Abstract
Mushrooms are rich in bioactive compounds. The potential health benefits associated with mushroom intake have gained recent research attention. We thus conducted a systematic review and meta-analysis to assess the association between mushroom intake and risk of cancer at any site. We searched MEDLINE, Web of Science, and Cochrane Library to identify relevant studies on mushroom intake and cancer published from 1 January, 1966, up to 31 October, 2020. Observational studies (n = 17) with RRs, HRs, or ORs and 95% CIs of cancer risk for ≥2 categories of mushroom intake were eligible for the present study. Random-effects meta-analyses were conducted. Higher mushroom consumption was associated with lower risk of total cancer (pooled RR for the highest compared with the lowest consumption groups: 0.66; 95% CI: 0.55, 0.78; n = 17). Higher mushroom consumption was also associated with lower risk of breast cancer (pooled RR for the highest compared with the lowest consumption groups: 0.65; 95% CI: 0.52, 0.81; n = 10) and nonbreast cancer (pooled RR for the highest compared with the lowest consumption groups: 0.80; 95% CI: 0.66, 0.97; n = 13). When site-specific cancers were examined, a significant association with mushroom consumption was only observed with breast cancer; this could be due to the small number of studies that were conducted with other cancers. There was evidence of a significant nonlinear dose–response association between mushroom consumption and the risk of total cancer (P-nonlinearity = 0.001; n = 7). Limitations included the potential for recall and selection bias in case-control designs, which comprised 11 out of the 17 studies included in this meta-analysis, and the large variation in the adjustment factors used in the final models from each study. The association between higher mushroom consumption and lower risk of cancer, particularly breast cancer, may indicate a potential protective role for mushrooms in the diet.
Keywords: mushroom, cancer risk, diet, epidemiology, dose-response, observational studies
Findings from this systematic review and meta-analysis of observational studies showed a significant association between higher mushroom consumption and lower risk of cancer.
Introduction
Cancer constitutes a major threat to public health in both high- and low-income countries. Globally, cancer is considered the second leading cause of death after cardiovascular diseases with an estimated 9.6 million deaths according to GLOBOCAN in 2018 (1). Modifiable risk factors such as a healthy diet are considered to play a significant role in the prevention of cancer (2). Mushrooms have been consumed as a functional food by many cultures for centuries because of their unique taste, subtle flavor, and role in a healthful diet, being low in calories, carbohydrates, sodium, and fats and cholesterol-free (3–6). Edible mushrooms are also rich in bioactive compounds, including phytochemicals (alkaloids, phenolic acids, flavonoids, carotenoids) (7, 8), fiber, polysaccharides (9), selenium (10, 11), vitamins (e.g., niacin, thiamin, riboflavin, ascorbic acid, and vitamins B and D) (12–14), and the crucial antioxidants ergothioneine and glutathione which may play a significant role in the prevention of cancer (15–19). Many of the protective effects of mushrooms are thought to be mediated through their antioxidant properties with the unique antioxidant, ergothioneine, thought to be playing an important role (6, 7, 20, 21). Ergothioneine concentrations vary by mushroom type with shiitake, oyster, maitake, and king oyster mushrooms which are widely consumed in Eastern Asian countries having higher concentrations than the white button, crimini, and portabellas mushrooms which are broadly distributed and eaten in the United States (17, 22). The growing body of evidence from various research groups across the globe regarding the potential health benefits associated with edible mushroom consumption, including reductions in the risk of chronic diseases including cancer, has gained increasing attention during the last few decades (3, 5, 7).
Even though mushrooms are often considered to be a vegetable, they actually belong to the fungal kingdom (22, 23). There are >2000 species of mushrooms in nature, ≥25 of which are widely accepted as functional foods for human consumption and commercially cultivated (5, 23). Overall, mushrooms have been reported to have anticancer capabilities and protective effects against tumor development (7) and laboratory studies have revealed these anticarcinogenic effects vary according to different types of mushrooms such as shiitake, maitake, and Agaricus bisporus (button mushroom) (8, 24, 25). Several epidemiological observational studies have also reported an inverse association between mushroom consumption and cancer risk (26–32). However, several other epidemiological studies that have examined the effects of mushroom intake on the risk of cancer have yielded nonsignificant associations (33–36). The previous meta-analysis that examined the association between mushroom consumption and cancer risk was limited to only breast cancer and included only a small number of articles (n = 7) (37). Given the inconsistent findings in the literature and lack of comprehensive studies including multiple cancers, we conducted a systematic review and meta-analysis of observational studies to examine the association between mushroom consumption and risk of cancer at any site. We hypothesized that higher mushroom consumption is associated with lower risk of cancer.
Methods
Search strategy
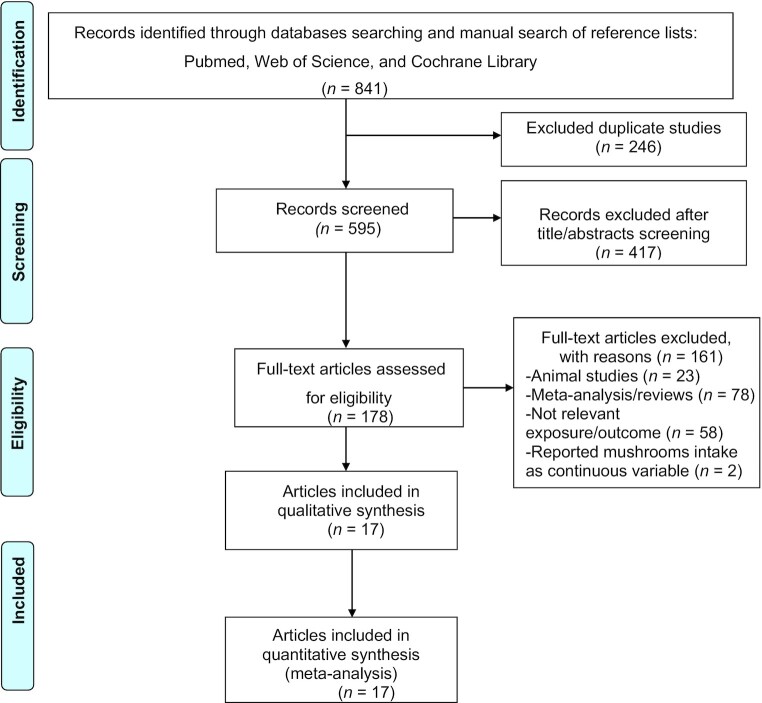
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines and the guidelines established for reporting nonrandomized studies in the Cochrane Library (38, 39) to select publications to be included in this meta-analysis and to extract data. The PRISMA approach follows a few key steps after conceptualization of the research question including identifying and selecting relevant studies, charting data, presenting summarized results, discussing the results and their limitations, and revealing funding sources (38). We performed a comprehensive systematic literature search of the PubMed (MEDLINE), Web of Science, and Cochrane Library databases to identify relevant observational studies on the association between mushroom consumption and the risk of cancer published from 1 January, 1966, up to 31 October, 2020. The following keywords were used: ((((mushroom OR Mushrooms)) OR “Agaricales”[Mesh])) AND ((((Prevention) OR (risk OR risks))) AND ((“Neoplasms”[Mesh]) OR (cancer OR cancers))). The reference lists of past systematic reviews (37, 40) and selected publications were manually searched and scrutinized to identify additional pertinent studies. Only articles written in the English language were included in this meta-analysis. Figure 1 depicts the search process.
FIGURE 1.
