Abstract
Alcohol misuse and smoking are risk factors for pneumonia, yet the impact of combined cigarette smoke and alcohol on pneumonia remains understudied. Smokers who misuse alcohol form lung malondialdehyde–acetaldehyde (MAA) protein adducts and have decreased levels of anti-MAA secretory IgA (sIgA). Transforming growth factor-β (TGF-β) down-regulates polymeric Ig receptor (pIgR) on mucosal epithelium, resulting in decreased sIgA transcytosis to the mucosa. It is hypothesized that MAA-adducted lung protein increases TGF-β, preventing expression of epithelial cell pIgR and decreasing sIgA. Cigarette smoke and alcohol co-exposure on sIgA and TGF-β in human bronchoalveolar lavage fluid and in mice instilled with MAA-adducted surfactant protein D (SPD-MAA) were studied herein. Human bronchial epithelial cells (HBECs) and mouse tracheal epithelial cells were treated with SPD-MAA and sIgA and TGF-β was measured. Decreased sIgA and increased TGF-β were observed in bronchoalveolar lavage from combined alcohol and smoking groups in humans and mice. CD204 (MAA receptor) knockout mice showed no changes in sIgA. SPD-MAA decreased pIgR in HBECs. Conversely, SPD-MAA stimulated TGF-β release in both HBECs and mouse tracheal epithelial cells, but not in CD204 knockout mice. SPD-MAA stimulated TGF-β in alveolar macrophage cells. These data show that MAA-adducted surfactant protein stimulates lung epithelial cell TGF-β, down-regulates pIgR, and decreases sIgA transcytosis. These data provide a mechanism for the decreased levels of sIgA observed in smokers who misuse alcohol.
Alcohol misuse has long been known to negatively affect innate lung defense against respiratory pathogens because individuals with alcohol use disorder (AUD) have an increased risk for pneumonia.1,2 Sustained alcohol exposure in cell and animal models had identified numerous alterations to lung immunity at the level of mucociliary clearance, cytokine responses, and macrophage function.3 Coincidentally, AUD is associated with cigarette smoking, and cigarette smokers also have an increased risk for pneumonia.4 Cigarette smoking causes chronic obstructive pulmonary disease (COPD), the third leading disease cause of death.5 Both cigarette smoking and alcohol misuse are implicated in the etiology of COPD, and COPD is a significant predisposing factor to pneumococcal pneumonia.6 Although reasons for these associations likely are multifactorial, lungs from patients with COPD are deficient in secretory immunoglobulin A (sIgA), an important antimicrobial mucosal agent that can attenuate the risk of pulmonary infection.7
Mucosal production of sIgA represents an important aspect of innate lung defense.8 Circulating monomeric and polymeric IgA antibodies bind to the polymeric Ig receptor (pIgR) basally located on lung epithelial cells, where they are internalized and processed into a dimeric form, and then released as antimicrobial sIgA to the mucosal surface.9 sIgA prevents bacterial and viral ligands from binding to epithelial cells. IgA also has an established relationship with COPD10 because lungs of patients with COPD have decreased levels of sIgA.11 This likely contributes to the decreased antimicrobial defense and increased pathogen-induced exacerbations12 observed in COPD patients. The COPD lung also has increased transforming growth factor β (TGF-β).13 Importantly, TGF-β has been shown to decrease airway epithelial IgA processing through the down-regulation of pIgR.14
Pathogenesis of alcohol and cigarette smoke co-exposure have been examined in a cohort of individuals (nonsmokers and smokers) with and without AUD.15 Individuals with alcohol and cigarette co-exposure showed significantly decreased levels of lung sIgA compared with individuals with only cigarette smoking, or individuals with AUD who did not smoke. At 1 week of alcohol cessation, the AUD smoker group showed increases in sIgA. In addition, the highest levels of malondialdehyde–acetaldehyde (MAA)-adducted protein was detected in lungs of smokers with AUD. MAA adducts have been implicated in altering several aspects of airway function such as proinflammatory cytokine release, ciliary beat frequency, and epithelial wound repair.16 A highly stable covalent modification, MAA has been shown to adduct to surfactant protein D (SPD) in the lung.17 MAA-adducted proteins bind to scavenger receptor A1 (also known as CD204) on the surface of both airway epithelial cells18 and macrophages.15 Because CD204 expression has been associated with an activated and profibrotic M2 macrophage phenotype,19 significant up-regulation of CD204 has been reported in COPD patients,20,21 a disease associated with decreased sIgA.
Because the lungs represent a unique target for MAA adduct formation in smokers who drink alcohol, the mechanism of smoke- and alcohol-generated MAA-adducted protein on the reduction of lung sIgA was investigated. It was hypothesized that SPD adducted to MAA (SPD-MAA) would decrease transcytosis of IgA across bronchial epithelium by increasing TGF-β, which in turn decreases expression of epithelial cell pIgR and sIgA in lung. To test this hypothesis, sIgA, pIgR, and TGF-β were measured using bronchoalveolar lavage (BAL) samples from human and mouse, as well as in vitro cell models of transcytosis.
Materials and Methods
AUD Bronchoalveolar Lavage Samples
Human BAL samples were obtained through the Colorado Pulmonary Alcohol Research Collaborative. The Institutional Review Board at the University of Colorado Anschutz Medical Campus and the University of Nebraska Medical Center approved this study. Previously obtained de-identified biospecimens from participants were selected to establish four defined groups consisting of nonsmoking non-AUD, non-AUD smokers, nonsmoking AUD, and smokers with AUD, as carefully demographically detailed in previous investigations.16,22 Aliquots of BAL were processed22 and delivered to the University of Nebraska Medical Center as previously described.16
Mouse Exposure Model
Mouse Model of Cigarette Smoke and Alcohol Co-Exposure
All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. Both wild-type C57BL/6J mice and scavenger receptor A (CD204) knockout (KO) mice obtained from Jackson Laboratory (Bar Harbor, ME) were used. Four exposure groups were generated, consisting of sham-treated mice, cigarette smoke–exposed mice, alcohol-fed mice, and the combination of smoke- and alcohol-treated mice as previously described in detail.19,20 BAL was collected and processed as described,19 and trachea were removed for the production of primary epithelial cells in culture.21
Mouse Model of MAA Adduct Nasal Instillation
SPD was purified from pulmonary alveolar proteinosis lung lavage fluid and proteins were adducted with malondialdehyde and acetaldehyde to generate SPD-MAA as described.17 As a control, bovine serum albumin (BSA) was MAA-adducted in the same manner and used in transcytosis assays. Purified proteins were sterile-filtered and delivered into the lungs of mice using a nasal instillation protocol previously described.17
Cell Culture and Treatments
Primary mouse tracheal epithelial cells (MTECs) were cultured for 4 weeks at an air–liquid interface (ALI) as described.23 Human bronchial epithelial cells (HBECs) were obtained from donor lungs with a minimum of three different donor source cell lines used.24 The murine alveolar macrophage cell line (MH-S; American Type Culture Collection, Manassas, VA) was cultured as described.25
Cell cultures were treated with media, SPD, SPD-MAA, BSA, or MAA-adducted BSA for 4 to 48 hours at various concentrations (1 to 50 μg/mL) on both apical and basal ALI compartments with 0.1 mg/mL dimeric IgA (Athens Research and Technology, Athens, GA) on the basolateral surface. Media were collected for subsequent protein analysis.
To control for any treatment injury leading to artifactual results, transepithelial electrical resistance measurements using electric cell impedance sensing (Applied Biosciences, Troy, NY) were made before and after all treatment conditions to rule out loss of barrier function in the cell monolayer.26 Cell viability was maintained under all conditions (>95% viable) as determined by a lactate dehydrogenase kit (Sigma, St. Louis, MO).
Enzyme-Linked Immunosorbent Assay
Both human and mouse BAL and ALI samples were collected and stored at −80°C for protein analyses using enzyme-linked immunosorbent assay (ELISA).
