Abstract
Background
This rapid review systematically evaluated the effects of honeybee products compared to controls for the prevention, duration, severity, and recovery of acute viral respiratory tract infections (RTIs), including SARS-CoV-2, in adults and children.
Methods
Cochrane rapid review methods were applied. Four English databases plus preprint servers and trial registries were searched for randomized controlled trials (RCTs). The evidence was appraised and synthesized using RoB 2.0 and GRADE.
Results
27 results were derived from 9 RCTs that included 674 adults and 781 children. In hospitalized adults with SARS-CoV-2, propolis plus usual-care compared to usual-care alone reduced the risk of shock, respiratory failure and kidney injury and duration of hospital admission. Honey was less effective than Guaifenesin for reducing cough severity at 60-minutes in adults with non-specific acute viral RTIs. Compared to coffee, honey plus coffee, and honey alone reduced the severity of post-infectious cough in adults. Honey reduced the duration of cough in children compared to placebo and salbutamol; and the global impact of nocturnal cough after one night compared to usual-care alone and pharmaceutical cough medicines.
Conclusion
More studies are needed to robustly assess honeybee's role in SARS-CoV-2 and non-specific viral respiratory infections.
Protocol registration
PROSPERO: CRD42020193847.
Keywords: Long-COVID, COVID-19, Coronavirus pandemic, TCIM, Complementary medicine
1. Introduction
This rapid review of honeybee products responds to calls from the World Health Organization and the World Naturopathic Federation to urgently review both direct and indirect evidence for traditional, complementary, and integrative medicine (TCIM) in the context of the COVID-19 pandemic.1
Honeybee products are traditional medicines used in many cultures for their health-promoting and medicinal properties, including for symptoms of viral respiratory tract infections (RTIs). Whilst the primary product is honey, other honeybee products include propolis, royal jelly, bee-pollen, beeswax, and bee-venom. These products contain numerous phenolic compounds, peptides, flavonoids, polysaccharides, vitamins, and minerals that have a wide range of pharmacological activity. To date, honeybee products have been demonstrated to have antiviral, antibacterial, antifungal, anti-inflammatory, antioxidant, immunomodulation, wound healing, and cardioprotective properties.2 The bioactivity and mechanisms of action of honeybee products, along with their potential to prevent or treat SARS-CoV-2 have been reviewed in detail elsewhere.3, 4, 5, 6, 7, 8
Honey alone and honey combined with other ingredients was evaluated for symptomatic relief of upper respiratory tract infections in a recent systematic review (2020) and meta-analyses.9 Included were 14 randomized controlled trials (RCTs) of 1,431 adults and children. The reviewers concluded that honey was superior to usual care for reducing cough frequency and severity. Similarly, a 2018 Cochrane Review of honey for acute cough in children, which included 6 RCTs (n=899 children) concluded that honey probably reduces cough symptoms more than placebo and salbutamol (a bronchodilator) when given for up to three days. It additionally demonstrated that honey was better than diphenhydramine (an antihistamine) at relieving and reducing children's cough, though no better than dextromethorphan (a cough suppressant) on its effect on all cough symptoms.10 Given the current evidence, clinical practice guidelines often recommend honey for the symptomatic management of cough.11, 12, 13 The clinical evidence for the use of honeybee products other than honey however, is yet to be synthesized for viral RTIs and no systematic reviews have included populations with SARS-CoV-2 infections.
In response, this rapid review aimed to systematically evaluate the effects of honeybee products on the prevention, duration, severity, and recovery of acute viral respiratory tract infections, including SARS-CoV-2 infections, in people of any age.
2. Method
2.1. Protocol registration
This rapid review (RR) conforms with the Interim Guidance from the Cochrane Rapid Reviews Methods Group14 and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).15 The protocol was registered in July 2020 with the International Prospective Register of Systematic Reviews, (PROSPERO) number CRD42020193847.16 No post protocol changes were made.
2.2. Search strategy
The search strategy was developed in collaboration with research librarians, (ZL & KA). As per recommendations for rapid reviews of traditional and complementary medicine,17 no restrictions on language or date were applied, however, for pragmatic reasons databases in languages other than English were not searched and records not published in English were not translated. The subject headings and text terms were for honeybee products, coronaviruses, respiratory tract infections, and RCTs in humans. PubMed, EMBASE, CENTRAL, AMED and Alt Health Watch databases were searched. The preprint servers: Research square, medRxiv, and bioRxiv, and the clinical trial registries: U.S. National Library of Medicine Register of Clinical Trials (ClinicalTrials.gov), International Standard Randomized Controlled Trial Number Register (ISRCTN), World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and Global Coronavirus COVID-19 Clinical Trial Tracker were searched for SARS-CoV-2 trials only. Searches were conducted between 29 April to 15 August 2020, repeated on 21st January 2021 and supplemented by bibliography searches of included articles and relevant systematic reviews.9,10 Full details of the searches are reported in Supplement 1.
2.3. Study eligibility criteria
Study design. Included were RCTs and quasi-RCTs.
Population. Included were people of any age and gender who had a laboratory confirmed acute upper or lower viral RTI, including SARS-CoV-2, or other respiratory illness where the cause is most likely a viral infection such as signs and/or consistent with the common cold, non-seasonal rhino-sinusitis, pharyngitis, laryngitis, flu-like illness, healthy people with acute bronchitis, or young children with pneumonia. Also included were people who had post-infectious symptoms that were likely to be caused by a viral RTI.
Excluded were people with upper or lower respiratory illnesses where the cause was confirmed not to be a viral infection, as well as respiratory illnesses where a non-viral cause is common, such as adolescents and adults with pneumonia, people of any age with bronchitis who have a concurrent underlying health problem /comorbidity, and people of any age with otitis externa/media infections.
Interventions and comparators. Any type of honeybee product including honey, propolis, royal jelly, bee-pollen, beeswax, and bee-venom, in any form, of single or multiple doses were included. Honeybee products in combination with another intervention were included if the control group also received the same co-intervention. All types of non-honeybee controls and comparator groups were included.
