Abstract
Background
Locoregional therapy (LRT) in de novo metastatic disease is controversial with inconsistent results from randomized control trials (RCTs).
Methods
RCTs comparing LRT and systemic therapy to standard therapy alone in de novo metastatic breast cancer were identified. Hazard ratios (HRs) and their associated 95% confidence intervals (CIs) were computed and pooled in a meta-analysis using generic inverse variance. Overall survival (OS) and time to locoregional progression data were extracted for the intention to treat (ITT) population. Data on OS for pre-specified subgroups defined by tumor subtype and by site of metastases were also extracted.
Results
Analyses included 4 trials comprising 970 patients. LRT included standard surgery to the primary breast tumor in all studies, and adjuvant radiation per standard of care was required in 3 studies. Compared to standard treatment, LRT was not associated with improved OS in the ITT population (HR 0.97, 95% CI 0.72–1.29, p = 0.81). However, LRT was associated with improved time to locoregional progression (HR 0.36, 95% CI 0.14–0.95, p = 0.04). LRT was not associated with improved OS in any tumor subtypes, including hormone receptor positive (HR 0.96, 95% CI 0.65–1.43), triple negative (HR 1.4, 95% CI 0.50–3.91) and human epidermal growth factor receptor 2 positive disease (HR 0.93, 95% CI 0.68–1.28). Additionally, LRT did not improve OS in bone only disease (HR 0.97, 95% CI 0.58–1.62) and in visceral disease (HR = 1.02, 95% CI 0.77–1.35). Our critical appraisal has identified some methodological problems in the design and conduct of the studies included that could affect the meta-analysis result.
Conclusions
LRT in de novo metastatic breast cancer is not associated with improved OS. Results are consistent among different breast cancer subgroups. However, this conclusion should be interpreted with caution in view of the limitations identified in meta-analysis.
Keywords: Breast cancer, de novo metastatic, Surgery, Locoregional treatment, Survival
Highlights
-
•
Locoregional treatment in de novo metastatic breast cancer does not improve survival.
-
•
The effect of locoregional therapy is similar between tumor subtypes.
-
•
Locoregional treatment does not improve survival in bone only or in visceral disease.
-
•
Time to locoregional progression is significantly longer with therapy.
1. Introduction
Despite changes to treatment of metastatic breast cancer over the last few decades, for most patients, the typical treatment is palliative systemic therapy with a median overall survival (OS) ranging between 1 and 5 years [1], depending on breast cancer subtype. De novo metastatic disease represents around 6% of breast cancer diagnosis [2].
Several retrospective studies have suggested a survival benefit for patients who underwent locoregional treatment (LRT) of the primary breast tumor in this setting [3,4]. Several mechanisms have proposed by which treatment of the primary tumor in metastatic disease may be beneficial. These include tumor burden reduction which may result in improved immunologic response [5,6], depletion of cancer stem cells and their tumor promoting functions [7] and disruption of self-seeding of the primary tumor [8].
In contrast to retrospective data, prospective data are limited and with conflicting results [[9], [10], [11], [12]]. A recent meta-analysis which included 216,066 patients from 42 studies, including also retrospective studies, demonstrated a reduction of 32% in the risk of mortality in patients receiving LRT (surgery and/or radiotherapy) to the primary breast tumor in de novo metastatic disease [13]. Retrospective studies suffer from inherent selection bias, which could explain the apparent survival benefit in patients treated with surgery in these studies. Additionally, patients achieving excellent response to systemic therapy, patients with locally advanced breast cancer mistakenly classified as metastatic disease and oligometastatic disease, may be overly represented in surgery arms in retrospective studies [14], resulting in improved outcomes compared to those with more widespread or more refractory disease.
Treatment of metastatic breast cancer has evolved over the years with the introduction of new systemic therapies resulting in meaningful improvements in OS [1,15]. There is variability in the treatment and prognosis between the different molecular sub-types. Patients with hormone receptor positive metastatic breast cancer have a favorable prognosis compared to hormone receptor negative breast cancer [16]. The treatment of human epidermal growth factor 2 (HER2) enriched breast cancers has evolved dramatically during the twenty years [[17], [18], [19]] with some patients with metastatic disease achieving long term remissions even in the absence of loco-regional treatment [17]. With the improvement in systemic therapies, the proposed benefit of the addition of LRT remains uncertain [20]. Furthermore, little is known about whether the different molecular subtypes may be impacted differentially by LRT.
Patients with bone only disease have a favorable prognosis compared to patients with visceral metastases [21,22]. Bone only disease is more likely to have luminal subtype [23,24] and typically, endocrine therapy-based regimens are utilized initially [25]. In contrast, visceral involvement at presentation is often associated with triple negative or HER2 positive disease [26,27] and treatment is more likely to comprise chemotherapy-based regimes [25,28]. Compared to endocrine therapy, peri-operative interruption with systemic therapy are more likely to occur with chemotherapy, possibly resulting with inferior control or metastatic disease. This raises the question of whether management of loco-regional disease may actually result in inferior outcomes in patients requiring chemotherapy.
