Abstract
Background
Prior studies have shown an association between myocardial injury after noncardiac surgery (MINS) and all‐cause mortality in patients following noncardiac surgery. However, the association between preoperative risk assessments, Revised Cardiac Risk Index and American College of Surgeons National Surgical Quality Improvement Program, and postoperative troponin elevations and long‐term mortality is unknown.
Methods and Results
A retrospective chart review identified 548 patients who had a troponin I level drawn within 14 days of noncardiac surgery that required an overnight hospital stay. Patients aged 40 to 80 years with at least 2 cardiovascular risk factors were included, while those with trauma, pulmonary embolism, and neurosurgery were excluded. Kaplan–Meier survival and odds ratio (OR) with sensitivity/specificity analysis were performed to assess the association between preoperative risk and postoperative troponin elevation and all‐cause mortality at 1 year. Overall, 69%/31% were classified as low‐risk/high‐risk per the Revised Cardiac Risk Index and 66%/34% per American College of Surgeons National Surgical Quality Improvement Program. Comparing the low‐risk versus high‐risk groups, preoperative risk assessment was not associated with either postoperative troponin elevation or 1‐year mortality. MINS portended a 1‐year mortality of OR, 3.9 (95% CI, 2.44–6.33) in the total population. Patients classified as low risk preoperatively with MINS had the highest risk of 1‐year mortality (OR, 9.6; 95% CI, 4.27–24.38), with a low prevalence of statin use.
Conclusions
Current preoperative risk stratification tools do not prognosticate the risk of postoperative troponin elevation and all‐cause mortality at 1 year. Interestingly, patients classified as low risk preoperatively with MINS had a markedly higher 1‐year mortality risk compared with the general population, and most of them are not taking a statin. Our results suggest that evaluating preoperatively low‐risk patients for MINS presents an opportunity for prognostication, risk reclassification, and initiating therapies such as statins to mitigate long‐term risk.
Keywords: all‐cause death, periprocedural myocardial infarction, risk stratification, secondary prevention, statin therapy, troponin
Nonstandard Abbreviations and Acronyms
- ACS NSQIP
American College of Surgeons National Surgical Quality Improvement Program
- MINS
myocardial injury after noncardiac surgery
- N0
low‐risk per American College of Surgeons National Surgical Quality Improvement Program
- N1
high‐risk per American College of Surgeons National Surgical Quality Improvement Program
- R0
low‐risk per Revised Cardiac Risk Index
- R0N0
low‐risk per Revised Cardiac Risk Index and American College of Surgeons National Surgical Quality Improvement Program
- R1
high‐risk per Revised Cardiac Risk Index
- RCRI
Revised Cardiac Risk Index
Clinical Perspective
What Is New?
Revised Cardiac Risk Index and American College of Surgeons National Surgical Quality Improvement Program preoperative risk stratification tools do not prognosticate the risk of postoperative troponin elevation and all‐cause mortality at 1 year.
Postoperative troponin elevation in patients classified as low‐risk preoperatively by Revised Cardiac Risk Index and American College of Surgeons National Surgical Quality Improvement Program portended a markedly increased 1‐year mortality compared with the general population (odds ratio, 10 versus 4).
This preoperatively low‐risk population with myocardial injury after noncardiac surgery had a low prevalence of statin use.
What Are the Clinical Implications?
Perioperative troponin screening might be an important opportunity for long‐term risk restratification and prognostication, even in preoperatively “low‐risk” patients if they have cardiovascular risk factors.
Risk mitigation strategies in this low‐risk population with myocardial injury after noncardiac surgery warrant further investigation and might include increased use of statins and antithrombotics.
Myocardial injury in asymptomatic patients is common after noncardiac surgery, estimated to occur in ≈12% to 20% of patients up to 1 week postoperatively and 27 million patients globally on an annual basis.1, 2, 3 Several studies have demonstrated poor outcomes for patients with myocardial injury after noncardiac surgery (MINS) detected by troponin elevations. One of those major studies, VISION (Vascular Events in Noncardiac Surgery Cohort Evaluation), demonstrated that among 15 000 patients, troponin T elevation within 3 days after surgery was strongly associated with a 2.4‐ to 10.5‐fold rise in all‐cause mortality at 30‐days.1 Additional studies,4, 5, 6, 7 including a meta‐analysis,8 have supported this idea that even a mild elevation in cardiac biomarkers (creatine kinase myocardial band, troponin I, or troponin T) after noncardiac surgery independently portends a high mortality risk (odds ratio [OR], 3.4) within the first year after surgery.