PRISMA flowchart of the systematic review and meta-analysis of mushroom consumption and risk of cancer. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Eligibility criteria
Studies were eligible for this systematic review if they met the following criteria: 1) they used an observational study design (case-control or cohort study design); 2) the exposure of interest was dietary intake of edible mushrooms; 3) the outcome was the occurrence of any cancer; 4) the authors reported associations in the form of relative risks (RRs), hazard ratios (HRs), or odds ratios (ORs) with 95% confidence intervals (CIs) for ≥2 categories of mushrooms intake. We identified 19 publications consisting of 8 cohort and 11 case-control studies: 17 publications (20, 26–31, 33–36, 41–46) were identified by searching PubMed (MEDLINE) and 2 publications were identified by the manual search of previous systematic reviews (47, 48). Two publications (44, 45) were excluded from the meta-analysis because mushroom intake had been treated as a continuous variable, not as discrete categories. The remaining 17 publications (20, 26–31, 33–36, 41–43, 46–48) were included in the meta-analysis (Table 1). Cross-sectional studies were excluded from the current meta-analysis.
TABLE 1.
Characteristics of the included observational studies reporting mushroom consumption and risk of cancer
| Authors (reference) | Sex | Sample size, n | Dietary assessment | Outcome assessment | Country | Study design | Cancer site | Mean age, y | Total cases, n | Mushroom consumption (quantity) | Reported effect sizes: HR/RR/OR (95% CI) | Follow-up, y | Covariates in the fully adjusted model |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al. (34) | M/F | Total: 112,991; M: 44,664; F: 68,327 | FFQ | Self-report diagnosis of cancer, verified by medical records to confirm the cancer diagnosis | US | Cohort study | Prostate cancerBreast cancerOvarian cancerStomach cancerLiver cancerColorectal cancer | 52.9 | 9561 | Never or almost never<1 time/wk1 time/wk2–4 times/wk≥5 times/wkNever or almost never<1 time/wk1 time/wk2–4 times/wk≥5 times/wkNever or almost never<1 time/wk1 time/wk2–4 times/wk≥5 times/wkNever or almost never<1 time/wk1 time/wk2–4 times/wk≥5 times/wkNever or almost never<1 time/wk1 time/wk2–4 times/wk≥5 times/wkNever or almost never<1 time/wk1 time/wk2–4 times/wk≥5 times/wk | ReferenceHR: 1.07 (0.90, 1.27)HR: 1.15 (0.95, 1.39)HR: 1.01 (0.80, 1.29)HR: 1.06 (0.69, 1.63)ReferenceHR: 1.04 (0.96, 1.12)HR: 1.01 (0.93, 1.09)HR: 1.03 (0.93, 1.13)HR: 0.89 (0.77, 1.04)ReferenceHR: 0.83 (0.66, 1.04)HR: 0.83 (0.65, 1.06)HR: 0.95 (0.70, 1.28)HR: 0.87 (0.54, 1.40)ReferenceHR: 1.12 (0.76, 1.64)HR: 1.16 (0.77, 1.77)HR: 0.96 (0.56, 1.64)HR: 1.37 (0.62, 3.02)ReferenceHR: 1.05 (0.60, 1.84)HR: 1.85 (1.05, 3.25)HR: 1.67 (0.84, 3.33)HR: 2.40 (0.91, 6.33)ReferenceHR: 0.97 (0.87, 1.09)HR: 1.06 (0.94, 1.19)HR: 0.89 (0.76, 1.04)HR: 0.85 (0.65, 1.10) | 26 | Age, race (white or nonwhite), height (continuous), BMI (quintiles), family history of cancer (yes or no), physical exam in past 2 y (yes or no), history of colonoscopy or sigmoidoscopy (yes or no), smoking in pack-years (never smoker, 1–4.9, 5–19.9, 20–39.9, or ≥40), physical activity (quintiles), regular aspirin use (yes or no), multivitamin use (yes or no), total energy intake (quintiles), alcohol consumption (0, 0.1–4.9, 5.0–14.9, 15.0–29.9, or ≥30 g/d), red and processed meat intake (quintiles), prudent diet pattern (quintiles), and Western diet pattern (quintiles); prostate-specific antigen test in past 2 y (yes or no) for men only; and menopausal status (premenopausal or postmenopausal), postmenopausal hormone use (never, past, or current), and mammogram in past 2 y (yes or no) for women only |
| van Gils et al. (35) | F | Total: 285,526 | FFQ | Cancer registries using ICD-O-2; C50 | 10 European countries | Cohort study | Breast cancer | 50.9 | 3505 | ≤0.3 g/d>0.3 to ≤1.8 g/d>1.8 to ≤5.2 g/d>5.2 to ≤11.1 g/d>11.1 g/d | ReferenceRR: 0.91 (0.80, 1.05)RR: 0.87 (0.76, 1.01)RR: 1.01 (0.88, 1.17)RR: 0.98 (0.85, 1.14) | 5.4 | Energy intake, alcohol intake, saturated fat, height, weight, age at menarche, parity, oral contraceptives, HRT, menopausal status, smoking status, physical activity, education |
| Masala et al. (33) | F | Total: 31,510 | FFQ | Cancer registries using ICD-O-2; C50 | Italy | Cohort study | Breast cancer | 50.2 | 1072 | <0.4 g/d0.4–0.9 g/d1.0–1.9 g/d2.0–4.0 g/d>4.0 g/d | ReferenceHR: 0.93 (0.77, 1.12)HR: 0.82 (0.67, 1.01)HR: 0.94 (0.79, 1.12)HR: 0.85 (0.69, 1.05) | 11.25 | Weight, height, education, number of children, age at menarche, menopausal status, energy intake except alcohol, alcohol intake, current use of hormone therapy, smoking status, physical activity |
| Lee et al. (41) | F | Total: 1000 | FFQ | Medical records and laboratory pathology reports, confirmed histopathologically | China | Case-control | Ovarian cancer | 59.0 | 500 | ≤14 g/d>14 g/d | ReferenceOR: 0.76 (0.58, 1.01) | Not applicable | Age at interview, age of menarche, BMI, physical activity, total energy intake (kcal/d), parity, oral contraceptive use, menopausal status, education level (none/primary, secondary, vocational/tertiary), smoking status, alcohol drinking, and family history of ovarian or breast cancer |
| Zhang et al. (42) | M/F | Total: 132,837; M: 60,351; F: 72,486 | FFQ | Medical records and/or histological slides of cancer | China | Cohort study | Liver cancer | 53.7 | 267 | ≤2.82 g/d≤5.88 g/d≤10.83 g/d>10.83 g/d | ReferenceHR: 0.71 (0.51, 0.99)HR: 0.73 (0.52, 1.03)HR: 0.66 (0.46, 0.95) | 10.9 | Age at enrollments, BMI, total energy intake, family income, education level, family history of liver cancer, history of chronic viral hepatitis, chronic liver disease, or cirrhosis, diabetes, cholelithiasis or cholecystectomy, vitamin C, E, or multivitamin supplementation |
| Zhang et al. (28) | F | Total: 2018 | FFQ | Histopathologically confirmed diagnosis | China | Case-control | Breast cancer | 48.4 | 1009 | 0 g/d<2 g/d2 to <10 g/d≥ 10 g/d | ReferenceOR: 0.88 (0.66, 1.19)OR: 0.81 (0.59, 1.10)OR: 0.36 (0.25, 0.51) | Not applicable | Age, residential area, education, BMI, age at menarche, oral contraceptive use, HRT, breast cancer in first-degree relatives, total energy intake, menopausal status, alcohol consumption, active smoking, passive smoking, tea drinking, and physical activity |
| Zhang et al. (27) | F | Total: 876 | FFQ | Breast cancer diagnosed and histologically confirmed | China | Case-control | Breast cancer | 47.1 | 438 | <0.8 g/d0.8–2.5 g/d2.5–7.1 g/d>7.1 g/d | ReferenceOR: 0.95 (0.66, 1.38)OR: 0.69 (0.45, 1.04)OR: 0.65 (0.43, 0.98) | Not applicable | Age at menarche, BMI, history of benign breast disease, family, physical activity, passive smoking, and total energy intake |
