Abstract
The circuitry of addiction comprises several neural networks including the midbrain- an expansive region critically involved in the control of motivated behaviors. Midbrain nuclei like the Edinger-Westphal (EW) and dorsal raphe (DR) contain unique populations of neurons that synthesize many understudied neuroactive molecules and are encircled by the periaqueductal gray (PAG). Despite the proximity of these special neuron classes to the ventral midbrain complex and surrounding PAG, functions of the EW and DR remain substantially underinvestigated by comparison. Spanning approximately −3.0 to −5.2mm posterior from bregma in the mouse, these various cell groups form a continuum of neurons that we refer to collectively as the subaqueductal paramedian zone. Defining how these pathways modulate affective behavioral states presents a difficult, yet conquerable challenge for today’s technological advances in neuroscience. In this review, we cover the known contributions of different neuronal subtypes of the subaqueductal paramedian zone. We catalogue these cell types based on their spatial, molecular, connectivity, and functional properties and integrate this information with the existing data on the EW and DR in addiction. We next discuss evidence that links the EW and DR anatomically and functionally, highlighting the potential contributions of an EW-DR circuit to addiction-related behaviors. Overall, we aim to derive an integrated framework that emphasizes the contributions of EW and DR nuclei to addictive states and describes how these cell groups function in individuals suffering from substance use disorders.
Keywords: Midbrain, Neuropeptide, Edinger-Westphal, Dorsal Raphe, Periaqueductal Gray, Addiction
1. Introduction
The circuitry of addiction comprises several neural networks including the midbrain- an expansive region critically involved in the control of motivated behaviors. The ventral midbrain complex - particularly the commonly studied ventral tegmental area (VTA) and substantia nigra - contains neurons that not only integrate information from ascending and descending sources, but also release neuromodulators like dopamine in sites with well-established roles in addiction, like the nucleus accumbens (NAc), amygdala, and prefrontal cortex. However, additional midbrain nuclei like the Edinger-Westphal (EW) and dorsal raphe (DR) are located dorsally and caudally to the VTA and contain unique populations of neurons that synthesize neuroactive molecules with vastly understudied functions compared with dopamine. Representing a parallel system that complements the architecture and actions of ventral midbrain outputs, these separate groups of neurons are encircled by the periaqueductal gray (PAG) and also send projections to the NAc, amygdala, and hypothalamus. Despite the proximity of these special neuron classes to the ventral midbrain complex and surrounding PAG, functions of the EW and DR remain substantially underinvestigated by comparison. Defining how these parallel streams of limbic circuitry interact to modulate affective behavioral states presents a difficult, yet conquerable challenge for today’s technological advances in neuroscience.
Within the ventral division of the midbrain PAG, a fascinating pattern emerges along the midline in which large clusters of cells rich with heterogeneous gene expression fill the space directly ventral to the tip of the cerebral aqueduct. Spanning approximately −3.0 to −5.2mm posterior from bregma in the mouse, these various cell groups form a continuum of neurons that we refer to collectively as the subaqueductal paramedian zone (Figure 1). Among these neuronal populations, the EW and DR nuclei stand out as intriguing and interrelated, particularly within the context of addiction. These two neuronal clusters are anatomically connected and both harbor diverse combinations of neuromodulators and receptors involved in regulating mood, appetite, stress, and reward processing.
FIGURE 1. Depiction of molecularly-defined subaqueductal paramedian zone neurons comprising the Edinger-Westphal and Dorsal Raphe nuclei.
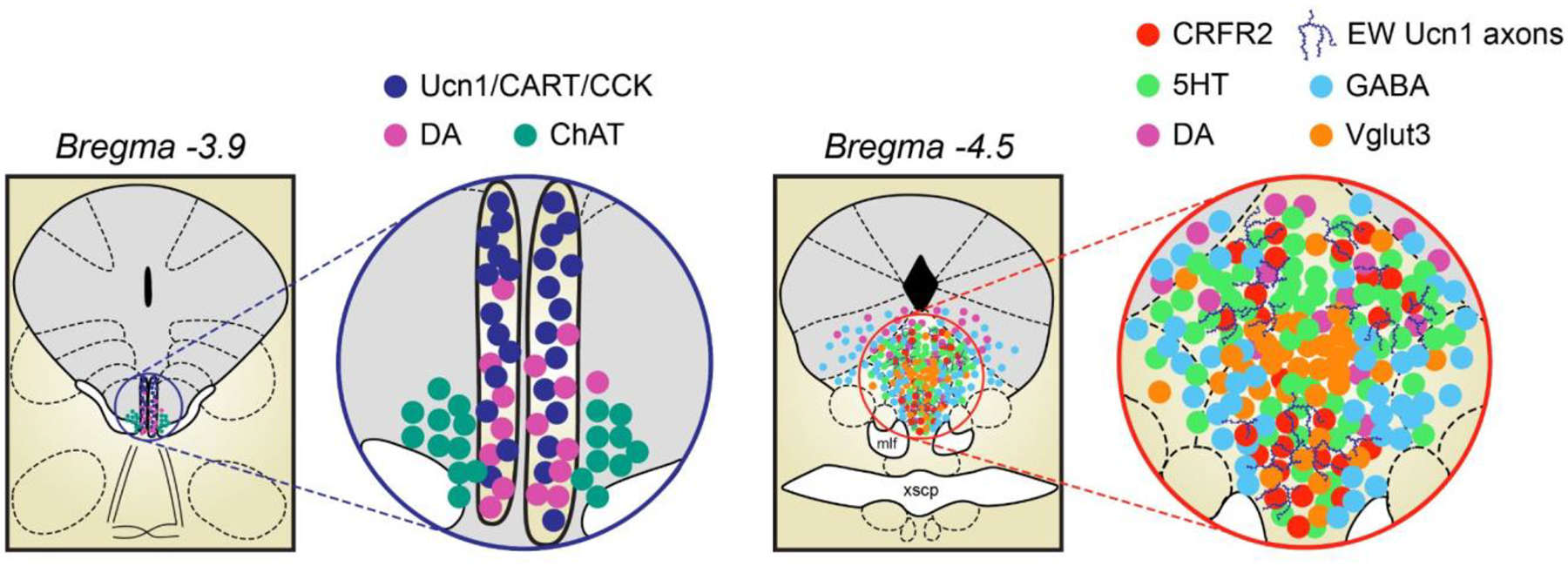
Left: Spanning approximately −3.0 to −4.0 mm posterior from bregma in the mouse brain, EWcp neuropeptide neurons (Ucn1/CART/CCK) are interspersed with EWpg cholinergic neurons (ChAT) and ventral PAG tyrosine hydroxylase neurons (DA). Right: Spanning approximately −4.0 to −5.00 mm posterior from bregma in the mouse brain, DR neurons containing 5HT are interspersed with those expressing DA, GABA, and Vglut3. In addition, DR neurons expressing CRFR2 are innervated by Ucn1-positive axons originating from EWcp neuropeptide neurons.
Notably, the DR contains the largest population of neurons that release serotonin (5HT) in forebrain and midbrain regions, thus making them key to the modulation of emotional behaviors. DR neurons project their axons to a plethora of forebrain regions involved in the regulation of motivation, including amygdala, striatum, and cortex. Much less is known about the EW neurons; however, evidence suggests they project to the hypothalamus, septum, and spinal cord, in addition to the ventral midbrain complex and the DR (da Silva et al., 2013; Dos Santos Junior et al., 2015; Kozicz et al., 2011a; Ryabinin et al., 2013). The EW contains a dense group of cells with high expression of urocortin 1 (Ucn1), a neuropeptide closely related to corticotropin-releasing factor (CRF) that displays high affinity for both CRF receptor subtypes (CRFR1, CRFR2) (Bittencourt et al., 1999; Ryabinin and Giardino, 2017; Ryabinin et al., 2005; Ryabinin et al., 2012). Nearby in the DR, a subpopulation of 5HT neurons express CRFR2, indicative of an adjacent substrate to support stress-related EW-Ucn1 release and signaling (Pernar et al., 2004; Van Pett et al., 2000). These cognate ligand-receptor cellular phenotypes across the EW and the DR raise the possibility of an underappreciated short-range circuit between these two structures that critically regulates affective processing in emotional states associated with substance use disorders (Bethea et al., 2013; Fabre et al., 2011; Sanchez et al., 2010; Staub et al., 2006; van der Doelen et al., 2017).
In this review, we cover the known contributions of different neuronal subtypes of the subaqueductal paramedian zone, focusing particularly on the EW and DR nuclei in addiction and motivated behavioral states. We catalogue these cell types based on their spatial, molecular, connectivity, and functional properties and integrate this information with the existing data on the EW and DR in addiction. We next discuss evidence that links the EW and DR anatomically and functionally, highlighting the potential contributions of an EW-DR circuit to addiction-related behaviors. We conclude by comparing these subaqueductal neuropeptide circuits with ventral midbrain pathways and propose a complementary system that co-modulates cortical and striatal circuitry. By synthesizing the extant literature, we aim to derive an integrated framework that emphasizes the contributions of EW and DR nuclei to addictive states and describes how these structures function in patients suffering from substance use disorders.
2. Edinger-Westphal nucleus cell types
The EW consists of two main juxtaposed and intermingled cell groups of the midbrain that have distinct connectivity, neurochemistry, and function (Kozicz et al., 2011a). The centrally projecting EW (EWcp) is composed of densely clustered peptidergic neurons that project throughout the CNS regulating stress adaptation and addiction (Xu et al., 2012; Zuniga and Ryabinin, 2020). In contrast, the preganglionic EW (EWpg) consists of cholinergic, preganglionic neurons projecting to the parasympathetic ciliary ganglion to control pupil dilation (Gamlin and Reiner, 1991). Given the selective activation of EWcp neurons by alcohol, psychostimulants, and opiates, EWpg neurons are unlikely to play a critical role in addiction, and we focus here primarily on neuropeptide-expressing EWcp neurons (Spangler et al., 2009; Zuniga and Ryabinin, 2020).
2.1. Ucn1/CART/CCK Edinger-Westphal neurons
Traditionally, the EWcp population is defined by expression of Ucn1, an endogenous neuropeptide ligand of CRF receptors. It is widely accepted that EWcp-Ucn1 neurons show near complete overlap with EWcp neurons expressing other anorexigenic neuropeptides including cocaine and amphetamine regulated transcript (CART) (Kozicz, 2003), Nesfatin (Bloem et al., 2012; Okere et al., 2010), and cholecystokinin (CCK) (Giardino et al., 2012; Li et al., 2018). There is some evidence that EW-Ucn1/CART/CCK cells are also glutamatergic and co-express the vesicular glutamate transporter type 2 (Vglut2; slc17a6) (Zhang et al., 2019), suggesting that the EWcp is a single neurochemically homogeneous population of Ucn1/CART/CCK/Vglut2 neurons. However, during the revision of this manuscript, two important preprint publications presented major advances on EWcp neurocircuitry, including data that challenge this idea. Topilko et al. showed that the vast majority of EWcp neuropeptide neurons do not co-express either Vglut2 nor Vgat, and Priest et al. found a similar result, showing that only a small percentage of EWcp neuropeptide neurons contain Vglut1, Vglut2, Vglut3, or Vgat (Priest et al., 2021; Topilko et al., 2021). Furthermore, Priest et al. found that while Ucn1 and CART are indeed co-expressed, this occurs in ~80% rather than 100% of EWcp neurons. Discrepancies in the reported overlap between neuropeptide and glutamate markers within the EW may be due to limitations in detection abilities of mRNA vs. protein, or due to nuances in expression that vary based on species, sex, age, and behavioral experience.
