Abstract
Background and aim
Obesity paradox has aroused increasing concern in recent years. However, impact of obesity on outcomes in intracerebral haemorrhage (ICH) remains unclear. This study aimed to evaluate association of body mass index (BMI) with in-hospital mortality, complications and discharge disposition in ICH.
Methods
Data were from 85 705 ICH enrolled in the China Stroke Center Alliance study. Patients were divided into four groups: underweight, normal weight, overweight and obese according to Asian-Pacific criteria. The primary outcome was in-hospital mortality. The secondary outcomes included non-routine discharge disposition and in-hospital complications. Discharge to graded II or III hospital, community hospital or rehabilitation facilities was considered non-routine disposition. Multivariable logistic regression analysed association of BMI with outcomes.
Results
82 789 patients with ICH were included in the final analysis. Underweight (OR=2.057, 95% CI 1.193 to 3.550) patients had higher odds of in-hospital mortality than those with normal weight after adjusting for covariates, but no significant difference was observed for patients who were overweight or obese. No significant association was found between BMI and non-disposition. Underweight was associated with increased odds of several complications, including pneumonia (OR 1.343, 95% CI 1.138 to 1.584), poor swallow function (OR 1.351, 95% CI 1.122 to 1.628) and urinary tract infection (OR 1.532, 95% CI 1.064 to 2.204). Moreover, obese patients had higher odds of haematoma expansion (OR 1.326, 95% CI 1.168 to 1.504), deep vein thrombosis (OR 1.506, 95% CI 1.165 to 1.947) and gastrointestinal bleeding (OR 1.257, 95% CI 1.027 to 1.539).
Conclusions
In patients with ICH, being underweight was associated with increased in-hospital mortality. Being underweight and obese can both increased risk of in-hospital complications compared with having normal weight.
Keywords: haemorrhage, stroke
Introduction
Obesity is now considered to be a global epidemic. The prevalence of obesity has increased globally over the past decades. Between 1980 and 2013, the proportion of adults with a body mass index (BMI) of ≥25 kg/m2 has increased from 29% to 37% in men and from 30% to 38% in women.1 Obesity is commonly associated with increased morbidity and mortality in the general population, also increased risk of stroke.2 Individuals are generally advocated to strive for a normal weight. However, a theory called obesity paradox has aroused increasing concern in recent years. It has been shown to be protective in several chronic disease states, such as heart failure3 and diabetes mellitus,4 those who are overweight or obese have lower mortality rates than patients of normal weight and underweight.
The impact of obesity on prognosis in patients with stroke is still under debate. The previous many studies5–8 mainly described the obesity paradox in ischaemic stroke. Intracerebral haemorrhage (ICH), accounting for 10%–15% of all stroke,9 10 is a common cause of permanent disability and mortality, and brings substantial burden on global healthcare systems and society.11 Presently, it still lacks the literature on obesity paradox in patients with ICH, who have different characteristics from ischaemic stroke. And the existence of an obesity paradox in ICH is controversial. Some previous studies in Korea12 and the USA13 showed the obesity paradox is present in ICH, but another study in Denmark14 found no evidence of an obesity paradox in patients who had a stroke. Their limitations, such as having a small sample size and being a single-centre study, need to be further investigated. Meanwhile, because no specific treatment is available for ICH, supportive medical care remains the main treatment. Therefore, it is also of significance to investigate the associated factors affecting the prognosis of patients with ICH. The previous study12 has shown a reduction of long-term mortality after ICH in obese patients compared with normal-weight patients. But few studies have investigated the relationship between body habitus and in-hospital mortality and complications in patients with ICH.
The present study aimed to evaluate association of BMI with in-hospital mortality, discharge disposition and in-hospital complications among patients with ICH from the China Stroke Center Alliance (CSCA).
Methods
Study cohort and participants
Data were derived from the CSCA. The CSCA was a national, hospital-based, multicentre, voluntary, quality assessment and improvement initiative performed in China.15 The aims of this trial were to (1) promote stroke centre development and organise the delivery of stroke care; (2) function as a national registry system to evaluate the features, managements and outcomes of patients hospitalised with acute stroke/transient ischaemic attack (TIA); (3) improve the management and outcomes of patients with acute stroke/TIA through pragmatic multifaceted quality improvement (QI) tools to translate evidence-based guideline recommendations into clinical practice. As of July 2019, the trial enrolled 1 006 798 consecutive patients with acute stroke or TIA from 1476 designated hospitals in China. Among all the enrolled patients in the registry, 85 705 were diagnosed with spontaneous ICH. The inclusion criteria in this study were as follows: (1) are aged 18 years or older; (2) have a primary diagnosis of ICH confirmed by brain CT or MRI; (3) are within 7 days of symptom onset; and (4) are admitted either directly to ward or through the emergency department. The exclusion criteria were as follows: (1) unavailable BMI data; (2) unknown in-hospital survival status and (3) incomplete information on admission time period. Trained hospital personnel use a web-based patient management tool (GaiDe, Beijing, China) to collect patient datum. The primary purpose of the CSCA is to facilitate QI. At the local level, data collection by the site is seen as a QI tool. Patient confidentiality will be protected in the following ways: (1) data are stripped of all identifiers before their use in research and (2) the use of data for these purposes is closely overseen by the China National Clinical Research Center for Neurological Diseases analytical centre.
