Abstract
There is limited evidence that non-leukaemic lymphoid malignancies are radiogenic. As radiation-related cancer risks are generally higher after childhood exposure, we analyzed pooled lymphoid neoplasm data in nine cohorts first exposed to external radiation aged <21 years using active bone marrow (ABM) and, where available, lymphoid system doses, and harmonized outcome classification. Relative and absolute risk models were fitted.
Years of entry spanned 1916-1981. At the end of follow-up (mean 42.1 years) there were 593 lymphoma (422 non-Hodgkin (NHL), 107 Hodgkin (HL), 64 uncertain subtype), 66 chronic lymphocytic leukaemia (CLL) and 122 multiple myeloma (MM) deaths and incident cases among 143,136 persons, with mean ABM dose 0.14 Gy (range 0–5.95 Gy) and mean age at first exposure 6.93 years. Excess relative risk (ERR) was not significantly increased for lymphoma (ERR/Gy=−0.001; 95%CI: −0.255, 0.279), HL (ERR/Gy=−0.113; 95%CI:−0.669, 0.709), NHL+CLL (ERR/Gy=0.099; 95%CI:−0.149, 0.433), NHL (ERR/Gy=0.068; 95%CI:−0.253, 0.421), CLL (ERR/Gy=0.320; 95%CI: −0.678, 1.712), or MM (ERR/Gy=0.149; 95%CI:−0.513, 1.063 (p-trend>0.4). In six cohorts with estimates of lymphatic tissue dose, borderline significant increased risks (p-trend=0.02-0.07) were observed for NHL+CLL, NHL, CLL.
Further pooled epidemiological studies are needed with longer follow-up, central outcome review by expert hematopathologists, and assessment of radiation doses to lymphoid tissues.
INTRODUCTION
Studies that associate radiation exposure from a variety of sources during childhood and adolescence with subsequent risk of hematopoietic neoplasms have established leukemia and myeloid neoplasms as clearly radiogenic, but only a limited number of studies have examined lymphoid malignancies1. Most studies of radiation exposure and subsequent risk of non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia (CLL), multiple myeloma (MM) and other lymphoid malignancies are based on adult exposures and have shown little or no evidence of elevated risks 1–13, with the exception of a few investigations of NHL 5, 6 and MM 3 in subsets of the Japanese atomic-bomb survivor Life Span Study (LSS) and in a small number of nuclear worker studies11, 12, and of NHL13 and CLL9 in Chernobyl emergency and clean-up workers.
Childhood radiation exposure confers the highest proportionally increased risks for many cancer types, in particular leukaemia 1, so it is the most promising setting for studying the risks of lymphoid malignancies. However, because of the relative rarity of lympho-haematopoietic malignancies, most individual cohort studies do not have enough cases to provide sufficient statistical power for a meaningful investigation.
We previously conducted a pooled analysis of myeloid neoplasms and leukaemia following low-level radiation exposure in young persons (age <21 years) 14. In the present paper we focused on the estimation of radiation-related risk of lymphoma (including NHL, CLL, HL, and MM) in a pooled analysis of nine of these cohorts exposed to radiation while aged <21 years. We assessed risks in relation to external exposure to radiation, excluding all groups treated for malignant disease. The primary analysis was conducted in terms of the active bone marrow (ABM) dose. Because of indications that ABM dose may not be optimal for lymphoma 15–18 in sensitivity analyses we evaluated dose-response in relation to lymphoid tissue doses19 for those cohorts for which these could be derived. We examined risks in subsets of the cohorts by type of outcome, nature of exposure, demographic and other factors.
MATERIALS AND METHODS
Cohort definition, incidence/mortality ascertainment
We examined all available radiation-exposed cohort studies from the most recent comprehensive summaries by international committees 1 combined with recent literature reviews 20 and PubMed literature searches. The PubMed searches were last performed on 29 May 2018, to find studies published before 30 June 2014. We chose cohorts without restriction by age at first exposure, but focused on cohort members receiving radiation under age 21 years. We restricted to groups with 5 or more haematopoietic malignancies receiving average cumulative ABM radiation doses >0.005 Gy of low-LET radiation. A complication in many medical studies, particularly after treatment for cancer, is the administration of certain types of concomitant chemotherapy, possibly associated with an elevated risk of NHL 10, 21. For this reason, we excluded any studies of patients treated for malignant disease. Those groups mainly exposed to internal sources of radiation, where dosimetry was inadequately described, or with very small numbers of cases were also excluded. We required that the cohorts had individual cumulative ABM dose estimates. The following nine cohorts met the eligibility criteria:
-
(a)
the paediatrically-exposed (age at treatment <21 years) patients of the Massachusetts TB fluoroscopy mortality study 22;
-
(b)
the paediatrically-exposed (age at treatment <21 years) patients of the Canadian TB fluoroscopy cohort 23;
-
(c)
the French haemangioma cohort 24;
-
(d)
the Göteborg haemangioma cohort 25;
- (e)
- (f)
-
(g)
the paediatrically-exposed (age at exposure <20 years) subjects of the Japanese atomic-bomb survivor Life Span Study (LSS) cohort 6;
- (h)
-
(i)
the US scoliosis cohort 32.
From the selected cohorts, we obtained individual-level data, specifying (among other things) restrictions by age at first exposure (generally <21 years).
Apart from the LSS, the datasets comprise medically exposed groups (for therapeutic or diagnostic purposes). Follow-up started generally at the end of treatment for most medically irradiated groups and continued until the earliest of date of cancer diagnosis, date of death, loss to follow-up or the end of the study. Follow-up in some groups (Canadian TB fluoroscopy study, Rochester thymus enlargement cohort, Swedish haemangioma cohorts) began on the date of establishment of the relevant national mortality or cancer registries, or the Japanese national census establishing the LSS cohort. Further details about subject identification are given in Appendix A; in particular, further details on follow-up in the individual cohorts are given in Table A1.