While many studies have implicated the EWcp in alcohol and drug consumption (recently reviewed by Zuniga and Ryabinin, 2020), only one study has examined the specific contribution of EWcp peptides in animal models of addiction. shRNA knockdown Ucn1 expression in the EWcp revealed a specific role for EWcp Ucn1 regulating intermittent alcohol consumption without impacting body weight, total fluid intake or food consumption (Giardino et al., 2017). Alcohol, both voluntarily consumed and non-voluntarily administered have been increase EWcp neural activation across rodent species and strains (Ryabinin et al., 1997; Ryabinin & Wang, 1998; Bachtell et al., 1999; 2002; Weitemier et al., 2001; Sharpe et al., 2005; Anacker et al., 2011; Giardino et al., 2017). Indeed, the amount of alcohol consumed positively correlates with the level of EWcp neural activity, as shown by cFos protein (Sharpe et al., 2005) and Fos mRNA expression (Giardino et al., 2017).
While alcohol, opioids (heroin & morphine) and psychostimulants (cocaine, amphetamine and methamphetamine) all induce neural activation of the EWcp (Singh et al., 2004; 2006; Spangler et al., 2009, Giardino et al., 2011), direct comparison across drug classes of shared vs. distinct neuronal ensembles recruited have not yet been defined. However, evidence that alcohol and cocaine act specifically upon Ucn1+, not TH+ populations (Spangler et al., 2009) suggest this is at least in part a shared activity profile of defined neuronal ensembles across drug classes.
Aside from neuropeptide ligands, EWcp cell bodies also express receptors for orexigenic and anorexigenic peptides, consistent with their roles in homeostatic regulation of metabolism through integration of peripheral and central signals. Receptors for “feeding peptides”, including growth hormone secretagogue receptor (Ghsr), leptin receptor (LepRb/ObR), hypocretin/orexin receptor (Hcrt1/OX1), and neuropeptide Y receptors (Y1/5) are primarily expressed on EWcp-Ucn1/CART neurons (Emmerzaal et al., 2013; Flak et al., 2014; Gaszner et al., 2007; Spencer et al., 2012; Xu et al., 2009; Xu et al., 2014) and can act to regulate Ucn1 and CART expression levels (Xu et al., 2011). With respect to their functions in addiction, a growing avenue of research is based on targeting many of these same feeding-related systems to treat alcohol use disorders (e.g., via ghrelin, leptin, glucagon-like peptide 1, amylin) (Bach et al., 2021; Farokhnia et al., 2021; Farokhnia et al., 2018; Haass-Koffler et al., 2019; Kalafateli et al., 2021; Vallof et al., 2020). It is not currently understood how these treatments exert their anti-addictive effects in the brain, but they likely involve modulation of EWcp neural activity. Indeed, in an animal model of addiction, systemic pharmacological blockade of the receptor for the pro-feeding hormone ghrelin (Ghsr) inhibited alcohol binge drinking and alcohol-induced neural activity in the EWcp (but not in the neighboring VTA, despite both regions having rich Ghsr expression) (Abizaid et al., 2006; Kaur and Ryabinin, 2010). Together, these findings suggest a specific role for EWcp-Ghsr signaling in promoting alcohol consumption.
2.2. EWcp Neurocircuitry
Although input/output tracing from a relatively small neuronal cluster may be imprecise without the use of methods for genetically encoded cell type specificity, neuroanatomical mapping studies in adult male rats detailed the afferent and efferent pathways of EWcp neurons (da Silva et al., 2013; Dos Santos Junior et al., 2015). Anterograde tracing methods identified widespread efferent projections from the EWcp to the forebrain, basal ganglia, extended amygdala, thalamus, hypothalamus, brainstem, trigeminal nucleus, and spinal cord (Dos Santos Junior et al., 2015). These findings are largely in line with earlier studies in rat (Bittencourt et al., 1999; Klooster et al., 1993) and cat (Loewy and Saper, 1978; Loewy et al., 1978). Further replicating early studies in cats that identified substantial hindbrain inputs to the EW (Breen et al., 1983), retrograde tracing experiments from the Bittencourt group found that the EWcp is innervated by neurons from specific subregions of the extended amygdala, hypothalamus, thalamus, midbrain, pons, and cerebellum (da Silva et al., 2013). In particular, axon terminals from the posterior hypothalamus (PH), paraventricular nucleus of the hypothalamus (PVN) and lateral hypothalamus (LH) make close appositions to EWcp-Ucn1 neurons (da Silva et al., 2013), highlighting potential pathways by which the EWcp receives information from feeding, stress and reward circuits.
Until recent advances from the Kozorovitskiy and Renier groups (Priest et al., 2021, Topilko, et al., 2021), the lateral septal nucleus (LS) was thought to comprise a major forebrain output of the EWcp-Ucn1 neurons, with fewer fibers observed within the prefrontal and cingulate cortices (Bachtell et al., 2003; Dos Santos Junior et al., 2015; Lim et al., 2006; Ryabinin and Weitemier, 2006; Weitemier et al., 2005). In line with evidence for strong Ucn1 innervation, CRFR2 is densely expressed in the ventrolateral LS (Bittencourt et al., 1999; Van Pett et al., 2000) and EWcp lesions reduce Ucn1 fibers within the LS (Bachtell et al., 2004). Several lines of evidence indicate that this pathway is critical for alcohol consumption. Selectively bred low alcohol preferring mice and rats differ in LS Ucn1 fiber expression and EWcp Ucn1 expression when compared to alcohol preferring counterparts, with higher alcohol preference generally associated with greater Ucn1 LS fiber and EWcp cell expression (Bachtell et al., 2003; Fonareva et al., 2009; Giardino et al., 2012; Giardino et al., 2017; Ryabinin and Giardino, 2017; Ryabinin et al., 2012; Ryabinin and Weitemier, 2006; Turek et al., 2005; Weitemier et al., 2005). Further, increased alcohol consumption in a limited access paradigm negatively correlates with Ucn1 reactivity in the LS and is associated with increased Ucn1 immunoreactivity in the EWcp, suggesting an association between greater alcohol drinking and increased release of Ucn1 peptide onto LS neurons (Bachtell et al., 2003).
CART immunoreactivity is also observed within axonal fibers innervating the LS (Kozicz, 2003, Neuroscience), however, their distribution differs from Ucn1 axons, with CART fibers observed throughout the ventromedial division, compared to Ucn1 fibers predominantly in the ventrolateral division (Kozicz, 2003). Given the high degree of CART/Ucn1 overlap within the EW cell bodies, the mismatch in downstream fiber expression would be initially surprising. However, CART fibers within the LS can arise from other regions, such as the LH, which also contains CART neurons and sends a strong projection to the LS (Swanson and Cowan, 1979). An alternative possibility is that EW-derived CART is differentially packaged, processed, and/or released in the LS terminal fields, compared with EW-Ucn1 axons innervating the LS (van den Pol, 2012; Zupanc, 1996).
Similar to the LS, EW-Ucn1 neurons strongly innervate the DR, particularly in dorsomedial and ventromedial zones, and EW lesions decrease Ucn1 axonal fibers in the DR (Bachtell et al., 2004). The DR expresses CRFR2 on both serotonergic and non-serotonergic neurons, and CRFR2 activation by urocortin peptides impact physiological activity of DR neurons in a dose-dependent manner (Pernar et al., 2004). CART innervation is also observed in close proximity to 5-HT cells in DR of female rhesus monkeys (Lima et al., 2008). CART peptide infusion in the DR increased serotonin efflux in the DR and downstream within the nucleus accumbens, a major target of DR serotonergic output (Ma et al., 2007).
Early findings showed EW-CCK projections to the spinal cord and trigeminal nucleus (Maciewicz et al., 1984; Phipps et al., 1983), and more recent studies showed EW-CCK projections to the paraventricular nucleus of the thalamus (PVT) (Otake, 2005), as well the LS, LH, and preoptic area (POA) (Zhang et al., 2019). One of these studies used cell-specific rabies tracing methods with optogenetics and slice electrophysiology to annotate microcircuit connectivity within the region, identifying reciprocal local connections between EW-CCK neurons and adjacent perioculomotor neurons marked by the calcitonin related polypeptide alpha (Calca) (Zhang et al., 2019). While these findings appear in a single publication and have yet to be replicated, the authors report EWcp-CCK/Vglut2 projections to the POA and the ventromedial medulla (but not to the LH or LS) are implicated in sleep regulation, acting to promote non-REM sleep (Zhang et al., 2019). Given the known interactions between sleep quality and alcohol intake (Thakkar et al., 2015), and the strong regulation of EWcp-Ucn1 neurons by alcohol (Bachtell et al., 2002a; Giardino et al., 2017; Ryabinin and Giardino, 2017; Ryabinin et al., 2012; Ryabinin and Weitemier, 2006; Schank et al., 2012; Spangler et al., 2009; Zuniga and Ryabinin, 2020; Zuniga et al., 2020),EWcp neuropeptide neurons may therefore be a critical site by which alcohol disrupts sleep.
Considering the visuomotor functions associated with cholinergic EWpg neurons, one publication reported surprising results that EWcp neuropeptide neurons are critical for visual task performance (Li et al., 2018). The authors showed that EW-CCK neurons receive direct inputs from 5HT 2c receptor (5HT2c) neurons in the ventral CA1 of the hippocampus, and that EW-CCK neurons project and synapse onto parvalbumin neurons of the medial prefrontal cortex (Li et al., 2018). While this assignment of EWcp neuropeptide cells as a conduit for cortico-hippocampal communication that modulates selective attention is unique among all other conceptions of the EW, it raises the possibility of higher-level executive functions that may be critically dependent upon EWcp neuropeptide circuit activity.
In addition to the POA, EW projections to the LH make close apposition with melanin concentrating hormone (MCH) neurons (Dos Santos Junior et al., 2015), suggesting another mechanism by which the EW can regulate sleep/wakefulness and feeding pathways. Elsewhere in the hypothalamus, EW neurons innervate parvocellular PVN neurons that express CRF, suggesting that the EW interfaces with hypothalamic-pituitary-adrenal (HPA) axis to coordinate the neuroendocrine stress response (Dos Santos Junior et al., 2015). Indeed, the EW also projects to CRF-rich sites in the oval nucleus of the bed nucleus of the stria terminalis (BNST) and central nucleus of the amygdala (CeA), indicating that it regulates stress responses through outputs to both hypothalamic and extra-hypothalamic CRF stress systems (Dos Santos Junior et al., 2015).
