Abstract
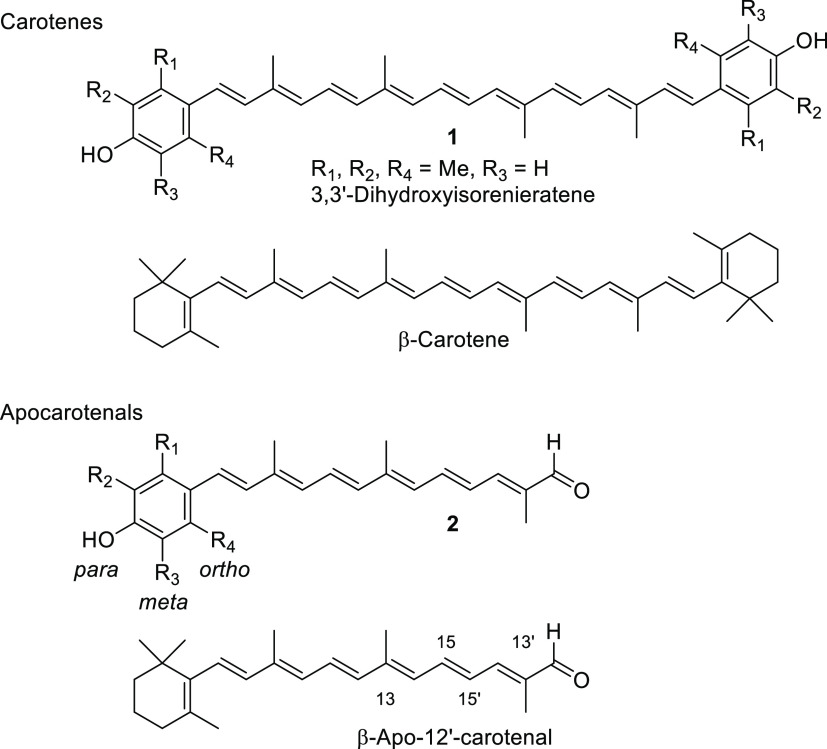
A series of para-phenolic carotenes 1 with ortho- and meta-substitutions were respectively prepared utilizing the benzenesulfonyl protection method, which demonstrated the importance of the ring substituents on their effective conjugation, evaluated by their UV absorption values. The corresponding apo-12′-carotenals 2 were devised to improve the conjugation effect of the para-phenolic radical with the polyene chain by the conjugated aldehyde group. Apo-12′-carotenals 2b and 2c without ortho-substituents exhibited superior antioxidant activities to their corresponding symmetrical carotenes 1 as well as β-carotene and apo-12′-β-carotenal in 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging assays.
Introduction
Carotenoids are secondary metabolites produced by plants, bacteria, and microalgae, which are indispensable for energy production in photosynthesis. Carotenoids not only absorb visible light complementarily to chlorophyll but also transfer the harvested light energy to the photosynthetic reaction center to generate nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine triphosphate (ATP).1 Excessive energy during this process is dissipated by carotenoids to reversibly form transient cationic radicals, which would form stable adducts or decay to more stabilized oxidative degradation products such as apocarotenals.2 Carotenoids react readily with any type of radicals, in which the main reaction profiles are adduct formation, electron transfer, and hydrogen abstraction.2 The readiness to undergo such reactions makes carotenoids outstanding antioxidants, especially for scavenging reactive oxygen species in biological systems.3
Isorenieratene is an unusual natural carotenoid containing polymethylated aromatic end groups.4 The para-phenolic 3,3′-dihydroxyisorenieratene (DHIR, Figure 1), isolated first from Streptomyces mediolani,5 was reported to exhibit superior antioxidant and photo-protective activities in vitro and in vivo assays.6 The aromatic end groups would increase the antioxidant ability independently or synergistically with the polyene chain. Methyl substituents in aromatic rings are reported to improve antioxidative property in the case of resveratrol,7 but the ortho-methyl substituents in isorenieratene and DHIR adversely distort coplanarity of the benzene ring to the polyene chain.8 Two-electron oxidation of the phenolic groups in DHIR, however, produces the quinoid structure, which would attain the full conjugation with the polyene chain to show a superior antioxidant activity.8
Figure 1.
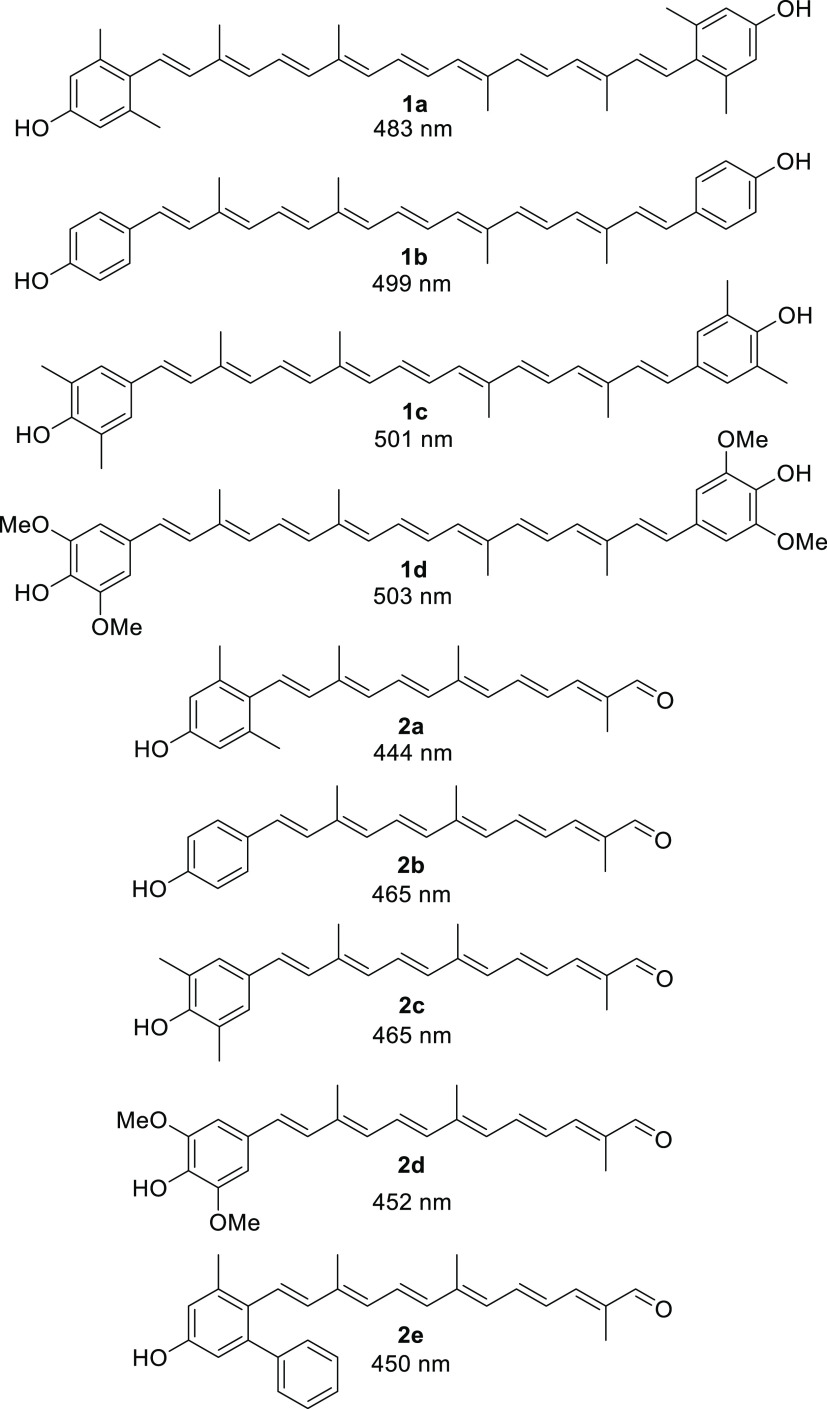
Proposed carotenes 1 and apo-12′-carotenals 2 with phenol end group(s) for superior antioxidant activities.
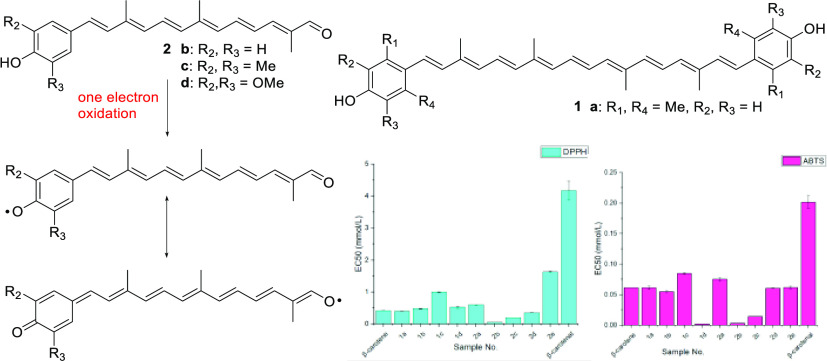
Apocarotenoids are the oxidative fragmentation products of the carotenoid polyene chain.9 Even though the biological function and the enzymatic mechanisms are relatively well known for some apocarotenoids such as retinal,10 bixin,11 and abscisic acid,12 those of the majority of apocarotenoids are still unclear.13 They may be formed nonspecifically by eccentric cleavage from photo- or radical oxidation, but their prevalence in a biological system is well documented for the antioxidant, stress signaling, and DNA-protective effects.14 Searching for carotenoids of superior antioxidant activities, we were interested in the apocarotenals with a terminal benzene ring. It was hypothesized that apocarotenals with a para-phenol end group would exhibit superior antioxidant activity. Facile one-electron oxidation (or hydrogen radical abstraction) would produce a phenolic radical having the quinoid resonance structure, which is fully conjugated with the polyene aldehyde unit, whereas two-electron oxidation is required for DHIR to furnish the fully conjugated quinoid structure.
We first prepared para-phenolic carotenoids 1 with substitutions, respectively, at ortho- and meta-positions (derivatives of DHIR) to explain the importance of conjugation effect of the terminal phenol ring with the polyene chain (Figure 1). The corresponding apo-12′-carotenals 2 of para-phenolic carotenoids were devised to prove our hypothesis: increased conjugation effect of the phenolic radical with the conjugated polyene aldehyde group. The antioxidant activities for the above novel carotenoids were then measured by the standard radical scavenging assays of 1,1-diphenyl-2-picryl-hydrazyl (DPPH)15 and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS).16 Disappearance of the UV peaks from the above radicals by carotenoids through the formation of carotenoid cationic radicals can be correlated with their antioxidant activities. The details of the syntheses and their antioxidant activities are herein described and compared with those of β-carotene and β-apo-12′-carotenal as references.
Results and Discussion
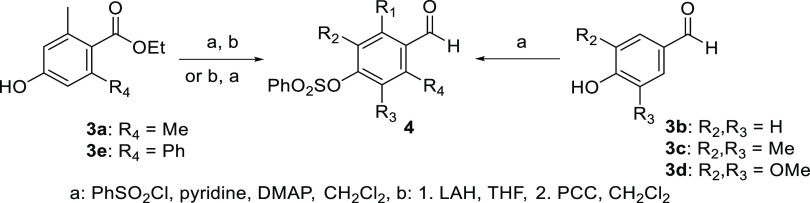
The syntheses of carotenes 1 and their apo-12′-carotenals 2 with diversely substituted para-phenol end group(s) were commenced from the preparation of the corresponding protected para-hydroxybenzaldehydes 4 (Scheme 1). The substituents at ortho- and meta-positions were selected among methyl, phenyl, and methoxy groups. para-Hydroxybenzaldehydes 3b (without substitution), 3c (meta-dimethyl), and 3d (meta-dimethoxy) were commercially available. ortho-Substituted para-hydroxybenzoates 3a (Me, Me) and 3e (Me, Ph) were prepared according to our previously reported procedure.17 Benzenesulfonyl protection for the para-hydroxyl group was utilized throughout the carotenoid syntheses, which was proven to survive the chain extension and olefination conditions and to be deprotected selectively in the presence of acid-sensitive polyene chain by KOH in t-BuOH.18
Scheme 1. Preparation of Protected 4-Hydroxybenzaldehydes 4 for the Phenol-Ending Carotenoids.
The para-hydroxybenzoate 3a with ortho-dimethyl substituents was protected first by a benzenesulfonyl group (step a), and the ester group was transformed into a formyl group (step b) after reduction (LAH) and then oxidation (PCC). The sterically hindered para-hydroxybenzoate 3e with ortho-phenyl substituent required a harsh condition for the ester reduction (LAH in refluxing tetrahydrofuran (THF)), which accompanied undesirable desulfonylation. The sequence of the reaction steps was thus reversed (steps b then a) in this case to overcome the premature deprotection problem. The para-hydroxybenzaldehydes 3b–3d were simply protected by a benzenesulfonyl group (step a) to produce the corresponding protected para-hydroxybenzaldehydes 4.
