Abstract
Background
Stroke is one of the leading causes of death worldwide. Cilostazol, an antiplatelet and phosphodiesterase 3 inhibitor, has not been clearly established for ischaemic stroke use. We aim to determine the efficacy and safety of cilostazol for secondary stroke prevention.
Methods
MEDLINE, EMBASE, Cochrane Library, Web of Science and ClinicalTrials.gov were searched from inception to 25 September 2020, for randomised trials comparing the efficacy and safety of cilostazol monotherapy or dual therapy with another antiplatelet regimen or placebo, in patients with ischaemic stroke. Version 2 of the Cochrane risk-of-bias tool for randomised trials (RoB 2) was used to assess study quality. This meta-analysis was reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Results
Eighteen randomised trials comprising 11 429 participants were included in this meta-analysis. Most trials possessed low risk of bias and were of low heterogeneity. Cilostazol significantly reduced the rate of ischaemic stroke recurrence (risk ratio, RR=0.69, 95% CI 0.58 to 0.81), any stroke recurrence (RR=0.64, 95% CI 0.54 to 0.74) and major adverse cardiovascular events (RR=0.67, 95% CI 0.56 to 0.81). Cilostazol did not significantly decrease mortality (RR=0.90, 95% CI 0.64 to 1.25) or increase the rate of good functional outcome (Modified Rankin Scale score of 0–1; RR=1.07, 95% CI 0.95 to 1.19). Cilostazol demonstrated favourable safety profile, significantly reducing the risk of intracranial haemorrhage (RR=0.46, 95% CI 0.31 to 0.68) and major haemorrhagic events (RR=0.49, 95% CI 0.34 to 0.70).
Conclusions
Cilostazol demonstrated superior efficacy and safety profiles compared with traditional antiplatelet regimens such as aspirin and clopidogrel for secondary stroke prevention but does not appear to affect functional outcomes. Future randomised trials can be conducted outside East Asia, or compare cilostazol with a wider range of antiplatelet agents.
Keywords: stroke, haemorrhage, platelets, stenosis
Background
Introduction
The rate of ischaemic stroke recurrence varies from 8%–14% at 1 year to 39% at 10 years,1 2 and is associated with poor outcomes.3 To reduce the recurrence of ischaemic strokes, antiplatelet therapies are widely adopted. Some established regimens include single antiplatelet therapy with aspirin,4 monotherapy with clopidogrel5 and combination aspirin and extended-release dipyridamole.6 Aspirin and clopidogrel combination can be considered for short-term stroke management. However, these antiplatelet therapies, especially long-term combination aspirin and clopidogrel, are known to increase the risk of haemorrhagic conversion and bleeding complications.7 8
Cilostazol is an approved treatment for intermittent claudication and thrombotic complications of coronary angioplasty in combination with aspirin or clopidogrel.9 It selectively inhibits phosphodiesterase 3, increasing activation of intracellular cyclic adenosine monophosphate and protein kinase A, thereby inhibiting platelet aggregation. Apart from its antiplatelet properties, cilostazol may prevent recurrent ischaemic stroke by improving endothelial function,10 reducing triglycerides and increasing high-density lipoproteins.11 The Chinese Guidelines for Secondary Prevention of Ischemic Stroke and Transient Ischemic Attack, Korean Clinical Practice Guidelines for Stroke, Japanese Guidelines for the Management of Stroke and other eastern institutions12–15 have accepted cilostazol as a second-line drug to aspirin and/or clopidogrel for secondary stroke prevention. However, cilostazol is neither widely recommended nor approved by most international guidelines.4
Objectives
Cilostazol is an accepted monotherapy for secondary stroke prevention in East Asian countries but is not commonly used outside Asia. Previous meta-analyses have also demonstrated the efficacy and safety of cilostazol to this end.16–18 However, those reviews do not include a new randomised trial evaluating the short-term use of cilostazol in acute ischaemic stroke. Since studies performed on these subgroups are relatively scarce, it is useful to perform an updated meta-analysis to (1) confirm the effect of cilostazol as monotherapy; and (2) explore the effect of cilostazol in combination therapy. To investigate the efficacy of cilostazol-based therapies in different situations, we performed subgroup analyses on intracranial arterial stenosis (ICAS), specific antiplatelet regimens, stroke chronicity and duration of treatment. Unlike previous meta-analyses, we graded the quality of evidence for each outcome measure.
Methods
Search strategy
We report the findings according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.19 The authors screened multiple comprehensive databases (MEDLINE, EMBASE, Cochrane Library, Web of Science and ClinicalTrials.gov) for studies comparing cilostazol and other antiplatelet regimens for secondary prevention of ischaemic stroke.
The literature search was conducted for all publications in the databases from inception to 25 September 2020. We used keywords related to stroke (eg, ‘stroke’, ‘cerebrovascular accident’ or ‘cerebral infarct’) and cilostazol (eg, ‘cilostazol’, ‘Pletal’ or ‘phosphodiesterase 3 inhibitor’). Medical Subject Headings (MeSH) and EMBASE subject headings (EMTREE) terms were used in conjunction with keywords to refine the search results (online supplemental data). Manual searches were also conducted on the reference lists of included studies and review articles to identify trials that were missed in the electronic search.
svn-2020-000737supp001.pdf (5.8MB, pdf)
Study selection
The inclusion criteria encompassed any randomised trials that compared the efficacy and safety of cilostazol used as monotherapy or combination therapy with other antiplatelet agents, placebo or best medical therapy for the secondary prevention of ischaemic stroke. Non-randomised observational studies and conference proceedings were excluded.
The primary clinical outcome was ischaemic stroke recurrence. Safety outcomes comprised intracranial haemorrhages (ICH) and major haemorrhagic events. Secondary outcomes included any stroke recurrence, all-cause mortality, major adverse cardiovascular events (MACE) and adverse drug events (ADEs) leading to treatment discontinuation. Most studies defined ICH as intracerebral haemorrhage or subarachnoid haemorrhage. The definition for ‘any stroke’ comprised ischaemic stroke and either haemorrhagic stroke or ICH but did not include transient ischaemic attacks. Good functional outcome was defined as a Modified Rankin Scale score of 0–1 (mRS 0–1) at 3–6 months.
Two reviewers (TCH and WGRA) independently performed title and abstract screening on studies retrieved from the search strategy, followed by full-text screening. Data extraction and quality assessment were performed independently. Any disagreements were resolved by consensus.
Data collection
The predesigned data extraction form included the following: (1) intervention and control; (2) participant characteristics, for example, age, National Institutes of Health Stroke Scale (NIHSS) on admission and country of study; (3) stroke subtypes; (4) time to randomisation or treatment; (5) duration of treatment and follow-up; and (6) outcome event rates.