| Zhang et al. (46) | M | Total: 36,499 | FFQ | ICD-O-3, coded as C61 | Japan | Cohort study | Prostate cancer | 55.7 | 1204 | <1 time/wk1–2 times/wk≥3 times/wk | ReferenceHR: 0.92 (0.81, 1.05)HR: 0.83 (0.70, 0.98) | 13.2 | Family history of cancer, BMI, education level, smoking status, alcohol drinking, time spent walking, 5-group meat consumption, vegetables, fruit, dairy products, coffee, and energy intakes |
| Mizoo et al. 2013 (31) | F | Total: 936 | Self-administered questionnaire | Japanese Single Nucleotide Polymorphism database | Japan | Case-control | Breast cancer | 54.1 | 472 | ≤1 time/wk2–4 times/wk≥5 times/wk | ReferenceOR: 0.73 (0.54, 0.98)OR: 0.60 (0.40, 0.91) | Not applicable | Age |
| Hara et al. (20) | M/F | Total: 781; M/F numbers not specified | FFQ | Histopathological grading and anatomical subsites of stomach cancers and colorectal cancers | Japan | Case-control | Stomach cancerColorectal cancer | 58.7 | 264 | Tertile 1 (3 g/1000 kcal)Tertile 2 (11 g/1000 kcal)Tertile 3 (28 g/1000 kcal)Tertile 1 (4 g/1000 kcal)Tertile 2 (11 g/1000 kcal)Tertile 3 (29 g/1000 kcal) | ReferenceOR: 0.76 (0.45, 1.29)OR: 0.71 (0.41, 1.23)ReferenceOR: 1.15 (0.51, 2.60)OR: 1.41 (0.60, 3.29) | Not applicable | For stomach cancer set, adjusted for smoking status (3 categories: never, ex, current), family history of stomach cancer, salt intake (3 categories), total energy intake (rank variable, 0–2), and JA Cooperatives membership; for colorectal cancer set, adjusted for smoking status (3 categories: never, ex, current), alcohol intake (3 categories), family history of colorectal cancer, total energy intake (rank variable, 0–2), and JA membership |
| Ko et al. (36) | M/F | Total: 9724; M: 3714; F: 6010 | Self-administered FFQ | Identified through record linkage with the Central Cancer Registry | Korea | Cohort study | Stomach cancer | 57.6 | 166 | Almost never1–4 times/mo1–4 times/wk≥1 time/d | ReferenceRR: 1.10 (0.79, 1.54)RR: 0.67 (0.37, 1.22)RR: 1.15 (0.46, 2.84) | 8.5 | Age, sex, cigarette smoking, BMI, alcohol drinking, and area of residence |
| Shin et al. (29) | F | Total: 718 | FFQ | Paraffin-embedded breast tumors by immunohistochemistry | Korea | Case-control | Breast cancer | 48.1 | 358 | <2.61 g/d2.62 to <5.36 g/d5.36 to <11.37 g/d≥11.37 g/d | ReferenceOR: 0.96 (0.57, 1.61)OR: 0.84 (0.48, 1.48)OR: 0.43 (0.21, 0.88) | Not applicable | Age, BMI, family history of breast cancer, current dietary supplements, education, job, smoking, alcohol intake, physical activity, menopausal status, age at menarche, parity, total energy intake, and vegetable intake |
| Hong et al. (26) | F | Total: 724 | FFQ | Mammography and histologically confirmed | Korea | Case-control | Breast cancer | 46.1 | 362 | 0 g/d2.45 g/d4.90 g/d9.80 g/d18.3 g/d | ReferenceOR: 0.48 (0.28, 0.81)OR: 0.53 (0.30, 0.92)OR: 0.64 (0.34, 1.18)OR: 0.55 (0.33, 0.94) | Not applicable | Education (y), family history for breast cancer, regular exercise (≥22.5 MET-h/wk), BMI, current smoker, current drinker, current multivitamin supplement, |
| number of children, menopausal status, energy intake, carbohydrate, soy protein, vitamin E, and folate | |||||||||||||
| Lee et al. (48) | F | Total: 262 | Not specified | Not specified | Korea | Case-control | Breast cancer | Not specified | 103 | ≤1 time/wk2–3 times/wk1 time/d | ReferenceOR: 1.00 (0.55, 1.81)OR: 1.11 (0.35, 3.59) | Not applicable | BMI, residence, occupation, family history, delivery miscarriage, breastfeeding, periods of breastfeeding, HRT |
| Lee et al. (30) | F | Total: 378 | Short FFQ method | First diagnosis of histopathologically confirmed | Korea | Case-control | Breast cancer | 63.0 | 189 | <1 time/wk≥1 time/wk | ReferenceOR: 0.40 (0.30, 0.70) | Not applicable | Age, education, BMI, and family history of breast cancer |
| Kim et al. (43) | M/F | Total: 272; M: 186; F: 86 | Quantitative food frequency method | Endoscopic examination confirmed by histologic method | Korea | Case-control | Stomach cancer | Not specified | 136 | Low (<25th percentile),Medium (25th–75th percentile),High (>75th percentile) | ReferenceOR: 0.38 (0.21, 0.66)OR: 0.30 (0.15, 0.62) | Not applicable | Age, sex, socioeconomic status, family history, and refrigerator use |
| Park et al. (47) | M/F | Total: 360; M: 222; F: 138 | FFQ | Confirmed by the histological diagnosis | Korea | Case-control | Stomach cancer | 51.5 | 126 | ≤4–6 times/y≥1 time/mo | ReferenceOR: 0.30 (0.20, 0.70) | Not applicable | Age, sex, education, economic status, and residence |
HRT, hormone replacement therapy; ICD-O, International Classification of Diseases for Oncology; JA, Japan Agricultural; MET, metabolic equivalents.
For the present meta-analysis, the primary outcome of interest was the risk of total cancer, and the secondary outcome of interest was the risk of site-specific cancer.
Data extraction
Two independent authors (DMB and PS) initially screened the titles and abstracts of all selected publications eligible for inclusion in the analysis. Disagreements were resolved by discussion to meet a consensus. If necessary, an available third author was consulted in order to reach a consensus. Only relevant observational studies were included in the current meta-analysis. The following data were extracted from each publication: the first author's name, the year of publication, sex, sample size, dietary assessment, outcome assessment, country in which the study was conducted, study design, cancer type, mean age of study participants, number of cases, categories of mushroom consumption, reported HRs, RRs, or ORs with corresponding 95% CIs, duration of follow-up for cohort studies, and the covariates for adjustments in the final multivariable regression models.
Meta-power analysis
The R software dmetar package was used to calculate the statistical power. With an expected average sample size of 12 individuals/group in the studies included in the meta-analysis, 10 as the expected number of studies, a significance level of 0.05, and high heterogeneity under a random-effect model, we had a 90% statistical power to detect an overall pooled effect size of RR = 0.66. For an Eastern compared with Western region subgroup analysis, we had a 90% statistical power to detect a minimum effect size difference of 0.34. However, in the current study, the effect size difference was 0.47, which is higher than the minimum effect size difference.
