Abstract
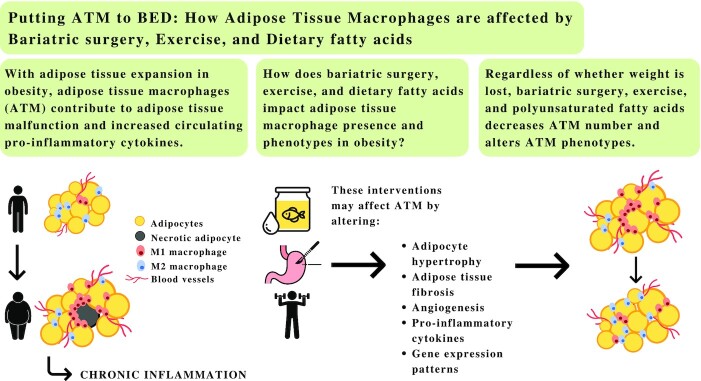
With increasing adiposity in obesity, adipose tissue macrophages contribute to adipose tissue malfunction and increased circulating proinflammatory cytokines. The chronic low-grade inflammation that occurs in obesity ultimately gives rise to a state of metainflammation that increases the risk of metabolic disease. To date, only lifestyle and surgical interventions have been shown to be somewhat effective at reversing the negative consequences of obesity and restoring adipose tissue homeostasis. Exercise, dietary interventions, and bariatric surgery result in immunomodulation, and for some individuals their effects are significant with or without weight loss. Robust evidence suggests that these interventions reduce chronic inflammation, in part, by affecting macrophage infiltration and promoting a phenotypic switch from the M1- to M2-like macrophages. The purpose of this review is to discuss the impact of dietary fatty acids, exercise, and bariatric surgery on cellular characteristics affecting adipose tissue macrophage presence and phenotypes in obesity.
Keywords: physical activity, dietary fatty acids, bariatric surgery, macrophages, adipose tissue characteristics, meta-inflammation
Adipose tissue macrophages play an important role in metabolic disease risk. This review discusses the effects of dietary fatty acids, exercise, and bariatric surgery on modulating adipose tissue macrophages.
Graphical Abstract
Introduction
According to the WHO, the number of individuals with obesity worldwide, adults and children, has nearly tripled since 1975 (1). This increased prevalence poses a significant threat to health as individuals with obesity are more likely to develop a myriad of related conditions such as type 2 diabetes, cardiovascular diseases (CVD), and certain types of cancers (2, 3). These health consequences, in part, stem from the negative effects of excess adipose tissue accumulation leading to morphologic and functional abnormalities (2). Subsequent, endocrine, metabolic, and immune derangements follow, which contribute to the obesity-associated inflammation that is, in part, mediated by macrophages (4, 5). Indeed, macrophages can be viewed as central to tissue stress, contributing to adipose tissue malfunction and increased circulating proinflammatory cytokines as obesity progresses (5, 6). The resulting chronic inflammatory state leads to adipocyte maladaptation and subsequent increases in angiogenesis, production of extracellular matrix (ECM), macrophage infiltration, and proinflammatory response (5–7); all of the aforementioned local consequences feed in a positive feedback loop exacerbating one another.
In both human and animal models, lifestyle and surgical interventions resulting in weight loss decreased macrophage infiltration and led to a phenotypic switch of the adipose tissue macrophages (ATM) (8–13). In the case of exercise, the beneficial effects were observed regardless of weight loss. Several mechanisms have been proposed to explain the anti-inflammatory properties of physical activity and the differential properties of dietary fatty acids culminating in beneficial quantitative and qualitative changes in ATM profiles (14). The aim of this review is to discuss the impact of dietary fatty acids, exercise, and bariatric surgery on the mechanisms that affect ATM presence and phenotype in obesity.
Macrophages and Obesity
What are macrophages?
Macrophages are innate immune cells that are typically found in every tissue and have the unique ability to sense and respond to pathogens and other environmental cues. Macrophages are particularly important for: tissue repair after an injury, clearance of foreign invaders and cellular debris through phagocytosis, and normal tissue development; they are especially efficient at integrating endocrine and paracrine signals in order to respond to stimuli (15, 16). Additionally, these phagocytes are prolific communicators as they interact directly with the receptors of other tissue-resident cells, immune cells recruited during injury (e.g. T cells), and extracellular proteins (15, 16). Other noteworthy characteristic features of these monocyte-derived cells are that they are heterogeneous and exhibit high levels of plasticity.
Macrophages are able to acquire different molecular and functional phenotypes after being exposed to different bioactive molecules and environments (16, 17). Indeed, macrophages can differentiate to proinflammatory M1 or anti-inflammatory M2 cell phenotypes, though for this process to occur macrophages need to be activated or polarized (7, 18). However, how M1 and M2 macrophages come to be in the adipose tissue remains ambiguous. It has been suggested that shifts in the M1: M2 macrophage ratio occurs from the transformation of resident macrophages during the course of resolution of an injury or from the continuous recruitment of monocytes in response to tissue stress (15).
The polarization of macrophages to M1 cells is mediated by type 1 T helper cells that secrete IFN-γ or with bacterial products (e.g. LPS). M1 macrophages produce proinflammatory cytokines such as TNF-α and IL-6 and they express inducible nitric oxide synthase (iNOS), reactive oxygen species (ROS), and nitrogen intermediates (7, 18). These proinflammatory molecules have been associated with the onset of numerous diseases such as CVD or type 2 diabetes (19–21). For example, TNF-α knockout mice had improved insulin sensitivity and lower concentrations of circulating free fatty acids (22). Conversely, the polarization of macrophages to M2 cells is mediated by type 2 T helper cells that secrete IL-4 and IL-13. M2 macrophages produce anti-inflammatory cytokines such as IL-4, IL-10, and TGF-β, which block the activity of iNOS and downregulate the synthesis of proinflammatory cytokines (7, 18, 23). M2 macrophages are more often associated with wound healing, resolution of inflammation, clearing of cellular debris, regulating proliferation, precursors of angiogenesis, and remodeling of the ECM, whereas M1-like macrophages appear to promote the opposite (6, 24). It should be noted that the M1/M2 paradigm is often seen as an oversimplified dichotomous division and should rather be considered as a continuum (6, 15, 24, 25). The identification of M1 and M2 cells is also challenging as phenotype markers are not specific and may indicate other cell types. The literature therefore identifies macrophage cells as M1-like and M2-like.
Macrophages in obesity
A plethora of immune cells accumulate within the expanding adipose tissue (6), although the macrophage population remains the predominant one (26). Macrophages make up ∼5–10% of the stromal vascular fraction (SVF) cells derived from adipose tissue of lean individuals, whereas in individuals with obesity, the SVF can consist of ≤40–50% macrophages (27). To preserve adipose tissue homeostasis and functionality, there has to be a balance between both populations of M1- and M2-like macrophages (7, 26). However, the phenotypic heterogeneity of macrophages is environment dependent (7, 18, 26). In the lean state, the balance of the macrophage population tends to shift towards the anti-inflammatory M2-like subpopulation (26). In comparison, in the obese state, the balance tilts toward the M1-like subpopulation, thus, creating a proinflammatory environment within the adipose tissue (26, 28–30). The accumulation of ATM in individuals with obesity has been linked with adipocyte and metabolic dysfunction (6, 24, 31).
Lifestyle and Surgical Interventions
Dietary fatty acids
The seminal work of Weisberg et al. (32) and Xu et al. (33) were the first to demonstrate that high-fat diets (HFDs) increase macrophage content and trafficking within the fatty depots that are associated with the development of obesity-induced insulin resistance. Indeed, fatty acids are thought to be immunomodulators of inflammatory pathways. However, not all fats are equal and different fats may have differential effects on macrophages and adipose tissue characteristics (34, 35).
Saturated Fatty Acids
Effects of saturated fatty acids on macrophage polarization and infiltration in rodents
Studies suggest that diets rich in saturated fatty acids (SFA) are associated with inflammation as they are considered naturally occurring ligands for the toll-like receptors (TLR), which activate downstream inflammatory pathways, on both adipocytes and macrophages/monocytes (36–38). Obese rodents, fed with diets rich in SFAs (mainly from lard), have increased expression of TLRs and markers associated with macrophage infiltration (38–45) (see Table 1). Moreover, the activation of the TLR inflammatory pathways by an increased flux of SFAs are thought to contribute to the classical polarization of M1-like macrophages. For example, Enos et al. (42) examined the effects of 3 HFDs, differing in the percentage of total calories from saturated fat (6%, 12%, and 24%) but identical in total fat (40%), on macrophage behavior. All HFDs increased adipose tissue inflammation, but the 12% and 24% saturated fat diets increased TLR2 expression, and led to the greatest increase in M1- and M2-like macrophages (42). Additionally, several murine studies reported that feeding of SFA-rich diets worsened ROS production, the expression of adipose tissue remodeling markers [e.g. TGF-β, tissue inhibitor matrix metalloproteinase 1 (TIMP1), collagen VI, hypoxia-inducible factor 1-α (HIF-1α), and PPARγ], decreased capillary density, and increased adipocyte size, proinflammatory cytokines [e.g. IL-6, TNF-α, monocyte chemoattractant protein-1 (MCP-1), C-reactive protein (CRP)] and the number of crown-like structures (39, 41, 42, 44–52). These changes may collectively prompt the aggregation of proinflammatory macrophages. Thus, it appears that a diet rich in SFAs may trigger the development of pathogenic remodeling processes in rodents in response to the accumulation of M1-like macrophages.
TABLE 1.
The effect of SFA-rich diets on macrophage infiltration and polarization in rodent and human studies
| Reference | N | Rodents or participants | Diet | Weight change | Macrophage/phenotype change |
|---|---|---|---|---|---|
| Coenen et al. (38, 39) | 48 and 58 | C57BL/6 mice | Western diet (42% fat + 0.15% cholesterol) vs. control diet12 wk | ↑ | ↑ infiltration of macrophages |
| Davis et al. (41) | 75 | Control male C57BL/10J mice and male Tlr-4-deficient C57BL/10ScN mice | 3 experimental diets: low-fat control (LFC) vs. high-fat control (HFC) vs. high-fat palmitate (HFP)16 wk | ↑ | ↑ % of macrophages |
| Enos et al. (42) | 45 | Male C57BL/6 mice | 5 treatment diets: 2 control diets vs. 3 HFDs (6% SF, 12% SF, and 24% SF)16 wk | ↑ | ↑ M1↑ M2 ↑ infiltration of macrophages |
| Prieur et al. (44) | 36 | Wild-type C57BL/6 male mice and ob/ob mice | HFD (45% fat) vs. control diet (11.5% fat)12 wk | Ø | ↑ M1 ↓ M2 |
| Cullberg et al. (40) | N/A | Cell culture | In vitro: 3T3-L1 adipocytes and THP-1 macrophages were incubated for 24 h with FFAs (oleic, palmitic, and elaidic acids) | Ø | ↑ 1.8-fold M1 |
| Nguyen et al. (43) | 40 | Wild-type male C57BL/mice and ob/obJ male mice Cell culture | HFD (40% fat) vs. control diet (12% fat)For 1, 12, or 20 wkIn vitro: RAW264.7 cells were cultured and treated with FFA (arachidonic, lauric, linoleic, oleic, and myristic acids) | Ø | ↑ M1 ↑ infiltration of macrophages |
| van Dijk et al. (53) | 20 | Abdominally overweight middle-aged adults (10 male and 10 female) | 2 experimental diets: SFA-rich diet (19% SFAs and 11% MUFAs) vs. MUFA-rich diet (11% SFAs and 20% MUFAs)8 wk | Ø | ↑ M1↑ M2 |
FFA, free fatty acids; HFD: High-fat diet; SF, saturated fat; lean (BMI ≤24.9); overweight (BMI 25–29.9); class I (BMI 30–34.9); class II (BMI 35–39.9); class III (BMI ≥40) ↓ : significant decrease; ↑ : significant increase; Ø: no significant change; N/A: not applicable.