Here, we report on a meta-analysis of prospective randomized trials evaluating the role of LRT in de novo metastatic breast cancer. We also aimed to define the role of LRT in the different breast cancer subtypes. We hypothesized that LRT will not be associated with significant improvement is OS despite improvement in local control.
2. Methods
2.1. Literature review and study identification
A literature search utilizing MEDLINE (Host: PubMed) identified randomized clinical trials published between January 1, 2010 and June 30, 2020 which compared standard systemic therapy to systemic therapy and LRT (comprising of surgery with or without radiation) in de novo metastatic breast cancer patients. The terms “breast cancer”, “metastatic”, “de novo” and “surgery” and similar terms were cross-searched, using the following search algorithm: (((((((((metastatic) OR (“stage 4″)) OR (“stage iv")) OR (advanced)) OR (metastases)) OR (“de novo")) OR (denovo)) AND ((((surgery) OR (local∗)) OR (lumpectomy)) OR (mastectomy))) AND (“breast cancer”) AND ((clinicaltrial OR randomizedcontrolledtrial) AND (english))) AND ((“2010/01/01": “2020/06/30″)). The search was restricted to the English language reports of prospective clinical trials. The search was supplemented by a review of abstracts from key conferences during the last 3 years (2018–2020) including the Annual Meetings of the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO) and San Antonio Breast Cancer Symposium (SABCS).
2.2. Data extraction
Data were collected independently by two reviewers (DR and RM). Discrepancies were resolved by a third reviewer (HG). All data were extracted from primary publications and their associated online appendices.
Collected data included year of publication, number of patients, median follow-up, median age, proportion of pre-menopausal patients and proportion of patients who underwent surgery in the control group. Trial level tumor characteristics were extracted including: proportions of HER2 positive, hormone receptor positive and triple negative disease and proportions of bone-only metastatic disease and visceral metastatic disease.
We then extracted the hazard ratios (HRs) and respective 95% confidence intervals (CI) for OS, time to locoregional progression and time to distant progression. When available, outcome data for subgroups were also collected based on tumor subtype and distribution of metastases (as categorized above).
2.3. Data synthesis and statistical analysis
The primary analysis compared OS between patients who were randomized to LRT to those who received standard therapy. The HR and associated 95% CI were computed for OS and then pooled in a meta-analysis using RevMan 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). Statistical significance was defined as p < 0.05. No adjustment was made for multiple significance testing. Statistical heterogeneity was reported using Cochran Q and I2 statistics. Statistically significant heterogeneity was defined as a Cochran Q P < 0.10 or I2 greater than 50%. In the intention to treat (ITT) population, a subgroup analysis was performed to explore the difference between studies that randomized to upfront LRT to studies that randomized to LRT after initial clinical benefit from systemic therapy was achieved. Subgroup analyses was performed using methods described by Deeks et al. [29]. Additionally, the effect of LRT on OS was analyzed in different subgroups based on tumor subtypes and distribution of metastases (as categorized above). Multiple sensitivity analyses were performed: excluding studies in which post-operative radiation was not provided as part of LRT, excluding studies stoped prematurely due to poor enrolment, excluding studies where results were available only in an abstract form and excluding studies in which HER2 targeted therapy was not available to the majority of HER2 positive patients. Additionally, for OS leave-one-out sensitivity analysis (excluding one study at a time) was conducted. For the analysis for hormone receptor positive disease, sensitivity analysis excluding the study that included hormone receptor positive and HER2 negative disease (rather than hormone receptor positive with any HER2 status) was also performed.
The effect of trial-level characteristics on the natural log of HR for OS was evaluated using meta-regression which comprised a univariable linear regression weighted by individual study sample size using the weighted least squares (mixed effect) function [30]. Meta-regression analysis was performed on the following variables: median age, median duration of follow-up, proportion of pre-menopausal patients, proportion of patients with visceral disease and bone only disease, proportion of patients who underwent surgery in the control group and proportions of the different tumor subtypes. Meta-regression was performed using SPSS version 25 (IBM Corp, Armonk, NY, USA) using the weighted least squares (mixed effect) function. Due to the small number of included studies, meta-regression analyses were evaluated quantitatively using the Burnand criteria [31] rather than to inferring associations based of the p-value [32,33].