Quality assessment
Quality assessment of the included studies was performed using Version 2 of the Cochrane tool for assessing risk of bias in randomised trials (RoB 2).20 For each included study, signalling questions were answered to assess the risk of bias arising from: (1) the randomisation process; (2) deviations from the intended interventions; (3) missing outcome data; (4) the measurement of outcomes; and (5) the selection of reported results. Each component was individually assessed as having low, some concerns or high risk of bias.
The quality of evidence for each outcome was determined using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system consisting of five components: risk of bias, imprecision, inconsistency, indirectness and publication bias.21
Statistical analysis
The random-effects model was used to generate risk ratios (RRs) and associated 95% CIs. Where possible, the intention-to-treat (ITT) population was used. Heterogeneity was evaluated using the chi square (χ2) and I2 test. I2<25%, 25%–75% and >75% indicated low, moderate and high degree of inconsistency, respectively. Meta-regression was performed using the mixed-effects model. Publication bias was assessed using funnel plots. A p value ≤0.05 was considered statistically significant. All analyses were performed using Review Manger V.5.3 (The Cochrane Collaboration, 2014) and meta package V.4.13-0 in R (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study selection and study characteristics
A total of 2415 unique records were retrieved. Of these, the full text of 81 studies were screened (online supplemental data).
We included 18 randomised trials comprising 11 429 patients in the ITT population22–39 (tables 1 and 2). Nine studies were conducted in Japan, four in Korea, two in China, two in multiple East Asian countries and one in the UK. Most studies explicitly excluded patients with cardioembolic stroke, but two studies23 33 did not specify this. Three studies30 31 39 recruited participants with symptomatic ICAS, while two studies23 26 included patients with lacunar infarction. Although one study recruited only patients with asymptomatic or previous intracerebral haemorrhage,29 most studies excluded such patients or participants with a bleeding diathesis. One study included patients with high-risk ischaemic stroke,38 while most others did not include patients with moderate-to-severe disability, for example, NIHSS ≥8,34 NIHSS ≥1625 30 32 or NIHSS ≥2022 36, mRS ≥425 27 or severe cerebral deficits.23 24 Some studies explicitly excluded patients with concurrent antiplatelet,22 24 26–28 30 32 33 37–39 anticoagulant,22–24 26 30 32–34 37–39 or thrombolytic/fibrinolytic24 31–34 36 37 use.
Table 1.
Characteristics of included studies
| Study ID | Country | Sample size, ITT (% male) | Age (intervention) | Age (control) | Intervention | Control |
| Aoki 2019 (ADS)*22 | Japan | 1201 (66%) | 69 (IQR 60–77) | 69 (IQR 61–78) | Cilostazol 200 mg + aspirin 81–200 mg | Aspirin 81–200 mg |
| Blair 2019 (LACI-1)23 | UK | 57 (68%) | Mean of all participants: 66.1 (11.1) | Cilostazol 100 mg BD + aspirin/clopidogrel | Aspirin/clopidogrel | |
| Gotoh 2000 (CSPS)24 | Japan | 1067 (66%) | 65.2 (8.7) | 65.1 (8.8) | Cilostazol 100 mg BD | Placebo |
| Guo 200925 | China | 68 (35%) | 59.44 (10.63) | 62.02 (11.12) | Cilostazol 100 mg BD | Aspirin 100 mg |
| Han 2013 (ECLIPse)26 | Korea | 182 (25%) | 64.63 (9.07) | 65.48 (9.92) | Cilostazol 100 mg BD + aspirin 100 mg | Aspirin 100 mg |
| Huang 2008 (CASISP)27 | China | 719 (69%) | 60.14 (10.05) | 60.31 (9.71) | Cilostazol | Aspirin |
| Johkura 201228 | Japan | 106 (43%) | 76.7 (9.8) | 73.7 (9.4) | Cilostazol 200 mg | Aspirin 100 mg |
| Kim 2018 (PICASSO)29 | South Korea, Hong Kong, Philippines | 1534 (62%) | 65.5 (10.9) | 65.8 (10.7) | Cilostazol 100 mg BD | Aspirin 100 mg |
| Kwon 2005 (TOSS)30 | South Korea | 135 (61%) | 62.28 (10.42) | 62.54 (8.97) | Cilostazol 100 mg BD + aspirin 100 mg | Aspirin 100 mg |
| Kwon 2011 (TOSS-2)31 | 4 East Asian countries | 457 (51%) | 66.42 (11.33) | 64.58 (11.11) | Cilostazol 100 mg BD + aspirin 75–150 mg | Clopidogrel 75 mg + aspirin 75–150 mg |
| Lee 2011 (CAIST)32 | Korea | 458 (61%) | 63 (12) | 63 (12) | Cilostazol 200 mg | Aspirin 300 mg |
| Lee 201733 | Korea | 80 (65%) | 57.4 (12.7) | 59.5 (11.7) | Cilostazol 100 mg BD | Aspirin 100 mg |
| Nakamura 201234 | Japan | 76 (74%) | 66 (12) | 67 (10) | Cilostazol 100 mg BD + aspirin 300 mg | Aspirin 300 mg |
| Ohnuki 201735 | Japan | 24 (71%) | 60.5 (10) | 63.6 (9.1) | Cilostazol 200 mg + aspirin 100 mg | Aspirin 100 mg |
| Shimizu 201336 | Japan | 507 (67%) | 66.2 (9.4) | 66.6 (8.9) | Cilostazol 200 mg + optimal medical treatment | Optimal medical treatment |
| Shinohara 2010 (CSPS 2)37 | Japan | 2716 (72%) | 63.5 (9.2) | 63.4 (9.0) | Cilostazol 100 mg BD | Aspirin 81 mg |
| Toyoda 2019 (CSPS.com)38 | Japan | 1879 (70%) | 69.6 (9.2) | 69.7 (9.2) | Cilostazol 100 mg BD + aspirin 81–100 mg/ clopidogrel 50–75 mg | Aspirin 81–100 mg/ clopidogrel 50–75 mg |
| Uchiyama 2015 (CATHARSIS)39 | Japan | 163 (66%) | 68.3 (range 45–84) | 68.3 (range 50–82) | Cilostazol 200 mg + aspirin 100 mg | Aspirin 100 mg |
All data are in mean (SD) or median (IQR/range).
*After 14 days, both arms were swapped to cilostazol 200 mg until 3 months. Where possible, we analysed data at 14 days instead of 3 months.
ITT, intention to treat.
Table 2.
