Abstract
BACKGROUND:
IgE-mediated food allergy (FA) affects children and adults with variable age of onset. Phenotype and quality of life (QoL) differences between childhood-onset FA (COFA) and adult-onset FA (AOFA) are not known.
OBJECTIVE:
To identify phenotypic and QoL differences between AOFA and COFA.
METHODS:
A cross-sectional study of adults (≥18 years old) seen at Northwestern Memorial HealthCare clinics between 2002–2017 with an ICD-9/ICD-10 diagnosis of FA were identified. Subjects were completed a FA history survey and Food Allergy Quality of Life Questionnaire (FAQLQ). FA characteristics and QoL scores were compared between groups.
RESULTS:
Among 294 consented subjects, 202 had a clinical history consistent with labeled IgE-mediated FA. The onset of FA symptoms occurred before age 18 years (COFA) in 80 subjects, and after age 18 years in 122 (AOFA) subjects. Shellfish reactions were most common in AOFA labeled subjects (28%) while tree nut reactions were the most common in COFA labeled subjects (55%) compared to other triggers. Hives (68% vs. 52%, P=0.03), facial swelling (69% vs 50%, P=0.009), wheezing (56% vs 29%, P<0.001), and vomiting (41% vs 22%, P=0.005) were more commonly observed in COFA compared to AOFA. Total QoL was significantly reduced in COFA compared to AOFA (3.6 vs. 3.0, P=0.003) along with specific domains related to: allergen avoidance/dietary restriction (3.7 vs. 3.1, P=0.006), emotional impact (3.9 vs. 3.2, P=0.003), and risk of accidental exposure (3.6 vs. 2.8, P=0.001).
CONCLUSIONS:
There are differences in specific food triggers and symptoms in adultonset and childhood-onset labeled FA. Adults labeled with childhood-onset FA have reduced QoL.
Keywords: food allergy, childhood-onset food allergy, adult-onset food allergy, quality of life (QoL), food allergy quality of life questionnaire – adult form (FAQLQ-AF)
Introduction
Food allergy (FA) affects nearly 32 million Americans with variable age of onset.1, 2 While most children outgrow their food allergies, it persists into adulthood for many, particularly those allergic to tree nuts and/or peanut.3 A population-based study reported the prevalence of self-reported food allergy in U.S. adults at 10.8%.2 Among this group, nearly half had a childhood food allergy with an additional acquired food allergy as an adult, while a quarter in this group had first food allergy as an adult. Thus, food allergy in adults is an emerging health problem. Few studies have investigated the clinical profile of adult-onset food allergy. The studies performed have been small or based on self-reported data and lack confirmatory testing such as oral food challenges.2, 4 As such, adult food allergy remains incompletely understood.
FA negatively impacts the quality of life (QoL), and adults with food allergy have significantly reduced QoL compared to the general population and even those with chronic medical conditions, such as type I diabetes.5–9 However, most published reports on QoL from the U.S. in adults with FA are relatively old or have utilized generic health-related QoL questionnaires, that may poorly estimate food allergy disease-specific effects.10, 11 Thus, there is limited data regarding disease-specific QoL in U.S. adults with FA relative to the pediatric FA population. The Food Allergy Quality of Life Questionnaire – Adult Form (FAQLQ-AF) is a validated QoL measure for adults with FA.12 Using the questionnaire, Goossens et al. found that U.S. adults with FA had significantly reduced QoL compared to Dutch adults with FA.11 However, it is unclear whether the age of onset of FA impacts QoL. We sought to determine differences in clinical characteristics and QoL metrics between adults with histories consistent with IgE-mediated reactions labeled with adult-onset food allergy and childhood-onset food allergy.
Methods
Participants and Recruitment
This was a cross-sectional study of adults (age ≥18 years old) recruited through an electronic database of medical records of patients with an ICD.9 or ICD.10 code associated with food allergy between 2002 and 2017 (558.3, 693.1, 995.3, 708.0, 995.6X, V13.81, V15.05, T78.00, T78.0XX, T78.1X, Z91,02, Z91.01X). Patients identified through this database were contacted by e-mail once in July 2017 and, if no response, were contacted once more in September 2017. Each participant could only participate once by clicking on the survey link unique to them, completing an e-consent, and completing the food allergy questionnaire and FAQLQ-AF survey.
