). Self-reported sleep quality and quantity were also controlled. The Hospital and Anxiety Depression Scale (HADS42) and the Mini Mental State Examination (MMSE43) were administered to measure anxiety, depression and global cognitive functioning, respectively. Following the test norms, a cut-off score 11 was used as a clinical score for the anxiety and depression subscales of the HADS, and a cut-off of < 27 was used for the MMSE. 8 participants were excluded based on these criteria. Among the young participants, 5 had a high depression or anxiety score and 1 had a BMI of > 35. One older adult had an elevated anxiety score and one had a score < 27 on the MMSE. For 16 more participants, there was a technical issue affecting physiological data acquisition which prevented us from analyzing the data. The final sample thus consisted of 47 participants, namely 25 young and 22 older adults (see Table 1).
Table 1.
Sample descriptive data.
| Overall | Young adults | Older adults | p | |
|---|---|---|---|---|
| n | 47 | 25 | 22 | |
| Age (mean (SD)) | 41.23 (23.63) | 19.56 (1.64) | 65.86 (5.05) | <0.001 |
| Sex = M (%) | 9 (19.1) | 4 (16.0) | 5 (22.7) | 0.831 |
| MMSE (mean (SD)) | 27.94 (1.41) | 27.76 (1.39) | 28.14 (1.42) | 0.365 |
| HAD (mean (SD)) | 3.04 (2.04) | 3.36 (2.18) | 2.68 (1.86) | 0.261 |
| HAD (mean (SD)) | 7.21 (3.51) | 7.92 (3.40) | 6.41 (3.54) | 0.143 |
| SleepH (mean (SD)) | 7.47 (1.33) | 7.44 (1.40) | 7.50 (1.28) | 0.886 |
| SleepQ (mean (SD)) | 6.03 (2.44) | 5.53 (2.47) | 6.60 (2.32) | 0.131 |
MMSE, Mini Mental State Examination; HAD Dep, Hospital Anxiety Scale, Depression subscale; HAD Anx, Hospital Anxiety Scale, Anxiety subscale; SleepH, Hours of sleep; SleepQ, Sleep quality.
Material
Perceived state anxiety
Spielberger’s State Anxiety Inventory (SAI) scale44 is a self-reported 20-item questionnaire assessing anxiety. The questionnaire focuses on the current state of anxiety, asking how participants feel “right now,” in response to items that measure subjective feelings of apprehension, tension, nervousness, worry, and activation/arousal of the autonomic nervous system.
Physiological stress
Stress response was assessed using the EMPATICA E4 Wristband (Empatica E4, Italy), which is a medical-grade wearable device that permits the acquisition of physiological data such as electrodermal activity, temperature and heart rate. The reliability and accuracy of EMPATICA E4 for research on HR and HRV have been previously demonstrated through comparisons with electrocardiography (ECG) measurements45. Heart rate was recorded in order to extract the inter-beat intervals (IBI). IBI were then used to obtain HRV outcomes using the Kubios HRV software (v.3.3.1). A visual check was performed to identify the presence of artefacts or occasional ectopic beats and a very low threshold filter was applied when judged necessary.
The two main HRV outcomes were the High Frequency band (HF) and the Root Mean Squared of Successive Differences (RMSSD) as they reflect parasympathetic activity through parasympathetic mediated changes in HRV46. The HF spectrum is a frequency domain measure (range from 0.15 to 0.4 Hz), called the respiratory band, that reflects parasympathetic or vagal activity on variations affecting the respiratory circle47. The HF power was computed in normal units as a percentage of the normalized total power which ranges from 0.04 to 0.4 Hz (total power minus the very low frequency)48. The remaining percent are therefore LF, which will not be directly taken into account in this study given that the interpretation of LF and thus of the LF/HF ratio is controversial28,47,49. The RMSSD is a time-domain measure obtained by calculating all the successive time differences between heartbeats, which are then squared and averaged before the squared root of the total is obtained47. As such, it reflects the beat-to-beat variance and therefore the fitness of the heart to adapt itself to sudden external or internal pressures. In order to specify the effect of DSB, its effects on RMSSD were also tested. While being correlated with HF50, RMSSD seems to be less affected by respiration51,52. Thus, no significant differences in RMSSD following DSB were expected among either young or older adults. The physiological data were recorded for the whole session in order to accustom participants to wearing the wristband. By placing tags (or triggers) on the recording, the pre-test and post test periods were timed to last between three and 5 min (the recommended range for assessing short-term HRV53) and took place, respectively, immediately before and after DSB, with the participants comfortably seated and not engaged in any cognitive demanding task.
Deep and slow breathing
The breathing exercise was guided by a video displayed on a computer screen on which a drop of water moved up and down in circles. When the drop went up, the participant had to inhale and when the drop went down the participant had to exhale. At first, the inhalation and exhalation duration were equal (4 seconds) and then, little by little, exhalation became longer than inhalation (4 seconds in and 6 seconds out). The whole exercise lasted 5 min.