ELISA for sIgA
Donor HBECs were expanded on porous Transwell inserts and subsequently differentiated into a pseudostratified mucociliary epithelium. ALI cultures were treated for 24 hours in both apical and basal compartments with 0.6 mL MAA-adducted protein with or without interferon-γ (IFN-γ), an inflammatory stimulus as a positive control,27 an equal amount of phosphate-buffered saline diluted in the culture media as a negative control, and nonadducted SPD as a carrier protein control. Commercially available polymeric IgA (pIgA) was placed in the basal compartment at 0.1 mg/mL and incubated for an additional 24 hours. Inserts were washed on the apical side at 48 hours to collect transported IgA. sIgA was measured by ELISA as previously described.11
ELISA for MAA Adducts
MAA-adducted proteins were assayed in human BAL samples by indirect competitive ELISA as previously described.20
ELISA for TGF-β
TGF-β1 and TGF-β2 were measured by sandwich ELISA in BAL and cell culture samples as previously described.14
Quantitative RT-PCR for pIgR mRNA
ALI cells were harvested for RNA isolation after the various treatment conditions. As previously described,11 total RNA from HBECs was extracted using the RNAqueous-Micro Kit (Applied Biosystems/Ambion, Austin, TX), according to the manufacturer's protocol. Total RNA from ALI-cultured cells was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA), according to the manufacturer's specifications. Primer sequences were as follows: pIgR forward: 5′-CTCTCTGGAGGACCACCGT-3′, reverse: 5′-CAGCCGTGACATTCCCTG-3′; hypoxanthine phosphoribosyltransferase 1 (HPRT) forward 5′-TGCTCGAGATGTGATGAAGGAG-3′, reverse 5′-TGATGTAATCCAGCAGGTCAGC-3′.
Immunolocalization of pIgR
Primary HBECs were grown on ALI and pretreated for 24 hours with 50 μg/mL control SPD or SPD-MAA. pIgA then was placed in the basal media for an additional 24 hours. Media were removed and cells were fixed and permeabilized in a blocking-permeabilization solution (0.05 mol/L Tris, 0.02% sodium azide, 0.5% Triton X-100 (Sigma), and 1% BSA) for 30 minutes. Cells were stained for pIgR (R&D Systems, Minneapolis, MN), acetylated α-tubulin (anti-rabbit; Santa Cruz (Dallas, TX): 6 to 11-B1), and cell nuclei (DAPI; Thermo Fisher, Waltham, MA) following a previously described method.28 The fluorochromes used were Alexa 488 and 540. Cell monolayers were imaged by confocal microscopy using a Zeiss LSM 510 confocal laser-scanning microscope (Göettingen, Germany), using an argon/krypton laser (488 nm/568 nm/647 nm) provided by the University of Nebraska Medical Center Confocal Microscopy Core.
Statistical Analysis
Data are presented as means ± SEM. To detect significant changes between groups, a one-way analysis of variance was used and a post hoc test (Tukey or nonparametric Mann-Whitney) was performed to account for multiple comparisons if the P value was <0.05. For all analyses, Prism software version 8.31 (GraphPad, La Jolla, CA) was used.
Results
Lung sIgA Levels Are Reduced in Smokers with AUD
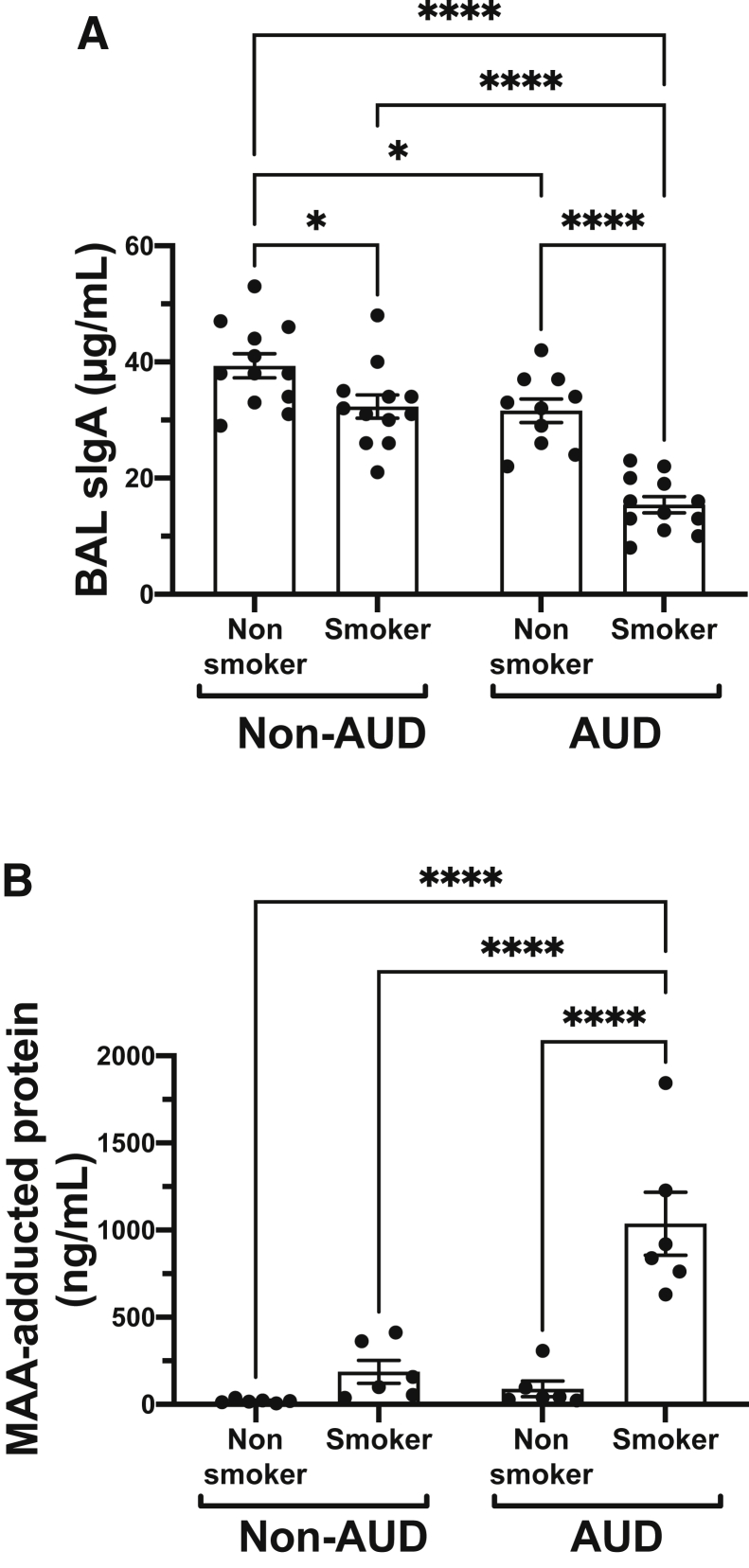
Total sIgA levels were measured using BAL fluid samples from a cohort of well-characterized individuals with and without cigarette smoking and alcohol use histories. The highest levels of sIgA detected in BAL were observed in the nonsmoking, non-AUD control group. A small but significant reduction in BAL sIgA was observed in both non-AUD smokers (P < 0.05) as well as nonsmoking AUD subjects (P < 0.05) compared with the aforementioned control group. However, a significantly large (P < 0.0001) decrease in BAL sIgA was measured in the AUD smoking group compared with the other three groups (Figure 1A). Likewise, the formation of smoke- and alcohol-generated MAA-adducted protein was significantly present (P < 0.0001) only in the AUD smoking group compared with the other three groups (Figure 1B).
Figure 1.
Lung secretory IgA (sIgA) and malondialdehyde–acetaldehyde (MAA) in smokers with alcohol use disorder (AUD). Bronchoalveolar lavage (BAL) fluid from nonsmoker non-AUD, smoker non-AUD, AUD nonsmoker, and AUD smoker subjects measured for sIgA (A) or MAA-adducted protein (B) by enzyme-linked immunosorbent assay. Values are presented as means ± SEM. n = 12 for sIgA (A); n = 6 for MAA (B). ∗P < 0.05, ∗∗∗∗P < 0.0001 (one-way analysis of variance with Tukey post hoc multiple comparisons).