Effects of saturated fatty acids on macrophage polarization and infiltration in humans
In human studies, SFAs also increased TLR genes and proinflammatory cytokines [e.g. IL-1β, IL-6, IL-8, TNF-α, chemokine (C-C motif) ligand 5 (CCL5)] in lean subjects, those with obesity, and in those with diabetes (54, 55) (see Table 1). van Dijk et al. (53) conducted a parallel controlled-feeding trial in 20 subjects who were abdominally overweight randomized to a SFA-based diet or a MUFA-based diet for 8 wk. Whole-genome microarray and histologic analysis of the adipose tissue showed that the consumption of SFAs increased proinflammatory obesity-linked gene expression including the downregulation of PPARγ and upregulation of the TLRs and macrophage marker genes (CD14 and CD163) (53). Of particular note, is that the participants’ weights were not significantly different between the diet groups and did not change throughout the intervention, ruling out the cofounding factor of weight gain. The direct effects of SFA on macrophage infiltration and polarization, cellular characteristics, and adipose tissue remodeling in individuals with obesity are still poorly documented and require further investigation.
Other contributing factors to inflammation in obesity
A diet rich in SFAs may also represent a crucial first step in disturbing the gut microbiota. This disruption in the gut microbiota results in alterations in the epithelial cells of the intestinal barrier while promoting the translocation of bacteria and their cellular components into the circulation (56, 57). Consequently, a diet high in SFAs may contribute to a rise in the systemic concentration of LPS, which can act as a ligand to the TLRs on the surface of ATM and adipocytes (58–63). As such, it has been hypothesized that the translocation of LPS and bacterial metabolites may increase the release of proinflammatory cytokines, promote macrophage infiltration, and prompt a phenotype switch towards the M1-like cells (64, 65). However, a phenotypic switch has yet to be demonstrated in humans, and the influence of LPS-mediated inflammation on adipose tissue characteristics is rather unknown. Moreover, recent studies suggest that lifestyle and surgical interventions may also partially revert gut microbiota dysbiosis to improve gut health and possibly inflammation (66–68). Overall, the gut microbiota-related inflammation represents a promising alternate pathway to explain the chronic low-grade inflammation seen in individuals with obesity.
n–3 Polyunsaturated Fatty Acids
Conversely to SFAs, n–3 PUFAs have the capacity to induce anti-inflammatory and insulin-sensitizing effects on adipocytes and their resident macrophages. These metabolic improvements have been predominantly observed with n–3 PUFA supplementation from fish oil (i.e. EPA and DHA). The anti-inflammatory n–3 PUFAs are known endogenous ligands to PPARγ and free fatty acid receptor 4 (FFAR4), have the ability to preferentially inhibit TLR-induced pathways, and reduce the expression of proinflammatory transcription factors (69, 70–72).
Numerous studies conducted on both humans and rodents alike have demonstrated the potential advantageous effects of n–3 PUFAs on macrophage infiltration and phenotypic shifts, culminating in the amelioration of adipose tissue homeostasis. Indeed, following a dietary regimen enriched in n–3 PUFAs, the number of macrophages and specific markers of macrophage polarization for the M1- and M2-like cells fluctuated favoring an M2-dominant ratio (11, 73–92) (see Table 2). Itariu et al. (75) conducted an 8-wk randomized trial on 55 nondiabetic individuals with class III obesity who received either 3.36 g EPA/DHA or the equivalent amount of butterfat each day. They found that, despite no changes in M2 macrophage markers [mannose receptor C type 1 (MRC1) and CD163], pan macrophage marker (CD68), and the total number of macrophages, the expression of CD40, an M1 marker, was downregulated by n–3 PUFA treatment. Another study demonstrated that after participants with class I obesity consumed 4 g of fish oil (∼3.6 g EPA and DHA) per day for 12 wk, significant decreases in total macrophage number and CD68 mRNA concentrations were observed (79). Further in vitro experiments showed that the addition of DHA to M1 macrophage cultures and cocultures with adipocytes markedly reduced the expression of MCP-1 (79). Therefore, fish oils may not only reduce macrophage abundance in adipose tissue, but also decrease the migration and infiltration of monocytes into adipose tissue (79). More recently, 3 other in vitro studies also supported these findings through similar observations and conclusions (74, 76, 77). On the other hand, in another study where individuals with overweight to class I obesity consumed 3.5% of their diet as fish oil, no difference in ATM gene expression (CD14 and CD206) was observed (93). The discrepancies in the findings may be explained by the differences in the n–3 PUFAs dose administered, the composition of the n–3 PUFAs used, or the weight status of the participants. The studies by Itariu and Spencer (75, 79) included individuals with more severe cases of obesity, which may suggest that the anti-inflammatory properties of n–3 PUFAs are more significant in individuals with greater obesity severity.
TABLE 2.
The effects of n–3 PUFAs on macrophage infiltration and polarization in rodent and human studies
| Reference | N | Rodents or participants | Diet | Weight change | Macrophage/phenotype change |
|---|---|---|---|---|---|
| Bashir et al. (80) | 25 | Male C57BL/6J mice | 3 experimental diets: control diet vs. HFD group (60% fat) vs. HFD + flaxseed oil (4, 8 or 16 mg/kg b.w.)18 wk | ↓ | ↓ M1↑ M2 |
| Fan et al. (84) | 47 | Male C57BL/6J mice | 3 experimental diets: HFD with ALA-enriched butter vs. HFD with butter lacking ALA and LA vs. HFD with ALA and LA-enriched margarine10 wk | Ø | ↓ M1↑ M2↓ infiltration of macrophages |
| Lopez-Vicario et al. (86) | 46 | Wild-type male mice and male hemizygous fat-1 mice | 3 experimental diets: control diet (13% fat) vs. HFD + placebo (60% fat) vs. HFD + sEH inhibitor16 wk | ↑ | ↑ M2↓ infiltration of macrophages |
| Titos et al. (87) | 37 | Male C57BL/6J mice | Control diet (13% fat) vs. HFD (60% fat)Animals then received a placebo or DHA (4 μg/g b.w.) every day for 10 d12 wk | Ø | ↓ M1↑ M2 Ø total ATM |
| Todoric et al. (88) | 49 | Male C57BL/KsJ-leprdb/leprdb diabetic (db/db) mice and nondiabetic mice (db/+) | 4 experimental diets: control diet vs. HFD + SFA + MUFA vs. HFD + n–6 PUFA vs. HFD + marine n–3 PUFA6 wk | ↑ | ↓ M1↓ infiltration of macrophages |
| White et al. (89) | 4–14 depending on measurement | Male hemizygous fat-1(+/−) | Control diet vs. HFD (55% fat)8 wk*Transgenic expression of fat-1 n–3 fatty acid desaturase was used to endogenously produce n–3 fatty acids in HF-fed mice | Ø | ↓ infiltration of macrophages↓ crown-like structures |
| Chan et al. (81) | Ø | Cell culture | Low-fat diet (10% fat) vs. HFD (60% fat)18 wkIn vitro: bone marrow-derived macrophages were cultured with palmitate or palmitoleate | Ø | ↑ M2↓ M1 |
| Chang et al. (82) | Ø | Cell culture | In vitro: murine macrophages and human T lymphocytes were cocultured and treated with DHA | Ø | ↑ M2↓ M1 |
| Colson et al. (83) | 24 | Male C57BL/6J miceCell culture | n–6-enriched control diet (12% fat) vs. n–3-enriched control diet (12% fat)12 wkIn vitro: THP-1 cells were cultured for differentiation experiments | Ø | ↑ M2 Ø M1 |
| De Boer et al. (91) | 32 | Male and female C57BL/6 miceCell culture | 4 experimental diets: HF control diet (34% fat) vs. HFD + FO (34% fat + 7.6% FO) vs. low-fat control diet (10% fat) vs. low fat + FO (10% fat + 3% FO)12 wkIn vitro: macrophages were cocultured with adipocytes | ↑ | ↓ M1 |
| De Boer et al. (92) | 10 | Male C57BL/6 miceCell culture | Control diet (10% SO) vs. LC n–3 PUFA-enriched diet (3% FO + 7% SO)4 wkIn vitro: visceral adipose tissue were collected to create adipose tissue conditioned media and challenged with LPS to mimic acute and chronic conditions | ↑ | ↓ M1↓ M2 |
| Liddle et al. (85) | 30 | Male and female C57BL/6 miceCell culture | Control diet (10% SO) vs. treatment diet (7% SO + 3% FO)4 wkIn vitro: RAW264.7 macrophages were cocultured with LPS-stimulated CD8+ T cells/adipocytes | Ø | ↓ M1↑ M2 |
| Baranowski et al. (11) | 21 | Male fa/fa Zucker rats and 7 lean Zucker rats | Control diet vs. ALA-rich flaxseed oil diet8 wk | Ø | Ø macrophage infiltration among groups |
| Itariu et al. (75) | 55 | Nondiabetic adults with class III obesity | 3.36 g long-chain n–3 PUFAs/d vs. 5 g of butter/d in addition to an isocaloric diet (55% carbohydrates, 15% protein, and 30% fat)8 wk | Ø | ↓ M1 Ø M2 Ø total ATM and infiltration |
| Kratz et al. (93) | 24 | Individuals with overweight to class I obesity (8 males and 16 females) | Control diet (0.5% n–3 PUFAs) vs. n–3 PUFA-rich diet (3.5%)14 wk | ↓ | Ø macrophage phenotypes and infiltration |
| Spencer et al. (79) | 33 | Adults with class I obesity (11 males and 22 females)Cell culture | 4 g of n–3 fatty acid ethyl esters vs. placebo (corn oil)12 wkIn vitro: M1 macrophage culture and M1 macrophage cocultured with adipocytes were treated with DHA | Ø | ↓ total ATM↓ crown-like structuresDHA decreased MCP-1 expression in cultured M1 macrophages and in cocultures of macrophages and adipocytes |
| Ferguson et al. (74) | N/A | Cell culture | In vitro: human SAT from lean and obese subjects were treated with EPA and/or DHA throughout differentiation or for 72 h postdifferentiation. THP-1 monocytes were added to adipocyte cocultures | Ø | ↑ M2↓ M1 |
| Pandurangan et al. (77) | Ø | Cell culture | In vitro: human adipocytes and macrophages were cocultured and treated with chia seed fatty acid (0–6.4 μg/mL) | Ø | ↓ M1↓ macrophage recruitment |
| Montserrat-de la Paz et al. (76) | 6 | Healthy adult malesCell culture | Participants were all given 3 times a meal rich in SFA, MUFA or MUFA + ω-3 LC PUFA with or without niacin.In vitro: monocytes were isolated to be differentiated into naïve macrophages; TLRs were also isolated | Ø | ↓ M1↑ M2 |
ALA, α-linoleic acid; ATM, adipose tissue macrophage; b.w., body weight; FO, fish oil; HF, high-fat; HFD, high-fat diet; LA, linoleic acid; LC, long chain; SAT, subcutaneous adipose tissue; sHE, soluble epoxide hydrolase; SO, safflower oil; TLR, toll-like receptor; %fat expressed based on total energy; lean (BMI ≤24.9); overweight (BMI 25–29.9); class I (BMI 30–34.9); class II (BMI 35–39.9); class III (BMI ≥40); ↓ : significant decrease; ↑ : significant increase; Ø: no significant change; N/A: not applicable.
Beneficial shifts in the M1-:M2-like macrophage ratio following n–3 PUFA supplementation may be due to a number of underlying mechanisms. Supplementation resulted in improvements in cellular stress (45, 66, 85, 94–98), metabolic profile (99), synthesis and release of anti-inflammatory mediators [i.e. IL-10, IL-4, arginase-1 (ARG-1) and adiponectin], while decreasing the secretion of proinflammatory mediators (i.e. IL-1β, IL-6, TNF-α, and MCP-1) (11, 73–75, 77, 79–81, 83–85, 100–105, 100), adipocyte enlargement (11, 12, 73, 77, 84, 95, 100, 106, 107), and the deposition of ECM and the expression of its associated markers (12, 75, 80, 85). Increased capillary density (79) and adipogenesis (73, 95, 106, 108, 109) have also been shown with supplementation. Moreover, supplementation of n–3 PUFAs downregulated the expression of important inflammatory transcription factors and receptors, such as NF-κB and TLR4, concomitant with an upregulation in adipogenic regulators [PPARγ and CCAAT-enhancer-binding protein α (C/EBPα)] (45, 73, 77, 80, 85, 90, 102, 103, 106, 109, 110). Additionally, some murine studies also observed changes in weight and fat mass loss with the previously mentioned improvements (73), whereas studies in humans showed the downregulation of inflammatory factors associated with PUFA consumption in the absence of changes in weight or body composition (73, 75, 93).