Procedure
This study was part of a larger project with multiple objectives. As such, several of the tests that the participants underwent are not taken into account in this study. Each participant was tested individually in a session lasting approximately 1 hour. Firstly, the experimenter fitted the Empatica wristband on the participant’s left wrist and turned the device on. The participant then completed the health and sociodemographic interview and inclusion/exclusion test (HADS and MMSE). Next, a 10-min computerized task was administrated (either an emotional categorization task or a false recognition task). After this, a trigger was set by quickly pressing the wristband switch to start recording the pre-test period during which the participants completed the state anxiety questionnaire (SAI). A new trigger was set to mark the end of pre-test and the beginning of the induction. The DSB exercise was then completed. A third trigger indicated the start of the post-test and the participant completed the state anxiety questionnaire (SAI) a second time. The fourth trigger marked the end of the post-test. Finally, the participant completed an interoception test (heart beat detection task54), échelle d’anxiété évaluation état (EAEE55), the second computerized task (counterbalanced with the first one described above), the emotion regulation questionnaire (ERQ56,57), the French National Reading Test (fNART58), the HADS42 and the White Bear Suppression Scale Inventory (WBSI59,60). Specifically, the order of the computerized tasks was counterbalanced so that half the participants undertook the emotional categorization task first (before the pre-test) and then the false recognition task (after the post-test) while the other half undertook the tasks in reverse order (first the false recognition task then the emotional categorization task). The session ended with a debriefing on the experiment.
Statistical analyses
All the statistical analyses were performed using RStudio software61. For all statistical analyses, the employed statistical significance threshold was set at p < 0.05. Pre-DSB and post-DSB anxiety scores were obtained from the SAI questionnaire. Since these variables were very left-skewed, the data did not meet the normality assumption. Therefore, Wilcoxon tests (non-parametric tests) were conducted to determine whether the reduction in self-reported anxiety occurred after DSB across all participants and within each age group. RMSSD and HF power were obtained from the HRV analyses performed with Kubios on the data from the Empatica E4 Wristband. A visual examination confirmed the normality of the distribution. A mixed analysis of variance (ANOVA) using a 2x (Group: young vs older participants) by 2x (Time : pre DSB vs post DSB) design was therefore conducted, with Group as between-subject variable and Time as within-subject variable. Post-hoc analyses were conducted to examine the interaction between the effect of DSB (Time: pre vs post) and age (Group: young vs older). More precisely, the differences between young and older adults in the pre- and post-DSB were investigated using the Tukey procedure (Bonferroni correction for multiple comparisons). A correlation analysis was performed to investigate the association between post-DSB anxiety scores and post-HF. As the post-DSB anxiety scores did not meet the normality assumption, the correlation test was computed using the Spearman method (non-parametric test). The Spearman correlation method is known to be resistant to outliers, making it a robust and effective way to conduct correlations on variables that deviate from normality62.