Outcomes. The a priori main and additional outcomes of interest were mapped against core outcome sets indexed for COVID-1918 and re-classified as critical or important to align with recommendations published post protocol registration.19 For studies evaluating the treatment of viral RTIs, the critically important (primary) outcomes of interest were duration of illness, symptom severity, complications, and recovery from post-viral illness. Important (secondary) outcomes of interest were duration of hospitalization, respiratory support, additional interventions, health related quality of life and adverse events.
2.4. Study selection and data extraction
The Covidence online platform was used to screen studies.20 In-line with recommended rapid review methods,14 the first 30 title-abstracts and 5 full-papers were jointly screened for calibration and consistency, the remaining were screened by single, experienced reviewers. To reduce the risk of missing eligible studies, a low threshold for including studies was applied and all studies excluded at full-paper screen were rescreened by a second reviewer. Similarly, following calibration, the data were extracted by a single reviewer and verified by a second reviewer.14 A piloted electronic spreadsheet was used to extract study design, participants, interventions, comparators, outcome measures, effect size and direction. When study authors did not respond to requests for further information, additional data were extracted from published systematic reviews and meta-analyses.9,10
2.5. Risk of bias assessment
For each eligible trial, after calibration against a pre-piloted rubric, reviewers used the revised version of the Cochrane risk of bias tool21 (RoB 2.0) for assessing risk of bias of each a priori outcome. As per rapid review recommendations,14 one reviewer appraised the RoB with verification by a seconder reviewer and any disagreements resolved through consensus.
2.6. Synthesis methods
Clinical and methodological diversity and statistical heterogeneity were considered prior to pooling two or more studies or combining two or more study arms. To determine which studies were eligible for syntheses, the population/condition and interventions of included studies were mapped against the critically important and important outcomes. The random-effects model was used for the synthesis of studies that were clinically and/or methodologically diverse. As per the preferred approach recommended in the Cochrane Handbook,22 multiple honeybee arms were combined for syntheses when the comparator arms were identical, and honeybee arms were divided by the number of comparator arms, when comparators were different. Comparative effectiveness of different types of honey was explored in subgroup analyses. Adverse events were synthesized per-participant when possible, otherwise as the most frequent adverse event.
2.7. Statistical methods
RevMan 5.423 and Microsoft Excel were used for statistical analyses that were conducted and interpreted according to the Cochrane Handbook.22 Missing standard deviations were calculated from data extracted from two published systematic reviews.9,10 Dichotomous outcomes were calculated using the Mantel-Haenszel method and reported as risk ratios and risk differences. For continuous outcomes, either the weighted mean or standardized mean differences were calculated using an inverse variance method. Statistical heterogeneity was assessed using the I² statistic and homogeneity assessed with the chi² test and interpreted according to published guidance.22 A priori subgroup analyses were conducted for different types of active controls and dose of honeybee products.
2.8. Certainty of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to rate the certainty (i.e. quality) of the evidence.24 As per rapid review recommendations, this was done by single reviewers and verified by at least one additional reviewer.14 Sensitivity analyses assessed the impact of individual studies on the overall RoB. Sub-group and sensitivity analyses assessed the degree to which statistical heterogeneity might be explained by clinical or methodological diversity. When grading imprecision, the optimal information size for effect estimates was based on single-study sample size calculations on a conventional 2-sided sample size calculation with 80% power and a type 1 error rate of 5%. The minimally important difference (MID) for mean composite scores of a validated global pediatric nocturnal cough questionnaire, was set at a decrease of 1-point lower, consistent with previous estimations.25,26 For standardized mean differences (SMD), the MID was set at an effect size magnitude of Cohens-d 0.5 and a large effect size was set at a magnitude of 0.8.27 The MID for duration of hospital admission with SARS-CoV was set as four days fewer. Publication bias was statistically assessed with funnel plots for meta-analyses that included ten or more RCTs.28
3. Results
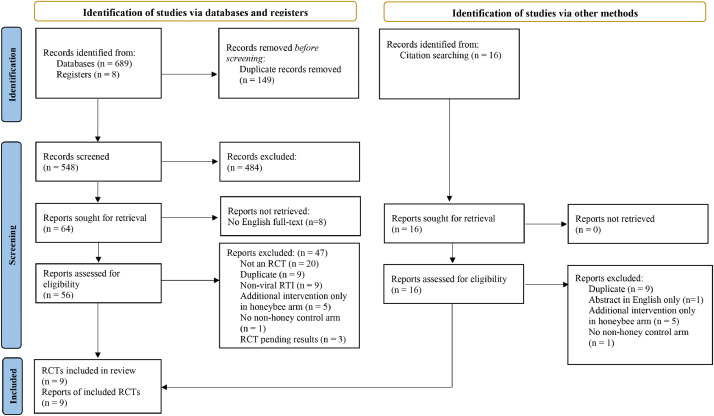
After screening 548 titles and abstracts, and 64 full-texts, nine unique RCTs that evaluated honeybee products for treatment of SARS-CoV-2 (1 RCT)29 or acute viral RTIs (8 RCTs) 29-37were included (Figure 1). Except for one RCT that published the results clinical trial registry only,37 they all were published in peer reviewed journals. Three other potentially eligible RCTs were identified from clinical trial registries and are yet to report results,38, 39, 40 one of which is investigating honey for SARS-CoV-2.40 Details of these ongoing trials and the studies excluded at full-text screen with reasons, are reported in Supplement 2.
Fig. 1.
PRIMSA flow diagram of search results.15
3.1. Study characteristics
The nine RCTs that included 674 adults and 781 children, compared honeybee products with and without usual care, against usual care, active, or placebo controls (Table 1). Propolis was evaluated in 124 adults participants admitted to hospital with a SARS-CoV-2 infection.29 Honey was evaluated in eight RCTs of 1,330 participants for symptoms consistent with a non-specific, community acquired viral RTI,30, 31, 32, 33, 34, 35, 36, 37 from which 84 adults had symptoms of persistent post-infectious cough,35 465 adults had an acute sore throat36 or cough,37 and 781 children had acute nocturnal cough.30, 31, 32, 33, 34
Table 1.