Lung sIgA Levels Are Reduced in a Mouse Model of Smoke+Alcohol Co-Exposure
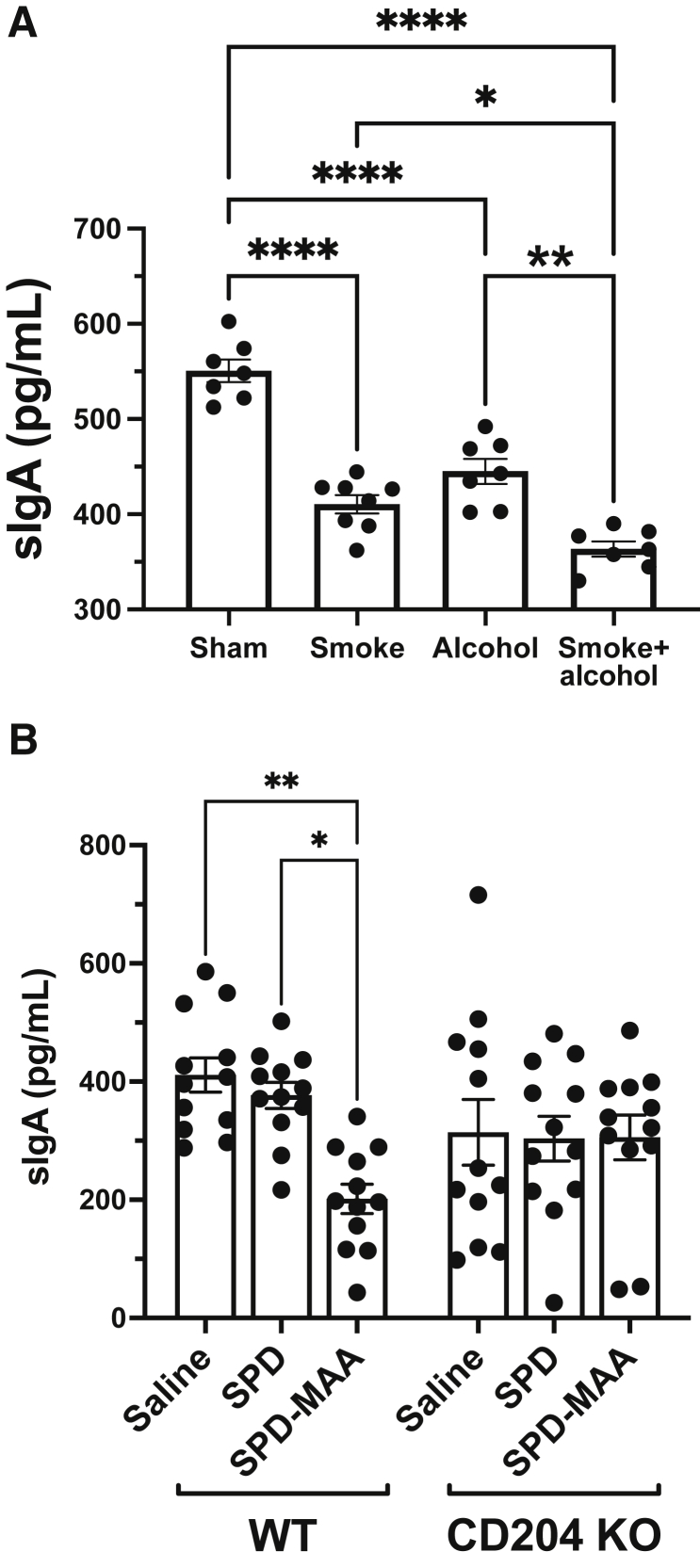
A mouse model of cigarette smoke and alcohol co-exposure was developed to minimize the exposomal variables present in human subjects.19 BAL samples were collected from smoke+alcohol co-exposed mice as well as individual smoke or alcohol mice to measure sIgA levels. Both smoke-treated (P < 0.0001) and alcohol-fed (P < 0.0001) mice showed significantly decreased levels of lung sIgA compared with sham-exposed control mice exposed only to room air and water (Figure 2A). Importantly, the smoke+alcohol co-exposure mice further showed significant reductions in sIgA compared with either smoke (P < 0.05) or alcohol (P < 0.0001) single-exposure groups. Because only co-exposure to smoke+alcohol results in the formation of large amounts of MAA-adducted protein in mouse lung,20 sterile MAA-adducted lung SPD was instilled repetitively into mouse lungs over a 3-week period and the effect on lung sIgA levels was evaluated. Nasal instillation of wild-type mice with 50 μg/mL SPD-MAA resulted in a significant (P < 0.01) reduction in BAL sIgA levels compared with mice instilled with sterile saline (Figure 2B). As a control, nonadducted SPD produced no significant reduction in lung sIgA levels after repetitive nasal instillation of mice. Because CD204 [scavenger receptor A (SRA)] is a receptor for MAA-adducted protein, the same nasal instillations also were performed using CD204 KO mice. Interestingly, although CD204 KO mice showed relatively diminished sIgA levels in BAL compared with wild type, no significant differences in sIgA levels were detected in SPD- or SPD-MAA–instilled mice lacking scavenger receptor A.
Figure 2.
A: Lung secretory IgA (sIgA) in mice exposed to cigarette smoke, alcohol, and malondialdehyde–acetaldehyde (MAA)-adducted protein. Mouse bronchoalveolar lavage (BAL) fluid representing handling control (Sham), cigarette smoke exposure (Smoke), ad libitum alcohol-fed (Alcohol), or co-exposure to both smoke and alcohol (Smoke+alcohol) for 8 weeks and assayed for sIgA. B: Wild-type (WT) or scavenger receptor A knock out (CD204 KO) mice nasally instilled for 3 weeks with either sterile phosphate-buffered saline (Saline), 50 μg/mL surfactant protein D (SPD), or 50 μg/mL MAA-adducted surfactant protein D (SPD-MAA) and BAL assayed for sIgA. Values are presented as means ± SEM. n = 7 (A); n = 12 (B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001 (one-way analysis of variance with Tukey post hoc multiple comparisons).
MAA-Adducted Protein Decreases Lung Epithelial Cell Transcytosis of sIgA
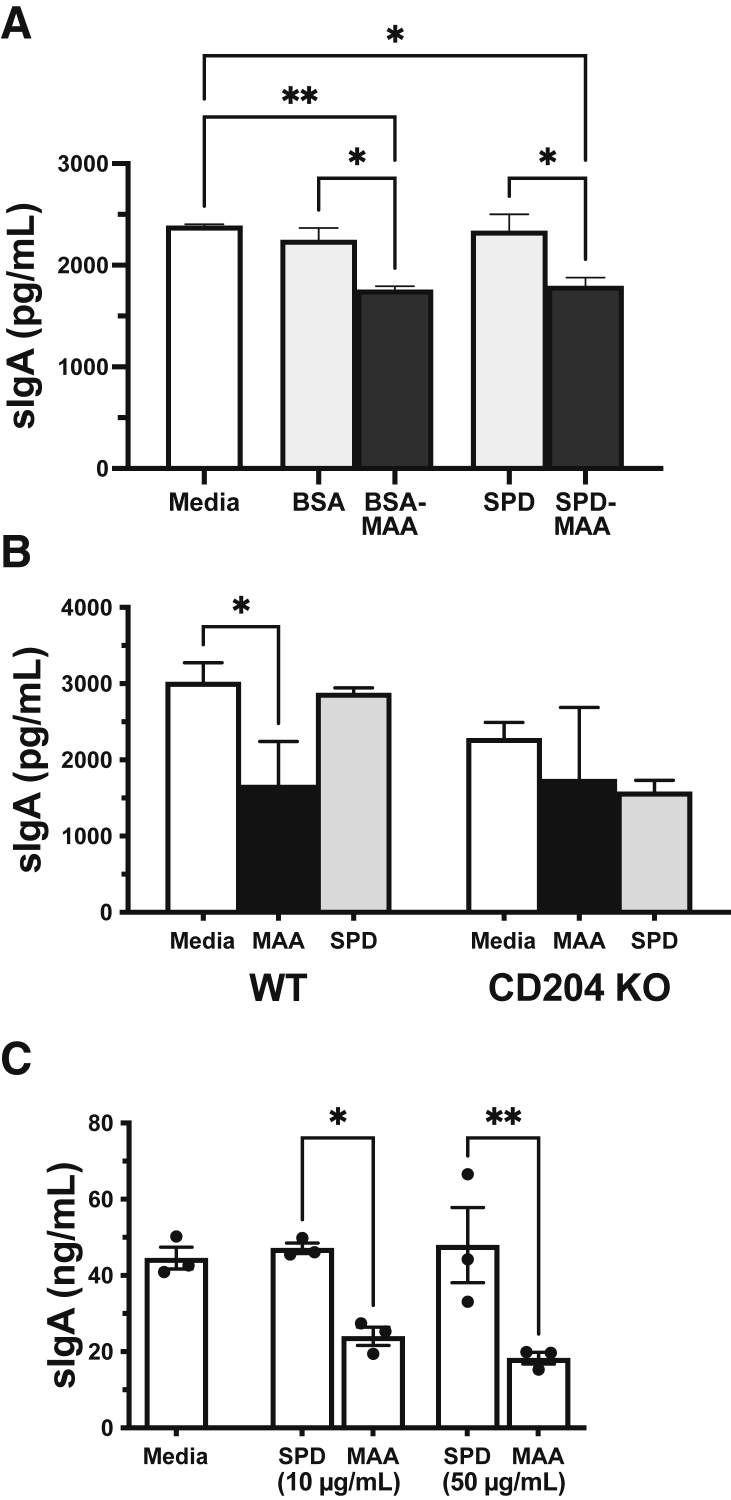
An in vitro ALI cell culture model of airway epithelial cell IgA transcytosis was used to better define epithelial cell sIgA responses to MAA-adducted protein. MTECs were isolated and cultured until confluent monolayers were established. Upon addition of 5 ng/mL pIgA to the basal media compartment only, control-media-only–treated MTECs processed and released maximal amounts of sIgA by 48 hours, while 50 μg/mL MAA-treated MTECs showed a significantly (P < 0.05) reduced level of pIgA transcytosis (Figure 3A). Treatment with either MAA-adducted SPD or MAA-adducted BSA reduced pIgA transcytosis regardless of the target adducted protein, while nonadducted SPD or BSA altered transcytosis of pIgA. As a positive control, 200 U/mL IFN-γ stimulated a significant increase in transcytosis of pIgA in MTEC ALI cultures over baseline media (Supplemental Figure S1). Similar to that seen with BAL sIgA levels, SPD-MAA treatment did not result in reduced sIgA in MTECs from scavenger receptor A KO mice compared with those cells cultured from wild-type mice (Figure 3B). None of the cell treatment conditions resulted in a loss of viability (not shown) or transepithelial electrical resistance (Supplemental Figure S2). Importantly, the same reduction in sIgA in response to SPD-MAA was observed in primary HBEC ALI cultures (n = 3 normal donors) (Figure 3C).