Results
Perceived state anxiety
A Wilcoxon test indicated that the anxiety level was lower after () the DSB exercise than before () among all participants (, ). This difference was observed in both young ( vs. ), , , and elderly adults ( vs. ), , .
Physiological stress
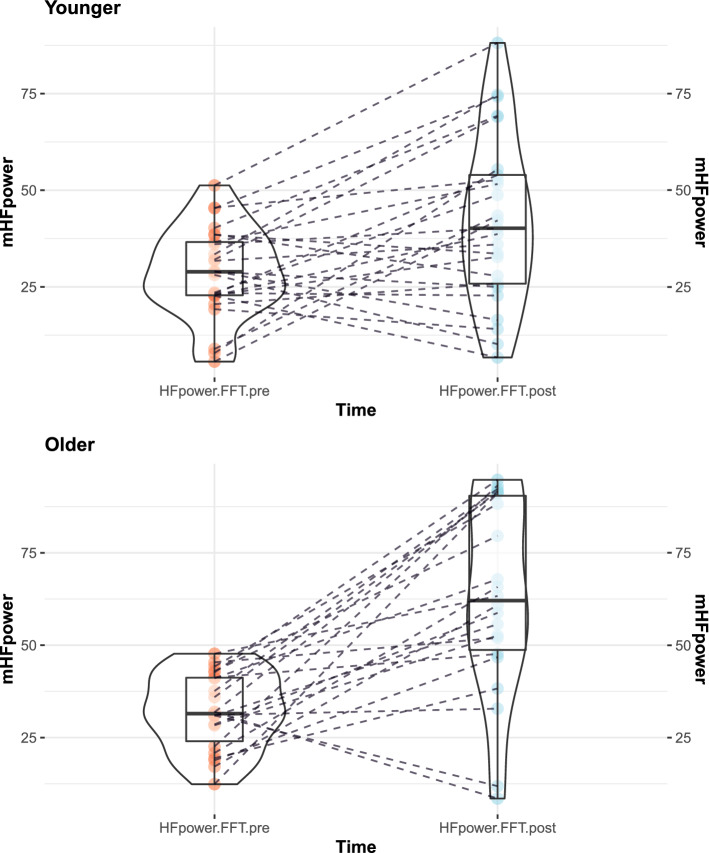
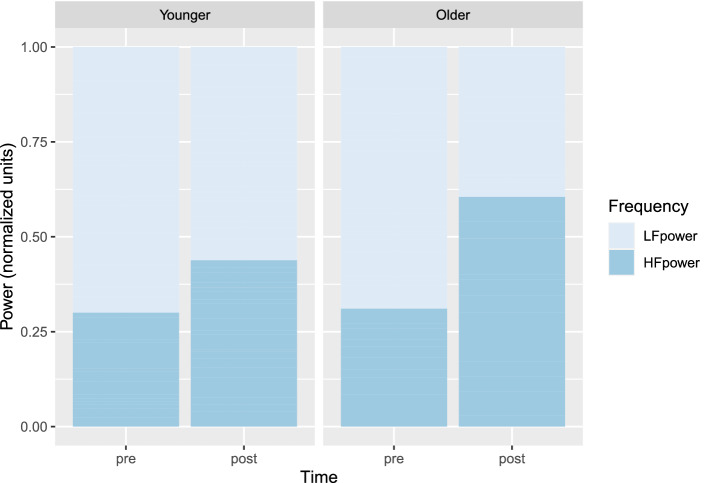
The ANOVA performed on HF power (see Fig. 1) revealed a main effect of Time (, , , ), a main effect of Group (, , , ) and a significant one-way interaction (, , , ). The increase in HF power among older adults ( vs. ) appeared to be significantly higher than that among young adults ( vs. ). Posthoc analyses revealed no significant difference between younger adults () and older ones () in pre-test HF power (, 95% CI , , ), whereas a significant difference was revealed at post-test (, 95% CI , , ) showing a higher HFpower among older () than younger adults (). Figure 2 provides a comparison between HF power and LF power (remaining percent of total power) according to Time and Group.
Figure 1.
Effect of the interaction between time and age on HFpower.
Figure 2.
HF and LF repartition according to time and age.
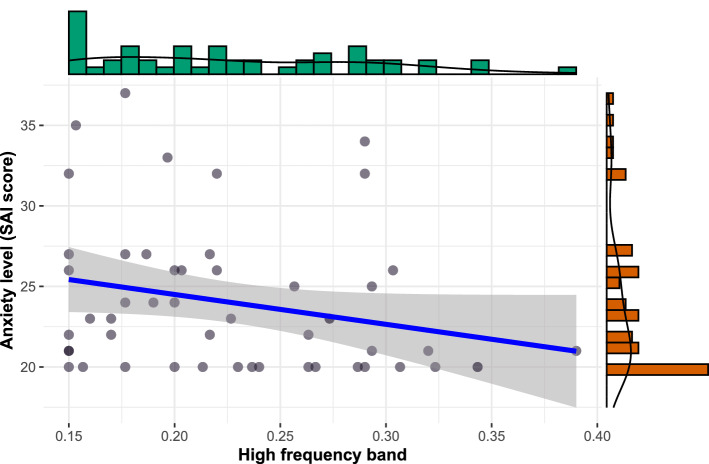
In order to test whether physiological stress is associated with state anxiety, a correlation between HF band and anxiety score was calculated (see Fig. 3). The HF band (Hz) was negatively associated with anxiety score after DSB (, , ) suggesting that the higher HF is, the lower the anxiety level.
Figure 3.
Negative association between HF and post-DSB anxiety scores.
In order to specify the effect of DSB, the RMSSD (another parasympathetic HRV index which is reportedly less affected by respiration) was also tested (for a summary of descriptive data, see Table 2). An ANOVA showed no main effect of Group (, , , ), no significant main effect of Time (, , , ) nor any interaction effect (, , , ) on RMSSD.
Table 2.
Mean (SD) for HF power and RMSSD across the young and older adult groups in the pre- and post-test.
| Time | Young adults | Older Adults | ||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| HF power | 29.97 (13.39) | 43.72 (21.53) | 31.01 (10.81) | 60.33 (28.20) |
| RMSSD | 61.29 (23.71) | 55.75 (15.90) | 65.10 (20.85) | 66.38 (26.10) |
Discussion
The aim of the present study was to assess the effect of a DSB exercise on both perceived state anxiety and physiological stress (HF-HRV) in older compared to young adults. We hypothesized that DSB would reduce the perceived anxiety level in both groups and decrease physiological stress more in the young than the older adults. Regarding HRV, it was expected that, specifically, HF power (reflecting parasympathetic activity and corresponding to the HR variations related to the respiratory cycle) would increase following the DSB exercise (the inhalation/exhalation ratio being low). By contrast, no difference was expected on the RMSSD, because it should be less affected by respiration.