Radiation dosimetry
We used ABM dose as the primary measure of target organ/tissue dose in analysis of lymphoid neoplasms risks since ABM dose was available for all cohorts and other dose-response analyses of radiation and lymphoid neoplasms have used ABM dose. As outlined in Appendix B, because of indications that ABM dose may not be optimal for lymphoma 15–18, for six cohorts for which the relevant information was available (both TB fluoroscopy, Rochester enlarged thymus, Israeli tinea capitis, LSS, scoliosis) we also conducted analyses using estimated doses to components of the lymphoid system, specifically to the two main components, lymphatic tissue (lymph nodes, spleen, thymus, small intestine), and to circulating lymphocytes derived from age category-specific computational phantoms developed by Lee et al 19. The dosimetry model used in the current analyses was an extension, also developed using age-specific computational phantoms, of a previously developed dosimetry model of lymph nodes 33. Mean cumulative ABM and lymphoid system doses were calculated for each subject in the cohorts according to methods described previously1 (see Appendix A). Doses were expressed as absorbed doses in Gy, which are predominantly photon doses, although for the LSS cohort the (relatively small) neutron component of absorbed dose is weighted (by a factor of 10) to account for the greater biological effectiveness of neutrons relative to photons. Overall, the pooled analysis generally used the most recently calculated set of doses described in these studies, although in some cases, for example in the Israeli tinea capitis data, modifications were applied to derive average whole-body ABM and lymphoid system doses from available skull ABM doses 29, 34.
Outcome classification
The methods/sources of case identification were study-specific. These include: (1) population-based tumour/cancer registries (LSS, Israeli tinea capitis, Göteborg and Stockholm haemangioma); (2) medically-validated self-reported information (French haemangioma); (3) national vital statistics registries (Canadian TB fluoroscopy, Massachusetts TB fluoroscopy, Rochester thymus, US scoliosis). Further details of disease ascertainment and follow-up methods for each cohort are given in Appendix A. These studies span several decades, with some cohorts recruiting subjects before 1920 and some recruiting after 1980, and follow-up extending beyond 2000 (Table 1), and include incidence and mortality data. Therefore, we carefully reviewed the lymphoid malignancy outcome data and developed ICD coding to harmonise outcomes across both incidence and mortality studies and over calendar time (see Appendix A). Owing to the large temporal range in the data and the notable changes in lymphoma classification during the past 60+ years 35–37, we had to concentrate on large subtype groups, and could not include more recent subtype classifications. We defined the following outcomes of interest with categories that have been used in other analyses of radiation-exposed populations and would allow us to categorize outcomes diagnosed over a 60+ year period:
Table 1.
Excess relative risk (ERR) per Gy (and 95% CI) of lymphoma and multiple myeloma obtained from fitting semi-parametric linear (in dose) excess relative risk models in relation to active bone marrow dose.a
| Endpoint | Cases | ERR/Gy (95% CI) | p-valueb | Inter-cohort heterogeneity p-value |
|---|---|---|---|---|
| All lymphoma | 593 | −0.001 (−0.255c, 0.279) | >0.999 | 0.197 |
| Non-Hodgkin lymphoma (NHL) + chronic lymphocytic leukaemia (CLL) | 488 | 0.099 (−0.149, 0.433) | 0.480 | 0.344 |
| Non-Hodgkin lymphoma (NHL) | 422 | 0.068 (−0.253c, 0.421) | 0.650 | 0.606 |
| Chronic lymphocytic leukaemia (CLL) | 66 | 0.320 (−0.678c, 1.712) | 0.445 | 0.912 |
| Hodgkin lymphoma (HL) | 107 | −0.113 (−0.669c, 0.709) | 0.737 | 0.995 |
| Multiple myeloma (MM) | 122 | 0.149 (−0.513c, 1.063) | 0.654 | 0.985 |
Models are as described by Appendix B (B2), stratifying by cohort, sex, age and year of follow-up (using intervals of age and year of follow-up defined by person-year table, as in Appendix A Table A1). Unless otherwise stated, all confidence intervals are based on the profile likelihood.
p-value of improvement in fit over null model (without dose trend).
Wald-based confidence limit.
-
a)
all lymphoma (NHL+HL, although a few lymphomas were not classified further as NHL or HL);
-
b)
NHL;
-
c)
CLL;
-
d)
NHL + CLL;
-
e)
Hodgkin lymphoma (HL);
-
f)
MM.
CLL, a form of NHL38, 39, was combined with NHL for analyses, but also considered separately for comparison with other studies. Deaths were coded to the International Classification of Diseases (ICD) revisions 6 through 10, and incident outcomes were generally coded to the International Classification of Diseases for Oncology (ICD-O) revisions 2 or 3 (see Appendix A for detailed ICD/ICD-O coding) 39.
Covariates
A unified set of covariates that was collected in a uniform way across the nine cohorts, was used for adjustment of radiation risk. These included sex, age at cohort entry, age first exposed, age last exposed, attained age, year of birth, years since first exposure, years since last exposure, two-year lagged mean ABM dose, accumulated in moving windows by time since exposure and age at exposure. Various dose lags have been used in the analysis of lymphoma and MM in other cohorts11 and while a dose-lag of two years has been adopted for our main analysis, because this is most frequently used in other studies 7–10, 40–42, we also evaluated longer and shorter latency periods in sensitivity analyses.
Randomisation and masking
All data received by the statistical analyst (MPL) was in fully anonymised form.
