Abstract
Cutting propagation is widely used in establishing poplar plantations, and this approach requires efficient adventitious root (AR) forming capacities. Although poplar species are considered to form roots easily, interspecific variations in AR formation are still observed. To better understand the gene regulatory network underlying the conserved modified pathways that are essential for AR formation in poplar species, comparative transcriptomic approaches were applied to identify the conserved common genes that were differentially expressed during the AR formation processes in two poplar species (Populus × euramericana and P. simonii) in woody plant medium (WPM). A total of 2146 genes were identified as conserved genes that shared similar gene expression profiles in at least one comparison. These conserved genes were enriched in diverse hormone signaling pathways, as well as the mitogen-associated protein kinase (MAPK) signaling pathway, suggesting an important role for signaling transduction in coordinating external stimuli and endogenous physiological status during AR regulation in poplar. Furthermore, the co-expression network analysis of conserved genes allowed identification of several co-expressed modules (CM) that are co-expressed with distinct biological functions, for instance, CM1 was enriched in defense response and hormone signaling, CM2 and CM3 were overrepresented in defense response-related pathways and for cell cycle, respectively. These results suggest that the AR formation processes in poplar were finely tuned at the transcriptomic level by integrating multiple biological processes essential for AR formation. Our results suggest conserved machinery for AR formation in poplar and generated informative gene co-expression networks that describe the basis of AR formation in these species.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01054-7.
Keywords: Adventitious root formation, Comparative transcriptome, Hormones, Poplar
Introduction
Asexual reproduction is a very efficient way to produce large amounts of plantlets in a short time, and maintains the good genetic background of parental plants (Duclercq et al. 2011). The vegetative organs like roots, stems, and leaves can be used as materials for asexual reproduction (Li et al. 2009; Xu 2018). The success of asexual reproduction relies on the formation of adventitious roots (ARs) and these are essential for new plants to forage for nutrients and water in soils (Bellini et al. 2014; Duclercq et al. 2011). Asexual reproduction using stems (i.e. cutting propagation) is widely used in forestry and horticultural production of numerous woody plants (Cao et al. 2018; Han et al. 2014a; Luo et al. 2015; Moriya et al. 2015; Thomas and Schiefelbein 2002; Xu et al. 2015; Zhang et al. 2015). In recent years, progress has been made to understand the physiological and molecular mechanisms underpinning AR formation in herbaceous plants, such as Arabidopsis and rice, however, relatively limited information is available for woody plants (Duclercq et al. 2011; Geiss et al. 2018; Steffens and Rasmussen 2016).
AR formation processes are sophisticated biological events that coordinate both exogenous and endogenous stimuli, including temperature, light, carbohydrate status, phenolic compounds and phytohormones (Eliasson 1978; Mauriat et al. 2014). Phytohormones can act as both exogenous and endogenous stimuli during AR formation, and exogenous application of hormones and their analogues can significantly increase the AR capacity of some plants (Cao et al. 2018; Druege et al. 2016; Zhang et al. 2015). Among the hormones known to participate in AR, auxin has been shown to play the central role in many plants (Druege et al. 2016; Pacurar et al. 2014). Generally, AR formation is promoted by high local IAA levels, which are facilitate by polar auxin transport with the help of a number of auxin transporters, such as AUXIN1/LIKE-AUX1 (AUX/LAX), PIN-FORMED (PIN), PIN-Like (PILS) and nitrate transporter 1.1 (NRT1.1/NPF6.3) (Druege et al. 2016; Zhou and Luo 2018). The asymmetric subcellular location of auxin within tissue can be sensed by auxin signaling modules, which trigger the gene reprogramming events implicated in cell fate determination and organogenesis during AR formation in plants (Duclercq et al. 2011; Luo et al. 2018). Besides IAA, a number of other hormones play roles in AR formation in plants. For instance, the dynamic changes in cytokinin (CK) levels during the AR induction processes in carnation are tightly linked to their roles in regulating cell division and differentiation (Agulló‐Antón et al. 2014). Abscisic acid (ABA), jasmonic acid (JA) and salicylic acid (SA) function as stress hormones (Luo et al. 2016, 2019b), and also participate in AR formation (Druege et al. 2016). Further, interactions between hormones also impose another layer that finely regulates cell determination and organogenesis (Druege et al. 2016; Lakehal and Bellini 2019; Sun et al. 2018). For instance, gibberellins negatively regulate AR formation in poplar and Arabidopsis by inhibiting polar auxin transport (Mauriat et al. 2014). However, there is limited molecular information available about regulation of hormone signaling during AR formation in woody plants, including poplar. Several typical studies revealed the underlying mechanisms for AR formation at physiological and molecular levels in forest tree species in recent years. Based on transcriptomic analyses, genes, phytohormones and phenolic compounds were found to be involved in AR formation in several tree species (Abu-Abied et al. 2012; De Klerk et al. 2011; Mauriat et al. 2014; Quan et al. 2017). Some genes, e.g., poplar BIG LEAF (Yordanov et al. 2017), Castanea sativa SCL1 gene (Vielba et al. 2011), and Olea europaea AOX2 gene (Hedayati et al. 2015) were proved to positively or negatively regulate AR formation.
In addition to hormones, several other types of molecules can act as signals during root development, such as reactive oxygen species (ROS), nitric oxide (NO), Ca2+, and cyclic guanosine monophosphate (cGMP) (Bai et al. 2014; Druege et al. 2016; Jiao et al. 2013; Li et al. 2015; Li and Xue 2010; Liao et al. 2012; Wang et al. 2010). The cGMP and mitogen-associated protein kinase (MAPK) cascades integrate the upstream signals from H2O2, Ca2+ and NO, and trigger the downstream gene reprogramming events to regulate AR formation in plants in an auxin-dependent manner (Astier et al. 2018; Han et al. 2015; Li and Xue 2010; Pagnussat et al. 2003). Furthermore, recent studies have also shown that H2O2 is implicated in other hormone signaling pathways, such as ethylene and ABA, to regulate AR formation in response to abiotic stresses (Li et al. 2018a; Qi et al. 2018). However, the roles of these signal molecules in AR formation are complicated, and information about the regulatory network in poplar remains scarce.
Poplar species are considered a model tree species, and are widely used in plantations (Luo et al. 2013a, 2019a; Plomion et al. 2016). The huge demands for plantlets for plantation establishment require efficient cutting propagation. Although poplar is considered a species that readily forms roots, the AR formation capacity varies among different poplar species (Krabel et al. 2015; Ronald Jr and Zalesny 2009). In recent years, great progress has been made in understanding the gene network that controls the capacity for AR formations in poplar (Li et al. 2017a; Ramírez-Carvajal et al. 2009; Rigal et al. 2012; Xu et al. 2012, 2015; Zhang et al. 2020). Furthermore, several key genes involved in AR formation in poplar have been identified, such as the Populus cytokinin type-B response regulator PtRR13 (Ramírez-Carvajal et al. 2009), two WUSCHEL-related HOMEOBOX genes (PeWOX11a and PeWOX11b) (Xu et al. 2015), AINTEGUMENTA LIKE1 (PtAIL1) (Rigal et al. 2012), PttGID1.3 (Mauriat et al. 2014), ROOT HAIR DEFECTIVE 3 (PeRHD3) (Xu et al. 2012), and PtaERF003 (Trupiano et al. 2013). The bZIP53–IAA4 module was uncovered to inhibit AR development in poplar in our recent study (Zhang et al. 2020). However, there are still many knowledge gaps in the transcriptomic gene network underlying the morphological and physiological changes that occur during AR formation in poplar.