Subjects were excluded if (1) no food trigger was identified, (2) the survey indicated a diagnosis of pollen-food syndrome but no other IgE-mediated food allergy, (3) the food allergy questionnaire was not completed, or (4) the clinical history was inconsistent with food allergy as assessed by two allergists (second year Allergy and Immunology fellow and an allergist/immunology attending with >10 years of clinical and research experience in food allergy). Allergist assessment included ensuring subjects had marked a symptom provided in the questionnaire that suggested a food-induced allergic reaction per the 2010 NIAID-Sponsored Expert Panel Food Allergy Guidelines.13 Based on this, patients were then excluded if symptom(s) included (1) isolated lower gastrointestinal symptom such as nausea, abdominal pain, and diarrhea that did not include emesis, (2) isolated upper airway symptoms such as sneezing, runny nose, and congestion, (3) exclusive oral pruritus, (4) isolated symptoms not provided in the questionnaire, or (5) foods without reproducible symptoms on each exposure. Any disagreements between the two allergists on the inclusion of subjects were discussed with a conservative approach to ensure subjects with a history consistent with allergy were included. If consensus was not reached, the participant was excluded from the analysis. The electronic medical record of each subject was reviewed to obtain additional information including comorbidities. This study was approved by institutional IRB (STU00202033).
Food Allergy Quality of Life Questionnaire
The validated food allergy quality of life (QoL) questionnaire “Food Allergy Quality of Life Questionnaire – Adult Form” (FAQLQ-AF) was utilized for this study, as previously described.12 It was developed in the Netherlands and has been validated across several languages, including English.11 The questionnaire includes 29 items using a 7 point Likert scale (0 = not, 1 = barely, 2 = slightly, 3 = moderately, 4 = quite, 5 = very, 6 = extremely), grouped into four domains: 1) allergen avoidance and dietary restrictions (AADR), 2) risk of accidental exposure (RAE), 3) emotional impact (EI), and 4) food allergy-related health (FAH). A higher score indicates greater impairment in QoL. The mean score was calculated for each domain and a total mean score of all four domains. Additionally, subjects completed the Food Allergy Independent Measures (FAIM). FAIM is composed of four questions regarding the expectation of outcome (i.e., what will happen following food exposure) and two additional independent measures (perceived number of foods one needs to avoid and impact on social life).12
Food Allergy History Questionnaire
Demographics were collected as part of the food allergy questionnaire (eTable 1). Subjects were asked to identify the foods that he or she is allergic to from a list: tree nuts, peanut, soy, milk, egg, wheat, sesame seed, avocado, sunflower seed, shellfish (i.e., crustacean and mollusk), fish and other. The less common food triggers listed were previously identified in a previous published study that assessed characteristics of adult-onset food allergic reactions.4 For each allergen, the individual was asked to provide the age of diagnosis, age of symptom onset, type of symptom, timing and duration of symptoms, frequency of reactions, frequency of previous consumption, the form of food consumed, the current strategy of avoidance, ownership, and use of epinephrine auto-injector (EAI) and emergency departments (ED) visits. Of note, individual tree nuts, shellfish, or other allergy (i.e., not provided by our list) were counted as one trigger if the subject was allergic to more than one in the category.
Statistical Analysis
Categorical data were analyzed using Fisher’s exact test for variables with two choices; Chi-square test of homogeneity was utilized for a variable with three or more levels. Mann-Whitney U test was used to compare continuous variables. Kruskal Wallis Test with Dunn’s post-hoc was used to compare mean scores of the four domains of the FAQLQ-AF. Construct validity and internal consistency were confirmed using Spearman’s correlation coefficient and Cronbach’s alpha.11 Univariable regression was used to identify variables that predicted FAQLQ-AF scores. Variables significantly associated with total FAQLQ-AF (P< 0.05) were utilized in a multivariable analysis. Age at survey and sex were also included as potential confounders. Statistical analyses were performed using IBM SPSS software, version 25.0 (IBM Co., Armonk, NY, USA). All analyses were 2-tailed, with a P-value of less than 0.05 considered statistically significant.
Results
Study Population Characteristics
Through electronic medical record identification of food allergy diagnosis, 3,777 emails were sent, 294 respondents consented to participate, and 202 were appropriate for analysis after applying exclusion criteria (Figure 1). Respondents were categorized by the age of symptom onset: 1) onset <18 years old was defined as childhood-onset food allergy (that persisted into adulthood) (COFA, n=80), or 2) onset ≥18 years old was defined as adult-onset food allergy (AOFA, n=122). Demographic characteristics are summarized in Table I. The mean age [±SD] at enrollment of study for COFA was 41±11 years, and the mean age for AOFA was 46±14 years (P=0.01). Both groups were predominantly female (75%) and were most likely to identify as Caucasian (73%) with no difference between groups. Asthma was the only comorbid condition significantly different between groups and was higher in COFA (63% COFA vs. 42% AOFA, P <0.01). The mean age of FA symptom onset for COFA was 6.5 years compared to 32 years in AOFA. The median number of food allergen trigger groups identified was increased in COFA compared to AOFA (2 vs. 1, P=0.004).
Figure 1.