Characteristics of studies.
| Study ID Country Setting* | Participants | Honeybee intervention (type) | Comparator | a priori Outcomes |
|---|---|---|---|---|
| Ayazi 2017 30 Iran Hospital outpatients | Children with acute cough, aged 1-12yrs (mean 3.5 years ± 1.6) | Honey (Kimina and Shahde-Golla) + usual care* 30 minutes before bed, 2 consecutive nights n=71 | DHM + usual care* 30 minutes before bed, 2 consecutive nights n=21 | Cough severity Global impact nocturnal cough† Adverse events |
| Cohen 201231 Israel Community clinics Multi-centre | Children with acute cough, aged 1-5 yrs (mean 26.1 months) | Honey (Eucalyptus, Citrus and Labiatae) + usual care* Single dose, 10g, 30 min before bedtime n=225 | Sicilian date extract + usual care* Single dose, 10g, 30 min before bedtime n=75 | Cough severity Global impact nocturnal cough† Adverse events |
| Nanda 201736 India Hospital outpatients | Adults and older adults with pharyngitis or tonsilitis | Honey (off the shelf) + antiseptic throat gargle antibiotics, anti-inflammatory medication 14mls twice per day, up to 15 days n=100 | Antiseptic throat gargle antibiotics, anti-inflammatory medication up to 15 days n=100 | Recovery Hospitalized Satisfaction Adverse events |
| Paul 200732 U.S.A. Hospital outpatients | Children with rhinorrhea and cough, aged 2-18 yrs (mean: 5.1 years) | Honey (Buckwheat) + usual care* Single dose 10ml at night n=35 | 1. DXM + usual care* Single dose 10ml at night n=33 2. Usual care* n=37 | Cough severity Global impact nocturnal cough† Adverse events |
| Raeessi 201135 Iran Hospital outpatients | Adults with persistent post-infectious cough, aged 21 to 65 years | 1. Honey (type not specified) 62.5g honey/day for 7 days n=16 2. Honey (type not specified) 62.5g honey/day & coffee 8.8g instant coffee (2.25g caffeine)/day for 7 days n=54 | Coffee 8.8g instant coffee (2.25g caffeine)/day for 7 days n=14 | Cough severity Sustained treatment effect |
| Shadkam 201033 Iran Paediatric clinic | Children with cough, rhinorrhea, sore throat and nasal congestion, aged 24-60 months | Honey (Kafi-Abad) + supportive care†, single dose 2.5ml, 30 minutes before bed n=33 | 1.DXM + supportive care‡ single dose 2.5ml, 30 minutes before bed n=36 2. DHM + supportive treatment single dose 2.5ml, 30 minutes before bed n=34 3. Supportive care‡ n=36 | Cough severity Global nocturnal cough† Adverse events |
| Silveira 202129 Brazil Hospital inpatients | Adults with SARS-CoV-2 infection confirmed with PCR, mean age 50.0 years (± 12.8) | 1.Propolis (Brazilian green propolis extract) 400mg/day for 7 days + standard care n=41 2.Propolis (Brazilian green propolis extract) 800mg/day for 7 days + standard care n=42 | Standard care n=42 | Complications (kidney injury respiratory failure, shock, mortality) Admission to ICU Length of hospital stay Duration oxygen dependency Renal replacement therapy Adverse events |
| Waris 201434 Kenya Hospital outpatients | Children with acute cough, aged 1 to 12 years | Honey (type not specified) 2.5ml (ages 1-2 yrs), 5ml (ages 2-6 yrs), 7.5ml (ages 6-12 yrs), 3x/day, 5 days n=57 | 1.Placebo syrup2.5ml (ages 1-2 yrs), 5ml (ages 2-6 yrs), 7.5ml (ages 6-12 yrs), 3x/day, 5 days n=45 2. Salbutamol syrup, 2.5ml (ages 1-2 yrs), 5ml (ages 2-6 yrs), 7.5ml (ages 6-12 yrs), 3x/day, 5 days n=43 | Cough severity Global impact nocturnal cough† Recovery Adverse events |
| Wyeth P/L 201337 U.S.A. Hospital outpatients | Adults with acute cough, mean age 32.3 years (±10.8) | Honey (Buckwheat) Single dose, 10ml n=103 | 1.Placebo Single dose 1 tablet n=55 2. Guaifenesin 400mg Single dose 1 tablet n=107 | Coughing bouts Cough severity Satisfaction Adverse events |
DXM, Dextromethorphan; DHM, Diphenhydramine.
Usual care, acetaminophen or ibuprofen as required.
Global impact nocturnal cough, combined score from parent rated cough frequency, severity and bothersomeness, and quality of parents’ and child's sleep.
Supportive care, saline nose drops, water vapor, cleaning a blocked nose, and acetaminophen for fever. See Supplement 3 for further details of study characteristics.
All RCTs reported at least one outcome of interest, from which 27 effect estimates were synthesized (13 critical outcomes and 14 important outcomes). None of the RCTs evaluated the prophylactic effects of honeybee products. Honeybee for treatment was evaluated for four of the critical, and five of the important a priori outcomes (Table 2). Further details of the characteristics of studies, including RCT protocol, HREC approval, funding and conflicts of interests are reported in Supplement 3.
Table 2.
Evaluated outcomes.
