Abstract
DNA sensing and timely activation of interferon (IFN)-mediated innate immunity are crucial for the defense against DNA virus infections and the clearance of abnormal cells. However, overactivation of immune responses may lead to tissue damage and autoimmune diseases; therefore, these processes must be intricately regulated. STING is the key adaptor protein, which is activated by cyclic GMP-AMP, the second messenger derived from cGAS-mediated DNA sensing. Here, we report that CCDC50, a newly identified autophagy receptor, tunes STING-directed type I IFN signaling activity by delivering K63-polyubiquitinated STING to autolysosomes for degradation. Knockout of CCDC50 significantly increases herpes simplex virus 1 (HSV-1)- or DNA ligand-induced production of type I IFN and proinflammatory cytokines. Ccdc50-deficient mice show increased production of IFN, decreased viral replication, reduced cell infiltration, and improved survival rates compared with their wild-type littermates when challenged with HSV-1. Remarkably, the expression of CCDC50 is downregulated in systemic lupus erythematosus (SLE), a chronic autoimmune disease. CCDC50 levels are negatively correlated with IFN signaling pathway activation and disease severity in human SLE patients. CCDC50 deficiency potentiates the cGAS-STING-mediated immune response triggered by SLE serum. Thus, our findings reveal the critical role of CCDC50 in the immune regulation of viral infections and autoimmune diseases and provide insights into the therapeutic implications of CCDC50 manipulation.
Keywords: CCDC50, STING, Type I IFN, HSV-1, Autophagy, Autoimmune diseases
Subject terms: Cell signalling, Pattern recognition receptors
Introduction
In the coevolution of viruses and their hosts, host cells have developed an innate immune system that is initiated through recognition of pathogen-associated molecular patterns or damage-associated molecular patterns (DAMPs) by pattern-recognition receptors (PRRs) [1, 2]. PRRs detect different but conserved elements of microorganisms, including nucleic acids, carbohydrates, and lipoproteins. Due to their difference in subcellular location and specification of substrates, these sensors are categorized into different classes. Toll-like receptors (TLRs) are mainly localized in the plasma membrane or endosomal membrane and recognize extracellular pathogen components such as bacterial lipopolysaccharide [3], whereas intracellular sensors such as cyclic GMP-AMP synthase (cGAS) and RIG‑I‑like receptors (RLRs) are responsible for the detection of cytosolic DNA and RNA, respectively [4–6]. Moreover, PRRs can also be activated by DAMPs released from infected or injured cells [7]. cGAS senses the double-stranded DNA (dsDNA) of many DNA viruses, retroviruses, and even host self-DNA and then signals to its adaptor STING [8, 9]. After binding to nonself or self-DNA, cGAS undergoes conformational changes and exposes its enzymatic active site to catalyze the formation of 2’3’-cGAMP by using ATP and GTP [10, 11]. Then, cGAMP binds to STING, also known as MITA [8], MPYS [12], or ERIS [13], which is localized in the endoplasmic reticulum (ER). Upon binding to cGAMP, STING undergoes dimerization and lys63‑linked polyubiquitination and then forms additional higher-order oligomers. After activation, STING translocates to the ER-Golgi intermediate compartment (ERGIC) and the Golgi apparatus, where it recruits TBK1 and is phosphorylated by TBK1 [14], which further triggers IRF3 and NF-κB activation [8]. IRF3- and NF-κB-mediated transcriptional activities induce the expression of type I IFN and various inflammatory genes, thus establishing an antiviral state.
In addition to pathogen-derived DNA, the IFN immune response can also be activated by self-DNA leaked from damaged mitochondria or the nucleus since the recognition of DNA by cGAS is sequence independent. Therefore, the immune system must be intensively regulated; otherwise, dysfunction of immunity will cause autoimmune diseases such as systemic lupus erythematosus (SLE). SLE is a heterogeneous multiorgan autoimmune disease with complicated manifestations and is characterized by overwhelming autoantibodies and deregulated inflammation due to the loss of tolerance against self-antigens [15]. To date, the pathogenesis of SLE remains poorly elucidated. However, it has been proven that type I IFN plays key roles in SLE pathogenesis [16]. Increased production or bioavailability of type I IFN in SLE patients can propagate the autoimmune response through promotion of DC maturation and T cell development, priming neutrophils to go through NETosis and enhancing B-cell responses [17]. In turn, SLE is characterized by overactivation of type I IFN signaling and increased expression of IFN-stimulated genes (ISGs) [18]. Given the importance of type I IFN in SLE pathogenesis, new treatments inhibiting the activation of the type I IFN system in SLE are now being studied in clinical trials [19].
Recent studies have suggested that an important role is played by autophagy defects in IFN responses and SLE disease susceptibility and progression [20–22]. Macroautophagy (hereafter referred to as autophagy) is recognized as a cellular scavenger that degrades intracellular components, such as misfolded proteins and damaged or senescent organelles [23]. Autophagy helps recycle nutrients and maintain host homeostasis. However, the functions of autophagy during microbial infection are controversial and complicated. On the one hand, it can degrade key components of invasive microorganisms, including viruses and bacteria [24–26]; on the other hand, the activation of PRRs can induce autophagy to restrict innate immune responses by delivering signaling proteins for degradation [27, 28]. Autophagy is also involved in the regulation of adaptive immunity, such as antigen presentation and T cell development and differentiation [29, 30]. Currently, mounting studies have provided evidence that autophagy deficiency is associated with the pathogenesis and progression of autoimmune diseases such as SLE [31–33]. However, how autophagy regulates the IFN response and autoimmune disease is not well defined.
CCDC50, a newly identified autophagic cargo adaptor by our group, recognizes K63-polyubiquitinated RLRs and mediates their subsequent autolysosome-dependent degradation, thereby negatively regulating RNA virus-induced RLR-mediated type I IFN production [22]. In the present study, we revealed an essential role of CCDC50 in STING-mediated immune responses in regulating DNA virus infection and SLE pathogenesis. We showed that CCDC50 is downregulated in human SLE and is negatively correlated with SLE disease progression, which is attributed to its alteration of STING-IFN activation.
Results
CCDC50 inhibits the dsDNA-induced type I IFN signaling pathway
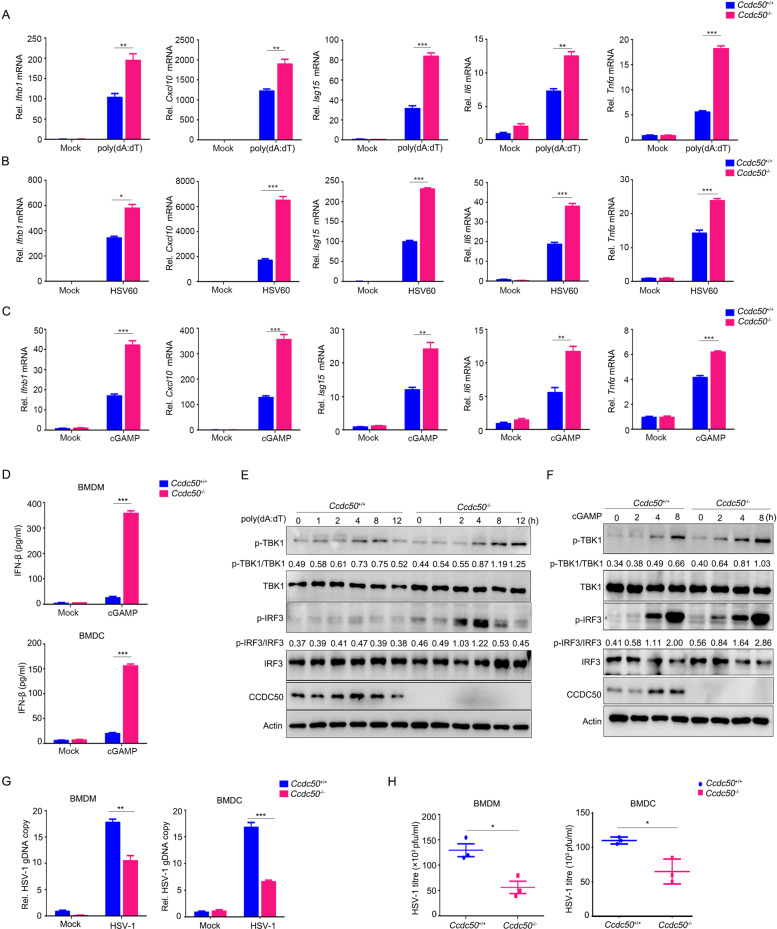
Our previous work demonstrated that CCDC50 negatively regulates RNA virus-induced IFN activation through autophagy [22]. To test whether endogenous CCDC50 is also involved in the regulation of the dsDNA-triggered signaling pathway, we generated CCDC50-knockout (KO) human acute monocytic leukemia THP-1 cells using the CRISPR/Cas9 system with single short guide RNAs (sgRNAs). The results showed that CCDC50 deficiency robustly enhanced dsDNA-induced transcription of IFNB1, CXCL10, ISG15, IL6, and TNFα in THP-1 cells (Fig. 1A, B and Supplementary Fig. S1A), including poly(dA:dT) and HSV60, 60-bp oligonucleotides derived from the HSV-1 genome; accordingly, knocking out CCDC50 also potentiated the production and secretion of IFN-β induced by poly(dA:dT) and HSV60 (Fig. 1C). In addition, the phosphorylation of TBK1 and IRF3, an activation hallmark of type I IFN downstream of PRRs, was markedly increased in CCDC50-KO THP-1 cells compared to CCDC50 wild-type (CCDC50-WT) cells when treated with DNA ligands or cGAMP (Fig. 1D, E and Supplementary Fig. S1B). To further investigate the potential role of the CCDC50-regulated immune response against DNA viruses, we challenged CCDC50-WT and CCDC50-KO THP-1 cells with HSV-1. We observed that the replication of HSV-1 was much lower in CCDC50-deleted cells than in CCDC50-WT cells, as determined by the relative copy number of HSV-1 genomic DNA (gDNA) (Fig. 1F). Consistently, knockout of CCDC50 significantly inhibited viral loads, as further determined by plaque assays (Fig. 1G and Supplementary Fig. S1C), suggesting that deficiency of CCDC50 inhibits viral infection and propagation. Similarly, the phosphorylation of TBK1 and IRF3 triggered by HSV-1 was enhanced in CCDC50-KO cells compared to CCDC50-WT cells (Fig. 1H). Taken together, these results indicated that CCDC50 acts as a negative regulator in cytosolic dsDNA- and DNA virus-triggered activation of the type I IFN signaling pathway.
