Abstract
Women have more difficulty maintaining smoking cessation than men, and experience greater withdrawal symptomatology as well as higher prevalence of relapse. Further, currently available treatments for smoking cessation, such as the nicotine patch and varenicline, have been shown to be less effective in women. Fluctuations in ovarian hormones across the menstrual cycle can affect craving and smoking relapse propensity. In addition, many women who smoke use some form of oral contraceptives, which most often contain ethinyl estradiol (EE), a synthetic, orally bio-available estrogen that is currently prescribed to women chronically and has been shown to alter smoking reward in women. The current study examined the impact of 17β-estradiol (E2), the prominent endogenous form of the steroid hormone estrogen, as well as EE, on nicotine self-administration, demand, and reinstatement following ovariectomy (OVX) or sham surgery. OVX vehicle-treated female rats consumed less nicotine, had lower intensity of demand, and reinstated less compared to sham vehicle-treated female rats. OVX-E2 and OVX-EE treatment groups showed a rebound of nicotine intake later in training, and Q0 levels of consumption were partially rescued in both groups. Further, E2 but not EE reversed the abolishment of reinstated nicotine seeking induced by OVX. Taken together, these results demonstrate that natural and synthetic estrogens play a critical role in mediating the neurobehavioral effects of nicotine, and future studies are essential for our understanding of how synthetic hormones contained within oral contraceptives interact with smoking.
Keywords: estrogen, 17β-estradiol, ethinyl estradiol, nicotine, demand, reinstatement
1. Introduction
Clinical trials indicate that long-term smoking cessation is more difficult to achieve in women as compared to men (Perkins et al., 1999; Piper et al., 2007). Menstrual cycle phase in women can affect cigarette craving and propensity to relapse to smoking following abstinence (Allen et al., 2008; Carpenter et al., 2006; Franklin et al., 2008). It is estimated that 35–45% of all women between 2008–2014 used hormone-containing contraceptives of some kind, with the most common being the oral contraceptive pill (Kavanaugh and Jerman, 2018), which can modify circulating ovarian hormone levels and the menstrual cycle overall. Despite negative health implications, nearly 50% of premenopausal women who smoke use some form of hormonal contraceptive (Allen et al., 2018). These studies illustrate that smoking is an important consideration in female reproductive health and highlight the need to better understand how ovarian hormones, endogenous and synthetic, interact with neurobehavioral mechanisms of nicotine use.
Fluctuating ovarian hormones impact nicotine use vulnerability, where high levels of progesterone are correlated with a decrease in cigarette smoking (Lynch, 2010), and high levels of estrogen are correlated with enhanced nicotine use (Wetherill et al., 2016). Further, exogenous progesterone treatment decreases smoking urges in women (Sofuoglu et al., 2009), and exogenous micronized progesterone treatment also significantly attenuates nicotine craving in women (Sofuoglu et al., 2001). Together, these studies indicate that circulating ovarian hormone levels can impact smoking cessation outcomes, and exogenous hormone administration can alter smoking urges, although the parameters driving these effects are as yet undetermined.
The most common estrogen in the contraceptive pill is ethinyl estradiol (EE), a synthetic form of endogenously circulating 17β-estradiol (E2; Mennenga et al., 2015; Shively, 1998). Importantly, users of EE-containing oral contraceptives have faster nicotine metabolism than both nonusers and men (Benowitz et al., 2006). One study examined smoking reward in women using the triphasic contraceptive Tri-Sprintec™, an EE-containing oral contraceptive, and found that smoking satisfaction was higher in oral contraceptive users during the low progesterone week of the pill pack compared to the weeks with high progesterone (Hinderaker et al., 2015). During acute smoking abstinence, users of EE-containing oral contraceptives reported significantly lower levels of positive affect than non-users (Hinderaker et al., 2015). Finally, nicotine has antiestrogenic effects whereby it results in lower levels of biologically active estrogens (Mueck and Seeger, 2005), which may alter nicotine-related behaviors. Together, these results suggest that women using EE-containing contraceptives have different patterns of smoking symptomatology, which could impact smoking cessation outcomes.
Given the large proportion of women using EE-containing oral contraceptives who concurrently smoke, this is an important experimental group to include when evaluating female-specific mechanisms of nicotine use. The current study examined the impact of E2 and EE on nicotine short-access self-administration, demand, extinction, and reinstatement. In order to study E2 and EE effects on female nicotine use, we utilized the ovariectomy (OVX) model thereby permitting evaluation of specific, isolated effects of E2 or EE exposure in animals with a “blank gonadal hormone” state (Koebele and Bimonte-Nelson, 2016). Female rats underwent either OVX or sham surgery, and received either daily vehicle (sesame oil), E2, or EE treatment during nicotine self-administration, extinction, and reinstatement. Demand analysis of nicotine self-administration has translational value, as it is utilized in both pre-clinical and clinical research (Bickel, 1999). We hypothesized that abrupt loss of circulating ovarian hormones via OVX would decrease nicotine intake and essential value, and would inhibit nicotine seeking behavior compared to intact, freely cycling females. Conversely, we hypothesized that daily, chronic delivery of E2 or EE treatment following OVX would increase nicotine self-administration, essential value, and nicotine seeking behavior.
2. Methods
2.1. Animals
Thirty-six sexually naïve female Long-Evans rats (Charles River; 200 – 250 g with an arrival age of approximately 8 weeks) were utilized in the current study. Rats were individually housed on a 12-hour reverse light cycle with ad libitum access to food and water prior to initiation of experimental procedures, and ad libitum access to water in the home cage throughout the study. Animals were handled daily upon arrival. One rat was excluded following the nicotine self-administration acquisition phase due to catheter patency failure. No other rats were excluded from behavioral experimentation. The total number of rats per group that completed all behavioral phases of the study were n=9 (sham vehicle), n=8 (OVX vehicle), n=9 (OVX E2), and n=9 (OVX EE). All animal use practices were approved by the Institutional Animal Care and Use Committee of Arizona State University (ASU; Protocol #18–1642R).
2.2. Apparatus
Experimental sessions occurred in operant chambers (MED Associates, St. Albans, VT) within sound-attenuating chambers. Two levers, one active and one inactive, were presented at the beginning of each session. Above each lever was a stimulus light and each chamber contained a house light. Infusion pumps (MED Associates) were located outside of each chamber. Ventilation fans were provided in each sound-attenuating chamber. Experimental events were recorded by MED-PC software (MED Associates) on a computer in the experimental room. All operant chambers have been previously described in detail (Leyrer-Jackson et al., 2020b; Overby et al., 2018).
2.3. Surgical Procedures, Hormone Treatment, and Food Training
One week following acclimation to facilities, rats either underwent OVX or sham surgery. Animals were anesthetized with intramuscular (i.m.) ketamine (80–100 mg/kg i.m.) and xylazine (8 mg/kg, i.m.). Two dorsolateral incisions approximately 1.5 cm long were made in the skin and peritoneum. Next, the ovaries and tips of the uterine horns were ligated with Vicryl suture and removed with scissors. The muscle was then sutured with absorbable Vicryl suture, and the skin was closed with surgical staples. Sham animals received skin and muscle incision, and appropriate respective surgical closure. Animals received daily injections of meloxicam (1 mg/kg, subcutaneous, s.c.) for three days for pain management, and three mL of saline (s.c.) to prevent dehydration during recovery on the day of surgery. Rats also received one injection of cefazolin (100 mg/mL, s.c.) on surgery day. Rats were given three weeks to recover prior to beginning experimental procedures. The three-week recovery period allowed for stabilization of the depleting ovarian hormone milieu prior to exogenous administration of specific hormone treatments (Mosquera et al., 2015; Turek et al., 2016; Yousefzadeh et al., 2020).
Three weeks following OVX or sham surgery, animals were again anesthetized with ketamine and xylazine as described above and implanted with an indwelling catheter in the jugular vein as described previously (Leyrer-Jackson et al., 2020a, 2020b). A small incision was made in the chest to isolate the jugular vein, and another small incision was made on the dorsal side between the scapulae. A 13 cm polyurethane catheter (Instech, Plymouth Meeting, PA, USA) with a silicone anchor 2 cm from one end was tunneled subcutaneously between the incisions, with the anchored side inserted into the jugular vein. The dorsal end of the catheter was attached to an indwelling back port (Instech, Plymouth Meeting, PA, USA). Dental cement (SNAP, Parkell, NY, USA or Ortho-Jet, Lang Dental, IL, USA) was used to adhere the catheter to the port. The indwelling port was sutured in place approximately 2 cm caudal from the shoulder blades using 4–0 Vicryl braided suture (Ethicon, Cincinnati, OH, USA). Following successful placement of the catheter, post-operative cefazolin (100 mg/kg, intravenous (i.v.)) and meloxicam (1 mg/kg, s.c.) were administered for seven and three days, respectively. Catheter patency was maintained throughout the experiments by daily administrations of 0.1 mL heparin saline (100 USP units/ml, i.v.), which were given both prior to and following nicotine self-administration sessions.