3. Results
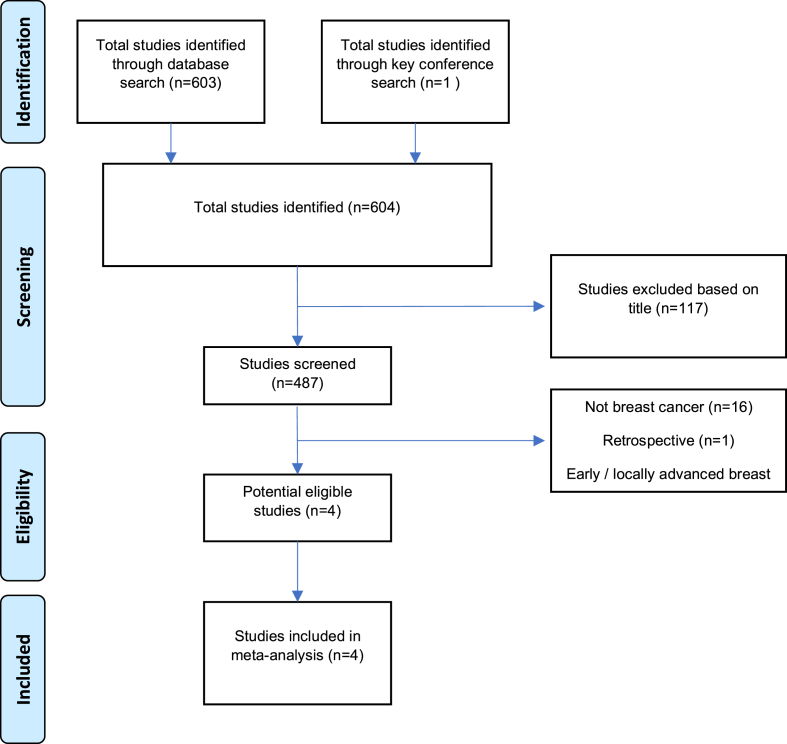
Our search identified 603 studies published between January 2010 and June 2020. After exclusions, 4 studies comprising 970 patients were included in the analysis [[9], [10], [11], [12]], see Fig. 1. The characteristics of the included studies in are shown in Table 1. In 2 studies patients who did not progress after systemic therapy were randomized between the experimental and the control arm [10,12] while in 2 studies no systemic treatment was given before randomization [9,11]. Post surgery adjuvant radiation in the experimental arm was mandatory per standard of care for early breast cancer in 3 studies [[10], [11], [12]]. One study was stopped prematurely due to poor enrollment [9]. Data on LRT in the control arm were reported in 3 studies [9,10,12] and it ranged between 11 and 19%. Most included patients had hormone receptor positive disease, and HER2 positive disease ranged between 22 and 32%. Visceral disease was present in the majority of the included patients in all studies.
Fig. 1.
Study selection scheme.
Table 1.
Trial characteristics.
| Trial | Median Follow up (months) | Number of patients | Treatment before randomization | Post- surgery adjuvant RT to breast (%) | HR positive | HER2 positive | Triple Negative | Pre-menopausal | Visceral disease | Bone only disease | Surgery in control arm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Badwe 2017 [10] | 23 | 350 | Systemic Therapy | 80% | 60% | 31% | NA | 46% | 71% | 29% | 11% |
| Soran 2018 [11] | 40 | 274 | Upfront surgery | 58% | 78% | 31% | 12% | 58% | 54% | 46% | 6% |
| Fitzal 2019 [9] | 37.5 | 90 | Upfront surgery | 20% | 81% | 22% | 9% | 13% | 62% | 38% | 15% |
| Khan 2020 [12] | 53 | 256 | Systemic Therapy | 60% | 58% | 32% | 8% | 36% | 65% | 38% | 19% |
Abbreviations: HER2 – human epidermal growth factor receptor 2, HR – hormone receptor, RT-radiotherapy.
3.1. Primary analysis
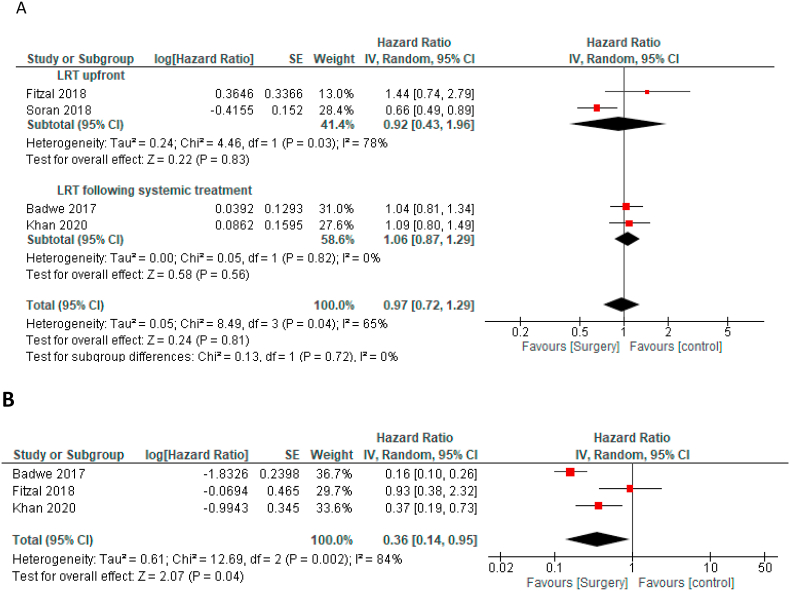
The mean weighted duration of follow-up was 37.1 (±0.38) months. Overall, in the 3 trials where number of deaths were reported, 60% (532/880) of patients had died during follow-up [[10], [11], [12]]. In the pooled analysis, compared to the control group, LRT was not associated with improved OS in the ITT population (HR = 0.97, 95% CI 0.72–1.29, Fig. 2A). There was a statistically significant heterogeneity (Cochran's Q p = 0.04, I2 = 65%). Multiple sensitivity analyses for OS and leave-one-out sensitivity analysis for OS have shown similar result, see Table 2 and supplementary Figure 1. Similar magnitude of effect was seen in studies were patients received LRT as an upfront treatment (HR 0.92 95% CI 0.43–1.96, p = 0.83) and in studies were patients received LRT after systemic therapy (HR 1.06 95% CI 0.87–1.29, p = 0.56), p value for the subgroup difference = 0.72.