As mentioned above, there are interspecific variations among poplar species, with some species sharing apparently similar AR forming capacities, but others displaying relatively low AR formation ratios in the absence of external treatments (Krabel et al. 2015; Ronald Jr and Zalesny 2009). Thus, we speculated that different poplar species might display considerably different gene reprogramming profiles during AR formation even with similar AR capacities, and also deploy a set of conserved genes that are implicated in AR formation. A number of transcriptomic studies associated with AR formation have been performed in recent years (Ramirez-Carvajal et al. 2009; Ribeiro et al. 2016; Rigal et al. 2012; Shu et al. 2019; Sun et al. 2019; Wei et al. 2020; Xiao et al. 2020; Zhang et al. 2019), however, the studies on comparable transcriptomic analysis uncovering the conserved genes during AR formation were rarely conducted (Bannoud and Bellini 2021). The conserved genes may represent the core machinery of the easy root-forming capacity in poplar, thus providing reliable candidates that can be used to improve AR formation in poplar and other plant species via genetic approaches. Here, we used two poplar species, i.e. P. × euramericana (“NL895”) and P. simonii, that form ARs easily in woody plant medium (WPM). Currently, the RNA-seq analysis was applied to dissect the transcriptomic reprogramming profiles of these two poplar species during the AR formation process, and comparative transcriptomic analysis was conducted to uncover the conserved biological events that contribute to their AR capacities. Our study has mapped the conserved comprehensive gene network that underlies the morphological and physiological modifications during AR formation, and has identified candidate genes that contribute to AR formation in poplar.
Materials and methods
Plant propagation, total RNA isolation and sequencing
Two hybrid varieties of poplar, “NL895” (P. × euramericana) and P. simonii were used as plant material in this study. Both poplars can easily form ARs in the field and in tissue culture. The strategy for plant propagation and RNA sample preparation was similar to previous reports with slight changes (Wang et al. 2017; Xia et al. 2017; Zhang et al. 2019). The outgrowth of ARs from the stem base mainly occurred during the first 8 days for both poplars in the WPM. Briefly, cuttings with 2–3 leaves and an apical bud excised from an individual tissue culture plantlet were propagated in WPM. The 0.5 cm base of each cutting was collected after 0, 2, 4, 6 and 8 days. These time points were termed DAE0 (0 days after excision), DAE2, DAE4, DAE6 and DAE8, respectively. At DAE0, the cuttings had not been subjected to any treatment but were simply excised from their mother plants. Ten to twenty stem bases (equal to 0.5 g) were mixed into one biological sample and three biological samples were prepared for each time point. Total RNA was isolated using a RNeasy Plant Mini Kit according to the manufacturer’s instructions (DP432, TIANGEN Biotech (Beijing) Co., Ltd., Beijing, China). RNA samples that met the required quality were sent for sequencing using a HiSeqX10 platform. All of the raw data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra/) under project number PRJNA516245.
Bioinformatic analysis
The raw data produced by the HiSeqX10 platform was quality trimmed and filtered using Trimmomatic software with the default parameter settings (Bolger et al. 2014). Clean reads were mapped onto the P. trichocarpa reference genome version 3.0 using TopHat software with the default parameters (Ghosh and Chan 2016; Trapnell et al. 2012). Subsequently, the read count for each sample was calculated using “htseq-count” software with parameters set to “unique mapping” (Anders et al. 2015). Only genes with average read counts larger than 2 in at least one time point were kept for further analysis. Finally, differential expression was analyzed using “EdgeR” software (Robinson et al. 2010), and genes with a false discovery rate (FDR) less than 0.05 and a fold change (FC) greater than 2.0 were selected as differentially expressed genes (DEGs).
The poplar genes were annotated based on information obtained from the Phytozome database (version 12.0), and the description of each gene was given according to its homologous gene from Arabidopsis. The overlapping genes between the two species were detected using Draw Venn Diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/), and the statistical significance of the overlaps were tested by SuperExactTest R package (Wang et al. 2015). The conserved genes were assigned into different functional categories with Mapman software (version 3.0.0) (Thimm et al. 2004). The R packages “clusterProfiler” and “pathview” were used to conduct GO and KEGG analysis (Luo and Brouwer 2013; Yu et al. 2012). Heatmap and cluster analysis were done in R with the “pheatmap” package (Kolde and Kolde 2015). The significant GO terms of conserved genes from different clusters were further processed with REVIGO (http://revigo.irb.hr/) (Supek et al. 2011), and the results were visualized in Cytoscape (version 3.5.0) (Shannon et al. 2003). Bubble diagrams of enriched GO terms were drawn in R with the “ggplot2” package (Ginestet 2011).
Co-expression analysis was performed similar to our previous work (Luo et al. 2019b). The values of fragments per kilobase per million mapped reads (FPKM) for the genes were calculated for biological samples from their read count using a python script. The WGCNA (weighted gene correlation network analysis) R package was employed to conduct the co-expression network construction with default parameters, except for soft thresholding, which was applied to conserved genes (Langfelder and Horvath 2008). The soft threshold was determined by a plot of parameters for “scale free topology model fit” in WGCNA. The co-expression network of conserved genes was visualized using Cytoscape (version 3.5.0) (Shannon et al. 2003).
To obtain the common DEGs in different poplar species during the AR formation, the lists of DEGs in previously published papers (Rigal et al. 2012; Ribeiro et al. 2016; Wei et al. 2020) were collected. The Venn and KEGG enrichment analysis were conducted as described above.
RT-qPCR validates the results of RNA-seq
Confirmation of the RNA-Seq results was performed with RT-qPCR assays. A total of ten genes were randomly selected, and the gene-specific primers designed for the RT-qPCR were reported previously (Zhang et al. 2019). RNA preparation was similar to the RNA-Seq experiment and a One-Step gDNA Removal and cDNA Synthesis SuperMix kit (AU311–02; Trans Biotech, Beijing, China) was used for cDNA synthesis. A FastStart Essential DNA Green Master mix (Roche Molecular Systems, Inc., China) was used for all RT-qPCR reactions and the LightCycler 96 (Roche) platform was used for subsequent assays. Three biological and technical replicates were used for each RNA sample. The quantification cycle (Cq) values of two reference genes (ACTIN and UBQL) were used for calculating the relative gene expression with the 2−ΔΔCq method (Livak and Schmittgen 2001).
Results
Identification of common genes under different time comparisons in two poplars
Although, the two poplar species can form AR easily, the AR were differentially observed on DAE6 and DAE8 for Ps and Pe, respectively (Fig. S1). The transcriptome analysis identified 5176 and 2472 DEGs during the initial stage (DAE2 vs DAE0) in Pe and Ps, respectively (Table S1 and Fig. 1A). There were 5489 and 4945 unique genes that were up- and down-regulated during the AR development in Pe, and comparable number of up- (4646) and down-regulated (5013) unique genes were identified in Ps (Table S1 and Fig. 1A-G). The highest number of DEGs was detected on comparing DAE2 vs DAE0 for Pe, and DAE4 vs DAE2 for Ps (Fig. 1H). Around 158–1061 DEGs were detected as common genes between Pe and Ps under these time comparisons, which accounted for 13%-35% and 4%-54% of the total DEGs for Pe and Ps, respectively (Fig. 1A-G). These two poplar species shared the lowest number of DEGs when DAE4 and DAE2 were compared (Fig. 1H). Furthermore, the overlaps between two species under different time comparisons were statistically significant (Table S2), suggesting the common gens detected here were with biological significances.
Fig. 1.
Venn diagrams representing the overlapping genes in P. × euramericana (Pe) and P. simonii (Ps) in different time comparisons during AR formation (A-E). The number of significantly expressed genes in Pe and Ps, as well as the common genes (CGs) between these two poplar species in the different time comparisons during AR formation (H). The red and blue numbers in the Venn diagrams indicate the numbers of significant up- and downregulated genes, respectively
There were 3843 and 2319 unique DEGs detected specially in Pe and Ps, respectively (Table S1). GO term analysis of the unique genes in these two poplar species uncovered large number of common significant terms (Table S3), in line with this, the exclusive genes in the two poplar species under the same time comparison were also commonly enriched in some pathways, such as response to chitin, response to organonitrogen compound, water transport, and systemic acquired resistance (Table S3), suggesting that these two species may deploy different genes that participate in similar biological processes. However, considering the most significant GO terms, unique genes of Pe were more pronounced enriched in metabolism involved in ribonucleotide metabolism, whereas the most significant enriched GO terms regards unique genes in Ps were relative to the responsive processes to light and stressors (Table S3). Besides, several unique enriched GO terms were found in the un-shared genes with the two poplar species under the same time comparison, such as auxin transport, auxin polar transport and pigment accumulation for Pe, whereas carbohydrate transport and cellular response to hypoxia for Ps (Table S3).