Fig. 1.
Deficiency of CCDC50 enhances dsDNA-induced activation of the type I IFN response. A, B qRT-PCR assay detection of the mRNA levels of IFNB1, CXCL10, ISG15, IL6, and TNFα in CCDC50-WT and CCDC50-KO THP-1 cells induced with poly(dA:dT) (A) and HSV60 (B) for the indicated time points. C ELISAs of IFN-β production in THP-1 cells treated with poly(dA:dT) and HSV60 for 8 h. D, E Immunoblot analysis of phosphorylated TBK1 and IRF3, total TBK1, IRF3, and CCDC50 in CCDC50-WT and CCDC50-KO THP-1 cells stimulated with poly(dA:dT) (D) or cGAMP (E) for the indicated time points. F qRT-PCR analysis of HSV-1 genomic DNA in CCDC50-WT and CCDC50-KO THP-1 cells infected with HSV-1 for 12 h. G Plaque assay of HSV-1 titers in the supernatant of CCDC50-WT and CCDC50-KO THP-1 cells infected with HSV-1 for 12 h. H Immunoblot analysis of cell lysates collected from CCDC50-WT and CCDC50-KO THP-1 cells infected with HSV-1 for the indicated time points; mRNA levels were normalized to GAPDH relative to those of untreated wild-type cells, and the relative copy number of HSV-1 genomic DNA was normalized to HBB; actin was used as a loading control. The expression levels of p-TBK1, p-IRF3, total TBK1, and IRF3 were quantitated using ImageJ software. Data are representative of three independent experiments, and error bars are shown as the mean with SEM (A–C, F, G). See also Supplementary Fig. S1. *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired Student’s t-test
Enhanced cellular immune responses against dsDNA in CCDC50-deficient primary cells
To further investigate the physiological significance of CCDC50 in dsDNA-induced antiviral immune responses, we generated CCDC50-KO mice on a C57BL/6J background by using CRISPR/Cas9-based technology (Supplementary Fig. S2A, B). The Ccdc50–/– mice were born normally in both sex ratios and numbers. These Ccdc50–/– mice grew normally and had no behavioral abnormalities compared with their wild-type littermates. We collected bone marrow-derived macrophages (BMDMs), dendritic cells (BMDCs), and various organs, including the brain, lung, spleen, and liver, from Ccdc50+/+ and Ccdc50–/– mice and confirmed the deletion of Ccdc50 (Supplementary Fig. S2C, D). Although CCDC50 is highly expressed in immune organs and cells, the cell number in the thymus was normal in 6- to 8-week-old Ccdc50–/– mice (Supplementary Fig. S2E), suggesting that CCDC50 did not affect thymus development. We then isolated BMDMs and BMDCs and stimulated the cells with cGAS inducers poly(dA:dT) and HSV60 and STING agonist cGAMP and found that both BMDMs and BMDCs from Ccdc50-deficient mice showed significantly upregulated transcription of Ifnb1, Cxcl10, and Isg15 and proinflammatory cytokines Il6 and Tnfα in comparison with those from Ccdc50+/+ mice (Fig. 2A–C and Supplementary Fig. S3A–C). Ccdc50–/– BMDMs and BMDCs also showed higher production and secretion of IFN-β, as shown by enzyme-linked immunosorbent assay (ELISA) (Fig. 2D). Moreover, the phosphorylation levels of TBK1 and IRF3 were higher in Ccdc50–/– BMDMs and BMDCs than in Ccdc50+/+ cells following poly(dA:dT) transfection or cGAMP induction, suggesting that Ccdc50 deficiency augmented the cGAS-STING pathway and increased IFN activation (Fig. 2E, F and Supplementary Fig. S3D, E). We then infected these primary immune cells with HSV-1 and observed that the replication of viral gDNA and viral titers of HSV-1 collected from cell culture supernatant significantly decreased in BMDMs and BMDCs from Ccdc50–/– mice in comparison with those from Ccdc50+/+ mice, suggesting that CCDC50 deficiency inhibits HSV-1 infection and propagation (Fig. 2G, H and Supplementary Fig. S3F). Taken together, these studies demonstrated that CCDC50 deficiency potentiates the dsDNA-induced immune response and suppresses viral infection in cells, indicating that CCDC50 is involved in the negative regulation of cGAS-STING-mediated type I IFN activation.
Fig. 2.
BMDMs from Ccdc50–/– mice produced higher levels of IFN and were more resistant to HSV-1 infection. A–C qRT-PCR analysis of Ifnb1, Cxcl10, Isg15, Il6, and Tnfα in BMDMs from Ccdc50+/+ and Ccdc50–/– mice following stimulation with poly(dA:dT) (A), HSV60 (B), and cGAMP (C). D ELISAs of IFN-β production in BMDMs and BMDCs from Ccdc50+/+ and Ccdc50–/– mice treated with cGAMP for 8 h. E, F Western blot analysis of cell lysates of BMDMs stimulated with poly(dA:dT) (E) and cGAMP (F) for the indicated time points. G qRT-PCR assay of HSV-1 genomic DNA in BMDMs and BMDCs from Ccdc50+/+ and Ccdc50–/– mice infected with HSV-1 for 12 h. H Viral titers in supernatant collected from BMDMs and BMDCs treated as in G. mRNA levels were normalized to GAPDH, and the relative copy number of HSV-1 genomic DNA was normalized to Tert and relative to those of mock-treated wild-type cells; actin was used as a loading control. The expression levels of p-TBK1, p-IRF3, total TBK1, and IRF3 were quantitated using ImageJ software. Data are representative of three independent experiments, and error bars are shown as the mean with SEM (A–D, G, H). See also Supplementary Fig. S2. *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired Student’s t-test