Fig. 2.
Forest plots - a: Overall survival in ITT group, b: Time to locoregional progression in ITT group.
Table 2.
Sensitivity analyses for outcomes.
| Analysis | Primary analysis HR, 95% CI | Excluding study radiotherapy was not mandatory/stopped prematurely HR, 95% CI | Excluding study available only in abstract form HR, 95% CI | Excluding study HER2 targeted therapy were not available HR, 95% CI |
|---|---|---|---|---|
| Overall survival | 0.97 (0.72–1.29) | 0.91 (0.67–1.24) | 0.94 (0.63–1.40) | 0.96 (0.62–1.48) |
| Time to locoregional progression | 0.36 (0.14–0.95) | 0.23 (0.10–0.53) | 0.37 (0.07–2.07) | 0.56 (0.23–1.37) |
Abbreviations: CI- confidence interval, HER2 – human epidermal growth factor receptor 2, HR-hazard ratio.
HRs data on time to locoregional progression were available in 3 studies [9,10,12]. Compared to the control group LRT was associated with significant improvement in time to loco-regional progression (HR = 0.36 95% CI0.14–0.95, p = 0.04, Fig. 2B). There was a statistically significant heterogeneity (Cochran's Q p = 0.002, I2 = 84%). Sensitivity analyses for time to locoregional progression are shown in Table 2. Overall, the magnitude of effect on time to loco-regional progression was lower after excluding the study in which HER2 targeted therapy was not available and was higher after excluding the study in which adjuvant radiation was not mandated by the protocol (this study was also stopped prematurely). In one study, the number of events on locoregional progression was higher in the systemic therapy arm compared to the LRT arm (11% vs 1%), but HR for locoregional progression was not reported [11]. Only 2 studies reported data on time to distant progression or distant-progression free survival [9,10]. Pooling theses results showed LRT was associated with significantly worse time to distant progression (HR = 1.47 95% CI 1.15–1.86, p = 0.002).
3.2. Subgroup analysis
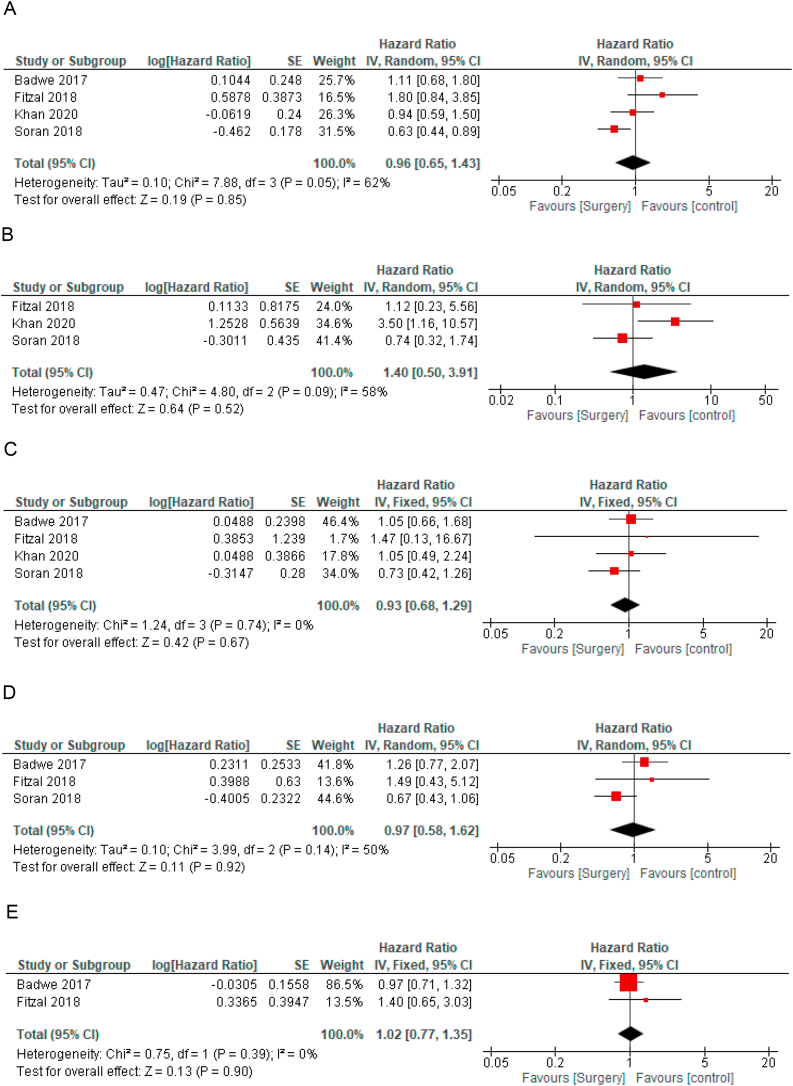
Data on OS by tumor subtype were available for hormone receptor positive disease and for HER2 positive disease in all studies [[9], [10], [11], [12]] and for triple negative disease in 3 studies [9,11,12]. LRT was not associated with improved OS in any tumor subtypes, including hormone receptor positive (HR for OS = 0.96, 95% CI 0.65–1.43, Fig. 3A), triple negative disease (HR 1.4, 95% CI 0.50–3.91, Fig. 3B) and HER2 positive disease (HR 0.93, 95% CI 0.68–1.28, Fig. 3C). In the subgroup of hormone receptor positive disease, sensitivity analysis excluding the study that included only hormone receptor positive and HER2 negative disease showed similar results (HR = 1.00 95% CI 0.56–1.79).
Fig. 3.
Forest plots for overall survival according to subgroups - a: Hormone positive disease, b: Triple negative disease, c: HER2 positive disease, d: Bone only disease, e: Visceral disease. Hazard ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval. The diamonds represent the estimated pooled effect. All P values are two-sided.
Data on OS for bone only disease were available in 3 studies [[9], [10], [11]] and for visceral disease in 2 studies [9,10]. Compared to the control arm, LRT was not associated with improved OS in bone only disease (HR 0.97, 95% CI 0.58–1.62, p = 0.92, Fig. 3D) and in visceral disease (HR = 1.02, 95% CI 0.77–1.35, p = 0.90, Fig. 3E).
3.3. Meta-regression