2.3. Sex Differences and Hormonal Influences on EWcp Neurons
Sex differences in drugs abuse and dependence are well documented; for recent reviews see (Becker and Chartoff, 2019; Quigley et al., 2021). The impact suffered by women is particularly problematic. Women are more likely to experience negative consequences of drinking, transitioning to dependence more quickly (Hernandez-Avila et al., 2004), show stronger alcohol cravings (Boykoff et al., 2010), experience worse medical and mental health outcomes (Peltier et al., 2019; Szabo, 2018) and are more sensitive to stress-induced relapse (Becker and Koob, 2016). The EWcp posits an attractive hub which is capable of mediating interactions between sex and alcohol/drug consumption. Almost all EWcp Ucn1/CART/CCK cells express estrogen receptors (ERβ, but not ERα) (Derks et al., 2009; Derks et al., 2010; Derks et al., 2007) and expression of progesterone receptors (PR) have also been reported (Lima et al., 2008). In line with the EWcp being sensitive to sex steroids, ovariectomy (OVX) decreased Ucn1 expression in the EW of Japanese macaques (Bethea and Reddy, 2012) and Ucn1 expression varies across the menstrual cycle of female rats (Derks et al., 2010). Further, reduced Ucn1 expression has been observed in late pregnancy, compared to virgin cycling rats in either estrous or non-estrous phase (Fatima et al., 2007), suggesting increased estrogen/progesterone associated with pregnancy to negatively regulate Ucn1 expression. ERβ and PR receptors also regulate EWcp CART expression in female rhesus monkeys, however, some discrepancies between mRNA and protein expression have been observed (Lima et al., 2008).
While studies suggest sex steroid regulation of EWcp neuropeptide populations, the functional implications are not well understood. Ucn1 and CART mRNA were upregulated after fasting in male, but not in female rats (Xu et al., 2009), however, addiction related studies that have included both male and female rodents suggest ethanol-induced activation of the EWcp is similar across sexes (Bachtell et al., 2002a, b) and while Ucn1 KO mice showed reduced alcohol consumption and preference in both intermittent and continuous two-bottle choice drinking procedures, effects were similar in males and females (Giardino et al., 2017). While the EW is the primary site of Ucn1 expression, sex specific actions within the EW remain unknown, and studies on the contribution of EWcp neuropeptides to sex differences associated with alcohol and drug addiction are warranted. Given the impact of stress in initiation, maintenance and relapse of drug and alcohol taking, and the role of the EWcp in stress related behaviors (Kozicz, 2007; Kozicz et al., 2001; Kozicz et al., 2011b), examining the intersect between sex, stress and drug/alcohol consumption requires attention.
2.4. Substance P Edinger-Westphal neurons
In addition to EWcp neurons containing Ucn1, CART, and CCK, a separate EW neuronal population contains the peptide substance P (generated from the gene tachykinin1; Tac1). Substance P-expressing EW neurons (EWSP) appear to span both EWcp and EWpg, with some expression observed in preganglionic cholinergic neurons (Erichsen et al., 1982a; Erichsen et al., 1982b) and some expression observed in centrally projecting pathways (Otake, 2005; Skirboll et al., 1983; Skirboll et al., 1982). EWSP neurons were reported to project to the spinal cord (Maciewicz et al., 1984; Phipps et al., 1983; Skirboll et al., 1983; Skirboll et al., 1982), as well as to the PVT (Otake, 2005).
Given the plethora of available transgenic animals, including major neuropeptide populations of the EW and advances in monosynaptic and transsynaptic tracing techniques (Xu et al., 2020), the tools are available to definitively answer questions surrounding discrepancies observed in EWcp connectivity and understand the contribution of distinct molecularly defined pathways in the development and maintenance of addictive behaviors.
3. Dorsal Raphe nucleus cell types
The DR is composed of a large collection of cell types that send strong projections across virtually the entire neuraxis. Decades of work has investigated the function of 5HT and the neurons that release it in various behaviors, physiologic functions, and disease states. Unsurprisingly, the DR has been most historically associated with 5HT. However, the DR contains many neurons that lack 5HT yet have the potential to release numerous other neurotransmitters and modulators with profound influences over cognition and behavior. In addition, the explosion of technological advances in transcriptomic sequencing and neural tract tracing have revealed dozens of previously unknown or underappreciated cellular phenotypes in the DR. This section will review some of the recent data on the most prominent DR cell types and their contributions to behavioral states related to substance use disorders. For more in-depth discussions on DR cell types, see (Okaty et al., 2019).
3.1. 5HT neurons
Perhaps the most prominent and well-studied feature of the DR is the presence of neurons that synthesize and release 5HT. The primary population of neurons supplying 5HT to the forebrain is the DR, with more minor contributions to specific structures such as septum and hippocampus emanating from the median raphe nuclei, just ventral to the DR. DR projections release 5HT in many brain regions involved in motivated behaviors, emotional states, and addiction phenotypes. Unlike dopamine, 5HT effects on behavior and physiology are more elusive, with recent evidence suggesting that they may be state-dependent. For example, while the release of dopamine in the striatum modulates motor behavior and is powerfully reinforcing, 5HT release is not reinforcing on its own but can influence specific forms of motivated behavior. 5HT release in the NAc promotes social behaviors and mediates MDMA’s prosocial effects (Dolen et al., 2013; Heifets et al., 2019; Nardou et al., 2019; Walsh et al., 2018). Interestingly, MDMA’s effects on sociability can be dissociated from its reinforcing effects. While at low doses MDMA promotes social behavior through 5HT release but is not reinforcing, larger doses are reinforcing and dependent on dopamine signaling (Heifets et al., 2019). Additionally, a recent study identified 5HT release in the anterior cingulate as critical to sociability and consolation behavior in monogamous mandarin voles (Li et al., 2021). 5HT release has also been proposed to mediate reward seeking and other appetitive responses occurring over long timescales (Fonseca et al., 2015; Miyazaki et al., 2014; Xu et al., 2017), reminiscent of “pausing” or “waiting” for future rewards. This property is remarkably distinct from dopamine kinetics which are more rapid and reinforcing in nature. Future studies are needed to unravel the complexities of how 5HT and dopamine interact to coordinate state-dependent motivated behavior.
Recent work also indicates deficits in 5HT release as a critical mechanism underlying autism spectrum disorder (ASD), as well as chronic social defeat stress-induced depression-like behaviors (Walsh et al., 2018; Zou et al., 2020). In the 16p11 deletion ASD mouse model, DR 5HT neurons exhibit reductions in excitability and are less responsive to encounters with a social stimulus (Walsh et al., 2018). Remarkably, Walsh et al. demonstrated that stimulation of DR 5HT axons in the NAc or activation of 5HT1b receptors in the NAc rescues social behaviors in ASD mice, rendering them indistinguishable from their healthy conspecifics. Recently, a 5HT circuit from the DR to the central amygdala (CeA) has been described where 5HT inhibits LHb-projecting CeA somatostatin neurons to prevent pain-induced depression (Zhou et al., 2019). 5HT inputs to the neighboring BLA promote fear memory formation through amplification of synchronized BLA cell firing by engagement of 5HT2a receptors (Sengupta and Holmes, 2019). In a similar manner, 5HT projections to CRF neurons in the BNST were found to potently modulate fear and anxiety-like states, such as associated with fluoxetine treatment (Marcinkiewcz et al., 2016). In an elegant set of studies, one recent paper reported a dissociation between DR 5HT projections where those to the CeA promote anxiety-like behavior and those to the orbital frontal cortex mediate active coping behaviors (Ren et al., 2018). Similar to the effects of many modulators and peptides, the structure in which 5HT is released can dictate its effects on affective behavior.
These data suggest that DR 5HT may limit reward seeking and contribute to the suppression of negative emotional states, such as those produced in drug withdrawal. This prediction is consistent with the finding that enhancing 5HT signaling in the NAc attenuates morphine seeking in morphine withdrawn rats (Harris and Aston-Jones, 2001). Similarly, 5HT dynamics were impaired in the DR during protracted abstinence from morphine, which was paralleled by antisocial and depressive behaviors (Goeldner et al., 2011). Chronic fluoxetine treatment prevented the negative affective behaviors during withdrawal. In addition to enhancing synaptic 5HT, activation of 5HT2c receptors with Lorcaserin suppressed behavioral sensitization and withdrawal symptoms in morphine-dependent mice (Zhang et al., 2016). A history of morphine has also been reported to sensitize GABA receptors on DR 5HT neurons, further implicating reduced 5HT function in the opioid withdrawal state (Staub et al., 2012). Cocaine increases 5HT in the ventral pallidum which inhibits NAc indirect medium spiny neuron transmission, disinhibiting pallidal output through 5HT1b receptors (Matsui and Alvarez, 2018). Whether 5HT signaling here is important for the behavioral effects of cocaine was not determined, although it may serve as a homeostatic mechanism to limit cocaine reward. In addition, repeated methamphetamine treatment reduced the number of 5HT cells in the DR (Sepulveda et al., 2021). Together these reports indicate that repeated drug exposure inhibits the activity of specific DR 5HT systems which may promote drug seeking, negative affect during drug withdrawal, and relapse.
It is clear that 5HT plays a profound, yet subtle role in modulating a spectrum of motivated behaviors. In addition to investigations on 5HT cell outputs, recent work has identified the brain-wide monosynaptic inputs to these cells. Retrograde tracing employing G-deleted rabies virus in serotonin transporter (SERT)-Cre mice revealed major inputs to DR 5HT cells from the medulla, hypothalamus, amygdala, lateral habenula (LHb), deep cerebellar nuclei, and cortex (Weissbourd et al., 2014). Many hypothalamic nuclei send direct projections to DR 5HT cells, with the strongest contributions coming from the lateral hypothalamus, POA, mammillary nuclei, and the PVN. In the PVN, DR 5HT projecting cells expressed oxytocin and vasopressin. Large inputs were also detected in the BNST and the CeA. Most of the input cells in these regions were GABAergic, although a minority were positive for Vglut2 in the anterior BNST. In addition to GABA in the CeA, input cells are also co-localized with Tac2, enkephalin and CRF, but not protein kinase C-δ. The major cortical regions sending direct inputs to DR 5HT neurons were the insular, orbitofrontal, prelimbic/cingulate, and motor cortices, essentially all excitatory. Most of these input regions have been previously implicated in the regulation of motivated behaviors, however the examination of discrete inputs to DR 5HT neurons has been relatively understudied. Projections from the medial prefrontal cortex to the DR in rats was previously shown to energize adaptive coping behaviors during forced swim challenges in rats (Warden et al., 2012). Projections from the LHb to the DR were recently shown to promote passive coping behaviors in rats (Coffey et al., 2020). LHb neurons projecting to the DR were also shown to reduce antisocial behaviors during naloxone-precipitated morphine withdrawal (Valentinova et al., 2019). It is likely that all of these inputs play important roles in regulating DR 5HT activity and the resulting behavioral outputs. It will be interesting to see whether direct manipulation of inputs specifically to 5HT neurons in the DR can be achieved and provide novel information on DR contributions to addictive behaviors in the coming years.