Ccdc50–/– mice were more resistant than Ccdc50+/+ mice to HSV-1 infection
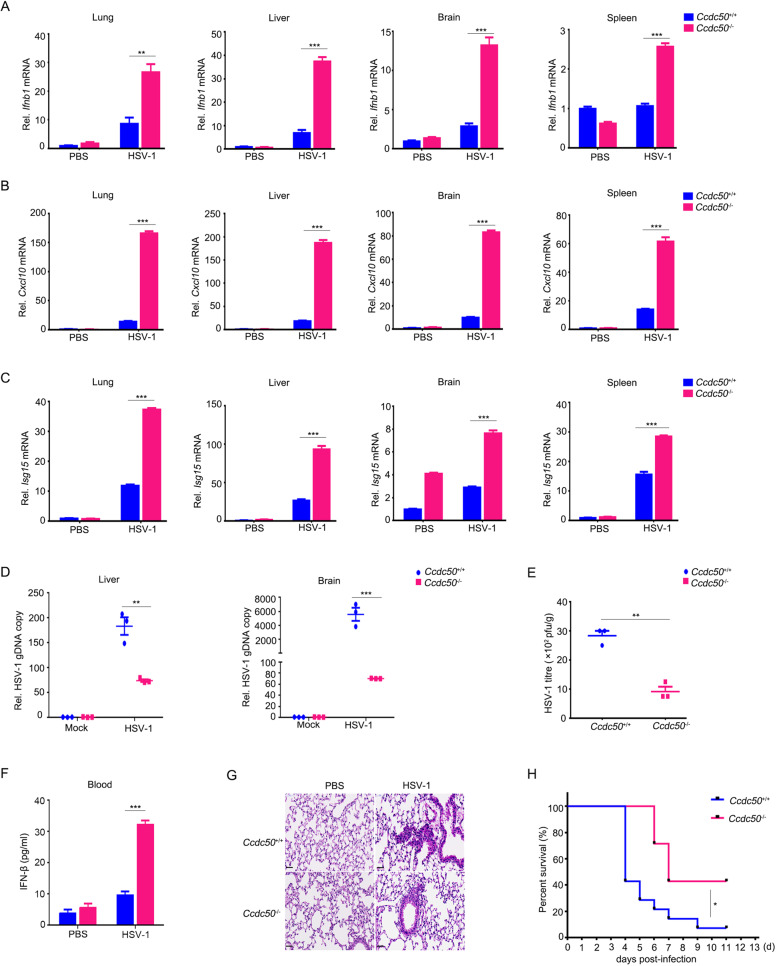
Next, we determined the biological functions of Ccdc50 in host defense against viral infection in vivo. Sex- and age-matched Ccdc50+/+ mice and Ccdc50–/– mice were challenged through intravenous injection of HSV-1 at a lethal dose (4 × 108 plaque-forming units per mouse). Remarkably, Ccdc50–/– mice showed substantially increased mRNA expression of Ifnb1, Cxcl10, and Isg15 in the lung, liver, brain, and spleen (Fig. 3A–C). In contrast, the replication of HSV-1 decreased as the relative gDNA copy number of HSV-1 was reduced in the brain and liver from Ccdc50–/– mice compared to those of Ccdc50+/+ littermates (Fig. 3D). As HSV-1 is a neurotropic virus, we dissociated the brain to determine the viral loads. The titers of HSV-1 decreased significantly in the brains isolated from Ccdc50–/– mice in comparison with those from Ccdc50+/+ control mice (Fig. 3E and Supplementary Fig. S3G). Consistent with the above result, ELISA showed significantly higher levels of IFN-β in serum from Ccdc50–/– mice than in serum from their wild-type counterparts (Fig. 3F). In addition, Ccdc50–/– mice showed an enhanced ability to eliminate virus and reduced inflammatory cell infiltration and tissue damage in the lung after HSV-1 infection (Fig. 3G). In accordance, Ccdc50–/– mice showed more resistance to HSV-1 infection with a higher overall survival rate than their wild-type littermates (Fig. 3H). Overall, these data demonstrate that CCDC50 deficiency enhances DNA virus-triggered innate immune responses, suggesting a physiological function of CCDC50 in the defense against viral infection.
Fig. 3.
Deficiency of CCDC50 protects mice from HSV-1 infection. A–C qRT-PCR analysis of Ifnb1 (A), Cxcl10 (B), and Isg15 (C) mRNAs in the lung, liver, brain, and spleen from Ccdc50+/+ and Ccdc50–/– mice infected with HSV-1 (4 × 108 PFU per mouse) by intravenous injection for 16 h. D qRT-PCR analysis of HSV-1 genomic DNA in the liver and brain of mice intravenously infected with HSV-1 for 3 days. E Plaque assay of HSV-1 titers in the brains of Ccdc50+/+ and Ccdc50–/– mice treated as in D. F ELISAs of IFN-β in serum from Ccdc50+/+ and Ccdc50–/– mice treated as in A. G Hematoxylin and eosin staining of lung sections isolated from Ccdc50+/+ and Ccdc50–/– mice infected with HSV-1 by intravenous injection for 16 h (scale bar: 100 μm). H Survival curves for sex- and age-matched Ccdc50+/+ and Ccdc50–/– mice infected with HSV-1 (Ccdc50+/+, n = 14; Ccdc50–/–, n = 7); mRNA levels are relative to those of PBS-treated wild-type mice; actin was used as a loading control. Data are representative of three independent experiments, and error bars are shown as the mean ± SEM (A–F). See also Supplementary Fig. S3. *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired Student’s t-test. The log-rank (Mantel–Cox) test was used for analysis of the survival curve of mice
CCDC50 associates with and targets STING for autophagic degradation
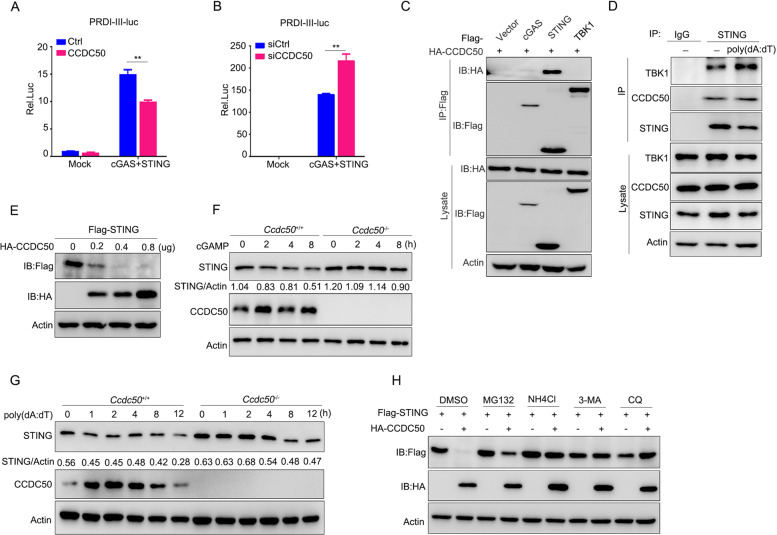
Next, we examined the molecular mechanism of CCDC50 in the regulation of dsDNA-induced activation of the cGAS-STING pathway. The results that both DNA ligand- and cGAMP-induced signaling activation could be altered by CCDC50 suggested that CCDC50 acts at steps downstream of cGAMP in the cGAS-cGAMP-STING pathway (Figs. 1 and 2). Furthermore, a dual-luciferase experiment demonstrated that transient expression of CCDC50 resulted in reduced PRDI-III reporter activity induced by cGAS-STING (Fig. 4A). Similarly, knockdown of CCDC50 enhanced the PRDI-III transcriptional activity triggered by cGAS-STING (Fig. 4B). Our previous work had already shown that CCDC50 had no effect on TBK1- and IRF3-induced transcriptional activation [22]. We further determined the association between CCDC50 and the components of cGAS, STING, and TBK1. Coimmunoprecipitation experiments showed that CCDC50 could interact with STING but had no interaction with cGAS or TBK1 (Fig. 4C). Endogenous immunoprecipitation assays revealed that CCDC50 had a strong association with STING even in resting cells (Fig. 4D). We constructed two truncations of STING, including the N-terminal transmembrane domain and C-terminal TBK1-binding domain, to identify which domain of STING is responsible for the interaction with CCDC50 (Supplementary Fig. S4A). A coimmunoprecipitation experiment with the truncated mutants showed that the N-terminal transmembrane domain of STING mediated the interaction (Supplementary Fig. S4B). We then generated five truncations of CCDC50 containing different domains and motifs and found that the N-terminal coiled-coil domain is indispensable for the interaction (Supplementary Fig. S4C, D). Taken together, these data indicate that CCDC50 can physically target STING through its own N-terminal coiled-coil domain.
Fig. 4.