Overall, n–3 PUFA supplementation may represent a potential therapeutic avenue to improve macrophage-mediated inflammation and adipose tissue characteristics, although the effects were less potent in vivo (73, 75, 93). The inconsistent results in human studies are likely to be attributed to variability in study design, weight status of participants, and adherence as most trials are outpatient studies and rely on self-reporting. Other factors may include differences in the amount of dosage administered or methods of calculating n–3 PUFA intake (71, 73, 111). Nonetheless, further studies should continue to explore the role of n–3 PUFAs in mediating ATM infiltration and phenotype.
Physical activity
Lack of exercise and prolonged sedentary behaviors are important catalysts for a cluster of metabolic and chronic diseases, whereas regular exercise may prevent or delay the progression of insulin resistance, hypertension, CVD, and diabetes (112, 113). Although numerous studies have denoted that the salutary effects of exercise are independent of weight loss (113–116); significant weight loss may amplify the exercise-induced benefits and have a greater impact on the inflammatory markers in humans (9, 117, 118). In fact, physical activity was shown to induce cellular and molecular changes in the adipose tissue in a way that alleviates the low-grade chronic inflammation that accompanies obesity (8–10, 119–121). The underlying mechanisms that contribute to the exercise-induced anti-inflammatory responses have not been completely elucidated. A major contributor to the reduction in inflammation accompanying exercise may reside in the mediation of ATM (14, 113, 116, 122, 123).
The effects of exercise on macrophage infiltration and phenotypes
Exercise, with or without weight loss, may decrease inflammation via promoting a phenotypic switching from M1 to M2 macrophages while simultaneously diminishing the trafficking of the macrophages within the adipose tissue. The early work of Kawanishi et al. (47) demonstrated that 16 wk of cardiovascular exercise training (12–20 m/min, 60 min/d, and 5 times/wk) in mice with obesity reduced M1-like and increased M2-like macrophage mRNA expression in adipose tissue such that the M1-:M2-like ratio was ∼50% lower with the exercise intervention relative to control. Quantification of macrophages in adipose tissue by flow cytometry also showed that exercise decreased both the proportion and absolute number of M1-like (CD11c+) macrophages (124). Another study found that in comparison to continuous training (steady state running at 20 m/min), aerobic interval training (3-min bouts at 40 m/min, interspersed by 3-min active recovery at 20 m/min on a treadmill with 15% incline, repeated 6 times per session) has been shown to result in greater increases in the number of M2-like macrophages (181% versus 122%) in mesenteric adipose tissue (48). More recent murine studies have demonstrated diminished infiltration and phenotypic shifts in macrophages (48, 125–130) (see Table 3).
TABLE 3.
The effects of exercise on macrophage infiltration and polarization in rodent and human studies
| Reference | N | Rodents or participants | Exercise intervention | Weight change | Macrophage/phenotype change |
|---|---|---|---|---|---|
| Kawanishi et al. (47, 124) | 40 | Male C57BL/6 mice | Treadmill running12–20 m/min × 60 min/d16 wk | Ø | ↓ M1↑ M2↓ number of macrophages |
| Macpherson et al. (126) | 27 | Male C57BL/6 mice | Treadmill running3 d × 15 min/d at 15 m/min acclimation2 h at 15 m/min with 5% incline | Ø | ↓ M1↑ M2↓ infiltration of M1-like macrophages |
| Linden et al. (125) | 113 | Male C57Bl6/J mice | Treadmill running40 min/d at 12 m/min with 8% incline4, 8, or 12 wk | Ø | ↓ M1↑ M2↓ infiltration of macrophages |
| Luo et al. (128) | 54 | Male C57BL/6H mice | Treadmill running45% of peak running speed, with 5% incline, 1 h/d, 6 d/wk8 wk | ↓ | ↓ M1↑ M2 |
| Baek et al. (129) | 49 | Male C57BL/6 J mice | Treadmill running at 10 m/min for 60 minMice ran at different intensities from week 2 to week 8 | ↓ | ↓ M1↑ M2 |
| Oliveira et al. (127) | 24 | Male Wistar rats | Swimming2-d swimming × 10 min/d acclimation3 h of exercise with a 45-min rest period | Ø | ↓ M1↑ M2 Ø in infiltration |
| Kolahdouzi et al. (48) | 48 | Male Wistar rats | Treadmill running5 d/wk3 groups: Sedentary vs. CT vs. AIT10 wk | CT: ↓ 30% weight loss AIT: ↓ 40% weight loss | ↓ M1↑ M2↓ number of macrophages |
| Shanaki et al. (130) | 45 | Male Wistar rats | Treadmill running (HIIT or CT)5 d/wk10 wk | ↓ | ↓ M1↑ M2 |
| Bruun et al. (9) | 27 | Individuals with class III obesity (15 females and 12 males) | 2–3 h of exercise5 d/wk15 wkIncluded a diet | ↓ ∼ 14% weight loss | ↓ ∼55% M1↓ ∼40% number of macrophages |
| Auerbach et al. (119) | 60 | Healthy and overweight adult men | Endurance training for 7 d/wk4 groups: training-induced weight loss (T) vs. diet-induced weight loss (D) vs. training and increased diet without weight loss (T-iD) vs. control (C)12 wk | ↓ 6% weight loss in the endurance training group (T) | ↑ 2.5-fold M2Ø macrophage number |
| yakeu et al. (132) | 17 | Healthy overweight adults (9 males and 8 females) | Walking on treadmill10,000 steps 3 times/wk for 75 min8 wk | Ø | ↓ M1↑ M2 |
| Ruffino et al. (146) | 19 | Overweight adult women | Walking on treadmill3 times/wk for 45 min8 wk | ↓ | ↓ M1↑ M2 |
| Lee et al. (131) | 26 | Sedentary lean or overweight men with or without dysglycemia | 2 sessions of strength training and 2 sessions of spinning4 h/wk12 wk | ↓ | Ø M1↓ M2↓ infiltration of macrophages |
AIT, aerobic-interval training; CT, continuous training; HIIT, high-intensity interval training; lean (BMI ≤24.9); overweight (BMI 25–29.9); class I (BMI 30–34.9); class II (BMI 35–39.9); class III (BMI ≥40); ↓ : significant decrease; ↑ : significant increase; Ø no significant change.
In humans, few studies have looked at the direct impact of exercise on the polarization of the macrophage populations (9, 119, 131) (see Table 3). These studies have found exercise-induced shifts towards a predominant M2-like phenotype. An 8-wk low-intensity exercise intervention (walking 10,000 steps 3 times/wk) in adults with overweight and class I obesity showed that exercise was associated with a ∼2.1-fold upregulation of M2 markers and a downregulation of M1 markers independent of weight loss (132). Additionally, Auerbach et al. (119) and Bruun et al. (9) corroborated the previous findings through an exercise-induced weight-loss protocol suggesting that pronounced weight loss may also further affect macrophage infiltration and phenotype resulting in an anti-inflammatory milieu within adipose tissue.
Mechanisms altering macrophage infiltration and phenotypes in exercise
Exercise decreases expression of proinflammatory and chemotactic signals
There is a growing body of evidence suggesting that exercise decreases the expression of proinflammatory and chemotactic cytokines involved in the recruitment of macrophages and monocytes (14, 113, 116). Among all the cytokines known to potentially contribute to inflammation within the adipose tissue and the chemotaxis of macrophages, TNF-α, IL-6, and MCP-1 appear to be the best studied and were consistently shown to have lower levels of expression following exercise treatment in humans, mice, and rats (8–10, 117, 120, 121, 124, 126, 127, 132, 133–144). Baturcam et al. (133) found that 3-mo supervised exercise {combination of moderate intensity [50–80% of max heart rate (HR)] aerobic exercise and resistance training using either a treadmill or cycling 3–5 times/wk} significantly reduced the expression of both CCL5 and C-C chemokine receptor type 5 (CCR5) in the adipose tissue of individuals with class I to class II obesity with decreases in the concentrations of the proinflammatory markers TNF-α, IL-6, and protein and c-jun NH2 terminal kinase (P-JNK). Complementing these findings, Barry et al. (8) demonstrated that both high-intensity interval training (at 90% of HRpeak, for 1 min interspersed with 1 min of low-intensity recovery periods, progressing from 4 to 10 intervals) and moderate-intensity continuous training (at 65% of HRpeak, progressing from 20 to 50 min) in humans, in the absence of weight and fat mass loss, altered leukocyte trafficking through the downregulation of inflammatory chemokine receptors such as CCR2, CCR5, and C-X-C chemokine receptor type 2 (CXCR2). In humans and rodents, exercise has also been associated with an increase in the expression and release of anti-inflammatory signals such as IL-10, IL-6, ARG-1, and adiponectin (9, 14, 115, 117, 126, 136, 137, 145). Despite the fact that the positive effects of exercise have been observed in the absence of weight loss, weight loss may compound the benefits. An exercise study (aerobic training, 60–75 min/session and 3 times/wk) found that compared with subjects in the lowest tertile (–3%) of weight loss, those in the highest tertile of weight loss (–14.5% weight loss) had larger decreases in macrophage inflammatory protein-1α (MIP-1α) and IL-15 and greater increases in adiponectin (117). Aside from TNF-α, IL-6, and MCP-1, several other cytokines associated with inflammation were shown to have a reduced expression following an exercise intervention such as CCL5, plasminogen activator inhibitor-1 (PAI-1), MIP-1α, CRP, chemerin, IFN-γ, IL-1, IL-8, IL-15, and IL-18, which may also further improve the chronic low-grade inflammation seen in adipose tissue (9, 117, 120, 121, 127, 133, 134, 136, 140).
Exercise affects adipose tissue characteristics
In addition to decreasing proinflammatory and chemotactic signals associated with macrophage recruitment, exercise also directly affects adipose tissue characteristics that are associated with the recruitment and phenotypic changes of ATM. A recent murine study by Kolahdouzi et al. (48) found that aerobic interval training improved adipose tissue dysfunction induced by a HFD through increasing the number of M2-like cells and capillary density while decreasing the total number of crown-like structures and mean adipocyte size (48). Multiple murine studies have also demonstrated beneficial changes in cellularity that may be associated with decreased ATMs. Moreover, several key features of dysfunctional adipose tissue are improved such as lipid and glucose metabolism (134, 135, 147), improved mitochondrial activity and biogenesis (147–150), decreased expression of apoptotic signals (151), decreased expression of angiogenesis precursors (152–157), increased capillary density (48), reduced accumulation of fibrotic depots (10, 46), and reduced adipocyte size (48, 134, 148, 158, 159). Similar findings were also made in human studies where lipid metabolism, mean adipocyte size, adipose tissue fibrosis, and proangiogenic responses were improved following exercise training with or without weight loss (139, 160, 161).
Exercise modifies gene expression patterns
Underlying the mechanisms of the potent anti-inflammatory properties of exercise are tremendous gene expression alterations that may have a direct impact on chronic low-grade inflammation as well as the ubiquitous proinflammatory macrophage infiltration seen in adipose tissue of individuals with obesity (148, 162). Aside from varying the gene expression of pro- and anti-inflammatory cytokines, angiogenic regulators, ECM precursors, markers of mitochondrial activity, and lipid and glucose metabolism (9, 46, 120, 147, 153), physical activity also affects the gene expression of key adipogenic regulators (such as PPARs) and well-characterized immune receptors that modulate inflammatory pathways (like the TLRs) (14).