Demographics and clinical characteristics
Baseline information on demographics, admission time period, severity of stroke, behavioural history, medical history, medication history, in-hospital laboratory examination, in-hospital interventions and complications, and discharge information was collected via the web-based patient data collection and management tool (Medicine Innovation Research Center, Beijing, China). Discharge information included status at discharge (death, survival), length of stay and hospital expenditure (renminbi). The CSCA recorded in-hospital data, and patients after discharge were not followed.
Assessment of BMI
In the acute phase, patients with ICH should absolutely rest in bed. Height and weight were self-reported by patients or legal proxies on admission. BMI was calculated as the ratio of weight (kg) and the square of height (m2). The patients were classified into four groups based on their BMI. BMI thresholds were adapted according to WHO Regional Office for the Western Pacific Region16 criteria, which were as follows: <18.5 kg/m2 for underweight, 18.5–22.9 kg/m2 for normal weight, 23.0–24.9 kg/m2 for overweight and ≥25.0 kg/m2 for obesity. The normal weight group was used as the reference group.
Outcomes
The primary outcome was in-hospital mortality. The secondary outcomes included non-routine discharge disposition and in-hospital complications. Disposition was dichotomised as routine or non-routine. Discharge to home was considered routine, while discharge to graded II or III hospital, community hospital or rehabilitation facilities was considered non-routine. We also investigated the effect of body habitus on various in-hospital complications, including haematoma expansion, pneumonia, poor swallow function, seizure, urinary tract infection, hydrocephalus, deep vein thrombosis (DVT) and gastrointestinal bleeding.
Subgroup analysis
The volume and location are the influencing factors for outcomes after ICH, but the CSCA database does not provide these variables. As we know, clinically surgical intervention may be necessary when the patients happen to have deterioration of nervous system function or the haematoma is large and superficial. The subgroup analysis for patients who underwent intracranial haematoma removal procedure may indirectly reflect patients’ haematoma volume and neurological examinations. In our study, the types of procedure include craniotomy evacuation, minimal invasive surgery, decompressive craniotomy, and brain ventricle puncture and drainage. Considering that the sample size of each procedure was relatively small, so we performed subgroup analysis of all patients who underwent intracranial haematoma removal procedure.
The average BMI of Asian population is relatively lower than non-Asian populations. The application of WHO criteria may underestimate obesity-related risks in Asian population,17 but we also made a sensitive analysis according to the WHO criteria.18 The WHO criteria were as follows: <18.5 kg/m2 for underweight, 18.5–24.9 kg/m2 for normal weight, 25.0–29.9 kg/m2 for overweight and ≥30.0 kg/m2 for obesity.
Statistical analysis
Baseline table was produced by %ggBaseline,19 a SAS macro which can analyse and report baseline characteristics automatically. Continuous variables are reported as mean±SD or median (IQR), when appropriate. Categorical variables are presented as frequency and percentage. Comparisons between groups for continuous variables were tested by analysis of variance or Kruskal-Wallis. Pearson Χ2 test was performed for comparison of categorical variables. Multivariable logistic regression analysis was used to determine the relationship between categorical BMI and outcomes. Covariates were included if they differed according to body habitus in the baseline analysis with a p value of <0.05. A two-sided p value of <0.05 was considered to be statistically significant. All analyses were performed with SAS software V.9.4.
Results
Characteristics of patients
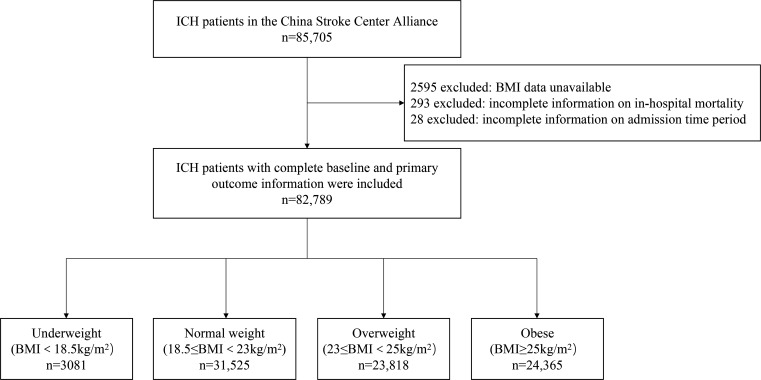
Between August 2015 and July 2019, a total of 85 705 patients with spontaneous ICH were registered in the CSCA. After excluding patients with unavailable BMI data (n=2595), unknown in-hospital survival status (n=293) and incomplete information on admission time period (n=28), 82 789 patients were involved in the current analysis. A flowchart of this study is shown in figure 1.
Figure 1.
Flowchart of patient selection from the China Stroke Center Alliance. BMI, body mass index; ICH, intracerebral haemorrhage.
The baseline characteristics of the patients are stratified by body habitus in table 1. Of these patients, 3.7% were underweight (mean age, 69.4 years), 38.1% had normal weight (mean age, 64.5 years), 28.8% were overweight (mean age, 62.3 years) and 29.4% were obese (mean age, 60.6 years). The mean age varied significantly between groups of BMI (p<0.0001). A greater proportion of men were obese than women (62.9% vs 37.1%, respectively). Compared with patients with normal weight, obese patients were younger, had more vascular risk factors (such as hypertension, diabetes mellitus, dyslipidaemia, drinking, smoking), more often had a medical history of ischaemic stroke and myocardial infarction, had higher low-density lipoprotein (LDL), glycated haemoglobin, fasting blood glucose (FBG), homocysteine (Hcy) and uric acid (UA). Meanwhile, the mean length of stay was greater and hospital expenditure was significantly higher in obese patients than normal-weight patients (p<0.0001). Underweight patients were older, more likely to be women, and more likely to have a medical history of TIA, ischaemic stroke, ICH, atrial fibrillation, heart failure and peripheral vascular disease (PVD), and had higher blood urea nitrogen. As for severity of stroke, there was no difference about National Institutes of Health Stroke Scale (NIHSS) among groups. However, more obese patients performed the haematoma evacuation.