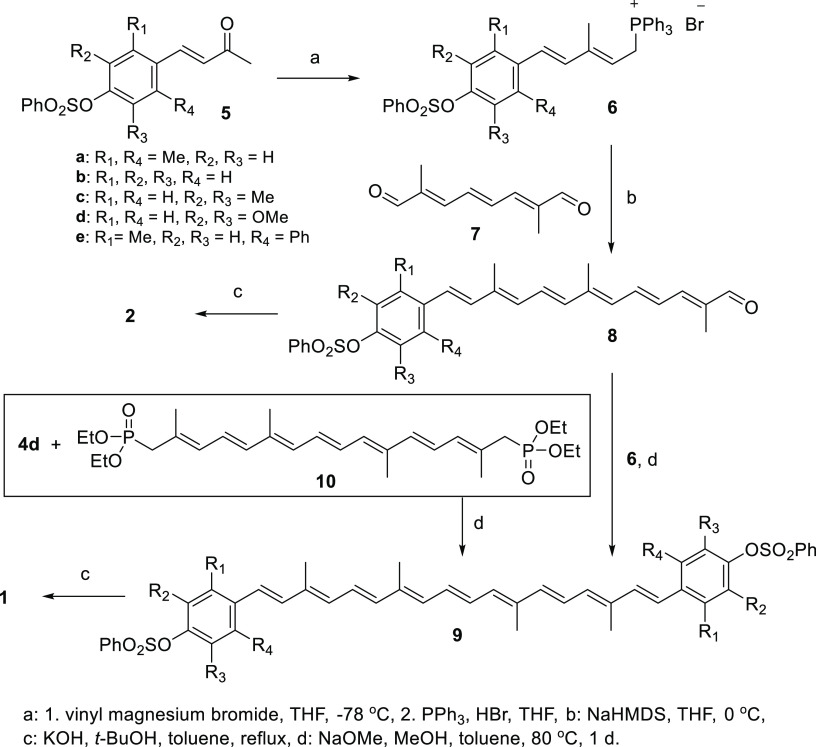
Aldol condensation of the protected para-hydroxybenzaldehydes 4 with acetone in 1 M NaOH solution proceeded uneventfully to produce the chain-extended conjugated ketones 5, from which the phosphonium salts 6 were prepared by the Grignard reaction with vinyl magnesium bromide, followed by HBr addition in the presence of PPh3 at 0 °C. The Wittig olefination of 6 with 2,7-dimethylocta-2,4,6-triendial (7) was sequentially carried out first to produce protected apo-12′-carotenal 8 with a phenol end group under the mild condition at 0 °C utilizing NaHMDS as a base in THF. The all-E carotenals 8 were mostly obtained (based on 1H NMR spectra) and further purified by trituration with Et2O.
The second Wittig reaction of 6 with the above-protected apo-12′-carotenal 8 required a harsh condition at 80 °C utilizing NaOMe as a base in MeOH/toluene to produce the protected phenol-ending carotenes 9. Nevertheless, carotene 9e with ortho-phenyl substituent was not produced presumably due to the steric reason. Expeditious one-pot synthesis of carotene 9d was demonstrated by the double Wadsworth–Emmons olefination of C20 polyene diphosphonate 10 with protected hydroxybenzaldehyde 4d.19 Carotenoids 9 were mostly obtained as all-E form (based on 1H NMR spectra) and further purified by recrystallization with a mixed solvent of MeOH and THF.
Deprotection of the benzenesulfonyl group from apo-12′-carotenals 8 and carotenes 9 was progressed smoothly using pulverized KOH in refluxing t-BuOH to afford the corresponding apo-12′-carotenals 2 and carotenes 1 with para-phenol end group(s), respectively. All-E configuration of the carotenoids was mostly maintained but deteriorated to 63% for apo-12′-carotenal 2b (see high-performance liquid chromatography (HPLC) data in the Supporting Information). The reaction yield in each step of the above carotenoid syntheses is summarized in Table 1 (Scheme 2).
Table 1. Yield of Each Compound in the Steps of Schemes 1 and 2.
| compd. | 4 (%) | 5 (%) | 6 (%) | 8 (%) | 2 (%) | 9 (%) | 1 (%) |
|---|---|---|---|---|---|---|---|
| a | 33 | 79 | 99 | 56 | 52 | 53 | 72 |
| b | 73 | 95 | 25 | 63 | 96a | 35 | 35 |
| c | 98 | 88 | 68 | 21 | 19 | 37 | 67 |
| d | 95 | 87 | 57 | 30 | 45 | 26b | 78 |
| e | 47 | 62 | 68 | 21 | 13 |
All-E configuration was deteriorated after deprotection (all-E:Z = 1.7:1).
The reaction was performed by one-pot double olefination between C20 diphosphonate 10 and 4d (2 equiv).
Scheme 2. Synthetic Routes for the Phenol-Ending Carotenes 1 and Apo-12′-carotenals 2.
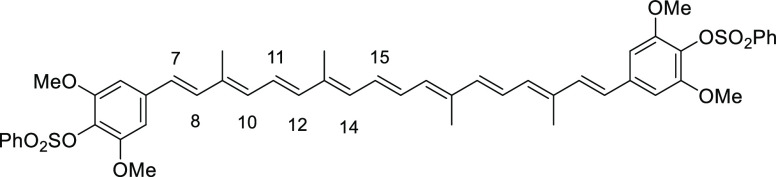
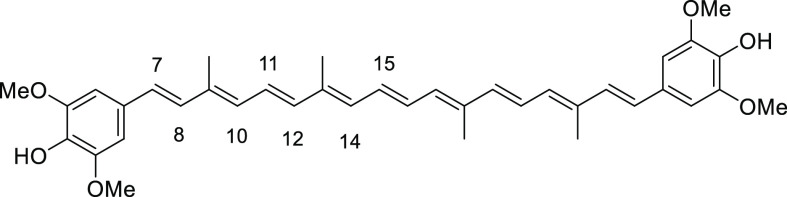
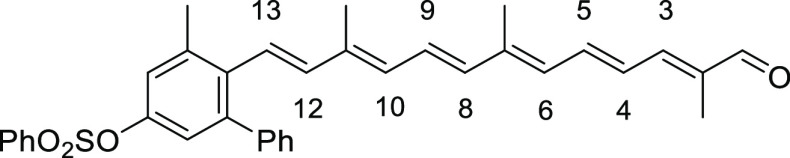
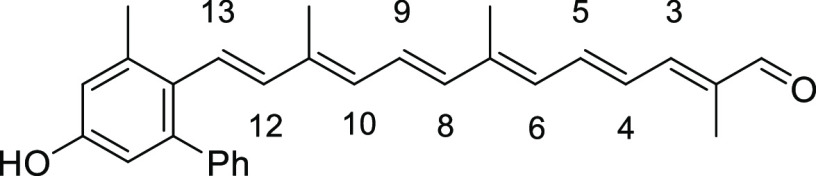
The structures of ortho- and meta-substituted carotenes 1 and their apo-12′-carotenals 2 with para-phenol end group(s) are listed in Figure 2 together with their UV absorption maxima (λmax) in dimethyl sulfoxide (DMSO), which indicate the extent of π-conjugation between para-phenol group and the conjugated polyene chain. The phenol end group of carotenoids 1a and 2a with ortho-dimethyl substitution would be deviated from coplanarity to the conjugated polyene chain due to the steric hindrance, thereby lowest UV absorption values (by ∼20 nm) were observed in each series. para-Phenolic carotenes 1 with no other substitution (1b) and meta-substitution (1c and 1d) showed a very specific UV absorption value at 501 ± 2 nm, which explained the effective conjugation of the para-phenol group to the polyene chain. Similarly, the highest UV absorption value (465 nm) was observed for para-phenolic apo-12′-carotenals 2b with no other substitution and 2c with meta-dimethyl substitution in the series, whereas a little lower value (452 nm) measured for 2d with meta-dimethoxy substitution was presumably due to an intramolecular hydrogen bonding of phenol with the neighboring methoxy group, which might slightly reduce the conjugation effect. The λmax difference between 2a and 2e can be ascribed to the auxochrome effect by the ortho-phenyl group in 2e, which exhibited a redshift by 6 nm.
Figure 2.
Carotenes 1 and apo-12′-carotenals 2 with para-phenol end group(s) and their UV absorption values (λmax) in DMSO.
Antioxidant activities of carotenoids 1 and 2 were measured by DPPH and ABTS radical scavenging assays. Disappearance of the UV absorption peak for relatively stable DPPH radical at 580 nm (instead of interfered 510 nm wavelength)15b by carotenoids was monitored to obtain EC50 values, which can be explained by quenching with hydrogen atom (H·) of phenol as well as a single π-electron from the polyene chain. ABTS cationic radical, which was in situ generated from the oxidation by potassium persulfate and exhibits the UV absorption maximum wavelength at 734 nm, can be quenched mainly by a single π-electron of the carotenoids. DPPH assay would thus be more suitable to incorporate the effect of phenol group in carotenoids with the para-phenol end group(s). The EC50 values of DPPH and ABTS assays for carotenoids 1 and 2 were measured quadruple and their mean and standard deviation values are listed in Table 2, which were compared with those of β-carotene and β-apo-12′-carotenal as references.
Table 2. EC50 of Carotenoids in Figure 2 for DPPH and ABTS Radical Scavenging Assays.
| DPPH |
ABTS |
|||
|---|---|---|---|---|
| rank | compound | EC50 (mM) | compound | EC50 (mM) |
| 1 | 2b | 0.0562 ± 0.0009 | 1d | 0.0019 ± 1.1E-5 |
| 2 | 2c | 0.1840 ± 0.0019 | 2b | 0.0030 ± 6.9E-5 |
| 3 | 2d | 0.3550 ± 0.0025 | 2c | 0.0144 ± 8.8E-5 |
| 4 | 1a | 0.3954 ± 0.0036 | 1b | 0.0552 ± 0.0014 |
| 5 | β-carotene | 0.4216 ± 0.0028 | 2d | 0.0606 ± 0.0007 |
| 6 | 1b | 0.4828 ± 0.0056 | 1a | 0.0614 ± 0.0028 |
| 7 | 1d | 0.5234 ± 0.0286 | β-carotene | 0.0614 ± 0.0002 |
| 8 | 2a | 0.5987 ± 0.0028 | 2e | 0.0616 ± 0.0021 |
| 9 | 1c | 0.9966 ± 0.0111 | 2a | 0.0752 ± 0.0032 |
| 10 | 2e | 1.6313 ± 0.0182 | 1c | 0.0849 ± 0.0010 |
| 11 | β-12′-carotenal | 4.1747 ± 0.2845 | β-12′-carotenal | 0.2017 ± 0.0101 |
The effects of the end groups in natural carotenoids on the antioxidant activity were studied theoretically and experimentally.20 The polarity of the terminal group affected the radical scavenging ability of natural carotenoids: carotenoids (lycopene and β-carotene) > hydroxy-carotenoids (zeaxanthin and lutein) > keto-carotenoids (astaxanthin and canthaxanthin) in phenoxy radical21 and ABTS cationic radical scavenging experiments.22 The effective conjugation length is also important in carotenoids’ antioxidant activity,23 and shorter β-apo-8′-carotenal was reported to exhibit poorer radical scavenging activity than β-carotene,21 which was also demonstrated by our referenced EC50 values of β-carotene and β-apo-12′-carotenal in Table 2.
Considering the above effects, the results in Table 2 are astonishing in that (1) apo-12′-carotenals 2 with a para-phenol end group are superior to the corresponding carotenes 1 in DPPH radical scavenging (except the sterically hindered ortho-substitution case a) and (2) polar hydroxy-carotenoids 1 are generally better than β-carotene in ABTS radical scavenging. In fact, apo-12′-carotenals 2b and 2c without ortho-substitution are superior in radical scavenging activities presumably by the effective conjugation of coplanar phenolic radical with the conjugated polyene aldehyde, which proves our hypothesis in this study. On the other hand, the radical scavenging abilities of carotenoids 1 are not correlated with the phenolic substitution patterns (or the UV absorption values). It could be explained that the resonance stabilized coplanar phenolic radicals of 2b and 2c without ortho-substitution were readily formed by facile one-electron oxidation, whereas two-electron oxidations would be necessary for carotenes 1 with para-phenol end groups to afford the fully conjugated coplanar quinoid structures.8 Density functional theory (DFT) calculation (Supporting Information) also supports the above results in that the singly occupied molecular orbital (SOMO) energy of 2b radical is lower by 6.41 kcal/mol than that of 1b radical. The energy gain by an electron transition from the highest occupied molecular orbital (HOMO) to the SOMO level is larger for 2b (3.13 kcal/mol) than that for 1b (2.72 kcal/mol). The strong ABTS radical scavenging activity of 1d can be ascribed to the charge-transfer complex formation of the electron-rich phenol rings with ABTS, which can be explained by prompt appearance of reddish-black color.
Conclusions
para-Phenolic carotenoids 1 and their apo-12′-carotenals 2 with ortho- and meta-substitutions were, respectively, prepared from the corresponding para-hydroxybenzaldehydes utilizing the benzenesulfonyl protection method. UV absorption values by the π-electronic transition of carotenoids 1 and 2 explained the effective conjugation length between the para-phenol group and the polyene chain by coplanarity, which was negatively affected by the sterically hindered ortho-substituents. Antioxidant activities of carotenoids 1 and 2, measured by DPPH and ABTS radical scavenging assays, indicated somewhat different trends in the stability of carotenoid radicals. The para-phenolic radicals of apo-12′-carotenals 2 with no ortho-substitution were effectively conjugated with the polyene aldehyde unit, which were even better stabilized than those of the corresponding carotenes 1. Superior radical scavenging activities were observed for para-phenolic apo-12′-carotenals 2b and 2c with no ortho-substitution in DPPH and ABTS assays.
Experimental Section
General Experimental
Reactions were generally performed in a well-dried flask under argon atmosphere unless noted otherwise. Solvents for extraction and chromatography were of reagent grade and used as received. The column chromatography was performed with silica gel 60 (70–230 mesh) using a mixture of EtOAc/hexane as an eluent. 1H- and 13C NMR spectra were, respectively, recorded on 400 and 100 MHz NMR spectrometers in deuterated chloroform (CDCl3) with tetramethylsilane (TMS) as an internal reference unless noted otherwise. The syntheses of carotenes 1 and apo-12′-carotenals 2 were described for the Series I of 2,6-dimethyl-4-hydroxyphenyl end group (a). Only the analytical data were given for the other Series II-V (b–e).
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 1,1-diphenyl-2-picryl-hydrazyl (DPPH), and potassium persulfate were purchased from Sigma-Aldrich, Inc. β-Carotene24 and β-apo-12′-carotenal25 used for ABTS and DPPH assays were freshly prepared according to the literature procedure. 2,7-Dimethyl-2,4,6-octatriendial (7) was obtained from BASF as a generous gift.