Current preoperative risk stratification tools are designed to predict the risk of major adverse cardiac events 30 days postoperatively and have been shown to be moderately accurate.9, 10 A large systemic (systematic) review of the Revised Cardiac Risk Index (RCRI) demonstrated only a moderate discrimination (sensitivity, 0.65; specificity, 0.76) of cardiac events within 30 days after surgery between patients classified as low versus high risk preoperatively.9 Interestingly, high‐risk RCRI did not correlate with risk of mortality, especially in patients who underwent vascular noncardiac surgery.
Currently, evaluating a troponin after noncardiac surgery is a grade I recommendation only if signs and symptoms of myocardial ischemia are present.11 The utility and role of routine postoperative troponin screening is thought to be uncertain even in high‐risk patients, and current guidelines advise a grade IIb recommendation for routine screening among patients deemed to be high‐risk without signs and symptoms of ischemia.11 Recently, there is a growing interest in investigating strategies to mitigate the cardiovascular risk in these patients12 and trends toward improved outcomes have been suggested with the use of aspirin, statin and antithrombotics in patients with MINS.
However, the correlation between preoperative risk assessments and postoperative troponin elevations and long‐term mortality is unknown. We sought to determine if postoperative troponin has prognostic value for the preoperatively determined “low‐risk” group as this represents the group that is less likely to have comprehensive cardiac testing or to be on medical therapy for known cardiovascular disease. We suspect that this low‐risk group would benefit from postoperative troponin screening to identify those at risk for major adverse cardiac events that would require further cardiac evaluation to mitigate risk.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. Our study is a single‐center retrospective chart review analysis of patients who underwent major noncardiac surgery from 2011 to 2016 who had a postoperative troponin drawn at the MetroHealth Medical Center. All patients entered in this study had a troponin drawn within 14 days after surgery. The assay that was used in our institution during the period of the study was the Beckman Coulter Access AccuTnI+3 Troponin I test kit. Patients were determined to have a positive troponin elevation if they had a troponin I level of 2 times the upper limit of normal (>0.03 ng/mL) as determined by our laboratory, which in our study is >0.06 ng/mL.
Patients were eligible for the study if they met the following inclusion criteria: underwent noncardiac surgery requiring an overnight hospital stay, a postoperative troponin I drawn within 14 days of the index surgery, aged 40 to 80 years, and those determined to be at risk for cardiovascular disease. Risk for cardiovascular disease was defined as either at least 1 major criterion: history of documented coronary artery disease, diabetes mellitus (type 1 or 2), peripheral arterial disease (documented by ankle‐brachial index), ischemic stroke, current smoker; or 2 minor criteria: hypertension, hyperlipidemia (low‐density lipoprotein >160 mg/dL) or current statin use, prior smoking history in the past decade, renal insufficiency (estimated glomerular filtration rate <59 mL/min). Exclusion criteria included pulmonary embolism, traumatic injury involving the chest cavity, or those who underwent neurosurgical intervention. Further details for patient enrollment can be found in the Standards for Reporting of Diagnostic Accuracy Studies guidelines flowchart shown in Figure S1.
The primary end point of this study was time to all‐cause mortality up to 1 year after surgery. All patient information was collected through retrospective chart review. Institutional Review Board approval was obtained for this study and is in accordance with the American Heart Associaton guidelines. Per the Institutional Review Board approval, only deidentified patient data were used in this study and did not require patient consent. The Ohio Department of Health provided official death records for those patients who were lost to follow‐up. This minimized the proportion of patients lost to follow‐up to only 3 patients overall.
Statistical Analysis
Individual American College of Surgeons National Surgical Quality Improvement Program (NSQIP) and RCRI preoperative risk scores were calculated for each patient. Kaplan‐Meier survival analysis and the log‐rank test was used to compare all‐cause mortality over a year between different preoperative risk groups and patients with and without postoperative troponin elevation. ORs were calculated, and sensitivity/specificity analyses was performed to assess the discrimination ability between preoperative risk and postoperative troponin elevation for all‐cause mortality at 1 year. We further evaluated, using these same methods in stratified analyses, the differential prognostic value of postoperative troponin elevation across all the combinations of preoperative risk classification groups as determined by the 2 tools toward all‐cause mortality at 1 year.
RESULTS
Our retrospective chart review identified 548 patients who met the inclusion and exclusion criteria for the study. A total of 211 patients were noted to have a troponin elevation, while 337 patients did not have a troponin elevation. As seen in Table 1, the most common surgical procedures for all patients were general (24%), orthopedic (22%), and vascular (18%). ECGs were obtained in >97% of the patients with troponin elevation. Less than 2% of patients had ST elevation, ≈30% of patients had ST segment or T‐wave changes consistent with ischemia, and ≈65% of the patients did not have ischemic changes (Table S1). While cardiology service was consulted for a majority of these patients, only 11% (n=24) were thought to need coronary angiography, of which only a total of 8 patients required coronary revascularization (Table S2).