Results of the meta-regression analysis are shown in Table 3. In quantitative assessment, higher proportions of pre-menopausal women, of triple negative disease and of bone-only disease were associated with highly quantitatively significant lower HR for OS (i.e. increased benefit from experimental therapy). Higher proportion of visceral disease were associated with highly quantitatively significant higher HR for OS (i.e. lower benefit or harm from experimental therapy). Older age was associated with substantially quantitatively significant higher magnitude of effect of OS and higher proportion of hormone receptor positive disease were associated with highly quantitatively significant lower magnitude of effect on OS.
Table 3.
Results of meta-regression analysis for overall survival.
| Variable | Β | P |
|---|---|---|
| Median age | 0.452 | 0.548 |
| Duration of follow-up | −0.056 | 0.944 |
| Proportion pre-menopausal | −0.903 | 0.097 |
| Proportion with visceral disease | 0.723 | 0.277 |
| Proportion with bone only | −0.651 | 0.349 |
| Proportion LRT in control group | 0.268 | 0.827 |
| Proportion of HR positive | −0.475 | 0.525 |
| Proportion of HER2 positive | −0.411 | 0.589 |
| Proportion of triple negative | −0.883 | 0.311 |
Abbreviations: HER2 – human epidermal growth factor receptor 2, HR – hormone receptor, LRT-locoregional therapy.
4. Discussion
The role of LRT of the breast in de novo metastatic breast cancer has been controversial with inconsistent results [34]. Numerous retrospective analyses have demonstrated a survival benefit in this setting [3,13,35,36], while results from prospective have been inconsistent [[9], [10], [11], [12]]. The improvement in systemic treatment further increases the uncertainty regarding to role of LRT in metastatic disease.
In this meta-analysis, comprising only randomized prospective trials, no survival advantage for LRT in de novo metastatic disease was identified, despite achieving significant improvement in locoregional control. Additionally, focusing on different subgroups, based on both cancer subtype by receptors expression and based on the site of metastases, did not reveal any subgroup that might benefit for LRT. Of note, as OS data for visceral disease were available only from 2 studies the interpretation that can be made for this subgroup is limited. Given the inconsistent results form prospective studies, our results suggest a limited role for LRT in de novo metastatic disease. In the presence of what seems to be robust retrospective data, our findings highlight the importance of randomized prospective trials to answer meaningful clinical decisions.
A possible explanation for the discrepancy between retrospective and prospective results is an inherent selection bias in retrospective data. Such bias is likely difficult to adjust/control for and will likely result in residual bias in most analyses. In retrospective studies patients who responded better to initial systemic therapy may have been more likely to be offered LRT, hence representing subpopulation with favorable prognosis compared to patients without initial response to systemic therapy. In some retrospective studies arms were not adequately adjusted and patients in surgery arms were also younger, had smaller tumors and lower metastatic burden [3], highlighting the selection of patients that were more likely to have longer survival regardless to treatment selection.