Several studies highlight the crucial immunomodulating role of PPARs, more specifically PPARγ, in regulating adipose tissue inflammation by promoting the infiltration of M2 macrophages in humans and mice (163–165). Macrophage-specific deletion of the PPARγ gene (163) and upregulation of PPARγ by rosiglitazone (164) in mice demonstrated the role of PPARγ in M2-like macrophage activation. Exercise upregulates PPARγ expression and its related signaling events in adipose tissue and monocytes/macrophages of humans, mice, and rats (146, 166–171), favoring a phenotypic shift towards the M2-like macrophages. In a human study, yakeu et al. (132) found that low-intensity exercise (walking 10,000 steps 3 times/wk) shares similar effects to the pharmacological activation of PPARγ and that a ∼4–5-fold increase in PPARγ activity and expression coincided with a ∼2.1-fold increase in the M2-like macrophage marker (CD14).
TLRs are a class of membrane proteins that play an important role in the innate immune system by initiating key downstream inflammatory pathways through recognition of exogenous and endogenous ligands (172). TLRs, especially TLR2 and TLR4, are present on the cell surface of adipocytes and macrophages [especially M1-like macrophages (173)] and play a pivotal role in obesity-related pathogenesis, including in the development of insulin resistance, and the metabolic syndrome (14, 172). The TLR family are activated by a vast array of ligands, many of which are higher in obesity such as LPS (a marker of gut permeability), oxidized LDLs, and SFAs. The binding of a bioactive molecule to TLRs, results in the activation of NF-κB and the release of proinflammatory cytokines (6, 113, 172). The pivotal role of TLR4 in obesity-associated pathogenesis was demonstrated from the observations that TLR4 knockout mice were protected from the adverse effects of high-fat feeding with attenuated inflammation and macrophage infiltration (113, 172). In parallel with TLR4 knockout mice, exercise training resulted in similar metabolic improvements by decreasing the expression of TLR4 on the cell surface of monocytes and macrophages (138, 174–178); in some cases, TLR4 expression and activity was reduced by ≤35% following exercise interventions (178). In mice and rats, the reduced expression of TLR4 on the surface of the adipocytes and/or SVF cells following exercise correlates with the phenotypic shift in ATM from the M1- to the M2-like phenotype, and reduced macrophage infiltration (47, 127, 177, 179, 180). However, despite the decreased expression of TLR4 activity following exercise training in humans (136, 178, 181–187), more questions remain to be explored regarding the role of TLR4 in macrophage polarization and infiltration. To our knowledge, most human studies on TLR4 expression following exercise examined monocytes rather than ATM directly and results were sometimes inconclusive (188). Given that monocytes are precursor cells of macrophages, it is plausible that the decreased TLR4 expression may also coincide with changes in the phenotype of macrophages as seen in rodents. Further investigations examining the effects of exercise on TLR4 expression in humans is required.
Overall, what remains unknown is which form of exercise training is best to mitigate ATM infiltration and phenotypes in obesity. Several studies indicate that higher intensities and combined training (e.g. combined aerobic and resistance training versus aerobic or resistance training alone) better improved obesity-associated inflammation (116, 189). However, the comparison of these training modalities has not been investigated in ATM infiltration. Future studies should further explore the mechanisms driving macrophage infiltration and polarization in response to exercise and focus on the training modalities (duration, type, volume, and intensity) that are best at mitigating ATM inflammation.
Bariatric surgery
Often, when first-line treatment options, such as dietary interventions and exercise programs, are not enough to induce significant weight loss or metabolic improvements in individuals with obesity, many turn to bariatric surgery. Indeed, bariatric surgery is one of the most powerful tools to induce weight loss; a worldwide study from 31 countries found that the surgeries induced an overall 1 y-weight loss of ∼30.5% (190). Aside from the effectiveness for weight loss, bariatric surgery is often accompanied with weight-loss-dependent metabolic improvements including the mitigation of ATM inflammation.
Effects of surgery on macrophage populations
Several studies observed significant reductions in macrophage number up to a year after surgery using the CD68 marker (30, 191–194) (see Table 4). Cancello et al. (30) found an ∼12% reduction in the number of ATM after surgery, which is likely due to the decreased expression of chemotactic genes. Bariatric surgery was also found to alter the phenotype of macrophages favoring a shift towards M2-like macrophages (195–201) (see Table 4). Aron-Wisnewsky et al. (195) found that in premenopausal women without diabetes, the ratio of M1-: M2-like (CD40+: CD206+) macrophages was 2-fold lower in subcutaneous adipose tissue (SAT) after 3 mo than before surgery due to a simultaneous decrease of CD40+ and an increase of CD206+ macrophages. Similarly, others have found an increased presence of M2- over M1-like macrophages in the adipose tissue ≤12 to 24 mo after surgery (198, 199). Altogether, these studies suggest that the immune and inflammatory profile of bariatric surgery patients may take years to reach new baseline levels. Overall, robust evidence indicates quantitative and qualitative changes in ATM populations following weight loss surgery.
TABLE 4.
The effects of bariatric surgery on macrophage infiltration and polarization at different time points after surgery in human studies
| Macrophage/phenotype change | |||||||
|---|---|---|---|---|---|---|---|
| Reference | N | Participants | ≤1 mo | 3 mo | 6 mo | 12 mo | 24 mo |
| Cancello et al. (30) | 24 | 17 women with class III obesity and 7 lean women | ↓ 12% ATM | ||||
| Aghamohammadzadeh et al. (191) | 22 | 15 adults with class III obesity and 7 lean individuals | ↓ ATM | ||||
| Haluzikova et al. (192) | 32 | 17 women with class III obesity and 15 lean women | ↑ ATM surpassing baseline concentrations (before surgery) | ↓ ATM | ↓ ATM (concentration similar to control) | ||
| Trachta et al. (193) | 31 | 13 nondiabetic women with class III obesity and 18 lean women | ↑ ATM | ↓ ATM | In SAT:↓ ATM (concentrations similar to baseline) | ||
| Liu et al. (198) | 118 | Individuals with class III obesity | ↓ ATM↑ M2 | ||||
| Aron-Wisnewsky et al. (195) | 26 | 16 women with class III obesity and 10 lean women | ↓ M1↑ M2↓ 2-fold M1/M2 | ||||
| Garcia-Rubio et al. (197) | 71 | 43 individuals with class III obesity and 28 exmorbidly obese individuals with class I obesity | ↓ M1-like cells in SAT and VAT | ||||
| Cinkajzlova et al. (196) | 83 | 32 nondiabetic individuals with class III obesity and 32 individuals with class III obesity and diabetes and 19 lean controls | In plasma:↓ M2In SVF of SAT: ↓ M2 | In plasma:↓ M2 In SVF of SAT: ↓ M2 | |||
| Moreno-Navarrete et al. (199) | 6 | Women with class III obesity and normal glucose metabolism | ↑ M2 | ||||
| Hagman et al. (200) | 17 | Men and women with obesity class II and III | ↑ M1↑ M2 | ↑ M1↑ M2 | |||
| Hess et al. (201) | 40 | 20 individuals with class III obesity and 20 lean controls | ↓ ∼1.1% M1↑ ∼10.1% M2 | ||||
ATM, adipose tissue macrophages; SAT, subcutaneous adipose tissue; SVF, stromal vascular fraction; VAT: visceral adipose tissue; lean (BMI ≤24.9); class I (BMI 30–34.9); class II (BMI 35–39.9); class III (BMI ≥40); ↓ : significant decrease; ↑ : significant increase; Ø: no significant change or not applicable.
Mechanisms affecting macrophage infiltration and polarization after bariatric surgery
Weight loss by bariatric surgery decreases expression of proinflammatory cytokines and chemotactic signals
An extensive amount of research has studied how bariatric surgery affects cytokine-related macrophage chemotaxis and polarization. Although unclear, current literature suggests that bariatric surgery may improve the inflammatory status of individuals with obesity. Two previous reviews (13, 202) listed several cytokines and their variations at different time points after bariatric surgery. An example being that CRP and leptin unanimously decreased, adiponectin constantly increased, and TNF-α remained unchanged at all time points after surgery. As for the other highly expressed cytokines during obesity such as IL-1β, IL-6, IL-10, and MCP-1, results are inconsistent even ≤2 y postoperation. For instance, 3 mo after surgery, Xu et al. (203) observed improved insulin sensitivity, increased AMP-activated protein kinase (AMPK) expression, and decreased oxidative stress with no changes in IL-1β, TNF-α, and IL-10 levels in patients. Such discrepancies were hypothesized to be the result of the presurgical presence of diabetes and the baseline level of insulin sensitivity (204–206). For example, a greater CRP reduction was observed after surgery in ex-obese patients with diabetes compared with those who were not diabetic (206). Moreover, the inflammatory state of visceral adipose tissue and the patients’ nutritional status presurgery were also suggested to influence the postsurgical inflammation state (202). Overall, the inconsistent effects of surgically induced weight loss on cytokine fluctuation remain unexplained due to a lack of convincing results. The long-term effects of cytokine secretion on health of individuals with obesity after surgery are unknown.
Effects of surgery on adipocyte morphology
Appreciable weight loss after bariatric surgery results in extensive adipose tissue remodeling on multiple levels, implicating mechanisms underlying adipose tissue plasticity. The architecture and homeostasis of the adipose tissue and the cells composing the SVF are tightly regulated by the equilibrium between hypertrophy and hyperplasia, which may be improved following weight loss resulting in diminished macrophage-mediated inflammation. Several studies analyzing either the volume or the area of the adipocytes found that postsurgery fat cells were smaller, ultimately approaching measurements similar to lean controls (191, 194, 207–209). For example, Casmatra et al. (210) and Löfgren et al. (211) reported a postsurgical reduction of fat cell area by 50% and volume by 43%, respectively. Additionally, Andersson et al. (212) reported significant adipocyte volume loss after surgery, but with no changes in cell number. Thus, suggesting that adipocyte atrophy is the main plastic event taking place during weight loss induced by surgery.
Effects of surgery on angiogenesis in adipose tissue
Adipose tissue expansion is intricately dependent on vasculature, which is increased during obesity. Indeed, angiogenesis is a response to adipose tissue hypoxia that results from its expansion and poor blood supply. As such, angiogenic markers like vascular endothelial growth factor (VEGF), angiopoietin-1 (ANG-1), ANG2, tyrosine-protein kinase receptor Tie-2 (Tie-2), and HIF-1α are overexpressed in obesity which potentiate proangiogenic responses to improve tissue blood supply, inflammation, and ultimately adipocyte dysfunction (213). Bariatric surgery may induce significant reduction of these angiogenic markers while concomitantly decreasing the recruitment of M1-like macrophages. Weiwiora et al. (214) studied the concentrations of circulating angiogenesis biomarkers [ANG-2, granulocyte colony-stimulating factor (G-CSF), hepatocyte growth factor (HGF), platelet endothelial cell adhesion molecule-1 (PECAM-1), VEGF, and follistatin] preoperatively and 12 mo after surgery in 24 patients with class III obesity. The expression levels of these angiogenic markers were all downregulated postsurgery and their changes were dependent upon the amount of weight loss. Similarly, Figueroa-Vega et al. (213) found that before surgery the concentrations of proangiogenic markers (ANG-1, ANG-2, Tie-2, and HIF-1α) were overexpressed (both in serum and adipose tissue), which correlated with an increased number of infiltrating M1-like macrophages expressing angiogenic receptor Tie-2 especially in SAT (213). At 6 mo after surgery, the expression of these markers was significantly reduced and correlated with a diminished number of infiltrating M1-like macrophages (213). Therefore, angiogenesis may not only be important for adipose tissue expansion, but it may also represent another pathway to explain the chronic inflammation observed in obesity, which is alleviated by weight loss surgery. However, the knowledge of angiogenic mechanisms and its impact on adipose tissue dysfunction and health postsurgery is still rudimentary and requires more research.
Effects of surgery on adipose tissue fibrosis
Fibrosis is a hallmark feature of adipose tissue inflammation as it is triggered and exacerbated by macrophage infiltration (215, 216). However, the reversibility of adipose tissue fibrosis after surgery-induced weight loss is unclear. To our knowledge, only 2 studies have directly examined fibrosis pre- and post-bariatric surgery and both have concluded that levels of fibrosis remained unchanged and persisted despite the significant weight loss in most participants from 6 mo to ≤2 y after surgery (194, 217). In contrast, Liu et al. (198) and Reggio et al. (218) observed a downregulation in the expression levels of genes encoding markers of adipose tissue fibrosis from 6 mo to 1 y postsurgery (198, 218). Moreover, Liu et al. (198) observed a positive relationship between collagen accumulation and the number of M2-like (CD163+) cells prior to surgery, indicating a role in the generation of fibrosis in obese SAT. However, this M2-to-pericellular collagen accumulation relation became a negative correlation at the 1-y-follow-up despite the moderate increase in the number of CD163+ cells. Overall, the evolution of fibrosis postsurgery, the role played by ECM proteins, and their link with ATM during weight loss are poorly documented.