Beginning on the day animals received jugular vein catheters, daily hormone injections of either E2 (3 μg/0.1 mL sesame oil), EE (0.3 μg/0.1 mL sesame oil), or vehicle (0.1 mL sesame oil) began; OVX Vehicle and Sham animals received vehicle injections. All hormone injections were administered subcutaneously. The dose of E2 was chosen to be within physiologically relevant endogenous plasma hormone concentrations (88–144 pg/mL; Butcher et al., 1974; Mennenga and Bimonte-Nelson, 2015). The EE dose utilized here corresponds to a 75–80 μg/day dose of EE in a woman, which has been shown to have biological impact (Devineni et al., 2007; Mennenga et al., 2015) and to result in serum levels of EE that are similar to that found in women taking oral contraceptives that contain 35 ug of EE early in their monthly cycle. Hormone treatment was given daily, 3 hrs following the beginning of the dark cycle (one hour prior to behavioral sessions) and continued until the end of behavioral testing. In accordance with other studies rats were weighed daily following OVX to provide further evidence of successful OVX as well as successful hormonal manipulations, as OVX induces weight gain in females, and hormonal supplementation should decrease this (Brutman et al., 2019; Rogers et al., 2009).
Following jugular vein catheter surgery and recovery, rats were food restricted to 20 g/d and trained to respond for food pellets in the apparatus described above. Food training sessions lasted 15 hours and the house light remained illuminated throughout training. Upon completion of a fixed ratio-1 (FR1) schedule of reinforcement on the active lever, a 45 mg food pellet (BioServ®, Flemington, NJ) was delivered into a food receptacle inside the operant chamber. No consequences were produced by pressing the inactive lever. Concurrent with the FR1, animals also received one 45 mg food pellet every 20 minutes, regardless of response. Animals were required to achieve an active to inactive lever press ratio ≥ 2. Any animals that did not reach this criterion underwent a second food training session the following day. All animals met the food training criterion after 1–2 sessions. Although the extension of the lights from 12h to 15h per day may affect circadian rhythms, animals only received 1 or 2 sessions of food training and time elapsed between food training and nicotine SA allowed animals to re-entrain to the 12h light cycle.
2.4. Self-Administration, Extinction, and Reinstatement Procedures
An experimental timeline is shown in Figure 1A. Rats remained food restricted to 20 g/d during all SA, extinction and reinstatement procedures (Donny et al., 1998). All animals were tested 4 hrs following the beginning of the dark cycle. Infusions of 0.06 mg/kg/infusion nicotine (0.1 mL/infusion) were delivered across 5.9 s following one response on the active lever (FR-1). Upon an active lever press, lights above both levers were illuminated, and a tone (2900 Hz) was presented simultaneously with drug infusion. Upon completion of the infusion, the tone and lights ceased. Infusions were followed by a 20-s timeout period, during which active lever responses were recorded but produced no consequences. An inactive lever was present at all times but produced no consequences when pressed. All sessions were 2 hr in duration. Rats completed a minimum of 12 sessions before moving into the demand phase, with criteria set at a minimum of a 2:1 ratio of active to inactive lever responses, and a minimum of 10 infusions. If these criteria were met, animals were moved to between-session dose-reduction procedures. If criteria were not met by 12 sessions, animals remained in nicotine self-administration acquisition until a maximum of 20 sessions prior to moving into the demand phase. Rats were moved into the demand phase after 20 sessions regardless of meeting criteria.
Figure 1. Acquisition of Nicotine Self-Administration.
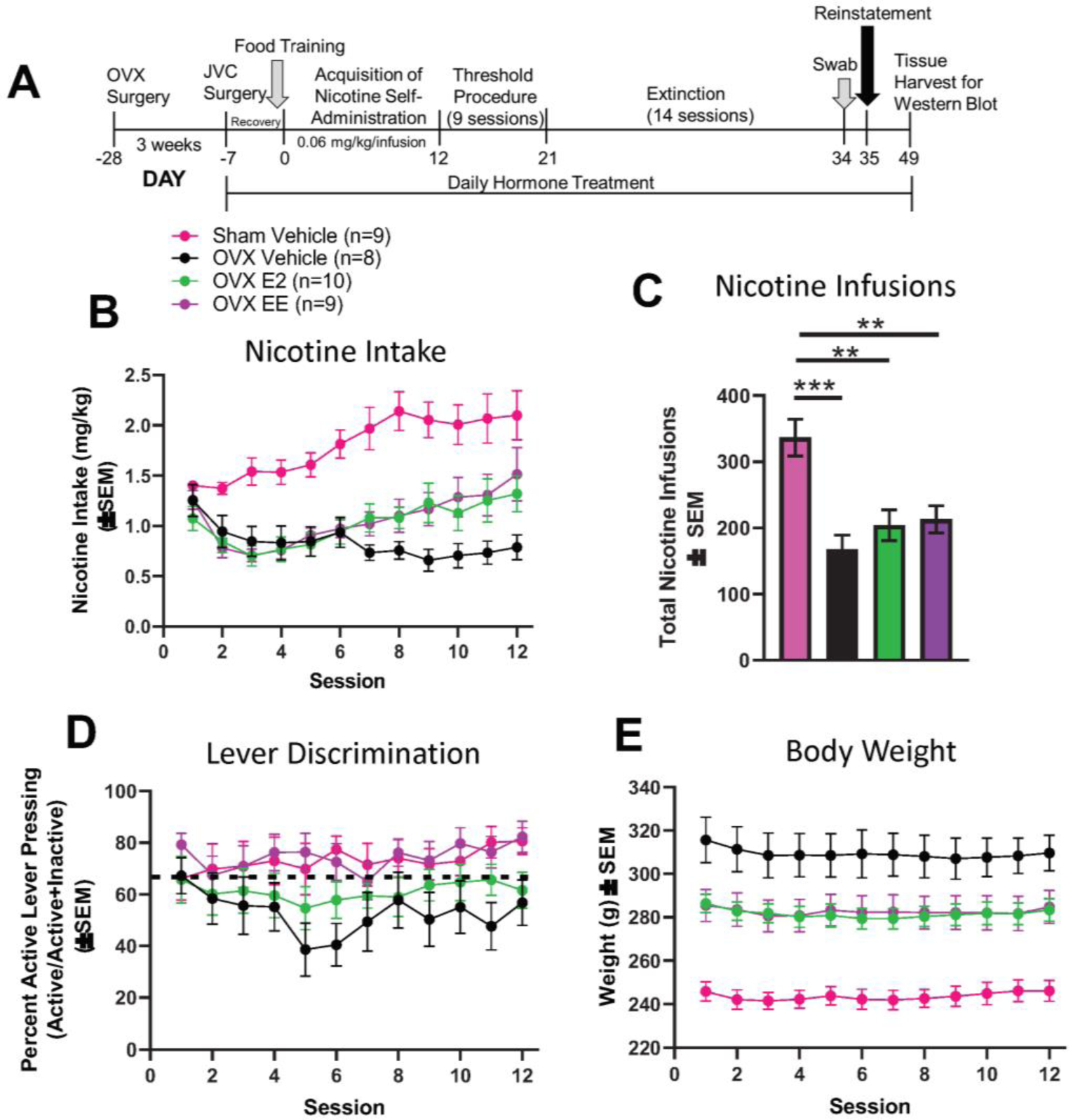
(A) Timeline of experimental events. (B) Nicotine intake per group. (C) Total nicotine infusions per group. (D) Proportion of active lever presses divided by active + inactive lever presses across the 12 sessions. The dotted line represents the 2:1 active:inactive lever press criterion. (E) OVX vehicle females weighed significantly more than the other three groups across the nicotine self-administration sessions. OVX-E2 and OVX-EE groups weighed significantly more than sham vehicle females. SEM = standard error of the mean; OVX = ovariectomized; JVC = jugular vein catheter; g = grams.