Statistical analysis
In our primary analyses we estimated the excess relative risk (ERR) per Gy of ABM dose (ERR/Gy) for each lymphoid malignancy outcome for pre-defined dose categories (see Table A5) using the unexposed group (0 mGy) as the reference category, and using linear-quadratic models to assess possible non-linearity in dose. We also fitted models of generalised absolute risk (GAM) to obtain estimates of excess absolute risk (EAR) 43. The models were fitted by Poisson maximum likelihood 44 using Epicure 45. The data were approximately Poisson distributed within each subcohort and age group. Central estimates were maximum likelihood estimates. All tests were based on the likelihood ratio statistic and were two-sided. There were no adjustments made for multiple comparisons. Confidence intervals (CI) were generally estimated using the profile likelihood, and when the profile CI did not converge were Wald-based. In particular, profile CI were used for Figures 1 and 2. The statistical power using a 1-sided Poisson trend test with type-I error α=0.05 46 and risk coefficients derived from the subset of the publicly available LSS dataset of Hsu et al6 with age at exposure <20 [ERR/Sv =0.2589 for NHL, =0.4537 for NHL+CLL, =0.406 for HL, =0.2543 for MM], with the given numbers of lymphomas and myelomas and using the dose distribution outlined in Table C1, was 37.9% for NHL, 75.9% for NHL+CLL, 25.2% for HL and 16.7% for MM. Further details are given in Appendix B. The primary analyses focused on all cohorts combined. Secondary analyses of heterogeneity, which compared each cohort against the remaining eight other cohorts, or assessing the differences between the LSS, the three diagnostically exposed cohorts and the five therapeutically exposed cohorts are less appropriate than the primary analysis of global heterogeneity (performed in Table 1), in part because of the problem of multiple testing in the absence of a clear hypothesis guiding the cohort or group of cohorts likely to be outliers.
Figure 1. Relative risk (and 95% CI) by active bone marrow dose for (a) non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukaemia (CLL), (b) non-Hodgkin lymphoma, (c) Hodgkin lymphoma, (d) multiple myeloma.
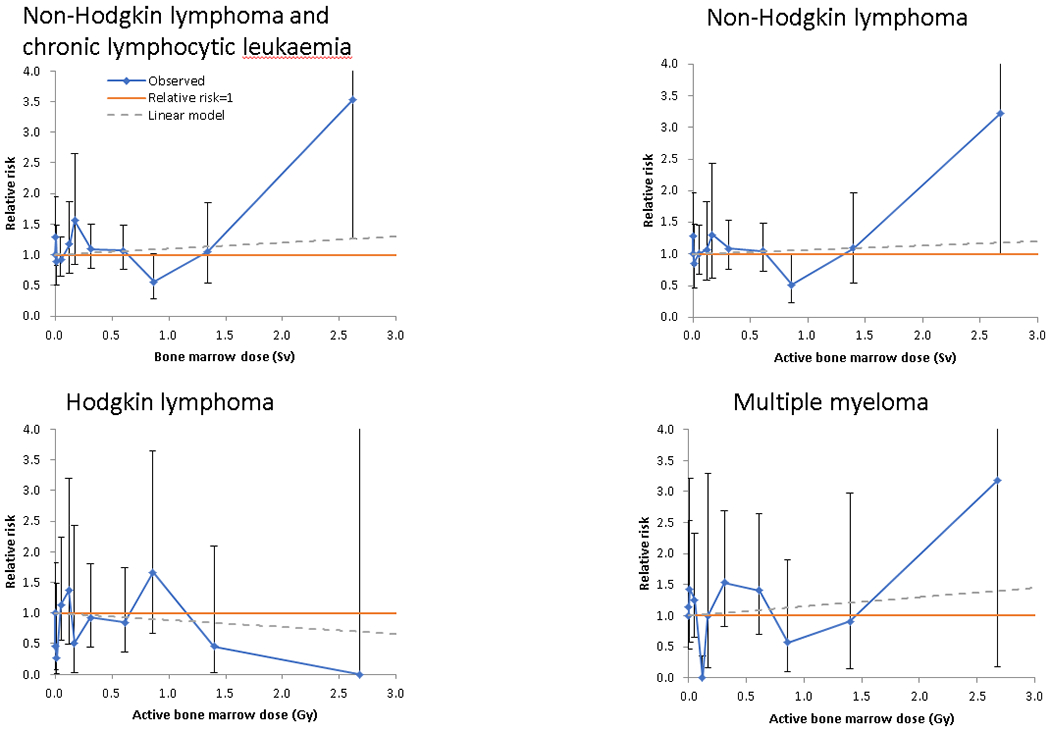
Solid blue line + symbols give the observed relative risk (and 95% CI), red line gives relative risk = 1, dashed green line the fitted linear relative risk model, with ERR/Gy taken from Table 1. Dose boundaries used for categories are 0, 0.005, 0.02, 0.10, 0.15, 0.20, 0.50, 0.75, 1.00, 2.00 Gy.
Figure 2.
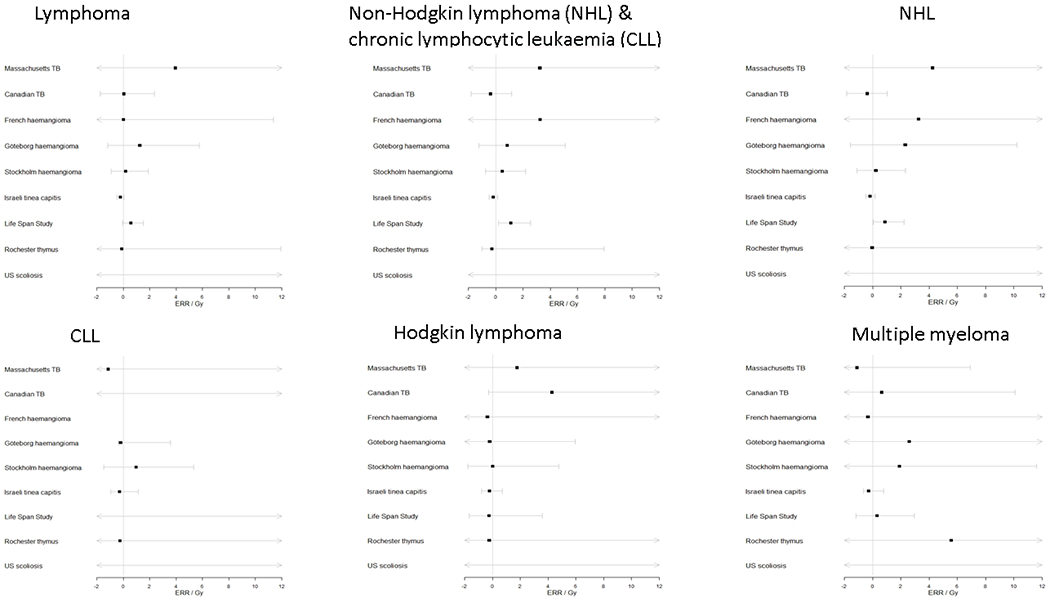
Forest plot of ERR/Gy and 95% CI by cohort and endpoint, for dose to the active bone marrow
We also conducted secondary analyses in six of the cohorts to evaluate lymphoid neoplasm risks in relation to estimated doses to total circulating lymphocytes and lymphatic tissue using a recent approach to estimating doses to these tissues 19. In other secondary analyses we assessed risk modifications by sex. An issue of potential importance was the impact of localized higher doses received from heterogeneous exposures, so we conducted certain analyses with the five therapeutic cohorts (Israeli tinea capitis, French/Göteborg/Stockholm haemangioma, Rochester thymus) considered separately. We contrasted these with the (effectively) uniformly exposed LSS cohort and those groups in which lower levels of exposure were used for diagnostic purposes (Canadian TB fluoroscopy, Massachusetts TB fluoroscopy, US scoliosis), and in which ages at exposure were generally higher.