CCDC50 associates with STING and targets it for autophagic degradation. A Dual-luciferase activity of PRDI-III-luc in HEK293 cells transfected with plasmids expressing cGAS plus STING along with CCDC50 or empty vector as a control (Ctrl) for 24 h. B Dual-luciferase activity of PRDI-III-luc in HEK293 cells transfected for 24 h with CCDC50 siRNA or nontargeting siRNA (siCtrl) and then transfected with plasmids expressing cGAS plus STING for another 24 h. C Coimmunoprecipitation analysis of the interaction between CCDC50 and cGAS, STING, and TBK1 in cotransfected HEK293 cells. D Immunoprecipitation analysis of the endogenous interaction between CCDC50 and STING in THP-1 cells stimulated with poly(dA:dT) for 4 h. E Immunoblot analysis of lysates from HEK293 cells cotransfected with Flag-STING and an increasing amount of HA-CCDC50. F, G Immunoblot analysis of endogenous STING in Ccdc50+/+ and Ccdc50–/– BMDMs treated with cGAMP (F) and poly(dA:dT) (G) for the indicated time points. H Immunoblot analysis of cell lysates from HEK293 cells cotransfected with Flag-STING and control vector or HA-CCDC50. Fourteen hours after transfection, the cells were treated with DMSO, MG132 (25 μM), NH4Cl (10 mM), 3-MA (5 mM), or CQ (20 μM) for 6 h; actin was used as a loading control. The expression levels of STING and actin were quantitated using ImageJ software. Data are representative of three independent experiments, and error bars are shown as the mean ± SEM (A, B). See also Supplementary Fig. S4. *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired Student’s t-test
CCDC50 is a novel autophagic adaptor recently identified by our group [22]. Therefore, we next examined the stability of STING and observed that ectopic expression of CCDC50 caused degradation of STING in a CCDC50 dose-dependent manner in HEK293 cells (Fig. 4E). Moreover, the turnover of STING induced by cGAMP and poly(dA:dT) was delayed in CCDC50-KO cells, where higher levels of STING were observed (Fig. 4F, G and Supplementary Fig. S4E, F). The cycloheximide chase assay showed that deficiency of CCDC50 decreased the degradation of STING, resulting in increased levels of IFN-induced cGAS (Supplementary Fig. S4G, H). We then used various inhibitors, such as the lysosome inhibitor NH4Cl, proteasome inhibitor MG132, and autophagy inhibitors 3-methyladenine (3-MA) and chloroquine (CQ), to block the two major intracellular degradation pathways. The results showed that NH4Cl, 3-MA, and CQ completely blocked CCDC50-mediated degradation of STING, while MG132 displayed a weak recovery effect, suggesting that CCDC50-mediated STING degradation took place via autophagic processes (Fig. 4H). Collectively, these results demonstrate that CCDC50 targets STING for autophagic degradation.
CCDC50 recognizes K63-polyubiquitinated STING and delivers it to LC3-positive autophagosomes
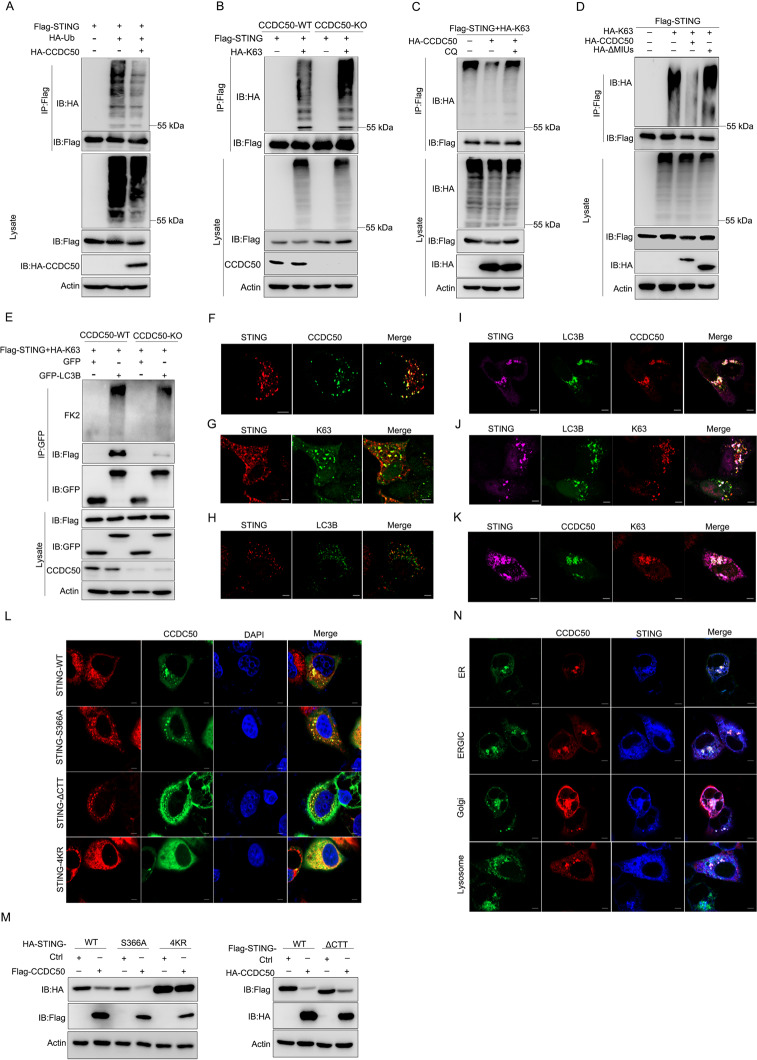
It has been proven that K63-linked polyubiquitination is essential for STING activation [34, 35], while CCDC50 recognizes K63-polyubiquitinated substrates using its MIU motifs, as shown in previous studies [22, 36, 37]. To investigate whether the ubiquitination of STING was required for CCDC50-directed degradation, we measured the level of polyubiquitinated STING. The results showed that CCDC50 mediated the degradation of ubiquitin-positive STING (Fig. 5A). We further found that CCDC50 induced degradation of K63-linked, but not K48-linked, polyubiquitinated, STING (Supplementary Fig. S4I). We also measured the level of K63-linked polyubiquitination of STING in CCDC50-WT and CCDC50-deficient HEK293 cells. The results showed that K63-polyubiquitinated STING was significantly increased in CCDC50-KO cells compared with wild-type control cells (Fig. 5B). Treatment with the autophagy inhibitor CQ blocked degradation and restored the level of K63-Ub-conjugated STING (Fig. 5C). The mutant of CCDC50, with deletion of MIU motifs that are responsible for binding of K63-polyubiquitin chains, also reversed the level of K63-polyubiquitinated STING (Fig. 5D), suggesting that K63-linked polyubiquitination is a prerequisite for CCDC50-mediated autophagic degradation of STING.
Fig. 5.
CCDC50 recognizes K63-polyubiquitinated STING. A Denature-IP (with anti-Flag) and immunoblot analysis of polyubiquitin-conjugated STING in HEK293 cells transfected with Flag-STING, HA-Ub (ubiquitin), or HA-CCDC50 or empty vector for 24 h. B Denature-IP (with anti-Flag) analysis of K63-linked polyubiquitination of STING in CCDC50 wild-type (CCDC50-WT) and CCDC50-knockout (CCDC50-KO) HEK293 cells transfected with Flag-STING and HA-K63-Ub. C Denature-IP analysis (with anti-Flag) of K63-linked polyubiquitination of STING in HEK293 cells transfected with plasmids expressing Flag-STING, HA-Ub-K63, and HA-CCDC50 or empty vector for 24 h and then left untreated or treated with CQ for 6 h. D Denature-IP (with anti-Flag) and immunoblot analysis of K63-linked polyubiquitinated STING in HEK293 cells cotransfected with Flag-STING, HA-K63-Ub, and HA-CCDC50-WT or HA-CCDC50-ΔMIUs for 24 h. E HEK293 cells were cotransfected with Flag-STING plus HA-K63-Ub and GFP-LC3B or GFP. GFP was immunoprecipitated to determine the coimmunoprecipitation of ubiquitinated proteins. F Confocal microscopy of colocalization between STING and CCDC50 in HeLa cells transfected with Flag-STING (red) and GFP-CCDC50 for 24 h; scale bars, 5 μm. G The association between STING and K63-Ub in HeLa cells scanned by confocal microscopy; scale bars, 5 μm. H–J Colocalization of STING and LC3B without (H) or with CCDC50 (I) or K63-Ub (J) in HeLa cells transfected with Flag-STING and GFP-LC3B along with control vector or HA-CCDC50 or HA-K63-Ub plasmids; scale bars, 5 μm. K Confocal microscopy analysis of HeLa cells transfected with STING (purple) and GFP-CCDC50 as well as HA-Ub-K63 (red); the scale bars shown are 5 μm. L HeLa cells were transfected with plasmids encoding GFP-CCDC50 and wild-type STING or its mutants and stimulated with cGAMP for 2 h. Formation of CCDC50-STING puncta was detected by confocal microscopy; scale bars, 5 μm. M Immunoblot analysis of lysates from HEK293 cells transfected with wild-type STING or its mutants as well as an empty control plasmid or a plasmid encoding CCDC50. N Confocal microscopy analysis of HeLa cells cotransfected with Flag-STING, HA-CCDC50, and GFP-tagged Rab9 (ER marker), p58 (ERGIC marker), GM130 (Golgi marker), or LAMP1 (lysosome marker) and then stimulated with cGAMP for 2 h; scale bars, 5 μm. Data are representative of three individual experiments. See also Supplementary Fig. S4
Next, we tested the interaction of phagophore membrane protein LC3 and polyubiquitinated STING in CCDC50-WT and CCDC50-KO cells. To monitor polyubiquitinated STING we used an FK2 monoclonal antibody that could recognize K63-linked polyubiquitylated proteins but not K48-linked polyubiquitinated proteins or free ubiquitin. Strikingly, we found that GFP-LC3B could coimmunoprecipitate substantial amounts of ubiquitylated proteins in CCDC50-WT cells but could barely coimmunoprecipitate ubiquitinated STING in CCDC50-KO cells, and the level of immunoprecipitated STING was also significantly reduced in CCDC50-KO cells compared to CCDC50-WT cells (Fig. 5E). All of these data suggest that CCDC50 delivers K63-Ub-conjugated STING to LC3-positive autophagosomes.