During the threshold procedure in the demand phase, 9 different doses of nicotine were given across 9 consecutive days of self-administration. Doses were altered by modulating the infusion length as previously published (Powell et al., 2020), with no timeout during these sessions. Nine different doses (0.06, 0.03, 0.017, 0.01, 0.0056, 0.003, 0.0017, 0.001, 0.00056 mg/kg/infusion nicotine) were tested, with the first dose being the same as the initial training dose (0.06 mg/kg/infusion). In addition to the reduction in infusion length, the duration of the associated cues was decreased as the nicotine dose decreased. Total session time for between-session self-administration remained at 2 hr.
Rats were then placed into extinction for 14 sessions, where neither nicotine nor cues were associated with active lever presses. All rats underwent the same number of extinction days to ensure an equal number of withdrawal days between treatments. Following the final extinction session, vaginal swabs were taken to examine the current stage of the estrous cycle in intact rats and assess vaginal cytology in OVX rats with and without hormone supplementation. The vaginal opening was swabbed (no more than 1 cm deep) with a sterile cotton swab that was dipped in sterile saline. The cells present on the swab were then pressed onto a microscope slide and examined at 10x magnification under a light microscope for analysis. Rats then underwent a cue-induced reinstatement session, in which previously paired nicotine cues (light and tone) were presented upon an active lever response; however, no nicotine infusions were delivered.
2.5. Drugs and chemicals
Nicotine tartrate salt (MP Biomedicals, LLC, Solon, OH, USA) was dissolved in 0.9% saline, and pH was adjusted to 7.4. All nicotine doses utilized were based on free base weight and expressed as mg/kg/infusion. Ketamine (Akorn Animal Health, Lake Forest, IL), xylazine (Akorn Animal Health, Lake Forest, IL), cefazolin (Qilu Pharmaceutical, Shandong, China), meloxicam (Norbrook Inc., Overbrook, KS), and heparin (Sagent Pharmaceuticals, Schaumburg, IL) were administered as discussed above. Hormones (E2 and EE) as well as sesame oil vehicle were purchased from Sigma Aldrich, St. Louis, MO.
2.6. Statistical Analysis
For self-administration acquisition, infusions, and lever presses (active and inactive) across the first 12 sessions were collected and analyzed using linear mixed effects (LME) modeling using JMP software from SAS. In all experiments, random factors were limited to subject. Total number of infusions per animal during acquisition was also summed across sessions and groups were compared using LME. LME was chosen in lieu of ANOVA based on Bayesian Information Criteria (BIC a metric of goodness of fit) and due to known efficiency of this model for handling continuous variable data such as those reported here(Young et al., 2009). For the demand phase following acquisition, daily infusions and responses were recorded. Consumption was calculated by multiplying the number of infusions earned by dose delivered, and demand curves were calculated according to the exponential demand equation (Hursh and Silberberg, 2008):
where Q represents consumption, Q0 represents the estimated maximum consumption at a zero-unit price point, αrepresents the rate of change of consumption, C is unit price, and k is a constant. Demand curves were fit to the data via nonlinear mixed effects (NLME) modeling in R using the ‘nlme’ package (Hofford et al., 2016; Powell et al., 2020). The global constant k had a best-fit value of 1.8 for all experiments. During extinction, lever pressing on active and inactive levers across sessions was compared between groups via LME. LME modeling was also used to analyze reinstatement active and inactive lever press data compared to session 14 of extinction. Session was treated as a continuous factor in all analyses. Post-hoc tests were conducted where appropriate and corrected using Tukey’s test (α = 0.05). Graphing was performed in Prism 8.1 (Graphpad Software, San Diego, CA). LME modeling was conducted in JMP software from SAS. Data are represented as means ± standard error of the mean (SEM) where appropriate.
3. Results
3.1. Nicotine Self-Administration Acquisition
Nicotine consumption data from all animals were included in LME modeling regardless of level of acquisition. An LME model with session (continuous) as a fixed within-subject factor, group (nominal) as a fixed between-subject factor, and subject as a random factor indicated significant main effects of session (F1,32 = 17.41, p < 0.001) and group (F3,32 = 12.50, p < 0.0001), as well as a significant session x group interaction (F3,32 = 7.52, p < 0.001; Figure 1B) were found. Post-hoc comparisons revealed that the change in the rate of intake in the OVX vehicle group was lower than all other groups (measured as a difference in slope). Next, the proportion of active lever presses [active/(active+inactive)] was compared between groups, and no significant main effects or interactions were found (p’s > 0.05; Figure 1C).
Body weights were monitored throughout acquisition and then compared between groups using LME modeling (Figure 1D). An LME model with session (continuous) as a fixed within-subject factor, group (nominal) as fixed between-subject factor and, subject as a random factor indicated a significant main effect of group (F3,32 = 15.79, p < 0.0001), but no main effect of session or group × session interaction (p’s > 0.05). The lack of interaction indicates that regardless of group, body weight did not change as a function of nicotine self-administration session. However, hormone supplemental treatment attenuated body weight gain after OVX as a function of hormone supplemental treatment, whereby vehicle-treated OVX females had the highest body weights, relative to EE- and E2- treated OVX females as well as sham rats. However, E2 and EE administration after OVX did not decrease body weights to the level of the ovary-intact, Sham group. Previous work has demonstrated robust weight gain in female rats after OVX compared to Sham (Brutman et al., 2019). Thus, weight gain by OVX animals and partial return to Sham weight by EE- and E2- treated OVX animals also served as confirmation of OVX and hormonal manipulation.
3.2. Nicotine Demand
Demand curves by group are shown in Figure 2A. NLME analysis revealed a significant main effect of group on demand intensity (Q0; F3,272 = 10.81; p < 0.0001), with increased demand intensity for the sham vehicle group compared to all OVX treatment groups, regardless of estrogen supplementation, and decreased demand intensity for the OVX vehicle group compared to all other treatment groups. For demand elasticity (α), no significant effect of group was found (p > 0.05). The proportion of active presses [active/(active + inactive)] during the demand phase was then analyzed by group. No effects or interactions were found (p’s > 0.05; Figure 2B), and all groups displayed higher proportions than chance (above 50%), indicating that all animals maintained the ability to discriminate between levers regardless of unit price. Specific Q0 and α values for each group are shown in the table in Figure 2C (mean ± SEM). These results indicate that hormone manipulation significantly impacted estimated maximum consumption at zero unit price without changing demand elasticity, and did not reduce lever press discriminability regardless of changes in nicotine unit price. Thus, cessation of cycling hormones via OVX decreases demand intensity for nicotine compared to intact, freely cycling females, and supplemental chronic EE or E2 treatment after OVX increases nicotine demand intensity above OVX vehicle levels. No differences in demand elasticity were found, indicating that the rate of change in nicotine consumption as unit price increases does not differ as a function of hormone manipulation.
Figure 2.