Table 1.
Baseline characteristics of patients with ICH according to BMI categories
| Variable | Underweight (n=3081) |
Normal weight (n=31 525) |
Overweight (n=23 818) |
Obese (n=24 365) |
P value |
| Age (years), mean±SD | 69.4±14.1 | 64.5±13.0 | 62.3±12.2 | 60.6±12.6 | <0.0001 |
| Male, n (%) | 1506 (48.9) | 19 067 (60.5) | 15 763 (66.2) | 15 325 (62.9) | <0.0001 |
| Level of education, n (%) | |||||
| Elementary or below | 1322 (42.9) | 11 017 (34.9) | 7474 (31.4) | 7199 (29.5) | <0.0001 |
| Middle school | 426 (13.8) | 5572 (17.7) | 4983 (20.9) | 5345 (21.9) | <0.0001 |
| High school or above | 266 (8.6) | 3329 (10.6) | 2803 (11.8) | 3338 (13.7) | <0.0001 |
| Health insurance status, n (%) | <0.0001 | ||||
| UEMRS | 540 (17.5) | 5756 (18.3) | 4803 (20.2) | 5424 (22.3) | |
| URMIS | 517 (16.8) | 5150 (16.3) | 3657 (15.4) | 3746 (15.4) | |
| NRCMS | 1687 (54.8) | 16 989 (53.9) | 12 708 (53.4) | 12 480 (51.2) | |
| Self-pay | 208 (6.8) | 2382 (7.6) | 1733 (7.3) | 1865 (7.7) | |
| Others | 129 (4.2) | 1248 (4.0) | 917 (3.9) | 850 (3.5) | |
| Admission time period, n (%) | <0.0001 | ||||
| 2015 | 86 (2.8) | 1111 (3.5) | 854 (3.6) | 912 (3.7) | |
| 2016 | 864 (28.0) | 7844 (24.9) | 5633 (23.7) | 6108 (25.1) | |
| 2017 | 759 (24.6) | 8488 (26.9) | 6447 (27.1) | 6363 (26.1) | |
| 2018 | 929 (30.2) | 9256 (29.4) | 7085 (29.7) | 7134 (29.3) | |
| 2019 | 443 (14.4) | 4826 (15.3) | 3799 (16.0) | 3848 (15.8) | |
| Pre-stroke mRS, n (%) | <0.0001 | ||||
| 0–2 | 2076 (67.4) | 22 110 (70.1) | 17 192 (72.2) | 17 728 (72.8) | |
| 3–5 | 784 (25.4) | 7707 (24.4) | 5579 (23.4) | 5341 (21.9) | |
| NIHSS, mean±SD | 9.2±9.3 | 8.5±8.7 | 8.2±8.3 | 8.4±8.8 | 0.0591 |
| GCS, mean±SD | 11.4±4.0 | 11.6±4.0 | 11.4±4.1 | 11.3±4.2 | <0.0001 |
| Behavioural history, n (%) | |||||
| Current smoking | 492 (16.0) | 5775 (18.3) | 4944 (20.8) | 5067 (20.8) | <0.0001 |
| Drinking | 556 (18.0) | 7110 (22.6) | 5999 (25.2) | 6574 (27.0) | <0.0001 |
| Medical history, n (%) | |||||
| Hypertension | 2000 (64.9) | 21 573 (68.4) | 17 076 (71.7) | 18 477 (75.8) | <0.0001 |
| Diabetes mellitus | 202 (6.6) | 2576 (8.2) | 2242 (9.4) | 2864 (11.8) | <0.0001 |
| Dyslipidaemia | 116 (3.8) | 1187 (3.8) | 933 (3.9) | 1293 (5.3) | <0.0001 |
| Atrial fibrillation | 67 (2.2) | 526 (1.7) | 318 (1.3) | 357 (1.5) | <0.0001 |
| TIA | 29 (0.9) | 191 (0.6) | 110 (0.5) | 172 (0.7) | <0.0001 |
| Ischaemic stroke | 423 (13.7) | 4005 (12.7) | 3046 (12.8) | 3313 (13.6) | <0.0001 |
| SAH | 17 (0.6) | 170 (0.5) | 130 (0.5) | 138 (0.6) | 0.9786 |
| ICH | 667 (21.6) | 5847 (18.5) | 4122 (17.3) | 4185 (17.2) | <0.0001 |
| Heart failure | 39 (1.3) | 166 (0.5) | 86 (0.4) | 101 (0.4) | <0.0001 |
| Myocardial infarction | 20 (0.6) | 271 (0.9) | 218 (0.9) | 237 (1.0) | 0.0003 |
| Peripheral vascular disease | 41 (1.3) | 301 (1.0) | 210 (0.9) | 248 (1.0) | <0.0001 |
| Medication history, n (%) | |||||
| Antiplatelet | 187 (6.1) | 2006 (6.4) | 1625 (6.8) | 1904 (7.8) | <0.0001 |
| Anticoagulation | 59 (1.9) | 593 (1.9) | 437 (1.8) | 485 (2.0) | <0.0001 |
| Antihypertensive | 1254 (40.7) | 13 989 (44.4) | 11 452 (48.1) | 12 459 (51.1) | <0.0001 |
| Antidiabetic | 139 (4.5) | 1919 (6.1) | 1627 (6.8) | 2085 (8.6) | <0.0001 |
| Cholesterol-reduce | 97 (3.1) | 933 (3.0) | 684 (2.9) | 745 (3.1) | 0.2620 |