DPPH Assay15
DPPH is a stable free radical with UV absorption maximum at 517 nm. Because of the interference of carotenoids at around 510 nm, the depletion of DPPH radical by carotenoid scavenging was measured at 580 nm.15b A 6.31 × 10–2 mM stock solution of DPPH in methanol was prepared, and it was diluted 1/100 with MeOH right before use. A 6.31 × 10–5 mM DPPH solution in MeOH (0.2 mL) was added to various carotenoid sample solutions (0.05 mL) in THF/DMSO (1:1). Keeping the mixture in the dark at room temperature for 2 h, an aliquot (0.05 mL) was placed in a 384-cell cuvette, in which the absorbance was determined at 580 nm by a UV–vis microplate spectrophotometer (Multiskan GO by Thermo Scientific Co.) in fourfold analyses.
ABTS Assay16
The ABTS cationic radical was freshly prepared by treating an aqueous solution of ABTS diammonium salt (2.5 mL, 7.4 mM) with an aqueous solution of potassium persulfate (2.5 mL, 2.6 mM). The mixture was kept in the dark at room temperature for 12 h and then diluted with methanol (about 1/50 of volume) until its absorbance value became 0.70±0.02 at 734 nm. Typically, fresh ABTS•+ stock solution (2 mL) was added to various carotenoid sample solutions (0.05 mL) in THF/DMSO (1:1). Keeping the mixture in the dark at room temperature for 2 h, an aliquot (0.05 mL) was placed in a 384-cell cuvette, in which the absorbance was determined at 734 nm by a UV–vis microplate spectrophotometer (Multiskan GO by Thermo Scientific Co.) in fourfold analyses.
Series I (a)
Ethyl 2,6-Dimethyl-4-((phenylsulfonyl)oxy)benzoate
To a stirred solution of ethyl 4-hydroxy-2,6-dimethylbenzoate (3a)17 (5.61 g, 28.87 mmol) and benzenesulfonyl chloride (6.12 g, 34.64 mmol) in CH2Cl2 (50 mL) were added pyridine (4.57 g, 57.74 mmol) and dimethylamino-pyridine (106 mg, 0.87 mmol). The mixture was stirred at room temperature under argon atmosphere for 1 day, diluted with CH2Cl2, washed with 1 M HCl, dried over anhydrous Na2SO4, filtered, and concentrated to give the crude product (9.95 g) as a brown oil. The crude product was purified by SiO2 flash column chromatography (eluent 10–35% EtOAc/hexane) to give the title compound (5.43 g, 16.24 mmol) in 56% yield as a light yellow solid. Data: Rf = 0.31 (1:4 EtOAc/hexane); m.p. = 75–76 °C; 1H NMR (CDCl3) δ 1.37 (t, J = 7.2 Hz, 3H), 2.24 (s, 6H), 4.38 (q, J = 7.2 Hz, 2H), 6.67 (s, 2H), 7.51–7.59 (m, 2H), 7.65–7.71 (m, 1H), 7.83–7.90 (m, 2H) ppm; 13C NMR (CDCl3) δ 14.1, 19.5, 61.1, 121.0, 128.3, 129.0, 132.9, 134.2, 135.2, 137.0, 149.3, 168.8 ppm; IR (KBr) 2982, 1722, 1595, 1469, 1446, 1372, 1260, 1185, 1133, 1088, 1021, 962, 872, 783, 753, 723, 686, 596, 574 cm–1; HRMS (EI) calcd for C17H18O5S 334.0875, found 334.0874.
4-Formyl-3,5-dimethylphenyl Benzenesulfonate (4a)
To a stirred solution of ethyl 2,6-dimethyl-4-((phenylsulfonyl)oxy)benzoate (5.48 g, 16.39 mmol) in THF (55 mL) was added LAH (622 mg, 16.39 mmol) at 0 °C. The mixture was slowly warmed to and stirred at room temperature for 15 h. The mixture was quenched with ice-water and extracted with EtOAc. The organic layer was washed with 1 M HCl, dried over anhydrous Na2SO4, filtered, and concentrated to give the crude benzylic alcohol (4.72 g, 16.13 mmol) as a yellow oil (Rf = 0.25, 2:3 EtOAc/hexane). Data: IR (KBr) 3422, 2960, 2922, 1722, 1595, 1476, 1446, 1372, 1275, 1185, 1126, 1096, 1021, 962, 872, 820, 753, 731, 686, 589, 574 cm–1.
The crude benzylic alcohol (4.72 g, 16.13 mmol) was dissolved in CH2Cl2 (35 mL), and silica gel (5.0 g) and PCC (4.17 g, 19.36 mmol) were added. The mixture was stirred at room temperature for 14 h under argon atmosphere and filtered through a sintered glass funnel under reduced pressure. The filtrate was diluted with CH2Cl2, washed with NaHCO3 solution, dried over anhydrous Na2SO4, filtered, and concentrated to give the crude product (3.79 g) as a brown oil, which was purified by SiO2 flash column chromatography to give benzaldehyde 4a (2.74 g, 9.45 mmol) in 59% yield as a yellow oil (Rf = 0.26, 1:4 EtOAc/hexane). Data: 1H NMR (CDCl3) δ 2.53 (s, 6H), 6.75 (s, 2H), 7.53–7.60 (m, 2H), 7.67–7.73 (m, 1H), 7.85–7.91 (m, 2H) ppm; 13C NMR (CDCl3) δ 20.4, 122.8, 128.3, 129.2, 131.0, 134.4, 135.1, 143.5, 151.6, 192.1 ppm; IR (KBr) 3066, 3032, 2978, 2932, 2870, 2770, 1694, 1591, 1450, 1373, 1276, 1193, 1128, 1095, 1026, 966, 878, 818, 748, 711, 688 cm–1; HRMS (EI) calcd for C15H14O4S 290.0613, found 290.0609.
(E)-3,5-Dimethyl-4-(3-oxobut-1-en-1-yl)phenyl Benzenesulfonate (5a)
To a stirred solution of benzaldehyde 4a (2.39 g, 8.21 mmol) in acetone (10 mL) was added 1 M NaOH solution (20 mL). The mixture was stirred at room temperature for 1 day, acidified with 1 M HCl (30 mL), and extracted with Et2O. The aqueous layer was extracted again with EtOAc, and the combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated to give the crude product (2.87 g) as a yellow oil, which was purified by SiO2 flash column chromatography (eluent 15–50% EtOAc/hexane) to give conjugated enone 5a (2.16 g, 6.52 mmol) in 79% yield as an off-white solid. Data: Rf = 0.13 (1:4 EtOAc/hexane); m.p. = 74–76 °C; 1H NMR (CDCl3) δ 2.23 (s, 6H), 2.36 (s, 3H), 6.27 (d, J = 16.4 Hz, 1H), 6.69 (s, 2H), 7.51–7.58 (m, 2H), 7.52 (d, J = 16.4 Hz, 1H), 7.64–7.70 (m, 1H), 7.82–7.88 (m, 2H) ppm; 13C NMR (CDCl3) δ 20.7, 27.2, 121.3, 128.0, 128.9, 132.7, 133.1, 134.0, 135.0, 138.2, 140.1, 148.4, 197.8 ppm; IR (KBr) 3064, 2967, 1692, 1670, 1588, 1476, 1446, 1364, 1152, 1185, 1126, 1096, 1021, 962, 880, 820, 753, 716, 686, 596, 567 cm–1; HRMS (CI) calcd for C18H19O4S 331.1004, found 331.0997.
((2E,4E)-5-(2,6-Dimethyl-4-((phenylsulfonyl)oxy)phenyl)-3-methylpenta-2,4-dien-1-yl)triphenylphosphonium Bromide (6a)
To a stirred solution of conjugated enone 5a (4.55 g, 13.77 mmol) in THF (25 mL) at −78 °C under argon atmosphere was added 1 M THF solution of vinyl magnesium bromide (21 mL, 21.0 mmol). The mixture was stirred at that temperature for 3 h and quenched with NH4Cl solution. The reaction mixture was diluted with H2O and extracted with Et2O. The aqueous layer was extracted again with EtOAc. The combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated to give the crude vinylic alcohol product (5.13 g) as a yellow oil. Data: 1H NMR (CDCl3) δ 1.48 (s, 3H), 2.18 (s, 6H), 5.13 (d, J = 10.8 Hz, 1H), 5.30 (s, 1H), 5.31 (d, J = 17.2 Hz, 1H), 5.76 (d, J = 16.0 Hz, 1H), 6.04 (dd, J = 17.2, 10.8 Hz, 1H), 6.47 (d, J = 16.0 Hz, 1H), 6.65 (s, 2H), 7.50–7.58 (m, 2H), 7.64–7.70 (m, 1H), 7.84–7.90 (m, 2H) ppm.
To a stirred solution of the above crude alcohol (5.13 g) in THF (50 mL) at 0 °C were added PPh3 and 48% HBr aqueous solution. The reaction mixture was stirred at 0 °C for 2 h and slowly warmed to and stirred at room temperature for 10 h. Most of solvent was removed under reduced pressure to give the crude product (10.39 g) as a light yellow solid. The crude product was purified by SiO2 flash column chromatography (eluent: 50% EtOAc/hexane 350 mL, CH2Cl2 250 mL, and then MeOH 300 mL) to give the Wittig salt 6a (9.36 g, 13.69 mmol) in 99% yield as an ivory solid. Data: m.p. = 66–68 °C; 1H NMR (CDCl3) δ 1.51 (d, J = 2.8 Hz, 3H), 2.13 (s, 6H), 5.01 (dd, J = 15.2, 8.0 Hz, 2H), 5.45 (dt, Jd = 6.8, Jt = 8.0 Hz, 1H), 6.11 (d, J = 16.4 Hz, 1H), 6.33 (d, J = 16.4 Hz, 1H), 6.63 (s, 2H), 7.43–7.95 (m, 20H) ppm; IR (KBr) 2924, 1589, 1481, 1435, 1373, 1188, 1119, 1018, 964, 910, 748, 687 cm–1; HRMS (FAB) calcd for C38H36O3PS 603.2123, found 603.2128.
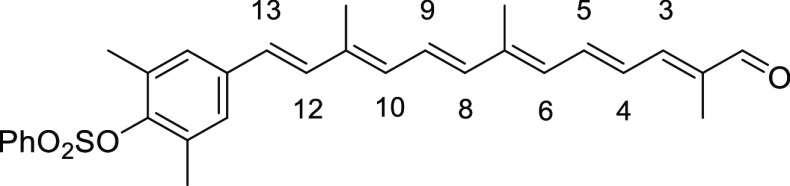
3,5-Dimethyl-4-((1E,3E,5E,7E,9E,11E)-3,7,12-trimethyl-13-oxotrideca-1,3,5,7,9,11-hexaen-1-yl)phenyl Benzenesulfonate (8a)
To a stirred suspension of the above Wittig salt 6a (3.00 g, 4.39 mmol, 3 equiv) in THF (30 mL) at 0 °C under argon atmosphere was added 1 M THF solution of NaHMDS (5.0 mL, 5.0 mmol). The resulting mixture turned to dark red and was stirred at 0 °C for 25 min, and a solution of C10 dialdehyde 7 (240 mg, 1.46 mmol) in THF (10 mL) was added. The reaction mixture was stirred at that temperature for 2 h and slowly warmed to and stirred at room temperature for 9 h. The mixture was diluted with Et2O and washed with NH4Cl solution. The aqueous layer was extracted with EtOAc, and the combined organic layer was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude product (3.53 g), which was purified by SiO2 flash column chromatography (eluent 15–50% EtOAc/hexane) to give the mono-coupled aldehyde 8a (401 mg, 0.82 mmol) in 56% yield as a red solid. Data: Rf = 0.18 (1:4 EtOAc/hexane); m.p. = 78–80 °C; 1H NMR (CDCl3) δ 1.89 (s, 3H), 2.06 (s, 3H), 2.07 (s, 3H), 2.23 (s, 6H), 6.24 (d, J = 11.6 Hz, 1H), 6.32 (d, J = 11.6 Hz, 1H), 6.33 (d, J = 16.0 Hz, 1H), 6.42 (d, J = 15.2 Hz, 1H), 6.51 (d, J = 16.0 Hz, 1H), 6.67 (s, 2H), 6.70 (dd, J = 15.2, 11.6 Hz, 1H), 6.80 (dd, J = 15.2, 11.6 Hz, 1H), 6.96 (d, J = 11.6 Hz, 1H), 7.03 (dd, J = 15.2, 11.6 Hz, 1H), 7.51–7.60 (m, 2H), 7.64–7.72 (m, 1H), 7.84–7.93 (m, 2H) ppm; 13C NMR (CDCl3) δ 9.5, 12.7, 13.0, 21.1, 121.2, 125.2, 127.1, 127.7, 128.4, 129.0, 131.5, 132.4, 134.0, 135.7, 136.2, 136.8, 137.0, 137.5, 137.6, 137.8, 139.2, 141.4, 147.4, 148.8, 194.4 ppm; UV (CHCl3, c = 2.98 × 10–6) λmax (ε) = 430 (231 088) nm; IR (KBr) 2915, 2728, 1662, 1588, 1469, 1446, 1372, 1275, 1185, 1126, 1096, 1014, 962, 872, 820, 745, 686, 663, 589, 567, 544 cm–1; HRMS (FAB) calcd for C30H32O4S 488.2021, found 488.2015.
