Abstract
Traditional wastewater-based epidemiology (W-BE) relying on SARS-CoV-2 RNA detection in wastewater is attractive for understanding COVID-19. Yet traditional W-BE based on centralized wastewaters excludes putative SARS-CoV-2 reservoirs such as: (i) wastewaters from shared on-site sanitation facilities, (ii) solid waste including faecal sludge from non-flushing on-site sanitation systems, and COVID-19 personal protective equipment (PPE), (iii) raw/untreated water, and (iv) drinking water supply systems in low-income countries (LICs). A novel hypothesis and decision-support tool based on Wastewater (on-site sanitation, municipal sewer systems), solid Waste, and raw/untreated and drinking Water-based epidemiology (WWW-BE) is proposed for understanding COVID-19 in LICs. The WWW-BE conceptual framework, including components and principles is presented. Evidence on the presence of SARS-CoV-2 and its proxies in wastewaters, solid materials/waste (papers, metals, fabric, plastics), and raw/untreated surface water, groundwater and drinking water is discussed. Taken together, wastewaters from municipal sewer and on-site sanitation systems, solid waste such as faecal sludge and COVID-19 PPE, raw/untreated surface water and groundwater, and drinking water systems in LICs act as potential reservoirs that receive and harbour SARS-CoV-2, and then transmit it to humans. Hence, WWW-BE could serve a dual function in estimating the prevalence and potential transmission of COVID-19. Several applications of WWW-BE as a hypothesis and decision support tool in LICs are discussed. WWW-BE aggregates data from various infected persons in a spatial unit, hence, putatively requires less resources (analytical kits, personnel) than individual diagnostic testing, making it an ideal decision-support tool for LICs. The novelty, and a critique of WWW-BE versus traditional W-BE are presented. Potential challenges of WWW-BE include: (i) biohazards and biosafety risks, (ii) lack of expertise, analytical equipment, and accredited laboratories, and (iii) high uncertainties in estimates of COVID-19 cases. Future perspectives and research directions including key knowledge gaps and the application of novel and emerging technologies in WWW-BE are discussed.
Keywords: Coronaviruses, COVID-19 prevalence, Decision-support tool, Environmental surveillance, Potential applications, SARS-CoV-2, Wastewater-based epidemiology
Graphical abstract
1. Introduction
SARS-CoV-2 is the etiologic agent causing the human coronavirus disease-2019 (COVID-19) now a global pandemic, with over 234 million confirmed cases and nearly 5 million deaths having been reported globally as of end of September 2021 (JHU, 2021). To date, COVID-19 has spread globally to include low-income countries (LICs) in Africa, the Caribbean region, south-east Asia, and Latin America (Ahmed et al., 2021a; Del Brutto et al., 2021; Fiesco-Sepúlveda and Serrano-Bermúdez, 2020; Kumar et al., 2020a; Miller et al., 2020; Nachega et al., 2020). In LICs, healthcare, social security and regulatory systems are weak, while financial resources and diagnostic facilities for mass individual testing, and data on COVID-19 prevalence are severely lacking (Gwenzi, 2020a, Gwenzi, 2020b; Gwenzi and Rzymski, 2021). Yet reliable data on COVID-19 burden and transmission are critical for the prioritization and deployment of scarce resources including protective equipment (PPE), and emergence response systems (Petrosino et al., 2021). Wastewater-based epidemiology (W-BE) entailing the detection of SARS-CoV-2 and its proxies in wastewater may provide cues on COVID-19 prevalence in cases where comprehensive surveillance data are lacking (Daughton, 2020a, Daughton, 2020b; Medema et al., 2020, Medema et al., 2020; Scott et al., 2021; Street et al., 2020). Recent evidence drawn largely from several high-income countries (e.g., Australia, Germany, France, Israel, Italy, Netherlands, Spain, the USA) shows that SARS-CoV-2 RNA in raw/untreated municipal wastewater anticipated COVID-19 outbreak before the first confirmed official cases (Ahmed et al., 2020; Hart and Halden, 2020; Medema et al., 2020, Medema et al., 2020; Randazzo et al., 2020; Scott et al., 2021; Wurtzer et al., 2020).
Barring data drawn from high-income countries, studies applying W-BE in LICs are scarce. Yet W-BE presents immense opportunities to understand COVID-19 in low-income settings (Bhattacharya et al., 2021). Currently, W-BE is predominantly limited to raw/untreated wastewaters from centralized municipal sewer systems (Ahmed et al., 2020; Hart and Halden, 2020; Medema et al., 2020, Medema et al., 2020). This is probably because this is the dominant sanitation system in developed countries where the tool was first developed, and later used for COVID-19 surveillance (Daughton, 2012; Medema et al., 2020, Medema et al., 2020). In LICs, other putative reservoirs of SARS-CoV-2 similar to municipal wastewaters include: (i) wastewaters/effluents, faecal sludge and bioaerosols from shared or decentralized on-site sanitation facilities (e.g., septic tanks, bucket latrines, pit latrines) (Adelodun et al., 2020; Amoah et al., 2021; Caruso and Freeman, 2020; Street et al., 2020), (ii) raw/untreated surface water and groundwater systems receiving raw/untreated or partially treated sewage (Fongaro et al., 2021; Guerrero-Latorre et al., 2020; Mahlknecht et al., 2021), (iii) solid wastes such as faecal sludge from non-flushing on-site sanitation systems and COVID-19 PPE, and (iv) unsafe drinking water sources.
Recent studies have detected SARS-CoV-2 RNA in environmental settings relevant to surveillance of COVID-19 in LICs including: (i) on-site sanitation and toilet systems (Amoah et al., 2021; Del Brutto et al., 2021; Liu et al., 2021; Meng et al., 2020; Peccia et al., 2020, Peccia et al., 2020; Zhang et al., 2020a), and (ii) raw/untreated surface water and groundwater systems receiving raw/untreated or partially treated sewage (Guerrero-Latorre et al., 2020; Kolarević et al., 2021; Rimoldi et al., 2020; Mahlknecht et al., 2021; Maidana-Kulesza et al., 2021). Such raw/untreated surface water and groundwater systems serve as drinking water sources in low-income settings, where such water is often consumed without treatment. Data also show that coronaviruses, SARS-CoV-2 and their proxies occur and persist on solid materials such as metals, paper, wood, plastic, cloth, and even COVID-19 PPE (Chin et al., 2020; Kasloff et al., 2021; Lui et al., 2020; Pastorino et al., 2020; van Doremalen et al., 2020). Hence, one may infer that SARS-CoV-2 may also persist on solid wastes including faecal sludge from non-flushing on-site sanitation systems and COVID-19 PPE from infected persons.
Traditional W-BE is narrow, because it is silent on application of wastewaters in on-site sanitation facilities, solid wastes such as faecal sludge from non-flushing on-site sanitation systems and COVID-19 PPE, raw/untreated surface water and groundwater, and raw/untreated drinking water as potential environmental media for SARS-CoV-2 monitoring. This omission makes traditional W-BE less relevant and applicable in low-income settings without centralized wastewater treatment systems, and where environmental surveillance tools are most needed due to severe lack of resources for comprehensive diagnostic testing of individuals. This exclusion is understandable, and attributed to the fact that, due to efficient multi-barrier systems such as solid waste management systems, advanced wastewater treatment systems, and the availability of clean drinking water from advanced treatment processes, such environmental media are not considered as key reservoirs of SARS-CoV-2 in developed countries (Nghiem et al., 2020; Randazzo et al., 2020). In contrast, LICs lack such multi-barrier systems such as proper and effective advanced solid waste management systems, and drinking water and wastewater treatment systems to effectively remove SARS-CoV-2. Moreover, treatment systems for both wastewater and drinking water are predominantly based on conventional processes, and are often poorly maintained, overloaded and inefficient. This is due to low investment in wastewater and water treatment infrastructure, coupled with rapid urban population growth and demand for services.
The present paper posits that wastewaters both from centralized municipal sewer and on-site sanitation facilities, solid wastes such as faecal sludge from non-flushing on-site sanitation systems and COVID-19 PPE, raw/untreated surface water and groundwater, and drinking water systems receive and harbour SARS-CoV-2 from various sources in a community or catchment, and then further transmit it to humans. Therefore, in addition to raw/untreated wastewaters from centralized systems, recent empirical and inferential evidence suggests that environmental surveillance in LICs should be extended to include four additional components; (i) raw/untreated wastewater/effluents from on-site sanitation systems, (ii) raw/untreated surface water and groundwater, (iii) drinking water systems, and (iv) solid wastes. Collectively, this extension of traditional W-BE constitutes the novel Wastewater, Waste and Water-based epidemiology (WWW-BE) hypothesis and decision-support tool.
Partitioning WWW-BE into three components, and the use of the acronym serve two functions: (i) to distinguish WWW-BE from traditional W-BE based solely on centralized wastewater systems and its limitations, and (ii) to highlight the need to consider the three components as separate but complementary SARS-CoV-2 monitoring media in low-income settings. As a hypothesis and decision support tool, WWW-BE could serve a dual function in estimating the prevalence and potential transmission of COVID-19. This potential dual function of WWW-BE is critical in understanding and mitigating COVID-19 in LICs.
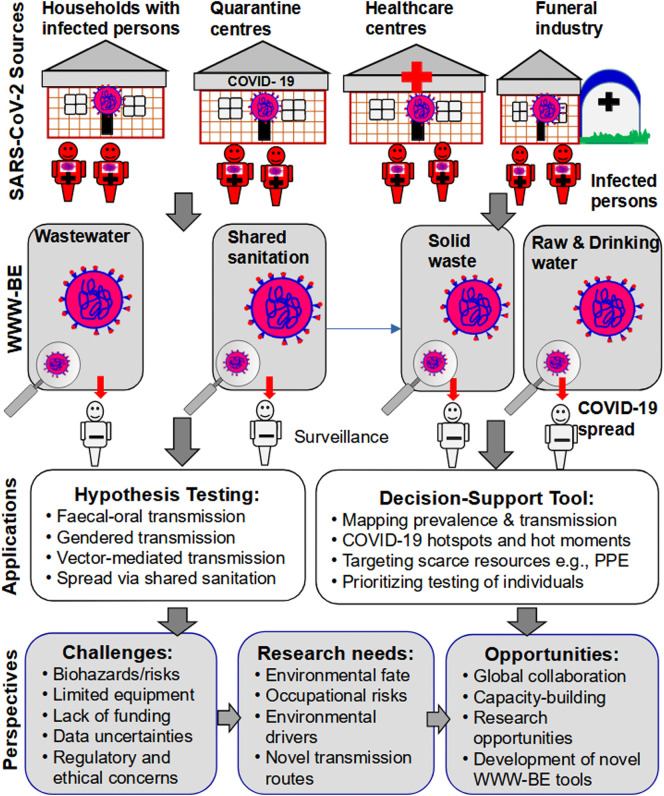
The purpose of the present paper is to draw the attention of the research community, governments, local and international development agencies, and practitioners to WWW-BE as a potential novel low-cost tool for understanding COVID-19. The specific objectives are: (i) to present the rationale and conceptual framework, including components and key principles of WWW-BE in LICs, (ii) to discuss the empirical and inferential evidence underpinning WWW-BE, (iii) to present the potential applications, novelty, critique, and challenges of WWW-BE as a hypothesis and decision-support tool (Table 1 ), and (iv) to propose future research directions, including key knowledge gaps, and application of emerging technologies. Fig. 1 depicts the focal points of the present paper, including the WWW-BE conceptual framework, and its potential applications, opportunities, challenges and research needs as a hypothesis and decision-support tool for understanding COVID-19 in LICs.
Table 1.
A summary of the potential merits, criticisms, counter-arguments, and possible solutions associated with the WWW-BE as a hypothesis and decision-support tool in low-income countries.
| Merits and opportunities | Potential criticisms and limitations | Counter-arguments and potential solutions |
|---|---|---|
| (1) WWW-BE builds on and extends W-BE, making WWW-BE potentially more ideal for low-income settings. | (1) Lack of global prior art and validation may lead to scepticism by the public, funders, and decision- and policy-makers. | This is a cross-cutting limitation, because no prior art and validation evidence exist in LICs even for W-BE. Research is required to validate, pilot test, and apply WWW-BE to develop the scientific evidence base to build confidence in the tool. |
| (2) WWW-BE modularity imparts potential flexibility and adaptability to diverse settings on a case-by-case basis. | (2) Heterogeneity, sampling difficulties, and low persistence of SARS-CoV-2 on solid waste may constrain the use of solid waste in epidemiology. | Solid media such as wastewater sludge has been sampled and used in epidemiology. SARS-CoV-2 and its proxies persist on solid materials. Sampling and sample preparation methods need to be developed and validated for solid waste. |
| (3) WWW-BE could serve a dual function to estimate the burden and potential transmission of COVID-19 in a spatial domain. | (3) Biosafety and human health risks associated with sampling, processing and disposal of WWW-BE media. | This is cross-cutting, and relevant to both WWW-BE and W-BE. Accredited laboratories with skilled personnel and high biosafety protocols are needed in LICs. |
| (4) Similar to W-BE, WWW-BE aggregates data, thus putatively requires less samples, time, and resources than conventional diagnostic individual testing. | (4) WWW-BE poses significant logistical and cost constraints in dispersed communities. | W-BE and diagnostic testing also face significant challenges in such settings. WWW-BE can be adapted to fill the gap by targeting on-site sanitation facilities. Mobile testing units, and rapid low-cost sensors need to be developed to support WWW-BE. |
| (5) WWW-BE could account for asymptomatic, oligosymptomatic and presymptomatic infected people, and those who may undergo self-isolation or quarantine without clinical testing. | (5) Bioethics and socio-cultural intrusion associated with sampling of WWW-BE media, and dissemination of results. | Similar to W-BE, WWW-BE is less intrusive than individual testing. WWW-BE outputs should presented as aggregated or clustered data rather than for individual households. Similar to other human health- related research, approvals and consent are required for WWW-BE. |
| (6) As a hypothesis and decision-support tool, WWW-BE has a potential to be extended beyond COVID-19 to other human infections in LICs such as cholera and typhoid. | (6) Lack of data, and validated tools for back-and forward-calculations to support WWW-BE. | Current W-BE tools, and those based on artificial intelligence and big data analytics can also be adapted, developed and validated through a comprehensive WWW-BE research programme entailing the acquisition of relevant data. |
| (7) WWW-BE could change the environmental surveillance paradigm in LICs, and presents translational research opportunities to pilot test, validate, and apply the hypothesis and decision-support tool. | (7) WWW-BE based COVID-19 estimates may entail high uncertainties due to sampling, analytical, and calculation errors. | This is cross-cutting, because uncertainity is also high for W-BE. This calls for further research to refine the analytical tools and address this potential limitation for both WWW-BE and W-BE. |
Fig. 1.
The Wastewater, Waste and Water-based epidemiology (WWW-BE) conceptual framework, and its potential applications, opportunities, challenges and research needs as a hypothesis and decision-support tool for understanding COVID-19 in low-income countries.
2. Moving beyond traditional W-BE to WWW-BE in LICs: A conceptual framework
2.1. Background and rationale
LICs have several risk drivers and factors predisposing its human population to the transmission and health risks of COVID-19, but lack capacity to effectively cope with infectious diseases of such magnitude. These risk factors include (Gwenzi, 2020a, Gwenzi, 2020b; Gwenzi and Rzymski, 2021): (i) weak and poorly-funded healthcare and social security systems, (ii) poor solid waste and wastewater management systems, (iii) weak research systems leading to a poor local evidence base, (iv) lack of environmental and public health surveillance systems, including diagnostic facilities, (v) lack of clean drinking water, and (vi) chronic shortages of essential goods and services including housing, leading to over-crowding and informal settlements lacking improved water and sanitation facilities. These risk factors are discussed in detailed in earlier papers focusing on low-income regions including Africa (Gwenzi, 2020a, Gwenzi, 2020b; Gwenzi and Rzymski, 2021). The weak research systems, and their impacts on the response of LICs to COVID-19 are discussed in an earlier paper focusing on Africa (Gwenzi and Rzymski, 2021).
Although a number of COVID-19 vaccines have been developed and are currently being administered in several countries, the coverage of vaccination programmes remains low in most LICs due to limited resources and lack of reliable cold chain systems for the storage, distribution, and transportation of vaccines (Acharya et al., 2021). Hence, COVID-19 control still relies mainly on social distancing, use of PPE, and frequent hand-washing to minimize transmission via human-human contact, fomites and respiratory droplets (WHO, 2020).
COVID-19 exerts excessive pressure on scarce resources (PPE, healthcare systems, healthcare workers) and even the supply chain systems for COVID-19 essential goods such as PPE and ventilators. Accurate data on the prevalence and transmission of COVID-19 are critical in the targeting and prioritization of scarce resources. Most LICs lack diagnostic equipment (i.e., PCR kits) for comprehensive mass testing, because such equipment is expensive, and the testing procedure is often time-consuming. For example, a typical COVID-19 PCR test costs approximately 50 US$/test (Atkeson et al., 2020), while reagents cost about 15 US$ per PCR kit (Hart and Halden, 2020). Based on data from developed countries, a COVID-19 PCR test has a turn-around time of about 48 h (Beeching et al., 2020). The cost of the PCR kits, reagents, and testing, and the time required for COVID-19 testing may vary among countries depending on levels of economic development and logistics (Hart and Halden, 2020). High testing costs and turn-around times are expected in LICs due to limited diagnostic testing capacity and logistical constraints (Gwenzi, 2020a, Gwenzi, 2020b; Gwenzi and Rzymski, 2021). Logistical constraints such as inaccessibility and poor transport systems make it difficult to reach rural communities in remote areas where awareness about COVID-19 and its health effects remain low. However, anecdotal evidence suggests that remote and inaccessible areas such as rural areas in Africa (e.g., Zimbabwe) seem to have low cases of COVID-19. The reasons for this trend are unclear, but this could be attributed to limited human-human interactions and low population densities in rural settings compared to urban areas. Others may also argue that indigenous populations seem to have limited COVID-19 outbreaks. The reasons for the limited COVID-19 outbreaks in rural areas, and among indigenous people in LICs require more detailed research. Once COVID-19 outbreaks occur in such remote and inaccessible area, the risks and impacts could be quite significant. This is because of a severe lack of essential services including healthcare facilities, isolation/quarantine centres, and PPE. Ironically, such remote communities may also lack access to COVID-19 vaccines and information on the prevention and control of COVID-19. Due to limited capacity for comprehensive COVID-19 testing, the exact prevalence or burden of COVID-19 in LICs remains unknown. Yet without data on COVID-19 prevalence, efficient planning and implementation of COVID-19 control measures present significant challenges.