Table 1.
Baseline Patient Characteristics and the Distribution of Surgery Types Within the Total Population in the Study
| Baseline Patient Characteristics in the Study | ||
|---|---|---|
| Total Population | ||
| Number | Percentage | |
| Total | 548 | |
| Age, y (mean) | 64.05 | |
| Men | 303 | 55.29 |
| Women | 245 | 44.71 |
| Hypertension | 419 | 76.46 |
| Known CAD | 173 | 31.57 |
| Smoker | 228 | 41.61 |
| PVD | 52 | 9.49 |
| CVA | 49 | 8.94 |
| Hyperlipidemia | 291 | 53.10 |
| DM | 200 | 36.50 |
| CKD (GFR <59) | 29 | 5.29 |
| 1‐y mortality | 85 | 15.51 |
| Distribution of Surgery Types Within the Total Population of the Study | |
|---|---|
| Surgery Type | % of Total Population |
| Ortho | 22.63 |
| General surgery | 24.64 |
| Vascular | 17.88 |
| Spinal | 14.78 |
| Ear, nose and throat | 4.20 |
| Other | 16.42 |
CAD indicates coronary artery disease; CKD, chronic kidney disease; CVA, cerebrovascular accident; DM, diabetes mellitus; GFR, glomerular filtration rate; and PVD, peripheral vascular disease.
Table 1 shows the baseline characteristics of the patients within the population in this study. Our study population had fair representation of female patients, about three‐fourths had hypertension, about one‐half were smokers or had hyperlipidemia, but only one‐third of the patients had diabetes mellitus or known coronary artery disease. The majority of patients did not have chronic kidney disease. The overall all‐cause mortality at 1 year in this study population was 16%.
Table 2 shows the characteristics of these patients when the total population is subdivided into patients with and without postoperative troponin elevation. Between the 2 groups, there is a comparable proportion of female patients and patients with coronary artery disease, cerebrovascular accident, diabetes mellitus, and chronic kidney disease. The positive troponin group had fewer patients with hypertension (12.6%), hyperlipidemia (19.3%), and smokers (9%). The positive troponin group had a higher proportion of patients with a known history of peripheral vascular disease (10.8%) and a significantly high 1‐year mortality (20.24%) compared with the group of patients without postoperative troponin elevation. This significant difference (OR, 3.9; 95% CI, 2.44–6.33) in long‐term mortality between patients with and without postoperative troponin elevation is seen early (within the first 3–6 months) in Figure with a persistent separation of the survival curves.
Table 2.
Comparing Baseline Characteristics of Patients With and Without Postoperative Troponin Elevation Within the Total Population
| Negative Troponin | Positive Troponin | P Value | |||
|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | ||
| Total | 337 | 61.50 | 211 | 38.50 | |
| Age, y (mean) | 64.18 | 63.85 | 0.70 | ||
| Men | 183 | 54.30 | 120 | 56.87 | 0.56 |
| Women | 154 | 45.70 | 91 | 43.13 | 0.56 |
| Hypertension | 274 | 81.31 | 145 | 68.72 | 0.73 E‐03* |
| CAD | 103 | 30.56 | 70 | 33.18 | 0.52 |
| Smoker | 152 | 45.10 | 76 | 36.02 | 0.04* |
| PVD | 18 | 5.34 | 34 | 16.11 | 2.82E‐05* |
| CVA | 30 | 8.90 | 19 | 9.00 | 0.97 |
| Hyperlipidemia | 204 | 60.53 | 87 | 41.23 | 1.05E‐05* |
| DM | 127 | 37.69 | 73 | 34.60 | 0.46 |
| CKD | 15 | 4.45 | 14 | 6.64 | 0.27 |
| 1‐y mortality | 26 | 7.72 | 59 | 27.96 | 1.88E‐10* |
Significance was determined using t test for continuous measures and χ2 test for binary measures. CAD indicates known coronary artery disease; CKD, chronic kidney disease; CVA, cerebrovascular accident; DM, diabetes mellitus; and PVD, peripheral vascular disease.
Statistically signficant (P<0.05).
Figure 1. Kaplan–Meier survival curves for patients with and without postoperative troponin elevation.