Conclusion and Future Prospects
In this review, we discussed the impact of dietary fatty acids, exercise, and bariatric surgery on cellular characteristics affecting ATM presence and phenotypes in obesity. We have shown that dietary fatty acids, exercise training, and bariatric surgery decrease ATM and induce a phenotypic switch to M2-like macrophages through modifying a number of potential mechanisms. In the case of the type of fat ingested and exercise, improvements in ATM occurred regardless of weight loss. These interventions modify ATM by affecting key adipose tissue characteristics such as adipocyte size, adipose tissue fibrosis, angiogenesis, and cytokine and adipokine secretion. Future studies should focus on gaining a better understanding of the underlying mechanisms and consequences of the reduction in macrophage presence and phenotypes, especially in humans. Understanding the events contributing to the pathogenesis of obesity may allow for the development of potential new therapies against obesity and its associated comorbidities.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—LT and SS were responsible for writing and editing the manuscript. All authors read and approved the final manuscript.
Notes
Supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant, SS is the recipient of a Canada Research Chair, Tier 2 in Clinical Nutrition.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: ANG, angiopoietin; ARG-1, arginase-1; ATM, adipose tissue macrophage; CCL5, chemokine (C-C motif) ligand 5; CCR, C-C chemokine receptor; CRP, C-reactive protein; CVD, cardiovascular disease; ECM, extracellular matrix; HFD, high-fat diet; HIF-1α, hypoxia-inducible factor 1-α; HR, heart rate; iNOS, inducible nitric oxide synthase; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; ROS, reactive oxygenic species; SAT, subcutaneous adipose tissue; SVF, stromal vascular fraction; Tie-2, tyrosine-protein kinase receptor Tie-2; TLR, toll-like receptor; VEGF, vascular endothelial growth factor.
Contributor Information
Laurent Turner, Department of Health, Kinesiology, and Applied Physiology, Concordia University, Montreal, Quebec, Canada; Metabolism, Obesity, and Nutrition Lab, PERFORM Centre, Concordia University, Montreal, Quebec, Canada.
Sylvia Santosa, Department of Health, Kinesiology, and Applied Physiology, Concordia University, Montreal, Quebec, Canada; Metabolism, Obesity, and Nutrition Lab, PERFORM Centre, Concordia University, Montreal, Quebec, Canada; Research Centre, Montreal North Island Integrated University Health and Social Services Centre, Montreal Sacré-Coeur Hospital (CIUSSS-NIM, HSCM), Montreal, Quebec, Canada.
References
- 1.World Health Organization . Obesity and overweight 2018. [Internet]. [Accessed 2019 Aug 25]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 2.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease?. J Am Coll Cardiol. 2011;57(25):2461–73. [DOI] [PubMed] [Google Scholar]
- 3.Canada Go . Tackling obesity in Canada: obesity and excess weight rates in Canadian adults 2018. [Internet]. [Accessed 2019 Aug 25]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/obesity-excess-weight-rates-canadian-adults.html. [Google Scholar]
- 4.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121(6):2111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suganami T, Ogawa y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 2010;88(1):33–9. [DOI] [PubMed] [Google Scholar]
- 6.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222(3):R113–27. [DOI] [PubMed] [Google Scholar]
- 7.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121(6):2094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barry JC, Simtchouk S, Durrer C, Jung ME, Little JP. Short-term exercise training alters leukocyte chemokine receptors in obese adults. Med Sci Sports Exerc. 2017;49(8):1631–40. [DOI] [PubMed] [Google Scholar]
- 9.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290(5):E961–7. [DOI] [PubMed] [Google Scholar]
- 10.Kawanishi N, yano H, Mizokami T, Takahashi M, Oyanagi E, Suzuki K. Exercise training attenuates hepatic inflammation, fibrosis and macrophage infiltration during diet induced-obesity in mice. Brain Behav Immun. 2012;26(6):931–41. [DOI] [PubMed] [Google Scholar]
- 11.Baranowski M, Enns J, Blewett H, yakandawala U, Zahradka P, Taylor CG. Dietary flaxseed oil reduces adipocyte size, adipose monocyte chemoattractant protein-1 levels and T-cell infiltration in obese, insulin-resistant rats. Cytokine. 2012;59(2):382–91. [DOI] [PubMed] [Google Scholar]
- 12.Huber J, Loffler M, Bilban M, Reimers M, Kadl A, Todoric J, Zeyda M, Geyeregger R, Schreiner M, Weichhart Tet al. . Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes. 2007;31(6):1004–13. [DOI] [PubMed] [Google Scholar]
- 13.Labrecque J, Laforest S, Michaud A, Biertho L, Tchernof A. Impact of bariatric surgery on white adipose tissue inflammation. Can J Diabetes. 2017;41(4):407–17. [DOI] [PubMed] [Google Scholar]
- 14.Goh J, Goh KP, Abbasi A. Exercise and adipose tissue macrophages: new frontiers in obesity research?. Front Endocrinol (Lausanne). 2016;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129(7):2619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185(10):2596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JK, Bettencourt R, Brenner D, Le TA, Barrett-Connor E, Loomba R. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One. 2012;7(4):e34218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soskic SS, Dobutovic BD, Sudar EM, Obradovic MM, Nikolic DM, Djordjevic JD, Radak DJ, Mikhailidis DP, Isenovic ER. Regulation of inducible nitric oxide synthase (iNOS) and its potential role in insulin resistance, diabetes and heart failure. TOCMJ. 2011;5:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb Haemost. 2006;95(3):511–8. [DOI] [PubMed] [Google Scholar]
- 22.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–4. [DOI] [PubMed] [Google Scholar]
- 23.Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. IJMS. 2018;19(6):1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113(9):1009–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res. 2016;119(3):414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care. 2011;14(4):341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chylikova J, Dvorackova J, Tauber Z, Kamarad V. M1/M2 macrophage polarization in human obese adipose tissue. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2018;162(2):79–82. [DOI] [PubMed] [Google Scholar]
- 28.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JDet al. . Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18(14):1657–69. [DOI] [PubMed] [Google Scholar]
- 29.Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes. 2007;31(9):1420–8. [DOI] [PubMed] [Google Scholar]
- 30.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JLet al. . Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–86. [DOI] [PubMed] [Google Scholar]
- 31.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59(5):879–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Barnes GT, yang Q, Tan G, yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LAet al. . Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Innis SM. Dietary lipids in early development: relevance to obesity, immune and inflammatory disorders. Curr Opin Endocrinol Diabetes Obes. 2007;14(5):359–64. [DOI] [PubMed] [Google Scholar]
- 35.Teng KT, Chang Cy, Chang LF, Nesaretnam K. Modulation of obesity-induced inflammation by dietary fats: mechanisms and clinical evidence. Nutr J. 2014;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dali-youcef N, Mecili M, Ricci R, Andres E. Metabolic inflammation: connecting obesity and insulin resistance. Ann Med. 2013;45(3):242–53. [DOI] [PubMed] [Google Scholar]
- 37.Rogero MM, Calder PC. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10(4):432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coenen KR, Gruen ML, Lee-young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52(2):318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56(3):564–73. [DOI] [PubMed] [Google Scholar]
- 40.Cullberg KB, Larsen JO, Pedersen SB, Richelsen B. Effects of LPS and dietary free fatty acids on MCP-1 in 3T3-L1 adipocytes and macrophages in vitro. Nutr & Diabetes. 2014;4:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. TLR-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring). 2008;16(6):1248–55. [DOI] [PubMed] [Google Scholar]
- 42.Enos RT, Davis JM, Velazquez KT, McClellan JL, Day SD, Carnevale KA, Murphy EA. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54(1):152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–92. [DOI] [PubMed] [Google Scholar]
- 44.Prieur X, Mok Cy, Velagapudi VR, Nunez V, Fuentes L, Montaner D, Ishikawa K, Camacho A, Barbarroja N, O'Rahilly Set al. . Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60(3):797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.yeop Han C, Kargi Ay, Omer M, Chan CK, Wabitsch M, O'Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59(2):386–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawanishi N, Niihara H, Mizokami T, yano H, Suzuki K. Exercise training attenuates adipose tissue fibrosis in diet-induced obese mice. Biochem Biophys Res Commun. 2013;440(4):774–9. [DOI] [PubMed] [Google Scholar]
- 47.Kawanishi N, yano H, yokogawa y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–18. [PubMed] [Google Scholar]
- 48.Kolahdouzi S, Talebi-Garakani E, Hamidian G, Safarzade A. Exercise training prevents high-fat diet-induced adipose tissue remodeling by promoting capillary density and macrophage polarization. Life Sci. 2019;220:32–43. [DOI] [PubMed] [Google Scholar]
- 49.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJet al. . Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. MCB. 2009;29(16):4467–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. MCB. 2009;29(6):1575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamer F, Ulug E, Akyol A, Nergiz-Unal R. The potential efficacy of dietary fatty acids and fructose induced inflammation and oxidative stress on the insulin signaling and fat accumulation in mice. Food Chem Toxicol. 2020;135:110914. [DOI] [PubMed] [Google Scholar]
- 52.Sharma M, Boytard L, Hadi T, Koelwyn G, Simon R, Ouimet M, Seifert L, Spiro W, yan B, Hutchison Set al. . Enhanced glycolysis and HIF-1alpha activation in adipose tissue macrophages sustains local and systemic interleukin-1beta production in obesity. Sci Rep. 2020;10(1):5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Dijk SJ, Feskens EJ, Bos MB, Hoelen DW, Heijligenberg R, Bromhaar MG, de Groot LC, de Vries JH, Muller M, Afman LA. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr. 2009;90(6):1656–64. [DOI] [PubMed] [Google Scholar]
- 54.Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33(4):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang H, Lum H, Alvarez A, Garduno-Garcia JJ, Daniel BJ, Musi N. A low dose lipid infusion is sufficient to induce insulin resistance and a pro-inflammatory response in human subjects. PLoS One. 2018;13(4):e0195810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Araujo JR, Tomas J, Brenner C, Sansonetti PJ. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie. 2017;141:97–106. [DOI] [PubMed] [Google Scholar]
- 57.Bleau C, Karelis AD, St-Pierre DH, Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev. 2015;31(6):545–61. [DOI] [PubMed] [Google Scholar]
- 58.Ni y, Ni L, Zhuge F, Xu L, Fu Z, Ota T. Adipose tissue macrophage phenotypes and characteristics: the key to insulin resistance in obesity and metabolic disorders. Obesity. 2020;28:225. [DOI] [PubMed] [Google Scholar]
- 59.Creely SJ, McTernan PG, Kusminski CM, Fisher f M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740–7. [DOI] [PubMed] [Google Scholar]
- 60.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo Cet al. . Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. [DOI] [PubMed] [Google Scholar]
- 61.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. [DOI] [PubMed] [Google Scholar]