The accuracy of RNA-Seq was validated by RT-qPCR in our recently published paper (Zhang et al. 2019) and a good correlation was observed between the results obtained from RNA-seq and RT-qPCR. The correlation coefficient between RNA-Seq and RT-qPCR for these 10 randomly selected genes was 0.80 (Fig. S2).
Cluster analysis of the conserved genes
There were 4336 genes that shared the same gene expression profiles within the time comparisons tested (Fig. 1). These common genes from the different time comparisons were combined for further analysis, and 2146 unique genes were identified as conserved genes during AR formation in these two poplar species (Table S4 and Fig. S3A). The cluster analysis clearly separated these conserved genes into two clusters, with Cluster 1 containing 1026 genes that were inhibited during the whole period of AR formation, whereas 1120 genes were assigned to Cluster 2 with the upregulated gene expression profiles compared to the ones from Cluster 1 (Fig. S3A). KEGG analysis revealed that genes from Cluster 1 had a larger number of enriched pathways compared to Cluster 2 (Fig. S3B). For instance, Cluster 1 was enriched in genes for phenylalanine metabolism, biosynthesis of amino acids, flavonoid biosynthesis, and alanine, aspartate and glutamate metabolism, whereas Cluster 2 contained genes that were globally up-regulated and enriched in only three KEGG pathways, i.e. plant hormone signal transduction, plant-pathogen interaction and MAPK signaling pathway (Fig. S3B).
At the same time, the GO term analysis also uncovered more significantly enriched GO terms in Cluster 1 compared to Cluster 2 (Table S5 and Fig. 2A). Specifically, Cluster 1 was most enriched in genes for phenylpropanoid metabolic and biosynthetic processes, secondary metabolite biosynthetic process, response to karrikin, and response to decreased oxygen levels (Table S5 and Fig. 2A). The genes from Cluster 2 were mainly enriched in GO terms relative to stress response (Table S5 and Fig. 2A). In addition, several GO terms were commonly enriched in respiratory burst, response to oxidative stress, regulation of reactive oxygen species (ROS) metabolism, ROS metabolism, response to wounding and intracellular signal transduction (Table S5 and Fig. 2A).
Fig. 2.
The significantly enriched GO terms in different clusters (A), and a radar map showing the numbers of genes assigned to the represented transcription factor (TF) families (B). For GO network, the nodes represent the significant GO terms, and highly similar GO terms (nodes) are linked by edges
Among these conserved genes, a total of 219 genes (around 10% of the conserved genes) were identified as transcription factors (TFs) (Table S6), according to the information obtained from PlantTFDB (http://planttfdb.cbi.pku.edu.cn/index.php) (Jin et al. 2016). There were six TF gene families with more than ten members in the conserved genes, i.e. ERF, WRKY, MYB, bHLH, NAC and C2H2 (Table S6 and Fig. 2B). There were 43, 29, 25 and 15 members that belonged to the ERF, WRKY, MYB and bHLH gene families, respectively (Table S6 and Fig. 2B).
Conserved genes involved in MAPK and hormone signaling pathways
The MAPK signaling pathway was involved in integrating multiple signaling to regulate the formation of ARs, as well as environmental responses (Han et al. 2014b; Li et al. 2009). In the MAPK signaling pathway, signal components such as MEKK1, MKK4, MKK9, MPK3, and MPK4, were globally inhibited (Fig. 3). However, the two genes encoding PR1, two ERF1 genes, several genes that belonged to ChiB gene family and CAT2 were strongly upregulated (Fig. 3).
Fig. 3.
Conserved genes assigned to the MAPK signaling pathway. This figure was
adapted from the MAPK signaling pathway in the KEGG pathway. The gene expression levels in P. × euramericana (Pe) and P. simonii (Ps) of each time point during AR formation are shown in different columns in the colored grids, and the different rows represent the different genes. The colors indicate the levels of gene expression with red representing upregulation and blue representing downregulation. The gene expression levels of Pe and Ps during the different time points were normalized to 0 days after excision (DAE0) of the corresponding poplar species
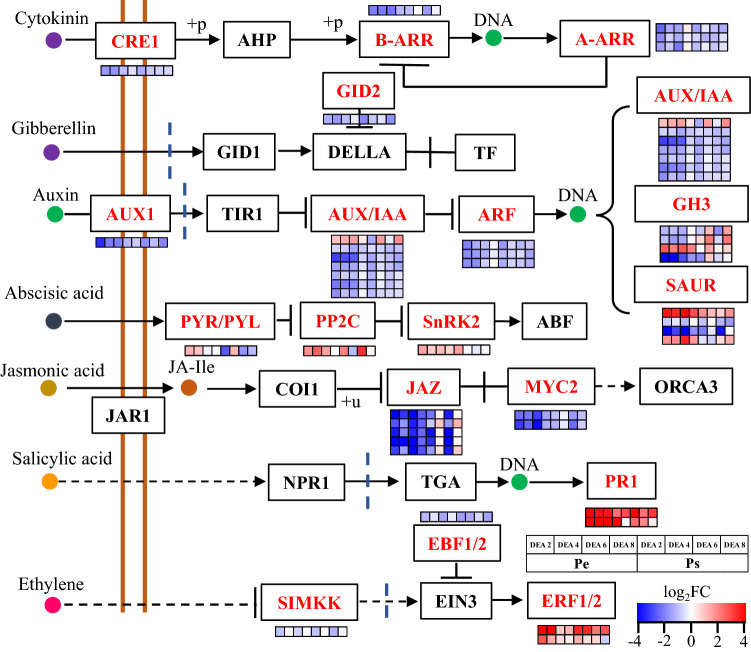
In addition to the MAPK signaling pathway, a substantial number of genes were assigned to plant hormone signal transduction pathways (Fig. 4). Pathways involved in regulating CK, gibberellin, auxin, ABA, JA and SA were significantly modified during AR formation (Fig. 4). For CK, the individual genes encoding CRE1 and B-ARR as well as several genes for A-ARR were inhibited in both poplar genotypes (Fig. 4). In the case of auxin, many genes encoding AUX/IAA, ARF, GH3 and SAUR were globally downregulated, except one of the AUX genes, one of the GH3 genes and two of the SAUR genes were induced during AR formation in poplar (Fig. 4). The pathways involved in SA were also generally inhibited; for instance, five genes encoding JAZ and two encoding MYC2 were inhibited (Fig. 4). However, some interspecific effects were observed between these two poplar species because the five genes encoding JAZ in Ps were slightly upregulated at some time points, whereas the same genes were totally inhibited at all the time points tested in Pe (Fig. 4). Furthermore, several genes that were involved in the ABA, SA and ethylene signaling pathways were slightly promoted (Fig. 3 and 4).
Fig. 4.
Conserved genes assigned to the phytohormone signaling pathways. This figure was
adapted from the phytohormone signaling pathways in the KEGG pathway. The gene expression levels in P. × euramericana (Pe) and P. simonii (Ps) of each time point during AR formation are shown in different columns in the colored grids, and the different rows represent the different genes. The colors indicate the levels of gene expression with red representing upregulation and blue representing downregulation. The gene expression levels of Pe and Ps during the different time points were normalized to 0 days after excision (DAE0) of the corresponding poplar species
Co-expression analysis of the conserved genes
It is well documented that genes involved in the same pathways tend to be co-expressed (Luo et al. 2019b). Thus, we used the data of these 2146 conserved genes to construct a co-expression gene network during AR formation (Fig. 5A). A total of 1111 genes (around 52% of the conserved genes) were co-expressed via 356,277 edges (Fig. 5A). Four co-expressed modules (CMs, subnetworks with more than 30 genes) were identified, and 537, 304, 186 and 38 genes were assigned into CMs 1, 2, 3 and 4, respectively (Fig. 5A). KEGG and GO analysis showed that these four modules displayed distinct biological functions with some overlapping and significantly enriched pathways (Table S7 and Fig. 5B-E).
Fig. 5.