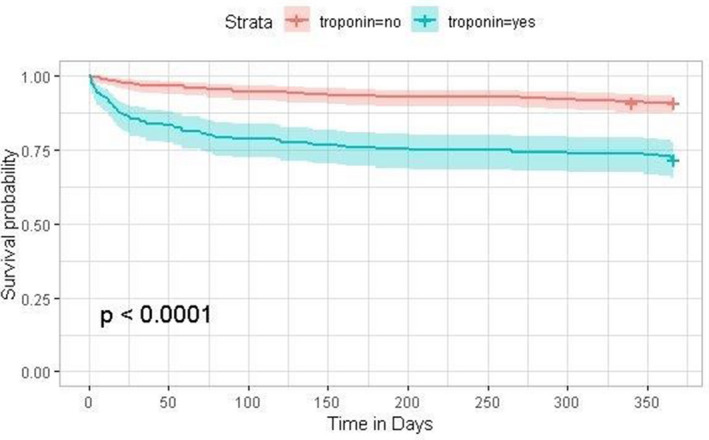
Consistent with previous studies such as VISION, Table S3 shows that an elevated troponin portends an ≈4 times higher risk of 1‐year mortality (OR, 3.9, 95% CI, 2.44–6.33) with moderate sensitivity (0.66) and specificity (0.67). Higher preoperative risk per either risk stratification tool (RCRI or NSQIP) did not portend a significantly higher 1‐year mortality.
Evaluating the association between RCRI and NSQIP and postoperative troponin elevations, Table S4 reveals that both risk stratification tools had poor sensitivity and did not correlate with postoperative troponin elevations.
Having shown that preoperative risk tools do not correlate with long‐term mortality or postoperative troponin elevation independently, we assessed whether there is differential prognostic value of postoperative troponin elevation across the risk groups (stratified by preoperative risk tools) toward long‐term mortality.
Table 3 shows the study population subdivided into the multiple risk groups. R0 is low risk per RCRI, and R1 is high risk per RCRI. N0 is low risk per NSQIP, and N1 is high risk per NSQIP. R0N0 corresponds to the group that is low risk per both RCRI and NSQIP. A majority of patients in our study were preoperatively classified as low risk (R0, 69% by RCRI; N0, 66% by NSQIP; R0N0, 52% per both RCRI and NSQIP). About one‐third of the population was classified as high‐risk preoperatively either by RCRI (R1) or NSQIP (N1) and only a small proportion (high‐risk per RCRI and NSQIP, 16% or 90 patients) were classified as high risk by both RCRI and NSQIP. A similar proportion of patients (≈40%) had postoperative troponin elevations in both low‐ and high‐risk groups regardless of the risk stratification tool. Though not significant, there is a trend toward higher mortality in the higher‐risk groups for each risk tool (R0 versus R1 and N0 versus N1). Compared with the low‐risk groups, there was a significantly higher mortality (23% versus 13%) noted in the group that was preoperatively classified as high risk by both RCRI and NSQIP.
Table 3.
Postoperative Troponin Elevation and 1‐Year Mortality Based on Preoperative Risk Stratification Subclasses
| Total: 548 | High Troponin | 1‐y Mortality | ||||
|---|---|---|---|---|---|---|
| Number | % of Total Population | Number | % Within Group | Number | % Within Group | |
| R0 | 380 | 69.34 | 141 | 37.11 | 52 | 13.68 |
| R1 | 168 | 30.66 | 70 | 41.67 | 33 | 19.64 |
| N0 | 362 | 66.06 | 151 | 41.71 | 50 | 13.81 |
| N1 | 186 | 33.94 | 60 | 32.26 | 35 | 18.82 |
| R0N0 | 284 | 51.82 | 114 | 40.14 | 38 | 13.38 |
| R0N1 | 96 | 17.52 | 27 | 28.13 | 14 | 14.58 |
| R1N0 | 78 | 14.23 | 37 | 47.44 | 12 | 15.38 |
| R1N1 | 90 | 16.42 | 33 | 36.67 | 21 | 23.33 |
N0 indicates American College of Surgeons National Surgical Quality Improvement Program (NSQIP) low risk; N1, NSQIP high risk; R0, Revised Cardiac Risk Index (RCRI) low risk; and R1, RCRI high risk. When used in combination, the values of both scales are indicated.
Table 4 shows that even in patients classified as low risk preoperatively by either or both risk tools, postoperative troponin elevation is associated with an increased risk of long‐term mortality. In low‐risk patients as classified by RCRI and NSQIP individually (R0 and N0), there was 7.6 times (95% CI, 3.98–15.22) and 6.7 times (95% CI, 3.4–14.12) risk of 1‐year mortality in patients with postoperative troponin elevation compared with those without. In patients classified as low risk by both risk stratification tools (R0N0), this risk was the highest at 9.6 times (95% CI, 4.27–24.38). Importantly, this significant discrimination of mortality by troponin in these low‐risk groups came with moderate specificity and a strong sensitivity: 0.76 in R0 and 0.79 in N0 that was further amplified to 0.83 in the R0N0 group (low risk by both risk tools).
Table 4.