The need for rapid and low-cost monitoring of the prevalence and trends of COVID-19 has been long recognized (Daughton, 2020a, Daughton, 2020b). Environmental surveillance, including the proposed WWW-BE is one novel tool for understanding the prevalence of COVID-19 at community level. To date, traditional W-BE has been used in several developed countries including Italy, Australia, the USA, Netherlands and Spain, among others because a large population in such countries has access to centralized wastewater facilities (Hart and Halden, 2020; Medema et al., 2020, Medema et al., 2020; Randazzo et al., 2020).
Data are still limited on the application of environmental surveillance in LICs. For example, in Africa a continent with 56 countries, an internet search of scholarly databases such as Google Scholar only gave two articles on W-BE of COVID-19 both by South African researchers; (i) a review or perspective paper focusing on Africa (Street et al., 2020), and (ii) a data-based paper reporting SARS-CoV-2 viral loads of between 0 and 7.32 × 105 copies/100 mL in wastewater influent in four wastewater treatment plants in Kwazulu-Natal (Pillay et al., 2021). Like studies conducted in developed countries, the study by Pillay et al. (2021) was limited to centralized wastewater treatment systems, because it excluded other components of WWW-BE.
The call for a shift from traditional W-BE to WWW-BE is motivated by several reasons unique to LICs. First, a large population in urban, peri-urban and rural areas in LICs lack access to centralized municipal wastewater systems, thus COVID-19 cases based on traditional W-BE will exclude a significant portion of the population. Second, several risk factors and drivers make WWW-BE more pertinent to such low-income settings than developed ones. These risk factors/drivers include: (i) lack of comprehensive and effective multi-barrier system such as engineered sanitary landfills, incinerators, and advanced wastewater and water treatment systems to safeguard public health, (ii) weak and poorly enforced environmental and public health regulations and policies leading to severe environmental pollution including that of aquatic systems, (iii) unhygienic recycling and reuse of post-consumer packaging materials collected from solid waste repositories, and (iv) over-reliance on raw/untreated drinking water from unsafe sources prone to faecal contamination. This is contrary to advanced multi-barrier systems and regulations in developed countries that reduce the risk of SARS-CoV-2 transmission through wastewater, solid wastes and drinking water (Nghiem et al., 2020; Randazzo et al., 2020). Therefore, wastewaters, solid wastes, raw/untreated water sources, and drinking water systems in LICs act as potential reservoirs that receive and harbour SARS-CoV-2 originating from several sources with infected persons in a community or catchment, including households, quarantine/isolation centres (Ahmed et al., 2021a, Ahmed et al., 2021b), funeral industry, and healthcare facilities, and then further transmit it to humans. This enables WWW-BE to serve a dual purpose of estimating both prevalence and potential transmission of COVID-19.
2.2. Fundamental principles of WWW-BE
As discussed earlier, WWW-BE has three complementary components (wastewater, solid waste, water) (Fig. 2 ). In the context of low-income settings, the partitioning of WWW-BE into the three components, and the use of the acronym serve a dual function: (i) to distinguish it from the traditional W-BE based on centralized sewer systems, and its limitations, and (2) to highlight and draw researchers' attention to the urgent need to consider the three components as separate but complementary monitoring media for SARS-CoV-2. Note that lumping the WWW-BE components, and referring to the framework as W-BE will negate the primary objectives of the proposed concept, and confound or blur the differences between WWW-BE and the traditional W-BE. The lumping of WWW-BE components under the umbrella term ‘wastewater’ may partly explain the apparent proliferation of studies on municipal wastewaters and traditional W-BE at the expense of other components of WWW-BE.
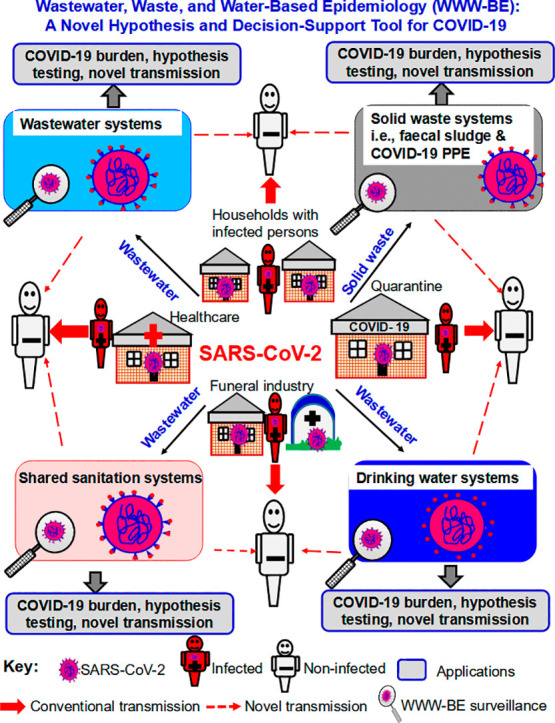
Fig. 2.
The Wastewater, Waste and Water-based epidemiology (WWW-BE) conceptual framework depicting: (1) Components: (i) wastewater-based epidemiology (W-BE) consisting of two sub-components, wastewaters from centralized sewer systems (i.e., traditional W-BE) and on-site sanitation systems, (ii) solid waste epidemiology (SW-E), (iii) raw/untreated water epidemiology (RW-E) including surface water and groundwater systems, and (iv) drinking water epidemiology (DW-E), (2) Dual function of WWW-BE in estimating: (i) COVID-19 prevalence via shedding from infected persons, and (ii) subsequent potential SARS-CoV-2 transmission from WWW-BE media to non-infected persons, and (3) Dissemination pathways and circulation of SARS-CoV-2 among the various WWW-BE compartments via hydrological processes, material flows, and vectors/vermin.
The fundamental principle underlying WWW-BE is that SARS-CoV-2 and its proxies such as viral RNA occur and persist in the four target environmental media: (i) solid wastes such as faecal sludge from non-flushing on-site sanitation systems, and COVID-19 PPE, (ii) raw/untreated wastewaters from municipal sewer systems, (iii) wastewaters/effluents from on-site sanitation facilities, and (iv) raw/untreated surface water, groundwater, and drinking water systems. In addition, SARS-CoV-2 or its proxies should occur in concentrations high enough and above the limit of detection of the existing analytical techniques such as qRT-PCR. Finally, the available analytical methods for SARS-CoV-2 and its proxies should be able to detected both viable and non-viable viral particles. This is important in order to account for both forms in case viable SARS-CoV-2 has a short half-life in one of the target WWW-BE environmental media.
The dual function of WWW-BE in estimating both the prevalence and potential transmission patterns of COVID-19 via environmental media at various spatio-temporal scales requires back- and forward-calculation of COVID-19 cases from WWW-BE data. A detailed discussion of the algorithms and techniques for back-and forward-calculation are beyond the scope of the present study. Briefly, generic tools for back-and forward-calculation and analysis of WWW-BE data may entail application of the following (Ahmed et al., 2020; Hart and Halden, 2020; Li et al., 2021; Pillay et al., 2021): (i) conventional univariate and multivariate statistics (e.g., Bayesian techniques), (ii) in silico or computational analysis or modelling, including the use of probabilistic or stochastic tools such as Monte Carlo simulation, and (iii) application of big data analytical tools (e.g., machine learning, artificial intelligence, data mining, network analysis), among others. Similar applications of these analytical tools in traditional W-BE (Ahmed et al., 2020; Daughton, 2018; Hart and Halden, 2020; Li et al., 2021) point to the feasibility to develop and adapt such tools for WWW-BE. The development and validation of WWW-BE is a non-trivial task that requires strong collaboration across traditionally disparate disciplines. These include those with expertise in analytical (bio)chemistry, immunochemistry, environmental/civil engineering, wastewater treatment operations, computer modelling, mathematics/statistics, clinical sciences, pharmacology and toxicology, infectious diseases and public health, microbiology (e.g., virology, epidemiology, social/behavioural sciences, and risk and science communication (Daughton, 2020a, Daughton, 2020b; Gwenzi and Rzymski, 2021).
Once developed and validated, WWW-BE has a number of potential merits relative to mass clinical surveillance (Table 1). First, it provides a snapshot of the COVID-19 outbreak situation in the entire spatial domain of interest by testing aggregate wastewater, solid waste, raw/untreated water sources, and drinking water samples, while clinical surveillance needs a large number of individual samples. This requires more time and resources for sample collection and testing, which is not always feasible in most LICs, and even in some developed countries. WWW-BE can also account for asymptomatic, oligosymptomatic and presymptomatic infected people, and those who may undergo self-isolation or quarantine without clinical testing. The inclusion of the asymptomatic infected persons is critical because studies show that the SARS-CoV-2 viral loads from asymptomatic infection are often similar to that of symptomatic patients (Hata and Honda, 2020; Tang et al., 2020). For SARS-CoV-2, the estimated ratio of asymptomatic infection is as high as 18–32% of total SARS-CoV-2 infections, which is similar to that of norovirus (Nishiura et al., 2020). WWW-BE can be considered as a more encompassing and flexible tool given its modularity while traditional W-BE is relatively rigid due to its focus only on raw/untreated wastewater from centralized systems.
The present proposal to develop, validate and apply WWW-BE for the surveillance of COVID-19 and other related future pandemics resonates well with earlier calls advocating for the widespread adoption of W-BE for addressing the COVID-19 pandemic (Daughton, 2020a, Daughton, 2020b; Hart and Halden, 2020; Orive et al., 2020; Street et al., 2020). Here, the summary evidence which forms the basis of WWW-BE, and the current application status of each epidemiology in COVID-19 surveillance are presented. For brevity, comprehensive reviews of the presence and behaviour of coronaviruses, SARS-CoV-2 and their proxies in the environmental are beyond the scope of the present study. Instead, reference is made to earlier studies and reviews on the presence of coronaviruses and their proxies in the environmental media relevant to WWW-BE in cases where they exist (e.g., Kitajima et al., 2020 in the case of wastewaters, Nghiem et al., 2020; Bueckert et al., 2020; Onakpoya et al., 2021 for solid waste).
In the present paper, and in the context of the potential applications, each of the (sub)-components of WWW-BE is presented as an ‘epidemiology’ (i.e., on-site sanitation wastewater epidemiology, solid waste epidemiology, raw/untreated and drinking water epidemiology) (Fig. 2). This notation corresponds to the current notion where the use of raw/untreated municipal wastewater in human disease surveillance is referred to as W-BE (Daughton, 2020a, Daughton, 2020b).
3. SARS-CoV-2 in WWW-BE environmental media: A summary of the empirical and inferential evidence
3.1. Wastewater epidemiology
The two types of W-BE, one based on raw/untreated wastewaters from centralized wastewater systems (traditional W-BE), and the other one on wastewaters/effluents in on-site sanitation facilities (septic tanks, pit latrines) share similar principles. However, salient differences exist, and these are summarized under each epidemiology.
-
(1)
The human gut and faeces as SARS-CoV-2 reservoirs
The two types of W-BE rely on the proliferation of SARS-CoV-2 in the human gut of infected persons (Pan et al., 2020; Xu et al., 2020; Zhang et al., 2020b). Subsequently, approximately 600,000 (Zhang et al., 2020b) to 30,000,000 (Wölfel et al., 2020) viral genomes of SARS-CoV-2 per mL of faecal material are shed in faeces of infected persons (oligosymptomatic, asymptomatic, symptomatic). The SARS-CoV-2-laden faeces are then discharged into wastewater and on-site sanitation systems (Medema et al., 2020, Medema et al., 2020; Hart and Halden, 2020; Zhang et al., 2020a). In fact, SARS-CoV-2 RNA detection and stability in wastewaters are some of the most studied environmental aspects of COVID-19 (Randazzo et al., 2020; Scott et al., 2021; Wurtzer et al., 2020; Zhang et al., 2020a; Westhaus et al., 2021). SARS-CoV-2 RNA detection in wastewaters has been reported in several locations, including Amsterdam, Netherlands (Medema et al., 2020, Medema et al., 2020), Paris, France (Wurtzer et al., 2020), Milan, Italy (La Rosa et al., 2020; Rimoldi et al., 2020), Brisbane, Australia (Ahmed et al., 2020), Massachusetts, Bozeman/Montana, Louisiana, USA (Nemudryi et al., 2020; Sherchan et al., 2020; Wu et al., 2020), different cities and wastewater facilities in Israel (Bar Or et al., 2020); Istanbul, Turkey (Kocameni et al., 2020), Valencia, Spain (Randazzo et al., 2020), and Yamanashi Prefecture, Japan (Haramoto et al., 2020). A few studies also observed SARS-CoV-2 RNA in secondary effluents from wastewater treatment plants. In Spain, 2 out of 18 secondary effluent samples tested positive (Randazzo et al., 2020), while in Paris, France, treated wastewater also tested positive (Wurtzer et al., 2020). A study in China observed SARS-CoV-2 RNA in hospital wastewater disinfected by chlorination in a septic tank, but no residual chlorine was detected in the effluent (Zhang et al., 2020a).
Existing evidence on SARS-CoV-2 RNA detection in wastewaters is dominated by studies drawn from developed regions, while those from LICs are comparatively very limited. Exceptions are: (i) one study from Ahmedabad, Gujarat, India which detected SARS-CoV-2 in 2 out of 2 influent wastewater samples, while the effluent samples tested negative (Kumar et al., 2020b), and (ii) a study reporting SARS-CoV-2 viral RNA in wastewater influent sampled from four wastewater treatment plants in Kwazulu-Natal (Pillay et al., 2021).
-
(2)
Global evidence on traditional wastewater-based COVID-19 epidemiology
The presence and stability of SARS-CoV-2 RNA in raw/untreated wastewater are the underlying principles for W-BE. Wastewater or sewage-based epidemiology (W-BE) was first reported in the 1970s, advancing steadily over the last 15 years to include: (i) licit and illicit drugs, (ii) human viral infections including polio and hepatitis A (Choi et al., 2018; Daughton, 2020a, Daughton, 2020b), and (iii) recently, SARS-CoV-2 (Ahmed et al., 2020; Kumar et al., 2020a, Kumar et al., 2020b; Randazzo et al., 2020; Scott et al., 2021). Similar to SARS-CoV-2 detection in wastewaters, the bulk of the studies on W-BE are limited to developed countries in Europe (e.g., Netherlands, Spain, Italy, France), Australia (Ahmed et al., 2020), and the USA (Hart and Halden, 2020) with a few recent exceptions in Asia and Latin America (Kumar et al., 2020a, Kumar et al., 2020b). In these earlier studies, the potential of W-BE as a COVID-19 early warning system has been reported in a number of studies using raw/untreated wastewater from centralized systems. For example, in three municipalities in Spain (Lorca, Cieza and Totana), SARS-CoV-2 RNA was reported in raw/untreated wastewater 12–16 days prior to the official reported cases of COVID-19 (Randazzo et al., 2020). In the USA, SARS-CoV-2 RNA data corrected for time lags were highly and positively correlated with the following COVID-19 data (r2 = 0.99): (i) local hospital admissions, and (ii) the epidemiological curve (Peccia et al., 2020, Peccia et al., 2020). As an early warning system or lead indicator, SARS-CoV-2 RNA concentrations in wastewaters were three and seven days ahead of COVID-19 data based on local hospital admissions and diagnostic testing, respectively (Peccia et al., 2020, Peccia et al., 2020). In Paris (France), the detection of viral RNA in wastewaters was ahead of the COVID-19 pandemic (Wurtzer et al., 2020), while in Italy, SARS-CoV-2 RNA was observed in wastewaters weeks before the first confirmed COVID-19 case (Randazzo et al., 2020). The capacity of W-BE to detect other human pathogens earlier than clinical data has also been reported in the case of norovirus and poliovirus (Hata and Honda, 2020). These lead times provide ample time for decision-makers and practitioners to activate and deploy COVID-19 emergency response systems. However, studies applying W-BE in LICs as a stand-alone tool or as part of a decision-support tool within the broader WWW-BE are still limited, but very few exceptions exist (Kumar et al., 2020a, Kumar et al., 2020b; Pillay et al., 2021).
3.1.1. Traditional centralized wastewater epidemiology in LICs
Based on the data on SARS-CoV-2 detection in wastewaters two inferences relevant to W-BE in LICs can be made: (i) SARS-CoV-2 RNA invariably occurs in raw/untreated wastewater from catchments with COVID-19 infected people with typical concentrations in the ranges of approximately 3 to 40 gene equivalents (Ahmed et al., 2020; Medema et al., 2020, Medema et al., 2020; Westhaus et al., 2021), and (ii) compared to advanced treatment processes used in developed countries, traditional wastewater treatment systems typical of those used in most LICs have low capacity to remove SARS-CoV-2 because they are often dilapidated, overloaded and hence inefficient. Raw/untreated and partially treated wastewater from conventional wastewater treatment plants are often discharged into surface water and groundwater systems supplying drinking water. These inferences are consistent with the general observation that raw/untreated wastewater often has high levels of pathogens, while secondary and tertiary treatment effluents often have medium and low levels of pathogens, respectively (Wang et al., 2019; Venugopal et al., 2020). Indeed, a few studies from South Africa and India applied traditional W-BE as a stand-alone tool to understand the prevalence of COVID-19 (Kumar et al., 2020a, Kumar et al., 2020b; Pillay et al., 2021).
3.1.2. On-site sanitation epidemiology
Globally, approximately 2 billion people, the bulk of them in low-income settings and informal settlements in rural, peri-urban, and urban areas including refugee camps, squatter camps, and slums rely on shared on-site sanitation facilities. For example, approximately 32% of urban sanitation facilities in sub-Saharan African is shared, one of the highest figures in the world (Caruso and Freeman, 2020). Shared sanitation facilities are also common in public institutions such as healthcare facilities, formal and informal markets, educational institutions (e.g., kindergartens, primary and secondary schools, colleges/universities), and COVID-19 quarantine centres. Like municipal wastewater systems, shared on-site sanitation facilities may harbour SARS-CoV-2 shed by various infected persons in a spatial unit, hence can be used for W-BE to understand the prevalence and dissemination of COVID-19. Currently, limited direct data are available on SARS-CoV-2 in environmental media from on-site sanitation facilities, and their use for COVID-19 surveillance in LICs. Here, the limited evidence relevant to WWW-BE is summarized.