Given that the degradation of STING in autophagosomes could be visualized by confocal microscopy, we first confirmed the colocalization between STING and CCDC50 (Fig. 5F). Furthermore, we found that STING strongly colocalized with K63-linked ubiquitin chains (Fig. 5G). We then used confocal microscopy to verify the interaction of STING and LC3B, a well-established hallmark of autophagosomes. STING poorly colocalized with LC3B in resting cells, but activation of CCDC50 and K63 polyubiquitination of STING markedly enhanced the association of STING-LC3B and led to the formation of typical autophagic puncta (Fig. 5H–J). As expected, confocal microscopy revealed that CCDC50 colocalized with K63-ubiquitin-positive STING (Fig. 5K). Upon cGAMP binding to STING, STING is translocated from the ER to the ER-Golgi and then the Golgi apparatus, during which STING is polyubiquitylated and recruits and activates TBK1 and IRF3. It was reported that ΔCTT deletion of the C-terminal domain of STING-ΔCTT abolished the phosphorylation of TBK1 and IRF3 and that the S366A mutation (STING-S366A) blocked IRF3 phosphorylation [38]. The K20, K150, K224, and K236 sites are essential for K63-linked polyubiquitination of STING, and simultaneous mutation of all four lysine residues (K20R/K150R/K224R/K236R, short for STING-4KR) abolished its ubiquitination [34, 35]. To further delineate how CCDC50 acts in the STING activation pathway, we examined the association between CCDC50 and a series of STING mutants. The results showed that CCDC50 could still bind to these mutants of STING. Similar to wild-type STING, STING-S366A and STING-ΔCTT still effectively promoted STING-CCDC50 puncta formation. Remarkably, the STING-4KR mutant reduced the formation of STING and CCDC50 puncta induced by cGAMP (Fig. 5L). We also determined the degradation of the STING mutants mediated by CCDC50, and the results showed that CCDC50 could promote the degradation of STING-S366A and STING-ΔCTT but not STING-4KR (Fig. 5M). To investigate whether the reduced K63-polyubiquitinated STING was due to the reduced level of the E3 ligase of STING, we expressed TRIM32 and CCDC50 in HEK293T cells. CCDC50 had no effect on the stability of TRIM32 (Supplementary Fig. S4J). These results suggested that CCDC50 delivers K63-polyubiquitination-activated STING for degradation and that the recruitment of TBK1 and IRF3 is dispensable for CCDC50-mediated autophagy.
We then examined whether CCDC50 was involved in STING trafficking using confocal microscopy. Under cGAMP stimulation, CCDC50-STING puncta also colocalized with markers for the ER, ERGIC, and Golgi. The compartments in the autophagosomes continued to traffic to the lysosome (Fig. 5N). Moreover, speck-like STING-positive structures were less positive for LC3 in CCDC50-KO cells than in CCDC50-WT cells (Supplementary Fig. S4K). Consistently, STING-positive puncta colocalized with Golgi and lysosome markers were also reduced in CCDC50-deficient cells compared to CCDC50-WT cells (Supplementary Fig. S4L). Thus, CCDC50 is involved in STING trafficking and is essential for engaging STING with the autophagy machinery following cGAMP stimulation. We also determined whether the function of CCDC50 is dependent on p62, a thoroughly studied autophagic adaptor protein. We used p62-KO cells, and the data showed that CCDC50 could still interact with STING (Supplementary Fig. S4M) and promote the degradation of STING without p62. Moreover, treatment with the autophagy inhibitor CQ restored the protein level of STING in p62-deleted cells (Supplementary Fig. S4N). Moreover, ectopic expression of CCDC50 promoted the degradation of K63-polyubiquitinated STING, and CQ treatment blocked its effect (Supplementary Fig. S4O) in the absence of p62, suggesting that the recognition and degradation of STING by CCDC50 is independent of p62. Taken together, these results suggested that CCDC50 promotes the delivery of K63-polyubiquitinated STING to LC3B-marked autophagosomes and sequential autophagic degradation independent of p62.
CCDC50 is downregulated in SLE patients, and its expression is negatively correlated with human SLE progression
SLE is characterized by an autoantigen complex of dsDNA and an antibody against DNA and enhanced activation of the cGAS-STING pathway. Therefore, we investigated the association between CCDC50 and SLE disease progression. We first analyzed CCDC50 expression levels in four different cohorts from the publicly available Gene Expression Omnibus (GEO) database (GSE: 61635, 121239, 72509, and 65391) [39–41]. As shown in Fig. 6A, CCDC50 mRNA levels were significantly lower in SLE patients than in healthy donors (HDs). We next examined the relationship between CCDC50 and type I IFN expression. The data showed that CCDC50 expression was negatively correlated with IFNα expression, especially in SLE patients (Fig. 6B). Although the causes of SLE are uncertain, defects in the negative regulation of the IFN response may contribute to disease progression. Therefore, we evaluated the association between CCDC50 and the IFN signaling pathway in SLE. As expected, CCDC50 expression was negatively related to the activation of the type I IFN pathway in SLE patients (Fig. 6C). To determine whether CCDC50 affected disease progression in human SLE patients, we reanalyzed three public datasets with the disease activity index (GSE: 49454, 72798, and 121239) [39, 42, 43]. Notably, CCDC50 mRNA levels were negatively correlated with the SLE disease activity index (SLEDAI) (Fig. 6D). Overall, these results indicated that CCDC50 is negatively correlated with human SLE pathogenesis.
Fig. 6.
The expression level of CCDC50 is negatively correlated with SLE disease progression. A Bee-swarm plots of the expression levels of CCDC50 in SLE patients and healthy donors (HD) in four SLE cohorts. One plot represents one GEO dataset. The mean and standard deviation (black dots and lines) are also plotted. Differences between the SLE and HD groups were quantified by limma-adjusted P values. B Correlation between the expression levels of CCDC50 and IFNα1 in the SLE and HD groups. Pearson’s correlation coefficients and the P values are also displayed. C Gene set enrichment analysis (GSEA) shows that CCDC50 is significantly associated with the type I IFN pathway in different GEO datasets. Enrichment scores (ES) are also displayed. D Correlations between CCDC50 expression and the SLE disease activity index (SLEDAI) in patients. Correlation coefficients r and P values were calculated using Pearson’s correlation; each dot represents a gene expression value from HD or SLE patients
CCDC50 inhibits the activation of the dsDNA-induced type I IFN pathway in SLE PBMCs
To further explore the potential functions of CCDC50 in SLE, we extracted and analyzed blood samples from HDs and SLE patients. In SLE, an overactive IFN signature and exacerbated inflammation are mainly induced by self-DNA, the inducer of the cGAS-STING pathway. Hence, after isolating peripheral blood mononuclear cells (PBMCs), we first determined the activation of the cGAS-STING-mediated DNA-sensing pathway, and we observed significantly higher expression of IFNB1, IFNα1, and cytokines IL6 and TNFα in SLE patients than in HDs after poly(dA:dT) and HSV60 stimulation (Fig. 7A), indicating increased activation of the cGAS-STING pathway in SLE patients. We then confirmed that the mRNA and protein levels of CCDC50 in PBMCs from SLE patients were significantly lower than those in PBMCs from HDs (Fig. 7B, C), which was consistent with the results we obtained from the database analysis (Fig. 6A). Notably, in SLE patients, lower levels of CCDC50 mRNA were associated with higher levels of serum IFN-β, further demonstrating that CCDC50 mRNA levels in SLE patients were negatively correlated with the production of IFN-β (Fig. 7D). More intriguingly, we found higher expression of type I IFNs and proinflammatory cytokines in CCDC50-KO THP-1 cells than in their wild-type control cells after treatment with SLE serum, suggesting that CCDC50 negatively regulates the DNA-sensing pathway in SLE patients (Fig. 7E). Collectively, these data indicated that CCDC50 may be involved in negative regulation in SLE disease progression because of its inhibition of the cGAS-STING signaling pathway.
Fig. 7.