Association Between Preoperative Risk Stratification Subclasses
| 1‐y Mortality: With MINS vs Without | Sensitivity | Specificity | ||
|---|---|---|---|---|
| OR | 95% CI | |||
| R0 | 7.55 | 3.98–15.22 | 0.76 | 0.70 |
| R1 | 1.43 | 0.67–3.02 | 0.49 | 0.60 |
| N0 | 6.67 | 3.40–14.12 | 0.79 | 0.64 |
| N1 | 2.42 | 1.16–5.03 | 0.47 | 0.73 |
| R0N0 | 9.55 | 4.27–24.38 | 0.83 | 0.67 |
| R0N1 | 5.50 | 1.76–18.42 | 0.60 | 0.79 |
| R1N0 | 2.28 | 0.65–9.26 | 0.67 | 0.53 |
| R1N1 | 1.26 | 0.47–3.30 | 0.39 | 0.66 |
N0 indicates American College of Surgeons National Surgical Quality Improvement Program (NSQIP) low risk; N1, NSQIP high risk; R0, Revised Cardiac Risk Index (RCRI) low risk; and R1, RCRI high risk. When used in combination, the values of both scales are indicated.
We further investigated how the risk stratification changes for different factor increases of troponin above upper limit of normal (1×, 2×, 3×) for our primary study groups (the low‐preoperative‐risk groups). Table S5 suggests that the high mortality risk in the low‐preoperative‐risk group is associated with any MINS (even when the troponin elevation threshold is 1× upper limit of normal) and persists with higher troponin elevations. This persistent trend observed across multiple thresholds adds further confidence in our results.
Table 5 shows the prevalence of aspirin and statin use in the preoperatively low‐risk groups. Among all patients classified as low preoperative risk by RCRI (R0), NSQIP (N0), or both (R0N0), about 60% of patients were taking aspirin, about 55% were taking a statin, of which ≈20% were taking a high‐intensity statin. This prevalence was similar whether or not the patient had MINS.
Table 5.
Prevalence of Aspirin and Statin Use in the Preoperatively Low‐Risk Groups
| Total Population | Positive Troponin | |||||
|---|---|---|---|---|---|---|
| Aspirin, n (%) | Low‐Moderate‐Intensity Statin, n (%) | High‐Intensity Statin, n (%) | Aspirin, n (%) | Low‐Moderate‐Intensity Statin, n (%) | High‐Intensity Statin, n (%) | |
| R0 | 301 (58) | 130 (34) | 68 (18) | 86 (60) | 49 (34) | 28 (20) |
| N0 | 214 (60) | 124 (35) | 85 (24) | 95 (71) | 54 (35) | 35 (23) |
| R0N0 | 156 (55) | 96 (34) | 58 (20) | 66 (58) | 96 (34) | 22 (19) |
N0 indicates American College of Surgeons National Surgical Quality Improvement Program (NSQIP) low risk; N1, NSQIP high risk; R0, Revised Cardiac Risk Index (RCRI) low risk; and R1, RCRI high risk. When used in combination, the values of both scales are indicated.
Table 6 shows that comparing the cause of 1‐year mortality, patients with a postoperative troponin elevation were noted to have 5.8 times (P<0.001) increased incidence of cardiovascular mortality compared with patients without a troponin elevation. There was no significant difference between the 2 populations for malignancy or respiratory‐related deaths.
Table 6.
Cause of Death for Patients With 1‐Year Mortality as Reported on Their Death Certificates for Patients With and Without Postoperative Troponin Elevation
| Cause of Death | Negative Troponin Group | Positive Troponin Group | P Value | ||
|---|---|---|---|---|---|
| Known: 25 | Unknown: 1 | Known: 57 | Unknown: 2 | ||
| Number | % of Total Population | Number | % of Total Population | ||
| Cardiac/Vascular | 5 | 1.48 | 18 | 8.53 | <0.001* |
| Malignancy | 12 | 3.56 | 11 | 5.21 | 0.347 |
| Respiratory | 1 | 0.30 | 4 | 1.90 | 0.055 |
| Other | 7 | 2.08 | 25 | 11.85 | 0.047* |
Significance was determined using t‐test for continuous measures and χ2 test for binary measures.
Statistically significant (P<0.05).
DISCUSSION
The relationship between postoperative myocardial injury in the general population and mortality has been established in the current literature. In our study, our population was purposefully enriched with patients with known cardiovascular risk factors as reflected in the baseline characteristics in Table 1. As expected, there was a high (38%) prevalence of postoperative troponin elevations and all‐cause mortality at 1 year (16%) in this patient population. Interestingly, there was no meaningfully significant difference in the prevalence of cardiovascular risk factors between patients who had a postoperative troponin elevation and those who did not, as seen in Table 2. This suggests that while cardiovascular risk factors might be useful to screen patients at a higher risk for postoperative myocardial injury and mortality, these risk factors by themselves do not adequately identify the patients specifically at risk for MINS or long‐term mortality.