As expected, subjective anxiety, significantly decreased among both young and older adults after only a 5-min DSB exercise. In addition, physiological stress also decreased after DSB, as indicated by a significant increase in HF power among younger and older adults. These results are consistent with the small number of other studies that have investigated the efficacy of DSB on vagal activity and on perceived anxiety level (e.g.,33–35 among young adults). For instance, one study compared DSB (low inhalation/exhalation ratio) and breathing with a high inhalation/exhalation ratio on HRV among undergraduate students and found that only the former specifically increased HF power and induced a relaxation state35. DSB has, surprisingly, never been studied among older adults. However, a few studies have shown that resonant breathing increases HRV indexes such as the RMSSD63,64. Nonetheless, RMSSD is probably not the best index of parasympathetic functioning because its calculation takes into account beat-to-beat variance and may thus reflect both parasympathetic and sympathetic activities65. Therefore, RMSSD should be more likely to be affected by resonant breathing (equal inhalation/exhalation ratio), which promotes a balance between the two branches of the autonomic nervous system, whereas DSB should more specifically target HF. Furthermore, RMSSD appears less susceptible to variations in respiratory frequency51,66. Therefore, it might be expected that RMSSD would not change following DSB in young or older adults as was observed in the present study.
The effects of DSB on the physiological and psychological state of the individual can be explained by psychophysiological models such as the polyvagal theory32. According to this theory, the autonomic nervous system evolved in order to influence cognitive information processing in response to contextual cues. Sympathetic activity is thought to be linked to stress responses that would trigger hypervigilance and anxiety. Conversely, parasympathetic activity, occurring in secure environments, would instead be associated with social cognition and emotion regulation in order to promote efficient cooperation that increases the odds of survival32. This theory predicts that a low inhale/exhale ratio should specifically increase vagal activity and not sympato-vagal balance, resulting in a relaxation state that promotes efficient social interactions. This is congruent with the increased HF power, the reduced state anxiety and the negative association between anxiety and HF band observed in the present study. Taken together, these empirical and theoretical data suggest that influencing vagal outflows by means of DSB is an efficient way to reduce anxiety level.
Our results go a step further by testing the effects of DSB on older adults in comparison to young ones. Unexpectedly, the increase in HF power was significantly greater in the older adults than in their younger counterparts. This difference occurred even though the participants in both groups showed an equivalent level of HF power at baseline, whereas, after DSB, the HF power level was significantly higher among older adults than younger ones. In other words, DSB seems to benefit vagal outflow more in older participants. This finding is congruent with studies investigating transcutaneous vagus nerve stimulation that have suggested that vagal stimulation could be particularly effective in healthy older compared to younger participants67,68. Such results might illustrate a greater benefit in terms of vagal tone increase in line with theories suggesting that older adults have enhanced emotion regulation skills69 which are mainly influenced by parasympathetic indexes such as HF70,71. With age, vagal modulations of heart rate should be maintained72 and parasympathetic HRV indexes follow a U-shaped curve with a reversal increase above 60 years old73,74. Similarly, studies have suggested that HRV is a marker of healthy aging associated with stress management among older individuals and that age-related decline in HRV is not inevitable75. By contrast, the HF band provides an index of psychological resilience, behavioral flexibility and the capacity to adapt to changing social demands76. As such, DSB, by promoting vagal nerve activity through a low inhale/exhale ratio, could be a catalyst for optimal anxiety management and emotion regulation. If this is indeed the case then a longer induction might not only greatly increase vagal tone, but it may also decrease state anxiety more greatly among older adults. The present results still report a similar reduction in anxiety level in the two age groups.
Another explanation may therefore be that greater vagal activation in older adults is required in order to effectively reduce perceived anxiety, in the same way as has been observed in younger individuals. Neuroimaging studies have revealed a similar mechanism, with greater activation (fMRI, functional magnetic resonance imaging) being observed in frontal regions in older people in order to achieve equivalent memory performances to those of younger adults77,78. In the same way, there might be an over-investment of vagal activity at ages over 60 years74 in order to counterbalance the age-related reduction in resources, for example by increasing the level of stress biomarkers7 to cope with anxiogenic situations9. If this is the case, the increase in the benefit brought about by the greater increase would not be specific to older adults, but rather an age-related compensation mechanism that maintains anxiety management with age.
