The prognostic value of additional copies of chromosome 1q remains debated. To address this uncertainty, we performed a validation and meta-analysis of gain(1q) (3 copies) and amp(1q) (≥4 copies) in 2,596 patients with newly diagnosed multiple myeloma (NDMM) from three phase III trials. Gain(1q) and amp(1q) were both associated with shorter progression-free survival (hazard ratio [HR]=1.50, 95% confidence interval [95% CI]: 1.16-1.95, P=0.002 and HR=1.65, 95% CI: 1.25-2.19, P=4.8x10-4, respectively) and overall survival (HR=1.85, 95% CI: 1.43-2.39, P=2.6x10-6 and HR=2.28, 95% CI: 1.42-3.64, P=5.8x10-4) by meta-analysis as well as in each trial individually; there was no statistically significant difference in outcome between the two copy number states. Gain(1q)/amp(1q) was independently prognostic in the context of the Revised International Staging System (R-ISS) and refined risk prediction by enabling identification of ultra high-risk tumors across trials.
Additional copies of 1q21 are one of the commonest genetic abnormalities in multiple myeloma;1 however their value as a prognostic marker remains controversial. While several studies showed that 1q21 gain is an independent poor prognostic factor, other studies have failed to support a relationship.2-7 Previous studies have often been small or conducted outside of clinical trials, thus having limited power to demonstrate a relationship, especially as assays can be complicated by heterogeneity in terms of copy number, i.e., gain versus amp(1q).6 In contrast to t(4;14) or del(17p), 1q21 status is not included among the high-risk markers listed by the International Myeloma Working Group’s R-ISS,8 and as a result it has invariably not been reported in most clinical trials over the past decade. Its prognostic relevance in the context of modern therapies is hence poorly defined.
To examine the relationship between gain(1q) and amp(1q) and prognosis and to address shortcomings in earlier studies we investigated 2,596 NDMM trial patients receiving controlled therapy with a proteasome inhibitor or an immunomodulatory drug.
We included patients from three independent phase III trials of NDMM with comparable baseline characteristics for validation purposes (Table 1), comprising the German-speaking Myeloma Multicenter Group (GMMG) HD4 trial (n=341, median follow-up 93 months; EudraCT 2004-000944-26), the GMMG MM5 trial (n=539, 58 months; EudraCT 2010-019173-16) and the UK National Cancer Research Institute (NCRI) Myeloma XI trial (MyXI, n=1,716, 65 months; NCT01554852); the designs and main outcomes of these trials have been previously reported.9-11 All patients provided written informed consent. GMMG trials were approved by ethics committees of the University of Heidelberg and all participating sites, and MyXI was approved by the UK South Central ethics committee (reference 09/H0604/79), research ethics committees at participating centers and the UK Medicines and Healthcare Products Regulatory Agency.
Table 1.
Clinical and laboratory characteristics in relation to 1q status in the GMMG and Myeloma XI trials.
Figure 1.
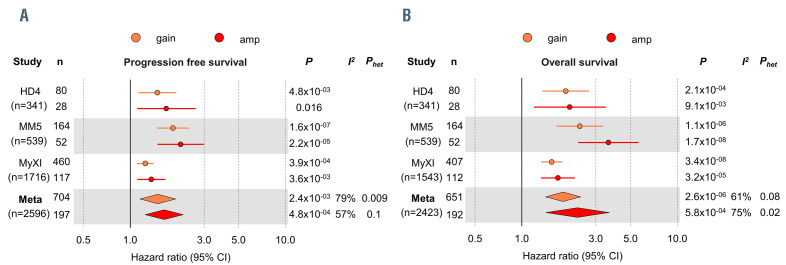
Prognostic impact of gain and amplification of 1q21 in multiple myeloma. Forest plot of a meta-analysis for (A) progression-free survival and (B) overall survival for gain(1q) (orange) and amp(1q) (red), validating the prognostic impact of both lesions in the independent GMMG HD4 and MM5 and the NCRI Myeloma XI trials. Column “n” shows the number of patients with gain(1q) or amp(1q) per trial. The total number of patients included per trial is shown in brackets in the column “study”. Circles show hazard ratio (HR) point estimates and lines indicate the 95% confidence intervals (95% CI). Diamonds depict summary hazard ratios computed under a random-effects model, with 95% confidence intervals given by their width. Unbroken vertical lines represent the null value (HR = 1.0).