The co-expression network of the conserved genes in P. × euramericana (Pe) and P. simonii (Ps) in the different time comparisons during AR formation (A). The co-expression network can be assigned to four major co-expression modules (CMs, subnetworks with more than 30 genes). The numbers of genes assigned to each module are indicated. The heatmap represent the gene expression profiles of the genes assigned into the same CM. The gene expression levels of Pe and Ps at different time points were normalized to the 0 days after excision (DAE0) time point of the corresponding poplar species. The selected significantly enriched GO terms of CM1 (B), CM2 (C), CM3 (D) and CM4 (E) have been indicated
For instance, CM1 was enriched in several defense response pathways like the response to wounding, respiratory burst involved in defense response, respiratory burst, defense response to fungus, regulation of immune response, systemic acquired resistance and host programmed cell death induced by symbiont (Table S7 and Fig. 5B). In addition to defense, several pathways that are involved in hormone metabolism, signaling and response were also enriched in CM1; these hormones included SA, JA, ABA, ethylene, and auxin (Table S7 and Fig. 5B). Further, several pathways involved in ROS were enriched in CM1, such as regulation of the hydrogen peroxide metabolic process, ROS metabolic process, response to ROS, response to oxidative stress and hydrogen peroxide metabolic process (Table S7 and Fig. 5B).
The genes assigned into CM2 were significantly enriched in several nitrogen transport-related pathways, including response to nitrate, nitrate transport, and amino acid transport (Table S7 and Fig. 5C). Pathways involved in ROS metabolism and the ROS response were also significantly enriched in CM2 (Table S7 and Fig. 5C). In contrast, CM3 was enriched in flavonoid metabolic and biosynthetic processes, several DNA replication-related pathways, and histone modification-related pathways (Table S7 and Fig. 5D). For CM4, genes from this module were enriched in lipid transport and localization, as well as secondary metabolite biosynthetic processes (Table S7 and Fig. 5E).
In addition to this, large number of TFs was also identified in these CMs, for instance, CM1 and CM2 had 99 and 21 TFs, respectively (Table S6). Moreover, the TFs identified in CM1 were highly connective with other genes, and twenty-four TFs from CM1 shared more than 200 edges with other genes (Table S6). These TFs with high connectivity in the CMs could be potential candidates for AR in the future study.
Common DEGs among different poplar species
To strengthen the understanding of conserved transcriptional regulation underlying AR formation in different poplar species, the DEGs of other poplars with similar time-point sampling method were combined with our data to find the common DEGs in different poplars (Fig. 6A). Although the culture methods and sampling time varied (Rigal et al. 2012; Ribeiro et al. 2016; Wei et al. 2020), there were still 1310 genes that differently changed among five poplars (Table S8 and Fig. 6A). These common genes were significantly enriched in nitrogen metabolism related pathway, plant hormone signal transduction, as well as MAPK signaling pathway (Fig. 6B), which were consisted with the results obtained from our data.
Fig. 6.
Venny analysis of the common DEGs in different poplar species during the AR formation (A), and the KEGG enrichment analysis of the common DEGs (B)
Discussion
The interspecific variations in gene reprogramming during AR formation in the two poplar species
Asexual reproduction of woody plants is a very convenient way to produce large numbers of saplings for nursery production and plantation establishment, and this relies on efficient AR formation. The capacities for AR formation are significantly different among woody plants, with species like R. pseudoacacia, Eucalyptus, Pinus and Morus alba being recalcitrant to AR formation, but application of exogenous stimuli (i.e. hormones) can be an efficient way to increase their root formation capacities (Du et al. 2017; Fett-Neto et al. 2001; Quan et al. 2017). Conversely, poplar species usually have strong abilities to form ARs without any treatment, and asexual reproduction of poplar via cuttings is widely used (Luo and Zhou 2019). Nevertheless, poplar species have also shown large interspecific variations in AR formation, for instance, poplar species like P. euramericana, P. popularis and P. cathayana to form ARs very easily, whereas, P. × canescens and P. alba × P. glandulosa usually have a relatively low ratio of ARs, according to our experience. In our previous study, the expression profiles for AR development in P. euramericana that had strong ability to form ARs were comprehensively investigated. Subsequent gene expression cluster analysis showed a number of biological processes involved in AR formation (Zhang et al. 2019). In this study, we mainly focused on investigating the difference and conservation between two poplar species, P. euramericana and P. simonii. These two species had strong abilities to form ARs in the field and with WPM, however, they still displayed distinct gene reprogramming patterns of AR development under WPM treatment. P. euramericana developed slower AR formation processes than P. simonii in WPM in the early stages of cutting development (Fig. S1). These different gene reprogramming events with similar AR abilities suggest that the processes of AR formation in these two poplar species largely depend on the genetic background. Additionally, the rooting response was not synchronized completely, these different gene reprogramming events might also be responsible for other traits variation occurred from DAE0 to DAE8 in the tested tissues between the two poplars.
In contrast with the similar observed phenotypes in these two poplars, it was revealed that large interspecific variations existed between these two poplar species at the transcriptome level. The number of DEGs decreased in Pe, whereas it increased in Ps during the AR formation, which was consistent with the facts that Ps priming the AR faster than Pe, and forming more root primordia than Pe. In addition, there was only a very small proportion of genes (around 4%-5%) that were common between the two poplar species, and the number of common genes decreased during the study, with the lowest number of common genes being detected between DAE4 and DAE2 and accounting for only around 13% and 4% of the total DEGs in Pe and Ps, respectively, suggesting that these two poplar species deployed different molecular strategies to form ARs. Indeed, many more root primordia were observed at DAE4 by the anatomical analysis, suggesting that the period between DAE2 and DAE4 was the key time to determine the root primordia, which may contribute to the interspecific variations in AR capacities between these two poplar species. Meanwhile, the DEGs detected on the comparison DAE2 vs DAE0 for Pe were more enriched in cell wall related processes, meanwhile the enriched GO terms of DEGs at the same comparison for Ps were more related to stress responses. Collectively, these results clearly demonstrated that these two poplar species deployed distinct gene reprogramming profiles to promote AR formation.
Poplar species shared common conserved pathways to regulate AR processes
Aside from the existing interspecific variation in the gene programming profiles there was still a considerable number of common genes between the two poplar species during each time comparison. A total of 2146 genes were identified as conserved genes that may represent the core conserved machinery that regulates AR formation. Indeed, these conserved genes can be clustered into two clusters with clear gene expression profiles. The uniformity of gene expression profiles between the two poplar species within each cluster suggests that these conserved genes may act in the same manner in both species, and represent the most highly conserved gene reprogramming events controlling AR formation in poplar. In addition to this, interspecific variation was also detected among these conserved genes, since the vertical clustering clearly separated the differences between Pe and Ps. Taken together, these results demonstrated that the conserved genes identified here may represent the core molecular machinery underlying AR formation in poplar species.
After excision from mother plants, the wounded surfaces of cuttings are exposed to the surrounding environment, and this induces plant defense responses (Da Costa et al. 2013). Indeed, the wounding response has been reported as being activated and accompanied by ethylene signal transduction (Da Costa et al. 2013; Xu 2018). In line with this, in the current work the plant-pathogen interaction was enriched in genes from Cluster 2, which were upregulated during AR formation. Moreover, two RP1 genes, which represented marker genes for plant defense, were strongly induced during all time points in both poplars alongside upregulation of six genes encoding for ChiB, which is implicated in ethylene signal transduction. Similar results have also been obtained in other woody plant species during AR formation. For instance, an AtERF3 homolog gene in apple was identified as a candidate gene for AR formation by a quantitative trait locus (QTL) approach (Moriya et al. 2015). Several genes encoding ERF have also been considered as positive effectors during AR formation in M. alba (Cao et al. 2018). In addition, PtaERF003 (also homologous to AtERF3) was identified by dominant tagging approaches as a positive factor in the regulation of ARs in P. tremula × P. alba (Trupiano et al. 2013). Interestingly, among the genes associated with AR formation in the two poplars we observed the induction of two genes that are homologous to AtERF3. Taken together, these data implied a role for ethylene signal transduction in coordinating the plant wounding response and AR formation in Populus.