Statistical analysis
We used the reported ORs, RRs, or HRs as the measures of the association between mushroom intake and the risk of cancer. According to a previous study, if the outcome (e.g., cancer) is rare in all populations and subgroups, the distinctions among different measures of RRs (e.g., ORs, rate ratios, and risk ratios) can be ignored (49); therefore, we combined RRs and HRs with ORs in the present meta-analysis and reported the pooled effect size as RRs as common risk estimates for all studies. Two publications (20, 34) reported separate ORs and HRs for a different type of cancer. In these circumstances, we extracted the ORs and HRs for each type of cancer and used random-effects models to pool the RRs within each study. In addition, 1 study (34) reported associations between mushroom intake and multiple site-specific cancers such as esophageal and endometrial. In this situation, we only selected cancers that were available in other studies because ≥2 studies are needed for meta-analysis (50). We used the reported RRs for the remaining studies which had only 1 type of cancer to assess the association between mushroom intake and the risk of cancer. We first log transformed all the reported effect sizes of the data to normalize the distributions. SEs were calculated by the following equations as described previously (51): lower = log (lower 95% CI) and upper = log (upper 95% CI), SE = (upper − lower)/3.92.
To assess the associations between mushroom intake and the risk of cancer, we pooled the RR data for the highest compared with the lowest mushroom intake category from each study, weighted by the inverse of their variances. We used the metagen function from the R package meta to calculate the pooled effect estimates using random-effects models (52). Random-effects models were pooled using DerSimonian and Laird's method for the association between mushroom intake and the risk of all cancer. The results derived were graphically presented in forest plots. Potential between-studies heterogeneity was quantified using the Cochran's Q test and I2 statistics expressed as a proportion (%) in the estimation of the random-effects models (53). Heterogeneity was determined with a significance of P < 0.05. Univariate meta-regression was used to examine the association between study designs, location of study (Western compared with Eastern), and breast cancer compared with nonbreast cancer. We selected breast cancer because it appeared to be the most affected site as significant associations with mushroom intake were only observed for cancers at this site. Location was examined because mushroom intake is much higher in Eastern countries such as China than in Western countries such as the United States (17, 22).
As a secondary analysis, to better understand the shape of the curve relating mushroom intake to cancer risk, we conducted a dose-response meta-analysis using the R software dosresmeta package. Studies included in the current meta-analysis used different units to report mushroom intake (e.g., grams, servings, and frequencies). To facilitate the dose-response meta-analysis, we included studies (n = 7) that reported ≥3 categories of mushroom intake in grams per day. In publications where the median intake values per mushroom intake category were not reported, we estimated the average intake in each category by calculating the midpoint of the upper and lower boundaries. When the upper boundary of the highest category was not provided, we assumed that it had the same amplitude of intake as the closet adjacent category (54, 55). We then examined the shape of the relation between mushroom intake and the risk of cancer with a 2-stage random-effects dose-response meta-analysis, using restricted cubic splines with 3 knots at fixed percentiles (25%, 50%, and 75%) of mushroom distribution as done in a previous study (37). It is worth noting that the 2-stage dose-response meta-analysis model requires data for ≥3 exposure categories, including the reference category, within each study.
A P value for the nonlinearity of the dose-response meta-analysis was tested using the Wald test. An influence sensitivity analysis was performed by removing 1 individual study at a time, to examine the effect of the excluded study on the pooled effect estimates.
Publication bias was investigated through the use of funnel plot asymmetry and tested by Egger's asymmetry test (56) and Begg's test (57). All analyses were conducted using R statistical software version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). A P value < 0.05 was considered statistically significant.
Results
Literature search and characteristics of studies
The first search from 1 January, 1966, up to 31 October, 2020, produced 450 studies from PubMed, 305 from Web of Science, 84 from the Cochrane Library databases, and 2 from a manual search of reference lists of the previous meta-analysis. After removing 246 duplicate records, a total of 595 potential related publications were screened for inclusion in the meta-analysis. Four hundred and seventeen records screened were excluded after review of titles and abstracts. One hundred and sixty-one full texts were further excluded because of the following reasons: animal studies (n = 23), meta-analysis/reviews (n = 78), not relevant exposure/outcome (n = 58), and mushroom intake treated as a continuous variable (n = 2). Finally, 17 studies (20, 26–31, 33–36, 41–43, 46–48) (Figure 1) were eligible for this meta-analysis consisting of 6 cohort studies (33–36, 42, 46) and 11 case-control studies (20, 26–31, 41, 43, 47, 48). The total number of cancer cases for this meta-analysis was 19,732. The majority of studies included in this meta-analysis were conducted in Asian countries (n = 14, referred to as “Eastern countries” in the article), whereas 2 studies were conducted in Europe and 1 in the United States (referred to as “Western countries” in the article). Table 1 shows characteristics of the studies included in the meta-analysis.
Mushroom consumption and risk of cancer
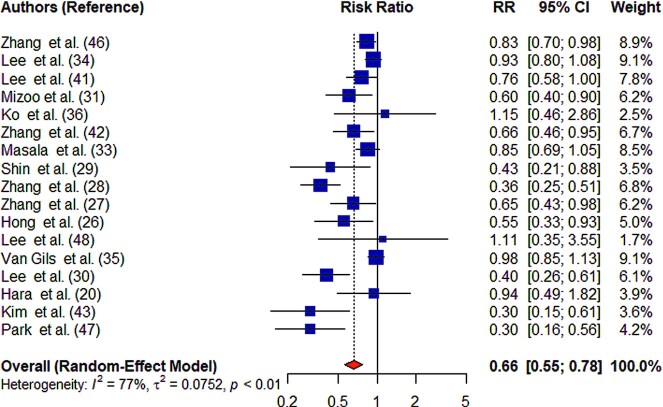
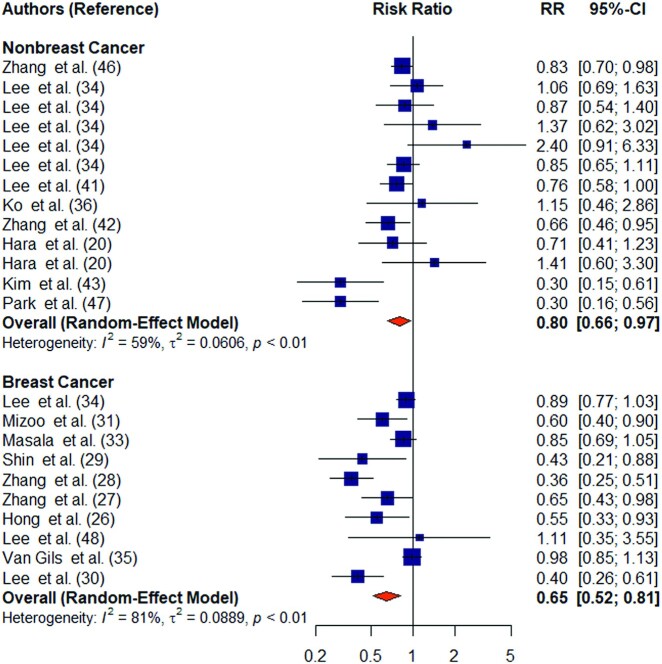
Higher mushroom consumption was associated with lower risk of total cancer (pooled RR for the highest compared with the lowest consumption groups: 0.66; 95% CI: 0.55, 0.78; n = 17) (Figure 2). There was substantial heterogeneity between studies (I2 = 77%; P-heterogeneity < 0.01). Higher mushroom consumption was associated with lower risk of total cancer in cohort studies (pooled RR for the highest compared with the lowest consumption groups: 0.89; 95% CI: 0.82, 0.97; n = 6) and case-control studies (pooled RR for the highest compared with the lowest consumption groups: 0.52; 95% CI: 0.41, 0.66; n = 11) (Supplemental Figure 1). Higher mushroom consumption was also associated with lower risk of breast cancer (pooled RR for the highest compared with the lowest consumption groups: 0.65; 95% CI: 0.52, 0.81; n = 10) and nonbreast cancer (pooled RR for the highest compared with the lowest consumption groups: 0.80; 95% CI: 0.66, 0.97; n = 13) (Figure 3).