Study flow chart. Patient inclusion based on food allergy related International Classification of Diseases (ICD), Ninth and Tenth revision clinical diagnosis codes, and review of survey history by two allergists.
Table 1.
Demographic and co-morbid characteristics between adults with childhood-onset food allergy (COFA) and adult-onset food allergy (AOFA).
| Characteristic | Total (n= 202) | Childhood-onset(COFA) (n= 80) | Adult-onset(AOFA) (n= 122) | P-value3 |
|---|---|---|---|---|
| Age at survey (years, mean ±SD) | 41 (11) | 46 (14) | 0.01 | |
| Sex, male (%)/female (%) | 51 (25)/151(75) | 21(26)/59 (74) | 30 (25)/92 (75) | 0.87 |
| Race/Ethnicity, n (%) | 0.80 | |||
| White | 148 (73) | 62 (78) | 86 (71) | |
| Black | 30 (15) | 9 (11) | 21 (17) | |
| Asian | 8 (4) | 3 (4) | 5 (4) | |
| Hispanic | 14 (7) | 5 (6) | 9 (7) | |
| Other | 2 (1) | 1 (1) | 1 (1) | |
| Co-morbidities, n (%) | ||||
| Eczema | 79 (39) | 33 (41) | 46 (38) | 0.66 |
| Allergic rhino-conjunctivitis | 168 (83) | 70 (88) | 98 (80) | 0.25 |
| Asthma | 101 (50) | 50 (63) | 51 (42) | <0.01 |
| OAS1 | 7 (4) | 3 (4) | 4 (3) | 1.0 |
| EoE2 | 11 (5) | 6 (8) | 5 (4) | 0.35 |
| Age of first FA symptom (years, mean ±SD | 6.5 (5.6) | 32 (13) | <0.001 | |
| Number of food allergy groups, median (IQR) | 2 (1, 3) | 1 (1, 2) | 0.004 |
OAS – Oral Allergy Syndrome
EoE – Eosinophilic Esophagitis
Continuous variables assessed by Mann-Whitney U test; dichotomous variables assessed by Fisher’s exact test; three or more variables by Chi-square test. Significant values are bolded.
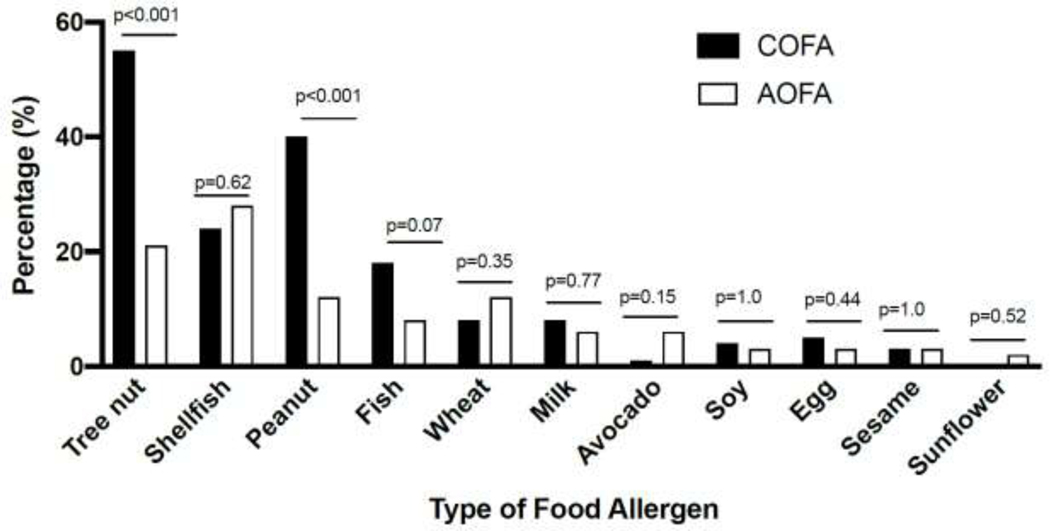
Food allergy clinical characteristics in COFA compared to AOFA
We assessed differences in the type of food allergy between the two groups. Compared to AOFA, COFA had an increased frequency of food allergy to tree nut (55% vs. 21%, P<0.001) and peanut (40% vs. 12%, P<0.001) (Figure 2). No other differences in common allergens were noted. The clinical presentation between COFA and AOFA were significantly different (Table 2). Typical organ system involvement for IgE-mediated food allergy was observed more commonly in COFA compared to AOFA including hives (68% vs. 52%, P=0.03), swelling of face, lips or tongue (69% vs. 50%, P<0.01), wheezing (56% vs. 28%, P<0.001), and vomiting (41% vs. 22%, P<0.01). In contrast, other symptoms were more prevalent in the AOFA group (15% vs. 30%, P<0.05). Other symptoms most commonly reported included itchy throat, pain in throat, and headache which were in addition to having symptoms consistent with IgE-mediated reactions. Patients with only non-classic IgE mediated symptoms were excluded.