Reduced levels of CCDC50 and increased activity of type I IFN signaling in SLE patients. A qRT-PCR analysis of the mRNA levels of IFNB1, IFNα1, IL6, and TNFα in PBMCs isolated from SLE patients and HD treated with poly(dA:dT) and HSV60. B The mRNA level of CCDC50 in PBMCs was significantly decreased in SLE patients (n = 11) compared to healthy donor controls (HD, n = 22). C Immunoblot analysis of the protein level of CCDC50 in PBMCs of SLE patients and HDs (n = 6). D Correlation analysis of CCDC50 mRNA expression with serum IFN-β levels in PBMCs from SLE patients. E qRT-PCR assay of mRNA levels of IFNB1, IFNα1, IL6, and CXCL10 in CCDC50 wild-type (WT) and CCDC50-knockout (KO) THP-1 cells treated with SLE sera; mRNA results were normalized to GAPDH and relative to those of untreated wild-type cells; actin was used as a loading control. All experiments were repeated one or two times and are shown as the mean ± SEM (A, B, D). *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired Student’s t-test. Correlation coefficients r and P values were calculated using Pearson’s correlation
Discussion
cGAS is a DNA sensor and conveys signals to STING by generating the second messenger cGAMP [44]. The production of IFN and proinflammatory cytokines establishes a stable antiviral state to eliminate the invaded intracellular pathogen. However, aberrant activation of the cGAS-STING pathway can cause severe autoimmune or autoinflammatory diseases. Therefore, this pathway must be tightly regulated. Posttranslational modifications such as phosphorylation, dephosphorylation, ubiquitination, and deubiquitination play key roles in the activation and regulation of nucleic acid sensing and the downstream signaling pathways [45]. It was reported that the E3 ubiquitin ligase tripartite motif proteins TRIM56 [34] and TRIM32 [35] can interact with STING and target it for K63-linked polyubiquitination, resulting in enhanced type I IFN activity. Therefore, K63-linked ubiquitination is crucial for the activation of STING and usually acts as an activation signal for STING. Here, our study reveals a role of CCDC50 in the negative regulation of the STING-directed response to dsDNA.
CCDC50 functions as an autophagic cargo receptor to recognize K63-polyubiquitinated STING and delivers the substrate to autolysosomes for degradation, thereby inhibiting the downstream signaling activation of STING. We found that ectopic expression of CCDC50 caused dose-dependent degradation of STING and that treatment with autophagy inhibitors blocked CCDC50-mediated degradation of STING. We also observed that CCDC50 promoted K63-polyubiquitinated STING to translocate to LC3-positive autophagosomes and that the level of K63-polyubiquitinated STING was increased in CCDC50-deficient cells. CCDC50 could recognize the K63-Ub activation signal and then direct activated STING for degradation. The likely role for such regulation may be to prevent overactivation of STING to maintain immune homeostasis and avoid damage to the host. We found that CCDC50 recognized K63-polyubiquitinated RLR [22], whose K63-E3 ligases were reported to be TRIM25 [46] and Riplet [47], while the K63-E3 ligases of STING were reported to be TRIM32 [35] and TRIM56 [34]. Therefore, we did not observe the preference of CCDC50 for a specific E3 ligase, and CCDC50 had no effect on the stability of TRIM32. Thus, CCDC50 delivers K63-polyubiquitination-activated substrates but has no E3 ligase preferences or site specificity. CCDC50 deficiency potentiates dsDNA- or HSV-1-induced cGAS-STING pathway activation and increases the production of type I IFN. We further found that Ccdc50-deficient mice showed an enhanced ability to eliminate the virus and an improved survival rate when challenged with HSV-1. Therefore, our study provides evidence that CCDC50 functions as a new negative regulator of the STING-mediated signaling pathway.
In addition to viral DNA, self-DNA released from damaged cells can also cause cGAS-STING activation. Therefore, cGAS-STING-driven IFN production can induce not only infectious immunity but also sterile autoimmunity. Self-dsDNA and its autoantibodies are considered diagnostic markers of SLE, which breaks down immune tolerance and triggers a robust type I IFN response [48, 49]. cGAS, the sensor of self-dsDNA, together with its adaptor STING, mediates signaling cascades, resulting in autoimmunity and autoinflammatory responses. Although the pathogenesis of SLE remains to be elucidated, the lack of negative regulation of the type I IFN pathway might contribute to SLE disease progression [18]. The cGAS-STING pathway is reported to participate in SLE disease occurrence and progression [50–53] through recognition of self-DNA leaked into the cytosolic compartment and promoting maturation of conventional dendritic cells and differentiation of plasmacytoid dendritic cells (pDCs) [54, 55]. Gain-of-function mutations of STING were also reported in STING-associated vasculopathy with onset in infancy and in patients with SLE-like syndromes or familial chilblain lupus syndrome [56–58]. However, it was also reported that the cGAS-STING pathway constrains TLR activation and thereby limits autoimmune manifestations in two models, Fas-deficient SLE-prone mice and a TMPD-induced SLE mouse model [59]. The authors also suggested that in such a mouse model the negative regulatory role of STING in SLE may be independent of its role in DNA sensing and represents a homeostatic role for STING resulting from stochastic dimerization [60]. It is possible that different DNA sensors and signaling pathways may function in a tissue- or mouse model-specific manner. Different mouse models may vary greatly, and clinical data should be more reliable. Moreover, there may be complicated interplay and crosstalk of several signaling pathways in a disease. In this study, we revealed that CCDC50 is downregulated in SLE patients (Fig. 6A). Low expression of CCDC50 was associated with increased activity of the type I IFN signaling pathway (Fig. 6C) and higher expression of IFN-α and IFN-β in PBMCs of SLE patients (Figs. 6B and 7D). Knockout of CCDC50 caused increased expression of IFN and proinflammatory cytokines when challenged with SLE serum (Fig. 7E). More importantly, the CCDC50 expression level was negatively correlated with the SLEDAI. Therefore, our study suggests a protective role of CCDC50 in SLE pathology, likely through inhibiting type I IFN activation.
Autophagy is a double-edged sword in immunity. Previously, there were reports on autolysosome-dependent degradation of STING and STING-involved initiation of autophagy membranes [61]. Following phosphorylation by TBK1, p62 binds to STING and directs it to autophagosomes for degradation. There were also reports that STING could induce autophagy through direct interaction with LC3 or promote ERGIC to serve as a membrane source for LC3 lipidation [62, 63,]. Interestingly, our work proved that STING could be degraded in a CCDC50-dependent manner in the absence of p62. Therefore, it is possible that there exists functional redundancy between CCDC50 and p62 and that they may have co-operative and synergetic effects. However, the expression of CCDC50 in immune organs and cells is much higher (https://www.proteinatlas.org/), especially in pDCs that produce the majority of type I IFN [64], whereas p62 is expressed broadly and highly in most tissues and cells but not immune cells. Taking the expression patterns of CCDC50 and p62 into consideration, it is tempting to suggest that CCDC50, rather than p62, may play a predominant role in the regulation of autophagy in the immune system. However, we also found that CCDC50 is highly expressed in some tumor cells and can also be activated by starvation, similar to p62, indicating that CCDC50 is not restricted to IFN-regulated pathways and may play a general role in autophagy. Therefore, CCDC50 may have broad substrate recognition, although K63-linked polyubiquitination of the substrate is crucial for the activation of CCDC50. However, there is no definitive conclusion on what specific mechanistic determinants specify the CCDC50-substrate interactions, and this needs to be further studied.
This study identified CCDC50 as a negative regulator of dsDNA-induced type I IFN activation and SLE pathogenesis. According to our results, we predict that under physiological conditions, CCDC50 is highly expressed in immune tissues and cells and helps to establish immune homeostasis by restricting the aberrant activation of the type I IFN pathway. CCDC50 is downregulated under certain pathological conditions, which likely contributes to autoinflammation in lupus-like diseases. Given the importance of IFN activation in autoimmune diseases such as SLE, therapeutic strategies targeting CCDC50 may be conducive to the treatment of related diseases.
Materials and methods
Mice
Ccdc50-KO mice were generated by GemPharmatech Co., Ltd. (Nanjing, China) using CRISPR/Cas9-based technology. Ccdc50-deficient mice were produced by microinjecting an RNP complex containing RNA of gRNAs and Cas9 protein into fertilized eggs. Under the guidance of gRNAs, the Cas9 protein binds to the target sites and then causes DNA double-strand breaks resulting in nonhomologous end joining-directed repair and finally achieves gene deletion. The genotype of the mice was confirmed by sequencing the PCR fragments, and the PCR primers were KO-F2 (Ccdc50-KO-tF2): AATGGATCACTGTCTGTCTGCCAG, KO-R2 (Ccdc50-KO-tR2): GAAATGTGTCCCTGGATTGACC and WT-F1 (Ccdc50-WT-tF1): CAGGATGAAATGAAACCTGCTG, WT-R1 (Ccdc50-WT-tR1): CTCAGAACAATCCACAATGTGGTG. The mice were bred according to previous guidelines [65]. All mice were maintained in a specific pathogen-free animal facility at Sun Yat-sen University. All animal experiments were supervised by the Institutional Animal Care and Use Committee of Sun Yat-sen University.