Details of included studies
| Study ID | Time to randomisation/treatment | Duration of treatment/ follow-up | Type of stroke | |
| Cilostazol | Control | |||
| Aoki 2019 (ADS)22 |
Within 48 hours Cilostazol: 10.1 hours (IQR 4.6–20.0) Control: 11.5 hours (IQR 4.8–20.9) |
14 days, 3 months |
Symptomatic ICAS (23%) Large-artery atherosclerosis (13%), LACI (46%), branch atheromatous disease (13%) |
Symptomatic ICAS (22%) Large-artery atherosclerosis (15%), LACI (43%), branch atheromatous disease (16%) |
| Blair 2019 (LACI-1)23 | Within 4 years Median: 203 days (range 6–920) |
Treatment: 6–9 weeks Follow-up: 11 weeks |
LACI | LACI |
| Gotoh 2000 (CSPS)24 |
1–6 months Cilostazol: 83.0 days (range 7–1805) Control: 82.4 days (range 8–1079) |
Mean follow-up duration: Cilostazol: 651.8 days Control: 569.7 days Treatment duration: Cilostazol: 632.2 (467.7) days Control: 695.1 (456.3) days |
Atherothrombotic (14.1%), LACI (75.0%), mixed (9.0%) | Atherothrombotic (13.1%), LACI (73.8%), mixed (11.4%) |
| Guo 200925 | 1–6 months | 12 months | ||
| Han 2013 (ECLIPse)26 |
Within 7 days Median: 5 days (78.3% of patients were randomised within 7 days) |
90 days | LACI | LACI |
| Huang 2008 (CASISP)27 |
1–6 months Cilostazol: 77.26 (50.05) days Control: 79.72 (51.96) days |
12–18 months (average: 740 person-years) | ||
| Johkura 201228 | 1–6 months | 6 months | ||
| Kim 2018 (PICASSO)*29 |
Within 180 days Cilostazol: 18 days (IQR 8–40) Control: 17 days (IQR 7–35) |
1.9 years (IQR 1.0–3.0) | Ischaemic stroke (95%), TIA (5%) | Ischaemic stroke (94%), TIA (6%) |
| Kwon 2005 (TOSS)30 | Within 2 weeks | 6 months | ICAS | ICAS |
| Kwon 2011 (TOSS-2)31 |
Within 2 weeks Cilostazol: 8.03 (3.34) days Control: 7.82 (3.15) days |
7 months | ICAS | ICAS |
| Lee 2011 (CAIST)32 |
Within 48 hours Cilostazol: 33 (12) hours Control: 35 (11) hours |
90 days | Large-artery disease (32%), small-vessel disease (55%), cardioembolism (1%), other determined aetiology (<1%), undetermined (12%) | Large-artery disease (25%), small-vessel disease (62%), cardioembolism (1%), other determined aetiology (<1%), undetermined (12%) |
| Lee 201733 | Within 7 days | 3 months | Atherosclerosis (9.4%), small-artery disease (87.5%), TIA (3.1%) | Atherosclerosis (2.9%), small-artery disease (87.5%), TIA (5.9%), unknown (8.8%) |
| Nakamura 201234 |
Within 48 hours Cilostazol: 25 (12) hours Control: 23 (14) hours |
6 months | Large-artery atherosclerosis (21%), small-vessel occlusion (47%), other determined or undetermined aetiology (32%) | Large-artery atherosclerosis (18%), small-vessel occlusion (47%), other determined or undetermined aetiology (34%) |
| Ohnuki 201735 | Within 7 days | 4 weeks | Atherothrombosis (15%), LACI (62%), TIA (8%), unknown (15%) | Atherothrombosis (45%), LACI (45%), TIA (9%) |
| Shimizu 201336 | Within 24 hours | 3 months | Atherothrombotic (30.7%), LACI (64.1%), others (5.2%) | Atherothrombotic (25.0%), LACI (70.7%), others (4.3%) |
| Shinohara 2010 (CSPS 2)37 | Within 26 weeks (6 months) | 1–5 years Mean: 29 (16) months |
Atherothrombotic (33%), LACI (65%), others (3%) | Atherothrombotic (31%), LACI (65%), others (3%) |
| Toyoda 2019 (CSPS.com)†38 |
8–180 days Cilostazol: 27 days (IQR 13–63) Control: 25 days (IQR 13–64) |
0.5–3.5 years Median: 1.4 (IQR 0.8–2.2) |
ICAS (30%), ECAS (12%) Atherothrombotic (42%), LACI (50%), others/unknown (8%) |
ICAS (29%), ECAS (14%) Atherothrombotic (42%), LACI (49%), others/unknown (9%) |
| Uchiyama 2015 (CATHARSIS)39 | 2 weeks to 6 months | 2 years Mean: 762 days |
ICAS | ICAS |
Data are in median (IQR), mean (range) or mean (SD) unless otherwise stated.
*Only patients with asymptomatic or previous intracerebral haemorrhage were included.
†Only patients with high-risk ischaemic stroke were included.
ICAS, intracranial arterial stenosis; LACI, lacunar infarction; TIA, transient ischaemic attack.
Four trials22 32 34 36 included patients with acute stroke (onset within 48 hours); three trials22 23 35 administered short-term antiplatelet therapy (<3 months). Studies compared cilostazol (CIL) single or dual antiplatelet therapy (SAPT or DAPT) against aspirin (ASA), clopidogrel (CLO), and placebo or best medical therapy (No CIL). For one study,22 we analysed data recorded at 14 days instead of 3 months if available—that is, for most outcomes except for major haemorrhagic events and good functional outcome—to minimise bias from the treatment regimen.
Quality assessment
On the basis of RoB 2, most trials were of high quality and possessed a low overall risk of bias (online supplemental data). One trial28 had a high risk of bias due to missing outcome data from a significant, asymmetric loss to follow-up.
Stroke recurrence and functional outcomes
Ischaemic stroke recurrence
Meta-analysis of all 18 randomised trials (figure 1) revealed cilostazol significantly reduced the risk of recurrent ischaemic stroke (RR=0.69, 95% CI 0.58 to 0.81, p<0.0001). There was low heterogeneity across all trials (I2=0%).
Figure 1.
Forest plot depicting risk of ischaemic stroke recurrence. ASA, aspirin; CIL, cilostazol; CLO, clopidogrel; No CIL, placebo or best medical therapy.
Subgroup analysis based on specific antiplatelet regimens indicated moderate heterogeneity between antiplatelet regimens (I2=42.9%; χ5 2=8.76, p=0.12). The greatest risk reduction was as follows: (1) CIL+CLO versus CLO (n=1116); followed by (2) CIL versus no CIL (n=1574); (3) CIL+ASA versus ASA (n=2544); and (4) CIL versus ASA (n=5681). Interestingly, CIL+ASA performed worse than ASA+CLO, although this effect was not statistically significant (n=457; RR=1.62, 95% CI 0.60 to 4.37, p=0.34). When the specific type of antiplatelet was disregarded, cilostazol DAPT had a greater risk reduction than cilostazol SAPT, both compared with an SAPT.
Any stroke recurrence
Meta-analysis of all 18 trials (figure 2) revealed cilostazol significantly reduced the recurrence of any stroke, both ischaemic and haemorrhagic (RR=0.64, 95% CI 0.54 to 0.74, p<0.00001). There was low heterogeneity across all trials (I2=0%).