| Lab tests, mean±SD | |||||
| LDL (mmol/L) | 2.8±1.7 | 2.8±1.5 | 2.8±1.5 | 2.9±1.7 | <0.0001 |
| Glycated haemoglobin (%) | 5.9±1.8 | 5.9±1.7 | 5.9±1.7 | 6.0±1.8 | <0.0001 |
| FBG (mmol/L) | 6.4±2.8 | 6.4±2.7 | 6.5±2.8 | 6.8±3.0 | <0.0001 |
| Hcy (μmol/L) | 13.7±8.4 | 13.9±8.0 | 14.2±8.0 | 14.3±8.1 | <0.0001 |
| Creatinine (μmol/L) | 141.7±1327.7 | 140.5±1270.3 | 141.9±1412.2 | 150.8±1399.6 | 0.828 |
| BUN (mmol/L) | 6.0±3.1 | 5.8±2.8 | 5.7±2.7 | 5.7±2.7 | <0.0001 |
| UA (μmol/L) | 268.8±140.1 | 284.1±133.2 | 289.0±132.4 | 295.9±142.8 | <0.0001 |
| Platelets (×109/L) | 199.6±74.5 | 201.1±71.1 | 202.6±69.1 | 206.2±69.6 | <0.0001 |
| SBP (mm Hg), mean±SD | 162.2±29.3 | 163.1±28.0 | 164.8±27.6 | 166.7±28.5 | <0.0001 |
| DBP (mm Hg), mean±SD | 91.2±16.8 | 93.9±16.6 | 95.6±16.5 | 97.2±17.2 | <0.0001 |
| In-hospital treatment, n (%) | |||||
| Haematoma evacuation | 202 (6.6) | 2798 (8.9) | 2640 (11.1) | 2915 (12.0) | <0.0001 |
| LOS (days), mean±SD | 15.9±11.9 | 16.4±11.5 | 16.6±11.7 | 16.8±11.8 | <0.0001 |
| Hospital expenditure (RMB), mean±SD | 17 444.2±16 541.5 | 17 910.8±16 480.4 | 18 461.0±17 184.0 | 18 777.8±17 486.9 | <0.0001 |
BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; FBG, fasting blood glucose; GCS, Glasgow Coma Scale; Hcy, homocysteine; ICH, intracerebral haemorrhage; LDL, low-density lipoprotein; LOS, length of stay; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; NRCMS, New Rural Cooperative Medical Scheme; RMB, renminbi; SAH, subarachnoid haemorrhage; SBP, systolic blood pressure; TIA, transient ischaemic attack; UA, uric acid; UEMRS, Urban Employee Medical Insurance; URMIS, Urban Residents Medical Insurance.
Outcomes
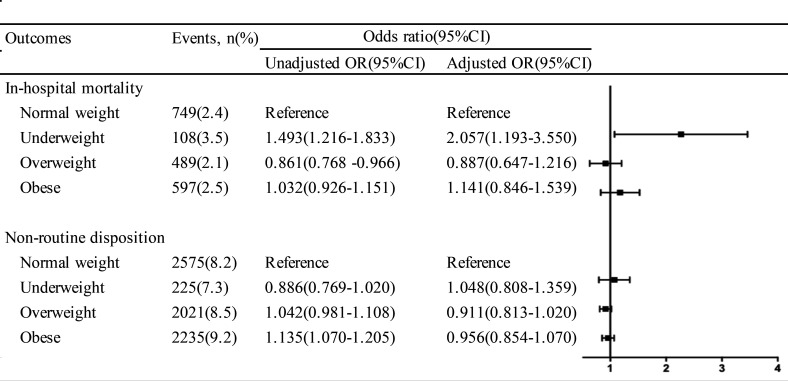
Multivariable logistic regression analysed the association of body habitus with in-hospital mortality rate, in-hospital complications and discharge disposition (figure 2). Covariates in the regression include age, sex, education, health insurance, admission time period, pre-stroke modified Rankin Scale (mRS), Glasgow Coma Scale, current smoking, drinking, previous hypertension, diabetes, dyslipidaemia, atrial fibrillation, TIA, ischaemic stroke, ICH, heart failure, myocardial infarction, PVD, antiplatelet drug, anticoagulation drug, antihypertensive drug, antidiabetic drug, LDL, FBG, glycated haemoglobin, Hcy, blood urea nitrogen, UA, platelets and haematoma evacuation. In the entire patient population, 1943 (2.3%) patients with ICH died during hospitalisation. As for body habitus, it was 3.5% for underweight patients, 2.4% for normal-weight patients, 2.1% for overweight patients and 2.5% for obese patients. In multivariable analysis, the adjusted odds of in-hospital mortality rate were significantly higher for underweight patients (OR 2.057, 95% CI 1.193 to 3.550, p=0.010), but no significant difference was observed for those who were overweight and obese (p=0.456 and p=0.388, respectively), compared with those normal-weight patients. A total of 7022 (8.5%) patients experienced a non-routine disposition, 7.3% for underweight patients, 8.2% for normal-weight patients, 8.5% for overweight patients and 9.2% for obese patients. A multivariable model indicated that there were no significant differences between body habitus and non-disposition.