Figure 3.
Secretory IgA (sIgA) transcytosis in airway epithelial cells treated with malondialdehyde–acetaldehyde (MAA)-adducted protein. Primary mouse tracheal epithelial cells (MTECs) (A and B) and human bronchial epithelial cells (HBECs) (C) grown on an air–liquid interface were pretreated with MAA-adducted protein in both basal and apical compartments for 24 hours followed by the addition of dimeric IgA in the basal compartment and apical sIgA release measured by enzyme-linked immunosorbent assay. Cells were treated with control media, nonadducted bovine serum albumin (BSA), MAA-adducted BSA (BSA-MAA), nonadducted surfactant protein D (SPD), or MAA-adducted SPD (SPD-MAA) (A). MTECs cultured from wild-type (WT) or scavenger receptor A knock out (CD204 KO) mice were treated with media, MAA-adducted SPD (MAA), or nonadducted SPD (SPD) (B). HBEC cultures were treated with 10 or 50 μg/mL of MAA-adducted SPD (MAA), or nonadducted SPD (SPD) (C). Values are presented as means ± SEM. n = 3 donors (A–C). ∗P < 0.05, ∗∗P < 0.01 (one-way analysis of variance with Tukey post hoc multiple comparisons).
MAA-Adducted Protein Decreases Airway Epithelial Cell pIgR Expression
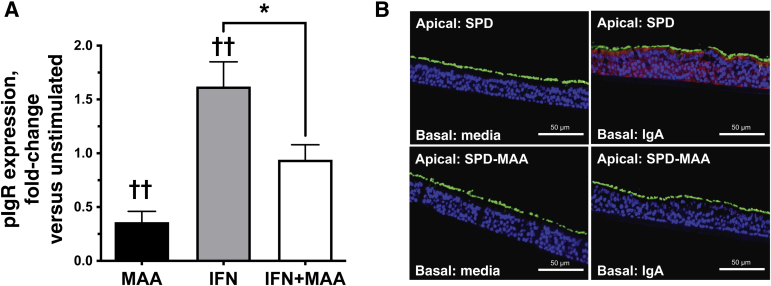
The mechanism for MAA-adducted protein-induced reduction of sIgA release was investigated in primary HBECs. HBECs (n = 3 normal donors) cultured on ALI were treated for 48 hours with either media, 200 U/mL IFN-γ, 50 μg/mL SPD-MAA, or both IFN-γ+MAA and pIgR mRNA measured by quantitative RT--PCR. Compared with control media-treated cells, SPD-MAA decreased significantly (P < 0.01) while IFN-γ increased pIgR mRNA expression (Figure 4A). Co-treatment with SPD-MAA also significantly reduced (P < 0.05) the IFN-γ–stimulated increase in pIgR mRNA. Protein expression and localization then were determined by confocal microscopy of pIgR in fully differentiated HBECs cultured on ALI. HBECs were pretreated apically for 24 hours with 50 μg/mL SPD or SPD-MAA, followed by the basal addition of 5 ng/mL pIgA for an additional 24 hours. Up-regulation of pIgR was detected in response to basal IgA in SPD-treated HBECs, but not in SPD-MAA–treated cells (Figure 4B). HBECs cultured for 48 hours in the absence of pIgA in the basal compartment showed little protein staining for pIgR.
Figure 4.
Polymeric Ig receptor (pIgR) expression in malondialdehyde–acetaldehyde (MAA)-adducted protein-treated airway epithelial cells. A: Primary human bronchial epithelial cells (HBECs) grown on an air–liquid interface and pretreated for 24 hours with 50 μg/mL MAA-adducted SPD (MAA), 200 U/mL interferon-γ (IFN; positive control), or both IFN and MAA in the apical and basal compartments. Cells were assayed for pIgR mRNA. Values are presented as means ± SEM. B: Representative confocal images from primary HBECs grown on an air–liquid interface and pretreated for 24 hours with 50 μg/mL control SPD or SPD-MAA. pIgA then was placed in the basal media for 24 hours and cells were stained for pIgR. pIgR is shown in red, cilia is shown in green, and nuclei are shown in blue. pIgR up-regulation was detected in response to IgA in the presence of SPD, but not when cells were pretreated with SPD-MAA. n = 3 donors (A). ∗P < 0.05 (one-way analysis of variance with Tukey post hoc multiple comparisons); ††P < 0.01 versus untreated control cells (one-way analysis of variance with Tukey post hoc multiple comparisons). Scale bars = 50 μm. SPD, surfactant protein D.
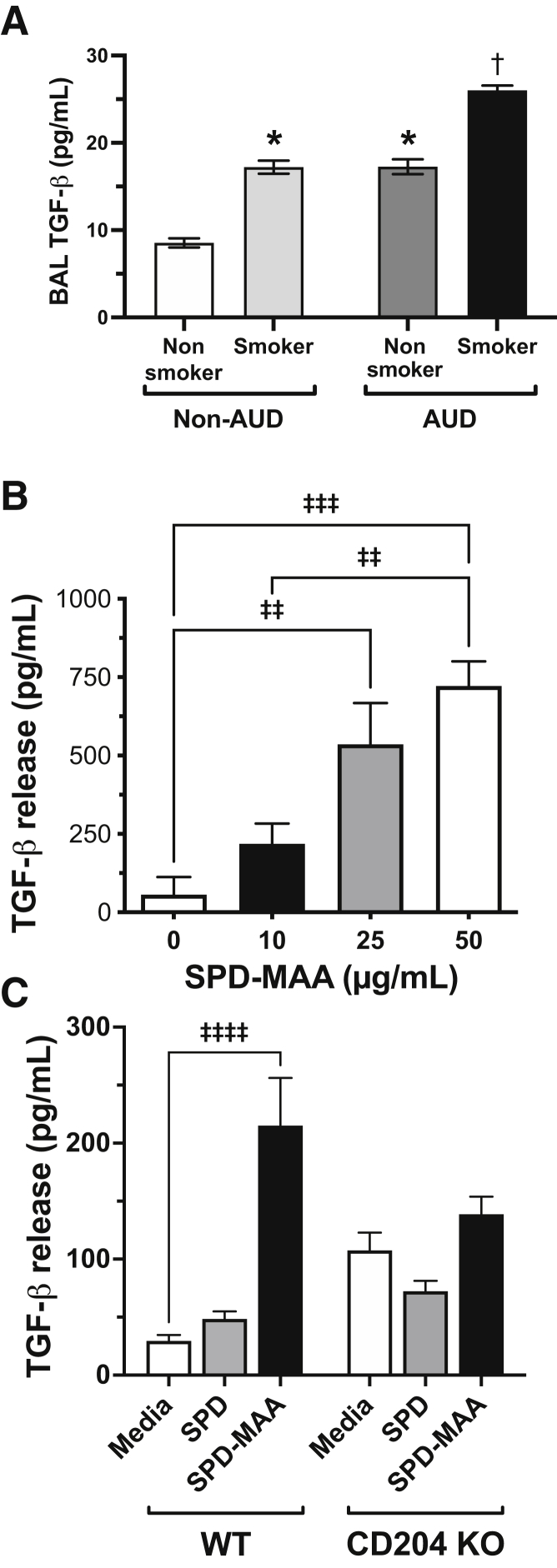
Lung TGF-β Levels Are Increased in Smokers with AUD
TGF-β has been shown to decrease pIgR in airway epithelium.14 BAL from a smoking and alcohol use cohort to measure TGF-β was examined. The highest levels of TGF-β detected in BAL were found in the smoking AUD group (Figure 5A). Smaller, but significantly increased (P < 0.05), levels of TGF-β also were observed in both non-AUD smokers as well as nonsmoking AUD subjects compared with the control nonsmoking, non-AUD group.