To further test the characteristics of our study population for generalizability to other studies, we assessed the association between MINS and mortality. Consistent with previous reports, our Kaplan–Meier survival curves in Figure clearly show that the MINS group had increased mortality, with separation of the curves within 30 days that stabilized after 6 months. Also consistent with previous reports, OR analysis of our study population revealed that postoperative troponin elevation portends a 4 times higher risk of all‐cause mortality at 1 year with moderate sensitivity and specificity (Table S3).
We then assessed whether NSQIP and RCRI correlate with MINS and 1‐year mortality. Tables S3 and S4 show that low versus high risk as determined by both RCRI and NSQIP preoperative risk tools did not portend a meaningful change in MINS or 1‐year mortality risk. In addition, while there was a moderate association between high‐risk patients, MINS, and 1‐year mortality, their sensitivity was noted to be poor.
We then assessed the correlation between troponin elevation and 1‐year mortality among all possible combinations of preoperative risk scores as classified by the 2 risk stratification tools. More than two‐thirds of the study population were classified as low risk preoperatively by either RCRI or NSQIP (R0 or N0), and about one‐half of the patients were classified as low risk by both RCRI and NSQIP (R0N0). A similar prevalence of postoperative troponin elevation was found in both the low‐ and high‐risk groups (RCRI and NSQIP). While there was a trend toward increased 1‐year mortality for all groups with MINS, only the high‐preoperative‐risk group (RCRI and NSQIP) was found to have a statistically significant increase in 1‐year mortality (10%) compared with the low‐risk group (RCRI and NSQIP).
Our results reveal that postoperative troponin elevation is associated with an increased risk of 1‐year mortality across all preoperative risk groups. Interestingly, in patients classified as low risk by both RCRI and NSQIP (R0N0), which was roughly one‐half of our study population, postoperative troponin elevation was associated with a 10 times higher risk of 1‐year mortality (compared with 4 times in the overall study population), with an improved sensitivity (0.83 versus 0.66) while still maintaining moderate specificity. Even if both scores are not used, patients classified as low risk per a single score (R0), which represented 69% of our patients, still revealed that postoperative troponin portended a marked rise in 1‐year mortality with increased sensitivity and specificity in this low‐risk group compared with the overall study population. Interestingly, this increased risk in the low preoperative risk group persisted at multiple troponin thresholds, including at the border of the upper limit of normal in our troponin I assay, suggesting that even small troponin elevations in this group are quite prognostic.
This is an important and nonintuitive finding because this preoperatively “low‐risk” group, which constitutes the majority of the patients undergoing noncardiac surgery, is shown to have highest risk of long‐term mortality with MINS. While classified as “low risk” preoperatively, these patients are noted to have several risk factors for cardiovascular disease. This suggests that our current preoperative risk stratification tools such as RCRI and NSQIP do not adequately capture the mortality risk specifically in the long term. There is then a need for improving risk stratification tools specifically for predicting long‐term mortality in these patients, and such a tool will likely benefit from using postoperative troponin for discrimination of risk.
We suspect this higher risk of mortality in the preoperatively “low‐risk” population is attributable to the unexplained postoperative troponin elevation in patients with likely untreated cardiovascular risk factors. Patients classified as high risk have known reasons for postoperative troponin elevation that are likely to have been medically intervened upon or optimized. This is to be compared with the heterogeneous patient population classified as low risk, many of whom have not had consistent prior medical care with likely unknown and untreated cardiovascular risk factors. The risk factors are not adequately captured when assessing for cardiovascular risk in the immediate perioperative period and patients are appropriately classified as “low‐risk” preoperatively, as the postoperative myocardial injury in the vast majority of these patients would not have been known if it were not for the troponin evaluation. Hence, MINS presents an efficient opportunity to reassess and reclassify the overall risks for these patients moving forward.
There is now growing interest toward various strategies to mitigate risk for major adverse cardiovascular events particularly in this population with MINS.12 While we do not know the underlying pathological mechanism of MINS in our population, prior studies with coronary angiography in patients with perioperative MINS suggest that the demand ischemia is the primary cause, especially in nonfatal myocardial infarction.13, 14, 15 Hence, it is no surprise that routine angiography and percutaneous coronary intervention of these patients did not prove to provide benefit.16 Instead, efforts to mitigating this risk might be best suited toward secondary prevention. Trials such as MANAGE (Management of Myocardial Injury After Noncardiac Surgery) suggest a trend to improving outcomes with antithrombotic therapy and have also highlighted the poor use of primary prevention strategies such as aspirin and statin in these patients. These preoperatively “low‐risk” patients with MINS likely stand to benefit from aggressive risk factor modifications through additional evaluations and therapies.