-
(1)
On-site sanitation facilities have putatively higher SARS-CoV-2 than municipal wastewaters
The proliferation and shedding of SARS-CoV-2 in the gut of infected persons (Tang et al., 2020) lead to the subsequent direct release of SARS-CoV-2-laden faeces into on-site sanitation facilities. On-site sanitation facilities (septic tanks, pit latrines) are not specifically designed to remove human pathogens including SARS-CoV-2, thus such systems are expected to attain low removal of SARS-CoV-2. Moreover, shared on-site sanitation facilities including septic tanks and non-flushing pit latrines have limited dilution effects, and have short travel distances between the source of the SARS-CoV-2-laden faeces (i.e., infected person using the sanitation facility) and the ultimate receptor (i.e., sanitation facility). This is contrary to centralized wastewater treatment systems where concentration of SARS-CoV-2 may be reduced by: (i) significant dilution via flushing and mixing with surface run-off/storm-water, and (ii) viral die-off due to relatively longer transit times in sewer systems. Thus, wastewaters/effluents from on-site sanitation systems are expected to have putatively higher concentrations of SARS-CoV-2 and its proxies than raw/untreated wastewater from centralized systems.
-
(2)
Presence of SARS-CoV-2 RNA in wastewaters and fomites from on-site sanitation and toilet environments
Shared on-site sanitation facilities including septic tanks, non-flushing pit latrines, and flushing toilets are potential SARS-CoV-2 reservoirs and transmission hotspots (Zhang et al., 2020a; Caruso and Freeman, 2020; Gormley et al., 2020). A few studies have investigated and reported SARS-CoV-2 RNA in on-site sanitation environments (Liu et al., 2020; Zhang et al., 2020a; Del Brutto et al., 2021). One case-control study conducted in a rural Ecuadorian village severely hit by COVID-19 showed that the inner and upper walls of 24 out of 48 latrines, and 12 out of 49 flushing toilets had significantly higher SARS-CoV-2 RNA than the paired control-houses, with a probability (p) equal to 0.014 (McNemar's test) (Del Brutto et al., 2021). A significantly higher number of SARS-CoV-2–seropositive persons was observed among those using latrines than flushing toilets and the control (p = 0.002). Comparison of data for latrines versus flushing toilets showed that the odds of detecting SARS-CoV-2 RNA in latrines were five times that of flushing toilets. A recent study published as a pre-print has applied W-BE to detect SARS-CoV-2 RNA in on-site sanitation facilities in Bangladesh (Jakariya et al., 2021). This study points to the possibility to use wastewater and faecal sludge in on-site sanitation systems for SARS-CoV-2 surveillance.
A study conducted in Thekwini Municipality in Durban, South Africa investigated the occurrence of SARS-CoV-2 viral loads on five contact surfaces (toilet seat, cistern handle, floor surface located in front of the toilet, tap in hand-wash basin, internal pull latch of the cubicle door) in eight shared toilets in two peri-urban informal settlements (Amoah et al., 2021). Results showed that 54 to 69% of the toilet contact surfaces tested positive for SARS-CoV-2 RNA with viral loads ranging between 28.1 and 132.7 gene copies (gc) per cm2. The mean (± standard deviation) concentration of SARS-CoV-2 RNA per area swabbed varied significantly among the contact surfaces (p ≤ 0.05) with the highest values being observed for the toilet seats (132.9 ± 39.8 gc/cm2), followed by the cistern handle (69.1 ± 21.6 gc/cm2), and then internal latch (60.1 ± 14.5 gc/cm2). The highest concentrations observed on the toilet seat indicate viral shedding in faeces. The overall pattern of SARS-CoV-2 contamination of contact surfaces was consistent with surfaces easily contaminated with faeces and/or touched by users of the shared toilet. Hence, for a one time use of the shared toilet, the risk of human infection with COVID-19 through the contact surfaces was greatest for the toilet seat (mean ± standard deviation: 1.76 × 10−4 ± 1.58 × 10−6). Note that the study was conducted when the reported active COVID-19 cases in South Africa were still low (circa 600,000), and the risk was estimated for a one time use of the shared toilet. Hence, one may expect the severity of contamination and potential risk of community transmission to increase with increasing COVID-19 cases, and frequency of use of the shared toilets. This is particularly true for women and girls given their more frequent use of shared sanitation facilities than their male counterparts.
At Wuchang Fangcang Hospital in China, effluent from a septic tank treating hospital wastewater tested positive for SARS-CoV-2 RNA after initial chlorination with sodium hypochlorite at a dosage of 800 g/m3 (Zhang et al., 2020a). The absence of free chlorine in the septic tank effluent may explain the presence of SARS-CoV-2 RNA. These findings point to the following: (i) depending on dosage, chlorination may not effectively remove SARS-CoV-2 in cases of high viral loads, and (ii) higher SARS-CoV-2 concentrations and longer persistence are expected in effluents from on-site sanitation systems in LICs. This is because, in most cases, no chlorination is practised given that chlorination reagents are not readily and freely available for typical low-income communities in LICs. The presence of SARS-CoV-2 in wastewaters in on-site sanitation facilities could be particularly high in healthcare facilities, quarantine centres (Ahmed et al., 2021a, Ahmed et al., 2021b), and funeral homes handling infected persons.
Due to limited land holding in low-income settings, shared on-site sanitation facilities are often closely located adjacent to drinking water supply systems such as shallow boreholes and wells, resulting in strong hydrological connectivity between the two. Besides the ingestion of contaminated raw/untreated drinking water, SARS-CoV-2 transmission in shared sanitation facilities may occur via fomites and bioaerosols (Caruso and Freeman, 2020; Gormley et al., 2020). Fomites are contaminated inanimate materials, including contact surfaces such as metals, plastics and wood, which may harbour and transmit SARS-CoV-2 (Caruso and Freeman, 2020). Evidence showing that coronaviruses may persist on such materials for three to nine days points to their potential role in COVID-19 transmission (Kampf, 2020; Kampf et al., 2020). Air-borne transmission via aerosols may occur during flushing of toilets or septic systems, and subsequent aerosolization of contaminated wastewater/effluents (Gormley et al., 2020). Given its stability in bioaerosols of about 30 min (van Doremalen et al., 2020), SARS-CoV-2 from an infected person may remain viable and infective in shared sanitation facilities and infect the next person using such facilities within 30 min. Air-borne transmission via bioaerosols has been advanced as the reason explaining the following: (i) super-spreading 2003 SARS outbreak in garden flats in Hong Kong (Gormley et al., 2020), and (ii) rapid spread of COVID-19 in confined spaces with dense populations such as among healthcare workers, air-plane passengers and cruise-ships (Mizumoto et al., 2020).
Insects and vermin such as cockroaches, houseflies, and rodents that frequent, and are attracted to shared on-site sanitation and wastewater facilities may harbour and transfer human pathogens on their external body and in their gut system (Bonwitt et al., 2017; Heller et al., 2020; Sarwar, 2015). SARS-CoV-2 transmission through insect-and rodent-mediated processes has not yet been confirmed, but the dissemination of SARS-CoV-2 via direct contact with faeces in shared on-site sanitation and wastewater facilities to other environmental compartments including households cannot be ruled out (Gwenzi, 2020b). Further research is required to confirm insect-and rodent-mediated transfer of SARS-CoV-2 and the mechanisms involved. In summary, wastewaters from both shared on-site sanitation and centralized wastewater facilities qualify to be used for W-BE in LICs, but the decay of SARS-CoV-2 and its proxies needs to be taken into account for each system. Yet to date, no studies have applied on-site sanitation epidemiology to understand COVID-19 in LICs.
3.1.3. SARS-CoV-2 in wastewaters and receiving waters in LICs versus developed countries: Cautionary remarks
Advanced wastewater treatment systems, including a combination of secondary and then tertiary treatment based on disinfection using chemicals or ultraviolet irradiation commonly used in developed countries have a higher potential to remove SARS-CoV-2 and its proxies (Randazzo et al., 2020; Rimoldi et al., 2020) than conventional systems dominant in LICs such as those based on aerobic digestion (Guerrero-Latorre et al., 2020; Kolarević et al., 2021; Mahlknecht et al., 2021; Maidana-Kulesza et al., 2021; Westhaus et al., 2021). For example, in Murcia, Spain, all tertiary and secondary effluents from wastewater treatment plants combining advanced treatment processes in the form of disinfection and ultra-violet irradiation tested negative for SARS-CoV-2 RNA (Randazzo et al., 2020). Similarly, in two provinces in Italy (Milan, Monza e Brianza), no SARS-CoV-2 was detected in wastewaters subjected to secondary treatment and tertiary disinfection using peracetic acid or high intensity ultraviolet lamps (Rimoldi et al., 2020). A few exceptions exist, pointing to the need for caution to avoid generalizations when comparing SARS-CoV-2 removal in wastewater treatments systems in LICs versus developed countries. This is because not all wastewater treatment systems in developed countries use advanced processes.
In cases where conventional wastewater treatment processes such as activated sludge are used, SARS-CoV-2 RNA has been detected in both raw/untreated and treated wastewater in nine wastewater treatment plants in North-Rhine Westphalia, Germany (Westhaus et al., 2021). The gene equivalents in the solid and aqueous phases of the effluent were similar to or even higher than that in the influent wastewater. The enrichment of gene equivalents in effluent aqueous phase was attributed to the repartitioning or mobilization of gene material from the solid to the liquid phase during wastewater treatment (Westhaus et al., 2021). In the study conducted by Rimoldi et al. (2020), although wastewater subjected to secondary treatment and tertiary disinfection tested negative for SARS-CoV-2 RNA, all the surface water samples tested positive for SARS-CoV-2. The SARS-CoV-2 detected in the receiving water was attributed to two possible sources: (i) discharges of non-treated or inefficiently treated wastewaters, and/or (ii) combined sewage overflows (Rimoldi et al., 2020). Incidental discharges of raw or partially treated wastewaters, and combined sewer outflows caused by malfunctioning urban drainage systems have been reported in other developed countries in Europe (e.g., Rizzo et al., 2020) and the USA (U.S. EPA, 2004). However, due to low levels of economic development and low investments in wastewater infrastructure in LICs, the practice is more prevalent in LICs than developed countries (Gwenzi and Rzymski, 2021).
In Japan, SARS-CoV-2 RNA (2.4 × 103 copies/L) was detected in a secondary-treated wastewater before chlorination (Haramoto et al., 2020). The SARS-CoV-2 RNA concentration in the secondary-treated wastewater sample was two orders of magnitude lower than that reported for secondary wastewater in Spain (2.5 × 105 copies/L) (Randazzo et al., 2020). This suggests that secondary wastewater treatment without tertiary disinfection has limited capacity to remove SARS-CoV-2 and its proxies. Surprisingly, in the study by Haramoto et al. (2020), no SARS-CoV-2 RNA in influent and river waters tested using the same analytical procedure. This apparent anomaly was attributed to differences in limits of detection associated with the filtration sample volumes used for influent versus treated wastewater. The filtration volume of the influent wastewater samples (200 mL) was 25 times smaller than that of the secondary-treated wastewater samples (5000 mL). Consequently, the limit of detection for influent (4.0 × 103–8.2 × 104 copies/L) was approximately one to two orders of magnitude larger than that of secondary-treated wastewater (1.4 × 102–2.5 × 103 copies/L). In addition, the influent and secondary-treated wastewaters were collected almost simultaneously without paying attention to potential differences in hydraulic retention time. The hydraulic retention time may affect the decay and repartitioning/mobilization of SARS-CoV-2 in wastewater.
In summary, these results suggest that, besides the wastewater treatment process (conventional versus advanced), the capacity to remove SARS-CoV-2 and its proxies may also depend on several other factors including: (i) the initial concentrations in raw/untreated wastewater, and (ii) operating conditions such as hydraulic loading rates and residence times, and dosages of the chemicals or ultra-violet radiation using in advanced treatment processes. Thus, even for the same conventional or advanced treatment process, SARS-CoV-2 removal may vary on a case-by-case basis. In this regard, a mere location of a country (low-income versus developed region) or the type of a wastewater treatment system (conventional versus advanced processes) cannot be used as a basis to infer the occurrence of SARS-CoV-2 in treated wastewater and receiving waters. Only direct analytical testing for SARS-CoV-2 and its proxies can provide unequivocal evidence on where such media can be used for WWW-BE. Finally, regardless of the wastewater treatment processes applied, the use of SARS-CoV-2 and its proxies in treated wastewater and receiving waters in WWW-BE will require accounting for any removal or decay, and enrichment occurring in such wastewater treatment systems.
3.2. Raw/untreated and drinking water epidemiology
Three lines of evidence motivate the use of raw/untreated and drinking water epidemiology in LICs.
-
(1)
SARS-CoV-2 RNA detection in groundwater and surface water systems in low-income settings
A few recent studies drawn from LICs and other low-income settings in east Europe, and Latin America provide direct evidence on SARS-CoV-2 RNA presence in raw/untreated surface water and groundwater impacted by sewage (Guerrero-Latorre et al., 2020; Maidana-Kulesza et al., 2021; Mahlknecht et al., 2021; Kolarevic et al., 2021). For example, during the COVID-19 peak in Quito, Ecuador, SARS-CoV-2 RNA was observed in surface water samples from three different sites of a river receiving untreated sewage (Guerrero-Latorre et al., 2020).
In Monterrey Metropolitan Area in Mexico, Mahlknecht et al. (2021) investigated SARS-CoV-2 RNA presence in groundwater, dam water, and river water. The results showed that 44% of groundwater sampled had SARS-CoV-2 viral loads ranging between 2.6 and 38.3 copies/mL. The viral loads were significantly correlated with the concentration of sucralose (an artificial sweetener) and E. coli in groundwater, indicating leaching and infiltration of effluent from the surface and/or failing sewage pipes. In the same study, 12% the dam water samples tested positive for viral RNA with concentrations ranging between 3.3 and 3.8 copies/mL. Lastly, 13% of the river samples tested positive for viral RNA, with concentrations ranging from 2.5 to 7.0 copies/mL. The viral loads in groundwater, river water and dam water were about three orders of magnitude lower than the viral loads of up to 3535 copies/mL detected in the corresponding raw/untreated wastewater samples. The difference in viral loads between water samples and wastewater was attributed to dilution effect and/or removal through wastewater treatment. The temporal trends of viral loads in the groundwater, river water, dam water and wastewater mirrored the reported trends of COVID19 infection cases.
In Argentina, SARS-CoV-2 RNA was observed in about half of the river water samples (75) impacted by wastewater from a wastewater treatment plants (Maidana-Kulesza et al., 2021). Moderate but significant positive correlations were also observed between SARS-CoV-2 RNA and faecal indicator bacteria (p ≤ 0.05; r2 = 0.40–0.75). The study by Maidana-Kulesza et al. (2021) is the first to apply raw/untreated water epidemiology to predict COVID-19 in LICs. The results of the study showed that SARS-CoV-2 RNA concentrations in river water samples accurately predicted the COVID-19 epidemiological curve (p = 00001–0.0084). Similar to most LICs, the rivers recording the high concentrations of SARS-CoV-2 received both raw/untreated and partially treated wastewaters.
Further evidence of SARS-CoV-2 contamination of surface water sources impacted by sewage is drawn from Europe. For example, SARS-CoV-2 RNA concentrations ranging from 5.97 × 103 to 1.32 × 104 copies/L were detected in surface water samples in the Danube River impacted by raw/untreated wastewater from Belgrade, Serbia (Kolarević et al., 2021). In metropolitan Milan, Italy, SARS-CoV-2 RNA was reported in receiving surface waters, and this was attributed to discharges of raw or partially treated wastewater, and/or combined sewer outflows (Rimoldi et al., 2020). Contrary, in Japan, no SARS-CoV-2 RNA was observed in three river samples collected between March and May 2020 (Haramoto et al., 2020). These studies clearly demonstrate that SARS-CoV-2 contamination of surface and groundwater systems is possible in cases where raw/untreated or partially treated wastewaters are discharged from conventional and inefficient treatment systems. As cautioned earlier, there is a need to avoid generalizations on the occurrence of SARS-CoV-2 and its proxies in wastewaters and receiving water in LICs versus developing countries (Section 3.1.3).
Currently missing in the literature are studies investigating SARS-CoV-2 presence in groundwater and surface water systems in densely populated high-risk areas in LICs (e.g., refugee camps, slums, squatter camps). One may infer that SARS-CoV-2 contamination of raw/untreated water and drinking water supply systems is highest under the following conditions: (i) on-site sanitation facilities located in close proximity with unprotected water and drinking sources from shallow wells, boreholes, and surface water bodies, (ii) congested informal settlements where shared on-site sanitation facilities are often overloaded and over-spilling, and (iii) groundwater systems in highly permeable and coarse-textured geological systems resulting in strong hydrological connectivity, and rapid travel times.
-
(2)
Strong hydrological connectivity between on-site sanitation/wastewater systems enhances SARS-CoV-2 contamination of drinking water supply systems
A key point to note in LICs is the strong hydrological connectivity between both on-site sanitation and centralized wastewater systems, and drinking water systems (Fig. 2). The hydrological connectivity, which is often coupled to faecal contamination of raw/untreated and drinking water supply systems occurs via: (i) wastewater/effluent spillages, (ii) surface run-off and erosion, (iii) groundwater recharge and infiltration, and (iv) interactions between groundwater and surface water. This strong hydrological connectivity is evidenced by several studies from LICs indicating high faecal coliform and indicator bacteria and human enteric pathogens in surface water and groundwater systems impacted by raw/untreated and partially treated wastewaters from on-site sanitation and wastewater facilities (Genter et al., 2021; Graham and Polizzotto, 2013; Potgieter et al., 2020). In these studies, faecal coliform and indicator bacteria act as ‘biotracers’ of wastewater contamination of aquatic systems. Thus, wastewaters/effluents coupled to hydrological processes act as reservoirs and vehicles for the SARS-CoV-2 contamination of raw/untreated and drinking water supply systems. The strong hydrological connectivity, and faecal contamination of raw/untreated surface water, groundwater, and drinking water systems account for recurrent outbreaks of water-borne diseases in LICs.