Figure 5.
Transforming growth factor-β (TGF-β) in smokers with alcohol use disorder (AUD). A: Bronchoalveolar lavage (BAL) fluid from nonsmoker non-AUD, smoker non-AUD, AUD nonsmoker, and AUD smoker subjects measured for TGF-β by enzyme-linked immunosorbent assay. Values are presented as the means ± SEM. B: Primary human bronchial epithelial cells (HBECs) were treated with 10 to 50 μg/mL malondialdehyde–acetaldehyde–adducted surfactant protein D (SPD-MAA) for 48 hours and supernatants were assayed for TGF-β. Mouse tracheal epithelial cells (MTECs) cultured from wild-type (WT) or scavenger receptor A knock-out (CD204 KO) mice were treated with media, MAA-adducted SPD (MAA), or nonadducted SPD (SPD) (C). n = 12 (A). ∗P < 0.05 versus non-AUD nonsmoker; †P < 0.05 versus non-AUD smoker and AUD nonsmoker (one-way analysis of variance with Tukey post hoc multiple comparisons); ‡‡P < 0.01, ‡‡‡P < 0.001, and ‡‡‡‡P < 0.0001.
MAA-Adducted Protein Increases Lung Epithelial Cell TGF-β
To further investigate the mechanism of MAA-adducted protein suppression of pIgR, TGF-β production after MAA exposure in primary HBECs was measured. SPD-MAA (10 to 50 μg/mL at 48 hours) treatment produced a dose-dependent increase (P < 0.001) in TGF-β release (Figure 5B). Identical treatment with nonadducted SPD produced no change in TGF-β compared with control media-treated HBECs (data not shown). Similarly, SPD-MAA stimulated a significant release (P < 0.0001) of TGF-β in MTECs, although fourfold to fivefold less than that observed in HBECs (Figure 5C). Under the same treatment conditions, but in MTECs from CD204 KO mice, SPD-MAA did not increase TGF-β release significantly. Levels of TGF-β after media and nonadducted SPD treatments were increased in CD204 KO MTECs, but were not significantly different from wild-type MTECs.
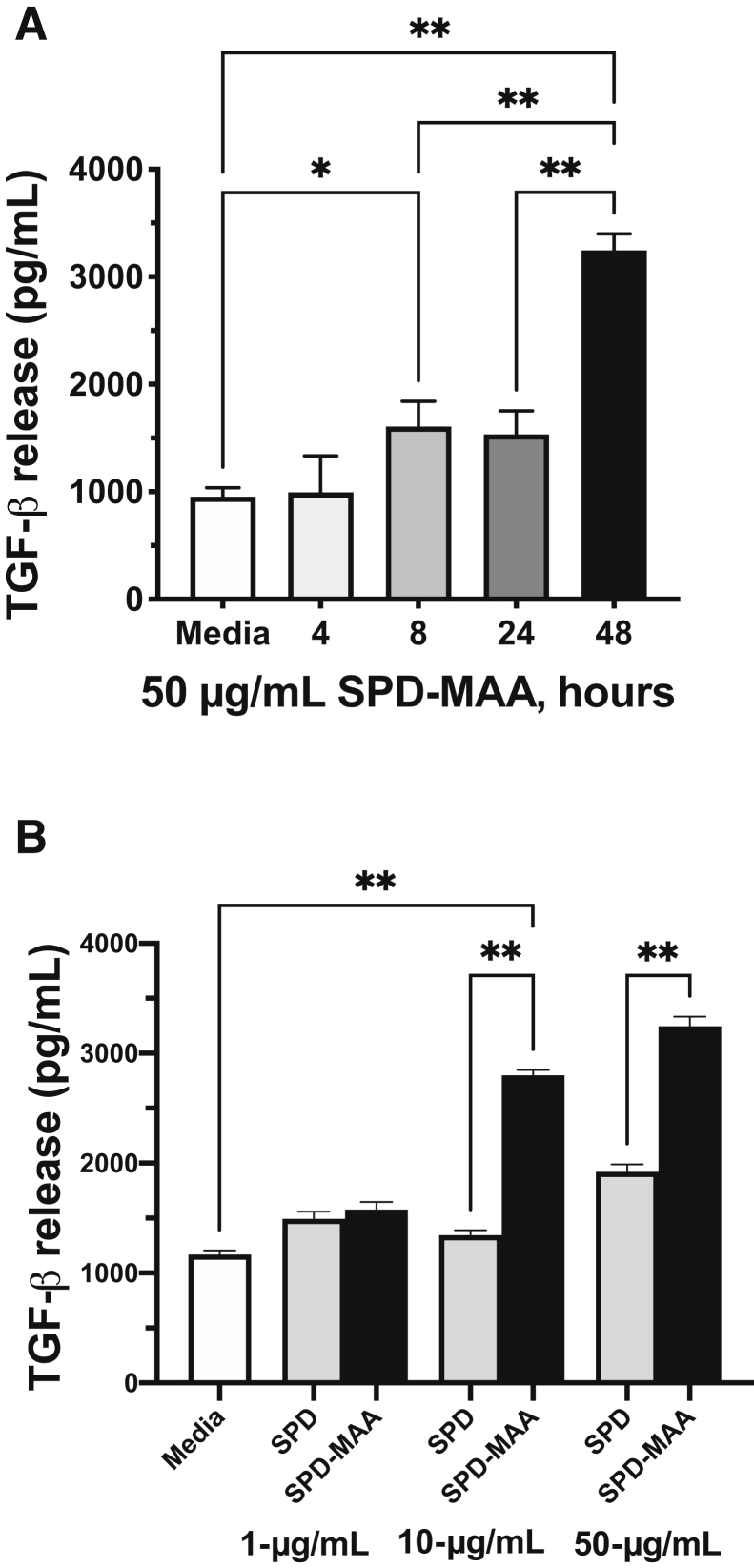
MAA-Adducted Protein Increases Lung Macrophage TGF-β
Because lung macrophages produce greater amounts of TGF-β than epithelial cells and MAA-adducted protein binds to macrophages,29 the effect of SPD-MAA on TGF-β release was examined using MH-S cells, a mouse lung macrophage cell line. SPD-MAA, but not nonadducted SPD, dose-dependently increased the release of TGF-β maximally by 48 hours (Figure 6A) at a treatment dose of 50 μg/mL (Figure 6B).
Figure 6.
Transforming growth factor-β (TGF-β) release from malondialdehyde–acetaldehyde (MAA)-adducted protein-treated alveolar macrophage cell line. Time course (A) and dose response (B) in an alveolar macrophage cell line (MH-S) treated with MAA-adducted surfactant protein and TGF-β measured by enzyme-linked immunosorbent assay. Values are presented as means ± SEM. ∗P < 0.05, ∗∗P < 0.0001 (one-way analysis of variance with Tukey post hoc multiple comparisons).