3.2. GABA and glutamate neurons
The DR also contains neurons that release fast-acting transmitters like GABA and glutamate. Many of these GABA cells synapse directly onto 5HT cells locally in the DR, therefore functioning as interneurons poised to regulate 5HT release (Weissbourd et al., 2014). These GABA neurons also likely express mu opioid receptors (MORs), rendering them sensitive to opioid agents, such as heroin and fentanyl, and thus are important players in opioid-induced modulation of the 5HT system (Lutz and Kieffer, 2013a, b). Remote GABA inputs to the DR, interestingly coming from the VTA, also express MORs, representing redundant modulatory mechanisms controlling DR output (Li et al., 2019). DR GABA neurons also project to the raphe pallidus deep in the medulla to regulate thermogenesis (Schneeberger et al., 2019). Distinct physiological properties have been described in DR GABA neurons. As visualized with GAD67-GFP reporter mice, these GABA neurons display depolarized resting membrane potentials, lower rheobases, and larger input resistance compared with non-GABA cells (Gocho et al., 2013). In response to increasing current injections, the input-output relationship curves were much steeper in GABA cells, but their firing frequencies saturated before non-GABA cells did (Gocho et al., 2013). These data indicate that DR GABA neurons have greater excitability than non-GABA neurons and are more likely to fire at sustained high frequencies, characteristic of many GABAergic interneuron types. Therefore, DR GABA neurons appear to play critical roles in gating the output of 5HT neurons.
There are at least two populations of glutamate neurons that reside in the DR - those expressing Vglut3 and those with Vglut2. Vglut3 neurons exhibit a partial co-localization with 5HT, particularly in the ventral aspect of the DR, with a Vglut3 ‘pure’ population right in the heart of the DR (central aspect) while completely avoiding dopamine markers (Cardozo Pinto et al., 2019). This population was recently found to preferentially project axons to the orbitofrontal cortex over subcortical regions like the CeA, where they promote active coping behaviors during stress challenges (Ren et al., 2018). These cells were also observed to exhibit increased activity during reward presentations and inhibition during punishing stimuli. Consistently, stimulation of DR Vglut3 fibers in the VTA supported instrumental reward behavior through excitation of NAc-projecting dopamine neurons (Qi et al., 2014). These DR Vglut3 inputs to the VTA were subsequently found to co-release 5HT and generate a positive valence through both 5HT and glutamate release onto NAc-projecting dopamine cells (Wang et al., 2019). Interestingly, while Wang et al. characterized a 5HT3 receptor-dependent mechanism, another study dissecting Vglut3 projections to the VTA and NAc found 5HT2a and 2c receptor activation as a result of optogenetic stimulation, leading to enhanced reward learning (Liu et al., 2014). These studies determine multiple circuit and pharmacological mechanisms underlying the influence of DR Vglut3 neurons over motivated behavior.
Distinct from Vglut3 are the Vglut2 neurons which are lower in number and do not localize with 5HT, though do overlap with dopamine markers (similar to VTA). More data is needed to determine the specific roles DR Vglut2 neurons play in motivated behaviors and addiction phenotypes. One paper recently identified Vglut2 projections from the median raphe to the LHb, medial VTA, and medial septum as critical to aversive memory formation (Szonyi et al., 2019). As mentioned above, DR neurons expressing markers for dopamine co-localize with Vglut2 and have been shown to release glutamate in the CeA and BNST upon optical stimulation (Matthews et al., 2016). The fact that a subpopulation of VTA dopamine neurons also expresses Vglut2 and modulates motivated behaviors further supports the idea that DR and VTA represent two different parallel modulatory systems. Interestingly, Cre-dependent rabies tracing in Vglut2-Cre mice revealed the DR as a major input to these VTA cells, suggesting that DR Vglut2 neurons may have a similar function. Overall, GABA and glutamate release from various DR cell types play essential, and perhaps reciprocal roles in gating motivated behaviors. Future studies focused on determining differences between the different glutamatergic cell types will be especially intriguing.
3.3. Dopamine neurons
DR dopamine neurons appear to comprise a separate modulatory system from 5HT neurons. These cells send axons primarily to the VTA, hypothalamus, CeA, and oval nucleus of the BNST (Cardozo Pinto et al., 2019). Notably, only light projections to the NAc and cortex have been observed, making these neurons a preferentially amygdala-projecting population. Recent data suggest that these dopamine neurons contribute to multiple forms of motivated behavior. After acute isolation, DR dopamine cells mediate the enhanced motivation to socialize through a negative reinforcement mechanism (Matthews et al., 2016). This paper also identified dense projections to the CeA and BNST, and elegantly demonstrated the co-release of dopamine and glutamate in these regions. Interestingly, when compared with VTA dopamine neurons in the same brain, the projection pattern appears exquisitely distinct by strong targeting of the extended amygdala while avoiding the striatum (Lin et al., 2020). This paper also found that DR dopamine neurons mediate the expression of incentive memories of both positive and negative valence. Furthermore, they show that DR dopamine neurons selectively control expression, while VTA dopamine neurons control the acquisition of reward memories associated with morphine.
Consistent with a key role for opioid-related behaviors, vlPAG/DR tyrosine hydroxylase (TH) expression is enhanced by heroin administration and selective 6-OHDA lesioning of these cells prevented the development of a heroin-paired conditioned place preference (CPP), without altering anxiety-like behaviors (Flores et al., 2006). However, in contrast to Ucn1, no difference in TH expression is observed between alcohol preferring and non-preferring strains of rats, nor does alcohol elicit a c-Fos response in vlPAG/DR dopamine neurons (Spangler et al., 2009). The effects of opioids and alcohol mediated by DR dopamine cells further suggests they may participate in pain-related behavioral states. Indeed, recent exciting work from the Kash Lab has identified key sex-dependent contributions of DR dopamine projections to the BNST to inflammatory pain states (Yu et al., 2021). This same group previously demonstrated that GABA transmission in DR dopamine neurons is negatively modulated by both MOR and kappa opioid receptor (KOR) signaling and that activation with Gq DREADDs produced antinociceptive effects, consistent with predictions of opioid effects on attenuating pain (Li and Kash, 2019; Li et al., 2016). Furthermore, DR dopamine cells are also implicated in states of arousal (Cho et al., 2017) and fear learning (Groessl et al., 2018). Altogether, these studies support the notion that multiple forms of motivated behavior are modulated by DR dopamine neurons.
3.4. Other DR cell-types
Advanced RNA sequencing techniques have further expanded our knowledge about DR neurons. Recent papers leveraging single-cell RNA sequencing (scRNA-seq) have identified numerous neuropeptides and G-protein coupled receptors (GPCRs) with well-established roles in motivated behaviors in DR neurons. These include, but are not limited to, thyrotropin releasing hormone (TRH), dynorphin, enkephalin, pituitary adenylate cyclase-activating peptide, galanin, somatostatin, CRF, MOR, KOR, neurokinin receptor 3, somatostatin receptor 1, oxytocin receptor, hypocretin receptor 2, CCK receptor, neuropeptide Y receptor 2, MET, and CRFR2 (Huang et al., 2019; Okaty et al., 2015; Okaty et al., 2020; Ren et al., 2018). For more comprehensive datasets of genes identified in DR cells, we encourage the reader to further explore the above RNA sequencing studies.
Although there are few studies investigating the specific roles each of these cell types play in motivated and addictive behaviors, a handful of recent pharmacology and genetic studies have started to assess the functions of some of the addiction-relevant genes in the DR. Activation of KORs has been reported to reduce extracellular 5HT in the DR and the NAc (Tao and Auerbach, 2002a, b), and systemic administration of KOR antagonists prevent social deficits and depressive behaviors produced by opioid withdrawal (Lalanne et al., 2017). Along similar lines, KOR activation in the DR reinstated conditioned place preference for cocaine (Land et al., 2009), although it is unclear whether local KORs or KORs on axonal inputs were responsible for this effect. In contrast to KOR, activation of MORs in the DR appears to increase 5HT release (Tao and Auerbach, 2005), yet also contribute to the development of negative emotional behaviors in heroin-abstinent mice (Lutz et al., 2014). The subpopulation of neurons expressing TRH was recently shown to respond to stimuli signaling both reward and punishment, yet promoted anxiety when activated (Ren et al., 2019).
The majority of studies assessing the role of DR neurons in addiction-like behavioral states have focused on the roles of 5HT, GABA, glutamate, or dopamine. As the arsenal of viral and genetic tools continues to expand (including Boolean logic viral vectors and Cre/Flp driver lines) (Fenno et al., 2014; Fenno et al., 2020), we expect more and more groups to begin dissecting the relative roles the cell types expressing these peptides, receptors, and other genetic markers (and combinations thereof) play in addiction states.
3.5. DR cell topography
The collection of diverse DR cell-types exists not as a random mixture of cells, but in an organized topographical arrangement that may predict some specific cell-type and circuit functions (Figure 1). The 5HT population takes up residence in the central aspect of the DR, with a stereotyped pattern across the AP axis (Cardozo Pinto et al., 2019; Huang et al., 2019; Okaty et al., 2019). In the rostral DR, 5HT cells appear as a vertical line spanning from the aqueduct down to the superior cerebellar peduncle. In the middle AP zone, they form two large clusters in the central aspect, one dorsal and one ventral with a gap in the middle, with bilateral clusters in the lateral wings. 5HT neurons in the lateral wings tend to have higher intrinsic excitability than other 5HT neurons (Okatay et al., 2019). 5HT neurons in the dorsal DR are modulated by rewarding and aversive stimuli, can drive acute anxiety-like responses, and send axons to limbic structures such as the CeA, BNST, and hypothalamus (Ren et al., 2018; Okatay et al., 2019). Neurons in the ventral cluster seem to project to the cortex, are activated by rewarding stimuli, and promote active coping behaviors (Ren et al., 2018).
In the caudal DR, 5HT cells again appear as a vertical column lining the aqueduct and dropping into the peduncle. These caudal cells project to the septum, ventral hippocampus, and striatum (Okaty et al., 2019; Ren et al., 2019), however less is currently known about their functions. Vglut3 neurons that are 5HT− occupy the gap where 5HT neurons are absent in the central aspect of the mid DR. Vglut3 5HT+ neurons are peppered throughout the ventral cluster of 5HT neurons with a few in the dorsal cluster, but not in the lateral wings (Cardozo Pinto et al., 2019; Okay et al., 2019). By contrast, GABA neurons tend to be distributed lateral to the central “stalk” or midline of the DR (where 5HT neurons are) at a similar density across the AP axis (Cardozo Pinto et al., 2019; Huang et al., 2019; Okaty et al., 2019). This unique positioning suggests a tight regulatory influence of these GABA cells over 5HT and glutamate population activity. Moreover, dopamine neurons are distributed more dorsally, just underneath the aqueduct with a gradient that extends down and around the dorsal 5HT cluster (Cardozo Pinto et al., 2019).