Figure 2.
ORs (95% CIs) for in-hospital mortality and non-routine disposition in patients with ICH according to BMI. Adjusted for age, sex, education, insurance, admission time period, pre-stroke mRS, GCS, current smoking, drinking, previous hypertension, diabetes, dyslipidaemia, atrial fibrillation, TIA, ischaemic stroke, ICH, heart failure, myocardial infarction, PVD, antiplatelet drug, anticoagulation drug, antihypertensive drug, antidiabetic drug, LDL, glycated haemoglobin, Hcy, FBG, BUN, UA, platelets and haematoma evacuation. BMI, body mass index; BUN, blood urea nitrogen; FBG, fasting blood glucose; GCS, Glasgow Coma Scale; Hcy, homocysteine; ICH, intracerebral haemorrhage; LDL, low-density lipoprotein; mRS, modified Rankin Scale; PVD, peripheral vascular disease; TIA, transient ischaemic attack; UA, uric acid.
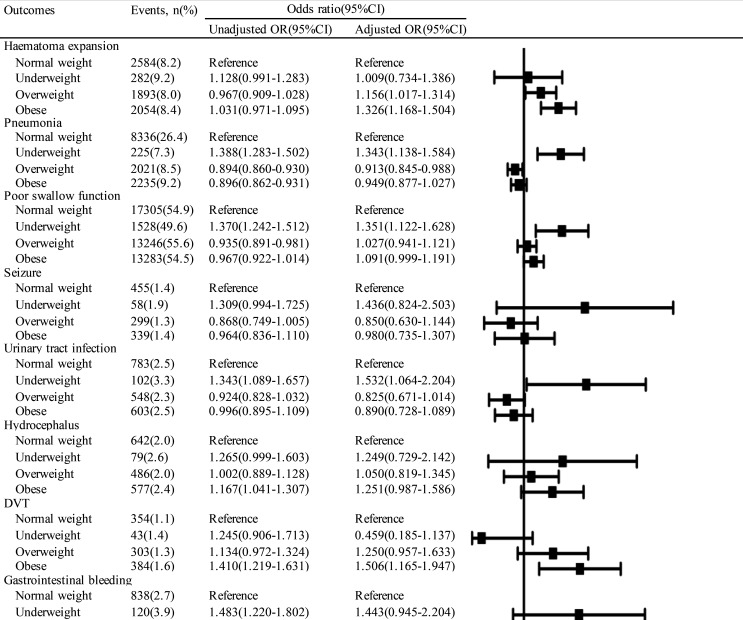
The relationship between body habitus and the various in-hospital complications is demonstrated in figure 3. Underweight was associated with significantly increased odds of several medical complications, including pneumonia (OR 1.343, 95% CI 1.138 to 1.584, p=0.001), poor swallow function (OR 1.351, 95% CI 1.122 to 1.628, p=0.002) and urinary tract infection (OR 1.532, 95% CI 1.064 to 2.204, p=0.022). Moreover, obese patients had higher odds of haematoma expansion (OR 1.326, 95% CI 1.168 to 1.504, p<0.0001), DVT (OR 1.506, 95% CI 1.165 to 1.947, p=0.002) and gastrointestinal bleeding (OR 1.257, 95% CI 1.027 to 1.539, p=0.027). Patients who were overweight had increased odds of haematoma expansion (OR 1.156, 95% CI 1.017 to 1.314, p=0.027) and decreased odds of pneumonia (OR 0.913, 95% CI 0.845 to 0.988, p=0.023).
Figure 3.
ORs (95% CIs) for in-hospital complications in patients with ICH according to BMI. Adjusted for age, sex, education, insurance, admission time period, pre-stroke mRS, GCS, current smoking, drinking, previous hypertension, diabetes, dyslipidaemia, atrial fibrillation, TIA, ischaemic stroke, ICH, heart failure, myocardial infarction, PVD, antiplatelet drug, anticoagulation drug, antihypertensive drug, antidiabetic drug, LDL, glycated haemoglobin, Hcy, FBG, BUN, UA, platelets and haematoma evacuation. BMI, body mass index; BUN, blood urea nitrogen; DVT, deep vein thrombosis; FBG, fasting blood glucose; GCS, Glasgow Coma Scale; Hcy, homocysteine; ICH, intracerebral haemorrhage; LDL, low-density lipoprotein; mRS, modified Rankin Scale; PVD, peripheral vascular disease; TIA, transient ischaemic attack; UA, uric acid.
Subgroup analysis
The subgroup analysis was conducted in patients with more severe haemorrhages as indicated by the need for intracranial haematoma removal procedure. As shown in online supplemental table 1, the adjusted odds of in-hospital mortality and non-routine disposition did not differ significantly by body habitus.
svn-2020-000534supp001.pdf (83.8KB, pdf)
According to the WHO criteria, as shown in the online supplemental table 2, the sensitive analysis did not change our results. In the multivariable analysis, the adjusted odds of in-hospital mortality rate were significantly higher for underweight patients (OR 1.688, 95% CI 1.136 to 2.510, p=0.010), but no significant difference was observed for those who were overweight and obese (p=0.324 and p=0.248, respectively), compared with those normal-weight patients. There were no significant differences between body habitus and non-disposition.