Despite the positive nature of the outcomes, the current study is not without limitations. Even though the total number of participants was adequate according to our pre-hoc power calculation, the results obtained in our male and female participants were analyzed together (the proportion of males in both age groups being equivalent). Nevertheless, a sexual dimorphism seems to influence autonomic functioning79 and its response to stress80. Moreover, the breathing exercise used in the present study was not individually adapted based on the spontaneous respiration of each participant81. We can nonetheless state that no age difference was found in the normal respiratory rate82. Even though we controlled for sleep quality and quantity and BMI, there are also potential confounds that may contribute to the present results, such as the practice of physical activity83, yoga84 or meditation85 and other lifestyle factors that are likely to influence HRV86,such as sedentariness87, a factor that is independent of physical inactivity88. Finally, there was no control group, which makes it difficult to rule out other possible explanations for the results obtained in the present study, such as test-retest effect despite the good psychometric properties of the SAI and physiological measures. Future studies addressing these limitations would be necessary to refine the conclusions drawn from these initial results.
Nonetheless, this study provides evidence that: 1) DSB seems to reduce anxiety level and increase vagal outflow (which are negatively correlated with each other) in both young and older adults; 2) DSB could have a greater effect on parasympathetic activity in older adults. This greater effect could reflect a greater benefit of vagal tone contributing to better emotional regulation strategies with age or possibly a compensation mechanism promoting efficient anxiety management. Whatever the case may be, these results suggest that DSB might be a useful and efficient intervention for acute anxiety management among young adults and, to an even greater extent, among older adults. Beyond acute anxiety, DSB may also constitute an interesting method for preventing the deleterious effects of long-term anxiety effects on health5 or might even contribute to successful aging. Indeed, the neuro-visceral integration89 and the psycho-physiological coherence model18 indicate that higher HRV (particularly HF band) is associated with better cognitive performance90. Higher vagal activity also predicts better emotion regulation strategies, such as avoidance of the negativity and positivity effect in aging91. As such, DSB represents a practical, low-cost exercise that can be performed anywhere in order to promote successful aging. Finally, to adopt a more clinical perspective, DSB would fit very well among the efficient techniques offered as part of Acceptance and Commitment Therapy (ACT) in order to reduce anxiety, stress, or pain92 in older adults, among other individuals93.
Supplementary information
Acknowledgements
The authors are supported by the European Union under the European Regional Development Fund (FEDER) in the context of a pluridisciplinary project “Aging, Chronic Illness and Cognitive Stimulation”. The authors would like to thank Pierre Chausse and Laurie Mondillon for designing the breathing task used in the present study as well as Stéphanie Picq and Anne Groenendaal for their assistance with the experimental trials.
Author contributions
Conceived and designed the experiments: V.M., G.T.V. Performed the experiments: V.M. Analyzed the data: V.M., G.T.V. Prepared first manuscript draft: V.M. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98736-9.
References
- 1.Jackson M. The stress of life: A modern complaint? Lancet. 2014;383:300–301. doi: 10.1016/S0140-6736(14)60093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015;17:327–335. doi: 10.31887/DCNS.2015.17.3/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta K, et al. Prevalence and correlates of anxiety symptoms in well-functioning older adults: Findings from the health aging and body composition study. J. Am. Geriatr. Soc. 2003;51:499–504. doi: 10.1046/j.1532-5415.2003.51158.x. [DOI] [PubMed] [Google Scholar]
- 4.Schneiderman N, Ironson G, Siegel S. Stress and health: Psychological, behavioral, and biological determinants. Annu. Rev. Clin. Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier SM, et al. Increased mortality among people with anxiety disorders: Total population study. Br. J. Psychiatry. 2016;209:216–221. doi: 10.1192/bjp.bp.115.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daviu N, Bruchas MR, Moghaddam B, Sandi C, Beyeler A. Neurobiological links between stress and anxiety. Neurobiol. Stress. 2019;11:100191. doi: 10.1016/j.ynstr.2019.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaffey A, Bergeman C, Clark L, Wirth M. Aging and the hpa axis: Stress and resilience in older adults. Neurosci. Biobehav. Rev. 2016;68:928–945. doi: 10.1016/j.neubiorev.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1038/138032a0. [DOI] [PubMed] [Google Scholar]
- 9.Yiallouris A, et al. Adrenal aging and its implications on stress responsiveness in humans. Front. Endocrinol. 2019 doi: 10.3389/fendo.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liguori I, et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 12.Karel M. Aging and depression: Vulnerability and stress across adulthood. Clin. Psychol. Rev. 1997;17:847–879. doi: 10.1016/S0272-7358(97)00053-6. [DOI] [PubMed] [Google Scholar]