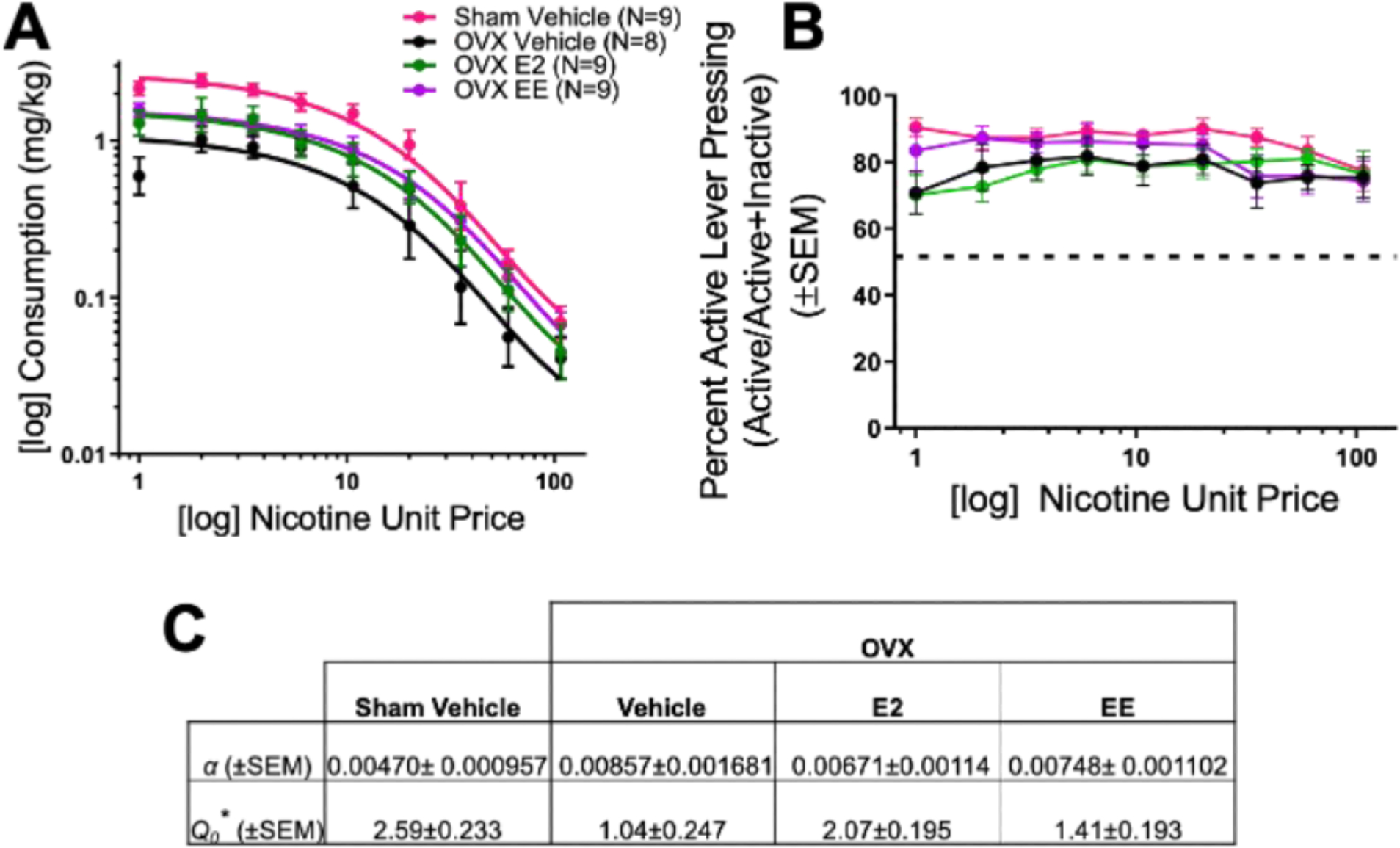
Nicotine demand data for sham and OVX groups are shown in (A), with the percent active lever pressing (active lever presses divided by active + inactive lever presses) across increasing unit price during the demand phase (B; the dotted line represents chance at 50%). (C) Parameter estimates for Q0 and α (asterisks indicates significant main effect of group, p < 0.0001). SEM = standard error of the mean; OVX = ovariectomized.
3.3. Extinction and Reinstatement
Following the threshold procedure, all subjects were placed into extinction (Figure 3A). An LME model with session (continuous) and lever (nominal) as a fixed within-subject factors, group (nominal) as a fixed between-subject factor, and subject as a random factor indicated a significant main effect of session (F1,31 = 15.53; p < 0.001), lever (F1,31 = 38.41; p < 0.0001), and session × lever interaction (F1,31 = 9.05; p < 0.01). No other main effects or interactions were significant (p’s > 0.05). Reinstatement data were analyzed using a 3-way LME model. Significant main effects of group (F3,31 = 9.33; p < 0.001), session (F1,31 = 81.30; p < 0.0001), and lever (F1,31 = 133.94; p < 0.0001) were found. Further, group × session (F3,31 = 7.31; p < 0.001), group × lever (F3,31 = 5.33; p < 0.01), and session × lever (F1,31 = 72.46; p < 0.0001) interactions were significant. Finally, the group × session × lever interaction was significant (F3,31 = 6.10; p < 0.01). Tukey post-hoc comparisons revealed numerous significant differences comparing all group, lever, and session conditions, some of which are depicted in Figures 3B and 3C. These figures demonstrate significant increases in active lever pressing in the sham vehicle and OVX E2 groups during reinstatement compared to their respective final extinction session (Figure 3B). Further, active lever pressing was significantly higher in the sham vehicle group versus all other groups, as well as in the OVX E2 group versus both the OVX vehicle and the OVX EE groups (Figure 3C). All groups pressed the active lever significantly more than the inactive lever during reinstatement. Taken together, OVX-induced ovarian hormone loss decreased cue-induced nicotine seeking behavior compared to intact, freely cycling females, and E2 administration after OVX rescued nicotine seeking behavior to sham vehicle levels, whereas EE administration after OVX did not.
Figure 3. Effects of Hormones on Extinction and Nicotine-Seeking Behavior.
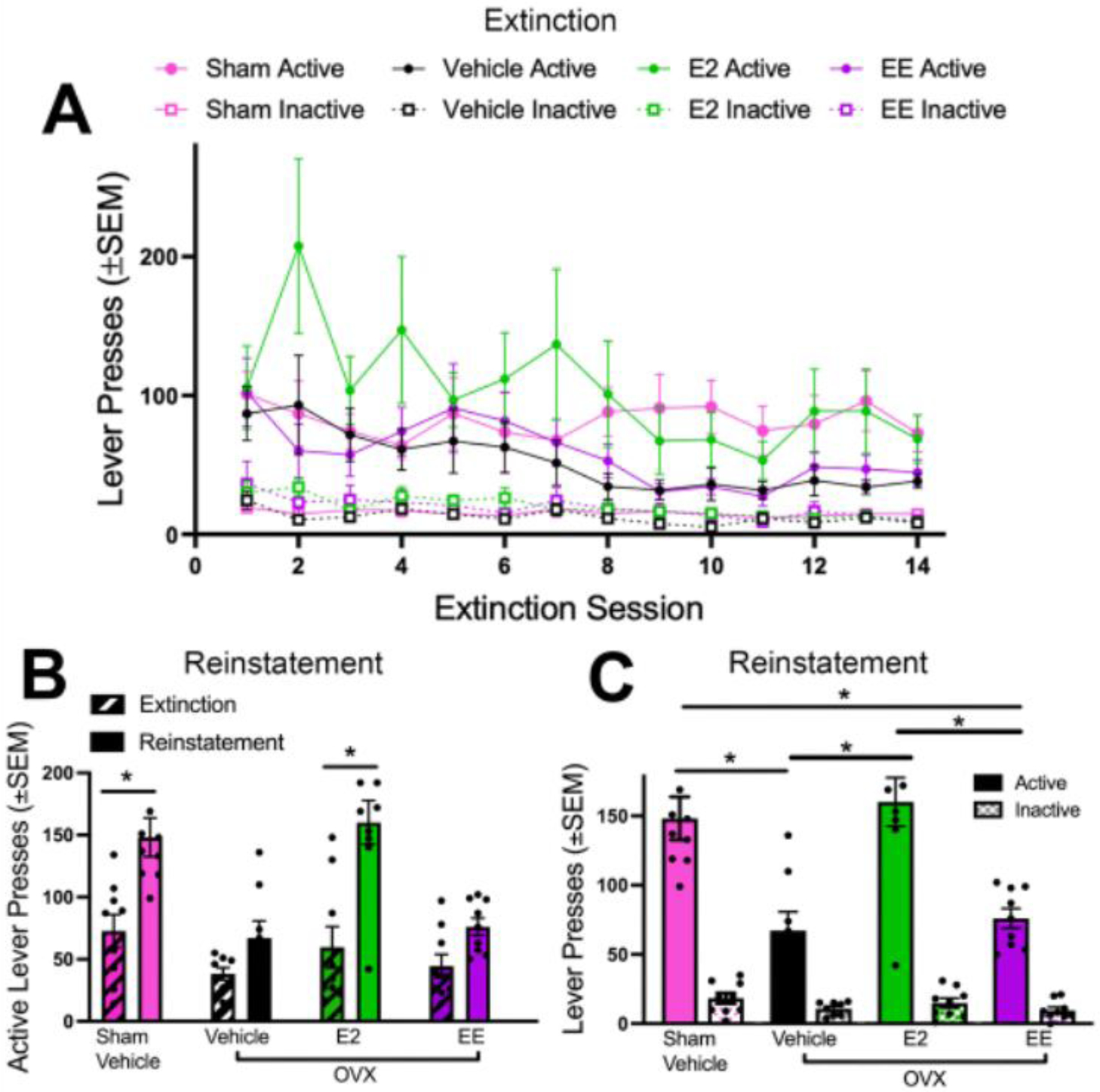
(A) Active and inactive lever pressing for all groups across extinction sessions. (B) Active lever pressing during the final extinction session (session 14) and reinstatement were compared for all groups. Only the sham vehicle and OVX E2 groups significantly reinstated above extinction levels. (C) Active and inactive lever pressing during reinstatement was compared across groups. Sham vehicle animals pressed the active lever significantly more than OVX vehicle and OVX EE groups. OVX E2 females pressed the active lever significantly more than OVX vehicle and OVX EE animals. No differences were found in inactive lever pressing between groups. All groups pressed the active lever significantly more than the inactive lever during reinstatement. *p < 0.05 in (B) and (C). SEM = standard error of the mean; OVX = ovariectomized.