Discussion
The present study showed that being underweight was associated with an increased risk of in-hospital mortality rate compared with being of normal weight. No significant association was found between body habitus and non-disposition. Being underweight and obese can both increase risk of in-hospital complications.
In our study, 1943 (2.3%) patients with ICH died during hospitalisation. The data about in-hospital mortality of ICH in China are scarce. This rate in our cohort may be lower than the previous data. The China National Stroke Registry (CNSR), a large nationwide stroke registry in China, indicated that in-hospital mortality was 9.3% for ICH.20 The following reasons may explain this inconsistent result. First, patients with ICH in CNSR had a higher proportion with NIHSS scale ≥15 (28.2%) than those with CSCA (18.0%). The severity of patients with ICH in our study was mild. Second, the CNSR was performed from 2007 to 2008. The patients with ICH from CSCA study were enrolled from 2015 to 2019. Over the last decade, with the popularisation of health insurance from 2011, Chinese healthcare system has undergone tremendous changes.21 In our study, only about 7.5% need self-pay. To some degree, with the improvement of above-mentioned national strategies in the healthcare system, evidence-based stroke care and patient outcomes also had got improved.22 Meanwhile, 58% participated hospitals in our study were tertiary hospitals. These may reduce the in-hospital mortality rate. Compared with Western patients with ICH, the mortality rate during hospitalisation in our cohort was much lower.23 This indicated an ethnic difference in the prognosis after ICH.
Obese patients had no lower odds of in-hospital mortality than those with normal weight after adjusting for the baseline covariates. At present, the existence of obesity paradox in ICH is controversial. Kim et al 12 and Sun et al 24 showed that obesity was associated with a lower risk of long-term death. Hoffman et al 23 and Persaud et al 25 also found that obesity is associated with decreased in-hospital mortality. But other studies12–14 found no evidence of obesity paradox in 30-day mortality after ICH. Datum from researches on obesity paradox in stroke needs to be discreetly interpreted. Investigating the obesity paradox, we think needs several key confounding factors to be considered. One is the time of follow-up. The Framingham study26 showed detrimental impact of obesity on cardiovascular outcomes which was evident after a follow-up of 8 years in men and 14 years in women. The time of follow-up may be too limited to evaluate the harmful effects of the obesity. Furthermore, age was also the significant affecting factors of outcomes. Some studies27 28 reported an age-dependent effect of obesity on post-stroke mortality. Higher BMI was related to a greater risk of death among young patients. Similarly, obese patients were younger in our study and had more vascular risk factors such as hypertension, diabetes mellitus, dyslipidaemia, drinking and smoking, which is consistent with previous research.29 It is possible that obese patients experienced ICH at a young age because of the presence of comorbidities. The obesity paradox was still not observed in our study. Third, the severity of stroke should also be considered. The previous study5 showed that there is an inverse association between BMI and severity of stroke. Kim et al found that the association between obesity and good outcome lost statistical difference after adjusting for the severity of stroke. Our study also adjusted for many possible confounding factors, for example age, severity of stroke and medical history. Currently there is not enough evidence available to suggest an association between obesity paradox and ICH.
Being underweight was associated with an increased risk of in-hospital mortality rate compared with being of normal weight. The previous studies also found underweight patients were related with the poor outcome after haemorrhagic stroke.12 The following two aspects may explain this phenomenon. On the one hand, in our cohort, underweight patients were older and more likely to have a medical history of TIA, ischaemic stroke, ICH, atrial fibrillation, heart failure and PVD. We speculated that the higher mortality in underweight patients is caused by these medical comorbidities. On the other hand, in our cohort, underweight patients were older and had the lowest rate of hypertension compared with other BMI subgroups. This might indicate a possible difference in the aetiology type of ICH. We speculated it may affect the outcome after ICH in the underweight patients. It reminded us that we should also give the same attention to underweight patients relative to obese patients.
The study about investigating the relationship between body habitus and in-hospital complications in patients with ICH is sparse. In our study, compared with normal-weight patients, underweight and obese patients were both associated with significantly increased odds of several medical complications. Some studies30 31 showed obesity is a significant risk factor for the development of venous thromboembolism. Indeed, inconsistent with the previous study,23 obese patients in our study had higher odds of DVT. The pathophysiological mechanism has not been fully explained. It may be related to low-grade inflammation, atherosclerosis, immobility and stasis in the obese people.32 These changes potentially lead to thrombosis. On the other hand, in our study, other interesting results were that haematoma expansion and gastrointestinal bleeding were also higher among obese patients. Currently, the relationship between obesity and bleeding events was uncertain. There are some studies revealed that obesity increased the risk of ICH.33 The SMART study, which investigated the relationship between obesity and risk of bleeding, also displayed that obesity was not associated with lower risk of bleeding.34 It might be because that the procoagulant profile in obese patients may not be enough to protect against clinically relevant bleeding events. The mechanism needs further research in the future. Being underweight increased odds of poor swallow function. Underweight was associated with significantly increased odds of pneumonia and urinary tract infection. Dysphagia is also a common complication of acute stroke.35 The presence of dysphagia may increase risk for pulmonary complications and infection, and lead to malnutrition and poor resistance. We recommend that patients with ICH should be included in comprehensive dysphagia screening protocols and receive prompt treatment when clinically appropriate.