For GMMG, interphase fluorescence in situ hybridization analysis was performed as described previously, with a cut-off of 10% for calling 1q abnormalities.12 For MyXI, multiplexed quantitative reverse transcriptase polymerase chain reaction analysis was used to determine translocation status, and multiplex ligation-dependent probe amplification (MRC Holland) to call copy number aberrations, with a cut-off equivalent to 20% for calling aberrations, as previously described.2
The association between categorical and continuous variables was examined using the Fisher exact test and the Wilcoxon rank test, respectively. Progression-free survival was defined as time from enrollment to progression, according to International Myeloma Working Group criteria, 13 or death of any cause. Overall survival was time from enrollment to death of any cause. The Kaplan- Meier method was used for survival analyses. Cox proportional hazards regression was used to estimate hazard ratios and 95% confidence intervals. Meta-analysis was performed using summary statistics under a random effect model. The Cochran Q and I2 statistics were calculated to test for heterogeneity, with I2 ≥75% being considered substantial heterogeneity. All analyses were performed using R version 3.6.3.
The frequencies of 1q21 abnormalities were consistent between GMMG (HD4 and MM5 combined) and MyXI trial patients, with gain(1q) being seen in 28% and 27%, and amp(1q) detectable in 9% and 7% of patients, respectively. Laboratory parameters indicative of aggressive disease were associated with both gain and amp(1q), including reduced hemoglobin and platelet levels, elevated plasma creatinine and stage III of the ISS and R-ISS (Table 1). Associations were stronger for amp(1q) for platelet levels, and stage III of the ISS and R-ISS. Translocations t(4;14) and t(14;16) were enriched in gain and amp(1q), the association between amp(1q) and t(4;14) being stronger.
Not surprisingly, given that amp(1q) was associated with aggressive disease, it had a negative impact on outcome (Figure 1). However, individually per trial and by meta-analysis gain(1q) was independently associated with poor outcome, too, with no discernible difference from amp(1q) and markedly overlapping confidence intervals, despite the significant size of the cohorts and long-term follow-up. For progression-free survival, the meta-analysis hazard ratios and 95% confidence intervals were 1.50 (1.16-1.95), P=0.002 for gain(1q), and 1.65 (1.25-2.19), P<0.001 for amp(1q). The respective values for overall survival were 1.85 (1.43-2.39), P<0.001 and 2.28 (1.42-3.64), P<0.001, respectively. We observed moderate to substantial heterogeneity, since the effect sizes differed between trials, with GMMG-MM5 showing the highest hazard ratios for both gain and amp(1q), yet consistent similarity in outcomes between the 1q copy number states, validating our finding in three independent datasets.
Our findings on gain(1q) are in contrast to recently published data suggesting that only amp(1q) is a prognostic marker, but in line with reports from other groups.4-6 Technical variability in calling 1q status and, in particular, differences in follow-up time may account for some of these discrepancies: for HD4, a previously published analysis with shorter follow-up suggested inferior outcome for amp(1q) over gain(1q).12 However, with extended follow-up shown here these differences levelled out as relapses in the gain(1q) group accumulated over time. Similar effects were observed for shorter versus extended observation time in MyXI. This is in line with the ongoing evolution of 1q aberrations, which have been shown to be of clinical significance.3,14 Of note, a recent study describing significant differences between amp(1q) and gain(1q) only had a median follow-up of less than 2 years, which is short for exploratory survival analyses in NDMM.6
To examine the impact of different therapies on 1q copy number aberrations, we performed landmark analyses from the start of maintenance (Online Supplementary Figure S1). There was no significant difference between gain and amp(1q) for arm A (thalidomide) or arm B (bortezomib) of HD4, both being associated with adverse outcome. The same held true for MM5 and MyXI, in which patients received lenalidomide maintenance for 2 years or until progression in the respective treatment arms. Together, gain and amp(1q21) had a similar prognostic impact and neither ongoing bortezomib nor immunomodulatory drug therapy could mitigate it. This is in keeping with reports on the significance of gain(1q) in the context of different induction therapies.4,5 Since in summary these results did not demonstrate a significant difference in outcome between gain and amp(1q), we subsequently analyzed 1q copy number aberrations under the overarching label ‘gain(1q)’.
Figure 2.