Heterogeneity between the included studies in this meta-analysis exists. In 2 studies randomization to LRT occurred after achieving clinical benefit from systemic therapy [10,12] while in 2 studies patients in the experimental arm were treated with an upfront surgery [9,11]. The strategy of delayed surgery has the advantage of preventing patients with rapidly progressive disease refractory to systemic treatment to undergo surgery further delaying exposure to potentially effective systemic therapy. Additionally, initial systemic treatment can potentially reduce tumor burden and prevent life threatening complications that could be associated with distant metastases. However, our analysis shows that timing of LRT had no impact on the magnitude of effect on OS in this meta-analysis. Compared to the control arm, LRT for triple negative disease had worse OS (HR = 1.4). Although this finding was not statistically significant, this trend might support the importance of systemic treatment, especially in a disease with aggressive biology, such as triple negative subtype. Of note, time to distant progression was significantly shorter with LRT, but this analysis is limited as only 2 studies reported these results [9,10].
The only positive trial which showed a survival advantage for LRT was that by Soran et al. [11] with a HR of 0.66 for survival and a 5 year overall survival improvement (46.4% versus 26.4%). However, this trial has limitations. Despite randomization, there were differences in characteristics between the treatment arms - patients in the surgical group had lower rates of triple negative disease (7% vs 17%), higher rates of hormone positive disease (85.5 vs 71.8%) and were more likely to have solitary bone metastases (34% vs 24%). These differences may have resulted in biased results. One other drawback of this study was that there was no histologic conformation of solitary bone metastases [11], which might increase the possibility of mis-diagnosis of metastatic disease and thereby result in substandard treatment in the control arm.
Other included studies in this meta-analysis also have several important limitations. Badwe et al. conducted a randomized prospective study in India [10] in patients who responded to systemic therapy and showed no survival benefit for LRT. They reported an improved locoregional control but with a significantly worse distant PFS. This may explain the results of comparable OS despite improvement in loco-regional control. The detrimental effect of LRT on distant PFS could be explained by an enhanced metastatic growth after tumor resection. This phenomenon has been demonstrated in animal and human studies [[37], [38], [39]]. Furthermore, potential delays in systemic therapy delivered perioperatively could also hamper systemic control. An important limitation of this study was that some patients may have been undertreated, for example only a small minority (8%) of patients with HER2 positive disease received directed anti-HER2 therapy. As there were more women with HER2 positive disease in the control arm (36% vs. 25%), this inferior systemic therapy is unlikely to explain lack of OS benefit with LRT therapy. Also, as this study enrolled women in a low-middle income country with low screening rates, it likely included patients with delayed presentation rather than tumor biology consistent with early metastatic potential. This may limit generalizability to higher income settings.
The study conducted by Fitzal et al. was planned to recruit 254 patients but was closed after 5 years when only 90 patients were recruited. Consequently, the study was under-powered to detect differences between treatment arms. However, despite not reaching statistical significance a trend for inferior OS was observed in the surgery arm with an absolute survival difference of 20.2 months [9]. Khan et al. presented the initial results of the phase 3 ECOG ACRIN 2108 trial during ASCO 2020 virtual conference [12]. Several concerns regarding the results of this trial have been raised [40]. Three-year survival rates were much higher than expected, decreasing the power of this study. Although one of the inclusion criteria was free surgical margins, only 80% of patients in the LRT arm achieved clear margins and a there was a high percentage of skin or fascia involvement. Some of the included patients had very short duration of follow-up, limiting the ability to capture a potential benefit from LRT in patients with relatively longer survival. Also, no subgroup analysis regarding metastatic sites were presented. To address the weakness of the includes studies multiple sensitivity analysis were done, with no effect of OS. Additionally, the results of leave-one-out sensitivity analysis support OS results are not driven by any one the individual study.
This study has limitations. First, this is a literature-based rather than an individual patient-based meta-analysis. Consequently, it is subject to publication bias. Second, there were several differences in the protocol of treatment of included studies. Systemic treatment prior randomization was not given in 2 studies, and there was both intra- and inter-study variability in the type of the systemic treatment that was given. Additionally, data of subsequent lines of systemic treatment were not reported and the impact of this on OS could not be assessed. Additionally, there was evidence of contamination with LRT given to some of the patients in the control arm. As only the minority in the control group was treated with LRT the impact on the results is limited. However, this was explored in meta-regression with no quantitive difference observed on the magnitude of effect on OS. Third, there was heterogeneity between studies populations, which may also affect the results, as there is a high variability in metastatic breast cancer outcome, mainly related to receptors expression and tumor burden. Of note, the effect of LRT was evaluated on different subgroups with overall similar magnitude of effect. Moreover, there was heterogeneity in the quality of data available from the studies, with one trial closed prematurely due to poor accrual and another trial which was presented only in abstract form. Finally, the studies that reported quality of life used different scales and therefore could not be pooled.