Code and data availability
The data and all analysis code is available from the principal author upon request.
Ethical approval
The study cohort has been declared exempt by the National Cancer Institute Special Studies Institution Review Board, because using pre-existing approved data. Obtaining informed consent from all study subjects was therefore not necessary.
RESULTS
There was a total of 143,136 persons in the combined cohort accumulating 6,020,619 person-years of follow-up, with mean follow-up 42.1 years; 60,886 (42.5%) of the cohort were male, 82,250 (57.5%) were female (Appendix C Tables C1, C2). Study entry occurred between the years of 1916 and 1981 (data not shown). There were 422 NHL, 66 CLL, 122 MM and 107 HL cases/deaths (Appendix C Table C1). Mean individual ABM dose in the combined cohort was 0.14 Gy (range 0–5.95 Gy), although there was considerable heterogeneity between cohorts: the highest mean dose, for the Israeli tinea capitis cohort, was 0.29 Gy whereas the lowest mean dose, for the US scoliosis cohort, was 0.008 Gy (Appendix C Table C2). Most of the cases/deaths received low or moderate ABM doses (<1 Gy) (Appendix C Table C1). Among the exposed, the mean age at first exposure was 6.93 years, the mean years since last exposure was 29.62 years, and the mean attained age at observation was 36.55 years (Appendix C Table C2).
Lymphoid neoplasm risks for all cohorts combined and individual cohorts using ABM dose
Table 1 shows that there was no excess risk for all lymphoma (ERR/Gy=−0.001; 95% CI: −0.255, 0.279, p-trend>0.999) or HL (ERR/Gy=−0.113; 95% CI: −0.669, 0.709, p-trend=0.737). We found small and statistically non-significantly raised risks for NHL+CLL (ERR/Gy=0.099; 95%CI:−0.149, 0.433, p-trend=0.480), NHL (ERR/Gy=0.068; 95% CI: −0.253, 0.421, p-trend=0.650), CLL (ERR/Gy=0.320; 95% CI: −0.678, 1.712, p-trend=0.445) and MM (ERR/Gy=0.149; 95% CI: −0.513, 1.063, p-trend=0.654). There was no evidence of heterogeneity between cohorts for any endpoint (p>0.1, Table 1), and no indications of curvature in the dose response, as measured by quadratic departures from linearity, for any endpoint (p>0.2) (results not shown), as illustrated by Figure 1 and Appendix C Table C3.
The individual risks by cohort and endpoint are shown in Figure 2. In Table C4 we formally assess the evidence for differences between each cohort and all other datasets combined as a secondary analysis of heterogeneity. There were indications that risks in the LSS and the Israeli tinea capitis cohorts were significantly different from the other cohorts, being higher and lower, respectively, particularly for NHL+CLL; otherwise no individual cohort differed significantly from the other eight (Appendix C Table C4). After omitting either the LSS or the Israeli tinea capitis cohorts the inter-cohort heterogeneity disappears entirely for all endpoints (data not shown). There was little difference in ERR between incidence and mortality data, although the HL mortality risk was raised to a marginally significant extent (ERR/Gy=4.156; 95% CI: −0.261, 20.440, p-trend=0.087), and was borderline significantly different (p=0.064) from the incidence risk (Appendix C Table C5).
Excess absolute risks (EAR) were generally small, in the range 0.0-0.65 cases/deaths per 10,000 person-years at 1 Gy (Appendix C Table C6); it should be noted that some of the models did not converge for the rarer endpoints. Of some interest is the significant EAR of NHL+CLL, with an EAR/104 PY.Gy of 0.153 (95% CI: 0.016, 0.337, p=0.023), largely driven by the trend for NHL, with an EAR/104 PY.Gy of 0.148 (95% CI: 0.011, 0.333, p=0.031) (Appendix C Table C6), which in turn is due to males in the LSS, with an EAR/104 PY.Gy of 0.901 (95% CI: 0.167, 2.027, p=0.005) (results not shown). Very similar EARs were found when analysis was restricted to the five incidence cohorts only, although the EARs in the four mortality cohorts were generally a little higher although based on fewer deaths (Appendix C Table C6).
There were few suggestions that varying latency in the range 0-10 years had an impact upon the results (Appendix C Table C7), and few indications of significant modifications of risk by age at exposure, time since exposure or attained age (results not shown).
Secondary analysis of heterogeneity suggested that there were indications of differences in risk between the LSS, diagnostic and therapeutic groups for NHL, at borderline levels of significance (p=0.066) , and also for CLL (p=0.012) (Appendix C Table C8); there were indications of a statistically significant elevation in NHL risk in the LSS (due to males, Appendix C Table C10). However, convergence problems complicate interpretation of all these findings. Although in the pooled data there were no suggestions of differences in ERR/Gy between males and females for any endpoint (Appendix C Table C9), if analysis was broken down by dataset there were indications in the LSS for all lymphoma, NHL+CLL and NHL of significantly higher (p<0.02) risks for men compared with women, primarily due to NHL, with risks for men generally statistically significantly elevated (Appendix C Table C10).