A meta‐analysis by Chopra et al17 and a prospective study by Feringa et al18 suggest that perioperative statin use, particularly at higher intensity, might be beneficial. Data regarding aspirin use is less clear as POISE‐2 (Perioperative Ischemic Evaluation 2)19 showed that perioperative aspirin use did not provide benefit at 30 days, but long‐term outcomes are unclear. In our population, evaluating the prevalence of aspirin and statin use in preoperatively low‐risk patients with MINS, we noted that while 60% were taking aspirin, about 45% of patients were not taking a statin and 80% were not taking a high‐intensity statin. While this suggests that statin use might present an opportunity for risk mitigation, we do not have evidence for statins to have benefit in this population toward mortality. Hence, postoperative troponin screening of patients with cardiovascular risk factors, especially if preoperative risk is low, might present an underused opportunity to identify patients with likely low stain prevalence with a high risk of 1‐year mortality.
CONCLUSIONS
Our work adds to the growing body of evidence that patients with MINS have clinically significant long‐term all‐cause mortality. In addition, our study demonstrates that currently used preoperative risk assessment tools, RCRI and NSQIP, were poorly associated with MINS and 1‐year mortality. Our results do, however, show that compared with the general population, postoperative troponin elevation in patients classified as low risk preoperatively by RCRI and NSQIP portended a markedly increased 1‐year mortality (10 times versus 4 times) with improved sensitivity of 0.83 and moderate specificity. These findings persisted at multiple abnormal troponin thresholds consistent with the theme that any abnormal troponin elevation in this “low‐risk” group might be important for screening.
Additionally, we noted that this preoperatively low‐risk population has a low prevalence of statin use, suggesting a potential avenue for risk mitigation. These results support the use of postoperative troponin screening in patients with the appropriate cardiovascular risk factors, even if the patients are determined to be low risk preoperatively, to better identify patients with an elevated risk of mortality who might best benefit from further evaluation and risk mitigation strategies.
Future Goals
Our work suggests the need for prospective trials that evaluate the role of routine postoperative MINS screening (including high‐sensitivity assays), even in preoperatively low‐risk patients if they have cardiovascular risk factors, toward mortality risk stratification and potential risk mitigation. In addition to the all‐cause mortality, a more rigorous evaluation of cardiac mortality, both in the short and the long term, is warranted to better assess if risk mitigation strategies including aspirin, statin, antithrombotics, and/or further ischemia evaluations and coronary interventions improve hard outcomes in these patients.
Limitations
Our study retrospectively evaluated all‐cause mortality in patients who had a postoperative troponin obtained by the primary teams after noncardiac surgery (for multiple reasons but the most common included hypotension, altered mental status, chest symptoms, and ECG or telemetry changes). Given that troponins were obtained for specific clinical indications, and both elective and urgent surgeries were enrolled, our study might represent a higher‐risk population than otherwise would be in a completely asymptomatic low‐risk group. Additionally, we acknowledge that we do not have preoperative baseline troponins to compare, and hence we cannot define confidently the underlying mechanism of myocardial infarction versus injury in our study population.
As a safety net hospital, we suspect that our patient population likely contains a high prevalence of patients without consistent prior medical care with likely unknown and untreated risk factors. These patients might be appropriately classified as low preoperative risk for the surgery using current preoperative risk stratification strategies but might be high risk for MINS and long‐term mortality, which might have amplified the mortality risk in the low‐risk group. Additionally, although several noncardiac etiologies for troponin elevation were excluded, not every cause could be excluded, and the proportion of cardiac etiology for the mortality noted is unknown.
Sources of Funding
This work was supported in part by grants from American Heart Association’s Clinical Scientist Training Program (17CPOST33650005) to Dr Vasireddi and NIH National Center for Advancing Translational Sciences (UL1 TR002548) to Dr Gunzler.
Disclosures
None.
Supporting information
Tables S1–S5
Figure S1
Acknowledgments
We thank the Ohio Department of Health for providing mortality information for patients lost to follow‐up, and the MetroHealth Institutional Review Board office for their guidance.