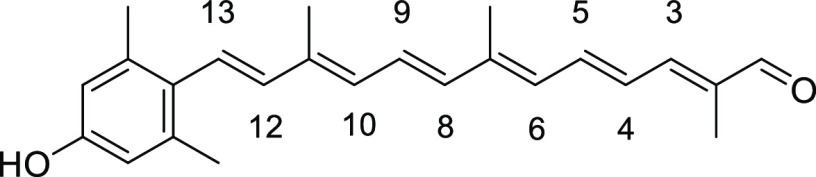
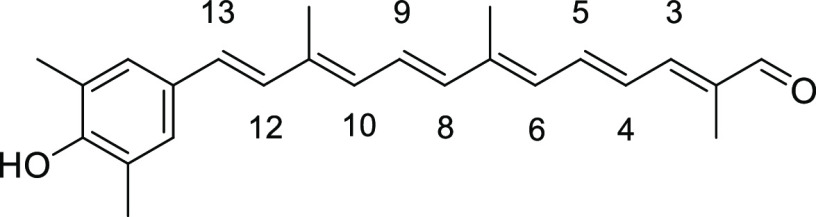
(2E,4E,6E,8E,10E,12E)-13-(4-Hydroxy-2,6-dimethylphenyl)-2,7,11-trimethyltrideca-2,4,6,8,10,12-hexaenal (2a)
To a stirred solution of sulfone-protected apo-carotenal 8a (0.30 g, 0.61 mmol) in t-BuOH/toluene (6 mL/24 mL) was added pulverized KOH (870 mg, 15.5 mmol). The mixture was then heated to reflux for 20 min and cooled to room temperature. Most of solvent was removed under reduced pressure. The crude product was dissolved in EtOAc, washed with 1 M HCl solution, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by SiO2 flash column chromatography to give 2a (111 mg, 0.32 mmol) in 52% yield as a red solid (triturated with Et2O). Data: Rf = 0.29 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.89 (s, 3H), 2.06 (s, 3H), 2.08 (s, 3H), 2.29 (s, 6H), 6.23 (d, J = 12.0 Hz, 1H; H-6), 6.31 (d, J = 11.6 Hz, 1H; H-10), 6.34 (d, J = 16.0 Hz, 1H; H-12), 6.41 (d, J = 14.8 Hz, 1H; H-8), 6.56 (s, 2H), 6.60 (d, J = 16.0 Hz, 1H; H-13), 6.69 (dd, J =14.8, 12.0 Hz, 1H; H-5), 6.83 (dd, J =14.8, 11.6 Hz, 1H; H-9), 6.97 (d, J =11.6 Hz, 1H; H-3), 7.04 (dd, J =14.8, 11.6 Hz, 1H; H-4), 9.46 (s, 1H) ppm; UV (DMSO, c = 6.38 × 10–6) λmax (ε) = 444 (60 843) nm; IR (KBr) 3358, 2956, 2934, 2853, 1663, 1606, 1544, 1448, 1378, 1309, 1262, 1215, 1187, 1150, 1029, 968, 910, 801, 761, 736, 650 cm–1; HRMS (EI) calcd for C24H28O2+Na 371.1982, found 371.1985. The 13C NMR spectrum could not be obtained due to lower solubility of 2a in any solvent.
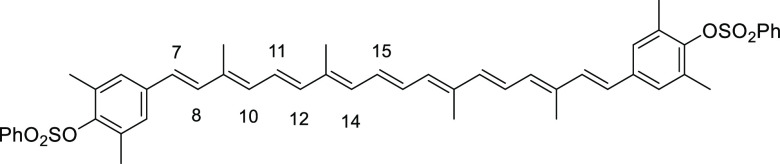
((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(3,5-dimethyl-4,1-phenylene) Dibenzenesulfonate (9a)
The mixture of the mono-coupled aldehyde 8a (600 mg, 1.30 mmol) and the above Wittig salt 6a (2.57 g, 3.76 mmol, 2.7 equiv) in toluene (30 mL) and MeOH (30 mL) was treated with NaOMe (406 mg, 7.52 mmol). The mixture was then heated to 80 °C for 1 day and cooled to room temperature. Most of solvent was removed under reduced pressure. The crude product was dissolved in CH2Cl2, washed with NH4Cl solution, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude product (2.89 g) as a red oil, which was purified by SiO2 flash column chromatography (eluent 15–60% EtOAc/hexane) to give carotene 9a (560 mg, 0.69 mmol) in 53% yield as a red solid (Rf = 0.17, 1:4 EtOAc/hexane). The analytical sample was prepared by recrystallization with MeOH and THF. Data: m.p. = 92–94 °C; 1H NMR (CDCl3) δ 1.97 (s, 6H), 2.03 (s, 6H), 2.21 (s, 12H), 6.21 (d, J = 11.2 Hz, 2H), 6.23–6.31 (m, 2H), 6.30 (d, J = 16.4 Hz, 2H), 6.38 (d, J = 14.8 Hz, 2H), 6.44 (d, J = 16.1 Hz, 2H), 6.59–6.67 (m, 2H), 6.64 (s, 4H), 6.65 (dd, J = 14.8, 11.2 Hz, 2H), 7.50–7.57 (m, 4H), 7.64–7.70 (m, 2H), 7.84–7.90 (m, 4H) ppm; 13C NMR (CDCl3) δ 12.7, 12.8, 21.2, 121.2, 124.2, 124.7, 128.5, 129.0, 130.3, 132.9, 133.1, 134.0, 135.1, 135.7, 136.4, 136.5, 137.8, 138.4, 139.4, 147.3 ppm; UV (CHCl3, c = 1.88 × 10–6) λmax (ε) = 465 (275 124) nm; IR (KBr) 3040, 2986, 2916, 2862, 1582, 1466, 1450, 1373, 1273, 1188, 1126, 1096, 1018, 972, 910, 880, 826, 772, 733, 679, 648, 617, 579 cm–1; HRMS (FAB) calcd for C50H52O6S2 812.3205, found 812.3205.
4,4′-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(3,5-dimethylphenol) (1a)
To a stirred solution of carotene (179 mg, 0.22 mmol) in toluene (20 mL) were added pulverized KOH (300 mg, 5.35 mmol, 25 equiv) and t-BuOH (5 mL). The mixture was heated to reflux for 1.5 h and cooled to room temperature. Most of solvent was removed under reduced pressure. The crude product was dissolved in EtOAc, washed with 1 M HCl, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude product (146 mg), which was purified by SiO2 flash column chromatography (eluent 25–45% EtOAc/hexane) to give the phenolic carotene 1a (84 mg, 0.16 mmol) in 72% yield as a red solid (Rf = 0.40, 2:3 EtOAc/hexane). Data: 1H NMR (acetone-d6) δ 1.99 (s, 6H), 2.06 (s, 6H), 2.24 (s, 12H), 6.28 (d, J = 11.2 Hz, 2H), 6.29–6.39 (m, 2H), 6.37 (d, J = 16.4 Hz, 2H), 6.43 (d, J = 14.8 Hz, 2H), 6.54 (s, 4H), 6.60 (d, J = 16.4 Hz, 2H), 6.69–6.79 (m, 2H), 6.78 (dd, J = 14.8, 11.2 Hz, 2H), 8.11 (br s, 2H) ppm; 13C NMR (acetone-d6) δ 12.7, 12.8, 21.4, 115.7, 126.0, 126.4, 129.2, 131.1, 132.7, 133.6, 136.6, 137.3, 138.1, 138.4, 138.5, 156.6 ppm; UV (DMSO, c = 1.40 × 10–6) λmax (ε) = 483 (369 197) nm; IR (KBr) 3356, 3032, 2916, 1736, 1597, 1466, 1373, 1304, 1204, 1150, 1026, 964, 910, 856, 733 cm–1; HRMS (FAB) calcd for C38H44O2 532.3341, found 532.3343.
Series II (b)
(E)-4-(3-Oxobut-1-en-1-yl)phenyl Benzenesulfonate (5b)
Yellow solid, 69% yield (5.73 g, 18.95 mmol) from 4-hydroxybenzaldehyde (3b). Data: Rf = 0.75 (hexane/acetone 1:1); 1H NMR (CDCl3) δ = 2.36 (s, 3H), 6.64 (d, J =16.4 Hz, 1H), 7.02 (d, J = 8.4 Hz, 2H), 7.44 (d, J = 16.4 Hz, 1H), 7.47 (d, J = 8.4 Hz, 2H), 7.52–7.58 (m, 2H), 7.66–7.72 (m, 1H), 7.82–7.86 (m, 2H) ppm; 13C NMR (CDCl3) δ = 27.5, 122.8, 127.8, 128.3, 129.2, 129.4, 133.4, 134.4, 135.0, 141.4, 150.6, 197.9 ppm; IR (KBr) 1691, 1667, 1611, 1598, 1583, 1501, 1449, 1415, 1373, 1315, 1296, 1257, 1199, 1178, 1150, 1092, 1015, 977, 862, 829, 746, 694 cm–1; HRMS (ESI) calcd for C16H14O4S+Na 325.0505, found 325.0508.
(E)-4-(3-Hydroxy-3-methylpenta-1,4-dien-1-yl)phenyl Benzenesulfonate
Reddish-yellow oil. Data: Rf = 0.75 (hexane/acetone 1:1); 1H NMR (CDCl3) δ 1.45 (s, 3H), 5.10 (dd, J =10.4, 0.8 Hz, 1H), 5.28 (dd, J = 17.2, 0.8 Hz, 1H), 6.00 (dd, J = 17.2, 10.4 Hz, 1H), 6.24 (d, J =16.0 Hz, 1H), 6.54 (d, J = 16.0 Hz, 1H), 6.89 (d, J =8.4 Hz, 2H), 7.26 (d, J = 8.4 Hz, 2H), 7.48–7.54 (m, 2H), 7.63–7.68 (m, 1H), 7.79–7.84 (m, 2H) ppm; IR (KBr) 3307, 2982, 1669, 1605, 1443, 1391, 1367, 1231, 1163, 1021, 961, 884, 813, 773 665 cm–1; HRMS (ESI) calcd for C18H18O4S+Na 353.0818, found 353.0822.
((2E,4E)-3-Methyl-5-(4-((phenylsulfonyl)oxy)phenyl)penta-2,4-dien-1-yl)triphenylphosphonium (6b)
Yellow solid, 25% yield (3.32 g, 5.17 mmol, E/Z = 3:1 by 1H NMR). Data: Rf = 0.15 (MC/MeOH 1:1); 1H NMR (CDCl3) δ 1.51 (dd, J = 4.0, 0.8 Hz, 3H), 4.82 (dd, J = 16.0, 8.0 Hz, 2H), 5.58 (q, J = 8.0 Hz, 1H), 6.44 (dd, J = 16.0, 2.8 Hz, 1H), 6.71 (dd, J = 16.0, 0.8 Hz, 1H), 7.18–7.23 (m, 1H), 7.25–7.31 (m, 2H), 7.35 (d, J = 8.0 Hz, 2H), 7.67–7.90 (m, 19H) ppm; IR (KBr) 1716, 1587, 1500, 1438, 1371, 1199, 1180, 1150, 1112, 1092, 1014, 997, 964, 912, 866, 798, 722, 687 cm–1; HRMS (ESI) calcd for C36H32O3PS 575.1804, found 575.1840.
4-((1E,3E,5E,7E,9E,11E)-3,7,12-Trimethyl-13-oxotrideca-1,3,5,7,9,11-hexaen-1-yl)phenyl Benzenesulfonate (8b)
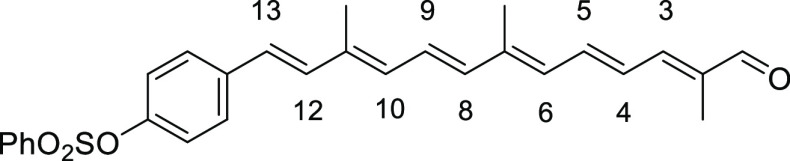
Red solid (recrystallized from MeOH), 63% yield (438 mg, 0.95 mmol). Data: Rf = 0.25 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.89 (s, 3H), 2.03 (s, 3H), 2.04 (s, 3H), 6.33 (d, J = 12.0 Hz, 2H; H-6, H-10), 6.43 (d, J = 14.8 Hz, 1H; H-8), 6.54 (d, J =16.0 Hz, 1H; H-12), 6.70 (dd, J = 14.8, 12.0 Hz, 1H; H-5), 6.79 (dd, J = 14.8, 12.0 Hz, 1H; H-9), 6.81 (d, J = 16.0 Hz, 1H; H-13), 6.92 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 12.0 Hz, 1H; H-3), 7.03 (dd, J =14.8, 12.0 Hz, 1H; H-4), 7.32 (d, J = 8.4 Hz, 2H), 7.50–7.56 (m, 2H), 7.64–7.69 (m, 1H), 7.82–7.86 (m, 2H), 9.45 (s, 1H) ppm; 13C NMR (CDCl3) δ 9.6, 12.9, 13.0, 29.6, 122.5, 126.6, 127.2, 127.3, 127.8, 128.4, 129.1, 131.7, 133.3, 134.2, 134.3, 135.3, 136.7, 136.8, 137.0, 137.5, 137.8, 141.4, 148.4, 148.7, 194.4 ppm; UV (CHCl3, c = 1.93 × 10–7) λmax (ε) = 442 (2 439 421) nm; IR (KBr) 3018, 2838, 1666, 1605, 1540, 1510. 1357, 1247, 1214, 1174, 1006, 964, 833, 751, 689, 667 cm–1; HRMS (ESI) calcd for C28H28O4S+Na 483.1601, found 483.1604.