We also explored the impact of body habitus on discharge disposition. There is no association between body habitus and disposition. This is inconsistent with Iwuchukwu et al’s36 database study of White patients with ICH in which discharge disposition did not differ according to body habitus. But Hoffman et al 23 identified obese patients but not morbidly obese patients had lower odds of non-routine discharge. The relationship between BMI and discharge disposition of a patient with ICH was not consistent and needed further research. Meanwhile, the previous study24 identified better functional status among obese patients with ICH. They adopted the mRS to define functional outcomes. However, our study did not provide validated measures of functional outcomes for ICH such as mRS. This is our limitation. In the future and prospective study, we will evaluate functional outcome of patients with ICH and investigate the relationship between mRS and BMI.
When we performed subgroup analysis to patients who underwent intracranial haematoma removal procedure, the in-hospital mortality and non-routine disposition did not differ significantly by body habitus. This result is consistent with the previous study.23 On the one hand, the subgroup patients with better homogeneity may reduce some confounding factors. It showed that the body habitus may not have an independent effect on in-hospital outcome. On the other hand, it is possible that the number of patients who underwent the haematoma evacuation was too small to indicate a difference in in-hospital outcome between patient stratified by body habitus. Further, the large and prospective studies on this subgroup of patients with ICH are necessary. We also performed the sensitivity analysis using the WHO criteria and did not change our results.
As a majority of Chinese hospitals participate in this programme, CSCA database with many important advantages provided us with the strength to investigate the association between body habitus and outcomes after ICH. The inclusion of patients from different hospitals across China limited bias inherent in studies. Compared with the single-centre study, multicentre study can evaluate the impact of body habits on outcomes. Data, including several medical complications, provide more detailed assessments of ICH outcomes beyond mortality and functional status. However, there are some limitations in our study. First, this study is a retrospective study in the single ethnic population, which limits the generalisation of findings. Further evaluation of patients in other races might also be required. Second, we could not have access to data in patients with ICH such as the volume, location of haematoma and the subtype of ICH. Although we performed the subgroup analysis concerning the patients who underwent the haematoma evacuation, further study is necessary to discuss the impact of these variables on the outcome of ICH. Third, we did not collect information on waist circumference or hip circumference in the CSCA study. Waist to hip ratio or waist circumference in addition to the BMI could be better correlated to the findings than BMI alone. Fourth, in the acute phase, patients with ICH should absolutely rest in bed. Height and weight were self-reported by patients or legal proxies on admission without measurement. Finally, the CSCA database includes only inpatient data without follow-up information. Long-term mortality rate and function outcome could not be evaluated.
Conclusions
In patients with ICH, being underweight was associated with increased in-hospital mortality. Being underweight and obese can both increased risk of in-hospital complications compared with having normal weight. Further prospective cohort studies are needed to evaluate the relationship between BMI and outcomes in patients with ICH. Clinically we should also focus on underweight patients, which is also an increasing group by older age with assumed high risk for medical complications and mortality.
Footnotes
Contributors: YW, XZ and ZL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. ZC and XL contributed to the study concept and design. HG and YJ analysed the data. ZC and XL drafted the manuscript. All authors have read and approved the final manuscript.
Funding: This research was supported by National Key R&D Program of China (2017YFC1310901, 2018YFC1705003), Beijing Municipal Science & Technology Commission (code: D171100003017002), National Science and Technology Major Project (2017ZX09304018), Beijing Municipal Administration of Hospitals’ Mission Plan (code: SML20150502), and Beijing Municipal Administration of Hospitals’ Ascent Plan (code: DFL20150501).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. All data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Participating hospitals received either healthcare quality assessment and research approval to collect data in the CSCA project without requiring individual patient informed consent under the common rule or a waiver of authorisation and exemption from subsequent review by their Institutional Review Board.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet 2014;384:766–81. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzano LA, Gu D, Whelton MR, et al. Body mass index and risk of stroke among Chinese men and women. Ann Neurol 2010;67:11–20. 10.1002/ana.21950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis 2018;61:151–6. 10.1016/j.pcad.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 4.Han SJ, Boyko EJ. The evidence for an obesity paradox in type 2 diabetes mellitus. Diabetes Metab J 2018;42:179–87. 10.4093/dmj.2018.0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryu W-S, Lee S-H, Kim CK, et al. Body mass index, initial neurological severity and long-term mortality in ischemic stroke. Cerebrovasc Dis 2011;32:170–6. 10.1159/000328250 [DOI] [PubMed] [Google Scholar]