Figure 2.
Forest plot depicting risk of any stroke recurrence. ASA, aspirin; CIL, cilostazol; CLO, clopidogrel; No CIL, placebo or best medical therapy.
Subgroup analysis based on specific antiplatelet regimens indicated moderate heterogeneity between antiplatelet regimens (I2=39.4%; χ4 2=6.61, p=0.16). The greatest risk reduction was as follows: (1) CIL+ASA/CLO versus ASA/CLO (n=1936); followed by (2) CIL versus no CIL (n=1574); and (3) CIL versus ASA (n=5681). Similar to ischaemic stroke recurrence, CIL+ASA conferred an insignificant increase in risk of any stroke recurrence compared with ASA+CLO (n=457; RR=1.78, 95% CI 0.67 to 4.73, p=0.25). However, unlike ischaemic stroke recurrence, CIL+ASA versus ASA did not entail a significant risk reduction for any stroke recurrence (p=0.30), which can be explained by the smaller sample size.
Intracranial haemorrhage
Meta-analysis of all 18 trials (figure 3) revealed cilostazol significantly reduced the risk of ICH (RR=0.46, 95% CI 0.31 to 0.68, p<0.0001). There was low heterogeneity across all trials (I2=0%).
Figure 3.
Forest plot depicting risk of intracranial haemorrhage. ASA, aspirin; CIL, cilostazol; CLO, clopidogrel; No CIL, placebo or best medical therapy.
Subgroup analysis of specific antiplatelet therapies indicated a substantial reduction in risk of ICH when cilostazol monotherapy was compared with aspirin (RR=0.36, 95% CI 0.22 to 0.59, p<0.0001). The addition of cilostazol to aspirin or clopidogrel did not significantly affect the risk of ICH.
Functional outcome
Meta-analysis of four trials (online supplemental data) showed cilostazol did not significantly increase the probability of a good functional outcome (RR=1.07, 95% CI 0.95 to 1.19, p=0.28). There was moderate heterogeneity among trials (I2=60%; χ3 2=7.49, p=0.06). One trial34 contributed significantly to the heterogeneity (n=76; RR=2.00, 95% CI 1.22 to 3.27); this trial measured patients’ mRS scores at 6 months compared with 3 months for the other trials. After removing this trial, I2 decreased to 0% and χ2 2=0.71 (p=0.70).
Adverse events and mortality
Major haemorrhagic events
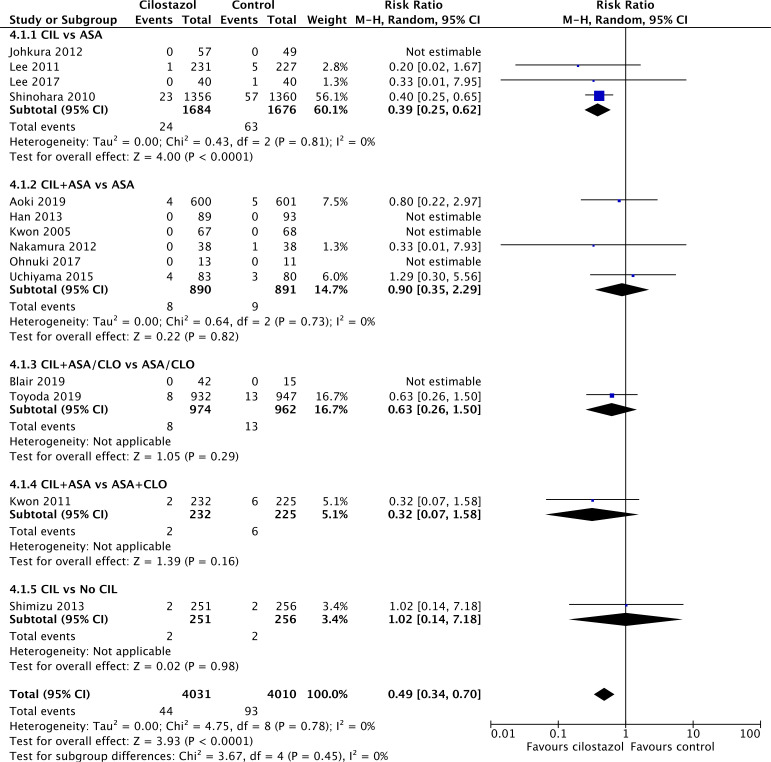
Meta-analysis of 14 trials (figure 4) revealed cilostazol reduced the risk of major haemorrhagic events, which included ICH (RR=0.49, 95% CI 0.34 to 0.70, p<0.0001). There was low heterogeneity across studies (I2=0%).
Figure 4.
Forest plot depicting risk of major haemorrhagic events. ASA, aspirin; CIL, cilostazol; CLO, clopidogrel; No CIL, placebo or best medical therapy.
Similar to ICH, cilostazol monotherapy substantially reduced the risk of major haemorrhagic events compared with aspirin (RR=0.39, 95% CI 0.25 to 0.62, p<0.0001), and the addition of cilostazol to aspirin or clopidogrel did not affect the risk of major haemorrhagic events.
Mortality
Meta-analysis of 15 trials (figure 5) revealed cilostazol did not significantly reduce the risk of mortality (RR=0.90, 95% CI 0.64 to 1.25, p=0.53). Two trials31 32 presented data on vascular deaths instead of all-cause mortality. There was low heterogeneity across studies (I2=0%). There was no significant difference between antiplatelet regimens (I2=0%; χ4 2=0.32, p=0.99); there was no statistically significant reduction in mortality for both cilostazol combination therapy and monotherapy.
Figure 5.
Forest plot depicting risk of mortality. ASA, aspirin; CIL, cilostazol; CLO, clopidogrel; No CIL, placebo or best medical therapy.
Major adverse cardiovascular events
Most studies defined MACE as a composite of strokes, ICH, myocardial infarctions and vascular deaths. Meta-analysis of 13 trials (online supplemental data) revealed cilostazol significantly reduced the risk of MACE (RR=0.67, 95% CI 0.56 to 0.81, p<0.0001). There was low heterogeneity across studies (I2=0%).
Subgroup analysis based on specific antiplatelet regimens indicated moderate heterogeneity between antiplatelet regimens (I2=54.6%; χ4 2=8.82, p=0.07). The greatest risk reduction was (1) CIL+ASA/CLO versus ASA/CLO (n=1879, from one trial)38; followed by (2) CIL versus no CIL (n=1574). However, CIL versus ASA and CIL+ASA versus ASA did not significantly reduce the risk of MACE (p=0.08 and 0.75, respectively). Similar to ischaemic stroke recurrence, CIL+ASA performed worse than ASA+CLO, but this effect was not statistically significant (n=457; RR=1.45, 95% CI 0.67 to 3.17, p=0.35). When the specific type of antiplatelet was disregarded, cilostazol DAPT had a greater risk reduction (n=3497; RR=0.58, 95% CI 0.42 to 0.80, p=0.0009) than cilostazol SAPT (n=2140; RR=0.77, 95% CI 0.57 to 1.03, p=0.08), both compared with an SAPT. Since one trial was able to considerably skew the results of CIL DAPT versus ASA SAPT, it suggests that the other studies are underpowered for MACE.