4. Discussion
Here, we show that the abrupt loss of ovarian hormones due to OVX significantly diminished nicotine intake relative to ovary-intact, freely cycling females. Further, supplemental E2 and EE treatment after OVX increased nicotine intake following several weeks of hormone exposure. Interestingly and contrary to our hypothesis, EE did not increase nicotine intake during initial acquisition above and beyond E2 levels. Demand curves demonstrated that OVX without estrogen supplementation significantly decreased Q0 compared to all other groups, indicating that circulating hormones are critical for driving maximum nicotine consumption. Importantly, this parameter provides an estimated amount of nicotine an animal would take if the commodity was free, demonstrating intensity of demand. During the demand phase, all subjects discriminated the active and inactive levers, demonstrating that regardless of hormone treatment group and nicotine unit price, discriminability was maintained. That all subjects showed systematically decreased consumption as unit price increased supports that all groups were equally sensitive to changes in nicotine unit price when it was manipulated by dose. Importantly, these data support that nicotine functioned as a reinforcer in all treatment groups. There were no differences in extinction learning between groups. All groups displayed higher active lever pressing than in other studies utilizing one unit price of nicotine (Goenaga et al., 2020; Namba et al., 2020); however, lever pressing was similar to our previous study utilizing the threshold procedure (Powell et al., 2020). Here we showed that OVX abolished reinstated nicotine seeking. Interestingly, there was a dissociation between nicotine intake measures and cue-driven nicotine seeking. Specifically, the sham vehicle and OVX E2 groups showed similar reinstatement levels and were the only two groups to show enhanced active lever pressing relative to their respective extinction active lever responding. Surprisingly, EE did not rescue nicotine seeking behavior as observed with E2 supplementation indicating that E2 and EE have unique effects on behavioral outcomes, despite both being characterized as estrogens.
4.1. Ovarian Hormones, EE, and Nicotine Self-Administration
Preclinical and clinical studies indicate that ovarian hormones play a large role in driving nicotine use. However, very little attention has been paid to the impact of ovarian hormones and synthetic estrogen on nicotine self-administration behavioral outcomes. Group differences in the current study are in line with a previous report that OVX females self-administer significantly less nicotine than intact or E2-supplemented groups (Flores et al., 2016). However, this previous study utilized supraphysiological doses of E2 as opposed to the dose utilized here (also see Martinez et al., 2016). In the current experiment estrogens were administered daily, which is more akin to a woman’s experience with the daily dosing of many oral contraceptives as compared to a regimen that is tonic. It is important to acknowledge that in addition to an estrogen, oral contraceptives contain a progestin, and that the intact female reproductive system involves fluctuations of estrogens as well as other steroid hormones, such as progesterone and androstenedione. Thus, future studies would benefit from systematically evaluating the contributions of these additional ovarian hormones to nicotine behavioral outcomes as well as examining the putative complex relationships amongst them. Although the beneficial results of progesterone in decreasing nicotine seeking in female rats is unclear (Swalve et al., 2016), progesterone supplementation has shown clinical utility in decreasing smoking-related outcomes such as craving (Sofuoglu et al., 2001). Thus, future studies evaluating the contribution of progestogens, both alone and in combination with E2 or EE, on nicotine behaviors will help elucidate the impact of endogenous hormone fluctuations associated with various reproductive shifts such as with the menstrual cycle and menopause, as well as the putative treatment utility of exogenous hormone applications such as hormone-including birth control.
Our results show that daily exposure to EE does not increase nicotine self-administration outcome measures in OVX females above and beyond levels of intact sham females. However, similar Q0 levels of nicotine consumption were observed in both the OVX EE and OVX E2 groups. In contrast, only E2 increased reinstatement above OVX vehicle levels, similar to reinstated nicotine seeking in the sham vehicle group. OVX abolished reinstatement of nicotine seeking, and EE administration after OVX did not reverse this effect. It is important to note that oral contraceptives are typically prescribed to reproductive tract intact, freely cycling women, and they typically contain a progestin component. Thus, it is unclear if a combination of EE plus a progestin in an intact system would alter nicotine-related outcomes, and future studies should methodically evaluate the most commonly used progestins (various forms of synthetic progesterone also found in oral contraceptives and hormone therapy) to provide additional translational value.
Nicotine demand was systematically evaluated as a function of ovary-intact status or hormone supplementation following OVX. Although demand intensity varied significantly as a function of hormone group (i.e., how much of the drug is preferred at a zero or very low cost), the price-dependent measure of elasticity (α) did not. These results are similar to other studies examining relationships between demand of other commodities such as food with biological processes such as body mass index (Epstein et al., 2018). Further, reductions in demand intensity predict drinking reductions one month after intervention, demonstrating the demand intensity measure as a potentially useful predictor for intervention response to other drugs of abuse such as alcohol (Murphy et al., 2015). Taken together, these results indicate that price-dependent measures such as elasticity of demand may not have utility in predicting individual differences in hormone interactions with nicotine, but measures of demand intensity may yield important translational results for studies examining smoking and endocrine interactions in women.
4.2. Reinstatement to Nicotine-Conditioned Cues Varies as a Function of Hormone Treatment
Our previous study demonstrated that intact, freely cycling female rats significantly reinstate to nicotine-conditioned cues (Goenaga et al., 2020). Here, we show that reinstatement of nicotine seeking behavior is significantly higher in ovary-intact, sham vehicle rats compared to OVX vehicle-treated rats. Further, there were differing results for nicotine reinstatement depending on hormone treatment group, contrary to our hypothesis that EE-treated OVX females would reinstate above and beyond all other groups. Rather, EE-treated females did not significantly reinstate to nicotine-conditioned cues, whereas E2-treated OVX females did. These results were surprising given their dissociation with nicotine intake outcomes, in which both nicotine self-administration acquisition and Q0 levels of consumption did not differ between these two groups. Further, there were no significant differences in extinction responding between the E2- and EE-treated OVX groups across sessions, although it is possible that differential impacts on extinction due to varied effects of E2 versus EE may be dose dependent. It is also possible that nicotine intake, extinction learning, and responding for nicotine-conditioned cues are gated by different neurobehavioral mechanisms, and further research is needed to understand these complex relationships. In sections 4.3 and 4.4, we describe potential biological and neurobiological mechanisms that may contribute to differences in nicotine seeking behavior found in the current study.
4.3. Biological Differences between E2 and EE
Here, we show differences in reinstatement of nicotine seeking between OVX animals treated with either E2 or EE. These hormones do have overlapping biological impacts, as both E2 and EE are rapidly absorbed following oral administration of therapeutic doses (Fishman, 1969; Fotherby, 1982), and both hormones bind to ERs via the same binding domain. However, there are pharmacokinetic differences between these two estrogens that may underlie differences seen between E2- and EE- treated groups. For example, it has been shown that EE is a more potent estrogen than E2 (Dickson and Eisenfeld, 1981; Fotherby, 1996). Further, previous work evaluating the binding mechanisms of E2 and EE shows that E2 is 100-fold less active compared with EE, and that higher doses of oral E2 are required to have the same effects as those seen with EE (Dickson and Eisenfeld, 1981). E2 and EE are also metabolized differently. E2 is converted into weaker estrogens, such as estrone (E1). This process is interconvertible, and E2 has been shown to be regenerated from pools of E1 and its downstream metabolite, E1 sulfate (E1S) (Stanczyk et al., 2013). Conversely, EE is not metabolized by the same mechanisms and is not converted into weaker estrogens. Instead of converting into biologically available E1, EE metabolites are not biologically active and are excreted (Stanczyk et al., 2013; Zhang et al., 2007). These differences in potency and metabolism of E2 versus EE may be contributing to the differences in reinstatement observed between EE- and E2- treated OVX animals. Additional research is needed to determine if variations in potency and metabolism between these two estrogens contribute to differences in nicotine seeking behavior.