FIGURE 2.
Summary forest plot of mushroom consumption (highest compared with lowest category) and cancer risk. The square represents the point estimate of each study and the size is proportional to its weight in the meta-analysis. The horizontal line through the square represents its 95% CI. The diamond represents the pooled risk ratio of the meta-analysis.
FIGURE 3.
Forest plot of mushroom consumption (highest compared with lowest category) and breast cancer risk. The square represents the point estimate of each study and the size is proportional to its weight in the meta-analysis. The horizontal line through the square represents its 95% CI. The diamond represents the pooled risk ratio of the meta-analysis.
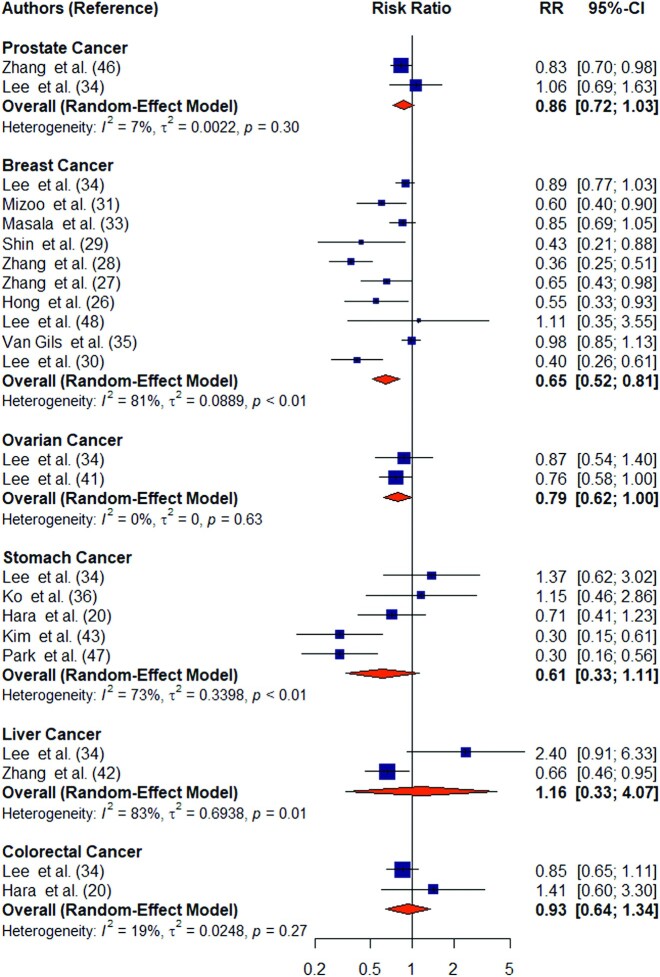
When site-specific cancer was examined, a significant association with mushroom consumption was only observed with breast cancer (Figure 4). The lack of association with other cancers could be due to the small number of studies which examined associations of mushroom intake with other site-specific cancers (<6 studies for each site-specific cancer compared with 10 studies for breast cancer).
FIGURE 4.
Forest plot of mushroom consumption (highest compared with lowest category) and risk of site-specific cancers. The square represents the point estimate of each study and the size is proportional to its weight in the meta-analysis. The horizontal line through the square represents its 95% CI. The diamond represents the pooled risk ratio of the meta-analysis.
Subgroup analyses, sensitivity analyses, and publication bias
We performed univariate meta-regression analysis to investigate the potential sources of heterogeneity. Subgroup analysis (Table 2) showed that the significant association between higher mushroom intake and total cancer risk was observed only in studies from Eastern regions (pooled RR for the highest compared with the lowest consumption groups: 0.58; 95% CI: 0.47, 0.71; P = 0.02; n = 14) (Supplemental Figure 2). When limiting to breast cancer studies only, higher mushroom intake was associated with lower risk of breast cancer only in case-control studies (pooled RR for the highest compared with the lowest consumption groups: 0.50; 95% CI: 0.40, 0.62) but not in cohort studies (Supplemental Figure 3).
TABLE 2.
Results of subgroup analysis and meta-regression for the association between mushroom intake and cancer risk
| Characteristic | Pooled RR (95% CI) | I2 (%) | P for heterogeneity between subgroups |
|---|---|---|---|
| Cancer type | 0.60 | ||
| Breast | 0.65 (0.52, 0.81) | 81 | |
| Prostate | 0.86 (0.72, 1.03) | 7 | |
| Ovarian | 0.79 (0.62, 1.00) | 0 | |
| Stomach | 0.61 (0.33, 1.11) | 73 | |
| Colorectal | 0.93 (0.64, 1.34) | 19 | |
| Liver | 1.16 (0.33, 4.07) | 83 | |
| Study design | 0.002 | ||
| Cohort | 0.89 (0.82, 0.97) | 13 | |
| Case-control | 0.52 (0.41, 0.66) | 59 | |
| Region | 0.02 | ||
| Western | 0.93 (0.85, 1.02) | 0 | |
| Eastern | 0.58 (0.47, 0.71) | 68 |
In the sensitivity analysis, omitting each study at a time did not indicate any substantial changes of the pooled RRs from the random-effects model. The pooled RRs ranged from 0.63 to 0.70 (P < 0.0001 for all) (Supplemental Figure 4). Inspection of the funnel plot (Figure 5), Egger's test for asymmetry (P = 0.08), and Begg's test (P = 0.16) did not indicate the presence of publication bias.
FIGURE 5.
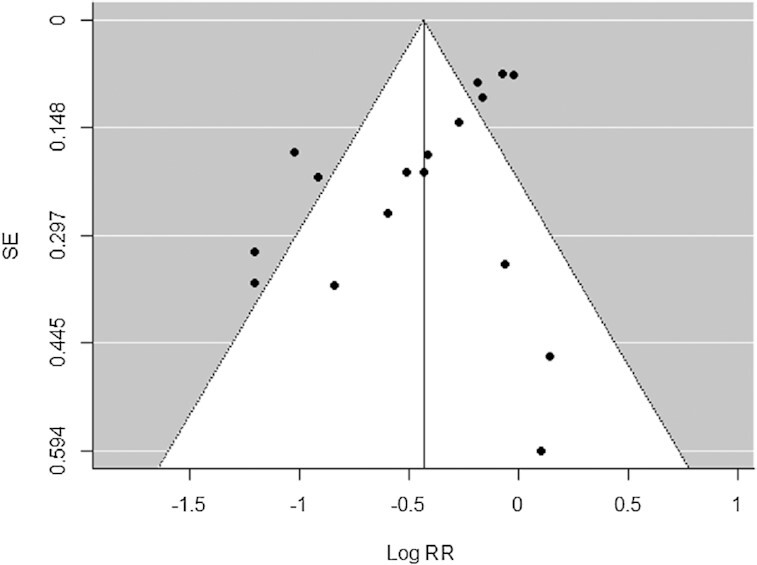
Funnel plot of mushroom consumption (highest compared with lowest category) and cancer risk from the 17 studies included in the meta-analysis. No significant publication bias was detected (P = 0.08 for Egger's test, P = 0.16 for Begg's test).
Nonlinear dose-response meta-analysis
Seven studies were included in the restricted cubic spline analysis to fit a 2-stage random-effects dose-response meta-analysis. There was evidence of a significant nonlinear dose–response association between mushroom consumption and the risk of total cancer (P-nonlinearity = 0.001; n = 7), with a 45% lower risk at higher intake of 18 g/d (RR: 0.55; 95% CI: 0.36, 0.86) than at intake of 0 g/d (Figure 6).
FIGURE 6.