| Outcomes a-priori | Adults SARS-CoV-2 | Adults viral RTI | Children viral RTI |
|---|---|---|---|
| Critical outcomes | |||
| Proportion with complications | Kidney injury29 Respiratory failure29 Shock29 Death29 | - | - |
| Duration of illness | - | Pharyngitis/tonsilitis recovery36 | Cough clearance34 |
| Symptom severity at the peak of illness | - | Cough severity 1 hour post intervention37 | Cough severity at day 1 of treatment30, 31, 32, 33, 34* |
| Recovery from post-viral symptoms | - | Severity# of persistent post-infectious cough35 | - |
| Important outcomes | |||
| Time spent in hospital | Days in hospital29 | - | - |
| Duration of respiratory support | Days with oxygen therapy29 Days ventilated29† | - | - |
| Proportion requiring additional interventions and health services | Admission to ICU29 Dialysis29 | Admission to hospital36 | - |
| Health Related Quality of Life | - | - | Global impact of nocturnal cough30, 31, 32, 33, 34† |
| Adverse events | Adverse events29 | Adverse events36 | Adverse events30, 31, 32, 33, 34 |
| Other relevant outcomes (not synthesized) | |||
| Symptom relief satisfaction | - | Pain relief36 Cough relief37 | - |
| Cough frequency | - | Observed number of acute cough bouts37 | Frequency acute cough30, 31, 32, 33, 34 |
| Other individual cough impact scores | - | - | Cough bothersome, parental & child sleep30, 31, 32, 33, 34 |
| Risk of signs not resolved after 5, 10 and 15 days | - | Fever36 Oropharyngeal congestion36 | - |
Unable to estimate means and/or SD for Warris 2014 34 and Cohen 2012.31
For persistent post-infectious cough, authors state that cough frequency is equivalent to cough severity “due to the positive relationship between cough frequency and cough reflex sensitivity”.35
Unable to estimate mean and/or SD for Silveira 2021 29 Warris 2014 34 and Shadkam 2010.33
3.2. Quality and certainty of the evidence
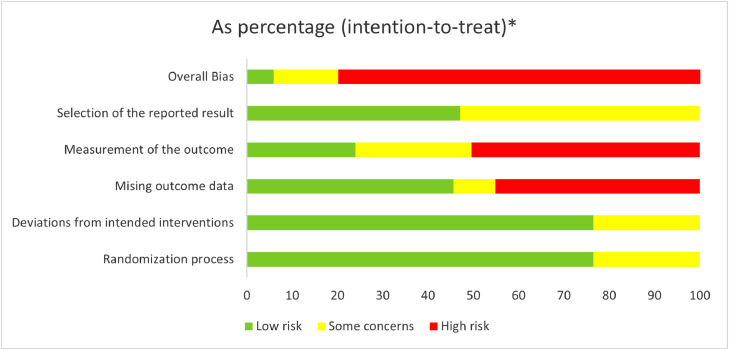
Thirty-seven outcomes from the nine RCTs were assessed for RoB (Figure 2 and Supplement 4). At least one outcome synthesized from each RCT was at a high RoB. Only three outcomes had a low RoB, all from the one RCT.32 Nine outcomes synthesized from two RCTs29,34 had some concerns about RoB. This included all six treatment outcomes evaluating propolis in participants with SARS-CoV-2 as there were concerns with possible selective reporting bias of the results due to insufficient information about the planned analysis reported in the protocol, and in the manuscript.29 Of the remaining 25 outcomes with a high RoB, synthesis of 13 outcomes from six RCTs had a high RoB due to missing data (domain 3)29,31,33, 34, 35, 36 and 19 outcomes from nine RCTs had a high risk of measurement bias (domain 4) due to unblinded, subjective measurements.
Fig. 2.
RoB 2.0 tool 21: Overall risk of bias for critical and important outcomes.
Honeybee arms were combined in three RCTs29, 30, 31 as comparators were identical. The honey arm of one RCT33 was divided, as comparators were different. (Supplement 5: Table 5.2). The type of honey was not specified in three RCTs34, 35, 36 and subgroup analyses comparing efficacy of different honey types was not conducted due to insufficient numbers. Adverse events were analyzed per-participant, except for honey when compared to placebo for the duration of cough in children, in which the most frequent adverse event (vomiting) was synthesized.34
Of the 27 effect estimates that were synthesized, the certainty (quality) of the evidence was graded as moderate certainty for one, low certainty for 12, and very low certainty for 14 (Supplement 6). All estimates were rated down at least one level for risk of bias, ten had serious RoB and 15 had very serious RoB. Serious or very serious imprecision was an issue for 22 estimates. For 21 estimates, this was due to the optimum information size not being met that in turn, reflected from small sample sizes and the small number of RCTs synthesized per estimate. Only one effect estimate was rated down for inconsistency. This was due to considerable statistical heterogeneity that was only partially explained by clinical and methodological diversity. None were rated down for indirectness; however, this assumed that the effects on honeybee would not be extrapolated to other populations or conditions that had not been evaluated. Publication bias was not strongly suspected for any estimate however, due to less than ten RCTs in each synthesis, no statistical tests for small study bias were conducted.
3.3. Effects of honeybee
Table 3 presents the summary of findings. Further details about the GRADE assessments and the statistical synthesis, subgroup and sensitivity analyses, effect sizes and additional calculations are reported in Supplement 5 and 6.
Table 3.
Summary of findings.
| Outcomes | Intervention/Comparison (number of participants) | Effect estimate (95% CI) | Certainty* | |
|---|---|---|---|---|
| Adults admitted to hospital for management of SARS-CoV-2 infection | ||||
| Critically important outcomes | ||||
| Risk of death for up to 28 days | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | No deaths (estimate of effect not estimable) | VERY LOWa,dd |
| Risk of shock requiring vasoactive drugs | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | RR 0.36 (0.15 to 0.87) 15 per 100 adults fewer (30 fewer to 1 fewer) | LOWa,d |
| Risk of respiratory failure requiring ventilation | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | RR 0.32 (0.11 to 0.92) 13 per 100 adults fewer (26 fewer to none fewer) | LOWa,d |
| Risk of acute kidney injury | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | RR 0.36 (0.15 to 0.87) 15 per 100 adults fewer (30 fewer to 1 fewer) | LOWa,d |
| Important outcomes | ||||
| Duration of oxygen therapy | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | MD -1.83 days (-4.82 to 1.16) | LOWa,d |
| Risk of requiring renal replacement therapy | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | RR 0.17 (0.02 to 1.59) 6 per 100 adults fewer (14 fewer to 2 more) | LOWa,d |