In addition to ethylene, ABA has been considered as the key hormone that increases rapidly in the early stage of root formation in response to water shortage when root primordia are just determined and the connections between the vascular tissues of the root and the stem have not been established yet (Agulló‐Antón et al. 2014). Coincidently, in our study, the genes encoding several ABA signaling components, including PYP/PYL, PP2C and SnRK2, were induced during the AR formation process. In line with this, two genes that are homologous to the EARLY-RESPONSIVE TO DEHYDRATION 9 (ERD9) gene in Arabidopsis were induced in the two poplar species throughout the entire AR formation process. In addition to this, 45–46 genes belonging to Cluster 2 were assigned into the significantly enriched GO terms “response to water deprivation” and “response to water”. Moreover, “water transport” and “fluid transport” were enriched in the genes that were assigned to CM2. These results implied a role for ABA in regulating water responses during the early period of root formation.
The MAPK signaling pathway has been shown to be intimately involved in AR formation through the integration of multiple signaling pathways such as H2O2, NO, cGMP and Ca2+ (Da Costa et al. 2013; Druege et al. 2016; Li and Xue 2010; Zhao et al. 2010). Several papers have demonstrated that H2O2 acts as a signaling molecule in auxin signaling-mediated AR formation in many plants (Li et al. 2018a, 2007; Li and Xue 2010; Qi et al. 2018). Furthermore, a recent study showed that the genes involved in H2O2 balance are consistently stimulated throughout AR formation in poplar (Zhang et al. 2019). Further pharmacological experiments also confirmed the negative roles played by H2O2 during AR formation in poplar (Zhang et al. 2019). Notably, cytosolic Ca2+ has been shown not only as being essential for adapting adverse environments in plants (Gao and Zhang 2019; Yang et al. 2019; Zhao et al. 2013), and also as the downstream component of H2O2 signaling during AR formation (Druege et al. 2016; Li and Xue 2010). NO is involved in the auxin-mediated signaling pathway, and positively regulates AR formation in cucumber (Lanteri et al. 2009). It has been postulated that the co-action of H2O2 and NO activates MAPK cascades to regulate auxin-mediated AR formation via independent but parallel pathways that are dependent on Ca2+ (Da Costa et al. 2013). In line with these earlier results, large numbers of conserved genes were assigned to the MAPK signaling pathway, together with the H2O2 and auxin signaling pathway, suggesting that poplar deployed the MAPK signaling pathway to integrate a suite of signaling molecules and hormones that regulate AR formation.
A number of biological pathways were uncovered from the conserved genes, which were consisted with the enriched pathways of the common DEGs detected from different poplars, suggesting poplar species shared common transcriptional regulation pathways to promote AR formation. However, we still should consider that it was possible that some conserved genes that were not involved in AR formation. For example, the conserved genes regulating root elongation, lateral root initiation and nutrition assimilation in root apex, would also be considered as conserved genes according to our strategy for gene expression comparison. However, all these biological processes occurred in the outgrowth ARs.
Coordination of the AR processes in poplar according to the gene co-expression network
In recent years, gene co-expression network analysis has been widely applied to dissect the transcriptomic reprograming profiles involved in diverse abiotic stresses in poplar. Li et al. (2017a) constructed a co-expression network from poplar that revealed that the PtoWOX5a gene acts as a repressor in AR formation, with most genes from the PtoWOX5a-centered network being down-regulated in the over-expression lines of this gene. Interestingly, the poplar PLASMA MEMBRANE INTRINSIC PROTEIN 2;4 (PtrPIP2;4), which may be involved in root development, was detected among the list of conserved genes in the PtoWOX5a co-expressed genes (Li et al. 2017a). Its homologous gene in Arabidopsis is implicated in early responses to phosphate deficiency in roots (Lin et al. 2011).
Among the 2146 conserved genes, approximately 52% conserved genes were co-expressed during AR formation in the two poplar species. Additionally, this gene network was separated into four distinguishable modules with specific biological functions. Moreover, a large number of common significant GO terms were detected among these different modules, suggesting that the modules might be finely co-regulated by their common pathways. For instance, CM1 was enriched in defense response and hormone signaling, suggesting that CM1 coordinates these processes during priming of AR formation in poplar. Several well-known stress-induced hormones including JA, ABA, and ethylene have been implicated in AR formation (Lakehal and Bellini 2019; Li et al. 2018b), implying an integration of these two biological processes (i.e. defense response and hormone signaling) during AR formation.
Although, a number of defense response-related GO terms, such as “response to wounding”, and “defense response to fungus”, were found in CM2, a few terms related to hypoxia and ROS were also detected in this CM. It is widely documented that the ROS and NO generated during hypoxia stress can act as signaling molecules (Kumar Patel et al. 2019), and roles for ROS (such as H2O2) and NO in AR formation have also been reported (Li et al. 2017b; Li and Xue 2010; Xuan et al. 2012). Hypoxia stress may be triggered by respiratory burst processes because both defense responses and root formation require a lot of energy and substrates that are derived from respiration. Furthermore, a lack of normal roots during AR formation may also hamper the O2 content in tissues. Indeed, most poplars are adapted to flooded soils, and forming ARs is an important strategy to overcome the hypoxia stress induced by waterlogging (Peng et al. 2018). Hypoxia stress in poplar has also been associated with inhibition of energy consummation processes, such as nitrogen uptake and transport (Liu et al. 2015; Peng et al. 2018). The uptake of both NO3− and NH4+ have been associated with the activities of PM H+-ATPase, which needs a lot of energy (Luo et al. 2013b). In agreement with this, genes assigned to CM2 and CM4 in the present study were enriched in many genes related to transport, with most of these transport-related GO terms being linked to nitrate and amino acid transport. These changes may be linked to the changes in O2 level in tissues. Another point to consider is that the major source of endogenous NO is produced by either L-arginine-dependent NO synthase (NOS)-like activity or nitrate reductase (NR) using L-arginine and NO3−, respectively (Astier et al. 2017). Changes in the quantities of NO3− and amino acids may modify the level of endogenous NO, thus rebalancing the relationships between H2O2 and NO in regulating AR formation in poplar. These data indicate the possible roles of H2O2 and NO generated from hypoxia stress in regulating AR formation.
In addition to this, genes assigned to CM3 were obviously related to the cell cycle, which is important for AR formation. For instance, many GO terms related to DNA replication, mitotic cell cycle processes, and histone modification were significantly enriched in CM3. Indeed, it is well known that histone modification is linked to changes in the structure of chromatin as well as the promotion/silencing of genes (Allfrey et al. 1964). In P. trichocarpa, several genes implicated in histone acetylation during root development have been identified (Ma et al. 2016) and a number of genes that govern the cell cycle are known to be induced during AR differentiation in M. alba (Cao et al. 2018). Here, the genes assigned to CM3 were clearly involved in the cell cycle by regulating histone modification and DNA replication.
Thus, it can be inferred from our results that the co-expression network of conserved genes plays important roles in coordinating multiple biological processes, such as defense responses, hormone signaling, ROS, NO, and the cell cycle, which are essential for AR formation in poplar (Fig. 7).
Fig. 7.

The regulation model for poplar AR formation. Genes related to biological processes in this figure were predicted to be invovled in poplar AR formation
Conclusion
The two poplar species examined displayed considerable interspecific variations in gene programming during AR formation. However, a set of conserved genes was identified that acted quite uniformly during the different time points of AR formation, representing the most conserved biological events that regulate the AR capacity of poplar. These conserved genes were enriched in diverse hormone signaling pathways and the MAPK signaling pathway, suggesting an important role for signal transduction in coordinating external stimuli and endogenous physiological status during AR formation. Furthermore, the co-expression network analysis of conserved genes allowed identification of several CMs that are co-expressed with distinct biological functions. This suggested that the AR formation processes in poplar were finely tuned at transcriptome level, integrating multiple biological processes that are essential for AR formation. All these biological processes were concluded in Fig. 7. In this concluded model, genes related to defense response, hormone metabolism and signaling, TFs, cell cycle, nitrogen uptake and transport, MAPK signaling pathway, Hypoxia and ROS production and Ca2+ signaling would be involved in poplar AR formation. This work should inform further research and development of poplar as a plantation tree and will form a baseline for understanding and modifying AR formation in recalcitrant woody species.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This Project was financially supported by the National Natural Science Foundation of China (NSFC accession No. 31901282), and the Fundamental Research Funds for the Central Universities (No. 2662019PY047).