-
(3)
Recurrent outbreaks of water-borne diseases linked to faecal contamination of raw/untreated drinking water supply systems in LICs
The potential to use drinking water for SARS-CoV-2 surveillance has been suggested in a few earlier studies (Guerrero-Latorre et al., 2020; Street et al., 2020), but data on SARS-CoV-2 detection in drinking water systems remain scarce. However, recurrent outbreaks of water-borne infections (e.g., cholera, typhoid) traced to the faecal contaminated drinking water supply systems including surface water and groundwater are well-documented in LICs (Islam et al., 2007; Ajayi and Smith, 2019; Gwenzi and Sanganyado, 2019). Human infections that induce diarrhea such as water-borne ones (e.g., cholera, typhoid) promote the risk of faecal contamination of drinking water (Islam et al., 2007). Similarly, given that COVID-19 induces diarrhea in some patients, this may increase the frequency of SARS-CoV-2 shedding in faeces, and risk of contamination of drinking water supply systems.
In LICs, faecal coliform and E. coli indicator bacteria, and viruses including human pathogens have been widely detected in faecal sludge and boreholes located close to pit latrines (Graham and Polizzotto, 2013; Capone et al., 2021; Liu et al., 2021; Otaki et al., 2021). Graham and Polizzotto (2013) present a systematic review of a global database on the role of pit latrines in faecal contamination of water supply systems. Two recent studies from Mexico and Argentina observed moderately significant and positive correlations (r2 = 0.40–0.75; p < 0.05;) between SARS-CoV-2 RNA concentration and corresponding faecal coliform bacteria in surface water and groundwater samples impacted by wastewaters (Mahlknecht et al., 2021; Maidana-Kulesza et al., 2021). Significant positive correlations between SARS-CoV-2 RNA and faecal coliform bacteria indicate potential co-shedding, co-occurrence and co-transport of the two in both faeces and wastewaters. However, more research is needed to determine the universal validity of the correlation between SARS-CoV-2 and its proxies, and faecal coliform bacteria, and other parameters.
In summary, a relatively strong evidence base largely drawn from LICs now exists to justify the use of water/drinking water supply systems in COVID-19 epidemiology (Guerrero-Latorre et al., 2020; Fongaro et al., 2021). Hence, in view of this recent research evidence, estimating COVID-19 prevalence in low-income settings using traditional W-BE based solely on centralized wastewater systems is flawed, and reflects a narrow understanding and application of the concept of environmental surveillance. Therefore, the WWW-BE framework explicitly broadens the traditional W-BE, which can be applied at differential spatial and temporal scales. One potential limitation of water and drinking water-based epidemiology is the dilution effects especially in river systems. For example, in cases where the initial concentration in the wastewater discharges are low, and the dilution in receiving water is high, this could a challenge in analytical detection of SARS-CoV-2. In turn, this may increase the likelihood of false-negative results, and potential under-estimation of COVID-19 prevalence. To address this potential limitation, there is a need to investigate and define the boundaries or limits for water and drinking water-based epidemiology.
Shared drinking water supply systems in LICs serve a wide community and population. Hence, drinking water systems may harbour SARS-CoV-2 emitted from the various sources, and further transmit it to non-infected persons. Human exposure to SARS-CoV-2 in shared drinking water supply systems may occur via: (i) ingestion of contaminated drinking water, (ii) fomites such as water abstraction devices on well and boreholes, and (iii) respiratory droplets and aerosols while queuing for water under overcrowded conditions (Gwenzi, 2020b). The analysis of data on SARS-CoV-2 occurrence in drinking water supply systems may provide insights on communities and households likely to be exposed to COVID-19 via drinking water. Moreover, purposive sampling at strategic points along the drinking water system may identify contamination hotspots and critical control points for safeguarding human health. Therefore, drinking water systems form a critical component of WWW-BE as part of a broader strategy to understand the prevalence of COVID-19 as well as water-borne infections (e.g., typhoid, cholera). Surprisingly, despite earlier calls made nearly a year ago to investigate SARS-CoV-2 presence and persistence in drinking water systems under such settings (Gwenzi, 2020a, Gwenzi, 2020b; Adelodun et al., 2020), no studies have been done so far. To bridge this gap, the proposed WWW-BE seeks to motivate the acquisition of such evidence, and its subsequent application to estimate COVID-19 prevalence and transmission in LICs. Note that the use of SARS-CoV-2 contaminated drinking water supply systems in epidemiology is not meant to undermine the need to terminate the use of such water sources for water supply or to treat the water before human consumption. Rather, the proposal is to use the data on SARS-CoV-2 monitoring in drinking water systems first, to safeguard human health, and second, in WWW-BE. Thus, no SARS-CoV-2 contaminated drinking water supply sources should be maintained solely for predicting COVID-19 burden and transmission trends.
3.3. Solid waste epidemiology
In the WWW-BE framework solid waste epidemiology can be conceived as having two components: (i) faecal sludge from non-flushing pit latrines with or without frequent emptying, and (ii) COVID-19-related solid waste such as PPE. For the purposes of sampling and analysis, faecal sludge from non-flushing on-site sanitation systems should be considered as solid waste rather than wastewater. This notion is consistent with recent studies classifying faecal sludge from such non-flushing on-site sanitation systems as solid waste (Capone et al., 2021). Primary faecal sludge from wastewater systems, and by inference on-site sanitation systems may harbour up to two to three orders of magnitude more SARS-CoV-2 than the raw/untreated wastewater/effluent itself (Peccia et al., 2020, Peccia et al., 2020). A study conducted in the New Haven, Connecticut in the USA during the peak of a COVID-19 outbreak showed that primary sludge contained two to three orders of magnitude higher SARS-CoV-2 RNA that values reported in literature for raw/untreated wastewater (Peccia et al., 2020, Peccia et al., 2020). Thus, faecal sludge in on-site sanitation systems could be ideal media for environmental surveillance of SARS-CoV-2. The use of faecal sludge from non-flushing on-site sanitation systems for SARS-CoV-2 monitoring has been proposed in an earlier study conducted in Malawi (Capone et al., 2021). A few studies conducted in developed countries have used wastewater sludge for SARS-CoV-2 surveillance (D'Aoust et al., 2021; Graham et al., 2020; Peccia et al., 2020, Peccia et al., 2020), pointing to the possibility of using faecal sludge for solid waste epidemiology.
The use of non-faecal solid waste such as COVID-19 PPE in epidemiology is relatively new, and no studies have been reported to date. The solid waste epidemiology is premised on the assumption that SARS-CoV-2 can be shed and persist on solid waste including household and institutional wastes, and COVID-19 PPE. The shedding of SARS-CoV-2 and contamination of solid wastes may occur via multiple routes including exhalation from infection persons, bioaerosols, and direct contact with contaminated materials and surfaces. Barring a few exceptions, a comprehensive global database on SARS-CoV-2 occurrence on solid wastes including faecal sludge from non-flushing on-site sanitation systems and COVID-19 PPE under real-life conditions is still lacking. The reason for the lack of comprehensive evidence is unclear, nearly two years after the first outbreak of COVID-19 towards the end of 2019. This is even surprising given the widespread global use and subsequent disposal of large quantities of COVID-19 PPE in households, public institutions, business facilities, healthcare facilities, quarantine centres, and the funeral industry. However, the few existing studies indicate presence and persistence of SARS-CoV-2 on solid waste including wood and COVID-19 PPE relevant to solid waste epidemiology (Kasloff et al., 2021; Liu et al., 2020; Pastorino et al., 2020).
In an inoculation experiment conducted with or without proteins (i.e., 3 g/L bovine serum albumin), SARS-CoV-2 was detected on aluminium, glass, and polystyrene plastic (Pastorino et al., 2020). The longevity of viral infectivity expressed as log 10 decrease was highest for polystyrene plastic (<1 log10 drop), followed by glass (3.5 log10 drop), and then aluminium (6 log10 drop), and in all cases, proteins prolonged infectivity. In Hong Kong, SARS-CoV-2 RNA was detected on disposable wooden chopsticks, indicating potential persistence on wood, but viral viability and infectivity were not determined (Liu et al., 2020). Kasloff et al. (2021) investigated the stability of SARS-CoV-2 on eight artificially inoculated PPE over a 21-day period. The PPE materials included respirator masks (N100, N-95), cotton, Tyvek, reinforced chemical resistant gloves, nitrile medical examination gloves, plastic, and stainless steel. Contrary to earlier findings suggesting short survival periods (van Doremalen et al., 2020), the study showed that, in the presence of a soil load, viable SARS-CoV-2 was detected for up to 21 days on the PPE. However, rapid viral degradation was observed when applied to 100% cotton fabric, and was not detected within 24 h using the TCID50 assay. These persistence data are particularly important, given that the PPE investigated included those used by the general public, and healthcare and funeral industry workers.
A number of earlier studies have shown that coronaviruses and their proxies, and by inference SARS-COV-2 may persist on typical waste materials such as metals, plastics, and paper for 3 to 9 days (Kampf, 2020; Kampf et al., 2020; Pastorino et al., 2020; van Doremalen et al., 2020). Solid materials tested to date include; cardboard, wood, plastics, fabric, printing and tissue paper, and metals, specifically copper and stainless steel (Chin et al., 2020; van Doremalen et al., 2020). This evidence provides some indicative values on survivorship of SARS-CoV-2 on solid waste materials. Studies on SARS-CoV-2 persistence showed that the virus was inactivated and undetectable on stainless steel and plastic after 4 days, while shorter survival times were observed for cardboard (2 days) and copper (4 h (Chin et al., 2020; van Doremalen et al., 2020). Additional data on the detection of SARS-CoV-2 on solid materials and surfaces are presented in studies including reviews (Bueckert et al., 2020; Dargahi et al., 2021; Onakpoya et al., 2021). Thus, based on the limited evidence on coronaviruses, coupled with data on SARS-CoV-2, it is reasonable to assume that, depending on environmental conditions, SARS-CoV-2 may survive for much longer periods than the 2–3 days on solid materials. In summary, the available data presented in earlier studies including reviews show that (Chin et al., 2020; van Doremalen et al., 2020; Nghiem et al., 2020): (i) survival times varied considerably among the different groups of materials, and even between various types of within the same group (e.g., copper versus stainless steel), (ii) SARS-CoV-1 persisted on PPE materials for 24 h and 48 h on cotton gown (fabric) and disposable polypropylene gown (plastic), respectively, (iii) under room temperature (circa 22 °C) and relative humidity of 40 to 50% SARS-CoV-1 survived for up to 9 days on a polystyrene petri dish, and for about 21 days also on plastic, and (iv) a study using transmissible gastroenteritis virus (TGEV) as a surrogate coronavirus showed that TGEV survived on N95 respirators for up to 24 h. However, longer persistence periods are possible under certain environmental conditions (e.g., presence of proteins, soil load) as shown in recent evidence (e.g., up to 21 days, Kasloff et al., 2021; Pastorino et al., 2020).
In the context of WWW-BE, the persistence of coronaviruses on solid waste material is significant in three respects. First, solid wastes may act as fomites, thereby increasing the risk of SARS-CoV-2 transmission to humans via contact surfaces and solid wastes. Secondly, SARS-CoV-2 RNA on raw/untreated or untreated solid waste materials could be used as a biomarker to estimate prevalence COVID-19. Finally, the persistence period may be used as a basis to determine the time needed to store solid waste to allow virus inactivation. For example, using such data one study estimated that combining a stand-down period of at least 4 days and subsequent disinfestation will allow maximum inactivation of the virus by subjecting it to a double hit (Derraik et al., 2020).
COVID-19 generates COVID-related waste such as used PPEs including face masks (Mol and Caldas, 2020; Peng et al., 2020). This is in addition to non-COVID-19 wastes such as domestic and municipal solid waste, and infectious solid wastes such as dressings, gloves and sharps. Sources of COVID-19 related solid waste include households with infected persons, healthcare facilities, quarantine centres, and the funeral industry. One study conducted in China showed that a hospital with total of 24 COVID-19 patients generated a daily output of medical waste of 2100 kg and COVID-related medical wastes of 150 kg. In Africa, estimates show that, 700 million face masks were used per day in just 15 countries implementing compulsory use of facemasks, which will ultimately end up as waste (Nzediegwu and Chang, 2020). This figure increases significantly when one also considers other PPE such as gloves, wipes and household and institutional wastes from healthcare facilities and quarantine centres such as waste paper and plastics.
In LICs, due to lack of waste separation and recycling systems, incinerators and engineered landfills, infectious medical wastes and COVID-19 PPE and related wastes are often co-mingled, and co-disposed of with general solid waste from households, and commercial activities in non-engineered solid waste repositories where they pose environmental pollution risks (Nzediegwu and Chang, 2020; World Bank, 2019; Gwenzi, 2020a, Gwenzi, 2020b). Workers in the solid waste industry, including sweepers, cleaners and bin collectors often work without appropriate PPE. Informal waste pickers from low-income communities often collect waste materials including used plastic bags, and bottles for personal use or for sale as packaging materials for vegetables, water and herbal medicines (Nzediegwu and Chang, 2020). Such waste pickers rarely use PPE during waste collection. Stray animals such as dogs and livestock often roam freely on such waste dumps, further disseminating the contaminated wastes. For example, in Abuja, Nigeria, a funeral industry worker dumped a used disposable safety overall in a public place following as burial of a COVID-19 patient at a cemetery (Nzediegwu and Chang, 2020). Such practices may promote the transmission of COVID-19 via solid wastes. Yet these aspects are currently not considered in current generic COVID-19 control measures, and traditional W-BE. Therefore, understanding the presence and behaviour of SARS-CoV-2 on solid waste including COVID-19 PPE should form part of a broader research programme on WWW-BE in LICs.
The lack of studies using non-faecal solid waste such as COVID-19 PPE in epidemiology seem to reflect the notion that such waste may be considered to be of limited epidemiological significance because the SARS-CoV-2 on such solid waste has limited mobility, giving rise to high heterogeneity. Hence, it appears the dominant notion has been that particular attention should be paid to the rational and appropriate disposal of potentially infectious solid wastes such as those from putative SARS-CoV-2 hotspots such as medical facilities, quarantine centres (Ahmed et al., 2021a, Ahmed et al., 2021b) and the funeral industry. As a hypothesis, there is a need to conduct research to generate the empirical data to confirm the (in)validity of using COVID-19-related solid waste in epidemiology, and highlight the associated opportunities and challenges.
3.4. WWW-BE versus traditional W-BE: a summary of the novelty
The novelty of the WWW-BE relative to traditional W-BE can summarized as follows (Table 1):
-
(1)
WWW-BE builds on the fundamental principles of traditional W-BE, and extends it to include wastewaters from on-site sanitation facilities, surface water and groundwater systems, drinking water supply systems, and solid wastes currently excluded in literature but relevant in LICs. The extension of W-BE to WWW-BE could potentially make the latter more ideal and relevant to LICs than the former.
-
(2)
WWW-BE is modular with four components (Fig. 2), unlike traditional W-BE limited to centralized wastewater systems. Thus, WWW-BE entails potential flexibility, where certain modules or components can be emphasized on a case-by-case basis.
-
(3)
WWW-BE could potentially serve a dual function, first for determining the prevalence or burden of COVID-19 in a spatial unit, and second, predicting potential subsequent SARS-CoV-2 transmission from WWW-BE media. By contrast, the traditional W-BE is currently limited to estimation of COVID-19 prevalence, while excluding potential application to estimate potential transmission.
-
(4)
WWW-BE could be a potential low-cost and appropriate tool for large-scale surveillance of COVID-19 and related future outbreaks, thereby overcoming the severe constraints of lack of diagnostic equipment and resources prevalent in LICs.
The novelties of WWW-BE, coupled with the lack of multi-barrier systems to safeguard human health provide a strong motivation for the research community, and decision and policy-makers including funding agencies to support research to develop and validate WWW-BE as a hypothesis and decision-support tool in LICs. Such research will provide a strong WWW-BE evidence base, which is currently missing.
4. Potential applications of WWW-BE
4.1. WWW-BE as a novel decision-support tool
Depending on research and operational objectives, WWW-BE can be potentially applied to acquire data on COVID-19 at various spatial and temporal resolutions. Spatial scales may include household, community, village, district, catchment, province/state and population levels. The sampling timescales may also range from once-off grab samples to repeated daily, weekly and monthly timescales, among others. Notably, the cost of WWW-BE data acquisition, analysis and interpretation is expected to increase with increasing temporal and spatial resolution. It should be emphasized that prediction of COVID-19 prevalence based on environmental surveillance still suffers from a number of limitations including high uncertainties. This is because SARS-CoV-2 transmission and progression dynamics are quite complex and depend on several anthropogenic and biophysical factors which may not be adequately captured by monitoring environmental media. Notwithstanding these challenges, here, potential generic applications of WWW-BE as a hypothesis and decision-support tool in LICs are discussed.
-
(1)
Identifying potential COVID-19 temporal ‘hot moments’ and spatial ‘hotspots’
Systematic spatial and temporal sampling of drinking water supply systems, solid waste, and wastewaters from municipal and shared on-site sanitation facilities may give information on COVID-19 temporal ‘hot moments’ and spatial ‘hotspots’. In this context, a spatial ‘hotspot’ is a distinct spatial domain or cluster with an exceptionally high transmission and infection rates while a temporal ‘hot moment’ is a temporal domain or cluster characterized by high transmission or infection rates. A number of analytical tools are available for assessing hotspots and hot moments, including; (i) geospatial tools such as geoinformatics and geostatistics, (ii) wavelet and spectral techniques, and (iii) artificial intelligence and network analysis (Charandabi and Gholami, 2021; Bwire et al., 2017; Pinheiro et al., 2021). Artificial intelligence, including the use of data mining techniques, artificial neural networks, and in silico techniques and networks analysis have capabilities to reveal trends and patterns in data that are not obvious using conventional statistical tools. WWW-BE data on potential SARS-CoV-2 contamination hotspots with respect to drinking water supply systems, solid wastes, and wastewater from municipal and on-site sanitation facilities can be used to safeguard public health through the choice of safe water sources, and/or as a basis to recommend drinking water disinfection.
-
(2)
Estimating the prevalence or burden of COVID-19
The use of W-BE data for estimating the prevalence or burden of COVID-19 is the most-documented application (Kumar et al., 2020a, Kumar et al., 2020b; Scott et al., 2021; Pillay et al., 2021). WWW-BE data may be disaggregated or aggregated to provide information on the prevalence or burden of COVID-19 at various spatial scales of interest. To achieve this, data on SARS-CoV-2 RNA titres in the solid waste, municipal wastewater, on-site sanitation facilities, raw/untreated water, and drinking water supply systems should be converted to the corresponding estimates of infected persons. Similar to traditional W-BE, scope exists to acquire WWW-BE data at the appropriate sampling scales to provide similar estimates of COVID-19 prevalence at various spatial and temporal scales.