- 13.Moreno-Peral P, et al. Risk factors for the onset of panic and generalised anxiety disorders in the general adult population: A systematic review of cohort studies. J. Affect. Disord. 2014;168:337–348. doi: 10.1016/j.jad.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Lenze EJ, Wetherell JL. A lifespan view of anxiety disorders. Dialogues Clin. Neurosci. 2011;13:381–399. doi: 10.31887/DCNS.2011.13.4/elenze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koen N, Stein D. Pharmacotherapy of anxiety disorders: A critical review. Dialogues Clin. Neurosci. 2011;13:423–437. doi: 10.31887/DCNS.2011.13.4/nkoen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hare BD, Ghosal S, Duman RS. Rapid acting antidepressants in chronic stress models: Molecular and cellular mechanisms. Chronic Stress. 2017 doi: 10.1177/2470547017697317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks SJ, Stein DJ. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin. Neurosci. 2015;17:261–279. doi: 10.31887/DCNS.2015.17.3/sbrooks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCraty R, Zayas M. Cardiac coherence, self-regulation, autonomic stability, and psychosocial well-being. Front. Psychol. 2014;5:1–13. doi: 10.3389/fpsyg.2014.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo, M. A., Santarelli, D. M. & O’Rourke, D. The physiological effects of slow breathing in the healthy human. Breathe13, 298–309. 10.1183/20734735.009817 (2017). [DOI] [PMC free article] [PubMed]
- 20.Wehrwein E, Orer H, Barman S. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous. Comp. Physiol. 2016;6:1239–1278. doi: 10.1002/cphy.c150037. [DOI] [PubMed] [Google Scholar]
- 21.Ogletree-Hughes M, et al. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation. 2001;104:881–886. doi: 10.1161/hc3301.094911. [DOI] [PubMed] [Google Scholar]
- 22.Hainsworth, R. Cardiovascular Reflexes from Ventricular and Coronary Receptors 157–174. Advances in Experimental Medicine and Biology (Springer, 1995). 10.1007/978-1-4615-1895-2_15. [DOI] [PubMed]
- 23.Eckberg D, Eckberg M. Human sinus node responses to repetitive, ramped carotid baroreceptor stimuli. Am. J. Physiol. 1982;242:H638–644. doi: 10.1152/ajpheart.1982.242.4.H638. [DOI] [PubMed] [Google Scholar]
- 24.Akselrod S, et al. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 25.Task Force Report Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 26.Kim H-G, Cheon E-J, Bai D-S, Lee YH, Koo B-H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018;15:235–245. doi: 10.30773/pi.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckberg D. Human sinus arrhythmia as an index of vagal cardiac outflow. J. Appl. Physiol. Respir. Environ. Exer. Physiol. 1983;54:961–966. doi: 10.1152/jappl.1983.54.4.961. [DOI] [PubMed] [Google Scholar]
- 28.Hayano J, Yuda E. Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthropol. 2019;38:3. doi: 10.1186/s40101-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKinnon S, Gevirtz R, McCraty R, Brown M. Utilizing heartbeat evoked potentials to identify cardiac regulation of vagal afferents during emotion and resonant breathing. Appl. Psychophysiol. Biofeedback. 2013;38:241–255. doi: 10.1007/s10484-013-9226-5. [DOI] [PubMed] [Google Scholar]
- 30.Steffen P, Austin T, DeBarros A, Brown T. The impact of resonance frequency breathing on measures of heart rate variability, blood pressure, and mood. Front. Public Health. 2017 doi: 10.3389/fpubh.2017.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grigorieva D, Dimitriev D, Saperova E. Effects of deep controlled breathing on heart rate variability in young adults. FASEB J. 2017;31:7242. doi: 10.1096/fasebj.31.1_supplement.724.2. [DOI] [Google Scholar]
- 32.Porges S. The polyvagal perspective. Biol. Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark ME, Hirschman R. Effects of paced respiration on anxiety reduction in a clinical population. Biofeedback Self Regul. 1990;15:273–284. doi: 10.1007/BF01011109. [DOI] [PubMed] [Google Scholar]
- 34.Strauss-Blasche G, et al. Relative timing of inspiration and expiration affects respiratory sinus arrhythmia. Clin. Exp. Pharmacol. Physiol. 2000;27:601–606. doi: 10.1046/j.1440-1681.2000.03306.x. [DOI] [PubMed] [Google Scholar]
- 35.Van Diest I, et al. Inhalation/exhalation ratio modulates the effect of slow breathing on heart rate variability and relaxation. Appl. Psychophysiol. Biofeedback. 2014;39:171–180. doi: 10.1007/s10484-014-9253-x. [DOI] [PubMed] [Google Scholar]
- 36.Paneni, F., Diaz Cañestro, C., Libby, P., Lüscher, T. & Camici, G. Understanding it at the cellular and clinical levels. The aging cardiovascular system. J. Am. College Cardiol.69, 1952–1967. 10.1016/j.jacc.2017.01.064 (2017). [DOI] [PubMed]
- 37.Hotta H, Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr. Gerontol. Int. 2010;10:S127–S136. doi: 10.1111/j.1447-0594.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 38.Lehrer P, Vaschillo E, Vidali V. Heart rate and breathing are not always in phase during resonance frequency breathing. Appl. Psychophysiol. Biofeedback. 2020;45:145–152. doi: 10.1007/s10484-020-09459-y. [DOI] [PubMed] [Google Scholar]
- 39.Laborde S, Mosley E, Thayer J. Heart rate variability and cardiac vagal tone in psychophysiological research-recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017 doi: 10.3389/fpsyg.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen, J. Statistical Power Analysis for the Behavioral Sciences (Routledge, 2013). 10.4324/9780203771587.