- 62.Moreno-Navarrete JM, Escote X, Ortega F, Serino M, Campbell M, Michalski MC, Laville M, Xifra G, Luche E, Domingo Pet al. . A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia. 2013;56(11):2524–37. [DOI] [PubMed] [Google Scholar]
- 63.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caesar R, Reigstad CS, Backhed HK, Reinhardt C, Ketonen M, Lunden GO, Cani PD, Backhed F. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61(12):1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hersoug LG, Moller P, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev. 2016;17(4):297–312. [DOI] [PubMed] [Google Scholar]
- 66.Bellenger J, Bellenger S, Escoula Q, Bidu C, Narce M. N-3 polyunsaturated fatty acids: an innovative strategy against obesity and related metabolic disorders, intestinal alteration and gut microbiota dysbiosis. Biochimie. 2019;159:66–71. [DOI] [PubMed] [Google Scholar]
- 67.Debedat J, Clement K, Aron-Wisnewsky J. Gut microbiota dysbiosis in human obesity: impact of bariatric surgery. Curr Obes Rep. 2019;8(3):229–42. [DOI] [PubMed] [Google Scholar]
- 68.Quiroga R, Nistal E, Estebanez B, Porras D, Juarez-Fernandez M, Martinez-Florez S, Garcia-Mediavilla MV, de Paz JA, Gonzalez-Gallego J, Sanchez-Campos Set al. . Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp Mol Med. 2020;52(7):1048–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan C, Zirpoli H, Qi K. n-3 fatty acids modulate adipose tissue inflammation and oxidative stress. Curr Opin Clin Nutr Metab Care. 2013;16(2):124–32. [DOI] [PubMed] [Google Scholar]
- 70.Martinez-Fernandez L, Laiglesia LM, Huerta AE, Martinez JA, Moreno-Aliaga MJ. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015;121(Pt A):24–41. [DOI] [PubMed] [Google Scholar]
- 71.Oliver E, McGillicuddy F, Phillips C, Toomey S, Roche HM. The role of inflammation and macrophage accumulation in the development of obesity-induced type 2 diabetes mellitus and the possible therapeutic effects of long-chain n-3 PUFA. Proc Nutr Soc. 2010;69(2):232–43. [DOI] [PubMed] [Google Scholar]
- 72.Oh Dy, Walenta E. Omega-3 fatty acids and FFAR4. Front Endocrinol (Lausanne). 2014;5:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Albracht-Schulte K, Kalupahana NS, Ramalingam L, Wang S, Rahman SM, Robert-McComb J, Moustaid-Moussa N. Omega-3 fatty acids in obesity and metabolic syndrome: a mechanistic update. J Nutr Biochem. 2018;58:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferguson JF, Roberts-Lee K, Borcea C, Smith HM, Midgette y, Shah R. Omega-3 polyunsaturated fatty acids attenuate inflammatory activation and alter differentiation in human adipocytes. J Nutr Biochem. 2019;64:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Itariu BK, Zeyda M, Hochbrugger EE, Neuhofer A, Prager G, Schindler K, Bohdjalian A, Mascher D, Vangala S, Schranz Met al. . Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am J Clin Nutr. 2012;96(5):1137–49. [DOI] [PubMed] [Google Scholar]
- 76.Montserrat-de la Paz S, Rodriguez D, Cardelo MP, Naranjo MC, Bermudez B, Abia R, Muriana FJG, Lopez S. The effects of exogenous fatty acids and niacin on human monocyte-macrophage plasticity. Mol Nutr Food Res. 2017;61(8):1600824. [DOI] [PubMed] [Google Scholar]
- 77.Pandurangan SB, Al-Maiman SA, Al-Harbi LN, Alshatwi AA. Beneficial fatty acid ratio of Salvia hispanica L. (Chia Seed) potentially inhibits adipocyte hypertrophy, and decreases adipokines expression and inflammation in macrophage. Foods. 2020;9(3):368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinel A, Morio-Liondore B, Capel F. n-3 Polyunsaturated fatty acids modulate metabolism of insulin-sensitive tissues: implication for the prevention of type 2 diabetes. J Physiol Biochem. 2014;70(2):647–58. [DOI] [PubMed] [Google Scholar]
- 79.Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LR, Lee J, Walton RG, Adu A, Erfani Ret al. . Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 2013;62(5):1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bashir S, Sharma y, Jairajpuri D, Rashid F, Nematullah M, Khan F. Alteration of adipose tissue immune cell milieu towards the suppression of inflammation in high fat diet fed mice by flaxseed oil supplementation. PLoS One. 2019;14(10):e0223070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chan KL, Pillon NJ, Sivaloganathan DM, Costford SR, Liu Z, Theret M, Chazaud B, Klip A. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J Biol Chem. 2015;290(27):16979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang Hy, Lee HN, Kim W, Surh yJ. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor gamma activation. Life Sci. 2015;120:39–47. [DOI] [PubMed] [Google Scholar]
- 83.Colson C, Ghandour RA, Dufies O, Rekima S, Loubat A, Munro P, Boyer L, Pisani DF. Diet supplementation in omega3 polyunsaturated fatty acid favors an anti-inflammatory basal environment in mouse adipose tissue. Nutrients. 2019;11(2):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan R, Kim J, you M, Giraud D, Toney AM, Shin SH, Kim Sy, Borkowski K, Newman JW, Chung S. alpha-Linolenic acid-enriched butter attenuated high fat diet-induced insulin resistance and inflammation by promoting bioconversion of n-3 PUFA and subsequent oxylipin formation. J Nutr Biochem. 2020;76:108285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liddle DM, Monk JM, Hutchinson AL, Ma DWL, Robinson LE. CD8(+) T cell/adipocyte inflammatory cross talk and ensuing M1 macrophage polarization are reduced by fish-oil-derived n-3 polyunsaturated fatty acids, in part by a TNF-alpha-dependent mechanism. J Nutr Biochem. 2020;76:108243. [DOI] [PubMed] [Google Scholar]
- 86.Lopez-Vicario C, Alcaraz-Quiles J, Garcia-Alonso V, Rius B, Hwang SH, Titos E, Lopategi A, Hammock BD, Arroyo V, Claria J. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci USA. 2015;112(2):536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol. 2011;187(10):5408–18. [DOI] [PubMed] [Google Scholar]
- 88.Todoric J, Loffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhausl W, Stulnig TM. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49(9):2109–19. [DOI] [PubMed] [Google Scholar]
- 89.White PJ, Arita M, Taguchi R, Kang JX, Marette A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes. 2010;59(12):3066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xue B, yang Z, Wang X, Shi H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One. 2012;7(10):e45990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Boer AA, Monk JM, Liddle DM, Hutchinson AL, Power KA, Ma DW, Robinson LE. Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross-talk between adipocytes and CD11b(+) macrophages. J Nutr Biochem. 2016;34:61–72. [DOI] [PubMed] [Google Scholar]
- 92.De Boer AA, Monk JM, Liddle DM, Power KA, Ma DWL, Robinson LE. Fish oil-derived long-chain n-3 polyunsaturated fatty acids reduce expression of M1-associated macrophage markers in an ex vivo adipose tissue culture model, in part through adiponectin. Front Nutr. 2015;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kratz M, Kuzma JN, Hagman DK, van yserloo B, Matthys CC, Callahan HS, Weigle DS. n-3 PUFAs do not affect adipose tissue inflammation in overweight to moderately obese men and women. J Nutr. 2013;143(8):1340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Mello AH, Schraiber RB, Goldim MPS, Mathias K, Mendes C, Correa M, Gomes ML, Silveira PCL, Schuck PF, Petronilho Fet al. . Omega-3 polyunsaturated fatty acids have beneficial effects on visceral fat in diet-induced obesity model. Biochem Cell Biol. 2019;97(6):693–701. [DOI] [PubMed] [Google Scholar]
- 95.LeMieux MJ, Kalupahana NS, Scoggin S, Moustaid-Moussa N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J Nutr. 2015;145(3):411–7. [DOI] [PubMed] [Google Scholar]
- 96.Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal N, Ruzickova J, Sponarova J, Drahota Z, Vlcek Cet al. . Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia. 2005;48(11):2365–75. [DOI] [PubMed] [Google Scholar]
- 97.Wang y, Huang F. N-3 Polyunsaturated fatty acids and inflammation in obesity: local effect and systemic benefit. Biomed Res Int. 2015;2015:581469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Belchior T, Paschoal VA, Magdalon J, Chimin P, Farias TM, Chaves-Filho AB, Gorjao R, St-Pierre P, Miyamoto S, Kang JXet al. . Omega-3 fatty acids protect from diet-induced obesity, glucose intolerance, and adipose tissue inflammation through PPARgamma-dependent and PPARgamma-independent actions. Mol Nutr Food Res. 2015;59(5):957–67. [DOI] [PubMed] [Google Scholar]
- 99.Van Name MA, Savoye M, Chick JM, Galuppo BT, Feldstein AE, Pierpont B, Johnson C, Shabanova V, Ekong U, Valentino PLet al. . A low omega-6 to omega-3 PUFA ratio (n-6:n-3 PUFA) diet to treat fatty liver disease in obese youth. J Nutr. 2020;150(9):2314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siriwardhana N, Kalupahana NS, Fletcher S, Xin W, Claycombe KJ, Quignard-Boulange A, Zhao L, Saxton AM, Moustaid-Moussa N. n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-kappaB-dependent mechanisms. J Nutr Biochem. 2012;23(12):1661–7. [DOI] [PubMed] [Google Scholar]
- 101.Murumalla RK, Gunasekaran MK, Padhan JK, Bencharif K, Gence L, Festy F, Cesari M, Roche R, Hoareau L. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012;11:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Flachs P, Rossmeisl M, Bryhn M, Kopecky J. Cellular and molecular effects of n-3 polyunsaturated fatty acids on adipose tissue biology and metabolism. Clin Sci (Lond). 2009;116(1):1–16. [DOI] [PubMed] [Google Scholar]
- 103.Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, yuan X, Tanaka M, Kawano H, yano T, Aoe S, Takeya Met al. . Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. ATVB. 2007;27(9):1918–25. [DOI] [PubMed] [Google Scholar]
- 104.Krebs JD, Browning LM, McLean NK, Rothwell JL, Mishra GD, Moore CS, Jebb SA. Additive benefits of long-chain n-3 polyunsaturated fatty acids and weight-loss in the management of cardiovascular disease risk in overweight hyperinsulinaemic women. Int J Obes. 2006;30(10):1535–44. [DOI] [PubMed] [Google Scholar]
- 105.Lee KR, Midgette y, Shah R. Fish oil derived omega 3 fatty acids suppress adipose NLRP3 inflammasome signaling in human obesity. J Endocr Soc. 2019;3(3):504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Samblas M, Carraro JC, Martinez JA, Milagro FI. The regulation of inflammation-related genes after palmitic acid and DHA treatments is not mediated by DNA methylation. J Physiol Biochem. 2019;75(3):341–9. [DOI] [PubMed] [Google Scholar]
- 107.Garaulet M, Hernandez-Morante JJ, Lujan J, Tebar FJ, Zamora S. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes. 2006;30(6):899–905. [DOI] [PubMed] [Google Scholar]
- 108.Kim HK, Della-Fera M, Lin J, Baile CA. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J Nutr. 2006;136(12):2965–9. [DOI] [PubMed] [Google Scholar]
- 109.Martins FF, Aguila MB, Mandarim-de-Lacerda CA. Eicosapentaenoic and docosapentaenoic acids lessen the expression of PPARgamma/Cidec affecting adipogenesis in cultured 3T3-L1 adipocytes. Acta Histochem. 2020;122:151504. [DOI] [PubMed] [Google Scholar]
- 110.Titos E, Claria J. Omega-3-derived mediators counteract obesity-induced adipose tissue inflammation. Prostaglandins Other Lipid Mediat. 2013;107:77–84. [DOI] [PubMed] [Google Scholar]
- 111.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease?. Prev Med. 2004;39(1):212–20. [DOI] [PubMed] [Google Scholar]
- 112.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lancaster GI, Febbraio MA. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014;35(6):262–9. [DOI] [PubMed] [Google Scholar]
- 114.de Matos MA, Garcia BCC, Vieira DV, de Oliveira MFA, Costa KB, Aguiar PF, Magalhaes FC, Brito-Melo GA, Amorim FT, Rocha-Vieira E. High-intensity interval training reduces monocyte activation in obese adults. Brain Behav Immun. 2019;80:818–24. [DOI] [PubMed] [Google Scholar]