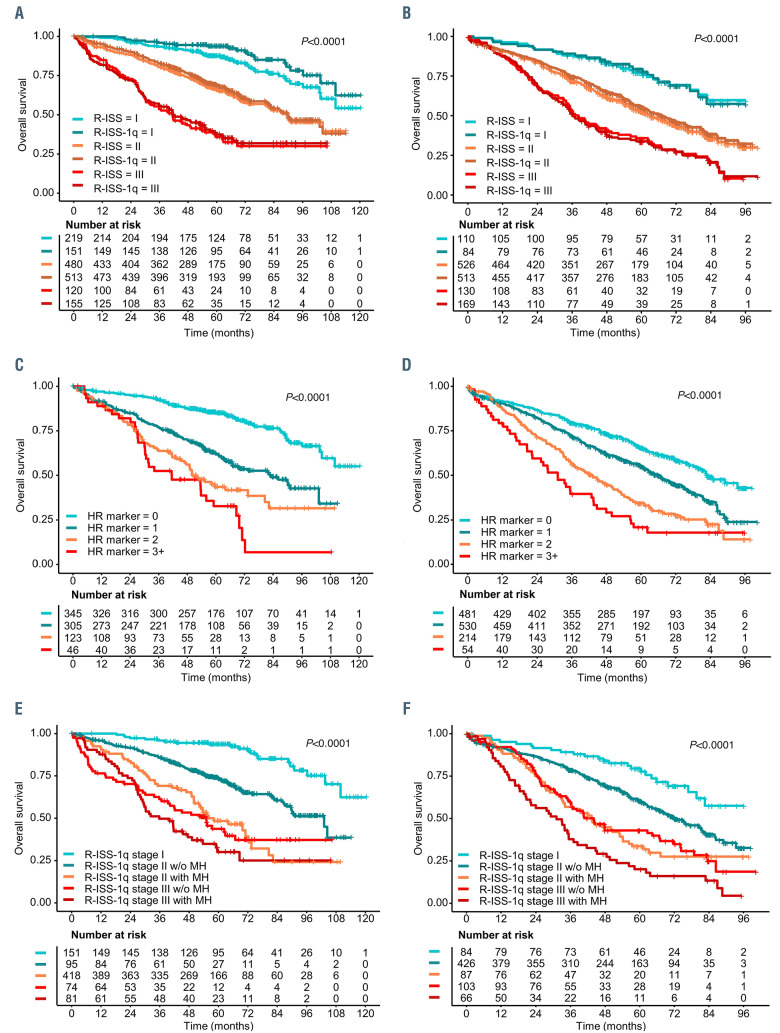
Prognostic impact of gain(1q) in the context of and in combination with other risk markers in multiple myeloma. (A, B) Kaplan-Meier overlay plots demonstrate the impact of including gain(1q) as a risk marker in the Revised International Staging System (R-ISS), termed R-ISS-1q. Plots show overall survival for R-ISS and for R-ISS-1q for the GMMG trials (A) and the Myeloma XI trial (B). (C, D) Kaplan-Meier plots are shown for overall survival of patients in the GMMG trials (C) and Myeloma XI trial (D) according to the number of risk markers present in these patients, including gain(1q) and R-ISS markers del(17p), t(4;14), t(14;16) or lactate dehydrogenase above the upper limit of normal. (E, F) Kaplan-Meier plots for overall survival showing discrimination of high- and ultra-highrisk groups by including information on co-occurrence of risk markers, called multi-hits (MH), for further subgrouping of R-ISS-1q stage II and stage III tumors in GMMG trials (E) and the Myeloma XI trial (F). The corresponding progression-free survival plots are presented in the Online Supplementary Data.
To examine if gain(1q) is independent of the R-ISS, we performed multivariate Cox-regression analyses, including R-ISS risk markers individually. By meta-analysis, gain(1q) was associated with both progression-free and overall survival (progression-free survival: HR=1.42 [95% CI: 1.11-1.81], P=0.005; overall survival HR=1.68 [95% CI: 1.21-2.32], P=0.002) (Online Supplementary Table S1). The same held true for all R-ISS markers. Having established its independent impact, we investigated the additional value gain(1q) could bring to the R-ISS. Considering gain(1q) as an equivalent risk marker in the R-ISS, termed R-ISS-1q, 68/219 GMMG and 29/125 MyXI patients were upstaged from stage I to stage II and 35/480 GMMG and 46/600 MyXI patients from stage II to stage III, with nearly identical outcome discrimination between groups compared to that based on the R-ISS. The median progression-free survival for R-ISS-1q was 55.4 (GMMG) and 45.3 (MyXI) months for stage I, 35.7 and 28.5 months for stage II, and 21.5 and 18.4 months for stage III. The respective overall survival values were not reached (stage I), 89.7/67.2 months (stage II) and 41.9/36.3 months (stage III) (Figure 2, Online Supplementary Figure S2).
In the current R-ISS, all patients with ISS II are assigned to stage II, irrespective of the presence or number of risk markers. However, consistently across trials and in line with other data,15 we found an increasingly adverse outcome, the more risk markers, including gain(1q), t(4;14), t(14;16), del(17p) and lactate dehydrogenase, a patient’s tumor showed (Online Supplementary Figure S2). Specifically, patients with two or more co-occurring tumor risk markers (also called hits) had significantly worse outcome than those with a single marker in isolation. Combining this information with the R-ISS-1q, cooccurrence of two or more markers identified ~18% of stage II patients with significantly poorer outcome than the general stage II group (GMMG: median progressionfree survival 26.4 [95% CI: 22.9-34.5] months; MyIX: 19.6 [95% CI: 17.0-29.4] months) (Figure 2, Online Supplementary Figure S2). R-ISS-1q stage III patients with multi-hits had very poor outcome (GMMG: median progression- free survival 18.5 [95% CI: 14.9-25.9] months; MyXI: 15.9 [95% CI: 11.8-20.0] months). Although multi-hit tumors have been recognized as a predictor of ultra high-risk disease,15 they have not been investigated in the context of R-ISS and are not assessed or reported in the majority of clinical trials to date. Our validation of multi-hits in multiple trial cohorts supports wider reporting, with all markers being accessible through standard fluorescence in situ hybridization diagnostics.