ADEs leading to treatment discontinuation
Meta-analysis of 13 trials (online supplemental data) revealed cilostazol increased the risk of ADEs leading to treatment discontinuation (RR=1.83, 95% CI 1.30 to 2.59, p=0.0006). There was high heterogeneity across studies (I2=81%). In three studies,26 29 32 the safety population comprising all participants who consumed the drug was used in place of the ITT population.
Subgroup analysis of the antiplatelet treatments demonstrated high heterogeneity among different regiments (I2=82.9%; χ3 2=17.58, p=0.0005). The greatest statistically significant increase in relative risk occurred in the CIL+ASA/CLO versus ASA/CLO subgroup (n=1936; RR=5.59, 95% CI 3.04 to 10.27), followed by the CIL versus No CIL subgroup (n=1067; RR=2.13, 95% CI 1.43 to 3.16). There was no significant increase in ADEs leading to drug discontinuation in the CIL versus ASA and CIL+ASA versus ASA subgroup (n=5476 and 1639, p=0.11 and 0.10, respectively).
Different administrative strategies
Acute versus delayed administration for stroke
Acute/subacute stroke was defined as stroke within 72 hours of symptom onset. Based on the time from onset to randomisation or treatment, 4 studies comprising 2242 patients presented data on cilostazol administration during acute/subacute stroke, while 14 studies comprising 9187 patients provided data for administration during chronic stroke (table 3). Cilostazol administration during the acute and chronic stroke phase demonstrated no significant difference in outcomes (I2≤4.8%; p>0.05).
Table 3.
Summary of outcomes
| Subgroup | N | Cilostazol | Control | RR (95% CI) | P value | I2 (%) | Test for subgroup differences | |
| Ischaemic stroke recurrence | ||||||||
| A | Overall | 18 | 217/5724 | 317/5705 | 0.69 (0.58–0.81) | <0.0001 | 0 | NA |
| B | CIL SAPT vs SAPT | 7 | 131/2844 | 168/2837 | 0.78 (0.62–0.97) | 0.03 | 0 | χ3 2=7.50, p=0.06, I2=60.0% |
| CIL DAPT vs SAPT | 8 | 43/1864 | 82/1853 | 0.52 (0.36–0.75) | 0.0005 | 0 | ||
| CIL DAPT vs DAPT | 1 | 10/232 | 6/225 | 1.62 (0.60–4.37) | 0.34 | NA | ||
| CIL vs No CIL | 2 | 33/784 | 61/790 | 0.54 (0.36–0.82) | 0.003 | 0 | ||
| C | Stroke onset <3 days | 4 | 16/1120 | 24/1122 | 0.67 (0.36–1.25) | 0.20 | 0 | χ1 2=0.01, p=0.93, I2=0% |
| Stroke onset >3 days | 14 | 201/4604 | 293/4583 | 0.69 (0.56–0.84) | 0.0002 | 8 | ||
| D | Short term (<3 months) | 3 | 7/655 | 8/627 | 0.78 (0.29–2.12) | 0.63 | 0 | χ1 2=0.07, p=0.80, I2=0% |
| Long term (≥3 months) | 15 | 210/5069 | 309/5078 | 0.68 (0.58–0.81) | <0.0001 | 0 | ||
| E | Symptomatic ICAS | 3 | 14/382 | 12/373 | 1.10 (0.45–2.68) | 0.84 | 24 | χ2 2=1.33, p=0.52, I2=0% |
| LACI | 2 | 2/131 | 1/108 | 1.08 (0.14–8.56) | 0.95 | 0 | ||
| Others/mixed/unknown | 13 | 201/5211 | 304/5224 | 0.67 (0.56–0.79) | <0.00001 | 0 | ||
| Any stroke recurrence | ||||||||
| A | Overall | 18 | 250/5724 | 394/5705 | 0.64 (0.54–0.74) | <0.00001 | 0 | NA |
| B | Stroke onset <3 days | 4 | 20/1120 | 29/1122 | 0.70 (0.39–1.23) | 0.21 | 0 | χ1 2=0.11, p=0.75, I2=0% |
| Stroke onset >3 days | 14 | 230/4604 | 365/4583 | 0.63 (0.54–0.74) | <0.00001 | 0 | ||
| C | Short term (<3 months) | 3 | 9/655 | 9/627 | 0.91 (0.37–2.24) | 0.83 | 0 | χ1 2=0.61, p=0.43, I2=0% |
| Long term (≥3 months) | 15 | 241/5069 | 385/5078 | 0.63 (0.54–0.74) | <0.00001 | 0 | ||
| D | Symptomatic ICAS | 3 | 15/382 | 14/373 | 0.96 (0.27–3.45) | 0.95 | 65 | χ2 2=0.71, p=0.70, I2=0% |
| LACI | 2 | 2/131 | 1/108 | 1.08 (0.14–8.56) | 0.95 | 0 | ||
| Others/mixed/unknown | 13 | 233/5211 | 379/5224 | 0.62 (0.53–0.73) | <0.00001 | 0 | ||
| Intracranial haemorrhage | ||||||||
| A | Overall | 18 | 37/5724 | 86/5705 | 0.46 (0.31–0.68) | <0.0001 | 0 | NA |
| B | Stroke onset <3 days | 4 | 4/1120 | 5/1122 | 0.91 (0.24–3.53) | 0.89 | 0 | χ1 2=1.05, p=0.31, I2=4.8% |
| Stroke onset >3 days | 14 | 33/4604 | 81/4583 | 0.43 (0.29–0.65) | <0.0001 | 0 | ||
| C | Short term (<3 months) | 3 | 2/655 | 1/627 | 2.00 (0.18–22.03) | 0.57 | NA | χ1 2=1.48, p=0.22, I2=32.3% |
| Long term (≥3 months) | 15 | 35/5069 | 85/5078 | 0.44 (0.30–0.66) | <0.0001 | 0 | ||
| D | Symptomatic ICAS | 3 | 1/382 | 3/373 | 0.46 (0.06–3.57) | 0.46 | 0 | χ1 2=0.00, p=1.00, I2=0% |
| LACI | 2 | 0/131 | 0/108 | NA | NA | NA | ||
| Others/mixed/unknown | 13 | 36/5211 | 83/5224 | 0.46 (0.31–0.68) | 0.0001 | 0 | ||
| Major haemorrhagic events | ||||||||
| A | Overall | 14 | 44/4031 | 93/4010 | 0.49 (0.34–0.70) | <0.0001 | 0 | NA |
| B | Stroke onset <3 days | 4 | 7/1120 | 13/1122 | 0.60 (0.24–1.52) | 0.28 | 0 | χ1 2=0.23, p=0.63, I2=0% |
| Stroke onset >3 days | 10 | 37/2911 | 80/2888 | 0.47 (0.32–0.69) | 0.0001 | 0 | ||