| Risk of admission to intensive care unit | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | RR 0.36 (0.12 to 1.05) 17 per 100 adults fewer (38 fewer to 3 more) | LOWa,d |
| Duration of hospital admission | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | MD -3.77 days (-6.14 to -1.39) | MODERATEa |
| Risk of non-serious adverse events | 1 RCT29 | Propolis + usual care (n = 82) vs. usual care (n = 42) | RR 0.26 (0.02 to 2.74) 4 per 100 adults fewer (10 fewer to 3 more) | VERY LOWaa,d |
| Adults in community settings with symptoms consistent with a non-specific viral RTI | ||||
| Critically important outcomes | ||||
| Likelihood of recovery within 5 days from acute pharyngitis/tonsilitis | 1 RCT36 | Honey + usual care (n = 45) vs. usual care (n = 38) | RR 1.20 (0.87 to 1.65) 8 per 100 adults more (6 fewer to 23 more) | VERY LOWaa,d |
| Acute RTI cough severity 1 hour after intervention | 1 RCT37 | Honey (n = 102) vs. placebo (n = 54) | MD 0.10 points (-0.08 to 0.28) | VERY LOWaa,d |
| 1 RCT37 | Honey (n = 102) vs. guaifenesin (n = 107) | MD 0.20 points (0.05 to 0.35) Favors Guaifenesin | VERY LOWaa,d | |
| Severity of persistent post-infectious cough | 1 RCT35 | Honey (n = 12) vs. coffee (n = 14) | MD -0.40 points (-0.75 to -0.05) | VERY LOWaa,d |
| 1 RCT35 | Honey + coffee (n = 48) vs. coffee (n = 14) | MD -1.40 points (-1.67 to -1.13) | LOWaa | |
| Important outcomes | ||||
| Risk of hospital admission for acute pharyngitis/tonsilitis | 1 RCT36 | Honey + usual care (n = 91) vs. usual care (n = 92) | RR 0.81 (0.22 to 2.92) 1 per 100 adults fewer (7 fewer to 5 more) | VERY LOWaa,d |
| Risk of non-serious adverse events | 1 RCT36 | Honey (n = 102) vs. placebo (n = 55) | RR 0.53 (0.11 to 2.58) 3 per 100 adults fewer (9 fewer to 4 more) | VERY LOWaa,d |
| 1 RCT36 | Honey + usual care (n = 91) vs. usual care (n = 92) | No adverse events (estimate of effect not estimable) | VERY LOWaa,d | |
| 1 RCT37 | Honey (n = 102) vs. guaifenesin (n = 107) | RR 0.79 (0.18 to 3.43) 1 per 100 children fewer (6 fewer to 4 more) | VERY LOWaa,d | |
| Children in community settings with symptoms consistent with a non-specific acute viral RTI | ||||
| Critically important outcomes | ||||
| Duration of acute cough | 1 RCT34 | Honey (n = 57) vs. placebo (n = 45) | MD -0.71 days (-1.15 to -0.28) | LOWaa |
| 1 RCT34 | Honey (n = 57) vs. salbutamol (n = 43) | MD -0.54 days (-0.99 to -0.09) | LOWaa | |
| Acute cough severity on day 1 | 2 RCT32,33 | Honey ± usual care (n = 68) vs. usual care (n = 71) | SMD -1.10 (-2.52 to 0.33) | VERY LOWaa,b,d |
| 3 RCT30,32,33 4 arms | Honey + usual care (n=135) vs. DXM or DHM + usual care (n=123) | SMD -0.65 (-0.97 to -0.32) | VERY LOWaa,d | |
| Important outcomes | ||||
| Global impact of nocturnal cough after 1 night | 1 RCT32 | Honey ± usual care (n = 35) vs. usual care (n = 37) | SMD -0.80 (-1.28 to -0.32) | VERY LOWaa,d |
| 3 RCT30, 31, 32 | Honey ± usual care (n = 301) vs. DXM, DHM or date extract ± usual care (n = 124) | SMD -0.74 (-1.18 to -0.30 | LOWaa | |
| Risk of vomiting as an adverse event | 1 RCT34 | Honey (n = 57) vs. placebo (n = 45) | RR 1.48 (0.69 to 3.18) 9 per 100 children more (7 fewer to 25 more) | LOWaa |
| Risk of any non-serious adverse event | 2 RCT32,33 | Honey ± usual care (n = 61) vs. usual care (n = 69) | RR 1.98 (0.40 to 9.87) 8 per 100 children more (11 fewer to 27 more) | VERY LOWaa,d |
| 4 RCT30, 31, 32, 33 | Honey + usual care (n = 332) vs. DXM, DHM or date extract + usual care (n = 194) | RR 1.57 (0.57 to 4.31) 1 per 100 children more (2 fewer to 4 more) | VERY LOWaa,d | |
GRADE 24 certainty (quality) assessments
serious risk of bias.
very serious risk of bias.
serious inconsistency; bbvery serious inconsistency; cserious indirectness; ccvery serious indirectness.
serious imprecision.
very serious imprecision; epublication bias strongly suspected. Further details of risk of bias are reported in Supplementary 5. The results of statistical tests for heterogeneity, and subgroup and sensitivity analyses are reported in Supplementary material. CI, Confidence interval; MD, Mean difference; RCT, Randomized controlled trial; RD, Risk difference / absolute relative risk; RR, Relative risk; RTI, Respiratory tract infection; SMD, Standard Mean Difference.
3.3.1. Propolis for treatment of SARS-CoV-2
In the one RCT that included 124 adults who were hospitalized with confirmed SARS-CoV-2 infections,29 the addition of propolis 400mg or 800mg daily to usual (standard) care reduced the risks of developing shock, respiratory failure, and kidney injury, as well as the duration of hospitalization. However, there were no significant effects from propolis on the duration of oxygen therapy, or the risks of requiring renal replacement therapy, being admitted to intensive care, or of non-serious adverse events such as nausea or headaches. Mortality was reported as zero for all participants over the 28 days of follow-up. Subgroup analyses found no significant differences according to dose for any of the outcomes (p = 0.83 to 0.32).
3.3.2. Honey for non-specific viral respiratory tract infections
Honey was evaluated for reduced symptom severity in three RCTs including 420 adults with acute RTI cough,37 pharyngitis/tonsilitis, 36 and persistent post-infectious cough.35 Honey was less effective than a guaifenesin tablet at reducing the severity of cough one-hour post intervention, there were no differences for honey compared to a placebo tablet, and there were no differences in the risks of non-serious adverse events when honey was compared to either guaifenesin or placebo.37 The addition of honey to usual care did not significantly reduce the likelihood of recovering from acute pharyngitis/tonsilitis at day-5, or the reduce the risk of hospital admission.36 In contrast, honey was more efficacious than coffee alone at reducing the severity of persistent post-infectious cough.35 The effects were largest when honey was combined with coffee rather than used on its own.(Supplementary 5: Figure 3 and Table 5.4).