As reported for traditional W-BE (Gibas et al., 2021; Scott et al., 2021), WWW-BE can also be used for COVID-19 surveillance at institutional or even at building levels including in educational (kindergartens, primary and secondary schools, colleges/universities), healthcare (clinics, hospitals), workplaces, commercial, and quarantine and isolation facilities. This is because even at institutional level, repeated mass diagnostic testing of individuals is not always feasible.
-
(3)
COVID-19 transmission patterns
Analysis of WWW-BE data using forward calculation techniques may give information on the subsequent COVID-19 transmission patterns from various reservoirs within a community or population. For example, analysis of high-resolution WWW-BE data on the occurrence of SARS-CoV-2 and its proxies in solid waste, municipal wastewater, on-site sanitation, and drinking water sources may indicate potential contributing localities. Moreover, information on municipal wastewater systems and shared contaminated sanitation systems and drinking water sources combined with the corresponding households using such systems may provide insights into the likely temporal and spatial progression of COVID-19 outbreaks. Scope also exist to use repeated WWW-BE data to provide information of the onset, peak and tail-end of a COVID-19 outbreak. This application is unique to LICs lacking engineered multi-barrier systems that effectively reduces the risk of SARS-CoV-2 transmission from the various WWW-BE media.
Note that bioaerosols and droplets are currently considered the dominant transmission routes for SARS-CoV-2 (WHO, 2020). The contribution of fomites and environmental transmission via wastewater and ingestion of contaminated drinking water is not yet known with certainty, and warrants further research (Gwenzi, 2020b). Thus, positive detection in environmental media indicates virus shedding and pollution, but may not be indicative of human exposure and health risks. This is because understanding human health risks and transmission trends require more data than mere detection of SARS-CoV-RNA. This includes data on dose-response relationships, infectious dose thresholds, and metrics to estimate viable and infective SARS-CoV-2 from viral RNA. These aspects are still poorly understood and should form part of research on the potential application of WWW-BE to estimate transmission patterns.
-
(4)
Targeting and prioritization of scarce resources
COVID-19 exerts excessive pressure on scare resources at all levels including, healthcare system, social security, logistics and the supply and cold chain systems. Therefore, data on COVID-19 prevalence and transmission patterns can be used for the following operational applications: (i) targeting and prioritizing the allocation of scarce resources such as PPE, comprehensive testing of individuals, and clean drinking water supplies, (ii) location and establishment of testing and quarantine centres, and (3) the deployment of emergency response systems and teams.
-
(5)
Enforcement and lifting of control measures
The enforcement and lifting of national control measures such as local and national lockdown requires accurate and timely data on the temporal and spatial progression of COVID-19. Similar to traditional W-BE, WWW-BE data could act as a leading indicator or an early warning system signalling the arrival of COVID-19 ahead of clinical data and hospital admissions. Conversely, absence of SARS-CoV-2 and its proxies following a number of consecutive periods of WWW-BE monitoring could be indicative of the end of a COVID-19 outbreak. In this regard, repeated negative SARS-CoV-2 in drinking water supply systems, solid waste, municipal wastewater, and shared on-site sanitation facilities could be used as a basis to cautiously lift lockdowns. Given that WWW-BE considers various environmental sources of SARS-CoV-2, it could potentially provide more reliable and robust COVID-19 estimates than traditional W-BE which relies on data from one medium (municipal wastewater).
4.2. Testing and validating hypotheses on COVID-19
-
(1)
Environmental source tracking of SARS-CoV-2
Source tracking of SARS-CoV-2 to determine its potential environmental sources and intermediate hosts in aquatic and terrestrial systems is a potential emerging research topic in environmental epidemiology. A number of genomic tools have been developed for the microbial source tracking of human pathogens (Meays et al., 2004; Sheludchenko, 2011). Thus, two questions may arise: (i) Could genomic analysis of SARS-CoV-2 in environmental media reveal its environmental sources and intermediate hosts?, and (ii) Can SARS-CoV-2 be used as a novel biological tracer of its environmental origin, including the nature of animal-human interactions, and how it jumped from its natural and intermediate hosts to humans? These questions have no simple answers, and addressing them is not a trivial task, but they highlight the potential contribution of environmental surveillance to our understanding of COVID-19.
-
(2)
Novel transmission via the faecal-oral route
A number of hypothesis on novel transmission of COVID-19 has been proposed, including faecal-oral route via water and food, and vector-mediated mechanisms, but these remain mere postulates until confirmed (Gwenzi, 2020b). WWW-BE could provide an opportunity to directly validate these hypotheses especially in LICs, where conditions are most conducive for such studies. In communities relying solely on shared raw/untreated water sources, drinking water-based epidemiology could provide essential data to minimize the risk of human exposure to SARS-CoV-2 through ingestion of contaminated drinking water. Specifically, drinking water-based epidemiology may address outstanding research questions raised in earlier reviews on COVID-19 (Gwenzi, 2020a, Gwenzi, 2020b; Gwenzi and Rzymski, 2021). Key questions include: (i) To what extent do SARS-CoV-2 and its proxies occur in raw/untreated water and drinking water supply systems in densely populated faecal contamination hotspots (e.g., refugee camps, squatter camps, slums)?, (ii) To what extent do low-cost drinking water treatment methods (e.g., biosand filtration, solar disinfection, biochar filters, chlorination, metallic iron filters, ceramic filters) used in such informal setting reduce the SARS-CoV-2 viral load?, (iii) How significant are the risk of exposure and human health effects of consumption of SARS-CoV-2 contaminated drinking water relative to other exposure pathways, and (iv) To what extent do vector-mediated and faecal transmission of SARS-CoV-2 from environmental reservoirs such as solid wastes, shared on-site sanitation facilities, and municipal wastewater systems contribute to COVID-19 outbreaks? Addressing these questions is critical in the development of potential control methods in informal human settlements and humanitarian emergencies.
-
(3)
Gendered COVID-19 exposure, transmission and mitigation
Shared drinking water supply and on-site sanitation facilities are potential hotspots for community transmission of COVID-19 especially among women and girls (Caruso and Freeman, 2020; Gwenzi, 2020a, Gwenzi, 2020b). This is because women and girls use shared sanitation systems more than their male counterparts. The risk of exposure to SARS-CoV-2 may occur during bathing, washing, cleaning, urination, defecation and mensuration. In addition, women and girls shoulder the burden of fetching and providing water for household uses, and such water is often collected from shared water points such as wells and boreholes. In LICs, women and girls also bear the burden of assisting and caring for (e.g., bathing) dependent family members such as children, the elderly and sick ones (Caruso and Freeman, 2020). Hence, the risk of COVID-19 transmission via fomites from drinking water systems is particularly higher among women and girls than men and boys. Therefore, WWW-BE may provide critical information on the presence and potential spread of SARS-CoV-2 from shared drinking water supply and on-site sanitation facilities via fomites and aerosols. Such data are currently missing, and current guidelines from international health agencies such as WHO (2020) are silent on the role of shared sanitation and drinking water sources as potential community transmission points especially for women and girls. Hence, appropriate sampling techniques need to be developed for fomites and bioaerosols as part of WWW-BE. Once SARS-CoV-2 transmission via shared sanitation and drinking water sources is confirmed, then mitigation measures will need to be developed to reduce the risk of transmission among women and girls. However, further research is required to provide comparative data on quantitative human exposure and health risks of women/girls versus their male counterparts in low-income settings. Quantitative tools such as Disability-Adjusted Life Years (DALYs), and quantitative microbial risk assessment similar to that reported by Amoah et al. (2021) may provide some early insights on this aspect.
4.3. WWW-BE versus traditional W-BE: Potential criticisms and counter-arguments
As an untested hypothesis and potential decision-support tool, the development and application of WWW-BE in LICs could face significant criticisms and rebuttals (Table 1). This is understandable for any emerging technique, and such criticisms should motivate further research to validate or refute the WWW-BE hypothesis. Here, the potential criticisms of WWW-BE versus traditional W-BE, and counter-arguments are discussed.
-
(1)
WWW-BE lacks global prior art and validation evidence
Critics may argue that unlike traditional W-BE which has been validated and applied in a number of developed countries (e.g., Australia, Ahmed et al., 2020), the WWW-BE hypothesis lacks prior art and validation anywhere in the world. Thus, the research community, funding agencies, and decision and policy-makers may be sceptical and lack trust and confidence in WWW-BE. To counter this criticism, proponents of the WWW-BE hypothesis may advance two arguments: (i) first, similar to WWW-BE, no prior art and validation evidence exists on W-BE in LICs, and (ii) traditional W-BE is silent on how the tool should be used in LICs where decentralized on-site sanitation systems are dominant. Moreover, W-BE relies on SARS-CoV-2 occurrence and stability data derived from predominantly temperate environments in developed countries, and cannot be extrapolated to LICs with different biophysical and socio-cultural environments, and lifestyles. For instance, contrary to temperate climates, the predominantly tropical environments characterized by high temperatures and episodic rainfall, may have an effect on the fate and behaviour of SARS-COV-2. Thus, proponents may further argue that both the traditional W-BE and the proposed WWW-BE warrant research and public attention in LICs since the two serve different but complementary functions. Finally, although WWW-BE is presented here as a single tool, it has a modular structure. Hence, it can be disaggregated into the three respective components (solid waste, wastewaters, raw/untreated and drinking water) to emphasize certain specific relevant aspects on a case-by-case basis. For instance, traditional W-BE will be ideal for high-income communities in urban settings with access to centralized wastewater, drinking water and solid waste management systems similar to those in developed countries. The proposed WWW-BE with an on-site sanitation components will be ideal for low-income and vulnerable households in urban, peri-urban and rural areas lacking access to centralized wastewater, drinking water and solid waste management systems. Ironically, the same population in LICs lacks access to and may not afford individual diagnostic testing. In cases where open defecation is prevalent, sampling of wastewaters may not be feasible, hence COVID-19 solid wastes and drinking water systems may need to be emphasized in WWW-BE. Given that LICs have a mix of water, sanitation and solid waste management systems, the modularity of the proposed WWW-BE could potentially make it more ideal for such settings than the traditional W-BE.
-
(2)
Solid waste is heterogeneous, and pose sampling and pre-treatment difficulties and biosafety risks
Some may argue that, unlike municipal wastewater used for traditional W-BE, the collection, separation, and heterogeneous nature of faecal sludge and COVID-19-related solid waste especially when mixed with other solid wastes could present significant challenges in its application in epidemiology. Hence, solid waste could pose significant difficulties in obtaining a representative sample for use in WWW-BE. To counter this criticism, in the case of COVID-19 and other respiratory infections, particular attention should be paid to high-risk household and institutional wastes such as COVID-19 PPE and related wastes such as tissue papers and wipes. Others may also argue that SARS-CoV-2 may have low persistence on solid wastes than in raw/untreated wastewaters used in traditional W-BE. However, data suggest that SARS-CoV-2 persist long enough on solid materials to enable the use of solid wastes in WWW-BE (Kasloff et al., 2021). Furthermore, the segregation and storage of high-risk solid wastes such as COVID-19 PPE from other general solid wastes could facilitate the use of such solid wastes in WWW-BE. Given that faecal sludge from non-flushing on-site sanitation systems and COVID-19 solid wastes may harbour biohazards, the segregation and subsequent use of COVID-19 solid wastes in WWW-BE could be coupled to the collection and safe disposal of such wastes. There is also a need to develop appropriate sampling and sample pretreatment protocols for solid waste samples to minimize aerosolization and SARS-CoV-2 transmission through to bioaerosols. Admittedly, as more WWW-BE validation evidence becomes available, it may turn out that certain components of the WWW-BE will be more valuable and relevant than others in some settings. Hence, the potential flexibility offered by the modular nature of WWW-BE allows the choice and prioritization of the relevant components on a case-by-case basis.
-
(3)
WWW-BE present logistical and cost challenges in dispersed populations
Critics may argue that WWW-BE pose logistical challenges in dispersed populations such as those in rural areas in LICs. This constraint is cross-cutting, and is even more severe in traditional W-BE because no centralized systems exists in highly dispersed low-income vulnerable households in rural communities. WWW-BE could be considered less intrusive and culturally acceptable than individual diagnostic testing especially in low-income settings with strong socio-cultural and religious norms. Due to severe logistical constraints (e.g., transport, accommodation), such households also lack access to diagnostic testing facilities which are often located several kilometres away in urban centres. Given that both traditional W-BE based on municipal wastewater analysis and mass diagnostic testing are not feasible in such settings, a question may then arise, ‘Besides WWW-BE, what alternative tools exist for COVID-19 surveillance for poor and vulnerable dispersed communities in low-income settings in the foreseeable future?’. Here, it is argued that the proposed WWW-BE could be adapted to fill this gap, where solid waste such as faecal sludge and wastewater or effluents from on-site sanitation systems substitute raw/untreated municipal wastewater in traditional W-BE.
Note even in LICs such as those in Africa, dispersed rural communities or households are often grouped according to traditional local administrative structures such as wards, villages, and chieftain-ships. Individuals within an administrative structure may be assumed to interact more, and have a higher chance of contracting SARS-CoV-2 via community transmission than those among various structures. However, transmission among different administrative structures such as villages cannot be ruled out. In this regard, sampling of selected households within such administrative structures using principles of spatial statistics such as geostatistics, and subsequent analysis of the data using geo-statistical tools such as kriging and (semi)variograms, and geoinformatics may provide data on COVID-19 clusters and hotspots. Such data collection should include potential explanatory variables such as travelling history, health status, and household demographics. Given that a typical rural household has at least 8 members, WWW-BE will be still putatively cheaper than individual testing especially if such testing can be conducted on-site using mobile testing units that do not require collection, storage and transport of samples to a central place. Hence, the development of rapid and low-cost testing kits include paper-based biomolecular sensors (Liu et al., 2020; Mao et al., 2020) will be critical in WWW-BE.
-
(4)
WWW-BE lacks data and established tools for back- and forward-calculations
As discussed earlier, WWW-BE is envisaged to serve a dual purpose of estimating the prevalence or burden of COVID-19 in a spatial unit, and subsequent potential transmission patterns via environmental media. The former requires back-calculation of COVID-19 cases from WWW-BE data while the latter entails a forward calculation or forecasting. Critics may argue that the techniques for back-and forward-calculation of COVID-19 cases based on WWW-BE data are still lacking. To overcome this limitation, one may argue that the tools currently used for back-calculation in traditional W-BE can be adapted and extended to WWW-BE. These back-and forward calculation can be developed based on artificial intelligence and big data analytics. This will also require data on per capita emissions of SARS-CoV-2, and its subsequent behaviour, persistence and fate in solid wastes, wastewaters and drinking water systems. Given that such data are currently lacking even for traditional W-BE, further research is required to address the data requirements for both traditional W-BE and WWW-BE.
-
(5)
WWW-BE may not guarantee better estimates of COVID-19 cases
Some may argue that the inclusion of three environmental media in WWW-BE may not guarantee better estimates of COVID-19 prevalence. However, it should be noted that, similar to traditional W-BE, WWW-BE gives estimates with upper and lower bounds or confidence limits of COVID-19 cases rather than a single absolute value. This is because of uncertainties associated with the analytical methods, input data and numerical techniques used for back-and forward- calculations. Similar to traditional W-BE based on raw/untreated municipal wastewaters, no data exist in LICs comparing back- or forward-calculated COVID-19 cases estimated using wastewaters from on-site sanitation systems versus those from solid wastes such as faecal sludge from non-flushing on-site sanitation systems and COVID-19 PPE or drinking water systems. Thus, comprehensive comparative research is required to determine the accuracy of COVID-cases estimates based each of the proposed WWW-BE environmental media versus data from diagnostic testing. Such comparative studies should also provide indicative resources (time, cots, personnel) required for each component of the WWW-BE.
5. Future perspectives: Looking ahead in low-income-countries and beyond
Notwithstanding the rationale and merits highlighted, WWW-BE, like traditional W-BE faces potential challenges in LICs (Table 1). Here, challenges and potential solutions are discussed.
-
(1)
Biohazards and biosafety concerns
The sampling, analysis and disposal of contaminated solid wastes, and raw/untreated and partially treated wastewater/sewage from municipal sewers and on-site sanitation present potential biohazards in the form of infectious pathogens (e.g., SARS-CoV-2). Researchers working in the field of environmental surveillance could be particularly at risk because LICs have poor and weakly enforced occupational health and safety guidelines. Moreover, most diagnostic and research laboratories in healthcare facilities and universities in most LICs are not well-equipped, certified and accredited to international biosafety and quality assurance and quality control standards (Street et al., 2020; Gwenzi and Rzymski, 2021). Ideally, laboratories handling infectious biohazards of the nature of SARS-CoV-2 require biosafety procedures and quality control and quality assurance protocols certified to at least biosafety level 2 (Won et al., 2020).
-
(2)
Lack of strong research systems and capacity
LICs especially those in Africa have the weakest research systems in public health, often characterized by: (i) poor research funding, (ii) lack of research infrastructure, (iii) lack of technical and research expertise, and (iv) lack of reliable basic services such as communication systems, and power and water supplies (Gwenzi and Rzymski, 2021). Thus, despite reporting significant COVID-19 cases and deaths, limited research exists on environmental surveillance of SARS-CoV-2 in LICs. In Africa, COVID-19 is regarded as an imported diseases, hence the limited resources available are reserved for the prevention and control of further spread of COVID-19, while research continue to receive a cursory attention. Similarly, international agencies including those from the United Nations systems focus on funding the control and mitigation of impacts of COVID-19, while paying limited attention to research to generate better understanding of COVID-19. Therefore, without international collaborations and local and international funding, research on WWW-BE could suffer the same fate.
-
(3)
Funding mechanisms and research biases
Most LICs especially in Africa spend less than 1% of the gross national product on research and development, and largely rely on external research funding from developed countries. Conventional research grants may even exclude certain thematic areas and geopolitical regions, and often have long approval times. Thus, granting agencies may need alternative funding models that cater for areas that warrant urgent research attention (e.g., COVID-19). Traditionally, epidemiology has been limited to the detection of causative pathogens and diseases transmission in humans and clinical settings while paying limited attention to environmental epidemiology such as the environment reservoirs of human pathogens (Gwenzi and Sanganyado, 2019). As the importance of environmental reservoirs of human pathogens including COVID-19 and other water-borne diseases (e.g., cholera, typhoid) becomes more apparent, this may need to change to better understand human disease dynamics and the detection of pathogens in environmental compartments outside clinical settings.