In addition to these major cell-types, the numerous genetic markers identified by RNA seq studies are distributed with some organizational principles. However, as these data have only started to be critically evaluated, these principles remain less well-defined, although some gross distributions are easy to observe. Neurons expressing TRH are mostly confined to the lateral wings of the DR, with a cluster in the center of the caudal DR (Huang et al., 2019; Ren et al., 2019). Huang et al. (2019) also observed that dynorphin neurons are clustered in the central and dorsal aspects of the DR, similar to some 5HT cells. MET cells appear very caudal while cells expressing calbindin 2 mostly exist in the ventromedial DR. Importantly, these distributions are also detected in the study performed by Ren et al. (2019). This group also found that neurons expressing the neurokinin receptor 3 are in the lateral caudal DR and neurons expressing CRFR2 exist across the A/P axis but are most dense in the ventromedial and dorsomedial central stalk of the DR. Interestingly, EW-Ucn1 neurons appear to send axons to this central zone. This strongly implicates a neurochemical interaction between the EW and DR, and supports the hypothesis that these structures act in concert to modulate affective and motivated behaviors. Notably, these genes all map onto distinct transcriptome clusters of DR cell-types and suggest unique functional roles of these cell-types based on gene expression, input-output wiring, and placement within the subaqueductal paramedian zone (Huang et al., 2019). Therefore the arrangements of these neurons imply that there may be “hot spots” throughout the subaqueductal paramedian zone that subserve unique physiological and behavioral functions. However more data is needed to rigorously determine this functional anatomy.
4. EW and DR: Crossing the Thin Gray Line
Given the spatial proximity of the EW and the DR, delineating the boundaries between these structures and corresponding cell types is not perfectly straightforward. The border of the DR is generally considered to be the outer edges of the space occupied by 5HT neurons. However, this characterization becomes ambiguous when considering the more lateral-dwelling GABA neurons, as well as the various mixtures of neuropeptide-expressing neurons distributed throughout the three-dimensional DR, and in many cases overlapping with 5HT markers. For example, Substance P-expressing neurons of the EW are well-documented, but additional reports describe a subpopulation of DR 5HT neurons also containing Substance P (Sergeyev et al., 1999), raising the possibility of multiple distinct Substance P neuron clusters within the subaqueductal paramedian zone.
4.1. EW v. DR Cell Type Distinctions
In many cases, the cell type under investigation is present in different gradients across the DR, and often when in the medial-lateral dimension can be considered present in the vPAG (e.g. dopamine neurons). Many of the markers present in the DR also spread anteriorly past the 5HT-rich zone, yet whether or not these areas are considered DR or not is debatable. Huang et al. (2019) used scRNA-seq to characterize DR neuronal subpopulations and recently acknowledged: “Peptidergic neurons were highly enriched for the genes encoding neuropeptides such as Cck, Cartpt, Ucn, and Postn. Inspection of ISHs from the Allen Brain Atlas indicated that these neurons were located in the Edinger-Westphal nucleus, which is adjacent to the DRN” (Huang et al., 2019). In other words, the distinction between bona fide EWcp and peptidergic DR neuronal subtypes based on scRNA-seq remains confounded by the inherent limitations of microdissecting small areas from the mouse brain. The fact that adjacent EW neurons express many molecular markers common to the DR raises the question of true borders between these structures (and whether or not it matters for behavioral function).
A range of functional roles in motivated behavior have been described for DR dopamine neurons (as discussed above). Interestingly, DR dopamine neurons appear less organized than DR 5HT neurons, raising the possibility of heterogeneity in function based on spatial location. These cells are widely distributed across the DR, albeit in lower numbers than 5HT cells, but occupy a much larger space with lower density. DR dopamine neurons overflow into the neighboring vlPAG as well as spread anteriorly into the rostral linear nucleus (RLi) and EW complex, making the true borders of these proximal structures nebulous. In the EW, dopaminergic neurons, as visualized by TH expression, are present in larger numbers in the ventral EW “stalk”, an extension of the RLi (Li et al., 2018). Consistent with border ambiguity, previous reports referred to TH neurons in this area as either “ventral PAG” or “DR”, but it remains to be seen whether functional heterogeneity exists among TH neurons spanning the RLi, EW complex, vlPAG, and DR. Nevertheless, dopamine neurons in this region are known to be distinct from cholinergic EWpg neurons (Li et al., 2018) and EWcp-Ucn1 neurons (Bachtell et al., 2002a; Spangler et al., 2009).
Along similar lines, while CCK neurons in the subaqueductal paramedian region are traditionally assigned to the EWcp, Huang et al. (2019) identified a distinct subpopulation of DR CCK neurons that co-express markers for dopamine transmission rather than other traditional EWcp neuropeptides (Huang et al., 2019). In summary, while expression of certain neuropeptides like Ucn1 and CART are limited to the EWcp and not the DR, other markers like Substance P, CCK, TH, and Vglut2 may be less restricted to a single cell group in the subaqueductal paramedian zone, highlighting the need for investigators to provide precise descriptions of their spatial targeting in this area, even when using genetically defined cell type-specific strategies. Intersectional strategies that require more than one marker (to control more than one orthogonal recombinase, e.g. Cre AND Flp) or exclude a specific marker (e.g. Cre BUT NOT Flp) for transgene expression will also facilitate precise targeting (Fenno et al., 2020). We predict that measurable gradients of genes exist across the EW and DR and an updated classification that incorporates these gradients will substantially refine our definitions of ‘structures’ like the EW, DR, and vlPAG.
4.2. EW and DR Circuit Interactions
Beyond the complexities of differentiating EW vs. DR spatial boundaries and cell type assignments, EWcp-Ucn1 neurons display a well-characterized short-range axonal pathway of DR innervation (Weitemier and Ryabinin, 2006). These original findings based on anterograde tracing and peptide immunostaining may be inadvertently supported by data from Weissbourd et al. (2014) who performed monosynaptic rabies retrograde tracing from defined DR neuron populations and presented images that appear to show EWcp neurons that directly synapse onto DR 5HT and DR GABA neurons (Weissbourd et al., 2014).
This innervation appears in the central aspect of the DR, right where CRFR2 neurons live, suggesting neurochemical communication through the Ucn1-CRFR2 system. In addition, Huang et al. (2019) reported that the CRF binding protein (Crhbp, CRFBP) was highly localized to a specific subset of DR neurons in the caudal linear nucleus marked by unique expression of both GABA and glutamate transporters. This suggests that Ucn1 modulation of CRF receptors in the DR may be abrogated (or augmented) through discrete interactions with CRFBP in a specified DR cell type. Regarding potential interactions between EWcp-Ucn1 neurons and DR neurons expressing CRFR1 (Crhr1), scant evidence suggests that both CRF receptor subtypes are co-localized in DR neurons (Fan et al., 2014), although CRFR1 may be expressed at extremely low levels (Day et al., 2004), and CRFR1 and CRFR2 appear to oppositely impact stress-induced serotonin synthesis in DR (Donner et al., 2016).
Considering the “volume transmission” form of peptide release (axonally, somatodendritically, and through fibers of passage), this raises the possibility that EW neurons communicate to the DR via neuropeptide volume diffusion, as well as via direct synaptic contacts, providing several parallel mechanisms for multiplexed circuit neuromodulation between the subaqueductal paramedian nuclei.
Given the evidence for EWcp→DR projections, many have hypothesized that EWcp neuropeptides like Ucn1 and CART act directly (or indirectly) via the DR to modulate emotional behaviors. Classic studies from the Maier/Watkins laboratory used intra-DR microinjections of CRF receptor ligands to conclude that CRFR2 signaling is essential for sensitization of DR 5HT neuron activity that results from repeated exposures to uncontrollable stress (Amat et al., 2004, Hammack et al., 2003, Maier and Watkins, 2005). In addition, a series of studies from the Lowry group found that central infusions of Ucn1 or Ucn2 (intracerebroventricular, intra-BLA, or intra-BNST) generated increased anxiety-like behaviors and increased cFos and mRNA for Tph2 (rate-limiting enzyme for 5HT synthesis) in DR 5HT neurons (Donner et al., 2020; Donner et al., 2012; Spiga et al., 2006; Staub et al., 2006; Staub et al., 2005). Follow-up studies further implicated 5HT signaling in the anxiety-promoting properties of Ucn1 and Ucn2. Genetic deletion of these peptides yielded an anxiolytic phenotype that was correlated with increased 5HT levels in the BLA, ventral hippocampus, and raphe nuclei (Neufeld-Cohen et al., 2010). Additional evidence shows that activation of CRFR2 in the DR increases 5HT levels in the NAc, although in this study CRFR2 was driven by microinfusion of CRF, and CRFR1 signaling was shown to decrease NAc 5HT at lower concentrations (Lukkes et al., 2008).
Though axonal projections from the DR innervating EW neurons are less well-documented, evidence hints at a regulatory role for 5HT signaling on EWcp neuropeptide expression. For example, Fabre and colleagues used a transgenic mouse model of SERT overexpression to identify significant upregulation of Ucn1 and CART in the EWcp, at the levels of mRNA as well as protein (Fabre et al., 2011). Conversely, genetic knockout of SERT resulted in downregulation of Ucn1 mRNA and protein expression, relative to wild-type controls (Fabre et al., 2011). Because SERT overexpression and knockout respectively reduce and elevate extracellular 5HT levels, these findings suggest that endogenous 5HT acts to inhibit EWcp-Ucn1 expression, and that EWcp-Ucn1 levels are elevated under conditions of low 5HT tone. This inverse 5HT/Ucn1 relationship is consistent with a general framework in which anti-stress properties of DR-5HT neurons act in opposition to stress-sensitive EWcp-Ucn1 neurons that promote states of anxiety contributing to the addiction process. As such, it suggests that 5HT performs a negative feedback function to properly calibrate EW-Ucn1 activity in response to stressful challenges and may mediate a ‘stress recovery’ process.
Regarding EW-DR circuit interactions in explicit addiction contexts, one previous report showed that repeated alcohol exposures increased CRFR2 expression and CRFR2 binding in DR (Weitemier and Ryabinin, 2006). However, intra-DR Ucn1 microinjections had unclear effects on motivation for alcohol consumption, reflected by an overall decrease in food and fluid consumption (Weitemier and Ryabinin, 2006), consistent with documented anorectic actions of central CRFR2 signaling (Fekete et al., 2011). Given the cyclical, phasic, and thus complex nature of substance use disorders, it remains unclear how EW-DR circuit interactions modulate addictive behaviors.