Discussion
Stable, hybrid MAA adducts are highly immunogenic and contribute to a robust antibody response.30 An increased quantity of IgA specific to MAA in the serum of AUD smokers has been reported, in parallel with decreased levels of secreted MAA IgA in BAL from these same individuals.15 A synergistic in vivo effect of cigarette smoke and alcohol feeding on the production of MAA-adducted protein (<100 ng/mL smoke or alcohol alone versus >300 ng/mL smoke+alcohol) is shown in the lungs of mice.20 Similar to the mouse model of MAA formation, wherein significant levels of MAA were observed only in the smoke and alcohol co-exposure group,20 MAA-adducted protein was most abundant in BAL from the AUD smoker group in the current investigation. Although both non-AUD smokers and AUD nonsmokers showed significant reductions in sIgA compared with the nonsmoking non-AUD control group, more striking decreases in total sIgA levels in BAL from AUD smokers was observed along with the greatest quantity of total MAA-adducted protein compared with either single-exposure group. The findings extend previous observations that cigarette smoking decreases lung sIgA31 by showing an additional impact of alcohol misuse. Recently, sIgA was reported to be highly effective at binding to the S-protein of severe acute respiratory syndrome coronavirus 2 and neutralizing binding to the angiotensin-converting enzyme 2 receptor in airway epithelium using a humanized mouse model of IgA expression,10 suggesting a role for sIgA in innate coronavirus disease-2019 defense.
The finding of reduced sIgA using an established mouse model of controlled cigarette smoke and alcohol exposure19 was recapitulated, whereby combination exposure resulted in further sIgA reduction compared with individual treatments. This alcohol feeding model uses the established ad libitum Meadows-Cook diet that already has shown an alcohol-mediated reduction in IFN-γ, a stimulator of IgA transcytosis.32 To determine if co-exposure to alcohol and smoke reduces sIgA in response to MAA-adducted protein formation, the previously characterized mouse model of purified MAA-adducted protein nasal instillation was used to deliver SPD-MAA into the lung airways.17 Repetitive doses of SPD-MAA (15 doses over 3 weeks) resulted in a significant reduction in mouse sIgA, but the same nonadducted SPD had no such effect, suggesting that alcohol and cigarette smoke co-exposure–derived MAA adduct formation represents one etiology of sIgA reduction. Because it was established previously that MAA-adducted protein binds to scavenger receptor A (CD204) in the lung,18 sIgA transcytosis in response to MAA using the CD204 KO mouse was evaluated. No significant sIgA reduction in SPD-MAA from control saline or SPD instilled mice was detected. Of note, levels of sIgA were quantitatively lower in the CD204 KO mice, which may explain previous reports of a decreased injury response in these mice.15,33
An established model of transcytosis was used to investigate the mechanism of MAA suppression of sIgA release.14 This model takes advantage of airway epithelial cells cultured on a permeabilized membrane to form a polarized ALI that can bind, internalize, and process dimeric IgA placed in the basal compartment, and release sIgA into the apical compartment. This model was used to recapitulate the in vivo findings that SPD-MAA reduces sIgA release, in experiments with primary mouse tracheal as well as human bronchial epithelial cells, and dissect specific transcytosis pathway responses to MAA. Consistent with the established role of pIgR in transcytosis,9 a MAA-induced down-regulation in mRNA and protein expression was observed in isolated epithelial cells treated in culture for 48 hours. Consistent with Gohy et al,14 it was found that SPD-MAA increases both MTEC and HBEC TGF-β, a mediator known to decrease IgA secretion via pIgR down-regulation. Previous studies support that TGF-β levels are increased in the BAL fluid of patients with ventilator-associated pneumonia34 and in the BAL fluid of experimental animal models of chronic alcohol use,35 however, until now, no study has shown that TGF-β levels are increased in the BAL fluid of patients with AUD. MAA-stimulated production of TGF-β was diminished in the CD204 KO mouse. Because the macrophage is a significant source for lung TGF-β, SPD-MAA was shown to stimulate significant release of TGF-β from a mouse lung macrophage cell line, suggesting an additional source of increased TGF-β for transcytosis suppression. These data support a role for MAA in the TGF-β–induced reduction of pIgR in the suppression of IgA transport across lung epithelium.
Smoking in the context of AUD is extremely common; however, downstream pathologic effects from combined exposures have been poorly described in the literature. Consistent with reported TGF-β reduction in the BAL of smokers,36 decreased TGF-β was detected in the non-AUD smoking group. However, the work is novel in its observations of enhanced TGF-β production with decreased sIgA in AUD smokers. The current study unraveled a novel mechanism for such TGF-β production in response to the reactive aldehydes generated from smoke and alcohol. Although such aldehydes can be derived from either tobacco pyrolysis or alcohol metabolism, the additive impact of combination use most certainly produces the unique formation of the MAA adduct that was not observed to form in the same concentrations from only smoke or only alcohol. The combination of smoke and alcohol is an additive effect that is required for the production of this bioactive aldehyde adduct. Recently, the up-regulation of the PIGR gene was identified in AUD active smokers.37 This supports the impact of combined smoke and alcohol on the pIgR pathway and suggests a potential feedback response to the observed reduction of transcytosed sIgA in the AUD smoker group. The study further suggests that MAA decreases pIgR expression to attain this effect.
MAA-adducted protein is likely not the only mediator of alcohol-associated dysfunctional IgA responses in lung. Existing roles for IL-17, IL-13, and IFN-γ in alcohol modulation of sIgA have also been reported. T-helper 17 cell production of IL-17 greatly stimulates pIgR.38 Trevejo-Nunez et al39 showed that alcohol decreases IL-17. A role for MAA-CD204 in down-regulating IL-17 needs to be explored. In addition, asthma and pIgR down-regulation via TGF-β40 establishes a role for IL-13 because exogenous IL-13 significantly inhibited secretory component release and pIgR expression. Increased serum IL-13 has been observed in AUD.41 Alcohol blunts IFN-γ release in mouse lung,42 although this does not appear to be the mechanism in MH-S macrophages. Chronic alcohol suppresses T-cell–derived IFN-γ in alcohol-fed mice.43 Spontaneous (basal) release of tumor necrosis factor α by alveolar macrophages was the same in AUD and non-AUD humans. Chronic alcohol consumption significantly suppresses lipopolysaccharide-stimulated alveolar macrophage production of tumor necrosis factor α. Because tumor necrosis factor α production is an important element in host defense, this may explain, in part, the susceptibility of chronic alcohol abusers to a variety of infections.44
The observations presented herein can have significant relevance to public health. Alcohol is an independent risk factor for COPD,45,46 and a large number of cigarette smokers misuse alcohol. COPD patients have decreased sIgA, an important antimicrobial mucosal agent against microbial infections.7,10 Patients with COPD have decreased levels of sIgA,11 and COPD lung has increased TGF-β levels.13 In addition to macrophages, the neutrophil is implicated in this TGF-β response in COPD.47 Future examination of paracrine neutrophil and macrophage responses to MAA should be explored in both healthy and COPD lungs. In addition, it has been proposed that COPD causes the reprogramming of bronchial epithelium in pIgR down-regulation.48 Furthermore, alcohol misuse has long been associated with a significant increase in lung infections. Alcohol consumption dose-dependently increases viral infections,49 and furthermore increases the risk of community-acquired infections.50 Given that infection plays such a major role in the life history of smoking alcoholic patients, these results translate beyond COPD.
Although the mouse and in vitro models corroborate findings in the human AUD cohort, limitations exist in the models that preclude conclusively understanding the contributing role for alcohol and smoking co-exposure in COPD. Barrier function was the same in all exposure groups before and after treatment, suggesting that a monolayer leak was not a contributing factor to changes in sIgA transcytosis. Vectoral transport integrity was maintained because no sIgA was detected in the basal compartment of ALI and no loss of viability occurred. However, differences in the total detectable amounts of sIgA in humans versus mice were noted even though overall responses were consistent. Human sIgA levels were robust and detectable whereas TGF-β levels were lower; the reverse was evident in mouse sIgA. In MTECs, treatment with MAA-adducted protein resulted in lower sIgA transcytosis from basal to apical. In mice exposed to cigarette smoke and fed alcohol, a significant decrease in BAL sIgA was observed compared with smoke or alcohol individual exposure. These data suggest that the combination of alcohol drinking and cigarette smoking may negatively impact sIgA-mediated lung innate defense. Although the antimicrobial nature of sIgA is established, the complexities of innate defense are comprised of multiple tissue injury aspects of drinking and smoking. The specific contribution of smoke- and alcohol-mediated sIgA suppression of transcytosis cannot be exactly determined owing to the complexities involved in regulating sIgA in the context of such functions as mucociliary transport, phagocytosis, and defensins, which collectively impact innate defense. Therefore, the focus of future studies should be determining the functional significance of such a compromise to mucosal immunity and should be characterized by bacterial clearance assays as a cumulative consequence of this multifaceted injury.