Lymphoid neoplasm risks for six-cohort subset using alternative dose measures
In a subset of six of the nine cohorts for which doses to two lymphoid system target tissues were available, the effect of using circulating lymphocyte or lymphatic tissue dose on the risk was to elevate and render borderline significant the ERR/Gy for NHL+CLL: using ABM dose, 0.050 (95% CI: −0.189, 0.387, p=0.726); using lymphocyte dose, 0.294 (95% CI: −0.114, 0.889, p=0.190); using lymphatic tissue dose, 0.790 (95% CI: 0.083, 1.882, p=0.022) (Table 2). Likewise, when lymphatic tissue dose was employed, trends in risk for NHL and CLL separately became borderline significant (p=0.074 and p=0.055, respectively) (Table 2). The trends both for NHL+CLL and NHL were largely driven by LSS males, although there were large (if non-significant) risks also in the Massachusetts TB and scoliosis cohorts (Appendix C Table C11, Appendix C Figure C1).
Table 2.
Assessment of significance and inter-cohort heterogeneity in excess relative risk (ERR) per Gy (and 95% CI) in relation to dose to active bone marrow (ABM), total circulating lymphocytes and lymphatic tissues, for the six cohorts for which doses to the lymphoid system are available.†
| Tissue dose used | Number of case/deaths | ERR/Gy (+95% CI) | p-valuea | Inter-cohort heterogeneity p-value |
|---|---|---|---|---|
| All lymphoma | ||||
| Active bone marrow dose | 474 | −0.031 (−0.237, 0.251) | 0.805 | 0.084 |
| Lymphocyte dose | 0.135 (−0.205, 0.621) | 0.498 | 0.105 | |
| Lymphatic tissue dose | 0.492 (−0.067, 1.332) | 0.096 | 0.340b | |
|
| ||||
| Non-Hodgkin lymphoma + CLL | ||||
| Active bone marrow dose | 376 | 0.050 (−0.189, 0.387) | 0.726 | 0.154b |
| Lymphocyte dose | 0.294 (−0.114, 0.889) | 0.190 | 0.377b | |
| Lymphatic tissue dose | 0.790 (0.083, 1.882) | 0.022 | 0.862b | |
|
| ||||
| Non-Hodgkin lymphoma | ||||
| Active bone marrow dose | 342 | 0.019 (−0.224, 0.370) | 0.896 | 0.333b |
| Lymphocyte dose | 0.219 (−0.189, 0.828) | 0.352 | 0.515b | |
| Lymphatic tissue dose | 0.631 (−0.045, 1.704) | 0.074 | 0.854b | |
|
| ||||
| Chronic lymphocytic leukemia | ||||
| Active bone marrow dose | 34 | 0.331 (−0.989c, 2.283) | 0.504 | 0.774b |
| Lymphocyte dose | 1.113b (−1.888c, 5.329) | 0.212b | 0.914b | |
| Lymphatic tissue dose | 4.511 (−0.031, 20.020) | 0.055 | 0.800b | |
|
| ||||
| Hodgkin lymphoma | ||||
| Active bone marrow dose | 71 | −0.080 (−0.711c, 0.846) | 0.825 | 0.900b |
| Lymphocyte dose | −0.024 (−1.309c, 1.858) | 0.975 | 0.896b | |
| Lymphatic tissue dose | 0.492 (−2.426c, 5.855) | 0.749 | 0.911b | |
|
| ||||
| Multiple myeloma | ||||
| Active bone marrow dose | 96 | −0.043 (−0.655c, 0.808) | 0.890 | 0.964a |
| Lymphocyte dose | 0.070 (−0.938c, 1.434) | 0.877 | 0.970a | |
| Lymphatic tissue dose | 0.281 (−1.130c, 2.489) | 0.640 | 0.983a | |
Obtained by fitting linear relative risk model, allowing for separate risk for diagnostically-exposed cohorts (Massachusetts TB, Canadian TB, US scoliosis), therapeutically-exposed cohorts (Israeli tinea capitis, Rochester thymus) and Japanese atomic bomb survivor Life Span Study (LSS), stratifying by cohort, sex, age and year of follow-up (using intervals of age and year of follow-up defined by person-year table, as in Appendix A Table A1). The six cohorts included in this analysis are those for which doses to the lymphoid system can be approximated from ABM doses (see Appendix B). Unless otherwise stated, all confidence intervals are based on the profile likelihood.
p-value of improvement in fit over null model (without dose trend).
indications of non-convergence
Wald-based CI
DISCUSSION
To our knowledge this pooled analysis is the first to focus on lymphoma and MM after radiation exposure at a young age 11. In this analysis of nine cohorts of children or adolescents exposed to radiation, we observed little evidence of radiation-related excess risk for most endpoints considered in our primary analyses of lymphoid neoplasms in all cohorts combined using ABM doses.
ABM dose has often been used for analyses of lymphoma and MM. However, the ABM constitutes a small fraction (3–7%)47, 48 of the lymphocyte distribution throughout the body, so that it may be a relatively poor target tissue for radiation exposure with respect to the lymphoid system, which could have reduced the power of previous epidemiological studies of non-uniform exposures to detect a dose-response for lymphomas. For this reason, we have explored in the six cohorts for which these calculations are possible use of lymphocyte or lymphatic tissue dose. In this analysis, we observed notable increases in ERR/Gy which became borderline significant (p=0.02 – 0.07) for NHL+CLL, NHL and CLL when lymphatic tissue dose was used (Table 2).
All the substantive studies of lymphoid malignant neoplasms after radiation exposure in childhood and adolescence are included here (see Appendix A for details of the few excluded studies) with the exception of therapeutic exposures for the treatment of malignant diseases and internal exposures from radionuclides. There have been a number of studies of lymphoma and MM in groups environmentally exposed to radiation in childhood, details of which are given in Table C12. Radiation risks in these studies were generally positive for all endpoints, but non-significantly so (Table C12), and therefore consistent with those of the present study.