In conclusion, gain(1q) is associated with inferior survival in NDMM, irrespective of current standard therapies, and should be considered as an independent risk factor. Whether additional risk factors may also refine risk prediction will be the subject of future studies and their useful integration subject to international consensus, taking accessibility to testing into account, which is well established for gain(1q). While the interaction of novel immunotherapies such as bispecific antibodies or chimeric antigen receptor T cells with tumor biology may differ, inclusion of gain(1q) testing should be considered in their clinical development. Our data support integration of gain(1q) and the concept of multi-hits in future consensus risk prediction frameworks for individualizing care and improving tailored management of NDMM patients.
Supplementary Material
Acknowledgments
The authors are grateful for the support of the Clinical Trials Research Unit (CTRU) at Leeds and the NCRI Haematological Oncology Clinical Studies Group. The GMMG thanks the Koordinierungszentrum für Klinische Studien (KKS) Heidelberg for support of the trial and data monitoring and all participating investigators, centers, patients and their families.
Funding Statement
Funding: NCRI Myeloma XI-related work was supported by research grants from Myeloma UK and infrastructure support from the National Institute of Health Biomedical Research Centre at the Royal Marsden Hospital and Institute of Cancer Research, London. Martin Kaiser was supported by a Jacquelin Forbes-Nixon Fellowship. Primary financial support for NCRI Myeloma XI was provided by Cancer Research UK (C1298/A10410). The GMMG MM5 trial was supported by Celgene, Chugai, Janssen-Cilag and The Binding Site. The GMMG HD4 trial corresponds to the German part of the joint HOVON-65/GMMG-HD4 trial, which was supported by the Dutch Cancer Foundation, by the German Federal Ministry of Education and Research, and by a grant from Janssen Cilag. The GMMG also received grants for this trial from Novartis, Amgen, Chugai, Roche, and the Tumorzentrum Heidelberg/Mannheim.
References
- 1.Manier S, Salem KZ, Park J, Landau DA, Getz G, Ghobrial IM. Genomic complexity of multiple myeloma and its clinical implications. Nat Rev Clin Oncol. 2017;14(2):100-113. [DOI] [PubMed] [Google Scholar]
- 2.Shah V, Sherborne AL, Walker BA, et al. Prediction of outcome in newly diagnosed myeloma: a meta-analysis of the molecular profiles of 1905 trial patients. Leukemia. 2018;32(1):102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft J, Ellis S, Sherborne AL, et al. Copy number evolution and its relationship with patient outcome-an analysis of 178 matched presentation- relapse tumor pairs from the Myeloma XI trial. Leukemia. 2021;35(7):2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt TM, Barwick BG, Joseph N, et al. Gain of chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J. 2019;9(12):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdallah N, Greipp P, Kapoor P, et al. Clinical characteristics and treatment outcomes of newly diagnosed multiple myeloma with chromosome 1q abnormalities. Blood Adv. 2020;4(15):3509-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker BA, Mavrommatis K, Wardell CP, et al. A high-risk, doublehit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia. 2019;33(1):159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrot A, Lauwers-Cances V, Tournay E, et al. Development and validation of a cytogenetic prognostic index predicting survival in multiple myeloma. J Clin Oncol. 2019;37(19):1657-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldschmidt H, Lokhorst HM, Mai EK, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018; 32(2):383-390. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt H, Mai EK, Dürig J, et al. Response-adapted lenalidomide maintenance in newly diagnosed myeloma: results from the phase III GMMG-MM5 trial. Leukemia. 2020;34(7):1853-1865. [DOI] [PubMed] [Google Scholar]
- 12.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940-948. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Harousseau J-L, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011; 117(18):4691-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinhold N, Ashby C, Rasche L, et al. Clonal selection and doublehit events involving tumor suppressor genes underlie relapse in myeloma. Blood. 2016;128(13):1735-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayo Clinic. Mayo Clinic mSMART: Risk adapted approach to management of multiple myeloma and related disorders. mSMART 3.0. https://www.msmart.org/s/Treatment-of-Newly-Diagnosed-Myelomav18_June-20FINAL.pdf (accessed January 18, 2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.