Figure 2.
Greater QOL impairment in childhood-onset food allergy (COFA) compared to adult-onset food allergy (AOFA). Overall QOL mean score analyzed by Mann-Whitney-U-test; sub-domain mean scores by Kruskal-Wallis Test with Dunn’s post-hoc. Boxplot shows median with interquartile range.
Table 2.
Reported individual symptoms between childhood-onset food allergy (COFA) and adult-onset food allergy (AOFA).
| Symptom | Total (n=202) | COFA (n=80) | AOFA (n=122) | P-value1 |
|---|---|---|---|---|
| Cutaneous, n (%) | 119 (95) | 79 (99) | 112 (92) | 0.05 |
| Hives | 117 (58) | 54 (68) | 63 (52) | 0.03 |
| Skin itching | 117 (58) | 55 (69) | 62 (51) | 0.01 |
| Flushing | 80 (40) | 41 (51) | 39 (32) | 0.008 |
| Swelling of face, lips or tongue | 116 (57) | 55 (69) | 61 (50) | 0.009 |
| Rash | 41 (20) | 20 (25) | 21 (17) | 0.21 |
| Mouth itching | 93 (46) | 46 (58) | 47 (39) | 0.01 |
| Swelling of throat | 125 (62) | 58 (73) | 67 (55) | 0.01 |
| Lower respiratory n (%) | 131 (65) | 61 (76) | 70 (57) | 0.007 |
| Trouble breathing | 109 (54) | 56 (70) | 53 (43) | <0.001 |
| Cough | 50 (25) | 24 (30) | 26 (21) | 0.18 |
| Wheezing | 80 (40) | 45 (56) | 35 (29) | <0.001 |
| Upper respiratory, n (%) | 64 (32) | 31 (39) | 33 (27) | 0.09 |
| Sneezing | 25 (12) | 11 (14) | 14 (12) | 0.67 |
| Runny nose | 34 (17) | 17 (21) | 17 (14) | 0.18 |
| Congestion | 46 (23) | 26 (33) | 20 (16) | 0.01 |
| Eye tearing, itchy, redness, n (%) | 58 (29) | 31 (39) | 27 (22) | 0.02 |
| Gastrointestinal, n (%) | 105 (52) | 53 (66) | 52 (43) | 0.001 |
| Nausea | 67 (33) | 35 (44) | 32 (26) | 0.01 |
| Vomiting | 60 (30) | 33 (41) | 27 (22) | 0.005 |
| Diarrhea | 48 (24) | 21 (26) | 27 (22) | 0.50 |
| Stomach pain | 70 (35) | 38 (48) | 32 (26) | 0.002 |
| Cardiovascular, n (%) | 46 (23) | 20 (25) | 26 (21) | 0.61 |
| Lightheadedness | 42 (21) | 18 (23) | 24 (20) | 0.72 |
| Loss of consciousness, fainting | 12 (6) | 6 (8) | 6 (5) | 0.55 |
| Other, n (%) ❈ | 49 (24) | 12 (15) | 37 (30) | 0.02 |
Fisher’s exact test,
included itchy throat, pain in throat, numb mouth, migraine/headaches, sweats, mouthwatering, ears itch, acid reflux, sleepy, fatigue, sore joints. Significant values are bolded.
We next evaluated prescription, carriage, and use of epinephrine auto-injector (EAI) as well as ED visits. Prescription (88% vs. 75%, P<0.05) and use of EAI (50% vs. 25%, P=0.001) were observed more frequently in COFA (Table 3). However, there was no difference in carrying EAI (odds ratio (CI): COFA 1.94 (0.99–3.78) vs AOFA 0.52 (CI 0.26–1.01); P=0.07). Emergency department visits for food allergy were observed more commonly in COFA (80% vs. 62%, P<0.01).
Table 3.
Epinephrine and hospital use between childhood-onset food allergy (COFA) and adult-onset food allergy (AOFA)
| EAI1 and hospital use | Total (n=202) | COFA (n=80) | AOFA(n=122) | P-value2 |
|---|---|---|---|---|
| Prescribed EAI, n (%) | 162 (80) | 70 (88) | 92 (75) | <0.05 |
| Needed to use EAI, n (%) | 71 (35) | 40 (50) | 31 (25) | 0.001 |
| Carry EAI, n (%) | 105 (64) | 51 (73) | 54 (58) | 0.07 |
| Hospital/ED visit for severe reaction, n (%) | 139 (69) | 64 (80) | 75 (62) | 0.005 |
| If yes, how many times? median (IQR) | 2 (1,4) | 3 (2, 6) | 2 (1,3) | <0.001 |
EAI – epinephrine auto-injector
Continuous variables assessed by Mann-Whitney U test, Dichotomous variables by Fisher’s exact test. Significant values are bolded.