In other studies of lymphoid malignancies in the literature, mostly focusing on adult radiation exposures, there is generally little evidence for radiation-related excess risk of any type of lymphoma or MM. 1, 4. There is some evidence of excess CLL incidence in the latest follow-up of the LSS, although based on only 12 cases 6, 3 of which are included in the present study (Appendix C Table C2), and some indications of this in the current analysis, particularly in the six-cohort subset using lymphatic tissue dose (Table 2, Appendix C Table C11, Appendix C Figure C1); it should be noted that two of the CLL cases analyzed by Hsu et al 6 were hairy cell leukemia (HCL), which should have been classified as NHL (Tables A3, A4). There are large and significant excess risks of NHL and MM incidence, although not of HL in a cohort of UK radiation workers 12. There are also large and borderline significant excess risks of CLL9 and NHL13 in two groups of Chernobyl liquidators, with magnitude equal to or exceeding that of non-CLL leukaemia 9. There is support in previous analysis of the LSS for a NHL dose-response for men, but none for women 6, which is reinforced by our own analyses of these data using ABM dose (Table 1, Appendix C Table C10). The evidence for excess risk for both sexes combined for NHL or NHL+CLL becomes stronger (in the subset of six of the nine cohorts) when lymphatic tissue dose is employed (Table 2, Appendix C Table C11, Appendix C Figure C1). There is slightly stronger evidence that MM might be radiogenic, as reviewed by UNSCEAR 1, but the evidence comes largely from studies of cancer mortality rather than cancer incidence, and in particular there is no evidence of a dose-related trend in the LSS incidence data 6.
Strengths of the study are the prospective designs used in all component sub-studies, high-quality dosimetry including evaluation of different dose metrics, close to 30 years mean follow-up and the large number of lymphoid malignancy cases or deaths among those exposed at a young age. Since incidence of CLL, MM and a substantial fraction of NHL is highest among the elderly, longer follow-up is needed to clarify whether radiation exposure during childhood or adolescence is linked with possible increases in risk of these lymphoid malignancies, which may only be apparent after many decades. A weakness is that the long period covered by the multiple studies included substantial changes in classification of lymphoid malignancies including misclassification over time within a particular study and among different studies, since different classifications were used worldwide before 200135. For example, earlier diagnoses of HL were found to be NHL upon re-review and initial diagnoses of CLL were later reclassified as HCL (and hence NHL). The effect of changes in classification are not clear-cut, but possibly result in risk coefficients being biased towards the null 49. We are aware of ongoing reclassification of lymphatic malignancies in the LSS, which may impact risks in this cohort.
Some heterogeneity in dosimetry between cohorts (see Appendix A) may have some bearing on the inter-cohort heterogeneity in risk that we observed for some outcomes. It is unclear if the ABM dose used in our primary analyses is the optimal target tissue dose. We have therefore evaluated lymphoid system doses in a subset of the cohorts in our secondary analyses. These may potentially be more relevant target tissue doses given the partial body exposures that are a feature particularly of the therapeutically-exposed cohorts considered here (Swedish/French haemangioma, Israeli tinea capitis, Rochester thymus irradiation). The higher risks for lymphoid system radiation doses in the subset of cohorts that we could evaluate raise questions about which target organs/tissues are most relevant. There are biological data to suggest that dose to lymphatic tissue may be the more appropriate dose to use for lymphomas, although ABM dose may be more likely optimal for MM 15–18.
Although most members of the cohorts are nominally exposed at low or moderate ABM doses (<1 Gy), consideration must be given to the heterogeneity in bone marrow dose (and lymphoid system dose) that is present in all cohorts apart from the LSS. Therefore, a mean ABM dose of, say, 1 Gy could, in some of the medically exposed cohorts, imply appreciably higher doses in certain bone marrow and lymphatic tissue compartments. This would only matter if there were substantial non-linearity in the dose response, in particular, if doses were sufficiently high that cell sterilisation could be significant. Among groups exposed to high-dose medical procedures, there is some evidence that this might occur for leukaemia 50, but we are not aware of such data for lymphoma and MM, although studies are limited.
The mixture of mortality and incidence data complicates interpretation; however, as we consider mainly relative risk models, one would not expect ERR/Gy estimates to differ appreciably in mortality compared with incidence. We found few indications of differences in ERR for mortality or incidence (Appendix C Table C5). Perhaps more surprisingly, we also found no appreciable difference in EAR between the full analysis and the analysis restricted to the incidence cohorts. Mortality and passive incidence collection studies may not be adequate for study of radiation risks of CLL, since mortality studies tend to underestimate CLL by as much as 38% 51, 52. In addition, several studies have shown that tumour registries under-report incident CLL by 12–38% 53, 54.
In summary, we found little evidence of radiation-related risk of lymphoma or MM in the primary analyses using bone marrow dose. However, the indications of excess risk for NHL and CLL when using lymphatic tissue dose in a six-cohort subset suggest that alternative lymphatic tissue doses should be further evaluated in future epidemiologic cohort studies assessing radiation-related risk of lymphoid malignancies following radiation exposure in childhood and adolescence. In addition, further follow-up of these nine cohorts is indicated. Future studies of childhood/adolescent radiation exposure should also use centralized expert pathology review and current systems of coding of lymphoid neoplasms.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan is a public interest foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the US Department of Energy (DOE). The research was also funded in part through DOE award DE-HS0000031 to the National Academy of Sciences. Dr Zablotska’s work was supported by National Cancer Institute of the National Institutes of Health under awards R03CA188614 and R01CA197422. This publication was supported by RERF Research Protocol A1-16. The views of the authors do not necessarily reflect those of the two governments.
Footnotes
COMPETING INTERESTS
Dr Wakeford receives a consultancy fee as a member of the Technical Working Party of the Compensation Scheme for Radiation-linked Diseases (http://www.csrld.org.uk). No other authors report conflicts of interest.