- 115.Flynn MG, McFarlin BK, Markofski MM. The anti-inflammatory actions of exercise training. Am J Lifestyle Med. 2007;1(3):220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.you T, Arsenis NC, Disanzo BL, Lamonte MJ. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med. 2013;43(4):243–56. [DOI] [PubMed] [Google Scholar]
- 117.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298(4):E824–31. [DOI] [PubMed] [Google Scholar]
- 118.Silverman NE, Nicklas BJ, Ryan AS. Addition of aerobic exercise to a weight loss program increases BMD, with an associated reduction in inflammation in overweight postmenopausal women. Calcif Tissue Int. 2009;84(4):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Auerbach P, Nordby P, Bendtsen LQ, Mehlsen JL, Basnet SK, Vestergaard H, Ploug T, Stallknecht B. Differential effects of endurance training and weight loss on plasma adiponectin multimers and adipose tissue macrophages in younger, moderately overweight men. Am J Physiol Regul Integr Comp Physiol. 2013;305(5):R490–8. [DOI] [PubMed] [Google Scholar]
- 120.Bradley RL, Jeon Jy, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295(3):E586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gomez-Merino D, Drogou C, Guezennec Cy, Chennaoui M. Effects of chronic exercise on cytokine production in white adipose tissue and skeletal muscle of rats. Cytokine. 2007;40(1):23–9. [DOI] [PubMed] [Google Scholar]
- 122.Ringseis R, Eder K, Mooren FC, Kruger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. 2015;21:58–68. [PubMed] [Google Scholar]
- 123.Woods JA, Vieira VJ, Keylock KT. Exercise, inflammation, and innate immunity. Immunol Allergy Clin North Am. 2009;29(2):381–93. [DOI] [PubMed] [Google Scholar]
- 124.Kawanishi N, Mizokami T, yano H, Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med Sci Sports Exerc. 2013;45(9):1684–93. [DOI] [PubMed] [Google Scholar]
- 125.Linden MA, Pincu y, Martin SA, Woods JA, Baynard T. Moderate exercise training provides modest protection against adipose tissue inflammatory gene expression in response to high-fat feeding. Physiol Rep. 2014;2(7):e12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Macpherson RE, Huber JS, Frendo-Cumbo S, Simpson JA, Wright DC. Adipose tissue insulin action and IL-6 signaling after exercise in obese mice. Med Sci Sports Exerc. 2015;47(10):2034–42. [DOI] [PubMed] [Google Scholar]
- 127.Oliveira AG, Araujo TG, Carvalho BM, Guadagnini D, Rocha GZ, Bagarolli RA, Carvalheira JB, Saad MJ. Acute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet-induced obese rats. Obesity (Silver Spring). 2013;21(12):2545–56. [DOI] [PubMed] [Google Scholar]
- 128.Luo W, Ai L, Wang B, Wang L, Gan y, Liu C, Jensen J, Zhou y. Eccentric exercise and dietary restriction inhibits M1 macrophage polarization activated by high-fat diet-induced obesity. Life Sci. 2020;243:117246. [DOI] [PubMed] [Google Scholar]
- 129.Baek KW, Lee DI, Kang SA, yu HS. Differences in macrophage polarization in the adipose tissue of obese mice under various levels of exercise intensity. J Physiol Biochem. 2020;76(1):159–68. [DOI] [PubMed] [Google Scholar]
- 130.Shanaki M, Khosravi M, Khoshdooni-Farahani A, Dadashi A, Heydari MF, Delfan M, Jafary H, Gorgani-Firuzjaee S. High-intensity interval training reversed high-fat diet-induced M1-macrophage polarization in rat adipose tissue via inhibition of NOTCH signaling. J Inflamm Res. 2020;13:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee S, Norheim F, Langleite TM, Gulseth HL, Birkeland KI, Drevon CA. Effects of long-term exercise on plasma adipokine levels and inflammation-related gene expression in subcutaneous adipose tissue in sedentary dysglycaemic, overweight men and sedentary normoglycaemic men of healthy weight. Diabetologia. 2019;62(6):1048–64. [DOI] [PubMed] [Google Scholar]
- 132.yakeu G, Butcher L, Isa S, Webb R, Roberts AW, Thomas AW, Backx K, James PE, Morris K. Low-intensity exercise enhances expression of markers of alternative activation in circulating leukocytes: roles of PPARgamma and Th2 cytokines. Atherosclerosis. 2010;212(2):668–73. [DOI] [PubMed] [Google Scholar]
- 133.Baturcam E, Abubaker J, Tiss A, Abu-Farha M, Khadir A, Al-Ghimlas F, Al-Khairi I, Cherian P, Elkum N, Hammad Met al. . Physical exercise reduces the expression of RANTES and its CCR5 receptor in the adipose tissue of obese humans. Mediators Inflamm. 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, Goodyear LJ. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab. 2009;297(2):E495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang P, Li S, Shao M, Qi Q, Zhao F, you J, Mao T, Li W, yan Z, Liu y. Calorie restriction and endurance exercise share potent anti-inflammatory function in adipose tissues in ameliorating diet-induced obesity and insulin resistance in mice. Nutr Metab (Lond). 2010;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. 2012;44(11):2099–110. [DOI] [PubMed] [Google Scholar]
- 137.Samaan MC, Marcinko K, Sikkema S, Fullerton MD, Ziafazeli T, Khan MI, Steinberg GR. Endurance interval training in obese mice reduces muscle inflammation and macrophage content independently of weight loss. Physiol Rep. 2014;2(5): e12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise?. J Leukoc Biol. 2008;84(5):1271–8. [DOI] [PubMed] [Google Scholar]
- 139.Van Pelt DW, Guth LM, Horowitz JF. Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J Appl Physiol. 2017;123(5):1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taherichadorneshin H, Cheragh-Birjandi S, Ahmadabadi F. The impact of high intensity interval training on serum chemerin, tumor necrosis factor-alpha and insulin resistance in overweight women. Obesity Medicine. 2019;14:100101. [Google Scholar]
- 141.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515(Pt 1):287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17(8):1. [DOI] [PubMed] [Google Scholar]
- 143.Jin CH, Rhyu HS, Kim Jy. The effects of combined aerobic and resistance training on inflammatory markers in obese men. J Exerc Rehabil. 2018;14(4):660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Souza DC, Matos VAF, Dos Santos VOA, Medeiros IF, Marinho CSR, Nascimento PRP, Dorneles GP, Peres A, Muller CH, Krause Met al. . Effects of high-intensity interval and moderate-intensity continuous exercise on inflammatory, leptin, IgA, and lipid peroxidation responses in obese males. Front Physiol. 2018;9:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008;2008:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ruffino JS, Davies NA, Morris K, Ludgate M, Zhang L, Webb R, Thomas AW. Moderate-intensity exercise alters markers of alternative activation in circulating monocytes in females: a putative role for PPARgamma. Eur J Appl Physiol. 2016;116(9):1671–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lehnig AC, Dewal RS, Baer LA, Kitching KM, Munoz VR, Arts PJ, Sindeldecker DA, May FJ, Lauritzen H, Goodyear LJet al. . Exercise training induces depot-specific adaptations to white and brown adipose tissue. iScience. 2019;11:425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dewal RS, Stanford KI. Effects of exercise on brown and beige adipocytes. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(1):71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khalafi M, Mohebbi H, Karimi P. High-intensity interval training increases mitochondria biogenesis in adipose tissue and improves insulin resistance in high fat diet-induced obese rat. International Journal of Applied Exercise Physiology. 2019;8(1):43–50. [Google Scholar]
- 150.Rocha-Rodrigues S, Rodriguez A, Becerril S, Ramirez B, Goncalves IO, Beleza J, Fruhbeck G, Ascensao A, Magalhaes J. Physical exercise remodels visceral adipose tissue and mitochondrial lipid metabolism in rats fed a high-fat diet. Clin Exp Pharmacol Physiol. 2017;44(3):386–94. [DOI] [PubMed] [Google Scholar]
- 151.Rocha-Rodrigues S, Goncalves IO, Beleza J, Ascensao A, Magalhaes J. Effects of endurance training on autophagy and apoptotic signaling in visceral adipose tissue of prolonged high fat diet-fed rats. Eur J Nutr. 2018;57(6):2237–47. [DOI] [PubMed] [Google Scholar]
- 152.Baynard T, Vieira-Potter VJ, Valentine RJ, Woods JA. Exercise training effects on inflammatory gene expression in white adipose tissue of young mice. Mediators Inflamm. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lee HJ. Exercise training regulates angiogenic gene expression in white adipose tissue. J Exerc Rehabil. 2018;14(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Min Sy, Learnard H, Kant S, Gealikman O, Rojas-Rodriguez R, DeSouza T, Desai A, Keaney JF Jr, Corvera S, Craige SM. Exercise rescues gene pathways involved in vascular expansion and promotes functional angiogenesis in subcutaneous white adipose tissue. IJMS. 2019;20(8): 2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nourshahi M, Bagheri B, Fallah HH. The effect of single bout of continuous and high intensity interval exercise on VEGF levels in adipose tissue in obese male wistar rats. Medical Journal of Tabriz University of Medical Sciences and Health Services. 2019;40(6):74–80. [Google Scholar]
- 156.Duggan C, Tapsoba JD, Wang Cy, Schubert KEF, McTiernan A. Long-term effects of weight loss and exercise on biomarkers associated with angiogenesis. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duggan C, Tapsoba Jde D, Wang Cy, McTiernan A. Dietary weight loss and exercise effects on serum biomarkers of angiogenesis in overweight postmenopausal women: a randomized controlled trial. Cancer Res. 2016;76(14):4226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haczeyni F, Barn V, Mridha AR, yeh MM, Estevez E, Febbraio MA, Nolan CJ, Bell-Anderson KS, Teoh NC, Farrell GC. Exercise improves adipose function and inflammation and ameliorates fatty liver disease in obese diabetic mice. Obesity. 2015;23(9):1845–55. [DOI] [PubMed] [Google Scholar]
- 159.Sandrini L, Ieraci A, Amadio P, Zara M, Mitro N, Lee FS, Tremoli E, Barbieri SS. Physical exercise affects adipose tissue profile and prevents arterial thrombosis in BDNF Val66Met mice. Cells. 2019;8(8):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.you T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int J Obes. 2006;30(8):1211–6. [DOI] [PubMed] [Google Scholar]
- 161.Ahn CR, Ryan BJ, Gillen JB, Ludzki A, Schleh MW, Reinheimer BA, Horowitz JF. Exercise training alters subcutaneous adipose tissue morphology in obese adults even without weight loss. Diabetes. 2019;68(Supplement 1)(12):721–P. [Google Scholar]
- 162.Fabre O, Ingerslev LR, Garde C, Donkin I, Simar D, Barres R. Exercise training alters the genomic response to acute exercise in human adipose tissue. Epigenomics. 2018;10(8):1033–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AWet al. . Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283(33):22620–7. [DOI] [PubMed] [Google Scholar]
- 165.Koppaka S, Kehlenbrink S, Carey M, Li W, Sanchez E, Lee DE, Lee H, Chen J, Carrasco E, Kishore Pet al. . Reduced adipose tissue macrophage content is associated with improved insulin sensitivity in thiazolidinedione-treated diabetic humans. Diabetes. 2013;62(6):1843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Butcher LR, Thomas A, Backx K, Roberts A, Webb R, Morris K. Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med Sci Sports Exerc. 2008;40(7):1263–70. [DOI] [PubMed] [Google Scholar]
- 167.Liu WX, Wang T, Zhou F, Wang y, Xing JW, Zhang S, Gu SZ, Sang LX, Dai C, Wang HL. Voluntary exercise prevents colonic inflammation in high-fat diet-induced obese mice by up-regulating PPAR-gamma activity. Biochem Biophys Res Commun. 2015;459(3):475–80. [DOI] [PubMed] [Google Scholar]
- 168.Stanford KI, Middelbeek RJ, Goodyear LJ. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015;64(7):2361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tanaka G, Kato H, Izawa T. Endurance exercise training induces fat depot-specific differences in basal autophagic activity. Biochem Biophys Res Commun. 2015;466(3):512–7. [DOI] [PubMed] [Google Scholar]