Validity
We confirmed construct validity, specifically convergent validity, between total and domain mean scores of FAQLQ-AF with the total FAIM score using Spearman’s correlation coefficient. Domains are categorized into questions related to allergen avoidance and dietary restriction (AADR), emotional impact (EI), risk of accidental exposure (RAE), and food allergy related health (FAH). There was a moderate to strong relationship between FAIM and each domain (n=187; AADR: r=0.62, P<0.001; EI: r=0.79, P<0.001; RAE: r=0.65, P<0.001; FAH: r=0.55, P<0.001) and total FAQLQ-AF score (r=0.74, P<0.001). The validity of our total score was similar to a previous study with a correlation coefficient of 0.76.12 Internal consistency was determined using Cronbach’s alpha with the expected goal to be alpha=0.70 indicating good internal consistency.7 Our total score was alpha=0.96 with the following alpha domain scores: AADR=0.94, EI=0.90, RAE=0.90, FAH=0.70, indicating high internal consistency. For comparison to the originally developed tool, the alpha reported for each included: total FAQLQ-AF=0.97, AADR = 0.95, EI=0.90, RAE=0.88, and FAH=0.77.12
Quality of Life (QoL) Findings
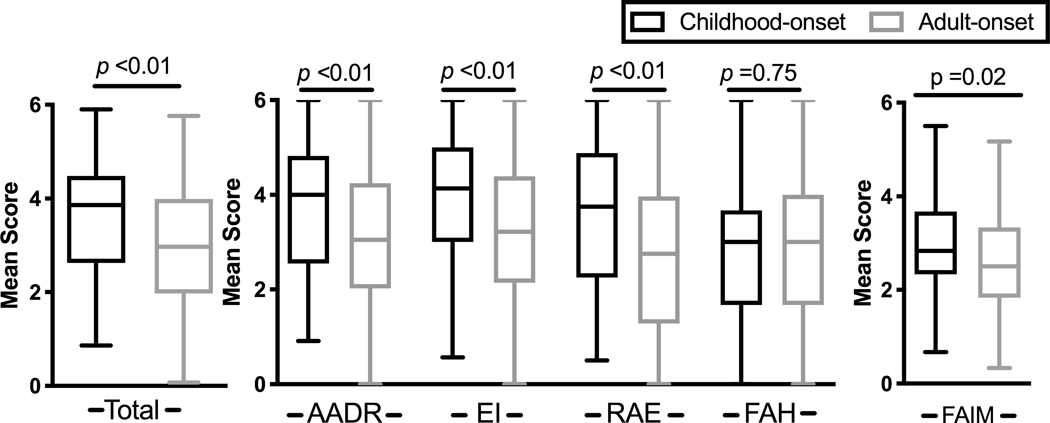
We investigated the differences in QoL between COFA and AOFA. Total burden of illness reflected by FAQLQ-AF mean score [±SD] was higher in COFA compared to AOFA (3.6 ±1.3 vs. 3.0 ±1.4, P=0.003) (Figure 3). There were also significant differences in sub-domains. COFA had higher scores in allergen avoidance and dietary restrictions (AADR, 3.7 ±1.4 vs. 3.1 ±1.5, P=0.006), emotional impact (EI, 3.9 ±1.3 vs. 3.2 ±1.5, P=0.003), and risk of accidental exposure (RAE, 3.6 ±1.6 vs. 2.8 ±1.6, P=0.001) compared to AOFA. The food allergy independent measure (FAIM) score was also higher in COFA compared to AOFA (3.0 ±1.0 vs. 2.6 ±1.1, P=0.018), although a difference of 0.5 between total and subdomain scores is considered clinically meaningful.14 There was no difference in food allergy related health (FAH) between groups (COFA 2.88±1.5 vs AOFA 2.96±1.6), P=0.75; small effect size of 0.05). Significant differences in responses to individual questions existed between AOFA and COFA (eTable 2).
Figure 3.
Greater quality of life (QOL) impairment in childhood-onset food allergy (COFA) compared to adult-onset food allergy (AOFA). Overall QOL mean score analyzed by Mann-Whitney-U-test; sub-domain mean scores by Kruskal-Wallis Test with Dunn’s post-hoc. Boxplot shows median with interquartile range. AADR, allergen avoidance and dietary restrictions; EI, Emotional impact; FAH, food allergy related health; FAIM, food allergy independent measure; RAE, risk of accidental exposure.