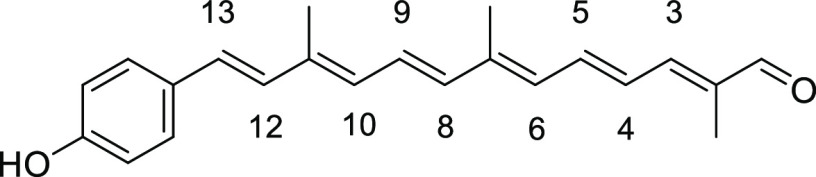
(2E,4E,6E,8E,10E,12E)-13-(4-Hydroxyphenyl)-2,7,11-trimethyltrideca-2,4,6,8,10,12-hexaenal (2b)
Dark red solid (triturated with Et2O), 96% yield (226 mg, 0.71 mmol, 1.7:1 all-E:Z). Data for all-E isomer: Rf = 0.19 (hexane/acetone 3:1); 1H NMR (CDCl3) δ = 1.89 (s, 3H), 2.06 (s, 6H), 4.92 (s, 1H), 6.31 (d, J = 11.6 Hz, 1H; H-10), 6.33 (d, J = 11.6 Hz, 1H; H-6), 6.42 (d, J = 14.8 Hz, 1H; H-8), 6.59 (d, J = 15.6 Hz, 1H; H-12), 6.70 (dd, J = 14.8, 11.6 Hz, 1H; H-5), 6.77 (d, J = 15.6 Hz, 1H; H-13), 6.80 (d, J = 8.4 Hz, 2H), 6.79–6.86 (m, 1H; H-9), 6.97 (d, J = 11.6 Hz, 1H; H-3), 7.04 (dd, J = 14.8, 11.6 Hz, 1H; H-4) 7.34 (d, J = 8.4 Hz, 2H), 9.46 (s, 1H) ppm; 13C NMR (acetone-d6) δ 10.3, 13.8, 13.9, 117.3, 129.4, 129.4, 129.5, 130.1, 131.1, 132.2, 133.0, 133.2, 138.4, 138.4, 139.2, 139.5, 143.3, 150.1, 159.0, 195.2 ppm; UV (DMSO, c = 2.23 × 10–6) λmax (ε) = 465 (300 796) nm; IR (KBr) 3268, 3031, 2921, 1694, 1654, 1605, 1538, 1512, 1437, 1379, 1357, 1256, 1209, 1187, 1170, 1151, 1050, 1013, 960, 846, 816, 755, 691 cm–1; HRMS (ESI) C22H24O2+Na 343.1669, found 343.1672.
((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(4,1-phenylene) Dibenzenesulfonate (9b)
Dark red solid (recrystallization from MeOH), 35% yield (550 mg, 0.89 mmol). Data: Rf = 0.20 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.99 (s, 6H), 2.02 (s, 6H), 6.28–6.33 (m, 2H), 6.33 (d, J = 11.6 Hz, 2H), 6.42 (d, J = 14.8 Hz, 2H), 6.49 (d, J = 16.0 Hz, 2H), 6.66 (dd, J = 14.8, 11.6 Hz, 2H), 6.64–6.68 (m, 2H), 6.81 (d, J = 16.0 Hz, 2H), 6.91 (d, J = 8.8 Hz, 4H), 7.32 (d, J = 8.8 Hz, 4H), 7.50–7.56 (m, 4H), 7.64–7.70 (m, 2H), 7.82–7.86 (m, 4H) ppm; 13C NMR (CDCl3) δ 12.8, 12.8, 122.6, 124.9, 125.7, 127.2, 128.5, 129.1, 130.4, 133.3, 133.9, 134.2, 134.7, 135.1, 135.3, 136.6, 137.0, 138.7, 148.3 ppm; UV (CHCl3, c = 1.00 × 10–6) λmax (ε) = 487 (499 829) nm; IR (KBr) 2919, 2850, 1498, 1367, 1219, 1199, 1173, 1150, 1093, 962, 872, 833, 773, 753, 700, 684 cm–1; HRMS (ESI) calcd for C46H44O6S2+Na 779.2472, found 779.2475.
4,4′-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)diphenol (1b)
Dark red solid (recrystallized from MeOH), 35% yield (75 mg, 0.16 mmol). Data: Rf = 0.10 (hexane/acetone 3:1), 1H NMR (DMSO-d6) δ 1.96 (s, 6H), 1.99 (s, 6H), 6.32–6.42 (m, 2H), 6.35 (d, J = 12.0 Hz, 2H), 6.42 (d, J = 14.4 Hz, 2H), 6.55 (d, J = 16.0 Hz, 2H), 6.68–6.78 (m, 2H), 6.70 (dd, J = 14.4, 12.0 Hz, 2H, calcd), 6.73 (d, J = 8.4 Hz, 4H), 6.81 (d, J = 16.0 Hz, 2H), 7.33 (d, J = 8.4 Hz, 4H), 9.53 (s, 2H) ppm; 13C NMR (DMSO-d6) δ 13.9, 14.0, 116.8, 126.6, 126.7, 128.9, 129.8, 131.5, 131.6, 132.9, 134.0, 137.1, 137.6, 138.5, 158.3 ppm; UV (DMSO, c = 2.10 × 10–6) λmax (ε) = 499 (309 910) nm; IR (KBr) 3263, 2923, 1654, 1603, 1582, 1538, 1512, 1438, 1360, 1211, 1187, 1170, 1156, 1104, 1012, 961, 832, 816, 772, 691 cm–1; HRMS (FAB) calcd for C34H36O2 476.2715, found 476.2706.
Series III (c)
4-Formyl-2,6-dimethylphenyl Benzenesulfonate (4c)
Light yellow oil, 98% yield (10.32 g, 35.55 mmol). Data: Rf = 0.55 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 2.21 (s, 6H), 7.57 (s, 2H), 7.58–7.64 (m, 2H), 7.71–7.77 (m, 1H), 7.97–8.02 (m, 2H), 9.92 (d, J = 1.6 Hz, 1H) ppm; 13C NMR (CDCl3) δ 17.3, 127.9, 129.4, 130.5, 133.5, 134.3, 134.4, 136,9, 151.7, 191.2 ppm; IR (KBr) 1697, 1597, 1476, 1449, 1371, 1303, 1194, 1167, 1116, 1088, 1036, 969, 949, 912, 876, 837, 752, 730, 710, 686 cm–1; HRMS (ESI) calcd for C15H14O4S+Na 313.0505, found 313.0509.
(E)-2,6-Dimethyl-4-(3-oxobut-1-en-1-yl)phenyl Benzenesulfonate (5c)
Yellow solid, 88% yield (12.30 g, 37.23 mmol). Data: Rf = 0.30 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 2.14 (s, 6H), 2.36 (s, 3H), 6.64 (d, J = 16.0 Hz, 1H), 7.22 (s, 2H), 7.40 (d, J = 16.0 Hz, 1H), 7.56–7.62 (m, 2H), 7.69–7.77 (m, 1H), 7.96–8.01 (m, 2H) ppm; 13C NMR (CDCl3) δ 17.2, 27.5, 127.5, 127.9, 129.0, 129.3, 132.7, 132.9, 134.2, 137.0, 142.0, 148,7, 198.1 ppm; IR (KBr) 2927, 1690, 1667, 1612, 1596, 1479, 1449, 1418, 1358, 1312, 1295, 1265, 1238, 1220, 1196, 1175, 1120, 1088, 1025, 976, 838, 772, 749, 729, 692 cm–1; HRMS (ESI) calcd for C18H18O4S+Na 353.0818, found 353.0820.
(E)-4-(3-Hydroxy-3-methylpenta-1,4-dien-1-yl)-2,6-dimethylphenyl Benzenesulfonate
Orange oil, Data: Rf = 0.32 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.47 (s, 3H), 2.10 (s, 6H), 5.12 (dd, J = 10.4, 1.2 Hz, 1H), 5.30 (dd, J = 17.6, 1.2 Hz, 1H), 6.02 (dd, J = 17.6, 10.4 Hz, 1H), 6.24 (d, J = 16.0 Hz, 1H), 6.50 (d, J = 16.0 Hz, 1H), 7.04 (s, 2H), 7.55–7.60 (m, 2H), 7.67–7.73 (m, 1H), 7.96–8.00 (m, 2H) ppm; IR (KBr) 3421, 2974, 1598, 1479, 1449, 1369, 1219, 1196, 1175, 1119, 1089, 970, 915, 847, 772, 749, 730, 689 cm–1; HRMS (ESI) calcd for C20H22O4S+Na 381.1131, found 381.1134
((2E,4E)-5-(3,5-Dimethyl-4-((phenylsulfonyl)oxy)phenyl)-3-methylpenta-2,4-dien-1-yl)triphenylphosphonium Bromide (6c)
Yellow solid, 68% yield (5.35 g, 7.99 mmol, E:Z = 4:1). Data for E,E-isomer: Rf = 0.13 (CH2Cl2/MeOH 1:1); 1H NMR (CDCl3) δ 1.48 (d, J = 4.0 Hz, 3H), 2.07 (s, 6H), 4.85 (dd, J = 16.0, 8.0 Hz, 2H), 5.59 (dt, Jd = 6.8, Jt = 8.0 Hz, 1H), 6.33 (dd, J = 16.0, 2.0 Hz, 1H), 6.64 (d, J = 16.0 Hz, 1H), 7.03 (s, 2H), 7.55–7.97 (m, 20H) ppm; IR (KBr) 2919, 1703, 1587, 1480, 1438, 1367, 1219, 1196, 1176, 1112, 1088, 1027, 997, 963, 922, 846, 807, 771, 744, 730, 689 cm–1; HRMS (ESI) calcd for C38H36O3PS 603.2117, found 603.2121.
2,6-Dimethyl-4-((1E,3E,5E,7E,9E,11E)-3,7,12-trimethyl-13-oxotrideca-1,3,5,7,9,11-hexaen-1-yl)phenyl Benzenesulfonate (8c)
Red solid (recrystallization from ether), 21% yield (555 mg, 1.14 mmol). Data: Rf = 0.42 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.89 (s, 3H), 2.03 (s, 3H), 2.05 (s, 3H), 2.11 (s, 6H), 6.33 (d, J = 11.6 Hz, 2H, H-6,H-10), 6.42 (d, J = 14.8 Hz, 1H, H-8), 6.51 (d, J = 16 Hz, 1H, H-12), 6.70 (dd, J =14.0, 11.6 Hz, 1H, H-5), 6.80 (dd, J = 14.8, 12.0 Hz, 1H, H-9), 6.82 (d, J =16.0 Hz, 1H, H-13), 6.96 (d, J = 11.6 Hz, 1H, H-3), 7.03 (dd, J = 14.0, 11.6 Hz, 1H, H-4), 7.09 (s, 2H), 7.55–7.61 (m, 2H), 7.67–7.73 (m, 1H), 7.96–8.01 (m, 2H), 9.45 (s, 1H) ppm; 13C NMR (CDCl3) δ 9.6, 12.9, 13.0, 17.3, 17.3, 127.1, 127.3, 127.7, 128.0, 129.2, 131.5, 132.3, 132.9, 133.9, 134.1, 136.0, 137.0, 137.1, 137.3, 137.5, 137.6, 141.5, 146.0, 146.7, 148.8, 194.4 ppm; UV (CHCl3, c = 8.26 × 10–7) λmax (ε) = 459 (899 101) nm; IR (KBr) 3032, 2919, 2862, 2815, 2711, 1659, 1609, 1585, 1540, 1477, 1448, 1406, 1354, 1311, 1271, 1198, 1174, 1106, 1088, 1007, 961, 844, 747, 733, 707, 687, 667 cm–1; HRMS (ESI) calcd for C30H32O4S+Na 511.1914, found 511.1912.
(2E,4E,6E,8E,10E,12E)-13-(4-Hydroxy-3,5-dimethylphenyl)-2,7,11-trimethyltrideca-2,4,6,8,10,12-hexaenal (2c)
Dark red solid (recrystallization from ether), 19% yield (73 mg, 0.21 mmol). Data: Rf = 0.35 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.89 (s, 3H), 2.04 (s, 3H), 2.05 (s, 3H), 2.25 (s, 6H), 4.78 (br s, 1H), 6.30 (d, J = 11.2 Hz, 1H, H-6), 6.32 (d, J = 11.2 Hz, 1H, H-10), 6.40 (d, J = 14.8 Hz, 1H, H-8), 6.54 (d, J = 16 Hz, 1H, H-12), 6.69 (dd, J = 14.0, 11.2 Hz, 1H, H-5), 6.76 (d, J = 16.0 Hz, 1H, H-13), 6.82 (dd, J = 14.8, 11.2 Hz, 1H, H-9), 6.97 (d, J = 11.2 Hz, 1H, H-3), 7.04 (dd, J = 14.0, 11.2 Hz, 1H, H-4), 7.09 (s, 2H), 9.45 (s, 1H) ppm; UV (DMSO, c = 2.29 × 10–6) λmax (ε) = 465 (213 261) nm; IR (KBr) 3389, 2923, 2855, 1663, 1603, 1540, 1489, 1407, 1379, 1356, 1310, 1261, 1209, 1187, 1153, 1021, 963, 910, 800, 761, 735 cm–1; HRMS (ESI) calcd for C24H28O2+Na 371.1982, found 371.1991. The 13C NMR spectrum could not be obtained due to lower solubility of 2c in any solvent.
((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(2,6-dimethyl-4,1-phenylene) Dibenzenesulfonate (9c)
Dark red solid (recrystallization from MeOH), 37% yield (498 mg, 0.74 mmol). Data: Rf = 0.20 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.99 (s, 6H), 2.02 (s, 6H), 2.11 (s, 12H), 6.26–6.36 (m, 2H, H-14), 6.33 (d, J = 12.0 Hz, 2H, H-10), 6.42 (d, J = 15.2 Hz, 2H, H-12), 6.46 (d, J = 16.0 Hz, 2H, H-8), 6.62–6.72 (m, 2H, H-15), 6.67 (dd, J =15.2, 12.0 Hz, 2H, H-11), 6.82 (d, J =16.0 Hz, 2H, H-7), 7.09 (s, 4H), 7.56–7.62 (m, 4H), 7.68–7.74 (m, 2H), 7.96–8.02 (m, 4H) ppm; UV (CHCl3, c = 2.11 × 10–6) λmax (ε) = 487 (181 129) nm; IR (KBr) 3022, 2914, 2856, 1719, 1590, 1478, 1446, 1350, 1215, 1193, 1174, 1115, 1089, 964, 908, 851, 756, 733, 688, 669, 650 cm–1; HRMS (ESI) calcd for C50H52O6S2+Na 835.3098, found 835.3102.