-
(4)
Potential uncertainties in WWW-BE COVID-19 data
Similar to traditional W-BE, estimates of COVID-19 prevalence based on WWW-BE may have high uncertainties (Li et al., 2021). These uncertainties arise from analytical errors including false positive and negative results (Katz et al., 2020; Wikramaratna et al., 2020). For example, using W-BE, Ahmed et al. (2020) estimated median values of between 171 and 1090 COVID-19 cases, which varied by about one factor of magnitude. Moreover, the use of a wide range of surveillance media in WWW-BE may add another layer of complexity to the interpretation of the WWW-BE data. Thus, further work is required to improve the accuracy of estimates of COVID-19 cases based on traditional W-BE and the proposed WWW-BE. The inclusion of wastewater from on-site sanitation systems, solid waste, and drinking water systems could provide additional data to constrain the model estimates based on traditional W-BE alone.
-
(5)
Integrating risk communication and mitigation in WWW-BE
The inappropriate communication of results of WWW-BE may cause unnecessary panic in locations identified to have COVID-19 clusters and hotspots, and complacency in those with low or no COVID-19 cases. Such behaviours could be counter-progressive and retrogressive in the fight against COVID-19. Therefore, there is a need for proper risk communication to stakeholders and the public. To maintain confidentiality and privacy, WWW-BE outputs should be presented as aggregated or clustered data (e.g., by village, community, catchment etc.) rather than for specific individual households. The risk communication strategy should be an integral part of a broader COVID-19 strategy including awareness campaigns and implementation of various control measures as outlined by the World Helath Organization (2020). The risk communication strategy may need to be jointly developed by experts in science communication, mass communication, and public health.
-
(6)
Regulatory and policy frameworks
COVID-19 is an emerging infectious human disease, representing the first significant outbreak of SARS in some of the LICs especially those in Africa. Hence, most countries still lack the regulatory and policy frameworks to govern research on COVID-19. In cases where such regulatory and policy frameworks exist, they are likely to be highly stringent given the health risks and infectious nature of COVID-19. Thus, conducting research on COVID-19 could take considerable time in terms of application and granting of approvals. Such regulatory and policy frameworks could even be more stringent for international researchers due to concerns over the fear of potential unethical research, including the testing of unapproved drugs and vaccines. These concerns are so strong in Africa and evidenced by the public outcry that occurred following a proposal by French scientists to conduct COVID-19 research entailing the development and testing of vaccines in Africa (BBC, 2020). Measures to overcome such resistance and perceptions are discussed in an earlier paper (Gwenzi, 2020b), and this include a transparent process entailing joint conceptualization, design and implementation of research, and subsequent co-sharing of results and benefits including intellectual property rights such as vaccines arising from such research. Therefore, the development of supportive policy and regulatory frameworks is critical in promoting COVID-19 research including WWW-BE in LICs.
-
(7)
The role of socio-cultural and religious norms and the human factor on COVID-19
LICs tend to have strong socio-cultural and religious norms that may have an impact on research on WWW-BE. For example, human health and sickness are often considered family secrets and are kept as confidential information not shared with strangers and outsiders. Moreover, ill-health or contracting diseases may be stigmatized and associated with certain superstitions and even witchcraft. Thus, any research entailing obtaining information on family health issues and COVID-19 could be considered as highly socially intrusive, hence respondents may not volunteer accurate information. In some socio-cultural and religious settings, sampling wastewater/effluent, sewage and faecal matter from on-site sanitation systems may not be acceptable. Although these issues may appear trivial, they cannot be simply addressed through obtaining consent and research approvals. The less or non-intrusive nature of WWW-BE may make it ideal for such settings.
A number of perceptions, attitudes and myths exist on the transmission and health risks of COVID-19 in LICs especially in Africa (Gwenzi, 2020a, Gwenzi, 2020b). Such perceptions, attitudes and myths affect human behaviour, in turn affect the spread and control of SARS-CoV-2. Human attitudes, perceptions and behaviours towards COVID-19 are currently not addressed in traditional W-BE, and may need attention in WWW-BE. These issues may have a significant bearing on the reliability of research results, and opportunities to conduct research on and apply WWW-BE in LICs.
-
(8)
The emerging ‘bandwagon/Matthew’ effect: A call to put COVID-19 research back on track
A rapid internet search and closer examination of the literature published in 2020/2021 after the early works documenting SARS-CoV-2 in the gut and faeces of infected persons and raw/untreated wastewaters (Tang et al., 2020; Medema et al., 2020, Medema et al., 2020) revealed the following emerging trends:
-
(1)
An increasing number of studies on SARS-CoV-2 detection in wastewaters, and use of W-BE to determine COVID-19 prevalence, albeit often in different countries from those initially reported (e.g., Nemudryi et al., 2020; Sherchan et al., 2020; Wu et al., 2020; Bar Or et al., 2020; Kocameni et al., 2020; Gibas et al., 2021; Haramoto et al., 2020; Hata et al., 2021; Hasan et al., 2021; Prado et al., 2021; Scott et al., 2021; Weidhaas et al., 2021; Hokajärvi et al., 2021; Westhaus et al., 2021; Gerrity et al., 2021; Ahmed et al., 2021a, Ahmed et al., 2021b; Hemalatha et al., 2021; D'Aoust et al., 2021; Carrillo-Reyes et al., 2021).
-
(2)
More recent reviews and perspectives based on analysis of relatively the same empirical evidence on SARS-CoV-2 detection in wastewaters and the potential health risks (e.g., Giacobbo et al., 2021; Anand et al., 2021; Hill et al., 2021; Kumar et al., 2020a, Kumar et al., 2020b, Kumar et al., 2021; Godini et al., 2021; Foladori et al., 2020; Bhatt et al., 2020; Achak et al., 2020; Hamouda et al., 2020; Heneghan et al., 2021; Hoseinzadeh et al., 2020; Lesimple et al., 2020; Patel et al., 2020).
By comparison, globally, since the outbreak of COVID-19 towards the end of 2019, only a handful of papers exist on SARS-CoV-2 detection in groundwater and surface water systems impacted by wastewaters (Fongaro et al., 2021; Kolarević et al., 2021; Rimoldi et al., 2020; Guerrero-Latorre et al., 2020; Mahlknecht et al., 2021; Haramoto et al., 2020). The situation is even more dire in the case of SARS-CoV-2 detection in drinking water supply systems and shared on-site sanitation facilities in LICs. For example, only two empirical studies were identified on SARS-CoV-2 detection in shared latrines/toilets (Amoah et al., 2021; Del Brutto et al., 2021), while no studies were found on drinking water supply systems.
Two reasons may account for the predominance of studies on wastewaters and W-BE relative to other environmental media: (i) the bandwagon or Matthew effect, and (ii) the North-South knowledge asymmetry. The bandwagon or Matthew effect occurs when one aspect is frequently investigated than other equally important ones simply because it has been widely reported in previous studies (Daughton, 2014; Gwenzi, 2020a, Gwenzi, 2020b). The North-South knowledge asymmetry relates to the lower research capacity and productivity in the global south where the bulk of the LICs are found relative to their developed counterparts in the global north. This knowledge asymmetry translates to less data being available on common on-site sanitation systems, surface water and groundwater, solid waste, and drinking water systems in LICs. These aspects are excluded in research on COVID-19 in developed countries because they are less relevant in such settings due to effective multi-barrier systems. Hence, the focus in such settings is raw/untreated wastewater from centralized systems, and the associated traditional W-BE.
While the increasing evidence may be indicative of a maturing field of study, the proliferation of field studies and reviews on wastewater and W-BE have a number of potential limitations. Some may argue that, besides validating earlier results in new geographical settings, subsequent studies may provide limited new insights relative to the pioneering ones. The bias induced by the bandwagon or Matthew effect may have adverse effects on decision-making in research priorities and even research funding (Daughton, 2014; Gwenzi, 2020c). Collectively, the bandwagon or Matthew effect, and the North-South knowledge asymmetry may account for the limited research progress on SARS-CoV-2 detection in shared on-site sanitation facilities, surface water and groundwater systems, and drinking water supply systems in LICs. This is despite a number of earlier calls made nearly a year ago for COVID-19 research to target these aspects (Gwenzi, 2020a, Gwenzi, 2020b; Adelodun et al., 2020; Street et al., 2020). One may then raise two questions: (i) whether or not the scientific community still takes time and effort to read each other’s work (i.e., prior art) before embarking on new research?, and/or (ii) as Nowakowska et al. (2020) pointed out that COVID-19 research has gone ‘viral’, could it be that there is now overwhelming and ever-increasing literature on the COVID-19 to the extent that research community could no longer cope?
These remarks are made to draw the attention of the research community, decision and policy-makers including funding agencies to this emerging research trend on COVID-19. The remarks are meant to be a wake-up call to the research community and other stakeholders to take corrective measures if need be, and put the research on COVID-19 back on track. While these remarks may invite a rebuke and disapproval from the scientific community working on COVID-19, one then wonders whether or not it is high time journals make it an editorial policy to limit the number of reviews on SARS-CoV-2 in wastewaters systems and W-BE, and field studies reporting the same generic findings as earlier ones. One hopes that this may encourage more innovative research and the submission of papers that provide new insights on COVID-19. One such research urgently needed in LICs is to track SARS-CoV-2 occurrence and fate along drinking water supply systems from the source/point of abstraction up to the point of human consumption, paying particular attention to refugee camps, squatter camps and slums. Such studies will provide the unequivocal evidence that will put to rest a number of untested hypotheses on novel SARS-CoV-2 transmission through the faecal-oral route (Gwenzi, 2020b).
-
(9)
Establishing regional research networks and accredited laboratories
The weak research systems coupled with poor research infrastructure in individual LICs especially those in Africa will continue to constrain research and innovation for the foreseeable future. One strategy to overcome this constraint is to establish regional research networks of research teams and accredited analytical laboratories. In the case of Africa, these regional laboratories can be organized according to the regional economic blocs – for example: (i) Economic Community of West African States, (ii) Southern African Development Community, (iii) Community of Sahel-Saharan States, (iv) Economic Community of Central African States, and (v) East African Community. Regional research networks entailing accredited analytical laboratories will facilitate the pooling of financial resources, research infrastructure, and research and technical expertise. Such laboratories should be linked to collaborative research entailing capacity-building and training of post-graduate research students. The regional laboratories can be designed to have specialized research units focusing on thematic areas of regional importance including human and animal health and epidemiology, among others. In this regard, the research unit on human and animal health may focus on development of surveillance tools (e.g., WWW-BE) for zoonoses including COVID-19, and antimicrobial resistance, among other issues. The establishment of such regional laboratories and research networks can be funded by the regional economic blocs, the Africa Union, and the international community including aid agencies and developed countries. The role of relevant international agencies such as those from the United Nations, and developed countries will include: (i) providing expertise for capacity-building and training of local experts, (ii) developing global best practices in research and laboratory procedures including biosafety standards, and quality assurance and quality control systems, (iii) facilitating global accreditation of such laboratories, and (iv) mobilizing of resources through joint research projects.
-
(10)
Global collaboration in WWW-BE research on COVID-19
Global research collaboration between LICs and their developed counterparts may address some of the research challenges highlighted, while facilitating the sharing and dissemination of results. Such collaborations may entail the following: (i) sharing of standardized research protocols, including biosafety and QA/QC procedures in WWW-BE, (ii) open and transparent dissemination of research findings and recommendations to promote scientific rigour and avoid unethical behaviour, (iii) synthesis of research results from WWW-BE across geographical regions, (iv) joint grant applications and research projects entailing pilot study in LICs to address cross-cutting research questions and hypotheses on WWW-BE, and (v) capacity-building through the training of technicians, researchers and post-graduate students, and development of research infrastructure on WWW-BE. One such global research network called the COVID-19 WBE Collaborative has been recently launched (https://www.covid19wbec.org/).
The COVID-19 WBE Collaborative is a research partnership with the Global Water Pathogen Project and the Sewage Analysis CORe group Europe (SCORE) network (Bivins et al., 2020). The goal of the COVID-19 WBE Collaborative is to act as a global hub for the coordination and promotion of research on W-BE. As the collaborative network matures, the database is likely to host a large dataset of experimental data from various sites, which may be conceived as ‘big’ data. Thus, the acquisition and archiving of data on the W-BE repositories should have in mind the possibility to apply big data analytics (e.g., artificial intelligence, machine learning) to extract emerging patterns and trends not apparent in individual studies. Moreover, the integration of genomics, including whole genome analysis of SARS-CoV-2 in such global research collaborations may yield insights on whether various strains of the virus occur in various regions. Although, LICs are currently under-represented in the network, they are free to join as research partners. Therefore, it is hoped that more researchers and institutions from LICs, including those in Africa will join the platform. This is particularly important because LICs could provide ideal sites for validating the WWW-BE as a hypothesis and decision support tool.
6. Future research directions
Future research to develop, validate and apply WWW-BE as a hypothesis and decision-support tool in LICs should address the following knowledge gaps:
-
(1)
Detection and fate of SARS-CoV-2 in WWW-BE-relevant media
SARS-CoV-2 detection and fate in WWW-BE-relevant media are still based on a limited evidence base including inferences. Hence, further investigations are needed to understand the following: (i) SARS-CoV 2 occurrence and fate in raw/untreated and drinking water supply systems, solid wastes, wastewaters, and shared on-site sanitation facilities in LICs, and (ii) the capacity of conventional and low-cost treatment processes (e.g., biosand filtration, solar disinfection (SODIS), biochar filters, ceramic filters) used in LICs to remove SARS-CoV-2. Such data is critical to understand the human exposure and health risks through solid waste, wastewaters, and contaminated drinking water supply systems, and the potential to use the various media in WWW-BE. Hence, research is required to address these gaps under environmentally relevant conditions in LICs.
-
(2)
Moving beyond SARS-CoV-2 RNA titres to predictive decision-support tools
The predictive capacity of traditional W-BE, and by inference WWW-BE, and the tools to convert SARS-CoV-2 RNA data to decision-support tools remain relatively weak, and have high uncertainties (Li et al., 2021). To provide accurate results, optimum sampling time and methods, sample preservation, analyses and interpretation should be developed considering SARS-CoV-2 survival dynamics in the monitoring media. There is also a need to develop and validate techniques for back- and forward calculation to estimate COVID-19 cases based on SARS-CoV-2 data for WWW-BE. Therefore, the next research frontier in WWW-BE should be on the development and field pilot testing of accurate predictive tools for the estimation of the COVID-19 prevalence, transmission dynamics, infection and fatality rates given data on SARS-CoV-2 and their proxies in WWW-BE media.
-
(3)
Potential SARS-CoV-2 intermediate hosts and environmental correlates
SARS-CoV-2 is not a bacteriophage, but it remains unclear whether or not some intermediate hosts for SARS-CoV-2 exist in solid wastes (e.g., rodents), and aquatic systems, including wastewaters and drinking water supply systems. For example, to what extent is SARS-CoV-2 correlated with other biological agents such as faecal coliforms and indicator bacteria in aquatic systems? To date, only two studies have investigated and observed significant moderate correlations between faecal coliform bacteria and SARS-CoV-2 RNA (Mahlknecht et al., 2021; Maidana-Kulesza et al., 2021), but the universal validity of such relationships needs further research. It is also currently unclear whether or not the presence, viability and infectivity of SARS-CoV-2 are correlated with high concentrations of organic, inorganic and biological contaminants commonly occurring in environmental systems in LICs. Such relationships can be used to develop proxy indicators of SARS-CoV-2 in the absence of diagnostic analytical equipment, and optimize water treatment processes to minimize human health risks.
-
(4)
Climatic and weather controls on COVID-19 and the validity of WWW-BE in LICs
Climatic and weather controls on COVID-19 dynamics, and the mechanisms involved are still poorly understood in most LICs. LICs experience a predominantly tropical climate with distinct wet and dry seasons and relatively higher temperatures than those in temperate developed regions. Some studies have reported significant correlations between climatic teleconnections and outbreaks of human diseases including vector-borne viral infections such as Chikungunya, and risk of hospitalization (Caldwell et al., 2021; Fisman et al., 2016). Yet the extent to which such climatic and weather drivers determine the validity of WWW-BE as a hypothesis and decision support tool remains unknown. It also remains unclear how climatic and weather dynamics are supposed to be accounted for in COVID-19 prevalence and transmission estimates based on WWW-BE?
-
(5)
SARS-CoV-2 occupational exposure and human health
Concerns have been raised about SARS-CoV-2 transmission in occupational settings in wastewater and solid waste management systems, but limited comparative information exists on the human exposure and health risks among various occupational workers. Thus, research is required to address the following: (i) Do workers in the solid waste management system (e.g., informal waste collectors), and wastewater and drinking water treatment systems have higher exposure and health risks, and infection rates than their counterparts in other industries?, (ii) what is the relative contribution of human exposure via solid waste, wastewater, drinking water and shared sanitation systems to COVID-19 cases and deaths in LICs?, and (iii) how long will SARS-CoV-2 persist in an infective state on workers' clothes, and what is risk of non-occupational exposure to family members? These potential novel transmission mechanisms are poorly understood and not accounted for in current evidence on environmental surveillance of COVID-19.
-
(6)
Defining the boundary conditions of WWW-BE
The application of traditional W-BE in developed countries is relatively straight-forward because a large number of the population are connected to the centralized wastewater systems. By contrast, in LICs, a diverse mixture of systems for drinking water supply, wastewater, solid waste, and on-site sanitation management are used to varying extents. Thus, SARS-COV-2 occurrence in these compartments may result in complex prevalence and transmission dynamics. Thus, it is critical to determine the biophysical limits for the application of WWW-BE for the current pandemic and even future ones i.e., under what biophysical and socio-cultural conditions will WWW-BE work as a hypothesis and decision support tool, and under what conditions is it invalid?