Authors' contributions
J. Luo and N. Wang designed experiments; J. Luo and T. Nvsvrot carried out experiments; J. Luo and N. Wang conducted the bioinformation analysis; J. Luo and N. Wang wrote and revised the manuscript; N. Wang supervised the entire work.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
There are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abu-Abied M, Szwerdszarf D, Mordehaev I, Levy A, Stelmakh OR, Belausov E, Yaniv Y, Uliel S, Katzenellenbogen M, Riov J, Ophir R, Sadot E. Microarray analysis revealed upregulation of nitrate reductase in juvenile cuttings of Eucalyptus grandis, which correlated with increased nitric oxide production and adventitious root formation. Plant J. 2012;71:787–799. doi: 10.1111/j.1365-313X.2012.05032.x. [DOI] [PubMed] [Google Scholar]
- Agulló-Antón MÁ, Ferrández-Ayela A, Fernández-García N, Nicolás C, Albacete A, Pérez-Alfocea F, Sánchez-Bravo J, Pérez-Pérez JM, Acosta M. Early steps of adventitious rooting: morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiol Plant. 2014;150:446–462. doi: 10.1111/ppl.12114. [DOI] [PubMed] [Google Scholar]
- Allfrey V, Faulkner R, Mirsky A. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier J, Gross I, Durner J. Nitric oxide production in plants: an update. J Exp Bot. 2017;69:3401–3411. doi: 10.1093/jxb/erx420. [DOI] [PubMed] [Google Scholar]
- Astier J, Besson-Bard A, Wawer I, Parent C, Rasul S, Jeandroz S, Dat J, Wendehenne D. Nitric oxide signalling in plants: cross-talk with Ca2+, protein kinases and reactive oxygen species. In: Roberts JA, editor. Annual Plant Reviews online. New York: Wiley Online Library; 2018. pp. 147–170. [Google Scholar]
- Bai L, Ma X, Zhang G, Song S, Zhou Y, Gao L, Miao Y, Song C-P. A receptor-like kinase mediates ammonium homeostasis and is important for the polar growth of root hairs in Arabidopsis. Plant Cell. 2014;26:1497–1511. doi: 10.1105/tpc.114.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannoud F, Bellini C. Adventitious rooting in Populus species: Update and perspectives. Front Plant Sci. 2021;12:668837. doi: 10.3389/fpls.2021.668837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol. 2014;65:639–666. doi: 10.1146/annurev-arplant-050213-035645. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Du W, Shang C, Shen Q, Liu L, Cheng J. Comparative transcriptome reveals circadian and hormonal control of adventitious rooting in mulberry hardwood cuttings. Acta Physiol Plant. 2018;40:197. doi: 10.1007/s11738-018-2772-y. [DOI] [Google Scholar]
- Da Costa C, De Almeida M, Ruedell C, Schwambach J, Maraschin F, Fett-Neto A. When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci. 2013;4:133. doi: 10.3389/fpls.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Klerk G-J, Guan H, Huisman P, Marinova S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul. 2011;63:175–185. doi: 10.1007/s10725-010-9555-9. [DOI] [Google Scholar]
- Druege U, Franken P, Hajirezaei MR. Plant hormone homeostasis, signaling, and function during adventitious root formation in cuttings. Front Plant Sci. 2016;7:381. doi: 10.3389/fpls.2016.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XL, Cao X, Yin CR, Tang Z, Du W, Ban YY, Cheng JL. Comprehensive analysis of R2R3-MYB genes during adventitious root formation in cuttings of Morus alba. J Plant Growth Reg. 2017;36:290–299. doi: 10.1007/s00344-016-9639-5. [DOI] [Google Scholar]
- Duclercq J, Sangwan-Norreel B, Catterou M, Sangwan RS. De novo shoot organogenesis: from art to science. Trends Plant Sci. 2011;16:597–606. doi: 10.1016/j.tplants.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Eliasson L. Effects of nutrients and light on growth and root formation in Pisum sativum cuttings. Physiol Plant. 1978;43:13–18. doi: 10.1111/j.1399-3054.1978.tb01560.x. [DOI] [Google Scholar]
- Fett-Neto AG, Fett JP, Goulart LWV, Pasquali G, Termignoni RR, Ferreira AG. Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol. 2001;21:457–464. doi: 10.1093/treephys/21.7.457. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang G. A calcium sensor calcineurin B-like 9 negatively regulates cold tolerance via calcium signaling in Arabidopsis thaliana. Plant Signal Behav. 2019;14:e1573099. doi: 10.1080/15592324.2019.1573099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss G, Gutierrez L, Bellini C. Adventitious root formation: new insights and perspectives. In: Roberts JA, editor. Annual plant reviews online. New York: Wiley Online Library; 2018. pp. 127–156. [Google Scholar]
- Ghosh S, Chan C-KK. Analysis of RNA-Seq data using TopHat and Cufflinks. In: Edwards D, editor. Plant bioinformatics. New York: Humana Press; 2016. pp. 339–361. [DOI] [PubMed] [Google Scholar]
- Ginestet C. ggplot2: elegant graphics for data analysis. J R Stat Soc A Stat. 2011;174:245–246. doi: 10.1111/j.1467-985X.2010.00676_9.x. [DOI] [Google Scholar]
- Han H, Sun X, Xie Y, Feng J, Zhang S. Transcriptome and proteome profiling of adventitious root development in hybrid larch (Larix kaempferi × Larix olgensis) BMC Plant Biol. 2014;14:305. doi: 10.1186/s12870-014-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Wang C-W, Jiang J. Mitogen-activated protein kinase 6 controls root growth in Arabidopsis by modulating Ca2+-based Na+ flux in root cell under salt stress. J Plant Physiol. 2014;171:26–34. doi: 10.1016/j.jplph.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Han S, Fang L, Ren X, Wang W, Jiang J. MPK6 controls H2O2-induced root elongation by mediating Ca2+ influx across the plasma membrane of root cells in Arabidopsis seedlings. New Phytol. 2015;205:695–706. doi: 10.1111/nph.12990. [DOI] [PubMed] [Google Scholar]
- Hedayati V, Mousavi A, Razavi K, Cultrera N, Alagna F, Mariotti R, Hosseini-Mazinani M, Baldoni L. Polymorphisms in the AOX2 gene are associated with the rooting ability of olive cuttings. Plant Cell Rep. 2015;34:1151–1164. doi: 10.1007/s00299-015-1774-0. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Sun L, Song Y, Wang L, Liu L, Zhang L, Liu B, Li N, Miao C, Hao F. AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signalling and auxin response of roots in Arabidopsis. J Exp Bot. 2013;64:4183–4192. doi: 10.1093/jxb/ert228. [DOI] [PubMed] [Google Scholar]
- Jin J, Tian F, Yang D-C, Meng Y-Q, Kong L, Luo J, Gao G. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016;2016:D1040–D1045. doi: 10.1093/nar/gkw982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolde R (2019) pheatmap: Pretty Heatmaps. R package version 1.0.12. https://CRAN.R-project.org/package=pheatmap
- Krabel D, Meyer M, Solger A, Müller R, Carvalho P, Foulkes J. Early root and aboveground biomass development of hybrid poplars (Populus spp.) under drought conditions. Can J Forest Res. 2015;45:1289–1298. doi: 10.1139/cjfr-2015-0126. [DOI] [Google Scholar]
- Kumar Patel M, Pandey S, Burritt DJ, Phan Tran L-S. Plant responses to low-oxygen stress: interplay between ROS and NO signaling pathways. Environ Exp Bot. 2019;161:131–142. [Google Scholar]
- Lakehal A, Bellini C. Control of adventitious root formation: insights into synergistic and antagonistic hormonal interactions. Physiol Plant. 2019;165:90–100. doi: 10.1111/ppl.12823. [DOI] [PubMed] [Google Scholar]
- Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri L, Pagnussat G, Laxalt A, Lamattina L. Nitric oxide is downstream of auxin and is required for inducing adventitious root formation in herbaceous and woody plants. In: Niemi K, Scagel C, editors. Adventitious root formation of forest trees and horticultural plants–from genes to applications. Kerala: Research Signpost; 2009. pp. 222–245. [Google Scholar]