It should also be noted that both E2 and EE have known interactions with G-protein estrogen receptors (GPERs), such as GPR30 (Yates et al., 2010). Specifically, EE has been previously shown to reduce severity of autoimmune encephalomyelitis (Subramanian et al., 2003), but a later study found that E2 but not EE was able to reduce autoimmune encephalomyelitis in GPR30 knockout mice. This study by Yates and colleagues therefore demonstrates that this receptor is critical in mediating EE’s ability to reduce disease. Although not measured in the current study, it is possible that OVX reduced GPER expression, and this may have impacted nicotine-related behavioral outcomes such as reinstatement. Further, given that there is evidence in the literature that differential outcomes can occur on disease states following E2 versus EE treatment, and this is mediated through GPERs, it is possible that the differential outcomes of E2 and EE on reinstatement reported here could be mediated through GPER signaling. In further support of this possibility, GPR30 has been shown to co-localize on cholinergic neurons and modulate acetylcholine release (Gibbs et al., 2014; Hammond et al., 2011; Hammond and Gibbs, 2011). Further, we have recently shown that cholinergic interneurons (ChIs) within the NAcore regulate nicotine seeking behavior as well as NAcore glutamate plasticity (Leyrer-Jackson et al., 2021). Thus, it is possible that OVX reduced acetylcholine release from ChIs through downregulation of GPR30 in the NAcore, and this contributed to reductions in nicotine-associated behaviors including consumption and seeking. It should be noted, however, that GPR30 expression in the NAcore is not always present between individual rats and it does not appear to be expressed in the VTA (Hazell et al., 2009), thus this may not be a viable possibility to explain our findings. Regardless, these are important questions for future research.
4.4. Potential Neurobiological Mechanisms
Our results show differences in nicotine demand behavior between Sham OVX, OVX vehicle, and OVX EE- or E2- treated groups. Nicotine activates nicotinic acetylcholine receptors (nAChRs) localized on dopamine cell bodies in the ventral tegmental area (VTA; Picciotto et al., 1998) and alters VTA dopaminergic signaling (Mansvelder et al., 2002; Mansvelder and McGehee, 2002). Within the core of the nucleus accumbens (NAcore), dopamine receptors (DRs) are expressed on medium spiny neurons and have been implicated in motivated behavior (Gallo et al., 2018), including drug seeking (Bock et al., 2013). Historically, studies designed to unravel the neural circuitry underlying drug use and seeking have relied on data collected from males, and do not typically focus on endocrine interactions with these neural pathways. Given that E2 has been shown to increase sensitivity of VTA dopaminergic neurons (Vandegrift et al., 2017) and directly regulates cell signaling within the striatum (Becker, 1990; Mermelstein et al., 1996; Xiao and Becker, 1998), it is biologically feasible that the female reproductive cycle and estrogens can impact vulnerability to nicotine use and related circuitry.
Estrogen receptors (ERs) are expressed throughout the brain, including on neurons within the striatum (Boulware et al., 2007; Grove-Strawser et al., 2010), and have the ability to alter reward circuitry and influence motivated behaviors (Eisinger et al., 2018). In supplemental studies included here, we show NAcore and VTA ER-β was significantly downregulated in the OVX vehicle-treated group compared to the sham vehicle and OVX E2 groups. Further, neither E2 nor EE rescued downregulated ER-β protein in OVX animals within the VTA compared to sham vehicle-treated animals, suggesting that other ovarian-derived factors or signaling may be important for sustaining ER-β levels in the VTA. No differences were found in ER-α protein expression across groups within either brain region. This result indicates that while ER-β expression in the NAcore is E2-specific, expression in the VTA may rely on additional factors. For example, previous research has shown that progesterone and E2 interactions are critical for ligand binding (Becker et al., 1984) and that progesterone can decrease smoking urges and prevent relapse in women (Saladin et al., 2014). Additional research is needed to determine the impact of various ovarian hormones, individually and in concert, on ER expression levels.
Here we show that OVX downregulated ER-β in critical nodes within the mesolimbic reward pathway, as noted above. Given that OVX occurred weeks prior to nicotine self-administration to allow for reductions in circulating ovarian hormones, it is likely that downregulation of ER-β occurred prior to nicotine consumption and played a role in decreasing consumption as well as other behaviors such as reinstatement. The justification for this timeline is to allow for systematic examination of specific estrogens on subsequent nicotine-related behavior in a system cleared of circulating ovarian hormones. It should be noted, however, that while OVX is generally accepted as a surgical model of menopause ((Koebele and Bimonte-Nelson, 2016); as is the 4-vinylcyclohexene diepoxide model of transitional menopause (Flaws et al., 1994)) as well as a frequently utilized technique to understand hormonal mechanisms on the brain and behavior (Hiroi et al., 2016; Mennenga et al., 2015), the use of OVX here as well as the extended timeline in which ovarian hormones significantly dropped may limit translational utility of the current study. Thus, future studies should examine contributions of specific ERs (e.g., through viral knockdown) on nicotine neurobiology and behavior in an ovary-intact system to enhance translational value.
4.5. Ovarian Hormones Regulate Body Weight
In accordance with other studies, OVX induced robust weight gain in females compared to ovary-intact shams (Brutman et al., 2019), regardless of nicotine self-administration session. E2 has been shown to negatively regulate food intake (Butera, 2010; Eckel, 2011); thus, depletion of E2 may enhance consumption and, subsequently, induce weight gain. However, recent studies suggest that other factors such as gut environmental changes also contribute to OVX-induced weight gain (Babaei et al., 2010; Choi et al., 2017; Cox-York et al., 2015; Leeners et al., 2017). Specifically, Babaei et al. found no differences in food intake between Sham and OVX animals, but they did observe increases in visceral fat in the OVX group that was reversed with E2 treatment (Babaei et al., 2010). We report similar results here; indeed, despite animals receiving and consuming the same amount of food throughout behavioral testing, only OVX animals gained weight as compared to the E2- and EE-treated OVX groups. These results suggest that OVX-induced weight gain may be, in part, controlled independently of caloric intake.
5. Potential Limitations
One potential limitation to this study is that limited access to nicotine was utilized (i.e., 2-hr sessions). Importantly, others have shown that extended access to nicotine under an intermittent access schedule paradigm may induce escalations in consumption (Cohen et al., 2012). Thus, the results presented here reflect hormonal influences on short access to nicotine and may not generalize to other models of nicotine addiction. Additionally, the present study utilized the OVX model to examine isolated contributions of specific hormones to nicotine consumption and reinstated nicotine seeking. It should be noted that EE is typically prescribed to ovary-intact women; thus, the questions addressed here should be evaluated in ovary-intact female rats to maximize translation. Moreover, if a woman has a uterus and is given estrogens, the hormone regimen must also contain a progestogen to protect against estrogen-induced endometrial hyperplasia and cancer (North American Menopause Society, 2017). Finally, it also important to note that studies suggest smoking increases estrogen metabolism in women (Mueck and Seeger, 2005). Although these specific effects were not evaluated here, this is an important area of research to be explored in future studies.
Finally, another potential limitation is the use of food restriction in the current study. It should be noted that food restriction enhances drug self-administration (De Vaca and Carr, 1998) including nicotine, and this is a frequently employed technique in nicotine self-administration paradigms (Donny et al., 1998) which impacts the motivational state of animals including rats. Thus, it is likely that food restriction in the current study impacted nicotine consumption behaviors, and additional studies are needed to determine if E2 and EE impact nicotine-related behavior in animals under free feeding conditions.
6. Conclusions and Future Directions
The results from the current study indicate complex interactions between natural and synthetic estrogens and nicotine-related behavior. It is known that in clinical trials for smoking, women are usually under-enrolled (Tomko et al., 2020), and are typically required to be on some form of birth control (Gray et al., 2010; Prado et al., 2015). Yet, our data show that chronic use of synthetic hormones affects nicotine demand and seeking in females, highlighting the need to fully characterize the complex interactions of estrogens, nicotine, and the reward pathway in females. This is especially important given the numerous putative women-specific factors which could contribute to sex differences in smoking.
Supplementary Material
Highlights.