| C | Short term (<3 months) | 3 | 4/655 | 5/627 | 0.80 (0.22–2.97) | 0.74 | NA | χ1 2=0.59, p=0.44, I2=0% |
| Long term (≥3 months) | 11 | 40/3376 | 88/3383 | 0.47 (0.32–0.68) | <0.0001 | 0 | ||
| D | Symptomatic ICAS | 3 | 6/382 | 9/373 | 0.67 (0.17–2.59) | 0.56 | 37 | χ1 2=0.24, p=0.62, I2=0% |
| LACI | 2 | 0/131 | 0/108 | NA | NA | NA | ||
| Others/mixed/unknown | 9 | 38/3518 | 84/3529 | 0.47 (0.32–0.68) | <0.0001 | 0 | ||
| Mortality | ||||||||
| A | Overall | 15 | 64/5029 | 72/5017 | 0.90 (0.64–1.25) | 0.53 | 0 | NA |
| B | Stroke onset <3 days | 2 | 3/482 | 1/483 | 2.33 (0.34–15.82) | 0.39 | 0 | χ1 2=0.98, p=0.32, I2=0% |
| Stroke onset >3 days | 13 | 61/4547 | 71/4534 | 0.87 (0.62–1.22) | 0.43 | 0 | ||
| C | Short term (<3 months) | 2 | 0/55 | 0/26 | NA | NA | NA | NA |
| Long term (≥3 months) | 13 | 64/4974 | 72/4991 | 0.90 (0.64–1.25) | 0.53 | 0 | ||
| D | Symptomatic ICAS | 3 | 2/382 | 3/373 | 0.67 (0.11–4.06) | 0.66 | 0 | χ1 2=0.11, p=0.74, I2=0% |
| LACI | 2 | 0/131 | 0/108 | NA | NA | NA | ||
| Others/mixed/unknown | 10 | 62/4516 | 69/4536 | 0.91 (0.65–1.27) | 0.58 | 0 | ||
| MACE | ||||||||
| A | Overall | 13 | 184/3826 | 276/3842 | 0.67 (0.56–0.81) | <0.0001 | 0 | NA |
| B | Stroke onset <3 days | 4 | 25/1120 | 33/1122 | 0.76 (0.45–1.27) | 0.30 | 0 | χ1 2=0.18, p=0.67, I2=0% |
| Stroke onset >3 days | 9 | 159/2706 | 243/2720 | 0.67 (0.52–0.86) | 0.002 | 20 | ||
| C | Short term (<3 months) | 2 | 12/613 | 12/612 | 1.00 (0.45–2.21) | 1.00 | NA | χ1 2=1.03, p=0.31, I2=3.0% |
| Long term (≥3 months) | 11 | 172/3213 | 264/3230 | 0.66 (0.55–0.79) | <0.0001 | 0 | ||
| D | Symptomatic ICAS | 2 | 17/299 | 12/293 | 1.38 (0.67–2.85) | 0.38 | 0 | χ2 2=4.22, p=0.12, I2=52.6% |
| LACI | 1 | 1/89 | 1/93 | 1.04 (0.07–16.45) | 0.98 | NA | ||
| Others/mixed/unknown | 10 | 166/3438 | 263/3456 | 0.64 (0.53–0.77) | <0.00001 | 0 | ||
CIL, cilostazol; DAPT, dual antiplatelet therapy; ICAS, intracranial arterial stenosis; LACI, lacunar infarction; No CIL, placebo or best medical therapy; RR, risk ratio; SAPT, single antiplatelet therapy.
Short-term versus long-term treatment
Long-term antiplatelet treatment was defined as drug administration for ≥3 months. Three studies comprising 1282 patients administered short-term antiplatelet therapy, while 15 studies comprising 10 147 patients administered treatment for ≥3 months. For most outcomes (ischaemic stroke recurrence, any stroke recurrence, major haemorrhagic events and MACE), there was no significant difference in the efficacy/safety of cilostazol for short-term and long-term administration (I2≤3.0%). For mortality, there was insufficient events for short-term cilostazol administration to make a comparison. However, for ICH, there was moderate heterogeneity between short-term and long-term cilostazol administration (I2=32.3%; χ1 2=1.48, p=0.22). Long-term cilostazol administration reduces the risk of ICH (RR=0.44, 95% CI 0.30 to 0.66, p<0.0001), while short-term cilostazol administration was underpowered and seemed to confer no benefit (RR=2.00, 95% CI 0.18 to 22.03, p=0.57).
Symptomatic ICAS
Three studies comprising 755 patients included only patients with symptomatic ICAS. Two studies22 25 that did not specify whether ICAS was symptomatic or was associated with the infarct territory were excluded. For most outcomes, there was no significant difference between the symptomatic ICAS and mixed/other stroke subgroup. Most strikingly, in the ICAS subgroup, cilostazol administration is associated with an insignificant increase in risk of MACE (RR=1.38, 95% CI 0.67 to 2.85, p=0.38), compared with a statistically significant decrease in MACE risk in studies which did not exclusively recruit patients with symptomatic ICAS. Furthermore, there was no significant decrease in risk of ICAS progression or worsening (RR=0.63, 95% CI 0.25 to 1.58, p=0.33) after cilostazol administration (online supplemental data).
Further analyses
Meta-regression of the mean or median: (1) duration of treatment or follow-up; (2) time from stroke onset to randomisation or treatment; or (3) proportion of patients with lacunar infarction did not reveal any significant effect on ischaemic stroke recurrence (online supplemental data). Funnel plots did not suggest publication bias for any outcome.
Using the GRADE tool, the quality of evidence was high for ischaemic stroke, any stroke, ICH, major haemorrhagic events and MACE; moderate for mortality and ADEs leading to treatment discontinuations; and low for good functional outcome.
Discussion
Overall, cilostazol demonstrated good efficacy in secondary stroke prevention, significantly reducing the rates of ischaemic stroke recurrence, any stroke recurrence and MACE.