5. Conclusions
The complex circuit dynamics of addiction remain a challenge to fully understand. The variable and cyclical nature of addictive behavior involves different brain systems at different times. We have presented an argument that an underappreciated circuit in the midbrain strongly participates in addictive behaviors and the sequelae of symptoms that accompany them. The EW-DR circuit contains a unique neuropeptide-receptor combination that has previously been implicated in emotional and stress-related behavioral states. Our synthesis of the existing literature and evidence produces an interesting hypothesis that the EW-DR Ucn1-CRFR2 system is critical to the addiction process. Future work should focus on this system and its potential complex roles in the cycles of addiction. As clinical interest already exists related to CRF peptides and receptors, this subaqueductal paramedian circuit could facilitate future knowledge of how these neuropeptides give rise to substance use disorders.
Highlights.
Edinger-Westphal and Dorsal Raphe nuclei are encircled by the periaqueductal gray
Unique neuronal populations that express many understudied molecules
A continuum of neurons that we name the subaqueductal paramedian zone
Cell type categorization by spatial, molecular, connectivity, and functional aspects
Highlight the contributions of EW-DR circuitry to addiction-related behaviors
Acknowledgements
Funding received:
NIH T32 DA035165 (M.B.P.), NHMRC 2002830 & Jack Brockhoff Foundation 4658 (L.C.W), NIH R00 AA025677 (W.J.G.), Stanford Psychiatry Innovator Grant (W.J.G.).
Abbreviations:
- 5HT
serotonin
- ASD
autism spectrum disorder
- BNST
bed nucleus of the stria terminalis
- Calca
calcitonin related polypeptide alpha
- CART
cocaine and amphetamine regulated transcript
- CCK
cholecystokinin
- CeA
central nucleus of the amygdala
- CPP
conditioned place preference
- CRF
corticotropin-releasing factor
- CRFBP
CRF binding protein
- CRFR
corticotropin-releasing factor receptor
- DR
dorsal raphe
- EW
Edinger-Westphal
- EWcp
centrally projecting EW
- EWpg
preganglionic EW
- ER
estrogen receptor
- Ghsr
growth hormone secretagogue receptor
- GPCR
G-protein coupled receptors
- Hcrt1
hypocretin receptor 1
- HPA
hypothalamic pituitary adrenal axis
- KOR
kappa opioid receptor
- LepRb/ObR
leptin receptor
- LH
lateral hypothalamus
- LHb
lateral habenula
- LS
lateral septal nucleus
- MCH
melanin concentrating hormone
- MOR
mu opioid receptors
- NAc
nucleus accumbens
- OVX
ovariectomy
- OX1
orexin receptor 1
- PAG
periaqueductal gray
- PH
posterior hypothalamus
- POA
preoptic area
- PR
progesterone receptors
- PVN
paraventricular nucleus of the hypothalamus
- PVT
paraventricular nucleus of the thalamus
- scRNA-seq
single-cell RNA sequencing
- SERT
serotonin transporter
- SP
substance P
- Tac1
tachykinin1
- TH
tyrosine hydroxylase
- TRH
thyrotropin releasing hormone
- Ucn1
urocortin 1
- Vglut
vesicular glutamate transporter
- VTA
ventral tegmental area
- Y1
neuropeptide Y receptor
- Y5
neuropeptide Y receptor 5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflicts of interest
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, et al. (2006). Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116, 3229–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF (2004). Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience 129, 509–19. [DOI] [PubMed] [Google Scholar]
- Bach P, Koopmann A, and Kiefer F (2021). The Impact of Appetite-Regulating Neuropeptide Leptin on Alcohol Use, Alcohol Craving and Addictive Behavior: A Systematic Review of Preclinical and Clinical Data. Alcohol Alcohol 56, 149–165. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, and Ryabinin AE (2002a). Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther 302, 516–524. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, and Ryabinin AE (2002b). Strain differences in urocortin expression in the Edinger-Westphal nucleus and its relation to alcohol-induced hypothermia. Neuroscience 113, 421–434. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, and Ryabinin AE (2003). The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci 23, 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, and Ryabinin AE (2004). Lesions of the Edinger-Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur J Neurosci 20, 1613–1623. [DOI] [PubMed] [Google Scholar]
- Becker JB, and Chartoff E (2019). Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, and Koob GF (2016). Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Phu K, Reddy AP, and Cameron JL (2013). The effect of short moderate stress on the midbrain corticotropin-releasing factor system in a macaque model of functional hypothalamic amenorrhea. Fertil Steril 100, 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, and Reddy AP (2012). The effect of long-term ovariectomy on midbrain stress systems in free ranging macaques. Brain Res 1488, 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, and Sawchenko PE (1999). Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol 415, 285–312. [PubMed] [Google Scholar]
- Bloem B, Xu L, Morava E, Faludi G, Palkovits M, Roubos EW, and Kozicz T (2012). Sex-specific differences in the dynamics of cocaine- and amphetamine-regulated transcript and nesfatin-1 expressions in the midbrain of depressed suicide victims vs. controls. Neuropharmacology 62, 297–303. [DOI] [PubMed] [Google Scholar]
- Boykoff N, Schneekloth TD, Hall-Flavin D, Loukianova L, Karpyak VM, Stevens SR, Biernacka JM, Mrazek DA, and Frye MA (2010). Gender differences in the relationship between depressive symptoms and cravings in alcoholism. Am J Addict 19, 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen LA, Burde RM, and Loewy AD (1983). Brainstem connections to the Edinger-Westphal nucleus of the cat: a retrograde tracer study. Brain Res 261, 303–306. [DOI] [PubMed] [Google Scholar]
- Cardozo Pinto DF, Yang H, Pollak Dorocic I, de Jong JW, Han VJ, Peck JR, Zhu Y, Liu C, Beier KT, Smidt MP, et al. (2019). Characterization of transgenic mouse models targeting neuromodulatory systems reveals organizational principles of the dorsal raphe. Nat Commun 10, 4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JR, Treweek JB, Robinson JE, Xiao C, Bremner LR, Greenbaum A, and Gradinaru V (2017). Dorsal Raphe Dopamine Neurons Modulate Arousal and Promote Wakefulness by Salient Stimuli. Neuron 94, 1205–1219 e1208. [DOI] [PubMed] [Google Scholar]
- Coffey KR, Marx RG, Vo EK, Nair SG, and Neumaier JF (2020). Chemogenetic inhibition of lateral habenula projections to the dorsal raphe nucleus reduces passive coping and perseverative reward seeking in rats. Neuropsychopharmacology 45, 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva AV, Torres KR, Haemmerle CA, Cespedes IC, and Bittencourt JC (2013). The Edinger-Westphal nucleus II: Hypothalamic afferents in the rat. J Chem Neuroanat 54, 5–19. [DOI] [PubMed] [Google Scholar]
- Day HEW, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S (2004) Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol 474, 364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks NM, Gaszner B, Bernhardt K, Roubos EW, and Kozicz T (2009). Sex-specific expression of BDNF and CART in the midbrain non-preganglionic Edinger-Westphal nucleus in the rat. Peptides 30, 2268–2274. [DOI] [PubMed] [Google Scholar]
- Derks NM, Gaszner B, Roubos EW, and Kozicz LT (2010). Sex differences in urocortin 1 dynamics in the non-preganglionic Edinger-Westphal nucleus of the rat. Neurosci Res 66, 117–123. [DOI] [PubMed] [Google Scholar]
- Derks NM, Roubos EW, and Kozicz T (2007). Presence of estrogen receptor beta in urocortin 1-neurons in the mouse non-preganglionic Edinger-Westphal nucleus. Gen Comp Endocrinol 153, 228–234. [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, and Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Davies SM, Fitz SD, Kienzle DM, Shekhar A, and Lowry CA (2020). Crh receptor priming in the bed nucleus of the stria terminalis (BNST) induces tph2 gene expression in the dorsomedial dorsal raphe nucleus and chronic anxiety. Prog Neuropsychopharmacol Biol Psychiatry 96, 109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, and Lowry CA (2012). Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety 29, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Siebler PH, Johnson DT, Villareal MD, Mani S, Matti AJ, Lowry CA (2016). Serotonergic systems in the balance: CRFR1 and CRHR2 differentially control stress-induced serotonin synthesis. Psychoneuroendocrinology. 63, 178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos Junior ED, Da Silva AV, Da Silva KR, Haemmerle CA, Batagello DS, Da Silva JM, Lima LB, Da Silva RJ, Diniz GB, Sita LV, et al. (2015). The centrally projecting Edinger-Westphal nucleus--I: Efferents in the rat brain. J Chem Neuroanat 68, 22–38. [DOI] [PubMed] [Google Scholar]
- Emmerzaal TL, vd Doelen RH, Roubos EW, and Kozicz T (2013). Orexinergic innervation of urocortin1 and cocaine and amphetamine regulated transcript neurons in the midbrain centrally projecting Edinger-Westphal nucleus. J Chem Neuroanat 54, 34–41. [DOI] [PubMed] [Google Scholar]
- Erichsen JT, Karten HJ, Eldred WD, and Brecha NC (1982a). Localization of substance P-like and enkephalin-like immunoreactivity within preganglionic terminals of the avian ciliary ganglion: light and electron microscopy. J Neurosci 2, 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen JT, Reiner A, and Karten HJ (1982b). Co-occurrence of substance P-like and Leu-enkephalin-like immunoreactivities in neurones and fibres of avian nervous system. Nature 295, 407–410. [DOI] [PubMed] [Google Scholar]
- Fabre V, Massart R, Rachalski A, Jennings K, Brass A, Sharp T, Lesch KP, Lanfumey L, and Hamon M (2011). Differential gene expression in mutant mice overexpressing or deficient in the serotonin transporter: a focus on urocortin 1. Eur Neuropsychopharmacol 21, 33–44. [DOI] [PubMed] [Google Scholar]
- Fan J-M, Chen X-Q, Wang X, Hao K, Du J-Z (2014). Corticotropin-releasing factor receptor type 1 colocalizes with type 2 in corticotropin-releasing factor-containing cellular profiles in rat brain. Neuro Endocrinol Lett. 35, 417–26. [PubMed] [Google Scholar]
- Farokhnia M, Abshire KM, Hammer A, Deschaine SL, Saravanakumar A, Cobbina E, You ZB, Haass-Koffler CL, Lee MR, Akhlaghi F, et al. (2021). Neuroendocrine response to exogenous ghrelin administration, combined with alcohol, in heavy-drinking individuals: Findings from a randomized, double-blind, placebo-controlled human laboratory study. Int J Neuropsychopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Lee MR, Farinelli LA, Ramchandani VA, Akhlaghi F, and Leggio L (2018). Pharmacological manipulation of the ghrelin system and alcohol hangover symptoms in heavy drinking individuals: Is there a link? Pharmacol Biochem Behav 172, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima A, Haroon MF, Wolf G, Engelmann M, and Spina MG (2007). Reduced urocortin 1 immunoreactivity in the non-preganglionic Edinger-Westphal nucleus during late pregnancy in rats. Regul Pept 143, 34–38. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Szucs A, Sabino V, Cottone P, Rivier J, Vale WW, Koob GF, and Zorrilla EP (2011). Systemic urocortin 2, but not urocortin 1 or stressin 1-A, suppresses feeding via CRF2 receptors without malaise and stress. Br J Pharmacol 164, 1959–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, et al. (2014). Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods 11, 763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno LE, Ramakrishnan C, Kim YS, Evans KE, Lo M, Vesuna S, Inoue M, Cheung KYM, Yuen E, Pichamoorthy N, et al. (2020). Comprehensive Dual- and Triple-Feature Intersectional Single-Vector Delivery of Diverse Functional Payloads to Cells of Behaving Mammals. Neuron 107(5), 836–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Patterson CM, Garfield AS, D’Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JT, Germani M, Jones JC, et al. (2014). Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci 17, 1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores JA, Galan-Rodriguez B, Ramiro-Fuentes S, and Fernandez-Espejo E (2006). Role for dopamine neurons of the rostral linear nucleus and periaqueductal gray in the rewarding and sensitizing properties of heroin. Neuropsychopharmacology 31, 1475–1488. [DOI] [PubMed] [Google Scholar]