Five RCTs evaluated the effects of honey in 714 children with acute RTI and cough.30, 31, 32, 33, 34 Honey reduced the duration of symptoms compared to placebo or salbutamol.34 Compared to the active controls Dextromethorphan (DXM) or Diphenhydramine (DHM), honey reduced nocturnal cough severity,30,32,33 however, there were no significant differences between honey and usual care.32,33 Considerable heterogeneity in pooled analyses (I2= 93%, p<0.01) was partially explained by diverse forms of usual care, (Supplementary 5: Outcome 3.2). In contrast, the global impact of nocturnal cough was reduced by honey compared to usual care32 and when honey was compared to the active controls DXM, DHM or date extract.30, 31, 32 Again, considerable heterogeneity (I2= 70%, p=0.04) was partially explained by diverse comparators, (Supplement 5: Outcome 4.4). The results from the subgroup analyses according to the type of active control were also inconsistent. There were no significant differences between honey and DXM or DHM on nocturnal cough severity (p=0.69), but significant differences between DXM, DHM and date extract on global impact of pediatric nocturnal cough were found (p=0.04). There were no significant differences between honey and placebo, usual care, or active controls on the risk of non-serious adverse events.
4. Discussion
This review is the first to report the effects of honeybee products for a SARS-CoV-2 population. There was low certainty evidence that for adults hospitalized with an acute SARS-CoV-2 infection, the addition of high dose propolis to standard care reduced the risks of shock requiring vasoactive drugs, respiratory failure requiring ventilation, and acute kidney injury, and moderate certainty evidence that the addition of propolis shortened the duration of hospitalization. These results were driven by one single-center RCT that enrolled in-patients with moderate to severe disease, including approximately 50% with known risk factors for poor SARS-CoV-2 outcomes.29 Notably, there is also emerging clinical evidence that propolis can improve renal function and reduce proteinuria in adults with chronic kidney disease,41 and reduce inflammation and fibrosis in adults with non-alcoholic fatty liver disease.42 Whilst the mechanisms of effect in SARS-CoV-2 were not investigated, the rationale for propolis as treatment for SARS-CoV-2 infections has been extensively described elsewhere.6,7 In short, propolis is a complex intervention with broad spectrum antiviral activity that includes inhibitory effects on cell-membrane proteins that mediate viral entry into cells (ACE2, TMPRSS2 and PAK1) as well as having other antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects.8 In this RCT, clinical outcomes for propolis plus usual care were supplemented by reductions in inflammation, suggesting propolis anti-inflammatory and immunomodulating mechanisms may reduce the risk of kidney injury and death from acute disease, as well as lower risks of post-infectious morbidity (long-covid), including ongoing needs for haemodialysis.43 Limitations to this RCT evidence included its open-label design and statistical imprecision as the sample size was only powered for determining the duration of hospital admission. Notwithstanding, the findings provide important preliminary evidence about the potential beneficial effects of propolis as an adjuvant to standard care for treating SARS-CoV-2 and deserve repetition in more rigorous RCT design.
This review builds on previous reviews about the effects of honey for RTIs.9,10 For adults with symptoms consistent with a non-specific acute viral RTI, an additional RCT was identified from which honey was found to be less efficacious than guaifenesin in reducing subjective cough severity after 60-minutes and no different from a placebo tablet.37 In keeping with another recently published systematic review,9 we also found no improvements in acute pharyngitis/tonsilitis outcomes when honey was used as an adjuvant to antiseptic throat gargle, antibiotics, and non-steroidal anti-inflammatory medications,36 and that the severity of post-infectious cough in adults was reduced after using honey with or without coffee for one week.35 These mixed findings may reflect the low to very low certainty in the evidence that was due to both a high risk of bias and imprecision due to small sample sizes. Further, there was considerable clinical and methodological diversity in the three RCTs.
For children with symptoms consistent with a non-specific acute viral RTI, the findings of this review mostly aligned with those of other systematic reviews.9,10 We found that honey reduced the duration of cough (low certainty) and the global impact of nocturnal cough (low to very low certainty). However, the effects of honey on nocturnal cough severity were mixed and of very low certainty. The key difference between our assessment and an earlier Cochrane review10 was that for all estimates of effect, we further downgraded the certainty of the evidence from moderate or low certainty to low and very low certainty due to greater concerns with the risk of bias. There were also differences in our estimates of effects for honey compared to DXM. This was due to discrepancies in the synthesized outcomes of the Cochrane review10. Specifically, in the Cochrane analyses 1.1.2 and 1.1.6,10 data had been incorrectly extracted in the opposite direction for Paul et al.32 Notwithstanding, in our subgroup analyses (Supplement 5: analyses 4.6.1 and 4.6.2), like the Cochrane review we found no significant differences between honey and DXM. Lastly, in contrast with previous reviews9,10 we reclassified date extract as an active control rather than placebo and acetaminophen or ibuprofen as required as usual care rather than no treatment. These decisions were informed by their potential therapeutic mechanisms in SARS-CoV-244 or RTI.45 In the sensitivity analyses, reclassifying these interventions as placebo or no treatment did not substantially impact our overall conclusions (Supplement 5: analyses 3.2, 4.7.6 and 4.8.1).
In contrast with a recent systematic review of honey for upper RTIs,9 we only included studies where the individual effects of honey could be ascertained and excluded studies when co-interventions (e.g. herbs, vitamins or milk)26,46, 47, 48, 49 were only given to the honey group. Traditional uses include combining honey with other potentially active ingredients due to their potential synergistic effects. Indeed, the findings from Raessi et al.35 suggest there may be significant synergistic effect when honey is combined with coffee that contains the xanthine alkaloid caffeine, a known bronchodilator.50 Study designs, such as that applied by Raessi are important for investigating potential agonistic and antagonistic effects of the components in combination therapies which are often utilized in traditional complementary and integrative medicine.
4.1. Strengths and limitations
Despite applying rapid review methods to the search strategy, an additional study missed by other reviewers was identified.37 However, for pragmatic reasons, only English databases were searched, and articles published in other languages were not translated. Honeybee products are widely used across the globe and only two of the nine studies were conducted countries where English is the official language. Therefore, it is likely that studies have been missed. Indeed, two potentially eligible articles were identified during title-abstract screening.51,52 The first was an RCT from Iran that reported honey was more efficacious than DHP for acute cough in young children.51 The second was a placebo-controlled RCT that evaluated the prophylactic and therapeutic effects of propolis for the common cold in healthy adult participants in Japan.52 The investigators reported no difference in the incidence of common cold or symptom severity. However, propolis shortened the duration of illness. To ensure other key studies are included, future reviews should strive to broaden their search of databases in languages other than English, including Chinese databases, and include a plan to retrieve and translate articles.