4,4′-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(2,6-dimethylphenol) (1c)
Dark red solid (recrystallization from MeOH), 67% yield (157 mg, 0.29 mmol). Data: Rf = 0.30 (hexane/acetone 3:1); 1H NMR (acetone-d6) δ 2.05 (s, 6H), 2.06 (s, 6H), 2.23 (s, 12H), 6.30–6.38 (m, 2H, H-14), 6.34 (d, J = 11.2 Hz, 2H, H-10), 6.43 (d, J = 14.8 Hz, 2H, H-12), 6.55 (d, J = 16.0 Hz, 2H, H-8), 6.73–6.80 (m, 4H, H-11,15), 6.84 (d, J = 16.0 Hz, 2H, H-7), 7.12 (s, 4H), 7.38 (s, 2H, -OH) ppm; UV (DMSO, c = 7.37 × 10–7) λmax (ε) = 501 (938 597) nm; IR (KBr) 3436, 3026, 2915, 1713, 1596, 1560, 1488, 1436, 1364, 1308, 1279, 1254, 1221, 1199, 1151, 1022, 957, 871, 836, 775, 723, 686 cm–1; HRMS (ESI) calcd for C38H44O2–H 531.3258, found 531.3261. The 13C NMR spectrum could not be obtained due to lower solubility of 1c in any solvent.
Series IV (d)
4-Formyl-2,6-dimethoxyphenyl Benzenesulfonate (4d)
Ivory solid, 95% yield (9.42 g, 29.22 mmol). Data: Rf = 0.70 (hexane/EtOAc 4:1); 1H NMR (CDCl3) δ 3.75 (s, 6H), 7.10 (s, 2H), 7.54–7.60 (m, 2H), 7.66–7.71 (m, 1H), 7.97–8.01 (m, 2H), 9.91 (s, 1H) ppm; 13C NMR δ 56.2, 106.0, 128.3, 128.7, 132.6, 133.8, 135.0, 137,6, 154.0, 190.8 ppm; IR (KBr) 1697, 1597, 1476, 1449, 1371, 1303, 1194, 1167, 1116, 1088, 969, 876, 837, 752, 730, 710, 686 cm–1; HRMS (ESI) calcd for C15H14O4S+Na 313.0505, found 313.0509.
(E)-2,6-Dimethoxy-4-(3-oxobut-1-en-1-yl)phenyl Benzenesulfonate (5d)
Yellow solid, 87% yield (7.13 g, 19.67 mmol). Data: Rf = 0.61 (hexane/acetone 1:1); 1H NMR (CDCl3) δ 2.38 (s, 3H), 3.68 (s, 6H), 6.64 (d, J = 16.0 Hz, 1H), 6.72 (s, 2H), 7.40 (d, J = 16.0 Hz, 1H), 7.53–7.59 (m, 2H), 7.65–7.70 (m, 1H), 7.96–8.00 (m, 2H) ppm; 13C NMR δ 27.5, 55.9, 104.7, 127.9, 128.2, 128.6, 129.4, 133.6, 133.7, 137.6, 142.5, 153.5, 198.0 ppm; IR (KBr) 1670, 1613, 1593, 1499, 1450, 1420, 1372, 1346, 1244, 1220, 1179, 1131, 1092, 985, 855, 772, 733, 695 cm–1; HRMS (ESI) calcd for C18H18O6S+Na 385.0716, found 385.0718.
(E)-4-(3-Hydroxy-3-methylpenta-1,4-dien-1-yl)-2,6-dimethoxyphenyl Benzenesulfonate
Orange oil, Data: Rf = 0.64 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.47 (s, 3H), 3.63 (s, 6H), 5.12 (dd, J =10.4, 1.2 Hz, 1H), 5.30 (dd, J = 17.2, 1.2 Hz, 1H), 6.02 (dd, J = 17.2, 10.4 Hz, 1H), 6.26 (d, J = 16.0 Hz, 1H), 6.52 (d, J = 16.0 Hz, 1H), 6.55 (s, 2H), 7.50–7.56 (m, 2H), 7.61–7.67 (m, 1H), 7.93–7.98 (m, 2H) ppm; IR (KBr) 3009, 2939, 2849, 1670, 1612, 1593, 1499, 1450, 1420, 1371, 1346, 1321, 1244, 1220, 1179, 1130, 1092, 980, 855, 771, 733, 695 cm–1.
((2E,4E)-5-(3,5-Dimethoxy-4-((phenylsulfonyl)oxy)phenyl)-3-methylpenta-2,4-dien-1-yl)triphenylphosphonium Bromide (6d)
Yellow solid, 57% yield (5.64 g, 8.04 mmol, E:Z = 5:1). Data for E,E-isomer: Rf = 0.15 (CH2Cl2/MeOH 20:1); 1H NMR (CDCl3) δ 1.46 (d, J = 3.6 Hz, 3H), 3.62 (s, 6H), 4.73 (dd, J = 16.0, 8.0 Hz, 2H), 5.74 (dt, Jd = 7.2, Jt = 8.0 Hz, 1H), 6.40 (dd, J = 16.0, 2.4 Hz, 1H), 6.61 (s, 2H), 6.76 (d, J = 16.0 Hz, 1H), 7.52–7.92 (m, 20H) ppm; IR (KBr) 1591, 1500, 1439, 1419, 1372, 1241, 1218, 1177, 1131, 1113, 1092, 907, 855, 723, 688 cm–1; HRMS (ESI) calcd for C38H36O5PS 635.2016, found 635.2053.
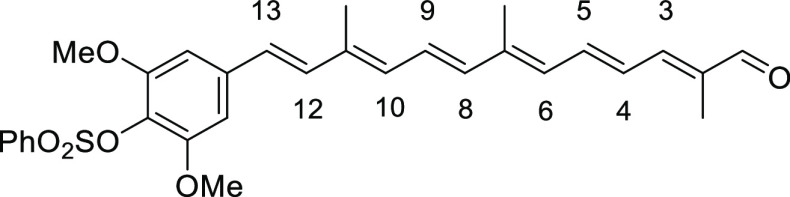
2,6-Dimethoxy-4-((1E,3E,5E,7E,9E,11E)-3,7,12-trimethyl-13-oxotrideca-1,3,5,7,9,11-hexaen-1-yl)phenyl Benzenesulfonate (8d)
Red solid (recrystallization from Et2O), 30% yield (370 mg, 0.71 mmol). Data: Rf = 0.16 (hexane/acetone 3:1); 1H NMR (CDCl3) δ 1.87 (s, 3H), 2.02 (s, 6H), 3.65 (s, 6H), 6.30 (d, J = 11.6 Hz, 1H, H-10), 6.36 (d, J = 11.6 Hz, 1H, H-6), 6.40 (d, J = 14.8 Hz, 1H, H-8), 6.51 (d, J = 16 Hz, 1H, H-12), 6.60 (s, 2H), 6.68 (dd, J =14.0, 11.6 Hz, 1H, H-5), 6.78 (dd, J = 14.8, 11.6 Hz, 1H, H-9), 6.82 (d, J =16.0 Hz, 1H, H-13), 6.95 (d, J = 12.0 Hz, 1H, H-3), 7.01 (dd, J = 14.0, 12.0 Hz, 1H, H-4), 7.49–7.57 (m, 2H), 7.62–7.68 (m, 1H), 7.94–8.01 (m, 2H), 9.43 (s, 1H) ppm; 13C NMR (CDCl3) δ 9.6, 12.9, 13.0, 55.8, 55.8, 102.9, 127.3, 127.3, 127.6, 127.8, 128.3, 128.6, 131.7, 133.5, 133.6, 134.4, 136.8, 136.9, 137.2, 137.6, 137.8, 137.8, 141.5, 148.9, 153.3, 194.4 ppm; UV (CHCl3, c = 1.45 × 10–6) λmax (ε) = 449 (386 736) nm; IR (KBr) 3007, 1656, 1586, 1541, 1500, 1449, 1417, 1370, 1351, 1274, 1243, 1182, 1129, 1091, 1006, 962, 909, 851, 751, 730, 710, 687, 667 cm–1; HRMS (ESI) calcd for C30H32O6S+Na 543.1812, found 543.1814.
(2E,4E,6E,8E,10E,12E)-13-(4-Hydroxy-3,5-dimethoxyphenyl)-2,7,11-trimethyltrideca-2,4,6,8,10,12-hexaenal (2d)
Dark red solid (recrystallization from Et2O), 45% yield (121 mg, 0.32 mmol). Data: Rf = 0.35 (hexane/acetone 2:1); 1H NMR (CDCl3) δ 1.89 (s, 3H), 2.06 (s, 6H), 3.93 (s, 6H), 5.63 (br s, 1H), 6.32 (d, J = 12.0 Hz, 1H, H-6), 6.34 (d, J = 11.2 Hz, 1H, H-10), 6.41 (d, J = 14.8 Hz, 1H, H-8), 6.56 (d, J = 16 Hz, 1H, H-12), 6.69 (s, 2H), 6.69 (dd, J = 14.0, 12.0 Hz, 1H, H-5), 6.77 (d, J = 16.0 Hz, 1H, H-13), 6.82 (dd, J = 14.8, 11.2 Hz, 1H, H-9), 6.96 (d, J = 11.6 Hz, 1H, H-3), 7.03 (dd, J = 14.0, 11.6Hz, 1H, H-4), 9.45 (s, 1H) ppm; 13C NMR δ 9.6, 13.0, 13.0, 56.2, 56.2, 103.2, 127.5, 127.5, 128.6, 129.1, 131.2, 131.5, 131.9, 134.7, 136.9, 136.9, 137.3, 137.6, 141.6, 147.2, 148.9, 194.4 ppm; UV (DMSO, c = 2.06 × 10–5) λmax (ε) = 452 (19 006) nm; IR (KBr) 3034, 2921, 1655, 1603, 1540, 1514, 1456, 1423, 1338, 1218, 1186, 1157, 1114, 1010, 962, 772, 668 cm–1; HRMS (ESI) calcd for C24H28O4+Na 403.1880, found 403.1882.
((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(2,6-dimethoxy-4,1-phenylene) Dibenzenesulfonate (9d)
To a stirred solution of C20 bis(diethyl phosphonate) (0.12 g, 0.21 mmol) and 4-benzenesulfonyl-3,5-dimethoxybenzaldehyde (0.21 g, 0.63 mmol) in MeOH (5 mL) and toluene (5 mL) was added NaH (0.17 g, 4.2 mmol). The mixture was heated at 100 °C for 12 h under argon atmosphere. The crude mixture was quenched with 10% NH4Cl solution and extracted with EtOAc. The organic layer was separated, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to give the crude red solid product, which was purified by recrystallization from MeOH/Et2O to give carotene (48 mg, 0.010 mmol) in 26% yield as a dark red solid. Data: Rf = 0.34 (2:1 hexane/acetone); 1H NMR (CDCl3) δ 1.99 (s, 6H), 2.03 (s, 6H), 3.68 (s, 12H), 6.25–6.36 (m, 2H, H-14), 6.36 (d, J = 12.0 Hz, 2H, H-10), 6.43 (d, J = 14.8 Hz, 2H, H-12), 6.47 (d, J = 16.0 Hz, 2H, H-8), 6.60 (s, 4H), 6.63–6.68 (m, 2H, H-15), 6.66 (dd, J =14.8, 12.0 Hz, 2H, H-11), 6.82 (d, J =16.0 Hz, 2H, H-7), 7.52–7.58 (m, 4H), 7.63–7.68 (m, 2H), 7.97–8.02 (m, 4H) ppm; 13C NMR δ 12.8, 12.9, 55.9, 56.0, 102.8, 103.2, 126.7, 127.3, 128.3, 128.5, 130.4, 133.4, 133.5, 134.0, 134.7, 135.1, 136.7, 137.4, 137.9, 138.7, 153.3 ppm; UV (CHCl3, c = 1.61 × 10–5) λmax (ε) = 486 (32 953) nm; IR (KBr) 2920, 2844, 1585, 1498, 1449, 1417, 1374, 1347, 1242, 1175, 1230, 1114, 1092, 1008, 968, 962, 852, 771, 756, 730, 711, 687 cm–1; HRMS (FAB) calcd for C50H52O10S2 876.3002, found 876.3004.
4,4′-((1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-Tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(2,6-dimethoxyphenol) (1d)
Dark red solid (recrystallized from MeOH), 78% yield (183 mg, 0.31 mmol). Data: Rf = 0.20 (hexane/acetone 2:1); 1H NMR (CDCl3) δ 2.00 (s, 6H), 2.04 (s, 6H), 3.93 (s, 12H), 6.25–6.34 (m, 2H, H-14), 6.33 (d, J = 10.8 Hz, 2H, H-10), 6.41 (d, J = 14.4 Hz, 2H, H-12), 6.51 (d, J = 16.0 Hz, 2H, H-8), 6.64–6.73 (m, 2H, H-15), 6.68 (s, 4H), 6.69 (dd, J = 14.4, 10.8 Hz, 2H, H-11), 6.77 (d, J = 16.0 Hz, 2H, H-7) ppm; UV (DMSO, c = 3.35 × 10–6) λmax (ε) = 503 (184 559) nm; IR (KBr) 3536, 3498, 3029, 2841, 1703, 1601, 1554, 1514, 1456, 1425, 1401, 1338, 1307, 1290, 1262, 1217, 1155, 1114, 1010, 987, 962, 856, 833, 809, 705, 623 cm–1; HRMS (FAB) calcd for C38H44O6 596.3138, found 596.3142. The 13C NMR spectrum could not be obtained due to lower solubility of 1d in any solvent.