We identified variables that could predict FAQLQ-AF scores in an univariable analysis. The variables significantly associated with total FAQLQ-AF (P< 0.05) were then utilized in a multivariate analysis. In this final analysis, variables that significantly contributed to the FAQLQ-AF score included EAI use, allergic rhino-conjunctivitis, and age at survey (Table 4). This model adjusted for age at survey, sex, age of onset (COFA vs. AOFA), food triggers, symptoms, comorbidities, EAI, and emergency department use history.
Table 4.
Predictors for total Food Allergy Quality of Life Questionnaire (FAQLQ-AF) score
| Predictor | Standardized Beta | P-value | 95% CI for B |
|---|---|---|---|
|
| |||
| Needed to use epinephrine auto-injector | 0.27 | 0.001 | 0.30–1.19 |
| History of allergic rhino-conjunctivitis | 0.18 | 0.02 | 0.12–1.22 |
| Age (at survey) | 0.20 | 0.01 | 0.01–0.04 |
N=154; R=0.584, Model prediction of outcome: 34.1%; Significant values are bolded.
Discussion
In this study, we assessed clinical characteristics and quality of life of adults with labeled food allergy based on the age of initial symptoms. In COFA, we identified a more severe clinical history of food allergy associated with peanut and tree nut allergy, history of epinephrine auto-injector usage, and ED visits. We identified a novel reduction in quality of life in subjects labeled with childhood-onset compared to adult-onset food allergy. We observed reduced scores in allergen avoidance and dietary restrictions, emotional impact, and risk of accidental exposure in adults with childhood-onset compared to adult-onset food allergy. This novel finding has the potential to impact care significantly for emerging adults with food allergy as it stresses the importance of evaluating the quality of life and the need for early supportive care.
Several interesting characteristics were noted in our cohort. Adults with food allergy (i.e., both COFA and AOFA) were more commonly female. This is similar to findings reported by Kamdar et al. and Gupta et al.2, 4 This contrasts to epidemiological reports on food allergy in the pediatric population, in which there is a male predominance.15, 16 This sex difference may illustrate a potential role of sex hormones as a biological modifier in promoting persistent food allergy and adult-onset food allergy or may reflect female survey reporting bias.17 Also, COFA more likely had tree nut or peanut allergy. This may be because natural tolerance is less likely to these foods and these allergies often persist into adulthood.3 However, adults also self-report development of tree nut and peanut allergy.2, 4 This illustrates that more diverse foods are reported in AOFA, which may explain why there are lower peanut and tree nut allergy in this group. With regards to clinical manifestations, lower respiratory symptoms, gastrointestinal symptoms, hives and angioedema, and ocular symptoms were more likely in subjects with COFA compared to AOFA. Further investigation in understanding the immune mechanism driving this phenotypic difference is warranted.
A key strength of our study was the use of a validated QoL survey with excellent construct validity. Notably, the FAQLQ-AF measures the most important aspects of concern as perceived by food-allergic adults, and FAQLQ-AF is not confounded by comorbid conditions.12 Three of the four domains—allergy avoidance/dietary restrictions, emotional impact, and risk of accidental exposure—were significantly diminished in COFA compared to AOFA. These domains reflect different concerns, some of which may not be easily modifiable by the patient. For example, COFA had greater QoL impairment when eating outside foods due to inconsistent ingredient use in foods (Q20), incomplete labeling practices (Q21), and labels that contain the statement “traces of..” (Q23) compared to AOFA (eTable 2). This suggests differences in ingredients in seemingly similar foods and the uncertainty of ingredients in purchased foods, may provoke certain feelings of anxiety for subjects. Patients with COFA may have more anxiety due to difficulty managing dietary avoidance, which could be related to several factors. Factors may include patient education level, dietary exposure (i.e., grocery store options), manufacture labeling practices, and/or may reflect phenotypic differences (such as more severe clinical history, increased anxiety due to repeated messaging of risks) in COFA compared to AOFA.
Previous studies have found that the top three items in overall importance associated with impaired QoL for children (ages 8–12 years) and adolescents (ages 13–17 years) include i) Q1, always [having to] be alert to what you are eating ii) Q2, ability to eat few products, and iii) Q20, changing ingredients of a product (eTable 2).18, 19 In our study, the COFA cohort had a more negative impact on these three items than the AOFA cohort. A recent study by Miller et al., observed that QoL worsens with increasing age, as they found adolescents had significantly more negative impact from their food allergy compared to younger children.20 These data suggest impaired quality of life in certain domains for food-allergic children or adolescents may persist into adulthood. It is possible that children and adolescents with a persistent food allergy may benefit from more support and resources before transitioning into adulthood.