- Fonareva I, Spangler E, Cannella N, Sabino V, Cottone P, Ciccocioppo R, Zorrilla EP, and Ryabinin AE (2009). Increased perioculomotor urocortin 1 immunoreactivity in genetically selected alcohol preferring rats. Alcohol Clin Exp Res 33, 1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca MS, Murakami M, and Mainen ZF (2015). Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Curr Biol 25, 306–315. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, and Reiner A (1991). The Edinger-Westphal nucleus: sources of input influencing accommodation, pupilloconstriction, and choroidal blood flow. J Comp Neurol 306, 425–438. [DOI] [PubMed] [Google Scholar]
- Gaszner B, Korosi A, Palkovits M, Roubos EW, and Kozicz T (2007). Neuropeptide Y activates urocortin 1 neurons in the nonpreganglionic Edinger-Westphal nucleus. J Comp Neurol 500, 708–719. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, Pastor R, Anacker AMJ, Spangler E, Cote DM, Li J, Stenzel-Poore MP, Phillips TJ, Ryabinin AE (2011). Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes, Brain and Behavior 10, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Cote DM, Li J, and Ryabinin AE (2012). Characterization of Genetic Differences within the Centrally Projecting Edinger-Westphal Nucleus of C57BL/6J and DBA/2J Mice by Expression Profiling. Front Neuroanat 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Rodriguez ED, Smith ML, Ford MM, Galili D, Mitchell SH, Chen A, and Ryabinin AE (2017). Control of chronic excessive alcohol drinking by genetic manipulation of the Edinger-Westphal nucleus urocortin-1 neuropeptide system. Transl Psychiatry 7, e1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocho Y, Sakai A, Yanagawa Y, Suzuki H, and Saitow F (2013). Electrophysiological and pharmacological properties of GABAergic cells in the dorsal raphe nucleus. J Physiol Sci 63, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, and Kieffer BL (2011). Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol Psychiatry 69, 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groessl F, Munsch T, Meis S, Griessner J, Kaczanowska J, Pliota P, Kargl D, Badurek S, Kraitsy K, Rassoulpour A, et al. (2018). Dorsal tegmental dopamine neurons gate associative learning of fear. Nat Neurosci 21, 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Long VM, Farokhnia M, Magill M, Kenna GA, Swift RM, and Leggio L (2019). Intravenous administration of ghrelin increases serum cortisol and aldosterone concentrations in heavy-drinking alcohol-dependent individuals: Results from a double-blind, placebo-controlled human laboratory study. Neuropharmacology 158, 107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF (2003). Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci 23, 1019–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, and Aston-Jones G (2001). Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology 24, 75–85. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Salgado JS, Taylor MD, Hoerbelt P, Cardozo Pinto DF, Steinberg EE, Walsh JJ, Sze JY, and Malenka RC (2019). Distinct neural mechanisms for the prosocial and rewarding properties of MDMA. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, and Kranzler HR (2004). Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend 74, 265–272. [DOI] [PubMed] [Google Scholar]
- Huang KW, Ochandarena NE, Philson AC, Hyun M, Birnbaum JE, Cicconet M, and Sabatini BL (2019). Molecular and anatomical organization of the dorsal raphe nucleus. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalafateli AL, Satir TM, Vallof D, Zetterberg H, and Jerlhag E (2021). An amylin and calcitonin receptor agonist modulates alcohol behaviors by acting on reward-related areas in the brain. Prog Neurobiol 200, 101969. [DOI] [PubMed] [Google Scholar]
- Kaur S, and Ryabinin AE (2010). Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res 34, 1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster J, Beckers HJ, Vrensen GF, and van der Want JJ (1993). The peripheral and central projections of the Edinger-Westphal nucleus in the rat. A light and electron microscopic tracing study. Brain Res 632, 260–273. [DOI] [PubMed] [Google Scholar]
- Kozicz T (2003). Neurons colocalizing urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neuroscience 116, 315–320. [DOI] [PubMed] [Google Scholar]
- Kozicz T (2007). On the role of urocortin 1 in the non-preganglionic Edinger-Westphal nucleus in stress adaptation. Gen Comp Endocrinol 153, 235–240. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PD, Palkovits M, Horn AK, Toledo CA, and Ryabinin AE (2011a). The Edinger-Westphal nucleus: a historical, structural, and functional perspective on a dichotomous terminology. J Comp Neurol 519, 1413–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T, Li M, and Arimura A (2001). The activation of urocortin immunoreactive neurons in the Einger-Westphal nucleus following stress in rats. Stress 4, 85–90. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Sterrenburg L, and Xu L (2011b). Does midbrain urocortin 1 matter? A 15-year journey from stress (mal)adaptation to energy metabolism. Stress 14, 376–383. [DOI] [PubMed] [Google Scholar]
- Lalanne L, Ayranci G, Filliol D, Gaveriaux-Ruff C, Befort K, Kieffer BL, and Lutz PE (2017). Kappa opioid receptor antagonism and chronic antidepressant treatment have beneficial activities on social interactions and grooming deficits during heroin abstinence. Addict Biol 22, 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, and Chavkin C (2009). Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A 106, 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, and Kash TL (2019). kappa-Opioid Receptor Modulation of GABAergic Inputs onto Ventrolateral Periaqueductal Gray Dopamine Neurons. Mol Neuropsychiatry 5, 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Sugam JA, Lowery-Gionta EG, McElligott ZA, McCall NM, Lopez AJ, McKlveen JM, Pleil KE, and Kash TL (2016). Mu Opioid Receptor Modulation of Dopamine Neurons in the Periaqueductal Gray/Dorsal Raphe: A Role in Regulation of Pain. Neuropsychopharmacology 41, 2122–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang LZ, He ZX, Ma H, Zhang YT, Xun YF, Yuan W, Hou WJ, Li YT, Lv ZJ, et al. (2021). Dorsal raphe nucleus to anterior cingulate cortex 5-HTergic neural circuit modulates consolation and sociability. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen W, Pan K, Li H, Pang P, Guo Y, Shu S, Cai Y, Pei L, Liu D, et al. (2018). Serotonin receptor 2c-expressing cells in the ventral CA1 control attention via innervation of the Edinger-Westphal nucleus. Nat Neurosci 21, 1239–1250. [DOI] [PubMed] [Google Scholar]
- Li Y, Li CY, Xi W, Jin S, Wu ZH, Jiang P, Dong P, He XB, Xu FQ, Duan S, et al. (2019). Rostral and Caudal Ventral Tegmental Area GABAergic Inputs to Different Dorsal Raphe Neurons Participate in Opioid Dependence. Neuron 101, 748–761 e745. [DOI] [PubMed] [Google Scholar]
- Lim MM, Tsivkovskaia NO, Bai Y, Young LJ, and Ryabinin AE (2006). Distribution of corticotropin-releasing factor and urocortin 1 in the vole brain. Brain Behav Evol 68, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima FB, Henderson JA, Reddy AP, Tokuyama Y, Hubert GW, Kuhar MJ, and Bethea CL (2008). Unique responses of midbrain CART neurons in macaques to ovarian steroids. Brain Res 1227, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Liang J, Wang R, Yan T, Zhou Y, Liu Y, Feng Q, Sun F, Li Y, Li A, et al. (2020). The Raphe Dopamine System Controls the Expression of Incentive Memory. Neuron 106, 498–514 e498. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang JE, Wang D, Zeng J, et al. (2014). Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81, 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy AD, and Saper CB (1978). Edinger-Westphal nucleus: projections to the brain stem and spinal cord in the cat. Brain Res 150, 1–27. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Saper CB, and Yamodis ND (1978). Re-evaluation of the efferent projections of the Edinger-Westphal nucleus in the cat. Brain Res 141, 153–159. [DOI] [PubMed] [Google Scholar]
- Lutz PE, and Kieffer BL (2013a). The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol 23, 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, and Kieffer BL (2013b). Opioid receptors: distinct roles in mood disorders. Trends Neurosci 36, 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Pearson E, and Tao R (2007). CART peptides increase 5-hydroxytryptamine in the dorsal raphe and nucleus accumbens of freely behaving rats. Neurosci Lett 417, 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciewicz R, Phipps BS, Grenier J, and Poletti CE (1984). Edinger-Westphal nucleus: cholecystokinin immunocytochemistry and projections to spinal cord and trigeminal nucleus in the cat. Brain Res 299, 139–145. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR (2005). Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 29, 829–41. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, et al. (2016). Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 7618, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, and Alvarez VA (2018). Cocaine Inhibition of Synaptic Transmission in the Ventral Pallidum Is Pathway-Specific and Mediated by Serotonin. Cell Rep 23, 3852–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, et al. (2016). Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 164, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Tanaka KF, Yamanaka A, Takahashi A, Tabuchi S, and Doya K (2014). Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr Biol 24, 2033–2040. [DOI] [PubMed] [Google Scholar]
- Nardou R, Lewis EM, Rothhaas R, Xu R, Yang A, Boyden E, and Dolen G (2019). Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature 569, 116–120. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Commons KG, and Dymecki SM (2019). Embracing diversity in the 5-HT neuronal system. Nat Rev Neurosci 20, 397–424. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Freret ME, Rood BD, Brust RD, Hennessy ML, deBairos D, Kim JC, Cook MN, and Dymecki SM (2015). Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron 88, 774–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Sturrock N, Escobedo Lozoya Y, Chang Y, Senft RA, Lyon KA, Alekseyenko OV, and Dymecki SM (2020). A single-cell transcriptomic and anatomic atlas of mouse dorsal raphe Pet1 neurons. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okere B, Xu L, Roubos EW, Sonetti D, and Kozicz T (2010). Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res 1317, 92–99. [DOI] [PubMed] [Google Scholar]
- Otake K (2005). Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neurosci Res 51, 383–394. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, and McKee SA (2019). Sex differences in stress-related alcohol use. Neurobiol Stress 10, 100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, and Valentino RJ (2004). Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci 24, 1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps BS, Maciewicz R, Sandrew BB, Poletti CE, and Foote WE (1983). Edinger-Westphal neurons that project to spinal cord contain substance P. Neurosci Lett 36, 125–131. [DOI] [PubMed] [Google Scholar]