SLE patient samples
Eleven adult SLE patients without any medical treatment and 22 adult HDs were enrolled. All human samples were supervised by the Institutional Medical Ethics Review Board of the School of Medicine, Sun Yat-sen University. PBMCs isolated from SLE patients or HDs were subjected to qPCR, western blotting assays, or stimulation with dsDNA ligands.
Data analysis of SLE patients
We downloaded the high-throughput data by the R package GEO query [66]. GSE72509 is the RPKM (reads per kilobase of transcript, per million mapped reads) value of RNA-seq experiments (we took log1p transformation of the RPKM values), and other datasets are the signal intensity values of microarray experiments. The retrieved gene expression values were used as the input into the limma package to conduct the differential expression gene analysis [67]. For the microarray datasets, if one gene had multiple probes, we used the most significant probe to represent the gene. The significantly differentially expressed genes were defined as the genes/probes with an adjusted P value < 0.05. The P values of limma were corrected using the Benjamini–Hochberg procedure [68]. The correlations between CCDC50 and IFNα1 were evaluated by the coefficient and P value of Pearson’s correlation. To evaluate the association between the CCDC50 and IFN pathways, we first constructed a list of correlation-based ranked genes (Pearson’s correlation between CCDC50 and other genes) and then entered the list as the input for the preranked GSEA [69]. Only SLE patients from each dataset were selected to calculate the correlations. Patients without treatment were selected if treatment might dramatically disturb the expression levels of genes. GSEA was conducted and visualized by the GO pathway enrichment module in the ClusterProfiler package [70].
Cells
CCDC50-KO HEK293 cells, HEK293T cells, and CCDC50-KO THP-1 cells were developed using CRISPR/Cas9-sgRNA. The target sequences in the CCDC50 genome were GAACGTTCAGCGGAACCGTT and ATTTGGCATCGAACGTTCAG. The sgRNA constructs were described previously [71]. BMDMs and BMDCs were isolated and cultured as previously described [72]. HEK293, HEK293T, Vero, and HeLa cells were cultured in DMEM supplemented with 10% FBS, 1% L-glutamine (200 mM), and 1% penicillin/streptomycin. THP-1 cells were maintained in RPMI 1640 supplemented with 10% FBS, 1% L-glutamine plus 1% penicillin/streptomycin, and 0.1% 2-mercaptoethanol.
Reagents
The dsDNA ligands poly(dA:dT) and HSV60 and the STING agonist 2’3’-cGAMP were purchased from Invivogen and were used at final concentrations of 1, 4, and 5 μg/ml, respectively. Recombinant murine M-CSF1 (Peprotech) and GM-CSF (Peprotech) were used at a final concentration of 50 ng/ml. Inhibitors of MG132 (Merck), NH4Cl (Sigma), 3-MA (Invitrogen), CQ (Invitrogen), and bafilomycin A1 (Sigma) were used at final concentrations of 25 μM, 25 mM, 5 mM, 50 μM, and 200 nM, respectively.
Plasmids and transfection
The full-length and truncations of STING were described previously [73]. The full-length, truncated, and site-directed mutants of CCDC50 and all the other plasmids used in this research were reported previously [22]. Transient transfection of plasmids into HEK293 cells and HEK293T cells was performed by Lipofectamine 2000 (Invitrogen) and polyethylenimine HCl MAX (PEI, Polysciences). Lipofectamine 2000 (Invitrogen) was also used for transfection of HeLa cells. RNAiMAX (Invitrogen) was used for siRNA duplex transfection.
Quantitative RT–PCR (qRT-PCR)
Total RNA was extracted with an RNA extraction kit (TIANGEN) or TRIzol reagent (Invitrogen). Prime Script™ RT Master Mix (TaKaRa) was used to synthesize cDNA. TaKaRa SYBR qPCR Master Mix and the ABI Q5 Detection System were used for qRT-PCR assays. The mRNA results were normalized to GAPDH expression. gDNA expression was normalized to HBB (Human) or Tert (Mouse) expression. Primers for qRT-PCR are listed in Supplementary Table S1.
Confocal microscopy
HeLa cells were seeded in glass coverslips the day before. Following transfection or stimulation, the cells were fixed for 30 min with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 for another 15 min, and then blocked with 1% bovine serum albumin in PBS for 30 min. After incubation with the indicated primary antibodies and the subsequent fluorescent dye-conjugated secondary antibodies, nuclei were stained with DAPI (Invitrogen). A Carl Zeiss laser-scanning confocal microscope was used for visualization. The primary and secondary antibodies used in this study are listed in Supplementary Table S2.
Immunoprecipitation and immunoblot
The cells were lysed in NP-40 lysis buffer supplemented with protease inhibitor cocktail (Roche) for 30 min. After centrifugation, the supernatants were collected and then incubated with anti-HA affinity gel or anti-Flag M2 affinity gel for immunoprecipitation. The cell lysates were boiled for 10 min at 95 °C with 1% SDS and then diluted to 10 volumes of lysis buffer before incubation with affinity gel for ubiquitination analysis. The whole-cell extracts were lysed in RIPA lysis buffer (Beyotime, P10013B) supplemented with protease inhibitor cocktail and phosphatase inhibitor PhosSTOP (Roche) for immunoblot analysis. All the samples were loaded onto SDS–PAGE gels, transferred onto PVDF membranes, and then blotted with the indicated antibodies. Detailed information about the antibodies used in this study is listed in Supplementary Table S2.
ELISA
Human IFN-β produced in THP-1 cell supernatants or sera of SLE patients was measured with a human IFN-β ELISA kit (R&D Systems). A mouse IFN-β ELISA kit (BioLegend) was used to detect proteins in mouse cell supernatants and mouse sera.
Lung histology
Sex- and age-matched Ccdc50+/+ and Ccdc50–/– mice were intravenously infected with HSV-1 (4 × 108 PFU per mouse) or PBS. The lungs were isolated, formalin-fixed, and paraffin-embedded. The slices were stained with hematoxylin and eosin solution and then subjected to histological analysis by light microscopy.
Plaque assay
Vero cells were plated on 6-well plates the day before and reached 90% confluence. The supernatants and brain-grinding fluid containing the virus were collected and gradient diluted to infect Vero cells. Two hours after infection, the virus was removed, the cells were washed with PBS several times, and methylcellulose was added. Seventy-two hours later, crystal violet was used to stain the cells, and plaques (pfu/ml) were counted.
Statistical analysis
The results are representative of three individual experiments and shown as the mean with standard error of the mean. Two-tailed unpaired Student’s t-tests were used for statistical significance. The correlations between human samples are evaluated by the coefficient and P value of Pearson’s correlation. The log-rank (Mantel–Cox) test was used to compare the survival rates of Ccdc50+/+ and Ccdc50–/– mice infected with HSV-1. A P value <0.05 was considered to be significant.
Supplementary information
Acknowledgements
This study is supported by the National Natural Science Foundation of China (#81620108020 to DG and #81801574 to PH), Guangdong Province “Pearl River Talent Plan” Innovation and Entrepreneurship Team Project (2019ZT08Y464 to CL), and Shenzhen Science and Technology Program (#JCYJ20200109142201695 and #KQTD20180411143323605 to DG and #JCYJ20190807161415336 to PH). DG is also supported by the Guangdong Zhujiang Talents Programme and the National Ten-thousand Talents Programme.
Author contributions
DG conceived and supervised the research; DG and PH designed the experiments and wrote the manuscript. PH, YL, ZL, RL, PJ, and YW performed the biochemical, cell biological, and in vitro experiments; ZL, PJ, and YW performed the mouse experiments and viral infections; TT analyzed the data and public datasets. LC, XZ, ZZ, CL, and JG helped with reagents, materials, patient samples, and discussions.
Data availability
Publicly available datasets were downloaded from the GEO database (GSE61635, GSE121239, GSE72509, GSE65391, GSE49454, and GSE72798) [39–43]. All of the other data supporting this research are included in the article and Supplementary information.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Panpan Hou, Yuxin Lin, Zibo Li.
Change history
10/15/2021
A Correction to this paper has been published: 10.1038/s41423-021-00777-7
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00758-w.