- Li S-W, Xue L. The interaction between H2O2 and NO, Ca2+, cGMP, and MAPKs during adventitious rooting in mung bean seedlings. Vitro Cell Dev-Pl. 2010;46:142–148. doi: 10.1007/s11627-009-9275-x. [DOI] [Google Scholar]
- Li S, Xue L, Xu S, Feng H, An L. Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Reg. 2007;52:173–180. doi: 10.1007/s10725-007-9188-9. [DOI] [Google Scholar]
- Li S-W, Xue L, Xu S, Feng H, An L. Mediators, genes and signaling in adventitious rooting. Bot Rev. 2009;75:230–247. doi: 10.1007/s12229-009-9029-9. [DOI] [Google Scholar]
- Li N, Sun L, Zhang L, Song Y, Hu P, Li C, Hao FS. AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta. 2015;241:591–602. doi: 10.1007/s00425-014-2204-1. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang J, Jia H, Liu B, Sun P, Hu J, Wang L, Lu M. The WUSCHEL-related homeobox 5a (PtoWOX5a) is involved in adventitious root development in poplar. Tree Physiol. 2017;38:139–153. doi: 10.1093/treephys/tpx118. [DOI] [PubMed] [Google Scholar]
- Li S-W, Leng Y, Shi R-F. Transcriptomic profiling provides molecular insights into hydrogen peroxide-induced adventitious rooting in mung bean seedlings. BMC Genomics. 2017;18:188. doi: 10.1186/s12864-017-3576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bian B, Gong T, Liao W. Comparative proteomic analysis of key proteins during abscisic acid-hydrogen peroxide-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J Plant Physiol. 2018;229:185–194. doi: 10.1016/j.jplph.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Li S-W, Zeng X-Y, Leng Y, Feng L, Kang X-H. Indole-3-butyric acid mediates antioxidative defense systems to promote adventitious rooting in mung bean seedlings under cadmium and drought stresses. Ecotox Environ Safe. 2018;161:332–341. doi: 10.1016/j.ecoenv.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Liao W-B, Zhang M-L, Huang G-B, Yu J-H. Ca2+ and CaM are involved in NO- and H2O2-induced adventitious root development in marigold. J Plant Growth Reg. 2012;31:253–264. doi: 10.1007/s00344-011-9235-7. [DOI] [Google Scholar]
- Lin W-D, Liao Y-Y, Yang TJ, Pan C-Y, Buckhout TJ, Schmidt W. Coexpression-based clustering of Arabidopsis root genes predicts functional modules in early phosphate deficiency signaling. Plant Physiol. 2011;155:1383–1402. doi: 10.1104/pp.110.166520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Rennenberg H, Kreuzwieser J. Hypoxia affects nitrogen uptake and distribution in young poplar (Populus × canescens) trees. PLoS ONE. 2015;10:e0136579. doi: 10.1371/journal.pone.0136579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29:1830–1831. doi: 10.1093/bioinformatics/btt285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Zhou JJ. Growth performance, photosynthesis, and root characteristics are associated with nitrogen use efficiency in six poplar species. Environ Exp Bot. 2019;164:40–51. doi: 10.1016/j.envexpbot.2019.04.013. [DOI] [Google Scholar]
- Luo J, Li H, Liu T, Polle A, Peng C, Luo Z-B. Nitrogen metabolism of two contrasting poplar species during acclimation to limiting nitrogen availability. J Exp Bot. 2013;64:4207–4224. doi: 10.1093/jxb/ert234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Qin J, He F, Li H, Liu T, Polle A, Peng C, Luo Z-B. Net fluxes of ammonium and nitrate in association with H+ fluxes in fine roots of Populus popularis. Planta. 2013;237:919–931. doi: 10.1007/s00425-012-1807-7. [DOI] [PubMed] [Google Scholar]
- Luo J, Zhou J, Li H, Shi W, Polle A, Lu M, Sun X, Luo Z-B. Global poplar root and leaf transcriptomes reveal links between growth and stress responses under nitrogen starvation and excess. Tree Physiol. 2015;35:1283–1302. doi: 10.1093/treephys/tpv091. [DOI] [PubMed] [Google Scholar]
- Luo J, Shi W, Li H, Janz D, Luo Z-B. The conserved salt-responsive genes in the roots of Populus × canescens and Arabidopsis thaliana. Environ Exp Bot. 2016;129:48–56. doi: 10.1016/j.envexpbot.2015.12.008. [DOI] [Google Scholar]
- Luo J, Zhou J-J, Zhang J-Z. Aux/IAA gene family in plants: molecular structure, regulation, and function. Int J Mol Sci. 2018;19:259. doi: 10.3390/ijms19010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Liang Z, Wu M, Mei L. Genome-wide identification of BOR genes in poplar and their roles in response to various environmental stimuli. Environ Exp Bot. 2019;164:101–113. doi: 10.1016/j.envexpbot.2019.04.006. [DOI] [Google Scholar]
- Luo J, Xia W, Cao P, Xiao ZA, Zhang Y, Liu M, Zhan C, Wang N. Integrated transcriptome analysis reveals plant hormones jasmonic acid and salicylic acid coordinate growth and defense responses upon fungal infection in poplar. Biomolecules. 2019;9:12. doi: 10.3390/biom9010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhang C, Zhang B, Yang C, Li S. Identification of genes regulated by histone acetylation during root development in Populus trichocarpa. BMC Genomics. 2016;17:96. doi: 10.1186/s12864-016-2407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriat M, Petterle A, Bellini C, Moritz T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014;78:372–384. doi: 10.1111/tpj.12478. [DOI] [PubMed] [Google Scholar]
- Moriya S, Iwanami H, Haji T, Okada K, Yamada M, Yamamoto T, Abe K. Identification and genetic characterization of a quantitative trait locus for adventitious rooting from apple hardwood cuttings. Tree Genet Genomes. 2015;11:59. doi: 10.1007/s11295-015-0883-9. [DOI] [Google Scholar]
- Pacurar DI, Perrone I, Bellini C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol Plant. 2014;151:83–96. doi: 10.1111/ppl.12171. [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Zhou Z, Zhang Z, Yu X, Zhang X, Du K. Molecular and physiological responses in roots of two full-sib poplars uncover mechanisms that contribute to differences in partial submergence tolerance. Sci Rep. 2018;8:12829. doi: 10.1038/s41598-018-30821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomion C, Bastien C, Bogeat-Triboulot M-B, Bouffier L, Déjardin A, Duplessis S, Fady B, Heuertz M, Le Gac A-L, Le Provost G, Legué V, Lelu-Walter M-A, Leplé J-C, Maury S, Morel A, Oddou-Muratorio S, Pilate G, Sanchez L, Scotti I, Scotti-Saintagne C, Segura V, Trontin J-F, Vacher C. Forest tree genomics: 10 achievements from the past 10 years and future prospects. An for Sci. 2016;73:77–103. doi: 10.1007/s13595-015-0488-3. [DOI] [Google Scholar]
- Qi X, Li Q, Ma X, Qian C, Wang H, Ren N, Shen C, Huang S, Xu X, Xu Q. Waterlogging-induced adventitious root formation in cucumber is regulated by ethylene and auxin through reactive oxygen species signalling. Plant Cell Environ. 2018;42:1458–1470. doi: 10.1111/pce.13504. [DOI] [PubMed] [Google Scholar]