As a monotherapy, our updated meta-analysis confirmed that cilostazol was superior to aspirin. However, cilostazol monotherapy was not compared with clopidogrel. As combination therapy, the performance of cilostazol is promising. While bleeding outcomes did not increase when cilostazol was added to aspirin or clopidogrel, its efficacy in secondary ischaemic stroke prevention requires confirmation. Compared with aspirin monotherapy, the risk reduction of cilostazol DAPT was greater but just missed significance; we were not able to comment on its efficacy against clopidogrel monotherapy as it was compared with clopidogrel monotherapy in only one trial.38 In contrast to ASA+CLO combination therapy whose use is typically limited to 3 months or less,40 our results suggest that cilostazol combination therapy is a promising option for long-term secondary stroke prevention, similar to aspirin plus dipyridamole.41 Finally, when compared with other DAPT, CIL combination versus ASA+CLO was limited by wide CI and the availability of only one study31 (which demonstrated possible inferiority in stroke recurrence); the CIL+CLO option was not tested.
The ‘No CIL’ subgroup is a heterogeneous classification. One study24 compared cilostazol with placebo. Another study36 compared cilostazol with best medical therapy—both arms were subjected to a variety of both oral and intravenous antiplatelets, antithrombin, anticoagulants and/or free radical scavengers—without a placebo. Resultantly, it is difficult to comment on this subgroup.
Previous studies have demonstrated high mortality rates after a secondary stroke.42 Interestingly, despite a reduction in stroke recurrence and haemorrhagic complications, cilostazol did not significantly reduce mortality rates. One potential explanation is that studies were underpowered to assess the benefits of cilostazol administration on all-cause mortality. Although there were 644 ‘any stroke’ recurrences, there were only 136 cases of mortality; given that the control mortality rate was only 1.44%, the optimal information size was not met for an assumed 25% relative risk reduction.43
Additionally, the rate of good functional outcomes did not increase with cilostazol use. Apart from the fact that only four trials reported mRS scores, treatment was only administered for 3 or 6 months, with the latter achieving better functional outcomes. Future studies could assess the rates of mRS 0–1 with longer duration of antiplatelet administration.
Cilostazol use was associated with an increase in drug discontinuations due to ADEs, especially when administered as combination therapy. Common adverse effects included headaches, dizziness, palpitations, arrythmias and gastrointestinal disturbances. However, this analysis was complicated by high heterogeneity, which may be attributed to the wide variation in definition among trials. Some trials included vascular events and deaths, while others included medication non-compliance. Nevertheless, existing literature suggests that most of these adverse events are mild and temporary.10
Unexpectedly, cilostazol did not further reduce the rates of efficacy and safety outcomes in patients with symptomatic ICAS. This does not corroborate with existing literature, which suggests that the antiplatelet and vasodilating effect44 of cilostazol could prevent ICAS progression, which, unlike extracranial artery stenosis, cannot be equilibrised by collateral circulation.45
Subgroup analysis did not reveal any significant effect of stroke chronicity, or duration of treatment or follow-up, on efficacy and safety outcomes. However, the risk reduction of ICH is greater when cilostazol is administered long term, which could be due to the increase in incidence of ICH over time when aspirin is employed. Surprisingly, there was a lower overall rate of ischaemic stroke recurrence when cilostazol is administered during the acute/subacute phase (1.78%) versus chronic phase (5.38%) of ischaemic stroke. This may be attributed to a shorter duration of follow-up (14 days to 6 months) in the former subgroup; alternatively, these four trials excluded patients with NIHSS ≥8, 16 or 20, which may reduce the risk of ischaemic stroke recurrence.
Limitations
All but one trial were performed in the East Asian population, thus the results of this meta-analysis may not be fully generalisable to other ethnicities. Additionally, the following analyses were underpowered: (1) mortality; (2) subgroup analysis for symptomatic ICAS; and (3) subgroup analysis for the duration of treatment. Moreover, there was moderate to high heterogeneity in the meta-analysis of (1) good functional outcome; and (2) ADEs leading to treatment discontinuation. Furthermore, due to the limited trials available, we were unable to compare the efficacy and safety of cilostazol with antiplatelet agents other than aspirin and clopidogrel. Finally, for one study,22 the time points at which the different outcomes were measured varied due to the limited information available.
Implications for future research
We identified knowledge gaps and areas for further research. For example, studies could perform trials on non-Asian populations. Furthermore, studies could compare the safety and efficacy profile of cilostazol with other antiplatelet regimens, in particular: (1) CIL versus CLO monotherapy; (2) CIL combination versus ASA+CLO, especially in the short term; and (3) newer antiplatelet agents, for instance, CIL versus ticagrelor (TIG) or CIL versus short-term ASA+TIG. While network meta-analyses may be performed to evaluate indirect evidence, further randomised controlled trials for direct comparisons are necessary.
Conclusions
Cilostazol SAPT and DAPT significantly reduced the risk of recurrent ischaemic stroke, any stroke and MACE but did not have a significant effect on functional outcomes. Cilostazol reduced ICH and major haemorrhagic complications compared with aspirin.
Footnotes
Contributors: All coauthors made significant contributions to the planning, conduct, reporting and final approval of the study. CHT and AGRW are co-first authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Mohan KM, Wolfe CDA, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 2011;42:1489–94. 10.1161/STROKEAHA.110.602615 [DOI] [PubMed] [Google Scholar]
- 2.Kauw F, Takx RAP, de Jong HWAM, et al. Clinical and imaging predictors of recurrent ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis 2018;45:279–87. 10.1159/000490422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albright KC, Huang L, Blackburn J, et al. Racial differences in recurrent ischemic stroke risk and recurrent stroke case fatality. Neurology 2018;91:e1741–50. 10.1212/WNL.0000000000006467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 5.CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–39. 10.1016/S0140-6736(96)09457-3 [DOI] [PubMed] [Google Scholar]
- 6.ESPRIT Study Group, Halkes PHA, van Gijn J, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet 2006;367:1665–73. 10.1016/S0140-6736(06)68734-5 [DOI] [PubMed] [Google Scholar]
- 7.Diener H-C, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331–7. 10.1016/S0140-6736(04)16721-4 [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Fox KAA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006;354:1706–17. 10.1056/NEJMoa060989 [DOI] [PubMed] [Google Scholar]