References
- 1.Chan YK, Gack MU. Viral evasion of intracellular DNA and RNA sensing. Nat Rev Microbiol. 2016;14:360–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol. 2020;15:493–518. [DOI] [PubMed] [Google Scholar]
- 3.Qian C, Cao X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann N Y Acad Sci. 2013;1283:67–74. [DOI] [PubMed] [Google Scholar]
- 4.Yoneyama M, Onomoto K, Jogi M, Akaboshi T, Fujita T. Viral RNA detection by RIG-I-like receptors. Curr Opin Immunol. 2015;32:48–53. [DOI] [PubMed] [Google Scholar]
- 5.Chan YK, Gack MU. RIG-I-like receptor regulation in virus infection and immunity. Curr Opin Virol. 2015;12:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nie Y, Wang YY. Innate immune responses to DNA viruses. Protein Cell. 2013;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. [DOI] [PubMed] [Google Scholar]
- 8.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–50. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Bai XC, Chen ZJ. Structures and mechanisms in the cGAS-STING innate. Immun Pathw Immun. 2020;53:43–53. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin L, Waterman PM, Jonscher KR, Short CM, Reisdorph NA, Cambier JC. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28:5014–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun W, Li Y, Chen L, Chen H, You F, Zhou X, et al. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci USA. 2009;106:8653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao B, Du F, Xu P, Shu C, Sankaran B, Bell SL, et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature. 2019;569:718–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertsias GK, Pamfil C, Fanouriakis A, Boumpas DT. Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat Rev Rheumatol. 2013;9:687–94. [DOI] [PubMed] [Google Scholar]
- 16.Zharkova O, Celhar T, Cravens PD, Satterthwaite AB, Fairhurst AM, Davis LS. Pathways leading to an immunological disease: systemic lupus erythematosus. Rheumatology (Oxford). 2017;56:i55–i66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronnblom L, Leonard D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med. 2019;6:e000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. 2020;382:211–21. [DOI] [PubMed] [Google Scholar]
- 20.Martinez J, Cunha LD, Park S, Yang M, Lu Q, Orchard R, et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Zhou XJ, Klionsky DJ, Zhang H. Podocytes and autophagy: a potential therapeutic target in lupus nephritis. Autophagy. 2019;15:908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou P, Yang K, Jia P, Liu L, Lin Y, Li Z, et al. A novel selective autophagy receptor, CCDC50, delivers K63 polyubiquitination-activated RIG-I/MDA5 for degradation during viral infection. Cell Res. 2021;31:62–79. [DOI] [PMC free article] [PubMed]
- 23.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41. [DOI] [PubMed] [Google Scholar]
- 24.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orvedahl A, MacPherson S, Sumpter R JR, Tallóczy Z, Zou Z, Levine B. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe. 2010;7:115–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurston TL, Ryzhakov G, Bloor S, von Muhlinen N, Randow F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat Immunol. 2009;10:1215–21. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki A. Role of autophagy in innate viral recognition. Autophagy. 2007;3:354–6. [DOI] [PubMed] [Google Scholar]
- 28.Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci USA. 2007;104:14050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–97. [DOI] [PubMed] [Google Scholar]
- 30.Bronietzki AW, Schuster M, Schmitz I. Autophagy in T-cell development, activation and differentiation. Immunol Cell Biol. 2015;93:25–34. [DOI] [PubMed] [Google Scholar]
- 31.Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564–76. [DOI] [PubMed] [Google Scholar]
- 32.Qi YY, Zhou XJ, Zhang H. Autophagy and immunological aberrations in systemic lupus erythematosus. Eur J Immunol. 2019;49:523–33. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Shen J, Ran Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy. 2020;16:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuchida T, Zou J, Saitoh T, Kumar H, Abe T, Matsuura Y, et al. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity. 2010;33:765–76. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Hu MM, Wang YY, Shu HB. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem. 2012;287:28646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penengo L, Mapelli M, Murachelli AG, Confalonieri S, Magri L, Musacchio A, et al. Crystal structure of the ubiquitin binding domains of rabex-5 reveals two modes of interaction with ubiquitin. Cell. 2006;124:1183–95. [DOI] [PubMed] [Google Scholar]
- 37.Bohgaki M, Tsukiyama T, Nakajima A, Maruyama S, Watanabe M, Koike T, et al. Involvement of Ymer in suppression of NF-kappaB activation by regulated interaction with lysine-63-linked polyubiquitin chain. Biochim Biophys Acta. 2008;1783:826–37. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya Y, Jounai N, Takeshita F, Ishii KJ, Mizuguchi K. Ligand-induced ordering of the C-terminal tail primes STING for phosphorylation by TBK1. EBioMedicine. 2016;9:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toro-Domínguez D, Martorell-Marugán J, Goldman D, Petri M, Carmona-Sáez P, Alarcón-Riquelme ME. Stratification of systemic lupus erythematosus patients into three groups of disease activity progression according to longitudinal gene expression. Arthritis Rheumatol. 2018;70:2025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung T, Pratt GA, Sundararaman B, Townsend MJ, Chaivorapol C, Bhangale T, et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science. 2015;350:455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, et al. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell. 2016;165:551–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiche L, Jourde-Chiche N, Whalen E, Presnell S, Gersuk V, Dang K, et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014;66:1583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ducreux J, Houssiau FA, Vandepapelière P, Jorgensen C, Lazaro E, Spertini F, et al. Interferon alpha kinoid induces neutralizing anti-interferon alpha antibodies that decrease the expression of interferon-induced and B cell activation associated transcripts: analysis of extended follow-up data from the interferon alpha kinoid phase I/II study. Rheumatology (Oxford). 2016;55:1901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9. [DOI] [PubMed] [Google Scholar]
- 46.Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–20. [DOI] [PubMed] [Google Scholar]
- 47.Hayman TJ, Hsu AC, Kolesnik TB, Dagley LF, Willemsen J, Tate MD, et al. RIPLET and not TRIM25 is required for endogenous RIG-I-dependent anti-viral responses. Immunol Cell Biol. 2019;97:840–52. [DOI] [PubMed]
- 48.Bai Y, Tong Y, Liu Y, Hu H. Self-dsDNA in the pathogenesis of systemic lupus erythematosus. Clin Exp Immunol. 2018;191:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soni C, Reizis B. DNA as a self-antigen: nature and regulation. Curr Opin Immunol. 2018;55:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci USA. 2012;109:19386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barber GN. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15:760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn J, Barber GN. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Curr Opin Immunol. 2014;31:121–6. [DOI] [PubMed] [Google Scholar]
- 53.Thim-Uam A, Prabakaran T, Tansakul M, Makjaroen J, Wongkongkathep P, Chantaravisoot N, et al. STING mediates lupus via the activation of conventional dendritic cell maturation and plasmacytoid dendritic cell differentiation. iScience. 2020;23:101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato Y, Park J, Takamatsu H, Konaka H, Aoki W, Aburaya S, et al. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann Rheum Dis. 2018;77:1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao D, Li T, Li XD, Chen X, Li QZ, Wight-Carter M, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci USA. 2015;112:E5699–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124:5516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez G, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.König N, Fiehn C, Wolf C, Schuster M, Cura Costa E, Tüngler V, et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann Rheum Dis. 2017;76:468–72. [DOI] [PubMed] [Google Scholar]
- 59.Motwani M, McGowan J, Antonovitch J, Gao KM, Jiang Z, Sharma S, et al. cGAS-STING pathway does not promote autoimmunity in murine models of SLE. Front Immunol. 2021;12:605930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma S, Campbell AM, Chan J, Schattgen SA, Orlowski GM, Nayar R, et al. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci USA. 2015;112:E710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prabakaran T, Bodda C, Krapp C, Zhang BC, Christensen MH, Sun C, et al. Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. EMBO J. 2018;37:e97858. [DOI] [PMC free article] [PubMed]
- 62.Gui X, Yang H, Li T, Tan X, Shi P, Li M, et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu D, Wu H, Wang C, Li Y, Tian H, Siraj S, et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019;26:1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Worah K, Mathan T, Vu Manh TP, Keerthikumar S, Schreibelt G, Tel J, et al. Proteomics of human dendritic cell subsets reveals subset-specific surface markers and differential inflammasome function. Cell Rep. 2016;16:2953–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron. 1997;19:755–9. [DOI] [PubMed]
- 66.Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–7. [DOI] [PubMed] [Google Scholar]
- 67.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300.
- 69.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hou P, Chen S, Wang S, Yu X, Chen Y, Jiang M, et al. Genome editing of CXCR4 by CRISPR/cas9 confers cells resistant to HIV-1 infection. Sci Rep. 2015;5:15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hou P, Jia P, Yang K, Li Z, Tian T, Lin Y, et al. An unconventional role of an ASB family protein in NF-kappaB activation and inflammatory response during microbial infection and colitis. Proc Natl Acad Sci USA. 2021;118:e2015416118. [DOI] [PMC free article] [PubMed]
- 73.Hu MM, Yang Q, Xie XQ, Liao CY, Lin H, Liu TT, et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity. 2016;45:555–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were downloaded from the GEO database (GSE61635, GSE121239, GSE72509, GSE65391, GSE49454, and GSE72798) [39–43]. All of the other data supporting this research are included in the article and Supplementary information.