- 6.Kim BJ, Lee S-H, Jung K-H, et al. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology 2012;79:856–63. 10.1212/WNL.0b013e318266fad1 [DOI] [PubMed] [Google Scholar]
- 7.Sun W, Huang Y, Xian Y, et al. Association of body mass index with mortality and functional outcome after acute ischemic stroke. Sci Rep 2017;7:2507. 10.1038/s41598-017-02551-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao L, Du W, Zhao X, et al. Favorable functional recovery in overweight ischemic stroke survivors: findings from the China national stroke Registry. J Stroke Cerebrovasc Dis 2014;23:e201–6. 10.1016/j.jstrokecerebrovasdis.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 9.Feigin VL, Lawes CMM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–69. 10.1016/S1474-4422(09)70025-0 [DOI] [PubMed] [Google Scholar]
- 10.Sacco S, Marini C, Toni D, et al. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 2009;40:394–9. 10.1161/STROKEAHA.108.523209 [DOI] [PubMed] [Google Scholar]
- 11.Krishnamurthi RV, Moran AE, Forouzanfar MH, et al. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart 2014;9:101–6. 10.1016/j.gheart.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 12.Kim BJ, Lee S-H, Ryu W-S, et al. Paradoxical longevity in obese patients with intracerebral hemorrhage. Neurology 2011;76:567–73. 10.1212/WNL.0b013e31820b7667 [DOI] [PubMed] [Google Scholar]
- 13.Dangayach NS, Grewal HS, De Marchis GM, et al. Does the obesity paradox predict functional outcome in intracerebral hemorrhage? J Neurosurg 2018;129:1125–9. 10.3171/2017.5.JNS163266 [DOI] [PubMed] [Google Scholar]
- 14.Dehlendorff C, Andersen KK, Olsen TS. Body mass index and death by stroke: no obesity paradox. JAMA Neurol 2014;71:978–84. 10.1001/jamaneurol.2014.1017 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Li Z, Wang Y, et al. Chinese stroke center alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol 2018;3:256–62. 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization WH . The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia. [Google Scholar]
- 17.WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 18.Carnethon MR, De Chavez PJD, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012;308:581–90. 10.1001/jama.2012.9282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu H-Q, Li D-J, Liu C, et al. %ggBaseline: a SAS macro for analyzing and reporting baseline characteristics automatically in medical research. Ann Transl Med 2018;6:326. 10.21037/atm.2018.08.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin H, Chen Y, Liu G, et al. Management characteristics and prognosis after stroke in China: findings from a large nationwide stroke Registry. Stroke Vasc Neurol 2020. 10.1136/svn-2020-000340. [Epub ahead of print: 22 Jun 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H. Universal health insurance coverage for 1.3 billion people: what accounts for China's success? Health Policy 2015;119:1145–52. 10.1016/j.healthpol.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Wang C, Zhao X, et al. Substantial progress yet significant opportunity for improvement in stroke care in China. Stroke 2016;47:2843–9. 10.1161/STROKEAHA.116.014143 [DOI] [PubMed] [Google Scholar]
- 23.Hoffman H, Jalal MS, Furst T, et al. The obesity paradox in spontaneous intracerebral hemorrhage: results from a retrospective analysis of the nationwide inpatient sample. Neurocrit Care 2020;32:765–74. 10.1007/s12028-019-00796-3 [DOI] [PubMed] [Google Scholar]
- 24.Sun W, Xian Y, Huang Y, et al. Obesity is associated with better survival and functional outcome after acute intracerebral hemorrhage. J Neurol Sci 2016;370:140–4. 10.1016/j.jns.2016.09.029 [DOI] [PubMed] [Google Scholar]
- 25.Persaud SR, Lieber AC, Donath E, et al. Obesity paradox in intracerebral hemorrhage. Stroke 2019;50:999–1002. 10.1161/STROKEAHA.119.024638 [DOI] [PubMed] [Google Scholar]
- 26.Hubert HB, Feinleib M, McNamara PM, et al. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham heart study. Circulation 1983;67:968–77. 10.1161/01.CIR.67.5.968 [DOI] [PubMed] [Google Scholar]
- 27.Towfighi A, Ovbiagele B. The impact of body mass index on mortality after stroke. Stroke 2009;40:2704–8. 10.1161/STROKEAHA.109.550228 [DOI] [PubMed] [Google Scholar]
- 28.Bell CL, LaCroix A, Masaki K, et al. Prestroke factors associated with poststroke mortality and recovery in older women in the women's health Initiative. J Am Geriatr Soc 2013;61:1324–30. 10.1111/jgs.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khaodhiar L, McCowen KC, Blackburn GL. Obesity and its comorbid conditions. Clin Cornerstone 1999;2:17–31. 10.1016/S1098-3597(99)90002-9 [DOI] [PubMed] [Google Scholar]
- 30.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med 2005;118:978–80. 10.1016/j.amjmed.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 31.Borch KH, Nyegaard C, Hansen J-B, et al. Joint effects of obesity and body height on the risk of venous thromboembolism: the Tromsø study. Arterioscler Thromb Vasc Biol 2011;31:1439–44. 10.1161/ATVBAHA.110.218925 [DOI] [PubMed] [Google Scholar]
- 32.Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–7. 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 33.Pezzini A, Grassi M, Paciaroni M, et al. Obesity and the risk of intracerebral hemorrhage: the multicenter study on cerebral hemorrhage in Italy. Stroke 2013;44:1584–9. 10.1161/STROKEAHA.111.000069 [DOI] [PubMed] [Google Scholar]
- 34.Braekkan SK, van der Graaf Y, Visseren FLJ, et al. Obesity and risk of bleeding: the smart study. J Thromb Haemost 2016;14:65–72. 10.1111/jth.13184 [DOI] [PubMed] [Google Scholar]
- 35.Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756–63. 10.1161/01.STR.0000190056.76543.eb [DOI] [PubMed] [Google Scholar]
- 36.Iwuchukwu I, Mahale N, Ryder J, et al. Racial differences in intracerebral haemorrhage outcomes in patients with obesity. Obes Sci Pract 2018;4:268–75. 10.1002/osp4.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2020-000534supp001.pdf (83.8KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.