Nicotine self-administration and demand intensity are sensitive to estrogens
17β-estradiol (E2) but not ethinyl estradiol (EE) promotes reinstatement of nicotine seeking
Abbreviations:
- EE
Ethinyl estradiol
- E2
17β-estradiol
- OVX
Ovariectomy
- VTA
Ventral tegmental area
- NAcore
Nucleus accumbens core
- DRs
Dopamine receptors
- ERs
Estrogen receptors
- ERα
Estrogen receptor alpha
- ERβ
Estrogen receptor beta
- FR1
Fixed ratio-1
- LME
linear mixed effects
- NLME
nonlinear mixed effects
- E1
Estrone
- E1S
E1 sulfate
- BIC
Bayesian Information Criteria
- ChIs
cholinergic interneurons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AM, Lundeen K, Eberly LE, Allen SS, al’Absi M, Muramoto M, Hatsukami D, 2018. Hormonal contraceptive use in smokers: Prevalence of use and associations with smoking motives. Addict. Behav 77, 187–192. 10.1016/j.addbeh.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Bade T, Center B, Finstad D, Hatsukami D, 2008. Menstrual phase effects on smoking relapse. Addiction 103, 809–821. 10.1111/j.1360-0443.2008.02146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei P, Mehdizadeh R, Ansar MM, Damirchi A, 2010. Effects of ovariectomy and estrogen replacement therapy on visceral adipose tissue and serum adiponectin levels in rats. Menopause Int. 16, 100–104. 10.1258/mi.2010.010028 [DOI] [PubMed] [Google Scholar]
- Becker JB, 1990. Direct effect of 17β-estradiol on striatum: Sex differences in dopamine release. Synapse 5, 157–164. 10.1002/syn.890050211 [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME, Robinson TE, 1984. Striatal dopamine release stimulated by amphetamine or potassium: influence of ovarian hormones and the light-dark cycle. Brain Res. 311, 157–160. 10.1016/0006-8993(84)91410-0 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P, 2006. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharmacol. Ther 79, 480–488. 10.1016/j.clpt.2006.01.008 [DOI] [PubMed] [Google Scholar]
- Bickel WK, 1999. THE ECONOMICS OF THE REINFORCING EFFECTS OF DRUGS. Behav. Pharmacol 10, S9. 10.1097/00008877-199908001-00023 [DOI] [Google Scholar]
- Bock R, Hoon Shin J, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA, 2013. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat. Neurosci 16, 632–638. 10.1038/nn.3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG, 2007. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J. Neurosci 27, 9941–9950. 10.1523/JNEUROSCI.1647-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutman JN, Sirohi S, Davis JF, 2019. Examining the Impact of Estrogen on Binge Feeding, Food-Motivated Behavior, and Body Weight in Female Rats. Obesity 27, 1617–1626. 10.1002/oby.22582 [DOI] [PubMed] [Google Scholar]
- Butcher R, Collins W, Fugo N, 1974. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94, 1704–1708. [DOI] [PubMed] [Google Scholar]
- Butera PC, 2010. ESTRADIOL AND THE CONTROL OF FOOD INTAKE. Physiol Behav. 99, 175. 10.1016/j.physbeh.2009.06.010.ESTRADIOL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT, 2006. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: A review. Nicotine Tob. Res 8, 627–638. 10.1080/14622200600910793 [DOI] [PubMed] [Google Scholar]
- Choi S, Hwang YJ, Shin MJ, Yi H, 2017. Difference in the gut microbiome between ovariectomy-induced obesity and diet-induced obesity. J. Microbiol. Biotechnol 27, 2228–2236. 10.4014/jmb.1710.10001 [DOI] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O, 2012. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology 37, 2153–2160. 10.1038/npp.2012.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-York KA, Sheflin AM, Foster MT, Gentile CL, Kahl A, Koch LG, Britton SL, Weir TL, 2015. Ovariectomy results in differential shifts in gut microbiota in low versus high aerobic capacity rats. Physiol. Rep 3, 1–14. 10.14814/phy2.12488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vaca SC, Carr KD, 1998. Food restriction enhances the central rewarding effect of abused drugs. J. Neurosci 18, 7502–7510. 10.1523/jneurosci.18-18-07502.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni D, Skee D, Vaccaro N, Massarella J, Janssens L, LaGuardia KD, Leung AT, 2007. Pharmacokinetics and pharmacodynamics of a transdermal contraceptive patch and an oral contraceptive. J. Clin. Pharmacol 47, 497–509. 10.1177/0091270006297919 [DOI] [PubMed] [Google Scholar]
- Dickson R, Eisenfeld A, 1981. 17 Alpha-ethinyl estradiol is more potent than estradiol in receptor interactions with isolated hepatic parenchymal cells. Endocrinology 108, 1511–1518. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF, 1998. Acquisition of nicotine self-administration in rats: The effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl). 136, 83–90. 10.1007/s002130050542 [DOI] [PubMed] [Google Scholar]
- Eckel LA, 2011. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 104, 517–524. 10.1016/j.physbeh.2011.04.014.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Carr KA, Temple JL, Bickel WK, MacKillop J, 2018. Reinforcing value and hypothetical behavioral economic demand for food and their relation to BMI. Eat. Behav 29, 120–127. 10.1016/j.eatbeh.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J, 1969. Intermediates in the transformation of oral estradiol. J. Endocrinol. Metab 29, 41–46. [DOI] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB, 1994. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reprod. Toxicol 8, 509–514. 10.1016/0890-6238(94)90033-7 [DOI] [PubMed] [Google Scholar]
- Flores RJ, Pipkin JA, Uribe KP, Perez A, O’Dell LE, 2016. Estradiol promotes the rewarding effects of nicotine in female rats. Behav. Brain Res 307, 258–263. 10.1016/j.bbr.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotherby K, 1996. Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy. Contraception 54, 59–69. 10.1016/0010-7824(96)00136-9 [DOI] [PubMed] [Google Scholar]
- Fotherby K, 1982. Pharmacokinetics of ethynyloestradiol in humans. Methods Find. Exp. Clin. Pharmacol 4, 133–141. [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR, 2008. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: A retrospective analysis. J. Women’s Heal 17, 287–292. 10.1089/jwh.2007.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS, Moore H, Javitch JA, Kellendonk C, 2018. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat. Commun 9. 10.1038/s41467-018-03272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Hammond R, 2014. Role of GPR30 in mediating estradiol effects on acetylcholine release in the hippocampus. Horm. Behav 66, 339–345. 10.1016/j.yhbeh.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenaga J, Powell GL, Leyrer-Jackson JM, Piña J, Phan S, Prakapenka AV, Koebele SV, Namba MD, McClure EA, Bimonte-Nelson HA, Gipson CD, 2020. N-acetylcysteine yields sex-specific efficacy for cue-induced reinstatement of nicotine seeking. Addict. Biol 25, 1–11. 10.1111/adb.12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Carpenter MJ, LaRowe SD, 2010. N-Acetylcysteine (NAC) in Young Marijuana Users: An Open-Label Pilot Study. Am. J. Addict 19, 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG, 2010. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170, 1045–1055. 10.1016/j.neuroscience.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Gibbs RB, 2011. GPR30 is Positioned to Mediate Estrogen Effects on Basal Forebrain Cholinergic Neurons and Cognitive Performance R. Brain Res. 1379, 53–60. 10.1016/j.brainres.2010.11.098.GPR30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB, 2011. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychone 36, 182–192. 10.1016/j.psyneuen.2010.07.007.GPR30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell GGJ, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ, 2009. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J. Endocrinol 202, 223–236. 10.1677/JOE-09-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderaker K, Allen AM, Tosun N, al’Absi M, Hatsukami D, Allen SS, 2015. The effect of combination oral contraceptives on smoking-related symptomatology during short-term smoking abstinence. Addict. Behav 41, 148–151. 10.1016/j.addbeh.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, Weyrich G, Koebele SV, Mennenga SE, Talboom JS, Hewitt LT, Lavery CN, Mendoza P, Jordan A, Bimonte-Nelson HA, 2016. Benefits of hormone therapy estrogens depend on estrogen type: 17ß-estradiol and conjugated equine estrogens have differential effects on cognitive, anxiety-like, and depressive-like behaviors and increase tryptophan hydroxylase-2 mRNA levels in dorsal raphe nucleus subregions. Front. Neurosci 10, 1–20. 10.3389/fnins.2016.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Beckmann JS, Bardo MT, 2016. Rearing environment differentially modulates cocaine self-administration after opioid pretreatment: A behavioral economic analysis. Drug Alcohol Depend. 167, 89–94. 10.1016/j.drugalcdep.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychol. Rev 115, 186–198. 10.1037/0033-295X.115.1.186 [DOI] [PubMed] [Google Scholar]