References
- 1.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2006 Report. Annex A. Epidemiological Studies of Radiation and Cancer. United Nations: New York, 2008, pp. 13–322. [Google Scholar]
- 2.Linet MS, Schubauer-Berigan MK, Weisenburger DD, Richardson DB, Landgren O, Blair A, et al. Chronic lymphocytic leukaemia: an overview of aetiology in light of recent developments in classification and pathogenesis. Br J Haematol 2007. 12/2007; 139(5): 672–686. [DOI] [PubMed] [Google Scholar]
- 3.Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950–1990. Radiat Res 1996. 7/1996; 146(1): 1–27. [PubMed] [Google Scholar]
- 4.Armstrong B, Brenner DJ, Baverstock K, Cardis E, Green A, Guilmette RA, et al. Radiation. Volume 100D. A review of human carcinogens. International Agency for Research on Cancer: Lyon, France, 2012, pp. 1–341. [Google Scholar]
- 5.Richardson DB, Sugiyama H, Wing S, Sakata R, Grant E, Shimizu Y, et al. Positive associations between ionizing radiation and lymphoma mortality among men. Am J Epidemiol 2009. 4/15/2009; 169(8): 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu W- L, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res 2013. 3/2013; 179(3): 361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CJ, Freedman DM, Curtis RE, Berrington de Gonzalez A, Morton LM. Risk of non-Hodgkin lymphoma after radiotherapy for solid cancers. Leukemia & Lymphoma 2013. 2013/08/01; 54(8): 1691–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O’Hagan JA, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol 2015; 2(7): e276–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zablotska LB, Bazyka D, Lubin JH, Gudzenko N, Little MP, Hatch M, et al. Radiation and the risk of chronic lymphocytic and other leukemias among Chornobyl cleanup workers. Environ Health Perspect 2013; 121(1): 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbron RW, Chapple CL, O’Sullivan JJ, Lee C, McHugh K, Higueras M, et al. Cancer incidence among children and young adults who have undergone x-ray guided cardiac catheterization procedures. Eur J Epidemiol 2018April; 33(4): 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harbron RW, Pasqual E. Ionising radiation as a risk factor for lymphoma: A review. J Radiol Prot 2020 Oct 5; 40: R151–R185. [DOI] [PubMed] [Google Scholar]
- 12.Hunter N, Haylock R. Radiation risks of lymphoma and multiple myeloma incidence in the updated NRRW-3 cohort in the UK: 1950–2011. J Radiol Prot 2021. Mar 15. [DOI] [PubMed] [Google Scholar]
- 13.Kesminiene A, Evrard AS, Ivanov VK, Malakhova IV, Kurtinaitis J, Stengrevics A, et al. Risk of hematological malignancies among Chernobyl liquidators. Radiat Res 2008. 12/2008; 170(6): 721–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little MP, Wakeford R, Borrego D, French B, Zablotska LB, Adams MJ, et al. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: a pooled analysis of nine historical cohort studies. Lancet Haematol 2018August; 5(8): e346–e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candéias SM, Kabacik S, Olsen A- K, Eide DM, Brede DA, Bouffler S, et al. Ionizing radiation does not impair the mechanisms controlling genetic stability during T cell receptor gene rearrangement in mice. Int J Radiat Biol 2018. 2018/03/07; 94(4): 357–365. [DOI] [PubMed] [Google Scholar]
- 16.Johnsen HE, Bogsted M, Schmitz A, Bodker JS, El-Galaly TC, Johansen P, et al. The myeloma stem cell concept, revisited: from phenomenology to operational terms. Haematologica 2016December; 101(12): 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Commission on Radiological Protection (ICRP). Stem cell biology with respect to carcinogenesis aspects of radiological protection. ICRP Publication 131. Ann ICRP 2015; 44(3-4): 1–357. [DOI] [PubMed] [Google Scholar]
- 18.Gault N, Verbiest T, Badie C, Romeo P- H, Bouffler S. Haematopoietic stem and progenitor cell responses to low radiation doses - implications for leukaemia risk. Int J Radiat Biol 2019: 1–26. [DOI] [PubMed] [Google Scholar]
- 19.Lee C, Morton LM, Berrington de Gonzalez A. A novel method to estimate lymphocyte dose and application to pediatric and young adult CT patients in the United Kingdom. Radiat Prot Dosimetry 2018; 178(1): 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little MP. Leukaemia following childhood radiation exposure in the Japanese atomic bomb survivors and in medically exposed groups. Radiat Prot Dosimetry 2008; 132(2): 156–165. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan B, Morgan GJ. Non-Hodgkin lymphoma secondary to cancer chemotherapy. Cancer Epidemiol Biomarkers Prev 2007March; 16(3): 377–380. [DOI] [PubMed] [Google Scholar]
- 22.Davis FG, Boice JD Jr., Hrubec Z, Monson RR. Cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients. Cancer Res 1989. 11/1/1989; 49(21): 6130–6136. [PubMed] [Google Scholar]
- 23.Zablotska LB, Little MP, Cornett RJ. Potential increased risk of ischemic heart disease mortality with significant dose fractionation in the Canadian fluoroscopy cohort study. Am J Epidemiol 2014January1; 179(1): 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dondon MG, de Vathaire F, Shamsaldin A, Doyon F, Diallo I, Ligot L, et al. Cancer mortality after radiotherapy for a skin hemangioma during childhood. Radiother Oncol 2004. 7/2004; 72(1): 87–93. [DOI] [PubMed] [Google Scholar]
- 25.Lindberg S, Karlsson P, Arvidsson B, Holmberg E, Lunberg LM, Wallgren A. Cancer incidence after radiotherapy for skin haemangioma during infancy. Acta Oncol 1995; 34(6): 735–740. [DOI] [PubMed] [Google Scholar]
- 26.Lundell M, Holm L- E. Mortality from leukemia after irradiation in infancy for skin hemangioma. Radiat Res 1996. 5/1996; 145(5): 595–601. [PubMed] [Google Scholar]