Exploration of the reasons for differences in QoL was beyond the scope of this article. However, our findings suggest that COFA have more indicators of severe disease. We found COFA subjects had had increased frequency of lower airway symptoms or multisystem involvement, and more epinephrine/ED use. These factors may drive negative quality of life, and we found the history of epinephrine auto-injector use and allergic rhino-conjunctivitis are associated with and predict a lower total QoL. The clinical significance of allergic rhino-conjunctivitis is not clear. Our multi-variate model suggests epinephrine use is associated with lower QoL, and COFA subjects had more epinephrine use. Our observation that clinical severity appears to be partly predictive of QoL suggests adults with a history of reaction requiring EAI may benefit from a multi-disciplinary approach to address the global impact of the disease. For example, those with history of EAI use may particularly benefit from screening for food allergy-related anxiety. If appropriate, these individuals can be referred to a mental health professional to help provide the skill sets needed to manage anxiety related to accidental ingestion and/or reactions. A nutritionist can work to provide further education on food restrictions and determine alternative food options. Another possible reason to explain differences in QoL could be the impact of an early food allergy diagnosis on their social development as a child or adolescent, although this was not explored in our study. The psychosocial effects of childhood FA are nicely described by Herbert and DunnGalvin.21 Food allergy diagnosis alters interaction with peers (e.g., sitting at an allergen-free table, eating different foods, etc.), which may affect QoL. In the home environment, parental protection regarding food allergy may create more anxiety.7 In a large study assessing QoL in parents of food-allergic children in the U.S. and Europe, U.S. caregivers had more impairment compared to European caregivers.9 This impairment could drive long-term anxiety in food-allergic children that was maintained into adulthood.
Our study has several limitations. Participation in this survey was voluntary and therefore, could lead to some selection bias as to who completed the survey. Only those with access to email were eligible for the study, which may represent a certain socioeconomic demographic of responders. The methodology of recruitment was chosen because it can limit cost and time, but it likely contributed to fewer survey responses as email addresses may be recorded incorrectly, outdated, or emails may be overlooked or deleted. This may be particularly true given the time frame of inclusion in the study. We also utilized broad inclusion criteria of physician-diagnosed food allergy or related ICD code diagnosis, which may have captured non-IgE mediated food hypersensitivity cases. Additionally, food allergy groups were not defined by double-blind, placebo-controlled oral food challenges. Nonetheless, rigorous exclusion criteria were used to minimize this. While diagnostic confirmation for IgE-mediated reactions is lacking in our study, our data supports that the label and perception of food allergy contribute significantly to reduced QOL. This suggests the importance to de-labeling inaccurate food allergies to potentially reduce food allergy related QoL consequences in both children and adults. Lastly, we did not include a survey question on the history of previous oral food challenges. This could affect QoL results as previous data has shown that undergoing oral food challenge procedures improve QoL regardless of results.22
In summary, we found clinical differences in AOFA vs. COFA. Additionally, this is the first study to assess QoL differences between childhood-onset (i.e., persistent) versus adult-onset food allergy. We found the quality of life is significantly more impaired in adults with childhood-onset compared to adult-onset food allergy. Adults with a childhood-onset food allergy may represent a distinct subgroup of adults with food allergy based on lower QoL metrics in addition to a more severe phenotype based on food triggers, lower airway or multisystem involvement, and comorbid asthma. Further studies are needed to determine if children with a persistent food allergy could benefit from transition programs that address the restrictions, limitations, and anxiety associated with their disease.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (K08DK097721 to JBW), National Institute of Allergy and Infectious Diseases (K23AI100995 to AMS), Northwestern University (anonymous grant #STU00200053 to AMS and PJB), National Institute of Health (T32AI083216 to GBP) and Ernest Bazley Foundation.
Disclosures: Paul Bryce is currently an employee of Sanofi-Genzyme but his involvement in this study is exclusively related to his employment at Northwestern University. Joshua Wechsler receives consulting fees from Allakos, Inc and serves (no fees) on the medical advisory board of Campaign Urging Research for Eosinophilic Diseases (CURED). The remaining authors have no relevant disclosures.