- 9.Clagett GP, Sobel M, Jackson MR, et al. Antithrombotic therapy in peripheral arterial occlusive disease: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 2004;126:609s–26. 10.1378/chest.126.3_suppl.609S [DOI] [PubMed] [Google Scholar]
- 10.Noma K, Higashi Y. Cilostazol for treatment of cerebral infarction. Expert Opin Pharmacother 2018;19:1719–26. 10.1080/14656566.2018.1515199 [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell ME, Badger SA, Sharif MA, et al. The vascular and biochemical effects of cilostazol in patients with peripheral arterial disease. J Vasc Surg 2009;49:1226–34. 10.1016/j.jvs.2008.11.098 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Liu M, Pu C. 2014 Chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack. Int J Stroke 2017;12:302–20. 10.1177/1747493017694391 [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Kwon SU, Uchiyama S. Cilostazol research in Asia: can it be applied to European and American patients? Int J Stroke 2015;10:1–9. 10.1111/ijs.12460 [DOI] [PubMed] [Google Scholar]
- 14.Toyoda K, Inoue M, Koga M. Small but steady steps in stroke medicine in Japan. J Am Heart Assoc 2019;8:e013306. 10.1161/JAHA.119.013306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kern R, Nagayama M, Toyoda K, et al. Comparison of the European and Japanese guidelines for the management of ischemic stroke. Cerebrovasc Dis 2013;35:402–18. 10.1159/000351753 [DOI] [PubMed] [Google Scholar]
- 16.Tan L, Margaret B, Zhang JH, et al. Efficacy and safety of cilostazol therapy in ischemic stroke: a meta-analysis. J Stroke Cerebrovasc Dis 2015;24:930–8. 10.1016/j.jstrokecerebrovasdis.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 17.Kim SM, Jung J-M, Kim BJ, et al. Cilostazol mono and combination treatments in ischemic stroke: an updated systematic review and meta-analysis. Stroke 2019;50:3503–11. 10.1161/STROKEAHA.119.026655 [DOI] [PubMed] [Google Scholar]
- 18.McHutchison C, Blair GW, Appleton JP, et al. Cilostazol for secondary prevention of stroke and cognitive decline: systematic review and meta-analysis. Stroke 2020;51:2374–85. 10.1161/STROKEAHA.120.029454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki J, Iguchi Y, Urabe T, et al. Acute aspirin plus cilostazol dual therapy for noncardioembolic stroke patients within 48 hours of symptom onset. J Am Heart Assoc 2019;8:e012652. 10.1161/JAHA.119.012652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blair GW, Appleton JP, Flaherty K, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: the LACunar Intervention-1 (LACI-1) trial, a randomised clinical trial. EClinicalMedicine 2019;11:34–43. 10.1016/j.eclinm.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotoh F, Tohgi H, Hirai S, et al. Cilostazol stroke prevention study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis 2000;9:147–57. 10.1053/jscd.2000.7216 [DOI] [PubMed] [Google Scholar]
- 25.Guo J-J, Xu E, Lin Q-Y, et al. Effect of cilostazol on cerebral arteries in secondary prevention of ischemic stroke. Neurosci Bull 2009;25:383–90. 10.1007/s12264-009-6192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han SW, Lee S-S, Kim SH, et al. Effect of cilostazol in acute lacunar infarction based on pulsatility index of transcranial Doppler (ECLIPse): a multicenter, randomized, double-blind, placebo-controlled trial. Eur Neurol 2013;69:33–40. 10.1159/000338247 [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Cheng Y, Wu J, et al. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol 2008;7:494–9. 10.1016/S1474-4422(08)70094-2 [DOI] [PubMed] [Google Scholar]
- 28.Johkura K, Yoshida TN, Kudo Y, et al. Cilostazol versus aspirin therapy in patients with chronic dizziness after ischemic stroke. Clin Neurol Neurosurg 2012;114:876–80. 10.1016/j.clineuro.2012.01.029 [DOI] [PubMed] [Google Scholar]
- 29.Kim BJ, Lee E-J, Kwon SU, et al. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. Lancet Neurol 2018;17:509–18. 10.1016/S1474-4422(18)30128-5 [DOI] [PubMed] [Google Scholar]
- 30.Kwon SU, Cho Y-J, Koo J-S, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 2005;36:782–6. 10.1161/01.STR.0000157667.06542.b7 [DOI] [PubMed] [Google Scholar]
- 31.Kwon SU, Hong K-S, Kang D-W, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke 2011;42:2883–90. 10.1161/STROKEAHA.110.609370 [DOI] [PubMed] [Google Scholar]
- 32.Lee Y-S, Bae H-J, Kang D-W, et al. Cilostazol in acute ischemic stroke treatment (CAIST trial): a randomized double-blind non-inferiority trial. Cerebrovasc Dis 2011;32:65–71. 10.1159/000327036 [DOI] [PubMed] [Google Scholar]
- 33.Lee S-J, Lee JS, Choi MH, et al. Cilostazol improves endothelial function in acute cerebral ischemia patients: a double-blind placebo controlled trial with flow-mediated dilation technique. BMC Neurol 2017;17:169. 10.1186/s12883-017-0950-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura T, Tsuruta S, Uchiyama S. Cilostazol combined with aspirin prevents early neurological deterioration in patients with acute ischemic stroke: a pilot study. J Neurol Sci 2012;313:22–6. 10.1016/j.jns.2011.09.038 [DOI] [PubMed] [Google Scholar]
- 35.Ohnuki Y, Ohnuki Y, Kohara S, et al. Dual therapy with aspirin and cilostazol may improve platelet aggregation in noncardioembolic stroke patients: a pilot study. Intern Med 2017;56:1307–13. 10.2169/internalmedicine.56.7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu H, Tominaga T, Ogawa A, et al. Cilostazol for the prevention of acute progressing stroke: a multicenter, randomized controlled trial. J Stroke Cerebrovasc Dis 2013;22:449–56. 10.1016/j.jstrokecerebrovasdis.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 37.Shinohara Y, Katayama Y, Uchiyama S, et al. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol 2010;9:959–68. 10.1016/S1474-4422(10)70198-8 [DOI] [PubMed] [Google Scholar]
- 38.Toyoda K, Uchiyama S, Yamaguchi T, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol 2019;18:539–48. 10.1016/S1474-4422(19)30148-6 [DOI] [PubMed] [Google Scholar]
- 39.Uchiyama S, Sakai N, Toi S, et al. Final results of Cilostazol-Aspirin therapy against recurrent stroke with intracranial artery stenosis (CATHARSIS). Cerebrovasc Dis Extra 2015;5:1–13. 10.1159/000369610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman H, Khan SU, Nasir F, et al. Optimal duration of aspirin plus clopidogrel after ischemic stroke or transient ischemic attack. Stroke 2019;50:947–53. 10.1161/STROKEAHA.118.023978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack. Stroke 2019;50:773–8. 10.1161/STROKEAHA.118.023954 [DOI] [PubMed] [Google Scholar]
- 42.Singh R-J, Chen S, Ganesh A, et al. Long-term neurological, vascular, and mortality outcomes after stroke. Int J Stroke 2018;13:787–96. 10.1177/1747493018798526 [DOI] [PubMed] [Google Scholar]
- 43.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 2011;64:1283–93. 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 44.Weintraub WS. The vascular effects of cilostazol. Can J Cardiol 2006;22:56B–60. 10.1016/S0828-282X(06)70987-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JS, Bang OY. Medical treatment of intracranial atherosclerosis: an update. J Stroke 2017;19:261–70. 10.5853/jos.2017.01830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000737supp001.pdf (5.8MB, pdf)
Data Availability Statement
Data are available upon reasonable request.