Twenty-seven effect estimates were presented. This relatively large number was due to wide clinical diversity in populations and comparisons, and methodological diversity in outcome measures, that prevented pooling many of the results. This was further exacerbated by relying on obtaining missing data for cough severity from other systematic reviews.10 However, discrepancies in the data extraction and SEs prevented reliable back computations of additional means and SDs31,34 that otherwise could have been synthesized. Consequently, 22 of the effect estimates reported were from only one RCT that in turn led to serious imprecision when grading the evidence certainty. Notwithstanding, the results from a broad range of critical and important outcomes were reported.
4.2. Conclusion
This review identified preliminary evidence of the potential beneficial effects of propolis as an adjunct to standard care for treating SARS-CoV-2 infections. However, more studies are needed to validate these findings. In adults with symptoms consistent with an acute viral RTI, honey was found to reduce the severity of post-infectious cough; however, honey did not provide immediate relief for acute cough, nor did honey improve recovery or reduce the risk of hospitalization from acute pharyngitis/tonsilitis. For children, honey was found to reduce the duration, severity, and impact of cough. The certainty (quality) of most evidence was low to very low. Higher quality RCTs, with larger sample sizes, are warranted.
Acknowledgments
The authors would like to thank the World Naturopathic Federation for organizing a response to the WHO call for TCIM evidence for COVID-19, and initiative that seeded this study.
Author contributions
Conceptualization: SA, JH, SM, BK, MM, RL. Methodology: SA, BK, JG, SM, JH. Investigation: ZL, KA, SA, BK, RL, MM. Formal Analysis: SA, BJ, MM, RL, BK, JH, JG. Writing – Original Draft: SA and JH. Writing – Review and Editing: all authors.
Conflict of interests
The authors declare that they have no conflicts of interest. SA and JH are editorial board members of this journal but had no role in the editorial process or decision of this paper.
Acknowledgments
Funding
No funding was received for this work.
Ethical statement
No ethical approval was required as this study did not involve human participants or laboratory animals.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version, at doi:10.1016/j.imr.2021.100779.
Appendix. Supplementary materials
References
- 1.Steel A, Wardle J, Lloyd I. The potential contribution of traditional, complementary and integrative treatments in acute viral respiratory tract infections: Rapid Reviews in response to the COVID-19 pandemic. Adv Integr Med. 2020;7(4):181–182. doi: 10.1016/j.aimed.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornara L, Biagi M, Xiao J, Burlando B. Therapeutic properties of bioactive compounds from different honeybee products. Front Pharmacol. 2017;8:412. doi: 10.3389/fphar.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Naggar Y, Giesy JP, Abdel-Daim MM, Javed Ansari M, Al-Kahtani SN, Yahya G. Fighting against the second wave of COVID-19: can honeybee products help protect against the pandemic? Saudi J Biol Sci. 2021;28(3):1519–1527. doi: 10.1016/j.sjbs.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hatamleh MAI, Hatmal MM, Sattar K, Ahmad S, Mustafa MZ, Bittencourt MC. Antiviral and immunomodulatory effects of phytochemicals from honey against COVID-19: potential mechanisms of action and future directions. Molecules. 2020;25(21) doi: 10.3390/molecules25215017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lima WG, Brito JCM, da Cruz, Nizer WS. Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2) Phytother Res. 2021;35(2):743–750. doi: 10.1002/ptr.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossain KS, Hossain MG, Moni A, Rahman MM, Rahman UH, Alam M. Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. Heliyon. 2020;6(12):e05798. doi: 10.1016/j.heliyon.2020.e05798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berretta AA, Silveira MAD, Cóndor Capcha JM, De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasupuleti VR, Sammugam L, Ramesh N, Gan SH. Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxidative Med Cellular Longevity. 2017:2017. doi: 10.1155/2017/1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abuelgasim H, Albury C, Lee J. Effectiveness of honey for symptomatic relief in upper respiratory tract infections: a systematic review and meta-analysis. BMJ Evid-Based Med. 2020 doi: 10.1136/bmjebm-2020-111336. [DOI] [PubMed] [Google Scholar]
- 10.Oduwole O, Udoh EE, Oyo-Ita A, Meremikwu MM. Honey for acute cough in children. Cochrane Database Syst Rev. 2018;(4) doi: 10.1002/14651858.CD007094.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. Common Cold: How to Feel Better, 〈https://www.cdc.gov/antibiotic-use/community/for-patients/common-illnesses/colds.html〉; Published 2020.
- 12.NICE. Cough (acute): antimicrobial prescribing, 〈https://www.nice.org.uk/guidance/ng120/resources/visual-summary-pdf-6664861405〉; Published 2019.
- 13.RACGP. The Handbook of Non-Drug interventions (HANDI): Honey and cough in children with URTI, 〈https://www.racgp.org.au/clinical-resources/clinical-guidelines/handi/conditions/children/honey-and-cough-in-children-with-urti〉; Published 2014.
- 14.Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C. Cochrane rapid reviews methods group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol. 2020;130:13–22. doi: 10.1016/j.jclinepi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arentz S, Hunter J, Goldenberg J, Yang G, Beardsley J. PROSPERO 2020 CRD42020182044. 2020. Protocol for a rapid review of zinc for the prevention or treatment of COVID-19 and other coronavirusrelated respiratory tract infections in humans. < www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020182044> Published. [Google Scholar]
- 17.Hunter J, Arentz S, Goldenberg J, Yang G, Beardsley J, Lee MS. Choose your shortcuts wisely: COVID-19 rapid reviews of traditional, complementary and integrative medicine. Integrat Med Res. 2020 doi: 10.1016/j.imr.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu R, Zhao C, Liang T, Hao X, Huang Y, Zhang X. Core outcome set for clinical trials of COVID-19 based on traditional Chinese and western medicine. medRxiv. 2020 doi: 10.3389/fphar.2020.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall JC, Murthy S, Diaz J, Adhikari N, Angus DC, Arabi YM. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192. doi: 10.1016/S1473-3099(20)30483-7. -e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covidence systematic review software, 〈www.covidence.org〉.