Abbreviations:
- AADR
Allergen avoidance and dietary restrictions
- AOFA
Adult-onset food allergy
- COFA
Childhood-onset food allergy
- ED
Emergency Department
- EI
Emotional impact
- EAI
Epinephrine auto-injector
- FA
Food allergy
- FAH
Food allergy related health
- FAIM
Food Allergy Independent Measure
- FAQLQ-AF
Food Allergy Quality of Life Questionnaire – Adult Form
- QoL
Quality of life
- HRQL
Health-related quality of life
- RAE
Risk of accidental exposure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. [DOI] [PubMed] [Google Scholar]
- 2.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw Open. 2019;2(1):e185630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savage J, Sicherer S, Wood R. The Natural History of Food Allergy. J Allergy Clin Immunol Pract. 2016;4(2):196–203; quiz 4. [DOI] [PubMed] [Google Scholar]
- 4.Kamdar TA, Peterson S, Lau CH, Saltoun CA, Gupta RS, Bryce PJ. Prevalence and characteristics of adult-onset food allergy. J Allergy Clin Immunol Pract. 2015;3(1):114–5.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleh-Langenberg J, Goossens NJ, Flokstra-de Blok BM, Kollen BJ, van der Meulen GN, Le TM, et al. Predictors of health-related quality of life of European food-allergic patients. Allergy. 2015;70(6):616–24. [DOI] [PubMed] [Google Scholar]
- 6.Flokstra-de Blok BM, Dubois AE, Vlieg-Boerstra BJ, Oude Elberink JN, Raat H, DunnGalvin A, et al. Health-related quality of life of food allergic patients: comparison with the general population and other diseases. Allergy. 2010;65(2):238–44. [DOI] [PubMed] [Google Scholar]
- 7.Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of Life Among Food Allergic Patients and Their Caregivers. Curr Allergy Asthma Rep. 2016;16(5):38. [DOI] [PubMed] [Google Scholar]
- 8.Goossens NJ, Flokstra-de Blok BM, van der Meulen GN, Arnlind MH, Asero R, Barreales L, et al. Health-related quality of life in food-allergic adults from eight European countries. Ann Allergy Asthma Immunol. 2014;113(1):63–8.e1. [DOI] [PubMed] [Google Scholar]
- 9.DunnGalvin A, Koman E, Raver E, Frome H, Adams M, Keena A, et al. An Examination of the Food Allergy Quality of Life Questionnaire Performance in a Countrywide American Sample of Children: Cross-Cultural Differences in Age and Impact in the United States and Europe. J Allergy Clin Immunol Pract. 2017;5(2):363–8.e2. [DOI] [PubMed] [Google Scholar]
- 10.Flokstra-de Blok BM, van der Velde JL, Vlieg-Boerstra BJ, Oude Elberink JN, DunnGalvin A, Hourihane JO, et al. Health-related quality of life of food allergic patients measured with generic and disease-specific questionnaires. Allergy. 2010;65(8):1031–8. [DOI] [PubMed] [Google Scholar]
- 11.Goossens NJ, Flokstra-de Blok BM, Vlieg-Boerstra BJ, Duiverman EJ, Weiss CC, Furlong TJ, et al. Online version of the food allergy quality of life questionnaire-adult form: validity, feasibility and cross-cultural comparison. Clin Exp Allergy. 2011;41(4):574–81. [DOI] [PubMed] [Google Scholar]
- 12.Flokstra-de Blok BM, van der Meulen GN, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, et al. Development and validation of the Food Allergy Quality of Life Questionnaire - Adult Form. Allergy. 2009;64(8):1209–17. [DOI] [PubMed] [Google Scholar]
- 13.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–15. [DOI] [PubMed] [Google Scholar]
- 15.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126(4):798–806.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Martinis M, Sirufo MM, Suppa M, Di Silvestre D, Ginaldi L. Sex and Gender Aspects for Patient Stratification in Allergy Prevention and Treatment. Int J Mol Sci. 2020;21(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly C, Gangur V. Sex Disparity in Food Allergy: Evidence from the PubMed Database. J Allergy (Cairo). 2009;2009:159845; 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flokstra-de Blok BM, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, Hourihane JO, et al. Development and validation of a self-administered Food Allergy Quality of Life Questionnaire for children. Clin Exp Allergy. 2009;39(1):127–37. [DOI] [PubMed] [Google Scholar]
- 19.Flokstra-de Blok BM, DunnGalvin A, Vlieg-Boerstra BJ, Oude Elberink JN, Duiverman EJ, Hourihane JO, et al. Development and validation of the self-administered Food Allergy Quality of Life Questionnaire for adolescents. J Allergy Clin Immunol. 2008;122(1):139–44, 44.e1–2. [DOI] [PubMed] [Google Scholar]
- 20.Miller J, Blackman AC, Wang HT, Anvari S, Joseph M, Davis CM, et al. Quality of life in food allergic children: Results from 174 quality-of-life patient questionnaires. Ann Allergy Asthma Immunol. 2020;124(4):379–84. [DOI] [PubMed] [Google Scholar]
- 21.Herbert L, DunnGalvin A. Psychotherapeutic Treatment for Psychosocial Concerns Related to Food Allergy: Current Treatment Approaches and Unmet Needs. J Allergy Clin Immunol Pract. 2021;9(1):101–8. [DOI] [PubMed] [Google Scholar]
- 22.van der Velde JL, Flokstra-de Blok BM, de Groot H, Oude-Elberink JN, Kerkhof M, Duiverman EJ, et al. Food allergy-related quality of life after double-blind, placebo-controlled food challenges in adults, adolescents, and children. J Allergy Clin Immunol. 2012;130(5):1136–43.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.