-
(7)
Linking WWW-BE to human health risks and outcomes
A number of open questions relating to methodological issues relevant to WWW-BE and even traditional W-BE still exist, and some of them are discussed in earlier studies (Kitajima et al., 2020; Westhaus et al., 2021). A key one is, ‘How do we develop quantitative relationships between WWW-BE data based on SARS-CoV-RNA, and the viability, and infectivity SARS-CoV-2 virions, and the risk of occurrence of adverse human acute infection cases?’. Answering this question is critical in improving the accuracy of any environmental surveillance tool including traditional W-BE and WWW-BE. Proposals have been made to apply quantitative microbial risk assessment (QMRA) based on dose-response relationships (Kitajima et al., 2020; Gwenzi, 2020b), but empirical evidence based on such techniques is still limited. A second question pertains to the lack of epidemiological evidence directly linking occupational and non-occupational exposure to SARS-CoV-2 in solid waste, municipal wastewater industry, drinking water and shared sanitation systems to human health outcomes. These aspects need further research combining QMRA, and case-control human toxicology and epidemiological studies.
-
(8)
Development and application of novel and emerging technologies
The development and applications of novel engineered materials with antimicrobial activities including capacity to inactivate human viral pathogens including SARS-CoV-2 are a potential research frontier in the fight against COVID-19. Such novel engineered materials can be based on redox active metals and metal complexes (Lemire et al., 2013) as well as nanomaterials (Imani et al., 2020) well-known to have antimicrobial activities. Potential applications of such materials may include surfaces and PPE for use by frontline workers in high-risk settings such as COVID-19 testing centres, quarantine centres, healthcare facilities, and the funeral industry. Similarly, the development, large-scale production and commercialization of novel sensors for detecting SARS-CoV-2 and its proxies require further research attention. Such novel sensors may include: (i) low-cost techniques such as paper-based bimolecular ones, (ii) those for use in mobile systems designed for low-income settings without grid power and cold chain systems, and (iii) automated and digital ones for high-resolution real-time environmental surveillance (e.g., wastewater monitoring).
The bulk of existing literature on COVID-19 in LICs is still limited to SARS-CoV-2 detection in surveillance media using predominantly conventional research tools (e.g., RT-PCR), while paying limited attention to several novel and emerging technologies. These emerging technologies include big data analytics (e.g., machine learning, data mining, artificial intelligence), genomics, in silico or computational techniques, game theory, and geospatial tools such as geostatistics and geoinformatics. The use of big data analytics is particularly attractive because WWW-BE will potentially generate large datasets (i.e., big data) that require better integration, synthesis and visualization. Analysis of such big data analytics using conventional univariate statistics presents significant challenges, which can be addressed using big data analytics. Hence, scope exists to harness emerging technologies to provide a comprehensive understanding of the occurrence, exposure routes, and human health risks of COVID-19.
7. Conclusion and outlook
The present paper proposed a WWW-BE relying on SARS-CoV-2 RNA detection in wastewaters, solid wastes, raw/untreated surface and groundwater, and drinking water systems as a novel hypothesis and decision-support tool for understanding COVID-19 prevalence and transmission in low-income settings. The rationale, framework and fundamental principles of WWW-BE were discussed in the context of LICs. The summary empirical and inferential evidence on SARS-CoV-2 detection and stability in drinking water supply systems, wastewaters, solid wastes, and raw/untreated groundwater and surface water forms the basis for WWW-BE.
WWW-BE could provide the following potential insights: (i) spatial clusters or ‘hotspots’ and temporal ‘hot moments’ of COVID-19, (ii) transmission patterns, and prevalence or burden of COVID-19 at village, community, catchment and population levels, and (iii) validate and determine the significance of human exposure to SARS-CoV-2 via the novel routes such as faecal-oral pathway, fomites and bioaerosols.
WWW-BE data can be used for the following operational applications: (i) prioritizing or targeting the allocation and deployment of scarce resources such as PPE, clean drinking water provision, comprehensive diagnostic testing of individuals, and location of quarantine or isolation centres, and (ii) determining whether and when to implement and relax COVID-19 control measures such as local and national lockdowns. WWW-BE integrates COVID-19 data from various sources, hence putatively requires less resources than traditional surveillance systems based on mass testing of individuals. Thus, WWW-BE is a potential low-cost tool for COVID-19 surveillance in LICs, where comprehensive individual testing is severely constrained by a critical shortage of resources and logistical challenges.
The novelty of the WWW-BE includes the following: (i) builds on and extends traditional W-BE to include surface water and groundwater, and drinking water supply systems, wastewaters from on-site sanitation systems, and solid waste such as faecal sludge from non-flushing on-site sanitation systems and COVID-19 PPE, which is currently excluded in traditional W-BE, (ii) its modularity based on the three sub-components confers it with potential flexibility unlike traditional W-BE limited to centralized wastewater systems, (iii) it could potentially serve a dual function in estimating the prevalence or burden of COVID-19 and subsequent potential transmission patterns via environmental media, and (iv) once fully developed and validated, WWW-BE could be a potential low-cost and appropriate tool of choice for large-scale surveillance of COVID-19 and related future outbreaks in LICs. Overall, the extension of W-BE to WWW-BE could potentially make the latter more ideal and relevant for understanding COVID-19 to LICs than the former.
The potential challenges of WWW-BE were highlighted, and these include: (i) biohazards and biosafety risks, (ii) lack of expertise and well-equipped accredited diagnostic and analytical laboratories, and (iii) high uncertainties in estimates of COVID-19 cases arising from analytical and simulation errors. Finally, several knowledge gaps are highlighted for further research. These challenges and knowledge gaps need to be addressed before the full potential of WWW-BE can be realized in LICs. Given the weak research systems, coupled with limited research expertise and funding in LICs, the need for regional and global research collaborations was highlighted. The next logical step is to initiate and implement research to develop and validate the WWW-BE hypothesis and decision-support tool in LICs. The current severe challenges faced by LICs in combating and estimating the prevalence of COVID-19 provide a strong motivation for WWW-BE. Collectively, these challenges and the proposed framework should stimulate the global research community to develop and validate appropriate low-cost tools to understand prevalence, prevention and control of COVID-19 and other related epidemics. The outputs of such research will provide the critical evidence to confirm or refute the WWW-BE hypothesis or part thereof. It is envisaged that, even a total rebuttal of the WWW-BE hypothesis may act as a precursor for the development of better hypotheses and decision-support tools for large-scale surveillance of COVID-19 and future related pandemics in LICs.
Declaration of competing interest
The author declares no conflict of interest, financially or otherwise, that could have influence his views and interpretation of the data.
Acknowledgements
The research received no external funding, and was solely funded by the author's personal resources. I acknowledge with thanks the comments from the three anonymous reviewers that greatly improved the manuscript. I am also indebted to two anonymous reviewers who provided comments on an earlier version of the manuscript. I thank Isabaella Sibongile Gwenzi for comments that improved Fig. 2.
Editor: Damia Barcelo
References
- Achak M., Alaoui S.B., Chhiti Y., Alaoui F.E.M.H., Barka N., Boumya W. SARS-CoV-2 in hospital wastewater during outbreak of COVID-19: a review on detection, survival and disinfection technologies. Sci. Total Environ. 2020:143192. doi: 10.1016/j.scitotenv.2020.143192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya K.P., Ghimire T.R., Subramanya S.H. Access to and equitable distribution of COVID-19 vaccine in low-income countries. npj Vaccines. 2021;6(1):1–3. doi: 10.1038/s41541-021-00323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelodun B., Ajibade F.O., Ibrahim R.G., Bakare H.O., Choi K.S. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F., Islam M.A., Kumar M., Hossain M., Bhattacharya P., Islam M.T., Hossen F., Hossain M.S., Islam M.S., Uddin M.M., Islam M.N. First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation Centre in Bangladesh: variation along the sewer network. Sci. Total Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Tscharke B., Bertsch P.M., Bibby K., Bivins A., Choi P., Clarke L., Dwyer J., Edson J., Nguyen T.M.H., O’Brien J.W. SARS-CoV-2 RNA monitoring in wastewater as a potential early warning system for COVID-19 transmission in the community: a temporal case study. Sci. Total Environ. 2021;761 doi: 10.1016/j.scitotenv.2020.144216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi A., Smith S.I. Recurrent cholera epidemics in Africa: which way forward? A literature review. 2019;47(3):341–349. doi: 10.1007/s15010-018-1186-5. [DOI] [PubMed] [Google Scholar]
- Amoah I.D., Pillay L., Deepnarian N., Awolusi O., Pillay K., Ramlal P., Kumari S., Bux F. Detection of SARS-CoV-2 RNA on contact surfaces within shared sanitation facilities. Int. J. Hyg. Environ. Health. 2021;236 doi: 10.1016/j.ijheh.2021.113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U., Adelodun B., Pivato A., Suresh S., Indari O., Jakhmola S., Jha H.C., Jha P.K., Tripathi V., Di Maria F. A review of the presence of SARS-CoV-2 RNA in wastewater and airborne particulates and its use for virus spreading surveillance. Environ. Res. 2021;196 doi: 10.1016/j.envres.2021.110929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkeson A., Droste M.C., Mina M., Stock J.H. Economic benefits of covid-19 screening tests (No. w28031) Natl. Bur. Econ. Res. 2020 Available at: https://www.nber.org/papers/w28031. [Google Scholar]
- Bar Or I., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson E., Shirazi R., Kramarsky-Winter E., Nir O., Abu-Ali H., Ronen Z., Rinott E., Lewis Y.E., Friedler E., Paitan Y., Bitkover E., Berchenko Y., Kushmaro A., Mannasse B. Regressing SARS-CoV-2 sewage measurements onto COVID-19 burden in the population: a proof-of-concept for quantitative environmental surveillance. medRxiv. 2020 doi: 10.1101/2020.04.26.20073569. (2020.04.26.20073569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- BBC (British Broadcasting Corporation) 2020 Coronavirus: France racism row over doctors' Africa testing comments. https://www.bbc.com/news/world-europe-52151722. 20 July 2021
- Beeching N.J., Fletcher T.E., Beadsworth M.B. Covid-19: testing times. BMJ. 2020;369 doi: 10.1136/bmj.m1403. m1403. [DOI] [PubMed] [Google Scholar]
- Bhatt A., Arora P., Prajapati S.K. Occurrence, fates and potential treatment approaches for removal of viruses from wastewater: a review with emphasis on SARS-CoV-2. J. Environ. Chem. Eng. 2020;8(5) doi: 10.1016/j.jece.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P., Kumar M., Islam M.T., Haque R., Chakraborty S., Ahmad A., Niazi N.K., Cetecioglu Z., Nilsson D., Ijumulana J., van der Voorn T., Jakariya M., Hossain M., Ahmed F., Rahman M., Akter N., Johnston D., Ahmed K.M. Prevalence of SARS-CoV-2 in communities through wastewater surveillance – a potential approach for estimation of disease burden. Curr. Pollut. Rep. 2021;7:160–166. doi: 10.1007/s40726-021-00178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G. 2020. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. [DOI] [PubMed] [Google Scholar]
- Bonwitt J., Sáez A.M., Lamin J., Ansumana R., Dawson M., Buanie J., Lamin J., Sondufu D., Borchert M., Sahr F., Fichet-Calvet E. 2017;96(4):935. doi: 10.4269/ajtmh.16-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueckert M., Gupta R., Gupta A., Garg M., Mazumder A. Infectivity of SARS-CoV-2 and other coronaviruses on dry surfaces: potential for indirect transmission. Materials. 2020;13(22):5211. doi: 10.3390/ma13225211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwire G., Ali M., Sack D.A., Nakinsige A., Naigaga M., Debes A.K., Ngwa M.C., Brooks W.A., Garimoi Orach C. Identifying cholera "hotspots" in Uganda: an analysis of cholera surveillance data from 2011 to 2016. PLoS Negl. Trop. Dis. 2017;11(12) doi: 10.1371/journal.pntd.0006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J.M., LaBeaud A.D., Lambin E.F., Stewart-Ibarra A.M., Ndenga B.A., Mutuku F.M., Krystosik A.R., Ayala E.B., Anyamba A., Borbor-Cordova M.J., Damoah R. Climate predicts geographic and temporal variation in mosquito-borne disease dynamics on two continents. Nat. Commun. 2021;12(1):1–13. doi: 10.1038/s41467-021-21496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone D., Chigwechokha P., de los Reyes F.L., III, Holm R.H., III, Risk B.B., III, Tilley E., III, Brown J., III Impact of sampling depth on pathogen detection in pit latrines. PLoS Negl. Trop. Dis. 2021;15(3) doi: 10.1371/journal.pntd.0009176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Reyes J., Barragán-Trinidad M., Buitrón G. Surveillance of SARS-CoV-2 in sewage and wastewater treatment plants in Mexico. J. Water Process Eng. 2021;40 doi: 10.1016/j.jwpe.2020.101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso B.A., Freeman M.C. Shared sanitation and the spread of COVID-19: risks and next steps. Lancet Planet. Health. 2020;4(5) doi: 10.1016/S2542-5196(20)30086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charandabi N.K., Gholami A. COVID-19 Pandemic, Geospatial Information, and Community Resilience. CRC Press; 2021. COVID-19 spatiotemporal hotspots and prediction based on wavelet and neural network; pp. 211–226. [Google Scholar]
- Chin A.W., Chu J.T., Perera M.R., Hui K.P., Yen H.L., Chan M.C., Peiris M., Poon L.L. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1) doi: 10.1016/S2666-5247(20)30003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi P.M., Tscharke B.J., Donner E., O'Brien J.W., Grant S.C., Kaserzon S.L., Mackie R., O'Malley E., Crosbie N.D., Thomas K.V., Mueller J.F. Wastewater-based epidemiology biomarkers: past, present and future. TrAC Trends Anal. Chem. 2018;105:453–469. doi: 10.1016/j.trac.2018.06.004. [DOI] [Google Scholar]
- D'Aoust P.M., Graber T.E., Mercier E., Montpetit D., Alexandrov I., Neault N., Baig A.T., Mayne J., Zhang X., Alain T., Servos M.R. Catching a resurgence: increase in SARS-CoV-2 viral RNA identified in wastewater 48 h before COVID-19 clinical tests and 96 h before hospitalizations. Sci. Total Environ. 2021;770 doi: 10.1016/j.scitotenv.2021.145319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargahi A., Jeddi F., Vosoughi M., Karami C., Hadisi A., Mokhtari S.A., Ghobadi H., Alighadri M., Haghighi S.B., Sadeghi H. Investigation of SARS CoV-2 virus in environmental surface. Environ. Res. 2021;195 doi: 10.1016/j.envres.2021.110765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Using biomarkers in sewage to monitor community-wide human health: isoprostanes as conceptual prototype. Sci. Total Environ. 2012;424:16–38. doi: 10.1016/j.scitotenv.2012.02.038. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. The Matthew effect and widely prescribed pharmaceuticals lacking environmental monitoring: case study of an exposure-assessment vulnerability. Sci. Total Environ. 2014;466:315–325. doi: 10.1016/j.scitotenv.2013.06.111. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. Monitoring wastewater for assessing community health: sewage chemical-information mining (SCIM) Sci. Total Environ. 2018;619–620:748–764. doi: 10.1016/j.scitotenv.2017.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. The international imperative to rapidly and inexpensively monitor community-wide Covid-19 infection status and trends. Sci. Total Environ. 2020;726 doi: 10.1016/j.scitotenv.2020.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C.G. Wastewater surveillance for population-wide Covid-19: the present and future. Sci. Total Environ. 2020;736(2020) doi: 10.1016/j.scitotenv.2020.139631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto O.H., Costa A.F., Mera R.M., Andrade-Molina D., Recalde B.Y., García H.H., Fernández-Cadena J.C. SARS-CoV-2 RNA in swabbed samples from latrines and Flushing toilets: a case-control study in a rural Latin American setting. Am.J.Trop.Med.Hyg. 2021;104(3):1045. doi: 10.4269/ajtmh.20-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derraik J.G., Anderson W.A., Connelly E.A., Anderson Y.C. Rapid review of SARS-CoV-1 and SARS-CoV-2 viability, susceptibility to treatment, and the disinfection and reuse of PPE, particularly filtering facepiece respirators. Int. J. Environ. Res. Public Health. 2020;17(17):6117. doi: 10.3390/ijerph17176117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16) doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiesco-Sepúlveda K.Y., Serrano-Bermúdez L.M. Contributions of Latin American 750 researchers in the understanding of the novel coronavirus outbreak: a literature review. PeerJ. 2020;751(8) doi: 10.7717/peerj.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisman D.N., Tuite A.R., Brown K.A. Impact of El Niño southern oscillation on infectious disease hospitalization risk in the United States. Proc. Natl. Acad. Sci. 2016;113(51):14589–14594. doi: 10.1073/pnas.1604980113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fongaro G., Rogovski P., Savi B.P., Cadamuro R.D., Pereira J.V.F., Anna I.H.S., Rodrigues I.H., Souza D.S.M., Saravia E.G.T., Rodríguez-Lázaro D., da Silva Lanna M.C. SARS-CoV-2 in human sewage and river water from a remote and vulnerable area as a surveillance tool in Brazil. Food Environ. Virol. 2021:1–4. doi: 10.1007/s12560-021-09487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genter F., Willetts J., Foster T. Faecal contamination of groundwater self-supply in low-and middle income countries: systematic review and meta-analysis. Water Res. 2021;201 doi: 10.1016/j.watres.2021.117350. [DOI] [PubMed] [Google Scholar]
- Gerrity D., Papp K., Stoker M., Sims A., Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in southern Nevada: methodology, occurrence, and incidence/prevalence considerations. Water Res. X. 2021;10 doi: 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbo A., Rodrigues M.A.S., Ferreira J.Z., Bernardes A.M., de Pinho M.N. A critical review on SARS-CoV-2 infectivity in water and wastewater. What do we know? Sci. Total Environ. 2021:145721. doi: 10.1016/j.scitotenv.2021.145721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibas C., Lambirth K., Mittal N., Juel M.A.I., Barua V.B., Brazell L.R., Hinton K., Lontai J., Stark N., Young I., Quach C. Implementing building-level SARS-CoV-2 wastewater surveillance on a university campus. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godini H., Hoseinzadeh E., Hossini H. Water and wastewater as potential sources of SARS-CoV-2 transmission: a systematic review. Rev. Environ. Health. 2021;36(3):309–317. doi: 10.1515/reveh-2020-0148. [DOI] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020;8(5) doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J.P., Polizzotto M.L. Pit latrines and their impacts on groundwater quality: a systematic review. Environ. Health Perspect. 2013;121(5):521–530. doi: 10.1289/ehp.1206028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K.E., Loeb S.K., Wolfe M.K., Catoe D., Sinnott-Armstrong N., Kim S., Yamahara K.M., Sassoubre L.M., Mendoza Grijalva L.M., Roldan-Hernandez L., Langenfeld K. SARS-CoV-2 RNA in wastewater settled solids is associated with COVID-19 cases in a large urban sewershed. Environ.Sci.Technol. 2020;55(1):488–498. doi: 10.1021/acs.est.0c06191. [DOI] [PubMed] [Google Scholar]