(J Am Heart Assoc. 2021;10:e019379. DOI: 10.1161/JAHA.120.019379.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019379
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1.Devereaux PJ, Chan MT, Alonso‐Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, et al. Association between postoperative troponin levels and 30‐day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. DOI: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 2.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009;119:2396–2444. DOI: 10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 3.Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, Weissman C, Mosseri M. Association of cardiac troponin, CK‐MB, and postoperative myocardial ischemia with long‐term survival after major vascular surgery. J Am Coll Cardiol. 2003;42:1547–1554. DOI: 10.1016/j.jacc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Kim LJ, Martinez EA, Faraday N, Dorman T, Fleisher LA, Perler BA, Williams GM, Chan D, Pronovost PJ. Cardiac troponin I predicts short‐term mortality in vascular surgery patients. Circulation. 2002;106:2366–2371. DOI: 10.1161/01.CIR.0000036016.52396.BB. [DOI] [PubMed] [Google Scholar]
- 5.Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol. 1996;57:37–44. DOI: 10.1016/S0167-5273(96)02769-6. [DOI] [PubMed] [Google Scholar]
- 6.Writing Committee for the VISION Study Investigators ; Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MTV, Srinathan SK, Walsh M, Abraham V, Pearse R, et al. Association of postoperative high‐sensitivity troponin levels with myocardial injury and 30‐day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317:1642–1651. DOI: 10.1001/jama.2017.4360. [DOI] [PubMed] [Google Scholar]
- 7.Croal BL, Hillis GS, Gibson PH, Fazal MT, El‐Shafei H, Gibson G, Jeffrey RR, Buchan KG, West D, Cuthbertson BH. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation. 2006;114:1468–1475. DOI: 10.1161/CIRCULATIONAHA.105.602370. [DOI] [PubMed] [Google Scholar]
- 8.Levy M, Heels‐Ansdell D, Hiralal R, Bhandari M, Guyatt G, Yusuf S, Cook D, Villar JC, McQueen M, McFalls E, et al. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta‐analysis. Anesthesiology. 2011;114:796–806. DOI: 10.1097/ALN.0b013e31820ad503. [DOI] [PubMed] [Google Scholar]
- 9.Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010;152:26–35. DOI: 10.7326/0003-4819-152-1-201001050-00007. [DOI] [PubMed] [Google Scholar]
- 10.Gordon HS, Johnson ML, Wray NP, Petersen NJ, Henderson WG, Khuri SF, Geraci JM. Mortality after noncardiac surgery: prediction from administrative versus clinical data. Med Care. 2005;43:159–167. DOI: 10.1097/00005650-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila‐Roman VG, Gerhard‐Herman MD, Holly TA, Kane GC, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215–2245. DOI: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 12.Devereaux PJ, Duceppe E, Guyatt G, Tandon V, Rodseth R, Biccard BM, Xavier D, Szczeklik W, Meyhoff CS, Vincent J, et al. Dabigatran in patients with myocardial injury after non‐cardiac surgery (MANAGE): an international, randomised, placebo‐controlled trial. Lancet. 2018;391:2325–2334. DOI: 10.1016/S0140-6736(18)30832-8. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. DOI: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 14.Duvall WL, Sealove B, Pungoti C, Katz D, Moreno P, Kim M. Angiographic investigation of the pathophysiology of perioperative myocardial infarction. Catheter Cardiovasc Interv. 2012;80:768–776. DOI: 10.1002/ccd.23446. [DOI] [PubMed] [Google Scholar]
- 15.Sandoval Y, Jaffe AS. Type 2 myocardial infarction. J Am Coll Cardiol. 2019;73:1846–1860. DOI: 10.1016/j.jacc.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Parashar A, Agarwal S, Krishnaswamy A, Sud K, Poddar KL, Bassi M, Ellis S, Tuzcu EM, Menon V, Kapadia SR, et al. Percutaneous intervention for myocardial infarction after noncardiac surgery: patient characteristics and outcomes. J Am Coll Cardiol. 2016;68:329–338. DOI: 10.1016/j.jacc.2016.03.602. [DOI] [PubMed] [Google Scholar]
- 17.Chopra V, Wesorick DH, Sussman JB, Greene T, Rogers M, Froehlich JB, Eagle KA, Saint S. Effect of perioperative statins on death, myocardial infarction, atrial fibrillation, and length of stay: a systematic review and meta‐analysis. Arch Surg. 2012;147:181–189. DOI: 10.1001/archsurg.2011.897. [DOI] [PubMed] [Google Scholar]
- 18.Feringa HH, Schouten O, Karagiannis SE, Brugts J, Elhendy A, Boersma E, Vidakovic R, van Sambeek MR, Noordzij PG, Bax JJ, et al. Intensity of statin therapy in relation to myocardial ischemia, troponin T release, and clinical cardiac outcome in patients undergoing major vascular surgery. J Am Coll Cardiol. 2007;50:1649–1656. DOI: 10.1016/j.jacc.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 19.Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso‐Coello P, Kurz A, Villar JC, Sigamani A, Biccard BM, Meyhoff CS, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–1503. DOI: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figure S1