Series V (e)
Ethyl 5-Hydroxy-3-methyl-[1,1′-biphenyl]-2-carboxylate (3e)17
Yellow oil, 42% yield (4.28 g, 16.70 mmol). Data: Rf = 0.50 (1:4 hexane/EtOAc); 1H NMR (CDCl3) δ 0.90 (t, J = 7.2 Hz, 3H), 2.32 (s, 3H), 4.00 (q, J = 7.2 Hz, 2H), 6.59 (d, J = 2.4 Hz, 1H), 6.61 (d, J = 2.4 Hz, 1H), 7.24–7.34 (m, 5H) ppm; 13C NMR δ 13.5, 19.9, 61.1, 114.3, 116.1, 125.2, 127.3, 128.0, 128.1, 138.1, 140.9, 142.7, 156.6, 170.7 ppm; HRMS (ESI) calcd for C16H16O3+Na 279.0992, found 279.0996.
6-(Hydroxymethyl)-5-methyl-[1,1′-biphenyl]-3-ol
To a stirred solution of the above benzenecarboxylic ester 3e (14.18 g, 55.34 mmol) in THF (20 mL) was added LAH (5.25 g, 138.35 mmol) at 0 °C. The mixture was heated at 90 °C for 20 min and cooled to room temperature. The mixture was quenched with 1 M HCl in ice-water, extracted with EtOAc, dried over anhydrous Na2SO4, filtered, and concentrated to give the crude product, which was purified by flash column chromatography (hexane/EtOAc from 8:1 to 1:1) to afford benzylic alcohol (6.86 g, 32 mmol) in 58% yield as a white solid. Data: Rf = 0.21 (4:1 hexane/EtOAc); 1H NMR (acetone-d6) δ 2.43 (s, 3H), 3.77 (br s, 1H), 4.42 (s, 2H), 6.58 (d, J = 2.4 Hz, 1H), 6.71 (d, J = 2.4 Hz, 1H), 7.30–7.35 (m, 1H), 7.35–7.40 (m, 2H), 7.42–7.46 (m, 2H), 8.32 (br s, 1H) ppm; 13C NMR (acetone-d6) δ 20.2, 59.8, 115.4, 117.9, 128.1, 129.2, 129.3, 130.6, 141.6, 143.3, 145.6, 157.5 ppm; IR (KBr) 3407, 3064, 1592, 1475, 1457, 1441, 1405, 1321, 1266, 1220, 1171, 1108, 1073, 1030, 1011, 972, 952, 855, 771, 717, 700 cm–1; HRMS (ESI) calcd for C14H14O2+Na 237.0886, found 237.0888.
5-Hydroxy-3-methyl-[1,1′-biphenyl]-2-carbaldehyde
To a stirred solution of the above crude benzylic alcohol (9.0 g, 42 mmol) in acetone (40 mL) were added silica gel (11.0 g) and PCC (10.86 g, 50.4 mmol). The mixture was stirred at room temperature for 5 h under argon atmosphere and filtered through a sintered glass funnel under reduced pressure. The filter cake was rinsed with 200 mL of hexane/EtOAc (v:v = 2:1). The filtrate was washed with NaHCO3 solution, dried over anhydrous Na2SO4, filtered, and concentrated to give the crude product, which was purified by SiO2 flash column chromatography (hexane/acetone 7:1–7:3) to give titled aldehyde (7.68 g, 36 mmol) in 86% as a light yellow solid. Data: Rf = 0.40 (hexane/acetone = 7:3); 1H NMR (CDCl3) δ 2.64 (s, 3H), 6.54 (br s, 1H), 6.70 (d, J = 1.6 Hz, 1H), 6.74 (d, J = 1.6 Hz, 1H), 7.28–7.34 (m, 2H), 7.38–7.44 (m, 3H), 9.81 (s, 1H) ppm; 13C NMR (CDCl3) δ 22.1, 115.3, 118.1, 125.8, 128.0, 128.2, 129.8, 138.7, 144.0, 151.0, 159.0, 193.5 ppm; IR (KBr) 3246, 1660, 1596, 1571, 1463, 1437, 1331, 1272, 1221, 1176, 1113, 866, 796, 771, 703, 662 cm–1; HRMS (ESI) calcd for C14H12O2+Na 235.0730, found 235.0733.
6-Formyl-5-methyl-[1,1′-biphenyl]-3-yl Benzenesulfonate (4e)
Claybank oil, 95% yield (5.69 g, 16.15 mmol). Data: Rf = 0.70 (hexane/EtOAc 4:1); 1H NMR (CDCl3) δ 2.58 (s, 3H), 6.91 (d, J = 3.2 Hz, 1H), 6.95 (d, J = 3.2 Hz, 1H), 7.18–7.24 (m, 2H), 7.39–7.45 (m, 3H), 7.55–7.60 (m, 2H), 7.69–7.74 (m, 1H), 7.88–7.94 (m, 2H), 9.86 (s, 1H) ppm; 13C NMR δ 21.5, 121.9, 124.4, 128.5, 128.7, 129.3, 128.8, 131.3, 134.5, 137.4, 142.8, 148.9, 151.1, 193.2 ppm; IR (KBr) 1750, 1607, 1449, 1373, 1316, 1285, 1238, 1220, 1189, 1115, 1091, 1060, 1041, 1003, 985, 952, 889, 871, 806, 773, 739, 684 cm–1; HRMS (ESI) calcd for C20H16O4S+Na 375.0662, found 375.0665.
(E)-5-Methyl-6-(3-oxobut-1-en-1-yl)-[1,1′-biphenyl]-3-yl Benzenesulfonate (5e)
White solid, 62% yield (6.31 g, 16.08 mmol). Data: Rf = 0.38 (hexane/EtOAc 4:1); 1H NMR (CDCl3) δ 2.13 (s, 3H), 2.39 (s, 3H), 6.07 (d, J = 16.4 Hz, 1H), 6.78 (d, J = 2.4 Hz, 1H), 6.96 (d, J = 2.4 Hz, 1H), 7.10–7.16 (m, 2H), 7.32–7.38 (m, 3H), 7.35 (d, J = 16.4 Hz, 1H), 7.54–7.59 (m, 2H), 7.67–7.73 (m, 1H), 7.88–7.93 (m, 2H) ppm; 13C NMR δ 21.5, 27.2, 121.6, 123.5, 127.8, 128.2, 128.4, 129.2, 129.5, 131.7, 133.6, 134.3, 135.4, 139.2, 139.7, 141.1, 144.1, 148.8, 198.1 ppm; IR (KBr) 3062, 1693, 1666, 1612, 1588, 1466, 1449, 1373, 1304, 1253, 1190, 1141, 1093, 974, 912, 884, 867, 820, 772, 753, 730, 716, 702, 686 cm–1; HRMS (ESI) calcd for C23H20O4S+Na 415.0975, found 415.0978.
(E)-6-(3-Hydroxy-3-methylpenta-1,4-dien-1-yl)-5-methyl-[1,1′-biphenyl]-3-yl Benzenesulfonate
Yellow oil. Data: Rf = 0.68 (hexane/EtOAc = 4:1); 1H NMR (CDCl3) δ 1.19 (s, 3H), 2.29 (s, 3H), 4.97 (dd, J = 10.4, 1.2 Hz, 1H), 5.04 (dd, J = 17.6, 1.2 Hz, 1H), 5.42 (d, J = 16.4 Hz, 1H), 5.79 (dd, J = 17.6, 10.4 Hz, 1H), 6.36 (d, J = 16.4 Hz, 1H), 6.69 (d, J = 2.8 Hz, 1H), 6.89 (d, J = 2.8 Hz, 1H), 7.08–7.12 (m, 2H), 7.22–7.33 (m, 3H), 7.52–7.57 (m, 2H), 7.65–7.71 (m, 1H), 7.86–7.92 (m, 2H) ppm; IR (KBr) 3421, 3059, 2974, 2928, 1588, 1466, 1449, 1372, 1307, 1220, 1190, 1141, 1093, 974, 917, 883, 772, 751, 687 cm–1; HRMS (ESI) calcd for C25H24O4S+Na 443.1288, found 443.1291.
((2E,4E)-3-Methyl-5-(3-methyl-5-((phenylsulfonyl)oxy)-[1,1′-biphenyl]-2-yl)penta-2,4-dien-1-yl)triphenylphosphonium Bromide (6e)
Yellow solid, 68% yield (7.79 g, 10.65 mmol, E:Z = 4:1). Data for E,E-isomer: Rf = 0.15 (CH2Cl2/MeOH=20:1); 1H NMR (CDCl3) δ 1.24 (s, 3H), 2.25 (s, 3H), 4.77 (dd, J = 12.8, 8.0 Hz, 2H), 5.18 (dt, Jd = 6.4, Jt = 8.0 Hz, 1H), 5.85 (d, J = 16.0 Hz, 1H), 6.21 (d, J = 16.0 Hz, 1H), 6.62 (br s, 1H), 6.84 (br s, 1H), 7.02–7.09 (m, 2H), 7.09–7.16 (m, 1H), 7.17–7.26 (m, 2H), 7.52–7.92 (m, 20H) ppm; IR (KBr) 3055, 1586, 1437, 1369, 1306, 1220, 1189, 1140, 1111, 1092, 972, 882, 773, 749, 721, 687 cm–1; HRMS (ESI) calcd for C43H38O3PS 665.2279, found 665.2283.
5-Methyl-6-((1E,3E,5E,7E,9E,11E)-3,7,12-trimethyl-13-oxotrideca-1,3,5,7,9,11-hexaen-1-yl)-[1,1′-biphenyl]-3-yl Benzenesulfonate (8e)
Red solid, 21% yield (288 mg, 0.52 mmol). Data: Rf = 0.32 (hexane/acetone = 3:1); 1H NMR (CDCl3) δ 1.84 (s, 3H), 1.88 (s, 3H), 2.01 (s, 3H), 2.37 (s, 3H), 6.03 (d, J = 11.2 Hz, 1H, H-6), 6.16 (d, J = 16.4 Hz, 1H, H-12), 6.29 (d, J = 11.6 Hz, 1H, H-10), 6.35 (d, J = 15.2 Hz, 1H, H-8), 6.37 (d, J =16.0 Hz, 1H, H-13), 6.68 (dd, J =14.8, 11.2 Hz, 1H, H-5), 6.70 (d, J = 2.4 Hz, 1H), 6.72 (dd, J = 15.2, 11.6 Hz, 1H, H-9), 6.92 (d, J = 2.4 Hz, 1H), 6.95 (d, J = 12.0 Hz, 1H, H-3), 7.01 (dd, J = 14.8, 12.0 Hz, 1H, H-4), 7.14–7.18 (m, 2H), 7.27–7.35 (m, 3H), 7.52–7.58 (m, 2H), 7.65–7.71 (m, 1H), 7.87–7.93 (m, 2H), 9.45 (s, 1H) ppm; 13C NMR (CDCl3) δ 9.6, 12.6, 13.0, 21.6, 121.4, 123.2, 125.8, 127.1, 127.2, 127.6, 127.9, 128.5, 129.1, 129.6, 131.4, 132.2, 134.1, 134.8, 135.6, 137.0, 137.1, 137.4, 137.5, 138.2, 139.7, 140.7, 141.4, 142.8, 147.4, 148.8, 194.4 ppm; UV (CHCl3, c = 1.27 × 10–6) λmax (ε) = 444 (419 628) nm; IR (KBr) 3032, 2918, 1660, 1609, 1594, 1544, 1465, 1448, 1407, 1374, 1355, 1304, 1211, 1188, 1141, 1093, 1006, 971, 884, 823, 752, 717, 702, 687, 667, 631 cm–1; HRMS (ESI) calcd for C35H34O4S+Na 573.2070, found 573.2069.
(2E,4E,6E,8E,10E,12E)-13-(5-Hydroxy-3-methyl-[1,1′-biphenyl]-2-yl)-2,7,11-trimethyltrideca-2,4,6,8,10,12-hexaenal (2e)
Red powder, 13% yield (80 mg, 0.19 mmol). Data: Rf = 0.28 (hexane/acetone = 3:1); 1H NMR (CDCl3) δ 1.85 (s, 3H), 1.88 (s, 3H), 2.02 (s, 3H), 2.41 (s, 3H), 5.46 (br s, 1H), 6.01 (d, J = 11.6 Hz, 1H, H-6), 6.13 (d, J = 16.0 Hz, 1H, H-12), 6.28 (d, J = 11.6 Hz, 1H, H-10), 6.33 (d, J = 14.8 Hz, 1H, H-8), 6.44 (d, J =16.0 Hz, 1H, H-13), 6.62 (d, J = 2.4 Hz, 1H), 6.67 (dd, J =14.8, 11.6 Hz, 1H, H-5), 6.73 (d, J = 2.4 Hz, 1H), 6.74 (dd, J = 14.8, 11.6 Hz, 1H, H-9), 6.96 (d, J = 12.0 Hz, 1H, H-3), 7.02 (dd, J = 14.8, 12.0 Hz, 1H, H-4), 7.26–7.38 (m, 5H), 9.44 (s, 1H) ppm; 13C NMR (CDCl3) δ 9.6, 12.7, 13.0, 21.8, 115.0, 116.8, 126.8, 127.1, 127.3, 127.6, 127.8, 128.0, 129.7, 129.7, 130.7, 130.9, 131.0, 136.6, 136.7, 137.9, 138.1, 141.9, 141.9, 143.2, 149.3, 154.2, 194.7 ppm; UV (DMSO, c = 2.66 × 10–6) λmax (ε) = 450 (164 286) nm; IR (KBr) 3310, 2921, 1655, 1595, 1541, 1465, 1407, 1356, 1318, 1260, 1210, 1183, 1010, 965, 907, 862, 754, 728, 701 cm–1; HRMS (ESI) calcd for C29H30O2+Na 433.2138, found 433.2140.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) through the grant funded by the Korean government, Ministry of Science and ICT (NRF-2020R1A2C1010724) and partly by Basic Science Research Program funded by the Ministry of Education (NRF-2020R1A6A1A03038817). The authors appreciate the generous gift of 2,7-dimethyl-2,4,6-octatriendial (7) from BASF.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c04432.