- Guerrero-Latorre L., Ballesteros I., Villacrés-Granda I., Genoveva Grandam M., Freire-Paspuel B., Ríos-Touma B. SARS-CoV-2 in river water: implications in low sanitation countries. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W. Dangerous liaisons?: As the COVID-19 wave hits Africa with potential for novel transmission dynamics. J. Public Health. 2020 doi: 10.1007/s10389-020-01467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W. Leaving no stone unturned in light of the COVID-19 faecal-oral hypothesis? A water, sanitation and hygiene (WASH) perspective targeting low-income countries. Sci. Total Environ. 2020;753 doi: 10.1016/j.scitotenv.2020.141751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W. The ‘thanato-resistome’-the funeral industry as a potential reservoir of antibiotic resistance: early insights and perspectives. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W., Rzymski P. When silence goes viral, Africa sneezes! A perspective on Africa's subdued research response to COVID-19 and a call for local scientific evidence. Environ. Res. 2021;194 doi: 10.1016/j.envres.2020.110637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwenzi W., Sanganyado E. Recurrent cholera outbreaks in Sub-Saharan Africa: moving beyond epidemiology to understand the environmental reservoirs and drivers. Challenges. 2019;10(1):1. [Google Scholar]
- Hamouda M., Mustafa F., Maraqa M., Rizvi T., Hassan A.A. Wastewater surveillance for SARS-CoV-2: Lessons learnt from recent studies to define future applications. Sci. Total Environ. 2020:143493. doi: 10.1016/j.scitotenv.2020.143493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;2020(730) doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.W., Ibrahim Y., Daou M., Kannout H., Jan N., Lopes A., Alsafar H., Yousef A.F. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: surveillance of COVID-19 epidemic in the United Arab Emirates. Sci. Total Environ. 2021;764 doi: 10.1016/j.scitotenv.2020.142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A., Honda R. Potential sensitivity of wastewater monitoring for SARS-CoV-2: comparison with norovirus cases. Environ. Sci. Technol. 2020;54(11):6451–6452. doi: 10.1021/acs.est.0c02271. [DOI] [PubMed] [Google Scholar]
- Hata A., Hara-Yamamura H., Meuchi Y., Imai S., Honda R. Detection of SARS-CoV-2 in wastewater in Japan during a COVID-19 outbreak. Sci. Total Environ. 2021;758 doi: 10.1016/j.scitotenv.2020.143578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: are we asking the right questions? Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemalatha M., Kiran U., Kuncha S.K., Kopperi H., Gokulan C.G., Mohan S.V., Mishra R.K. Surveillance of SARS-CoV-2 spread using wastewater-based epidemiology: comprehensive study. Sci. Total Environ. 2021;768 doi: 10.1016/j.scitotenv.2020.144704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneghan C.J., Spencer E.A., Brassey J., Plüddemann A., Onakpoya I.J., Evans D., Conly J.M., Jefferson T. SARS-CoV-2 and the role of orofecal transmission: a systematic review. F1000Research. 2021;10(231):231. doi: 10.12688/f1000research.51592.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K., Zamyadi A., Deere D., Vanrolleghem P.A., Crosbie N.D. SARS-CoV-2 known and unknowns, implications for the water sector and wastewater-based epidemiology to support national responses worldwide: early review of global experiences with the COVID-19 pandemic. Water Qual. Res. J. 2021;56(2):57–67. [Google Scholar]
- Hokajärvi A.M., Rytkönen A., Tiwari A., Kauppinen A., Oikarinen S., Lehto K.M., Kankaanpää A., Gunnar T., Al-Hello H., Blomqvist S., Miettinen I.T. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki, Finland. 2021;770 doi: 10.1016/j.scitotenv.2021.145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseinzadeh E., Javan S., Farzadkia M., Mohammadi F., Hossini H., Taghavi M. An updated min-review on environmental route of the SARS-CoV-2 transmission. Ecotoxicol. Environ. Saf. 2020:111015. doi: 10.1016/j.ecoenv.2020.111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imani S.M., Ladouceur L., Marshall T., Maclachlan R., Soleymani L., Didar T.F. Antimicrobial nanomaterials and coatings: current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano. 2020;14(10):12341–12369. doi: 10.1021/acsnano.0c05937. [DOI] [PubMed] [Google Scholar]
- Islam M.Sirajul, Brooks A., Kabir M.S., Jahid I.K., Shafiqul Islam M., Goswami D., Nair G.B., Larson C., Yukiko W., Luby S. Faecal contamination of drinking water sources of Dhaka City during the 2004 flood in Bangladesh and use of disinfectants for water treatment. J. Appl. Microbiol. 2007;103(80–87):422. doi: 10.1111/j.1365-2672.2006.03234.x. [DOI] [PubMed] [Google Scholar]
- Jakariya M. Jakariya F. Ahmed M.A. Islam T. Ahmed A. Al Marzan M. Hossain H.M. Reza P. Bhattacharya A. Hossain T. Nahla N.M. Bahadur M.N. Hasan M.T. Islam M.F. Hossen M. Didar-ul-Alam Nowrin Mow N. Jahan 2021 Wastewater based surveillance system to detect SARS-CoV-2 genetic material for countries with on-site sanitation facilities: an experience from Bangladesh medRxiv (preprint) 10.1101/2021.07.30.212613
- JHU (John Hopkins University) COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2021. https://coronavirus.jhu.edu/map.html
- Kampf G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect. Prev. Pract. 2020;2(2) doi: 10.1016/j.infpip.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasloff S.B., Leung A., Strong J.E., Funk D., Cutts T. Stability of SARS-CoV-2 on critical personal protective equipment. Sci. Rep. 2021;11(1):1–7. doi: 10.1038/s41598-020-80098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A.P., Civantos F.J., Sargi Z., Leibowitz J.M., Nicolli E.A., Weed D., Moskovitz A.E., Civantos A.M., Andrews D.M., Martinez O., Thomas G.R. False-positive reverse transcriptase polymerase chain reaction screening for SARS-CoV-2 in the setting of urgent head and neck surgery and otolaryngologic emergencies during the pandemic: clinical implications. Head Neck. 2020;42(7):1621–1628. doi: 10.1002/hed.26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocameni B.A., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. SARS-CoV-2 detection in Istanbul wastewater treatment plant sludges. medRxiv. 2020 doi: 10.1101/2020.05.12.20099358. (Preprint) [DOI] [Google Scholar]
- Kolarević S., Micsinai A., Szántó-Egész R., Lukács A., Kračun-Kolarević M., Lundy L., Kirschner A.K., Farnleitner A.H., Djukic A., Čolić J., Nenin T. Detection of SARS-CoV-2 RNA in the Danube River in Serbia associated with the discharge of untreated wastewaters. Sci. Total Environ. 2021;783 doi: 10.1016/j.scitotenv.2021.146967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Thakur A.K., Mazumder P., Kuroda K., Mohapatra S., Rinklebe J., Ramanathan A.L., Cetecioglu Z., Jain S., Tyagi V.K., Gikas P. Frontier review on the propensity and repercussion of SARS-CoV-2 migration to aquatic environment. J. Hazard. Mater. Lett. 2020;1 doi: 10.1016/j.hazl.2020.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Kumar Patel A., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Alamin M., Kuroda K., Dhangar K., Hata A., Yamaguchi H., Honda R. Potential discharge, attenuation and exposure risk of SARS-CoV-2 in natural water bodies receiving treated wastewater. npj Clean Wate. 2021;4(1):1–11. [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J.A., Harrison J.J., Turner R.J. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013;11(6):371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- Lesimple A., Jasim S.Y., Johnson D.J., Hilal N. The role of wastewater treatment plants as tools for SARS-CoV-2 early detection and removal. Journal of Water Process Engineering. 2020;38 doi: 10.1016/j.jwpe.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang S., Shi J., Luby S.P., Jiang G. Uncertainties in estimating SARS-CoV-2 prevalence by wastewater-based epidemiology. Chem. Eng. J. 2021;415 doi: 10.1016/j.cej.2021.129039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Fu A., Deng Z., Li Y., Liu T. Promising methods for detection of novel coronavirus SARS-CoV-2. View. 2020;1(1) doi: 10.1002/viw2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Hu J., Hou Y., Tao Z., Chen Z., Chen K. Pit latrines may be a potential risk in rural China and low-income countries when dealing with COVID-19. Sci. Total Environ. 2021;761 doi: 10.1016/j.scitotenv.2020.143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui G., Lai C.K., Chen Z., Tong S.L., Ho W.C., Yeung A.C., Boon S.S., Ng R.W., Chan P.K. SARS-CoV-2 RNA detection on disposable wooden chopsticks, Hong Kong. Emerg.Infect.Dis. 2020;26(9):2274. doi: 10.3201/eid2609.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht J., Reyes D.A.P., Ramos E., Reyes L.M., Álvarez M.M. The presence of SARS-CoV-2 RNA in different freshwater environments in urban settings determined by RT-qPCR: implications for water safety. Sci. Total Environ. 2021;784 doi: 10.1016/j.scitotenv.2021.147183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidana-Kulesza M.N., Poma H.R., Sanguino-Jorquera D.G., Reyes S.I., del Milagro Said-Adamo M., Remis M.M., Gutierrez-Cacciabue D., Cristobal H.A., Cruz M.C., Gonzalez M.A., Rajal V.B. Tracking SARS-CoV-2 in rivers as a tool for epidemiological surveillance. medRxiv. 2021 doi: 10.1101/2021.06.17.21259122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K., Zhang H., Yang Z. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environ. Sci. Technol. 2020;54(7):3733–3735. doi: 10.1021/acs.est.0c011743733-3735. [DOI] [PubMed] [Google Scholar]
- Meays C.L., Broersma K., Nordin R., Mazumder A. Source tracking fecal bacteria in water: a critical review of current methods. J. Environ. Manag. 2004;73(1):71–79. doi: 10.1016/j.jenvman.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Medema G., Been F., Heijnen L., Petterson S. Curr. Opin. Environ. Sci. Health. 2020. Implementation of environmental surveillance for SARS-CoV-2 virus to support public health decisions: opportunities and challenges. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Huang X., Zhou P., Li C., Wu A. Alert for SARS-CoV-2 infection caused by fecal aerosols in rural areas in China. Infect. Control Hosp. Epidemiol. 2020:1–4. doi: 10.1017/ice.2020.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Loaiza J.R., Takyar A., Gilman R.H. COVID-19 in Latin America: novel transmission dynamics for a global pandemic? PLoS Negl. Trop. Dis. 2020;14(5) doi: 10.1371/journal.pntd.0008265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25(10):1–5. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol M.P.G., Caldas S. Can the human coronavirus epidemic also spread through solid waste? Waste Manag. Res. 2020;38(5):485–486. doi: 10.1177/0734242X20918312. [DOI] [PubMed] [Google Scholar]
- Nachega J., Seydi M., Zumla A. The late arrival of coronavirus disease 2019 (COVID-19) in Africa: mitigating pan-continental spread. Clin. Infect. Dis. 2020;71(15):875–878. doi: 10.1093/cid/ciaa353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Rep. Med. 2020;1(6) doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud. Therm. Eng. 2020;1 doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S.-M., Hayashi K., Kinoshita R., Yang Y., Yuan B., Akhmetzhanov A.R., Linton N.M. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int. J. Infect. Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowska J., Sobocińska J., Lewicki M., Lemańska Ż., Rzymski P. When science goes viral: the research response during three months of the COVID-19 outbreak. Biomed. Pharmacother. 2020;129:110451. doi: 10.1016/j.biopha.2020.110451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzediegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020;161 doi: 10.1016/j.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onakpoya I.J., Heneghan C.J., Spencer E.A., Brassey J., Plüddemann A., Evans D.H., Conly J.M., Jefferson T. 2021;F1000Research:10. doi: 10.12688/f1000research.51590.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020;732 doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaki Y., Otaki M., Chaminda T., Kishimoto Y., Nakazawa Y., Gimhana K. Hygiene risk of waterborne pathogenic viruses in rural communities using onsite sanitation systems and shallow dug wells. Sci. Total Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.141775. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 270 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg. Infect. Dis. 2020;26(9):2256. doi: 10.3201/eid2609.201788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., Chaubey A.K., Pittman C.U., Jr., Mlsna T., Mohan D. Sci. Total Environ. 2020. Coronavirus (SARS-CoV-2) in the environment: occurrence, persistence, analysis in aquatic systems and possible management; p. 142698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. MedRxiv. 2020 doi: 10.1101/2020.05.19.20105999. [DOI] [Google Scholar]
- Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., Warren J.L. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020;38(10):1164–1167. doi: 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J., Wu X., Wang R., Li C., Zhang Q., Wei D. Medical waste management practice during the 2019–2020 novel coronavirus pandemic: experience in a general hospital. Am. J. Infect. Control. 2020;48(8):918–921. doi: 10.1016/j.ajic.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino F., Mukherjee D., Coppola G., Gaudio M.T., Curcio S., Calabro V., Marra F., Bhattacharya P., Pal U., Khélifi N., Chakraborty S. Transmission of SARS-Cov-2 and other enveloped viruses to the environment through protective gear: a brief review. EuroMediterr. J. Environ. Integr. 2021;6(2):1–13. doi: 10.1007/s41207-021-00251-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay L., Amoah I.D., Deepnarain N., Pillay K., Awolusi O.O., Kumari S., Bux F. Monitoring changes in COVID-19 infection using wastewater-based epidemiology: a south african perspective. Sci. Total Environ. 2021;786 doi: 10.1016/j.scitotenv.2021.147273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C.A.R., Galati M., Summerville N., Lambrecht M. Using network analysis and machine learning to identify virus spread trends in COVID-19. Big Data Res. 2021:100242. [Google Scholar]
- Potgieter N., Karambwe S., Mudau L.S., Barnard T., Traore A. Human enteric pathogens in eight rivers used as rural household drinking water sources in the northern region of South Africa. Int. J. Environ. Res. Public Health. 2020;17(6):2079. doi: 10.3390/ijerph17062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado T., Fumian T.M., Mannarino C.F., Resende P.C., Motta F.C., Eppinghaus A.L.F., do Vale V.H.C., Braz R.M.S., de Andrade J.D.S.R., Maranhão A.G., Miagostovich M.P. Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Res. 2021;191:116810. doi: 10.1016/j.watres.2021.116810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA titers in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181 doi: 10.1016/j.Watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Moja L., Gismondo M.R., Salerno F. Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo A., Tondera K., Pálfy T.G., Dittmer U., Meyer D., Schreiber C., Zacharias N., Ruppelt J.P., Esser D., Molle P., Troesch S., Masi F. Constructed wetlands for combined sewer overflow treatment: a state-of-the-art review. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138618. [DOI] [PubMed] [Google Scholar]
- Sarwar M. Insect vectors involving in mechanical transmission of human pathogens for serious diseases. Int. J. Bioinformatics Biomed. Eng. 2015;1(3):300–306. [Google Scholar]
- Scott L.C., Aubee A., Babahaji L., Vigil K., Tims S., Aw T.G. Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation. Environ. Res. 2021;200 doi: 10.1016/j.envres.2021.111374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheludchenko M. Queensland University of Technology; 2011. [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street R., Malema S., Mahlangeni N., Mathee A. Wastewater surveillance for Covid-19: an african perspective. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A.N., Tong Z.D., Wang H.L., Dai Y.X., Li K.F., Liu J.N., Wu W.J., Yuan C., Yu M.L., Li P., Yan J.B. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. 2020;26(6):1337. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (EPA), 2004U.S. Environmental Protection Agency (EPA) 2004 Report to Congress on Impacts and Control of Combined Sewer Overflows and Sanitary Sewer Overflows. Water Office of Water Washington, DC, USA Available at: 10.1227/01.NEU.0000028680.51299.00. Accesssed 19 September 2021
- Venugopal A., Ganesan H., Raja S.S.S., Govindasamy V., Arunachalam M., Narayanasamy A., Sivaprakash P., Rahman P.K., Gopalakrishnan A.V., Siama Z., Vellingiri B. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. Curr. Opin. Environ. Sci. Health. 2020;17:8–13. doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2019;2020(262) doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidhaas J., Aanderud Z.T., Roper D.K., VanDerslice J., Gaddis E.B., Ostermiller J., Hoffman K., Jamal R., Heck P., Zhang Y., Torgersen K. Correlation of SARS-CoV-2 RNA in wastewater with COVID-19 disease burden in sewersheds. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany–suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Helath Organization) WHO, 2020. Water, sanitation, hygiene, and waste management for the COVID-19 virus. Interim guidance March 19th 2020. 2020. https://www.who.int/publications-detail/water-sanitation-hygiene-and-waste-management-for-covid-19
- Wikramaratna P.S., Paton R.S., Ghafari M., Lourenço J. Estimating the false-negative test probability of SARS-CoV-2 by RT-PCR. Eurosurveillance. 2020;25(50):2000568. doi: 10.2807/1560-7917.ES.2020.25.50.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;1–10 doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Won J., Lee S., Park M., Kim T.Y., Park M.G., Choi B.Y., Kim D., Chang H., Kim V.N., Lee C.J. Development of a laboratory-safe and low-cost detection protocol for SARS-CoV-2 of the coronavirus disease 2019 (COVID-19) Exp. Neurobiol. 2020;29(2):107. doi: 10.5607/en20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank World development indicators. Solid waste management. 2019. https://www.worldbank.org/en/topic/urbandevelopment/brief/solid-waste-management Available online at:
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J., Maday Y., Teyssou R., Richard E., Almayrac J.L., Moulin L. Evaluation o flockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. medRxiv. 2020 doi: 10.1101/2020.04.12.20062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARSCoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X. Potential spreading risks and disinfection challenges of medical wastewater bythe presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Gong Y., Meng F., Bi Y., Yang P., Wang F. Virus shedding patterns in nasopharyngeal and fecal specimens of COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.03.28.20043059. press. [DOI] [PMC free article] [PubMed] [Google Scholar]