- 41.Chinagudi S, et al. Immediate effect of short duration of slow deep breathing on heart rate variability in healthy adults. Natl. J. Physiol. Pharm. Pharmacol. 2014;4:233–235. doi: 10.5455/njppp.2014.4.060520141. [DOI] [Google Scholar]
- 42.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 43.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 44.Spielberger, C. D. Inventaire d’anxiété état-trait forme Y (Éditions du centre de psychologie appliquée, 1993).
- 45.Schuurmans A, et al. Validity of the empatica e4 wristband to measure heart rate variability (hrv) parameters: A comparison to electrocardiography (ecg) J. Med. Syst. 2020;44:190. doi: 10.1007/s10916-020-01648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaffer F, Ginsberg J. An overview of heart rate variability metrics and norms. Front. Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaffer, F., McCraty, R. & Zerr, C. L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol.10.3389/fpsyg.2014.01040 (2014). [DOI] [PMC free article] [PubMed]
- 48.Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV-heart rate variability analysis software. Comput. Methods Programs Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Billman G. The lf/hf ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013 doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleiger R, Stein P, Bigger J. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schipke J, Arnold G, Pelzer M. Effect of respiration rate on short-term heart rate variability. J. Clin. Basic Cardiol. 1999;2:92–95. [Google Scholar]
- 52.Penttilä J, et al. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: Effects of various respiratory patterns. Clin. Physiol. (Oxford, England) 2001;21:365–376. doi: 10.1046/j.1365-2281.2001.00337.x. [DOI] [PubMed] [Google Scholar]
- 53.Li K, Rüdiger H, Ziemssen T. Spectral analysis of heart rate variability: Time window matters. Front. Neurol. 2019 doi: 10.3389/fneur.2019.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 2015;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Beaudoin M, Desrichard O. Validation of a short French state test worry and emotionality scale. Revue internationale de psychologie sociale Tome. 2009;22:79–105. [Google Scholar]
- 56.Christophe V, Antoine P, Leroy T, Delelis G. Évaluation de deux stratégies de régulation émotionnelle?: La suppression expressive et la réévaluation cognitive. Eur. Rev. Appl. Psychol. 2009;59:59–67. doi: 10.1016/j.erap.2008.07.001. [DOI] [Google Scholar]
- 57.Ioannidis C, Siegling A. Criterion and incremental validity of the emotion regulation questionnaire. Front. Psychol. 2015;6:247. doi: 10.3389/fpsyg.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mackinnon A, Ritchie K, Mulligan R. The measurement properties of a French language adaptation of the National Adult Reading Test. Int. J. Methods Psychiatr. Res. 1999;8:27–38. doi: 10.1002/mpr.54. [DOI] [Google Scholar]
- 59.Wegner D, Zanakos S. Chronic thought suppression. J. Person. 1994;62:615–640. doi: 10.1111/j.1467-6494.1994.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt R, et al. Anatomy of the white bear suppression inventory (WBSI): A review of previous findings and a new approach. J. Pers. Assess. 2009;91:323–330. doi: 10.1080/00223890902935738. [DOI] [PubMed] [Google Scholar]
- 61.RStudio Team. RStudio: Integrated Development Environment for R. RStudio, PBC., Boston, MA (2020).