- Quan J, Meng S, Guo E, Zhang S, Zhao Z, Yang X. De novo sequencing and comparative transcriptome analysis of adventitious root development induced by exogenous indole-3-butyric acid in cuttings of tetraploid black locust. BMC Genomics. 2017;18:179. doi: 10.1186/s12864-017-3554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Carvajal GA, Morse AM, Dervinis C, Davis JM. The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol. 2009;150:759–771. doi: 10.1104/pp.109.137505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro CL, Silva CM, Drost DR, Novaes E, Novaes CRDB, Dervinis C, Kirst M. Integration of genetic, genomic and transcriptomic information identifies putative regulators of adventitious root formation in Populus. BMC Plant Biol. 2016;16:66. doi: 10.1186/s12870-016-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigal A, Yordanov YS, Perrone I, Karlberg A, Tisserant E, Bellini C, Busov VB, Martin F, Kohler A, Bhalerao R. The AINTEGUMENTA LIKE1 homeotic transcription factor PtAIL1 controls the formation of adventitious root primordia in poplar. Plant Physiol. 2012;160:1996–2006. doi: 10.1104/pp.112.204453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald S, Jr, Zalesny JA. Selecting Populus with different adventitious root types for environmental benefits, fiber, and energy. In: Niemi K, Seagel C, editors. Adventitious root formation of forest trees and horticultural plants-from genes to applications. Kerala: Research Signpost; 2009. pp. 359–384. [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens B, Rasmussen A. The physiology of adventitious roots. Plant Physiol. 2016;170:603–617. doi: 10.1104/pp.15.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LR, Wang YB, He SB, Hao FS. Mechanisms for abscisic acid inhibition of primary root growth. Plant Signal Behav. 2018;13:e1500069. doi: 10.1080/15592324.2018.1500069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Jia HX, Zhang YH, Li JB, Lu MZ, Hu JJ. Deciphering genetic architecture of adventitious root and related shoot traits in Populus using QTL mapping and RNA-Seq data. Int J Mol Sci. 2019;20:6114. doi: 10.3390/ijms20246114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE. 2011;6:21800. doi: 10.1371/journal.pone.0021800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004;37:914–939. doi: 10.1111/j.1365-313X.2004.02016.x. [DOI] [PubMed] [Google Scholar]
- Thomas P, Schiefelbein J. Cloning and characterization of an actin depolymerizing factor gene from grape (Vitis vinifera L.) expressed during rooting in stem cuttings. Plant Sci. 2002;162:283–288. doi: 10.1016/S0168-9452(01)00569-6. [DOI] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protoc. 2012;7:562. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupiano D, Yordanov Y, Regan S, Meilan R, Tschaplinski T, Scippa GS, Busov V. Identification, characterization of an AP2/ERF transcription factor that promotes adventitious, lateral root formation in Populus. Planta. 2013;238:271–282. doi: 10.1007/s00425-013-1890-4. [DOI] [PubMed] [Google Scholar]
- Vielba JM, Díaz-Sala C, Ferro E, Rico S, Lamprecht M, Abarca D, Ballester A, Sánchez C. CsSCL1 is differentially regulated upon maturation in chestnut microshoots and is specifically expressed in rooting-competent cells. Tree Physiol. 2011;31:1152–1160. doi: 10.1093/treephys/tpr086. [DOI] [PubMed] [Google Scholar]
- Wang P, Du Y, Li Y, Ren D, Song C-P. Hydrogen peroxide-mediated activation of MAP Kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell. 2010;22:2981–2998. doi: 10.1105/tpc.109.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhao Y, Zhang B. Efficient test and visualization of multi-set intersections. Sci Rep. 2015;5:16923. doi: 10.1038/srep16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Cao P, Xia W, Fang L, Yu H. Identification and characterization of long non-coding RNAs in response to early infection by Melampsora larici-populina using genome-wide high-throughput RNA sequencing. Tree Genet Genomes. 2017;13:34. doi: 10.1007/s11295-017-1116-1. [DOI] [Google Scholar]
- Wei M, Liu QG, Wang ZC, Yang JL, Li WL, Chen YX, Lu H, Nie JF, Liu BG, Lv KW, Mao XL, Chen S, Sanders J, Wei HR, Li CH. PuHox52-mediated hierarchical multilayered gene regulatory network promotes adventitious root formation in Populus ussuriensis. New Phytol. 2020;228:1369–1385. doi: 10.1111/nph.16778. [DOI] [PubMed] [Google Scholar]
- Xia W, Yu H, Cao P, Luo J, Wang N. Identification of TIFY family genes and analysis of their expression profiles in response to phytohormone treatments and Melampsora larici-populina infection in poplar. Front Plant Sci. 2017;8:493. doi: 10.3389/fpls.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZA, Zhang Y, Liu MF, Zhan C, Yang XQ, Nvsvrot T, Yan ZG, Wang N. Coexpression analysis of a large-scale transcriptome identified a calmodulin-like protein regulating the development of adventitious roots in poplar. Tree Physiol. 2020;40:1405–1419. doi: 10.1093/treephys/tpaa078. [DOI] [PubMed] [Google Scholar]
- Xu L. De novo root regeneration from leaf explants: wounding, auxin, and cell fate transition. Curr Opin Plant Biol. 2018;41:39–45. doi: 10.1016/j.pbi.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Xu M, Xie W, Huang M. Overexpression of PeRHD3 alters the root architecture in Populus. Biochem Bioph Res Co. 2012;424:239–244. doi: 10.1016/j.bbrc.2012.06.083. [DOI] [PubMed] [Google Scholar]
- Xu M, Xie W, Huang M. Two WUSCHEL-related HOMEOBOX genes, PeWOX11a and PeWOX11b, are involved in adventitious root formation of poplar. Physiol Plant. 2015;155:446–456. doi: 10.1111/ppl.12349. [DOI] [PubMed] [Google Scholar]
- Xuan W, Xu S, Li M, Han B, Zhang B, Zhang J, Lin Y, Huang J, Shen W, Cui J. Nitric oxide is involved in hemin-induced cucumber adventitious rooting process. J Plant Physiol. 2012;169:1032–1039. doi: 10.1016/j.jplph.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wu Y, Ma L, Yang Z, Dong Q, Li Q, Ni X, Kudla J, Song C, Guo Y. The Ca2+ sensor SCaBP3/CBL7 modulates plasma membrane H+-ATPase activity and promotes alkali tolerance in Arabidopsis. Plant Cell. 2019;31:1367–1384. doi: 10.1105/tpc.18.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanov YS, Ma C, Yordanova E, Meilan R, Strauss SH, Busov VB. BIG LEAF is a regulator of organ size and adventitious root formation in poplar. PLoS ONE. 2017;12:e0180527. doi: 10.1371/journal.pone.0180527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics J Integr Biol. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhao Z, Zhang L, Zhou Q. Comparative proteomic analysis of tetraploid black locust (Robinia pseudoacacia L.) cuttings in different phases of adventitious root development. Trees. 2015;29:367–384. doi: 10.1007/s00468-014-1116-9. [DOI] [Google Scholar]
- Zhang Y, Xiao ZA, Zhan C, Liu M, Xia W, Wang N. Comprehensive analysis of dynamic gene expression and investigation of the roles of hydrogen peroxide during adventitious rooting in poplar. BMC Plant Biol. 2019;19:99. doi: 10.1186/s12870-019-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang X, Cao P, Xiao Z, Zhan C, Liu M, Nvsvrot T, Wang N. The bZIP53-IAA4 module negatively regulates adventitious root development in poplar. J Exp Bot. 2020;71:3485–3498. doi: 10.1093/jxb/eraa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhao X-W, He H, Wang Y-X, Zhang X. Mechanisms of extracellular NO and Ca2+ regulating the growth of wheat seedling roots. Journal Plant Biol. 2010;53:275–281. doi: 10.1007/s12374-010-9114-y. [DOI] [Google Scholar]
- Zhao X, Wang Y-L, Qiao X-R, Wang J, Wang L-D, Xu C-S, Zhang X. Phototropins function in high-intensity blue light-induced hypocotyl phototropism in Arabidopsis by altering cytosolic calcium. Plant Physiol. 2013;162:1539–1551. doi: 10.1104/pp.113.216556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J-J, Luo J. The PIN-FORMED auxin efflux carriers in plants. Int J Mol Sci. 2018;19:2759. doi: 10.3390/ijms19092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.