- 27.Lundell M, Mattsson A, Karlsson P, Holmberg E, Gustafsson A, Holm L- E. Breast cancer risk after radiotherapy in infancy: a pooled analysis of two Swedish cohorts of 17,202 infants. Radiat Res 1999. 5/1999; 151(5): 626–632. [PubMed] [Google Scholar]
- 28.Sadetzki S, Chetrit A, Lubina A, Stovall M, Novikov I. Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. J Clin Endocrinol Metab 2006. 12/2006; 91(12): 4798–4804. [DOI] [PubMed] [Google Scholar]
- 29.Ron E, Modan B, Boice JD. Mortality after radiotherapy for ringworm of the scalp. Am J Epidemiol 1988. 4/1988; 127(4): 713–725. [DOI] [PubMed] [Google Scholar]
- 30.Adams MJ, Dozier A, Shore RE, Lipshultz SE, Schwartz RG, Constine LS, et al. Breast cancer risk 55+ years after irradiation for an enlarged thymus and its implications for early childhood medical irradiation today. Cancer Epidemiol Biomarkers Prev 2010. 1/2010; 19(1): 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams MJ, Shore RE, Dozier A, Lipshultz SE, Schwartz RG, Constine LS, et al. Thyroid cancer risk 40+ years after irradiation for an enlarged thymus: an update of the Hempelmann cohort. Radiat Res 2010. 12/2010; 174(6): 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronckers CM, Land CE, Miller JS, Stovall M, Lonstein JE, Doody MM. Cancer mortality among women frequently exposed to radiographic examinations for spinal disorders. Radiat Res 2010. 7/2010; 174(1): 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C, Lamart S, Moroz BE. Computational lymphatic node models in pediatric and adult hybrid phantoms for radiation dosimetry. Phys Med Biol 2013. Mar 7; 58(5): N59–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadetzki S, Chetrit A, Mandelzweig L, Nahon D, Freedman L, Susser E, et al. Childhood exposure to ionizing radiation to the head and risk of schizophrenia. Radiat Res 2011. 11/2011; 176(5): 670–677. [DOI] [PubMed] [Google Scholar]
- 35.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood 2008. Dec 1; 112(12): 4384–4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007 7/15/2007; 110(2): 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood 2010. Nov 18; 116(20): e90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu E- M, Kittai A, Tabbara IA. Chronic lymphocytic leukemia: current concepts. Anticancer Res 2015. October 1; 35(10): 5149–5165. [PubMed] [Google Scholar]
- 39.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 1–352. [Google Scholar]
- 40.Kendall GM, Little MP, Wakeford R, Bunch KJ, Miles JCH, Vincent TJ, et al. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980–2006. Leukemia 2013. 1/2013; 27(1): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spycher BD, Lupatsch JE, Zwahlen M, Roosli M, Niggli F, Grotzer MA, et al. Background ionizing radiation and the risk of childhood cancer: a census-based nationwide cohort study. Environ Health Perspect 2015June; 123(6): 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krestinina LY, Davis FG, Schonfeld S, Preston DL, Degteva M, Epifanova S, et al. Leukaemia incidence in the Techa River Cohort: 1953-2007. Br J Cancer 2013. Nov 26; 109(11): 2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hastie TJ, Tibshirani RJ. Generalized additive models. Chapman & Hall/CRC: Boca Raton, FL, 1990, pp. 1–350. [Google Scholar]
- 44.McCullagh P, Nelder JA. Generalized linear models. 2nd edition. Chapman and Hall/CRC: Boca Raton, FL, 1989, pp. 1–526. [Google Scholar]
- 45.Risk Sciences International. Epicure version 2.0.1.0. 55 Metcalfe, K1P 6L5, Canada: Risk Sciences International; 2015. [Google Scholar]
- 46.Little MP, Wakeford R, Lubin JH, Kendall GM. The statistical power of epidemiological studies analyzing the relationship between exposure to ionizing radiation and cancer, with special reference to childhood leukemia and natural background radiation. Radiat Res 2010. 9/2010; 174(3): 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.International Commission on Radiological Protection (ICRP). Basic anatomical and physiological data for use in radiological protection: reference values. ICRP Publication 89. Ann ICRP 2001; 32(3-4): i-xi, 1–265. [PubMed] [Google Scholar]
- 48.International Commission on Radiological Protection (ICRP). Report of the Task Group on reference man. ICRP Publication 23. Ann ICRP; 1975; 23(1): 1–480. [Google Scholar]
- 49.Linet MS, Schubauer-Berigan MK, Berrington de Gonzalez A. Outcome Assessment in Epidemiological Studies of Low-Dose Radiation Exposure and Cancer Risks: Sources, Level of Ascertainment, and Misclassification. J Natl Cancer Inst Monogr 2020. Jul 1; 2020(56): 154–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little MP, Weiss HA, Boice JD Jr., Darby SC, Day NE, Muirhead CR. Risks of leukemia in Japanese atomic bomb survivors, in women treated for cervical cancer, and in patients treated for ankylosing spondylitis. Radiat Res 1999. 9/1999; 152(3): 280–292. [PubMed] [Google Scholar]
- 51.Richardson DB, Wing S, Schroeder J, Schmitz-Feuerhake I, Hoffmann W. Ionizing radiation and chronic lymphocytic leukemia. Environ Health Perspect 2005. 1/2005; 113(1): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schubauer-Berigan MK, Daniels RD, Fleming DA, Markey AM, Couch JR, Ahrenholz SH, et al. Chronic lymphocytic leukaemia and radiation: findings among workers at five US nuclear facilities and a review of the recent literature. Br J Haematol 2007. 12/2007; 139(5): 799–808. [DOI] [PubMed] [Google Scholar]
- 53.Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer 2001. Sep 1; 92(5): 1325–1330. [DOI] [PubMed] [Google Scholar]
- 54.Turesson I, Linet MS, Bjorkholm M, Kristinsson SY, Goldin LR, Caporaso NE, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964–2003. Int J Cancer 2007. 11/15/2007; 121(10): 2260–2266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and all analysis code is available from the principal author upon request.