- 62.Croux C, Dehon C. Influence functions of the Spearman and Kendall correlation measures. Statist. Methods Appl. 2010;19:497–515. doi: 10.1007/s10260-010-0142-z. [DOI] [Google Scholar]
- 63.Lehrer P, et al. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom. Med. 2003;65:796–805. doi: 10.1097/01.psy.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues G, Gurgel J, Gonçalves T, Porto F, Soares P. Influence of breathing patterns and orthostatic stress on postural control in older adults. Geriatr. Gerontol. Int. 2018;18:692–697. doi: 10.1111/ggi.13231. [DOI] [PubMed] [Google Scholar]
- 65.Berntson G, Lozano D, Chen Y. Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology. 2005;42:246–252. doi: 10.1111/j.1469-8986.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 66.Quintana D, et al. Resting-state high-frequency heart rate variability is related to respiratory frequency in individuals with severe mental illness but not healthy controls. Sci. Rep. 2016;6:37212. doi: 10.1038/srep37212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bretherton B, Deuchars J, Deuchars S. What impact could transcutaneous vagal nerve stimulation have on an aging population? Bioelectron. Med. 2019 doi: 10.2217/bem-2019-0022. [DOI] [Google Scholar]
- 68.Bretherton B, et al. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: Potential benefits of daily stimulation. Aging. 2019;11:4836–4857. doi: 10.18632/aging.102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurera JW, Isaacowitz DM. Emotion regulation and emotion perception in aging: A perspective on age-related differences and similarities. Prog. Brain Res. 2019;247:329–351. doi: 10.1016/bs.pbr.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 70.Mather M, Thayer J. How heart rate variability affects emotion regulation brain networks. Curr. Opin. Behav. Sci. 2018;19:98–104. doi: 10.1016/j.cobeha.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: Potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front. Psychol. 2020 doi: 10.3389/fpsyg.2020.01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reardon M, Malik M. Changes in heart rate variability with age. Pacing Clin. Electrophysiol. 1996;19:1863–1866. doi: 10.1111/j.1540-8159.1996.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 73.Almeida-Santos M, et al. Aging, heart rate variability and patterns of autonomic regulation of the heart. Arch. Gerontol. Geriatr. 2016;63:1–8. doi: 10.1016/j.archger.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 74.Geovanini GR, et al. Age and sex differences in heart rate variability and vagal specific patterns-Baependi Heart Study. Glob. Heart. 2020;15:71. doi: 10.5334/gh.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tan JPH, Beilharz JE, Vollmer-Conna U, Cvejic E. Heart rate variability as a marker of healthy ageing. Int. J. Cardiol. 2019;275:101–103. doi: 10.1016/j.ijcard.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Beauchaine, T. Vagal tone, development, and gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Dev. Psychopathol.13, 183–214. 10.1017/s0954579401002012 (2001). [DOI] [PubMed]
- 77.Cabeza R, Anderson N, Locantore J, McIntosh A. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 78.Cabeza R, et al. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex (New York, N.Y.: 1991) 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- 79.Koenig J, Thayer J. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016;64:288–310. doi: 10.1016/j.neubiorev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Adjei T, Xue J, Mandic D. The female heart: Sex differences in the dynamics of ecg in response to stress. Front. Physiol. 2018 doi: 10.3389/fphys.2018.01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quintana D, Heathers J. Considerations in the assessment of heart rate variability in biobehavioral research. Front. Psychol. 2014;5:805. doi: 10.3389/fpsyg.2014.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodríguez-Molinero A, Narvaiza L, Ruiz J, Gálvez-Barrón C. Normal respiratory rate and peripheral blood oxygen saturation in the elderly population. J. Am. Geriatr. Soc. 2013;61:2238–2240. doi: 10.1111/jgs.12580. [DOI] [PubMed] [Google Scholar]
- 83.Luisa S-M, et al. Physical activity and heart rate variability in older adults. Circulation. 2014;129:2100–2110. doi: 10.1161/CIRCULATIONAHA.113.005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tyagi A, Cohen M. Yoga and heart rate variability: A comprehensive review of the literature. Int. J. Yoga. 2016;9:97–113. doi: 10.4103/0973-6131.183712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rådmark L, Sidorchuk A, Osika W, Niemi M. A systematic review and meta-analysis of the mpact of mindfulness based interventions on heart rate variability and inflammatory markers. J. Clin. Med. 2019 doi: 10.3390/jcm8101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jandackova VK, Shaun S, Annie B, Andrew S. Healthy lifestyle and cardiac vagal modulation over 10 years: Whitehall II cohort study. J. Am. Heart Assoc. 2019;8:e012420. doi: 10.1161/JAHA.119.012420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zaffalon Júnior J, Viana A, de Melo G, De Angelis K. The impact of sedentarism on heart rate variability (hrv) at rest and in response to mental stress in young women. Physiol. Rep. 2018 doi: 10.14814/phy2.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Magnon V, Dutheil F, Auxiette C. Sedentariness: A need for a definition. Front. Public Health. 2018 doi: 10.3389/fpubh.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 90.Forte G, Favieri F, Casagrande M. Heart rate variability and cognitive function: A systematic review. Front. Neurosci. 2019 doi: 10.3389/fnins.2019.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mantantzis K, Schlaghecken F, Maylor EA. Heart rate variability predicts older adults avoidance of negativity. J. Gerontol. Ser. B. 2020;75:1679–1688. doi: 10.1093/geronb/gby148. [DOI] [PubMed] [Google Scholar]
- 92.Dindo L, Van Liew J, Arch J. Acceptance and commitment therapy: A transdiagnostic behavioral intervention for mental health and medical conditions. Neurotherapeutics. 2017;14:546–553. doi: 10.1007/s13311-017-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petkus A, Wetherell J. Acceptance and commitment therapy with older adults: Rationale and considerations. Cogn. Behav. Pract. 2013;20:47–56. doi: 10.1016/j.cbpra.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.