1H/13C NMR spectra, HPLC data for 9a, 2b, 1b, and 1d; graphs of DPPH and ABTS assay results; and DFT calculations for the energy levels of 2b and 1b (PDF)
Author Contributions
G.S. contributed to the synthesis (lead), DPPH and ABTS assays (lead), and DFT calculation (lead). L.G. performed synthesis (supporting) and investigation (supporting). H.J. performed synthesis (supporting) and investigation (supporting). W.-J.C. carried out data curation (supporting) and DFT calculation (supporting). S.K. contributed to data curation (lead), funding acquisition (lead), formal analysis (lead), investigation (lead), project administration (lead), synthesis (supporting), and manuscript writing (lead).
National Research Foundation of Korea NRF-2020R1A2C1010724; NRF-2020R1A6A1A03038817.
The authors declare no competing financial interest.
Dedication
† This paper is dedicated to my dear Professor Lanny S. Liebeskind.
Supplementary Material
References
- a Polívka T.; Sundström V. Ultrafast Dynamics of Carotenoid Excited States–From Solution to Natural and Artificial Systems. Chem. Rev. 2004, 104, 2021–2071. 10.1021/cr020674n. [DOI] [PubMed] [Google Scholar]; b Holt N. E.; Zigmantas D.; Valkunas L.; Li X.-P.; Niyogi K. K.; Fleming G. R. Carotenoid Cation Formation and the Regulation of Photosynthetic Light Harvesting. Science 2005, 307, 433–436. 10.1126/science.1105833. [DOI] [PubMed] [Google Scholar]; c Wormit M.; Harbach P. H. P.; Mewes J. M.; Amarie S.; Wachtveitl J.; Dreuw A. Excitation energy transfer and carotenoid radical cation formation in light harvesting complexes – A theoretical perspective. Biochim. Biophys. Acta, Bioenerg. 2009, 1787, 738–746. 10.1016/j.bbabio.2009.01.021. [DOI] [PubMed] [Google Scholar]
- a Krinsky N. I.; Yeum K.-J. Carotenoid–radical interactions. Biochem. Biophys. Res. Commun. 2003, 305, 754–760. 10.1016/S0006-291X(03)00816-7. [DOI] [PubMed] [Google Scholar]; b El-Agamey A.; Lowe G. M.; McGarvey D. J.; Mortensen A.; Phillip D. M.; Truscott T. G.; Young A. J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- a Burton G. W.; Ingold K. U. β-Carotene: An Unusual Type of Lipid Antioxidant. Science 1984, 224, 569–573. 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]; b Terao J. Antioxidant Activity of β-Carotene-Related Carotenoids in Solution. Lipids 1989, 24, 659–661. 10.1007/BF02535085. [DOI] [PubMed] [Google Scholar]; c Stahl W.; Sies H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. 10.1016/S0098-2997(03)00030-X. [DOI] [PubMed] [Google Scholar]
- a Yamaguchi M. On Carotenoids of a Sponge “Reniera japonica”. Bull. Chem. Soc. Jpn. 1957, 30, 111–114. 10.1246/bcsj.30.111. [DOI] [Google Scholar]; b Liaaen-Jensen S. Bacterial Carotenoids. Acta Chem. Scand. 1965, 19, 1025–1030. 10.3891/acta.chem.scand.19-1025. [DOI] [PubMed] [Google Scholar]
- Arcamone F.; Camerino B.; Cotta E.; Franceschi G.; Grein A.; Penco S.; Spalla C. New Carotenoids from Streptomyces mediolani n. sp. Experientia 1969, 25, 241–242. 10.1007/BF02034366. [DOI] [PubMed] [Google Scholar]
- a Lutter K.; De Spirt S.; Kock S.; Kröncke K.-D.; Martin H.-D.; Wagener T.; Stahl W. 3,3′-Dihydroxyisorenieratene prevents UV-induced formation of reactive oxygen species and the release of protein-bound zinc ions in human skin fibroblasts. Mol. Nutr. Food. Res. 2010, 54, 285–291. 10.1002/mnfr.200900044. [DOI] [PubMed] [Google Scholar]; b Spirt S. D.; Lutter K.; Stahl W. Carotenoids in Photooxidative Stress. Curr. Nutr. Food Sci. 2010, 6, 36–43. 10.2174/157340110790909572. [DOI] [Google Scholar]; c Wagener S.; Völker T.; De Spirt S.; Ernst H.; Stahl W. 3,3′-Dihydroxyisorenieratene and isorenieratene prevent UV-induced DNA damage in human skin fibroblasts. Free Radical Biol. Med. 2012, 53, 457–463. 10.1016/j.freeradbiomed.2012.05.022. [DOI] [PubMed] [Google Scholar]; d Martin H.-D.; Kock S.; Scherrers R.; Lutter K.; Wagener T.; Hundsdörfer C.; Frixel S.; Schaper K.; Ernst H.; Schrader W.; Görner H.; Stahl W. 3,3′-Dihydroxyisorenieratene, a Natural Carotenoid with Superior Antioxidant and Photoprotective Properties. Angew. Chem., Int. Ed. 2009, 48, 400–403. 10.1002/anie.200803668. [DOI] [PubMed] [Google Scholar]
- a Fukuhara K.; Nakanishi I.; Matsuoka A.; Matsumura T.; Honda S.; Hayashi M.; Ozawa T.; Miyata N.; Saito S.; Ikota N.; Okuda H. Effect of Methyl Substitution on the Antioxidative Property and Genotoxicity of Resveratrol. Chem. Res. Toxicol. 2008, 21, 282–287. 10.1021/tx7003008. [DOI] [PubMed] [Google Scholar]; b Kang Y.-F.; Yan W.-J.; Zhou T.-W.; Dai F.; Li X.-Z.; Bao X.-Z.; Du Y.-T.; Yuan C.-H.; Wang H.-B.; Ren X.-R.; Liu Q.; Jin X.-L.; Zhou B.; Zhang J. Tailoring 3,3′-Dihydroxyisorenieratene to Hydroxystilbene: Finding a Resveratrol Analogue with Increased Antiproliferation Activity and Cell Selectivity. Chem. – Eur. J. 2014, 20, 8904–8908. 10.1002/chem.201403024. [DOI] [PubMed] [Google Scholar]
- Marian C. M.; Kock S. C.; Hundsdörfer C.; Martin H.-D.; Stahl W.; Ostroumov E.; Müller M. G.; Holzwarth A. R. Spectroscopic properties of phenolic and quinoid carotenoids: a combined theoretical and experimental study. Photochem. Photobiol. Sci. 2009, 8, 270–278. 10.1039/B814713B. [DOI] [PubMed] [Google Scholar]
- a Walter M. H.; Strack D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. 10.1039/c0np00036a. [DOI] [PubMed] [Google Scholar]; b Carail M.; Caris-Veyrat C. Carotenoid oxidation products: From villain to saviour?. Pure Appl. Chem. 2006, 78, 1493–1503. 10.1351/pac200678081493. [DOI] [Google Scholar]; c Ramel F.; Birtic S.; Cuiné S.; Triantaphylidès C.; Ravanat J.-L.; Havaux M. Chemical Quenching of Singlet Oxygen by Carotenoids in Plants. Plant Physiol. 2012, 158, 1267–1278. 10.1104/pp.111.182394. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zoccali M.; Giuffrida D.; Salafia F.; Socaciu C.; Skjånes K.; Dugo P.; Mondello L. First Apocarotenoids Profiling of Four Microalgae Strains. Antioxidants 2019, 8, 209. 10.3390/antiox8070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X.; Kiser P. D.; Che T.; Carey P. R.; Golczak M.; Shi W.; von Lintig J.; Palczewski K. Analysis of Carotenoid Isomerase Activity in a Prototypical Carotenoid Cleavage Enzyme, Apocarotenoid Oxygenase (ACO). J. Biol. Chem. 2014, 289, 12286–12299. 10.1074/jbc.M114.552836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bouvier F.; Dogbo O.; Camara B. Biosynthesis of the Food and Cosmetic Plant Pigment Bixin (Annatto). Science 2003, 300, 2089–2091. 10.1126/science.1085162. [DOI] [PubMed] [Google Scholar]; b Tao S.; Park S. L.; de la Vega M. R.; Zhang D. D.; Wondrak G. T. Systemic administration of the apocarotenoid bixin protects skin against solar UV-induced damage through activation of NRF2. Free Radical Biol. Med. 2015, 89, 690–700. 10.1016/j.freeradbiomed.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. H.; Tan B. C.; Gage D. A.; Zeevaart J. A. D.; McCarty D. R. Specific Oxidative Cleavage of Carotenoids by VP14 of Maize. Science 1997, 276, 1872–1874. 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- Eroglu A.; Harrison E. H. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J. Lipid Res. 2013, 54, 1719–1730. 10.1194/jlr.R039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Méndez-Robles M. D.; Permady H. H.; Jaramillo-Flores M. E.; Lugo-Cervantes E. C.; Cardador-Martínez A.; Canales-Aguirre A. A.; López-Dellamary F.; Cerda-García-Rojas C. M.; Tamariz J. C-26 and C-30 Apocarotenoids from Seeds of Ditaxis heterantha with Antioxidant Activity and Protection against DNA Oxidative Damage. J. Nat. Prod. 2006, 69, 1140–1144. 10.1021/np050489f. [DOI] [PubMed] [Google Scholar]; b Ramel F.; Birtic S.; Ginies C.; Soubigou-Taconnat L.; Triantaphylidès C.; Havaux M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 5535–5540. 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Harrison E. H.; dela Sena C.; Eroglu A.; Fleshman M. K. The formation, occurrence, and function of β-apocarotenoids: β-carotene metabolites that may modulate nuclear receptor signaling. Am. J. Clin. Nutr. 2012, 96, 1189S–1192S. 10.3945/ajcn.112.034843. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Sharoni Y.; Linnewiel-Hermoni K.; Khanin M.; Salman H.; Veprik A.; Danilenko M.; Levy J. Carotenoids and apocarotenoids in cellular signaling related to cancer: A review. Mol. Nutr. Food Res. 2016, 56, 259–269. 10.1002/mnfr.201100311. [DOI] [PubMed] [Google Scholar]; e Havaux M. Carotenoid oxidation products as stress signals in plants. Plant J. 2014, 79, 597–606. 10.1111/tpj.12386. [DOI] [PubMed] [Google Scholar]
- a Sánchez-Moreno C.; Larrauri J. A.; Saura-Calixto F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. . [DOI] [Google Scholar]; b Jiménez-Escrig A.; Jiménez-Jiménez I.; Sánchez-Moreno C.; Saura-Calixto F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690. . [DOI] [Google Scholar]; c Sharma O. P.; Bhat T. K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. 10.1016/j.foodchem.2008.08.008. [DOI] [Google Scholar]
- a Li X.; Lin J.; Gao Y.; Han W.; Chen D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. 10.1186/1752-153X-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang Y.; Fang H.; Xie Q.; Sun J.; Liu R.; Hong Z.; Yi R.; Wu H. Comparative Evaluation of the Radical-Scavenging Activities of Fucoxanthin and Its Stereoisomers. Molecules 2014, 19, 2100–2113. 10.3390/molecules19022100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.; Kim D.; In I. J.; Koo S. Efficient preparation method of 4-hydroxybenzoic esters – Oxidation of substituted Hagemman’s ester. Tetrahedron Lett. 2017, 58, 2264–2266. 10.1016/j.tetlet.2017.04.090. [DOI] [Google Scholar]
- Alam M. S.; Koo S. Deprotection of durable benzenesulfonyl protection for phenols −efficient synthesis of polyphenols. Synth. Commun. 2018, 48, 247–254. 10.1080/00397911.2017.1393088. [DOI] [Google Scholar]
- Kim D.; Shi G.; Kim Y.; Koo S. Fast Assembly and High-Throughput Screening of Structure and Antioxidant Relationship of Carotenoids. Org. Lett. 2019, 21, 714–718. 10.1021/acs.orglett.8b03915. [DOI] [PubMed] [Google Scholar]
- Liao F.-X.; Hu C.-H. A density functional theory study for the role of end groups on the antioxidative potency of carotenoids. Theor. Chem. Acc. 2013, 132, 1357 10.1007/s00214-013-1357-5. [DOI] [Google Scholar]
- Mortensen A.; Skibsted L. H. Importance of Carotenoid Structure in Radical-Scavenging Reactions. J. Agric. Food Chem 1997, 45, 2970–2977. 10.1021/jf970010s. [DOI] [Google Scholar]
- Miller N. J.; Sampson J.; Candeias L. P.; Bramley P. M.; Rice-Evans C. A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. 10.1016/0014-5793(96)00323-7. [DOI] [PubMed] [Google Scholar]
- Focsan A. L.; Bowman M. K.; Molnár P.; Deli J.; Kispert L. D. Carotenoid Radical Formation: Dependence on Conjugation Length. J. Phys. Chem. B 2011, 115, 9495–9506. 10.1021/jp204787b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.; Ji M.; Park M.; Yun I.-K.; Oh S.-S.; Baik W.; Koo S. Diallylic Sulfides as Key Structures for Carotenoid Syntheses. J. Org. Chem. 1999, 64, 8051–8053. 10.1021/jo990987r. [DOI] [Google Scholar]
- Paust J.Technical Syntheses. In Carotenoids; Britton G.; Liaaen-Jensen S.; Pfander H., Eds.; Birkhauser: Basel, Switzerland, 1996; Vol. 2, pp 259–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.