- Priest MF, Freda SN, Badong D, Dumrongprechachan V, Kozorovitskiy Y (2021). Peptidergic modulation of fear responses by the Edinger-Westphal nucleus. bioRxiv. https://www.biorxiv.org/content/10.1101/2021.08.05.455317v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang HL, Wang H, de Jesus Aceves Buendia J, Hoffman AF, Lupica CR, Seal RP, and Morales M (2014). A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat Commun 5, 5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley JA, Logsdon MK, Turner CA, Gonzalez IL, Leonardo NB, and Becker JB (2021). Sex differences in vulnerability to addiction. Neuropharmacology 187, 108491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C, Pun A, Sun Y, et al. (2018). Anatomically Defined and Functionally Distinct Dorsal Raphe Serotonin Sub-systems. Cell 175, 472–487 e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Isakova A, Friedmann D, Zeng J, Grutzner SM, Pun A, Zhao GQ, Kolluru SS, Wang R, Lin R, et al. (2019). Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Cocking DL, and Kaur S (2013). Inhibition of VTA neurons activates the centrally projecting Edinger-Westphal nucleus: evidence of a stress-reward link? J Chem Neuroanat 54, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, and Giardino WJ (2017). Contribution of Urocortin to the Development of Excessive Drinking. Int Rev Neurobiol 136, 275–291. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsivkovskaia NO, and Ryabinin SA (2005). Urocortin 1-containing neurons in the human Edinger-Westphal nucleus. Neuroscience 134, 1317–1323. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsoory MM, Kozicz T, Thiele TE, Neufeld-Cohen A, Chen A, Lowery-Gionta EG, Giardino WJ, and Kaur S (2012). Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol 46, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, and Weitemier AZ (2006). The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res Rev 52, 368–380. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, and Bethea CL (2010). Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience 171, 893–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, and Heilig M (2012). Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron 76, 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Parolari L, Das Banerjee T, Bhave V, Wang P, Patel B, Topilko T, Wu Z, Choi CHJ, Yu X, et al. (2019). Regulation of Energy Expenditure by Brainstem GABA Neurons. Cell 178, 672–685 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, and Holmes A (2019). A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory. Neuron 103, 489–505 e487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda M, Manning EE, Gogos A, Hale M, and van den Buuse M (2021). Long-term effects of young-adult methamphetamine on dorsal raphe serotonin systems in mice: Role of brain-derived neurotrophic factor. Brain Res 1762, 147428. [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Hokfelt T, and Hurd Y (1999). Serotonin and substance P co-exist in dorsal raphe neurons of the human brain. Neuroreport 10, 3967–3970. [DOI] [PubMed] [Google Scholar]
- Skirboll L, Hokfelt T, Dockray G, Rehfeld J, Brownstein M, and Cuello AC (1983). Evidence for periaqueductal cholecystokinin-substance P neurons projecting to the spinal cord. J Neurosci 3, 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirboll L, Hokfelt T, Rehfeld J, Cuello AC, and Dockray G (1982). Coexistence of substance P- and cholecystokinin-like immunoreactivity in neurons of the mesencephalic periaqueductal central gray. Neurosci Lett 28, 35–39. [DOI] [PubMed] [Google Scholar]
- Spangler E, Cote DM, Anacker AM, Mark GP, and Ryabinin AE (2009). Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience 160, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, Kozicz T, and Andrews ZB (2012). Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry 72, 457–465. [DOI] [PubMed] [Google Scholar]
- Spiga F, Lightman SL, Shekhar A, and Lowry CA (2006). Injections of urocortin 1 into the basolateral amygdala induce anxiety-like behavior and c-Fos expression in brainstem serotonergic neurons. Neuroscience 138, 1265–1276. [DOI] [PubMed] [Google Scholar]
- Staub DR, Evans AK, and Lowry CA (2006). Evidence supporting a role for corticotropin-releasing factor type 2 (CRF2) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res 1070, 77–89. [DOI] [PubMed] [Google Scholar]
- Staub DR, Lunden JW, Cathel AM, Dolben EL, and Kirby LG (2012). Morphine history sensitizes postsynaptic GABA receptors on dorsal raphe serotonin neurons in a stress-induced relapse model in rats. Psychoneuroendocrinology 37, 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub DR, Spiga F, and Lowry CA (2005). Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res 1044, 176–189. [DOI] [PubMed] [Google Scholar]
- Swanson LW, and Cowan WM (1979). The connections of the septal region in the rat. J Comp Neurol 186, 621–655. [DOI] [PubMed] [Google Scholar]
- Szabo G (2018). Women and alcoholic liver disease - warning of a silent danger. Nat Rev Gastroenterol Hepatol 15, 253–254. [DOI] [PubMed] [Google Scholar]
- Szonyi A, Zicho K, Barth AM, Gonczi RT, Schlingloff D, Torok B, Sipos E, Major A, Bardoczi Z, Sos KE, et al. (2019). Median raphe controls acquisition of negative experience in the mouse. Science 366. [DOI] [PubMed] [Google Scholar]
- Tao R, and Auerbach SB (2002a). GABAergic and glutamatergic afferents in the dorsal raphe nucleus mediate morphine-induced increases in serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther 303, 704–710. [DOI] [PubMed] [Google Scholar]
- Tao R, and Auerbach SB (2002b). Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther 303, 549–556. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Sharma R, and Sahota P (2015). Alcohol disrupts sleep homeostasis. Alcohol 49, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko T, Diaz SL, Pacheco CM, Verny F, Deleuze C, Gaspar P, Renier N Midbrain Peptidergic Neurons Enable Maternal Nesting. (2021). Cell Press Sneak Peek. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3878409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek VF, Tsivkovskaia NO, Hyytia P, Harding S, Le AD, and Ryabinin AE (2005). Urocortin 1 expression in five pairs of rat lines selectively bred for differences in alcohol drinking. Psychopharmacology (Berl) 181, 511–517. [DOI] [PubMed] [Google Scholar]
- Valentinova K, Tchenio A, Trusel M, Clerke JA, Lalive AL, Tzanoulinou S, Matera A, Moutkine I, Maroteaux L, Paolicelli RC, et al. (2019). Morphine withdrawal recruits lateral habenula cytokine signaling to reduce synaptic excitation and sociability. Nat Neurosci 22, 1053–1056. [DOI] [PubMed] [Google Scholar]
- Vallof D, Kalafateli AL, and Jerlhag E (2020). Long-term treatment with a glucagon-like peptide-1 receptor agonist reduces ethanol intake in male and female rats. Transl Psychiatry 10, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN (2012). Neuropeptide transmission in brain circuits. Neuron 76, 98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Doelen RHA, Robroch B, Arnoldussen IA, Schulpen M, Homberg JR, and Kozicz T (2017). Serotonin and urocortin 1 in the dorsal raphe and Edinger-Westphal nuclei after early life stress in serotonin transporter knockout rats. Neuroscience 340, 345–358. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, and Sawchenko PE (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428, 191–212. [DOI] [PubMed] [Google Scholar]
- Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, Deisseroth K, and Malenka RC (2018). 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 560, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Zhang S, Qi J, Wang H, Cachope R, Mejias-Aponte CA, Gomez JA, Mateo-Semidey GE, Beaudoin GMJ, Paladini CA, et al. (2019). Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons. Cell Rep 26, 1128–1142 e1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, and Deisseroth K (2012). A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, and Luo L (2014). Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83, 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, and Ryabinin AE (2006). Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience 137, 1439–1445. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Tsivkovskaia NO, and Ryabinin AE (2005). Urocortin 1 distribution in mouse brain is strain-dependent. Neuroscience 132, 729–740. [DOI] [PubMed] [Google Scholar]
- Xu L, Bloem B, Gaszner B, Roubos EW, and Kozicz T (2009). Sex-specific effects of fasting on urocortin 1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 expression in the rat Edinger-Westphal nucleus. Neuroscience 162, 1141–1149. [DOI] [PubMed] [Google Scholar]
- Xu L, Janssen D, van der Knaap N, Roubos EW, Leshan RL, Myers MG Jr., Gaszner B, and Kozicz T (2014). Integration of stress and leptin signaling by CART producing neurons in the rodent midbrain centrally projecting Edinger-Westphal nucleus. Front Neuroanat 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Scheenen WJ, Leshan RL, Patterson CM, Elias CF, Bouwhuis S, Roubos EW, Myers MG Jr., and Kozicz T (2011). Leptin signaling modulates the activity of urocortin 1 neurons in the mouse nonpreganglionic Edinger-Westphal nucleus. Endocrinology 152, 979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Scheenen WJ, Roubos EW, and Kozicz T (2012). Peptidergic Edinger-Westphal neurons and the energy-dependent stress response. Gen Comp Endocrinol 177, 296–304. [DOI] [PubMed] [Google Scholar]
- Xu S, Das G, Hueske E, and Tonegawa S (2017). Dorsal Raphe Serotonergic Neurons Control Intertemporal Choice under Trade-off. Curr Biol 27, 3111–3119 e3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Holmes TC, Luo MH, Beier KT, Horwitz GD, Zhao F, Zeng W, Hui M, Semler BL, and Sandri-Goldin RM (2020). Viral Vectors for Neural Circuit Mapping and Recent Advances in Trans-synaptic Anterograde Tracers. Neuron 107, 1029–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Pati D, Pina MM, Schmidt KT, Boyt KM, Hunker AC, Zweifel LS, McElligott ZA, and Kash TL (2021). Periaqueductal gray/dorsal raphe dopamine neurons contribute to sex differences in pain-related behaviors. Neuron 109, 1365–1380 e1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wu X, Zhang YM, Liu H, Jiang Q, Pang G, Tao X, Dong L, and Stackman RW Jr. (2016). Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology 101, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhong P, Hu F, Barger Z, Ren Y, Ding X, Li S, Weber F, Chung S, Palmiter RD, et al. (2019). An Excitatory Circuit in the Perioculomotor Midbrain for Non-REM Sleep Control. Cell 177, 1293–1307 e1216. [DOI] [PubMed] [Google Scholar]
- Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, Mao Y, Wang L, Xie W, Zhong H, et al. (2019). A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci 22, 1649–1658. [DOI] [PubMed] [Google Scholar]
- Zuniga A, and Ryabinin AE (2020). Involvement of Centrally Projecting Edinger-Westphal Nucleus Neuropeptides in Actions of Addictive Drugs. Brain Sci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga A, Ryabinin AE, and Cunningham CL (2020). Effects of pharmacological inhibition of the centrally-projecting Edinger-Westphal nucleus on ethanol-induced conditioned place preference and body temperature. Alcohol 87, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc GK (1996). Peptidergic transmission: from morphological correlates to functional implications. Micron 27, 35–91. [DOI] [PubMed] [Google Scholar]