In conclusion, this meta-analysis shows no survival advantage for LRT in de novo metastatic breast cancer despite significant improvement in locoregional control. Comparable OS results were consistent among different subgroups based on tumor biology or burden of disease. Systemic treatment remains the core treatment in metastatic breast cancer and efforts to improve anti -cancer drugs in order to improve survival and quality of life should continue. LRT for de novo metastatic disease should not be offered routinely. Current data did not identify specific subgroups that may benefit form LRT treatment. However, further research is desired to investigate to role of local treatment in some clinical circumstances such as oligometastatic disease or when the primary site is the sole active site after systemic therapy.
Declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
Dr. Yerushalmi reports personal fees from: Roche (Consulting, Invited speaker), Pfizer (Consulting), Novartis (Consulting), Teva (Invited speaker), Medison (Invited speaker), MSD (Invited speaker), Astra-Zeneca (Invited speaker) and Novartis (Invited speaker), all outside the submitted work.
Dr. Moore reports personal fees from Roche (invited speaker), MSD (invited speaker), all outside the submitted work.
Dr. Amir reports personal fees from Genentech/Roche (expert testimony), Apobiologix (consulting). Novartis (consulting), Sandoz (consulting), Exact Sciences (consulting) and Agendia (consulting).
Dr. Goldvaser reports personal fees from: Roche (honorarium), Pfizer (honorarium), Novartis (honorarium and consulting) and Oncotest (honorarium) all outside the submitted work.
All other authors have no conflicts of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
Daniel Reinhorn: Investigation, data curation, writing–original draft, writing–review and editing, final approval of the version to be submitted.
Raz Mutai: Data curation, validation, writing–review and editing, final approval of the version to be submitted.
Rinat Yerushalmi: Investigation, writing–review and editing, final approval of the version to be submitted.
Assaf Moore: Investigation Writing–original draft, and writing–review and editing, final approval of the version to be submitted.
Eitan Amir: methodology, Investigation writing–original draft, and writing–review and editing, final approval of the version to be submitted.
Hadar Goldvaser: Conceptualization, methodology, formal analysis, investigation, validation, writing–original draft, and writing–review and editing, final approval of the version to be submitted.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.05.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Caswell-Jin J.L., Plevritis S.K., Tian L. 2018. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Canc J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Harris E., Barry M., Kell M.R. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann Surg Oncol. 2013;20:2828–2834. doi: 10.1245/s10434-013-2998-2. [DOI] [PubMed] [Google Scholar]
- 4.Warschkow R., Guller U., Tarantino I. Improved survival after primary tumor surgery in metastatic breast cancer a propensity-adjusted, population-based SEER trend analysis. Ann Surg. 2016;263:1188–1198. doi: 10.1097/SLA.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 5.Rashid O.M., Nagahashi M., Ramachandran S. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surg (United States) 2013;153:771–778. doi: 10.1016/j.surg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danna E.A., Sinha P., Gilbert M. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Canc Res. 2004;64:2205–2211. doi: 10.1158/0008-5472.CAN-03-2646. [DOI] [PubMed] [Google Scholar]
- 7.Liu H., Patel M.R., Prescher J.A. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci U S A. 2010;107:18115–18120. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M.Y., Oskarsson T., Acharyya S. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzal F., Bjelic-Radisic V., Knauer M. Impact of breast surgery in primary metastasized breast cancer: outcomes of the prospective randomized phase III ABCSG-28 POSYTIVE trial. Ann Surg. 2019;269:1163–1169. doi: 10.1097/SLA.0000000000002771. [DOI] [PubMed] [Google Scholar]
- 10.Badwe R., Hawaldar R., Nair N. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 2015;16:1380–1388. doi: 10.1016/S1470-2045(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 11.Soran A., Ozmen V., Ozbas S. Randomized trial comparing resection of primary tumor with No surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann Surg Oncol. 2018;25:3141–3149. doi: 10.1245/s10434-018-6494-6. [DOI] [PubMed] [Google Scholar]
- 12.Khan S.A., Zhao F., Solin L.J. A randomized phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: a trial of the ECOG-ACRIN Research Group (E2108) J Clin Oncol. 2020;38 doi: 10.1200/jco.2020.38.18_suppl.lba2. LBA2–LBA2. [DOI] [Google Scholar]