Acknowledgments
We thank Michael Price, M.D., Ph.D., for technical assistance with confocal microscopy, and Lisa Chudomelka for editorial assistance.
Footnotes
Supported by Central States Center for Agricultural Safety and Health grant U54OH010162 (T.A.W.), Department of Veterans Affairs Merit Review grants I01BX003635 (T.A.W.) and I01BX005413 (T.A.W., K.L.B.), National Institute on Alcohol Abuse and Alcoholism grants R01AA026086 (S.M.Y.) and R24AA019661 (E.L.B.), NIH/National Center for Advancing Translational Sciences (NCATS) grant UL1TR001082 (E.L.B.), Department of Veterans Affairs Research Career Scientist Award IK6BX003781 (T.A.W.), and Department of Veterans Affairs Career Development Award IK2BX004219 (K.J.W.).
Disclosures: None declared.
Supplemental material for this article can be found at http://doi.org/10.1016/j.ajpath.2021.06.007.
Supplemental Data
Secretory IgA (sIgA) release in mouse tracheal epithelial cells (MTECs) treated with interferon-γ (IFN-γ) as a positive control. MTECs cultured on an air–liquid interface were treated with 200 U/mL IFN-γ in both the apical and basal compartments for 24 hours before the addition of dimeric IgA to the basal compartment for an additional 24 hours and apical media collected for sIgA enzyme-linked immunosorbent assay. Values are presented as the means ± SEM; analyzed by one-way analysis of variance with Tukey post hoc multiple comparisons. ∗P < 0.05, ∗∗P < 0.01 versus media control. SPD-MAA, malondialdehyde-acetaldehyde–adducted surfactant protein D.
Barrier function in mouse tracheal epithelial cells (MTECs) treated with MAA-adducted protein. MTECs cultured on an air-liquid interface were treated with both MAA-adducted and nonadducted proteins and transepithelial resistance measured daily for 9 days in culture. No significant differences between treatment groups were observed at any time point. BSA, bovine serum albumin; SPD-MAA, malondialdehyde-acetaldehyde–adducted surfactant protein D.
References
- 1.Gupta N.M., Lindenauer P.K., Yu P.C., Imrey P.B., Haessler S., Deshpande A., Higgins T.L., Rothberg M.B. association between alcohol use disorders and outcomes of patients hospitalized with community-acquired pneumonia. JAMA Netw Open. 2019;2:e195172. doi: 10.1001/jamanetworkopen.2019.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolley S.E., Welsh D.A. Substance use is independently associated with pneumonia severity in persons living with the human immunodeficiency virus (HIV) Subst Abus. 2019;40:256–261. doi: 10.1080/08897077.2019.1576088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeligar S.M., Chen M.M., Kovacs E.J., Sisson J.H., Burnham E.L., Brown L.A. Alcohol and lung injury and immunity. Alcohol. 2016;55:51–59. doi: 10.1016/j.alcohol.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garmendia J., Morey P., Bengoechea J.A. Impact of cigarette smoke exposure on host-bacterial pathogen interactions. Eur Respir J. 2012;39:467–477. doi: 10.1183/09031936.00061911. [DOI] [PubMed] [Google Scholar]
- 5.Croft J.B., Wheaton A.G., Liu Y., Xu F., Lu H., Matthews K.A., Cunningham T.J., Wang Y., Holt J.B. Urban-rural county and state differences in chronic obstructive pulmonary disease — United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67:205–211. doi: 10.15585/mmwr.mm6707a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins I.T.T. The epidemiology of chronic respiratory disease. Prev Med. 1973;2:14–33. doi: 10.1016/0091-7435(73)90005-4. [DOI] [PubMed] [Google Scholar]
- 7.Pilette C., Godding V., Kiss R., Delos M., Verbeken E., Decaestecker C., Paepe K.D., Vaerman J.-P., Decramer M., Sibille Y. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:185–194. doi: 10.1164/ajrccm.163.1.9912137. [DOI] [PubMed] [Google Scholar]
- 8.Burnett D. Immunoglobulins in the lung. Thorax. 1986;41:337–344. doi: 10.1136/thx.41.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loman S., Radl J., Jansen H.M., Out T.A., Lutter R. Vectorial transcytosis of dimeric IgA by the Calu-3 human lung epithelial cell line: upregulation by IFN-gamma. Am J Physiol. 1997;272:L951–L958. doi: 10.1152/ajplung.1997.272.5.L951. [DOI] [PubMed] [Google Scholar]
- 10.Pilette C., Durham S.R., Vaerman J.P., Sibille Y. Mucosal immunity in asthma and chronic obstructive pulmonary disease: a role for immunoglobulin A? Proc Am Thorac Soc. 2004;1:125–135. doi: 10.1513/pats.2306032. [DOI] [PubMed] [Google Scholar]
- 11.Polosukhin V.V., Cates J.M., Lawson W.E., Zaynagetdinov R., Milstone A.P., Massion P.P., Ocak S., Ware L.B., Lee J.W., Bowler R.P., Kononov A.V., Randell S.H., Blackwell T.S. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:317–327. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richmond B.W., Brucker R.M., Han W., Du R.H., Zhang Y., Cheng D.S., Gleaves L., Abdolrasulnia R., Polosukhina D., Clark P.E., Bordenstein S.R., Blackwell T.S., Polosukhin V.V. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat Commun. 2016;7:11240. doi: 10.1038/ncomms11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak J.C., Chan-Yeung M.M., Ho S.P., Chan K.S., Choo K., Yee K.S., Chau C.H., Cheung A.H., Ip M.S., Members of Hong Kong Thoracic Society CSG Elevated plasma TGF-beta1 levels in patients with chronic obstructive pulmonary disease. Respir Med. 2009;103:1083–1089. doi: 10.1016/j.rmed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Gohy S.T., Detry B.R., Lecocq M., Bouzin C., Weynand B.A., Amatngalim G.D., Sibille Y.M., Pilette C. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-beta. Am J Respir Crit Care Med. 2014;190:509–521. doi: 10.1164/rccm.201311-1971OC. [DOI] [PubMed] [Google Scholar]
- 15.Sapkota M., Burnham E.L., DeVasure J.M., Sweeter J.M., Hunter C.D., Duryee M.J., Klassen L.W., Kharbanda K.K., Sisson J.H., Thiele G.M., Wyatt T.A. Malondialdehyde-acetaldehyde (MAA) protein adducts are found exclusively in the lungs of smokers with alcohol use disorders and are associated with systemic anti-MAA antibodies. Alcohol Clin Exp Res. 2017;41:2093–2099. doi: 10.1111/acer.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapkota M., Wyatt T.A. Alcohol, aldehydes, adducts and airways. Biomolecules. 2015;5:2987–3008. doi: 10.3390/biom5042987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyatt T.A., Kharbanda K.K., McCaskill M.L., Tuma D.J., Yanov D., Devasure J., Sisson J.H. Malondialdehyde-acetaldehyde-adducted protein inhalation causes lung injury. Alcohol. 2012;46:51–59. doi: 10.1016/j.alcohol.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger J.P., Simet S.M., DeVasure J.M., Boten J.A., Sweeter J.M., Kharbanda K.K., Sisson J.H., Wyatt T.A. Malondialdehyde-acetaldehyde (MAA) adducted proteins bind to scavenger receptor A in airway epithelial cells. Alcohol. 2014;48:493–500. doi: 10.1016/j.alcohol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elliott M.K., Sisson J.H., Wyatt T.A. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol. 2007;36:452–459. doi: 10.1165/rcmb.2005-0440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCaskill M.L., Kharbanda K.K., Tuma D.J., Reynolds J.D., DeVasure J.M., Sisson J.H., Wyatt T.A. Hybrid malondialdehyde and acetaldehyde protein adducts form in the lungs of mice exposed to alcohol and cigarette smoke. Alcohol Clin Exp Res. 2011;35:1106–1113. doi: 10.1111/j.1530-0277.2011.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You Y., Richer E.J., Huang T., Brody S.L. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 22.Burnham E.L., Kovacs E.J., Davis C.S. Pulmonary cytokine composition differs in the setting of alcohol use disorders and cigarette smoking. Am J Physiol Lung Cell Mol Physiol. 2013;304:L873–L882. doi: 10.1152/ajplung.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wyatt T.A., Bailey K.L., Simet S.M., Warren K.J., Sweeter J.M., DeVasure J.M., Pavlik J.A., Sisson J.H. Alcohol potentiates RSV-mediated injury to ciliated airway epithelium. Alcohol. 2019;80:17–24. doi: 10.1016/j.alcohol.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey K.L., Kharbanda K.K., Katafiasz D.M., Sisson J.H., Wyatt T.A. Oxidative stress associated with aging activates protein kinase Cepsilon, leading to cilia slowing. Am J Physiol Lung Cell Mol Physiol. 2018;315:L882–L890. doi: 10.1152/ajplung.00033.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole J.A., Kielian T., Wyatt T.A., Gleason A.M., Stone J., Palm K., West W.W., Romberger D.J. Organic dust augments nucleotide-binding oligomerization domain expression via an NF-{kappa}B pathway to negatively regulate inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2011;301:L296–L306. doi: 10.1152/ajplung.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen-Gipson D.S., Zimmerman M.C., Zhang H., Castellanos G., O'Malley J.K., Alvarez-Ramirez H., Kharbanda K., Sisson J.H., Wyatt T.A. Smoke extract impairs adenosine wound healing: implications of smoke-generated reactive oxygen species. Am J Respiratory Cell Mol Biol. 2013;48:665–673. doi: 10.1165/rcmb.2011-0273OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sollid L.M., Kvale D., Brandtzaeg P., Markussen G., Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987;138:4303–4306. [PubMed] [Google Scholar]