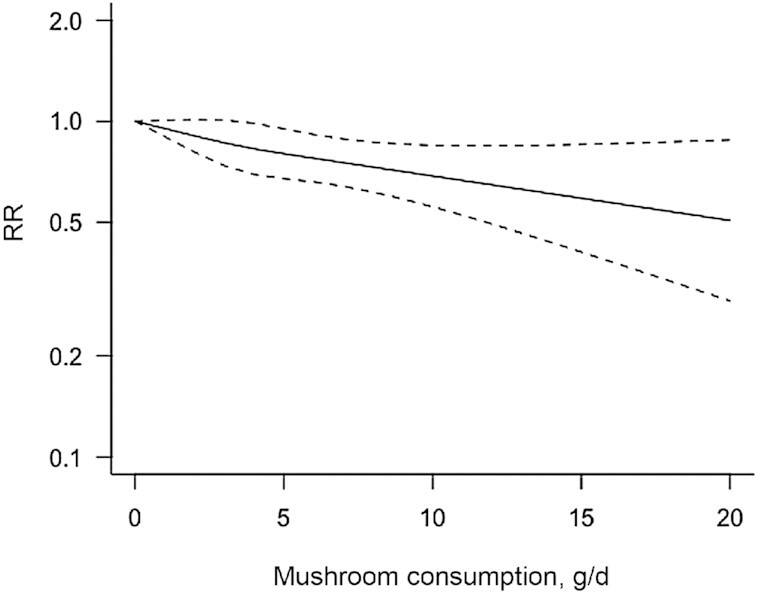
Dose–response associations of mushroom consumption and cancer risk. The solid lines represent the best-fitting cubic spline and the dotted lines represent the 95% CIs.
Discussion
In this systematic review and meta-analysis of observational studies, we found that higher mushroom consumption was associated with lower risk of cancer. In particular, breast cancer appeared to be the most affected site because a significant association with mushroom intake was only observed for cancers at this site. Importantly, mushroom consumption was associated with lower risk of cancer in both cohort and case-control studies. The effect was much stronger in case-control studies than in cohort studies. Our meta-analysis is generally consistent with results from a previous meta-analysis of observational studies that indicated an inverse association between mushroom intake and the risk of breast cancer (37). Evidence from epidemiological studies has shown that regular consumption of fruits and vegetables is associated with reduced risk of chronic diseases such as cancer and all-cause mortality (58–61). A dose-response meta-analysis indicated that higher mushroom consumption of 18 g/d was associated with a 45% lower risk of total cancer than an intake of 0 g/d.
The potential biological mechanisms underlying the association between mushroom consumption and lower risk of cancer may stem from their antioxidant properties due to the specific mushroom components ergothioneine and glutathione. Oxidative damage due to an excess of free radicals is an important causal factor in the aging process and diseases of aging including many cancers, with amounts being related to poor lifestyle habits such as an unhealthy diet (62). Reactive oxygen and nitrogen species (RONS) are produced by some endogenous and exogenous processes, and their negative effects are neutralized by antioxidant defenses. Oxidative stress occurs from the imbalance between RONS production and these antioxidant defenses and has been associated with several chronic diseases such as cancer that account for a vital portion of death today (62, 63). Given the significant role of oxidative stress in the pathogenesis of many chronic diseases, antioxidants may play a significant role in the control and prevention of chronic diseases (62). Mushrooms are a potent source of key antioxidants that can mitigate oxidative stress and improve human health and promote quality of life (16). In particular, ergothioneine, which is found in very high concentrations in mushrooms, is a sulfur-containing amino acid that has strong antioxidant activity and is obtained exclusively through dietary sources (4, 16, 19, 22, 64, 65). In a recent review, it was proposed that ergothioneine could be used as a therapeutic to reduce the severity of and mortality from coronavirus infectious disease 2019 (COVID-19) (66). In addition, Paul and Snyder (19) indicated that ergothioneine is an important physiological cytoprotectant and should be designated as a new vitamin. Furthermore, Bruce Ames (67) has suggested that ergothioneine is a “longevity vitamin” as defined by his Triage Theory, with multiple potential functions in the body (e.g., antioxidant, cytoprotective, and antiaging). A very recent study has also suggested that higher plasma ergothioneine was associated with reduced risk of cardiometabolic disease and mortality (68). Researchers from a previous study have also revealed that mushroom species such as oyster mushrooms contain ∼10 times more ergothioneine than other dietary sources such as chicken liver and black beans (64). Mushrooms also contain other bioactive compounds including polysaccharides such as β-glucans that have been implicated as having antitumor and immunomodulation properties (4, 7, 69–71). Laboratory experiment studies have revealed that anticarcinogenic effects of mushrooms vary with different types of mushrooms such as shiitake, maitake, and A. bisporus (8, 24, 25). Furthermore, in vitro and in vivo studies have suggested that extracts of mushrooms such as A. bisporus (8) and Agaricus blazei Murill (72) were more likely to inhibit the growth of prostate tumor cell lines in immune-deficient mice and to block prostate tumorigenic progression. Based on their findings, the authors suggested that mushrooms might be effective for the prevention and treatment of human prostate cancer (8, 72).
When site-specific cancer was examined, a significant association with mushroom consumption was only observed with breast cancer, not other cancers. The lack of association with other cancers could be due to the small number of studies which examined associations of mushroom intake with other site-specific cancers (<6 studies for each site-specific cancer compared with 10 studies for breast cancer). It could be also due to the fact that dietary antioxidant phytochemicals from mushrooms are inhibitors of aromatase activity and possess antiestrogen properties which may suppress breast cancer proliferation.
An interesting finding in the present meta-analysis is that studies conducted in Eastern regions were inversely associated with the risk of all cancer compared with those in Western regions. The differences in risk between Eastern and Western countries may result from differences in the amounts and types of mushrooms consumed between these regions. Total amounts of mushroom consumption tend to be greater in Eastern countries (26, 29, 41).
Our meta-analysis has several strengths. To the best of our knowledge, this is the most comprehensive meta-analysis to assess the association between mushroom intake and the risk of cancer at any site. The previous meta-analysis was limited to only breast cancer and included a small number of articles. The majority of studies in the present meta-analysis (12 out of 17) used validated dietary assessment methods such as FFQs. Moreover, we have tested the robustness of our results by conducting several sensitivity analyses and testing for potential publication bias. Lastly, we conducted a dose-response meta-analysis to better understand the shape of the curve relating to mushroom intake and cancer using a restricted cubic spline.
Notwithstanding, our study has several limitations that should be noted. First, combining studies from distinct populations increases the sample size and statistical power; however, it may also result in heterogeneity because of inequality in the characteristics of the study populations. Second, the majority of the studies (11 out of 17) included in the present analyses used a case-control design, which is subject to recall and selection bias. A third limitation is that publication bias is inevitable in any meta-analysis and our study was limited by the inclusion of only those studies that were published in English, thus, relevant non-English published studies may have been missed. A fourth limitation is that the adjustment factors used in the final models from each study were not the same.
In conclusion, the current meta-analysis showed a significant inverse association between higher mushroom consumption and lower risk of cancer. In particular, breast cancer appeared to be the most affected site because a significant association with mushroom intake was only observed for cancers at this site. A lack of significant association for site-specific cancers in this study could be due to the low numbers of studies specifically for these cancers. Our findings may have important public health implications in the prevention of chronic diseases and mortality. In addition, the results are useful for policy makers, contributing to increasing public awareness about the role of the diet on health, and potential protective effects of mushrooms in lowering the risk of cancer. Lastly, findings from this study will also provide useful directions for future epidemiological studies about the nutritional benefits of mushrooms and health. Future clinical studies for site-specific cancers are warranted.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Esther Dell at the Penn State College of Medicine Libraries for her systematic review consultation about the database and keyword searches. We also thank Dr. Vernon M Chinchilli for providing helpful feedback. The authors’ responsibilities were as follows—DMB, XG, and JPR: designed the research (project conception, development of the overall research plan, and study oversight); DMB and PS: conducted the systematic review and performed the statistical analysis; DMB: analyzed the data, managed the systematic review and meta-analysis, and wrote the first draft of the manuscript; and all authors: review, editing, and approved the final version of the manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
XG is an Editorial Board member for Advances in Nutrition but played no role in the Journal's evaluation of this manuscript.
Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Contributor Information
Djibril M Ba, Department of Public Health Sciences, Penn State College of Medicine, Hershey, PA, USA.
Paddy Ssentongo, Department of Public Health Sciences, Penn State College of Medicine, Hershey, PA, USA.
Robert B Beelman, Center for Plant and Mushroom Foods for Health, Department of Food Science, College of Agricultural Sciences, Pennsylvania State University, University Park, PA, USA.
Joshua Muscat, Department of Public Health Sciences, Penn State College of Medicine, Hershey, PA, USA.
Xiang Gao, Department of Nutritional Sciences, Pennsylvania State University, State College, PA, USA.
John P Richie, Jr, Department of Public Health Sciences, Penn State College of Medicine, Hershey, PA, USA.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–40. [DOI] [PubMed] [Google Scholar]
- 3.Roncero-Ramos I, Delgado-Andrade C. The beneficial role of edible mushrooms in human health. Curr Opin Food Sci. 2017;14:122–8. [Google Scholar]
- 4.Feeney MJ, Dwyer J, Hasler-Lewis CM, Milner JA, Noakes M, Rowe S, Wach M, Beelman RB, Caldwell J, Cantorna MTet al. Mushrooms and Health Summit Proceedings. J Nutr. 2014;144(7):1128S–36S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valverde ME, Hernández-Pérez T, Paredes-López O. Edible mushrooms: improving human health and promoting quality life. Int J Microbiol. 2015:376387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarikurkcu C, Tepe B, Yamac M. Evaluation of the antioxidant activity of four edible mushrooms from the Central Anatolia, Eskisehir – Turkey: Lactarius deterrimus, Suillus collitinus, Boletus edulis, Xerocomus chrysenteron. Bioresour Technol. 2008;99(14):6651–5. [DOI] [PubMed] [Google Scholar]
- 7.Patel S, Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2012;2(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams LS, Phung S, Wu X, Ki L, Chen S. White button mushroom (Agaricus bisporus) exhibits antiproliferative and proapoptotic properties and inhibits prostate tumor growth in athymic mice. Nutr Cancer. 2008;60(6):744–56. [DOI] [PubMed] [Google Scholar]
- 9.Meng X, Liang H, Luo L. Antitumor polysaccharides from mushrooms: a review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr Res. 2016;424:30–41. [DOI] [PubMed] [Google Scholar]
- 10.Beelman RB, Royse DJ. Selenium enrichment of Pleurotus cornucopiae (Paulet) Rolland and Grifola frondosa (Dicks.:Fr.) S.F. Gray mushrooms. Int J Med Mushr. 2006;8(1):77–84. [Google Scholar]
- 11.Werner AR, Beelman RB. Growing high-selenium edible and medicinal button mushrooms (Agaricus bisporus (J. Lge) Imbach) as ingredients for functional foods or dietary supplements. Int J Med Mushr. 2002;4:6. [Google Scholar]
- 12.Kalaras MD, Beelman RB, Elias RJ. Effects of postharvest pulsed UV light treatment of white button mushrooms (Agaricus bisporus) on vitamin D2 content and quality attributes. J Agric Food Chem. 2012;60(1):220–5. [DOI] [PubMed] [Google Scholar]
- 13.Kalaras MD, Beelman RB, Holick MF, Elias RJ. Generation of potentially bioactive ergosterol-derived products following pulsed ultraviolet light exposure of mushrooms (Agaricus bisporus). Food Chem. 2012;135(2):396–401. [DOI] [PubMed] [Google Scholar]
- 14.Mattila P, Suonpää K, Piironen V. Functional properties of edible mushrooms. Nutrition. 2000;16(7–8):694–6. [DOI] [PubMed] [Google Scholar]
- 15.Dubost N, Ou B, Beelman R. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007;105(2):727–35. [Google Scholar]
- 16.Kalaras MD, Richie JP, Calcagnotto A, Beelman RB. Mushrooms: a rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017;233:429–33. [DOI] [PubMed] [Google Scholar]
- 17.Dubost NJ, Beelman RB, Royse DJ. Influence of selected cultural factors and postharvest storage on ergothioneine content of common button mushroom Agaricus bisporus (J. Lge) Imbach (Agaricomycetideae). Int J Med Mushr. 2007;9(2):163–76. [Google Scholar]
- 18.Calvo MS, Mehrotra A, Beelman RB, Nadkarni G, Wang L, Cai W, Goh BC, Kalaras MD, Uribarri J. A retrospective study in adults with metabolic syndrome: diabetic risk factor response to daily consumption of Agaricus bisporus (white button mushrooms). Plant Foods Hum Nutr. 2016;71(3):245–51. [DOI] [PubMed] [Google Scholar]
- 19.Paul BD, Snyder SH. The unusual amino acid l-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010;17(7):1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara M, Hanaoka T, Kobayashi M, Otani T, Adachi HY, Montani A, Natsukawa S, Shaura K, Koizumi Y, Kasuga Yet al. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr Cancer. 2003;46(2):138–47. [DOI] [PubMed] [Google Scholar]
- 21.Stajić M, Vukojević J, Knežević A, Laušević SD, Milovanović I. Antioxidant protective effects of mushroom metabolites. Curr Top Med Chem. 2013;13(21):2660–76. [DOI] [PubMed] [Google Scholar]
- 22.Beelman RB, Kalaras MD, Richie JPJ. Micronutrients and bioactive compounds in mushrooms: a recipe for healthy aging?. Nutrition Today. 2019;54(1):16–22. [Google Scholar]
- 23.Jo Feeney M, Miller AM, Roupas P. Mushrooms—biologically distinct and nutritionally unique: exploring a “third food kingdom.”. Nutrition Today. 2014;49(6):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HX, Liu WK, Ng TB, Ooi VEC, Chang ST. The immunomodulatory and antitumor activities of lectins from the mushroom Tricholoma mongolicum. Immunopharmacology. 1996;31(2–3):205–11. [DOI] [PubMed] [Google Scholar]
- 25.Borchers AT, Stern JS, Hackman RM, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med. 1999;221(4):281–93. [DOI] [PubMed] [Google Scholar]
- 26.Hong SA, Kim K, Nam S-J, Kong G, Kim MK. A case–control study on the dietary intake of mushrooms and breast cancer risk among Korean women. Int J Cancer. 2008;122(4):919–23. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C-X, Ho SC, Chen Y-M, Fu J-H, Cheng S-Z, Lin F-Y. Greater vegetable and fruit intake is associated with a lower risk of breast cancer among Chinese women. Int J Cancer. 2009;125(1):181–8. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Huang J, Xie X, Holman CD. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int J Cancer. 2009;124(6):1404–8. [DOI] [PubMed] [Google Scholar]
- 29.Shin A, Kim J, Lim S-Y, Kim G, Sung M-K, Lee E-S, Ro J. Dietary mushroom intake and the risk of breast cancer based on hormone receptor status. Nutr Cancer. 2010;62(4):476–83. [DOI] [PubMed] [Google Scholar]
- 30.Lee S-A, Kang D, Nishio H, Lee MJ, Kim D-H, Han W, Yoo K-Y, Ahn S-H, Choe K-J, Hirvonen Aet al. Methylenetetrahydrofolate reductase polymorphism, diet, and breast cancer in Korean women. Exp Mol Med. 2004;36(2):116–21. [DOI] [PubMed] [Google Scholar]
- 31.Mizoo T, Taira N, Nishiyama K, Nogami T, Iwamoto T, Motoki T, Shien T, Matsuoka J, Doihara H, Ishihara Set al. Effects of lifestyle and single nucleotide polymorphisms on breast cancer risk: a case-control study in Japanese women. BMC Cancer. 2013;13:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spolar MR, Schaffer EM, Beelman RB, Milner JA. Selenium-enriched Agaricus bisporus mushrooms suppress 7,12-dimethlybenz[a]anthracene bioactivation in mammary tissue. Cancer Lett. 1999;138(1–2):145–50. [DOI] [PubMed] [Google Scholar]