- 13.Gera R., Chehade H.E.L.H., Wazir U. Locoregional therapy of the primary tumour in de novo stage IV breast cancer in 216 066 patients: a meta-analysis. Sci Rep. 2020 doi: 10.1038/s41598-020-59908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cady B., Nathan N.R., Michaelson J.S. Matched pair analyses of stage IV breast cancer with or without resection of primary breast site. Ann Surg Oncol. 2008;15:3384–3395. doi: 10.1245/s10434-008-0085-x. [DOI] [PubMed] [Google Scholar]
- 15.Chia S.K., Speers C.H., D'Yachkova Y. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 16.Waks A.G., Winer E.P. Breast cancer treatment: a review. JAMA, J Am Med Assoc. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 17.Swain S.M., Miles D., Kim S.B. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 18.Murthy R.K., Loi S., Okines A. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/nejmoa1914609. [DOI] [PubMed] [Google Scholar]
- 19.Diéras V., Miles D., Verma S. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong Y., Raghavendra A.S., Hatzis C. Long-term survival of de novo stage IV human epidermal growth receptor 2 (HER2) positive breast cancers treated with HER2-targeted therapy. Oncol. 2019;24:313–318. doi: 10.1634/theoncologist.2018-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R., Zhu Y., Liu X. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Canc. 2019;19 doi: 10.1186/s12885-019-6311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leone B.A., Vallejo C.T., Romero A.O. Prognostic impact of metastatic pattern in stage IV breast cancer at initial diagnosis. Breast Canc Res Treat. 2017;161:537–548. doi: 10.1007/s10549-016-4066-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.J., Park S., Ahn H.K. Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res Treat. 2011;43:89–95. doi: 10.4143/crt.2011.43.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkes A., Clifton K., Al-Awadhi A. Characterization of bone only metastasis patients with respect to tumor subtypes. npj Breast Canc. 2018;4 doi: 10.1038/s41523-018-0054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NCCN clinical practice guidelines in Oncology (NCCN Guidelines®) breast cancer. NCCN. 2020:2020. Version 3. [Google Scholar]
- 26.Kast K., Link T., Friedrich K. Impact of breast cancer subtypes and patterns of metastasis on outcome. Breast Canc Res Treat. 2015;150:621–629. doi: 10.1007/s10549-015-3341-3. [DOI] [PubMed] [Google Scholar]
- 27.Park S., Koo J.S., Kim M.S. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21:50–57. doi: 10.1016/j.breast.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Barrios C.H., Sampaio C., Vinholes J., Caponero R. What is the role of chemotherapy in estrogen receptor-positive, advanced breast cancer? Ann Oncol. 2009;20:1157–1162. doi: 10.1093/annonc/mdn756. [DOI] [PubMed] [Google Scholar]
- 29.Deeks J.J. Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001 doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanley T.D., Doucouliagos H. Neither fixed nor random: weighted least squares meta-analysis. Stat Med. 2015 doi: 10.1002/sim.6481. [DOI] [PubMed] [Google Scholar]
- 31.Burnand B., Kernan W.N., Feinstein A.R. Indexes and boundaries for "quantitative significance" in statistical decisions. J Clin Epidemiol. 1990;43 doi: 10.1016/0895-4356(90)90093-5. 1273–84. [DOI] [PubMed] [Google Scholar]
- 32.Wasserstein R.L., Lazar N.A. The ASA statement on p -values: context, process, and purpose. Am Statistician. 2016;70:129–133. doi: 10.1080/00031305.2016.1154108. [DOI] [Google Scholar]
- 33.Wasserstein R.L., Schirm A.L., Lazar N.A. Moving to a world beyond “ p< 0.05.”. Am Statistician. 2019;73:1–19. doi: 10.1080/00031305.2019.1583913. [DOI] [Google Scholar]
- 34.Khan S.A. Primary tumor resection in stage IV breast cancer: consistent benefit, or consistent bias? Ann Surg Oncol. 2007;14:3285–3287. doi: 10.1245/s10434-007-9547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arciero C., Liu Y., Gillespie T., Subhedar P. Surgery and survival in patients with stage IV breast cancer. Breast J. 2019;25:644–653. doi: 10.1111/tbj.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fields R.C., Jeffe D.B., Trinkaus K. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol. 2007;14:3345–3351. doi: 10.1245/s10434-007-9527-0. [DOI] [PubMed] [Google Scholar]
- 37.Al-Sahaf O., Wang J.H., Browne T.J. Surgical injury enhances the expression of genes that mediate breast cancer metastasis to the lung. Ann Surg. 2010;252:1037–1043. doi: 10.1097/SLA.0b013e3181efc635. [DOI] [PubMed] [Google Scholar]
- 38.Ceelen W., Pattyn P., Mareel M. Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit Rev Oncol Hematol. 2014;89:16–26. doi: 10.1016/j.critrevonc.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Coffey J.C., Wang J.H., Smith M.J.F. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4:760–768. doi: 10.1016/s1470-2045(03)01282-8. [DOI] [PubMed] [Google Scholar]
- 40.Soran A., Ozbas S., Dogan L. Loco-regional treatment for intact primary tumor in patient with de novo metastatic breast cancer; comments and concerns of ECOG-ACRIN 2108 trial. Eur J Breast Heal. 2020;16:158–159. doi: 10.5152/ejbh.2020.080620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.