- Kavanaugh ML, Jerman J, 2018. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception 97, 14–21. 10.1016/j.contraception.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson HA, 2016. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 87, 5–17. 10.1016/j.maturitas.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeners B, Geary N, Tobler PN, Asarian L, 2017. Ovarian hormones and obesity. Hum. Reprod. Update 23, 300–321. 10.1093/humupd/dmw045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyrer-Jackson JM, Holter M, Overby PF, Newbern JM, Scofield MD, Foster Olive M, Gipson CD, 2021. Accumbens cholinergic interneurons mediate cue-induced nicotine seeking and associated glutamatergic plasticity. eNeuro 8, 1–14. 10.1523/ENEURO.0276-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyrer-Jackson JM, Overby PF, Bull A, Marusich JA, Gipson CD, 2020a. Strain and sex matters: Differences in nicotine self-administration between outbred and recombinase-driver transgenic rat lines. Exp. Clin. Psychopharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyrer-Jackson JM, Piña JA, McCallum J, Foster Olive M, Gipson CD, 2020b. Direct administration of ifenprodil and citalopram into the nucleus accumbens inhibits cue-induced nicotine seeking and associated glutamatergic plasticity. Brain Struct. Funct 225, 1967–1978. 10.1007/s00429-020-02103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, 2010. Role of Progesterone in Nicotine Addiction: Evidence From Initiation to Relapse. Exp. Clin. Psychopharmacol 18, 451–461. 10.1037/a0021265.Role [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS, 2002. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33, 905–919. 10.1016/S0896-6273(02)00625-6 [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS, 2002. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol 53, 606–617. 10.1002/neu.10148 [DOI] [PubMed] [Google Scholar]
- Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Foster Olive M, Carroll ME, Meisel RL, Mermelstein PG, 2016. Estradiol facilitation of cocaine Self-Administration in female rats requires activation of mglur5. eNeuro 3. 10.1523/ENEURO.0140-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennenga S, Bimonte-Nelson HA, 2015. The importance of incorporating both sexes and embracing hormonal diversity when conducting rodent behavioral assays. In: The Maze Book: Theories, Practice, and Protocols for Testing Rodent Cognition., Springer Protocols. Humana Press: New York. [Google Scholar]
- Mennenga SE, Gerson JE, Koebele SV, Kingston ML, Tsang CWS, Engler-Chiurazzi EB, Baxter LC, Bimonte-Nelson HA, 2015. Understanding the cognitive impact of the contraceptive estrogen Ethinyl Estradiol: Tonic and cyclic administration impairs memory, and performance correlates with basal forebrain cholinergic system integrity. Psychoneuroendocrinology 54, 1–13. 10.1016/j.psyneuen.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Backer JB, Surmeier DJ, 1996. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci 16, 595–604. 10.1523/jneurosci.16-02-00595.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera L, Shepherd L, Torrado AI, 2015. Comparison of Two Methods of Estradiol Replacement: their Physiological and Behavioral Outcomes. J. Vet. Sci. Technol 06. 10.4172/2157-7579.1000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueck AO, Seeger H, 2005. Smoking, estradiol metabolism and hormone replacement therapy. Curr. Med. Chem. Hematol. Agents 3, 45–54. [DOI] [PubMed] [Google Scholar]
- Murphy JG, Dennhardt AA, Martens MP, Yurasek AM, Skidmore JR, MacKillop J, McDevitt-Murphy ME, 2015. Behavioral Economic Predictors of Brief Alcohol Intervention Outcomes. J Consult Clin Psychol 83, 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba MD, Kupchik YM, Spencer SM, Garcia-Keller C, Goenaga JG, Powell GL, Vicino IA, Hogue IB, Gipson CD, 2020. Accumbens neuroimmune signaling and dysregulation of astrocytic glutamate transport underlie conditioned nicotine-seeking behavior. Addict. Biol 25, 1–13. 10.1111/adb.12797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American Menopause Society, 2017. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause 24, 728–753. 10.1097/GME.0000000000000921 [DOI] [PubMed] [Google Scholar]
- Overby PF, Daniels CW, Del Franco A, Goenaga J, Powell GL, Gipson CD, Sanabria F, 2018. Effects of nicotine self-administration on incentive salience in male Sprague Dawley rats. Psychopharmacology (Berl). 235, 1121–1130. 10.1007/s00213-018-4829-4 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR, 1999. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine Tob. Res 1, 301–315. 10.1080/14622299050011431 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP, 1998. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. 10.1038/34413 [DOI] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker Timothy B., 2007. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine Tob. Res 9, 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell GL, Beckmann JS, Marusich JA, Gipson CD, 2020. Nicotine reduction does not alter essential value of nicotine or reduce cue-induced reinstatement of nicotine seeking. Drug Alcohol Depend. 212. 10.1016/j.drugalcdep.2020.108020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado E, Maes M, Piccoli LG, Baracat M, Barbosa DS, Franco O, Dodd S, Berk M, Nunes SOV, 2015. N-acetylcysteine for therapy-resistant tobacco use disorder: A pilot study. Redox Rep. 20, 215–222. 10.1179/1351000215Y.0000000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NH, Li JWP, Strissel KJ, Obin MS, Greenberg AS, 2009. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150, 2161–2168. 10.1210/en.2008-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, McClure EA, Baker NL, Carpenter MJ, Ramakrishnan V, Hartwell KJ, Gray KM, 2014. Increasing progesterone levels are associated with smoking abstinence among free-cycling women smokers who receive brief pharmacotherapy. Nicotine Tob. Res 17, 398–406. 10.1093/ntr/ntu262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, 1998. Behavioral and neurobiological effects of estrogen replacement therapy and a history of triphasic oral contraceptive exposure. Psychoneuroendocrinology 23, 713–732. 10.1016/S0306-4530(98)00039-0 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK, 2001. Progesterone treatment during the early follicular phase of the menstrual cycle: Effects on smoking behavior in women. Pharmacol. Biochem. Behav 69, 299–304. 10.1016/S0091-3057(01)00527-5 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Mooney M, 2009. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. J. Hum. Psychopharmacol 24, 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ, Archer DF, Bhavnani BR, 2013. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: Pharmacokinetics, pharmacodynamics and risk assessment. Contraception 87, 706–727. 10.1016/j.contraception.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H, 2003. Oral Feeding with Ethinyl Estradiol Suppresses and Treats Experimental Autoimmune Encephalomyelitis in SJL Mice and Inhibits the Recruitment of Inflammatory Cells into the Central Nervous System. J. Immunol 170, 1548–1555. 10.4049/jimmunol.170.3.1548 [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME, 2016. Sex differences in attenuation of nicotine reinstatement after individual and combined treatments of progesterone and varenicline. Behav. Brain Res 308, 46–52. 10.1016/j.bbr.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RL, Baker NL, Hood CO, Gilmore AK, McClure EA, Squeglia LM, McRae-Clark AL, Sonne SC, Gray KM, 2020. Depressive symptoms and cannabis use in a placebo-controlled trial of N-Acetylcysteine for adult cannabis use disorder. Psychopharmacology (Berl). 237, 479–490. 10.1007/s00213-019-05384-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn Eisinger KR, Larson EB, Boulware MI, Thomas MJ, Mermelstein PG, 2018. Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids 133, 53–59. 10.1016/j.steroids.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek A, Olakowska E, Borecka A, Janeczek H, Sobota M, Jaworska J, Kaczmarczyk B, Jarząbek B, Gruchlik A, Libera M, Liśkiewicz A, Jędrzejowska-Szypułka H, Kasperczyk J, 2016. Shape-Memory Terpolymer Rods with 17-β-estradiol for the Treatment of Neurodegenerative Diseases: an In Vitro and In Vivo Study. Pharm. Res 33, 2967–2978. 10.1007/s11095-016-2019-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift BJ, You C, Satta R, Brodie MS, Lasek AW, 2017. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS One 12, 1–18. 10.1371/journal.pone.0187698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Franklin TR, Allen SS, 2016. Ovarian Hormones, Menstrual Cycle Phase, and Smoking: a Review with Recommendations for Future Studies. Curr. Addict. Reports 3, 1–8. 10.1007/s40429-016-0093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB, 1998. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse 29, 379–391. [DOI] [PubMed] [Google Scholar]
- Yates MA, Li Y, Chlebeck PJ, Offner H, 2010. GPR30, but not estrogen receptor-α, is crucial in the treatment of experimental autoimmune encephalomyelitis by oral ethinyl estradiol. BMC Immunol. 11. 10.1186/1471-2172-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Clark MH, Goffus A, Hoane MR, 2009. Mixed effects modeling of Morris water maze data: Advantages and cautionary notes. Learn. Motiv 40, 160–177. 10.1016/j.lmot.2008.10.004 [DOI] [Google Scholar]
- Yousefzadeh N, Kashfi K, Jeddi S, Ghasemi A, Physiology E, Sciences B, Education B, Physiology E, Street A, Blvd D, 2020. Review article : ovariectomised rat model of osteoporosis. EXCLI J. 19, 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cui D, Wang B, Han YH, Balimane P, Yang Z, Sinz M, Rodrigues AD, 2007. Pharmacokinetic drug interactions involving 17α-ethinylestradiol: A new look at an old drug. Clin. Pharmacokinet 46, 133–157. 10.2165/00003088-200746020-00003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