- 21.Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:366. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ WV. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 23.Review Manager (RevMan). 5.3 ed. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014
- 24.Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach: The GRADE Working Group; 2013. Available from: https://gradepro.org/cite/guidelinedevelopment.org/handbook.
- 25.Ayazi P. Comparison of the effect of two kinds of Iranian honey and diphenhydramine on nocturnal cough and sleep quality in coughing children and their parents. 2013. [DOI] [PMC free article] [PubMed]
- 26.Peixoto DM, Rizzo JA, Schor D, Silva AR, DCd Oliveira, Solé D. Use of honey associated with Ananas comosus (Bromelin) in the treatment of acute irritative cough. Revista Paulista de Pediatria. 2016;34(4):412–417. doi: 10.1016/j.rppede.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical power analysis for the behavioral sciences: Academic press; 2013.
- 28.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 29.Silveira MAD, De Jong D, Berretta AA, dos Santos Galvão EB, Ribeiro JC, Cerqueira-Silva T. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayazi P, Mahyar A, Yousef-Zanjani M, Allami A, Esmailzadehha N, Beyhaghi T. Comparison of the effect of two kinds of Iranian honey and diphenhydramine on nocturnal cough and the sleep quality in coughing children and their parents. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen HA, Rozen J, Kristal H, Laks Y, Berkovitch M, Uziel Y. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics. 2012;130(3):465–471. doi: 10.1542/peds.2011-3075. [DOI] [PubMed] [Google Scholar]
- 32.Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161(12):1140–1146. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 33.Shadkam MN, Mozaffari-Khosravi H, Mozayan MR. A comparison of the effect of honey, dextromethorphan, and diphenhydramine on nightly cough and sleep quality in children and their parents. J Alternat Complement Med. 2010;16(7):787–793. doi: 10.1089/acm.2009.0311. [DOI] [PubMed] [Google Scholar]
- 34.Waris A, Macharia W, Njeru E, Essajee F. Randomised Double Blind study to compare effectiveness of honey, salbutamol and placebo in treatment of cough in children with common cold. East Afr Med J. 2014;91(2):50–56. [PubMed] [Google Scholar]
- 35.Raeessi M-A, Aslani J, Gharaie H, Karimi Z, Raeessi N, Assari S. Honey with Coffee: A new finding in the treatment of Persistent Postinfectious Cough. 2011.
- 36.Nanda MS, Mittal SP, Gupta V. Role of honey as adjuvant therapy in patients with sore throat. Nat J Physiol, Pharmacy Pharmacol. 2017;7(4):412. [Google Scholar]
- 37.Wyeth_Pharmaceuticals Pty Ltd. Study of Count Coughs in Subjects With Acute Upper Respiratory Tract Infections NCT01062256, 〈https://clinicaltrials.gov/ct2/show/results/NCT01062256?view=results〉; Published 2013.
- 38.Hamidi GA. The effect of honey on duration of pediatric pneumonia, 〈https://www.irct.ir/trial/11474〉; Published 2016.
- 39.Nobahar M. Effect of Propolis mouthwash on incidence of Ventilator-Associated Pneumonia in hospitalized patient in critical care unit, 〈https://en.irct.ir/trial/37046〉; Published 2019.
- 40.Tantawy M. Efficacy of Natural Honey Treatment in Patients With Novel Coronavirus, 〈https://clinicaltrials.gov/ct2/show/NCT04323345〉; Published 2020.
- 41.Silveira MAD, Teles F, Berretta AA, Sanches TR, Rodrigues CE, Seguro AC. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial. BMC Nephrol. 2019;20(1):1–12. doi: 10.1186/s12882-019-1337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soleimani D, Rezaie M, Rajabzadeh F, Gholizadeh Navashenaq J, Abbaspour M, Miryan M. Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real-time two-dimensional shear wave elastography: A randomized clinical trial. Phytother Res. 2021;35(3):1669–1679. doi: 10.1002/ptr.6937. [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2021;32(1):161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takdehghan G, Dorchin M, Amin M, Ghorbanzadeh B, Assarian AR, Amirgholami N, et al. Efficacy of date palm (Phoenix Dactylifera L.) leaf extract in COVID-19 infection: a randomized double-blind clinical trial. 2021.
- 45.Sarrell EM, Wielunsky E, Cohen HA. Antipyretic treatment in young children with fever: acetaminophen, ibuprofen, or both alternating in a randomized, double-blind study. Arch Pediatr Adolesc Med. 2006;160(2):197–202. doi: 10.1001/archpedi.160.2.197. [DOI] [PubMed] [Google Scholar]
- 46.Cohen HA, Hoshen M, Gur S, Bahir A, Laks Y, Blau H. Efficacy and tolerability of a polysaccharide-resin-honey based cough syrup as compared to carbocysteine syrup for children with colds: a randomized, single-blinded, multicenter study. World J Pediatr. 2017;13(1):27–33. doi: 10.1007/s12519-016-0048-4. [DOI] [PubMed] [Google Scholar]
- 47.Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled, multicenter study. Arch Pediatr Adolesc Med. 2004;158(3):217–221. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 48.Sopo SM, Greco M, Monaco S, Varrasi G, Di Lorenzo G, Simeone G. Effect of multiple honey doses on non-specific acute cough in children. An open randomised study and literature review. Allergol Immunopathol (Madr) 2015;43(5):449–455. doi: 10.1016/j.aller.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Ashraf S, Ashraf S, Imran MA, Ashraf M, Kalsoom L, Siddiqui UN, et al. Efficacy of honey and Nigella sativa against COVID-19: HNS-COVID-PK Trial. medRxiv 2020.
- 50.Welsh EJ, Bara A, Barley E, Cates CJ. Caffeine for asthma. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD001112.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmadi M, Moosavi S, Zakeri S. Comparison of the effect of honey and diphenhydramine on cough alleviation in 2-5-year-old children with viral upper respiratory tract infection. J Gorgan Univ Med Sci. 2013;15(2) [Google Scholar]
- 52.Ohkuma A, Kanno T, Asama T, Doi-Takaki S, Kawaguchi M, Tatefuji T. Effect of dietary supplement containing brazilian propolis on the common cold. Pharmacometrics. 2010;79(3-4):43–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.