- 33.Masala G, Assedi M, Bendinelli B, Ermini I, Sieri S, Grioni S, Sacerdote C, Ricceri F, Panico S, Mattiello Aet al. Fruit and vegetables consumption and breast cancer risk: the EPIC Italy study. Breast Cancer Res Treat. 2012;132(3):1127–36. [DOI] [PubMed] [Google Scholar]
- 34.Lee DH, Yang M, Keum N, Giovannucci EL, Sun Q, Chavarro JE. Mushroom consumption and risk of total and site-specific cancer in two large U.S. prospective cohorts. Cancer Prev Res. 2019;12(8):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Gils CH, Peeters PH, Bueno-de-Mesquita HB, Boshuizen HC, Lahmann PH, Clavel-Chapelon F, Thiébaut A, Kesse E, Sieri S, Palli Det al. Consumption of vegetables and fruits and risk of breast cancer. JAMA. 2005;293(2):183–93. [DOI] [PubMed] [Google Scholar]
- 36.Ko K-P, Park SK, Yang JJ, Ma SH, Gwack J, Shin A, Kim Y, Kang D, Chang S-H, Shin H-Ret al. Intake of soy products and other foods and gastric cancer risk: a prospective study. J Epidemiol. 2013;23(5):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Zou L, Chen W, Zhu B, Shen N, Ke J, Lou J, Song R, Zhong R, Miao X. Dietary mushroom intake may reduce the risk of breast cancer: evidence from a meta-analysis of observational studies. PLoS One. 2014;9(4):e93437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 39.Reeves BC, Deeks JJ, Higgins JPT, Shea B, Tugwell P, Wells GA. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). [Internet]. Cochrane; 2000; (Accessed 2021 Feb 17). Available from: https://training.cochrane.org/handbook/current/chapter-24. [Google Scholar]
- 40.Woo HD, Park S, Oh K, Kim HJ, Shin HR, Moon HK, Kim J. Diet and cancer risk in the Korean population: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(19):8509–19. [DOI] [PubMed] [Google Scholar]
- 41.Lee AH, Pasalich M, Su D, Tang L, Tran VD, Binns CW. Mushroom intake and risk of epithelial ovarian cancer in southern Chinese women. Int J Gynecol Cancer. 2013;23(8):1400–5. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Xiang Y-B, Li H-L, Yang G, Cai H, Ji B-T, Gao Y-T, Zheng W, Shu X-O. Vegetable-based dietary pattern and liver cancer risk: results from the Shanghai Women's and Men's Health Studies. Cancer Sci. 2013;104(10):1353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HJ, Chang WK, Kim MK, Lee SS, Choi BY. Dietary factors and gastric cancer in Korea: a case-control study. Int J Cancer. 2002;97(4):531–5. [DOI] [PubMed] [Google Scholar]
- 44.Dunneram Y, Greenwood DC, Cade JE. Diet and risk of breast, endometrial and ovarian cancer: UK Women's Cohort Study. Br J Nutr. 2019;122(5):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Vegetable and fruit consumption and prostate cancer risk: a cohort study in The Netherlands. Cancer Epidemiol Biomarkers Prev. 1998;7(8):673–80. [PubMed] [Google Scholar]
- 46.Zhang S, Sugawara Y, Chen S, Beelman RB, Tsuduki T, Tomata Y, Matsuyama S, Tsuji I. Mushroom consumption and incident risk of prostate cancer in Japan: a pooled analysis of the Miyagi Cohort Study and the Ohsaki Cohort Study. Int J Cancer. 2020;146(10):2712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HS, Kim HS, Choi SY, Chung CK. Effect of dietary factors in the etiology of stomach cancer. Korean J Epidemiol. 1998;20(1):82–101. [Google Scholar]
- 48.Lee EJ, Suh SW, Lee WK, Lee HS. Reproductive factor and food intake pattern influencing on the breast cancer risk in Daegu Gyungbuk Area, Korea. Korean J Nutr. 2007;40(4):334–46. [Google Scholar]
- 49.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- 50.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. [Internet]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch Veditors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane; 2020. [Accessed 2020 Oct 20]. Available from: https://training.cochrane.org/handbook/current/chapter-10. [Google Scholar]
- 51.Mathias H, Pim C, David E. Doing meta-analysis in R. Zenodo, version 1.0.0. 2019; Jan 28. [Google Scholar]
- 52.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Cham (Switzerland): Springer International; 2015. [Google Scholar]
- 53.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 54.Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005;97(23):1768–77. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289:579–88. [DOI] [PubMed] [Google Scholar]
- 56.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 58.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Temple NJ. Antioxidants and disease: more questions than answers. Nutr Res. 2000;20:449–59. [Google Scholar]
- 60.Willett WC. Diet and health: what should we eat?. Science. 1994;264:532–7. [DOI] [PubMed] [Google Scholar]
- 61.Willett WC. Balancing life-style and genomics research for disease prevention. Science. 2002;296(5568):695–8. [DOI] [PubMed] [Google Scholar]
- 62.Willcox JK, Ash SL, Catignani GL. Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr. 2004;44(4):275–95. [DOI] [PubMed] [Google Scholar]
- 63.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce Det al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ey J, Schömig E, Taubert D. Dietary sources and antioxidant effects of ergothioneine. J Agric Food Chem. 2007;55(16):6466–74. [DOI] [PubMed] [Google Scholar]
- 65.Weigand-Heller AJ, Kris-Etherton PM, Beelman RB. The bioavailability of ergothioneine from mushrooms (Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev Med. 2012;54 Suppl:S75–S8. [DOI] [PubMed] [Google Scholar]
- 66.Cheah IK, Halliwell B. Could ergothioneine aid in the treatment of coronavirus patients?. Antioxidants. 2020;9(7):595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ames BN. Prolonging healthy aging: longevity vitamins and proteins. Proc Natl Acad Sci U S A. 2018;115(43):10836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith E, Ottosson F, Hellstrand S, Ericson U, Orho-Melander M, Fernandez C, Melander O. Ergothioneine is associated with reduced mortality and decreased risk of cardiovascular disease. Heart. 2020;106(9):691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen S-N, Chang C-S, Hung M-H, Chen S, Wang W, Tai C-J, Lu C-L. The effect of mushroom beta-glucans from solid culture of Ganoderma lucidum on inhibition of the primary tumor metastasis. Evid Based Complement Altern Med. 2014:252171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hetland G, Johnson E, Lyberg T, Kvalheim G. The mushroom Agaricus blazei Murill elicits medicinal effects on tumor, infection, allergy, and inflammation through its modulation of innate immunity and amelioration of Th1/Th2 imbalance and inflammation. Adv Pharm Sci. 2011:157015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friedman M. Mushroom polysaccharides: chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods. 2016;5(4):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu C-H, Kan S-F, Shu C-H, Lu T-J, Sun-Hwang L, Wang PS. Inhibitory mechanisms of Agaricus blazei Murill on the growth of prostate cancer in vitro and in vivo. J Nutr Biochem. 2009;20(10):753–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.