- 28.Gupta S., Heacock M., Perez A., Davis P.B. Antibodies to the polymeric immunoglobulin receptor with different binding and trafficking patterns. Am J Respir Cell Mol Biol. 2005;33:363–370. doi: 10.1165/rcmb.2005-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sapkota M., Kharbanda K.K., Wyatt T.A. Malondialdehyde-acetaldehyde-adducted surfactant protein alters macrophage functions through scavenger receptor A. Alcohol Clin Exp Res. 2016;40:2563–2572. doi: 10.1111/acer.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiele G.M., Tuma D.J., Willis M.S., Miller J.A., McDonald T.L., Sorrell M.F., Klassen L.W. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res. 1998;22:1731–1739. [PubMed] [Google Scholar]
- 31.Gotoh T., Ueda S., Nakayama T., Takishita Y., Yasuoka S., Tsubura E. Protein components of bronchoalveolar lavage fluids from non-smokers and smokers. Eur J Respir Dis. 1983;64:369–377. [PubMed] [Google Scholar]
- 32.Song K., Coleman R.A., Zhu X., Alber C., Ballas Z.K., Waldschmide T.J., Cook R.T. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- 33.Beamer C.A., Holian A. Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L186–L195. doi: 10.1152/ajplung.00474.2004. [DOI] [PubMed] [Google Scholar]
- 34.Overgaard C.E., Schlingmann B., Dorsainvil White S., Ward C., Fan X., Swarnakar S., Brown L.A., Guidot D.M., Koval M. The relative balance of GM-CSF and TGF-beta1 regulates lung epithelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1212–L1223. doi: 10.1152/ajplung.00042.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bechara R.I., Brown L.A., Roman J., Joshi P.C., Guidot D.M. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med. 2004;170:188–194. doi: 10.1164/rccm.200304-478OC. [DOI] [PubMed] [Google Scholar]
- 36.Koli K., Myllarniemi M., Keski-Oja J., Kinnula V.L. Transforming growth factor-beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal. 2008;10:333–342. doi: 10.1089/ars.2007.1914. [DOI] [PubMed] [Google Scholar]
- 37.Bailey K.L., Smith H., Mathai S.K., Huber J., Yacoub M., Yang I.V., Wyatt T.A., Kechris K., Burnham E.L. Alcohol use disorders are associated with a unique impact on airway epithelial cell gene expression. Alcohol Clin Exp Res. 2020;44:1571–1584. doi: 10.1111/acer.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaffar Z., Ferrini M.E., Herritt L.A., Roberts K. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol. 2009;182:4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trevejo-Nunez G., Chen K., Dufour J.P., Bagby G.J., Horne W.T., Nelson S., Kolls J.K. Ethanol impairs mucosal immunity against Streptococcus pneumoniae infection by disrupting interleukin 17 gene expression. Infect Immun. 2015;83:2082–2088. doi: 10.1128/IAI.02869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ladjemi M.Z., Gras D., Dupasquier S., Detry B., Lecocq M., Garulli C., Fregimilicka C., Bouzin C., Gohy S., Chanez P., Pilette C. Bronchial epithelial IgA secretion is impaired in asthma. Role of IL-4/IL-13. Am J Respir Crit Care Med. 2018;197:1396–1409. doi: 10.1164/rccm.201703-0561OC. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Reimers E., Santolaria-Fernandez F., Medina-Garcia J.A., Gonzalez-Perez J.M., de la Vega-Prieto M.J., Medina-Vega L., Martin-Gonzalez C., Duran-Castellon M.C. TH-1 and TH-2 cytokines in stable chronic alcoholics. Alcohol Alcohol. 2012;47:390–396. doi: 10.1093/alcalc/ags041. [DOI] [PubMed] [Google Scholar]
- 42.Happel K.I., Rudner X., Quinton L.J., Movassaghi J.L., Clark C., Odden A.R., Zhang P., Bagby G.J., Nelson S., Shellito J.E. Acute alcohol intoxication suppresses the pulmonary ELRNegative CXC chemokine response to lipopolysaccharide. Alcohol. 2007;41:325–333. doi: 10.1016/j.alcohol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemann E.A., McGill J.L., Legge K.L. Chronic ethanol exposure selectively inhibits the influenza-specific CD8 T cell response during influenza a virus infection. Alcohol Clin Exp Res. 2014;38:2403–2413. doi: 10.1111/acer.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omidvari K., Casey R., Nelson S., Olariu R., Shellito J.E. Alveolar macrophage release of tumor necrosis factor-a in chronic alcoholics without liver disease. Alcohol Clin Exp Res. 1998;22:567–572. doi: 10.1111/j.1530-0277.1998.tb04294.x. [DOI] [PubMed] [Google Scholar]
- 45.Sisson J.H., Stoner J.A., Romberger D.J., Spurzem J.R., Wyatt T.A., Owens-Ream J., Mannino D.M. Alcohol intake is associated with altered pulmonary function. Alcohol. 2005;36:19–30. doi: 10.1016/j.alcohol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Tabak C., Smit H.A., Rasanen L., Fidanza F., Menotti A., Nissinen A., Feskens E.J., Heederik D., Kromhout D. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology. 2001;12:239–245. doi: 10.1097/00001648-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Ratajczak C., Guisset A., Detry B., Sibille Y., Pilette C. Dual effect of neutrophils on pIgR/secretory component in human bronchial epithelial cells: role of TGF-beta. J Biomed Biotechnol. 2010;2010:428618. doi: 10.1155/2010/428618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sankineni S., Cho Y., Hosseinian N., Kolliputi N. Does pIgR down-regulation in COPD cause reprogramming of bronchial epithelium? Lung. 2015;193:1–2. doi: 10.1007/s00408-014-9668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Happel K.I., Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2:428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- 50.Simou E., Britton J., Leonardi-Bee J. Alcohol and the risk of pneumonia: a systematic review and meta-analysis. BMJ Open. 2018;8:e022344. doi: 10.1136/bmjopen-2018-022344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Secretory IgA (sIgA) release in mouse tracheal epithelial cells (MTECs) treated with interferon-γ (IFN-γ) as a positive control. MTECs cultured on an air–liquid interface were treated with 200 U/mL IFN-γ in both the apical and basal compartments for 24 hours before the addition of dimeric IgA to the basal compartment for an additional 24 hours and apical media collected for sIgA enzyme-linked immunosorbent assay. Values are presented as the means ± SEM; analyzed by one-way analysis of variance with Tukey post hoc multiple comparisons. ∗P < 0.05, ∗∗P < 0.01 versus media control. SPD-MAA, malondialdehyde-acetaldehyde–adducted surfactant protein D.
Barrier function in mouse tracheal epithelial cells (MTECs) treated with MAA-adducted protein. MTECs cultured on an air-liquid interface were treated with both MAA-adducted and nonadducted proteins and transepithelial resistance measured daily for 9 days in culture. No significant differences between treatment groups were observed at any time point. BSA, bovine serum albumin; SPD-MAA, malondialdehyde-acetaldehyde–adducted surfactant protein D.