- 170.Zheng F, Cai y. Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-gamma and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis. 2019;18(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Silveira LS, Batatinha HAP, Castoldi A, Camara NOS, Festuccia WT, Souza CO, Rosa Neto JC, Lira FS. Exercise rescues the immune response fine-tuned impaired by peroxisome proliferator-activated receptors gamma deletion in macrophages. J Cell Physiol. 2019;234(4):5241–51. [DOI] [PubMed] [Google Scholar]
- 172.Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab. 2014;99(1):39–48. [DOI] [PubMed] [Google Scholar]
- 173.Ito A, Suganami T, yamauchi A, Degawa-yamauchi M, Tanaka M, Kouyama R, Kobayashi y, Nitta N, yasuda K, Hirata yet al. . Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem. 2008;283(51):35715–23. [DOI] [PubMed] [Google Scholar]
- 174.Oliveira M, Gleeson M. The influence of prolonged cycling on monocyte toll-like receptor 2 and 4 expression in healthy men. Eur J Appl Physiol. 2010;109(2):251–7. [DOI] [PubMed] [Google Scholar]
- 175.Simpson RJ, McFarlin BK, McSporran C, Spielmann G, ó Hartaigh B, Guy K. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav Immun. 2009;23(2):232–9. [DOI] [PubMed] [Google Scholar]
- 176.Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise?. Exerc Sport Sci Rev. 2006;34(4):176–81. [DOI] [PubMed] [Google Scholar]
- 177.Silveira LS, Biondo LA, de Souza Teixeira AA, de Lima Junior EA, Castoldi A, Camara NOS, Festuccia WT, Rosa-Neto JC, Lira FS. Macrophage immunophenotype but not anti-inflammatory profile is modulated by peroxisome proliferator-activated receptor gamma (PPARgamma) in exercised obese mice. Exerc Immunol Rev. 2020;26:10–22. [PubMed] [Google Scholar]
- 178.Soltani N, Esmaeil N, Marandi SM, Hovsepian V, Momen T, Shahsanai A, Kelishadi R. Assessment of the effect of short-term combined high-intensity interval training on TLR4, NF-kappaB and IRF3 expression in young overweight and obese girls. Public Health Genomics. 2020;; 23:26–36. [DOI] [PubMed] [Google Scholar]
- 179.Jeong JH, Lee yR, Park HG, Lee WL. The effects of either resveratrol or exercise on macrophage infiltration and switching from M1 to M2 in high fat diet mice. J Exerc Nutrition Biochem. 2015;19(2):65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Oliveira AG, Carvalho BM, Tobar N, Ropelle ER, Pauli JR, Bagarolli RA, Guadagnini D, Carvalheira JB, Saad MJ. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60(3):784–96. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 181.Flynn MG, McFarlin BK, Phillips MD, Stewart LK, Timmerman KL. Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J Appl Physiol. 2003;95(5):1833–42. [DOI] [PubMed] [Google Scholar]
- 182.Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. The physiological regulation of toll-like receptor expression and function in humans. J Physiol. 2005;563(Pt 3):945–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc. 2004;36(11):1876–83. [DOI] [PubMed] [Google Scholar]
- 184.Robinson E, Durrer C, Simtchouk S, Jung ME, Bourne JE, Voth E, Little JP. Short-term high-intensity interval and moderate-intensity continuous training reduce leukocyte TLR4 in inactive adults at elevated risk of type 2 diabetes. J Appl Physiol. 2015;119(5):508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Niemiro GM, Allen JM, Mailing LJ, Khan NA, Holscher HD, Woods JA, De Lisio M. Effects of endurance exercise training on inflammatory circulating progenitor cell content in lean and obese adults. J Physiol. 2018;596(14):2811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cavalcante PAM, Gregnani MF, Henrique JS, Ornellas FH, Araujo RC. Aerobic but not resistance exercise can induce inflammatory pathways via toll-like 2 and 4: a systematic review. Sports Med - Open. 2017;3(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu y, Liu SX, Cai y, Xie KL, Zhang WL, Zheng F. Effects of combined aerobic and resistance training on the glycolipid metabolism and inflammation levels in type 2 diabetes mellitus. J Phys Ther Sci. 2015;27(7):2365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rada I, Deldicque L, Francaux M, Zbinden-Foncea H. Toll like receptor expression induced by exercise in obesity and metabolic syndrome: a systematic review. Exerc Immunol Rev. 2018;24:60–71. [PubMed] [Google Scholar]
- 189.Soltani N, Marandi SM, Kazemi M, Esmaeil N. The exercise training modulatory effects on the obesity-induced immunometabolic dysfunctions. Diabetes Metab Syndr Obes. 2020;13:785–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, Ramos A, Vage V, Al-Sabah S, Brown Wet al. . Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg. 2019;29(3):782–95. [DOI] [PubMed] [Google Scholar]
- 191.Aghamohammadzadeh R, Greenstein AS, yadav R, Jeziorska M, Hama S, Soltani F, Pemberton PW, Ammori B, Malik RA, Soran Het al. . Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013;62(2):128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Haluzikova D, Lacinova Z, Kavalkova P, Drapalova J, Krizova J, Bartlova M, Mraz M, Petr T, Vitek L, Kasalicky Met al. . Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity. 2013;21(7):1335–42. [DOI] [PubMed] [Google Scholar]
- 193.Trachta P, Dostalova I, Haluzikova D, Kasalicky M, Kavalkova P, Drapalova J, Urbanova M, Lacinova Z, Mraz M, Haluzik M. Laparoscopic sleeve gastrectomy ameliorates mRNA expression of inflammation-related genes in subcutaneous adipose tissue but not in peripheral monocytes of obese patients. Mol Cell Endocrinol. 2014;383(1–2):96–102. [DOI] [PubMed] [Google Scholar]
- 194.Cancello R, Zulian A, Gentilini D, Mencarelli M, Della Barba A, Maffei M, Vitti P, Invitti C, Liuzzi A, Di Blasio AM. Permanence of molecular features of obesity in subcutaneous adipose tissue of ex-obese subjects. Int J Obes. 2013;37(6):867–73. [DOI] [PubMed] [Google Scholar]
- 195.Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clement K. Human adipose tissue macrophages: M1 and M2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94(11):4619–23. [DOI] [PubMed] [Google Scholar]
- 196.Cinkajzlova A, Lacinova Z, Klouckova J, Kavalkova P, Trachta P, Kosak M, Kratky J, Kasalicky M, DoleZalova K, Mraz Met al. . An alternatively activated macrophage marker CD163 in severely obese patients: the influence of very low-calorie diet and bariatric surgery. Physiol Res. 2017;66(4):641–52. [DOI] [PubMed] [Google Scholar]
- 197.Garcia-Rubio J, Leon J, Redruello-Romero A, Pavon E, Cozar A, Tamayo F, Caba-Molina M, Salmeron J, Carazo A. Cytometric analysis of adipose tissue reveals increments of adipocyte progenitor cells after weight loss induced by bariatric surgery. Sci Rep. 2018;8(1):15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu y, Aron-Wisnewsky J, Marcelin G, Genser L, Le Naour G, Torcivia A, Bauvois B, Bouchet S, Pelloux V, Sasso Met al. . Accumulation and changes in composition of collagens in subcutaneous adipose tissue after bariatric surgery. J Clin Endocrinol Metab. 2016;101(1):293–304. [DOI] [PubMed] [Google Scholar]
- 199.Moreno-Navarrete JM, Ortega F, Gomez-Serrano M, Garcia-Santos E, Ricart W, Tinahones F, Mingrone G, Peral B, Fernandez-Real JM. The MRC1/CD68 ratio is positively associated with adipose tissue lipogenesis and with muscle mitochondrial gene expression in humans. PLoS One. 2013;8(8):e70810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hagman DK, Larson I, Kuzma JN, Cromer G, Makar K, Rubinow KB, Foster-Schubert KE, van yserloo B, Billing PS, Landerholm RWet al. . The short-term and long-term effects of bariatric/metabolic surgery on subcutaneous adipose tissue inflammation in humans. Metabolism. 2017;70:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hess DA, Trac JZ, Glazer SA, Terenzi DC, Quan A, Teoh H, Al-Omran M, Bhatt DL, Mazer CD, Rotstein ODet al. . Vascular risk reduction in obesity through reduced granulocyte burden and improved angiogenic monocyte content following bariatric surgery. Cell Rep Med. 2020;1(2):100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang C, Zhang J, Liu Z, Zhou Z. More than an anti-diabetic bariatric surgery, metabolic surgery alleviates systemic and local inflammation in obesity. Obes Surg. 2018;28(11):3658–68. [DOI] [PubMed] [Google Scholar]
- 203.Xu XJ, Apovian C, Hess D, Carmine B, Saha A, Ruderman N. Improved insulin sensitivity 3 months after RyGB surgery is associated with increased subcutaneous adipose tissue AMPK activity and decreased oxidative stress. Diabetes. 2015;64(9):3155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Moreno-Castellanos N, Guzman-Ruiz R, Cano DA, Madrazo-Atutxa A, Peinado JR, Pereira-Cunill JL, Garcia-Luna PP, Morales-Conde S, Socas-Macias M, Vazquez-Martinez Ret al. . The effects of bariatric surgery-induced weight loss on adipose tissue in morbidly obese women depends on the initial metabolic status. Obes Surg. 2016;26(8):1757–67. [DOI] [PubMed] [Google Scholar]
- 205.Swarbrick MM, Stanhope KL, Austrheim-Smith IT, Van Loan MD, Ali MR, Wolfe BM, Havel PJ. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-y gastric bypass surgery. Diabetologia. 2008;51(10):1901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, Ikramuddin S. Adipokine response in diabetics and nondiabetics following the Roux-en-y gastric bypass: a preliminary study. J Surg Res. 2007;142(2):295–300. [DOI] [PubMed] [Google Scholar]
- 207.Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Kloting N, Gregoire C, Lolmede K, Bluher M, Clement K. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab. 2014;99(8):E1466–70. [DOI] [PubMed] [Google Scholar]
- 208.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JDet al. . Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Frikke-Schmidt H, O'Rourke RW, Lumeng CN, Sandoval DA, Seeley RJ. Does bariatric surgery improve adipose tissue function?. Obes Rev. 2016;17(9):795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Camastra S, Vitali A, Anselmino M, Gastaldelli A, Bellini R, Berta R, Severi I, Baldi S, Astiarraga B, Barbatelli Get al. . Muscle and adipose tissue morphology, insulin sensitivity and beta-cell function in diabetic and nondiabetic obese patients: effects of bariatric surgery. Sci Rep. 2017;7(1):9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Löfgren P, Andersson I, Adolfsson B, Leijonhufvud BM, Hertel K, Hoffstedt J, Arner P. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J Clin Endocrinol Metab. 2005;90(11):6207–13. [DOI] [PubMed] [Google Scholar]
- 212.Andersson DP, Eriksson Hogling D, Thorell A, Toft E, Qvisth V, Naslund E, Thorne A, Wiren M, Lofgren P, Hoffstedt Jet al. . Changes in subcutaneous fat cell volume and insulin sensitivity after weight loss. Diabetes Care. 2014;37(7):1831–6. [DOI] [PubMed] [Google Scholar]
- 213.Figueroa-Vega N, Jordan B, Perez-Luque EL, Parra-Laporte L, Garnelo S, Malacara JM. Effects of sleeve gastrectomy and rs9930506 FTO variants on angiopoietin/Tie-2 system in fat expansion and M1 macrophages recruitment in morbidly obese subjects. Endocrine. 2016;54(3):700–13. [DOI] [PubMed] [Google Scholar]
- 214.Wiewiora M, Mertas A, Gluck M, Nowowiejska-Wiewiora A, Czuba Z, Piecuch J. Effect of weight loss surgery on biomarkers of angiogenesis in obese patients. Obes Surg. 2020; 303:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Datta R, Podolsky MJ, Atabai K. Fat fibrosis: friend or foe?. JCI Insight. 2018;3(19):e122289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Keophiphath M, Achard V, Henegar C, Rouault C, Clement K, Lacasa D. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23(1):11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chabot K, Gauthier MS, Garneau Py, Rabasa-Lhoret R. Evolution of subcutaneous adipose tissue fibrosis after bariatric surgery. Diabetes Metab. 2017;43(2):125–33. [DOI] [PubMed] [Google Scholar]
- 218.Reggio S, Rouault C, Poitou C, Bichet JC, Prifti E, Bouillot JL, Rizkalla S, Lacasa D, Tordjman J, Clement K. Increased basement membrane components in adipose tissue during obesity: links with TGFbeta and metabolic phenotypes. J Clin Endocrinol Metab. 2016;101(6):2578–87. [DOI] [PubMed] [Google Scholar]